Abstract
Background
Globally, the respiratory syncytial virus (RSV) is the most common etiologic agent of acute respiratory illnesses in children. However, its burden has not been well addressed in developing countries. We aimed to estimate the molecular epidemiology of RSV in children less than 18 years of age with acute respiratory infections in Africa by conducting a systematic review and meta-analysis.
Methods
We systematically searched PubMed, Scopus, CINAHL, and Global Index Medicus databases to identify studies published from January 1, 2002, to April 27, 2022, following the PRISMA 2020 guideline. We assessed the study quality using the Joanna Brigg’s Institute (JBI) critical appraisal checklists. We conducted a qualitative synthesis by describing the characteristics of included studies and performed the quantitative synthesis with random effects model using STATA-14. We checked for heterogeneity with Q statistics, quantified by I2, and determined the prediction interval. We performed subgroup analyses to explain the sources of heterogeneity and assessed publication biases by funnel plots augmented with Egger’s test.
Results
Eighty-eight studies with 105 139 participants were included in the review. The overall pooled prevalence of RSV in children <18 years of age was 23% (95% confidence interval (CI) = 20, 25%). Considerable heterogeneity was present across the included studies. The adjusted prediction interval was found to be 19%-27%. Heterogeneities were explained by subgroups analyses. The highest prevalence of RSV was found among inpatients, 28% (95% CI = 25, 31%) compared with inpatients/outpatients and outpatients, with statistically significant differences (P < 0.01). The RSV estimate was also highest among those with acute lower respiratory tract illnesses (ALRTIs), 28% (95% CI = 25, 31%) compared with acute upper respiratory tract illnesses (AURTIs) and both acute upper/lower respiratory manifestations, with statistically different prevalence (P < 0.01). RSV infection estimates in each sub-region of Africa were statistically different (P < 0.01). There were no statistically significant differences in RSV infections by designs, specimen types, and specimen conditions, despite them contributing to heterogeneity.
Conclusions
We found a high prevalence of RSV in pediatric populations with acute respiratory tract illnesses in Africa, highlighting that the prevention and control of RSV infections in children deserve more attention.
Registration
PROSPERO CRD42022327054
Acute respiratory tract infections caused by a range of respiratory viruses are linked to a spectrum of upper and lower respiratory tract syndromes [1,2]. The burden of acute respiratory infections is increasing as novel respiratory pathogens are being discovered by molecular methods [3]. Consequently, over 200 different virus types have been found to cause acute respiratory infections, with the respiratory syncytial virus (RSV), influenza viruses, human metapneumovirus, parainfluenza viruses, adenoviruses, rhinoviruses, coronaviruses, and bocaviruses being the most prevalent [4].
RSV is a single-stranded ribonucleic acid (RNA) virus affecting respiratory epithelial cells; it has two subtypes, RSV-A and RSV-B, with antigenic differences. These differences are found in the attachment glycoprotein G, and the RSV G protein shows the highest degree of divergence, both between and within the two groups [5,6].
RSV is one of the most common etiologic agents of acute respiratory infections causing severe disease and mortality, particularly in very young children, but also in other age groups and in at-risk groups [7]. Globally, the virus has led to 33 million episodes of acute lower respiratory tract infection, 3.6 million hospital admissions, 26 300 in-hospital deaths, and 101 400 RSV-attributable overall deaths in children younger than five years [8]. RSV is also the most prevalent cause of severe lower respiratory tract infection in the first six months of life. Most deaths occur in low- and middle-income countries [8,9]. Ninety-nine percent (99%) of these deaths occurred in developing countries, though the actual mortality rate due to RSV infection is higher than reported [10,11].
Currently, ribavirin is the only one licensed antiviral medication for treating RSV infection; it has a very limited efficacy and high toxicity. Its use is usually reserved for severely immunocompromised children. To date, only the maternal antibody palivizumab is available for preventing RSV infection; it has been shown to reduce hospital admission due to RSV infection in some high-risk infants. There are several RSV vaccines under development or undergoing clinical trials in humans. Understanding the disease burden caused by RSV will facilitate the study of the effectiveness of antivirals and vaccines [12].
Generally, approaches for detecting RSV have not been widely accessible [13] and its exact burden in different geographical regions has not been fully determined, despite evidence that RSV was the most widely detected virus in children [14]. Lack of data on disease burden is especially problematic in developing countries with high numbers of severe cases and deaths, as the true burden of the disease can only be estimated with adequate epidemiological data on RSV infections in different settings [15]. For these reasons, we conducted a systematic review and meta-analysis on molecular epidemiology of RSV in children under 18 years of age with acute respiratory infections in Africa.
METHODS
Databases and search strategies
We registered the review protocol in the International Prospective Register of Systematic Reviews (PROSPERO, registration number CRD42022327054). We comprehensively searched PubMed, Scopus, CINAHL, and Global Index Medicus (GIM) databases with keywords, medical subject headings (MeSH), and related terms (full search strategy is available in Appendix S1 in the Online Supplementary Document). We also searched for gray literature through Mednar, worldwide science, and Grey Literature Report. Additionally, we searched articles from reference lists of included studies and related systematic reviews and meta-analyses through Google scholar and African Journal Online (AJOL).
Inclusion and exclusion criteria
We screened titles and abstracts and evaluate full texts for eligibility based on pre-defined inclusion and exclusion criteria.
Inclusion criteria
We included observational studies (including cross-sectional, case-control, and cohort studies) and studies addressing the molecular epidemiology of respiratory syncytial virus in children less than 18 years of age reporting RSV positive cases and/or RSV subgroups from Africa. Children less than 18 years of age with clinical diagnosis of acute respiratory tract infections (acute upper respiratory tract infection and/or acute lower respiratory tract infections) were included, while only considering reverse transcriptase-polymerase chain reaction (RT-PCR) diagnosed/confirmed RSV cases (including rapid molecular tests, multiplex molecular tests, and conventional PCR of respiratory specimens). We included only studies conducted in Africa and published in the English language between January 01, 2002, and April 27, 2022.
Exclusion criteria
We excluded original studies performed outside of Africa, review papers, books, letters, brief report, case reports, meeting reports, poster presentations, and studies published before January 01, 2002, during the screening of titles and abstracts, while articles with irretrievable full texts, records with unrelated outcome measures, or articles with missing outcomes were excluded during full text screening. We excluded RSV cases identified through viral culture, rapid antigen detection tests, or direct immunofluorescent antibody tests and studies of asymptomatic RSV infections.
Study selection and quality assessment
We imported all retrieved studies to Endnote X9 and deduplicated, after which two reviewers (BTR and LAG) independently screened all their titles and abstracts for eligibility according to inclusion and exclusion criteria. Discrepancies were discussed and until a consensus was reach and the full text was accessed if necessary. Then, the two reviewers assessed the full texts of the remaining articles for eligibility. After this step, two investigators assessed the study quality independently using the Joanna Brigg’s Institute (JBI) critical appraisal checklist adapted for observational studies (checklist for cross-sectional studies, case-control studies, and cohort studies containing 8, 10 and 11 items, respectively). If discrepancies in the rating occurred, the two reviewers discussed and resolved the issues. If the issue was not resolved through discussion, the third reviewer’s decision was accepted. Accordingly, studies with the number of positive responses (yes) greater than or equal to half (≥50%) of the number of checklist items relevant for the specific study were included in the systematic review and meta-analysis.
Data extraction and management
Data extractions were performed on excel spreadsheet containing the following items: study first author and publication year, study population, number of cases positive for RSV, sample size, RSV subgroups, clinical manifestations (upper respiratory illness, and/or lower respiratory illness), patient categories (inpatients, outpatients or both), study design, sampling method, specimen type, specimen condition (stored or fresh), study setting (rural, urban or both), country, and sub-region of Africa (Table S1 in the Online Supplementary Document). Extractions were done by two independent reviewers. Where results were published multiple times, the data was used only once. Uncertainties during the extraction process were resolved by joint discussion between the reviewers.
Strategy for data synthesis
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines [16]. We used a flow diagram to illustrate the literature search and article selection processes and a table to provide an overview of the included articles’ characteristics, which we described using a qualitative synthesis performed with the STATA software (Version 14.0, StataCorp, Texas, USA). A random effects model was also applied. We checked for heterogeneity across studies and performed subgroup analyses for age groups, patient categories, clinical manifestations, sub-regions of Africa, study designs, specimen types as well as specimen conditions to identify the sources of heterogeneity. Heterogeneity was evaluated by the χ2 test on Cochrane’s Q statistic, which was quantified by I2 values. The I2 statistic estimates the percentage of total variation across studies due to true between-study differences rather than chance. We used comprehensive meta-analysis (CMA) prediction intervals program (Biostat Inc., New Jersey, USA) to determine the adjusted prediction interval to indicate how much the effect sizes vary in 95% of all comparable populations. By omitting one study at a time using STATA software, we conducted a sensitivity analysis to check the stability of summary estimate. We assessed publication biases by visually inspecting funnel plots, augmented with statistical testing using the Egger’s test.
RESULTS
Review processes and findings
We retrieved a total of 7595 articles from the databases (PubMed = 1767, Scopus = 5053, CINAHL = 626, Global Index Medicus = 149), limiting our search to the period from January 1, 2002, to April 27, 2022. After deduplication (n = 1646) using Endnote, 5949 records remained. We then screened the titles and abstracts and removed 5816 irrelevant studies. Of the 133 full texts retrieved and assessed for eligibility, we excluded 58 and retained 75 as relevant. Additionally, records were sought from other sources such as free web search engines (African Journal Online and Google Scholar) and Grey Literature, from which we retrieved 65 full text records, 52 of which were not eligible and 13 were found retained and included. At the end, 88 articles were found to be relevant for this systematic review and meta-analysis (Figure 1).
Figure 1.
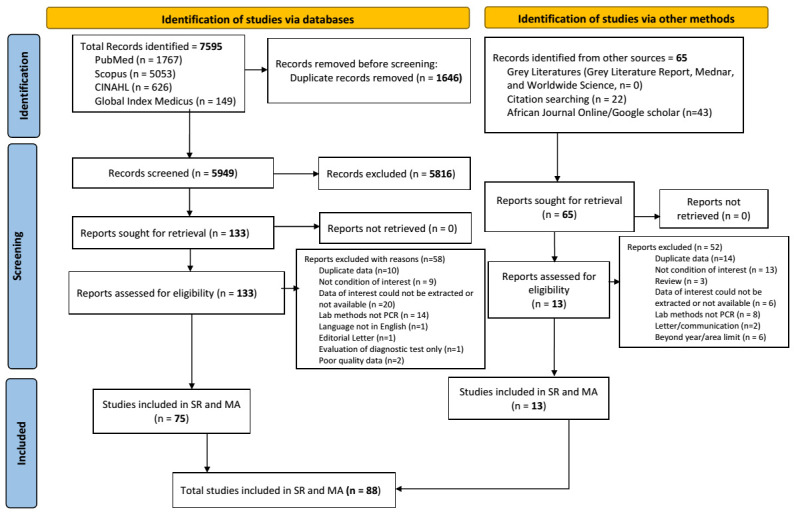
PRISMA 2020 flow diagram for literature search and selection.
Characteristics of the included studies
Eighty-eight studies with 105 139 study participants from 25 African countries were included in this systematic review and meta-analysis. JBI quality assessment tools were applied and all included studies satisfied the required level of methodological quality. The minimum and maximum sample sizes utilized were 51 [17] and 9969 [18], respectively. Considering the number of studies included from sub-regions of Africa, 24 reports were from Northern, 23 from Eastern, 18 from Southern, 16 from Western, and six from Middle Africa, while one study presented data from three African sub-regions (Table 1).
Table 1.
Studies included in SR and MA by country and sub-regions of Africa
| African sub-regions | Countries | Number of studies included |
|---|---|---|
| Northern Africa | Algeria |
1 |
| Egypt |
17 |
|
| Morocco |
3 |
|
| Sudan |
1 |
|
| Tunisia |
2 |
|
|
|
Total |
24 |
| Eastern Africa | Kenya |
12 |
| Madagascar |
5 |
|
| Malawi |
1 |
|
| Mozambique |
2 |
|
| Tanzania |
1 |
|
| Zambia |
2 |
|
|
|
Total |
23 |
| Southern Africa | South Africa |
17 |
| Botswana |
1 |
|
|
|
Total |
18 |
| Western Africa | Burkina Faso |
1 |
| Côte d'Ivoire |
2 |
|
| Gambia |
2 |
|
| Ghana |
3 |
|
| Mali |
2 |
|
| Niger |
2 |
|
| Nigeria |
2 |
|
| Senegal |
2 |
|
|
|
Total |
16 |
| Middle Africa | Cameroon |
2 |
| Central African Republic |
2 |
|
| DR Congo |
1 |
|
| Gabon |
1 |
|
|
|
Total |
6 |
| *Ghana, Gabon, Tanzania, Burkina Faso | 1 |
*Research reported from multi-countries of different sub-regions.
Referring to the specific countries, 17 studies were reported from Egypt, 17 from South Africa, 12 from Kenya, five from Madagascar, three from Ghana, and three from Morocco. The remaining 22 studies were reported from the 11 other countries, with two studies from each. Eight studies were reported from eight countries, with one study reported from each, while one study was reported from four African countries (Table 1).
Most included studies (n = 73 (82.95%)) used a cross-sectional study design, while the rest were case-control (n = 11 (12.50%)) and cohort studies (n = 4, (4.55%)). Most studies used consecutive sampling methods with prospective time courses. Regarding the study population, two thirds of the included studies (n = 60 (68.2%)) were done on under five children or presented accessible data of the under five children; the remaining 28 studies were performed on children less than 18 years of age or presented data for the group. Approximately one third of the included studies (35.23%) described the study settings described as rural, urban, or both. Studies indicated that single types or combined respiratory specimens were taken where nasopharyngeal swabs were most utilized. All the respiratory specimens in the included studies were analyzed by reverse transcriptase PCR. All the studies described the conditions of specimens analyzed as fresh or stored, except for three studies which did not describe the specimen condition. Most included studies (n = 48 (54.55%)) had inpatients as participants. Regarding the participants’ clinical manifestations, half of the studies dealt with acute lower respiratory tract illnesses (ALRTI), while 39 (44.32%) of the studies dealt with participants with both acute upper respiratory tract illnesses (AURTI) and ALRTI. The included studies’ profiles are available in Table 2.
Table 2.
Profile of studies included in the systematic review and meta-analysis
| Study profile | Articles included (n (%)) | |
|---|---|---|
| Study design | Cross-sectional |
73 (82.95) |
| Case-control |
11 (12.50) |
|
|
|
Cohort |
4 (4.55) |
| Sampling method | Consecutive |
80 (90.91) |
| Random |
4 (4.55) |
|
|
|
Systematic |
4 (4.55) |
| Time course of study | Prospective |
76 (86.36) |
| Retrospective |
10 (11.36) |
|
|
|
Both |
2 (2.27) |
| Study population | <5 y |
60 (68.18) |
|
|
<18 y |
28 (31.82) |
| Specimen type | NPS |
29 (32.95) |
| NPA |
18 (20.45) |
|
| NS |
6 (6.82) |
|
| Mixed* |
28 (31.82) |
|
| Others† |
5 (5.68) |
|
|
|
Not described |
2 (2.27) |
| Specimen condition | Fresh |
43 (48.86) |
| Stored |
42 (47.73) |
|
|
|
Not described |
3 (3.41) |
| Study setting | Rural |
12 (13.64) |
| Urban |
3 (3.41) |
|
| Rural and urban |
16 (18.18) |
|
|
|
Not described |
57 (64.77) |
| Patient categories | Inpatients |
48 (54.55) |
| Inpatients and outpatients |
26 (29.55) |
|
| Outpatients |
10 (11.36) |
|
|
|
Not described |
4 (4.55) |
| Clinical manifestations | ALRTI |
44 (50.00) |
| AURTI |
5 (5.68) |
|
| AURTI/ALRTI | 39 (44.32) |
NPS – nasopharyngeal swab, NPA – nasopharyngeal aspirate, NS – nasal swab, ALRTI – acute lower respiratory tract infections, AURTI – acute upper respiratory tract infections, y – years
*Nasal wash/NPS, or NS/NPS, or NPS/OPS, or NPS/NPA, or NPS/NPA/bronchoalveolar lavage.
†Bronchoalveolar lavage, oropharyngeal swab, throat swab.
Proportions of RSV infections
The proportion of RSV infections in the studies ranged from 0.5 to 86%; the minimum was reported from the study conducted in Nigeria [19] while the highest infection proportion, 86%, was reported from Egypt [20]. The proportion of RSV for most studies (n = 28 (31.5%)) was 21%-30% [18,21-47], followed by 24 (27.3%) studies with the proportion of 11%-20% [14,17,48-69], 17 (19.3%) with 31%-50% [70-86], and 17 (19.3%) with 0.5%-10% [19,87-102]. The remaining two studies reported the highest proportions of infections, 85.3% [103] and 86% [20] (Table S1 in the Online Supplementary Document).
Referring to RSV sub-grouping, 25 studies reported the subgroups of RSV (Table S1 in the Online Supplementary Document). From the total 4095 RSV samples that underwent sub-grouping, RSV-A was identified in 2074 cases (51%), RSV-B was identified in 1812 (44%) and RSV-A and B co-infections were identified in 209 cases (5%).
The pooled prevalence of RSV
The point estimates of RSV infections among children from the individual studies ranged from 0 (95% confidence interval (CI) = 0, 3%) to 86% (95% CI = 76, 92%). The overall pooled prevalence of RSV in children under 18 years of age was 23% (95% CI = 20, 25%). Considerable heterogeneity was present for the overall combined effect size (Q = 8633.54 (degrees of freedom (df) = 87), P < 0.01; I2 = 98.99% and Τ2 = 0.01) (Figure 2). The adjusted prediction interval was found to be from 19% to 27%.
Figure 2.
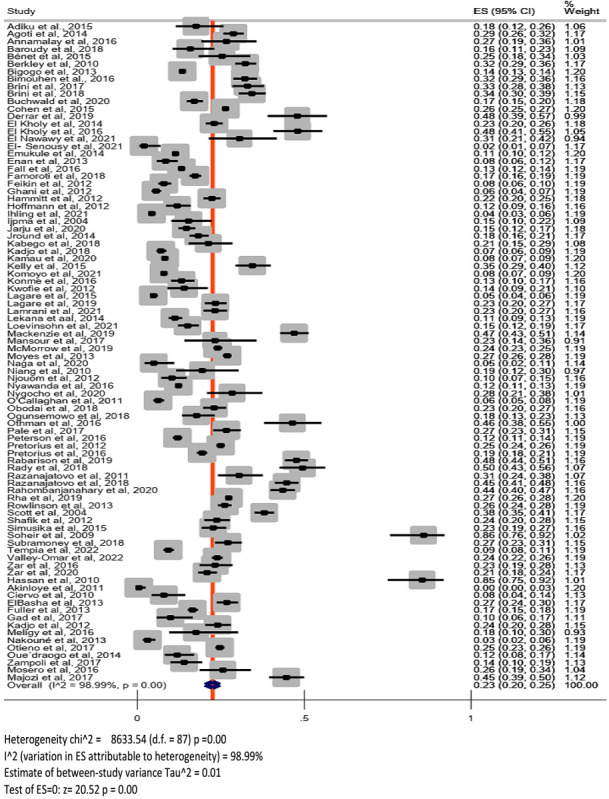
Forest plot.
By visually inspecting the forest plots, we found that some studies were outliers (Figure 2). Checking for the distribution of the proportions of included studies, we found that it was skewed to the right. Using a graph box, we identified two studies [20,104] as outliers. After removing them, we performed the meta-analysis and computed the pooled prevalence of RSV infections of 21% (95% CI = 19, 23%) with considerable heterogeneity (Q = 8083.69 (df = 85), P < 0.01; I2 = 98.95%; T2 = 0.01). The prediction interval was also found to range from 17% to 25%. We found no major difference when comparing this with the combined effect and heterogeneity statistics of the previous analysis (including all the 88 studies). We thus retained all 88 studies in the analysis.
Subgroup analysis
The results of the subgroup analyses are presented in Table 3. We found substantial heterogeneity among the individual effect sizes of the included studies, so we performed subgroups analyses to explore the potential sources of heterogeneity among them. We found that the differences in age groups, clinical manifestations, patient categories, and sub-regions of Africa most likely contributed to the heterogeneity between individual studies, as did specimen type, and specimen condition.
Table 3.
Subgroup analyses results for the positive rate of RSV infection in children
| Heterogeneity tests |
||||||||
|---|---|---|---|---|---|---|---|---|
|
Groups
|
Number of studies
|
Number of RSV positive
|
Number of total participants
|
RSV positive rate (95% CI)
|
Q-value
|
P
h
|
I2 (%)
|
P-difference
|
|
Overall
|
88 |
21 178 |
105 139 |
23% (20, 25%) |
8633.54 |
<0.01 |
98.99 |
- |
| Subgroup analyses |
|
|
|
|
|
|
|
|
|
Age groups
|
|
|
|
|
|
|
|
<0.001 |
| <5 y |
60 |
17 154 |
77 219 |
25% (23, 28%) |
5098.51 |
<0.01 |
98.84 |
|
| <18 y |
28 |
4024 |
27 920 |
17% (13, 20%) |
1917.58 |
<0.01 |
98.59 |
|
|
Patient category
|
|
|
|
|
|
|
|
<0.001 |
| Inpatients |
48 |
14 086 |
57 453 |
28% (25, 31%) |
3834.98 |
<0.01 |
98.77 |
|
| Inpatients and outpatients |
26 |
5544 |
33 505 |
18% (14, 21%) |
163.72 |
<0.01 |
98.95 |
|
| Outpatients |
10 |
1401 |
12 239 |
14% (11, 17%) |
2381.55 |
<0.01 |
94.50 |
|
| Not described |
4 |
147 |
1942 |
7% (4, 10%) |
19.97 |
<0.01 |
84.98 |
|
|
Clinical manifestations
|
|
|
|
|
|
|
|
<0.001 |
| ALRTI |
44 |
13 578 |
53 795 |
28% (25, 31%) |
3452.63 |
<0.01 |
98.75 |
|
| AURTI |
5 |
333 |
1828 |
19% (13, 25%) |
41.71 |
<0.01 |
90.41 |
|
| AURTI/ALRTI |
39 |
7267 |
49 516 |
17% (15, 19%) |
2687.58 |
<0.01 |
98.59 |
|
|
Sub-regions of Africa*
|
|
|
|
|
|
|
|
<0.001 |
| Northern |
24 |
2313 |
8605 |
30% (24, 36%) |
1131.84 |
<0.01 |
97.97 |
|
| Southern |
18 |
10 273 |
42 178 |
23% (19, 26%) |
1212.75 |
<0.01 |
98.60 |
|
| Eastern |
24 |
6363 |
36 858 |
22% (18, 26%) |
2267.36 |
<0.01 |
98.99 |
|
| Western |
17 |
1696 |
11 420 |
17% (12, 21%) |
1053.22 |
<0.01 |
98.48 |
|
| Middle |
7 |
533 |
6078 |
9% (6, 12%) |
89.24 |
<0.01 |
93.28 |
|
|
Design
|
|
|
|
|
|
|
|
0.944 |
| Cross-sectional |
73 |
18 328 |
89 353 |
22% (20, 25%) |
8213.42 |
<0.01 |
99.12 |
|
| Case-control |
11 |
2401 |
13 641 |
23% (19, 27%) |
340.87 |
<0.01 |
97.07 |
|
| Cohort |
4 |
449 |
2145 |
22% (16, 28%) |
36.35 |
<0.01 |
91.75 |
|
|
Specimen type
|
|
|
|
|
|
|
|
0.625 |
| NPS |
29 |
5877 |
30 921 |
22% (18, 26%) |
3059.09 |
<0.01 |
99.08 |
|
| NPA |
18 |
6482 |
28 914 |
23% (18, 28%) |
1625.36 |
<0.01 |
98.95 |
|
| NS |
6 |
565 |
2544 |
16% (5, 28%) |
668.64 |
<0.01 |
99.25 |
|
| Mixed† |
28 |
7606 |
40 054 |
24% (21, 27%) |
1814.32 |
<0.01 |
98.51 |
|
| Others‡ |
5 |
340 |
1450 |
17% (4, 29%) |
170.32 |
<0.01 |
97.65 |
|
| Not described |
2 |
308 |
1256 |
22% (20, 24%) |
- |
- |
- |
|
|
Specimen condition
|
|
|
|
|
|
|
|
0.437 |
| Fresh |
43 |
11 443 |
57 859 |
21% (19, 24%) |
3870.40 |
<0.01 |
98.91 |
|
| Stored |
42 |
9038 |
44 087 |
23% (20, 27%) |
4580.07 |
<0.01 |
99.10 |
|
| Not described | 3 | 697 | 3193 | 28% (17, 39%) | - | - | ||
CI – confidence interval, NPS – nasopharyngeal swab, NPA – nasopharyngeal aspirate, NS – nasal swab, ALRTI – acute lower respiratory tract infections, AURTI – acute upper respiratory tract infections
*One study reported data from three sub-regions.
†Nasal wash/NPS, or NS/NPS, or NPS/OPS, or NPS/NPA, or NPS/NPA/bronchoalveolar lavage.
‡Bronchoalveolar lavage, oropharyngeal swab, throat swab.
Among the 88 studies included in the meta-analysis, 60 reported the prevalence of RSV in children under five years of age, while 28 reported the prevalence of RSV in children under 18 years age. The pooled estimates of RSV were found to be 25% (95% CI = 23, 28%) in the age group of 0-5-year-old age group and 17% (95% CI = 13, 20%) in the 0-18-year-old age group. Looking at the heterogeneity summary reported in each group, the heterogeneities were not reduced (Q = 5098.51 with df = 59, P < 0.01, I2 = 98.84% for children under five years, and Q = 1917.58 with df = 27, P < 0.01, I2 = 98.59% for children under 18 years of age). The test of group differences (Q = 18.60, (df = 1), P < 0.01) indicated that the group-specific overall effect sizes were statistically different.
Considering patients’ category, the prevalence of RSV was stratified into “inpatients”, “outpatients”, “inpatients/outpatients”, and “not described” categories. Looking at the heterogeneity summary reported in each group, the heterogeneities were not reduced as compared with the result of overall estimate. The highest prevalence of RSV was found among inpatients (28%; 95% CI = 25, 31%), followed by inpatients/outpatients (18%; 95% CI = 14, 21%), outpatients (14%; 95% CI = 11, 17%), and the “not described” category (7%; 95% CI = 4, 10%). The test of group differences showed that prevalence specific to patient categories were statistically different (Q = 87.90 (df = 3), P < 0.01).
Stratifying patients according to their respiratory syndromes (AURTI, ALRTI, and both), the highest estimate of RSV was reported among those with lower respiratory tract illnesses, 28% (95% CI = 25, 31%), with statistically different pooled prevalence between the groups (Q = 29.90 (df = 2), P < 0.01). We found considerable heterogeneity in each group.
The pooled prevalence of RSV was also determined by sub-regions of Africa. The highest prevalence of RSV was from Northern Africa (30%; 95% CI = 24, 36%), Southern Africa, (23%; 95% CI = 19, 26%), and Eastern Africa (22%; 95% CI = 18, 26%). The prevalence in Western and Middle Africa were 17% (95% CI = 12, 21%) and 9% (95% CI = 6, 12%), respectively. The overall estimates in each sub-region were statistically different (Q = 57.07 with df = 4, P < 0.01). Heterogeneity statistics also showed variations among sub-regions.
Subgroup analyses by study design, specimen type, and specimen condition also indicated that they contributed to the heterogeneity between the individual studies. However, there were no statistically significant differences in the rates of RSV infections by designs (P = 0.944), specimen types (P = 0.625), and specimen conditions (P = 0.437).
Sensitivity analysis and publication bias
Performing the sensitivity analysis, the overall results noticeably changed after each individual study was omitted, and the combined effect was found to be 31.11 (95% CI = -4.83, 67.05%). For the overall meta-analysis of the pooled prevalence of RSV among children with acute respiratory illnesses, the funnel plot revealed evidence of publication bias (Figure 3). Egger’s linear regression test was performed to test for publication bias, which was detected among the publications that reported RSV positive rates for all patients (Intercept, Bo = 8.76; 95% CI = 1.48, 16.03, standard error (SE) = 3.66, P < 0.001).
Figure 3.
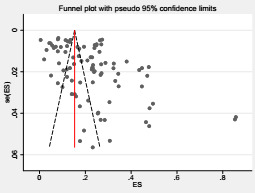
Funnel plot.
DISCUSSION
RSV is the most common viral cause of ARTIs in developed and developing countries [11,104]. The available epidemiological data on RSV in Africa has not been systematically summarized among the pediatric population; it requires data on the epidemiology of RSV infection in different settings to determine the disease’s true burden. We thus conducted a systematic review and meta-analysis in children under 18 years of age in Africa. After rigorous screening, 88 articles, including 105 139 patients, were deemed eligible and included in the systematic review and meta-analysis.
We found that the distribution of RSV was not uniform in the continent. Its proportion varied from 0.5 to 86% across countries. This was consistent with the previous review from Africa, which was done on all age groups [105]. This might indicate that RSV was associated with a significant burden of ARTIs in the pediatric population in Africa. The overall pooled prevalence of RSV in the current review was 23% (95% CI = 20, 25%). This result was higher than the report of the previous meta-analysis from Africa, which found the prevalence to be 14.6% (95% CI = 13.0, 16.4) [105]. Both studies had similar the methods of detecting RSV (reverse transcriptase PCR). The variation might be due to the study population, as the previous study included participants of all ages, and our study only included children. The overall positive rate of RSV infection among children from China (16.0%; 95% CI = 12.9, 19.6%) [106] was lower than the pooled prevalence in our meta-analysis. The possible reasons for the variations between the results could be geographical differences and detection methods – the study from China used both PCR and immunofluorescence assays (IFA), while we only included studies using PCR as a detection method. RSV is identified less frequently by immunofluorescent detection techniques. Compared to immunofluorescence, molecular diagnostics are more sensitive and specific [107,108]. There is evidence that the introduction of nucleic acid-based diagnostic tests has markedly improved the understanding of viral etiology among ARTI patients [109]. Therefore, the real burden of RSV in China would likely be comparable to our findings if molecular technique had been used in all included studies, though the geographical variations should also be considered.
We also found considerable heterogeneity in this meta-analysis. The Q test statistic was 8633.54 (df = 87) with P < 0.01, from which we found considerable variations of effect sizes between the individual studies. The I2 heterogeneity statistic also indicates the presence of heterogeneity between studies, highlighting that about 98.99% of the variability in the effect-size estimates came from the real differences between studies. This result was consistent with reports from Africa [105] and China [106] and supported by the global and national evidence that the burden of RSV varied substantially from year to year in any given population and setting [11,110]. In the current meta-analysis, the pooled prevalence was 23% (95% CI = 20, 25%). Since the T2 value was greater than zero (T2 = 0.01), the prediction interval had to be determined to indicate how much the effect sizes vary in the population of interest. We found that the adjusted prediction interval ranged from 19% to 27%, suggesting that the true effect size in 95% of all comparable populations falls in this interval.
We conducted subgroup analyses based on the age groups, clinical manifestations, patient categories, and sub-regions, to explore the potential sources of heterogeneity between the individual studies and found that these factors were the sources for the variations observed in the effect sizes.
Additionally, the subgroup analyses by study design, specimen type, and specimen condition indicated that they contributed to the heterogeneity between the individual studies. Similarly, subgroup analyses in previous studies also identified sources of heterogeneity; they were explained by age group, patient category, and sample types [106] and within all groups [105]. These variations might suggest that the burden of respiratory syncytial viral infections differs between studies from country to country, region to region, even within a county, due to several factors such as socio-demographic characteristics, geographical differences, climatic variations, and cultural differences. The heterogeneity could also result from clinical conditions of participants, methodological differences, or both.
We found the prevalence of RSV to be highest among inpatients (28%; 95% CI = 25, 31%), followed by inpatients/outpatients (18%; 95% CI = 14, 21%) and outpatients (14%; 95% CI = 11, 17%), with statistically significant differences between the groups (P < 0.001). This result was consistent with the results of other meta-analyses [111,112]; a higher prevalence of RSV was seen in inpatients than in outpatients. In contrast, no significant difference was reported from patient categories (inpatients and in/outpatients) in the meta-analysis from China [106]. In both developed and developing countries, RSV infections are a major cause of hospitalizations and in-hospital deaths among children [113,114]. RSV has been associated with 12%-63% of all acute respiratory infections (ARIs) and 19%-81% of viral ARIs causing hospitalization in infants and children [15]. These all indicate that RSV is the most common cause of ARIs and a major cause of hospital admissions in children, resulting in a substantial burden on health care services. There was also evidence to put the burden of RSV into this context. Compared to influenza, retrospective analyses showed that RSV causes up to 16 times more hospitalizations and emergency department visits in children aged <5 years [115-117].
We performed sensitivity analyses, and the overall results were noticeably changed after each individual study was omitted (31.11%; 95% CI = -4.83, 67.05%); this might indicate the instability of the meta-analysis results.
Funnel plot is a plot that measures the study size (usually standard error or precision) on the vertical axis as a function of effect size on the horizontal axis. In the absence of publication bias, we would expect the studies to be distributed symmetrically about the combined effect size. In the presence of bias, we would expect a higher concentration of studies on one side of the combined effect size. In this study, the funnel plot showed evidence of publication bias for the pooled prevalence of RSV in children less than 18 years of age with ARIs. Furthermore, we performed Egger’s linear regression test to check for publication bias, which was detected among the publications that reported RSV positive rates for all patients (Intercept, Bo = 8.76; 95% CI = 1.48, 16.03; SE = 3.66, P < 0.001). This result might show that the missing studies differ systematically from the observed studies. However, the funnel plot asymmetry may be caused by factors other than publication bias, such as a presence of a moderator correlated with the study effect and study size or, more generally, the presence of substantial between-study heterogeneity.
Our review indicated that less than one third of the included studies (n = 25 (28.4%)) performed subgroupings of RSV. From a total of 4095 RSV samples that underwent subgrouping, 51% were RSV type A, 44% were type B, and the remaining 5% were RSV type A and B co-infections. The analysis of nucleotide and amino acid sequences in detected RSV help with characterizing the pathogen’s genetic variability, tracing of its evolution, and clarification of the mechanisms required for immune escape [4]. The clinical impact of viral factors during RSV infection is still controversial, as there are conflicting reports regarding the associations of different groups and genotypes with severity of infection [118]. Regarding the relationship between virulence and RSV strains, evidence shows that specific genotypes of subtype A are related to increased illness severity [119-121]. Linking specific RSV strains to human disease severity with confidence is challenging and no consistent picture of virulent strains has yet emerged [118]. Determination of circulating RSV subgroups and genotypes in different countries is important in terms of the development of effective vaccine.
This review has some limitations. One of the concerns is that we found substantial heterogeneity in estimating the prevalence of RSV infection in children with ARI across the included studies. Consequently, some sources of heterogeneity were identified. For example, sub-regions, clinical syndromes and patient categories were the investigated potential sources of heterogeneity in our study. However, there may still be other sources of heterogeneity that have not yet been determined. We had difficulty in classifying the children’s age groups due to incommensurability of ages in these studies. Therefore, the interpretation of the results should be with cautious due to the difficulties in comparing some of these studies. Lastly, the amount of data available per country is variable with multiple studies from certain countries, which may bias the data.
Despite the above-mentioned limitations, this study has several strengths. This systematic review and meta-analysis used a predefined and registered protocol. The systematic searches performed in this review included many medical literature databases, so numerous studies were retrieved spanning a large time period. Additionally, this was complemented with a gray literature search using additional search engines. Two independent investigators were involved in all stages of the review process. Studies that identified RSV using reverse transcriptase PCR, with the highest sensitivity and specificity, were included. Sufficient data existed in the original studies to answer the objective of our review. Most of the included studies were prospective, where testing bias could not have occurred.
CONCLUSIONS
We found a high prevalence of RSV in the pediatric population with ARTI in Africa. Consequently, the prevention and control of RSV infections in children deserves more attention from health care providers, researchers, policymakers, and stakeholders for detection, management, and efficient control. Like other infectious diseases, surveillance systems for RSV should be widely initiated or incorporated into existing surveillance programs. Furthermore, efforts to address this burden could mainly focus on primary prevention including development and implementation of vaccines against RSV.
Additional material
Acknowledgments
Data availability: The study data are available from the corresponding author upon reasonable request.
Footnotes
Funding: None.
Authorship contributions: conceptualization of the work (BTR); data extraction, article screening and quality assessment (BTR, LAG, WTM); analysis (BTR, LAG, NAK); Interpretation (BTR, LAG, WTM, NAK), development of initial draft of manuscript (BTR); critical revisions for intellectual content of manuscript (BTR, NAK); study supervision (BTR, NAK). All authors reviewed and approved the final draft of manuscript. The corresponding author (BTR) had full access to all the data in the study and was responsible for the decision to submit the manuscript for publication.
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and declare no relevant interests.
REFERENCES
- 1.McIntosh K.Community-acquired pneumonia in children. N Engl J Med. 2002;346:429-37. 10.1056/NEJMra011994 [DOI] [PubMed] [Google Scholar]
- 2.Piedimonte G, Perez MK.Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev. 2014;35:519. 10.1542/pir.35.12.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pierangeli A, Gentile M, Di Marco P, Pagnotti P, Scagnolari C, Trombetti S, et al. Detection and typing by molecular techniques of respiratory viruses in children hospitalized for acute respiratory infection in Rome, Italy. J Med Virol. 2007;79:463-8. 10.1002/jmv.20832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korsun N, Angelova S, Tzotcheva I, Georgieva I, Lazova S, Parina S, et al. Prevalence and genetic characterisation of respiratory syncytial viruses circulating in Bulgaria during the 2014/15 and 2015/16 winter seasons. Pathog Glob Health. 2017;111:351-61. 10.1080/20477724.2017.1375708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellegrinelli L, Galli C, Bubba L, Cereda D, Anselmi G, Binda S, et al. Respiratory syncytial virus in influenza-like illness cases: epidemiology and molecular analyses of four consecutive winter seasons (2014-2015/2017-2018) in Lombardy (Northern Italy). J Med Virol. 2020;92:2999-3006. 10.1002/jmv.25917 [DOI] [PubMed] [Google Scholar]
- 6.Qin X, Zhang C, Zhao Y, Zhao X.Genetic variability of subgroup A and B respiratory syncytial virus strains circulating in southwestern China from 2009 to 2011. Arch Virol. 2013;158:1487-95. 10.1007/s00705-012-1552-z [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. WHO Strategy for the Global Respiratory Syncytial Virus Surveillance Project Based on the Influenza Platform. 2019. Available: https://wwwwhoint/publications/i/item/who-strategy-for-global-respiratory-syncytial-virus-surveillance-project-based-on-the-influenza-platform. Accessed: 30 June 2022.
- 8.Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399:2047-64. 10.1016/S0140-6736(22)00478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheltema NM, Gentile A, Lucion F, Nokes DJ, Munywoki PK, Madhi SA, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. 2017;5:e984-91. 10.1016/S2214-109X(17)30344-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson LJ, Dormitzer P, Nokes D, Rappuoli R, Roca A, Graham BJV.Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31:B209-15. 10.1016/j.vaccine.2012.11.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545-55. 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mammas IN, Drysdale SB, Rath B, Theodoridou M, Papaioannou G, Papatheodoropoulou A, et al. Update on current views and advances on RSV infection. Int J Mol Med. 2020;46:509-20. 10.3892/ijmm.2020.4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths C, Drews SJ.Marchant DJJCmr. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30:277-319. 10.1128/CMR.00010-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Famoroti T, Sibanda W.Ndung’u TJBp. Prevalence and seasonality of common viral respiratory pathogens, including Cytomegalovirus in children, between 0–5 years of age in KwaZulu-Natal, an HIV endemic province in South Africa. BMC Pediatr. 2018;18:240. 10.1186/s12887-018-1222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bont L, Checchia PA, Fauroux B, Figueras-Aloy J, Manzoni P, Paes B, et al. Defining the epidemiology and burden of severe respiratory syncytial virus infection among infants and children in western countries. Infect Dis Ther. 2016;5:271-98. 10.1007/s40121-016-0123-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meligy B, Sayed A, Ismail DK, Kamal D, Abdel-Latif W, Erfan DMJEPAG.Detection of viral acute lower respiratory tract infection in hospitalized infants using real-time PCR. Gaz Egypt Paediatr Assoc. 2016;64:13-9. 10.1016/j.epag.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rha B, Dahl RM, Moyes J, Binder AM, Tempia S, Walaza S, et al. Performance of surveillance case definitions in detecting respiratory syncytial virus infection among young children hospitalized with severe respiratory illness—South Africa, 2009–2014. J Pediatric Infect Dis Soc. 2019;8:325-33. 10.1093/jpids/piy055 [DOI] [PubMed] [Google Scholar]
- 19.Akinloye OM, Rönkkö E, Savolainen-Kopra C, Ziegler T, Iwalokun BA, Deji-Agboola MA, et al. Specific viruses detected in Nigerian children in association with acute respiratory disease. J Trop Med. 2011;2011:690286. 10.1155/2011/690286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soheir H, Gehan H, Maha G, Azza Ahmad A.Frequency and detection of respiratory syncytial virus pneumonia in children by nested reverse transcriptase polymerase chain reaction. Med J Cairo Univ. 2009;77:41-46 [Google Scholar]
- 21.Zar HJ, Nduru P, Stadler JA, Gray D, Barnett W, Lesosky M, et al. Early-life respiratory syncytial virus lower respiratory tract infection in a South African birth cohort: epidemiology and effect on lung health. Lancet Glob Health. 2020;8:e1316-25. 10.1016/S2214-109X(20)30251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabego L, Balol’Ebwami S, Kasengi JB, Miyanga S, Bahati YL, Kambale R, et al. Human respiratory syncytial virus: prevalence, viral co-infections and risk factors for lower respiratory tract infections in children under 5 years of age at a general hospital in the Democratic Republic of Congo. J Med Microbiol. 2018;67:514-22. 10.1099/jmm.0.000713 [DOI] [PubMed] [Google Scholar]
- 23.Hammitt LL, Kazungu S, Morpeth SC, Gibson DG, Mvera B, Brent AJ, et al. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis. 2012;54 suppl_2:S190-9. 10.1093/cid/cir1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simusika P, Bateman AC, Theo A, Kwenda G, Mfula C, Chentulo E, et al. Identification of viral and bacterial pathogens from hospitalized children with severe acute respiratory illness in Lusaka, Zambia, 2011–2012: a cross-sectional study. BMC Infect Dis. 2015;15:52. 10.1186/s12879-015-0779-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Kholy AA, Mostafa NA, Ali AA, El-Sherbini SA, Ismail RI, Magdy RI, et al. Risk factors of prolonged hospital stay in children with viral severe acute respiratory infections. J Infect Dev Ctries. 2014;8:1285-93. 10.3855/jidc.4682 [DOI] [PubMed] [Google Scholar]
- 26.Lagare A, Ousmane S, Dano ID, Issaka B, Issa I, Mainassara HB, et al. Molecular detection of respiratory pathogens among children aged younger than 5 years hospitalized with febrile acute respiratory infections: A prospective hospital-based observational study in Niamey, Niger. Health Sci Rep. 2019;2:e137. 10.1002/hsr2.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamrani Hanchi A, Guennouni M, Rachidi M, Benhoumich T, Bennani H, Bourrous M, et al. Epidemiology of Respiratory Pathogens in Children with Severe Acute Respiratory Infection and Impact of the Multiplex PCR Film Array Respiratory Panel: A 2-Year Study. Int J Microbiol. 2021;2021. 10.1155/2021/2276261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansour MGE, Albendary S.Comparison of multiplex reverse transcription-PCR-enzyme hybridization assay with immunofluorescence techniques for the detection of four viral respiratory pathogens in pediatric community acquired pneumonia. Egypt J Med Hum Genet. 2017;18(4):355–358. 10.1016/j.ejmhg.2017.04.001 [DOI] [Google Scholar]
- 29.Obodai E, Odoom JK, Adiku T, Goka B, Wolff T, Biere B, et al. The significance of human respiratory syncytial virus (HRSV) in children from Ghana with acute lower respiratory tract infection: a molecular epidemiological analysis, 2006 and 2013-2014. PLoS One. 2018;13:e0203788. 10.1371/journal.pone.0203788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zar HJ, Barnett W, Stadler A, Gardner-Lubbe S, Myer L, Nicol MP.Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir Med. 2016;4:463-72. 10.1016/S2213-2600(16)00096-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agoti CN, Otieno JR, Gitahi CW, Cane PA, Nokes DJ.Rapid spread and diversification of respiratory syncytial virus genotype ON1, Kenya. Emerg Infect Dis. 2014;20:950. 10.3201/eid2006.131438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annamalay AA, Abbott S, Sikazwe C, Khoo S-K, Bizzintino J, Zhang G, et al. Respiratory viruses in young South African children with acute lower respiratory infections and interactions with HIV. J Clin Virol. 2016;81:58-63. 10.1016/j.jcv.2016.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bénet T, Sylla M, Messaoudi M, Sánchez Picot V, Telles J-N, Diakite A-A, et al. Etiology and factors associated with pneumonia in children under 5 years of age in Mali: a prospective case-control study. PLoS One. 2015;10:e0145447. 10.1371/journal.pone.0145447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen C, Walaza S, Moyes J, Groome M, Tempia S, Pretorius M, et al. Epidemiology of viral-associated acute lower respiratory tract infection among children< 5 years of age in a high HIV prevalence setting, South Africa, 2009–2012. Pediatr Infect Dis J. 2015;34:66. 10.1097/INF.0000000000000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ElBasha N, El Rifai N, Draz I, El Kholy A.Contribution of viruses to severe pneumonia in children. Gaz Egypt Paediatr Assoc. 2013;61:73-7. 10.1016/j.epag.2013.07.001 [DOI] [Google Scholar]
- 36.Kadjo HA, Ekaza E, Coulibaly D, Kouassi DP, Nzussouo NT, Kouakou B, et al. Sentinel surveillance for influenza and other respiratory viruses in Cote d’Ivoire, 2003–2010. Influenza Other Respir Viruses. 2013;7:296-303. 10.1111/j.1750-2659.2012.00389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMorrow ML, Tempia S, Walaza S, Treurnicht FK, Moyes J, Cohen AL, et al. The role of human immunodeficiency virus in influenza-and respiratory syncytial virus–associated hospitalizations in South African children, 2011–2016. Clin Infect Dis. 2019;68:773-80. 10.1093/cid/ciy532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosero L, Khamadi S, Oundo J, Ndegwa L.Potential health-care associated respiratory syncytial virus in three referral Hospitals in Kenya, 2009-2011. Afr J Health Sci. 2016;29:1-12. [Google Scholar]
- 39.Moyes J, Cohen C, Pretorius M, Groome M, von Gottberg A, Wolter N, et al. Epidemiology of respiratory syncytial virus–associated acute lower respiratory tract infection hospitalizations among HIV-infected and HIV-uninfected South African children, 2010–2011. J Infect Dis. 2013;208 suppl_3:S217-26. 10.1093/infdis/jit479 [DOI] [PubMed] [Google Scholar]
- 40.Ngocho JS, Minja L, van der Gaast–de CE, Rahamat-Langendoen JC, Langereis JD, Mmbaga BT, et al. Viral-bacterial (co-) occurrence in the upper airways and the risk of childhood pneumonia in resource-limited settings. J Infect. 2020;81:213-20. 10.1016/j.jinf.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otieno JR, Kamau EM, Agoti CN, Lewa C, Otieno G, Bett A, et al. Spread and evolution of respiratory syncytial virus A genotype ON1, Coastal Kenya, 2010–2015. Emerg Infect Dis. 2017;23:264. 10.3201/eid2302.161149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pale M, Nacoto A, Tivane A, Nguenha N, Machalele L, Gundane F, et al. Respiratory syncytial and influenza viruses in children under 2 years old with severe acute respiratory infection (SARI) in Maputo, 2015. PLoS One. 2017;12:e0186735. 10.1371/journal.pone.0186735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pretorius MA, Madhi SA, Cohen C, Naidoo D, Groome M, Moyes J, et al. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness—South Africa, 2009–2010. J Infect Dis. 2012;206 suppl_1:S159-65. 10.1093/infdis/jis538 [DOI] [PubMed] [Google Scholar]
- 44.Rowlinson E, Dueger E, Taylor T, Mansour A, Van Beneden C, Abukela M, et al. Incidence and clinical features of respiratory syncytial virus infections in a population-based surveillance site in the Nile Delta Region. J Infect Dis. 2013;208 suppl_3:S189-96. 10.1093/infdis/jit457 [DOI] [PubMed] [Google Scholar]
- 45.Shafik CF, Mohareb EW, Yassin AS, Amin MA, El Kholy A, El-Karaksy H, et al. Viral etiologies of lower respiratory tract infections among Egyptian children under five years of age. BMC Infect Dis. 2012;12:350. 10.1186/1471-2334-12-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramoney K, Hellferscee O, Pretorius M, Tempia S, McMorrow M, von Gottberg A, et al. Human bocavirus, coronavirus, and polyomavirus detected among patients hospitalised with severe acute respiratory illness in South Africa, 2012 to 2013. Health Sci Rep. 2018;1:e59. 10.1002/hsr2.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valley-Omar Z, Tempia S, Hellferscee O, Walaza S, Variava E, Dawood H, et al. Human respiratory syncytial virus diversity and epidemiology among patients hospitalized with severe respiratory illness in South Africa, 2012–2015. Influenza Other Respir Viruses. 2022;16:222-35. 10.1111/irv.12905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adiku TK, Asmah RH, Rodrigues O, Goka B, Obodai E, Adjei AA, et al. Aetiology of acute lower respiratory infections among children under five years in Accra, Ghana. Pathogens. 2015;4:22-33. 10.3390/pathogens4010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bigogo GM, Breiman RF, Feikin DR, Audi AO, Aura B, Cosmas L, et al. Epidemiology of respiratory syncytial virus infection in rural and urban Kenya. J Infect Dis. 2013;208 suppl_3:S207-16. 10.1093/infdis/jit489 [DOI] [PubMed] [Google Scholar]
- 50.Buchwald AG, Tamboura B, Tennant SM, Haidara FC, Coulibaly F, Doumbia M, et al. Epidemiology, risk factors, and outcomes of respiratory syncytial virus infections in newborns in Bamako, Mali. Clin Infect Dis. 2020;70:59-66. 10.1093/cid/ciz157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baroudy NR, El Refay AS, Hamid TAA, Hassan DM, Soliman MS, Sherif L.Respiratory viruses and atypical bacteria co-infection in children with acute respiratory infection. Open Access Maced J Med Sci. 2018;6:1588. 10.3889/oamjms.2018.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emukule GO, Khagayi S, McMorrow ML, Ochola R, Otieno N, Widdowson M-A, et al. The burden of influenza and RSV among inpatients and outpatients in rural western Kenya, 2009–2012. PLoS One. 2014;9:e105543. 10.1371/journal.pone.0105543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fall A, Dia N, Cisse EHAK, Kiori DE, Sarr FD, Sy S, et al. Epidemiology and molecular characterization of human respiratory syncytial virus in Senegal after four consecutive years of surveillance, 2012–2015. PLoS One. 2016;11:e0157163. 10.1371/journal.pone.0157163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuller JA, Njenga MK, Bigogo G, Aura B, Ope MO, Nderitu L, et al. Association of the CT values of real-time PCR of viral upper respiratory tract infection with clinical severity, Kenya. J Med Virol. 2013;85:924-32. 10.1002/jmv.23455 [DOI] [PubMed] [Google Scholar]
- 55.Hoffmann J, Rabezanahary H, Randriamarotia M, Ratsimbasoa A, Najjar J, Vernet G, et al. Viral and atypical bacterial etiology of acute respiratory infections in children under 5 years old living in a rural tropical area of Madagascar. PLoS One. 2012;7:e43666. 10.1371/journal.pone.0043666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.IJpma FF, Beekhuis D, Cotton MF, Pieper CH, Kimpen JL, van den Hoogen BG, et al. Human metapneumovirus infection in hospital referred South African children. J Med Virol. 2004;73:486-93. 10.1002/jmv.20116 [DOI] [PubMed] [Google Scholar]
- 57.Jarju S, Greenhalgh K, Wathuo M, Banda M, Camara B, Mendy S, et al. Viral etiology, clinical features and antibiotic use in children< 5 years of age in The Gambia presenting with influenza-like illness. Pediatr Infect Dis J. 2020;39:925-30. 10.1097/INF.0000000000002761 [DOI] [PubMed] [Google Scholar]
- 58.Jroundi I, Mahraoui C, Benmessaoud R, Moraleda C, Tligui H, Seffar M, et al. The epidemiology and aetiology of infections in children admitted with clinical severe pneumonia to a university hospital in Rabat, Morocco. J Trop Pediatr. 2014;60:270-8. 10.1093/tropej/fmu010 [DOI] [PubMed] [Google Scholar]
- 59.Kenmoe S, Tchendjou P, Vernet MA, Moyo-Tetang S, Mossus T, Njankouo-Ripa M, et al. Viral etiology of severe acute respiratory infections in hospitalized children in Cameroon, 2011–2013. Influenza Other Respir Viruses. 2016;10:386-93. 10.1111/irv.12391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwofie TB, Anane YA, Nkrumah B, Annan A, Nguah SB, Owusu M.Respiratory viruses in children hospitalized for acute lower respiratory tract infection in Ghana. Virol J. 2012;9:78. 10.1186/1743-422X-9-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lekana-Douki SE, Nkoghe D, Drosten C, Ngoungou EB, Drexler JF, Leroyi EM.Viral etiology and seasonality of influenza-like illness in Gabon, March 2010 to June 2011. BMC Infect Dis. 2014;14:373. 10.1186/1471-2334-14-373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Loevinsohn G, Hamahuwa M, Sinywimaanzi P, Fenstermacher KZ, Shaw-Saliba K, Pekosz A, et al. Facility-based surveillance for influenza and respiratory syncytial virus in rural Zambia. BMC Infect Dis. 2021;21:986. 10.1186/s12879-021-06677-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niang MN, Diop OM, Sarr FD, Goudiaby D, Malou-Sompy H, Ndiaye K, et al. Viral etiology of respiratory infections in children under 5 years old living in tropical rural areas of Senegal: The EVIRA project. J Med Virol. 2010;82:866-72. 10.1002/jmv.21665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nyawanda BO, Mott JA, Njuguna HN, Mayieka L, Khagayi S, Onkoba R, et al. Evaluation of case definitions to detect respiratory syncytial virus infection in hospitalized children below 5 years in Rural Western Kenya, 2009–2013. BMC Infect Dis. 2016;16:218. 10.1186/s12879-016-1532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogunsemowo O, Olaleye D, Odaibo G.Human respiratory syncytial virus subtypes A and B infection among children attending primary and secondary health care facilities in Ibadan, Nigeria. Arch Basic Appl Med. 2018;6:73. [PMC free article] [PubMed] [Google Scholar]
- 66.Ouédraogo S, Traoré B, Nene Bi ZAB, Yonli FT, Kima D, Bonané P, et al. Viral etiology of respiratory tract infections in children at the pediatric hospital in Ouagadougou (Burkina Faso). PLoS One. 2014;9:e110435. 10.1371/journal.pone.0110435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peterson I, Bar-Zeev N, Kennedy N, Ho A, Newberry L, SanJoaquin MA, et al. Respiratory virus–associated severe acute respiratory illness and viral clustering in Malawian children in a setting with a high prevalence of HIV infection, malaria, and malnutrition. J Infect Dis. 2016;214:1700-11. 10.1093/infdis/jiw426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pretorius MA, Tempia S, Walaza S, Cohen AL, Moyes J, Variava E, et al. The role of influenza, RSV and other common respiratory viruses in severe acute respiratory infections and influenza-like illness in a population with a high HIV sero-prevalence, South Africa 2012–2015. J Clin Virol. 2016;75:21-6. 10.1016/j.jcv.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zampoli M, Mukuddem-Sablay Z.Adenovirus-associated pneumonia in South African children: Presentation, clinical course and outcome. S Afr Med J. 2017;107:123-6. 10.7196/SAMJ.2017.v107i2.11451 [DOI] [PubMed] [Google Scholar]
- 70.Berkley JA, Munywoki P, Ngama M, Kazungu S, Abwao J, Bett A, et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303:2051-7. 10.1001/jama.2010.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bimouhen A, El Falaki F, Ihazmad H, Regragui Z, Benkerroum S, Barakat A.Circulation of Respiratory Syncytial Virus in Morocco during 2014-2016: Findings from a sentinel-based virological surveillance system for influenza. East Mediterr Health J. 2016;22:483-490. 10.26719/2016.22.7.482 [DOI] [PubMed] [Google Scholar]
- 72.Brini I, Guerrero A, Hannachi N, Bouguila J, Orth-Höller D, Bouhlel A, et al. Epidemiology and clinical profile of pathogens responsible for the hospitalization of children in Sousse area, Tunisia. PLoS One. 2017;12:e0188325. 10.1371/journal.pone.0188325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brini Khalifa I, Hannachi N, Guerrero A, Orth-Höller D, Bhiri S, Bougila J, et al. Demographic and seasonal characteristics of respiratory pathogens in neonates and infants aged 0 to 12 months in the Central-East region of Tunisia. J Med Virol. 2019;91:570-81. 10.1002/jmv.25347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Derrar F, Izri K, Kaddache C, Boukari R, Hannoun D.infections n. Virologic study of acute lower respiratory tract infections in children admitted to the paediatric department of Blida University Hospital, Algeria. New Microbes New Infect. 2019;30:100536. 10.1016/j.nmni.2019.100536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El Kholy AA, Mostafa N, Ali A, Soliman M, El-Sherbini S, Ismail R, et al. The use of multiplex PCR for the diagnosis of viral severe acute respiratory infection in children: a high rate of co-detection during the winter season. Eur J Clin Microbiol Infect Dis. 2016;35:1607-13. 10.1007/s10096-016-2698-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El-Nawawy A, Antonios MA, Meheissen MA, Fahim M.Respiratory viruses associated with severe mechanically ventilated pneumonia in children. J Med Virol. 2022;94:461-8. 10.1002/jmv.27284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelly MS, Smieja M, Luinstra K, Wirth KE, Goldfarb DM, Steenhoff AP, et al. Association of respiratory viruses with outcomes of severe childhood pneumonia in Botswana. PLoS One. 2015;10:e0126593. 10.1371/journal.pone.0126593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mackenzie GA, Vilane A, Salaudeen R, Hogerwerf L, van den Brink S, Wijsman LA, et al. Respiratory syncytial, parainfluenza and influenza virus infection in young children with acute lower respiratory infection in rural Gambia. Sci Rep. 2019;9:17965. 10.1038/s41598-019-54059-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Majozi N, Nkwanyana N, Thula S, Coutsoudis A.Association between HIV and proven viral lower respiratory tract infection in paediatric intensive care unit patients at Inkosi Albert Luthuli Central Hospital, Durban, South Africa. SAJCH. 2017;11:154-8. [Google Scholar]
- 80.Othman HT, Elhamed WASA, Hassan DM, Soliman MS, Baset R.Respiratory syncytial virus and human metapneumovirus in severe lower respiratory tract infections in children under two. J Infect Dev Ctries. 2016;10:283-9. 10.3855/jidc.7087 [DOI] [PubMed] [Google Scholar]
- 81.Rabarison JH, Tempia S, Harimanana A, Guillebaud J, Razanajatovo NH, Ratsitorahina M, et al. Burden and epidemiology of influenza-and respiratory syncytial virus-associated severe acute respiratory illness hospitalization in Madagascar, 2011-2016. Influenza Other Respir Viruses. 2019;13:138-47. 10.1111/irv.12557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rady HI, El Kholy A.Prevalence of Human rhinovirus infection in young children with acute wheezing. Gaz Egypt Paediatr Assoc. 2018;66:35-8. 10.1016/j.epag.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Razanajatovo Rahombanjanahary NH, Rybkina K, Randriambolamanantsoa TH, Razafimanjato H, Heraud J-M.Genetic diversity and molecular epidemiology of respiratory syncytial virus circulated in Antananarivo, Madagascar, from 2011 to 2017: Predominance of ON1 and BA9 genotypes. J Clin Virol. 2020;129:104506. 10.1016/j.jcv.2020.104506 [DOI] [PubMed] [Google Scholar]
- 84.Razanajatovo NH, Guillebaud J, Harimanana A, Rajatonirina S, Ratsima EH, Andrianirina ZZ, et al. Epidemiology of severe acute respiratory infections from hospital-based surveillance in Madagascar, November 2010 to July 2013. PLoS One. 2018;13:e0205124. 10.1371/journal.pone.0205124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Razanajatovo NH, Richard V, Hoffmann J, Reynes J-M, Razafitrimo GM, Randremanana RV, et al. Viral etiology of influenza-like illnesses in Antananarivo, Madagascar, July 2008 to June 2009. PLoS One. 2011;6:e17579. 10.1371/journal.pone.0017579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scott PD, Ochola R, Ngama M, Okiro EA, Nokes DJ, Medley GF, et al. Molecular epidemiology of respiratory syncytial virus in Kilifi district, Kenya. J Med Virol. 2004;74:344-54. 10.1002/jmv.20183 [DOI] [PubMed] [Google Scholar]
- 87.Ciervo A, Mancini F, Puzelli S, Interisano M, Vescio MF, Farchi F, et al. Detection and correlates of Chlamydophila pneumoniae among children with acute respiratory infections. J Pediatr Infect Dis. 2010;5:249-54. [Google Scholar]
- 88.El-Senousy WM, Shouman MJF, Virology E.Human Coronavirus NL63 Among Other Respiratory Viruses in Clinical Specimens of Egyptian Children and Raw Sewage Samples. Food Environ Virol. 2021;13:322-8. 10.1007/s12560-021-09479-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Enan KA, Nabeshima T, Kubo T, Buerano CC, El Hussein ARM, Elkhidir IM, et al. Survey of causative agents for acute respiratory infections among patients in Khartoum-State, Sudan, 2010–2011. Virol J. 2013;10:312. 10.1186/1743-422X-10-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feikin DR, Njenga MK, Bigogo G, Aura B, Aol G, Audi A, et al. Etiology and Incidence of viral and bacterial acute respiratory illness among older children and adults in rural western Kenya, 2007–2010. PLoS One. 2012;7:e43656 10.1371/journal.pone.0043656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gad M, Refaay D, Gad N, Mohamed A.Viral infections in Egyptian hospitalized children with acute respiratory tract infections. J Clin Cell Immunol. 2017;8:5. 10.4172/2155-9899.1000526 [DOI] [Google Scholar]
- 92.Ghani ASA, Morrow BM, Hardie DR, Argent ACJPCCM.An investigation into the prevalence and outcome of patients admitted to a pediatric intensive care unit with viral respiratory tract infections in Cape Town, South Africa. Pediatr Crit Care Med. 2012;13:e275-81. 10.1097/PCC.0b013e3182417848 [DOI] [PubMed] [Google Scholar]
- 93.Ihling CM, Schnitzler P, Heinrich N, Mangu C, Sudi L, Souares A, et al. Molecular epidemiology of respiratory syncytial virus in children in sub-Saharan Africa. Trop Med Int Health. 2021;26:810-22. 10.1111/tmi.13573 [DOI] [PubMed] [Google Scholar]
- 94.Kadjo HA, Adjogoua E, Dia N, Adagba M, Abdoulaye O, Daniel S, et al. Detection of non-influenza viruses in acute respiratory infections in children under five-year-old in Cote D’Ivoire (January–December 2013). Afr J Infect Dis. 2018;12:78-88. 10.21010/ajid.v12i2.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamau E, Otieno JR, Murunga N, Oketch JW, Ngoi JM, de Laurent ZR, et al. Genomic epidemiology and evolutionary dynamics of respiratory syncytial virus group B in Kilifi, Kenya, 2015–17. Virus Evol. 2020;6:veaa050. 10.1093/ve/veaa050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Komoyo GF, Yambiyo BM, Manirakiza A, Gody JC, Muller CP, Hübschen JM, et al. Epidemiology and genetic characterization of respiratory syncytial virus in children with acute respiratory infections: Findings from the influenza sentinel surveillance network in Central African Republic, 2015 to 2018. Health Sci Rep. 2021;4:e298. 10.1002/hsr2.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lagare A, Maïnassara HB, Issaka B, Sidiki A, Tempia S.Viral and bacterial etiology of severe acute respiratory illness among children< 5 years of age without influenza in Niger. BMC Infect Dis. 2015;15:515. 10.1186/s12879-015-1251-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naga IS, Elsawaf GE, Elzalabany M, Eltalkhawy MY, Kader O.Human coronavirus OC43 and other respiratory viruses from acute respiratory infections of Egyptian children. Acta Microbiol Immunol Hung. 2020;67:112-9. 10.1556/030.2020.01059 [DOI] [PubMed] [Google Scholar]
- 99.Nakouné E, Tricou V, Manirakiza A, Komoyo F, Selekon B, Gody JC, et al. First introduction of pandemic influenza A/H1N1 and detection of respiratory viruses in pediatric patients in Central African Republic. Virol J. 2013;10:49. 10.1186/1743-422X-10-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Njouom R, Yekwa EL, Cappy P, Vabret A, Boisier P, Rousset D.Viral etiology of influenza-like illnesses in Cameroon, January–December 2009. J Infect Dis. 2012;206 suppl_1:S29-35. 10.1093/infdis/jis573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O’Callaghan-Gordo C, Bassat Q, Morais L, Díez-Padrisa N, Machevo S, Nhampossa T, et al. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: a malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr Infect Dis J. 2011;30:39-44. 10.1097/INF.0b013e3181f232fe [DOI] [PubMed] [Google Scholar]
- 102.Tempia S, Moyes J, Cohen AL, Walaza S, McMorrow ML, Treurnicht FK, et al. The national burden of influenza-like illness and severe respiratory illness overall and associated with nine respiratory viruses in South Africa, 2013–2015. Influenza Other Respir Viruses. 2022;16:438-51. 10.1111/irv.12949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hassan ZK, Hafez MM, Helal S, Gaafar M, Hussein G, Kamel MM, et al. Prevalence of Respiratory Syncytial Virus in Egyptian Pediatric Patients suffering from Pneumonia. Egypt J Med Microbiol. 2010;19:55-62. [Google Scholar]
- 104.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588-98. 10.1056/NEJMoa0804877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kenmoe S, Bigna JJ, Well EA, Simo FBN, Penlap VB, Vabret A, et al. Prevalence of human respiratory syncytial virus infection in people with acute respiratory tract infections in Africa: A systematic review and meta-analysis. Influenza Other Respir Viruses. 2018;12:793-803. 10.1111/irv.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xie Z, Qin Q, Shen K, Fang C, Li Y, Deng T.The burden of respiratory syncytial virus associated with acute lower respiratory tract infections in Chinese children: a meta-analysis. Transl Pediatr. 2020;9:496. 10.21037/tp-20-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahony JB.Nucleic acid amplification-based diagnosis of respiratory virus infections. Expert Rev Anti Infect Ther. 2010;8:1273-92. 10.1586/eri.10.121 [DOI] [PubMed] [Google Scholar]
- 108.Beck ET.Henrickson KJJFm. Molecular diagnosis of respiratory viruses. Future Microbiol. 2010;5:901-16. 10.2217/fmb.10.48 [DOI] [PubMed] [Google Scholar]
- 109.Mahony JB.Detection of respiratory viruses by molecular methods. Clin Microbiol Rev. 2008;21:716-47. 10.1128/CMR.00037-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shi T, Balsells E, Wastnedge E, Singleton R, Rasmussen ZA, Zar HJ, et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta–analysis. J Glob Health. 2015;5:020416 10.7189/jogh.05.020416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pangesti KN, Abd El Ghany M, Kesson AM, Hill-Cawthorne G.Respiratory syncytial virus in the Western Pacific Region: a systematic review and meta-analysis. J Glob Health. 2019;9:020431. 10.7189/jogh.09.020431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y, Yuan L, Zhang Y, Zhang X, Zheng M, Kyaw M.Burden of respiratory syncytial virus infections in China: Systematic review and meta–analysis. J Glob Health. 2015;5:020417. 10.7189/jogh.05.020417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tao Y, Tang M, Luo L, Xiang L, Xia Y, Li B, et al. Identification of etiologic agents and clinical characteristics for patients suspected of having pertussis in a large Children’s Hospital in China. Ann Transl Med. 2019;7:443 10.21037/atm.2019.08.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gkentzi D, Dimitriou G, Karatza A.Non-pulmonary manifestations of respiratory syncytial virus infection. J Thorac Dis. 2018;10 Suppl 33:S3815. 10.21037/jtd.2018.10.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou H, Thompson WW, Viboud CG, Ringholz CM, Cheng P-Y, Steiner C, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis. 2012;54:1427-36. 10.1093/cid/cis211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fleming DM, Pannell RS, Elliot AJ, Cross K.Respiratory illness associated with influenza and respiratory syncytial virus infection. Arch Dis Child. 2005;90:741-6. 10.1136/adc.2004.063461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bourgeois FT, Valim C, McAdam AJ, Mandl KDJP.Relative impact of influenza and respiratory syncytial virus in young children. Pediatrics. 2009;124:e1072-80. 10.1542/peds.2008-3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vandini S, Biagi C, Lanari M.Respiratory syncytial virus: the influence of serotype and genotype variability on clinical course of infection. Int J Mol Sci. 2017;18:1717. 10.3390/ijms18081717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Esposito S, Piralla A, Zampiero A, Bianchini S, Di Pietro G, Scala A, et al. Characteristics and their clinical relevance of respiratory syncytial virus types and genotypes circulating in Northern Italy in five consecutive winter seasons. PLoS One. 2015;10:e0129369. 10.1371/journal.pone.0129369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoshihara K, Le MN, Okamoto M, Wadagni ACA, Nguyen HA, Toizumi M, et al. Association of RSV-A ON1 genotype with increased pediatric acute lower respiratory tract infection in Vietnam. Sci Rep. 2016;6:27856. 10.1038/srep27856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martinello RA, Chen MD, Weibel C, Kahn J.Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis. 2002;186:839-42. 10.1086/342414 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


