Abstract
Some studies suggest that individuals with ASD have reduced emotional empathy (e.g. Bos & Stokes, 2018; Sucksmith et al., 2013) while others do not (e.g. Bellebaum et. al, 2014; Deschamps et al., 2014). The presence of co-occurring alexithymia in ASD (e.g. Bird et al., 2010) and differences in interoception (e.g. Fukushima, Terasawa, & Umeda, 2011) have been associated with reductions in empathic ability. To fully explore the relationships between interoception, alexithymia, and emotional empathy, we collected self-report and interview data in 35 youths with ASD and 40 TD controls (ages 8–17). The ASD sample had increased alexithymia and physiological hyperarousal compared to TD controls, but there were no group differences in interoception or emotional empathy. Alexithymia severity correlated with higher personal distress in both groups, and with lower empathic concern in the ASD group. Within the ASD group, higher incidence of reports of bodily sensation when describing emotional experience correlated with lower personal distress and lower alexithymia. Additionally, although empathic concern was negatively correlated with alexithymia in the ASD group, across groups, the alexithymia hypothesis was supported in only the personal distress domain of emotional empathy. These results suggest emotional empathy, personal distress in particular, is not intrinsically impaired in ASD.
Keywords: Alexithymia, Autism, ASD, Interoception, Empathy, Personal Distress
Lay Abstract:
Empathy, the ability to understand and share the emotions of others, is a necessary skill for social functioning and can be categorized into cognitive and emotional empathy. There is evidence to suggest that individuals with autism spectrum disorder (ASD) have difficulties with cognitive empathy, the ability to imagine how another person is thinking or feeling. However, it is unclear if individuals with ASD struggle with emotional empathy, the ability to share and feel emotions other’s are experiencing. Self-report and interview data were collected to explore relationships between interoception (individuals self-reported awareness of sensation from their body like thirst, heartbeat etc), alexithymia (an individual’s ability to describe and distinguish between their own emotions), and emotional empathy, in 35 youth with ASD and 40 typically developing (TD) youth. Greater personal distress to others emotions, and greater difficulty describing and recognizing self emotions was associated with reporting fewer physical sensations in the body when experiencing emotion in the ASD group. The results of the present study suggest that while ASD youth with concomitant alexithymia may experience emotional empathy differently, it should not be characterized as an absence of a capacity for emotional empathy.
Introduction
Autism spectrum disorder (ASD) is a condition defined by difficulties in social communication, social interaction, and restricted or repetitive behaviors and interests (American Psychiatric Association, 2013). Individuals with ASD may have difficulties with intention understanding and empathic processing, which are important skills for adaptive social emotional functioning. Empathy can be divided into two dimensions 1) cognitive empathy; and 2) emotional empathy. Cognitive empathy is the ability to imagine how another person is thinking or feeling (de Waal & Preston, 2017); emotional empathy describes the ability to share and experience the feelings of others (Davis et al., 1994) through embodied simulation (Gallese & Sinigaglia, 2011).
While there is considerable evidence that individuals with ASD have reduced situation understanding, mentalizing, and cognitive empathy ability (e.g. Castelli et al., 2002; Frith & Happé, 2005; Jolliffe & Baron-Cohen, 1997; Zalla et al., 2009), there is no consensus on how emotional empathy is impacted in ASD. Some studies suggest that individuals with ASD have reduced emotional empathy ability (Bos & Stokes, 2018; Kasari et al., 1990; Lombardo et al., 2007; Mathersul et al., 2013; Mazza et al., 2014; Minio-Paluello et al., 2009; Schulte-Rüther et al., 2011; Shamay-Tsoory et al., 2002; Sigman et al., 1992; Sucksmith et al.; 2013; Trimmer et al., 2017; Yirmiya et al., 1992), while other studies do not (Bellebaum et al., 2014; Deschamps et al., 2014; Dziobek et al., 2008; Hadjikhani et al., 2014; Markram et al., 2007; Smith, 2009; Rogers et al., 2007; Rueda et al., 2015; Schwenck et al., 2012). Inconsistent findings regarding emotional empathy in individuals with ASD may be understood from two prominent theories: 1) the interoception hypothesis (Fukushima et al., 2011; Grynberg & Pollatos, 2015); and/or 2) the alexithymia hypothesis (Bird, et al., 2010).
Interoception, Alexithymia, and Emotional Empathy
Interoception is the sense of the physiological condition of all internal tissues in the body (Craig, 2003). Embodied simulation theories of emotion processing stipulate that changes in bodily states produce feeling states in the brain, which can modulate empathic behavior (i.e., James, 1884; Schachter & Singer, 1962; Damasio, 1994). Interoceptive ability is more frequently hypothesized to be related to understanding emotional states, rather than cognitive states (Grynberg & Pollatos, 2015; Herbert, et al., 2007; Shah et al., 2017). Thus, the presence of interoception differences may account for discrepant social processing in ASD, including emotional empathy (the interoception hypothesis; Quattrocki & Friston, 2014). Individuals with ASD show variability in interoceptive sensory processing (see DuBois et al., 2016 for review), with some studies showing reduced interoceptive ability (Fiene & Brownlow, 2015; Garfinkel et al., 2016), others showing enhanced interoceptive ability (Schauder et al., 2015), and still others finding no differences or links between interoception and autistic traits (Nicholson et al., 2018, Yang et al., 2021a). However, research indicates that alexithymia – characterized by difficulties in recognizing, describing, and distinguishing one’s own emotions – is linked to difficulties in regulation of physical and emotional arousal (Cox et al. 1995), interoceptive ability (Herbert et al., 2011), and is highly common in individuals with ASD (~50%; Hill et al., 2004). Others have found that interoceptive impairments should not be considered a feature of ASD, but instead due to co-occurring alexithymia (Shah et al., 2016a).
The second hypothesis poses that emotional empathy reductions observed in ASD may be accounted for by the presence of co-occurring alexithymia (the alexithymia hypothesis; Bird & Cook, 2013). Co-occurring alexithymia in ASD has previously been found to account for difficulties in various emotion processing tasks including: neural responses to empathy paradigms (Bird et al., 2010), skin conductance responses to emotional pictures (Gaigg et al., 2018), identification of emotions in faces (Cook et al., 2013; Milosavljevic et. al., 2016), understanding of vocal affect (Heaton et al., 2012), moral decision-making (Brewer et al., 2015), and eye gaze fixation on faces (Bird et al., 2011). Two studies have explored the relationship between alexithymia and emotional and cognitive empathy functioning as separate constructs, with conflicting findings. In a sample of adults with ASD, alexithymia scores predicted an empathic emotion recognition outcome (reading the mind in the eyes), but not scores on a theory of mind task (Movie for Assessment of Social Cognition; Oakley et al., 2016). However, another study using the Interpersonal Reactivity Index (IRI) self-report measure in an adult ASD sample observed that even after controlling for alexithymia, there was no difference in empathic concern scores compared with control participants (Bernhardt et. al, 2014).
The common co-occurrence of interoception abnormalities and alexithymia in ASD, and prior findings showing bodily information is important for the interpretation of feeling states (Damasio & Carvalho, 2013), suggest it is important to explore the relationship between these constructs in ASD. Some argue that alexithymia is the result of a general impairment in interoception (Brewer et al., 2016), while others support that interoceptive difficulties are a symptom of alexithymia (Shah et al., 2016a, 2017). In typical samples, others have found that high autistic traits are associated with higher levels of alexithymia, but not empathy or interoception (Yang et al., 2021b). To our knowledge, only one study to date has examined the impact of both interoception and alexithymia on emotional empathy in individuals with ASD. Mul and colleagues (2018) directly compared TD controls and two groups of adults with ASD: those with and without alexithymia. Participants with ASD and alexithymia demonstrated lower emotional empathy on the Multifaceted Empathy Test (MET) than participants with ASD without alexithymia. Participants with ASD showed reduced interoceptive sensibility (self-report) and accuracy (heartbeat tracking) than TD controls, and alexithymia scores mediated the relationship between interoceptive sensibility and emotional empathy (on the MET; Mul et al., 2018). Anxiety was not assessed in Mul et al. (2018). Due to the high incidence of anxiety disorders in individuals with ASD (Simonoff et al., 2008), and the association between anxiety and interoceptive sensibility (Ehlers & Breuer, 1992), anxiety symptoms also were investigated in the current study.
Given the discrepant findings of alexithymia and interoception influences on emotional empathy in ASD, the purpose of the present study is to: 1) compare youth with ASD to TD youth on levels of interoceptive sensibility, alexithymia, and empathy; and 2) examine which behavioral variables most strongly influence emotional empathy ability, with a particular focus on interoception and alexithymia. We hypothesized that: 1) compared to TD controls, participants with ASD would have lower interoceptive sensibility, higher alexithymia, and lower cognitive and emotional empathy ability; 2) interoceptive sensibility would be positively associated with empathy ability, while alexithymia severity would be negatively associated with empathy ability; and 3) alexithymia may account for differences in emotional empathy ability in the ASD group.
Methods
Participants
This study was part of a larger study with additional brain imaging and behavioral components (Kilroy et al., 2020) and some of the inclusion criteria reflect inclusion criteria for the larger study (e.g., right handed, IQ and age restrictions). For the current study, participants included youths ages 8 to 17 years (mean age = 11.90 ± 2.16) who were typically developing (TD; n = 40, 12 female, mean age = 11.86 ± 2.17) or had a diagnosis of ASD (n = 35, 7 female, mean age = 11.95 ± 2.19). Participants were recruited from clinics, local schools, word-of-mouth, and social media advertising. A measure of interoceptive sensibility (26 TD, 20 ASD) and an interview measure (25 TD, 24 ASD) were collected in a subset of individuals because these measures were added after the larger study had been initiated. Inclusion criteria for all groups included: IQ > 80 on at least one composite score (Verbal Comprehension Index, Perceptual Reasoning Index, Full Scale IQ–2) and composite score of at least 75 on the Full Scale Intelligence Quotient (Full Scale IQ–4) as assessed by the Wechsler Abbreviated Scale of Intelligence, 2nd edition (WASI-II; Wechsler, 2011); and English fluency of child and parent. All participants were right-handed and were born after 36 weeks of gestation. Each family was informed about study procedures in accordance with the protocol approved by the University of Southern California Institutional Review Board and written child assent and parental consent were provided. ASD community members were not involved in the conception or execution of this study.
TD participants were excluded if they had any psychological or neurological disorder, including attention deficit hyperactivity disorder (ADHD) and generalized anxiety disorder. They were also excluded if they had a first degree relative with an ASD diagnosis, or scored above a T-score of 60 on the Social Responsiveness Scale, Second Edition (SRS-2; Constantino & Gruber, 2012). Eligible participants with ASD had a previous clinical diagnosis and met criteria on the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2; Lord et al., 2000), the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994), or both, which were administered at the time of the study. Individuals in the ASD group were excluded if they had another diagnosis of neurological or psychological disorder with the exception of Developmental Coordination Disorder, ADHD, or generalized anxiety disorder, due to the high comorbidity of those conditions with ASD (Mazzone et al., 2012). Twelve participants with ASD had prescriptions for psychotropic medication at the time of the study (ADHD and anxiety), and no TD participants reported any prescription medication use.
Study Measures
The 20-item version of the Childhood Adolescent Symptom Inventory (CASI-Anx; Sprafkin et al., 2002), was used to assess symptoms of DSM-IV defined anxiety disorders. On the CASI-Anx, among school children, test–retest correlation for the total anxiety sensitivity score was 0.76, with a Cronbach’s alpha of 0.87 on two occasions. The 10-item Conners third edition ADHD Index (Conners-3AI; Conners, 2008), was used to characterize levels of ADHD symptoms. Reliability analyses demonstrate high levels of internal consistency, with Cronbach’s alpha ranging from 0.77 to 0.97 (mean Cronbach’a alpha = 0.90), and excellent temporal stability, with test-retest correlations ranging from 0.71 to 0.98 (mean r = 0.83, all correlations, p < 0.001; Conners, 2008).
Empathy ability was assessed using a modified version of the self-report Interpersonal Reactivity Index (IRI; Davis, 1983), with child-appropriate language (Pfeifer et al., 2008). On the IRI, original estimates of Cronbach’s alpha coefficients ranged from .71 to .77 (Davis, 1980) and internal consistency within this range for the four subscales: PT: .63–.81; EC: .68–.81; PD: .70–.88; FS: .70–.86 (e.g. Baldner & McGinley, 2014). This work focuses on the two aspects of emotional empathy measured by the IRI — empathic concern (IRI EC) and personal distress (IRI PD). Trained research staff administered two subtests which make up the Social Perception domain: (1) Theory of Mind (ToM) and; (2) Affect Recognition (AR) from the Developmental Neuropsychological Assessment (NEPSY-II; Korkman et al., 2007). The Social Perception domain has adequate internal reliability for 7 to 16-year-olds (r = .80 or greater), and test-retest reliability correlations are all above 0.5 for ages 8–16 (Korkman, Kirk, & Kemp, 2007).
Alexithymia was measured using the 20-item self-report Alexithymia Questionnaire for Children (AQC; Rieffe et al., 2006), an adapted version of the Toronto Alexithymia Scale. Cronbach’s alphas for difficulty identifying feelings, difficulty describing feelings, and externally oriented thinking were .73, .75, and .29, respectively (Rieffe et al., 2006). Given the low alpha in externally oriented thinking, three scores were used to assess alexithymia: difficulty identifying feelings (AQC ID), difficulty describing and communicating feelings (AQC COMM), and the total of these two factors (AQC total; Loas et. al, 2017).
For interoceptive sensibility, the 12-item self-report Body Perception Questionnaire-Body Awareness Very Short Form (BPQ-VSF; Porges, 1993, Cabrera et al., 2018) was used to assess the subjective awareness of target organs and structures innervated by the autonomic nervous system. The 12-item BPQ-VSF was developed by assessing items with the highest factor loadings to generate scores with high fidelity to the previous 26-item score (criterion Rho = .90). Good internal consistency for an American sample was observed (categorical ω = .91), and test–retest reliability was high (ICC = .97; Cabrera et al., 2017). The Physiological Hyperarousal Scale for Children (PH-C; Laurent et al., 2004) was used as a measure of frequency of physical experiences associated with physiological hyperarousal (alpha coefficient for the scale is .87). Additionally, a semi-structured interview using the emBODY tool (Nummenmaa et al., 2014) was developed and administered. This interview assessed a participant’s ability to identify somatotopic patterns of physical feeling associated with each of the six basic emotions. Each instance of “bodily sensations of emotion” (e.g. “I know I am angry because I feel my head getting hot and chest-pounding”) was 1) collapsed across all negatively valenced emotions (BSE-neg), and 2) collapsed across all emotions (BSE).
Analysis
Group differences between TD and ASD groups were assessed using either two-tailed independent samples t-tests or chi-square tests of independence. Pairwise Pearson partial correlation coefficients assessed relationships between variables in all participants, the ASD group alone, and the TD group alone, while controlling for age, gender, and verbal IQ. In order to assess the previously documented influence of alexithymia in relationships between interoception and emotional empathy variables in ASD (Mul et al., 2018), additional analyses were run which also included AQC total as a control variable in Pearson partial correlations. Anxiety symptoms were also controlled for in secondary analyses in the ASD group. Scatter plots of correlations were visually analyzed to prevent reporting results driven by the presence of outliers. Additionally, Rouder’s Bayes Factors (BF) were calculated for each group comparison and correlation to compute the ratio of the likelihood of the null to the alternative hypothesis, which can be interpreted as a measure of the strength or weight of evidence in favor of a given hypothesis. In other words, where there is an absence of evidence (i.e., null p-values) we report Rouder’s Bayes Factors to test if there is evidence of the absence of these relationships.
To test both the alexithymia and interoception hypotheses, hierarchical multiple linear regression models were used across all participants. Data was centered and behavioral variables were entered into a hierarchical multiple linear regression model. The alexithymia hypothesis test was entered in the following order: age, sex, WASI-II: VCI, AQC total, group. The interoception hypothesis test was entered in the following order: age, sex, WASI-II: VCI, BPQ-VSF, group. Significance of alexithymia or interoception as a predictor was assessed before and after the addition of “Group” to each model. Homoscedasticity and normality of residuals were assessed using scatterplots of residuals and normal P-P plots, and tolerance statistic was used to assess independent variables for multicollinearity.
Results
Group Differences: ASD & TD
As expected, significant group differences were observed on the verbal IQ (WASI-II VCI), social impairment (SRS-2), ADHD symptomatology (Conners 3AI), anxiety (CASI-Anx), and the ToM ability (NEPSY-II ToM total; ps < .05; Table 1). For additional variables of interest, significant differences were observed (Table 1) in the NEPSY-II Affect Recognition (p = .035), measures of alexithymia (AQC total [p = .039], AQC COMM [p = .034]) and in physiological hyperarousal (PH-C total [p = .012]). In the cognitive empathy domain, the ASD group showed nearly significantly less IRI Perspective Taking ability compared to the TD group (p = .052; BF = .972). There were no significant group differences, or Bayes Factors supporting the alternative hypothesis, in either emotional empathy subscale (IRI Empathic Concern, p = .380, BF = 3.965; IRI Personal Distress, p = .247, BF = 3.038), measures of interoception (BPQ-VSF, p = .891, BF = 4.501), or in the use of sensation responses when describing experiences of emotion (BSE: p = .960, BF = 4.936; BSE-neg: p = .453, BF = 3.607). In summary, the ASD group had reduced verbal IQ, facial affect recognition, and cognitive empathy/ToM; increased social impairment, alexithymia severity, ADHD symptomatology, anxiety, and physiological hyperarousal; and intact interoception and emotional empathy skill. Due to the fact that cognitive empathy differences in ASD are well established in the literature, all other analyses were performed on relationships in the emotional empathy domain, in keeping with the study’s aims.
Table 1.
Descriptive Statistics and Group Comparisons of Behavioral Data
| Variable | TD | ASD | t | p | BF | ||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Age | 11.86 | 2.17 | 11.95 | 2.17 | −0.18 | 0.86 | 5.60 |
| WASI-II: FSIQ-4 | 114.85 | 12.9 | 109.29 | 17.76 | 1.57 | 0.12 | 3.55 |
| WASI-II: VCI | 114.95 | 12.29 | 105.80 | 19.12 | 2.43 | 0.02* | 0.35 |
| SRS-2 | 46.63 | 5.00 | 75.83 | 8.89 | −17.79 | <0.00* | 0.00 |
| Conners 3AI | 47.65 | 9.01 | 84.91 | 8.45 | −18.22 | <0.00* | 0.00 |
| AQC ID | 0.45 | 0.34 | 0.62 | 0.46 | −1.76 | 0.08 | 1.40 |
| AQC COMM | 0.62 | 0.46 | 0.86 | 0.49 | −2.17 | 0.03* | 0.68 |
| AQC total | 6.28 | 4.21 | 8.40 | 4.53 | −2.10 | 0.04* | 0.77 |
| IRI PT | 15.40 | 5.44 | 12.86 | 5.70 | 1.98 | 0.05 | 0.97 |
| IRI FS | 17.43 | 5.54 | 16.57 | 5.74 | 0.66 | 0.52 | 4.66 |
| IRI EC | 18.33 | 5.04 | 17.23 | 5.71 | 0.88 | 0.38 | 3.97 |
| IRI PD | 12.55 | 5.08 | 13.94 | 5.25 | −1.17 | 0.247 | 3.04 |
| IRI Total | 63.95 | 13.78 | 60.60 | 14.57 | 1.02 | 0.310 | 3.51 |
| NEPSY-II AR | 11.15 | 2.48 | 9.88 | 2.51 | 2.15 | 0.04* | 0.69 |
| NEPSY-II ToM | 25.08 | 1.65 | 23.17 | 3.06 | 2.98 | 0.01* | 0.08 |
| CASI-Anx | 24.90 | 4.91 | 36.97 | 8.59 | −7.08 | <0.00* | 0.00 |
| PH-C | 25.68 | 5.88 | 30.81 | 9.50 | −2.62 | 0.01* | 0.20 |
| BPQ-VSF | 27.46 | 13.11 | 26.95 | 11.68 | 0.14 | 0.89 | 4.50 |
| BSE | 3.24 | 1.99 | 3.27 | 2.13 | −0.05 | 0.96 | 4.94 |
| BSE-neg | 2.48 | 1.09 | 2.74 | 1.29 | −0.76 | 0.45 | 3.61 |
Note. Group differences between TD and ASD groups. WASI-II: FSIQ-4 = Wechsler Abbreviated Scale of Intelligence, Second Edition: Full Scale IQ; WASI-II: VCI = Wechsler Abbreviated Scale of Intelligence, Second Edition: Verbal Comprehension Index; WASI-II: PRI = Wechsler Abbreviated Scale of Intelligence, Second Edition: Perceptual Reasoning Index; SRS-2 = Social Responsiveness Scale, Second Edition; Conners-3AI = Conners 3 ADHD Index; AQC ID = Alexithymia Questionnaire for Children Identifying Emotions Subscale; AQC COMM = Alexithymia Questionnaire for Children Communicating Emotions Subscale; AQC total = Alexithymia Questionnaire for Children 2 factor total; IRI PT = Interpersonal Reactivity Index Perspective Taking Subscale; IRI FS = Interpersonal Reactivity Index Fantasy Subscale; IRI EC = Interpersonal Reactivity Index Empathic Concern Subscale; IRI PD = Interpersonal Reactivity Index Personal Distress Subscale; IRI Total = Interpersonal Reactivity Index Total Score; NEPSY-II AR = The Developmental Neuropsychological Assessment Affect Recognition Subscale; NEPSY-II ToM Total = The Developmental Neuropsychological Assessment Theory of Mind Subscale; CASI-Anx = Childhood Adolescent Symptom Inventory Total; PH-C = Physiological Hyperarousal Scale for Children Total; BPQ-VSF = Body Perception Questionnaire-Body Awareness Very Short Form Total; BSE = bodily sensation during emotion; BSE-neg = bodily sensation during negative emotion.
p < .05
Partial Correlation: Self-Report Data
Emotional Empathy
Across all participants, alexithymia (AQC) was related to emotional empathy in both domains (IRI Personal Distress, IRI Empathic Concern), but not always in the hypothesized direction. Across all participants, AQC Communication scores were negatively correlated with IRI Empathic Concern (r = −.360, p = .002). This relationship was observed again in the ASD group (r = −.470, p = .007; Table 2). However, an opposite pattern emerged in the IRI Personal Distress subscale. Across all participants, alexithymia scores were positively correlated with IRI Personal Distress (AQC ID: r = .390, p = .001; AQC COMM: r = .359, p = .002; AQC total: r = .444, p = .000). This relationship was seen in both the TD group (AQC total: r = .367, p = .026; AQC COMM: r = .354, p = .032; Table 2) and in the ASD group (AQC ID: r = .399, p = .024; AQC COMM: r = .385, p = .030; AQC total: r = .516, p = .002; Table 2). Thus, the presence of alexithymia was associated with lower empathic concern, but higher personal distress to others’ pain in ASD (Figure 1).
Table 2.
Correlations With Emotional Empathy Scores
| Variables | Empathic Concern | Personal Distress | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TD | ASD | TD | ASD | |||||||||
| r | p | BF | r | p | BF | r | p | BF | r | p | BF | |
| SRS-2 | 0.21 | 0.22 | 5.55 | −0.02 | 0.93 | 7.57 | −0.05 | 0.78 | 8.02 | 0.08 | 0.68 | 4.69 |
| Conners-3AI | 0.06 | 0.71 | 7.37 | 0.12 | 0.54 | 5.91 | 0.13 | 0.46 | 3.85 | −0.27 | 0.15 | 3.43 |
| AQC ID | −0.04 | 0.83 | 7.93 | −0.10 | 0.58 | 6.91 | 0.23 | 0.17 | 1.51 | 0.41 | 0.02* | 0.09 |
| AQC COMM | −0.25 | 0.13 | 3.19 | −0.47 | <0.01* | 0.64 | 0.32 | 0.05 | 1.12 | 0.40 | 0.02* | 0.75 |
| AQC total | −0.16 | 0.35 | 5.62 | −0.20 | 0.27 | 4.97 | 0.36 | 0.02* | 0.84 | 0.52 | 0.00* | 0.04 |
| NEPSY-II AR | 0.08 | 0.65 | 5.69 | 0.07 | 0.72 | 6.81 | 0.19 | 0.27 | 1.03 | 0.00 | 1.00 | 7.28 |
| CASI-Anx | 0.22 | 0.19 | 3.73 | 0.18 | 0.34 | 4.52 | 0.28 | 0.10 | 2.34 | 0.29 | 0.12 | 1.12 |
| PH-C | 0.13 | 0.47 | 6.10 | 0.16 | 0.41 | 4.78 | 0.37 | 0.02* | 0.54 | 0.04 | 0.84 | 6.16 |
| BPQ-VSF | −0.01 | 0.97 | 6.48 | 0.02 | 0.93 | 5.82 | −0.16 | 0.46 | 3.37 | −0.30 | 0.25 | 3.43 |
| BSE | −0.26 | 0.20 | 5.55 | 0.21 | 0.34 | 3.62 | −0.19 | 0.37 | 6.10 | −0.45 | 0.03* | 1.89 |
| BSE-neg | −0.33 | 0.13 | 4.55 | 0.19 | 0.42 | 4.12 | −0.11 | 0.62 | 6.49 | −0.41 | 0.07 | 2.86 |
Note: Correlations with emotional empathy subscales. SRS-2 = Social Responsiveness Scale, Second Edition; Conners-3AI = Conners 3 ADHD Index; AQC ID = Alexithymia Questionnaire for Children Identifying Emotions Subscale; AQC COMM = Alexithymia Questionnaire for Children Communicating Emotions Subscale; AQC total = Alexithymia Questionnaire for Children 2 factor total; NEPSY-II AR = The Developmental Neuropsychological Assessment Affect Recognition Subscale; CASI-Anx = Childhood Adolescent Symptom Inventory Total; PH-C = Physiological Hyperarousal Scale for Children Total; BPQ-VSF = Body Perception Questionnaire-Body Awareness Very Short Form Total; BSE = bodily sensation during emotion; BSE-neg = bodily sensation during negative emotion.
p < .05
Figure 1.
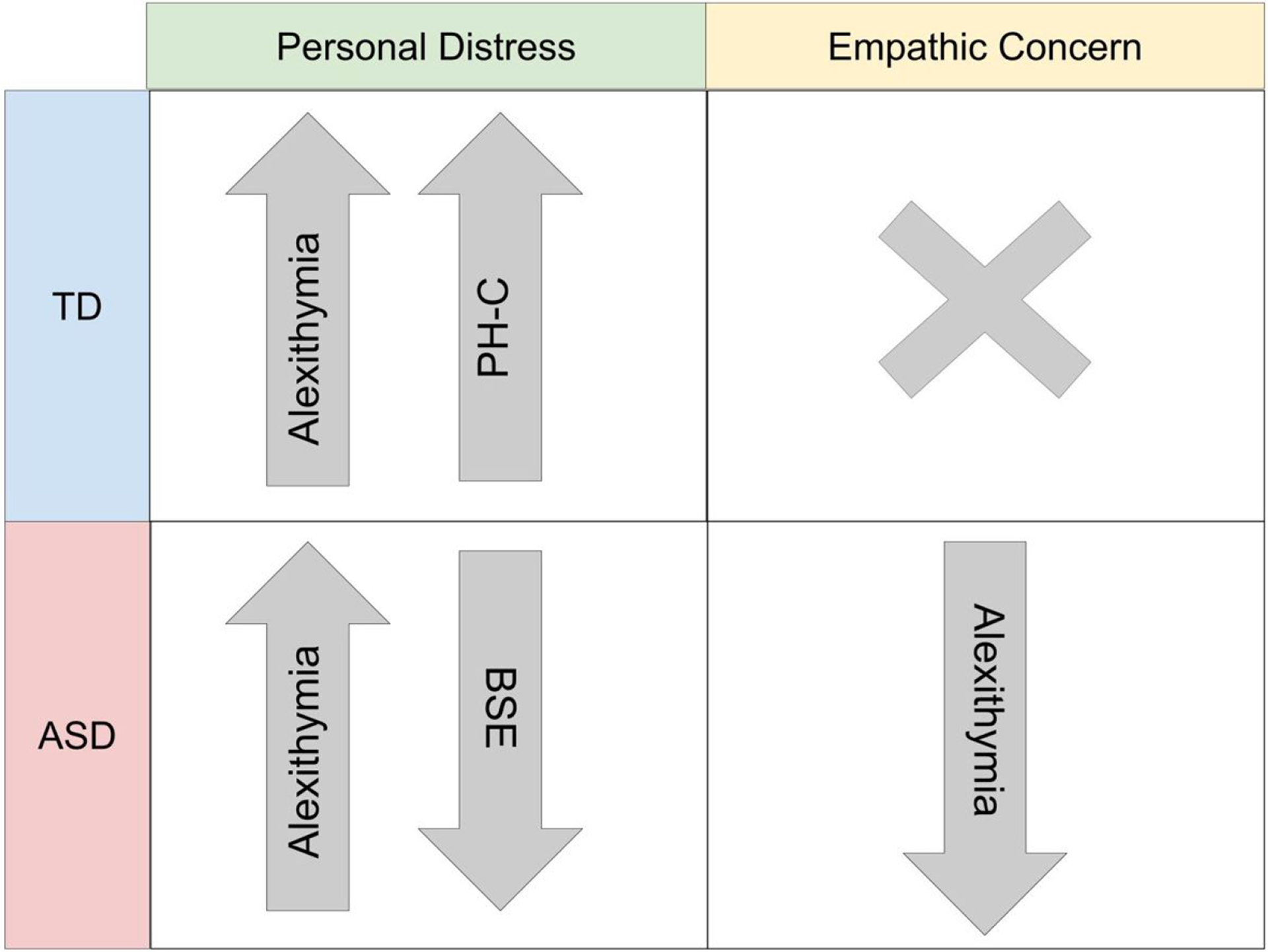
Summary of Relationships with Emotional Empathy Domains. PH-C = Physiological Hyperarousal Scale for Children Total; BSE = bodily sensation during emotion.
Embodied Variables
There were no significant relationships between interoception (BPQ-VSF) and alexithymia (AQC) or empathy (IRI) variables across all participants or in either individual group (ps > .05; BFs: 2.42 – 8.66). When AQC total was controlled for in the partial correlation, there were no significant relationships between interoceptive sensibility and emotional empathy. Across all participants, physiological arousal (PH-C) was positively correlated with alexithymia (AQC ID: r = .347, p = .004; AQC COMM: r = .293, p = .017; AQC total: r = .391, p = .001), and with IRI PD (r = .255, p = .039). In the TD group, the PH-C was positively correlated with both alexithymia (AQC ID: r = .370, p = .028; AQC total: r = .384, p = .023; Table 2) and IRI Personal Distress (r = .373, p = .027; Table 2). In individuals with ASD, there were no significant relationships between the PH-C and emotional empathy variables (ps > .05; BFs: 4.78, 6.16), this remained true when AQC total was controlled for in the partial correlation. In the ASD group, there were no significant relationships between the PH-C and alexithymia COMM variables (ps > .05; AQC COMM: BF = 6.03). However, Bayes Factors did not give substantial evidence for the null hypothesis in the identifying emotions subscale and the total score (AQC ID: BF = 2.0; AQC total: BF = 1.68).
There were no correlations found in the TD group between bodily sensation during emotion (BSE or BSE-neg) and emotional empathy variables (ps > .05; BFs: 4.55 – 6.49). In the ASD group, BSE was significantly negatively correlated with IRI Personal Distress (r = −.448. p = .032; Table 2). When the emotions were grouped into a negative valence category, a negative correlation was observed with alexithymia (AQC total; BSE-neg: r = −.471, p = .036). Results suggest that in the ASD group, greater alexithymia severity and higher personal distress were associated with fewer descriptions of physical sensations when experiencing emotions. The results are summarized in Figure 1.
Relationships with anxiety
There were no significant correlations between anxiety (CASI-Anx scores) and emotional empathy, alexithymia, or interoception in the TD group (ps > .05; BFs: 2.34 – 6.37). However, in the ASD group, CASI-Anx scores were positively correlated with alexithymia (AQC ID; r = .417, p = .025; AQC total r = .415, p = .025). After controlling for anxiety, the relationships between alexithymia and emotional empathy variables remained significant. These results suggest that anxiety in the ASD group does not account for the differing relationships observed between alexithymia and the two emotional empathy subscales. Additionally, no significant relationships between interoception (BPQ-VSF) and emotional empathy outcomes in ASD were observed when anxiety or alexithymia were controlled for.
Hierarchical Regression Analysis
Hierarchical linear regression models were run across all participants to assess whether interoceptive sensibility (BPQ-VSF) or alexithymia (AQC total) explained variance in emotional empathy outcomes above and beyond the influence of diagnostic group. All tolerance statistics for individual predictors were above .50, suggesting little concern for multicollinearity. Scatterplots of residuals and normal P-P plots did not indicate the need for adjustments based on normality or homoscedasticity.
Variance in neither empathic concern nor personal distress was significantly explained by the model, including interoceptive sensibility or any of its individual predictors. This result suggests that interoceptive sensibility is not significantly contributing to variance in personal distress or emotional empathy scores across all participants.
Variance in empathic concern was not significantly explained by the model including alexithymia or any of its individual predictors. However, the model including: age, sex, verbal IQ (WASI-II VCI), and alexithymia (AQC total) explained 28.3% of the variance in personal distress across participants. The addition of group to this model added a negligible amount to the R2 value of model 1 (.003) and group was not a significant individual predictor (Table 3). This result suggests that diagnostic group status is not significantly contributing to variance in personal distress scores above and beyond alexithymia severity.
Table 3.
Summary of Hierarchical Regression Analysis for IRI Personal Distress Across Groups
| Variable | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| B | SE B | β | p | B | SE B | β | p | |
| Age | −0.16 | 0.24 | −0.07 | 0.51 | −0.17 | 0.25 | −0.07 | 0.50 |
| Sex | 3.28 | 1.22 | 0.28 | 0.01 | 3.36 | 1.24 | 0.29 | 0.01 |
| WASI-II: VCI | −0.04 | 0.03 | −0.12 | 0.25 | −0.03 | 0.03 | −0.10 | 0.35 |
| AQC total | 0.48 | 0.12 | 0.42 | <0.01 | 0.46 | 0.12 | 0.41 | <0.01 |
| Group | 0.59 | 1.14 | 0.06 | 0.61 | ||||
| Model p | <0.01 | <0.01 | ||||||
| R 2 | 0.283 | 0.286 | ||||||
| Change in R2 | 0.003 | |||||||
Note: WASI-II: VCI = Wechsler Abbreviated Scale of Intelligence, Second Edition: Verbal Comprehension Index; AQC = Alexithymia Questionnaire for Children.
Discussion
Interoception, Alexithymia and Emotional Empathy in ASD
In this study, no significant differences in interoceptive sensibility were observed between TD and ASD youth. These results are inconsistent with others who find reduced interoceptive sensibility (Elwin et al., 2012; Fiene & Brownlow, 2015), or increased interoceptive sensibility in ASD (Garfinkel et al., 2016), although these prior studies were completed in adults and methodology for measuring interoceptive sensibility differed in some cases. Additionally, no relationships between interoceptive sensibility and empathy in ASD were found, even if alexithymia or anxiety was controlled for. Thus, it appears that interoceptive sensibility does not differ between ASD and TD youth and does not correlate with empathic ability in ASD.
Further, in emBody interviews, youths with ASD were able to describe bodily experiences of emotion at the same rate as TD youth, supporting the notion that interoceptive sensibility was not impaired in this ASD sample. In the ASD group, higher personal distress to others’ emotions was associated with lower descriptions of bodily sensations for negative emotions (BSE-neg). Thus, although there may not be reductions in interoceptive sensibility in ASD, there is evidence that reduced awareness of interoceptive information during actual emotion experience was related to increased alexithymia and personal distress in our ASD sample. This aligns with previous studies which find perhaps alexithymia, rather than ASD, is related to interoceptive difficulties (Shah et al., 2016a; Yang et al., 2021b).
These results contradict a study by Mul and colleagues (2018), which was completed in an adult sample, but used a similar protocol for defining ASD clinical diagnosis and IQ inclusion criteria. In their study, participants with ASD showed reduced interoceptive sensibility, and that alexithymia scores mediated the relationship between interoceptive sensibility and emotional empathy (Mul et al., 2018). By contrast, here we found no relationship between either empathic concern or personal distress with interoceptive sensibility in the ASD sample, alone, or when accounting for the influence of alexithymia. One possible explanation for the discrepancy between the current study and Mul et al. (2018) is differences in the measurement of interoceptive ability. Another crucial difference was that the current study separated domains of emotional empathy into empathic concern and personal distress, while the study by Mul and colleagues (2018) did not distinguish between subdomains of emotional empathy. These issues are discussed further below.
Interoception in ASD
While many studies have found no significant differences in cardiac interoceptive accuracy between TD and ASD groups (Mash et al., 2017; Nicholson et al., 2018; Schauder et al., 2015; Shah et al., 2016a; Shah et al., 2016b), several reports suggest differences in self-reported interoceptive awareness, reflecting diminished attention to and interpretation of interoceptive cues (Fiene et al., 2018; Fiene & Brownlow, 2015; Garfinkel et al., 2016; Mul et al., 2018). Garfinkel et al. (2016) found that individuals with ASD actually had an inverse relationship between self-reported interoceptive awareness and experimentally measured interoceptive accuracy on a heart-beat tracking task; the more confident a rater was about their interoceptive awareness, the worse they performed on an interoceptive accuracy task. These results indicate that individuals with ASD may have a particular difficulty in accurately reporting their interoceptive ability on questionnaires, which may impact inconsistencies in results. This discrepancy could impact social–emotional functioning in ASD by increasing the likelihood of incorrect interpretation of one’s own interoceptive cues (Failla et al., 2020). Self-reported global interoceptive awareness may be related to impaired multisensory integration and cognitive-affective control, resulting in increased hyper-vigilance toward bodily signals (Wang et al., 2020), which aligns with our finding of higher physiological hyperarousal in the ASD group. Interestingly, the interview assessment – which uses experimenter-led questioning about frequency, location, and intensity of physical sensation experienced during multiple emotional states – is negatively correlated with personal distress and alexithymia. The assessment format itself, which probes a participant to recall situations that elicited emotions and to reflect on specific emotionally salient experiences rather than global body awareness, may allow for easier and more accurate reporting of awareness of bodily signals.
Personal Distress and Empathic Concern
In this study, no significant differences between TD and ASD groups were observed in either emotional empathy subscore. An unexpected outcome of this study was the two inverse patterns observed with alexithymia across the different domains of emotional empathy. A key difference between the current study and the work of Mul and colleagues (2018) (and others e.g. Shah et al., 2019) was that these studies did not separate emotional empathy into the two components of personal distress and empathic concern, as was performed here. Mul and colleagues (2018) results indicate that those with alexithymia have lower emotional empathy than participants with ASD without alexithymia. Here, when including two seperate emotional empathy domains, we find that while correlation analyses show that alexithymia is related to reduced emotional empathy in ASD in the empathic concern domain, the opposite pattern is true in the personal distress component of emotional empathy. The divergent patterns in personal distress and empathic concern could account for previous discrepant findings regarding emotional empathy ability in ASD. The results point to a measurement and operational definition issue when combining the two subscales as part of one construct. Some studies that refer to emotional empathy in ASD are only referring to the empathic concern domain (e.g. Dzobiek et al., 2008; Trimmer et al., 2017), while other studies consider characteristics of both personal distress and empathic concern together as a measure of emotional empathy ability on measures like the Multifaceted Empathy Test (e.g. Mazza et al., 2014, Mul et al., 2018).
Hierarchical regression demonstrated that alexithymia predicted personal distress above and beyond ASD group membership. Although this is the opposite direction of the original alexithymia hypothesis (higher alexithymia, lower emotional empathy), it does support the idea that alexithymia is associated with potentially maladaptive experiences of empathy. Personal distress is an index of emotional empathy that refers to the tendency to feel personal pain when exposed to the tension, pain, or suffering of others. While this is an aspect of greater emotional empathy, it is also associated with maladaptive outcomes, such as ruminative coping, neuroticism, depression, self-criticism, and negative self-concept (Kim & Han, 2018). Similar to the results of the current study, Kim and Han (2018) suggest that personal distress could block empathic interaction and prosocial behavior by encouraging avoidance of overwhelming emotions from others’ suffering. Although, to our knowledge, the relationship between alexithymia and personal distress has not been specifically explored previously in individuals with ASD, the link between alexithymia severity and higher personal distress has been observed in non-clinical samples with schizotypy and autism traits (Aaron et al., 2015), TD participants (Grynberg et al., 2010; Moriguchi et al., 2007), and individuals diagnosed with either major depressive disorder (Banzhaf et al., 2018) or eating disorders (Brewer et al., 2019). Consistent with these findings, the current results suggest that alexithymia is marked by reduced ability to engage in other-oriented empathic concern, and an increased tendency towards self-oriented personal distress when exposed to another’s suffering. These findings reveal the need for specificity about what aspects of emotional empathy are being measured in ASD, and for a larger conversation regarding the narrative used to characterize empathy ability in ASD, which is reviewed below.
Empathy in ASD
The current study found intact emotional empathy ability, and reduced cognitive empathy in a sample of ASD youth. In a recent editorial written by Fletcher-Watson and Bird (2019), the authors argue that significant effort should be made to separate the social, emotion processing, and normative social behavioral processes that surround the phenomenon of empathy. Conflation of these terms can result in the belief that reduced capacity for emotional empathy is a central feature of ASD. Based on this study and previous studies, atypical emotional empathy may actually be a feature of alexithymia and not ASD (e.g. Bird & Cook, 2013; Brewer et al., 2015; Oakley et al., 2016). Additionally, the results of the current study suggest that for those with alexithymia and ASD, while we do see lower empathic concern, at the same time we find an increase in personal distress to others’ pain. Thus, individuals with alexithymia and ASD may have an increased resonance with other people’s distress, but less other-oriented empathic concern that may be prosocially expressed.
A major point reviewed by Fletcher-Watson and Bird (2019) is how ingroup-outgroup status of individuals with ASD impacts the way empathic ability in ASD is conceptualized. What has been described as the “double empathy problem” (Milton, 2012) is supported by evidence that TD individuals less accurately judge emotional expressions of individuals with ASD (Edey et al., 2016; Sheppard et al., 2016) and that interactions of two individuals with ASD are rated higher in terms of rapport than interactions than with ASD/TD pairs, by both the participants and diagnosis-blind observers (Crompton et al., 2020). Normative behavioral responses to emotional signaling of others are dictated by societal expectations defined by the non-ASD majority. In situations of others’ emotional display, individuals with ASD may appear to lack feelings of empathy, when in reality they are experiencing empathy, but not following the same rules of behavioral responding as a typically developing person (Fletcher-Watson & Bird, 2019). In fact, writings by individuals on the spectrum describe intense empathic hyperarousal (Elcheson et al., 2018; Williams, 1998), and other work indicates that object personification and anthropomorphism in individuals with ASD could cause empathic responses to more targets than in TD individuals (Clutterbuck et al., 2021; White & Remington, 2019). The consequences of these misattributions can have harmful effects on the ASD community. The belief in lack of empathy has facilitated work associating autism with extremist terrorism (Palermo, 2013), and with experiences of dehumanization in individuals with ASD (Yergeau, 2013). The findings of this study are supported by many of the issues raised by Fletcher-Watson and Bird (2019) about emotional empathy in ASD, and implicate a need for clear definitions, greater use of measures that probe individual experiences of empathy, characterization of potential subpopulations within ASD samples, and require reflection on the part of the researchers responsible for disseminating work perpetuating the narrative that empathy is unilaterally absent in ASD.
Limitations and Future Directions
There are many limitations to consider when interpreting the current findings. First, individuals diagnosed with ASD make-up a very heterogeneous population; the relatively small sample size in this study limits generalization to the whole spectrum of ASD. This study included participants ages 8–18 years with ASD who are verbal and have normal range IQ; therefore, the results may or may not apply to individuals on the spectrum with less verbal or intellectual abilities or to those in other age groups. Since some of our main variables of interest can only be measured in individuals who are verbal, future studies should further investigate the influence of verbal IQ on the relationships reported here. Additionally, the AQC was used to measure alexithymia in youth; however, like the TAS-20 (Bagby et al., 1994), this questionnaire captures more cognitive aspects of alexithymia, including identifying and communicating emotions, but does not measure additional emotionalizing aspects which should also be explored in ASD.
Secondly, many of the measures used to capture interoception, alexithymia, and empathy ability, are self-report measures. Accurate self-report can be particularly for individuals with ASD who may have difficulty with metacognition and self-referential insight. For this reason, we also included the experimenter-led emBody interview in the study. Future studies should examine these questions in a larger and more heterogeneous sample of participants with ASD, employ more observational and standardized measures when possible, and include the multidimensional emotional aspects of alexithymia, including emotionalizing and fantasizing.
Conclusions
The current study found intact emotional empathy ability and interoceptive sensibility, increased alexithymia severity, and reduced cognitive empathy/ToM ability in a sample of ASD youth. Major findings include: 1) alexithymia was associated with lower empathic concern and higher personal distress in individuals with ASD; 2) In the TD group, physiological hyperarousal (but not interoceptive sensibility) was positively correlated with both alexithymia and personal distress and 3) in the ASD group, higher personal distress and greater alexithymia severity, was associated with reporting fewer physical sensations in the body during emotion experience; 4) alexithymia predicted personal distress above and beyond ASD group membership, showing support for the alexithymia hypothesis for the personal distress domain of emotional empathy. An important takeaway from this work is the need for exploring the two domains of empathic concern and personal distress separately when examining alexithymia and interoception in ASD. The divergent patterns in personal distress and empathic concern may account for previous discrepant findings regarding emotional empathy ability in ASD. Future work should be cautious of conflation of empathy with other social cognitive processes, and dissemination of work should use care when reporting the implications of the results for the ASD community. The results of the present study suggest that while ASD youth with concomitant alexithymia report lower empathic concern, they do not lack empathy altogether, but rather experience an increase in personal distress to others’ pain; this may result in avoidance rather than prosocial action, but should not be characterized as an absence of a capacity for empathy.
Supplementary Material
Acknowledgements:
We would like to thank Dr. Jonas Kaplan, Dr. Sharon Cermak, Dr. Marian Williams, and Dr. Grace Baranek for their thoughtful feedback and edits to prior versions of this manuscript. Additionally, we are grateful to the participants and their families, we could not do this work without them. We also thank prior lab members, Alyssa Concha, Elisabeth Goo, Anusha Hossain, Alexis Nalbach, Ryann MacMurdo, Priscilla Ring, Cristin Zeisler, and research assistants Gabriel Abrams, Michelle Canales, Daisy Duong, Anastasiya Kats, Sharada Krishnan, Mariam Mnatsakanian, Samantha Noor, Jessie Tien, Lamoni Lucas, Corinne Archer, and Vanessa Yu for their contributions to participant recruitment, data collection and scoring.
Funding:
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD079432. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the Department of Defense through the Idea Development Award under award number AR170062. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. In the conduct of research involving hazardous organisms or toxins, the investigators adhered to the CDC-NIH Guide for Biosafety in Microbiological and Biomedical Laboratories.
Footnotes
Conflicts of Interest: We have no known conflicts of interest to disclose.
References
- Aaron RV, Benson TL, & Park S (2015). Investigating the role of alexithymia on the empathic deficits found in schizotypy and autism spectrum traits. Personality and Individual Differences, 77, 215–220. 10.1016/j.paid.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Publisher. [Google Scholar]
- Bagby RM, Parker JD, & Taylor GJ (1994). The twenty-item Toronto Alexithymia Scale—I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research, 38(1), 23–32. 10.1016/0022-3999(94)90005-1 [DOI] [PubMed] [Google Scholar]
- Baldner C, & McGinley JJ (2014). Correlational and exploratory factor analyses (EFA) of commonly used empathy questionnaires: New insights. Motivation and Emotion, 38(5), 727–744. 10.1007/s11031-014-9417-2 [DOI] [Google Scholar]
- Banzhaf C, Hoffmann F, Kanske P, Fan Y, Walter H, Spengler S, Schreiter S, Singer T, & Bermpohl F (2018). Interacting and dissociable effects of alexithymia and depression on empathy. Psychiatry Research, 270, 631–638. 10.1016/j.psychres.2018.10.045 [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Brodmann K, & Thoma P (2014). Active and observational reward learning in adults with autism spectrum disorder: Relationship with empathy in an atypical sample. Cognitive Neuropsychiatry, 19(3), 205–225. 10.1080/13546805.2013.823860 [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Valk SL, Silani G, Bird G, Frith U, & Singer T (2014). Selective Disruption of Sociocognitive Structural Brain Networks in Autism and Alexithymia. Cerebral Cortex, 24(12), 3258–3267. 10.1093/cercor/bht182 [DOI] [PubMed] [Google Scholar]
- Bird G, & Cook R (2013). Mixed emotions: The contribution of alexithymia to the emotional symptoms of autism. Translational Psychiatry, 3(7), e285. 10.1038/tp.2013.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G, Press C, & Richardson DC (2011). The Role of Alexithymia in Reduced Eye-Fixation in Autism Spectrum Conditions. Journal of Autism and Developmental Disorders, 41(11), 1556–1564. 10.1007/s10803-011-1183-3 [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, & Singer T (2010). Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain, 133(5), 1515–1525. 10.1093/brain/awq060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J, & Stokes MA (2018). Cognitive empathy moderates the relationship between affective empathy and wellbeing in adolescents with autism spectrum disorder. European Journal of Developmental Psychology, 1–14. 10.1080/17405629.2018.1444987 [DOI] [Google Scholar]
- Brewer R, Cook R, & Bird G (2016). Alexithymia: A general deficit of interoception. Open Science, 3(10), 150664. 10.1098/rsos.150664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer R, Cook R, Cardi V, Treasure J, Catmur C, & Bird G (2018). Alexithymia explains increased empathic personal distress in individuals with and without eating disorders. Quarterly Journal of Experimental Psychology. 10.1177/1747021818816051 [DOI] [PubMed] [Google Scholar]
- Brewer R, Happé F, Cook R, & Bird G (2015). Commentary on “Autism, oxytocin and interoception”: Alexithymia, not Autism Spectrum Disorders, is the consequence of interoceptive failure. Neuroscience & Biobehavioral Reviews, 56, 348–353. 10.1016/j.neubiorev.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Cabrera A, Kolacz J, Pailhez G, Bulbena‐Cabre A, Bulbena A, & Porges SW (2018). Assessing body awareness and autonomic reactivity: Factor structure and psychometric properties of the Body Perception Questionnaire-Short Form (BPQ-SF). International Journal of Methods in Psychiatric Research, 27(2), e1596. 10.1002/mpr.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happé F, & Frith U (2002). Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain: A Journal of Neurology, 125(Pt 8), 1839–1849. [DOI] [PubMed] [Google Scholar]
- Clutterbuck RA, Shah P, Leung HS, Callan MJ, Gjersoe N, & Livingston LA (2021). Anthropomorphic tendencies in autism: A conceptual replication and extension of White and Remington (2019) and preliminary development of a novel anthropomorphism measure. Autism: The International Journal of Research and Practice, 13623613211039387. Advance online publication. 10.1177/13623613211039387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK (2008). Conners 3rd edition: Manual (Vol. 14). Toronto, Ontario, Canada: Multi-Health Systems. [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social responsiveness scale: SRS-2. Torrance, CA: Western Psychological Services. [Google Scholar]
- Cook R, Brewer R, Shah P, & Bird G (2013). Alexithymia, Not Autism, Predicts Poor Recognition of Emotional Facial Expressions. Psychological Science, 24(5), 723–732. 10.1177/0956797612463582 [DOI] [PubMed] [Google Scholar]
- Cox BJ, Swinson RP, Shulman ID, & Bourdeau D (1995). Alexithymia in panic disorder and social phobia. Comprehensive Psychiatry, 36(3), 195–198. 10.1016/0010-440X(95)90081-6 [DOI] [PubMed] [Google Scholar]
- Craig A (2003). Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology, 13(4), 500–505. 10.1016/S0959-4388(03)00090-4 [DOI] [PubMed] [Google Scholar]
- Crompton CJ, Ropar D, Evans-Williams CV, Flynn EG, & Fletcher-Watson S (2020). Autistic peer-to-peer information transfer is highly effective. Autism, 24(7), 1704–1712. 10.1177/1362361320919286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR (1994). Descartes’ Error: Emotion, Rationality and the Human Brain. New York: Putnam, 352. [Google Scholar]
- Damasio A, Carvalho G (2013). The nature of feelings: evolutionary and neurobiological origins. Nature Reviews Neuroscience, 14, 143–152. 10.1038/nrn3403 [DOI] [PubMed] [Google Scholar]
- Davis M (1983). Measuring individual differences in empathy: Evidence for a multidemensional approach. Journal of Personality and Social Psychology, 44, 113. [Google Scholar]
- de Waal FBM, & Preston SD (2017). Mammalian empathy: Behavioural manifestations and neural basis. Nature Reviews Neuroscience, 18(8), 498–509. 10.1038/nrn.2017.72 [DOI] [PubMed] [Google Scholar]
- Deschamps PKH, Been M, & Matthys W (2014). Empathy and empathy induced prosocial behavior in 6- and 7-year-olds with autism spectrum disorder. Journal of Autism and Developmental Disorders, 44(7), 1749–1758. 10.1007/s10803-014-2048-3 [DOI] [PubMed] [Google Scholar]
- DuBois D, Ameis SH, Lai M-C, Casanova MF, & Desarkar P (2016). Interoception in Autism Spectrum Disorder: A review. International Journal of Developmental Neuroscience, 52, 104–111. 10.1016/j.ijdevneu.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Dziobek I, Rogers K, Fleck S, Bahnemann M, Heekeren HR, Wolf OT, & Convit A (2008). Dissociation of cognitive and emotional empathy in adults with asperger syndrome using the Multifaceted Empathy Test (MET). Journal of Autism and Developmental Disorders, 38(3), 464–473. 10.1007/s10803-007-0486-x [DOI] [PubMed] [Google Scholar]
- Edey R, Cook J, Brewer R, Johnson MH, Bird G, & Press C (2016). Interaction takes two: Typical adults exhibit mind-blindness towards those with autism spectrum disorder. Journal of Abnormal Psychology, 125(7), 879–885. 10.1037/abn0000199 [DOI] [PubMed] [Google Scholar]
- Ehlers A, & Breuer P (1992). Increased cardiac awareness in panic disorder. Journal of Abnormal Psychology, 101(3), 371–382. 10.1037/0021-843X.101.3.371 [DOI] [PubMed] [Google Scholar]
- Elcheson J, Stewart C, Lesko A, Willey LH, Craft S, Purkis Y, Campbell M (2018). Spectrum women: Walking to the beat of autism. London, UK: Jessica Kingsley. [Google Scholar]
- Elwin M, Ek L, Schröder A, & Kjellin L (2012). Autobiographical accounts of sensing in asperger syndrome and high-functioning autism. Archives of Psychiatric Nursing, 26(5), 420–429. 10.1016/j.apnu.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Failla MD, Bryant LK, Heflin BH, Mash LE, Schauder K, Davis S, Gerdes MB, Weitlauf A, Rogers BP, & Cascio CJ (2020). Neural correlates of cardiac interoceptive focus across development: implications for social symptoms in autism spectrum disorder. Autism Research: Official Journal of the International Society for Autism Research, 13(6), 908–920. 10.1002/aur.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiene L, & Brownlow C (2015). Investigating interoception and body awareness in adults with and without autism spectrum disorder. Autism Research, 8(6), 709–716. 10.1002/aur.1486 [DOI] [PubMed] [Google Scholar]
- Fiene L, Ireland MJ & Brownlow C The Interoception Sensory Questionnaire (ISQ): A Scale to Measure Interoceptive Challenges in Adults. J Autism Dev Disord 48, 3354–3366 (2018). 10.1007/s10803-018-3600-3 [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S, & Bird G (2019). Autism and empathy: What are the real links? Autism. http://journals.sagepub.com/doi/10.1177/1362361319883506 [DOI] [PubMed] [Google Scholar]
- Frith U, & Happé F (2005). Autism spectrum disorder. Current Biology, 15(19), R786–R790. 10.1016/j.cub.2005.09.033 [DOI] [PubMed] [Google Scholar]
- Fukushima H, Terasawa Y, & Umeda S (2011). Association between interoception and empathy: Evidence from heartbeat-evoked brain potential. International Journal of Psychophysiology, 79(2), 259–265. 10.1016/j.ijpsycho.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Gaigg SB, Cornell AS, & Bird G (2018). The psychophysiological mechanisms of alexithymia in autism spectrum disorder. Autism, 22(2), 227–231. 10.1177/1362361316667062 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Tiley C, O’Keeffe S, Harrison NA, Seth AK, & Critchley HD (2016). Discrepancies between dimensions of interoception in autism: Implications for emotion and anxiety. Biological Psychology, 114, 117–126. 10.1016/j.biopsycho.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Grynberg D, Luminet O, Corneille O, Grèzes J, & Berthoz S (2010). Alexithymia in the interpersonal domain: A general deficit of empathy? Personality and Individual Differences, 49(8), 845–850. 10.1016/j.paid.2010.07.013 [DOI] [Google Scholar]
- Grynberg D, & Pollatos O (2015). Perceiving one’s body shapes empathy. Physiology & Behavior, 140, 54–60. 10.1016/j.physbeh.2014.12.026 [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Zürcher NR, Rogier O, Hippolyte L, Lemonnier E, Ruest T, Ward N, Lassalle A, Gillberg N, Billstedt E, Helles A, Gillberg C, Solomon P, Prkachin KM, & Gillberg C (2014). Emotional contagion for pain is intact in autism spectrum disorders. Translational Psychiatry, 4(1), e343. 10.1038/tp.2013.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, Ronald A, & Plomin R (2006). Time to give up on a single explanation for autism. Nature Neuroscience, 9(10), 1218–1220. 10.1038/nn1770 [DOI] [PubMed] [Google Scholar]
- Heaton P, Reichenbacher L, Sauter D, Allen R, Scott S, & Hill E (2012). Measuring the effects of alexithymia on perception of emotional vocalizations in autistic spectrum disorder and typical development. Psychological Medicine, 42(11), 2453–2459. 10.1017/S0033291712000621 [DOI] [PubMed] [Google Scholar]
- Herbert BM, Herbert C, & Pollatos O (2011). On the relationship between interoceptive awareness and alexithymia: is interoceptive awareness related to emotional awareness? Journal of Personality, 79(5), 1149–1175. 10.1111/j.1467-6494.2011.00717.x [DOI] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O, & Schandry R (2007). Interoceptive sensitivity and emotion processing: an EEG study. International Journal of Psychophysiology, 65(3), 214–227. [DOI] [PubMed] [Google Scholar]
- Hill E, Berthoz S, & Frith U (2004). Brief report: Cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. Journal of Autism and Developmental Disorders, 34(2), 229–235. 10.1023/B:JADD.0000022613.41399.14 [DOI] [PubMed] [Google Scholar]
- James W (1884). What is emotion? Mind, IX(34), 188–205. 10.1093/mind/os-IX.34.188 [DOI] [Google Scholar]
- Jolliffe T, & Baron‐Cohen S (1997). Are people with autism and asperger syndrome faster than normal on the embedded figures test? Journal of Child Psychology and Psychiatry, 38(5), 527–534. 10.1111/j.1469-7610.1997.tb01539.x [DOI] [PubMed] [Google Scholar]
- Kasari C, Sigman M, Mundy P, & Yirmiya N (1990). Affective sharing in the context of joint attention interactions of normal, autistic, and mentally retarded children. Journal of Autism and Developmental Disorders, 20(1), 87–100. 10.1007/BF02206859 [DOI] [PubMed] [Google Scholar]
- Kim H, & Han S (2018). Does personal distress enhance empathic interaction or block it? Personality and Individual Differences, 124, 77–83. 10.1016/j.paid.2017.12.005 [DOI] [Google Scholar]
- Korkman M, Kirk U, & Kemp S (2007). NEPSY II: Clinical and interpretive manual. Harcourt Assessment, PsychCorp. https://researchportal.helsinki.fi/en/publications/nepsy-ii-clinical-and-interpretive-manual [Google Scholar]
- Laurent J, Catanzaro SJ, & Joiner TE Jr. (2004). Development and preliminary validation of the physiological hyperarousal scale for children. Psychological Assessment, 16(4), 373–380. 10.1037/1040-3590.16.4.373 [DOI] [PubMed] [Google Scholar]
- Loas G, Braun S, Delhaye M, & Linkowski P (2017). The measurement of alexithymia in children and adolescents: Psychometric properties of the Alexithymia Questionnaire for Children and the twenty-item Toronto Alexithymia Scale in different non-clinical and clinical samples of children and adolescents. PLOS ONE, 12(5), e0177982. 10.1371/journal.pone.0177982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, & Baron-Cohen S (2007). Self-Referential Cognition and Empathy in Autism. PLOS ONE, 2(9), e883. 10.1371/journal.pone.0000883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, DiLavore P, Pickles A, & Rutter M (2000). The Autism Diagnostic Observation Schedule—Generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. 10.1023/A:1005592401947 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- Markram H, Rinaldi T, & Markram K (2007). The intense world syndrome—An alternative hypothesis for autism. Frontiers in Neuroscience, 1. 10.3389/neuro.01.1.1.006.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash LE, Schauder KB, Cochran C, Park S, & Cascio CJ (2017). Associations between interoceptive cognition and age in autism spectrum disorder and typical development. Journal of Cognitive Education and Psychology: JCEP, 16(1), 23–37. 10.1891/1945-8959.16.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathersul D, McDonald S, & Rushby JA (2013). Understanding advanced theory of mind and empathy in high-functioning adults with autism spectrum disorder. Journal of Clinical and Experimental Neuropsychology, 35(6), 655–668. 10.1080/13803395.2013.809700 [DOI] [PubMed] [Google Scholar]
- Mazza M, Pino MC, Mariano M, Tempesta D, Ferrara M, De Berardis D, … & Valenti M (2014). Affective and cognitive empathy in adolescents with autism spectrum disorder. Frontiers in Human Neuroscience, 8, 791. 10.3389/fnhum.2014.00791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone L, Ruta L, & Reale L (2012). Psychiatric comorbidities in asperger syndrome and high functioning autism: Diagnostic challenges. Annals of General Psychiatry, 11(1), 16. 10.1186/1744-859X-11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosavljevic B, Carter Leno V, Simonoff E, Baird G, Pickles A, Jones CRG, Erskine C, Charman T, & Happé F (2016). Alexithymia in adolescents with autism spectrum disorder: its relationship to internalising difficulties, sensory modulation and social cognition. Journal of Autism and Developmental Disorders, 46(4), 1354–1367. 10.1007/s10803-015-2670-8 [DOI] [PubMed] [Google Scholar]
- Milton DEM (2012). On the ontological status of autism: The ‘double empathy problem.’ Disability & Society, 27(6), 883–887. 10.1080/09687599.2012.710008 [DOI] [Google Scholar]
- Minio-Paluello I, Baron-Cohen S, Avenanti A, Walsh V, & Aglioti SM (2009). Absence of embodied empathy during pain observation in asperger syndrome. Biological Psychiatry, 65(1), 55–62. 10.1016/j.biopsych.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, Matsuda H, & Komaki G (2007). Empathy and Judging other’s pain: An fmri study of alexithymia. Cerebral Cortex, 17(9), 2223–2234. 10.1093/cercor/bhl130 [DOI] [PubMed] [Google Scholar]
- Mul C, Stagg SD, Herbelin B, & Aspell JE (2018). The feeling of me feeling for you: interoception, alexithymia and empathy in autism. Journal of Autism and Developmental Disorders, 48(9), 2953–2967. 10.1007/s10803-018-3564-3 [DOI] [PubMed] [Google Scholar]
- Nicholson TM, Williams DM, Grainger C, Christensen JF, Calvo-Merino B, & Gaigg SB (2018). Interoceptive impairments do not lie at the heart of autism or alexithymia. Journal of Abnormal Psychology, 127(6), 612–622. 10.1037/abn0000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Glerean E, Hari R, & Hietanen JK (2014). Bodily maps of emotions. Proceedings of the National Academy of Sciences, 111(2), 646–651. 10.1073/pnas.1321664111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BFM, Brewer R, Bird G, & Catmur C (2016). Theory of mind is not theory of emotion: a cautionary note on the reading the mind in the eyes test. Journal of Abnormal Psychology, 125(6), 818–823. 10.1037/abn0000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo MT (2013). Developmental disorders and political extremism: A case study of asperger syndrome and the neo-nazi subculture. Journal of Forensic Psychology Practice, 13(4), 341–354. 10.1080/15228932.2013.817890 [DOI] [Google Scholar]
- Porges S (1993). Body perception questionnaire. Laboratory of Developmental Assessment, University of Maryland. [Google Scholar]
- Quattrocki E, & Friston K (2014). Autism, oxytocin and interoception. Neuroscience and Biobehavioral Reviews, 47, 410–430. 10.1016/j.neubiorev.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieffe C, Oosterveld P, & Terwogt MM (2006). An alexithymia questionnaire for children: Factorial and concurrent validation results. Personality and Individual Differences, 40(1), 123–133. [Google Scholar]
- Rogers K, Dziobek I, Hassenstab J, Wolf OT, & Convit A (2007). Who cares? Revisiting empathy in asperger syndrome. Journal of Autism and Developmental Disorders, 37(4), 709–715. 10.1007/s10803-006-0197-8 [DOI] [PubMed] [Google Scholar]
- Rueda P, Fernández-Berrocal P, & Baron-Cohen S (2015). Dissociation between cognitive and affective empathy in youth with Asperger Syndrome. European Journal of Developmental Psychology, 12(1), 85–98. 10.1080/17405629.2014.950221 [DOI] [Google Scholar]
- Schachter S, & Singer J (1962). Cognitive, social, and physiological determinants of emotional state. Psychological Review, 69(5), 379–399. 10.1037/h0046234 [DOI] [PubMed] [Google Scholar]
- Schauder KB, Mash LE, Bryant LK, & Cascio CJ (2015). Interoceptive ability and body awareness in autism spectrum disorder. Journal of Experimental Child Psychology, 131, 193–200. 10.1016/j.jecp.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Rüther M, Greimel E, Markowitsch HJ, Kamp-Becker I, Remschmidt H, Fink GR, & Piefke M (2011). Dysfunctions in brain networks supporting empathy: An fMRI study in adults with autism spectrum disorders. Social Neuroscience, 6(1), 1–21. 10.1080/17470911003708032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenck C, Mergenthaler J, Keller K, Zech J, Salehi S, Taurines R, Romanos M, Schecklmann M, Schneider W, Warnke A, & Freitag CM (2012). Empathy in children with autism and conduct disorder: Group-specific profiles and developmental aspects. Journal of Child Psychology and Psychiatry, 53(6), 651–659. 10.1111/j.1469-7610.2011.02499.x [DOI] [PubMed] [Google Scholar]
- Shah P, Catmur C, & Bird G (2016b). Emotional decision-making in autism spectrum disorder: The roles of interoception and alexithymia. Molecular Autism, 7(1), 43. 10.1186/s13229-016-0104-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Catmur C, & Bird G (2017). From heart to mind: Linking interoception, emotion, and theory of mind. Cortex, 93, 220–223. 10.1016/j.cortex.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Hall R, Catmur C, & Bird G (2016a). Alexithymia, not autism, is associated with impaired interoception. Cortex, 81, 215–220. 10.1016/j.cortex.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Livingston LA, Callan MJ, & Player L (2019). Trait autism is a better predictor of empathy than alexithymia. Journal of Autism and Developmental Disorders, 49(10), 3956–3964. 10.1007/s10803-019-04080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Yaniv S, Aharon-Peretz J. Empathy deficits in Asperger syndrome: a cognitive profile. Neurocase. 2002;8(3):245–52. doi: 10.1093/neucas/8.3.245. [DOI] [PubMed] [Google Scholar]
- Sheppard E, Pillai D, Wong GT-L, Ropar D, & Mitchell P (2016). How easy is it to read the minds of people with autism spectrum disorder? Journal of Autism and Developmental Disorders, 46(4), 1247–1254. 10.1007/s10803-015-2662-8 [DOI] [PubMed] [Google Scholar]
- Sigman MD, Kasari C, Kwon J-H, & Yirmiya N (1992). Responses to the negative emotions of others by autistic, mentally retarded, and normal children. Child Development, 63(4), 796–807. 10.1111/j.1467-8624.1992.tb01662.x [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry, 47(8), 921–929. 10.1097/CHI.0b013e318179964f [DOI] [PubMed] [Google Scholar]
- Smith A (2009). The empathy imbalance hypothesis of autism: A theoretical approach to cognitive and emotional empathy in autistic development. The Psychological Record, 59(3), 489–510. 10.1007/BF03395675 [DOI] [Google Scholar]
- Sprafkin J, Gadow KD, Salisbury H, Schneider J, Loney J. (2002). Further evidence of reliability and validity of the Child Symptom Inventory-4: parent checklist in clinically referred boys. Journal of Clinical Child Adolescent Psychology, (4):513–24. 10.1207/S15374424JCCP3104_10. [DOI] [PubMed] [Google Scholar]
- Sucksmith E, Allison C, Baron-Cohen S, Chakrabarti B, & Hoekstra RA (2013). Empathy and emotion recognition in people with autism, first-degree relatives, and controls. Neuropsychologia, 51(1), 98–105. 10.1016/j.neuropsychologia.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer E, McDonald S, & Rushby JA (2017). Not knowing what I feel: Emotional empathy in autism spectrum disorders. Autism, 21(4), 450–457. https://doi-org.libproxy1.usc.edu/10.1177/1362361316648520 [DOI] [PubMed] [Google Scholar]
- Wang X, Tan Y, Van den Bergh O, von Leupoldt A, & Qiu J (2020). Intrinsic functional brain connectivity patterns underlying enhanced interoceptive sensibility. Journal of Affective Disorders, 276, 804–814. 10.1016/j.jad.2020.07.032 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011). WASI-II: Wechsler abbreviated scale of intelligence. PsychCorp. [Google Scholar]
- Williams D (1998). Autism and sensing: The unlost instinct. London, UK: Jessica Kingsley. [Google Scholar]
- White RC, & Remington A (2019). Object personification in autism: This paper will be very sad if you don’t read it. Autism, 23(4), 1042–1045. 10.1177/1362361318793408 [DOI] [PubMed] [Google Scholar]
- Yang HX, Zhou HY, Wei Z, Wan GB, Wang Y, Wang YY, Yang TX, Lui S, & Chan R (2021a). Multidimensional interoception and autistic traits across life stages: evidence from a novel eye-tracking task. Journal of Autism and Developmental Disorders, 10.1007/s10803-021-05155-w. Advance online publication. 10.1007/s10803-021-05155-w [DOI] [PubMed] [Google Scholar]
- Yang HX, Zhou HY, Zheng H, Wang Y, Wang YY, Lui SS, & Chan RC (2021b). Individuals with autistic traits exhibit heightened alexithymia but intact interoceptive-exteroceptive sensory integration. Journal of Autism and Developmental Disorders, 1–11. 10.1007/s10803-021-05199-y [DOI] [PubMed] [Google Scholar]
- Yergeau M (2013). Clinically significant disturbance: On theorists who theorize theory of mind. Disability Studies Quarterly, 33(4). 10.18061/dsq.v33i4.3876 [DOI] [Google Scholar]
- Yirmiya N, Sigman MD, Kasari C, & Mundy P (1992). Empathy and cognition in high-functioning children with autism. Child Development, 63(1), 150–160. 10.1111/j.1467-8624.1992.tb03603.x [DOI] [PubMed] [Google Scholar]
- Zaki J, & Ochsner KN (2012). The neuroscience of empathy: Progress, pitfalls and promise. Nature Neuroscience, 15(5), 675–680. 10.1038/nn.3085 [DOI] [PubMed] [Google Scholar]
- Zalla T, Sav A-M, Stopin A, Ahade S, & Leboyer M (2009). Faux pas detection and intentional action in Asperger Syndrome: A replication on a French sample. Journal of Autism and Developmental Disorders, 39(2), 373–382. 10.1007/s10803-008-0634-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


