Abstract
Background
Chronic cough is a common troublesome condition and accounts for a high burden on quality of life. Previous data investigating the mortality associated with chronic cough has been derived in patients with chronic bronchitis. No data exists on chronic dry cough. Therefore, we investigated if chronic dry and productive cough is independently associated with increased mortality.
Methods
The Canadian Longitudinal Study on Ageing (CLSA) is a prospective, nationally generalizable, stratified random sample of adults aged 45–85 years at baseline recruited between 2011–2015 and followed up three years later. Chronic cough was identified based on a self-reported daily cough in the last 12 months. Deaths were confirmed by the Ministry of Health and/or completion of descendent questionnaire by a family member. Models were investigated for dry and productive chronic cough and was adjusted for age, sex, smoking, body mass index (BMI), and respiratory diseases.
Results
Of the 30,016 participants, 4,783 (15.9%) reported chronic cough at baseline; 2,724 (57%) had a dry cough, and 2,059 (43%) had productive chronic cough. There was a total of 561 deaths between baseline and follow-up-1 (3 years later). There was a 49% higher risk of death in participants with chronic productive cough {adjusted odds ratio (aOR) 1.49 [95% confidence intervals (CI): 1.08–2.07]}, but not dry chronic cough [aOR 0.85 (0.60–1.20)]. The effects of chronic productive cough on mortality were persistent in those with no airflow obstruction [chronic productive cough aOR 1.90 (1.09–3.31)].
Conclusions
Chronic productive cough is associated with a higher risk of death, while chronic dry cough has no impact on mortality risk of death in middle-aged and older adults. This highlights the importance of careful evaluation of patients with chronic cough.
Keywords: Chronic cough, mortality, epidemiology, Canadian Longitudinal Study on Ageing (CLSA)
Highlight box.
Key findings
• There was a 49% higher risk of death in participants with chronic productive cough but not dry chronic cough. The effects of chronic productive cough on mortality were persistent in those with no airflow obstruction.
What is known and what is new?
• Previous data on mortality on chronic cough was extrapolated from data collected based on the Medical Research Council definition of chronic bronchitis. This new data examines the impact of both dry and productive chronic cough on mortality after adjusting for age, sex, smoking, body mass index, asthma and chronic obstructive pulmonary disease.
What is the implication, and what should change now?
• This highlights the importance of careful evaluation of the type of cough in patients with chronic cough.
Introduction
Cough is the leading cause for ambulatory and primary care visits to physicians (1,2). Chronic cough is defined as a daily cough lasting greater than eight weeks affecting approximately 10% of the general population and is associated with significant impairment in quality of life (3-9). Chronic cough is also one of the most common reasons for referral to a specialist in secondary care, representing a significant burden on the health care system (10,11). Although these are predominantly dry chronic cough, approximately 25% have a productive chronic cough (12). Primary and secondary care physicians may consider this a benign condition with no substantial risk of death, but there is no data investigating the risk of death in patients with dry and productive chronic cough separately.
Prior to the introduction of effective anti-microbial therapy, deaths due to acute, sub-acute and chronic cough with sputum was commonly associated with pulmonary tuberculosis, pneumonia and other respiratory tract infections (13,14). However, after the world-wars, there was an increase in deaths following the mechanisation of coal mines (15,16). In December 1952, there was also a sharp increase in deaths in London (The Great Smog of London) with men dying with cough and sputum (17). This led the Medical Research Council (MRC) in the UK to setup the Bronchitis Research Committee led by Sir Charles Fletcher. This ultimately led to the MRC Chronic Bronchitis definition of cough which is productive sputum on most days for three months in two consecutive years (18). Often forgotten is that the definition also required exclusion of other conditions associated with cough such as tuberculosis, bronchiectasis, pneumoconiosis and pulmonary oedema.
A number of cohort studies showed chronic bronchitis was associated with increased mortality in men, smokers, chronic obstructive pulmonary disease (COPD) and in those with lower lung function (19-26). However, these earlier studies had limitations as the focus was on the inclusion of participants with COPD, smokers, and use of occupational cohorts. Secondly, the definition of chronic bronchitis definition was typically synonymous with productive coughing during the winter months. Therefore, there is still an unmet need to understand the impact of chronic cough, both productive and dry, on mortality in a broad contemporary population from the general community beyond the 3-month seasonal definition.
The objective of this study was to estimate the impact of daily productive and dry chronic cough on the risk of death in a national sample of adults from the Canadian Longitudinal Study on Ageing (CLSA) who were between the ages of 45 and 85 years at baseline and followed up three years later. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1306/rc).
Methods
Study design and population
The CLSA is a large, nationally generalizable, stratified random sample of 51,338 Canadian men and women aged 45 to 85 years at baseline (recruited 2011–2015) from the 10 Canadian provinces (27). Eligible participants had to be physically and have cognitive capacity to participate independently and not live in institutions such as long-term care facilities. Participants were recruited in the tracking cohort (n=21,241) and the comprehensive cohort (n=30,097). Tracking cohort participants were randomly selected from the 10 provinces and completed interviews by phone. Participants in the comprehensive cohort were randomly selected from within 25–50 km of 11 data collection sites located in seven provinces (n=30,097). Participants in the comprehensive cohort completed in-depth questionnaires in person and physical assessments. Details on the study design have been described elsewhere (28). Each participant is followed every three years for 20 years or until death. The first follow-up was conducted between 2015 and 2018 with a retention rate of 95%. The comprehensive data from baseline and first follow-up were included in the current analyses. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Hamilton Integrated Research Ethics Board and the CLSA scientific advisory board (Project ID: 1909024) and individual consent for this retrospective analysis was waived.
Chronic cough definitions
Participants who self-identified as having “daily cough over the last 12 months” were classified as having chronic cough at baseline. Chronic cough was further classified as a productive cough if participants reported “bringing up phlegm in the morning or most days during the year”. Participants who reported chronic cough withing bringing up phlegm were categorized as having a dry cough.
Respiratory symptoms and chronic conditions
Self-reported presence of respiratory symptoms, including chest pain, shortness of breath upon exertion, and wheezing, were assessed. Participants were asked, “Do you wheeze with mild to moderate exertion? Do you become short of breath climbing stairs or walking up a small hill?” Disease definitions were based on self-reported physician diagnosis on direct questioning of participants by trained research assistants at baseline and follow-up. Participants were asked if they had ever been diagnosed with asthma, COPD or both by a physician. A history of infectious diseases such as pneumonia or influenza in the last 12 months was also surveyed.
Spirometry measurements
Lung function was measured with the TruFlow Easy-On Air Spirometer (ndd Medical Technologies, Switzerland) and categorized based on the American Thoracic Society requirements and criteria. Participants who screened positive for major contra-indications were excluded (21). The highest forced expiratory volume in 1 second (FEV1) and FVC from 3 acceptable maximal efforts were selected. Only grades A and B were accepted for analysis. The FEV1, FVC, and the ratio of FEV1/FVC was recorded without bronchodilator therapy.
Chronic Airflow Obstruction (CAO) was defined as an FEV1/FVC ratio of <0.7 as well as using the lower limit of normal (LLN). Age, height, and sex were used to develop CLSA specific prediction reference values for this total population. These were based on standard allometric principles, FEV1 and FVC increase in a positively accelerating manner with height (y = k × HeightK1). There was a proportionate increase in males relative to females at the same height (y = k × HeightK1 × (1 + K2 × Males(1))); and decreasing by a constant proportion with age (y = k × HeightK1 × (1 + K2 × Males(1) × (1 − K3 × (Age)))). Grade A and B spirometry data were available in 22,547 participants.
Mortality events
Deaths were confirmed by the Ministry of Health, Canada, in participants recruited at baseline but did not attend the follow-up 1 visit three years later. The exact date and cause of death were not made available by the Ministry of Health to maintain anonymity. Information about deaths were also collected from family members who completed a descendent questionnaire. Participants who had not died and did not attend for follow-up 1 were assumed to be alive.
Statistical analysis
The CLSA provides inflation weights and analytical weights, which were used for prevalence estimates and regression modelling respectively, that allow the results to reflect the population of Canada (28). As the exact date of death and withdrawal are missing, Logistic Regression (LR) models were used to estimate the odds ratios (OR) and 95% confidence intervals (95% CI) for the outcome of mortality. Potential confounding covariates associated with chronic cough and mortality were identified from prior literature describing associations, clinical relevance and mechanistic plausibility (19,29). The univariate association between each variable and mortality was assessed; a set of pre-defined variables were considered candidates for the model. Age and sex were automatically included in the model, and other potential covariates were added one at a time based on statistical significance. A model including age group (45–54, 55–64, 65–74, 75+ years), sex, smoking status (non-smoker, former smoker, and current smoker), body mass index (BMI) category (underweight, <18.5 kg/m2; normal weight, 18.5 to 24.9 kg/m2; overweight, 25.0–29.9 kg/m2; and obese >30 kg/m2). The presence of respiratory diseases including asthma, COPD, influenza and pneumonia in the past 12 months were included in the model. The model was performed for dry and productive chronic cough.
Variables for which the deviance statistic was statistically significant (P value of <0.05) or those which impacted the strength of the association between chronic cough and mortality were kept in the model (30). Finally, the mutually adjusted model is presented along with 95% CI are shown.
As chronic cough and mortality are known to be associated with lower lung function, we also conducted sub-group stratified analyses in participants with or without airflow obstruction (FEV1/FVC <0.7), and when the FEV1% predicted is below or above 80% predicted. All statistical analyses were completed using SAS (Version 12.3).
Results
Study population
The comprehensive cohort included 30,097 participants. A total of 30,016 completed the chronic cough question at baseline. There were 967 participants who withdrew from the study after the baseline visit, and in 804 participants, there was missing data at the first follow-up who were assumed to be alive. Of the remaining, 4,783 (15.9%) participants reported chronic cough at baseline; 2,724 (57%) had a dry cough, and 2,059 (43%) had a productive chronic cough. There was a total of 561 deaths between baseline and follow-up 1, which were confirmed by the Ministry of Health or by a family member completing the descendent questionnaire.
Baseline characteristics
The proportion of participants who died was highest in those with chronic productive cough (3.27%), with identical proportions with chronic dry cough (1.22%) and no chronic cough (1.22%) (Table 1). Participants with wheeze and shortness of breath on exertion, self-reported physician diagnosis of COPD, pneumonia or influenza in the last 12 months experienced a higher proportion of deaths (Table 2). The proportion of deaths was higher in those with productive chronic cough across all respiratory symptoms and diseases compared to those with dry chronic cough; however, the proportion of deaths in those with chronic dry cough was similar or lower compared with those without chronic cough.
Table 1. Baseline demographics of participants who died.
| Variables | Subtypes | Deaths: no chronic cough | Deaths: dry chronic cough | Deaths: productive chronic cough | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Weighted % | N | Weighted % | N | Weighted % | ||||
| Deaths | 419 | 1.22 | 55 | 1.22 | 85 | 3.27 | |||
| Age category (years) | 45–54 | 22 | 0.37 | 3 | 0.68 | 5 | 2.62 | ||
| 55–64 | 69 | 0.92 | 6 | 0.45 | 17 | 2.08 | |||
| 65–74 | 102 | 1.66 | 19 | 1.88 | 28 | 4.34 | |||
| 75–85 | 226 | 4.84 | 27 | 3.69 | 35 | 5.44 | |||
| Sex | Male | 245 | 1.37 | 39 | 1.47 | 55 | 3.89 | ||
| Female | 174 | 1.07 | 16 | 0.98 | 30 | 2.41 | |||
| Body mass index | Under-weight | 10 | 3.64 | 2 | 6.30 | 4 | 11.44 | ||
| Normal | 121 | 1.25 | 13 | 0.80 | 15 | 1.99 | |||
| Overweight | 153 | 1.01 | 17 | 1.42 | 30 | 3.15 | |||
| Obese | 130 | 1.34 | 20 | 1.09 | 34 | 3.93 | |||
| Smoking status | Current | 49 | 2.25 | 8 | 0.96 | 27 | 3.81 | ||
| Previous | 203 | 1.51 | 27 | 1.66 | 39 | 3.70 | |||
| Never | 164 | 0.90 | 19 | 1.04 | 19 | 2.37 | |||
Demographics shown in those with no chronic cough, dry chronic cough and productive. Numbers and weighted % shown. Missing data for BMI (n=5) and smoking status (n=3).
Table 2. Respiratory co-morbidities of participants who died.
| Variables | Subtypes | Deaths: no chronic cough | Deaths: dry chronic cough | Deaths: productive chronic cough | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Weighted % | N | Weighted % | N | Weighted % | ||||
| Wheeze | No | 343 | 1.16 | 40 | 1.39 | 40 | 2.50 | ||
| Yes | 75 | 1.57 | 15 | 0.86 | 45 | 4.13 | |||
| Shortness of breath up hill | No | 398 | 1.15 | 53 | 1.25 | 70 | 3.01 | ||
| Yes | 21 | 3.87 | 2 | 1.01 | 15 | 5.66 | |||
| Chest pain | No | 277 | 1.13 | 34 | 1.21 | 42 | 2.19 | ||
| Yes | 75 | 0.92 | 10 | 0.95 | 23 | 4.02 | |||
| Asthma only | 30 | 0.90 | 3 | 0.91 | 7 | 2.79 | |||
| COPD only | 35 | 4.13 | 4 | 2.38 | 24 | 7.77 | |||
| Asthma and COPD | 15 | 3.87 | 5 | 2.46 | 6 | 2.50 | |||
| No asthma or COPD | 335 | 1.14 | 43 | 1.18 | 47 | 2.75 | |||
| Pneumonia | No | 394 | 1.16 | 51 | 1.23 | 73 | 3.17 | ||
| Yes | 24 | 3.63 | 4 | 1.40 | 12 | 4.75 | |||
| Influenza | No | 388 | 1.20 | 52 | 1.29 | 73 | 3.11 | ||
| Yes | 29 | 2.41 | 3 | 0.49 | 12 | 4.66 | |||
| FEV1/FVC | <0.7 | 52 | 2.47 | 10 | 2.05 | 13 | 2.18 | ||
| ≥0.7 | 179 | 2.54 | 19 | 0.63 | 26 | 2.54 | |||
| FEV1 % predicted | <80% | 65 | 2.60 | 13 | 1.74 | 19 | 3.81 | ||
| ≥80% | 166 | 2.66 | 16 | 0.61 | 20 | 1.98 | |||
Data shown in those with no chronic cough, dry chronic cough and productive. COPD, obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Mortality
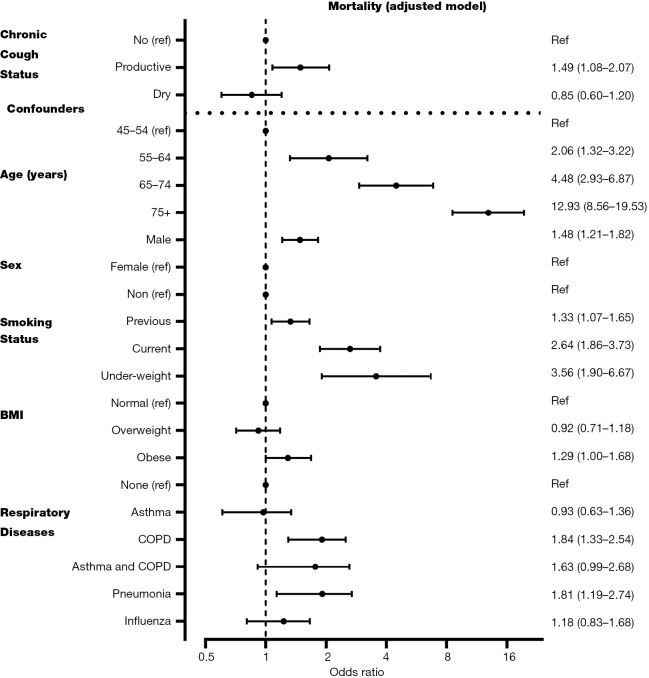
Chronic productive cough was independently associated with a 49% higher risk of death [adjusted OR (aOR) 1.49 (95% CI: 1.08–2.07)], Figure 1], after adjusting for age, sex, smoking status, BMI, asthma, COPD, prior pneumonia and influenza. Chronic dry cough was not associated with a higher risk of death [aOR 0.85 (0.60–1.20), Figure 1] in the fully adjusted model.
Figure 1.
Mortality model for dry and productive cough. Data shown as estimated mean odds ratio and 95% CI and adjusted for age, sex, BMI, smoking status, respiratory diseases (asthma, COPD, influenza in the past 12 months, pneumonia in the past 12 months). BMI, body mass index; COPD, obstructive pulmonary disease.
Impaired lung function
The impact of chronic cough on mortality was stratified based on normal or low FEV1% predicted and the presence of airflow obstruction (FEV1/FVC <0.7). Mortality was also higher in participants with productive chronic cough and no airflow obstruction (FEV1/FVC ≥0.7) compared with chronic dry cough [productive chronic cough OR 1.90 (1.09–3.31) vs. dry chronic cough adj OR 0.72 (0.41–1.28), Table 3]. There were no significant effects of chronic productive or dry chronic cough in patients with an FEV1 <80% or ≥80% predicted (Table 3).
Table 3. Fully adjusted mortality model stratified by FEV1% predicted and airflow obstruction.
| Chronic cough status | FEV1 <80% pred (n=3,516) | FEV1 ≥80% pred (n=19,303) | FEV1/FVC <0.7 (n=2,660) | FEV1/FVC ≥0.7 (n=20,162) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| No chronic cough | Ref | Ref | Ref | Ref | |||||||
| Dry chronic cough | 0.80 | (0.40–1.63) | 0.72 | (0.39–1.34) | 1.00 | (0.47–2.13) | 0.72 | (0.41–1.28) | |||
| Productive chronic cough | 1.14 | (0.58–2.21) | 1.72 | (0.92–3.22) | 0.85 | (0.41–1.77) | 1.90 | (1.09–3.31) | |||
Data shown as estimated mean odds ratio and 95% CI and adjusted for age, sex, BMI, smoking status, respiratory diseases (asthma, COPD, influenza in the past 12 months, pneumonia in the past 12 months). Number of participants for each category showed in brackets. FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; OR, odds ratio; CI, confidence interval; BMI, body mass index; COPD, obstructive pulmonary disease.
Discussion
To our knowledge, this is the first study to investigate the risk of death associated with dry and productive chronic cough in the general community over a 3-year period. Chronic productive cough was associated with an estimated 49% higher risk of death whilst chronic dry cough had no impact on the risk of death. Importantly, these associations were independent of other important risk factors and potential confounders including age, sex, smoking, low BMI, COPD, pneumonia and influenza. The effects of chronic productive cough on mortality persisted in those with no airflow obstruction.
The results of this study are difficult to directly compare with other studies because no studies have investigated chronic dry cough. Most studies have focused on chronic bronchitis, chronic mucus hypersecretion or coughing up phlegm in winter mornings. The most recent National Heart Lung and Blood Institute (NHLBI) pooled study of 9 cohorts of 22,235 recruited between 1971–2007 and followed-up for nearly 40 years, showed that non-obstructive chronic bronchitis was associated with a 50% increase in all-cause mortality in ever-smokers, but not in never smokers (31). A Danish study of over 100,000 randomly selected individuals from the community with a median follow-up of 9 years, showed a similar 34% increased risk of death in those presenting with chronic mucus hypersecretion, after adjusting for potential confounders (29). However, over 10,000 subjects with pre-existing asthma and COPD were excluded. The Tucson study recruited 1,412 subjects with normal spirometry between 1972–1973 and, after following for 30 years, also demonstrated a 31% increased risk of death with chronic bronchitis (32). Similar hazard ratios of death with chronic productive cough ranging between 1.23 to 1.56 have been reported in older cohort studies from Norway, UK, Poland, Oregon, and Paris (19,33-37). Whilst the estimated 49% increased risk of death with chronic productive cough in this study is consistent with previous data, it is also slightly disappointing. One might have hoped and expected an improvement in the risk of death over the last 40–50 years in developed countries. This raises broader questions about the need to provide better access and quality of health care, availability, compliance with medication, diagnosing and monitoring diseases, and primary and secondary prevention programmes which target high risk individuals at the population level.
Data from our mutually adjusted model (Figure 1) also reveals broader public health insights. The biggest modifiable risk factor for death is smoking established and propagated by Doll et al. (26). Our data shows that current smokers have a OR of 2.64, but for previous smokers the OR is 1.33, more than a 50% reduction. This underlines the importance of smoking cessation and public health measures to reduce its uptake. The banning of smoking in public spaces in Canada occurred between 1994 and 2006 with different regulations and timing across the provinces. However, all provinces had a public ban initiated before recruitment of the CLSA started. The long-term beneficial effects of this public smoking ban on chronic cough and mortality in the general community may take longer to detect, particularly as the CLSA recruited patients over the age of 45 who may have started or been exposed to smoking much earlier. The benefits of the public smoking ban also need to be balanced with the legalisation of vaping and cannabis in Canada since 2018. This requires further study in the context of the CLSA, which aims to follow patients for 20 years or death.
These findings also have important clinical implications for healthcare providers investigating and managing patients with chronic cough. It is important to differentiate productive compared to chronic dry cough. The presence of daily mucus and phlegm should alert the physician to a potentially more serious underlying disorder such as bronchiectasis, uncontrolled asthma, COPD, unresolved pneumonia, or recurrent lower respiratory tract infections due to immunodeficiency. This study also underlines the importance of the American College of Chest Physicians (ACCP) and European Respiratory Society (ERS) guidelines recommending performing a chest X-ray and spirometry in all patients with a chronic cough greater than eight weeks (3,38,39). In secondary care, physicians may consider more detailed testing such as a high-resolution CT (HRCT) scan of the chest, sputum cytology and culture to quantify eosinophils and neutrophils and rule out bacterial, fungal or viral infections. This stratification of productive and dry chronic cough is important for triaging referrals and developing more individualised management pathways for productive and dry chronic cough.
Clinical trials of novel anti-tussives typically require a normal chest X-ray, the absence of airflow obstruction and any serious cardio-pulmonary diseases. Two large 52-week phase 3 studies of gefapixant, a peripherally acting, non-opioid, P2X3 antagonist, demonstrated 45 mg BID reduced objective cough frequency by 15% and 18% over placebo (40). However, most studies on refractory and unexplained chronic cough have yet to provide a detailed clinical description of whether the cough was productive or dry or a sub-group analysis to ascertain if treatment is equally effective in both types of chronic cough. Current sub-group analysis suggests gefapixant is equally effective in refractory and unexplained chronic cough. However, further classification of productive (and, if so, volume) and chronic dry cough would be helpful to clinicians.
There are limitations to this study. First, the CLSA recruited an older population over the age of 45, as the main focus of the study was on aging; hence mortality rates may be higher. Second, acceptable and reproducible pre-bronchodilator spirometry was available only in 22,547 subjects at baseline, and we were unable to confirm airway reversibility or post-bronchodilator airflow obstruction. Third, diagnoses were based on self-reported physician diagnosis, thus possibly resulting in under or over-diagnosis. Fourth, there was missing data in 804 participants who were not seen at follow-up 1, and death could not be confirmed with the Ministry of Health, who were assumed to be alive. Fifth, we do not have data on the exact cause of death and are unable to differentiate between respiratory and non-respiratory mortality due to chronic cough. Sixth, we were unable to estimate conventional hazard ratios as the exact date of death was unknown.
Conclusions
Chronic productive chronic cough was associated with a higher risk of death after adjusting for potential confounding factors such as age, sex, smoking, BMI and respiratory co-morbidities. This highlights the importance of carefully evaluating, investigating, and monitoring patients presenting with chronic cough.
Supplementary
The article’s supplementary files as
Acknowledgments
This research was made possible using the data/biospecimens collected by the Canadian Longitudinal Study on Aging (CLSA). Funding for the Canadian Longitudinal Study on Aging (CLSA) is provided by the Government of Canada through the Canadian Institutes of Health Research (CIHR) under grant reference: LSA 94473 and the Canada Foundation for Innovation as well as the following provinces, Newfoundland, Nova Scotia, Quebec, Ontario, Manitoba, Alberta, and British Columbia. This research has been conducted using the CLSA dataset, Baseline and Follow-up 1 Comprehensive Dataset, under Application Number 1909024. The CLSA is led by Drs. Parminder Raina, Christina Wolfson and Susan Kirkland. The opinions expressed in this manuscript are the author’s own and do not reflect the views of the Canadian Longitudinal Study on Aging. The final manuscript was reviewed and approved by the Publication Review Committee of the Canadian Longitudinal Study for Ageing (CLSA).
Funding: The study was funded by Merck Canada. I.S. is currently supported by the E.J. Moran Campbell Early Career Award, Department of Medicine, McMaster University. The study sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Hamilton Integrated Research Ethics Board and the CLSA scientific advisory board (Project ID: 1909024) and individual consent for this retrospective analysis was waived.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editors (Woo-Jung Song and Kian Fan Chung) for the series “Novel Insights Into Chronic Cough” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1306/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1306/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1306/coif). The series “Novel Insights into Chronic Cough” was commissioned by the editorial office without any funding or sponsorship. IS reports grants and personal fees from Merck Canada and GSK, grants from Bayer and Bellus, personal fees from Respiplus, Genentech, and AstraZeneca, outside the submitted work; PMOB reports grants and personal fees from AstraZeneca, personal fees from GSK, grants from Novartis, grants and personal fees from Medimmune, personal fees from Chiesi, outside the submitted work. The other authors have no conflicts of interest to declare.
References
- 1.Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician 2018;64:832-40. [PMC free article] [PubMed] [Google Scholar]
- 2.Burt CW, Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments; United States, 1999-2000: data from the National Health Care Survey; 2004. [PubMed]
- 3.Morice AH, Millqvist E, Bieksiene K, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J 2020;55:1901136. 10.1183/13993003.01136-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irwin RS, French CL, Chang AB, et al. Classification of Cough as a Symptom in Adults and Management Algorithms: CHEST Guideline and Expert Panel Report. Chest 2018;153:196-209. 10.1016/j.chest.2017.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French CL, Irwin RS, Curley FJ, et al. Impact of chronic cough on quality of life. Arch Intern Med 1998;158:1657-61. 10.1001/archinte.158.15.1657 [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain SA, Garrod R, Douiri A, et al. The impact of chronic cough: a cross-sectional European survey. Lung 2015;193:401-8. 10.1007/s00408-015-9701-2 [DOI] [PubMed] [Google Scholar]
- 7.Satia I, Mayhew AJ, Sohel N, et al. Prevalence, incidence and characteristics of chronic cough among adults from the Canadian Longitudinal Study on Aging. ERJ Open Res 2021;7:e00160-2021. 10.1183/23120541.00160-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satia I, Mayhew AJ, Sohel N, et al. Language and geographical location influence the incidence of chronic cough in the Canadian Longitudinal Study on Aging. ERJ Open Res 2022;8:e00721-2021. 10.1183/23120541.00721-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015;45:1479-81. 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 10.Irwin RS, Curley FJ, French CL. Chronic cough. The spectrum and frequency of causes, key components of the diagnostic evaluation, and outcome of specific therapy. Am Rev Respir Dis 1990;141:640-7. 10.1164/ajrccm/141.3.640 [DOI] [PubMed] [Google Scholar]
- 11.Zeiger RS, Schatz M, Butler RK, et al. Burden of Specialist-Diagnosed Chronic Cough in Adults. J Allergy Clin Immunol Pract 2020;8:1645-1657.e7. 10.1016/j.jaip.2020.01.054 [DOI] [PubMed] [Google Scholar]
- 12.King J, Digby JW, Hennessey S, et al. Productive Cough, a Forgotten Phenotype of Refractory Chronic Cough. Eur Respiratory Soc 2021;58:PA1941. [DOI] [PubMed]
- 13.GRIGG ER. The arcana of tuberculosis with a brief epidemiologic history of the disease in the U.S.A. Am Rev Tuberc 1958;78:151-72 contd. [DOI] [PubMed]
- 14.Potter CW. A history of influenza. J Appl Microbiol 2001;91:572-9. 10.1046/j.1365-2672.2001.01492.x [DOI] [PubMed] [Google Scholar]
- 15.Stewart A. Pneumoconiosis of coal-miners; a study of the disease after exposure to dust has ceased. Br J Ind Med 1948;5:120-40. 10.1136/oem.5.3.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cotes JE. The Medical Research Council Pneumoconiosis Research Unit, 1945-1985: a short history and tribute. Occup Med (Lond) 2000;50:440-9. 10.1093/occmed/50.6.440 [DOI] [PubMed] [Google Scholar]
- 17.LOGAN WP . Mortality in the London fog incident, 1952. Lancet 1953;1:336-8. 10.1016/s0140-6736(53)91012-5 [DOI] [PubMed] [Google Scholar]
- 18.Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet 1965;1:775-9. [PubMed] [Google Scholar]
- 19.Fortis S, Shannon ZK, Garcia CJ, et al. Association of Nonobstructive Chronic Bronchitis With All-Cause Mortality: A Systematic Literature Review and Meta-analysis. Chest 2022;162:92-100. 10.1016/j.chest.2022.02.003 [DOI] [PubMed] [Google Scholar]
- 20.Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J 1995;8:1333-8. 10.1183/09031936.95.08081333 [DOI] [PubMed] [Google Scholar]
- 21.Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med 2002;166:329-32. 10.1164/rccm.2112048 [DOI] [PubMed] [Google Scholar]
- 22.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996;153:1530-5. 10.1164/ajrccm.153.5.8630597 [DOI] [PubMed] [Google Scholar]
- 23.Peto R, Speizer FE, Cochrane AL, et al. The relevance in adults of air-flow obstruction, but not of mucus hypersecretion, to mortality from chronic lung disease. Results from 20 years of prospective observation. Am Rev Respir Dis 1983;128:491-500. 10.1164/arrd.1983.128.3.491 [DOI] [PubMed] [Google Scholar]
- 24.Wiles FJ, Hnizdo E. Relevance of airflow obstruction and mucus hypersecretion to mortality. Respir Med 1991;85:27-35. 10.1016/s0954-6111(06)80207-6 [DOI] [PubMed] [Google Scholar]
- 25.Fletcher C, Peto R, Tinker C, et al. The natural history of chronic bronchitis and emphysema. An eight-year study of early chronic obstructive lung disease in working men in London. 1976:272.
- 26.Doll R, Peto R, Boreham J, et al. Mortality from cancer in relation to smoking: 50 years observations on British doctors. Br J Cancer 2005;92:426-9. 10.1038/sj.bjc.6602359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raina PS, Wolfson C, Kirkland SA, et al. The Canadian longitudinal study on aging (CLSA). Can J Aging 2009;28:221-9. 10.1017/S0714980809990055 [DOI] [PubMed] [Google Scholar]
- 28.Raina P, Wolfson C, Kirkland S, et al. Cohort Profile: The Canadian Longitudinal Study on Aging (CLSA). Int J Epidemiol 2019;48:1752-1753j. 10.1093/ije/dyz173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Çolak Y, Nordestgaard BG, Vestbo J, et al. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur Respir J 2019;54:1900734. 10.1183/13993003.00734-2019 [DOI] [PubMed] [Google Scholar]
- 30.David W, Hosmer SL. Applied Logistic Regression. Second ed: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 31.Balte PP, Chaves PHM, Couper DJ, et al. Association of Nonobstructive Chronic Bronchitis With Respiratory Health Outcomes in Adults. JAMA Intern Med 2020;180:676-86. 10.1001/jamainternmed.2020.0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerra S, Sherrill DL, Venker C, et al. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax 2009;64:894-900. 10.1136/thx.2008.110619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putcha N, Drummond MB, Connett JE, et al. Chronic productive cough is associated with death in smokers with early COPD. COPD 2014;11:451-8. 10.3109/15412555.2013.837870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hewitt J, Smeeth L, Bulpitt CJ, et al. Respiratory symptoms in older people and their association with mortality. Thorax 2005;60:331-4. 10.1136/thx.2004.029579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krzyzanowski M, Wysocki M. The relation of thirteen-year mortality to ventilatory impairment and other respiratory symptoms: the Cracow Study. Int J Epidemiol 1986;15:56-64. 10.1093/ije/15.1.56 [DOI] [PubMed] [Google Scholar]
- 36.Annesi I, Kauffmann F. Is Respiratory Mucus Hypersecretion Really an Innocent Disorder? A 22−Year Mortality Survey of 1,061 Working Men. American Review of Respiratory Disease 1986;134:688-93. 10.1164/arrd.1986.134.4.688 [DOI] [PubMed] [Google Scholar]
- 37.Pelkonen M, Notkola IL, Nissinen A, et al. Thirty-year cumulative incidence of chronic bronchitis and COPD in relation to 30-year pulmonary function and 40-year mortality: a follow-up in middle-aged rural men. Chest 2006;130:1129-37. 10.1378/chest.130.4.1129 [DOI] [PubMed] [Google Scholar]
- 38.Satia I, Wahab M, Kum E, et al. Chronic cough: Investigations, management, current and future treatments. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine 2021;5:404-16. [Google Scholar]
- 39.Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006;129:1S-23S. 10.1378/chest.129.1_suppl.1S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGarvey LP, Birring SS, Morice AH, et al. Efficacy and safety of gefapixant, a P2X(3) receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet 2022;399:909-23. 10.1016/S0140-6736(21)02348-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as