Abstract
The aim of the present study is to investigate the preventive effect of water extract of Mori Ramulus (MRWE) on oxidative stress-mediated cellular damages in rat skeletal L6 myoblasts. Our results demonstrated that MRWE pretreatment markedly improved cell survival and suppressed cell cycle arrest at the G2/M phase and apoptosis in hydrogen peroxide (H2O2)-treated L6 cells. H2O2-triggered DNA damage was also notably reduced by MRWE, which since it was correlated with protection of reactive oxygen species (ROS) production. Additionally, H2O2 stimulated cytosolic release of cytochrome c and up-regulation of Bax/Bcl-2 ratio, whereas MRWE suppressed these changes following by H2O2. Moreover, MRWE inhibited the cleavage of poly(ADP-ribose) polymerase as well as the activity of caspase-3 by H2O2. Furthermore, MRWE enhanced H2O2-mediated expression of nuclear factor erythroid 2-associated factor 2 (Nrf2) and its representative downstream enzyme, heme oxygenase-1 (HO-1). However, the protective effects of MRWE on H2O2-induced ROS production, cell cycle arrest and apoptosis were significantly attenuated by HO-1 inhibitor. In conclusion, our present results suggests that MRWE could protect L6 myoblasts from H2O2-induced cellular injury by inhibiting ROS generation along with Nrf2-mediated activation of HO-1, indicating this finding may expand the scope of application of Mori Ramulus in medicine.
Keywords: Mori Ramulus, ROS, DNA damage, Apoptosis, Nrf2/HO-1
Introduction
Morus alba Linn (Mulberry), a perennial woody tree belonging to the family Morus, is native to China, and all parts of this tree have excellent pharmacological actions [1–3]. Among them, Mori Ramulus, which refers to the dry branches of this tree, has been reported to have beneficial effects such as relieving diabetes, lowering cholesterol and blood glucose, improving insulin sensitivity, and preventing liver damage [4–6]. This pharmacological activity is believed to be at least due to its antioxidant effect. For example, Mori Ramulus extract reduced the replication of herpes simplex virus by blocking the production of free radicals including reactive oxygen species (ROS), suggesting that its antiviral effect was associated with ROS scavenging activity [7]. In addition, this herb improved insulin sensitivity while preventing ROS generation in high-fat diet-induced diabetic mice, thereby preserving mitochondrial function and preventing apoptosis in hepatocytes [4]. To date, more than 20 phytochemicals have been identified from M. alba such as flavonoids, astragalin, maclurin and caffeoylquinic acid [8–11]. Recently, Jiang et al. [9] isolated several phenolic components, such as morin, isoquercitrin, luteolin and rutin, with antioxidant properties, from Mori Fructus and Mori Ramulus. In addition, 13 marker compounds, including neochlorogenic acid, rutin, and astragalin were identified from Ramulus Mori, Cortex Mori, Fructus Mori and Folium Mori [10]. In a comparative study of the effects of different extraction methods on the antioxidant activity of Ramulus Mori, the 50% ethanol extraction method showed a relatively higher radical scavenging activity with greater total flavonoid contents than hot water extraction method [12]. However, the comparative functional effects of these method-based extracts have not been evaluated in vitro or in vivo. We also demonstrated that water extract of Mori Ramulus (MRWE) contained astragalin and rutin hydrate in the previous study [11]. Moreover, Mori Ramulus-derived polysaccharides (RMP) was able to block kidney damage in diabetic mice through activation of antioxidant enzymes, including glutathione peroxidase and superoxide dismutase [13]. Similar to these results, Guo et al. [14] reported that RMP normalized pancreatic function by attenuating the inflammatory response and suppressing oxidative stress, which was associated with increased expression of heme oxygenase-1 (HO-1), a downstream enzyme of nuclear factor E2-related factor 2 (Nrf2). Moreover, mulberrin, a major compound of Mori Ramulus, significantly blocked spinal cord injury-induced neuronal cell apoptosis by reducing oxidative damage through the activation of HO-1/Nrf-2 pathway [15]. Consistent with this result, 10-oxomornigrol F, a type of flavonoid identified from Mori Ramulus, exerted an alleviating effect on the inflammatory response by activating this signaling pathway [16]. These results indicate that the components contained in Mori Ramulus can act as an Nrf2 activator to counteract oxidative and inflammatory disorders. Additionally, several recent studies have demonstrated that Mori Ramulus as well as other parts of M. alba, including leaves, fruits and roots, have strong antioxidant activity and that the Nrf-2-dependent HO-1 activation plays a key role in this process [17–19].
Although ROS is produced during normal cellular metabolism, excessive production of ROS results in progressive oxidative injury to organelles [20, 21]. In particular, skeletal muscle produced excessive ROS by contractile activity in mitochondria due to consumes more oxygen that other tissues [22–24]. As in other tissues, oxidative stress resulting from excessive accumulation of ROS in the muscle ultimately leads to the death of muscle cells [25, 26]. Moreover, excess ROS induce depolarization of mitochondrial membrane potential (Δψm), which is a hallmark of mitochondrial dysfunction. In subsequence, cytochrome c is released from mitochondria to the cytoplasm, which activates the caspase cascade, ultimately leading to apoptosis [20, 27]. More recently, we demonstrated that Mori Ramulus suppressed oxidative stress-mediated cell death through the up-regulation of ROS-dependent AMP‐activated protein kinase in C2C12 myoblasts, and Mori Ramulus intake suppressed muscle loss in dexamethasone‐induced muscle atrophy rodent [28]. However, this previous study has some limitations that the further studies are need to elucidate other pathways involved in the recovery of mitochondrial dysfunction and ROS production in myoblasts. Therefore, in this study, we evaluated the inhibitory effect of MRWE on hydrogen peroxide (H2O2)-induced cellular damages in rat skeletal L6 myoblasts.
Materials and methods
MRWE preparation and cell culture
The dried powder of Mori Ramulus was provided by the Bohyeonsan Clean Herb Farming Association (Yeongcheon, Korea), and extracted in water, as previous described [28]. The extraction yield of MRWE was 2.74%, and the samples were kept in deep freezer. L6 myoblasts purchased from the American Type Culture Collection (CRL-1458™, Manassas, VA, USA) were cultured in minimal essential medium containing 15% fetal bovine serum and 1% antibiotics at 37 °C and 5% CO2. All materials needed for cell culture were obtained from WelGENE Inc. (Gyungsan, Korea). MRWE and H2O2 (Sigma-Aldrich Chemical Co., St. Louis, MO, USA) were dissolved in distilled water to make a 200 mg/ml and 100 mM stock solution, respectively. The stock solutions were diluted in the medium before utilization.
Cell viability
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay is used to measure cell viability [29]. Briefly, L6 cells were stimulated with the different concentrations of MRWE or H2O2 alone for 24 h, or exposed to MRWE for 1 h and then treated with H2O2 for 24 h. At the end time, the MTT reaction was run and absorbance was measured with a microplate reader (Beckman Coulter Inc., Brea, CA, USA) at the Core Facility Center for Tissue Regeneration (Dong-eui University, Busan, Korea).
Cell cycle analysis
MRWE-treated or untreated L6 cells with or without H2O2 or zinc protoporphyrin IX (ZnPP, Enzo Life Sciences Inc., Farmingdale, NY, USA) were analyzed for cell cycle analysis. After treatment, both adherent and detached cells were washed with phosphate-buffered saline (PBS) and then fixed by ethanol [29]. After that, cells were exposed with RNAase and propidium iodide (PI) (Thermo Fisher Scientific., Waltham, MA, USA) for 20 min at 4 °C. Cell cycle distributions were calculated after appropriate gating of cell populations using flow cytometry (Becton Dickinson, San Jose, CA, USA).
Comet assay
A comet assay® kit obtained from Trevigen, Inc. (Gaithersburg, MD, USA) was used to evaluate DNA damage according to the manufacturer’s protocols [30].
Western blotting for protein expression analysis
Whole cell lysates for immunoblotting were prepared according to a previous method [31]. In order to isolate the cytoplasmic and mitochondrial fractions, a mitochondrial/cytoplasmic fractionation kit (Active Motif, Inc., Carlsbad, CA, USA) was used. For Western blot analysis, the same amount of protein for each treatment group was used, and the antibodies used in this study are as follows; actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), Bax (Santa Cruz Biotechnology, Inc.), Bcl-2 (Santa Cruz Biotechnology, Inc.), cytochrome c oxidase subunit IV (COX IV) (Cell Signaling Technology, Danvers, MA, USA), cytochrome c (Santa Cruz Biotechnology, Inc.), HO-1 (Abcam, Inc., Cambridge, UK), Kelch-like ECH-associated protein 1 (Keap1) (Thermo Fisher Scientific), Nrf2 (Thermo Fisher Scientific), phosphorylated (p)-Nrf2 (Ser40, Thermo Fisher Scientific), poly(ADP-ribose) polymerase (PARP) (Cell Signaling Technology), caspase-3 (Santa Cruz Biotechnology, Inc.), and nuclear histone H2A.X protein (γH2A.X) (Ser139, Cell Signaling Technology). Subsequently, the membranes that had finished reacting with the primary antibodies reacted with the correlated secondary antibodies (Santa Cruz Biotechnology, Inc.). The membranes were then exposed enhanced chemiluminescence solution (Thermo Fisher Scientific) and visualized using a Fusion FX Imaging System (Vilber Lourmat, Torcy, France).
Immunofluorescence assay for detection of γH2A.X and p-Nrf2
To observe intracellular expression of γH2A.X and p-Nrf2, L6 cells grown on 4-well chamber slide were stimulated with or without MRWE for 1 h and then treated with H2O2 for additional 24 h. After fixing with 4% paraformaldehyde solution at RT for 20 min, cells were incubated with ice-cold PBS containing 1% bovine serum albumin (Sigma-Aldrich Chemical Co.) and 1% Triton X-100 (Sigma-Aldrich Chemical Co.) for 1 h. Subsequently, immunostaining was performed using antibodies against γH2A.X and p-Nrf2 at 4 °C overnight and then reacted with Alexa Fluor® 647-conjugated secondary antibody (Abcam, Inc.) for 1 h at RT. Additionally, nuclei were also stained with 4′,6′-diamidino-2-phenylindole (DAPI) solution (1 μg/ml, Sigma-Aldrich Chemical Co) for 20 min at RT. The cells were immediately observed under a fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Analysis of intracellular ROS
To measure the levels of ROS, cells were cultured in medium containing the indicated concentrations of MRWE of compound C (Sigma-Aldrich Chemical Co.) for 1 h and then treated with H2O2 (0.5 mM) for 1 h. The cells were stained with 2’,7’-dichlorofluorescein diacetate (DCF-DA) dye (Sigma-Aldrich Chemical Co.). The fluorescence intensity was observed under a fluorescence microscope or quantified using flow cytometry.
Apoptosis analysis
To observe whether apoptosis was induced, DAPI staining was performed according to a published method [29]. Briefly, L6 cells were stimulated with H2O2 for 24 h with or without MRWE, washed with PBS and then fixed with 4% paraformaldehyde solution for 15 min. Subsequently, cells were stained with DAPI solution for 10 min, and then images were observed under a fluorescence microscope. To quantitatively assess the degree of apoptosis, cells were stained with annexin V-fluorescein isothiocyanate (FITC)/PI (Becton Dickinson), and annexin V-positive cells were quantified as cells induced by apoptosis using a flow cytometer.
Caspase-3 activity assay
The activity of caspase-3 was determined using a caspase-3 activity enzyme-linked immunosorbent assay kit (Abcam, Inc.), as previous described [32].
Statistical analysis
All experiments were independently repeated at least three times to determine significance. Results were presented as mean and standard deviation (SD) using SPSS 25.0 (SPSS Inc., Chicago, IL, USA), and differences (p < 0.05) were considered statistically using ANOVA-Tukey’s post-hoc test.
Results
MRWE restored the loss of L6 cell viability caused by H2O2 treatment
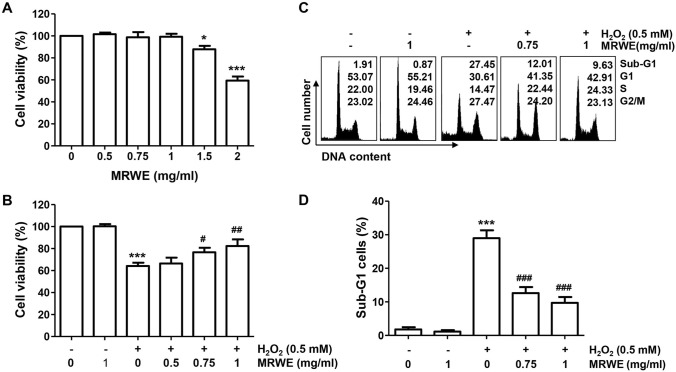
As indicated in Fig. 1A, MRWE has been no cytotoxicity up to 1 mg/ml of concentration in L6 cells. However, cells exposed to more than 1.5 mg/ml MRWE showed a significant inhibition in cell viability. Therefore, 1 mg/ml was selected the maximum concentration of MRWE to establish its efficacy, we performed further experiments. The cell viability in L6 cells treated with 0.5 mM H2O2 was significantly decreased to approximately 60% compared that in the untreated control cells. However, pretreatment of MRWE significantly prevented H2O2-induced reduction of cell viability in a dose-dependent manner (Fig. 1B). Especially, exposure to 1 mg/ml of MRWE restored the cell viability as control levels in H2O2-stimulated L6 cells.
Fig. 1.
Inhibitory effects of water extracts of Mori Ramulus (MRWE) on hydrogen peroxide (H2O2)-mediated cytotoxicity and cell cycle arrest at the G2/M phase in L6 cells. Cells were treated with different concentrations of MRWE for 24 h (a) or treated with MRWE for 1 h, and then stimulated with H2O2 for 24 h (b–d). a and b The results of quantitative analysis of cell viability according to MTT assay were presented. c and d Cells were collected and analysis of cell cycle distribution was performed. c Representative flow histograms were presented. d The frequency of sub-G1 cells were presented. a, b and d The data were represented as mean ± standard deviation (SD) of three independent experiments. Significant differences compared to the control cells (*p < 0.05, **p < 0.01 and ***p < 0.001) or H2O2‑treated cells (#p < 0.05, ##p < 0.01 and ###p < 0.001) were shown
MRWE prevented cell cycle arrest and apoptotic cell death in H2O2-treated L6 cells
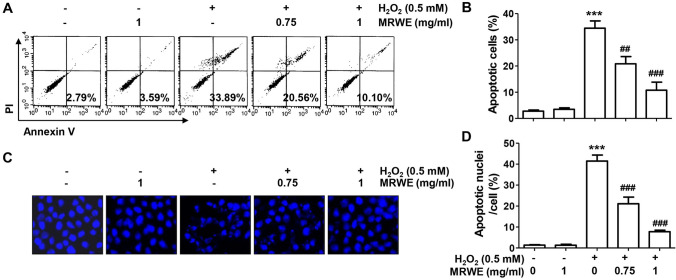
As shown in Fig. 1C, the population of cells belonging to the G2∕M phase in H2O2-treated cells was significantly increased compared to the untreated control group, which was concentration-dependently attenuated by MRWE pretreatment. The proportion of cells with sub-G1 DNA content, which indicates the frequency of apoptosis, also greatly increased after H2O2 treatment compared with control cells, which significantly reduced by pretreatment of 1 mg/ml of MRWE (Fig. 1D). In addition, the population of annexin V-positive cells was also significantly increased in H2O2-treated cells (Fig. 2A, B). Consistent with this, it was found that the frequency of chromatin condensation and apoptotic body-forming nuclei increased in cells treated with H2O2 alone through DAPI staining (Fig. 2C, D). However, these indicators of apoptosis were markedly suppressed by MRWE pretreatment. The results indicate that MRWE substantially attenuated cell cycle arrest and apoptotic cell death following by H2O2, thereby restoring cell viability.
Fig. 2.
Attenuation of apoptosis by MRWE in H2O2-treated L6 cells. L6 cells were incubated in medium containing various concentrations of MRWE for 1 h, and then exposed to H2O2 (0.5 mM) for 24 h. a and b Cells were then double-stained with annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI), and analyzed by flow cytometry. a The population of apoptotic cells were shown in the lower right panel of representative histograms. b The averages of the frequencies of apoptotic cells. c and d After staining with 4′,6′-diamidino-2-phenylindole (DAPI), the morphology of the nucleus was observed. Representative photomicrographs of nuclei after DAPI staining (c) and quantified results (d) are shown. b and d Significant differences compared to the control cells (***p < 0.001) or H2O2‑treated cells (#p < 0.05 and ###p < 0.001) were shown
MRWE suppressed ROS production in H2O2-stimulated L6 cells
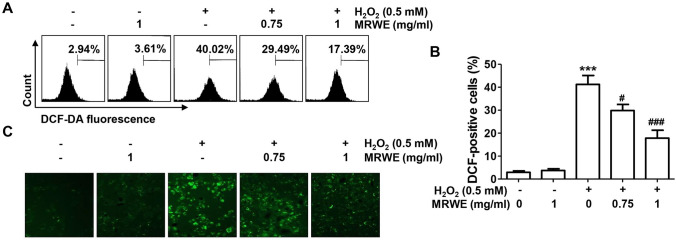
The results of flow cytometry after DCF-DA staining showed that the level of intracellular ROS production was markedly increased in L6 cells treated with H2O2 (Fig. 3A, B). Consistent with this result, the DCF-fluorescence image in H2O2-treated cells was markedly increased compared to that of control cells (Fig. 3C). However, pretreatment with MRWE significantly decreased H2O2-induced ROS generation, indicating that the inhibitory effect of MRWE on the cytotoxicity of H2O2 in L6 cells was related to its antioxidant activity.
Fig. 3.
Inhibitory effects of MRWE on reactive oxygen species (ROS) generated by H2O2 in L6 cells. L6 cells were cultured in medium containing different concentrations of MRWE for 1 h, treated with H2O2 (0.5 mM) for 1 h and stained with 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA). a and b Intracellular ROS levels were calculated using a flow cytometer (a and b) or observed under a fluorescence microscope (c). b Significant differences compared to the control cells (*p < 0.05 and ***p < 0.001) or H2O2‑treated cells (###p < 0.001) were shown
MRWE inhibited DNA damage in H2O2-treated L6 cells
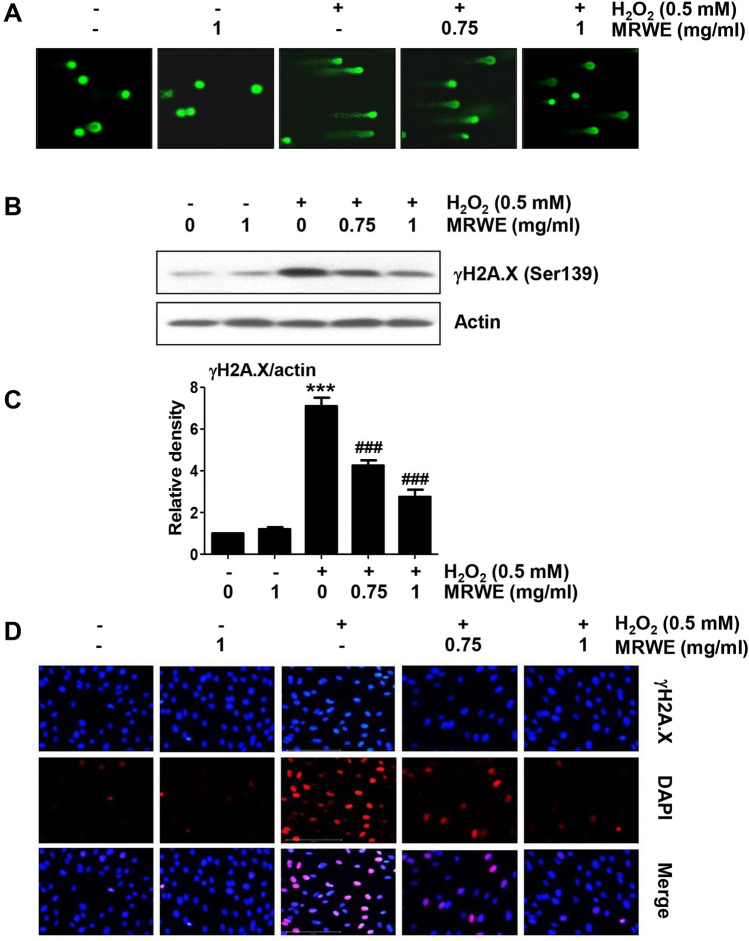
Next, we investigated the inhibitory effect of MRWE on DNA damage induced by H2O2-treatment. According to the comet assay results, DNA tails caused by damaged DNA fragments were greatly enhanced in H2O2-treated L6 cells (Fig. 4A). However, these tails were concentration-dependently reduced in cells pretreated with MRWE. In addition, phosphorylation of γH2A.X was strongly induced in H2O2-treated cells (Fig. 4B, C). Immunofluorescence also indicated that H2O2 significantly increased the number of γH2A.X positive-stained cells compared to control cells (Fig. 4D). However, its expression was attenuated by MRWE pretreatment, indicating that the inhibitory effect of MRWE against H2O2-induced DNA damage was related to inhibition of ROS generation.
Fig. 4.
Inhibition of DNA damage by MRWE in H2O2-treated L6 cells. L6 cells were stimulated with indicated concentration of MRWE for 1 h, and then exposed to H2O2 (0.5 mM) for 24 h. a Representative images of comet assay. b The protein expression of γH2A.X was investigated using Western blotting. Actin was used as the reference. c Expression values of each protein were normalized to actin levels by ImageJ software (***p < 0.001 vs. untreated cells; ###p < 0.001 vs. H2O2-treated cells). d After treatment, cells were double-stained with γH2A.X (red) and DAPI (blue), and representative immunofluorescence images observed are shown
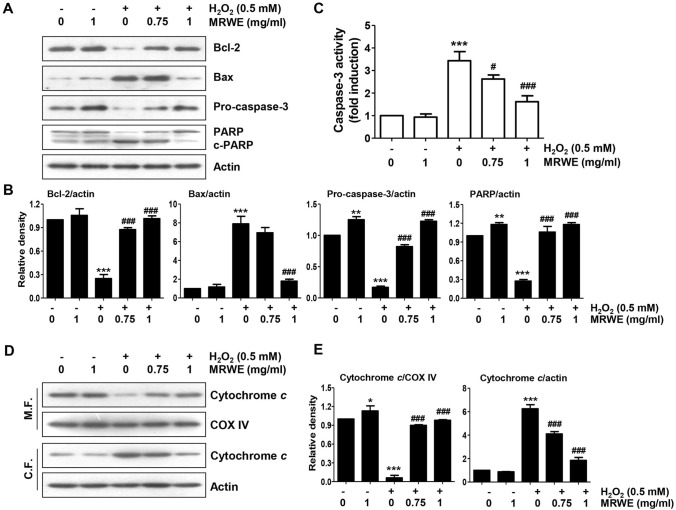
MRWE alleviated the change of apoptosis regulators expression in H2O2-treated L6 cells
As indicated in Fig. 5A, the protein level of Bcl-2 was down-regulated in H2O2-treated L6 cells, while that of Bax was up-regulated. In addition, Fig. 5A and B showed that H2O2 inhibited the level of pro-caspase-3 protein and enhanced the degradation of PARP. Moreover, H2O2 substantially enhanced caspase-3 activity compared with control cells (Fig. 5C), whereas its activity was markedly inhibited by pretreatment with MRWE. Furthermore, the level of cytochrome c expression in the mitochondria of cells treated with H2O2 was diminished, but its expression in the cytoplasm was increased (Fig. 5D, E). However, H2O2-induced these alterations were remarkably suppressed with increasing MRWE pretreatment concentration.
Fig. 5.
Inhibitory effect of MRWE on changes of mitochondria-mediated apoptosis regulatory factors in H2O2-treated L6 cells. L6 cells were pretreated with indicated concentration of MRWE for 1 h, and then incubated with H2O2 (0.5 mM) for additional 24 h. a Protein expression of apoptosis-related mediators (c-PARP, cleaved PARP). b Expression values of each protein were normalized to actin levels by ImageJ software (**p < 0.01 and ***p < 0.001 vs. untreated cells; ###p < 0.001 vs. H2O2-treated cells). c Activity of caspase-3. Significant differences compared to the control cells (**p < 0.01 and ***p < 0.001) or H2O2‑treated cells (##p < 0.01) were shown. d Expression of cytochrome c in mitochondrial and cytoplasmic fractions. Actin and cytochrome c oxidase subunit IV (COX IV) were used as the reference genes for cytosolic and mitochondrial fractions (M.F. mitochondrial fraction; C.F. cytoplasmic fraction). e Expression values of each protein were normalized to COX IV or actin levels by ImageJ software (*p < 0.05 and ***p < 0.001 vs. untreated cells; ###p < 0.001 vs. H2O2-treated cells)
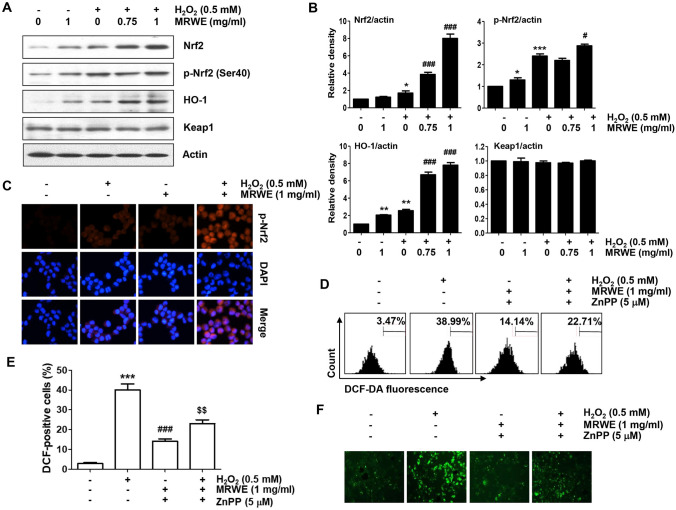
The Nrf2-mediated activation of HO-1 was enhanced by MRWE in H2O2-treated L6 cells
We next examined whether the activation of HO-1 was associated with the antioxidant activity of MRWE in L6 cells. As indicated in Fig. 6A and B the result of immunoblotting showed that the expression of HO-1 as well as Nrf2 was partially up-regulated in cells treated with MRWE and H2O2 alone. However, their expression was greatly improved when MRWE and H2O2 were treated together. Additionally, the level of p-Nrf2 expression showed a similar tendency and its expression was predominantly expressed in the nucleus (Fig. 6C), indicating that the Nrf2-mediated HO-1 activation was more activated in L6 cells treated with H2O2 and MRWE together compared to cells exposed to each alone.
Fig. 6.
Enhancement of Nrf2-mediated HO-1 antioxidant signaling activation by MRWE in H2O2-treated L6 cells. a and b Cells were stimulated with different concentrations of MRWE for 1 h, and then treated with H2O2 (0.5 mM) for additional 24 h. c After treatment, cells were double-stained with p-Nrf2 (red) and DAPI (blue), and representative immunofluorescence images observed are shown. d–f Cells were treated with zinc protoporphyrin IX (ZnPP, 5 μM) in the presence or absence of MRWE for 1 h, and then exposed to H2O2 (0.5 mM) for 1 h. a Western blotting results were presented. (B) Expression values of each protein were normalized to actin levels by ImageJ software (*p < 0.05, **p < 0.01 and ***p < 0.001 vs. untreated cells; #p < 0.05 and ###p < 0.001 vs. H2O2-treated cells). d–f Intracellular ROS levels using DCF-DA staining were investigated using a flow cytometer (d and e) or a fluorescence microscope (f). e The percentages of DCF-positive cells by flow cytometry were quantified. Significant differences compared to the control cells (*p < 0.05, **p < 0.01 and ***p < 0.001), H2O2‑treated cells (#p < 0.01 and ###p < 0.001) or H2O2 and MRWE-treated cells (&&p < 0.01) were shown
Activation of Nrf2-mediated HO-1 by MRWE was associated with the inhibitory effect against H2O2-induced cytotoxicity in L6 cells
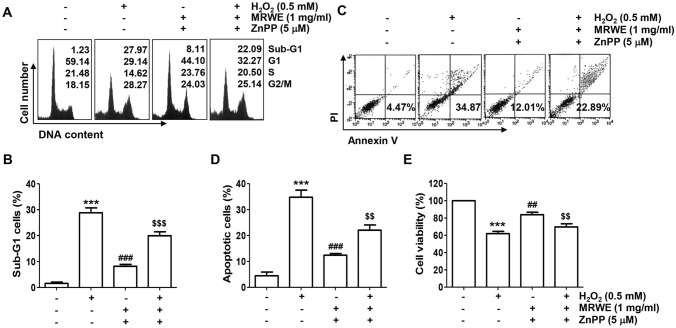
In order to further elucidate the involvement of Nrf2-mediated HO-1 antioxidant pathway in the inhibitory effect of MRWE against H2O2-induced cytotoxicity, L6 cells were cultured in medium containing a competitive inhibitor of HO-1, ZnPP, and then treated with MRWE and H2O2. As shown in Fig. 6D–F, the inhibitory effect of MRWE against H2O2-induced ROS accumulation was markedly disappeared in the presence of ZnPP. In addition, the protective effect of MRWE on cell cycle arrest induced by H2O2 was abrogated when cells were pre-incubated with ZnPP (Fig. 7A). Furthermore, the protective effects of MRWE against apoptosis and growth inhibition induced by H2O2 were also reduced by ZnPP (Fig. 7B–E). Therefore, the present results indicate that MRWE protected L6 cells from oxidative damage caused by H2O2 through Nrf2-mediated activation of HO-1.
Fig. 7.
Protection against H2O2-induced cytotoxicity by MRWE was related to activation of HO-1 in L6 cells. L6 cells were incubated with ZnPP with or without MRWE for 1 h, and then stimulated with H2O2 (0.5 mM) for additional 24 h. a Representative results showing cell cycle distribution were presented. b The frequencies of sub-G1 cells were presented. c and d After staining with annexin V/PI, flow cytometry was performed. c Representative results showing the frequency of apoptosis were presented. d The results of quantitative analysis on apoptotic cells were presented. e The results of quantitative analysis of cell viability according to MTT assay were presented. b, d and e Significant differences compared to the control cells (***p < 0.001), H2O2‑treated cells (##p < 0.01 and ###p < 0.001) or H2O2 and MRWE-treated cells (&&p < 0.01 and &&&p < 0.001) were shown
Discussion
Here in, we examined the efficacy of MRWE on oxidative damage in H2O2-stimulated L6 myoblasts. The present finding demonstrated that MRWE markedly inhibited H2O2-induced cellular dysfunctions, including cell cycle arrest at the G2/M phase, DNA damage and apoptotic cell death, which was caused by blocking of ROS accumulation. Furthermore, we showed that the antioxidant potential of MRWE was accompanied by stimulation of Nrf2-mediated activation of HO-1.
As is well known, oxidative stressors, including H2O2, induce cell cycle arrest at the G2/M phase in most cells, including muscle cells, causing to DNA damage as well as cell death [33–35]. In this study, suppression of cell survival in H2O2-treated L6 cells was accompanied by inhibition of cell cycle progression at the G2/M phase, which was in good agreement with the previous findings using C2C12 skeletal myoblasts and H9c2 cardiomyoblasts [36, 37]. However, these effects were significantly reversed by MRWE pretreatment. We also demonstrated that MRWE had potent antioxidant activity by significantly inhibiting H2O2-induced ROS generation. Oxidative stress induces damage to intracellular macromolecules such as nucleic acids, contributing to DNA damage and apoptosis [27, 38]. The results of the comet assay, a commonly used method to measure DNA strand breaks [39], showed that MRWE effectively inhibited H2O2-induced comet tail formation. Additionally, in the immunoblotting results for analyzing the phosphorylation level of H2A.X, which indicates that the DNA double-strand is broken by oxidative stress [40], H2O2 induced expression of γH2A.X was largely blocked in the presence of MRWE. These findings indicated that MRWE has a remarkable ameliorating effect for H2O2-induced DNA damage in L6 myoblasts. In our previous study, the antioxidant effect of Mori Ramulus extract was investigated in C2C12 myoblast cells [28]. The results showed that Mori Ramulus could prevent C2C12 cells from oxidative damage through AMP-activated protein kinase (AMPK) signaling activation. As our current study demonstrates that MRWE exerted antioxidant action through Nrf2/HO-1 activation, the antioxidant signaling pathway of MRWE may be associated with AMPK activation and could contribute to maintaining oxidative homeostasis and attenuating ROS-related diseases. A recent study demonstrated that AMPK can act as a transcription factor by directly phosphorylating Nrf2 and mediating its translocation to the nucleus [41]. Therefore, we investigated the association between MRWE-mediated Nrf2 activation and AMPK activation using compound C, an AMPK inhibitor. Our results showed that compound C did not exhibit significant antioxidant activity on H2O2-induced intracellular ROS generation (data not shown), suggesting that Nrf2/HO-1 activation regulated by MRWE is not dependent on AMPK signaling.
According to the results of previous studies, H2O2 -induced apoptosis in L6 myoblasts was strongly correlated with the cytosolic release of apoptogenic factors, including cytochrome c, which is initiates intrinsic apoptosis pathway [42, 43]. In the cytoplasm, cytochrome c increases the activity of effector caspases such as caspase-3 and -7 through the activation of caspase-9, which induce degradation of matrix proteins including PARP to terminate apoptosis [44, 45]. In this study, the expression of cytochrome c was up-regulated in the cytoplasm in H2O2-stimulated cells, consistent with previous studies [42, 43]. However, its expression was reversed in the presence of MRWE, suggesting that mitochondrial integrity in H2O2-treated L6 myoblasts was maintained in the presence of MRWE. Subsequently, the increase of Bax/Bcl-2 ratio by H2O2 was also counteracted by MRWE pretreatment, which was correlated with to suppressing the cleavage of PARP via blocking the caspase-3 activity. Bcl-2 family members control the release of apoptogenic factors through regulation of mitochondrial membrane permeability [43, 45]. Therefore, it is presumed that the reduction of the Bax/Bcl-2 expression ratio by MRWE plays a critical role in attenuating H2O2-induced L6 cell apoptosis. Based on this finding, we considered that MRWE may be a potential antioxidant to prevent mitochondria-mediated intrinsic apoptosis by blocking ROS accumulation.
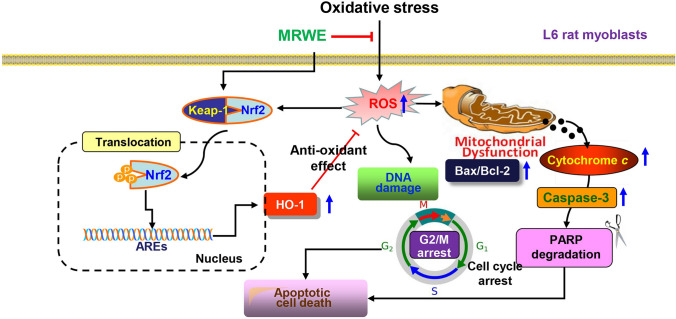
Nrf2 is a major redox sensing transcription factor, and under stress conditions or in the presence of Nrf2 activators, Nrf2 dissociates from its inhibitor Keap1 in the cytoplasm and then trans-locates to the nucleus [46–48]. Recently, several reports have suggested that various components of M. alba act as Nrf2 activators to inhibit oxidative stress-mediated damage [7, 15–18]. Supporting these results, our data showed that MRWE further activated Nrf2 in H2O2-treated L6 cells. Therefore, the increased expression of HO-1 by MRWE in the presence of H2O2 is probably due to the activation of Nrf2. Based on the results, we further investigated whether the blocking effect of MRWE against H2O2-mediated oxidative injury was associated with activation of HO-1 using ZnPP, a selective antagonist of HO-1. Interestingly, ZnPP pretreatment abolished the inhibitory effect of MRWE on H2O2-induced ROS accumulation. Additionally, ZnPP interrupted the inhibitory effect of MRWE on H2O2-induced cytotoxicity. Therefore, our data revealed that MRWE protected L6 cells from H2O2-induced cytotoxicity through Nrf2-mediated activation of HO-1 (Fig. 8).
Fig. 8.
Schematic diagram representing the protective mechanism of MRWE against oxidative stress-induced oxidative damage in L6 cells. MRWE markedly inhibited H2O2-induced cellular dysfunctions, including DNA damage, cell cycle arrest at the G2/M phase and apoptosis, which was caused by blocking of ROS accumulation
In the current study, we evaluated the efficacy of MRWE on H2O2-mediated oxidative damage in L6 myoblasts. Our data showed that MRWE markedly attenuated cell cycle arrest at the G2/M phase, DNA damage and apoptosis in H2O2-stimulated L6 cells, which was linked to its ability to suppress ROS accumulation. Additionally, our finding indicated that the anti-apoptotic effect by MRWE was a result of blockade of caspase cascade activation, which correlated with inhibition of cytoplasmic release of cytochrome c due to inhibition of increased Bax/Bcl-2 ratio. Moreover, our data showed that the antioxidant activity of MRWE was achieved through Nrf2-mediated HO-1 activation.
Funding
This research was funded by the National Research Foundation of Korea Grant (NRF-2021R1A2C201471711 and 2021R1A2C200954911) and Korea Basic Science Institute Grant funded (2020R1A6C101A201)
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributor Information
Cheol Park, Email: parkch@deu.ac.kr.
Hyesook Lee, Email: lhyes0219@pusan.ac.kr.
Sung Ok Kim, Email: sokim@ks.ac.kr.
Eun-Woo Lee, Email: ewlee@deu.ac.kr.
Hyun-Tai Lee, Email: htlee@deu.ac.kr.
Hyun Ju Kwon, Email: hjkwon@deu.ac.kr.
Byung Woo Kim, Email: bwkim@deu.ac.kr.
Gi-Young Kim, Email: immunkim@jejunu.ac.kr.
Mi Ryeo Kim, Email: mrkim@dhu.ac.kr.
Yung Hyun Choi, Email: choiyh@deu.ac.kr.
References
- 1.Chen C, Mohamad Razali UH, Saikim FH, Mahyudin A, Mohd Noor NQI. Morus alba L. plant: Bioactive compounds and potential as a functional food ingredient. Foods. 2021;10:689. doi: 10.3390/foods10030689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan EW, Lye PY, Wong SK. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin J Nat Med. 2016;14:17–30. doi: 10.3724/SP.J.1009.2016.00017. [DOI] [PubMed] [Google Scholar]
- 3.He X, Fang J, Ruan Y, Wang X, Sun Y, Wu N, Zhao Z, Chang Y, Ning N, Guo H, Huang L. Structures, bioactivities and future prospective of polysaccharides from Morus alba (white mulberry): a review. Food Chem. 2018;245:899–910. doi: 10.1016/j.foodchem.2017.11.084. [DOI] [PubMed] [Google Scholar]
- 4.Han T, Ko E, Kim M, Choi M, Lee C, Kim IH, Shin S, Um MY. Mori Ramulus inhibits pancreatic beta-cell apoptosis and prevents insulin resistance by restoring hepatic mitochondrial function. Antioxidants (Basel) 2021;10:901. doi: 10.3390/antiox10060901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SY, Jin B, Shin JH, Adisakwattana S, Kwon O. Standardized Mori ramulus extract improves insulin secretion and insulin sensitivity in C57BLKS/J db/db mice and INS-1 cells. Biomed Pharmacother. 2017;92:308–315. doi: 10.1016/j.biopha.2017.05.080. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Yang F, Wang J, Huang H, Huang Y. Anti-diabetic effect mediated by Ramulus mori polysaccharides. Carbohydr Polym. 2015;117:63–69. doi: 10.1016/j.carbpol.2014.09.052. [DOI] [PubMed] [Google Scholar]
- 7.Kim TI, Kwon EB, Oh YC, Go Y, Choi JG. Mori ramulus and its major component morusin inhibit herpes simplex virus type 1 replication and the virus-induced reactive oxygen species. Am J Chin Med. 2021;49:163–179. doi: 10.1142/S0192415X21500099. [DOI] [PubMed] [Google Scholar]
- 8.Wan LZ, Ma B, Zhang YQ. Preparation of morusin from Ramulus mori and its effects on mice with transplanted H22 hepatocarcinoma. BioFactors. 2014;40:636–645. doi: 10.1002/biof.1191. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Q, Li X, Tian Y, Lin Q, Xie H, Lu W, Chi Y, Chen D. Lyophilized aqueous extracts of Mori Fructus and Mori Ramulus protect mesenchymal stem cells from ⋅OH-treated damage: bioassay and antioxidant mechanism. BMC Complement Altern Med. 2017;17:242. doi: 10.1186/s12906-017-1730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Du X, Yang Y, Cui X, Zhang Z, Li Y. Comparative study of chemical composition and active components against α-glucosidase of various medicinal parts of Morus alba L. Biomed Chromatogr. 2018;32:e4328. doi: 10.1002/bmc.4328. [DOI] [PubMed] [Google Scholar]
- 11.Kwon DH, Jeong JW, Choi EO, Lee HW, Lee KW, Kim KY, Kim SG, Hong SH, Kim GY, Park C, Hwang HJ, Son CG, Choi YH. Inhibitory effects on the production of inflammatory mediators and reactive oxygen species by Mori folium in lipopolysaccharide-stimulated macrophages and zebrafish. An Acad Bras Cienc. 2017;89:661–674. doi: 10.1590/0001-3765201720160836. [DOI] [PubMed] [Google Scholar]
- 12.Park HM, Hong JH. Effect of extraction methods on antioxidant activities of Mori Ramulus. J Korean Soc Food Sci Nutr. 2014;43:1709–1715. doi: 10.3746/jkfn.2014.43.11.1709. [DOI] [Google Scholar]
- 13.Li X, Wang L, Gao X, Li G, Cao H, Song D, Cai S, Liang T, Zhang B, Du G. Mechanisms of protective effect of Ramulus Mori polysaccharides on renal injury in high-fat diet/streptozotocin-induced diabetic rats. Cell Physiol Biochem. 2015;37:2125–2134. doi: 10.1159/000438570. [DOI] [PubMed] [Google Scholar]
- 14.Guo C, Li R, Zheng N, Xu L, Liang T, He Q. Anti-diabetic effect of ramulus mori polysaccharides, isolated from Morus alba L., on STZ-diabetic mice through blocking inflammatory response and attenuating oxidative stress. Int Immunopharmacol. 2013;16:93–99. doi: 10.1016/j.intimp.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Xia P, Gao X, Duan L, Zhang W, Sun YF. Mulberrin (Mul) reduces spinal cord injury (SCI)-induced apoptosis, inflammation and oxidative stress in rats via miroRNA-337 by targeting Nrf-2. Biomed Pharmacother. 2018;107:1480–1487. doi: 10.1016/j.biopha.2018.07.082. [DOI] [PubMed] [Google Scholar]
- 16.Tran PL, Tran PT, Tran HNK, Lee S, Kim O, Min BS, Lee JH. A prenylated flavonoid, 10-oxomornigrol F, exhibits anti-inflammatory effects by activating the Nrf2/heme oxygenase-1 pathway in macrophage cells. Int Immunopharmacol. 2018;55:165–173. doi: 10.1016/j.intimp.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Gao XH, Zhang SD, Wang LT, Yu L, Zhao XL, Ni HY, Wang YQ, Wang JD, Shan CH, Fu YJ. Anti-inflammatory effects of neochlorogenic acid extract from mulberry leaf (Morus alba L.) against LPS-stimulated inflammatory response through mediating the AMPK/Nrf2 signaling pathway in A549 cells. Molecules. 2020;25:1385. doi: 10.3390/molecules25061385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu D, Bao T, Lu Y, Su H, Ke H, Chen W. Polysaccharide from mulberry fruit (Morus alba L.) protects against palmitic-acid-induced hepatocyte lipotoxicity by activating the Nrf2/ARE signaling pathway. J Agric Food Chem. 2020;68:13016–13024. doi: 10.1021/acs.jafc.9b03335. [DOI] [PubMed] [Google Scholar]
- 19.Wen L, Shi D, Zhou T, Tu J, He M, Jiang Y, Yang B. Identification of two novel prenylated flavonoids in mulberry leaf and their bioactivities. Food Chem. 2020;315:126236. doi: 10.1016/j.foodchem.2020.126236. [DOI] [PubMed] [Google Scholar]
- 20.He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 21.Benhar M. Oxidants, antioxidants and thiol redox switches in the control of regulated cell death pathways. Antioxidants (Basel) 2020;9:309. doi: 10.3390/antiox9040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouviere J, Fortunato RS, Dupuy C, Werneck-de-Castro JP, Carvalho DP, Louzada RA. Exercise-stimulated ROS sensitive signaling pathways in skeletal muscle. Antioxidants (Basel) 2021;10:537. doi: 10.3390/antiox10040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosca N, Petrillo S, Bortolani S, Monforte M, Ricci E, Piemonte F, Tasca G. Redox homeostasis in muscular dystrophies. Cells. 2021;10:1364. doi: 10.3390/cells10061364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qaisar R, Bhaskaran S, Premkumar P, Ranjit R, Natarajan KS, Ahn B, Riddle K, Claflin DR, Richardson A, Brooks SV, Van Remmen H. Oxidative stress-induced dysregulation of excitation-contraction coupling contributes to muscle weakness. J Cachexia Sarcopenia Muscle. 2018;9:1003–1017. doi: 10.1002/jcsm.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majumder A, Singh M, George AK, Tyagi SC. Restoration of skeletal muscle homeostasis by hydrogen sulfide during hyperhomocysteinemia-mediated oxidative/ER stress condition (1) Can J Physiol Pharmacol. 2019;97:441–456. doi: 10.1139/cjpp-2018-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lian D, Chen MM, Wu H, Deng S, Hu X. The role of oxidative stress in skeletal muscle myogenesis and muscle disease. Antioxidants (Basel) 2022;11:755. doi: 10.3390/antiox11040755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brillo V, Chieregato L, Leanza L, Muccioli S, Costa R. Mitochondrial dynamics, ROS, and cell signaling: a blended overview. Life (Basel) 2021;11:332. doi: 10.3390/life11040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park C, Ji SY, Lee H, Choi SH, Kwon CY, Kim SY, Lee ET, Choo ST, Kim GY, Choi YH, Kim MR. Mori Ramulus suppresses hydrogen peroxide-induced oxidative damage in murine myoblast C2C12 cells through activation of AMPK. Int J Mol Sci. 2021;22:11729. doi: 10.3390/ijms222111729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim KH, Shu MS, Kim S, Kim JY, Choi BH, Lee YJ. Cilostazol induces apoptosis and inhibits proliferation of hepatocellular carcinoma cells by activating AMPK. Biotechnol Bioprocess Eng. 2021;26:776–785. doi: 10.1007/s12257-021-0002-8. [DOI] [Google Scholar]
- 30.Park C, Lee H, Hong SH, Kim JH, Park SK, Jeong JW, Kim GY, Hyun JW, Yun SJ, Kim BW, Kim WJ, Choi YH. Protective effect of diphlorethohydroxycarmalol against oxidative stress-induced DNA damage and apoptosis in retinal pigment epithelial cells. Cutan Ocul Toxicol. 2019;38:298–308. doi: 10.1080/15569527.2019.1613425. [DOI] [PubMed] [Google Scholar]
- 31.Choi MJ, Mukherjee S, Yun JW. Loss of ADAMTS15 promotes browning in 3T3-L1 white adipocytes via activation of β3-adrenergic receptor. Biotechnol Bioprocess Eng. 2021;26:188–200. doi: 10.1007/s12257-021-0036-y. [DOI] [Google Scholar]
- 32.Liang Y, Kong D, Zhang Y, Li S, Li Y, Ramamoorthy S, Ma J. Fisetin inhibits cell proliferation and induces apoptosis via JAK/STAT3 signaling pathways in human thyroid TPC 1 cancer cells. Biotechnol Bioprocess Eng. 2020;25:197–205. doi: 10.1007/s12257-019-0326-9. [DOI] [Google Scholar]
- 33.Shi T, van Soest DMK, Polderman PE, Burgering BMT, Dansen TB. DNA damage and oxidant stress activate p53 through differential upstream signaling pathways. Free Radic Biol Med. 2021;172:298–311. doi: 10.1016/j.freeradbiomed.2021.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Beyfuss K, Hood DA. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep. 2018;23:100–117. doi: 10.1080/13510002.2017.1416773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panieri E, Gogvadze V, Norberg E, Venkatesh R, Orrenius S, Zhivotovsky B. Reactive oxygen species generated in different compartments induce cell death, survival, or senescence. Free Radic Biol Med. 2013;57:176–187. doi: 10.1016/j.freeradbiomed.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Santa-Gonzalez GA, Gomez-Molina A, Arcos-Burgos M, Meyer JN, Camargo M. Distinctive adaptive response to repeated exposure to hydrogen peroxide associated with upregulation of DNA repair genes and cell cycle arrest. Redox Biol. 2016;9:124–133. doi: 10.1016/j.redox.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oyama K, Takahashi K, Sakurai K. Hydrogen peroxide induces cell cycle arrest in cardiomyoblast H9c2 cells, which is related to hypertrophy. Biol Pharm Bull. 2011;34:501–506. doi: 10.1248/bpb.34.501. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Q, Yin J, Chen J, Ma X, Wu M, Liu G, Yao K, Tan B, Yin Y. Mitochondria-targeted antioxidants: A step towards disease treatment. Oxid Med Cell Longev. 2020;2020:8837893. doi: 10.1155/2020/8837893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordelli E, Bignami M, Pacchierotti F. Comet assay: a versatile but complex tool in genotoxicity testing. Toxicol Res (Camb) 2021;10:68–78. doi: 10.1093/toxres/tfaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raavi V, Perumal V, Paul SFD. Potential application of γ-H2AX as a biodosimetry tool for radiation triage. Mutat Res Rev Mutat Res. 2021;787:108350. doi: 10.1016/j.mrrev.2020.108350. [DOI] [PubMed] [Google Scholar]
- 41.Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol. 2016;36:1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W, Zhang YN, Jia QQ, Ji A, Shao SX, Zhang L, Gong M, Yin Q, Huang XL. MicroRNA-214 protects L6 skeletal myoblasts against hydrogen peroxide-induced apoptosis. Free Radic Res. 2020;54:162–172. doi: 10.1080/10715762.2020.1730828. [DOI] [PubMed] [Google Scholar]
- 43.Dam AD, Mitchell AS, Quadrilatero J. Induction of mitochondrial biogenesis protects against caspase-dependent and caspase-independent apoptosis in L6 myoblasts. Biochim Biophys Acta. 2013;1833:3426–3435. doi: 10.1016/j.bbamcr.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Dadsena S, King LE, García-Sáez AJ. Apoptosis regulation at the mitochondria membrane level. Biochim Biophys Acta Biomembr. 2021;1863:183716. doi: 10.1016/j.bbamem.2021.183716. [DOI] [PubMed] [Google Scholar]
- 45.Xiong S, Mu T, Wang G, Jiang X. Mitochondria-mediated apoptosis in mammals. Protein Cell. 2014;5:737–749. doi: 10.1007/s13238-014-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Lv YF, Zhao JL, You QD, Jiang ZY. Regulation of Nrf2 by phosphorylation: Consequences for biological function and therapeutic implications. Free Radic Biol Med. 2021;168:129–141. doi: 10.1016/j.freeradbiomed.2021.03.034. [DOI] [PubMed] [Google Scholar]
- 47.Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu C, Xiao JH. The Keap1-Nrf2 system: a mediator between oxidative stress and aging. Oxid Med Cell Longev. 2021;2021:6635460. doi: 10.1155/2021/6635460. [DOI] [PMC free article] [PubMed] [Google Scholar]