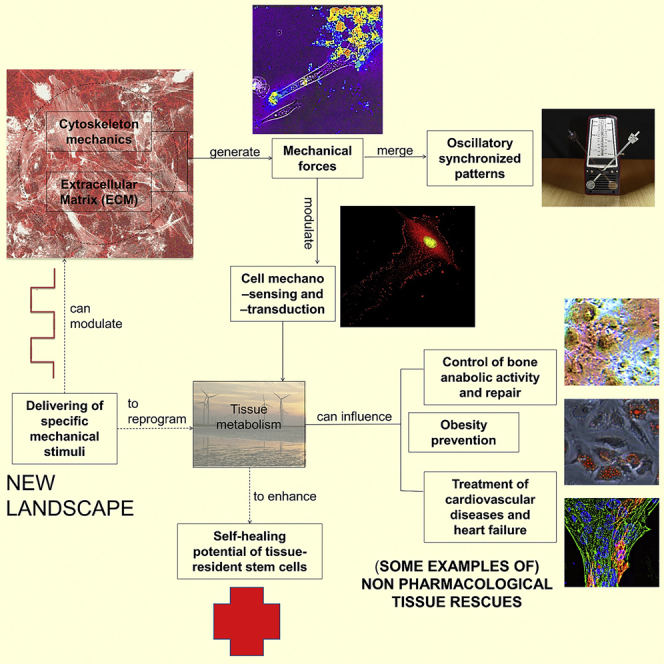
Summary
Mechanical forces play a fundamental role in cellular dynamics from the molecular level to the establishment of complex heterogeneity in somatic and stem cells. Here, we highlight the role of cytoskeletal mechanics and extracellular matrix in generating mechanical forces merging into oscillatory synchronized patterns. We discuss how cellular mechanosensing/-transduction can be modulated by mechanical forces to control tissue metabolism and set the basis for nonpharmacologic tissue rescue. Control of bone anabolic activity and repair, as well as obesity prevention, through a fine-tuning of the stem cell morphodynamics are highlighted. We also discuss the use of mechanical forces in the treatment of cardiovascular diseases and heart failure through the fine modulation of stem cell metabolic activity and regenerative potential. We finally focus on the new landscape of delivering specific mechanical stimuli to reprogram tissue-resident stem cells and enhance our self-healing potential, without the need for stem cell or tissue transplantation.
Subject areas: Mechanobiology, Cell biology
Graphical abstract
Mechanobiology; Cell biology
Introduction
Biophysical stimuli are constantly present in the human body and play a critical role in tissue formation from the earliest stages of embryogenesis and throughout life. Even before implantation, self-organization of the embryo and specification of the germ layers into the blastocyst rely on the cooperation between contractile mechanical forces1 and biochemical signaling. Afterward, biochemical and biophysical cues continue to cooperate to give rise to proper organ form, and in case of atypical mechanical loading the embryonic rudiments face an asymmetric, deranged development.2 Mechanosensing and mechanotransduction are fundamental attributes of developmental and adaptive pathways.3 The (stem) cell property of transducing mechanical forces into complex and interconnected motions is essential in the emergence of forms, and cell-to-cell variability, thus contributing to cellular heterogeneity, an immanent property of living organisms.4 Both pluripotent stem cells, including embryonic and induced pluripotent stem cells, as well as adult stem cells, including hematopoietic and mesenchymal stem cells obtained from different tissue sources, exhibit remarkable heterogeneity in their pluripotency, self-renewal ability and differentiating potential.5,6,7 Heterogeneity may even arise within single-cell originated stem cell lines.8 Growing evidence shows that stem cell fate can be predicted by cellular mechanical properties.9,10,11 The apparent Young’s modulus, which estimates the substrates stiffness and cytoskeletal organization in living cells, as measured by atomic force microscopy (AFM), was found to change dynamically during stem cell passaging and proliferation on substrates with different stiffness.10 This, in turn, resulted in significant difference in the maintenance of pluripotency and/or lineage-specific characteristics in embryonic stem cells and their derived progenitor cells. In particular, stem cell culturing on extremely rigid or soft substrates resulted in Young’s modulus fluctuations and loss in lineage specificity throughout cell passaging and proliferation.10 The same study revealed that stem cell growing onto defined substrate stiffness around 3.5 kPa ensued in a constant level Young’s modulus consistent with preservation of lineage specificity during cell proliferation throughout multiple passaging. Adding further complexity to the role of mechanobiology in stem cell heterogeneity, asymmetry in integrin-mediated mechanical forces between the periphery and interior of a mesenchymal stem cell (MSC) population can spatially direct osteogenic-adipogenic12,13,14 or chondrogenic-myogenic15 differentiation decisions. E-cadherin adhesion is essential to preserve stem cell pluripotency,16,17 whereas mechanical forces associated with increased integrin-mediated adhesion have been found to promote differentiating processes.18 Cell micropatterning can be exploited to control and modulate the relative spatial/temporal and strength distribution of integrin and E-cadherin adhesions within a given (stem) cell colony.14 By the aid of this approach, spatially polarized integrin adhesion in a cohesive colony of human pluripotent stem cells was found to compete in recruiting Rho-ROCK activated myosin II away from E-cadherin mediated cell-to-cell junctions, to promote differentiation at that locality, thus resulting in cell population heterogeneity. These findings indicate that mechanical asymmetry emerging from spatial and temporal polarization in cell-to-cell, and cell-to-matrix interactions play an important role in determining stem cell heterogeneity. As an additional proof of concept of this view, exposure to a ROCK inhibitor (leading to inhibition of Rho-ROCK-myosin II activation) phenocopied E-cadherin rather than integrin inhibition, leading to uniformly differentiated colonies.14 These results proved that E-cadherin-mediated mechanical patterning acted as the primary gatekeeper to differentiation progression. Intriguingly, these observations pave the way to the development of tailored biomaterials capable to orchestrate mechanical cues for human stem cell maintenance, or differentiation, with minimal heterogeneity.
Overall, the above reported findings suggest that defined cellular structures behave as actuators capable of coupling and transforming the sensed mechanical forces into instructive messages and building blocks of biological information.
To this end, the capability of AFM of probing the nanomechanics of cellular processes19,20,21,22 holds promise for stem cell characterization at the nanomechanical level, with the potential for deciphering specific mechanical vibration profiles during the progression throughout different metabolic states and fate decisions. This perspective may also provide further insights into how the rhythmic unfolding of mechanical forces may interplay with chemical oscillatory patterns, as those underlying cytosolic Ca2+ and pH homeostasis,23,24,25,26 in the shaping of complex cell signaling networks.
Mechanobiology is a rapidly developing transdisciplinary field, encompassing cell and developmental biology, bioengineering and biophysics.27 This field concerns the reciprocity of biological and mechanical interactions, and gains more and more interest as biochemistry and genetics alone are not sufficient to explain the heterogeneity of biological forms and functions. Mechanobiology may also be seen as the landscape for posing a number of fundamental unanswered questions:
-
-
How are multiple, multifaceted mechanical oscillatory patterns connecting and acting in a coordinated fashion for the storage of information over time?
-
-
How are mechanical oscillatory patterns integrated within, or contributing to the subcellular and cellular environment?
-
-
What is the implication of cellular mechanical actuators and mechanical forces in the emergence of simple-to-complex multifaceted forms?
-
-
Which fundamental dynamics may connect the cellular landscape of mechanical forces (mechanoscape) to onset and progression of morphogenetic pathways up to the emergence of large-scale anatomy?
From these questions and the analysis of currently available data it is evident that we are still at a partial, analytical stage of a journey attempting to explore the mechanistic underpinning through which shaping, self-organization, morphogenesis and regeneration may occur.
The purpose of this review is to provide an update of how mechanical cues govern the heterogeneity of (stem) cell fate and constitute an interesting mechanoscape28 for the development of unprecedented strategies and tools in regenerative medicine. We focus on the capability of mechanical forces to emerge as defined oscillatory patterns and spread from the subcellular to a multicellular tissue environment, entraining tenets of connectedness, which may offer the chance of using simple mechanical manipulations to cause complex and long-ranging responses. We report on the chance of using mechanical vibrations to afford a fine-tuning of morphodynamic processes, including stem cell controlled adipogenesis and osteogenesis, paving the way to nonpharmacologic approaches in prevention of obesity. We also discuss the use of extracorporeal shock waves (ESW) as a mechanotransduction strategy in the treatment of cardiovascular diseases and heart failure, through the fine modulation of stem cell metabolic activity and rescuing potential. We finally tackle the new perspective of using specific mechanical stimuli to directly control the heterogeneity of tissue-resident stem cells in situ, thus enhancing their rescuing capabilities and our self-healing potential, without the needs for stem cell or tissue transplantation.
Cytoskeletal mechanics and (stem) cell heterogeneity
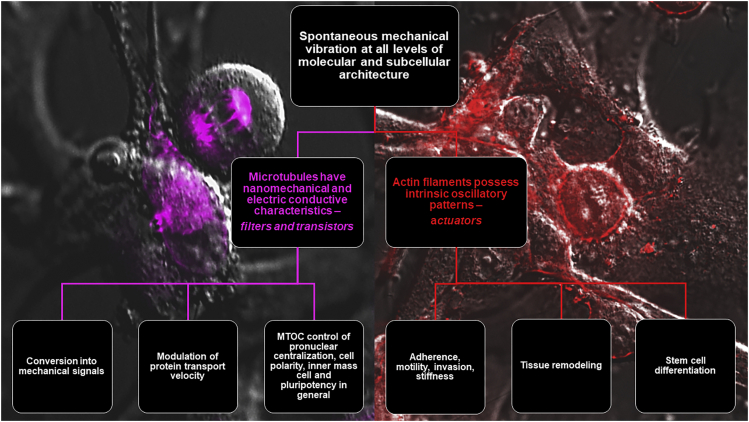
Spontaneous mechanical vibrations have been theoretically predicted and experimentally demonstrated in virtually all levels of the molecular and subcellular architecture. As we reviewed elsewhere,29 the repetitive helix–loop–helix domains in single peptide molecules behave as intrinsically oscillatory modules, that owing to the electrically charged features of their composing amino acids, acquire the identity of electromechanical actuators. The hierarchical self-assembly of peptide moieties into larger cytoskeletal structures entails a major outcome of self-organization, implying that the resultant assembly exhibits traits that are way beyond the sum of the features of the composing building blocks (Figure 1). Thus, it happens that the nanomechanical (as well as the electric conductivity) characteristics of microtubule are by far different of those exhibited by a tubulin dimer, up to the chance of developing a mechano-electric actuator providing features of mechanical and electromagnetic connectedness with radiation characteristics.29,30,31 The spreading of mechanical signals through cells entails particular complexity. In the attempt to elucidate new mechanotransduction pathways, Koch et al.32 investigated the frequency-dependent transport of mechanical stimuli by single microtubuli and small microtubular networks in a bottom-up approach, using optically trapped beads as anchor points. By interconnecting microtubuli to form linear and triangular geometries, microrheology could be performed by defined oscillations of the beads relative to each other. These studies revealed a substantial stiffening in single microtubuli on mechanical stimulation above a characteristic transition frequency ranging from 1 to 30 Hz, depending on the filament’s molecular composition (i.e., variations in microtubular length induced by stabilizing agents), with smaller transition frequencies for longer microtubule. Below this frequency range, microtubule elasticity appeared to solely depend on its intrinsic flexural features (including the polymer length ad maximum physically possible extension, and the bending stiffness, referrable to as the contour and persistence length). Of interest, this elastic behavior was found to be transferable to small networks, where linear two-filament connections acted as transistor-like, angle dependent momentum filters, whereas triangular networks behaved as stabilizing elements. These findings implicate that cells may be able of tuning mechanical signals by temporal and spatial filtering dynamics. Such “mechanic transistor” function of microtubular networks, also suggests that small mechanical forces may control a large amount of momentum transport. Further analysis of these features, and of the mechanisms underlying the establishment of multivariate geometrical assembly of microtubuli may prove rewarding in the comprehension of how cells can control the directionality of mechanical signaling, a relevant issue in the mechanics of somatic and stem cell integration into organs, and possible of organs into organisms.33 Common views of mechanotransduction processes have considered the conversion of a physical stimulus into a chemical signaling through the intervention of membrane proteins, including integrins, followed by transportation of regulatory players to targeted subcellular domains, such as the nucleus, either by passive diffusion, or actively by molecular motors. These are relatively slow processes that cannot account for the extremely fast speed at which cells often execute their tasks. To this end, a direct spreading of mechanical signaling by stress waves through a variably stiff cytoskeleton, bridging the cell surface with the nucleus, would enable transport routes operating on microsecond time scales, for a quasi-real-time integrations of cellular responses. Future studies focusing on bottom-up approaches may elucidate the transmission of mechanical forces within networks of increasing complexity, hopefully helping to understand how cellular mechanosensing/-transduction may contribute in shaping biological patterns up to the macroscopic level.
Figure 1.
Cytoskeletal mechanics and (stem) cell heterogeneity
Diagram showing a recap of the functions that involved tubulin and actin filaments depicted in the chapter.
The remarkable role of microtubular dynamics in subcellular self-organization has been relatively understudied until quite recently,34 when the availability of super-resolution microscopy has enabled to monitor single microtubule dynamics within the context of complex cytoskeletal networks. Thus, innovative live imaging is providing evidence for the emergence of non-centrosomal microtubular assembly and networking as a crucial causative determinant in morphological and functional cell heterogeneity and specialization throughout differentiating processes.35 This aspect has received substantial confirmation from studies showing that the formation of specific intracellular bridging geometries in the microtubular network is playing a pivotal role in the establishment and regulation of cell pluripotency.36,37 To this end, microtubule organizing centers (MTOCs), particularly non-centrosomal MTOCs, have been shown to act as major conductors of cell potency, namely the (stem) cell capability of exhibiting heterogeneous, multifaceted features.34 Recently, live cell confocal and super-resolution microscopy38 has provided evidence for microtubule-dependent mechanisms in early mouse embryogenesis, with peculiar involvement in multiple essential steps, including (1) pronuclear centralization, (2) subcellular reorganization patterning and cell polarity modulation occurring at 8- and 16-cell stage, and (3) inner cell mass formation. Additional studies are needed to further dissect the analogies in microtubular dynamics among the embryonic development and the modulation of pluripotency in isolated stem cells in vitro. Addressing the molecular underpinning of these similarities may likely aid developing novel strategies and tools to control stem cell heterogeneity, pluripotency and rescuing potential through the application of defined physical forces capable of modulating the mechanical properties of cellular microtubuli.
Akin to microtubuli, actin filaments can also be viewed as mechano-electric actuators, coupling intrinsic oscillatory patterns to the capability of sensing and generating changes in cellular behavior.39 Two distinct spontaneous oscillatory patterns have been recorded in crawling microglial cells, exhibiting zigzag motile behaviors that appear as temporally structured sequences of runs and turns.40 One type of intrinsic actin oscillation manifested as fast peripheral ruffles, spontaneously emerging with an oscillating period of about 6 s at the leading edge of crawling microglia, where the vigorously biased peripheral ruffles appear to define the direction for a new turn (in 2D free space).40 When the cell turning events are inhibited with a physical confinement (in 1D track), the peripheral ruffles still exist at the leading edge with no bias but showing phase coherence in the cell crawling direction.40 The other type of spontaneous oscillatory pattern was referred to as dorsal actin waves, exhibiting a much longer oscillatory period of about 2 min, and likely associated with cell migration.40 On the whole, these findings indicated that the slow dorsal actin waves and the fast peripheral ruffles were connected with microglial run and turn stages, respectively. Spontaneous pulsation and flow of actomyosin cytoskeleton has also been proposed as relevant dynamics in tissue remodeling to promote heterogeneous developmental contexts.41
The involvement of intrinsic vibrational properties of actin filaments in cellular and tissue remodeling is consonant with the observed interconnection between actin-regulated cytoskeletal stiffness and stem cell differentiation. A significant contribution in this field has been brought about by the use of agents capable of disrupting actin filaments, thus altering their mechanical properties. Among these agents, Cytochalasin D (CD) and B (CB) have been shown to reversibly interfere with actin dynamics. In fact, a less-organized and less-stiff actin cytoskeleton has been found to act as a necessary prerequisite to differentiate mesenchymal stem cells into adipocytes.42,43,44 By the aid of AFM, coupled with gene expression and metabolic pattern analyses, we have recently shown that CB could be used to afford a fine-tuning of nanomechanical properties in human, adipose-derived MSCs (AD-MSCs), deeply affecting their metabolism, proliferation, and morphology. In particular, CB elicited a dose-dependent reduction in metabolic activity, associated with remarkable changes in acting organization, focal adhesions subcellular redistribution patterning, and stem cell shape.42 The functional implications in the stem cell heterogeneity potential of such tuning of cell stiffness and viscoelastic properties could be inferred by the capability of CB of inducing a marked metabolic switch toward an adipogenesis program, highlighted by the expression and subcellular localization of PPAR-γ, and C/EBP-α, by the overexpression of perilipin, and by the appearance of typical lipid vacuoles, positively stained with ORO solution.42 Modulation of biomechanical dynamics at the level of actomyosin network and adhesion complexes has proven effective in the orchestration of multicellular development of human embryonic stem cells and neuroprogenitor cells. Plexin-B2 manipulations has been shown to affect actomyosin contractility, changing stem cell stiffness and cytoskeletal tension, as well as cell-cell and cell-matrix adhesion.45 It has been observed that the functional domains of Plexin-B2, RAP1/2 effectors, and the signaling association with ERK1/2, calcium activation, and YAP mechanosensory, provide a mechanistic link between Plexin-B2-mediated cytoskeletal tension and stem cell physiology. Plexin-B2-deficient stem cells exhibited premature lineage commitment, whereas a balanced level in Plexin-B2 activity was essential in preserving the cytoarchitectural integrity of the developing neuroepithelium, as it was modeled in cerebral organoids. These findings demonstrate the essential role of Plexin-B2, together with cell-cell/cell-matrix adhesion, in the orchestration of cytoskeletal tension, and solidify the relevance of collective cell mechanics in guiding stem cell physiology and tissue morphogenesis.45 Tissue spatial organization is a complex phenomenon, and attempts to generate competent tissues in vitro have often failed owing to the difficulty of faithfully reproducing the interplay between gene regulatory networks, cell-cell communication, and physical interactions mediated by mechanical forces. Most of the adopted strategies have relied on extracellular matrix manipulation which responds to, rather than imposing mechanical forces, thus failed to recapitulate experimentally the dynamical ensemble of physical forces linking stem cell dynamics to tissue morphogenesis. This important task has been recently afforded through a dedicated platform enabling a precise actuation of gel-embedded organoids, where the gel was made of a synthetic poly(ethyleneglycol (PEG)-based extracellular matrix whose composition and stiffness matched specific organoid properties, and the actuation was operated through a specially designed mechanical stretcher equipped with programmable equibiaxially actuated arms.46 Under these experimental conditions, active mechanical forces increased growth and lead to enhanced patterning in an organoid model of the neural tube derived from single human pluripotent stem cells (hPSCs). Single-cell transcriptomics and immunohistochemistry revealed the feasibility of actuating organoid mechanoregulation in a temporally restricted competence window, and that organoid response to stretch was mediated extracellularly by matrix stiffness and intracellularly by cytoskeleton contractility and planar cell polarity.46 The chance of delivering mechanical forces to organoids by controlling the spatial and temporal profile of the actuating dynamics opens the new avenue of a regenerative medicine executed through a precise guiding of biological patterning and morphogenesis.47
On more general terms, the involvement of cytoskeletal stiffness regulation in morphodynamic processes can be further inferred through the vibrational profiling of normal and diseased/malignant cells. For this purpose, Nelson et al.48 have designed a contact-free method and analysis protocol to convert an atomic force microscope into an ultra-sensitive microphone, capable of sensing and recording from live biological samples. This study investigated the vibrational profiles of normal neuronal cells and tissues, as well as brain tumors and neocortical specimens. A major frequency of 3.4 Hz was deciphered in both cultured rat hippocampal neurons and tissues, and the detected vibration pattern could be modulated by chemical agents. Following high-K+-induced depolarization, whereas the ∼3.4 Hz major frequency peak observed at rest persisted in the depolarized hippocampus, the same major frequency peak of ∼3.4 Hz could not be detected in resting cerebellum but emerged within this tissue in the depolarized state. Of interest, vibrational analyses performed following other chemical manipulations, including Ca2+ removal or exposure to Tetrodotoxin revealed that in both conditions the dominant major frequency of 3.4 Hz disappeared. Even more intriguingly, samples of malignant astrocytoma were found to vibrate with a significantly different frequency profile and amplitude, when compared to meningioma or lateral temporal cortex.48 These observations indicate the chance of using a peculiar AFM technology to uncover tissue-specific, stiffness-related vibrational modes, as well as vibrational patterning traceable for healthy, diseased/non-malignant, or malignant tissues. In cancer cells, biomechanics features, in particular stiffness or elasticity, have been identified as important determinants relating to cancer cell function, adherence, motility, transformation, and invasion.49,50 Moreover, analysis of surface cell adhesion inherent to tumor and normal cells harvested from patients revealed that cancer cells were ∼33% less adhesive compared to normal cells, suggesting that biomechanical-based functional analysis may provide novel assessment and diagnostic platforms for cancer in the future.50
The extent of biophysical signaling afforded by the actin cytoskeleton is not merely restricted to the mechanical level. Like microtubuli, actin filaments are highly electrostatically charged.51,52 Thus, the head-to-head arrangement of actin monomers into dimeric structures, produces an alternate patterning of electric dipole moments along the filament.53 The participation of actin filaments to a sort of intracellular bioelectronic circuit is theoretically conceived on the assumption that ions may be subjected to a helical distribution winding around the filament at approximately one Bjerrum length, and it is also suggested by experimental evidence showing that actin filaments are able to conduct ionic currents through a swarm-like arrangement of surrounding counterions.52 Agents interfering with the structure and polymerization dynamics of actin filaments are expected not only to affect the mechano-sensing and-transduction, but even the bioelectric circuitries of the cells, thus influencing normal morphogenetic decisions, and precisely controlling stem cell fate, and larger scale anatomy.54,55,56,57,58
Although the role of actin filaments in sensing and transducing physical stimuli has polarized a large body of investigations, there are also relevant actin-dependent biochemical changes that, albeit being discovered over 40 years ago, have gained renewed consideration only quite recently. The actin cytoskeleton has long been shown to bind many metabolic enzymes and to be tightly connected to cell metabolism. Actin-bound ATP enhances filament polymerization and affects binding affinity for players controlling actin polymerization and branching.59,60,61 Moreover, the assembly of actin cytoskeleton represents a major energetic drain itself. In different cell types, about 50% of total ATP is consumed to sustain actin cytoskeletal dynamics, as it has been shown by the observation that blocking actin assembly or disassembly in platelets or neurons, resulted in about 50% decline in ATP consumption.62,63 These findings imply that about half of ATP is produced cellularly to maintain actin remodeling, and that precise information has to be conveyed by actin cytoskeleton to the biochemical machinery, to receive a timely metabolic support. Glycolytic enzymes, including active, phosphorylated phosphofruttokinase-1 (PFK-1),64 aldolase and GAPDH, both in their inactive forms,65,66,67,68 have been shown to bind filamentous actin. Insulin has been reported to activate PI3K and Rac signaling to trigger cytoskeleton rearrangements, causing release of actin-bound aldolase, which in turn enhances aldolase activity, which contributes in supporting the glycolytic flux (for a comprehensive review see De-Wane et al.69). There is compelling evidence linking actin binding of glycolytic enzymes, and energy consumption to the large actin rearrangements occurring throughout cell migration, aggregation, cell coping with mechanical deformations, or malignant metastatic dissemination.69 In this regard, cell sensing of external mechanical forces has been shown to reinforce actin cytoskeleton, increasing the number and thickness of in stress fibers. In particular, in response to external forces, AMPK and its activator LKB1 are recruited and activated at the level of the E-cadherin adhesion complex. Activated AMPK leads to enhanced phosphorylation and activation of vinculin, which in turn leads to the activation of the RhoA–ROCK–MLCK–MLC pathway, which regulates the myosin light chain (MLC) of non-muscle myosin II, causing an increase in actomyosin contractility, associated with actin cytoskeletal remodeling and reinforcement.69 The metabolic fuel for such cytoskeletal requirements is provided by AMPK itself which promotes increased glucose uptake.69 This is likely connected to the above-mentioned interaction of glycolytic enzymes with actin filaments, where PFK-1 is already bound in its active form, whereas aldolase, which is bound in its inactive form may undergo release from actin cytoskeleton and subsequent activation by insulin. Such release is relevant for actin rearrangement and increase in glycolytic flux in the presence of insulin.70 Similar considerations apply to GAPDH, another key glycolytic enzyme, which is inactive in its actin-bound configuration, under low metabolic states, as in non-proliferating cells, being released from cytoskeletal actin and acquiring an active configuration under high metabolic activities. Compounding this intricate picture, on soft extracellular matrices, active E3 ubiquitin ligase TRIM21 has been reported to bind and ubiquitinylate (degrading) PFK-1 in the cytosol, keeping the glycolytic pathway in a low state. On the contrary, cell growth onto stiff substrates is associated with increased contractility within the actin cytoskeleton, as well as enhanced F-actin binding to talin-bound integrins,71 which leads to actin bundling and TRIM21 sequestration and inactivation. Within this context, PFK-1 accumulates in the cytosol and stimulates glycolysis. Overall, there is progressive evidence that the mechanical patterning of actin cytoskeleton requires substantial metabolic fueling, and that actin mechanics itself is shaped to finely signal such request to the metabolic machinery. In such interplay, the boundaries between cellular mechanics and metabolism vanish, posing the needs for further studies aimed at elucidating this complex coordination. Further studies are also desirable to specifically direct the heterogeneity potential, including the differentiating and paracrine properties, of different stem cell populations, by modulating the nanomechanical dynamics of actin filaments and microtubuli, or by affecting their interaction with other cytoskeletal players, molecular motors, and the overall intracellular environment.
Conceivably, novel cell therapy strategies may be developed, coupling the manipulation of the mechanical and electric properties of microtubule and actin filaments by the aid of natural or synthetic compounds, or by cell/tissue exposure to physical energies, such as defined mechanical stimuli or electromagnetic radiation (including light).
These studies should carefully consider the metabolic expenditure (stem) cells will undergo when subjected to physical manipulations leading to increased cytoskeletal dynamics and stiffness. Thus, attaining a given differentiating pathway by the aid of mechanical forces should take into account the corresponding metabolic drain, and the needs for consonant metabolic compensation.
The extracellular matrix, a relevant nanomechanical cue in framing stem cell heterogeneity
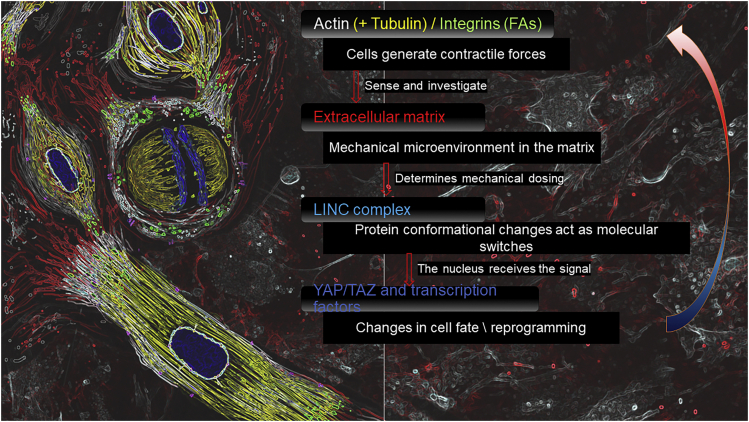
The extracellular matrix (ECM) is a complex 3D network of macromolecules, growth factors and bioactive peptides providing both physical support and dynamic microenvironment for cells, modulating their behavior and fate. ECM is a key component of the stem cell niche.72 The variegated, tissue-specific composition and arrangement of the ECM accounts for the observation that each tissue possesses its peculiar stiffness (also called elastic modulus). Interpretation of mechanical inputs (including substrate stiffness) requires the generation of contractile forces in the cytoskeleton through which the cell actively interrogates its surroundings73,74,75 (Figure 2).
Figure 2.
The extracellular matrix, a relevant nanomechanical cue in framing stem cell heterogeneity
Diagram focusing on the role of the continuous mechanical feedback sensing performed by the cell to compute and respond to the physical external stimuli leading, in the end, to morphogenetic reprogramming.
The key determinants of rigidity sensing relate to the integrin family (receptors) and linked proteins,76 including: (1) the strength of integrin-ECM bonds, (2) the rates of cell protrusion/retraction, (3) the force produced by cells during protrusion/retraction, and (4) the elasticity of associated mechanosensitive proteins.
Stiffness varies markedly between organs and tissues, representing a key element in the unfolding of (stem) cell and tissue heterogeneity: soft tissues (e.g., brain) have a low elastic modulus of approximately 1 kPa, muscle tissue has an intermediate elastic modulus of ∼10 kPa, chondrogenic tissue has an elastic modulus of ∼70 kPa and more rigid structures (e.g., bones) exhibit a relatively high elastic modulus of ∼100 kPa.77 It has been shown that stiffness alone is sufficient to direct MSC heterogeneity through the attainment of variegated differentiation outcomes: MSCs acquire neurogenic/adipogenic phenotypes on soft substrates (<1 kPa), whereas on stiff ones (>10 kPa) they undergo myogenic/osteogenic differentiation.78,79 Intriguingly, Yang et al.80 demonstrated that if MSCs were seeded on a phototunable hydrogel, therefore osteogenic patterns of gene expression persist even after decreasing the substrate stiffness. They concluded that stem cells possess a sort of mechanical memory, and that YAP/TAZ acts as an intracellular mechanical rheostat storing information from past physical environments (depending on both culture time and substrate stiffness), thus orchestrating stem cell heterogeneity and multilineage repertoire. In this regard, Peng et al.81 simulated gene expression dynamics under different mechanical loadings, using an in silico approach describing MSCs cultured in two passages: a first, and a second seeding. Their mathematical model predicted that memory regions can exist for neurogenic, adipogenic, myogenic and osteogenic lineages. They found that the substrate stiffness for the first seeding and the duration of the first seeding were remarkable determinants in stem cell fate specification. Stiffness throughout the second seeding was also relevant in these simulation studies, further contributing in mechanical memory characterization. This mathematical model enabled to assess when stem cell fates are determined, not only by the current substrate stiffness but also by a past exposure. This approach emphasized the role of temporal and spatial accumulation of mechanical loadings, introducing the issue of “mechanical dosings” as major determinants in shaping cell memory.81
An important feature of cell-matrix adhesion, which makes it more versatile than any individual surface receptor, is the large repertoire of mechanosensitive and signaling components in its cytoplasmic scaffold. Mechanotransduction most likely occurs through protein conformational changes that act as molecular switches leading to major transcriptional and signaling reprogramming and heterogeneity.82,83
Undoubtedly, the main hub for cell-matrix interaction is represented by focal adhesions (FAs), which are complex plasma membrane-associated macromolecular assemblies, through which the direct contact cell-ECM occurs, transmitting mechanical force and regulatory signals.84 FAs undergo a regulated turnover at any given time, governed by diverse environmental factors, including adherens and gap junctions, integrins and ECM related proteins.84 There is now compelling evidence that the microenvironmental interaction between FAs, cytoskeleton, and the Linker between Cytoskeleton and Nucleoskeleton (LINC) creates the context for a major heterogeneity event: the reprogramming of a somatic cell into a stem cell. As demonstrated by the pioneer work of Yamanaka et al.,85 this phenomenon was first induced by viral-vector mediated delivery of a set of defined genes. Noteworthy, even in the absence of gene delivery, mature adipocytes have been reprogrammed in vitro into dedifferentiated cells (DFAT cells), undergoing loss of lineage-specific transcripts and gain of stem cell-specific transcripts, solely after exposure to mechanical stress, including high osmotic pressure, traction forces, and ceiling culture.86 DFAT cells lack lipid droplets and acquire a fibroblast-like phenotype,87 even expressing similar surface markers to those of ESCs, bone marrow-, and AD-MSCs.88,89 DFAT cells have proliferation traits similar to those in MSCs,90,91 and undergo a major heterogeneity shift, because they show multi-directional differentiation both in vitro and in vivo, mainly depending on physical stimuli applied in culture. Such a physical mechano-sensing/-transduction pathway triggers a chemical pathway involving YAP/TAZ, through the intervention of the ENH protein, acting as a connector between the two pathways. These findings lay the foundation for the use of mechanical forces in the modulation of somatic-to-stem cell reprogramming.86
Accordingly, it has been recently shown that the capability of Sox2 to promote a pro-regenerative behavior of Schwann cells, the main glial cells responsible for axonal post-injury re-growth in the peripheral nervous system, relies on a direct regulatory action of the stemness gene on integrin-based cell-ECM adhesion, through the activation of fibronectin expression and fibrillogenesis.92,93 These results show that stemness activation in Schwann cells and axonal regeneration are coupled by cell-ECM mediated reprogramming. The relevance of defined, mechanically applied vibrational modes in governing stem cell heterogeneity is shown by the observation that cyclic strain dominates over microtopography in regulating cytoskeletal and FAs remodeling in human MSCs through the transcriptional activation of the related coding genes.94 This finding indicates that defined vibrational patterns are essential for the stem cells’ ability to decipher their physical environmental cues, suggesting that targeted external mechanical stimuli may be fashioned to optimize the stem cell niche for regenerative medicine strategies. Corroborating the relevance of ECM/FAs-regulated mechanotransduction in determining biological heterogeneity is the evidence that ECM/FAs patterning not only operates within in vitro settings, but it is also essential in modulating metabolic dynamics and the onset of complex forms, up to the establishment of large-scale anatomy in vivo. In this regard, studies on cardiogenesis have been particularly informative. During heart development, epithelial-to-mesenchymal transition (EMT) of epicardial cells and their migration into the underlying myocardium represent a crucial step in supporting organ metabolism, growth, and morphogenesis, as it is indicated by the fact that disrupting epicardial EMT results in embryonic lethality.95,96 Ultra-high resolution scanning electron microscopy combined with immunofluorescence analysis revealed an essential role of ECM, mainly the basement membrane-associated proteoglycan agrin, in directing the whole EMT process. Deletion of agrin led to impaired EMT and cardiac development. Conversely, in human ESC-derived epicardial-like cells agrin enhanced EMT via localization of the phosphorylated form of Focal Adhesion Kinase at FAs.97
Thus, ECM/FAs orchestrate not only specific developmental pathways in isolated cells but provide essential morphogenetic signaling for the acquirement of organ shapes.
Oscillatory patterns in mechanobiology: A cue for controlling (stem) cell heterogeneity
Overall, the data discussed above concur with the notion that cellular, and on a larger scale, tissue morphogenesis require force-generating mechanisms, and that these mechanisms are essential to the appearance of complex structures. If so, a fundamental question arises: how are mechanical forces arranged over space and time? Can we consider these forces moving as discrete phenomena, rising from a baseline threshold, reaching a stable sustained plateau, then declining toward baseline again? Rather, should we think of mechanical forces as oscillatory phenomena, exerting their biological effects on the basis of the frequency at which mechano-sensing and-transduction are intertwined within a given cellular, or inter-cellular context? The data up to now discussed in terms of single molecule, and larger subcellular structures, including microtubule and actin filaments argue in favor of this last hypothesis, and prompt consideration for cellular nanomechanics as a context where mechanical forces emerge as ensembles of oscillatory patterns, characterized by defined frequencies and amplitudes. We have previously highlighted how nanomechanical and electromagnetic oscillations may coexist in signaling molecules and supramolecular assemblies, owing to their electrically charged nature.29 Although the mechanical and electric/electromagnetic (including light) oscillations can hardly be separated, as they emerge within the context of the same subcellular structure, it is conceivable that a thorough dissection of the mechanical and electrical components of these oscillations may provide relevant knowledge on how these patterns are generated and on the chance of using mechanical and electric stimulation alone, or in various combinations, to efficiently direct the heterogeneity and rescuing potential of stem cells. A major premise in light of future attempts along this line is establishing whether oscillatory patterns are occurring not only at the subcellular level in cytoskeletal elements, but they are also evident in complex in vitro systems recapitulating relevant developmental pathways, as well as in in vivo settings, and whether, in the affirmative, such oscillatory patterning may have any causal role in morphogenetic decisions. Focusing on mechanical oscillations, a number of interconnected observations may be gathered to confirm such hypotheses. Exploring cell matching, in which individual cells establish targeted inter-cellular connections between partner cells, within the complex context of cardiogenesis, revealed periodical oscillations of non-muscle Myosin II within cardioblasts, with about 4-min intervals, during the Drosophila embryogenesis.98 In this setting, filopodia dynamics, including protrusions, retraction, binding stabilization, and binding separation, correlated with the periodic (oscillatory) positioning of Myosin II clusters at the level of the cell leading edge, conveying mechanical oscillations to the leading-edge actin cytoskeleton.98 These dynamics enable precise cell alignment and represent a major developmental cue in the heart, like other organs’ formation. The causal role of the oscillatory interplay of Myosin II and actin filaments in correctly shaping cardiac morphogenesis was inferred by the observation that perturbing Myosin II activity and oscillatory patterns resulted both in the derangement of filopodia dynamics, and in mismatched cardioblasts, up to the appearance of severe heart defects.98 In the same study, evidence was produced that load bearing filopodia drove the heart closure, suggesting that the oscillatory profile associated with Myosin II and actin filaments was essential in imparting a timely pattern of forces acting as a sort of nanomechanical code for the execution of proper cardiogenesis. These findings indicate the existence of a mechanical proofreading machinery for robust cell matching and morphogenetic decisions, where rhythmic oscillatory mechanical patterns are featured to modulate (stem) cell heterogeneity by ensuring correct cell-cell connection formation. Actomyosin flow has been found to represent another important phenomenon in driving morphogenetic processes, including cytokinesis, migration, polarization, metabolic activity, and wound healing.99 During embryogenesis, cytoskeletal fluctuations and mechanical instability are fashioned to define the axis of symmetry breaking, that is the key morphogenetic event where symmetry along a particular axis is lost to establish a polarity.99
These studies aid placing oscillatory patterns within the context of “morphodynamics”, to elucidate how cellular heterogeneity may be orchestrated to define the changes of a subject’s form over time. Morphodynamic processes necessarily entail a collective behavior, as they require consistent coordination of mechano-sensing/-transduction in individual cells, which are often too far apart to communicate by chemical diffusion of chemical moieties. Complex computational/modeling approaches, coupled with ad hoc designed experiments in the Drosophila embryo have been performed to elucidate whether, and how oscillatory mechanics may contribute to the systems’ collective morphodynamics. These studies identified the tensile stress as the key activator that switches the collective oscillations on and off.100 This regulatory role was shown analytically using the Hopf bifurcation theory. The same study showed that the physical properties of the tissue boundary are directly responsible for synchronizing the oscillatory intensity and polarity of all inner cells and for orchestrating the spatial oscillation patterns in the tissue. Further experimental observations indicate that oscillatory morphodynamics also involves the collective behavioral interplay of phase-coherent synchronization and desynchronization of cytoskeletal elements with tissue fluidics, emerging from pressure inflation/deflation dynamics, shear-stress induced waves, and the pressure generated by rhythmic variation in cell shape and proliferation rates.101
On this basis, oscillatory morphodynamics may be viewed as a field of investigation designed to assess the unfolding of mechanical cues in multicellular process-driven collective behavior, with the perspective of using defined vibrational signatures to modulate cell heterogeneity and afford efficient regenerative medicine approaches in the treatment of complex diseased states, including metabolic and cardiovascular diseases, as discussed below.
The application of mechanical forces to supporttissue regeneration
Major evidence supporting the relevance of mechano-sensing/-transduction in controlling stem cell heterogeneity would result from the chance of using defined mechanical stimuli to afford a targeted direction of stem cell fate along different lineages in vitro on the basis of the characteristics of the applied forces. Additional, even more conclusive evidence would come from the possibility to perform the same manipulation in vivo, exploiting the diffusive features of mechanical vibrations to target tissue resident stem cells and enhance their rescuing potential in multiple diseased states without the need for cell or tissue transplantation.
Bone and cartilage: From tissue rescue to metabolic remodeling
On the basis of the currently available literature, we can affirm that both conditions are satisfied (Table 1). Sometimes, evidence was produced in a sort of reverse approach, being firstly gathered in vivo, and then brought to a deeper mechanistic understanding through in vitro experiments. This was the case of the pioneer studies of Rubin et al., showing that long-term (one year) mechanical stimulation of hindlimbs in adult sheep on a daily basis with 20-min, low-amplitude, high-frequency vibration, remarkably increased the density of the trabecular bone in the proximal femur, as compared with untreated controls.102 The strain levels generated by this treatment were three orders of magnitude below those known to damage the bone tissue, indicating that this soft approach of mechano-stimulation acted as an anabolic, non-invasive stimulus, holding the potential for treating skeletal conditions such as osteoporosis. The same team showed that in adult female rats the anabolic activity of bone tissue suppressed by disuse could be normalized by a brief exposure to extremely low-amplitude mechanical stimuli. These studies provided evidence that mechanical vibrations could effectively restore bone mass in vivo, setting the basis for clinical intervention in conditions plagued by bone loss, such as long-term space flight, bed rest, or immobilization caused by paralysis.103,104 Exposure to high-frequency mechanical signals, induced at a magnitude well below that which would arise during walking, was found to inhibit adipogenesis in C57BL/6J mice.105 Exposure to mechanical vibrations also reduced key risk factors in the onset of type II diabetes, non-esterified free fatty acid and triglyceride content in the liver.105 Over 9 weeks of exposure, the same mechanical signals suppressed fat production in the C3H.B6-6T congenic mouse strain that exhibits accelerated age-related changes in body composition. This compelling in vivo evidence could be related to a mechano-transduction effect on tissue resident stem cells, when in the aim of elucidating the underlying mechanism for the observed inhibition of fat depots, the Authors decided to repeat the experiments in irradiated mice receiving bone marrow transplants from heterozygous GFP+ mice.105 In this way, they could prove that the exposure to low-magnitude mechanical signals reduced MSC differentiation into adipocytes, indicating that adipose tissue formation was deterred by a marked reduction in MSC adipogenesis. These studies set the basis for mechanotransduction approaches in nonpharmacologic prevention of obesity that could be achieved through developmental pathways executed at the stem cell morphodynamic level, rather than intervening on metabolic pathways. These perspectives entail further implications in the clinical handling of obesity. Such a condition is paralleled by impairment in both skeletal and immune systems. In a murine model of diet-induced obesity, leading to accelerated age-related loss of trabecular bone, and marked reduction in bone marrow and circulating B-cells, in vivo exposure to low-magnitude mechanical signals (0.2 x g at 90 Hz, 15 min/day, 5 days/week) restored both bone structure and B cells to the levels detected in control mice fed a regular diet.106 These outcomes resulted at least in part from the downregulation of osteoclastic activity and a biasing of hematopoietic stem cell commitment toward the lymphoid B-cell lineage at the expenses of a myeloid fate decision.106 These results demonstrate that mechanical signals and mechanotransduction can act in vivo at the stem cell level to operate major developmental metabolic switches within the context of a fragile interplay between adiposity and osteoimmunology. These conclusions are corroborated by additional molecular evidence in in vitro experiments, showing that in bone marrow derived murine MSCs cultured under strong adipogenic conditions, mechanical load (3600 cycles/day, 2% strain) inactivated GSK3β in a Wnt-independent fashion.107 GSK3β inhibition led to the activation of both β-catenin and NFATc1 signaling, thus limiting MSC adipogenesis through the former mechanism (β-catenin upregulation) and promoting osteoblastic differentiation via NFATc1/COX2. Hence, cell exposure to mechanical vibrations evocates complex and interrelated molecular and metabolic pathways, and the reiterated application of a defined mechanical load results in the regulation of multiple downstream effectors controlling stem cell heterogeneity.107 When translated to an in vivo setting, the observed osteogenic response of stem cells to mechanical vibrations could reflect a cellular response to a fluid shear stress and/or cytoskeletal remodeling.108 Highlighting the major role of cytoskeletal dynamics in cellular response to mechanical stimulation, exposure of hMSCs to multiple vibration frequencies and acceleration magnitudes revealed that hMSC proliferation was greater in the presence of the frequency/acceleration combination that induced the smallest level of fluid shear stress. Actin remodeling by mechanical vibrations was essential to achieve upregulation in the expression of the osteogenic gene RUNX-2, depending on the magnitude of the applied acceleration.108 Further insights into the multifaceted intracellular mechanisms, transducing stem cell response to mechanical vibrations, came from the comparative analysis of the molecular patterning elicited by high-magnitude substrate strain (HMS ≥2%) and extremely low magnitude signals (LMS). Although HMS suppressed murine MSC adipogenesis through FAK/mTORC2 signaling generated at FAs, LMS suppressed MSC adipogenesis, as well, despite virtual absence of substrate strain (<0.001%).109 This intriguing response occurred through mechanical coupling of the cytoskeleton and the cell nucleus, with LMS-induced actin remodeling being concentrated at the perinuclear domain. Disruption of the LINC complexes prevented nuclear-actin cytoskeleton mechano coupling, thus abrogating LMS-mediated repression of adipogenic differentiation, highlighting that LINC connections are critical for sensing LMS. In contrast, FAK activation by HMS was unaffected by LINC decoupling, consistent with signal initiation at the FA mechanosome.109
Table 1.
Bone and cartilage: mechanical force applications, from tissue rescue to metabolic remodeling
| Aim/findings | Biomechanical stimulus | Vitro/vivo | Cell/tissue type | Observed effect | Reference |
|---|---|---|---|---|---|
| Treating skeletal conditions such as osteoporosis | Daily, long-term (1 year), 20-min bursts of very-low-magnitude, high-frequency vibration | vivo | Hind limbs of adult sheep | Significantly increased (by 34.2%) density of the trabecular bone in the proximal femur, compared to controls | Rubin et al.102 |
| Clinical intervention in conditions plagued by bone loss (long-term space flight, bed rest, or immobilization caused by paralysis) | 10 min/day, for 28 days, extremely low-magnitude (<10 microstrain), high-frequency mechanical signals | vivo | adult female rats | Restored anabolic bone cell activity inhibited by disuse | Rubin et al.103 |
| Setting the basis for nonpharmacologic prevention of obesity and its sequelae | 15 weeks of brief, daily exposure to high-frequency mechanical signals, induced at a magnitude well below that which would arise during walking | vivo | C57BL/6J mice | Inhibited adipogenesis by 27%; reduced key risk factors in the onset of type II diabetes, non-esterified free fatty acid (by 43%) and triglyceride content (by 39%), in the liver | Rubin et al.105 |
| Over 9 weeks, same LMS | vivo | C3H.B6-6T congenic mouse strain with accelerated age-related changes in body composition | Suppressed fat production by 22% | ||
| 6 weeks of LMS | vivo | irradiated mice receiving BM transplants from heterozygous GFP+ mice | Reduced commitment of MSC differentiation into adipocytes by 19% | ||
| Mechanical signals can act in vivo at the stem cell level to operate major developmental metabolic switches | Brief daily exposure to LMS (0.2gat 90 Hz, 15 min/day, 5 days/week) | vivo | murine model of diet-induced obesity | Restored bone structure and B cells to the levels detected in control mice fed a regular diet | Chan et al.106 |
| Mechanical loading regulates MSC differentiation through inhibition of GSK3β, which in turn regulates multiple downstream effectors | Mechanical load (3600 cycles/day, 2% strain) | vitro | murine BM-MSCs cultured under strong adipogenic conditions | Inactivation of GSK3β in a Wnt-independent fashion, leading to the activation of both b-catenin and NFATc1 signaling, thus limiting MSC adipogenesis and promoting osteoblastic differentiation | Sen et al.107 |
| Major role of cytoskeletal dynamics in cellular response to mechanical stimulation | MSCs were subjected to vibration frequencies (100 Hz and 30 Hz) and acceleration magnitudes (0.15 g, 1 g, and 2 g) that induced fluid shear stress ranging from 0.04 Pa to 5 Pa | vitro | AD-hMSCs | Vibration-induced increase in the osteogenic commitment and proliferation of MSCs does not depend on fluid shear; the mechanically driven osteogenic commitment of undifferentiated MSCs was influenced by the level of cytoskeletal remodeling | Uzer et al.108 |
| MSCs respond to dynamical physical environment not only with “outside-in” signaling primed by HMS, but even through matrix independent “inside-inside” signaling conducted by LMS through the LINC complex | LMS: vibrations applied to MSCs at peak magnitudes of 0.7gat 90 Hz for 20 min (two 20 min bouts separated by 2 h rest). HMS: uniform 2% biaxial strain delivered at 10 cycles per min for 20 min | vitro | murine BM-MSCs | While HMS suppressed MSC Adipogenesis through FAK/mTORC2 signaling generated at focal adhesions, LMS suppressed MSC adipogenesis despite virtual absence of substrate strain (<0.001%); this response occurred through mechanical coupling of the cytoskeleton and the cell nucleus | Uzer et al.109 |
| Gene and protein expression of BMPs is implicated in the bone healing action exerted by ESW | ESW treatment using 500 impulses at 0.16 mJ/mm2 | vivo | Rats with a 5-mm segmental femoral defect | Intensive MSC aggregation, hypertrophic chondrogenesis, and endochondral/intramembrane ossification, resulting in the healing of segmental defect; ESW promoted BMP-2, BMP-3, BMP-4, and BMP-7 mRNA expression in callus (tissue-rescuing pattern) | Wang et al.110 |
| SW-promoted bone healing is associated with significant increases in serum NO level and osteogenic growth factors | 6000 impulses of SW at 28 kV in a single session | clinical trial | Patients affected by long bone non-unions | At 6 months radiographically confirmed bony union in 78.6% of treated patients; in these patients after 1 month of treatment higher serum levels of NO, TGF-beta1, VEGF and BMP-2 were measured | Wang et al.111 |
| ESWT strategy is feasible, well tolerated, and suitable to be evaluated in a Phase III trial for acute traumatic wounds | Debridement, outpatient ESWT (100–1000 shocks/cm2 at 0.1 mJ/mm2, according to wound size, every 1 to 2 weeks over mean 3 treatments), and moist dressings | clinical trial | Patients with complicated, non-healing, acute and chronic soft-tissue wounds | Feasibility and safety of ESWT for acute and chronic soft-tissue wounds | Schaden et al.112 |
| Application of a single defocused ESWT immediately after skin graft harvest can accelerate donor site epithelialization | Standard topical therapy and antiseptic gel to graft donor sites with or without defocused ESWT (100 impulses/cm2 at 0.1 mJ/mm2) applied once to the donor site, immediately after skin harvest | clinical trial | Patients with acute traumatic wounds and burns requiring skin grafting | Mean times to complete graft donor site epithelialization for patients who did and did not undergo ESWT were 13.9 ± 2.0 days and 16.7 ± 2.0 days, respectively (p = 0.0001) | Ottomann et al.113 |
BM: bone marrow, MSCs: mesenchymal stem cells, AD-hMSCs: adipose derived human MSCs, HMS: high magnitude strain, LMS: low magnitude mechanical signals, BMPs: bone morphogenetic proteins, ESW: extracorporeal shock waves, SW: shock wave, ESWT: extracorporeal shock wave therapy.
A complex mechanoscape emerges from these results, indicating that MSC heterogeneity can be regulated by mechanical vibrations not only by “outside-in” dynamics primed by HMS, but even through “inside-inside” signaling conducted by the LMS through the LINC complex.
An important modality of releasing mechanical signals and afford therapeutic outcomes is quite recently emerging as the result of tissue-resident stem cell reprogramming, is represented by the tissue application of ESW. These are high-energy carrying acoustic waves, including sound pulses, exhibiting a peculiar waveform, involving a rapid peak of positive pressure followed by a rapid phase of negative pressure, able to produce direct mechanical stimulation. When ESW are generated in an aqueous environment and conveyed onto defined “focuses”, they transmit a measurable amount of energy, providing precise therapeutic effects.114
In 2003, Wang et al.110 provided the first evidence that the gene and protein expression of bone morphogenetic proteins (BMPs), playing an essential role in bone development and fracture healing, were implicated in the bone healing action exerted by ESW in segmented femoral defects induced in rats. Fractured femurs receiving ESW underwent intensive MSC aggregation, hypertrophic chondrogenesis, and endochondral/intramembranous ossification, resulting in the healing of segmental defects.110 Moreover, ESW significantly promoted BMP-2, BMP-3, BMP-4, and BMP-7 mRNA expression in callus, with MSCs and immature chondroblasts mainly expressing BMP-2, BMP-3, BMP-4 proteins, and osteoblasts exhibiting BMP-7 immunoreactivity throughout the whole regeneration period. These tissue-rescuing patterns were associated with a highly proliferative state of osteoblasts adjunct to newly formed woven bone.110
These studies set the basis for an important clinical trial in patients affected by long bone non-unions.111 For each of the patients enrolled in the study the long bone non-union was subjected to 6000 pulses of ESW at 28 kV within a single session. At 6 months, bony union was radiographically confirmed in about 80% of treated patients. Intriguing parallel biochemical experiments performed on peripheral blood samples, revealed that patients with successful bony union had significantly higher serum levels of nitric oxide (NO), TGF-beta1, VEGF and BMP-2, as compared with patients with persistent non-union. The increase in NO levels and osteogenic growth factors was evident as early as after 1 month of treatment, a period by far preceding the onset of bone healing.111 These findings were further refined in experimental animal models showing that ESW promoted bone formation in segmental defects in rats through the activation of extracellular signal-regulated kinase (ERK) and p38 kinase. ESW rapidly promoted [3H]-thymidine uptake in 1 day and progressively increased alkaline phosphatase activity, collagen I, II, and osteocalcin synthesis in callus organ cultures within 14 days after treatment. Analysis of [γ−32P]-phosphotransferase activity revealed that ERK and p38 in calluses were rapidly activated 1 day and 7 days after ESW treatment, respectively. MSC aggregates were detected in segmental defects subjected to ESW treatment, along with hypertrophic cartilage, and endochondral/intramembranous ossification. Intensive bone formation coincided with evident expression of phosphorylated ERK and p38. Moreover, persisting expression of phosphorylated ERK was found in MSCs, as well as in chondral, and osteoblastic cells at newly developed bone and cartilage sites, and the expression of activated p38 was evident on chondral cells located at hypertrophic cartilage.111
In the following years, ESW also proved to act efficiently in soft tissue wound healing in patients with complicated, non-healing, acute and chronic soft-tissue wounds,112 as well as in accelerating the re-epithelialization of skin graft donor sites.113
Cardiovascular system: From peripheral tissue revascularization to cardiac repair
Tissue damage very often involves, and results from impaired vascularization, especially at the microvessel level, representing at the same time the best site for tissue perfusion and oxygen supply, and the worst context for a surgical intervention, given the size limits of the affected vasculature. One of the first evidence of ESW effectiveness in peripheral vascular diseases (Table 2) came from the observation that exposure to low-energy ESW of non-ischemic hindlimb adductor muscles in nude rats enhanced by itself the tissue expression of the chemoattractant stromal cell-derived factor 1 (SDF-1), with subsequent increase in local number of vascular endothelial growth factor (VEGF)-positive endothelial cells per myocyte.115 Such preconditioning resulted in a remarkable enhancement in muscle recruitment and homing of endothelial precursor cells (EPCs) infused 24 h after ESW exposure. To explore the implications of these results, in the same animal model, ESW were applied after the induction of unilateral chronic hindlimb ischemia, then followed by the intravenous infusion of EPCs. This strategy resulted in significantly increased blood flow recovery, compared to untreated limb, or the ischemic limb only receiving ESW, or EPC alone.115 Another relevant finding in this field was contributed by the observation that ESW could restore tissue perfusion following hindlimb ischemia in mice.116 Macrophages are crucial for angiogenic responses following an ischemic damage, with the M2 macrophage being the main conductors. ESW treated muscles manifested increased expression of monocyte chemotactic protein 1 (MCP-1), an essential factor in macrophage recruiting.116 Supporting the effectiveness of the ESW treatment, increased numbers of macrophages, in the M2 polarization, releasing anti-inflammatory chemokines were found in the exposed tissue. Elevated gene expression of the M2 scavenger receptor CD163, contributing to the macrophage transition from the M1 (releasing proinflammatory factors) to the M2 phenotype (releasing anti-inflammatory chemokines), were also detected. These effects were associated with increased number of capillaries and arterioles, with significant improvement in limb perfusion.116
Table 2.
Cardiovascular system: mechanical force applications, from peripheral tissue revascularization to cardiac repair
| Aim/findings | Biomechanical stimulus | Vitro/vivo | Cell/tissue type | Observed effect | Reference |
|---|---|---|---|---|---|
| Giving evidence of ESW effectiveness in peripheral vascular diseases | (1) Hindlimb adductor muscles of nude rats were treated with 500, 1000, and 2000 impulses of focused low-energy SW (flux density level: 0.05 mJ/mm2); (2) SW were applied after the induction of unilateral chronic hindlimb ischemia, followed by IV infusion of EPCs | vivo | nude rats: (1) non-ischemic, (2) chronic ischemic tissue | (1) Enhanced expression of SDF-1, with subsequent increase in local number of VEGF-positive endothelial cells per myocyte; (2) significantly increased blood recovery | Aicher et al.115 |
| ESW could restore tissue perfusion following HLI, therefore represents a promising new treatment option for ischaemic heart disease | ESW | vivo | C57BL/6 mice subjected to HLI | In muscles increased expression of MCP-1, essential in macrophage recruitment; increased number of macrophages in the M2 polarization (releasing anti-inflammatory chemokines); increased number of capillaries and arterioles | Tepeköylü et al.116 |
| Investigation of ESW effect in animal models of myocardial ischemia | ESW | vitro | HUVECs | Increased gene expression of VEGF and its receptor Flt-1 | Nishida et al.117 |
| ESW therapy for 4 weeks to the ischemic myocardial region | vivo | Porcine model of chronic myocardial ischemia | Complete recovery of LVEF, wall thickening fraction, regional myocardial blood flow, myocardial upregulation of VEGF | ||
| Investigation of safety and efficacy of SWT in a large preclinical model of AMI | 4 weeks after ligation of LAD coronary artery, animals were treated in the absence or presence of epicardial SW delivered through 300 impulses at 0.38 mJ/mm2 at the level of the infarcted wall | vivo | Porcine model of ischemic heart disease | Cardiac contractility assessed as LVEF remarkably improved in ESW-exposed animals, and was associated with increased angiogenesis at the infarcted and border zone | Holfeld et al.118 |
| Potential mechanism for the observed in vivo effects | ESW | vitro | HCAECs | Elicited stimulation of VEGF receptors | |
| ESW prevented the onset of heart failure after AMI | ESW delivered to the heart through the thorax, with 300 impulses of 0.38 mJ/mm2 at a frequency of 5 Hz | vivo | Mice subjected to AMI | Significant improvement of the myocardial function as well as hemodynamic pressure-volume measurements, increased number of capillaries and arterioles, marked reduction of fibrosis and scar formation | Tepeköylü et al.119 |
| Cell treatment with a preconditioned medium derived from previously ESW-exposed HUVECs (250 impulses with an energy flux density of 0.08 mJ/mm2 at a frequency of 3 Hz) | vitro | HUVECs | Higher proliferation rates, ESW action was owing to a factor released in the culture medium | ||
| Central role of the innate immune system, TLR3, to mediate angiogenesis | SW | vivo | Murine HLI model | Induced angiogenesis and arteriogenesis, improved blood perfusion of treated limbs | Holfeld et al.120 |
| Cardiac regeneration promoted by ESW therapy may occur through endogenous mobilization of vasculogenic progenitors from the BM | Animals received SWT 3 weeks after LAD ligation: 300 impulses were delivered to the ischemic area with an energy flux density of 0.38 mJ/mm2 at a frequency of 4 Hz | vivo | Murine model of chronic myocardial ischemia | Induced local cardiac sprouting of pre-existing vessels through increased in vivo tissue gene expression of pivotal angiogenic factors; enhanced expression of SDF-1 and recruitment of BM resident endothelial cells to the damaged myocardium | Gollmann-Tepeköylü et al.121 |
| ESWT is an effective and non-invasive treatment for end-stage coronary artery disease, although further careful evaluation is needed | Cardiac SWT (200 shots/spot at 0.09 mJ/mm2 for 20–40 spots, 3 times a week/series) | clinical trial | Patients with severe coronary artery disease | Improved symptoms, reduced nitroglycerin use, improved myocardial perfusion | Fukumoto et al.122 |
| Effectiveness and safety of cardiac ESWT for severe angina pectoris | SW | double-blind and placebo-controlled study | Patients with severe angina pectoris | Improved chest pain symptoms and cardiac function, without any complications or side effects | Kikuchi et al.123 |
| SW therapy is feasible and may ameliorate postmyocardial infarction LV remodeling in patients with AMI as an adjunctive therapy to primary PCI | SW | first-in-human study | Patients with AMI who successfully underwent primary PCI | Reverse remodeling and enhanced myocardial performance | Kagaya et al.124 |
ESW: extracorporeal shock waves, SW: shockwave, IV: intravenous infusion, EPCs: endothelial progenitor cells, HLI: hindlimb ischemia, HUVECs: human umbilical vein endothelial cells, LVEF: left ventricular ejection fraction, SWT: shock wave therapy, LAD: left anterior descending artery, HCAECs: human coronary artery endothelial cells, AMI: acute myocardial infarction, TLR3: Toll-like receptor 3, BM: bone marrow, LV: left ventricle, PCI: percutaneous coronary intervention.
These observations are highly consonant with the first studies investigating the ESW effect in animal models of myocardial ischemia. ESW treatment first applied to cultured human umbilical vein endothelial cells (HUVECs) markedly increased the gene expression of VEGF and that of its receptor Flt-1 in vitro.117 In concomitant in vivo experiments in a porcine model of chronic myocardial ischemia, with sustained myocardial dysfunction in the absence of myocardial infarction, ESW therapy applied for 4 weeks to the ischemic myocardial region induced a complete recovery of left ventricular ejection fraction (LVEF), wall thickening fraction, and regional myocardial blood flow.117 These effects were also accompanied by a significant myocardial upregulation of VEGF in vivo.117
Holfeld et al.118 investigated the safety and the efficacy of shock wave treatment in a large, preclinical animal model of acute myocardial infarction. SW were delivered through the epicardium of pigs subjected to the ligation of the left anterior descending (LAD) coronary artery. Four weeks later, animals underwent a re-thoracotomy and were treated in the absence, or presence of epicardial SW delivered through 300 impulses at 0.38 mJ/mm2 at the level of the infarcted wall. Cardiac contractility assessed as left ventricular ejection fraction remarkably improved in ESW-exposed animals.118 This rescuing action occurred without acute or chronic adverse events and was associated with a notable increase in angiogenesis at the infarcted and border zone. Concomitant in vitro experiments, aiming at providing a potential mechanism for the observed effects in vivo, showed that ESW elicited stimulation of VEGF receptors in human coronary artery endothelial cells.118 In subsequent studies conducted in mice subjected to acute myocardial infarction through LAD ligation, ESW were delivered to the heart through the thorax, aiming at the ischemic area with 300 impulses of 0.38 mJ/mm2 at a frequency of 5 Hz.119 This treatment elicited a significant improvement of the myocardial function, assessed through echocardiography, as well as hemodynamic pressure-volume measurements,119 showing that ESW prevented the onset of heart failure after acute myocardial infarction. ESW treatment resulted in significantly increased numbers of capillaries, and arterioles, which accounted for the marked decrease in the amount of fibrosis and scar formation. In parallel in vitro experiments, ESW enhanced proliferation in HUVECs. Cell treatment with a preconditioned medium derived from previously ESW-exposed HUVECs resulted in higher proliferation rates, indicating that the ESW action was because of a factor released in the culture medium.119 At this level, the authors found enhanced amounts of total RNA, which was also subjected to reuptake, following ESW treatment, as it was revealed by the aid of pre-marked RNA. To elucidate whether RNA release and uptake may have a causative role in the endothelial proliferation induced by ESW, RNase, or proteinase, or a combination of both were added to the endothelial cells before shock wave treatment. Although RNase alone did not affect the shock wave effect, this was instead abolished in the presence of proteinase. In actual fact, the expression of the antimicrobial peptide LL37 resulted to be enhanced following ESW treatment in both HUVECs, and preconditioned supernatants.119 In previous experiments, the same group described that ESW-mediated rescue of ischemic skeletal muscle occurred via RNA release and TollLike Receptor3 (TLR3) activation, but it went lost in TLR3−/− animals.120 The fact that extracellular self-RNA escapesits degradation by forming RNA-protein complexes,125 and that self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLRs,126 led to the hypothesis that the observed increase in LL37 following ESW exposure may have fulfilled similar requirements, by protecting RNA from degradation and forming RNA-TLRs complexes.119 Thus, differently from untreated controls, the supernatant from HUVECs that had been exposed to ESW was found to cause TLR3 activation when transferred onto TLR3 reporter cells. Similar to the in vivo experiments, this effect could not be abolished by RNase, but only by additional proteinase treatment, or by the sole proteinase itself.119 These data strongly suggest that ESW-mediated prevention of left ventricular remodeling and failure occurred through an angiogenic mechanism, involving LL37 release, its ability to form complexes with RNA, and the subsequent cellular uptake of such complexes, ensuing in RNA binding and activation of TLR3 at the intracellular level.
The possibility that cardiac regeneration promoted by ESW therapy in infarcted mice may occur through endogenous mobilization of vasculogenic progenitors from the bone marrow has been confirmed by recent studies in an animal model of chronic myocardial ischemia, using wild-type mice receiving bone marrow transplantation from green fluorescent protein (GFP) donor mice.121 These experiments showed that ESW induced local cardiac sprouting of pre-existing vessels (angiogenesis) through an increased in vivo tissue gene expression of pivotal angiogenic factors, including VEGF, its receptor VEGFR2, placental growth factor and fibroblast growth factor (FGF). Although VEGF and FGF stimulate capillary sprouting by induction of endothelial proliferation and migration, placental growth factor is known for inducing maturation and stabilization of vessels contributing to arteriogenesis, an essential process optimizing tissue oxygen supply.121 Even more intriguingly, ESW triggered an enhanced expression of SDF-1 in the ischemic myocardium and serum, and led to the recruitment of bone marrow resident endothelial cells to the damaged myocardium, an event associated with the de novo vessel formation (vasculogenesis).121In vitro studies in HUVECs confirmed that ESW induced endothelial cell proliferation, their enhanced survival, and capillary sprouting, and that this vasculogenic program was VEGFR2-, and heparan sulfate proteoglycan (HSPG)-dependent.121 In fact, ECM contains growth factors from the VEGF and FGF families bound to HSPGs. Based on the observation that HUVEC pretreatment with heparinase or heparin, leading to growth factor depletions of the ECM, abolished ESW induced cell proliferation and in vitro vasculogenesis, it has been proposed that the SW action may have involved the release of VEGF from an HSPG-bound reservoir.121 This hypothesis is consistent with a more general mechano-sensing/-transduction context for the exploitation of the ESW effects. In fact, the polysaccharides composed of a core protein and glucosamine chains have been described as mechanosensors directing major cellular decisions through growth factor release and angiogenesis on applied mechanical forces, or changes in cellular viscoelastic properties arising from tissue injuries and diseased states.127
These observations may also be recalled as a reverse from - the bed - to - the bench side story, because they helped to elucidate the potential molecular and biophysical mechanisms underlying a number of earlier studies performed in subjects with severe coronary artery disease. These clinical investigations showed that ESW therapy was able to ameliorate myocardial ischemia in patients with severe coronary artery disease,122 also improving the myocardial function and affording chest pain relief in patients with severe angina, without any complication or sideeffects.123 More recently, a first in human study revealed that SW therapy afforded a reverse remodeling and enhanced myocardial performance in patients with acute myocardial infarction.124
Conclusions and future directions
Deciphering nanomechanical signatures in morphodynamic pathways has been boosted by the continuous upgrading in AFM technology.49,128,129,130 Within this context, we have established the ability of (stem) cells to express “vibrational” (nanomechanical) signatures of their healthy/non-healthy status, as well as nanomechanical signatures of their differentiation pattern along defined lineages.129 The term “sonocytology” was coined to describe a new field of study based on the fact that cell vibrations could be converted into audible sounds following a thorough amplification process, providing comprehensive morphodynamic analyses.129 These sounds have been shown to emerge as well-defined sequences of pitch, timing and structure of audible vibrations, providing an accurate characterization of the extreme complexity of the nanomechanical activity of somatic and stem cells, as a function of the specific cellular tasks executed within a given experimental setting.48
Now the tremendous informational potential offered by nanoscale measurement of vibrational forces in living systems poses an intriguing chance for future biomedical development. Mechanical, like electromagnetic forces, entail diffusive properties, meaning that their energy can be conveyed to cells and tissues to elicit local and non-local precise mechanotransducting responses, capable of effective molecular and functional reprogramming, even for therapeutic purposes. The story and outcomes from the use of ESW and vibrational actuators reviewed herein is a clear example of such perspective. There is anyway an additional putative level of understanding that may now emerge, with dense biomedical and therapeutic implications. The evidence for defined signatures of nanomechanical patterning and (stem) cell morphodynamics48,129,131,132 raises the more general question as to whether they not only recapitulate a specific biological journey, but whether they also entail causative roles in the adaptogenic potential of somatic and stem cells. If so, we may assume that within the challenging context of a deranged pathological environment, (stem) cells may also express decipherable signatures of their effort to counteract, at least in part, the non-coherent environment, or signatures that drive specific fates within their multilineage stem cell repertoire. We are currently working to extract these vibrational signatures by the aid of advanced AFM recordings, also developing multifrequency mechanical and electromagnetic actuators capable of conveying the acquired signatures back to targeted stem cells and tissues, in the effort to specifically orchestrate their self-healing and differentiating potential. Hopefully, these lines of investigation will pave the way to the development of a new generation of wearables, embedding ad hoc designed and versatile mechanical/electromagnetic actuators, making available a regenerative medicine without the needs for stem cell/tissue transplantation, but primarily based on the in situ reprogramming of tissue-resident stem cells to boost our inherent self-healing potential.
Acknowledgments
This work was supported by Eldor Lab, via Vittor Pisani 16, 20124, Milan, Italy.
Author contributions
R.T. and E.O. equally contributed to this work with literature review and analysis. C.V. conceived and designed the study and wrote the paper. All authors equally contributed to this paper with drafting, critical revision and editing, and approval of the final version.
Declaration of interests
The authors declare that they have no competing interests.
References
- 1.Maître J.L., Turlier H., Illukkumbura R., Eismann B., Niwayama R., Nédélec F., Hiiragi T. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature. 2016;536:344–348. doi: 10.1038/nature18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mammoto T., Ingber D.E. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver V.M. Cell and tissue mechanics: the new cell biology Frontier. Mol. Biol. Cell. 2017;28:1815–1818. doi: 10.1091/mbc.E17-05-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Facchin F., Canaider S., Tassinari R., Zannini C., Bianconi E., Taglioli V., Olivi E., Cavallini C., Tausel M., Ventura C. Physical energies to the rescue of damaged tissues. World J. Stem Cells. 2019;11:297–321. doi: 10.4252/wjsc.v11.i6.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa L.A., Eiro N., Fraile M., Gonzalez L.O., Saá J., Garcia-Portabella P., Vega B., Schneider J., Vizoso F.J. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell. Mol. Life Sci. 2021;78:447–467. doi: 10.1007/s00018-020-03600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas S., Trumpp A., Milsom M.D. Causes and consequences of hematopoietic stem cell heterogeneity. Cell Stem Cell. 2018;22:627–638. doi: 10.1016/j.stem.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi Y., Ohnuma K., Furue M.K. Pluripotent stem cell heterogeneity. Adv. Exp. Med. Biol. 2019;1123:71–94. doi: 10.1007/978-3-030-11096-3_6. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y., Dai W., Liu Z., Liu J., Cheng J., Li Y., Li X., Hu J., Lü J. Single-cell infrared microspectroscopy quantifies dynamic heterogeneity of mesenchymal stem cells during adipogenic differentiation. Anal. Chem. 2021;93:671–676. doi: 10.1021/acs.analchem.0c04110. [DOI] [PubMed] [Google Scholar]
- 9.Bongiorno T., Kazlow J., Mezencev R., Griffiths S., Olivares-Navarrete R., McDonald J.F., Schwartz Z., Boyan B.D., McDevitt T.C., Sulchek T. Mechanical stiffness as an improved single-cell indicator of osteoblastic human mesenchymal stem cell differentiation. J. Biomech. 2014;47:2197–2204. doi: 10.1016/j.jbiomech.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo A., Wang B., Lyu C., Li W., Wu Y., Zhu L., Bi R., Huang C., Li J.J., Du Y. Consistent apparent Young's modulus of human embryonic stem cells and derived cell types stabilized by substrate stiffness regulation promotes lineage specificity maintenance. Cell Regen. 2020;9:15. doi: 10.1186/s13619-020-00054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labriola N.R., Darling E.M. Temporal heterogeneity in single-cell gene expression and mechanical properties during adipogenic differentiation. J. Biomech. 2015;48:1058–1066. doi: 10.1016/j.jbiomech.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilian K.A., Bugarija B., Lahn B.T., Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz S.A., Chen C.S. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cell. 2008;26:2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toh Y.C., Xing J., Yu H. Modulation of integrin and E-cadherin-mediated adhesions to spatially control heterogeneity in human pluripotent stem cell differentiation. Biomaterials. 2015;50:87–97. doi: 10.1016/j.biomaterials.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Gao L., McBeath R., Chen C.S. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cell. 2010;28:564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harb N., Archer T.K., Sato N. The Rho-Rock-Myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS One. 2008;3:e3001. doi: 10.1371/journal.pone.0003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D., Zhou J., Wang L., Shin M.E., Su P., Lei X., Kuang H., Guo W., Yang H., Cheng L., et al. Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J. Cell Biol. 2010;191:631–644. doi: 10.1083/jcb.201006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury F., Na S., Li D., Poh Y.C., Tanaka T.S., Wang F., Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahimou F., Touhami A., Dufrêne Y.F. Real-time imaging of the surface topography of living yeast cells by atomic force microscopy. Yeast. 2003;20:25–30. doi: 10.1002/yea.923. [DOI] [PubMed] [Google Scholar]
- 20.Müller D.J., Dufrêne Y.F. Atomic force microscopy: a nanoscopic window on the cell surface. Trends Cell Biol. 2011;21:461–469. doi: 10.1016/j.tcb.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Pelling A.E., Sehati S., Gralla E.B., Valentine J.S., Gimzewski J.K. Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science. 2004;305:1147–1150. doi: 10.1126/science.1097640. [DOI] [PubMed] [Google Scholar]
- 22.Radmacher M. Studying the mechanics of cellular processes by atomic force microscopy. Methods Cell Biol. 2007;83:347–372. doi: 10.1016/S0091-679X(07)83015-9. [DOI] [PubMed] [Google Scholar]
- 23.Hashimura H., Morimoto Y.V., Hirayama Y., Ueda M. Calcium responses to external mechanical stimuli in the multicellular stage of Dictyostelium discoideum. Sci. Rep. 2022;12:12428. doi: 10.1038/s41598-022-16774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ventura C., Capogrossi M.C., Spurgeon H.A., Lakatta E.G. Kappa-opioid peptide receptor stimulation increases cytosolic pH and myofilament responsiveness to Ca2+ in cardiac myocytes. Am. J. Physiol. 1991;261:H1671–H1674. doi: 10.1152/ajpheart.1991.261.5.H1671. [DOI] [PubMed] [Google Scholar]
- 25.Ventura C., Spurgeon H., Lakatta E.G., Guarnieri C., Capogrossi M.C. Kappa and delta opioid receptor stimulation affects cardiac myocyte function and Ca2+ release from an intracellular pool in myocytes and neurons. Circ. Res. 1992;70:66–81. doi: 10.1161/01.res.70.1.66. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q.Y., Bai J.D., Wu X.A., Liu X.N., Zhang M., Chen W.Y. Microniche geometry modulates the mechanical properties and calcium signaling of chondrocytes. J. Biomech. 2020;104:109729. doi: 10.1016/j.jbiomech.2020.109729. [DOI] [PubMed] [Google Scholar]
- 27.Jansen K.A., Donato D.M., Balcioglu H.E., Schmidt T., Danen E.H.J., Koenderink G.H. A guide to mechanobiology: where biology and physics meet. Biochim. Biophys. Acta. 2015;1853:3043–3052. doi: 10.1016/j.bbamcr.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Holle A.W., Young J.L., Van Vliet K.J., Kamm R.D., Discher D., Janmey P., Spatz J.P., Saif T. Cell-extracellular matrix mechanobiology: forceful tools and emerging needs for basic and translational research. Nano Lett. 2018;18:1–8. doi: 10.1021/acs.nanolett.7b04982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tassinari R., Cavallini C., Olivi E., Facchin F., Taglioli V., Zannini C., Marcuzzi M., Ventura C. Cell responsiveness to physical energies: paving the way to decipher a morphogenetic code. Int. J. Mol. Sci. 2022;23:3157. doi: 10.3390/ijms23063157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahu S., Ghosh S., Hirata K., Fujita D., Bandyopadhyay A. Multi-level memory-switching properties of a single brain microtubule. Appl. Phys. Lett. 2013;102:123701. doi: 10.1063/1.4793995. [DOI] [Google Scholar]
- 31.Sahu S., Ghosh S., Fujita D., Bandyopadhyay A. Live visualizations of single isolated tubulin protein self-assembly via tunneling current: effect of electromagnetic pumping during spontaneous growth of microtubule. Sci. Rep. 2014;4:7303. doi: 10.1038/srep07303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch M.D., Schneider N., Nick P., Rohrbach A. Single microtubules and small networks become significantly stiffer on short time-scales upon mechanical stimulation. Sci. Rep. 2017;7:4229. doi: 10.1038/s41598-017-04415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamant O., Heisler M.G., Jönsson H., Krupinski P., Uyttewaal M., Bokov P., Corson F., Sahlin P., Boudaoud A., Meyerowitz E.M., et al. Developmental patterning by mechanical signals in Arabidopsis. Science. 2008;322:1650–1655. doi: 10.1126/science.1165594. [DOI] [PubMed] [Google Scholar]
- 34.Hawdon A., Aberkane A., Zenker J. Microtubule-dependent subcellular organisation of pluripotent cells. Development. 2021;148:dev199909. doi: 10.1242/dev.199909. [DOI] [PubMed] [Google Scholar]
- 35.Akhmanova A., Hoogenraad C.C. Microtubule minus-end-targeting proteins. Curr. Biol. 2015;25:R162–R171. doi: 10.1016/j.cub.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 36.Chaigne A., Labouesse C., White I.J., Agnew M., Hannezo E., Chalut K.J., Paluch E.K. Abscission couples cell division to embryonic stem cell fate. Dev. Cell. 2020;55:195–208.e5. doi: 10.1016/j.devcel.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zenker J., White M.D., Templin R.M., Parton R.G., Thorn-Seshold O., Bissiere S., Plachta N. A microtubule-organizing center directing intracellular transport in the early mouse embryo. Science. 2017;357:925–928. doi: 10.1126/science.aam9335. [DOI] [PubMed] [Google Scholar]
- 38.Scheffler K., Uraji J., Jentoft I., Cavazza T., Mönnich E., Mogessie B., Schuh M. Two mechanisms drive pronuclear migration in mouse zygotes. Nat. Commun. 2021;12:841. doi: 10.1038/s41467-021-21020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plaçais P.Y., Balland M., Guérin T., Joanny J.F., Martin P. Spontaneous oscillations of a minimal actomyosin system under elastic loading. Phys. Rev. Lett. 2009;103:158102. doi: 10.1103/PhysRevLett.103.158102. [DOI] [PubMed] [Google Scholar]
- 40.Yang T.D., Park K., Park J.S., Lee J.H., Choi E., Lee J., Choi W., Choi Y., Lee K.J. Two distinct actin waves correlated with turns-and-runs of crawling microglia. PLoS One. 2019;14:e0220810. doi: 10.1371/journal.pone.0220810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banerjee D.S., Munjal A., Lecuit T., Rao M. Actomyosin pulsation and flows in an active elastomer with turnover and network remodeling. Nat. Commun. 2017;8:1121. doi: 10.1038/s41467-017-01130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bianconi E., Tassinari R., Alessandrini A., Ragazzini G., Cavallini C., Abruzzo P.M., Petrocelli G., Pampanella L., Casadei R., Maioli M., et al. Cytochalasin B modulates nanomechanical patterning and fate in human adipose-derived stem cells. Cells. 2022;11:1629. doi: 10.3390/cells11101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo W., Shitaye H., Friedman M., Bennett C.N., Miller J., MacDougald O.A., Hankenson K.D. Disruption of cell matrix interaction by heparin enhances mesenchymal progenitor adipocyte differentiation. Exp. Cell Res. 2008;314:3382–3391. doi: 10.1016/j.yexcr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., Chen C.S. Cell shape, cytoskeletal tension and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 45.Junqueira Alves C., Dariolli R., Haydak J., Kang S., Hannah T., Wiener R.J., DeFronzo S., Tejero R., Gusella G.L., Ramakrishnan A., et al. Plexin-B2 orchestrates collective stem cell dynamics via actomyosin contractility, cytoskeletal tension and adhesion. Nat. Commun. 2021;12:6019. doi: 10.1038/s41467-021-26296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdel Fattah A.R., Daza B., Rustandi G., Berrocal-Rubio M.Á., Gorissen B., Poovathingal S., Davie K., Barrasa-Fano J., Cóndor M., Cao X., et al. Actuation enhances patterning in human neural tube organoids. Nat. Commun. 2021;12:3192. doi: 10.1038/s41467-021-22952-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tortorella I., Argentati C., Emiliani C., Martino S., Morena F. The role of physical cues in the development of stem cell-derived organoids. Eur. Biophys. J. 2022;51:105–117. doi: 10.1007/s00249-021-01551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson S.L., Proctor D.T., Ghasemloonia A., Lama S., Zareinia K., Ahn Y., Al-Saiedy M.R., Green F.H., Amrein M.W., Sutherland G.R. Vibrational profiling of brain tumors and cells. Theranostics. 2017;7:2417–2430. doi: 10.7150/thno.19172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cross S.E., Jin Y.S., Rao J., Gimzewski J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 50.Cross S.E., Jin Y.S., Tondre J., Wong R., Rao J., Gimzewski J.K. AFM-based analysis of human metastatic cancer cells. Nanotechnology. 2008;19:384003. doi: 10.1088/0957-4484/19/38/384003. [DOI] [PubMed] [Google Scholar]
- 51.Cantiello H.F., Patenaude C., Zaner K. Osmotically induced electrical signals from actin filaments. Biophys. J. 1991;59:1284–1289. doi: 10.1016/S0006-3495(91)82343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin E.C., Cantiello H.F. A novel method to study the electrodynamic behavior of actin filaments. Evidence for cable-like properties of actin. Biophys. J. 1993;65:1371–1378. doi: 10.1016/S0006-3495(93)81188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobayasi S., Asai H., Oosawa F. Electric birefringence of actin. Biochim. Biophys. Acta. 1964;88:528–540. doi: 10.1016/0926-6577(64)90096-8. [DOI] [PubMed] [Google Scholar]
- 54.Adams D.S., Levin M. General principles for measuring resting membrane potential and ion concentration using fluorescent bioelectricity reporters. Cold Spring Harb. Protoc. 2012;2012:385–397. doi: 10.1101/pdb.top067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams D.S., Levin M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb. Protoc. 2012;2012:459–464. doi: 10.1101/pdb.prot067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Law R., Levin M. Bioelectric memory: modeling resting potential bistability in amphibian embryos and mammalian cells. Theor. Biol. Med. Model. 2015;12:22. doi: 10.1186/s12976-015-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pai V.P., Willocq V., Pitcairn E.J., Lemire J.M., Paré J.F., Shi N.Q., McLaughlin K.A., Levin M. HCN4 ion channel function is required for early events that regulate anatomical left-right patterning in a nodal and lefty asymmetric gene expression-independent manner. Biol. Open. 2017;6:1445–1457. doi: 10.1242/bio.025957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pai V.P., Pietak A., Willocq V., Ye B., Shi N.Q., Levin M. HCN2 rescues brain defects by enforcing endogenous voltage pre-patterns. Nat. Commun. 2018;9:998. doi: 10.1038/s41467-018-03334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blanchoin L., Pollard T.D. Mechanism of interaction of Acanthamoeba actophorin (ADF/Cofilin) with actin filaments. J. Biol. Chem. 1999;274:15538–15546. doi: 10.1074/jbc.274.22.15538. [DOI] [PubMed] [Google Scholar]
- 60.Cai L., Makhov A.M., Bear J.E. F-actin binding is essential for coronin 1B function in vivo. J. Cell Sci. 2007;120:1779–1790. doi: 10.1242/jcs.007641. [DOI] [PubMed] [Google Scholar]
- 61.Cai L., Marshall T.W., Uetrecht A.C., Schafer D.A., Bear J.E. Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge. Cell. 2007;128:915–929. doi: 10.1016/j.cell.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daniel J.L., Molish I.R., Robkin L., Holmsen H. Nucleotide exchange between cytosolic ATP and F-actin-bound ADP may be a major energy-utilizing process in unstimulated platelets. Eur. J. Biochem. 1986;156:677–684. doi: 10.1111/j.1432-1033.1986.tb09631.x. [DOI] [PubMed] [Google Scholar]
- 63.Bernstein B.W., Bamburg J.R. Actin-ATP hydrolysis is a major energy drain for neurons. J. Neurosci. 2003;23:1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roberts S.J., Somero G.N. Properties of the interaction between phosphofructokinase and actin. Arch. Biochem. Biophys. 1989;269:284–294. doi: 10.1016/0003-9861(89)90110-0. [DOI] [PubMed] [Google Scholar]
- 65.Schindler R., Weichselsdorfer E., Wagner O., Bereiter-Hahn J. Aldolase-localization in cultured cells: cell-type and substrate-specific regulation of cytoskeletal associations. Biochem. Cell. Biol. 2001;79:719–728. doi: 10.1139/o01-137. [DOI] [PubMed] [Google Scholar]
- 66.Wang J., Morris A.J., Tolan D.R., Pagliaro L. The molecular nature of the F-actin binding activity of aldolase revealed with site-directed mutants. J. Biol. Chem. 1996;271:6861–6865. doi: 10.1074/jbc.271.12.6861. [DOI] [PubMed] [Google Scholar]
- 67.Forlemu N.Y., Njabon E.N., Carlson K.L., Schmidt E.S., Waingeh V.F., Thomasson K.A. Ionic strength dependence of F-actin and glycolytic enzyme associations: a Brownian dynamics simulations approach. Proteins. 2011;79:2813–2827. doi: 10.1002/prot.23107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poglazov B.F., Livanova N.B. Interaction of actin with the enzymes of carbohydrate metabolism. Adv. Enzyme Regul. 1986;25:297–305. doi: 10.1016/0065-2571(86)90020-8. [DOI] [PubMed] [Google Scholar]
- 69.DeWane G., Salvi A.M., DeMali K.A. Fueling the cytoskeleton - links between cell metabolism and actin remodeling. J. Cell Sci. 2021;134:jcs248385. doi: 10.1242/jcs.248385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hu H., Juvekar A., Lyssiotis C.A., Lien E.C., Albeck J.G., Oh D., Varma G., Hung Y.P., Ullas S., Lauring J., et al. Phosphoinositide 3-kinase regulates glycolysis through mobilization of aldolase from the actin cytoskeleton. Cell. 2016;164:433–446. doi: 10.1016/j.cell.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park J.S., Burckhardt C.J., Lazcano R., Solis L.M., Isogai T., Li L., Chen C.S., Gao B., Minna J.D., Bachoo R., et al. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature. 2020;578:621–626. doi: 10.1038/s41586-020-1998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Novoseletskaya E., Grigorieva O., Nimiritsky P., Basalova N., Eremichev R., Milovskaya I., Kulebyakin K., Kulebyakina M., Rodionov S., Omelyanenko N., et al. Mesenchymal stromal cell-produced components of extracellular matrix potentiate multipotent stem cell response to differentiation stimuli. Front. Cell Dev. Biol. 2020;8:555378. doi: 10.3389/fcell.2020.555378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geiger B., Spatz J.P., Bershadsky A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 74.Kumar A., Placone J.K., Engler A.J. Understanding the extracellular forces that determine cell fate and maintenance. Development. 2017;144:4261–4270. doi: 10.1242/dev.158469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vining K.H., Mooney D.J. Mechanical forces direct stem cells behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017;18:728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moore S.W., Roca-Cusachs P., Sheetz M.P. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. Dev. Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Selig M., Lauer J.C., Hart M.L., Rolauffs B. Mechanotransduction and stiffness-sensing: mechanisms and opportunities to control multiple molecular aspects of cell phenotype as a design cornerstone of cell-instructive biomaterials for articular cartilage repair. Int. J. Mol. Sci. 2020;21:5399. doi: 10.3390/ijms21155399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang C., Dai J., Zhang X.A. Environmental physical cues determine the lineage specification of mesenchymal stem cells. Biochim. Biophys. Acta. 2015;1850:1261–1266. doi: 10.1016/j.bbagen.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang P., Mao Z., Gao C. Combinational effect of matrix elasticity and alendronate density on differentiation of rat mesenchymal stem cells. Acta Biomater. 2015;19:76–84. doi: 10.1016/j.actbio.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 80.Yang C., Tibbitt M.W., Basta L., Anseth K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peng T., Liu L., MacLean A.L., Wong C.W., Zhao W., Nie Q. A mathematical model of mechanotransduction reveals how mechanical memory regulates mesenchymal stem cell fate decisions. BMC Syst. Biol. 2017;11:55. doi: 10.1186/s12918-017-0429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roy B., Venkatachalapathy S., Ratna P., Wang Y., Jokhun D.S., Nagarajan M., Shivashankar G.V. Laterally confined growth of cells induces nuclear reprogramming in the absence of exogenous biochemical factors. Proc. Natl. Acad. Sci. USA. 2018;115:E4741–E4750. doi: 10.1073/pnas.1714770115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roy B., Yuan L., Lee Y., Bharti A., Mitra A., Shivashankar G.V. Fibroblast rejuvenation by mechanical reprogramming and redifferentiation. Proc. Natl. Acad. Sci. USA. 2020;117:10131–10141. doi: 10.1073/pnas.1911497117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martino F., Perestrelo A.R., Vinarský V., Pagliari S., Forte G. Cellular mechanotransduction: from tension to function. Front. Physiol. 2018;9:824. doi: 10.3389/fphys.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamanaka S. Pluripotent stem cell-based cell therapy-promise and challenges. Cell Stem Cell. 2020;27:523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 86.Liu L., Liu M., Xie D., Liu X., Yan H. Role of the extracellular matrix and YAP/TAZ in cell reprogramming. Differentiation. 2021;122:1–6. doi: 10.1016/j.diff.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 87.Lessard J., Pelletier M., Biertho L., Biron S., Marceau S., Hould F.S., Lebel S., Moustarah F., Lescelleur O., Marceau P., Tchernof A. Characterization of dedifferentiating human mature adipocytes from the visceral and subcutaneous fat compartments: fibroblast-activation protein alpha and dipeptidyl peptidase 4 as major components of matrix remodeling. PLoS One. 2015;10:e0122065. doi: 10.1371/journal.pone.0122065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jumabay M., Abdmaulen R., Ly A., Cubberly M.R., Shahmirian L.J., Heydarkhan-Hagvall S., Dumesic D.A., Yao Y., Boström K.I. Pluripotent stem cells derived from mouse and human white mature adipocytes. Stem Cells Transl. Med. 2014;3:161–171. doi: 10.5966/sctm.2013-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murata D., Yamasaki A., Matsuzaki S., Sunaga T., Fujiki M., Tokunaga S., Misumi K. Characteristics and multipotency of equine dedifferentiated fat cells. J. Equine Sci. 2016;27:57–65. doi: 10.1294/jes.27.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poloni A., Maurizi G., Babini L., Serrani F., Berardinelli E., Mancini S., Costantini B., Discepoli G., Leoni P. Human mesenchymal stem cells from chorionic villi and amniotic fluid are not susceptible to transformation after extensive in vitro expansion. Cell Transplant. 2011;20:643–654. doi: 10.3727/096368910X536518. [DOI] [PubMed] [Google Scholar]
- 91.Poloni A., Maurizi G., Leoni P., Serrani F., Mancini S., Frontini A., Zingaretti M.C., Siquini W., Sarzani R., Cinti S. Human dedifferentiated adipocytes show similar properties to bone marrow-derived mesenchymal stem cells. Stem Cell. 2012;30:965–974. doi: 10.1002/stem.1067. [DOI] [PubMed] [Google Scholar]
- 92.Seo C.H., Jeong H., Feng Y., Montagne K., Ushida T., Suzuki Y., Furukawa K.S. Micropit surfaces designed for accelerating osteogenic differentiation of murine mesenchymal stem cells via enhancing focal adhesion and actin polymerization. Biomaterials. 2014;35:2245–2252. doi: 10.1016/j.biomaterials.2013.11.089. [DOI] [PubMed] [Google Scholar]
- 93.Torres-Mejía E., Trümbach D., Kleeberger C., Dornseifer U., Orschmann T., Bäcker T., Brenke J.K., Hadian K., Wurst W., López-Schier H., et al. Sox2 controls Schwann cell self-organization through fibronectin fibrillogenesis. Sci. Rep. 2020;10:1984. doi: 10.1038/s41598-019-56877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doroudian G., Curtis M.W., Gang A., Russell B. Cyclic strain dominates over microtopography in regulating cytoskeletal and focal adhesion remodeling of human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2013;430:1040–1046. doi: 10.1016/j.bbrc.2012.11.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao J., Poss K.D. The epicardium as a hub for heart regeneration. Nat. Rev. Cardiol. 2018;15:631–647. doi: 10.1038/s41569-018-0046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang J.T., Rayburn H., Hynes R.O. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 97.Sun X., Malandraki-Miller S., Kennedy T., Bassat E., Klaourakis K., Zhao J., Gamen E., Vieira J.M., Tzahor E., Riley P.R. The extracellular matrix protein agrin is essential for epicardial epithelial-to-mesenchymal transition during heart development. Development. 2021;148:dev197525. doi: 10.1242/dev.197525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang S., Teng X., Toyama Y., Saunders T.E. Periodic oscillations of myosin-II mechanically proofread cell-cell connections to ensure robust formation of the cardiac vessel. Curr. Biol. 2020;30:3364–3377.e4. doi: 10.1016/j.cub.2020.06.041. [DOI] [PubMed] [Google Scholar]
- 99.Levayer R., Lecuit T. Oscillation and polarity of E-cadherin asymmetries control actomyosin flow patterns during morphogenesis. Dev. Cell. 2013;26:162–175. doi: 10.1016/j.devcel.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 100.Lin S.Z., Li B., Lan G., Feng X.Q. Activation and synchronization of the oscillatory morphodynamics in multicellular monolayer. Proc. Natl. Acad. Sci. USA. 2017;114:8157–8162. doi: 10.1073/pnas.1705492114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chan C.J., Costanzo M., Ruiz-Herrero T., Mönke G., Petrie R.J., Bergert M., Diz-Muñoz A., Mahadevan L., Hiiragi T. Hydraulic control of mammalian embryo size and cell fate. Nature. 2019;571:112–116. doi: 10.1038/s41586-019-1309-x. [DOI] [PubMed] [Google Scholar]
- 102.Rubin C., Turner A.S., Bain S., Mallinckrodt C., McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603–604. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 103.Rubin C., Xu G., Judex S. The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J. 2001;15:2225–2229. doi: 10.1096/fj.01-0166com. [DOI] [PubMed] [Google Scholar]
- 104.Ozcivici E., Luu Y.K., Adler B., Qin Y.X., Rubin J., Judex S., Rubin C.T. Mechanical signals as anabolic agents in bone. Nat. Rev. Rheumatol. 2010;6:50–59. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rubin C.T., Capilla E., Luu Y.K., Busa B., Crawford H., Nolan D.J., Mittal V., Rosen C.J., Pessin J.E., Judex S. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc. Natl. Acad. Sci. USA. 2007;104:17879–17884. doi: 10.1073/pnas.0708467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chan M.E., Adler B.J., Green D.E., Rubin C.T. Bone structure and B-cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals. FASEB J. 2012;26:4855–4863. doi: 10.1096/fj.12-209841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sen B., Styner M., Xie Z., Case N., Rubin C.T., Rubin J. Mechanical loading regulates NFATc1 and beta- catenin signaling through a GSK3beta control node. J. Biol. Chem. 2009;284:34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uzer G., Pongkitwitoon S., Ete Chan M., Judex S. Vibration induced osteogenic commitment of mesenchymal stem cells is enhanced by cytoskeletal remodeling but not fluid shear. J. Biomech. 2013;46:2296–2302. doi: 10.1016/j.jbiomech.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Uzer G., Thompson W.R., Sen B., Xie Z., Yen S.S., Miller S., Bas G., Styner M., Rubin C.T., Judex S., et al. Cell mechanosensitivity to extremely low-magnitude signals is enabled by a LINCed nucleus. Stem Cell. 2015;33:2063–2076. doi: 10.1002/stem.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang F.S., Yang K.D., Kuo Y.R., Wang C.J., Sheen-Chen S.M., Huang H.C., Chen Y.J. Temporal and spatial expression of bone morphogenetic proteins in extracorporeal shock wave-promoted healing of segmental defect. Bone. 2003;32:387–396. doi: 10.1016/s8756-3282(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 111.Wang C.J., Yang K.D., Ko J.Y., Huang C.C., Huang H.Y., Wang F.S. The effects of shockwave on bone healing and systemic concentrations of nitric oxide (NO), TGF-beta1, VEGF and BMP-2 in long bone non-unions. Nitric Oxide. 2009;20:298–303. doi: 10.1016/j.niox.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 112.Schaden W., Thiele R., Kölpl C., Pusch M., Nissan A., Attinger C.E., Maniscalco-Theberge M.E., Peoples G.E., Elster E.A., Stojadinovic A. Shock wave therapy for acute and chronic soft tissue wounds: a feasibility study. J. Surg. Res. 2007;143:1–12. doi: 10.1016/j.jss.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 113.Ottomann C., Hartmann B., Tyler J., Maier H., Thiele R., Schaden W., Stojadinovic A. Prospective randomized trial of accelerated re-epithelization of skin graft donor sites using extracorporeal shock wave therapy. J. Am. Coll. Surg. 2010;211:361–367. doi: 10.1016/j.jamcollsurg.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 114.Mittermayr R., Antonic V., Hartinger J., Kaufmann H., Redl H., Téot L., Stojadinovic A., Schaden W. Extracorporeal shock wave therapy (ESWT) for wound healing: technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012;20:456–465. doi: 10.1111/j.1524-475X.2012.00796.x. [DOI] [PubMed] [Google Scholar]
- 115.Aicher A., Heeschen C., Sasaki K.i., Urbich C., Zeiher A.M., Dimmeler S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114:2823–2830. doi: 10.1161/CIRCULATIONAHA.106.628623. [DOI] [PubMed] [Google Scholar]
- 116.Tepeköylü C., Lobenwein D., Urbschat A., Graber M., Pechriggl E.J., Fritsch H., Paulus P., Grimm M., Holfeld J. Shock wave treatment after hindlimb ischaemia results in increased perfusion and M2 macrophage presence. J. Tissue Eng. Regen. Med. 2018;12:e486–e494. doi: 10.1002/term.2317. [DOI] [PubMed] [Google Scholar]
- 117.Nishida T., Shimokawa H., Oi K., Tatewaki H., Uwatoku T., Abe K., Matsumoto Y., Kajihara N., Eto M., Matsuda T., et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004;110:3055–3061. doi: 10.1161/01.CIR.0000148849.51177.97. [DOI] [PubMed] [Google Scholar]
- 118.Holfeld J., Zimpfer D., Albrecht-Schgoer K., Stojadinovic A., Paulus P., Dumfarth J., Thomas A., Lobenwein D., Tepeköylü C., Rosenhek R., et al. Epicardial shock-wave therapy improves ventricular function in a porcine model of ischaemic heart disease. J. Tissue Eng. Regen. Med. 2016;10:1057–1064. doi: 10.1161/JAHA.118.010025. [DOI] [PubMed] [Google Scholar]
- 119.Tepeköylü C., Primessnig U., Pölzl L., Graber M., Lobenwein D., Nägele F., Kirchmair E., Pechriggl E., Grimm M., Holfeld J. Shockwaves prevent from heart failure after acute myocardial ischaemia via RNA/protein complexes. J. Cell Mol. Med. 2017;21:791–801. doi: 10.1111/jcmm.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holfeld J., Tepeköylü C., Reissig C., Lobenwein D., Scheller B., Kirchmair E., Kozaryn R., Albrecht-Schgoer K., Krapf C., Zins K., et al. Toll-like receptor 3 signalling mediates angiogenic response upon shock wave treatment of ischaemic muscle. Cardiovasc. Res. 2016;109:331–343. doi: 10.1093/cvr/cvv272. [DOI] [PubMed] [Google Scholar]
- 121.Gollmann-Tepeköylü C., Lobenwein D., Theurl M., Primessnig U., Lener D., Kirchmair E., Mathes W., Graber M., Pölzl L., An A., et al. Shock wave therapy improves cardiac function in a model of chronic ischemic heart failure: evidence for a mechanism involving VEGF signaling and the extracellular matrix. J. Am. Heart Assoc. 2018;7:e010025. doi: 10.1161/JAHA.118.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fukumoto Y., Ito A., Uwatoku T., Matoba T., Kishi T., Tanaka H., Takeshita A., Sunagawa K., Shimokawa H. Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coron. Artery Dis. 2006;17:63–70. doi: 10.1097/00019501-200602000-00011. [DOI] [PubMed] [Google Scholar]
- 123.Kikuchi Y., Ito K., Ito Y., Shiroto T., Tsuburaya R., Aizawa K., Hao K., Fukumoto Y., Takahashi J., Takeda M., et al. Double-blind and placebo-controlled study of the effectiveness and safety of extracorporeal cardiac shock wave therapy for severe angina pectoris. Circ. J. 2010;74:589–591. doi: 10.1253/circj.cj-09-1028. [DOI] [PubMed] [Google Scholar]
- 124.Kagaya Y., Ito K., Takahashi J., Matsumoto Y., Shiroto T., Tsuburaya R., Kikuchi Y., Hao K., Nishimiya K., Shindo T., et al. Low-energy cardiac shockwave therapy to suppress left ventricular remodeling in patients with acute myocardial infarction: a first-in-human study. Coron. Artery Dis. 2018;29:294–300. doi: 10.1097/MCA.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 125.Barton G.M., Kagan J.C., Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self-DNA but facilitates access to viral DNA. Nat. Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 126.Ganguly D., Chamilos G., Lande R., Gregorio J., Meller S., Facchinetti V., Homey B., Barrat F.J., Zal T., Gilliet M. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moon J.J., Matsumoto M., Patel S., Lee L., Guan J.L., Li S. Role of cell surface heparan sulfate proteoglycans in endothelial cell migration and mechanotransduction. J. Cell. Physiol. 2005;203:166–176. doi: 10.1002/jcp.20220. [DOI] [PubMed] [Google Scholar]
- 128.Ding Y.X., Cheng Y., Sun Q.M., Zhang Y.Y., You K., Guo Y.L., Han D., Geng L. Mechanical characterization of cervical squamous carcinoma cells by atomic force microscopy at nanoscale. Med. Oncol. 2015;32:71. doi: 10.1007/s12032-015-0507-0. [DOI] [PubMed] [Google Scholar]
- 129.Gimzewski J.K., Pelling A., Ventura C. International Patent WO; 2008. Nanomechanical Characterization of Cellular Activity. [Google Scholar]
- 130.Yu W., Lu Q.-Y., Sharma S., Ly C., Di Carlo D., Rowat A.C., LeClaire M., Kim D., Chow C., Gimzewski J.K., Rao J. Single cell mechanotype and associated molecular changes in urothelial cell transformation and progression. Front. Cell Dev. Biol. 2020;8:601376. doi: 10.3389/fcell.2020.601376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fu X., Liu P., Zhao D., Yuan B., Xiao Z., Zhou Y., Yang X., Zhu X., Tu C., Zhang X. Effects of nanotopography regulation and silicon doping on angiogenic and osteogenic activities of hydroxyapatite coating on titanium implant. Int. J. Nanomedicine. 2020;15:4171–4189. doi: 10.2147/IJN.S252936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Möllmert S., Kharlamova M.A., Hoche T., Taubenberger A.V., Abuhattum S., Kuscha V., Kurth T., Brand M., Guck J. Zebrafish spinal cord repair is accompanied by transient tissue stiffening. Biophys. J. 2020;118:448–463. doi: 10.1016/j.bpj.2019.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]