Abstract
The American Society of Colon and Rectal Surgeons (ASCRS) and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) are dedicated to ensuring high-quality innovative patient care for surgical patients by advancing the science, prevention, and management of disorders and diseases of the colon, rectum, and anus as well as minimally invasive surgery. The ASCRS and SAGES society members involved in the creation of these guidelines were chosen because they have demonstrated expertise in the specialty of colon and rectal surgery and enhanced recovery. This consensus document was created to lead international efforts in defining quality care for conditions related to the colon, rectum, and anus and develop clinical practice guidelines based on the best available evidence. While not proscriptive, these guidelines provide information on which decisions can be made and do not dictate a specific form of treatment. These guidelines are intended for the use of all practitioners, healthcare workers, and patients who desire information about the management of the conditions addressed by the topics covered in these guidelines. These guidelines should not be deemed inclusive of all proper methods of care nor exclusive of methods of care reasonably directed toward obtaining the same results. The ultimate judgment regarding the propriety of any specific procedure must be made by the physician in light of all the circumstances presented by the individual patient. This clinical practice guideline represents a collaborative effort between the American Society of Colon and Rectal Surgeons (ASCRS) and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) and was approved by both societies.
Keywords: Enhanced recovery, Colorectal surgery, ERAS
Statement of the problem
Colorectal surgery has historically been associated with long postoperative hospital stays, high costs, and surgical site infection rates approaching 20% [1, 2]. In addition, the incidence of in-hospital perioperative nausea and vomiting (PONV) may be as high as 80% [3] and readmission rates are as high as 35% [4]. Enhanced recovery protocols (ERPs) are a set of standardized perioperative processes, the content of which may vary significantly, that are applied to patients undergoing elective surgery. In general, these protocols are not intended for non-elective cases, but components of ERPs could certainly be applied to the emergent/urgent patient [5, 6]. Also known as “fast track” or “enhanced recovery after surgery” (ERAS) protocols, enhanced recovery protocols are designed to improve patient outcomes [7]. Outcomes of interest include alleviating nausea and pain, achieving early return of bowel function, and decreasing rates of wound infection and length of hospital stay [8]. Although numerous perioperative protocols exist, this clinical practice guideline will evaluate the evidence in support of individual measures to improve patient outcomes after elective colon and rectal resections.
Implementation of ERPs in colorectal surgery has been shown to reduce morbidity rates and decrease length of stay without increasing readmission rates [9–13]. A 2011 Cochrane review found that ERPs were associated with reduced overall complication rates and length of stay when compared to conventional perioperative patient management [14]. Subsequent studies have shown that ERPs are associated with reduced healthcare costs, improved patient satisfaction, lower rates of complications, and reduced mortality [2, 10, 15–20]. ERPs are also associated with improved outcomes regardless of whether patients undergo laparoscopic or open surgery [21]. In addition, multiple studies have shown that ERPs are safe and efficacious in elderly patient populations [22–30]. Studies also support that ERPs should not be implemented and maintained dogmatically, but rather require ongoing compliance evaluation and continual quality improvement [31–34]. Greater adherence to ERPs is associated with decreased complications and shorter length of stay (LOS) [35–38].
There are many different preoperative, intraoperative, and postoperative components of a typical ERP and it is difficult to identify which are most beneficial within the “bundle” of simultaneously implemented measures. This clinical practice guideline will evaluate the evidence pertaining to different components of ERPs for colorectal surgery. While ostomy surgery, deep vein thrombosis (DVT) prevention, bowel preparation, and frailty are discussed in this CPG, a detailed review of these topics is beyond the scope of this Clinical Practice Guideline; these topics are addressed in depth in other ASCRS Clinical Practice Guidelines [39–42].
Methodology
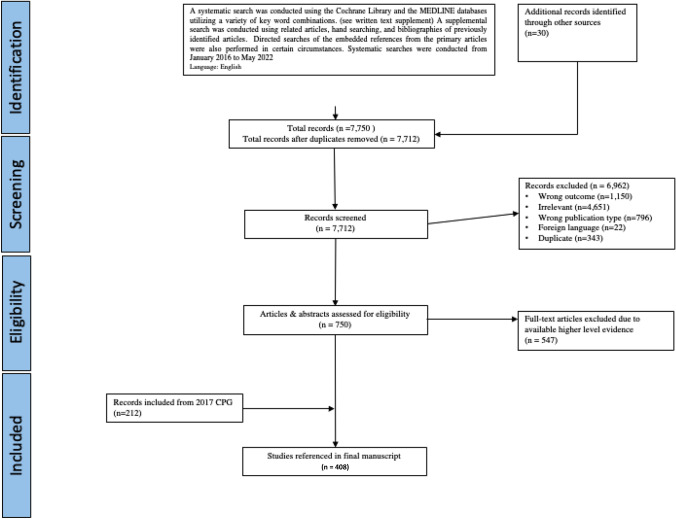
The original clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and the Society of American Gastrointestinal and Endoscopic surgeons were published in 2017 [43]. The present guideline was constructed using the 2017 guidelines as a platform. Compared with 2017, this guideline has 3 new recommendations and 5 statements with updated levels of evidence. All other statements have been reviewed and updated with current evidence (Table 1). A systematic search was conducted under the guidance of a librarian. The details of specific search strategies including search terms, inclusion criteria, exclusion criteria, and total number of studies identified, and tables of evidence for each statement are available in the digital supplemental materials. In brief, a systematic search from January 1, 2016 to May 1, 2022 was conducted using the Cochrane Library, EMBASE, and the MEDLINE databases utilizing a variety of key word combinations. A supplemental search was conducted using related articles and bibliographies of previously identified articles. Directed searches of the embedded references from the primary articles were also performed in certain circumstances. Prospective, randomized controlled trials (RCTs) and meta-analyses were given preference. A total of 7,712 abstracts were identified; 6,962 articles were excluded and a total of 750 full-text articles were evaluated. Of those, 547 were excluded, and along with 212 articles from the 2017 guidelines, a total of 415 articles were included in the final document (Fig. 1). The final grade of recommendation was performed using the Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) system (Table 2) [44]. When agreement was incomplete regarding the evidence base or treatment guideline, consensus from the committee chair, vice chair, and 2 assigned reviewers determined the outcome. Members of the ASCRS Clinical Practice Guidelines Committee worked together with members of the SAGES Colorectal Committee from inception to publication. Recommendations formulated by the subcommittee were reviewed by the entire Clinical Practice Guidelines Committee of ASCRS and the Colorectal Committee of SAGES. The submission was approved by both the ASCRS and SAGES executive council and then peer reviewed by the Diseases of the Colon & Rectum and Surgical Endoscopy. In general, each ASCRS Clinical Practice Guideline (including joint guidelines) is updated every 5 years. No funding was received for preparing this guideline and the authors have declared no competing interests related this material. This guideline conforms to the Appraisal of Guidelines for Research and Evaluation (AGREE) checklist.
Table 1.
What is New in the 2022 ASCRS Enhanced Recovery After Colon and Rectal Surgery Clinical Practice Guidelines?
| New recommendations | |
| Preoperative interventions | 5. Oral nutritional supplementation is recommended in malnourished patients prior to elective colorectal surgery. Weak recommendation based on moderate quality evidence, 2B |
| Preadmission nutrition and bowel prep | |
| Perioperative interventions | 15. Hypotension should be avoided as even short durations of MBP < 65 are associated with adverse outcomes, in particular myocardial injury, and acute kidney injury. Grade of recommendation: Strong recommendation based on moderate quality evidence, 1B |
| Intraoperative fluid management | |
| Postoperative interventions | 26. Early discharge prior to return of bowel function may be feasible in low-risk patients undergoing minimally invasive colectomy when coupled with close outpatient communication and follow-up. Grade of Recommendation: Weak recommendation based on moderate quality evidence, 2B |
| Discharge criteria | |
| Updated recommendations | |
| Preadmission | 6. Mechanical bowel preparation combined with preoperative oral antibiotics is typically recommended prior to elective colorectal resection. Grade of Recommendation: Strong recommendation based on moderate-quality evidence, 1B |
| Pain control | 11. Thoracic epidural analgesia, while not recommended for routine use in laparoscopic colorectal surgery, is an option for open colorectal surgery if a dedicated acute pain team is available for postoperative management. Recommendation: Strong recommendation based on moderate-quality evidence, 1B |
| Fluid management | 13. Fluid administration should be tailored to avoid excessive fluid administration and volume overload or undue fluid restriction and hypovolemia. Grade of Recommendation: Strong recommendation based on high-quality evidence, 1A |
| Fluid management | 14. Balanced chloride-restricted crystalloid solutions should be used for maintenance infusions and fluid boluses in patients undergoing colorectal surgery. There is no benefit to the routine use of colloid solutions for fluid boluses. Grade of Recommendation: Strong recommendation based on moderate-quality evidence, 1B |
| Fluid management | 16. In high-risk patients and in patients undergoing colorectal surgery with significant intravascular losses, the use of goal-directed hemodynamic therapy may be considered. Grade of Recommendation: Weak recommendation, based on moderate-quality evidence, 2B |
| Postoperative management | 25. Urinary catheters should typically be removed within 24–48 h after mid/lower rectal resection. Grade of Recommendation: Strong recommendation based on moderate quality evidence, 1B |
Fig. 1.
PRISMA literature search flow sheet. PRISMA preferred reporting items for systematic reviews and meta-analysis
Table 2.
The GRADE system—grading recommendations
| Grade | Description | Benefit versus risk and burdens | Methodologic quality of supporting evidence | Implications |
|---|---|---|---|---|
| 1A | Strong recommendation, high-quality evidence | Benefits clearly outweigh risk and burdens or vice versa | RCTs without important limitations or overwhelming evidence from observational studies | Strong recommendation can apply to most patients in most circumstances without reservation |
| 1B | Strong recommendation, moderate-quality evidence | Benefits clearly outweigh risk and burdens or vice versa | RCTs with important limitations (inconsistent results, methodologic flaws, indirect or imprecise) or exceptionally strong evidence from observational studies | Strong recommendation, can apply to most patients in most circumstances without reservation |
| 1C | Strong recommendation, low- or very low-quality evidence | Benefits clearly outweigh risk and burdens or vice versa | Observational studies or case series | Strong recommendation but may change when higher-quality evidence becomes available |
| 2A | Weak recommendation, high-quality evidence | Benefits closely balanced with risks and burdens | RCTs without important limitations or overwhelming evidence from observational studies | Weak recommendation, best action may differ depending on circumstances or patients’ or societal values |
| 2B | Weak recommendations, moderate-quality evidence | Benefits closely balanced with risks and burdens | RCTs with important limitations (inconsistent results, methodologic flaws, indirect or imprecise) or exceptionally strong evidence from observational studies | Weak recommendation, best action may differ depending on circumstances or patients’ or societal values |
| 2C | Weak recommendation, low- or very low-quality evidence | Uncertainty in the estimates of benefits, risks, and burden; benefits, risk, and burden may be closely balanced | Observational studies or case series | Very weak recommendations; other alternatives may be equally reasonable |
GRADE Grades of Recommendation, Assessment, Development, and Evaluation, RCT randomized controlled trial. Adapted from Guyatt G, Gutermen D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest. 2006;129:174-181. Used with permission
Preoperative interventions
Preadmission counseling
A preoperative discussion regarding clinical milestones and discharge criteria should typically be performed prior to surgery. Grade of recommendation: strong recommendation based on low-quality evidence, 1C
Preadmission counseling regarding milestones and discharge criteria are a well-established cornerstone of ERPs [7, 45–50]. Single-center case series, prospective cohort studies, systematic reviews, and RCTs have reported the benefits of using an ERP that includes preoperative education describing milestones and discharge criteria. [2, 51–72]. Furthermore, compliance with an ERP that includes preoperative patient education is associated with decreased length of stay and decreased complication rates [31, 73–79]. Despite the benefit, in-person preoperative counseling can be resource intensive, which may limit its widespread use; prescripted phone calls may provide sufficient counseling while saving resources [78, 79].
Patients undergoing ileostomy creation should receive stoma teaching and counseling regarding how to avoid dehydration. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
The creation of an ostomy is an independent risk factor for prolonged length of stay after colorectal surgery [50, 80–83]. Several single-center and multicenter studies as well as a systematic review have shown that structured patient stoma education significantly improves quality of life, facilitates psychosocial adjustment, and reduces hospital length of stay and hospital costs [84–94].
Ostomy education can also impact readmission rates [80, 95–97]. As dehydration is the most common cause of readmission following ileostomy creation [98, 99], counseling patients regarding dehydration avoidance is an important element of ERP [98, 99]. In a prospective study of 42 patients versus 168 historical controls, implementation of an ileostomy pathway in which patients were directly engaged in ostomy management, discharged with supplies for measuring input/output, and set up with visiting nurse services reduced the readmission rate for dehydration from 15.5 to 0% (p = 0.02) [4]. Others have reported similar reductions in readmission rates for dehydration when utilizing an ERP focused on ostomy education [100–102]. Gonella et al. in a retrospective study of 296 patients showed that the hospital readmission rate within 30-day postdischarge for dehydration dropped from 9 to 3.9% after protocol application [101].
Preadmission nutrition and bowel preparation
Clear liquids may be continued up to 2 h prior to general anesthesia. Grade of recommendation: strong recommendation based on high-quality evidence, 1A
Drinking clear fluids up to 2 h before induction of anesthesia, according to data from multiple RCTs, is safe and improves patients’ sense of well-being [103–111]. The same RCTs have also reported that ingesting clear liquids within 2–4 h of surgery versus > 4 h is associated with smaller gastric volumes and higher gastric pH at the time of surgery. The current practice guidelines of both the American Society of Anesthesiologists and the European Society of Anesthesiology support this recommendation [111–113].
Carbohydrate loading should be encouraged prior to surgery in non-diabetic patients. Grade of recommendation: weak recommendation based on moderate-quality evidence, 2B
The use of carbohydrate (CHO)-rich beverages should be encouraged to attenuate insulin resistance induced by surgery and starvation [114–116]. The focus is not on avoiding glycogen depletion, but rather on converting the patient from a fasting state to a fed stated to impact insulin resistance. A 2014 Cochrane review of 27 international trials, including 1,976 patients undergoing elective operations, concluded carbohydrate loading was associated with a 0.3-day reduction in length of hospital stay when compared with placebo or fasting (95% CI 0.56 to 0.0) but no difference in overall perioperative complications [114]. Of note, most beverages consumed in these studies contained complex carbohydrates (e.g., maltodextrin) as opposed to the monosaccharides (e.g., fructose) or disaccharides (e.g., sucrose) found in fruit juice or sports drinks. Another meta-analysis of 21 randomized studies including 1,685 patients showed no overall difference in length of stay; however, the subgroup of patients undergoing major abdominal surgery had a shorter length of stay associated with carbohydrate loading ([mean difference, 95% confidence interval: − 1.08 days (− 1.87 to − 0.29), p = 0.007]) [117]. Another meta-analysis including 43 RCTs with 3,110 elective surgery patients found that high-dose carbohydrate loading (≥ 45 g) was associated with a reduced length of hospital stay compared to fasting (− 1.7 days [− 3.2, − 0.1]) or placebo/water (− 1.4 days [− 2.7, − 0.1], p < 0.05), but there were no differences in complication rates or other secondary endpoints [118]. This recommendation applies to non-diabetic patients because diabetic patients were not included in the trials.
Oral nutritional supplementation is recommended in malnourished patients prior to elective colorectal surgery. Grade of recommendation: weak recommendation based on moderate-quality evidence, 2B
In malnourished patients planning elective gastrointestinal (GI) surgery, oral nutritional supplementation targeting a protein intake of 1.2–1.5 g/kg/day for a period of 1–2 weeks has been associated with reduced postoperative complications and is endorsed by several national and international guidelines [119–122]. Meanwhile, the efficacy of immunonutrition, supplementation containing immune-modulating nutrients such as arginine, fish oil (ω-3 fatty acids), nucleotides, and glutamine, over standard high-protein oral nutritional supplements remains controversial. Meta-analyses have demonstrated reduced complications and infectious complications and shortened LOS associated with preoperative immunonutrition [123, 124]. However, other studies have reported conflicting results depending on whether or not patients were malnourished, the degree of industry support (more positive results reported in industry sponsored trials) and the type of control used for comparison (standard isonitrogenous, iso-caloric non-enhancing nutritional supplement versus normal diet without any supplementation) [123–126].
Mechanical bowel preparation combined with preoperative oral antibiotics is typically recommended prior to elective colorectal resection. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
A 2011 Cochrane review of RCTs showed no benefit to mechanical bowel prep (MBP) alone in colorectal surgery in reducing anastomotic leak or complications [127]. Meanwhile, a meta-analysis of seven RCTs including 1769 patients comparing MBP with oral antibiotics to MBP alone showed a reduction in total surgical site infection (7.2% vs 16.0%, p < 0.001) and incisional site infection (4.6% vs 12.1%, p < 0.001), with no difference in the rate of organ/space infection after elective colorectal surgery [128]. These trial findings are consistent with population level data. In a retrospective analysis of a nationwide database from the USA, MBP plus oral antibiotic prep in left colon resection was associated with decreased overall morbidity, superficial surgical site infection, anastomotic leakage, and intra-abdominal infections [129]. Similar retrospective studies in different populations (Veterans Administration database [130] and a Polish hospital database [131]) have also shown a reduction in surgical site infection with the addition of OBP to MBP. The Michigan Surgical Quality Collaborative database showed reductions in surgical site infection and in postoperative C. difficile colitis in patients who received MBP and OBP versus patients who received no bowel prep [132]. These kinds of data supported the ASCRS 2019 Clinical Practice Guideline on Bowel Preparation recommending the use of a mechanical bowel prep combined with preoperative oral antibiotics in elective colorectal surgery [40].
Preadmission optimization
Multimodal prehabilitation prior to elective colorectal surgery may be considered for patients with multiple co-morbidities or significant deconditioning. Grade of recommendation: weak recommendation based on moderate-quality evidence, 2B
Prehabilitation, defined as enhancement of the patient’s preoperative condition, has been proposed as a possible strategy for improving postoperative outcomes [133–135]. Several recent RCTs [136–143] and systematic reviews have demonstrated that prehabilitation improves physical function prior to colorectal or major abdominal surgery [135, 144–148]. However, whether better physical function translates into improved postoperative outcomes remains debateable [135–139, 147, 149]. A meta-analysis of 35 studies evaluating 3402 patients undergoing major abdominal surgery found that patients who received prehabilitation experienced significantly lower rates of overall complications (p = 0.005), pulmonary complications (p < 0.001), and cardiac complications (p = 0.044) [150]. Another meta-analysis of 8 trials with 442 patients undergoing major liver, colorectal, gastroesophageal, and general abdominal surgery demonstrated significant reductions in postoperative pulmonary complications and overall postoperative morbidity in the prehabilitation group versus controls and no differences in LOS [151]. While the available data remain limited due to many underpowered studies, patients with lower baseline functional capacity undergoing open surgery may achieve the greatest benefit from prehabilitation [137–139, 141, 142, 152].
Preadmission orders
Standardized order sets should be utilized in enhanced recovery pathways. Grade of recommendation: weak recommendation based on low-quality evidence, 2C
Comprehensive, multifaceted enhanced recovery protocols are complex and require a multidisciplinary collaboration between stakeholders including nursing teams, anesthesiologists, social workers, and surgeons. Increased compliance with ERP components has repeatedly been associated with improved perioperative outcomes [153–156]. Dedicated order sets standardize care and are considered essential for improving compliance with ERP elements [2, 13, 157, 158]. The use of order sets has also been proven effective in reducing the risk of surgical site infection [157, 159, 160].
Perioperative interventions
Surgical site infection (SSI)
A bundle of measures should be in place to reduce surgical site infection. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
Various SSI prevention bundles have been described to decrease surgical site infections in colorectal surgery. While there are many commonalities between SSI bundles, there is no universal standardization of elements and it is rare for the impact of any one component to be specifically evaluated [161–167]. Preoperative measures incorporated into bundles include a chlorhexidine shower, mechanical bowel preparation with oral antibiotics, intravenous antibiotics within one hour of incision, and standardization of the surgical field preparation with chlorhexidine/alcohol [168]. Operative measures typically found in SSI prevention bundles include the use of a wound protector, gown, and glove changes before fascial closure, using a dedicated wound closure tray, antimicrobial sutures, limiting OR traffic, and maintaining euglycemia and normothermia [169–171].
A meta-analysis evaluating SSI prevention bundles including 17,557 patients reported risk reductions of 40% in the overall SSI rate, 44% in the superficial infection rate, and 34% in the deep/organ space infection rate. This analysis also reported that utilization of sterile wound closure trays, mechanical bowel preparation with oral antibiotics, and glove changes before fascial closure were considered most important to implement [170]. Another meta-analysis of 20,701 patients found that while there was significant heterogeneity in SSI reduction bundle component elements and compliance rates (ranging from 19 to 90% in the included studies), the OR of SSI was 0.56 with a bundle compared to without [171]. Higher rates of compliance with specific bundle elements within SSI prevention bundles have repeatedly been associated with significantly lower SSI rates [159, 160].
Pain control
A multimodal, opioid-sparing, pain management plan should be implemented before the induction of anesthesia. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
Multiple studies have demonstrated that minimizing opioids after colorectal surgery is associated with earlier return of bowel function and shorter length of stay [2, 31, 172]. One of the simplest techniques to limit opioid use is to schedule non-narcotic alternatives, such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), rather than administering them on an as-needed basis [173–178]. There have been ongoing concerns about the postoperative safety profile of NSAIDs in this setting. However, a 2007 Cochrane review concluded that NSAIDs can cause a clinically unimportant transient reduction in renal function in the early postoperative period and should therefore not be withheld from adults with normal preoperative renal function [179]. In addition, experimental and observational clinical studies have shown that NSAIDs may increase the risk of anastomotic leak [180–183] and subsequent research has demonstrated that this potential effect on anastomotic leak appears to be molecule and class specific [184]; diclofenac has been associated with the highest risk of leak in this setting. In a retrospective cohort study of 856 patients undergoing elective colorectal surgery, the risk of anastomotic leak was 11.8% versus 6.0% (p = 0.01) in patients receiving diclofenac, but there was no differences in leak rates related to other nonsteroidals [185]. Additionally, two meta-analyses have demonstrated an overall increased risk of anastomotic leak with NSAIDs but no increase in the risk of anastomotic leak with the use of selective NSAIDs (such as cyclooxygenase 2 inhibitors) [180, 181]. In these studies nonselective NSAID diclofenac use was associated with an increased leak rate (OR 2.79 [1.96, 3.96], p < 0.001 and pooled OR 2.02, 95% CI 1.62–2.50, p < 0.001), while ketorolac and selective NSAIDS were not associated with anastomotic leak. In addition, a large multicenter cohort study in Europe showed no differences in anastomotic leak rate with nonselective NSAIDs [174].
Perioperative gabapentinoids, ketamine, lidocaine, magnesium, and α2-agonists have also been administered to improve analgesia and reduce opioid consumption and postoperative hyperalgesia. The role of gabapentinoids is controversial as two large database studies reported that gabapentinoid use after colorectal or orthopedic surgery was associated with increased postoperative pulmonary complications and no reduction in postoperative opioid consumption [186, 187]. A meta-analysis evaluating the perioperative use of gabapentinoids also reported no clinically significant analgesic effect from gabapentinoid use and stated that the routine use of these medications cannot be recommended [188]. Meanwhile, a perioperative low-dose ketamine infusion can be especially useful in patients with chronic pain [189, 190]. However, psychotropic adverse effects, dizziness, and sedation may impair immediate recovery, particularly in elderly patients [191]. Magnesium either as a bolus or infusion is also associated with a decrease in postoperative opioid consumption and can be a useful adjunct [192].
Analgesic blocks and wound infiltration have shown benefit in opioid reduction among patients undergoing open and laparoscopic colorectal surgery [190, 193]. There are an increasing number of block options, including but not limited to transversus abdominis plane (TAP), quadratus lumborum (QL), erector spinae, and rectus sheath blocks. Two meta-analyses of TAP blocks demonstrated decreased length of stay compared with systemic opioid use in laparoscopic colorectal surgery [193, 194]. A recent systematic review and meta-analysis demonstrated that laparoscopic-guided TAP block is safe and effective for pain management in minimally invasive surgery and seems to be as effective as US-guided TAP blocks with respect to early pain control and reducing postoperative opioid use [195]. Data remain controversial regarding the purported extended duration of benefit with long-acting local anesthetics such as liposomal bupivacaine in reducing postoperative opioid consumption [196–199].
Another option, spinal analgesia with intrathecal morphine administration, can be utilized in the perioperative setting. Studies and meta-analyses have shown that intrathecal morphine is more effective than intravenous opioids in laparoscopic surgery and is associated with lower pain scores [2, 200–202]. The concern about delayed respiratory depression related to this analgesia has not been substantiated and guidelines for postoperative monitoring have been published [203].
Thoracic epidural analgesia, while not recommended for routine use in laparoscopic colorectal surgery, is an option for open colorectal surgery if a dedicated acute pain team is available for postoperative management. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
Thoracic epidural analgesia (TEA; T6–T12) has shown efficacy (versus patient controlled analgesia or simple parenteral opioids) in controlling pain and limiting opioids in patients undergoing open colorectal surgery [204, 205]. However, epidurals have no analgesic benefit over multimodal analgesia and abdominal wall blocks in laparoscopic surgery. In addition, evidence shows that the analgesic benefits provided by TEA do not translate into faster recovery in either laparoscopic or open colorectal surgery [206, 207]. In fact, TEA may actually delay hospital discharge after laparoscopic surgery [208] due to the higher incidence of hypotension and urinary tract infections that necessitate additional postoperative care [207–210].
Perioperative nausea and vomiting
Pre-emptive, multimodal anti-emetic prophylaxis reduces perioperative nausea and vomiting. Grade of recommendation: strong recommendation based on high-quality evidence, 1A
Several validated scoring systems have been developed to identify patients at higher risk for PONV [211–216]. Risk factors for developing PONV include female sex, history of PONV and/or motion sickness, nonsmoking status, young age, laparoscopic surgery, use of volatile anesthesia, prolonged operative time, and opioid analgesia. Strategies to reduce the risk of PONV include using regional anesthesia or propofol-based total intravenous anesthesia (TIVA), avoiding volatile anesthetics, and minimizing perioperative opioids by employing multimodal analgesia [207, 217–220]. Although TIVA has been associated with reduced PONV and significantly better patient satisfaction compared to volatile anesthetics, its high cost has precluded widespread adoption [221, 222].
One guideline updated in 2020 supports preoperative risk assessment in all patients undergoing anesthesia and recommends subsequent tailored multimodal therapy to prevent and treat PONV [220]. Combining risk assessment with a specific recommendation for anti-emetic intervention has been associated with significant reduction in PONV in randomized and non-randomized trials [223–226]. Given the low cost and minimal risk associated with anti-emetics, the liberal use of a multimodal anti-emetic protocol for all patients (regardless of risk) has been advocated [227, 228].
ERPs, which include multimodal PONV prophylaxis, are associated with reduced rates of PONV and readmission in colorectal surgery [229–231]. Multiple prospective and observational studies demonstrate that combination therapy using two or more anti-emetics for preventing PONV is superior over a single agent [232–269]. A description of all the available prophylactic agents is beyond the scope of this CPG. However, a common intervention for patients determined to be high risk for PONV that has been studied in randomized controlled fashion is the administration of dexamethasone and ondansetron (or other 5-hydroxytryptamine 3 (5-HT3) antagonist) [225–270]. A meta-analysis of 9 RCTs, including 1,089 patients, demonstrated that dexamethasone combined with other anti-emetics provided significantly better PONV prophylaxis than single anti-emetics and decreased the need for rescue therapy [271]. In addition, several meta-analyses found that dexamethasone did not increase postoperative infections or significantly impact glycemic control [272, 273].
Fluid management
Fluid administration should be tailored to avoid excessive fluid administration and volume overload or undue fluid restriction and hypovolemia. Grade of recommendation: strong recommendation based on high-quality evidence, 1A
Both intravenous fluid overload and hypovolemia can significantly impair organ function, increase postoperative morbidity, and prolong hospital stay [274, 275]. Intraoperative infusion regimens based on definitions such as liberal, restrictive, or supplemental should typically be avoided because of the variability in the volumes of fluid infused among different studies using these qualifiers [276]. However, more recently within ERP literature the term “restrictive fluid management” has gained popularity and the amount of fluid recommended with restrictive fluid management has gradually decreased. The term “zero-balance” fluid management was introduced to describe a restrictive fluid regimen aiming to avoid postoperative fluid retention (as indicated by weight gain) [277]. However, while a zero-balance approach might improve postoperative GI function, it is associated with a slightly increased risk of acute kidney injury (8.6% versus 5.0% in an RCT of 3000 patients undergoing major abdominal surgery) [278].
Based on these considerations, the overall goal of fluid management should typically be a positive fluid balance at the end of surgery of ~ 1 L. This should be sufficient to avoid hypovolemia and AKI, while limiting substantial postoperative weight gain (> 2.5 kg/days) which is associated with increased morbidity and prolonged hospital stay [279].
Balanced chloride-restricted crystalloid solutions should be used for maintenance infusions and fluid boluses in patients undergoing colorectal surgery. There is no benefit to the routine use of colloid solutions for fluid boluses. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
Results from studies conducted in healthy volunteers and from meta-analyses of small RCTs indicate that balanced chloride-restricted crystalloid solutions should be preferred to normal saline to decrease the risk of hyperchloremic metabolic acidosis [280, 281]. Large propensity-matched observational studies have reported an association between the use of normal saline and an increased incidence of renal dysfunction, postoperative morbidity, and mortality in surgical patients [282, 283]. A large cluster randomized trial of 15,000 critically ill adults showed similar results, with lower rates of death and renal dysfunction attributed to the use of balanced crystalloids [284]. Based on the evidence from this trial, the current recommendation was upgraded from a 1C in 2017 to a 1B.
There is little evidence that colloids offer any benefit over crystalloids for fluid boluses, either during abdominal surgery or postoperatively in intensive care [285–289]. Meanwhile, there may be some benefit in individual cases, particularly in the setting of blood loss or when rapid resuscitation is needed [290, 291]. Colloids restore circulating volume faster than crystalloids and with a lower fluid volume (although this difference is less than traditionally taught with a ratio of around 1:1.5) [292]. Given that the evidence does not show an outcome benefit with colloids and that colloids are significantly more expensive, their routine use should be discouraged.
Intraoperative hypotension should be avoided as even short durations of MAP < 65 are associated with adverse outcomes, in particular myocardial injury and acute kidney injury. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
In a recent retrospective analysis of 4282 patients undergoing noncardiac surgery, intraoperative hypotension defined as MAP < 65 mmHg occurred in 71% of patients [293]. Approximately one-third of these hypotensive events occurred before the surgical incision. There is increasing evidence from large retrospective database reviews showing that even a short duration of hypotension is associated with myocardial injury and acute kidney injury [293–295] and that the severity of injury is associated with both the duration and degree of hypotension [294, 296]. One major prospective interventional trial showed a significant reduction in complications (38% versus 51%, p = 0.02) with individualized blood pressure management (n = 147) compared with standard pressure management (n = 245) [297]. In this study, patients in the intervention group had their fluid status optimized and then had a vasopressor infused to maintain their SBP within 10% of their resting blood pressure. In patients with an epidural block, crystalloid or colloid preloading does not typically prevent hypotension induced by the neuraxial blockade because total blood volume is unchanged after neuraxial blockade [298]; in these circumstances, a low dose of vasopressors, not intravenous fluids, restores colonic perfusion in normovolemic hypotensive patients [299].
In high-risk patients and in patients undergoing colorectal surgery with anticipated significant intravascular losses, the use of goal-directed hemodynamic therapy is recommended. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
Objective measures of hypovolemia such as cardiac output, stroke volume, oxygen delivery, oxygen extraction, and mixed venous oxygen saturation and dynamic indices of fluid responsiveness (e.g., pulse pressure variation or stroke volume variation) can help physicians decide whether to administer intravenous fluids for purposes of resuscitation. Several meta-analyses of RCTs have shown that goal-directed fluid therapy (GDFT) reduces postoperative morbidity and length of hospital stay, especially in high-risk patients undergoing major surgery [300–302]. High-risk patients have been variably defined as patients with a history of severe cardiorespiratory illness (e.g., acute myocardial infarction, chronic obstructive pulmonary disease, stroke), a prolonged planned surgery (> 8 h), age > 70 years with limited physiologic reserve, respiratory failure, and aortic vascular disease. However, it must be acknowledged that advancements in perioperative and surgical care seem to have offset the previously demonstrated benefits of GDFT, especially in low–moderate-risk patients [303]. The largest multicenter RCT studying these issues, included 734 high-risk patients undergoing major abdominal surgery (45% colorectal surgery and the majority in the context of an ERP) and showed a decrease in complications and mortality in patients treated with GDFT though this difference did not meet statistical significance (relative risk = 0.84 [95% CI 0.71–1.01], p = 0.07) [304].
Recent studies have focused on goal-directed hemodynamic therapy, rather than goal-directed fluid therapy and showed an improvement in outcomes even in low–moderate-risk patients [305]. These treatment algorithms first optimize stroke volume with fluid boluses and then, if hypotension persists, add a vasopressor to maintain MBP > 65 mmHg. This management reflects the increasing evidence that perioperative hypotension is associated with harm and should be avoided [294, 296, 297].
In the absence of surgical complications or hemodynamic instability, intravenous fluids should be routinely discontinued in the early postoperative period. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
A few small, heterogeneous randomized controlled trials support discontinuation of intravenous fluids in the early postoperative period [279, 306, 307]. Traditional surgical practice recommends maintaining a minimal urine output target of 0.5 mL/kg/h in the postoperative period. However, a small prospective study of 40 low-risk patients undergoing a variety of elective colorectal resections randomized subjects to a minimum urine output target of 0.2 mL/kg/h or 0.5 mL/kg/h in the perioperative period, using intravenous fluid administration to achieve targets [308]. In this study, there were no differences in postoperative serum creatinine or other markers of acute renal tubular damage. Another RCT of patients undergoing elective colorectal surgery with an ERP evaluated the use of diuretics to achieve a euvolemic state in diuretically naïve patients and found no difference in postoperative length of stay or complications [309].
Surgical approach
A minimally invasive surgical approach should be employed when the expertise is available and when appropriate. Grade of recommendation: strong recommendation based on high-quality evidence, 1A.
High-quality evidence from RCTs and large database studies supports the use of laparoscopy in colorectal surgery. Two separate multicenter RCTs of patients with colon cancer, the ALCCaS trial from Australia and the COLOR trial from the Netherlands, showed laparoscopy to be superior to open resection regarding short-term outcomes (e.g., return of bowel function, blood loss, postoperative pain, and hospital length of stay) [310, 311]. Several other RCTs have reported improved perioperative morbidity, including total morbidity, wound morbidity, and non-surgical morbidity, following laparoscopic compared to open colonic resection [312–315]. Other RCTs showed that patients undergoing laparoscopy have decreased time to pulmonary recovery, reduced use of narcotics, and improved short-term quality of life [316–318]. These results are consistent with large, database studies that relied on data from the National Surgical Quality Improvement Program and the National Inpatient Sample which support the use of laparoscopy [319–322]. High-quality Cochrane reviews have evaluated short- and long-term outcomes as well and support the laparoscopic approach in colorectal surgery [323–325].
The use of robotics in colorectal surgery has increased exponentially over the last decade [326] and multiple studies have demonstrated the feasibility and safety of robotic colorectal surgery [326–330]. However, the benefits of the robotic approach over standard laparoscopy with regard to short- and long-term surgical outcomes have yet to be fully elucidated. Meta-analyses of RCTs suggest lower conversion rates with a robotic approach [326, 328–330]; however, operative times and costs are consistently higher with robotic surgery compared to laparoscopy, while complication rates are similar between the two approaches [327, 329]. Notably, many of the included studies in these meta-analyses and systematic reviews were of moderate to poor methodological quality.
Combining minimally invasive surgery with an ERP is associated with optimal outcomes, as demonstrated in the 4-arm LAFA trial which randomized 427 patients to open versus laparoscopic surgery with an ERP versus a traditional care pathway. In this study, patients undergoing laparoscopic surgery within an ERP had the shortest LOS and morbidity compared to either laparoscopy within a traditional care pathway or open surgery [331]. As such, a minimally invasive approach is recommended when appropriate to optimize postoperative recovery within an ERP.
The routine use of nasogastric tubes and intra-abdominal drains for colorectal surgery should be avoided. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
Small RCTs evaluating elective colorectal surgery have failed to demonstrate an impact from the routine use of nasogastric tube decompression on nausea, vomiting, time to return of bowel function or length of stay [332–334]. Alternatively, the routine use of nasogastric tube decompression delays the tolerance of oral intake by an average of two days and has been associated with significantly higher risk of associated complications, notably pharyngolaryngitis [332, 335, 336].
Similarly, there is no benefit to the routine use of intra-abdominal drains in colorectal surgery. RCTs show no significant differences in mortality, leak, or a composite of postoperative complications in patients who had drains placed [51, 337–339]. The lack of benefit from operative drains has been demonstrated across a variety of colorectal anastomoses as well as low pelvic anastomoses specifically [337, 338, 340–345]. Meanwhile, a review of the US Rectal Cancer Consortium data found a nonstatistically significant association between drains and higher leak rates but there was no difference in the rate of intervention for leak between patients with and without drains [346]. Notably, this was a retrospective review and drain placement was left to the discretion of the operating surgeons; drain use was likely a surrogate for patients with a higher risk for leak due to other factors. Contrary to these studies, a retrospective analysis of the Dutch TME data suggested that intra-abdominal drains in the presence of a diverting stoma may be associated with lower rates of surgical intervention in patients with anastomotic failure [347].
Postoperative interventions
Patient mobilization
Early and progressive patient mobilization are associated with shorter length of stay. Grade of recommendation: strong recommendation based on low-quality evidence, 1C
Complications of prolonged immobility include skeletal muscle loss and weakness, atelectasis, insulin resistance, thromboembolic disease, and decreased exercise capacity [348, 349]. It is estimated that muscle mass decreases by 1.5–2% for every day of bedrest [350]. However, the deconditioning associated with bedrest can be minimized or avoided by engaging in physical activity. Definitions of early mobilization within a colorectal ERP vary significantly, from any mobilization at all within 24 h of operation to 8 h of activity per day by the second postoperative day [31, 351]. Compliance with mobilization targets within ERPs varies significantly between centers, but early ambulation has been associated with faster recovery and fewer complications after colorectal surgery [35, 352–354]. In a prospective cohort study of 100 patients, individuals who had a higher step count on the first postoperative day after major abdominal or thoracic surgery were more likely to have a shorter length of stay [355].
There are limited data about interventions that specifically increase mobilization with regard to their effects on postoperative outcomes. A randomized trial compared facilitated supervised mobilization (n = 49) on postoperative days 0 to 3 versus conventional care (n = 50) after colorectal surgery within the construct of an ERP. [356]. In this study, step counts were higher in the intervention group but there were no differences between the two groups in functional recovery, length of stay, complications, or return of gastrointestinal function. A subgroup analysis of this trial also did not find any differences in pulmonary function or postoperative pulmonary complications between the two arms [357]. These data suggest that additional resources to increase mobilization are not associated with improved outcomes within an established colorectal ERP. However, importantly, no studies have reported harm associated with early mobilization, even after perineal reconstruction following abdominoperineal resection [358].
Ileus prevention
Patients should be offered a regular diet within 24 h after elective colorectal surgery. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
A 2019 Cochrane systematic review and meta-analysis evaluated 17 RCTs that compared early feeding (i.e., within 24 h of surgery) versus “later commencement” after lower gastrointestinal surgery [359]. In this review, early feeding was associated with a two-day decrease in length of hospital stay (weighted mean difference 1.95, 95% CI 0.91–2.99). However, perioperative management strategies varied significantly within the included trials and the mean length of stay in the control group ranged from 6 to 24 days. Furthermore, the risk of complications such as anastomotic leak, wound infection, pneumonia, and mortality were not affected by early feeding. Even symptoms of nausea and vomiting were not significantly higher in the early feeding group in this review. Early enteral feeding is associated with faster return of gastrointestinal function and with shorter time to flatus and first bowel movement [360]. While there is heterogeneity between trials, the overall body of evidence supports the benefits of early feeding.
Sham feeding (i.e., chewing gum for ≥ 10 min 3–4 times daily) after colorectal surgery is safe, results in small improvements in GI recovery, and may be associated with a reduction in length of hospital stay. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
Sham feeding such as gum chewing has been hypothesized to hasten recovery of gastrointestinal function through increased saliva production and vagal cholinergic stimulation that increases bowel peristalsis [361]. Eighteen RCTs have evaluated chewing gum after colorectal surgery [362]. The majority of these trials used sugar-free gum chewed for at least 5–10 min 3 times daily. However, the majority of these trials were not performed in the context of an ERP were of low quality and had a high risk of bias. A meta-analysis of all 18 randomized trials reported that chewing gum was associated with shorter time to first flatus (weighted mean difference (WMD) − 8.81 h, 95% CI − 13.45 to − 4.17), shorter time to first bowel movement (WMD − 16.43 h, 95% CI − 22.68 to − 10.19), and a reduction in length of stay (WMD − 0.89 days, 95% CI − 1.72 to − 0.07) [362]. The pooled outcome of “postoperative ileus” was also lower in the chewing gum arm (RR 0.41, 95% CI 0.23–0.73). Other outcomes, including complications, readmission, and reoperations, were not significantly different between the two groups. Subgroup analysis of laparoscopic and open approaches maintained these significant associations. However, subgroup analysis of trials performed within the context of an ERP reported that chewing gum was no longer associated with significant decreases in the time to flatus and length of stay.
In another systematic review and meta-analysis that only included ten randomized trials that were deemed “high quality,” [363] the use of chewing gum was associated with a lower incidence of postoperative ileus (RR 0.55, 95% CI 0.39–0.79) and faster time to first flatus (WMD − 0.31 days, 95% CI − 0.36 to − 0.26) and bowel movement (WMD − 0.47, 95% CI − 0.60 to − 0.34), but no difference in length of stay. However, the trials included in this meta-analysis suffered from many of the same limitations pertaining to heterogeneity and variable perioperative management strategies that were present in the prior studies. Nonetheless, the overall body of literature suggests that chewing gum may only have a small effect on gastrointestinal recovery without a clear effect on length of stay but is safe and not costly.
There are even some data to support the use of coffee to facilitate gastrointestinal recovery after colorectal surgery [364–366]. Caffeine and coffee may stimulate the lower gastrointestinal tract and can potentially reduce postoperative ileus. A meta-analysis of 7 randomized trials including 606 patients reported that drinking coffee decreased the time to first bowel movement and toleration of oral intake, but did not reduce time to flatus, overall complications, or length of stay [367].
Alvimopan is recommended to hasten recovery after open colorectal surgery. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
Alvimopan, an oral peripheral acting mu-opioid antagonist that minimizes the effect of opioids on postoperative gastrointestinal function, was first approved by the US Food and Drug Administration in 2008. A systematic review of all relevant studies published up to May 2020 identified 31 studies that investigated the effect of alvimopan on gastrointestinal function in colorectal surgery, of which 23 demonstrated a positive effect and 8 reported no effect [368]. Of the 6 randomized trials, 4 were positive and 2 showed no effect related to the medication.
Most of the available data supporting alvimopan in the setting of colorectal surgery is limited to open surgery. Several RCTs and pooled post hoc analyses reported accelerated time to recovery of gastrointestinal function with alvimopan 6-mg and 12-mg doses compared to placebo and a significantly shorter hospital length of stay in the alvimopan 12-mg group compared with placebo for patients undergoing open surgery [369–378]. A Cochrane review of nine studies affirmed that alvimopan was better than placebo in reversing opioid-induced increased gastrointestinal transit time and constipation and that alvimopan was safe and efficacious in decreasing postoperative ileus, but the studies were limited to open surgery patients without an ERP in place [379].
There have been no randomized trials evaluating alvimopan after laparoscopic surgery [380–382]. Most of the non-randomized studies have shown modest benefits in favor of alvimopan for laparoscopic resection albeit within traditional care pathways [383–385]. Given the low quality of the available evidence, it may be difficult to justify the use and cost of alvimopan in laparoscopic surgery in the setting of an ERP.
Urinary catheters
Urinary catheters should typically be removed within 24 h of elective colonic or upper rectal resection irrespective of thoracic epidural analgesia use. Grade of recommendation: strong recommendation, based on moderate-quality evidence, 1B
Urinary catheterization is routinely used in abdominal and pelvic colorectal surgery for intraoperative bladder decompression and monitoring urinary output. Assessing whether or not to remove catheters early should consider the risk of postoperative urinary retention requiring subsequent catheter reinsertion as well as the risk of urinary tract infection (UTI) related to prolonged use of a catheter. Postoperative urinary retention is associated with decreased functional recovery (e.g., less mobility, more postoperative pain) and longer length of stay [386]. UTIs are also associated with increased morbidity, longer length of stay, and mortality [387]. However, longer duration of catheter use is associated with higher rates of UTI and in-and-out (i.e., straight catheterization) catheterization in the setting of postoperative urinary retention is not associated with an increased risk of UTI [388, 389].
Overall, the evidence suggests that early urinary catheter removal within 24 h of surgery is safe. In a large multicenter study of 2927 surgery patients (1897 colonic procedures), early catheter removal was associated with a higher incidence of catheter reinsertion compared to later removal (4.9% versus 1.9%, p < 0.001) but a lower rate of UTIs (0.8% versus 4.1%, p = 0.003) [388]. Length of stay in this study was also shorter in the early catheter removal group by 1 day and other studies have reported similar results [390, 391]. There are increasing data suggesting that catheters can be removed even earlier (e.g., within 6 h after surgery) or avoided altogether [392–394].
In the context of thoracic epidural analgesia, randomized controlled trials have investigated early urinary catheter removal compared with removal at the time of epidural discontinuation and found lower UTI rates after early catheter removal and no differences in re-catherization rates [395, 396]. In an RCT of 215 patients undergoing abdominal or thoracic surgery with a thoracic epidural that randomized patients to early catheter removal on POD 1 or following epidural removal, the incidence of recatheterization was similar between groups but the incidence of UTI was much lower in the early removal group (2% versus 24%, p = 0.004). [396].
Urinary catheters should typically be removed within 24–48 h after mid/lower rectal resection. Grade of recommendation: strong recommendation based on moderate-quality evidence, 1B
Retracting on the bladder and dissecting in close proximity to the lateral pelvic nerves during proctectomy may increase the risk of postoperative urinary retention. There have been 4 randomized controlled trials comparing outcomes between early and late catheter removal specifically in the setting of proctectomy [397–400]. A meta-analysis of these 4 trials examined the non-inferiority of early removal (before postoperative day 2) versus late (postoperative day 2 and after) catheter removal and concluded that the data were insufficient to conclude non-inferiority of early catheter removal after proctectomy in terms of the development of postoperative urinary retention [401]. However, this meta-analysis showed that early catheter removal decreased the risk of UTI (9.7% versus 21.1%; absolute risk difference − 11%, 95% CI − 17, − 4). Another systematic review and meta-analysis compared POD1 catheter removal versus POD3 or POD5 removal and found lower UTI rates of in the earlier removal groups [402]. There may be some subgroups of patients that were not included in the clinical trials such as pelvic exenteration patients or patients with a difficult hand-sewn coloanal anastomosis, and management of these patients is up to the best clinical judgment of the surgeon balancing the risk of urinary tract infection vs. urinary retention.
Discharge criteria
Hospital discharge prior to return of bowel function may be offered for selected patients. Grade of recommendation: weak recommendation based on moderate-quality evidence, 2B
Traditional discharge criteria following colorectal surgery include demonstrating return of bowel function along with tolerance of oral intake, adequate pain control with oral analgesia, and the ability to mobilize in the absence of complications [403]. Many patients meet these criteria by the first or second postoperative day [57, 58, 62]. However, there are increasing reports of same-day discharge which hinges on the feasibility of discharging patients prior to return of bowel function.
The concept of the “ambulatory” or “outpatient” colectomy was first introduced over a decade ago and was initially reported in small case series [62, 404, 405]. In these early reports, low-risk patients undergoing colorectal resection were successfully discharged home after an observation period of 24 h without undue complications [57, 62]. An RCT of patients undergoing minimally invasive colorectal resection for cancer randomized 30 patients to discharge on postoperative day 1 regardless of bowel function with telemedicine follow-up on postoperative day 2 versus standard postoperative care with discharge after return of bowel function (RecoverMI trial) [406]. In this study, the median LOS was 28.3 h in the study arm and 51.5 h in the control arm (p = 0.041) and there were no differences in adverse events or quality of life as measured by EQ-5D-5L™ between the 2 groups. Exclusion criteria included patient-reported history of severe postoperative nausea/vomiting. Patients with a serum creatinine above 1.5 ng/ml, measured within 30 days of surgery, or a history of congestive heart failure, defined as ejection fraction of 40% or less, or of more than 40% with systemic signs of heart failure were also excluded. Finally and patients requiring conversion to open surgery or in whom an ostomy was necessary at completion of the study were removed from the study and not randomized.
Other retrospective cohort studies have reported that same-day discharge after colorectal surgery was associated with low rates of readmission [128, 407]. The largest of these retrospective cohort studies included 157 consecutive patients undergoing laparoscopic right, transverse, total, or left colectomy (left colectomy accounted for the majority of cases) [407]. In this study, same-day discharge was possible in 93% of patients with an associated readmission rate of 6% [408]. These studies demonstrate that same-day discharge is feasible within an ERP in selected patients with acceptable complication rates [408]. Success of these initiatives depends on patients having adequate support at home, close outpatient surveillance, and the ability to tolerate clear liquids in the postoperative recovery unit [128]. This is an area with limited but evolving evidence. Recommendations could change as more evidence becomes available.
Acknowledgements
We thank Elaine Attridge, MLS, Quality and Performance Improvement Librarian in the Claude Moore Health Sciences Library at the University of Virginia for her invaluable expertise and guidance.
Funding
The funding bodies (ASCRS and SAGES) did not influence the content of this work and no other specific funding was received from other entities.
Disclosure
I. Paquette have no financial disclosure.
Footnotes
This publication was approved by both the ASCRS and SAGES executive council and then peer reviewed by the Diseases of the Colon & Rectum and Surgical Endoscopy And Other Interventional Techniques. The articles are identical except for minor stylistic and spelling differences in keeping with each journal’s style. Either citation can be used when citing this article. In order to encourage its wide dissemination this article is freely accessible on Surgical Endoscopy And Other Interventional Techniques and Diseases of the Colon & Rectum journal web sites.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jennifer L. Irani and Traci L. Hedrick are co-first authors.
References
- 1.Kang CY, Chaudhry OO, Halabi WJ, et al. Outcomes of laparoscopic colorectal surgery: data from the Nationwide Inpatient Sample 2009. Am J Surg. 2012;204:952–957. doi: 10.1016/j.amjsurg.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 2.Thiele RH, Rea KM, Turrentine FE, et al. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 2015;220:430–443. doi: 10.1016/j.jamcollsurg.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 3.Eberhart LH, Mauch M, Morin AM, Wulf H, Geldner G. Impact of a multimodal anti-emetic prophylaxis on patient satisfaction in high-risk patients for postoperative nausea and vomiting. Anaesthesia. 2002;57:1022–1027. doi: 10.1046/j.1365-2044.2002.02822.x. [DOI] [PubMed] [Google Scholar]
- 4.Nagle D, Pare T, Keenan E, Marcet K, Tizio S, Poylin V. Ileostomy pathway virtually eliminates readmissions for dehydration in new ostomates. Dis Colon Rectum. 2012;55:1266–1272. doi: 10.1097/DCR.0b013e31827080c1. [DOI] [PubMed] [Google Scholar]
- 5.Liska D, Novello M, Cengiz BT, et al. Enhanced recovery pathway benefits patients undergoing nonelective colorectal surgery. Ann Surg. 2021;273:772–777. doi: 10.1097/SLA.0000000000003438. [DOI] [PubMed] [Google Scholar]
- 6.Lohsiriwat V, Jitmungngan R, Chadbunchachai W, Ungprasert P. Enhanced recovery after surgery in emergency resection for obstructive colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2020;35:1453–1461. doi: 10.1007/s00384-020-03652-5. [DOI] [PubMed] [Google Scholar]
- 7.Gustafsson UO, Scott MJ, Schwenk W, European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN) et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg. 2013;37:259–284. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 8.Hughes M, Coolsen MM, Aahlin EK, et al. Attitudes of patients and care providers to enhanced recovery after surgery programs after major abdominal surgery. J Surg Res. 2015;193:102–110. doi: 10.1016/j.jss.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Ni X, Jia D, Chen Y, Wang L, Suo J. Is the enhanced recovery after surgery (ERAS) program effective and safe in laparoscopic colorectal cancer surgery? A meta-analysis of randomized controlled trials. J Gastrointest Surg. 2019;23:1502–1512. doi: 10.1007/s11605-019-04170-8. [DOI] [PubMed] [Google Scholar]
- 10.Jung AD, Dhar VK, Hoehn RS, et al. Enhanced recovery after colorectal surgery: can we afford not to use it? J Am Coll Surg. 2018;226:586–593. doi: 10.1016/j.jamcollsurg.2017.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Lemini R, Spaulding AC, Naessens JM, et al. ERAS protocol validation in a propensity-matched cohort of patients undergoing colorectal surgery. Int J Colorectal Dis. 2018;33:1543–1550. doi: 10.1007/s00384-018-3133-4. [DOI] [PubMed] [Google Scholar]
- 12.Shah PM, Johnston L, Sarosiek B, et al. Reducing readmissions while shortening length of stay: the positive impact of an enhanced recovery protocol in colorectal surgery. Dis Colon Rectum. 2017;60:219–227. doi: 10.1097/DCR.0000000000000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedrick TL, Thiele RH, Hassinger TE, et al. Multicenter observational study examining the implementation of enhanced recovery within the virginia surgical quality collaborative in patients undergoing elective colectomy. J Am Coll Surg. 2019;229:374–382.e3. doi: 10.1016/j.jamcollsurg.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 14.Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011;2011(2):CD007635. doi: 10.1002/14651858.CD007635.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Joliat G-R, Hübner M, Roulin D, Demartines N. Cost analysis of enhanced recovery programs in colorectal, pancreatic, and hepatic surgery: a systematic review. World J Surg. 2020;44:647–655. doi: 10.1007/s00268-019-05252-z. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Jensen CC. Patient satisfaction and quality of life with enhanced recovery protocols. Clin Colon Rectal Surg. 2019;32:138–144. doi: 10.1055/s-0038-1676480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Najjar PA, Fields AC, Maldonado LJ, Ward A, Bleday R. Differential index-hospitalization cost center impact of enhanced recovery after surgery program implementation. Dis Colon Rectum. 2020;63:837–841. doi: 10.1097/DCR.0000000000001662. [DOI] [PubMed] [Google Scholar]
- 18.Stone AB, Grant MC, Pio Roda C, et al. Implementation costs of an enhanced recovery after surgery program in the united states: a financial model and sensitivity analysis based on experiences at a quaternary academic medical center. J Am Coll Surg. 2016;222:219–225. doi: 10.1016/j.jamcollsurg.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Esper SA, Holder-Murray J, Subramaniam K, et al. Enhanced recovery protocols reduce mortality across eight surgical specialties at academic and university-affiliated community hospitals. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004642. [DOI] [PubMed] [Google Scholar]
- 20.Thiele RH, Sarosiek BM, Modesitt SC, et al. Development and impact of an institutional enhanced recovery program on opioid use, length of stay, and hospital costs within an academic medical center: a cohort analysis of 7774 patients. Anesth Analg. 2021;132:442–455. doi: 10.1213/ANE.0000000000005182. [DOI] [PubMed] [Google Scholar]
- 21.Currie AC, Malietzis G, Jenkins JT, et al. Network meta-analysis of protocol-driven care and laparoscopic surgery for colorectal cancer. Br J Surg. 2016;103:1783–1794. doi: 10.1002/bjs.10306. [DOI] [PubMed] [Google Scholar]
- 22.Forsmo HM, Erichsen C, Rasdal A, Körner H, Pfeffer F. Enhanced recovery after colorectal surgery (ERAS) in elderly patients is feasible and achieves similar results as in younger patients. Gerontol Geriatr Med. 2017;3:2333721417706299. doi: 10.1177/2333721417706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joris J, Hans G, Coimbra C, Decker E, Kaba A. Elderly patients over 70 years benefit from enhanced recovery programme after colorectal surgery as much as younger patients. J Visc Surg. 2020;157:23–31. doi: 10.1016/j.jviscsurg.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Launay-Savary M-V, Mathonnet M, Theissen A, Ostermann S, Raynaud-Simon A, Slim K, GRACE (Groupe francophone de Réhabilitation Améliorée après Chirurgie) Are enhanced recovery programs in colorectal surgery feasible and useful in the elderly? A systematic review of the literature. J Visc Surg. 2017;154:29–35. doi: 10.1016/j.jviscsurg.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Ostermann S, Morel P, Chalé J-J, et al. Randomized controlled trial of enhanced recovery program dedicated to elderly patients after colorectal surgery. Dis Colon Rectum. 2019;62:1105–1116. doi: 10.1097/DCR.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 26.Pirrera B, Lucchi A, Gabbianelli C, Alagna V, Martorelli G, Berti P, Panzini I, Fabbri E. Garulli G.E.R.A.S. pathway in colorectal surgery in elderly: our experience: a retrospective cohort study. Int J Surg (Lond, Engl) 2017;43:101–106. doi: 10.1016/j.ijsu.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Slieker J, Frauche P, Jurt J, et al. Enhanced recovery ERAS for elderly: a safe and beneficial pathway in colorectal surgery. Int J Colorectal Dis. 2017;32:215–221. doi: 10.1007/s00384-016-2691-6. [DOI] [PubMed] [Google Scholar]
- 28.Tan JKH, Ang JJ, Chan DKH. Enhanced recovery program versus conventional care after colorectal surgery in the geriatric population: a systematic review and meta-analysis. Surg Endosc. 2021;35:3166–3174. doi: 10.1007/s00464-020-07673-7. [DOI] [PubMed] [Google Scholar]
- 29.Tejedor P, Pastor C, Gonzalez-Ayora S, Ortega-Lopez M, Guadalajara H, Garcia-Olmo D. Short-term outcomes and benefits of ERAS program in elderly patients undergoing colorectal surgery: a case-matched study compared to conventional care. Int J Colorectal Dis. 2018;33:1251–1258. doi: 10.1007/s00384-018-3057-z. [DOI] [PubMed] [Google Scholar]
- 30.Hallam S, Rickard F, Reeves N, Messenger D, Shabbir J. Compliance with enhanced recovery protocols in elderly patients undergoing colorectal resection. Ann R Coll Surg Engl. 2018;100:570–579. doi: 10.1308/rcsann.2018.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakker N, Cakir H, Doodeman HJ, Houdijk AP. Eight years of experience with enhanced recovery after surgery in patients with colon cancer: impact of measures to improve adherence. Surgery. 2015;157:1130–1136. doi: 10.1016/j.surg.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 32.McLeod RS, Aarts MA, Chung F, et al. Development of an enhanced recovery after surgery guideline and implementation strategy based on the knowledge-to-action cycle. Ann Surg. 2015;262:1016–1025. doi: 10.1097/SLA.0000000000001067. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed J, Khan S, Lim M, Chandrasekaran TV, MacFie J. Enhanced recovery after surgery protocols—compliance and variations in practice during routine colorectal surgery. Colorectal Dis. 2012;14:1045–1051. doi: 10.1111/j.1463-1318.2011.02856.x. [DOI] [PubMed] [Google Scholar]
- 34.Day RW, Fielder S, Calhoun J, Kehlet H, Gottumukkala V, Aloia TA. Incomplete reporting of enhanced recovery elements and its impact on achieving quality improvement. Br J Surg. 2015;102:1594–1602. doi: 10.1002/bjs.9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berian JR, Ban KA, Liu JB, Ko CY, Feldman LS, Thacker JK. Adherence to enhanced recovery protocols in NSQIP and association with colectomy outcomes. Ann Surg. 2019;269:486–493. doi: 10.1097/SLA.0000000000002566. [DOI] [PubMed] [Google Scholar]
- 36.Messenger DE, Curtis NJ, Jones A, Jones EL, Smart NJ, Francis NK. Factors predicting outcome from enhanced recovery programmes in laparoscopic colorectal surgery: a systematic review. Surg Endosc. 2017;31:2050–2071. doi: 10.1007/s00464-016-5205-2. [DOI] [PubMed] [Google Scholar]
- 37.Arrick L, Mayson K, Hong T, Warnock G. Enhanced recovery after surgery in colorectal surgery: impact of protocol adherence on patient outcomes. J Clin Anesth. 2019;55:7–12. doi: 10.1016/j.jclinane.2018.12.034. [DOI] [PubMed] [Google Scholar]
- 38.Ripollés-Melchor J, Ramírez-Rodríguez JM, Casans-Francés R, POWER Study Investigators Group for the Spanish Perioperative Audit and Research Network (REDGERM) et al. Association between use of enhanced recovery after surgery protocol and postoperative complications in colorectal surgery: the postoperative outcomes within enhanced recovery after surgery protocol (POWER) study. JAMA Surg. 2019;154:725–736. doi: 10.1001/jamasurg.2019.0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleming F, Gaertner W, Ternent CA, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guideline for the Prevention of Venous Thromboembolic Disease in Colorectal Surgery. Dis Colon Rectum. 2018;61:14–20. doi: 10.1097/DCR.0000000000000982. [DOI] [PubMed] [Google Scholar]
- 40.Migaly J, Bafford AC, Francone TD, Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Use of Bowel Preparation in Elective Colon and Rectal Surgery. Dis Colon Rectum. 2019;62:3–8. doi: 10.1097/DCR.0000000000001238. [DOI] [PubMed] [Google Scholar]
- 41.Saur NM, Davis BR, Montroni I, Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Perioperative Evaluation and Management of Frailty Among Older Adults Undergoing Colorectal Surgery. Dis Colon Rectum. 2022;65:473–488. doi: 10.1097/DCR.0000000000002410. [DOI] [PubMed] [Google Scholar]
- 42.Davis BR, Valente MA, Goldberg JE, Lightner AL, Feingold DL, Paquette IM. Prepared on behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for Ostomy Surgery. Dis Colon Rectum. 2022;65:1173–1190. doi: 10.1097/DCR.0000000000002498. [DOI] [PubMed] [Google Scholar]
- 43.Carmichael JC, Keller DS, Baldini G, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum. 2017;60:761–784. doi: 10.1097/DCR.0000000000000883. [DOI] [PubMed] [Google Scholar]
- 44.Guyatt G, Guterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest. 2006;129:174–181. doi: 10.1378/chest.129.1.174. [DOI] [PubMed] [Google Scholar]
- 45.Gustafsson UO, Scott MJ, Schwenk W, Enhanced Recovery After Surgery Society et al. Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149:830–840. doi: 10.1016/j.surg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630–641. doi: 10.1016/S0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 49.Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–198. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 50.Delaney CP, Zutshi M, Senagore AJ, Remzi FH, Hammel J, Fazio VW. Prospective, randomized, controlled trial between a pathway of controlled rehabilitation with early ambulation and diet and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum. 2003;46:851–859. doi: 10.1007/s10350-004-6672-4. [DOI] [PubMed] [Google Scholar]
- 51.Merad F, Yahchouchi E, Hay JM, Fingerhut A, Laborde Y, Langlois-Zantain O, French Associations for Surgical Research Prophylactic abdominal drainage after elective colonic resection and suprapromontory anastomosis: a multicenter study controlled by randomization. Arch Surg. 1998;133:309–314. doi: 10.1001/archsurg.133.3.309. [DOI] [PubMed] [Google Scholar]
- 52.Alvarez MP, Foley KE, Zebley DM, Fassler SA. Comprehensive enhanced recovery pathway significantly reduces postoperative length of stay and opioid usage in elective laparoscopic colectomy. Surg Endosc. 2015;29:2506–2511. doi: 10.1007/s00464-014-4006-8. [DOI] [PubMed] [Google Scholar]
- 53.Chand M, De’Ath HD, Rasheed S, Mehta C, Bromilow J, Qureshi T. The influence of peri-operative factors for accelerated discharge following laparoscopic colorectal surgery when combined with an enhanced recovery after surgery (ERAS) pathway. Int J Surg. 2016;25:59–63. doi: 10.1016/j.ijsu.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 54.Gash KJ, Goede AC, Chambers W, Greenslade GL, Dixon AR. Laparoendoscopic single-site surgery is feasible in complex colorectal resections and could enable day case colectomy. Surg Endosc. 2011;25:835–840. doi: 10.1007/s00464-010-1275-8. [DOI] [PubMed] [Google Scholar]
- 55.Joh YG, Lindsetmo RO, Stulberg J, Obias V, Champagne B, Delaney CP. Standardized postoperative pathway: accelerating recovery after ileostomy closure. Dis Colon Rectum. 2008;51:1786–1789. doi: 10.1007/s10350-008-9399-9. [DOI] [PubMed] [Google Scholar]
- 56.Lawrence JK, Keller DS, Samia H, et al. Discharge within 24 to 72 hours of colorectal surgery is associated with low readmission rates when using enhanced recovery pathways. J Am Coll Surg. 2013;216:390–394. doi: 10.1016/j.jamcollsurg.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Gash KJ, Greenslade GL, Dixon AR. Enhanced recovery after laparoscopic colorectal resection with primary anastomosis: accelerated discharge is safe and does not give rise to increased readmission rates. Colorectal Dis. 2012;14:1287–1290. doi: 10.1111/j.1463-1318.2012.02969.x. [DOI] [PubMed] [Google Scholar]
- 58.Schwenk W, Günther N, Wendling P, “Fast-track” Colon II Quality Assurance Group et al. “Fast-track” rehabilitation for elective colonic surgery in Germany–prospective observational data from a multi-centre quality assurance programme. Int J Colorectal Dis. 2008;23:93–99. doi: 10.1007/s00384-007-0374-z. [DOI] [PubMed] [Google Scholar]
- 59.Christensen HK, Thaysen HV, Rodt SÅ, Carlsson P, Laurberg S. Short hospital stay and low complication rate are possible with a fully implemented fast-track model after elective colonic surgery. Eur Surg Res. 2011;46:156–161. doi: 10.1159/000324406. [DOI] [PubMed] [Google Scholar]
- 60.Delaney CP, Fazio VW, Senagore AJ, Robinson B, Halverson AL, Remzi FH. ‘Fast track’ postoperative management protocol for patients with high co-morbidity undergoing complex abdominal and pelvic colorectal surgery. Br J Surg. 2001;88:1533–1538. doi: 10.1046/j.0007-1323.2001.01905.x. [DOI] [PubMed] [Google Scholar]
- 61.Delaney CP. Outcome of discharge within 24 to 72 hours after laparoscopic colorectal surgery. Dis Colon Rectum. 2008;51:181–185. doi: 10.1007/s10350-007-9126-y. [DOI] [PubMed] [Google Scholar]
- 62.Delaney CP, Brady K, Woconish D, Parmar SP, Champagne BJ. Towards optimizing perioperative colorectal care: outcomes for 1,000 consecutive laparoscopic colon procedures using enhanced recovery pathways. Am J Surg. 2012;203:353–355. doi: 10.1016/j.amjsurg.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 63.Keller DS, Tahilramani RN, Flores-Gonzalez JR, Ibarra S, Haas EM. Pilot study of a novel pain management strategy: evaluating the impact on patient outcomes. Surg Endosc. 2016;30:2192–2198. doi: 10.1007/s00464-015-4459-4. [DOI] [PubMed] [Google Scholar]
- 64.Miller TE, Thacker JK, White WD, Enhanced Recovery Study Group et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118:1052–1061. doi: 10.1213/ANE.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 65.Rawlinson A, Kang P, Evans J, Khanna A. A systematic review of enhanced recovery protocols in colorectal surgery. Ann R Coll Surg Engl. 2011;93:583–588. doi: 10.1308/147870811X605219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neville A, Lee L, Antonescu I, et al. Systematic review of outcomes used to evaluate enhanced recovery after surgery. Br J Surg. 2014;101:159–170. doi: 10.1002/bjs.9324. [DOI] [PubMed] [Google Scholar]
- 67.Khoo CK, Vickery CJ, Forsyth N, Vinall NS, Eyre-Brook IA. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg. 2007;245:867–872. doi: 10.1097/01.sla.0000259219.08209.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ihedioha U, Vaughan S, Mastermann J, Singh B, Chaudhri S. Patient education videos for elective colorectal surgery: results of a randomized controlled trial. Colorectal Dis. 2013;15:1436–1441. doi: 10.1111/codi.12348. [DOI] [PubMed] [Google Scholar]
- 69.Kaya E, Paksoy E, Ozturk E, Sigirli D, Bilgel H. Subcutaneous closed-suction drainage does not affect surgical site infection rate following elective abdominal operations: a prospective randomized clinical trial. Acta Chir Belg. 2010;110:457–462. doi: 10.1080/00015458.2010.11680655. [DOI] [PubMed] [Google Scholar]
- 70.Forsmo HM, Pfeffer F, Rasdal A, et al. Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: results of a randomized controlled trial. Colorectal Dis. 2016;18:603–611. doi: 10.1111/codi.13253. [DOI] [PubMed] [Google Scholar]
- 71.Forsmo HM, Erichsen C, Rasdal A, Tvinnereim JM, Körner H, Pfeffer F. Randomized controlled trial of extended perioperative counseling in enhanced recovery after colorectal surgery. Dis Colon Rectum. 2018;61:724–732. doi: 10.1097/DCR.0000000000001007. [DOI] [PubMed] [Google Scholar]
- 72.Tweed TTT, Woortman C, Tummers S, Bakens MJAM, van Bastelaar J, Stoot JHMB. Reducing hospital stay for colorectal surgery in ERAS setting by means of perioperative patient education of expected day of discharge. Int J Colorectal Dis. 2021;36:1535–1542. doi: 10.1007/s00384-021-03948-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pędziwiatr M, Kisialeuski M, Wierdak M, et al. Early implementation of Enhanced Recovery After Surgery (ERAS®) protocol—Compliance improves outcomes: a prospective cohort study. Int J Surg. 2015;21:75–81. doi: 10.1016/j.ijsu.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 74.Wolk S, Distler M, Müssle B, Söthje S, Weitz J, Welsch T. Adherence to ERAS elements in major visceral surgery-an observational pilot study. Langenbecks Arch Surg. 2016;401:349–356. doi: 10.1007/s00423-016-1407-2. [DOI] [PubMed] [Google Scholar]
- 75.Nelson G, Kiyang LN, Crumley ET, et al. Implementation of enhanced recovery after surgery (ERAS) across a provincial healthcare system: the ERAS Alberta colorectal surgery experience. World J Surg. 2016;40:1092–1103. doi: 10.1007/s00268-016-3472-7. [DOI] [PubMed] [Google Scholar]
- 76.Francis NK, Mason J, Salib E, et al. Factors predicting 30-day readmission after laparoscopic colorectal cancer surgery within an enhanced recovery programme. Colorectal Dis. 2015;17:O148–O154. doi: 10.1111/codi.13002. [DOI] [PubMed] [Google Scholar]
- 77.Simpson JC, Moonesinghe SR, Grocott MP, National Enhanced Recovery Partnership Advisory Board et al. Enhanced recovery from surgery in the UK: an audit of the enhanced recovery partnership programme 2009–2012. Br J Anaesth. 2015;115:560–568. doi: 10.1093/bja/aev105. [DOI] [PubMed] [Google Scholar]
- 78.Cavallaro PM, Milch H, Savitt L, et al. Addition of a scripted pre-operative patient education module to an existing ERAS pathway further reduces length of stay. Am J Surg. 2018;216:652–657. doi: 10.1016/j.amjsurg.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 79.Mata J, Pecorelli N, Kaneva P, et al. A mobile device application (app) to improve adherence to an enhanced recovery program for colorectal surgery: a randomized controlled trial. Surg Endosc. 2020;34:742–751. doi: 10.1007/s00464-019-06823-w. [DOI] [PubMed] [Google Scholar]
- 80.Chaudhri S, Brown L, Hassan I, Horgan AF. Preoperative intensive, community-based vs. traditional stoma education: a randomized, controlled trial. Dis Colon Rectum. 2005;48:504–509. doi: 10.1007/s10350-004-0897-0. [DOI] [PubMed] [Google Scholar]
- 81.Cartmell MT, Jones OM, Moran BJ, Cecil TD. A defunctioning stoma significantly prolongs the length of stay in laparoscopic colorectal resection. Surg Endosc. 2008;22:2643–2647. doi: 10.1007/s00464-008-9776-4. [DOI] [PubMed] [Google Scholar]
- 82.Ulrich AB, Seiler C, Rahbari N, Weitz J, Büchler MW. Diverting stoma after low anterior resection: more arguments in favor. Dis Colon Rectum. 2009;52:412–418. doi: 10.1007/DCR.0b013e318197e1b1. [DOI] [PubMed] [Google Scholar]
- 83.King PM, Blazeby JM, Ewings P, et al. The influence of an enhanced recovery programme on clinical outcomes, costs and quality of life after surgery for colorectal cancer. Colorectal Dis. 2006;8:506–513. doi: 10.1111/j.1463-1318.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 84.Danielsen AK, Burcharth J, Rosenberg J. Patient education has a positive effect in patients with a stoma: a systematic review. Colorectal Dis. 2013;15:e276–e283. doi: 10.1111/codi.12197. [DOI] [PubMed] [Google Scholar]
- 85.Altuntas YE, Kement M, Gezen C, et al. The role of group education on quality of life in patients with a stoma. Eur J Cancer Care (Engl) 2012;21:776–781. doi: 10.1111/j.1365-2354.2012.01360.x. [DOI] [PubMed] [Google Scholar]
- 86.Hughes MJ, Cunningham W, Yalamarthi S. The effect of preoperative stoma training for patients undergoing colorectal surgery in an enhanced recovery programme. Ann R Coll Surg Engl. 2020;102:180–184. doi: 10.1308/rcsann.2019.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Danielsen AK, Rosenberg J. Health related quality of life may increase when patients with a stoma attend patient education–a case-control study. PLoS ONE. 2014;9:e90354. doi: 10.1371/journal.pone.0090354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bass EM, Del Pino A, Tan A, Pearl RK, Orsay CP, Abcarian H. Does preoperative stoma marking and education by the enterostomal therapist affect outcome? Dis Colon Rectum. 1997;40:440–442. doi: 10.1007/BF02258389. [DOI] [PubMed] [Google Scholar]
- 89.Person B, Ifargan R, Lachter J, Duek SD, Kluger Y, Assalia A. The impact of preoperative stoma site marking on the incidence of complications, quality of life, and patient’s independence. Dis Colon Rectum. 2012;55:783–787. doi: 10.1097/DCR.0b013e31825763f0. [DOI] [PubMed] [Google Scholar]
- 90.McKenna LS, Taggart E, Stoelting J, Kirkbride G, Forbes GB. The impact of preoperative stoma marking on health-related quality of life: a comparison cohort study. J Wound Ostomy Continence Nurs. 2016;43:57–61. doi: 10.1097/WON.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 91.Baykara ZG, Demir SG, Karadag A, et al. A multicenter, retrospective study to evaluate the effect of preoperative stoma site marking on stomal and peristomal complications. Ostomy Wound Manage. 2014;60:16–26. [PubMed] [Google Scholar]
- 92.Millan M, Tegido M, Biondo S, García-Granero E. Preoperative stoma siting and education by stomatherapists of colorectal cancer patients: a descriptive study in twelve Spanish colorectal surgical units. Colorectal Dis. 2010;12:e88–e92. doi: 10.1111/j.1463-1318.2009.01942.x. [DOI] [PubMed] [Google Scholar]
- 93.Goldblatt J, Buxey K, Paul E, Foot-Connolly R, Leech T, Bell S. Study on the time taken for patients to achieve the ability to self-care their new stoma. ANZ J Surg. 2018;88:E503–E506. doi: 10.1111/ans.14195. [DOI] [PubMed] [Google Scholar]
- 94.Younis J, Salerno G, Fanto D, Hadjipavlou M, Chellar D, Trickett JP. Focused preoperative patient stoma education, prior to ileostomy formation after anterior resection, contributes to a reduction in delayed discharge within the enhanced recovery programme. Int J Colorectal Dis. 2012;27:43–47. doi: 10.1007/s00384-011-1252-2. [DOI] [PubMed] [Google Scholar]
- 95.Grahn SW, Lowry AC, Osborne MC, et al. System-wide improvement for transitions after ileostomy surgery: can intensive monitoring of protocol compliance decrease readmissions? A randomized trial. Dis Colon Rectum. 2019;62:363–370. doi: 10.1097/DCR.0000000000001286. [DOI] [PubMed] [Google Scholar]
- 96.Stokes AL, Tice S, Follett S, et al. Institution of a preoperative stoma education group class decreases rate of peristomal complications in new stoma patients. J Wound Ostomy Continence Nurs. 2017;44:363–367. doi: 10.1097/WON.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 97.Rashidi L, Long K, Hawkins M, Menon R, Bellevue O. Stoma creation: does onset of ostomy care education delay hospital length of stay? Am J Surg. 2016;211:954–957. doi: 10.1016/j.amjsurg.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 98.Messaris E, Sehgal R, Deiling S, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum. 2012;55:175–180. doi: 10.1097/DCR.0b013e31823d0ec5. [DOI] [PubMed] [Google Scholar]
- 99.Hayden DM, Pinzon MC, Francescatti AB, et al. Hospital readmission for fluid and electrolyte abnormalities following ileostomy construction: preventable or unpredictable? J Gastrointest Surg. 2013;17:298–303. doi: 10.1007/s11605-012-2073-5. [DOI] [PubMed] [Google Scholar]
- 100.Halverson AL, Sellers MM, Bilimoria KY, et al. Identification of process measures to reduce postoperative readmission. J Gastrointest Surg. 2014;18:1407–1415. doi: 10.1007/s11605-013-2429-5. [DOI] [PubMed] [Google Scholar]
- 101.Gonella F, Valenti A, Massucco P, et al. A novel patient-centered protocol to reduce hospital readmissions for dehydration after ileostomy. Updates Surg. 2019;71:515–521. doi: 10.1007/s13304-019-00643-2. [DOI] [PubMed] [Google Scholar]
- 102.Iqbal A, Raza A, Huang E, Goldstein L, Hughes SJ, Tan SA. Cost effectiveness of a novel attempt to reduce readmission after ileostomy creation. JSLS. 2017;21:21. doi: 10.4293/JSLS.2016.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maltby JR, Sutherland AD, Sale JP, Shaffer EA. Preoperative oral fluids: is a five-hour fast justified prior to elective surgery? Anesth Analg. 1986;65:1112–1116. doi: 10.1213/00000539-198611000-00003. [DOI] [PubMed] [Google Scholar]
- 104.Sutherland AD, Maltby JR, Sale JP, Reid CR. The effect of preoperative oral fluid and ranitidine on gastric fluid volume and pH. Can J Anaesth J Can d'anesth. 1987;34:117–121. doi: 10.1007/BF03015327. [DOI] [PubMed] [Google Scholar]
- 105.Hutchinson A, Maltby JR, Reid CR. Gastric fluid volume and pH in elective inpatients. Part I: coffee or orange juice versus overnight fast. Can J Anaesth J Can d'anesth. 1988;35:12–15. doi: 10.1007/BF03010537. [DOI] [PubMed] [Google Scholar]
- 106.McGrady EM, Macdonald AG. Effect of the preoperative administration of water on gastric volume and pH. Br J Anaesth. 1988;60:803–805. doi: 10.1093/bja/60.7.803. [DOI] [PubMed] [Google Scholar]
- 107.Agarwal A, Chari P, Singh H. Fluid deprivation before operation. The effect of a small drink. Anaesthesia. 1989;44:632–634. doi: 10.1111/j.1365-2044.1989.tb13581.x. [DOI] [PubMed] [Google Scholar]
- 108.Read MS, Vaughan RS. Allowing pre-operative patients to drink: effects on patients’ safety and comfort of unlimited oral water until 2 hours before anaesthesia. Acta Anaesthesiol Scand. 1991;35:591–595. doi: 10.1111/j.1399-6576.1991.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 109.Phillips S, Hutchinson S, Davidson T. Preoperative drinking does not affect gastric contents. Br J Anaesth. 1993;70:6–9. doi: 10.1093/bja/70.1.6. [DOI] [PubMed] [Google Scholar]
- 110.Yagci G, Can MF, Ozturk E, et al. Effects of preoperative carbohydrate loading on glucose metabolism and gastric contents in patients undergoing moderate surgery: a randomized, controlled trial. Nutrition. 2008;24:212–216. doi: 10.1016/j.nut.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 111.Practice Guidelines for Preoperative Fasting and the Use of Pharmacologic Agents to Reduce the Risk of Pulmonary Aspiration Application to healthy patients undergoing elective procedures: an updated report by the american society of anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology. 2017;126:376–393. doi: 10.1097/ALN.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 112.American Society of Anesthesiologists Committee Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: an updated report by the American Society of Anesthesiologists Committee on Standards and Practice Parameters. Anesthesiology. 2011;114:495–511. doi: 10.1097/ALN.0b013e3181fcbfd9. [DOI] [PubMed] [Google Scholar]
- 113.Smith I, Kranke P, Murat I, European Society of Anaesthesiology et al. Perioperative fasting in adults and children: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:556–569. doi: 10.1097/EJA.0b013e3283495ba1. [DOI] [PubMed] [Google Scholar]
- 114.Smith MD, McCall J, Plank L, Herbison GP, Soop M, Nygren J. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev. 2014;8:CD009161. doi: 10.1002/14651858.CD009161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rizvanović N, Nesek Adam V, Čaušević S, Dervišević S, Delibegović S. A randomised controlled study of preoperative oral carbohydrate loading versus fasting in patients undergoing colorectal surgery. Int J Colorectal Dis. 2019;34:1551–1561. doi: 10.1007/s00384-019-03349-4. [DOI] [PubMed] [Google Scholar]
- 116.Shi M, Hu Z, Yang D, Cai Q, Zhu Z. Preoperative oral carbohydrate reduces postoperative insulin resistance by activating AMP-activated protein kinase after colorectal surgery. Dig Surg. 2020;37:368–375. doi: 10.1159/000505515. [DOI] [PubMed] [Google Scholar]
- 117.Awad S, Varadhan KK, Ljungqvist O, Lobo DN. A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin Nutr. 2013;32:34–44. doi: 10.1016/j.clnu.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 118.Amer MA, Smith MD, Herbison GP, Plank LD, McCall JL. Network meta-analysis of the effect of preoperative carbohydrate loading on recovery after elective surgery. Br J Surg. 2017;104:187–197. doi: 10.1002/bjs.10408. [DOI] [PubMed] [Google Scholar]
- 119.Weimann A, Braga M, Harsanyi L, DGEM (German Society for Nutritional Medicine); ESPEN (European Society for Parenteral and Enteral Nutrition) et al. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clin Nutr. 2006;25:224–244. doi: 10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 120.Wischmeyer PE, Carli F, Evans DC, Perioperative Quality Initiative (POQI) 2 Workgroup et al. American Society for Enhanced recovery and perioperative quality initiative joint consensus statement on nutrition screening and therapy within a surgical enhanced recovery pathway. Anesth Analg. 2018;126:1883–1895. doi: 10.1213/ANE.0000000000002743. [DOI] [PubMed] [Google Scholar]
- 121.Kong SH, Lee HJ, Na JR, et al. Effect of perioperative oral nutritional supplementation in malnourished patients who undergo gastrectomy: a prospective randomized trial. Surgery. 2018;164:1263–1270. doi: 10.1016/j.surg.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 122.Kabata P, Jastrzębski T, Kąkol M, et al. Preoperative nutritional support in cancer patients with no clinical signs of malnutrition–prospective randomized controlled trial. Support Care Cancer. 2015;23:365–370. doi: 10.1007/s00520-014-2363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Adiamah A, Skořepa P, Weimann A, Lobo DN. The impact of preoperative immune modulating nutrition on outcomes in patients undergoing surgery for gastrointestinal cancer: a systematic review and meta-analysis. Ann Surg. 2019;270:247–256. doi: 10.1097/SLA.0000000000003256. [DOI] [PubMed] [Google Scholar]
- 124.Hegazi RA, Hustead DS, Evans DC. Preoperative standard oral nutrition supplements vs immunonutrition: results of a systematic review and meta-analysis. J Am Coll Surg. 2014;219:1078–1087. doi: 10.1016/j.jamcollsurg.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 125.Probst P, Ohmann S, Klaiber U, et al. Meta-analysis of immunonutrition in major abdominal surgery. Br J Surg. 2017;104:1594–1608. doi: 10.1002/bjs.10659. [DOI] [PubMed] [Google Scholar]
- 126.Lee SY, Lee J, Park HM, Kim CH, Kim HR. Impact of preoperative immunonutrition on the outcomes of colon cancer surgery: results from a randomized controlled trial. Ann Surg. 2022 doi: 10.1097/SLA.0000000000005140. [DOI] [PubMed] [Google Scholar]
- 127.Güenaga KF, Matos D, Wille-Jørgensen P. Mechanical bowel preparation for elective colorectal surgery. Cochrane Database Syst Rev. 2011;9:CD001544. doi: 10.1002/14651858.CD001544.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen M, Song X, Chen LZ, Lin ZD, Zhang XL. Comparing mechanical bowel preparation with both oral and systemic antibiotics versus mechanical bowel preparation and systemic antibiotics alone for the prevention of surgical site infection after elective colorectal surgery: a meta-analysis of randomized controlled clinical trials. Dis Colon Rectum. 2016;59:70–78. doi: 10.1097/DCR.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 129.Moghadamyeghaneh Z, Hwang GS, Hanna MH, et al. Surgical site infection impact of pelvic exenteration procedure. J Surg Oncol. 2015;112:533–537. doi: 10.1002/jso.24023. [DOI] [PubMed] [Google Scholar]
- 130.Toneva GD, Deierhoi RJ, Morris M, et al. Oral antibiotic bowel preparation reduces length of stay and readmissions after colorectal surgery. J Am Coll Surg. 2013;216:756–762. doi: 10.1016/j.jamcollsurg.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 131.Mik M, Berut M, Trzcinski R, Dziki L, Buczynski J, Dziki A. Preoperative oral antibiotics reduce infections after colorectal cancer surgery. Langenbecks Arch Surg. 2016;401:1153–1162. doi: 10.1007/s00423-016-1513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim EK, Sheetz KH, Bonn J, et al. A statewide colectomy experience: the role of full bowel preparation in preventing surgical site infection. Ann Surg. 2014;259:310–314. doi: 10.1097/SLA.0b013e3182a62643. [DOI] [PubMed] [Google Scholar]
- 133.Le Roy B, Selvy M, Slim K. The concept of prehabilitation: what the surgeon needs to know? J Visc Surg. 2016;153:109–112. doi: 10.1016/j.jviscsurg.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 134.Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care. 2005;8:23–32. doi: 10.1097/00075197-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 135.Bruns ER, van den Heuvel B, Buskens CJ, et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Dis. 2016;18:O267–O277. doi: 10.1111/codi.13429. [DOI] [PubMed] [Google Scholar]
- 136.Carli F, Bousquet-Dion G, Awasthi R, et al. Effect of multimodal prehabilitation vs postoperative rehabilitation on 30-day postoperative complications for frail patients undergoing resection of colorectal cancer: a randomized clinical trial. JAMA Surg. 2020;155:233–242. doi: 10.1001/jamasurg.2019.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bousquet-Dion G, Awasthi R, Loiselle SE, et al. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial. Acta Oncol. 2018;57:849–859. doi: 10.1080/0284186X.2017.1423180. [DOI] [PubMed] [Google Scholar]
- 138.Minnella EM, Bousquet-Dion G, Awasthi R, Scheede-Bergdahl C, Carli F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: a five-year research experience. Acta Oncol. 2017;56:295–300. doi: 10.1080/0284186X.2016.1268268. [DOI] [PubMed] [Google Scholar]
- 139.Fulop A, Lakatos L, Susztak N, Szijarto A, Banky B. The effect of trimodal prehabilitation on the physical and psychological health of patients undergoing colorectal surgery: a randomised clinical trial. Anaesthesia. 2021;76:82–90. doi: 10.1111/anae.15215. [DOI] [PubMed] [Google Scholar]
- 140.Chen BP, Awasthi R, Sweet SN, et al. Four-week prehabilitation program is sufficient to modify exercise behaviors and improve preoperative functional walking capacity in patients with colorectal cancer. Support Care Cancer. 2017;25:33–40. doi: 10.1007/s00520-016-3379-8. [DOI] [PubMed] [Google Scholar]
- 141.Barberan-Garcia A, Ubré M, Roca J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg. 2018;267:50–56. doi: 10.1097/SLA.0000000000002293. [DOI] [PubMed] [Google Scholar]
- 142.Berkel AEM, Bongers BC, Kotte H, et al. Effects of community-based exercise prehabilitation for patients scheduled for colorectal surgery with high risk for postoperative complications: results of a randomized clinical trial. Ann Surg. 2022;275:e299–e306. doi: 10.1097/SLA.0000000000004702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Onerup A, Andersson J, Angenete E, et al. Effect of short-term homebased pre- and postoperative exercise on recovery after colorectal cancer surgery (PHYSSURG-C): a randomized clinical trial. Ann Surg. 2022;275:448–455. doi: 10.1097/SLA.0000000000004901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gillis C, Buhler K, Bresee L, et al. Effects of nutritional prehabilitation, with and without exercise, on outcomes of patients who undergo colorectal surgery: a systematic review and meta-analysis. Gastroenterology. 2018;155:391–410.e4. doi: 10.1053/j.gastro.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 145.Teo JYK, Turner R, Self M. Effect of exercise prehabilitation on functional status of patients undergoing bowel resection: a systematic review. ANZ J Surg. 2020;90:693–701. doi: 10.1111/ans.15659. [DOI] [PubMed] [Google Scholar]
- 146.van Rooijen SJ, Engelen MA, Scheede-Bergdahl C, et al. Systematic review of exercise training in colorectal cancer patients during treatment. Scand J Med Sci Sports. 2018;28:360–370. doi: 10.1111/sms.12907. [DOI] [PubMed] [Google Scholar]
- 147.Boereboom C, Doleman B, Lund JN, Williams JP. Systematic review of pre-operative exercise in colorectal cancer patients. Tech Coloproctol. 2016;20:81–89. doi: 10.1007/s10151-015-1407-1. [DOI] [PubMed] [Google Scholar]
- 148.Piraux E, Caty G, Reychler G. Effects of preoperative combined aerobic and resistance exercise training in cancer patients undergoing tumour resection surgery: a systematic review of randomised trials. Surg Oncol. 2018;27:584–594. doi: 10.1016/j.suronc.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 149.Looijaard SMLM, Slee-Valentijn MS, Otten RHJ, Maier AB. Physical and nutritional prehabilitation in older patients with colorectal carcinoma: a systematic review. J Geriatr Phys Ther. 2018;41:236–244. doi: 10.1519/JPT.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 150.Kamarajah SK, Bundred J, Weblin J, Tan BHL. Critical appraisal on the impact of preoperative rehabilitation and outcomes after major abdominal and cardiothoracic surgery: a systematic review and meta-analysis. Surgery. 2020;167:540–549. doi: 10.1016/j.surg.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 151.Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL. A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366) J Gastrointest Surg. 2020;24:1375–1385. doi: 10.1007/s11605-019-04287-w. [DOI] [PubMed] [Google Scholar]
- 152.Minnella EM, Awasthi R, Gillis C, et al. Patients with poor baseline walking capacity are most likely to improve their functional status with multimodal prehabilitation. Surgery. 2016;160:1070–1079. doi: 10.1016/j.surg.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 153.Group EC; ERAS Compliance Group The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg. 2015;261:1153–1159. doi: 10.1097/SLA.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 154.Aarts MA, Rotstein OD, Pearsall EA, iERAS group et al. Postoperative ERAS interventions have the greatest impact on optimal recovery: experience with implementation of ERAS across multiple hospitals. Ann Surg. 2018;267:992–997. doi: 10.1097/SLA.0000000000002632. [DOI] [PubMed] [Google Scholar]
- 155.Ahmed J, Khan S, Gatt M, Kallam R, MacFie J. Compliance with enhanced recovery programmes in elective colorectal surgery. Br J Surg. 2010;97:754–758. doi: 10.1002/bjs.6961. [DOI] [PubMed] [Google Scholar]
- 156.Maessen J, Dejong CH, Hausel J, et al. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg. 2007;94:224–231. doi: 10.1002/bjs.5468. [DOI] [PubMed] [Google Scholar]
- 157.Carter F, Kennedy RH. Setting up an enhanced recovery programme. In: Francis N, editor. Manual of fast-track recovery for colorectal surgery London. London: Springer; 2012. pp. 131–142. [Google Scholar]
- 158.Wei IH, Pappou EP, Smith JJ, et al. Monitoring an ongoing enhanced recovery after surgery (ERAS) program: adherence improves clinical outcomes in a comparison of three thousand colorectal cases. Clin Surg. 2020;5:2909. [PMC free article] [PubMed] [Google Scholar]
- 159.Lohsiriwat V. High Compliance with surgical site infection (SSI) prevention bundle reduces incisional SSI after colorectal surgery. Ann Coloproctol. 2021;37:146–152. doi: 10.3393/ac.2020.04.10.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vu JV, Collins SD, Seese E, et al. Evidence that a regional surgical collaborative can transform care: surgical site infection prevention practices for colectomy in Michigan. J Am Coll Surg. 2018;226:91–99. doi: 10.1016/j.jamcollsurg.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 161.Albert H, Bataller W, Masroor N, et al. Infection prevention and enhanced recovery after surgery: a partnership for implementation of an evidence-based bundle to reduce colorectal surgical site infections. Am J Infect Control. 2019;47:718–719. doi: 10.1016/j.ajic.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 162.D’Souza K, Choi JI, Wootton J, Wallace T. Impact of sequential implementation of multimodal perioperative care pathways on colorectal surgical outcomes. Can J Surg. 2019;62:25–32. doi: 10.1503/cjs.015617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hajirawala LN, Legare TB, Tiu SPT, et al. The impact of a colorectal care bundle for surgical site infections at an academic disproportionate share hospital with a level I trauma center. Am Surg. 2020;86:848–855. doi: 10.1177/0003134820940240. [DOI] [PubMed] [Google Scholar]
- 164.Hoang SC, Klipfel AA, Roth LA, Vrees M, Schechter S, Shah N. Colon and rectal surgery surgical site infection reduction bundle: to improve is to change. Am J Surg. 2019;217:40–45. doi: 10.1016/j.amjsurg.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 165.Martinez C, Omesiete P, Pandit V, et al. A protocol-driven reduction in surgical site infections after colon surgery. J Surg Res. 2020;246:100–105. doi: 10.1016/j.jss.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 166.Rumberger LK, Vittetoe D, Cathey L, Bennett H, Heidel RE, Daley BJ. Improving outcomes in elective colorectal surgery: a single-institution retrospective review. Am Surg. 2016;82:325–330. doi: 10.1177/000313481608200417. [DOI] [PubMed] [Google Scholar]
- 167.Weiser MR, Gonen M, Usiak S, Memorial Sloan Kettering Multidisciplinary Surgical-Site Infection Reduction Team et al. Effectiveness of a multidisciplinary patient care bundle for reducing surgical-site infections. Br J Surg. 2018;105:1680–1687. doi: 10.1002/bjs.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Keenan JE, Speicher PJ, Thacker JK, Walter M, Kuchibhatla M, Mantyh CR. The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg. 2014;149:1045–1052. doi: 10.1001/jamasurg.2014.346. [DOI] [PubMed] [Google Scholar]
- 169.Ruiz-Tovar J, Boermeester MA, Bordeianou L, Colorectal Delphi Facilitating Group et al. Delphi consensus on intraoperative technical/surgical aspects to prevent surgical site infection after colorectal surgery. J Am Coll Surg. 2022;234:1–11. doi: 10.1097/XCS.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zywot A, Lau CSM, Stephen Fletcher H, Paul S. Bundles prevent surgical site infections after colorectal surgery: meta-analysis and systematic review. J Gastrointest Surg. 2017;21:1915–1930. doi: 10.1007/s11605-017-3465-3. [DOI] [PubMed] [Google Scholar]
- 171.Pop-Vicas AE, Abad C, Baubie K, Osman F, Heise C, Safdar N. Colorectal bundles for surgical site infection prevention: A systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2020;41:805–812. doi: 10.1017/ice.2020.112. [DOI] [PubMed] [Google Scholar]
- 172.De Roo AC, Vu JV, Regenbogen SE. Statewide utilization of multimodal analgesia and length of stay after colectomy. J Surg Res. 2020;247:264–270. doi: 10.1016/j.jss.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chapman SJ, Garner JJ, Drake TM, Aldaffaa M, Jayne DG. Systematic review and meta-analysis of nonsteroidal anti-inflammatory drugs to improve GI recovery after colorectal surgery. Dis Colon Rectum. 2019;62:248–256. doi: 10.1097/DCR.0000000000001281. [DOI] [PubMed] [Google Scholar]
- 174.Collaborative E. EuroSurg Collaborative Safety and efficacy of non-steroidal anti-inflammatory drugs to reduce ileus after colorectal surgery. Br J Surg. 2020;107:e161–e169. doi: 10.1002/bjs.11326. [DOI] [PubMed] [Google Scholar]
- 175.Remy C, Marret E, Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94:505–513. doi: 10.1093/bja/aei085. [DOI] [PubMed] [Google Scholar]
- 176.Aryaie AH, Lalezari S, Sergent WK, et al. Decreased opioid consumption and enhance recovery with the addition of IV Acetaminophen in colorectal patients: a prospective, multi-institutional, randomized, double-blinded, placebo-controlled study (DOCIVA study) Surg Endosc. 2018;32:3432–3438. doi: 10.1007/s00464-018-6062-y. [DOI] [PubMed] [Google Scholar]
- 177.Apfel CC, Turan A, Souza K, Pergolizzi J, Hornuss C. Intravenous acetaminophen reduces postoperative nausea and vomiting: a systematic review and meta-analysis. Pain. 2013;154:677–689. doi: 10.1016/j.pain.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 178.Wu CL, Rowlingson AJ, Partin AW, et al. Correlation of postoperative pain to quality of recovery in the immediate postoperative period. Reg Anesth Pain Med. 2005;30:516–522. doi: 10.1097/00115550-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 179.Lee A, Cooper MG, Craig JC, Knight JF, Keneally JP. Effects of nonsteroidal anti-inflammatory drugs on postoperative renal function in adults with normal renal function. Cochrane Database Syst Rev. 2007;2:CD002765. doi: 10.1002/14651858.CD002765.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang Y, Tang SR, Young CJ. Nonsteroidal anti-inflammatory drugs and anastomotic dehiscence after colorectal surgery: a meta-analysis. ANZ J Surg. 2018;88:959–965. doi: 10.1111/ans.14322. [DOI] [PubMed] [Google Scholar]
- 181.Modasi A, Pace D, Godwin M, Smith C, Curtis B. NSAID administration post colorectal surgery increases anastomotic leak rate: systematic review/meta-analysis. Surg Endosc. 2019;33:879–885. doi: 10.1007/s00464-018-6355-1. [DOI] [PubMed] [Google Scholar]
- 182.Bhangu A, Singh P, Fitzgerald JE, Slesser A, Tekkis P. Postoperative nonsteroidal anti-inflammatory drugs and risk of anastomotic leak: meta-analysis of clinical and experimental studies. World J Surg. 2014;38:2247–2257. doi: 10.1007/s00268-014-2531-1. [DOI] [PubMed] [Google Scholar]
- 183.Hakkarainen TW, Steele SR, Bastaworous A, et al. Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP) JAMA Surg. 2015;150:223–228. doi: 10.1001/jamasurg.2014.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gorissen KJ, Benning D, Berghmans T, et al. Risk of anastomotic leakage with non-steroidal anti-inflammatory drugs in colorectal surgery. Br J Surg. 2012;99:721–727. doi: 10.1002/bjs.8691. [DOI] [PubMed] [Google Scholar]
- 185.Bakker N, Deelder JD, Richir MC, et al. Risk of anastomotic leakage with nonsteroidal anti-inflammatory drugs within an enhanced recovery program. J Gastrointest Surg. 2016;20:776–782. doi: 10.1007/s11605-015-3010-1. [DOI] [PubMed] [Google Scholar]
- 186.Ohnuma T, Krishnamoorthy V, Ellis AR, et al. Association between gabapentinoids on the day of colorectal surgery and adverse postoperative respiratory outcomes. Ann Surg. 2019;270:e65–e67. doi: 10.1097/SLA.0000000000003317. [DOI] [PubMed] [Google Scholar]
- 187.Ohnuma T, Raghunathan K, Moore S, et al. Dose-dependent association of gabapentinoids with pulmonary complications after total hip and knee arthroplasties. J Bone Joint Surg Am. 2020;102:221–229. doi: 10.2106/JBJS.19.00889. [DOI] [PubMed] [Google Scholar]
- 188.Verret M, Lauzier F, Zarychanski R, Canadian Perioperative Anesthesia Clinical Trials (PACT) Group et al. Perioperative use of gabapentinoids for the management of postoperative acute pain: a systematic review and meta-analysis. Anesthesiology. 2020;133:265–279. doi: 10.1097/ALN.0000000000003428. [DOI] [PubMed] [Google Scholar]
- 189.Edwards DA, Hedrick TL, Jayaram J, POQI-4 Working Group et al. American Society for enhanced recovery and perioperative quality initiative joint consensus statement on perioperative management of patients on preoperative opioid therapy. Anesth Analg. 2019;129:553–566. doi: 10.1213/ANE.0000000000004018. [DOI] [PubMed] [Google Scholar]
- 190.Brinck EC, Tiippana E, Heesen M, et al. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev. 2018;12:CD012033. doi: 10.1002/14651858.CD012033.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.McKay WP, Donais P. Bowel function after bowel surgery: morphine with ketamine or placebo; a randomized controlled trial pilot study. Acta Anaesthesiol Scand. 2007;51:1166–1171. doi: 10.1111/j.1399-6576.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 192.De Oliveira GS, Jr, Castro-Alves LJ, Khan JH, McCarthy RJ. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2013;119:178–190. doi: 10.1097/ALN.0b013e318297630d. [DOI] [PubMed] [Google Scholar]
- 193.Hamid HKS, Marc-Hernández A, Saber AA. Transversus abdominis plane block versus thoracic epidural analgesia in colorectal surgery: a systematic review and meta-analysis. Langenbecks Arch Surg. 2021;406:273–282. doi: 10.1007/s00423-020-01995-9. [DOI] [PubMed] [Google Scholar]
- 194.Peltrini R, Cantoni V, Green R, et al. Efficacy of transversus abdominis plane (TAP) block in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol. 2020;24:787–802. doi: 10.1007/s10151-020-02206-9. [DOI] [PubMed] [Google Scholar]
- 195.Hamid HK, Emile SH, Saber AA, Ruiz-Tovar J, Minas V, Cataldo TE. Laparoscopic-guided transversus abdominis plane block for postoperative pain management in minimally invasive surgery: systematic review and meta-analysis. J Am Coll Surg. 2020;231:376–386.e15. doi: 10.1016/j.jamcollsurg.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 196.Ilfeld BM, Eisenach JC, Gabriel RA. Clinical effectiveness of liposomal bupivacaine administered by infiltration or peripheral nerve block to treat postoperative pain. Anesthesiology. 2021;134:283–344. doi: 10.1097/ALN.0000000000003630. [DOI] [PubMed] [Google Scholar]
- 197.Keller DS, Pedraza R, Tahilramani RN, Flores-Gonzalez JR, Ibarra S, Haas EM. Impact of long-acting local anesthesia on clinical and financial outcomes in laparoscopic colorectal surgery. Am J Surg. 2017;214:53–58. doi: 10.1016/j.amjsurg.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 198.Byrnes KG, Sahebally SM, Burke JP. Effect of liposomal bupivacaine on opioid requirements and length of stay in colorectal enhanced recovery pathways: a systematic review and network meta-analysis. Colorectal Dis. 2021;23:603–613. doi: 10.1111/codi.15377. [DOI] [PubMed] [Google Scholar]
- 199.Truong A, Fleshner PR, Mirocha JM, Tran HP, Shane R, Zaghiyan KN. A prospective randomized trial of surgeon-administered intraoperative transversus abdominis plane block with bupivacaine against liposomal bupivacaine: the TINGLE Trial. Dis Colon Rectum. 2021;64:888–898. doi: 10.1097/DCR.0000000000002008. [DOI] [PubMed] [Google Scholar]
- 200.Koning MV, Teunissen AJW, van der Harst E, Ruijgrok EJ, Stolker RJ. Intrathecal morphine for laparoscopic segmental colonic resection as part of an enhanced recovery protocol: a randomized controlled trial. Reg Anesth Pain Med. 2018;43:166–173. doi: 10.1097/AAP.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Meylan N, Elia N, Lysakowski C, Tramèr MR. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. Br J Anaesth. 2009;102:156–167. doi: 10.1093/bja/aen368. [DOI] [PubMed] [Google Scholar]
- 202.Xu W, Varghese C, Bissett IP, O’Grady G, Wells CI. Network meta-analysis of local and regional analgesia following colorectal resection. Br J Surg. 2020;107:e109–e122. doi: 10.1002/bjs.11425. [DOI] [PubMed] [Google Scholar]
- 203.Practice Guidelines for the Prevention Detection, and management of respiratory depression associated with neuraxial opioid administration: an updated report by the American Society of anesthesiologists task force on neuraxial opioids and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2016;124:535–552. doi: 10.1097/ALN.0000000000000975. [DOI] [PubMed] [Google Scholar]
- 204.Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst Rev. 2018;8:CD010434. doi: 10.1002/14651858.CD010434.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Block BM, Liu SS, Rowlingson AJ, Cowan AR, Cowan JA, Jr, Wu CL. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003;290:2455–2463. doi: 10.1001/jama.290.18.2455. [DOI] [PubMed] [Google Scholar]
- 206.Al-Mazrou AM, Kiely JM, Kiran RP. Epidural analgesia in the era of enhanced recovery: time to rethink its use? Surg Endosc. 2019;33:2197–2205. doi: 10.1007/s00464-018-6505-5. [DOI] [PubMed] [Google Scholar]
- 207.Torgeson M, Kileny J, Pfeifer C, Narkiewicz L, Obi S. Conventional epidural vs transversus abdominis plane block with liposomal bupivacaine: a randomized trial in colorectal surgery. J Am Coll Surg. 2018;227:78–83. doi: 10.1016/j.jamcollsurg.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 208.Halabi WJ, Kang CY, Nguyen VQ, et al. Epidural analgesia in laparoscopic colorectal surgery: a nationwide analysis of use and outcomes. JAMA Surg. 2014;149:130–136. doi: 10.1001/jamasurg.2013.3186. [DOI] [PubMed] [Google Scholar]
- 209.Borzellino G, Francis NK, Chapuis O, Krastinova E, Dyevre V, Genna M. Role of epidural analgesia within an ERAS program after laparoscopic colorectal surgery: a review and meta-analysis of randomised controlled studies. Surg Res Pract. 2016;2016:7543684. doi: 10.1155/2016/7543684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hübner M, Blanc C, Roulin D, Winiker M, Gander S, Demartines N. Randomized clinical trial on epidural versus patient-controlled analgesia for laparoscopic colorectal surgery within an enhanced recovery pathway. Ann Surg. 2015;261:648–653. doi: 10.1097/SLA.0000000000000838. [DOI] [PubMed] [Google Scholar]
- 211.Pierre S, Benais H, Pouymayou J. Apfel's simplified score may favourably predict the risk of postoperative nausea and vomiting. Can J Anaesth J Can d'anesth. 2002;49:237–242. doi: 10.1007/BF03020521. [DOI] [PubMed] [Google Scholar]
- 212.Pierre S, Corno G, Benais H, Apfel CC. A risk score-dependent antiemetic approach effectively reduces postoperative nausea and vomiting–a continuous quality improvement initiative. Can J Anaesth J Can d'anesth. 2004;51:320–325. doi: 10.1007/BF03018235. [DOI] [PubMed] [Google Scholar]
- 213.Apfel CC, Korttila K, Abdalla M, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–2451. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Apfel CC, Läärä E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 215.Koivuranta M, Läärä E, Snåre L, Alahuhta S. A survey of postoperative nausea and vomiting. Anaesthesia. 1997;52:443–449. doi: 10.1111/j.1365-2044.1997.117-az0113.x. [DOI] [PubMed] [Google Scholar]
- 216.Apfel CC, Kranke P, Eberhart LH, Roos A, Roewer N. Comparison of predictive models for postoperative nausea and vomiting. Br J Anaesth. 2002;88:234–240. doi: 10.1093/bja/88.2.234. [DOI] [PubMed] [Google Scholar]
- 217.Lee SH, Sim WS, Kim GE, et al. Randomized trial of subfascial infusion of ropivacaine for early recovery in laparoscopic colorectal cancer surgery. Korean J Anesthesiol. 2016;69:604–613. doi: 10.4097/kjae.2016.69.6.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.McIsaac DI, Cole ET, McCartney CJ. Impact of including regional anaesthesia in enhanced recovery protocols: a scoping review. Br J Anaesth. 2015;115(suppl 2):ii46–ii56. doi: 10.1093/bja/aev376. [DOI] [PubMed] [Google Scholar]
- 219.Schraag S, Pradelli L, Alsaleh AJO, et al. Propofol vs. inhalational agents to maintain general anaesthesia in ambulatory and in-patient surgery: a systematic review and meta-analysis. BMC Anesthesiol. 2018;18:162. doi: 10.1186/s12871-018-0632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gan TJ, Belani KG, Bergese S, et al. Fourth Consensus Guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2020;131:411–448. doi: 10.1213/ANE.0000000000004833. [DOI] [PubMed] [Google Scholar]
- 221.Malhotra R, Kumar N, Jain A. Cost identification analysis of general anesthesia. J Anaesthesiol Clin Pharmacol. 2020;36:219–226. doi: 10.4103/joacp.JOACP_77_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kampmeier T, Rehberg S, Omar Alsaleh AJ, Schraag S, Pham J, Westphal M. Cost-effectiveness of propofol (Diprivan) versus inhalational anesthetics to maintain general anesthesia in noncardiac surgery in the United States. Value Health. 2021;24:939–947. doi: 10.1016/j.jval.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 223.Kappen TH, Vergouwe Y, van Wolfswinkel L, Kalkman CJ, Moons KG, van Klei WA. Impact of adding therapeutic recommendations to risk assessments from a prediction model for postoperative nausea and vomiting. Br J Anaesth. 2015;114:252–260. doi: 10.1093/bja/aeu321. [DOI] [PubMed] [Google Scholar]
- 224.Kolanek B, Svartz L, Robin F, et al. Management program decreases postoperative nausea and vomiting in high-risk and in general surgical patients: a quality improvement cycle. Miner Anestesiol. 2014;80:337–346. [PubMed] [Google Scholar]
- 225.Kooij FO, Vos N, Siebenga P, Klok T, Hollmann MW, Kal JE. Automated reminders decrease postoperative nausea and vomiting incidence in a general surgical population. Br J Anaesth. 2012;108:961–965. doi: 10.1093/bja/aes024. [DOI] [PubMed] [Google Scholar]
- 226.Mayeur C, Robin E, Kipnis E, et al. Impact of a prophylactic strategy on the incidence of nausea and vomiting after general surgery. Ann Fr Anesth Reanim. 2012;31:e53–e57. doi: 10.1016/j.annfar.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 227.Eberhart LH, Morin AM. Risk scores for predicting postoperative nausea and vomiting are clinically useful tools and should be used in every patient: con–‘life is really simple, but we insist on making it complicated’. Eur J Anaesthesiol. 2011;28:155–159. doi: 10.1097/EJA.0b013e3283427f4f. [DOI] [PubMed] [Google Scholar]
- 228.Gupta R, Soto R. Prophylaxis and management of postoperative nausea and vomiting in enhanced recovery protocols: expert opinion statement from the American Society for Enhanced Recovery (ASER) Perioper Med (Lond) 2016;5:4. doi: 10.1186/s13741-016-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sarin A, Litonius ES, Naidu R, Yost CS, Varma MG, Chen LL. Successful implementation of an enhanced recovery after surgery program shortens length of stay and improves postoperative pain, and bowel and bladder function after colorectal surgery. BMC Anesthesiol. 2016;16:55. doi: 10.1186/s12871-016-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Dickinson KJ, Taswell JB, Allen MS, et al. Factors influencing length of stay after surgery for benign foregut disease. Eur J Cardiothorac Surg. 2016;50:124–129. doi: 10.1093/ejcts/ezv453. [DOI] [PubMed] [Google Scholar]
- 231.Feldheiser A, Aziz O, Baldini G, et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60:289–334. doi: 10.1111/aas.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ahsan K, Abbas N, Naqvi SM, Murtaza G, Tariq S. Comparison of efficacy of ondansetron and dexamethasone combination and ondansetron alone in preventing postoperative nausea and vomiting after laparoscopic cholecystectomy. J Pak Med Assoc. 2014;64:242–246. [PubMed] [Google Scholar]
- 233.Aghadavoudi O, Mirkheshti M. Evaluating the effect of intravenous haloperidol and midazolam on postoperative nausea and vomiting after strabismus surgery. Majallah-i Danishkadah-i Pizishki-i Isfahan. 2014;32:470–476. [Google Scholar]
- 234.Bala I, Bharti N, Murugesan S, Gupta R. Comparison of palonosetron with palonosetron-dexamethasone combination for prevention of postoperative nausea and vomiting in patients undergoing laparoscopic cholecystectomy. Miner Anestesiol. 2014;80:779–784. [PubMed] [Google Scholar]
- 235.Benevides ML, Oliveira SS, de Aguilar-Nascimento JE. The combination of haloperidol, dexamethasone, and ondansetron for prevention of postoperative nausea and vomiting in laparoscopic sleeve gastrectomy: a randomized double-blind trial. Obes Surg. 2013;23:1389–1396. doi: 10.1007/s11695-013-0923-1. [DOI] [PubMed] [Google Scholar]
- 236.Cho E, Kim DH, Shin S, Kim SH, Oh YJ, Choi YS. Efficacy of palonosetron-dexamethasone combination versus palonosetron alone for preventing nausea and vomiting related to opioid-based analgesia: a prospective, randomized, double-blind trial. Int J Med Sci. 2018;15:961–968. doi: 10.7150/ijms.24230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cho JS, Kim EJ, Lee JH, et al. Betahistine reduces postoperative nausea and vomiting after laparoscopic gynecological surgery. Miner Anestesiol. 2016;82:649–656. [PubMed] [Google Scholar]
- 238.Desai S, Santosh MC, Annigeri R, Santoshi VB, Rao R. Comparison of the antiemetic effect of ramosetron with the combination of dexamethasone and ondansetron in middle ear surgery: a double-blind, randomized clinical study. Saudi J Anaesth. 2013;7:254–258. doi: 10.4103/1658-354X.115328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gupta R, Srivastava S, Dhiraaj S, Chovatiya PP. Minimum effective dose of dexamethasone in combination with midazolam as prophylaxis against postoperative nausea and vomiting after laparoscopic cholecystectomy. Anesth Essays Res. 2018;12:396–401. doi: 10.4103/aer.AER_19_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bhattarai B, Shrestha S, Singh J. Comparison of ondansetron and combination of ondansetron and dexamethasone as a prophylaxis for postoperative nausea and vomiting in adults undergoing elective laparoscopic surgery. J Emerg Trauma Shock. 2011;4:168–172. doi: 10.4103/0974-2700.82200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Honarmand A, Safavi M, Chegeni M, Hirmanpour A, Nazem M, Sarizdi SH. Prophylactic antiemetic effects of Midazolam, Ondansetron, and their combination after middle ear surgery. J Res Pharm Pract. 2016;5:16–21. doi: 10.4103/2279-042X.176556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Honarmand A, Safavi M, Khalili G, Mohammadnejad F. Prophylactic administration of haloperidol plus midazolam reduces postoperative nausea and vomiting better than using each drug alone in patients undergoing middle ear surgery. Saudi J Anaesth. 2012;6:145–151. doi: 10.4103/1658-354X.97028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Imeh A, Olaniyi O, Simeon O, Omotola O. Dexamethasone versus a combination of dexamethasone and ondansetron as prophylactic antiemetic in patients receiving intrathecal morphine for caesarean section. Afr Health Sci. 2014;14:453–459. doi: 10.4314/ahs.v14i2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Joo J, Park YG, Baek J, Moon YE. Haloperidol dose combined with dexamethasone for PONV prophylaxis in high-risk patients undergoing gynecological laparoscopic surgery: a prospective, randomized, double-blind, dose-response and placebo-controlled study. BMC Anesthesiol. 2015;15:99. doi: 10.1186/s12871-015-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kawano H, Matsumoto T, Hamaguchi E, et al. Antiemetic efficacy of combined aprepitant and dexamethasone in patients at high-risk of postoperative nausea and vomiting from epidural fentanyl analgesia. Miner Anestesiol. 2015;81:362–368. [PubMed] [Google Scholar]
- 246.Kim KM, Huh J, Lee SK, Park EY, Lee JM, Kim HJ. Combination of gabapentin and ramosetron for the prevention of postoperative nausea and vomiting after gynecologic laparoscopic surgery: a prospective randomized comparative study. BMC Anesthesiol. 2017;17:65. doi: 10.1186/s12871-017-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kim WJ, Kang H, Shin HY, et al. Ramosetron, midazolam, and combination of ramosetron and midazolam for prevention of postoperative nausea and vomiting: a prospective, randomized, double-blind study. J Int Med Res. 2013;41:1203–1213. doi: 10.1177/0300060513485864. [DOI] [PubMed] [Google Scholar]
- 248.Kiran A, Panchaksharimath P, Sharvani R. Comparison of the efficacy of ondansetron versus ondansetron and dexamethasone in the prevention/ reduction of post-operative nausea & vomiting after elective surgeries under general anaesthesia. J Chem Pharm Res. 2013;5:1126–1130. [Google Scholar]
- 249.Kranke P, Bergese SD, Minkowitz HS, et al. Amisulpride prevents postoperative nausea and vomiting in patients at high risk: a randomized, double-blind, placebo-controlled trial. Anesthesiology. 2018;128:1099–1106. doi: 10.1097/ALN.0000000000002133. [DOI] [PubMed] [Google Scholar]
- 250.Kumar A, Patodia M, Pandove P, Sharda V. A randomized, placebo controlled study evaluating preventive role of ondansetron, dexamethasone and ondansetron plus dexamethasone for postoperative nausea and vomiting (PONV) in patients undergoing laparoscopic cholecystectomy. J Int Med Sci Acad. 2013;26:217–218. [Google Scholar]
- 251.Lee MJ, Lee KC, Kim HY, Lee WS, Seo WJ, Lee C. Comparison of ramosetron plus dexamethasone with ramosetron alone on postoperative nausea, vomiting, shivering and pain after thyroid surgery. Korean J Pain. 2015;28:39–44. doi: 10.3344/kjp.2015.28.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lee SJ, Lee SM, Kim SI, et al. The effect of aprepitant for the prevention of postoperative nausea and vomiting in patients undergoing gynecologic surgery with intravenous patient controlled analgesia using fentanyl: aprepitant plus ramosetron vs ramosetron alone. Korean J Anesthesiol. 2012;63:221–226. doi: 10.4097/kjae.2012.63.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lim CS, Ko YK, Kim YH, et al. Efficacy of the oral neurokinin-1 receptor antagonist aprepitant administered with ondansetron for the prevention of postoperative nausea and vomiting. Korean J Anesthesiol. 2013;64:212–217. doi: 10.4097/kjae.2013.64.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mansour E. Postoperative nausea and vomiting prophylaxis: the efficacy of a novel antiemetic drug (palonosetron) combined with dexamethasone. Egypt J Anaesth. 2013;29:117–123. doi: 10.1016/j.egja.2012.11.001. [DOI] [Google Scholar]
- 255.Matsota P, Angelidi M, Pandazi A, Tzirogiannis KN, Panoutsopoulos GI, Kostopanagiotou G. Ondansetron-droperidol comhyubination vs. ondansetron or droperidol monotherapy in the prevention of postoperative nausea and vomiting. Arch Med Sci. 2015;11:362–370. doi: 10.5114/aoms.2015.50968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Misra S, Parthasarathi G, Vilanilam GC. The effect of gabapentin premedication on postoperative nausea, vomiting, and pain in patients on preoperative dexamethasone undergoing craniotomy for intracranial tumors. J Neurosurg Anesthesiol. 2013;25:386–391. doi: 10.1097/ANA.0b013e31829327eb. [DOI] [PubMed] [Google Scholar]
- 257.Mukhopadhyay S, Niyogi M, Ray R, Mukhopadhyay BS, Dutta M, Mukherjee M. Betahistine as an add-on: the magic bullet for postoperative nausea, vomiting and dizziness after middle ear surgery? J Anaesthesiol Clin Pharmacol. 2013;29:205–210. doi: 10.4103/0970-9185.111725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Narayanappa AB, Gurulingaswamy S, Prabhakaraiah UN, Gurushanth SR, Sapare V, Goud N. Intravenous palonosetron compared with a combination of ramosetron and dexamethasone in preventing post operative nausea and vomiting in patients undergoing gynaecological surgeries under spinal anaesthesia, a randomised study. Indian J Anaesth. 2017;61:144–149. doi: 10.4103/0019-5049.199851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Park EY, Lee SK, Kang MH, et al. Comparison of ramosetron with combined ramosetron and midazolam for preventing postoperative nausea and vomiting in patients at high risk following laparoscopic gynaecological surgery. J Int Med Res. 2013;41:654–663. doi: 10.1177/0300060513487627. [DOI] [PubMed] [Google Scholar]
- 260.Vallejo MC, Phelps AL, Ibinson JW, et al. Aprepitant plus ondansetron compared with ondansetron alone in reducing postoperative nausea and vomiting in ambulatory patients undergoing plastic surgery. Plast Reconstr Surg. 2012;129:519–526. doi: 10.1097/PRS.0b013e31822b6932. [DOI] [PubMed] [Google Scholar]
- 261.Wang PK, Tsay PJ, Huang CC, et al. Comparison of dexamethasone with ondansetron or haloperidol for prevention of patient-controlled analgesia-related postoperative nausea and vomiting: a randomized clinical trial. World J Surg. 2012;36:775–781. doi: 10.1007/s00268-012-1446-y. [DOI] [PubMed] [Google Scholar]
- 262.Yu Q, Gao L, Gu MH, et al. Antiemetic effects of combined methylprednisolone and tropisetron in mastectomy. Miner Anestesiol. 2013;79:130–136. [PubMed] [Google Scholar]
- 263.Ryoo SH, Yoo JH, Kim MG, Lee KH, Kim SI. The effect of combination treatment using palonosetron and dexamethasone for the prevention of postoperative nausea and vomiting versus dexamethasone alone in women receiving intravenous patient-controlled analgesia. Korean J Anesthesiol. 2015;68:267–273. doi: 10.4097/kjae.2015.68.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Som A, Bhattacharjee S, Maitra S, Arora MK, Baidya DK. Combination of 5-HT3 antagonist and dexamethasone is superior to 5-HT3 antagonist alone for PONV prophylaxis after laparoscopic surgeries: a meta-analysis. Anesth Analg. 2016;123:1418–1426. doi: 10.1213/ANE.0000000000001617. [DOI] [PubMed] [Google Scholar]
- 265.Zhou H, Xu H, Zhang J, Wang W, Wang Y, Hu Z. Combination of dexamethasone and tropisetron before thyroidectomy to alleviate postoperative nausea, vomiting, and pain: randomized controlled trial. World J Surg. 2012;36:1217–1224. doi: 10.1007/s00268-011-1363-5. [DOI] [PubMed] [Google Scholar]
- 266.Papadima A, Gourgiotis S, Lagoudianakis E, et al. Granisetron versus tropisetron in the prevention of postoperative nausea and vomiting after total thyroidectomy. Saudi J Anaesth. 2013;7:68–74. doi: 10.4103/1658-354X.109817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kovac AL. Update on the management of postoperative nausea and vomiting. Drugs. 2013;73:1525–1547. doi: 10.1007/s40265-013-0110-7. [DOI] [PubMed] [Google Scholar]
- 268.Sirajuddin M, Naqvi S, Murtaza G, Abbas N. Metoclopramide alone and metoclopramide with dimenhydrinate for prophylaxis of post operative nausea & vomiting in patients admitted in day care for breast surgery | M. Sirajuddin | Request PDF. Med Channel. 2014;20:39–42. [Google Scholar]
- 269.White PF, O’Hara JF, Roberson CR, Wender RH, Candiotti KA, POST-OP Study Group The impact of current antiemetic practices on patient outcomes: a prospective study on high-risk patients. Anesth Analg. 2008;107:452–458. doi: 10.1213/ane.0b013e31817b842c. [DOI] [PubMed] [Google Scholar]
- 270.McKenzie R, Tantisira B, Karambelkar DJ, Riley TJ, Abdelhady H. Comparison of ondansetron with ondansetron plus dexamethasone in the prevention of postoperative nausea and vomiting. Anesth Analg. 1994;79:961–964. doi: 10.1213/00000539-199411000-00024. [DOI] [PubMed] [Google Scholar]
- 271.Si XY, Wu LP, Li XD, Li B, Zhou YM. Dexamethasone combined with other antiemetics for prophylaxis after laparoscopic cholecystectomy. Asian J Surg Asian Surg Assoc. 2015;38:21–27. doi: 10.1016/j.asjsur.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 272.Toner AJ, Ganeshanathan V, Chan MT, Ho KM, Corcoran TB. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2017;126:234–248. doi: 10.1097/ALN.0000000000001466. [DOI] [PubMed] [Google Scholar]
- 273.Polderman JA, Farhang-Razi V, Van Dieren S, et al. Adverse side effects of dexamethasone in surgical patients. Cochrane Database Syst Rev. 2018;8:CD011940. doi: 10.1002/14651858.CD011940.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Thacker JKM, Mountford WK, Ernst FR, Krukas MR, Mythen MMG. Perioperative fluid utilization variability and association with outcomes: considerations for enhanced recovery efforts in sample US surgical populations. Ann Surg. 2016;263:502–510. doi: 10.1097/SLA.0000000000001402. [DOI] [PubMed] [Google Scholar]
- 275.Miller TE, Mythen M, Shaw AD, et al. Association between perioperative fluid management and patient outcomes: a multicentre retrospective study. Br J Anaesth. 2021;126:720–729. doi: 10.1016/j.bja.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 276.Miller TE, Myles PS. Perioperative fluid therapy for major surgery. Anesthesiology. 2019;130:825–832. doi: 10.1097/ALN.0000000000002603. [DOI] [PubMed] [Google Scholar]
- 277.Brandstrup B, Svendsen PE, Rasmussen M, et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? Br J Anaesth. 2012;109:191–199. doi: 10.1093/bja/aes163. [DOI] [PubMed] [Google Scholar]
- 278.Myles PS, Bellomo R, Corcoran T, Australian and New Zealand College of Anaesthetists Clinical Trials Network and the Australian and New Zealand Intensive Care Society Clinical Trials Group et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378:2263–2274. doi: 10.1056/NEJMoa1801601. [DOI] [PubMed] [Google Scholar]
- 279.Brandstrup B, Tønnesen H, Beier-Holgersen R, Danish Study Group on Perioperative Fluid Therapy et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–648. doi: 10.1097/01.sla.0000094387.50865.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Chowdhury AH, Cox EF, Francis ST, Lobo DN. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 281.Burdett E, Dushianthan A, Bennett-Guerrero E, et al. Perioperative buffered versus non-buffered fluid administration for surgery in adults. Cochrane Database Syst Rev. 2012;12:CD004089. doi: 10.1002/14651858.CD004089.pub2. [DOI] [PubMed] [Google Scholar]
- 282.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 283.McCluskey SA, Karkouti K, Wijeysundera D, Minkovich L, Tait G, Beattie WS. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg. 2013;117:412–421. doi: 10.1213/ANE.0b013e318293d81e. [DOI] [PubMed] [Google Scholar]
- 284.Semler MW, Self WH, Wanderer JP, SMART Investigators and the Pragmatic Critical Care Research Group et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kabon B, Sessler DI, Kurz A, Crystalloid–Colloid Study Team Effect of intraoperative goal-directed balanced crystalloid versus colloid administration on major postoperative morbidity: a randomized trial. Anesthesiology. 2019;130:728–744. doi: 10.1097/ALN.0000000000002601. [DOI] [PubMed] [Google Scholar]
- 286.Futier E, Garot M, Godet T, FLASH Trial Group et al. Effect of hydroxyethyl starch vs saline for volume replacement therapy on death or postoperative complications among high-risk patients undergoing major abdominal surgery: the FLASH randomized clinical trial. JAMA. 2020;323:225–236. doi: 10.1001/jama.2019.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Feldheiser A, Pavlova V, Bonomo T, et al. Balanced crystalloid compared with balanced colloid solution using a goal-directed haemodynamic algorithm. Br J Anaesth. 2013;110:231–240. doi: 10.1093/bja/aes377. [DOI] [PubMed] [Google Scholar]
- 288.Yates DR, Davies SJ, Milner HE, Wilson RJ. Crystalloid or colloid for goal-directed fluid therapy in colorectal surgery. Br J Anaesth. 2014;112:281–289. doi: 10.1093/bja/aet307. [DOI] [PubMed] [Google Scholar]
- 289.Heming N, Lamothe L, Jaber S, et al. Morbidity and mortality of crystalloids compared to colloids in critically ill surgical patients: a subgroup analysis of a randomized trial. Anesthesiology. 2018;129:1149–1158. doi: 10.1097/ALN.0000000000002413. [DOI] [PubMed] [Google Scholar]
- 290.Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–740. doi: 10.1097/ALN.0b013e3181863117. [DOI] [PubMed] [Google Scholar]
- 291.Roger C, Muller L, Deras P, et al. Does the type of fluid affect rapidity of shock reversal in an anaesthetized-piglet model of near-fatal controlled haemorrhage? A randomized study. Br J Anaesth. 2014;112:1015–1023. doi: 10.1093/bja/aet375. [DOI] [PubMed] [Google Scholar]
- 292.Orbegozo Cortés D, Gamarano Barros T, Njimi H, Vincent JL. Crystalloids versus colloids: exploring differences in fluid requirements by systematic review and meta-regression. Anesth Analg. 2015;120:389–402. doi: 10.1213/ANE.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 293.Maheshwari K, Turan A, Mao G, et al. The association of hypotension during non-cardiac surgery, before and after skin incision, with postoperative acute kidney injury: a retrospective cohort analysis. Anaesthesia. 2018;73:1223–1228. doi: 10.1111/anae.14416. [DOI] [PubMed] [Google Scholar]
- 294.Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126:47–65. doi: 10.1097/ALN.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 295.Abbott TEF, Pearse RM, Archbold RA, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg. 2018;126:1936–1945. doi: 10.1213/ANE.0000000000002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Maheshwari K, Pu X, Rivas E, et al. Association between intraoperative mean arterial pressure and postoperative complications is independent of cardiac index in patients undergoing noncardiac surgery. Br J Anaesth. 2021;127:e102–e104. doi: 10.1016/j.bja.2021.06.024. [DOI] [PubMed] [Google Scholar]
- 297.Futier E, Lefrant JY, Guinot PG, INPRESS Study Group et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318:1346–1357. doi: 10.1001/jama.2017.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Holte K, Foss NB, Svensén C, Lund C, Madsen JL, Kehlet H. Epidural anesthesia, hypotension, and changes in intravascular volume. Anesthesiology. 2004;100:281–286. doi: 10.1097/00000542-200402000-00016. [DOI] [PubMed] [Google Scholar]
- 299.Gould TH, Grace K, Thorne G, Thomas M. Effect of thoracic epidural anaesthesia on colonic blood flow. Br J Anaesth. 2002;89:446–451. doi: 10.1093/bja/89.3.446. [DOI] [PubMed] [Google Scholar]
- 300.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–1402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- 301.Grocott MP, Dushianthan A, Hamilton MA, Mythen MG, Harrison D, Rowan K, Optimisation Systematic Review Steering Group Perioperative increase in global blood flow to explicit defined goals and outcomes after surgery: a Cochrane systematic review. Br J Anaesth. 2013;111:535–548. doi: 10.1093/bja/aet155. [DOI] [PubMed] [Google Scholar]
- 302.Benes J, Giglio M, Brienza N, Michard F. The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trials. Crit Care. 2014;18:584. doi: 10.1186/s13054-014-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Xu C, Peng J, Liu S, et al. Goal-directed fluid therapy versus conventional fluid therapy in colorectal surgery: a meta analysis of randomized controlled trials. Int J Surg. 2018;56:264–273. doi: 10.1016/j.ijsu.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 304.Pearse RM, Harrison DA, MacDonald N, OPTIMISE Study Group et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–2190. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 305.Calvo-Vecino JM, Ripollés-Melchor J, Mythen MG, FEDORA Trial Investigators Group et al. Effect of goal-directed haemodynamic therapy on postoperative complications in low-moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial) Br J Anaesth. 2018;120:734–744. doi: 10.1016/j.bja.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 306.Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc. 2010;69:488–498. doi: 10.1017/S0029665110001734. [DOI] [PubMed] [Google Scholar]
- 307.Cook JA, Fraser IA, Sandhu D, Everson NW, Rossard DP. A randomised comparison of two postoperative fluid regimens. Ann R Coll Surg Engl. 1989;71:67–69. [PMC free article] [PubMed] [Google Scholar]
- 308.Puckett JR, Pickering JW, Palmer SC, et al. Low versus standard urine output targets in patients undergoing major abdominal surgery: a randomized noninferiority trial. Ann Surg. 2017;265:874–881. doi: 10.1097/SLA.0000000000002044. [DOI] [PubMed] [Google Scholar]
- 309.Danelich IM, Bergquist JR, Bergquist WJ, et al. Early diuresis after colon and rectal surgery does not reduce length of hospital stay: results of a randomized trial. Dis Colon Rectum. 2018;61:1187–1195. doi: 10.1097/DCR.0000000000001183. [DOI] [PubMed] [Google Scholar]
- 310.Hewett PJ, Allardyce RA, Bagshaw PF, et al. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248:728–738. doi: 10.1097/SLA.0b013e31818b7595. [DOI] [PubMed] [Google Scholar]
- 311.Veldkamp R, Kuhry E, Hop WC, COlon cancer Laparoscopic or Open Resection Study Group (COLOR) et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 312.Braga M, Frasson M, Zuliani W, Vignali A, Pecorelli N, Di Carlo V. Randomized clinical trial of laparoscopic versus open left colonic resection. Br J Surg. 2010;97:1180–1186. doi: 10.1002/bjs.7094. [DOI] [PubMed] [Google Scholar]
- 313.Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, Dellabona P, Di Carlo V. Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg. 2002;236:759–766. doi: 10.1097/00000658-200212000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 315.Yamamoto S, Inomata M, Katayama H, Japan Clinical Oncology Group Colorectal Cancer Study Group et al. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg. 2014;260:23–30. doi: 10.1097/SLA.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 316.Milsom JW, Böhm B, Hammerhofer KA, Fazio V, Steiger E, Elson P. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg. 1998;187:46–54. doi: 10.1016/S1072-7515(98)00132-X. [DOI] [PubMed] [Google Scholar]
- 317.Stage JG, Schulze S, Møller P, et al. Prospective randomized study of laparoscopic versus open colonic resection for adenocarcinoma. Br J Surg. 1997;84:391–396. [PubMed] [Google Scholar]
- 318.Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G, Clinical Outcomes of Surgical Therapy (COST) Study Group Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287:321–328. doi: 10.1001/jama.287.3.321. [DOI] [PubMed] [Google Scholar]
- 319.Feroci F, Kröning KC, Lenzi E, Moraldi L, Cantafio S, Scatizzi M. Laparoscopy within a fast-track program enhances the short-term results after elective surgery for resectable colorectal cancer. Surg Endosc. 2011;25:2919–2925. doi: 10.1007/s00464-011-1643-z. [DOI] [PubMed] [Google Scholar]
- 320.Levack M, Berger D, Sylla P, Rattner D, Bordeianou L. Laparoscopy decreases anastomotic leak rate in sigmoid colectomy for diverticulitis. Arch Surg. 2011;146:207–210. doi: 10.1001/archsurg.2010.325. [DOI] [PubMed] [Google Scholar]
- 321.Senagore AJ, Stulberg JJ, Byrnes J, Delaney CP. A national comparison of laparoscopic vs. open colectomy using the National Surgical Quality Improvement Project data. Dis Colon Rectum. 2009;52:183–186. doi: 10.1007/DCR.0b013e31819ad4a4. [DOI] [PubMed] [Google Scholar]
- 322.Vaid S, Tucker J, Bell T, Grim R, Ahuja V. Cost analysis of laparoscopic versus open colectomy in patients with colon cancer: results from a large nationwide population database. Am Surg. 2012;78:635–641. doi: 10.1177/000313481207800614. [DOI] [PubMed] [Google Scholar]
- 323.Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005;2005(3):CD003145. doi: 10.1002/14651858.CD003145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008;2008(2):CD003432. doi: 10.1002/14651858.CD003432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Vennix S, Pelzers L, Bouvy N, et al. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev. 2014;4:CD005200. doi: 10.1002/14651858.CD005200.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Sheetz KH, Norton EC, Dimick JB, Regenbogen SE. Perioperative outcomes and trends in the use of robotic colectomy for medicare beneficiaries from 2010 through 2016. JAMA Surg. 2020;155:41–49. doi: 10.1001/jamasurg.2019.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Solaini L, Bazzocchi F, Cavaliere D, Avanzolini A, Cucchetti A, Ercolani G. Robotic versus laparoscopic right colectomy: an updated systematic review and meta-analysis. Surg Endosc. 2018;32:1104–1110. doi: 10.1007/s00464-017-5980-4. [DOI] [PubMed] [Google Scholar]
- 328.Roh HF, Nam SH, Kim JM. Robot-assisted laparoscopic surgery versus conventional laparoscopic surgery in randomized controlled trials: a systematic review and meta-analysis. PLoS ONE. 2018;13:e0191628. doi: 10.1371/journal.pone.0191628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Huang YJ, Kang YN, Huang YM, Wu AT, Wang W, Wei PL. Effects of laparoscopic vs robotic-assisted mesorectal excision for rectal cancer: an update systematic review and meta-analysis of randomized controlled trials. Asian J Surg. 2019;42:657–666. doi: 10.1016/j.asjsur.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 330.Giuliani G, Guerra F, Coletta D, et al. Robotic versus conventional laparoscopic technique for the treatment of left-sided colonic diverticular disease: a systematic review with meta-analysis. Int J Colorectal Dis. 2022;37:101–109. doi: 10.1007/s00384-021-04038-x. [DOI] [PubMed] [Google Scholar]
- 331.Vlug MS, Wind J, Hollmann MW, LAFA study group et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study) Ann Surg. 2011;254:868–875. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 332.Lei WZ, Zhao GP, Cheng Z, Li K, Zhou ZG. Gastrointestinal decompression after excision and anastomosis of lower digestive tract. World J Gastroenterol. 2004;10:1998–2001. doi: 10.3748/wjg.v10.i13.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Feo CV, Romanini B, Sortini D, et al. Early oral feeding after colorectal resection: a randomized controlled study. ANZ J Surg. 2004;74:298–301. doi: 10.1111/j.1445-1433.2004.02985.x. [DOI] [PubMed] [Google Scholar]
- 334.Petrelli NJ, Stulc JP, Rodriguez-Bigas M, Blumenson L. Nasogastric decompression following elective colorectal surgery: a prospective randomized study. Am Surg. 1993;59:632–635. [PubMed] [Google Scholar]
- 335.Li K, Zhou Z, Chen Z, Zhang Y, Wang C. “Fast Track” nasogastric decompression of rectal cancer surgery. Front Med. 2011;5:306–309. doi: 10.1007/s11684-011-0154-6. [DOI] [PubMed] [Google Scholar]
- 336.Ortiz H, Armendariz P, Yarnoz C. Is early postoperative feeding feasible in elective colon and rectal surgery? Int J Colorectal Dis. 1996;11:119–121. doi: 10.1007/s003840050032. [DOI] [PubMed] [Google Scholar]
- 337.Brown SR, Seow-Choen F, Eu KW, Heah SM, Tang CL. A prospective randomised study of drains in infra-peritoneal rectal anastomoses. Tech Coloproctol. 2001;5:89–92. doi: 10.1007/s101510170005. [DOI] [PubMed] [Google Scholar]
- 338.Merad F, Hay JM, Fingerhut A, et al. Is prophylactic pelvic drainage useful after elective rectal or anal anastomosis? A multicenter controlled randomized trial. Surgery. 1999;125:529–535. doi: 10.1016/S0039-6060(99)70205-9. [DOI] [PubMed] [Google Scholar]
- 339.Sagar PM, Couse N, Kerin M, May J, MacFie J. Randomized trial of drainage of colorectal anastomosis. Br J Surg. 1993;80:769–771. doi: 10.1002/bjs.1800800640. [DOI] [PubMed] [Google Scholar]
- 340.Cavaliere D, Popivanov G, Cassini D, et al. Is a drain necessary after anterior resection of the rectum? A systematic review and meta-analysis. Int J Colorectal Dis. 2019;34:973–981. doi: 10.1007/s00384-019-03276-4. [DOI] [PubMed] [Google Scholar]
- 341.Guerra F, Giuliani G, Coletta D, et al. A meta-analysis of randomized controlled trials on the use of suction drains following rectal surgery. Dig Surg. 2018;35:482–490. doi: 10.1159/000485139. [DOI] [PubMed] [Google Scholar]
- 342.Matsuda K, Yokoyama S, Hotta T, et al. Pelvic drain after laparoscopic low anterior resection for rectal cancer in patients with diverting stoma. Surg Laparosc Endosc Percutan Tech. 2018;28:82–85. doi: 10.1097/SLE.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 343.Podda M, Di Saverio S, Davies RJ, et al. Prophylactic intra-abdominal drainage following colorectal anastomoses. A systematic review and meta-analysis of randomized controlled trials. Am J Surg. 2020;219:164–174. doi: 10.1016/j.amjsurg.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 344.Zhang HY, Zhao CL, Xie J, et al. To drain or not to drain in colorectal anastomosis: a meta-analysis. Int J Colorectal Dis. 2016;31:951–960. doi: 10.1007/s00384-016-2509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Denost Q, Rouanet P, Faucheron JL, French Research Group of Rectal Cancer Surgery (GRECCAR) et al. To drain or not to drain infraperitoneal anastomosis after rectal excision for cancer: the GRECCAR 5 randomized trial. Ann Surg. 2017;265:474–480. doi: 10.1097/SLA.0000000000001991. [DOI] [PubMed] [Google Scholar]
- 346.Lee RM, Gamboa AC, Turgeon MK, et al. Revisiting the value of drains after low anterior resection for rectal cancer: a multi-institutional analysis of 996 patients. J Gastrointest Surg. 2021;25:2000–2010. doi: 10.1007/s11605-020-04781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Peeters KC, Tollenaar RA, Marijnen CA, Dutch Colorectal Cancer Group et al. Risk factors for anastomotic failure after total mesorectal excision of rectal cancer. Br J Surg. 2005;92:211–216. doi: 10.1002/bjs.4806. [DOI] [PubMed] [Google Scholar]
- 348.Brower RG. Consequences of bed rest. Crit Care Med. 2009;37(suppl):S422–S428. doi: 10.1097/CCM.0b013e3181b6e30a. [DOI] [PubMed] [Google Scholar]
- 349.Convertino VA, Bloomfield SA, Greenleaf JE. An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190. doi: 10.1097/00005768-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 350.Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol. 2003;95:2185–2201. doi: 10.1152/japplphysiol.00346.2003. [DOI] [PubMed] [Google Scholar]
- 351.Feroci F, Lenzi E, Baraghini M, et al. Fast-track colorectal surgery: protocol adherence influences postoperative outcomes. Int J Colorectal Dis. 2013;28:103–109. doi: 10.1007/s00384-012-1569-5. [DOI] [PubMed] [Google Scholar]
- 352.van Zelm R, Coeckelberghs E, Sermeus W, et al. Variation in care for surgical patients with colorectal cancer: protocol adherence in 12 European hospitals. Int J Colorectal Dis. 2017;32:1471–1478. doi: 10.1007/s00384-017-2863-z. [DOI] [PubMed] [Google Scholar]
- 353.Pecorelli N, Hershorn O, Baldini G, et al. Impact of adherence to care pathway interventions on recovery following bowel resection within an established enhanced recovery program. Surg Endosc. 2017;31:1760–1771. doi: 10.1007/s00464-016-5169-2. [DOI] [PubMed] [Google Scholar]
- 354.Pisarska M, Pędziwiatr M, Małczak P, et al. Do we really need the full compliance with ERAS protocol in laparoscopic colorectal surgery? A prospective cohort study. Int J Surg. 2016;36:377–382. doi: 10.1016/j.ijsu.2016.11.088. [DOI] [PubMed] [Google Scholar]
- 355.Daskivich TJ, Houman J, Lopez M, et al. Association of wearable activity monitors with assessment of daily ambulation and length of stay among patients undergoing major surgery. JAMA Netw Open. 2019;2:e187673. doi: 10.1001/jamanetworkopen.2018.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Fiore JF, Jr, Castelino T, Pecorelli N, et al. Ensuring early mobilization within an enhanced recovery program for colorectal surgery: a randomized controlled trial. Ann Surg. 2017;266:223–231. doi: 10.1097/SLA.0000000000002114. [DOI] [PubMed] [Google Scholar]
- 357.Balvardi S, Pecorelli N, Castelino T, et al. Impact of facilitation of early mobilization on postoperative pulmonary outcomes after colorectal surgery: a randomized controlled trial. Ann Surg. 2021;273:868–875. doi: 10.1097/SLA.0000000000003919. [DOI] [PubMed] [Google Scholar]
- 358.Calotta NA, Coon D, Bos TJ, et al. Early ambulation after colorectal oncologic resection with perineal reconstruction is safe and effective. Am J Surg. 2019;218:125–130. doi: 10.1016/j.amjsurg.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 359.Herbert G, Perry R, Andersen HK, et al. Early enteral nutrition within 24 hours of lower gastrointestinal surgery versus later commencement for length of hospital stay and postoperative complications. Cochrane Database Syst Rev. 2019;7:CD004080. doi: 10.1002/14651858.CD004080.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Hogan S, Steffens D, Rangan A, Solomon M, Carey S. The effect of diets delivered into the gastrointestinal tract on gut motility after colorectal surgery-a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr. 2019;73:1331–1342. doi: 10.1038/s41430-019-0474-1. [DOI] [PubMed] [Google Scholar]
- 361.Meyer JP, Fawcett D. The use of chewing gum for preventing postoperative ileus. BJU Int. 2008;101:1–2. doi: 10.1111/j.1464-410X.2007.07278.x. [DOI] [PubMed] [Google Scholar]
- 362.Liu Q, Jiang H, Xu D, Jin J. Effect of gum chewing on ameliorating ileus following colorectal surgery: a meta-analysis of 18 randomized controlled trials. Int J Surg. 2017;47:107–115. doi: 10.1016/j.ijsu.2017.07.107. [DOI] [PubMed] [Google Scholar]
- 363.Roslan F, Kushairi A, Cappuyns L, Daliya P, Adiamah A. The impact of sham feeding with chewing gum on postoperative ileus following colorectal surgery: a meta-analysis of randomised controlled trials. J Gastrointest Surg. 2020;24:2643–2653. doi: 10.1007/s11605-019-04507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Dulskas A, Klimovskij M, Vitkauskiene M, Samalavicius NE. Effect of coffee on the length of postoperative ileus after elective laparoscopic left-sided colectomy: a randomized, prospective single-center study. Dis Colon Rectum. 2015;58:1064–1069. doi: 10.1097/DCR.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 365.Müller SA, Rahbari NN, Schneider F, et al. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br J Surg. 2012;99:1530–1538. doi: 10.1002/bjs.8885. [DOI] [PubMed] [Google Scholar]
- 366.Piric M, Pasic F, Rifatbegovic Z, Konjic F. The effects of drinking coffee while recovering from colon and rectal resection surgery. Med Arh. 2015;69:357–361. doi: 10.5455/medarh.2015.69.357-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Cornwall HL, Edwards BA, Curran JF, Boyce S. Coffee to go? The effect of coffee on resolution of ileus following abdominal surgery: a systematic review and meta-analysis of randomised controlled trials. Clin Nutr. 2020;39:1385–1394. doi: 10.1016/j.clnu.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 368.Alhashemi M, Hamad R, El-Kefraoui C, et al. The association of alvimopan treatment with postoperative outcomes after abdominal surgery: a systematic review across different surgical procedures and contexts of perioperative care. Surgery. 2021;169:934–944. doi: 10.1016/j.surg.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 369.Wolff BG, Michelassi F, Gerkin TM, Alvimopan Postoperative Ileus Study Group et al. Alvimopan, a novel, peripherally acting mu opioid antagonist: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg. 2004;240:728–734. doi: 10.1097/01.sla.0000141158.27977.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Viscusi ER, Goldstein S, Witkowski T, et al. Alvimopan, a peripherally acting mu-opioid receptor antagonist, compared with placebo in postoperative ileus after major abdominal surgery: results of a randomized, double-blind, controlled study. Surg Endosc. 2006;20:64–70. doi: 10.1007/s00464-005-0104-y. [DOI] [PubMed] [Google Scholar]
- 371.Delaney C, Weese J, Hyman N, Bauer J, Techner L, Gabriel K, Du W, Schmidt W, Wallin B. Phase III trial of alvimopan, a novel, peripherally acting, mu opioid antagonist, for postoperative ileus after major abdominal surgery. Dis Colon Rectum. 2005;48:1114–1125. doi: 10.1007/s10350-005-0035-7. [DOI] [PubMed] [Google Scholar]
- 372.Wolff BG, Weese JL, Ludwig KA, et al. Postoperative ileus-related morbidity profile in patients treated with alvimopan after bowel resection. J Am Coll Surg. 2007;204:609–616. doi: 10.1016/j.jamcollsurg.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 373.Irving G, Pénzes J, Ramjattan B, et al. A randomized, placebo-controlled phase 3 trial (Study SB-767905/013) of alvimopan for opioid-induced bowel dysfunction in patients with non-cancer pain. J Pain. 2011;12:175–184. doi: 10.1016/j.jpain.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 374.Delaney CP, Senagore AJ, Viscusi ER, et al. Postoperative upper and lower gastrointestinal recovery and gastrointestinal morbidity in patients undergoing bowel resection: pooled analysis of placebo data from 3 randomized controlled trials. Am J Surg. 2006;191:315–319. doi: 10.1016/j.amjsurg.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 375.Delaney CP, Wolff BG, Viscusi ER, et al. Alvimopan, for postoperative ileus following bowel resection: a pooled analysis of phase III studies. Ann Surg. 2007;245:355–363. doi: 10.1097/01.sla.0000232538.72458.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Bell TJ, Poston SA, Kraft MD, Senagore AJ, Delaney CP, Techner L. Economic analysis of alvimopan in North American Phase III efficacy trials. Am J Health Syst Pharm. 2009;66:1362–1368. doi: 10.2146/ajhp080329. [DOI] [PubMed] [Google Scholar]
- 377.Ludwig K, Viscusi ER, Wolff BG, Delaney CP, Senagore A, Techner L. Alvimopan for the management of postoperative ileus after bowel resection: characterization of clinical benefit by pooled responder analysis. World J Surg. 2010;34:2185–2190. doi: 10.1007/s00268-010-0635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Senagore AJ, Bauer JJ, Du W, Techner L. Alvimopan accelerates gastrointestinal recovery after bowel resection regardless of age, gender, race, or concomitant medication use. Surgery. 2007;142:478–486. doi: 10.1016/j.surg.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 379.McNicol ED, Boyce D, Schumann R, Carr DB. Mu-opioid antagonists for opioid-induced bowel dysfunction. Cochrane Database Syst Rev. 2008;2:CD006332. doi: 10.1002/14651858.CD006332.pub2. [DOI] [PubMed] [Google Scholar]
- 380.Vaughan-Shaw PG, Fecher IC, Harris S, Knight JS. A meta-analysis of the effectiveness of the opioid receptor antagonist alvimopan in reducing hospital length of stay and time to GI recovery in patients enrolled in a standardized accelerated recovery program after abdominal surgery. Dis Colon Rectum. 2012;55:611–620. doi: 10.1097/DCR.0b013e318249fc78. [DOI] [PubMed] [Google Scholar]
- 381.McNicol E, Boyce DB, Schumann R, Carr D. Efficacy and safety of mu-opioid antagonists in the treatment of opioid-induced bowel dysfunction: systematic review and meta-analysis of randomized controlled trials. Pain Med. 2008;9:634–659. doi: 10.1111/j.1526-4637.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 382.Tan EK, Cornish J, Darzi AW, Tekkis PP. Meta-analysis: alvimopan vs placebo in the treatment of post-operative ileus. Aliment Pharmacol Ther. 2007;25:47–57. doi: 10.1111/j.1365-2036.2006.03150.x. [DOI] [PubMed] [Google Scholar]
- 383.Delaney CP, Craver C, Gibbons MM, et al. Evaluation of clinical outcomes with alvimopan in clinical practice: a national matched-cohort study in patients undergoing bowel resection. Ann Surg. 2012;255:731–738. doi: 10.1097/SLA.0b013e31824a36cc. [DOI] [PubMed] [Google Scholar]
- 384.Simorov A, Thompson J, Oleynikov D. Alvimopan reduces length of stay and costs in patients undergoing segmental colonic resections: results from multicenter national administrative database. Am J Surg. 2014;208:919–925. doi: 10.1016/j.amjsurg.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 385.Steele SR, Brady JT, Cao Z, et al. Evaluation of healthcare use and clinical outcomes of alvimopan in patients undergoing bowel resection: a propensity score-matched analysis. Dis Colon Rectum. 2018;61:1418–1425. doi: 10.1097/DCR.0000000000001181. [DOI] [PubMed] [Google Scholar]
- 386.Grass F, Slieker J, Frauche P, et al. Postoperative urinary retention in colorectal surgery within an enhanced recovery pathway. J Surg Res. 2017;207:70–76. doi: 10.1016/j.jss.2016.08.089. [DOI] [PubMed] [Google Scholar]
- 387.Sheka AC, Tevis S, Kennedy GD. Urinary tract infection after surgery for colorectal malignancy: risk factors and complications. Am J Surg. 2016;211:31–39. doi: 10.1016/j.amjsurg.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Okrainec A, Aarts MA, Conn LG, members of the iERAS Group et al. Compliance with urinary catheter removal guidelines leads to improved outcome in enhanced recovery after surgery patients. J Gastrointest Surg. 2017;21:1309–1317. doi: 10.1007/s11605-017-3434-x. [DOI] [PubMed] [Google Scholar]
- 389.Hung LY, Benlice C, Jia X, et al. Outcomes after early versus delayed urinary bladder catheter removal after proctectomy for benign and malignant disease in 2,429 patients: an observational cohort study. Surg Infect (Larchmt) 2021;22:310–317. doi: 10.1089/sur.2020.159. [DOI] [PubMed] [Google Scholar]
- 390.Schreiber A, Aydil E, Walschus U, et al. Early removal of urinary drainage in patients receiving epidural analgesia after colorectal surgery within an ERAS protocol is feasible. Langenbecks Arch Surg. 2019;404:853–863. doi: 10.1007/s00423-019-01834-6. [DOI] [PubMed] [Google Scholar]
- 391.Eriksen JR, Munk-Madsen P, Kehlet H, Gögenur I. Postoperative urinary retention after laparoscopic colorectal resection with early catheter removal: a prospective observational study. World J Surg. 2019;43:2090–2098. doi: 10.1007/s00268-019-05010-1. [DOI] [PubMed] [Google Scholar]
- 392.Roberts ST, Patel K, Smith SR. Impact of avoiding post-operative urinary catheters on outcomes following colorectal resection in an ERAS programme: no IDUC and ERAS programmes. ANZ J Surg. 2018;88:E390–E394. doi: 10.1111/ans.13916. [DOI] [PubMed] [Google Scholar]
- 393.Van Backer J, Ahn N, Chan R, et al. Early urinary catheter removal in patients undergoing colorectal surgery with an enhanced recovery after surgery pathway. Am Surg. 2019;85:e139–e141. doi: 10.1177/000313481908500309. [DOI] [PubMed] [Google Scholar]
- 394.Alyami M, Lundberg P, Passot G, Glehen O, Cotte E. Laparoscopic colonic resection without urinary drainage: is it “feasible”? J Gastrointest Surg. 2016;20:1388–1392. doi: 10.1007/s11605-016-3160-9. [DOI] [PubMed] [Google Scholar]
- 395.Zaouter C, Kaneva P, Carli F. Less urinary tract infection by earlier removal of bladder catheter in surgical patients receiving thoracic epidural analgesia. Reg Anesth Pain Med. 2009;34:542–548. doi: 10.1097/AAP.0b013e3181ae9fac. [DOI] [PubMed] [Google Scholar]
- 396.Wald HL, Ma A, Bratzler DW, Kramer AM. Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data. Arch Surg. 2008;143:551–557. doi: 10.1001/archsurg.143.6.551. [DOI] [PubMed] [Google Scholar]
- 397.Patel DN, Felder SI, Luu M, Daskivich TJ, Zaghiyan NK, Fleshner P. Early urinary catheter removal following pelvic colorectal surgery: a prospective, randomized, noninferiority trial. Dis Colon Rectum. 2018;61:1180–1186. doi: 10.1097/DCR.0000000000001206. [DOI] [PubMed] [Google Scholar]
- 398.Benoist S, Panis Y, Denet C, Mauvais F, Mariani P, Valleur P. Optimal duration of urinary drainage after rectal resection: a randomized controlled trial. Surgery. 1999;125:135–141. doi: 10.1016/S0039-6060(99)70256-4. [DOI] [PubMed] [Google Scholar]
- 399.Zmora O, Madbouly K, Tulchinsky H, Hussein A, Khaikin M. Urinary bladder catheter drainage following pelvic surgery—is it necessary for that long? Dis Colon Rectum. 2010;53:321–326. doi: 10.1007/DCR.06013e3181c7525c. [DOI] [PubMed] [Google Scholar]
- 400.Coyle D, Joyce KM, Garvin JT, et al. Early post-operative removal of urethral catheter in patients undergoing colorectal surgery with epidural analgesia—a prospective pilot clinical study. Int J Surg. 2015;16:94–98. doi: 10.1016/j.ijsu.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 401.Castelo M, Sue-Chue-Lam C, Kishibe T, Acuna SA, Baxter NN. Early urinary catheter removal after rectal surgery: systematic review and meta-analysis. BJS Open. 2020;4:545–553. doi: 10.1002/bjs5.50288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Lee Y, McKechnie T, Springer JE, Doumouras AG, Hong D, Eskicioglu C. Optimal timing of urinary catheter removal following pelvic colorectal surgery: a systematic review and meta-analysis. Int J Colorectal Dis. 2019;34:2011–2021. doi: 10.1007/s00384-019-03404-0. [DOI] [PubMed] [Google Scholar]
- 403.Fiore JF, Jr, Bialocerkowski A, Browning L, Faragher IG, Denehy L. Criteria to determine readiness for hospital discharge following colorectal surgery: an international consensus using the Delphi technique. Dis Colon Rectum. 2012;55:416–423. doi: 10.1097/DCR.0b013e318244a8f2. [DOI] [PubMed] [Google Scholar]
- 404.Levy BF, Scott MJ, Fawcett WJ, Rockall TA. 23-hour-stay laparoscopic colectomy. Dis Colon Rectum. 2009;52:1239–1243. doi: 10.1007/DCR.0b013e3181a0b32d. [DOI] [PubMed] [Google Scholar]
- 405.Gignoux B, Pasquer A, Vulliez A, Lanz T. Outpatient colectomy within an enhanced recovery program. J Visc Surg. 2015;152:11–15. doi: 10.1016/j.jviscsurg.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 406.Bednarski BK, Nickerson TP, You YN, et al. Randomized clinical trial of accelerated enhanced recovery after minimally invasive colorectal cancer surgery (RecoverMI trial) Br J Surg. 2019;106:1311–1318. doi: 10.1002/bjs.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Gignoux B, Gosgnach M, Lanz T, et al. Short-term outcomes of ambulatory colectomy for 157 consecutive patients. Ann Surg. 2019;270:317–321. doi: 10.1097/SLA.0000000000002800. [DOI] [PubMed] [Google Scholar]
- 408.McKenna NP, Bews KA, Shariq OA, et al. Is same-day and next-day discharge after laparoscopic colectomy reasonable in select patients? Dis Colon Rectum. 2020;63:1427–1435. doi: 10.1097/DCR.0000000000001729. [DOI] [PubMed] [Google Scholar]