Abstract
Lung cancer is the leading cause of cancer death. Although docetaxel has been used as a second- or third-line treatment for non-small cell lung cancer (NSCLC), the objective response rate is less than 10%. Hence, there is a need to improve the clinical efficacy of docetaxel monotherapy; combination therapy should be considered. Here, we show that CKD-516, a vascular disruption agent, can be combined with docetaxel to treat epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI)-resistant NSCLC. CKD-516 was orally bioavailable; neither CKD-516 nor docetaxel affected the mean plasma concentration–time profile or pharmacokinetic parameters of the other drug. CKD-516 and docetaxel synergistically inhibited the growth of H1975 (with an L858R/T790M double mutation of EGFR) and A549 (with a KRAS mutation) lung cancer cell lines. In addition, docetaxel plus CKD-516 delayed tumor growth in—and extended the lifespan of—tumor-bearing mice. Thus, combination CKD-516 and docetaxel therapy could be used to treat EGFR-TKI-resistant NSCLC.
Keywords: CKD-516, Docetaxel, TKI-resistant NSCLC, Vascular disrupting agent
Introduction
Lung cancer is the leading cause of cancer death and the second most common cancer worldwide [1]. Non-small cell lung cancer (NSCLC) constitute approximately 85% of all lung cancers [1]. Novel therapeutic agents including epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors improve the clinical outcomes of NSCLC patients with specific biomarkers (e.g., EGFR mutation and PD-L1 expression) [2]. Because new therapeutic options fail in NSCLC patients lacking such biomarkers, chemotherapy remains important.
Docetaxel, a microtubule-stabilizing agent, is effective in patients with advanced NSCLC [3]. Docetaxel significantly improved the median overall survival (OS) compared to best supportive care (BSC) in second-line treatment [4]. However, the objective response rate (ORR) to docetaxel monotherapy was less than 10% [3]. Hence, it is important to prolong survival in NSCLC patients who do not respond to EGFR-TKIs.
Vascular disrupting agents (VDAs) are a class of anti-cancer drugs targeting tumor vasculature [5]. They induce selective collapse of tumor vessel and subsequent central necrosis of the tumor mass [6]. The cytotoxic agents mainly affect the well-perfused peripheral rim, while the VDAs affect the tumor center which is poorly responsive to chemotherapy [7]. Therefore, the anti-cancer effect of chemotherapy would be improved by combination with VDAs [7].
We previously reported that CKD-516 (a VDA) exhibited potent activity against various types of cancer [8]. There are two vascular targeting strategies: anti-angiogenic approach that inhibits formation of new capillaries and vascular disrupting approach that targets already existing tumor blood vessels [9]. CKD-516 significantly decreased tumor blood flow investigated by dynamic contrast-enhanced (DCE)-MRI, and tumor vessel collapse was confirmed by immunohistochemistry with CD31 in allograft model [10]. Therefore, CKD-516 affected tumor vessel as VDA rather than angiogenesis inhibitor. Here, we investigated the effects of CKD-516 plus docetaxel with a focus on pharmacological efficacy and pharmacokinetics (PKs); we found that CKD-516 plus docetaxel may effectively treat EGFR-TKI-resistant NSCLC.
Materials and methods
Chemicals
CKD-516 and S516 (an active metabolite of CKD-516) were supplied from CKD Pharmaceuticals (Seoul, Korea). Docetaxel, propidium iodide and triton X-100 were purchased from Sigma-Aldrich (St. Louis, MO, USA). RNase A was supplied from Thermo Fisher Scientific (Waltham, MA, USA). For animal experiments, CKD-516 was dissolved in saline (for injection) or purified water (for oral gavage). Docetaxel was dissolved in ethanol:Tween80:saline (1:1:8).
Cancer cell growth inhibition
A549 and H1975 cell lines were cultured as previously described [8]. The phase confluence of the cells was scanned by IncuCyte® S3 Live Cell Analysis System (Essen Bioscience MI, USA) for 48 h.
Cell cycle analysis
A549 cells were treated with S516 and/or docetaxel for 24 h. Cells were harvested and fixed in 70% ethanol in Dulbecco’s phosphate buffered saline (DPBS) overnight, followed by 30 min incubation in 1 mL solution containing propidium iodide, RNase A, and Triton X-100 in DPBS. The cell cycle analysis was processed using flow cytometry (Agilent, NovoCyte 2060R, Santa Clara, CA, USA).
Pharmacokinetic study
The time-dependent inhibition of cytochrome P450s (CYP) were evaluated in human liver microsomes, and metabolites of the substrate probes were analyzed using a LC/MS/MS. IC50s were analyzed using WinNonlin (Pharsight, Mountain view, CA, USA). All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of CKD Research Institute or LSI Medience Corporation. Sprague–Dawley rats (7-week-old) were obtained from Charles River Lab. (Yokohama, Japan) or Orient Bio. (Sungnam, Korea). Plasma samples were analyzed using LC–MS/MS. The plasma concentration data were analyzed using WinNonlin.
Xenograft study
In vivo anti-cancer effects in xenografts were performed as previously described [8]. For lifespan experiment, male Hsd:Athymic Nude-Foxn1nu mice were purchased from Envigo RMS, Inc. (Indianapolis, IN, USA) and A549 cells (5 × 105/head) were orthotopically implanted into the lung. After 3 weeks, the lung tumor mass was confirmed by magnetic resonance imaging (MRI, Magnetom Essenza 1.5 Tesla, Siemens Healthineers, Germany).
Statistical analysis
One-way ANOVA was used to determine the differences in all experiments except tumor xenograft experiment. For tumor xenograft experiment, two-way ANOVA was used. Data were analyzed using Prism (Irvine, CA, USA), and differences were considered statistically significant at p < 0.05.
Results
Absorption of CKD-516
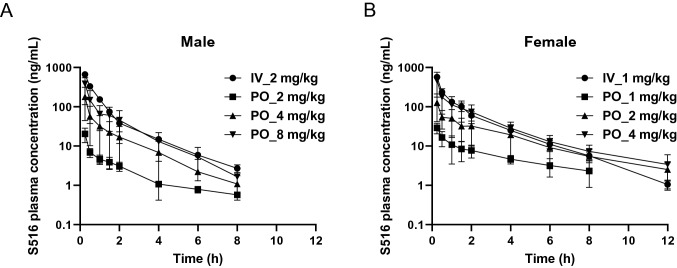
PK parameters, including the oral bioavailabilities (BAs) of CKD-516 and S516 (i.e., the active metabolite of CKD-516), were determined in rats. Figure 1A and B show the mean plasma S516 concentration–time profiles in male and female rats, respectively. The PK parameters of CKD-516 and S516 are summarized in Table 1. After intravenous or oral administration, CKD-516 was rapidly metabolized to the active metabolite S516. The S516 AUClast after intravenous injection was 2.3-fold higher in female rats than in male rats. The oral BAs of S516 in male and female rats administered with 4 mg/kg CKD-516 were 18.0 and 29.2, respectively (Table 1). The S516 AUClast was 3.2–5.3-fold higher in female rats than in male rats after oral CKD-516 administration; a sex difference was thus apparent, possibly because CYP2C11—which metabolizes CKD-516 and S516—is expressed only by male rats. Such a sex difference has not been observed in other species, including humans [11].
Fig. 1.
Mean plasma concentration–time profiles of S516 after intravenous or oral administration of CKD-516 in (a) male and (b) female SD rats. Mean plasma concentration–time profiles of S516 (c) or docetaxel (d). Vehicle or docetaxel (30 mg/kg) was intraperitoneally injected 0.5 h after intraperitoneal injection of CKD-516 (5 mg/kg). Data represent mean ± SD (n = 3)
Table 1.
Pharmacokinetic parameters (mean ± SD, n = 3) of CKD-516 and S516 in SD rats
| Sex | Analyte | Dose | Route | C0 | Cmax | Tmax | t1/2 | AUClast | AUCinf | BA |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg/kg/day) | (ng/mL) | (ng/mL) | (h) | (h) | (ng·h/mL) | (ng·h/mL) | (%) | |||
| Male | CKD-516 | 2 | i.v | 1648 ± 623 | n.d | n.d | 1.1 ± 0.6 | 194 ± 37 | 194 ± 37 | n.d |
| 2 | p.o | n.d | 16.63 ± 9.97 | 0.3 ± 0.0 | 1.2 ± 0.3 | 8.56 ± 4.79 | 10.1 ± 4.5 | n.d | ||
| 4 | p.o | n.d | 95.87 ± 51.29 | 0.3 ± 0.0 | 0.8 ± 0.3 | 32.8 ± 13.3 | 33.9 ± 13.6 | n.d | ||
| 8 | p.o | n.d | 218.6 ± 126.8 | 0.3 ± 0.0 | 1.8 ± 1.2 | 87.2 ± 39.3 | 89.5 ± 37.1 | n.d | ||
| S516 | 2 | i.v | n.d | 663.1 ± 115.8 | 0.1 ± 0.0 | 1.7 ± 0.2 | 366 ± 20 | 373 ± 21 | n.d | |
| 2 | p.o | n.d | 20.8 ± 8.16 | 0.3 ± 0.0 | 3.7 ± 1.6 | 19.6 ± 4.5 | 22.8 ± 4.4 | 5.4 | ||
| 4 | p.o | n.d | 178.6 ± 134.0 | 0.3 ± 0.0 | 1.3 ± 0.2 | 133 ± 111 | 134 ± 111 | 18.0 | ||
| 8 | p.o | n.d | 385.5 ± 272.8 | 0.3 ± 0.0 | 1.7 ± 0.7 | 312 ± 212 | 317 ± 210 | 21.1 | ||
| Female | CKD-516 | 1 | i.v | 1102 ± 476 | n.d | n.d | 0.5 ± 0.0 | 101 ± 34 | 102 ± 34 | n.d |
| 1 | p.o | n.d | 3.250 ± 1.284 | 0.3 ± 0.0 | 0.6 | 1.59 ± 1.27 | 2.05 ± 1.09 | n.d | ||
| 2 | p.o | n.d | 13.06 ± 13.57 | 0.4 ± 0.1 | n.c | 4.72 ± 3.35 | n.c | n.d | ||
| 4 | p.o | n.d | 71.60 ± 39.11 | 0.3 ± 0.0 | 1.2 ± 0.2 | 26.3 ± 10.6 | 27.6 ± 10.5 | n.d | ||
| S516 | 1 | i.v | n.d | 569.2 ± 50.1 | 0.1 ± 0.0 | 1.7 ± 0.1 | 426 ± 47 | 429 ± 48 | n.d | |
| 1 | p.o | n.d | 29.13 ± 8.69 | 0.3 ± 0.0 | 5.0 ± 4.7 | 50.7 ± 17.4 | 72.9 ± 28.5 | 12.2 | ||
| 2 | p.o | n.d | 126.4 ± 85.0 | 0.3 ± 0.0 | 2.9 ± 1.5 | 212 ± 129 | 224 ± 119 | 25.2 | ||
| 4 | p.o | n.d | 464.6 ± 288.5 | 0.3 ± 0.0 | 3.0 ± 0.1 | 499 ± 260 | 512 ± 245 | 29.2 |
n.c. not calculated, n.d. not determined, BA oral bioavailability
Drug–drug interactions (DDIs) between CKD-516 and docetaxel
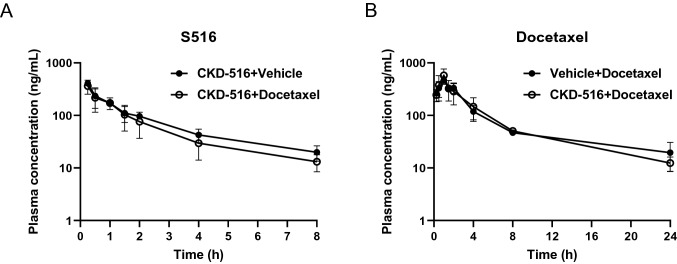
DDIs significantly affect the efficacy and safety of combination therapy [12]. Because female rats exhibited greater S516 systemic exposure than did male rats, we used female rats to study DDIs. We performed intraperitoneal injection of CKD-516 to guarantee higher exposure. The mean S516 plasma concentration–time profiles and PK parameters in the presence or absence of docetaxel are shown in Fig. 2A and Table 2. The S516 and docetaxel PK parameters were not affected by co-treatment (Fig. 2B, Table 3).
Fig. 2.
Mean plasma concentration–time profiles of S516 (a) or docetaxel (b). Vehicle or docetaxel (30 mg/kg) was intraperitoneally injected 0.5 h after intraperitoneal injection of CKD-516 (5 mg/kg) in SD female rats. Data represent mean ± SD (n = 5)
Table 2.
PK parameters of CKD-516 and S516 after administration of CKD-516 and docetaxel in female SD rats
| PK parameter | CKD-516 | S516 | ||
|---|---|---|---|---|
| CKD-516 + vehicle group | CKD-516 + docetaxel group | CKD-516 + vehicle group | CKD-516 + docetaxel group | |
| T1/2(hr) | n.c.a | 5.7 | 2.6 | 2.2 |
| Tmax(hr) | 8.0 | 1.0 | 0.3 | 0.3 |
| Cmax(ng/mL) | 25.6 | 18.8 | 407 | 362 |
| AUC0~8 h(ng·hr/mL) | 110.0 | 65.4 | 601 | 504 |
| AUCinf(ng·hr/mL) | n.c | 105 | 677 | 547 |
an.c. not calculated
Table 3.
PK parameters of doxetaxel after administration of CKD-516 and docetaxel in female SD rats
| PK parameter | Docetaxel | |
|---|---|---|
| Vehicle + docetaxel group | CKD-516 + docetaxel group | |
| T1/2(hr) | 8.7 | 5.0 |
| Tmax(hr) | 1.0 | 1.0 |
| Cmax(ng/mL) | 469 | 578 |
| AUC0~24 h(ng·hr/mL) | 1893 | 1933 |
| AUCinf(ng·hr/mL) | 2137 | 2022 |
Docetaxel has been reported as a CYP3A4 substrate [13]. In fact, pharmacokinetics of docetaxel is affected by chemical inducers or inhibitors of CYP3A4 [14]. When we determined in vitro inhibitory effects of CKD-516 and S516 on the activities of CYP isoforms, both the compounds inhibited the enzyme activities of CYP2B6, CYP2C8, CYP2C9 and CYP3A (Table 4). However, CKD-516 and docetaxel did not alter the plasma concentration of each drug (Fig. 2A, B).
Table 4.
Effects of CKD-516 and S516 on the activities of CYP isoforms
| Isoform | IC50 (μM) | |||
|---|---|---|---|---|
| CKD-516 | S516 | |||
| −NADPH | + NADPH | −NADPH | + NADPH | |
| 1A2 | No inhibition at 100 μM | No inhibition at 100 μM | ||
| 2B6 | 48.8 | 39.6 | 97.2 | 35.4 |
| 2C8 | 2.5 | 1.82 | 12.2 | 1.42 |
| 2C9 | 16.4 | 13.8 | 22.9 | 18.0 |
| 2C19 | 84.9 | 70.5 | No inhibition at 100 μM | |
| 2D6 | No inhibition at 100 μM | No inhibition at 100 μM | ||
|
3A (midazolam) |
18.0 | 16.4 | 13.6 | 1.76 |
|
3A (testosterone) |
17.6 | 14.3 | 6.27 | 0.65 |
Anti-cancer activity of combined CKD-516 and docetaxel in TKI-resistant cancer cells
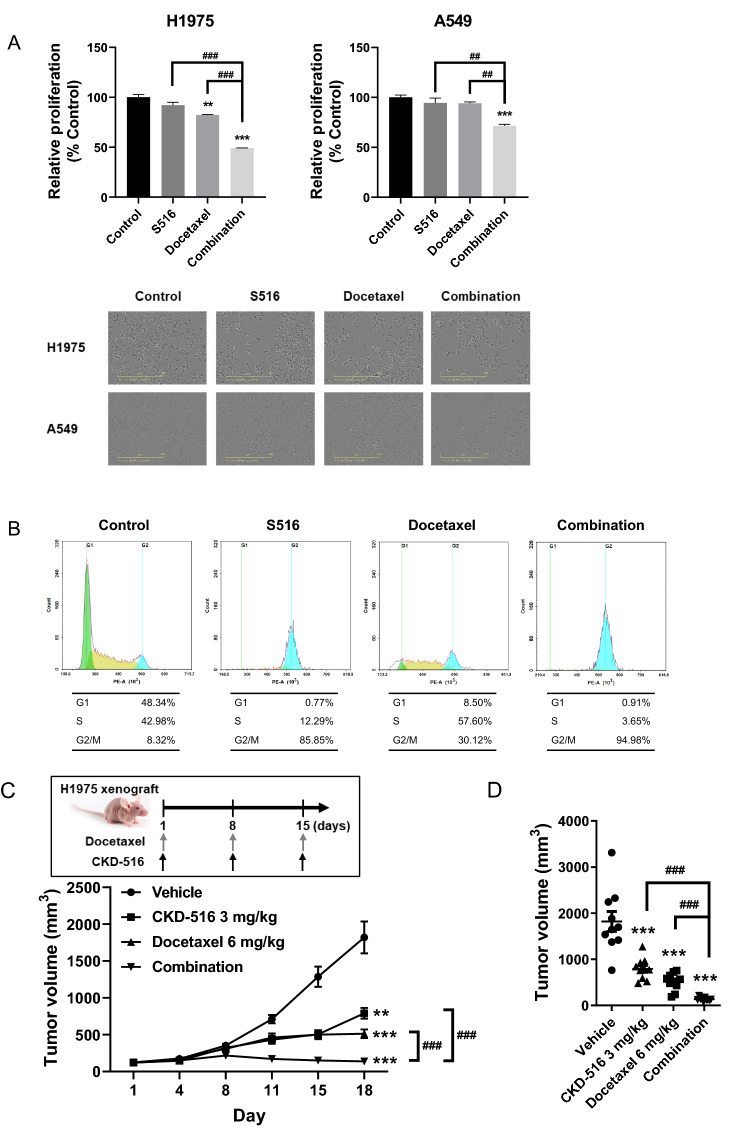
To investigate the combined effects of S516 and docetaxel on cancer cell proliferation, EGFR-TKI-resistant A549 and H1975 cells were treated with S516 and/or docetaxel for 48 h. Cell proliferation was only marginally inhibited by 1.5 nM (H1975) or 2 nM (A549) S516 or docetaxel (Fig. 3A). However, the drug combination more potently inhibited cell proliferation than did either drug alone (Fig. 3A).
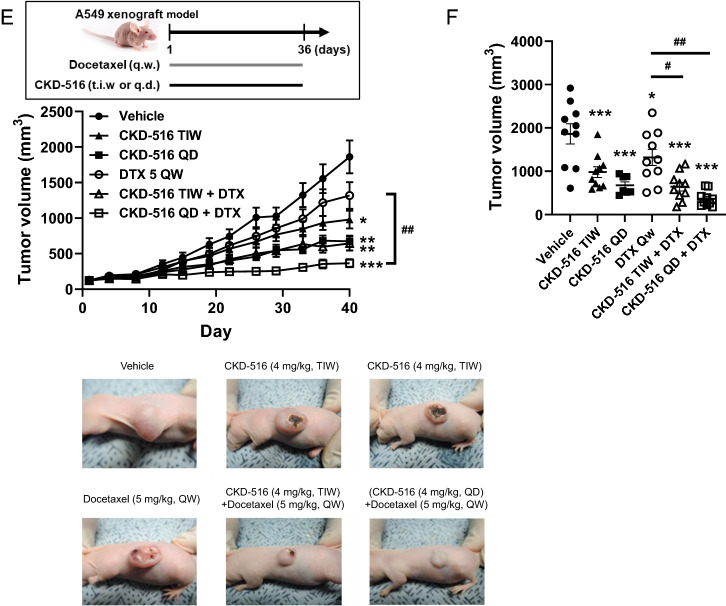
Fig. 3.
Anti-proliferation effects of S516 and docetaxel in EGFR-TKI-resistant lung cancer cell lines. a Synergistic anti-proliferating effects of S516 with docetaxel. Cell proliferation was monitored in H1975 and A549 lung cancer cells by using Incucyte® real-time monitoring system. H1975 cells were treated with 1.5 nM S516 and/or 1 nM docetaxel. A549 cells were treated with 2 nM S516 and/or 1 nM docetaxel. b G2/M arrest induction by S516 and docetaxel. A549 cells were treated with 5 nM S516 and/or 10 nM docetaxel for 24 h, and cell cycle was analyzed by flow cytometry. c, d H1975 tumor-bearing mice were intraperitoneally injected with CKD-516 and/or docetaxel once a week. c Tumor growth curve and (d) individual tumor size (on day 18). e, f A549 tumor-bearing mice were administrated with 4 mg/kg CKD-516 (p.o., t.i.w. or q.d.) and/or 5 mg/kg docetaxel (i.p., q.w.) according to the described dosing schedules. e Tumor growth curve and f individual tumor volume on day 40. All data represent mean ± SEM (significant vs. vehicle treated group; *p < 0.05, **p < 0.01, ***p < 0.001, significant vs. each treatment groups; #p < 0.05, ##p < 0.01, ###p < 0.001)
It is known that docetaxel and S516 induce cell cycle arrest at G2/M phase [15, 16]. Therefore, we tested the effect of S516 and/or docetaxel on cell cycle arrest. A549 cells were treated with 5 nM S516 and/or 10 nM docetaxel for 24 h. Although there were some differences in their effect on the cell cycle, both S516 and docetaxel arrested cells in the G2/M phase (Fig. 3B). And the combination of S516 and docetaxel increased the G2/M phase portion of A549 cells more than each single agent. Therefore, the reduced proliferation by docetaxel and S516 treatment would be related to cell cycle arrest (Fig. 3A and B).
We used an H1975 xenograft model to explore the possible synergistic anti-cancer effects of CKD-516 plus docetaxel. H1975 is a human EGFR-TKI-resistant NSCLC cell line, with the L858R/T790M double-mutation of the EGFR gene [17]. Intraperitoneal injection of docetaxel or CKD-516 significantly decreased both the tumor growth rate and tumor volume of H1975 xenografts (Fig. 3C and D). The tumor size was significantly smaller in the combination group than in the docetaxel- or CKD-516-monotherapy groups (Fig. 3C and D).
Because CKD-516 is orally bioavailable (Fig. 1A and B, Table S1), we optimized the oral CKD-516 administration schedule in an A549 xenograft model. The A549 human lung cancer cell line harbors the KRAS mutation and does not respond to EGFR-TKIs [17]. The A549 xenograft tumor growth rate is slow; the model thus allows long-term assessment. CKD-516 was orally administered three times weekly (t.i.w.) or once daily (q.d.); docetaxel was intraperitoneally injected once weekly. The anti-cancer effect of CKD-516 increased as the administration frequency increased; the anti-cancer activity of docetaxel was enhanced when combined with frequent oral CKD-516 (Fig. 3E and F).
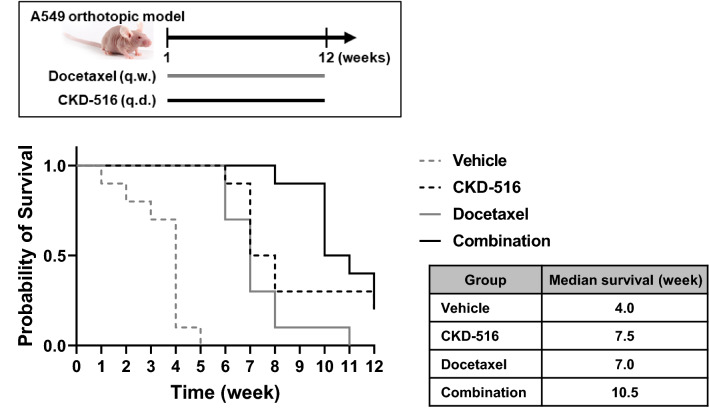
We used an A549 lung orthotopic model to explore the effect of the drug combination on mouse lifespan. Monotherapy with docetaxel or CKD-516 extended the lifespan, but combination therapy increased the lifespan afforded by either monotherapy (Fig. 4).
Fig. 4.
Median survival of tumor bearing mice. A549 orthotopic lung cancer-bearing mice were administered with 4 mg/kg CKD-516 (p.o., q.d.) and/or 5 mg/kg docetaxel (i.p., q.w.). Median survival week was depicted in right table
Discussion
Although docetaxel is very useful when NSCLC patients do not respond to first-line therapies, its low clinical efficacy requires improvement [3]. We found that CKD-516 significantly potentiated the anti-cancer activity of docetaxel in terms of both tumor growth inhibition and lifespan extension. CKD-516 (a VDA) has shown anti-cancer activities in multiple lung cancer models [8]. VDAs effectively inhibit tumor growth, but viable tumor rims remain [5]. VDAs and chemotherapeutic agents have distinct therapeutic targets (tumor blood vessels and cancer cells, respectively) and affect different spatial compartments (central and peripheral regions, respectively) [18]. Thus, chemotherapeutic agents and VDAs can be combined. Although microtubules are targeted by both CKD-516 and docetaxel, the drug-binding sites differ. CKD-516 binds to the colchicine site while docetaxel binds to the taxane site, thus enhancing the inhibition of abnormal microtubule dynamics [19].
As shown in Fig. 3B, the combination of S516 and docetaxel arrested cells in G2M phase. CDK1/cyclin B complex is essentially required for mitosis, and the activity of the CDK1/cyclin B complex is regulated by its phosphorylation status [20]. Docetaxel increased the phosphorylated CDK1, an inactive form, and subsequently inhibited entry into mitosis [21]. Hence, it could be possible that S516 potentiated docetaxel-induced cell cycle arrest via CDK1 inactivation or the regulation of cyclin B in cancer cells. It has been also reported that docetaxel-induced apoptosis is dependent on the c-Jun N-terminal kinase (JNK) pathway [15]. And VDAs caused cancer cell death via JNK activation [22]. Hence, docetaxel combination with CKD-516 may potentiate the activation of JNK and subsequent cancer cell death.
The anti-cancer effect of VDAs is based on the unique features of tumor vessels in vivo. In contrast, in vitro cancer cell culture condition could not reflect the potential effects on tumor vessels. Therefore, difference of anti-cancer effects in cell culture (Fig. 3A) and animal models (Fig. 3C to F and 4) would be due to the presence of absence of tumor vessels. In addition, according to “cancer-immunity cycle”, the release of cancer antigens and capture by dendritic cells (DCs) are the first step in an anti-cancer immune response [23]. Then, DCs present the captured antigen on MHCI and MHCII molecules [23]. It has been reported that microtubule destabilizers, including VDAs activate DCs and subsequently lead to effective priming of CD8 T cells [24]. S516, an active metabolite of CKD-516 induces the activation of DCs through the Rho signaling pathway in bone marrow-derived DCs [25]. Thus, immune checkpoint blocker would be a good combination partner for VDAs.
In most clinical trials, VDAs were administered once weekly or once every 3 weeks because of concerns regarding cardiac toxicity and patient compliance [26]. Figure 1A and B show that CKD-516 is orally bioavailable; the drug is safe [11]. Hence, preclinical and clinical dosing schedules can be flexible; compliance and safety issues are of minimal concern. Here, we explored the anti-cancer activity of CKD-516 according to the dosing schedule. Compared to t.i.w. dosing, q.d. administration was more effective. Daily oral CKD-516 potentiated the anti-cancer activities of docetaxel in terms of both tumor growth inhibition and lifespan extension (Fig. 4). There was no DDI between CKD-516 and docetaxel (Fig. 2A and B; Tables 2 and 3). CKD-516 can be combined with docetaxel. This may aid patients with EGFR-TKI-resistant NSCLC.
Acknowledgements
This research was funded by the National Research Foundation of Korea (NRF) grant 2021R1A2C2093196.
Abbreviations
- ANOVA
Analysis of variance
- AUC
Area under the curve
- BA
Bioavailability
- BSC
Best supportive care
- CD31
Cluster of differentiation 31
- CDK
Cyclin-dependent kinase
- CYP
Cytochrome P450
- DC
Dendritic cell
- DCE
Dynamic contrast-enhanced
- DDI
Drug-drug interaction
- EGFR
Epidermal growth factor receptor
- FACS
Fluorescence activated cell sorting
- IACUC
Institutional animal care and use committee
- ICI
Immune checkpoint inhibitor
- i.p.
Intraperitoneal
- i.v.
Intravenous
- JNK
C-Jun N-terminal kinase
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- LC–MS/MS
Liquid chromatography tandem-mass spectrometry
- MHC
Major histocompatibility complex
- MRI
Magnetic resonance imaging
- NADPH
Nicotinamide adenine dinucleotide phosphate
- n.c.
Not calculated
- n.d.
Not determined
- NSCLC
Non-small cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- PK
Pharmacokinetics
- p.o.
Per os
- q.d.
Quaque die
- SD
Sprague–Dawley
- TKI
Tyrosine kinase inhibitor
- VDA
Vascular disrupting agent
Funding
The authors have not disclosed any funding.
Declaration
Conflict of interest
Soo Jin Kim, Kyunghyeon Lee, U Ji Kim, Se-mi Kim and Keun Ho Ryu works in CKD Research Institution, Chong Kun Dang Pharmacuetical Corporation.
References
- 1.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 2.Alexander M, Kim SY, Cheng H. Update 2020: Management of non-small cell lung cancer. Lung. 2020;198:897–907. doi: 10.1007/s00408-020-00407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 4.Weiss JM, Stinchcombe TE. Second-Line Therapy for Advanced NSCLC. Oncologist. 2013;18:947–953. doi: 10.1634/theoncologist.2013-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinnen P, Eskens FA. Vascular disrupting agents in clinical development. Br J Cancer. 2007;96:1159–1165. doi: 10.1038/sj.bjc.6603694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollebecque A, Massard C, Soria JC. Vascular disrupting agents: a delicate balance between efficacy and side effects. Curr Opin Oncol. 2012;24:305–315. doi: 10.1097/CCO.0b013e32835249de. [DOI] [PubMed] [Google Scholar]
- 7.Mita MM, Sargsyan L, Mita AC, Spear M. Vascular-disrupting agents in oncology. Expert Opin Investig Drugs. 2013;22:317–328. doi: 10.1517/13543784.2013.759557. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Jegal KH, Im JH, Park G, Kim S, Jeong HG, Cho IJ, Kang KW. Involvement of ER stress and reactive oxygen species generation in anti-cancer effect of CKD-516 for lung cancer. Cancer Chemother Pharmacol. 2020 doi: 10.1007/s00280-020-04043-x. [DOI] [PubMed] [Google Scholar]
- 9.Smolarczyk R, Czapla J, Jarosz-Biej M, Czerwinski K, Cichon T. Vascular disrupting agents in cancer therapy. Eur J Pharmacol. 2021;891:173692. doi: 10.1016/j.ejphar.2020.173692. [DOI] [PubMed] [Google Scholar]
- 10.Kim KW, Lee JM, Jeon YS, Lee IJ, Choi Y, Park J, Kiefer B, Kim C, Han JK, Choi BI. Vascular disrupting effect of CKD-516: preclinical study using DCE-MRI. Invest New Drugs. 2013;31:1097–1106. doi: 10.1007/s10637-012-9915-6. [DOI] [PubMed] [Google Scholar]
- 11.Oh DY, Kim TM, Han SW, Shin DY, Lee YG, Lee KW, Kim JH, Kim TY, Jang IJ, Lee JS, Bang YJ. Phase I study of CKD-516, a novel vascular disrupting agent, in patients with advanced solid tumors. Cancer Res Treat. 2016;48:28–36. doi: 10.4143/crt.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcath LA, Coe TD, Hoylman EK, Redman BG, Hertz DL. Prevalence of drug-drug interactions in oncology patients enrolled on national clinical trials network oncology clinical trials. BMC Cancer. 2018;18:1155. doi: 10.1186/s12885-018-5076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Royer I, Monsarrat B, Sonnier M, Wright M, Cresteil T. Metabolism of docetaxel by human cytochromes P450: interactions with paclitaxel and other antineoplastic drugs. Cancer Res. 1996;56:58–65. [PubMed] [Google Scholar]
- 14.Engels FK, Ten Tije AJ, Baker SD, Lee CK, Loos WJ, Vulto AG, Verweij J, Sparreboom A. Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharmacol Ther. 2004;75:448–454. doi: 10.1016/j.clpt.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Mhaidat NM, Zhang XD, Jiang CC, Hersey P. Docetaxel-induced apoptosis of human melanoma is mediated by activation of c-Jun NH2-terminal kinase and inhibited by the mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway. Clin Cancer Res. 2007;13:1308–1314. doi: 10.1158/1078-0432.CCR-06-2216. [DOI] [PubMed] [Google Scholar]
- 16.Moon CH, Lee SJ, Lee HY, le Dung TK, Cho WJ, Cha H, Park JW, Min YJ. CKD-516 displays vascular disrupting properties and enhances anti-tumor activity in combination with chemotherapy in a murine tumor model. Invest New Drugs. 2014;32:400–411. doi: 10.1007/s10637-013-0043-8. [DOI] [PubMed] [Google Scholar]
- 17.Onodera K, Sakurada A, Notsuda H, Watanabe T, Matsuda Y, Noda M, Endo C, Okada Y. Growth inhibition of KRAS and EGFRmutant lung adenocarcinoma by cosuppression of STAT3 and the SRC/ARHGAP35 axis. Oncol Rep. 2018;40:1761–1768. doi: 10.3892/or.2018.6536. [DOI] [PubMed] [Google Scholar]
- 18.Wang ES, Pili R, Seshadri M (2012) Modulation of chemotherapeutic efficacy by vascular disrupting agents: optimizing the sequence and schedule. J Clin Oncol 30:760–761; author reply 761–763. 10.1200/JCO.2011.39.3934 [DOI] [PubMed]
- 19.Clemenson C, Chargari C, Deutsch E. Combination of vascular disrupting agents and ionizing radiation. Crit Rev Oncol Hematol. 2013;86:143–160. doi: 10.1016/j.critrevonc.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 21.Nehme A, Varadarajan P, Sellakumar G, Gerhold M, Niedner H, Zhang Q, Lin X, Christen RD. Modulation of docetaxel-induced apoptosis and cell cycle arrest by all- trans retinoic acid in prostate cancer cells. Br J Cancer. 2001;84:1571–1576. doi: 10.1054/bjoc.2001.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh AV, Bandi M, Raje N, Richardson P, Palladino MA, Chauhan D, Anderson KC. A novel vascular disrupting agent plinabulin triggers JNK-mediated apoptosis and inhibits angiogenesis in multiple myeloma cells. Blood. 2011;117:5692–5700. doi: 10.1182/blood-2010-12-323857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Kashyap AS, Fernandez-Rodriguez L, Zhao Y, Monaco G, Trefny MP, Yoshida N, Martin K, Sharma A, Olieric N, Shah P, Stanczak M, Kirchhammer N, Park SM, Wieckowski S, Laubli H, Zagani R, Kasenda B, Steinmetz MO, Reinecker HC, Zippelius A. GEF-H1 signaling upon microtubule destabilization is required for dendritic cell activation and specific anti-tumor responses. Cell Rep. 2019;28(3367–3380):e3368. doi: 10.1016/j.celrep.2019.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SJ, Kim HK, Ryu KH, Hong CI. Abstract 3216: CKD-516, a novel vascular disrupting agent, enhances anticancer activity of anti-PD-1 antibody in SMAD4-deficient colon cancer model. Can Res. 2019;79:3216–3216. doi: 10.1158/1538-7445.AM2019-3216. [DOI] [Google Scholar]
- 26.Spear MA, LoRusso P, Mita A, Mita M. Vascular disrupting agents (VDA) in oncology: advancing towards new therapeutic paradigms in the clinic. Curr Drug Targets. 2011;12:2009–2015. doi: 10.2174/138945011798829366. [DOI] [PubMed] [Google Scholar]