Abstract
Alcohol-related liver disease (ARLD) remains one of the leading causes of chronic liver disease and the prevalence of alcohol-related cirrhosis is still increasing worldwide. Thus, ARLD is one of the leading indications for liver transplantation (LT) worldwide especially after the arrival of direct-acting antivirals for chronic hepatitis C infection. Despite the risk of alcohol relapse, the outcomes of LT for ARLD are as good as for other indications such as hepatocellular carcinoma (HCC), with 1-, 5-, and 10- year survival rates of 85%, 74%, and 59%, respectively. Despite these good results, certain questions concerning LT for ARLD remain unanswered, in particular because of persistent organ shortages. As a result, too many transplantation centers continue to require 6 months of abstinence from alcohol for patients with ARLD before LT to reduce the risk of alcohol relapse even though compelling data show the poor prognostic value of this criterion. A recent pilot study even observed a lower alcohol relapse rate in patients receiving LT after less than 6 months of abstinence as long as addictological follow-up is reinforced. Thus, the question should not be whether LT should be offered to patients with ARLD but how to select patients who will benefit from this treatment.
Keywords: alcohol, alcohol-related liver disease, alcohol-related hepatitis, liver transplantation, survival
Abbreviations: AH, alcohol-related hepatitis; ARLD, Alcohol-related liver disease; AUDIT, Alcohol Use Disorders Identification Test; CLD, chronic liver disease; ELTR, European Liver Transplant Registry; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; LT, liver transplantation; NASH, non-alcoholic steatohepatitis; NIAAA, National Institute on Alcohol Abuse and Alcoholism; UNOS, United Network for Organ Sharing
Since 2005, even though global alcohol consumption has been stable or even decreased worldwide, especially in Western countries, total per capita consumption remains high.1 For example, in Europe, alcohol consumption has decreased from 12.3 L in 2005 to 9.8 L in 2016. Thus alcohol-related liver disease (ARLD) is one of the most prevalent causes of chronic liver disease (CLD) worldwide. In 2017, nearly 123 million individuals were suspected of having alcohol-related cirrhosis with more than 2 million in the United States.2 Alcohol-related cirrhosis or alcohol-related hepatitis (AH) are responsible for approximately 60% of hospitalizations from the complications of cirrhosis or acute-on-chronic liver failure (ACLF),3 and mortality is twice as high in patients with alcohol-related cirrhosis as than in those from cirrhosis from other causes.4 In fact, excessive alcohol consumption accounts for approximately 25% of liver-related mortality5, 6, 7 with 607.000 deaths and 22.2 million disability-adjusted life years in 2016.1 Furthermore, a recent study in the United States has shown that the cost of alcohol-related cirrhosis exceeds all other etiologies of cirrhosis combined.8
Except for abstinence, very few medical options are available to patients with decompensated alcohol-related cirrhosis. When liver decompensation persists despite symptomatic measures and alcohol withdrawal, liver transplantation (LT) is the only option to improve survival. As a result, since 1983 when ARLD became a recognised indication for LT, it has been one of the leading indications for LT.9 In recent years, the percentage of ARLD patients in LT recipients has increased significantly from 24.2% in 2002 to 36.7%10 in 2016 in the United States.11
Which candidates with alcohol-related liver disease should be considered for liver transplantation?
Because of the poor survival of patients with decompensated cirrhosis, LT should be considered in abstinent patients with a MELD score >17.12 Indeed, the higher the MELD score, the poorer the 3-month survival, and LT improves survival when the MELD score is higher than this cut-off compared with a wait-and-watch strategy.12 It is important to remember that patients with decompensated alcohol-related cirrhosis can rapidly develop several complications and some patients will develop two liver events or more with a marked decrease in short-term survival. Indeed, one Danish study showed that one-year mortality in patients with alcohol-related cirrhosis was 20% following variceal bleeding alone, 29% following ascites alone, 49% following ascites and variceal bleeding and 64% following hepatic encephalopathy.13 Thus, LT should be considered an option in case of severe decompensation in patients with alcohol-related cirrhosis because it saves lives, especially in the presence of multiple organ failures.14 Conversely, patients who develop a single liver decompensation event (e.g., refractory ascites) without major liver dysfunction may not need to be candidates for LT since alternative treatment (e.g., TIPSS) is available. Moreover, a multicenter randomized controlled trial has shown that patients with Child-Pugh B alcohol-related cirrhosis did not benefit from LT compared with standard medical treatment.15,16 However, since liver function before TIPSS placement is a strong predictor of survival after TIPSS, LT should be discussed in patients with significant liver failure. Prognostic indices can help make the final decision to perform LT or TIPSS.17,18 In some patients, TIPSS can also be considered as a bridge to LT.
The time frame when considering LT in patients with decompensated alcohol-related cirrhosis is crucial. Indeed, liver function may improve in some patients with alcohol-related cirrhosis after alcohol is discontinued. Improvement mostly occurs within the first 3–6 months, and numerous patients are no longer at risk of short-term mortality because of an important decrease in the MELD score after alcohol is withdrawn.19 Because most improvement is seen after 3–6 months, it is tempting to consider a wait-and-watch strategy without LT during this period. This strategy is thought to avoid unnecessary LT in ARLD.20 On univariate analysis, Giard et al. observed that adults with ARLD in the United States listed for LT for 6 months had only a 13% lower risk of removal for improvement than patients with non-ARLD causes (P = 0.11). This percentage increases significantly at 2 years and is estimated to be 139% higher in ARLD patients than in non-ARLD patients (P < 0.001). This shows that the wait-and-watch strategy can be useful in some patients (less severely ill patients).20 A recent Spanish study confirmed that compared with other etiologies the probability of being taken off the list due to improvement was more frequent in patients with alcohol-related cirrhosis. Approximately 9% of patients with alcohol-related decompensated cirrhosis were taken off the list due to improvement after being listed compared with less than 2% of patients listed for cholestatic diseases or NASH.21 It is not surprising that the patients with the lowest MELD score had a higher probability of being taken off the list for improvement than patients with a high MELD score.
On the other hand, a long waiting period is not ethical and not relevant for the most severely ill patients who are good candidates for LT because they could die rapidly. The classic 6-month rule before being listed has been a policy for decades (see below). Strict application of this rule to patients with ARLD and high MELD scores is associated with mortality rates as high as 70%.22, 23–24
Compared with other indications, access to LT is limited in candidates with decompensated ARLD.16,20 In the 1990s, numerous surveys in both the United States and the United Kingdom confirmed that the general public considered that patients with self-inflicted illness especially ARLD should have lower priority access to available liver grafts.25,26 Like NAFLD, a disease that can also be considered to be self-inflicted because it is related to poor habits and lifestyle, the decision to list a patient with ARLD for LT should not only be influenced by the cause of decompensation but by the benefit that can be provided by LT. Fortunately, public opinion has gradually improved toward LT for ARLD patients. In a US survey published in 2015, only 13.7% of respondents thought that patients with end-stage ARLD should not receive LT.27 This shift in public opinion is accompanied by an increase in the percentage of ARLD patients among all LT recipients.11
Evaluation of addiction before liver transplantation for alcohol-related liver disease
A 6-month period (the so-called “6-month rule”) has been suggested as a prerequisite for LT to select patients who will continue to remain abstinent.28 Indeed, the longer the withdrawal period, the higher the probability of being abstinent after LT.29 A recent study showed that if patients were well selected, there was no difference in the outcomes of patients with more or less than 6 months of abstinence. Indeed, among 163 patients with ARLD, 54% underwent early LT (defined by a period of pre-LT abstinence of less than 6 months) and 46% standard LT (after 6 months of abstinence).30 Patients receiving early LT had not only similar 1-year survival (94.1% vs. 95.9%, respectively, P = 0.60) but also similar relapse-free survival (80.4% vs. 83.5%, respectively, P = 0.41) and allograft survival (92.7% vs. 90.5%, respectively, P = 0.42)30 than patients receiving standard LT. Furthermore, the duration of abstinence <6 months before LT alone does not accurately predict the risk of relapse after LT compared with other factors such as the presence of psychiatric comorbidities or a High-Risk Alcoholism Relapse score >3.31, 32, 33–34 A recent pilot study even observed a lower alcohol relapse rate in patients receiving LT after less than 6 months of abstinence as long as addictological follow-up was reinforced.33
Despite these encouraging data, certain individuals in the public and even certain philosophers and physicians view ARLD as a self-inflicted disease and feel that these patients should be given lower priority for LT.25,35, 36–37 In addition, certain physicians are reluctant to transplant patients with ARLD because of the risk of alcohol relapse after LT. Because of these fears, many transplantation centers use the 6-month rule before considering LT even though the validity of this tool to identify post-LT abstinence is weak.28 To ensure equal access to LT for patients with ARLD, the US and European guidelines do not propose 6-month abstinence as a mandatory selection criterion.38, 39–40
It must be kept in mind that there is no consensus on the addiction evaluation protocol before LT and that this is strongly influenced by local resources in the transplantation centre. Several studies have found that psychiatric disorders, other addictions and lack of close family support negatively influence the probability of being abstinent after LT (see below). Thus, the addiction evaluation before LT should at least analyse the presence or absence of these drivers of relapse before placing a patient on the waiting list. Since no particular driver is automatically associated with alcohol consumption after LT, the final decision must be made in a consensus meeting.
Somatic evaluation before liver transplantation for alcohol-related cirrhosis
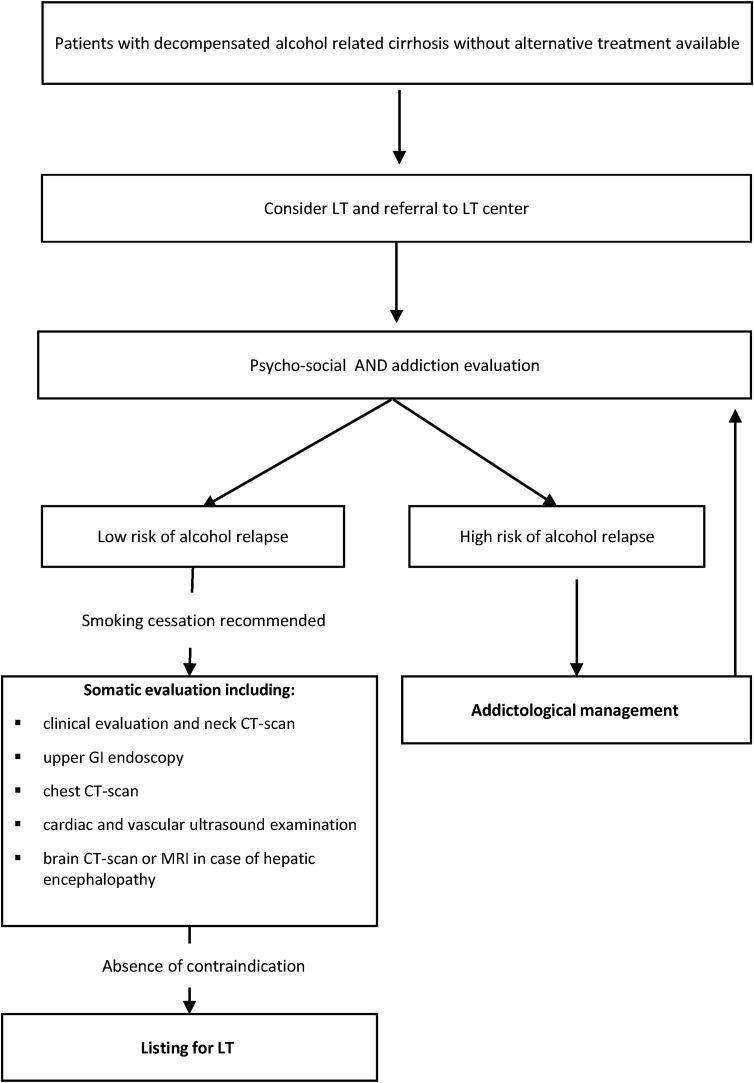
Since long-term ethanol abuse has multisystemic effects and is responsible for comorbidities such as neurological complications, osteoporosis, infections, cancers, and cardiovascular disease,41, 42, 43, 44–45 the pre-LT evaluation should be adapted to patients with ARLD. In addition, patients with alcohol-related cirrhosis often have a history of tobacco consumption (about 50% are active smokers),46 which significantly increases the risk of cancer and cardiovascular events. Despite the lack of specific guidelines for the pre-LT evaluation, the following examinations can be considered: screening for carcinomas of the aerodigestive tract (clinical evaluation, neck CT-scan), upper GI endoscopy to detect oesophageal cancers, chest CT-scan, cardiac and vascular ultrasound examination. In addition, when considering the prevalence of intracranial hemorrhage or ischemic events in patients with a history of alcohol consumption,47 systematic brain CT-scan or MRI and screening for cognitive impairment should be discussed, especially in patients with encephalopathy (search for alternative diagnoses).48,49
Patients who are active smokers before LT for ARLD often return to smoking after LT at addictive levels.50 Patients should, therefore, stop smoking before LT, especially because immunosuppressive drugs are associated with an increased risk of cardiovascular complications and cancers especially carcinomas of the aerodigestive tract.51, 52, 53–54 Five and ten years after LT, alcohol- and tobacco-related cancers are observed in about 5% and 13% of cases, respectively,51 and are responsible for more than 50% of deaths at 5 years.51,52 Figure 1 presents a decision-making algorithm based on our practices.
Figure 1.
Liver transplantation for alcohol related cirrhosis.
It is important to bear in mind that screening before LT must be adapted to the patient's condition (i.e., severity of liver failure) to avoid a prolonged period of evaluation in the most severely ill patients who have a high probability of early death without LT.
Outcomes of liver transplantation for alcohol-related liver disease: from the waiting list to the period following liver transplantation
In a US study in 83,348 eligible patients on the waiting list, 22.9% were listed for indications associated with ARLD and 77.1% for non-ARLD.20 During follow-up, candidates with ARLD had a lower risk of waiting list removal due to death or worsening of disease (HR 0.84, 95% CI 0.81–0.86, P < 0.001) and a higher risk of removal for improvement (HR 2.91, 95% CI 2.35–3.61 P < 0.001) than patients with non-ARLD. Moreover, after adjustment for the diagnosis of ARLD and other potential confounders, women had a higher risk of removal due to death or worsening of disease (HR 1.10, 95% CI 1.06–1.14, P < 0.001) or improvement (HR 1.33, 95% CI 1.19–1.47, P <0.001) than men.20 This study shows that patients listed for ARLD benefit from abstinence and identifies the restrictive criteria used for adding patients with ARLD on the waiting list. Despite the significant strengths of this study, there are certain limitations to the database, which lacks information on the patient's alcohol history or the duration of abstinence. More recently, a Spanish study investigated the probability of recovery and delisting due to improvement in patients with alcohol-related decompensated cirrhosis on the waiting list for LT. In total, 1001 patients were included, 37% with alcohol-related decompensated cirrhosis. Compared with other etiologies, nearly 9% of these patients had the probability of being taken off the list due to improvement a median 29 months after admission on the waiting list. Moreover, 5 years after being taken off the list, the cumulative probability of remaining free from liver-related death or LT was 76%, which is similar to patients with HCV-related decompensated cirrhosis who are removed after improvement. Factors independently associated with delisting following improvement were a lower MELD score, higher platelet count and women or lower height. However, the length of abstinence was not statistically different on multivariate analysis (P = 0.055) confirming the need to discontinue the 6-month rule.
When patients with ARLD have access to LT, post-transplant survival is good and has steadily improved in recent years. In fact, they are now at least as good or even better than those of other indications of LT (i.e., hepatocellular carcinoma [HCC] or decompensated HCV-related cirrhosis). The 1-, 5-, and 10- year survival rates are 85%, 74%, and 59%, respectively.38,39,55,56 Despite these results, with the persistent organ shortage, the main barrier to access to LT for patients with ARLD remains the risk of relapse after LT in many centres.
Alcohol relapse after liver transplantation
The rate of alcohol relapse after LT varies from 6 to 50% in the literature.31,57,58 This difference is due to an absence of consensus on the definition of alcohol relapse.59,60 In a recent review, Arab et al. propose a three-tier definition of relapse: occasional relapse (less than once per month), mild relapse (continuous drinking at daily and weekly doses within recommended standards of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) ≤ 4 drinks per day for men, ≤ 3 drinks per day for women; and ≤14 drinks per week for men, and ≤7 drinks per week for women) and severe relapse (regular use above recommended standards of the NIAAA or with associated morbidity or mortality, which includes alcohol-related pancreatitis, acute AH, graft loss, or other medical problems directly associated with return to drinking).61
No consumption of alcohol at all in the years following LT approaches 50% in patients transplanted for ARLD. In a prospective study, DiMartini et al. observed four different patterns of alcohol relapse after LT: low amounts occasionally, early-onset relapse with moderate alcohol intake that decreases over time, later-onset of relapse with moderate alcohol intake that increases over time and finally early-onset, heavy, increasing pattern of use.57 Occasional or moderate drinking does not seem to influence graft or patient survival in the short term,62,63 but long-term data are needed and occasional and moderate drinking can later result in an increase in alcohol consumption as observed in non-LT patients.64 On the other hand, relapse to heavy drinking results in the rapid progression of fibrosis leading to recurrent alcohol-related cirrhosis,65, 66–67 which influences both graft and patient survival.68,69 In addition to the amount of alcohol consumed, the timing of the relapse is also important. In fact, moderate alcohol use early after LT was associated with more adverse results than a later relapse.57
Thus, screening for alcohol relapse is crucial, especially to provide patient support. There are numerous tools to detect unhealthy alcohol use.61 Simple and quick clinical tools such as the Alcohol Use Disorders Identification Test (AUDIT) or its simplified version (AUDIT-c)70 can be used to screen excessive drinking. Biological markers of alcohol consumption, and in particular direct markers of alcohol metabolism such as ethyl glucuronide71 or phosphatidylethanol72 are more specific to detect alcohol consumption and are especially helpful in difficult situations such as in the presence of hepatic encephalopathy or cognitive dysfunction. Although these tools can help identify heavy or persistent alcohol use, they should not replace the essential role of experts in addictology in the transplant unit.61,73 There is evidence to support the role of the medical interview especially with a psychiatrist74 or an addiction specialist.75 This emphasises the need for addiction experts not only before but also during patient follow-up after LT to reduce the risk of relapse. This strategy has now been integrated in the recent European and American guidelines.38,56
Certain analytic studies have analyzed factors associated with the probability of drinking after LT and identified many factors including addiction history, younger age, psychosomatic status, social support etc.76 Thus, Lee et al. proposed a score (the SALT score ranging from 0 to 11) that includes the consumption of more than 10 drinks per day before LT, addiction histories requiring multiple rehabilitation visits, previous legal issues associated with alcohol and previous illicit substance abuse.77 A SALT score ≥5 had a 25% positive predictive value and a SALT score of <5 had a 95% negative predictive value for sustained alcohol use early post-LT for AH. However, this score did not identify patients who resumed heavy drinking after LT, thus this decision-making tool should be used with caution. Other scores have been developed to predict alcohol relapse especially after LT, for example, the High-Risk Alcoholism Relapse (HRAR).78 This score is based on three variables: duration of heavy drinking, number of drinks per day, and number of prior alcoholism treatment experiences. The study by De Gottardi et al.32 showed on multivariate logistic regression that an HRAR score >3 was one of the three independent factors of alcohol relapse. However, the conclusion of another was different. In the cohort published by DiMartini et al.79 HRAR scores did not distinguish patients listed for transplant from those who were not, or those who drank after transplantation from those who did not. The Alcohol Relapse Risk Assessment, ARRA,80 assesses nine variables (absence of HCC, tobacco dependence, continued alcohol use after liver disease diagnosis, low motivation for alcohol treatment, poor stress management skills, no rehabilitation relationship, limited social support, lack of nonmedical behavioural consequences, and continued participation in social activities with alcohol present) and categorized patients into four groups. Patients in groups ARRA III (4–6 points) and ARRA IV (7–9 points) had significantly higher rates of alcohol relapse in the pilot study, however The ARRA scale has not been validated by other studies. Finally, we can also mention the University of Michigan Alcoholism Prognosis Score (MAPS), although there was no significant association between pre-LT MAPS and post-LT alcohol use in a retrospective study.81
Overall, the main drawbacks of the scores designed to predict alcohol relapse after LT are their relatively poor positive predictive value, the lack of external validation for most of them and the fact that they do not originate from LT cohorts.
Liver transplantation for severe alcohol-related hepatitis?
Compelling evidence has confirmed that corticosteroids significantly decrease the risk of death within 28 days compared with placebo in patients with severe AH defined by a Maddrey score ≥ 32.82, 83, 84, 85, 86, 87–88 No other pharmacological option has been shown to be effective until now. Nevertheless, steroids do not improve medium- or long-term survival and response to treatment only occurs in 60% of patients. Treatment response can be evaluated by a change in bilirubin89 or a MELD score and by the Lille score calculated seven days after beginning steroids.90 Patients who have a Lille score >0.45 patients are considered to be non-responders to steroids90 and have a risk of 6-month mortality as high as 70%.91 No medical option has been shown to be effective in these patients and only LT can improve survival in highly selected patients22, 23–24,92, 93–94 (Table 1).
Table 1.
Main Studies Evaluating Early Liver Transplantation (LT) for Severe Alcohol-related Hepatits (AH).
| Study | N (patients with severe AH transplanted) | Study design | Control group | Survival rate in patients transplanted (in bold) and in patients not transplanted (light) |
Any alcohol relapse post-LT | |||
|---|---|---|---|---|---|---|---|---|
| 6-month survival | 1-year survival | 2-year survival | 3-year survival | |||||
| Mathurin et al., 201122 | 26 | Prospective | Patients with severe AH not transplanted |
77% 23% |
71% 23% |
12% | ||
| Im et al., 201624 | 9 | Retrospective | Patients with severe AH not transplanted |
89% 11% |
22% | |||
| Lee et al., 201793 | 17 | Retrospective | Alcohol-related cirrhosis with ≥6 months abstinence |
100% 88% |
23.5% | |||
| Weeks et al., 201894 | 46 | Retrospective | Alcohol-related cirrhosis who received liver transplants under standard protocols with at least 6 months sobriety. |
97% 100% |
−28% in patients with severe AH −24% in patients with alcohol-related cirrhosis |
|||
| Lee et al., 201823 | 147 | Retrospective | None | 94% | 84% | −25% (at one year) −34% (at 3 years) |
||
| Louvet et al., 202292 | 68 | Prospective |
|
89.7% 88,2% |
−34% at 2 years (patients with AH) −25% at 2 years (in patients with alcohol related cirrhosis) |
|||
| Germani et al., 202296 | 16 | Retrospective | Patients with severe AH not transplanted |
100% 41% |
100% 41% |
100% 38% |
100% 35% |
12.5% |
Abbreviations: AH, Alcohol-related Hepatitis; LT, Liver Transplantation.
Before 2005, AH was considered to be a contraindication for LT.95 Application of the 6-month rule was harmful to patients with severe AH who do not respond to steroids because abstinence did not improve their liver function resulting in a 6-month mortality of approximately 70–80%.91 Therefore in 2005, a consensus conference on the indications for LT held in Lyon97 strongly recommended testing innovative strategies in these patients.
Early LT was first evaluated in a French–Belgian case–control study by Mathurin et al.22 In this study, certain patients were selected during LT committee meetings with members of the medical, surgical teams, and nursing staff based on the following criteria: an absence of comorbidities or psychiatric disorders, no history of underlying cirrhosis, strong family support, and the patient's commitment to lifelong total abstinence from alcohol. It is important to note that, 80% of the patients on the waiting list have a probability of death within 30 days if they are not transplanted. Survival at 6 months was significantly higher in early transplanted patients (77 ± 8%) than in patients who did not receive a liver transplant (23 ± 8%). This benefit to survival persisted 2 years after LT: 71 ± 9% and 23 ± 8% in transplanted and non-transplanted patients, respectively. Two years after LT, 12% of early transplanted patients had alcohol relapse, which was considered to be acceptable compared with the rate observed after LT in ARLD patients who had achieved at least 6 months of sobriety.57,58 Nevertheless, validation and long-term data were needed.
Several studies that were performed after this pilot study confirmed the increase in survival following early LT in patients with severe AH who do not respond to steroids23,24 (Table 1). Between 2005 and 2018, the percentage of transplant centres in France that considered early LT to be an option in patients with severe AH who do not respond to steroids increased from 31% to 100%.28 In countries where LT from deceased donors is not available, living-donor LT can be proposed for AH with encouraging results.98 However, this indication raises important ethical issues, which differ among countries, and more data are needed. In most Western countries, LT for ARLD or AH is performed for patients with severe liver dysfunction from deceased donors and the benefits provided by LT largely compensate the disadvantages such as postoperative mortality, drug toxicity from immunosuppressant drugs or even costs. However, in other places in the world such as Asia where living-donor LT is preferred, the decision for LT must also be weighed against specific ethical issues and the disadvantages mentioned above.
With a selection protocol close to that used in the French–Belgian pilot study,22 Im et al. confirmed a higher 6-month survival rate in patients transplanted for AH compared with matched controls (89% vs. 11%, P < 0.001).24 After LT, the risk of relapse was 25%. Another retrospective American multicenter analysis of 147 patients evaluated early LT for severe AH in 12 liver transplant programs in the United States.23 One and 3-year survival rates were 94% and 84%, respectively, 34% of recipients relapsed to any alcohol use and 11% relapsed to sustained alcohol use.
Despite the good results of early LT for severe AH, certain questions regarding a potential increase in this indication should be discussed. A recent study from the United States99 has questioned the resources and financial costs associated with LT for AH in a series of 193 patients. They observed that the need for psychosocial support increases with the growing number of patients transplanted for AH and that the median length of stay also increased along with this indication. Their conclusion is that specific resources are needed which implies that centres should question their practices and develop structured care pathways to improve the quality of care in a cost-effective fashion.100
Despite the encouraging results of early LT for AH in Europe and the United States, heterogeneities still persist for this indication around the world. In Canada, all transplant centers require 6 month of abstinence before listing patients with ARLD and early LT is still contraindicated in patients with severe AH.101 However, there is a desire to relax the criteria in selected patients who are unlikely to survive without transplantation, such as those with severe AH who do not respond to steroids.101,102 The Ontario ARLD Pilot Program, which was launched in 2018 to challenge the “6-month abstinence rule”33 observed no significant differences in survival between patients transplanted through the program or after more than 6 months of abstinence and 6.8% of patients returned to alcohol use an average of 260 days after transplantation. On multivariate analysis, younger age and lower Model for End-Stage Liver Disease scores at listing but not the length of abstinence were predictors of a return to alcohol use.33
In 2015 in Germany, alcohol-related cirrhosis was the first indication of LT.103 However, before the rules were updated, strict German regulations required 6 months of abstinence in all patients with ARLD confirmed by at least two negative urine ethyl glucuronide (ETG) tests during the 6 months before possible wait-listing. Since 2016, legal transplant regulations allow transplant centers to request exceptional wait-listing for patients who have been abstinent for less than 6 months but each application needs to be approved by a committee of specialists at the Bundesärztekammer.103 This is a first step toward early LT in patients with severe AH.
In Italy, experts proposed mandatory early LT based on strict criteria in patients with severe AH who do not respond to medical treatment even if they are not abstinent.104,105 The results of the first pilot study evaluating early LT in patients with severe AH reported by Germani et al. are encouraging with 6-month and 24-month survival of 100% versus 45% and 100% versus 36%, in transplanted patients versus non-transplanted patients, respectively (P < 0.001).96
In 2021, as a result of an important reduction in the number of patients awaiting LT in Spain (the number has halved from 2015 to 2019), the Spanish Society of LT106 produced a consensus statement on potential areas to expand the indications for LT. One of these areas was “acute AH”. They stated that “patients with a first episode of severe acute AH (Maddrey score >32) who do not respond to corticosteroid therapy (Lille model score ≥0.45 at day 7) could be considered for LT unless otherwise contraindicated.”
In the United Kingdom, there is no absolute rule on the period of abstinence before listing a patient for LT.107,108 In recent UK guidelines,108 AH is considered to be a cause of ACLF and was not a contraindication for LT. A pilot program for transplanting patients with a first episode of AH was proposed in the United Kingdom, but the results are not available.
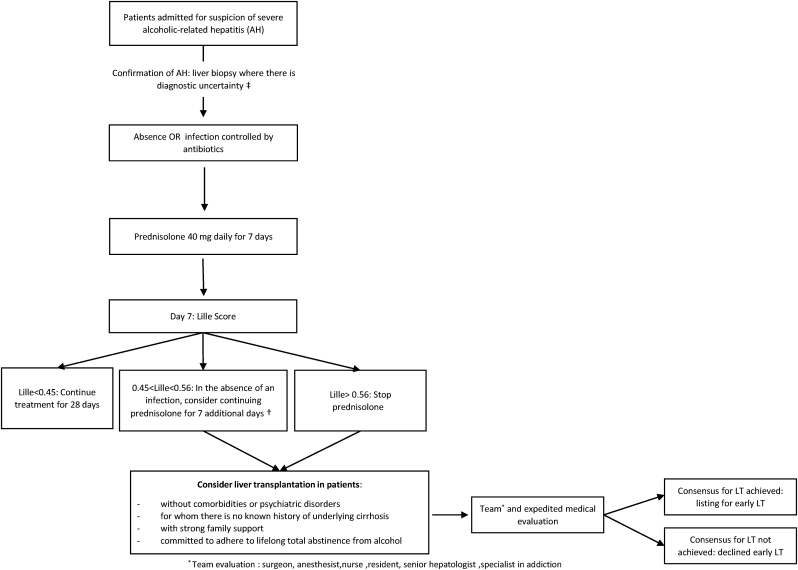
To reduce these heterogeneities, international consensus guidelines should be encouraged and well-designed, prospective, controlled studies are needed.109 A prospective non-inferiority French–Belgian controlled trial was designed to provide reliable data to experts. This study compared three groups of patients; group A: patients with severe AH not responding to medical treatment selected for early LT using a specific score (≥220/250) based on social and addiction parameters; group B: patients with alcohol-related cirrhosis with at least 6 months of abstinence, candidates for LT; group C: patients with severe AH not responding to medical treatment denied for early LT (score <220). 78 patients with severe AH were selected for early LT (group A) and 77 were denied early LT (group C). A total of 129 patients were included in group B. A total of 68 and 93 patients, respectively, were transplanted and included in groups A and B. In the primary analysis of transplanted patients, the non-inferiority of A versus B was not demonstrated, with a 2-year alcohol relapse of 34% in group A and 25% in group B. The 2-year heavy drinking relapse rate was also higher in group A (22%) than in group B (5%). However, 2-year survival was similar in groups A and B (89.7% and 88.2%, respectively) and was significantly higher in the early transplantation group, whether patients were transplanted or not (70.6%), compared with patients with severe AH who were not eligible for early transplantation (28.3%, P < 0.001).92 These results confirm the importance of addiction experts in the multidisciplinary teams in liver transplant centres and emphasize the need for addictological follow-up. Early identification of alcohol relapse is important to implement appropriate measures. It is also important to note that post-LT follow-up was not specified in the study protocol and was center-based. Thus, although these patients may require closer follow-up and/or adapted medication to decrease the risk of relapse, no specific management of addiction was proposed after LT for AH. Future studies and expert opinions should define optimal addiction management for patients transplanted for AH. Additional data are also needed to evaluate how AH as an indication for LT affects the waiting lists. We propose a decision-making algorithm based on our practices in Figure 2.
Figure 2.
Algorithm for management of patients with severe alcoholic hepatitis. †There is no consensus in patients with 0.45 < Lille < 0.56 to continue or stop prednisolone. In patients with no sign of infection, we propose to consider a further challenge by steroids for 7 additional days, but this is not evidence-based and this is only an expert opinion. ‡Liver biopsy can also have a prognostic value (Altamirano et al. Gastroenterology 2014). ∗Team evaluation: surgeon, anesthesist, nurse, resident, senior hepatologist, specialist in addiction.
Based on the current levels of alcohol consumption, ARLD will remain one of the leading causes of CLD. Medical options are limited in patients with decompensated ARLD, except for severe AH for which steroids have been shown to improve short-term survival. LT is, therefore, the most effective therapy in patients with persistent liver failure. Despite the good results for survival, access to LT is still limited in candidates with ARLD. The main argument for this reticence in the presence of organ shortages is the risk of alcohol relapse and its consequences on graft and patient survival. Moreover, numerous studies have confirmed the positive impact of the participation of an addiction specialist in the reduction of alcohol relapse even in patients after early LT. Thus, there is an urgent need to change attitudes toward the indication for LT in patients with ARLD. Patient selection, rather than the indication, should be the actual purpose of the debate, especially for LT candidates with severe AH.
Credit authorship contribution statement
Conceptualization: PM, AL. Supervision: PM, AL. Validation: LCNW, MN, GL, SD, EB, PM, AL. Roles/Writing – original draft: LCNW, AL. Writing – review & editing: LCNW, PM, AL.
Conflicts of interest
None.
Funding
None.
References
- 1.Organization WH . WHO; Geneva: 2018. Global Status Report on Alcohol and Health 2018. [Google Scholar]
- 2.Collaborators GBDC The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245–266. doi: 10.1016/S2468-1253(19)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singal A.K., Ahmed Z., Axley P., et al. Hospitalizations for acute on chronic liver failure at academic compared to non-academic centers have higher mortality. Dig Dis Sci. 2021;66:1306–1314. doi: 10.1007/s10620-020-06263-w. [DOI] [PubMed] [Google Scholar]
- 4.Hirode G., Saab S., Wong R.J. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paik J.M., Golabi P., Younossi Y., Mishra A., Younossi Z.M. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72:1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 6.Disease GBD. Injury I. Prevalence C Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborators GBDCoD Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barritt ASt, Jiang Y., Schmidt M., Hayashi P.H., Bataller R. Charges for alcoholic cirrhosis exceed all other etiologies of cirrhosis combined: a national and state inpatient survey analysis. Dig Dis Sci. 2019;64:1460–1469. doi: 10.1007/s10620-019-5471-7. [DOI] [PubMed] [Google Scholar]
- 9.Kwong A., Kim W.R., Lake J.R., et al. OPTN/SRTR 2018 annual data report: liver. Am J Transplant. 2020;20(suppl 1):193–299. doi: 10.1111/ajt.15674. [DOI] [PubMed] [Google Scholar]
- 10.Lee B.P., Vittinghoff E., Dodge J.L., Cullaro G., Terrault N.A. National trends and long-term outcomes of liver transplant for alcohol-associated liver disease in the United States. JAMA Intern Med. 2019;179:340–348. doi: 10.1001/jamainternmed.2018.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim W.R., Lake J.R., Smith J.M., et al. OPTN/SRTR 2017 annual data report: liver. Am J Transplant. 2019;19(suppl 2):184–283. doi: 10.1111/ajt.15276. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner R., Edwards E., Freeman R., et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 13.Jepsen P., Ott P., Andersen P.K., Sorensen H.T., Vilstrup H. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675–1682. doi: 10.1002/hep.23500. [DOI] [PubMed] [Google Scholar]
- 14.Artru F., Louvet A., Ruiz I., et al. Liver transplantation in the most severely ill cirrhotic patients: a multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67:708–715. doi: 10.1016/j.jhep.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Vanlemmens C., Di Martino V., Milan C., et al. Immediate listing for liver transplantation versus standard care for Child-Pugh stage B alcoholic cirrhosis: a randomized trial. Ann Intern Med. 2009;150:153–161. doi: 10.7326/0003-4819-150-3-200902030-00004. [DOI] [PubMed] [Google Scholar]
- 16.Kotlyar D.S., Burke A., Campbell M.S., Weinrieb R.M. A critical review of candidacy for orthotopic liver transplantation in alcoholic liver disease. Am J Gastroenterol. 2008;103:734–743. doi: 10.1111/j.1572-0241.2007.01691.x. quiz 44. [DOI] [PubMed] [Google Scholar]
- 17.Bettinger D., Sturm L., Pfaff L., et al. Refining prediction of survival after TIPS with the novel Freiburg index of post-TIPS survival. J Hepatol. 2021;74:1362–1372. doi: 10.1016/j.jhep.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Heinzow H.S., Lenz P., Kohler M., et al. Clinical outcome and predictors of survival after TIPS insertion in patients with liver cirrhosis. World J Gastroenterol. 2012;18:5211–5218. doi: 10.3748/wjg.v18.i37.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldt B.J., Laine F., Guillygomarc'h A., et al. Indication of liver transplantation in severe alcoholic liver cirrhosis: quantitative evaluation and optimal timing. J Hepatol. 2002;36:93–98. doi: 10.1016/s0168-8278(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 20.Giard J.M., Dodge J.L., Terrault N.A. Superior wait-list outcomes in patients with alcohol-associated liver disease compared with other indications for liver transplantation. Liver Transplant. 2019;25:1310–1320. doi: 10.1002/lt.25485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pose E., Torrents A., Reverter E., et al. A notable proportion of liver transplant candidates with alcohol-related cirrhosis can be delisted because of clinical improvement. J Hepatol. 2021;75:275–283. doi: 10.1016/j.jhep.2021.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Mathurin P., Moreno C., Samuel D., et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–1800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 23.Lee B.P., Mehta N., Platt L., et al. Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology. 2018;155:422–430 e1. doi: 10.1053/j.gastro.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im G.Y., Kim-Schluger L., Shenoy A., et al. Early liver transplantation for severe alcoholic hepatitis in the United States--A single-center experience. Am J Transplant. 2016;16:841–849. doi: 10.1111/ajt.13586. [DOI] [PubMed] [Google Scholar]
- 25.Neuberger J. Public and professional attitudes to transplanting alcoholic patients. Liver Transplant. 2007;13(11 suppl 2):S65–S68. doi: 10.1002/lt.21337. [DOI] [PubMed] [Google Scholar]
- 26.Neuberger J., Adams D., MacMaster P., Maidment A., Speed M. Assessing priorities for allocation of donor liver grafts: survey of public and clinicians. BMJ. 1998;317:172–175. doi: 10.1136/bmj.317.7152.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroh G., Rosell T., Dong F., Forster J. Early liver transplantation for patients with acute alcoholic hepatitis: public views and the effects on organ donation. Am J Transplant. 2015;15:1598–1604. doi: 10.1111/ajt.13176. [DOI] [PubMed] [Google Scholar]
- 28.Antonini T.M., Guillaud O., Dumortier J., et al. Impact of a first study of early transplantation in acute alcoholic hepatitis: results of a nationwide survey in French liver transplantation programs. Liver Transplant. 2018;24:841–844. doi: 10.1002/lt.25039. [DOI] [PubMed] [Google Scholar]
- 29.Tandon P., Goodman K.J., Ma M.M., et al. A shorter duration of pre-transplant abstinence predicts problem drinking after liver transplantation. Am J Gastroenterol. 2009;104:1700–1706. doi: 10.1038/ajg.2009.226. [DOI] [PubMed] [Google Scholar]
- 30.Herrick-Reynolds K.M., Punchhi G., Greenberg R.S., et al. Evaluation of early vs standard liver transplant for alcohol-associated liver disease. JAMA Surg. 2021;156:1026–1034. doi: 10.1001/jamasurg.2021.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim J., Curry M.P., Sundaram V. Risk factors and outcomes associated with alcohol relapse after liver transplantation. World J Hepatol. 2017;9:771–780. doi: 10.4254/wjh.v9.i17.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Gottardi A., Spahr L., Gelez P., et al. A simple score for predicting alcohol relapse after liver transplantation: results from 387 patients over 15 years. Arch Intern Med. 2007;167:1183–1188. doi: 10.1001/archinte.167.11.1183. [DOI] [PubMed] [Google Scholar]
- 33.Carrique L., Quance J., Tan A., et al. Results of early transplantation for alcohol-related cirrhosis: integrated addiction treatment with low rate of relapse. Gastroenterology. 2021;161:1896–18906 e2. doi: 10.1053/j.gastro.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Pfitzmann R., Schwenzer J., Rayes N., Seehofer D., Neuhaus R., Nussler N.C. Long-term survival and predictors of relapse after orthotopic liver transplantation for alcoholic liver disease. Liver Transplant. 2007;13:197–205. doi: 10.1002/lt.20934. [DOI] [PubMed] [Google Scholar]
- 35.Moss A.H., Siegler M. Should alcoholics compete equally for liver transplantation? JAMA. 1991;265:1295–1298. [PubMed] [Google Scholar]
- 36.Glannon W. Responsibility and priority in liver transplantation. Camb Q Healthc Ethics. 2009;18:23–35. doi: 10.1017/S0963180108090051. [DOI] [PubMed] [Google Scholar]
- 37.Zambrano A. Why alcoholics ought to compete equally for liver transplants. Bioethics. 2016;30:689–697. doi: 10.1111/bioe.12274. [DOI] [PubMed] [Google Scholar]
- 38.European Association for the Study of the Liver. Electronic Address eee. European Association for the Study of the L EASL Clinical Practice Guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69:154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 39.Singal A.K., Bataller R., Ahn J., Kamath P.S., Shah V.H. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113:175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucey M.R., Im G.Y., Mellinger J.L., Szabo G., Crabb D.W. Introducing the 2019 American Association for the Study of Liver Diseases guidance on Alcohol-associated Liver Disease. Liver Transplant. 2020;26:14–16. doi: 10.1002/lt.25600. [DOI] [PubMed] [Google Scholar]
- 41.Martin P., DiMartini A., Feng S., Brown R., Jr., Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 42.Gaglio P.J., Jr., Gaglio P.J., Sr. Complications in patients with alcohol-associated liver disease who undergo liver transplantation. Clin Liver Dis. 2012;16:865–875. doi: 10.1016/j.cld.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Addolorato G., Bataller R., Burra P., et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100:981–987. doi: 10.1097/TP.0000000000001156. [DOI] [PubMed] [Google Scholar]
- 44.Dumortier J., Guillaud O., Adham M., et al. Negative impact of de novo malignancies rather than alcohol relapse on survival after liver transplantation for alcoholic cirrhosis: a retrospective analysis of 305 patients in a single center. Am J Gastroenterol. 2007;102:1032–1041. doi: 10.1111/j.1572-0241.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- 45.Madhwal S., Atreja A., Albeldawi M., Lopez R., Post A., Costa M.A. Is liver transplantation a risk factor for cardiovascular disease? A meta-analysis of observational studies. Liver Transplant. 2012;18:1140–1146. doi: 10.1002/lt.23508. [DOI] [PubMed] [Google Scholar]
- 46.van der Heide F., Dijkstra G., Porte R.J., Kleibeuker J.H., Haagsma E.B. Smoking behavior in liver transplant recipients. Liver Transplant. 2009;15:648–655. doi: 10.1002/lt.21722. [DOI] [PubMed] [Google Scholar]
- 47.Gronbaek H., Johnsen S.P., Jepsen P., et al. Liver cirrhosis, other liver diseases, and risk of hospitalisation for intracerebral haemorrhage: a Danish population-based case-control study. BMC Gastroenterol. 2008;8:16. doi: 10.1186/1471-230X-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ritz L., Lannuzel C., Boudehent C., et al. Validation of a brief screening tool for alcohol-related neuropsychological impairments. Alcohol Clin Exp Res. 2015;39:2249–2260. doi: 10.1111/acer.12888. [DOI] [PubMed] [Google Scholar]
- 49.Pelletier S., Alarcon R., Ewert V., Forest M., Nalpas B., Perney P. Comparison of the MoCA and BEARNI tests for detection of cognitive impairment in in-patients with alcohol use disorders. Drug Alcohol Depend. 2018;187:249–253. doi: 10.1016/j.drugalcdep.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 50.DiMartini A., Javed L., Russell S., et al. Tobacco use following liver transplantation for alcoholic liver disease: an underestimated problem. Liver Transplant. 2005;11:679–683. doi: 10.1002/lt.20385. [DOI] [PubMed] [Google Scholar]
- 51.Herrero J.I., Pardo F., D'Avola D., et al. Risk factors of lung, head and neck, esophageal, and kidney and urinary tract carcinomas after liver transplantation: the effect of smoking withdrawal. Liver Transplant. 2011;17:402–408. doi: 10.1002/lt.22247. [DOI] [PubMed] [Google Scholar]
- 52.Watt K.D., Pedersen R.A., Kremers W.K., Heimbach J.K., Sanchez W., Gores G.J. Long-term probability of and mortality from de novo malignancy after liver transplantation. Gastroenterology. 2009;137:2010–2017. doi: 10.1053/j.gastro.2009.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duvoux C., Delacroix I., Richardet J.P., et al. Increased incidence of oropharyngeal squamous cell carcinomas after liver transplantation for alcoholic cirrhosis. Transplantation. 1999;67:418–421. doi: 10.1097/00007890-199902150-00014. [DOI] [PubMed] [Google Scholar]
- 54.Carenco C., Faure S., Herrero A., et al. Incidence of solid organ cancers after liver transplantation: comparison with regional cancer incidence rates and risk factors. Liver Int. 2015;35:1748–1755. doi: 10.1111/liv.12758. [DOI] [PubMed] [Google Scholar]
- 55.Ursic-Bedoya J., Dumortier J., Altwegg R., et al. Alcohol consumption the day of liver transplantation for alcohol-associated liver disease does not affect long-term survival: a case-control study. Liver Transplant. 2021;27:34–42. doi: 10.1002/lt.25904. [DOI] [PubMed] [Google Scholar]
- 56.Crabb D.W., Im G.Y., Szabo G., Mellinger J.L., Lucey M.R. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–333. doi: 10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- 57.DiMartini A., Dew M.A., Day N., et al. Trajectories of alcohol consumption following liver transplantation. Am J Transplant. 2010;10:2305–2312. doi: 10.1111/j.1600-6143.2010.03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogal S., Shenai N., Kruckenberg K., Rosenberger E., Dew M.A., DiMartini A. Post-transplant outcomes of persons receiving a liver graft for alcoholic liver disease. Alcohol Alcohol. 2018;53:157–165. doi: 10.1093/alcalc/agx100. [DOI] [PubMed] [Google Scholar]
- 59.Grant B.F., Chou S.P., Saha T.D., et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatr. 2017;74:911–923. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crabb D.W., Bataller R., Chalasani N.P., et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. 2016;150:785–790. doi: 10.1053/j.gastro.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arab J.P., Izzy M., Leggio L., Bataller R., Shah V.H. Management of alcohol use disorder in patients with cirrhosis in the setting of liver transplantation. Nat Rev Gastroenterol Hepatol. 2022;19:45–59. doi: 10.1038/s41575-021-00527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mathurin P., Lucey M.R. Liver transplantation in patients with alcohol-related liver disease: current status and future directions. Lancet Gastroenterol Hepatol. 2020;5:507–514. doi: 10.1016/S2468-1253(19)30451-0. [DOI] [PubMed] [Google Scholar]
- 63.Pageaux G.P., Bismuth M., Perney P., et al. Alcohol relapse after liver transplantation for alcoholic liver disease: does it matter? J Hepatol. 2003;38:629–634. doi: 10.1016/s0168-8278(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 64.Lemoine M., Gmel G., Foster S., Marmet S., Studer J. Multiple trajectories of alcohol use and the development of alcohol use disorder: do Swiss men mature-out of problematic alcohol use during emerging adulthood? PLoS One. 2020;15 doi: 10.1371/journal.pone.0220232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erard-Poinsot D., Dharancy S., Hilleret M.N., et al. Natural history of recurrent alcohol-related cirrhosis after liver transplantation: fast and furious. Liver Transplant. 2020;26:25–33. doi: 10.1002/lt.25647. [DOI] [PubMed] [Google Scholar]
- 66.Erard-Poinsot D., Guillaud O., Hervieu V., et al. Severe alcoholic relapse after liver transplantation: what consequences on the graft? A study based on liver biopsies analysis. Liver Transplant. 2016;22:773–784. doi: 10.1002/lt.24425. [DOI] [PubMed] [Google Scholar]
- 67.Dumortier J., Dharancy S., Cannesson A., et al. Recurrent alcoholic cirrhosis in severe alcoholic relapse after liver transplantation: a frequent and serious complication. Am J Gastroenterol. 2015;110:1160–1166. doi: 10.1038/ajg.2015.204. quiz 7. [DOI] [PubMed] [Google Scholar]
- 68.Kodali S., Kaif M., Tariq R., Singal A.K. Alcohol relapse after liver transplantation for alcoholic cirrhosis-impact on liver graft and patient survival: a meta-analysis. Alcohol Alcohol. 2018;53:166–172. doi: 10.1093/alcalc/agx098. [DOI] [PubMed] [Google Scholar]
- 69.Faure S., Herrero A., Jung B., et al. Excessive alcohol consumption after liver transplantation impacts on long-term survival, whatever the primary indication. J Hepatol. 2012;57:306–312. doi: 10.1016/j.jhep.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 70.Bradley K.A., DeBenedetti A.F., Volk R.J., Williams E.C., Frank D., Kivlahan D.R. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 71.Staufer K., Andresen H., Vettorazzi E., Tobias N., Nashan B., Sterneck M. Urinary ethyl glucuronide as a novel screening tool in patients pre- and post-liver transplantation improves detection of alcohol consumption. Hepatology. 2011;54:1640–1649. doi: 10.1002/hep.24596. [DOI] [PubMed] [Google Scholar]
- 72.Fleming M.F., Smith M.J., Oslakovic E., et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857–862. doi: 10.1111/acer.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Addolorato G., Mirijello A., Leggio L., et al. Liver transplantation in alcoholic patients: impact of an alcohol addiction unit within a liver transplant center. Alcohol Clin Exp Res. 2013;37:1601–1608. doi: 10.1111/acer.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DiMartini A., Day N., Dew M.A., et al. Alcohol use following liver transplantation: a comparison of follow-up methods. Psychosomatics. 2001;42:55–62. doi: 10.1176/appi.psy.42.1.55. [DOI] [PubMed] [Google Scholar]
- 75.Donnadieu-Rigole H., Olive L., Nalpas B., et al. Follow-up of alcohol consumption after liver transplantation: interest of an addiction team? Alcohol Clin Exp Res. 2017;41:165–170. doi: 10.1111/acer.13276. [DOI] [PubMed] [Google Scholar]
- 76.McCallum S., Masterton G. Liver transplantation for alcoholic liver disease: a systematic review of psychosocial selection criteria. Alcohol Alcohol. 2006;41:358–363. doi: 10.1093/alcalc/agl033. [DOI] [PubMed] [Google Scholar]
- 77.Lee B.P., Vittinghoff E., Hsu C., et al. Predicting low risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: the sustained alcohol use post-liver transplant score. Hepatology. 2019;69:1477–1487. doi: 10.1002/hep.30478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yates W.R., Booth B.M., Reed D.A., Brown K., Masterson B.J. Descriptive and predictive validity of a high-risk alcoholism relapse model. J Stud Alcohol. 1993;54:645–651. doi: 10.15288/jsa.1993.54.645. [DOI] [PubMed] [Google Scholar]
- 79.DiMartini A., Magill J., Fitzgerald M.G., et al. Use of a high-risk alcohol relapse scale in evaluating liver transplant candidates. Alcohol Clin Exp Res. 2000;24:1198–1201. [PubMed] [Google Scholar]
- 80.Rodrigue J.R., Hanto D.W., Curry M.P. The Alcohol Relapse Risk Assessment: a scoring system to predict the risk of relapse to any alcohol use after liver transplant. Prog Transplant. 2013;23:310–318. doi: 10.7182/pit2013604. [DOI] [PubMed] [Google Scholar]
- 81.Lucey M.R., Carr K., Beresford T.P., et al. Alcohol use after liver transplantation in alcoholics: a clinical cohort follow-up study. Hepatology. 1997;25:1223–1227. doi: 10.1002/hep.510250526. [DOI] [PubMed] [Google Scholar]
- 82.Maddrey W.C., Boitnott J.K., Bedine M.S., Weber F.L., Jr., Mezey E., White R.I., Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 83.Rambaldi A., Saconato H.H., Christensen E., Thorlund K., Wetterslev J., Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis--a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomized clinical trials. Aliment Pharmacol Ther. 2008;27:1167–1178. doi: 10.1111/j.1365-2036.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 84.Ramond M.J., Poynard T., Rueff B., et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507–512. doi: 10.1056/NEJM199202203260802. [DOI] [PubMed] [Google Scholar]
- 85.Mathurin P., Duchatelle V., Ramond M.J., et al. Survival and prognostic factors in patients with severe alcoholic hepatitis treated with prednisolone. Gastroenterology. 1996;110:1847–1853. doi: 10.1053/gast.1996.v110.pm8964410. [DOI] [PubMed] [Google Scholar]
- 86.Mathurin P., O'Grady J., Carithers R.L., et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–260. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 87.Thursz M.R., Forrest E.H., Ryder S., investigators S. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;373:282–283. doi: 10.1056/NEJMc1506342. [DOI] [PubMed] [Google Scholar]
- 88.Louvet A., Thursz M.R., Kim D.J., et al. Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo-a meta-analysis of individual data from controlled trials. Gastroenterology. 2018;155:458–468 e8. doi: 10.1053/j.gastro.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 89.Mathurin P., Abdelnour M., Ramond M.J., et al. Early change in bilirubin levels is an important prognostic factor in severe alcoholic hepatitis treated with prednisolone. Hepatology. 2003;38:1363–1369. doi: 10.1016/j.hep.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 90.Louvet A., Naveau S., Abdelnour M., et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 91.Lucey M.R., Mathurin P., Morgan T.R. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 92.Louvet A., Labreuche J., Moreno C., et al. Early liver transplantation for severe alcohol-related hepatitis not responding to medical treatment: a prospective controlled study. Lancet Gastroenterol Hepatol. 2022;7:416–425. doi: 10.1016/S2468-1253(21)00430-1. [DOI] [PubMed] [Google Scholar]
- 93.Lee B.P., Chen P.H., Haugen C., et al. Three-year results of a pilot program in early liver transplantation for severe alcoholic hepatitis. Ann Surg. 2017;265:20–29. doi: 10.1097/SLA.0000000000001831. [DOI] [PubMed] [Google Scholar]
- 94.Weeks S.R., Sun Z., McCaul M.E., et al. Liver transplantation for severe alcoholic hepatitis, updated lessons from the world's largest series. J Am Coll Surg. 2018;226:549–557. doi: 10.1016/j.jamcollsurg.2017.12.044. [DOI] [PubMed] [Google Scholar]
- 95.Bathgate A.J., Units U.K.L.T. Recommendations for alcohol-related liver disease. Lancet. 2006;367:2045–2046. doi: 10.1016/S0140-6736(06)68904-6. [DOI] [PubMed] [Google Scholar]
- 96.Germani G., Angrisani D., Addolorato G., et al. Liver transplantation for severe alcoholic hepatitis: a multicenter Italian study. Am J Transplant. 2022;22:1191–1200. doi: 10.1111/ajt.16936. [DOI] [PubMed] [Google Scholar]
- 97.Consensus Conference: indications for liver transplantation, January 19 and 20, 2005, Lyon-Palais des Congres: text of recommendations (long version) Liver Transplant. 2006;12:998–1011. doi: 10.1002/lt.20765. [DOI] [PubMed] [Google Scholar]
- 98.Choudhary N.S., Saigal S., Gautam D., et al. Good outcome of living donor liver transplantation for severe alcoholic hepatitis not responding to medical management: a single center experience of 39 patients. Alcohol. 2019;77:27–30. doi: 10.1016/j.alcohol.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 99.Im G.Y., Vogel A.S., Florman S., et al. Extensive health care utilization and costs of an early liver transplantation program for alcoholic hepatitis. Liver Transplant. 2022;28:27–38. doi: 10.1002/lt.26215. [DOI] [PubMed] [Google Scholar]
- 100.Aby E.S., Lake J.R. The rush to early liver transplantation for alcoholic hepatitis-potential financial implications. Liver Transplant. 2022;28:9–10. doi: 10.1002/lt.26282. [DOI] [PubMed] [Google Scholar]
- 101.Chandok N., Aljawad M., White A., Hernandez-Alejandro R., Marotta P., Yoshida E.M. Liver transplantation for alcoholic liver disease among Canadian transplant centres: a national study. Can J Gastroenterol. 2013;27:643–646. doi: 10.1155/2013/897467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong E., Mullins P.D., Wallach J.P., et al. Patients' perspectives on early liver transplantation in alcohol-related liver disease. Hepatol Commun. 2019;3:1022–1031. doi: 10.1002/hep4.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tacke F., Kroy D.C., Barreiros A.P., Neumann U.P. Liver transplantation in Germany. Liver Transplant. 2016;22:1136–1142. doi: 10.1002/lt.24461. [DOI] [PubMed] [Google Scholar]
- 104.Testino G., Burra P., Bonino F., et al. Acute alcoholic hepatitis, end stage alcoholic liver disease and liver transplantation: an Italian position statement. World J Gastroenterol. 2014;20:14642–14651. doi: 10.3748/wjg.v20.i40.14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burra P., Belli L.S., Ginanni Corradini S., et al. Common issues in the management of patients in the waiting list and after liver transplantation. Dig Liver Dis. 2017;49:241–253. doi: 10.1016/j.dld.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 106.Rodriguez-Peralvarez M., Gomez-Bravo M.A., Sanchez-Antolin G., et al. Expanding indications of liver transplantation in Spain: consensus statement and recommendations by the Spanish Society of Liver Transplantation. Transplantation. 2021;105:602–607. doi: 10.1097/TP.0000000000003281. [DOI] [PubMed] [Google Scholar]
- 107.Neuberger J. Liver transplantation in the United Kingdom. Liver Transplant. 2016;22:1129–1135. doi: 10.1002/lt.24462. [DOI] [PubMed] [Google Scholar]
- 108.Millson C., Considine A., Cramp M.E., et al. Adult liver transplantation: a UK clinical guideline - part 1: pre-operation. Frontline Gastroenterol. 2020;11:375–384. doi: 10.1136/flgastro-2019-101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thursz M., Allison M. Liver transplantation for alcoholic hepatitis: being consistent about where to set the bar. Liver Transplant. 2018;24:733–734. doi: 10.1002/lt.25188. [DOI] [PubMed] [Google Scholar]