Abstract
Alcohol-related liver disease (ALD) represents one of the leading causes of chronic liver disease and is a major cause of liver-related deaths worldwide. ALD encompasses a range of disorders including simple steatosis, alcoholic steatohepatitis, fibrosis, cirrhosis, and hepatocellular carcinoma. Patients with underlying ALD and continued heavy alcohol consumption can also develop an episode of acute-on-chronic liver injury called alcohol-associated hepatitis, the most severe form of the disease, which portends a poor prognosis. The most important risk factor for the development of ALD is the amount of alcohol consumed. Individual susceptibility to progression to advanced fibrosis among heavy drinkers is likely determined by a combination of behavioral, environmental, genetic, and epigenetic factors, but the mechanisms are largely unknown. The only effective therapy for ALD is prolonged alcohol abstinence. Diagnosis of ALD involves assessing patients for alcohol use disorder and signs of advanced liver disease. In clinical practice, the histological assessment for ALD diagnosis is uncommon, and it is usually based on the medical history, clinical manifestations, and laboratory and imaging tests. Several promising biomarkers that can have both diagnostic and prognostic value in patients with ALD have been identified in recent years. This review provides an overview of the clinical spectrum of ALD, the diagnostic approach of the disease from different perspectives as well as current diagnostic and prognostic biomarkers.
Keywords: alcohol-associated hepatitis, alcohol-related liver disease, alcoholic steatohepatitis, alcohol-related liver cirrhosis
Abbreviations: AH, alcohol-associated hepatitis; ALD, alcohol-related liver disease; ASH, alcoholic steatohepatitis; AST, aspartate aminotransferase; AUD, alcohol use disorder; AUDIT, Alcohol Use Disorders Identification Test; CAGE, Cut down, Annoyed, Guilty, and Eye-opener; DSM-5, Diagnostic and Statistical Manual of Mental Disorders Fifth edition; GGT, gamma-glutamyl transferase; HCC, hepatocellular carcinoma; INR, international normalized ratio; LSM, liver stiffness measurement; NAFLD, non-alcoholic fatty liver disease; PCF, pericellular fibrosis; SFS, SALVE fibrosis stages; SHG, SALVE Histopathology Group; TE, transient elastography; WHO, World Health Organization
Spectrum and natural history of alcohol-related liver disease
Burden of Alcohol-related Liver Disease
Alcohol-related liver disease (ALD) is a leading causes of chronic liver disease worldwide. The Global Status Report on Alcohol and Health 2018 from the World Health Organization reported that alcohol abuse comprises 50% of cirrhosis causes and 50% of liver deaths worldwide.1, 2, 3 Recent data on a population level stratified by country and drinking pattern have also shown that ALD is a frequent etiology of advanced liver fibrosis globally.4 In the United States, the prevalence of advanced fibrosis in ALD patients has significantly increased in the last 20 years, as has the number of hospitalizations for decompensated alcoholic cirrhosis and all causes of alcohol-related mortality.5,6 This increase seems to be especially pronounced in the younger population groups.7,8 Patterns of alcohol consumption have also experienced a change of trend along with the increased prevalence of the disease. Binge drinking, defined as heavy episodic drinking for a short time, has become increasingly frequent, and a more elevated liver disease risk has been proved to be associated, regardless of the average alcohol intake of each individual.9
ALD is often identified as decompensated cirrhosis onset as proven by a global study attested that ALD is detected at the latest stages (ratio of early/late referral negative eight-fold) more than any other liver disease.10 This may impede the implementation of specific therapy and lead to suboptimal surveillance of liver-related complications, delaying the diagnosis of potential life-threatening conditions.11 The specific indicators of silent underlying liver disease are unclear in patients with alcohol use disorder (AUD). Non-alcoholic fatty liver disease (NAFLD) studies show that nearly 20% of patients with histologically proven non-alcoholic steatohepatitis have normal ALT values, which increases to 25% when the full spectrum of NAFLD is considered.12 Given that ALD and NAFLD share many features, such as histologic characteristics and pathways that lead to disease progression,13 studies aimed at assessing the prevalence of ALD among heavy drinkers with normal liver biochemistry are needed. This reality has a major impact not only in terms of clinical outcomes for the patients but also in the economic burden of ALD. Between 2002 and 2014, the total charges for admissions related to alcohol cirrhosis complications in the United States increased by 95.7%. Said charges comprised of 59.9% of all admissions-related charges in cirrhotic patients in 2014 (alcohol vs. other etiologies). Financially, in-patient episodes due to alcoholic cirrhosis generated $28 billion total aggregate charges, with an increase from $1.4 billion in 2002 to $2.8 billion by 2014.14 These results strongly suggest an urgent need for early detection of ALD.
Natural History of ALD
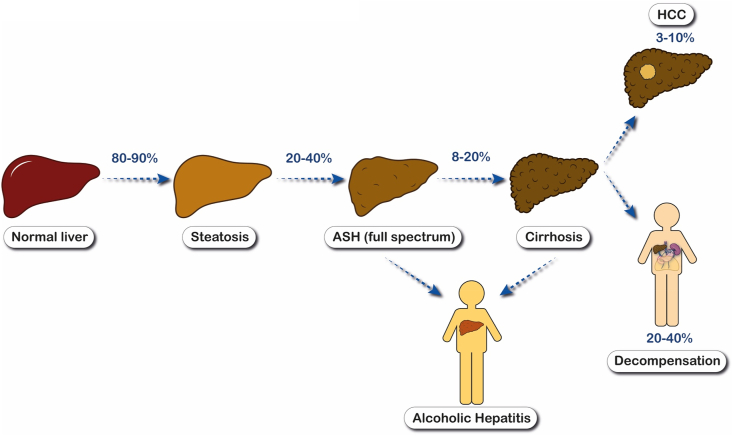
ALD comprises a wide spectrum of conditions that range from the early disease with steatosis or alcoholic steatohepatitis (ASH) to a more advance stages when fibrosis, cirrhosis, and even hepatocellular carcinoma (HCC) are developed. Patients with underlying ALD and active drinking may develop an episode of acute-on-chronic liver injury known as alcohol-associated hepatitis (AH), which can precede a poor prognosis15 (Figure 1).
Figure 1.
Natural history of alcoholic-related liver disease. Abbreviations: ASH, alcoholic steatohepatitis; HCC, Hepatocellular carcinoma.
Surprisingly, the natural history of ALD has been the focus of only a few studies. At least the 90% of patients who keep a chronic heavy drinking will develop steatosis, which may be reversible with alcohol abstinence, but in 20–40% of patients with sustained heavy alcohol consumption, the liver injury will progress, developing inflammation and fibrosis deposition; 10–20% of the patients with steatohepatitis and different degrees of fibrosis will progress to alcohol cirrhosis, which confers a worse prognosis and a significant risk of liver-related complications and death.15, 16, 17 Early forms of ALD are typically silent and thus are often overlooked and not diagnosed in a timely manner. High rates of advanced fibrosis were described in patients with ALD in a recent study.4 The presence of fibrosis, even in the absence of cirrhosis, negatively influences long-term survival with an increase of nearly three times the risk of death weighed against the general population.17 Therefore, early ALD is not well characterized, and a better definition of natural history, trustworthy biomarkers, and prognosis is crucial. In addition, there is a dire need to implement strategies to promote early detection.
A superimposed episode of AH, the most severe disease form, can be developed in patients with ALD who keep heavily drinking alcohol. AH is clinically characterized by the rapid onset of jaundice and other liver-related complications, with a histopathologic correlation consisting of polymorphonuclear leukocytes infiltration and hepatocellular damage.18 Patients may develop an intense systemic inflammatory response and multiorgan failure in the most severe cases.19 The AH prognosis can be approached by multiple predictive models,20, 21, 22, 23 but MELD score has been the most successful model for predicting short-term mortality.24 Severe from non-severe form of the disease can be differentiated by using a cut-off of 21 points in MELD score.21 Patients with severe AH have a short-term mortality up to 30% at 28 days, with long-term mortality exceeding 50% at 1 year.25 The moderate form of the disease still has a non-negligible mortality of up to 3–7% in the short-to medium-term and as high as 13–20% at 1 year.26 Therapies other than supportive measures have not demonstrated a benefit in long-term survival.27,28 Alcohol abstinence is the only independent factor associated with long-term survival improvement, regardless of the severity of the disease.29,30 Only a selected subset of patients with severe AH is candidate for an early liver transplantation when they are non-responders to medical therapy. Transplantations have increased over the 10 years with acceptable relapse rates.31,32
HCC in alcoholic cirrhosis has a lower incidence when compared to other causes, such as viral hepatitis and NAFLD, but it is often diagnosed at more advanced stages with lower survival rates.33,34 Therefore, the risk of HCC in ALD patients remains significant with an annual incidence of 2.9%.35 Due to that, it is important that the early detection of patients who are more prone to develop HCC. In addition to the amount of alcohol consumption,3 the presence of concomitant risk factors such as obesity,36 viral hepatitis,37 or smoking,38 may increase HCC risk through a synergistic mechanism. Specific genetic polymorphisms such as PNPLA3 gene polymorphism (allele G) have also been shown to affect the predisposition to HCC in ALD.36 Therefore, ALD patients should undergo HCC screening regularly and should be encouraged by physicians to engage in these preventive strategies.39
Alcohol consumption has been identified as the more significant risk factor for the development of ALD.40 However, the individual variability plays a crucial role, as the development of cirrhosis happens only in 10–20% of chronic drinkers. A combination of behavioral (pattern of drinking, beverage type), environmental (concomitant obesity, viral hepatitis, smoking), and genetic or epigenetic factors are likely to determine an individual's susceptibility to progression to advanced fibrosis among heavy drinkers, but the mechanisms are largely unknown. The global increase of obesity and metabolic syndrome have lead to pay attention to the additive effect of metabolic factors and excessive alcohol use.41 Mounting evidence suggests that moderate drinking (2–3 drinks per day) in patients with BMI >30 and/or diabetes mellitus may promote the development of cirrhosis. Studies of patients with non-alcoholic steatohepatitis-related compensated cirrhotic reveal an accelerated advance of liver disease and an increased risk of clinical decompensation, HCC, and death.42, 43, 44 The most extensively studied genetic factor is the gene coding for Patatin-like phospholipase domain-containing 3 (PNPLA3). Recent data suggest that PNPLA3 gene variations raise the risk of ALD progression, including the subsequent development of HCC.45, 46, 47 Along with PNPLA3, other genetic polymorphisms in genes HSD17B13 and TM6F2 have been shown to strongly predict cirrhosis development in alcohol users, especially in patients with diabetes.44 Other factors such as binge drinking,9 sex,48 and ethnicity49 may increase the risk of advanced ALD.
Educating heavy alcohol users about the precise risk factors that drive the development of liver disease (from subclinical to fibrosis and cirrhosis) is key to empowering patients to make informed decisions relating to alcohol use and abstinence.
Fibrosis stage is the main predictor of long-term mortality in patients with ALD. A recent study demonstrated that fibrosis stage, but not clinical or biochemical variables, determines long-term mortality (45% vs. 0% for F3-4 vs. F0-2, P < 0.001).50 Another important predictor of long-term mortality is the presence of alcohol withdrawal, which appears to have an impact across all disease stages.51,52 As recently reported, up to 30% of patients with ALD and advanced fibrosis will develop liver-related complications within 5 years.53
Screening of ALD
Unrecognized liver disease with advanced fibrosis or cirrhosis of different etiology is a frequent condition that entails a non-negligible risk of short-term, liver-related outcomes, such as gastroesophageal varices and HCC, especially when clinical indicators of liver disease are absent.11 As previously mentioned, ALD is usually detected at late phase of the disease.10 Currently, few active screening programs seek to identify individuals with early stage disease in any healthcare setting. Because ALD is one of the most frequent etiologies of liver-related mortality and cirrhosis worldwide,4 general practice settings and subsequent behavioral interventions should be urged to implement early detection programs. Moreover, it has been recently reported that implementing liver disease screening tests in the primary care setting using non-invasive methods is a cost-effective intervention.54,55
A blood test with abnormal liver biochemistry should be further investigated, regardless of the healthcare setting, and the existence of AUD should be ruled out. The current definition of AUD based on the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5) criteria encompasses both alcohol abuse and alcohol dependence.56 In order to aid in the diagnosis of AUD, well-structured and standardized questionnaires have been developed.
There is a wide range of available tools to assess alcohol misuse, but no single test presents the ideal screening method. The combined use of questionnaires and biomarkers that estimate persistent alcohol intake has been demonstrated to improve the sensitivity of alcohol use detection methods compared to self-report methods.57 An appropriate combination of diagnostic tests is the best approach for an accurate and early detection of ALD.
History of Significant Alcohol Intake
Self-reported questionnaires are the first tool used to assess alcohol abuse, but alcohol consumption is often underreported. Moreover, cognitively impaired individuals (e.g. hepatic encephalopathy in end-stage ALD patients) may not accurately complete self-reported questionnaires.58
Validated Questionnaires
DSM-5 Criteria
Mental disorders professionals in United States use the standardized classification of the Diagnostic and Statistical Manual of Mental Disorders. The manual includes criteria that determine the AUD severity according to the symptoms of the patient as well as factors that may rule out the existence of AUD in the patient. AUD is indicated by the presence of at least two symptoms. The severity of AUD is categorized as mild (2–3 symptoms), moderate (4–5 symptoms), or severe (6 or more symptoms).56
AUDIT and AUDIT-C
The Alcohol Use Disorders Identification Test (AUDIT) uses a specific scoring system to assess 10 questions.59 Based on the score, AUD diagnosis is established if >8 and >20 suggests alcohol dependence.60 The AUDIT–Concise (AUDIT-C) is a shortened form of the original questionnaire that minimizes the time required to perform the test with the same reliability. It comprises only three questions with a specific score each that sums from 0 to 12. A score ≥3 for women and ≥4 for men is considered diagnostic of AUD. The AUDIT-C has 73% sensitivity and 91% specificity for AUD and 85% sensitivity and 89% specificity for detecting alcohol dependence.61
CAGE
The Cut down, Annoyed, Guilty, Eye opener (CAGE) questionnaire is a practical, easy-to-use tool. The test is made up of four questions. Two or more positive responses constitute a result that may raise suspicion of AUD and should be investigated further.62 The CAGE questionnaire was evaluated by a meta-analysis; a cutoff of more than two positive answers found a sensitivity of 71% and specificity of 90%.63
Table 1 summarizes the current screening tools and diagnostic criteria for AUD.
Table 1.
Screening Tools and Diagnostic Criteria for Alcohol Use Disorder.
| Test/criteria | Items/points | Interpretation |
|---|---|---|
| DSM-5 |
|
The presence of at least 2 of these symptoms indicates Alcohol Use Disorder (AUD). The severity of the AUD is defined as:
|
| AUDIT |
|
|
| CAGE |
C Have you ever felt you should cut down on your drinking? A Have people annoyed you by criticizing your drinking? G Have you ever felt bad or guilty about your drinking? E Eye opener: Have you ever had a drink first thing in the morning to steady your nerves or to get rid of a hangover? |
Scores of 2 or more are considered as “screening positive” An abnormal or positive screening result may thus “raise suspicion” about the presence of an alcohol use problem, while a normal or negative result should suggest a low probability of an alcohol use problem. |
Abbreviations: AUD, alcohol use disorder; AUDIT, Alcohol Use Disorders Identification Test; CAGE, Cut down, annoyed, guilty, and eye-opener; DSM, Diagnostic and statistical manual of mental disorders.
Alcohol Biomarkers
Liver Biochemistry
Several laboratory parameters may be used to estimate persistent alcohol intake. Gamma-glutamyl transferase (GGT), the most employed marker to detect former alcohol consumption, is not specific. The carbohydrate-deficient transferrin is another indirect indicator of alcohol use that has a very low specificity, and it is considered useless to accurately identify alcohol consumption.64
Abnormalities in basic laboratory tests, such as elevated mean corpuscular volume and mild to moderate increase of aspartate aminotransferase (AST), may reflect the existence of underlying ALD. These routine laboratory tests are relatively inexpensive and accessible, but their specificity and usefulness for the early detection of ALD are limited.
Biomarkers of Alcohol Use
In recent years, an array of biomarkers of alcohol use has been comprehensively studied in patients with AUD and ALD65,66 although their usefulness in patients with ALD is largely unknown.67 The ideal biomarker does not exist, and the pros and cons of every parameter should be considered according to every clinical setting and availability across centers (Table 2).
-
-
Direct alcohol biomarkers: These biomarkers are the standard method of alcohol detection and include the ethanol detection in serum, urine, breath, and other body fluids. Because alcohol is quickly cleared, these biomarkers are reliable for testing recent alcohol intake. Disadvantages associated with these tests include potential false positives, risk of poor sample storage, inappropriate sampling, and post-sampling ethanol production by microbes.58
-
-
Indirect alcohol biomarkers: These tests measure ethanol metabolites and are being increasingly used due to the narrow time window for ethanol detection. When transferases in the liver metabolize alcohol, two molecules, ethyl glucuronide and ethyl sulfate, are generated. The molecules are nonvolatile water-soluble metabolites that can be detected in urine within at least 90 h after alcohol ingestion, with a reported sensitivity of 62–89% and specificity of 93–99%. Ethyl-glucuronide and ethyl-sulfate can be also measured in hair and blood. No issues in metabolites performance have been reported regarding to liver disease.
Table 2.
Diagnostic and Prognostic Biomarkers in Patients With Alcohol-Related Liver Disease.
| Biomarkers | Biology | Significance |
|---|---|---|
| Patients with active alcohol consumption | ||
| GGT | Increased synthesis or accelerated release from damaged or dead hepatocytes | Marker of heavy alcohol use |
| AST/ALT ratio | Reduced hepatic ALT activity, alcohol-induced depletion of hepatic pyridoxal 5′-phosphate and an increased hepatic mitochondrial AST | Marker of heavy alcohol use |
| Alcohol detection in body fluids (blood, urine, breath) | Ethanol | Recent alcohol intake |
| Ethyl-glucuronide Ethyl sulfate Phosphatidylethanol |
Alcohol-derive metabolites | Recent alcohol intake |
| Alcohol-related liver disease | ||
| FIB-4, Forns' index, APRI | Markers of fibrogenesis | Fibrosis assessment |
| Fibrometer®, Hepascore®, Fibrotest® | Markers of fibrogenesis | Fibrosis assessment |
| PNPLA3 | Genetic polymorphism | Disease progression Prognostic HCC carcinoma development |
| HSD17B13 TM6F2 |
Genetic polymorphisms | Disease progression Prognostic |
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transferase; PNPLA3: Patatin-like phospholipase domaincontaining 3.
The interaction of alcohol with lipids generates fatty acid ethyl esters and phosphatidylethanol, a phospholipid that is only synthesized if alcohol is present and that can be detected in blood within 28 days of alcohol consumption. Phosphatidylethanol has been reported with a sensitivity of 90–99% and a specificity of 100%.68 There is an accurate correlation between serum phosphatidylethanol and the consumption of alcohol, but the role that may play age, gender, concurrent diseases, or other confounders are unclear.
Recent technological advances have led to the use of devices, usually bracelets, that perform continuous alcohol monitoring by 24/7 transdermal alcohol testing to promote long-term abstinence and avoid testing gaps (e.g., Secure Continuous Remote Alcohol Monitor, SCRAM CAM).69
Diagnosis of ALD
Diagnosing ALD relies on a relevant alcohol drinking history, compatible clinical signs, abnormal laboratory test, and the exclusion of another alternative causes of liver damage. The diagnosis of ALD is clinicopathological, but a liver biopsy may establish the diagnosis and exclude coexisting conditions when there are confounders or when a consistent history of heavy alcohol consumption cannot be established. In clinical practice, the histological assessment for ALD diagnosis is uncommon, and it is often based on the medical history, clinical manifestations, and laboratory and imaging tests.8
Clinical Features
The early stages of ALD are usually detected through laboratory and/or imaging examinations,61 but physical examination at early stages may also be helpful. Patients with early ALD may display stigmata of alcohol abuse, including bilateral parotid gland or lacrimal gland hypertrophy, muscle wasting, malnutrition, Dupuytren's contractures, and peripheral neuropathy.70
Once the disease progresses to cirrhosis, some physical findings characteristic of this phase (although not pathognomonic of ALD) may help to establish a clinical diagnosis. The presence of ascites, peripheral edema, cognitive impairment in the context of hepatic encephalopathy, and visible collateral vessels on abdominal skin is common in the latest stage of liver disease. Other typical findings of cirrhosis in the physical examination, such gynecomastia and wide spider angiomas, are more frequently related with alcoholic etiology.
A subset of patients with ALD and sustained heavy drinking may develop an episode of AH clinically characterized by rapidly progressive jaundice, frequently associated with other signs and symptoms, such as malaise with anorexia and weight loss, fever, and right upper quadrant abdominal pain due to tender hepatomegaly. Given that most patients present already cirrhosis at AH onset,18,30 the development of cirrhosis decompensations (e.g., ascites and peripheral edema, liver encephalopathy, portal hypertensive bleeding, bacterial infection) at presentation or during the episode is also characteristic. Renal function impairment should be closely monitored, as is a common complication in patients with AH that associates poor outcomes.19,71, 72, 73
An episode of sudden jaundice and decompensation in ALD-cirrhotic patients can be due to other reasons, such as severe sepsis (particularly in a spontaneous bacterial peritonitis scenario), bile duct obstruction, diffuse HCC, drug-induced liver injury, and ischemic injury (usually secondary to cocaine use or massive bleeding). The differential diagnosis workup of AH should also include a serological study to rule out viral or autoimmune hepatitis.
Despite of an AH episode should only be suspected if the individual has a consistent history of heavy alcohol use, drinking in the immediate time preceding the admission can be variable. There is often a daily heavy ethanol intake, sometimes with periods of increased consumption the weeks and months before presentation, though some patients may have intermittently abstained from alcohol consumption for up to 8 weeks prior to presentation. The progression to acute-on-chronic liver failure may occur in the most severe cases, with a 1-month mortality that ranges from 18 to 50%, depending on organ failures number.74
Alcohol abuse may promote the development of extrahepatic alcohol-induced organ injury such as malnutrition, cardiomyopathy, pancreatitis, IgA nephropathy, cognitive dysfunction, neuropathy, or myopathy that should be investigated in the setting of ALD.75,76
Non-invasive Tests
Blood-based Tests
Mild elevations of GGT may be associated with steatosis, the earliest hepatic manifestation of excessive alcohol consumption, that can develop within 3–7 days of heavy alcohol intake. AST may be mildly or moderately increased (2–6 times the upper limit of normality), but serum levels are usually below 300–400 IU/L. Patients with ALD typically have an AST to alanine aminotransferase (ALT) ratio greater than 177. This ratio is usually greater than 2 in AH but can also appear advanced cirrhosis, regardless of the etiology. This ratio indicates reduced hepatic ALT activity, alcohol-induced depletion of hepatic pyridoxal 5′-phosphate, and increased hepatic mitochondrial AST.77 The diagnosis of advanced ALD should be always suspected in the presence of simultaneous increase of the international normalized ratio, elevated serum bilirubin, or decrease in serum albumin or platelet count.
The differential diagnosis between ALD and NAFLD may suppose a challenge. In patients with NAFLD, the AST/ALT ratio is usually is less than 1 (vs. greater than 1 in ALD), but it may rise as liver damage progresses.78 A cross-sectional cohort study reported that mean corpuscular volume, AST/ALT ratio, body mass index, and the gender were the most relevant factors to discern ALD against patients with NAFLD. The combination of these variables was used to create the ALD/NAFLD Index that allows differentiation of ALD from NAFLD with a C-index of 0.98.79 This is especially relevant since ALD and NAFLD frequently coexist in clinical practice. To refine the diagnostic criteria and avoid overlapping between the two entities, a new nomenclature for NAFLD has been recently proposed. To more accurately describe the pathogenesis of the disease and help in a correct patient stratification for further management, the term metabolic-associated fatty liver disease has been coined.80
In the clinical context of an AH episode, along with the clinical manifestations previously described, a rapid onset of hyperbilirubinemia (bilirubin levels greater than 3), leukocytosis, and increase of acute-phase reactants, including ferritin and C-reactive protein, are frequently observed. AST levels are typically elevated (2–6 times the upper limit of normality) with an AST/ALT ratio >2. In 2016, a experts panel from the National Institute on Alcohol Abuse and Alcoholism Alcoholic Hepatitis Consortia conceived of a set of standard definitions aimed at addressing diagnostic criteria of AH: (1) heavy alcohol use for >6 months (typically > 5years); (2) active alcohol use until <60 days prior to presentation; (3) sudden onset or worsening of jaundice; (4) AST/ALT ratio >1.5:1 with levels <400 IU/L; and (5) absence of other causes of liver disease.81
Combined Serum Biomarkers of Fibrosis
Fibrosis, along with sustained drinking, is one of the most relevant factors for ALD prognosis. Liver biopsy is the standard technique to evaluate alcohol-induced liver injury and fibrosis grade, but it is an invasive method that is not exempt from risks and sampling errors. To overcome these drawbacks, some non-invasive methods have been developed for the assessment of fibrosis in ALD scenario.82
Commercially available tests, such as FibroTest®, Fibrometer®, and HepaScore® (originally developed for detecting fibrosis in patients with chronic hepatitis C), have been proven to be helpful for staging liver fibrosis in ALD.
-
•
Fibrotest® is a patented formula (Biopredictive, Paris, France) combining α-2-macroglobulin, GGT, apolipoprotein A1, haptoglobin, total bilirubin, age, and gender. It was the first algorithm to combine several parameters.83
-
•
Fibrometer® (proprietary formula: Echosens, Paris, France) combines platelet count, prothrombin index, AST, α-2-macroglobulin, hyaluronate, urea, and age.
-
•
HepaScore® is a formula (PathWest, University of Western Australia, Australia) combining bilirubin, GGT, hyaluronate, α-2-macroglobulin, age, and gender.
These commercial tests have demonstrated a diagnostic accuracy higher than other easy-to-obtain, cheaper tests (e.g., APRI or AST-to-platelet ratio index, Forns Index, FIB4),18 but they are not widely available. Despite the evident advantages of the non-commercial serological tests, they do not improve the diagnostic performance of the commercial ones either alone or in combination.84
Methods to Assess Liver Stiffness
Currently, the most frequent non-invasive method for liver disease screening relies on the assessment of liver stiffness through transient elastography (TE). The development of more compact and portable models allows for point-of-care testing in any clinical setting. TE has proven to be a cost-effective intervention to detect patients with alcohol and NAFLD fibrosis in the primary care setting, which could benefit healthcare systems.54 Liver stiffness measurement (LSM) is closely related with the fibrosis stage, making it a highly reliable method to rule out the presence of significant fibrosis in patients with suspected ALD .85,86 Evidence in literature supports the use of TE to diagnose advanced fibrosis. Since 2015, the LSM usefulness in ALD has been confirmed in six single-etiology studies,85,87,88 one Cochrane meta-analysis,89 and two meta-analyses of individual patient data.90,91 TE is extremely accurate for the diagnosis of advanced fibrosis (as indicated by an AUROCs >0.90) and still good enough for substantial fibrosis (AUROCs around 0.85).92,93 However, the standard used as a reference in most of the earlier diagnostic studies was the fibrosis degree, based on scoring systems developed for chronic viral hepatitis (METAVIR). While these scores likely underestimate early stages of alcohol-related fibrosis, they may be reliable for bridging fibrosis and cirrhosis diagnostic estimation across histological fibrosis scoring systems. Existing evidence recommends against any non-invasive test either for the AH94,95 or ASH diagnosis.86
The cutoff values put forth to identify significant and advanced fibrosis are considerably greater in ALD when compared to other etiologies (viral hepatitis or NAFLD). Based on the latest individual patient data meta-analysis,86 advanced fibrosis may be considered in patients with ALD with a TE ≥12–15 kPa, if others potential factors for false positives are excluded. An increase in AST levels more than twice, recent alcohol intake, failure to fast before the procedure, and the presence of cholestasis89 are all factors associated with spurious elevation of LSM. In patients with elevated LSM (and any confounding factor), it is recommended to perform a new measurement following at least 1 week after quitting or reduced drinking, alongside a new liver function retesting.
Magnetic resonance elastography has been recently demonstrated to be a very good tool to accurately evaluate the liver stiffness with an equal or higher preciseness than TE for the diagnosis of relevant and advanced fibrosis with any etiology. Compared to other techniques, magnetic resonance elastography can evaluate all liver tissue using both two-dimensional and = three-dimensional methods.96 Despite this, it is not widely available due to its high cost.
Imaging Methods
Imaging studies are typically non-specific for the diagnosis of ALD. The standard imaging techniques commonly used in clinical practice in this setting are abdominal ultrasound, contrast enhanced computed tomography, and magnetic resonance imaging. The presence of an enlarged liver and fatty infiltration are common findings in abdominal ultrasound. Computed tomography and magnetic resonance imaging are helpful techniques to confirm ultrasound findings. A thorough evaluation of the liver anatomy, as along with other adjacent abdominal organs and vascular structures, is essential for the screening and assessment of liver-related complications. Standard imaging methods help to characterize and confirm findings of cirrhosis, portal hypertension, and HCC and to exclude other disorders from the differential diagnosis such as biliary obstruction or vascular thrombosis.
Histology
ALD's histological features vary, largely dependent on the extension and stage of liver injury. Even if they are characteristic, they are not pathognomonic of ALD. The earliest and most frequently seen feature is steatosis. The steatosis pattern in ALD is usually macrovesicular, with typical fat accumulation (specifically in centrilobular areas). Isolated steatosis has been associated with more severe forms of the disease and should not be considered a benign condition.
Continuous, excessive alcohol consumption may precede the development of ASH. This is a histopathological hallmark distinguished by steatosis, hepatocellular damage (characterized by ballooned hepatocytes that typically contain cytoplasmic hyaline inclusions called Mallory–Denk bodies), inflammatory infiltrates (predominantly neutrophilic), and variable degrees of fibrosis. A predominantly pericellular pattern of fibrosis is peculiar to alcohol-related liver damage.
Histologically, the pathological features that define ASH do not essentially vary from non-alcoholic steatohepatitis,97 but a more serious histological features, along with a worse clinical course, are usually described in ASH.
In the context of superimposed AH, intraparenchymal cholestasis or bilirubinostasis is frequently seen.18 AH is also associated with other histologic findings, including the so-called foamy degeneration in hepatocytes, the presence of megamitochondria, and an acute sclerosing hyaline necrosis.61,98 There is a correlation between the histological findings and the prognosis in individuals with AH. The Alcoholic Hepatitis Histologic Score is a scoring system developed and further validated in patients with histologically proven AH. The Alcoholic Hepatitis Histologic Score accurately classified patients according to the risk of death at 90 days allocating as low if 0–3 points, moderate if 4–5 points, and high if 6–9 points, with a survival of 100, 83, and 64%, respectively.18 The scoring system is based on the fibrosis stage, the type of bilirrubinostasis, the severity of polymorphonuclear infiltration, and megamitochondria presence (Table 3). Abundant polymorphonuclear infiltration and presence of megamitochondria were the only independent factors associated with a good outcome, while canalicular/ductular bilirubinostasis, bridging fibrosis, and cirrhosis were reported to be related with poor outcomes.
Table 3.
AHHS for Prognostic Stratification of AH.18
| Points | |
|---|---|
| Stage of fibrosis | |
| No fibrosis or portal fibrosis | 0 |
| Expansive fibrosis | 0 |
| Bridging fibrosis or cirrhosis | +3 |
| Bilirubinostasis | |
| No | 0 |
| Hepatocellular only | 0 |
| Canalicular or ductular | +1 |
| Canalicular or ductular plus hepatocellular | +2 |
| PMN infiltration | |
| No/Mild | +2 |
| Severe | 0 |
| Megamitochondria | |
| No megamitochondria | +2 |
| Megamitochondria | 0 |
Note. The AHHS categories are as follows: mild, 0–3; intermediate, 4–5; severe, 6–9.
A new histologic grading and staging system with prognostic relevance has been recently developed by the SALVE Histopathology Group (SHG). The SALVE staging system99 describes seven SALVE fibrosis stages (SFS) comprising four precirrhotic stages (SFS 0, 1, 2, and 3), similar to the Clinical Research Network staging system for NAFLD, and three cirrhotic stages (SFS 4A, 4B, and 4C), similar to Laennec staging system (Table 4). The histological cirrhosis substages100,101 and the extension of pericellular fibrosis (PCF)18 have a prognostic relevance in ALD. Because of the latter, the inclusion and grading of PCF in this new score system is very important. SFS staging can be stratified in a five-level system according to survival: no fibrosis (SFS 0); mild and moderate fibrosis (SFS 1 and 2); severe fibrosis (SFS 3); cirrhosis with thin or broad septa (SFS 4A and 4B); and cirrhosis with very broad septa (severe cirrhosis; SFS 4C) with a respective 10-year survival probabilities of 100%, 89%, 65%, 43%, and 32%. The more PCF (progressively higher as the stages go: SFS 3P, 4AP, 4BP, 4CP), the worse long-term outcomes than those without severe PCF.
Table 4.
SALVE Staging.99
| SFS | Description | Morphological changes |
|---|---|---|
| 0 | No fibrosis | Fibrosis is absent |
| 1 | Mild fibrosis | Periportal fibrosis only or PCF in zone(s) 3 ± 2
|
| 2 | Moderate fibrosis | Periportal fibrosis and PCF in zone(s) 3 ± 2 |
| 3 | Severe fibrosis | ≥1 complete septum bridging portal tracts and/or central veins, ±PCF
|
| 4A | Cirrhosis thin septa | ≥1 parenchymal nodule, thin septa, ± 1 broad septum ± PCF
|
| 4B | Cirrhosis broad septa | Parenchymal nodules, >1 broad septum, ± 1 very broad septum, ±PCF
|
| 4C | Cirrhosis very broad septa | Parenchymal nodules, >1 very broad septum, ±PCF
|
Abbreviations: PCF, pericellular fibrosis; SALVE, Consortium for the Study of Alcohol-related LiVer disease in Europe; SFS, SALVE fibrosis stage.
Because ALD is a clinicopathological diagnosis, liver biopsy is not mandatory. However, a transjugular liver biopsy is recommended in specific clinical scenarios (e.g., suspected AH along with potential confounding factors,81 uncertain ALD diagnosis despite of non-invasive evaluation or for the assessment of hepatic venous pressure gradient52).
Credit authorship contribution statement
-
-
Conceptualization: Ramon Bataller, Ana Clemente-Sanchez, Maria Hernandez-Tejero.
-
-
Roles/Writing – original draft: Ana Clemente-Sanchez, Maria Hernandez-Tejero.
-
-
Writing – review and editing: Ramon Bataller.
Conflicts of interest
No conflict of interest.
Funding
Ramon Bataller was supported by NIH/NIAAA grants RO1AA018873, 1U01AA026978-01, 1U01AA026972-01, 1U01AA026264-01 and NIDDK grant 1R01DK117881-01. Ana Clemente-Sanchez was supported by a scholarship grant for study extension abroad, sponsored by the Spanish Association for the Study of the Liver (AEEH) and NIH/NIDKK (US).
References
- 1.Hammer J.H.P.M., Spiker D.A., World Health Organization . World Health Organization (WHO); 2018. Global Status Report on Alcohol and Health 2018. [Google Scholar]
- 2.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Rehm J., Samokhvalov A.V., Shield K.D. Global burden of alcoholic liver diseases. J Hepatol. 2013;59:160–168. doi: 10.1016/j.jhep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Stein E., Cruz-Lemini M., Altamirano J., et al. Heavy daily alcohol intake at the population level predicts the weight of alcohol in cirrhosis burden worldwide. J Hepatol. 2016;65:998–1005. doi: 10.1016/j.jhep.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Dang K., Hirode G., Singal A.K., Sundaram V., Wong R.J. Alcoholic liver disease epidemiology in the United States: a retrospective analysis of 3 US databases. Am J Gastroenterol. 2020;115:96–104. doi: 10.14309/ajg.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 6.White A.M., Castle I.P., Hingson R.W., Powell P.A. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44:178–187. doi: 10.1111/acer.14239. [DOI] [PubMed] [Google Scholar]
- 7.Case A., Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A. 2015;112:15078–15083. doi: 10.1073/pnas.1518393112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arab J.P., Roblero J.P., Altamirano J., et al. Alcohol-related liver disease: clinical practice guidelines by the Latin American association for the study of the liver (ALEH) Ann Hepatol. 2019;18:518–535. doi: 10.1016/j.aohep.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Åberg F., Helenius-Hietala J., Puukka P., Jula A. Binge drinking and the risk of liver events: a population-based cohort study. Liver Int. 2017;37:1373–1381. doi: 10.1111/liv.13408. [DOI] [PubMed] [Google Scholar]
- 10.Shah N.D., Ventura-Cots M., Abraldes J.G., et al. Alcohol-related liver disease is rarely detected at early stages compared with liver diseases of other etiologies worldwide. Clin Gastroenterol Hepatol. 2019;17:2320–2329.e12. doi: 10.1016/j.cgh.2019.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T., Wong R., Wong P., et al. Occult cirrhosis diagnosed by transient elastography is a frequent and under-monitored clinical entity. Liver Int. 2015;35:2285–2293. doi: 10.1111/liv.12802. [DOI] [PubMed] [Google Scholar]
- 12.Ma X., Liu S., Zhang J., et al. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: a systematic review and meta-analysis. BMC Gastroenterol. 2020;20:10. doi: 10.1186/s12876-020-1165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanyal A.J., Mathurin P., Nagy L.A. Commonalities and distinctions between alcoholic and nonalcoholic fatty liver disease. Gastroenterology. 2016;150:1695–1697. doi: 10.1053/j.gastro.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barritt ASt, Jiang Y., Schmidt M., Hayashi P.H., Bataller R. Charges for alcoholic cirrhosis exceed all other etiologies of cirrhosis combined: a National and State Inpatient Survey Analysis. Dig Dis Sci. 2019;64:1460–1469. doi: 10.1007/s10620-019-5471-7. [DOI] [PubMed] [Google Scholar]
- 15.Altamirano J., Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 16.Adachi M., Brenner D.A. Clinical syndromes of alcoholic liver disease. Dig Dis. 2005;23:255–263. doi: 10.1159/000090173. [DOI] [PubMed] [Google Scholar]
- 17.Hagström H., Thiele M., Roelstraete B., Söderling J., Ludvigsson J.F. Mortality in biopsy-proven alcohol-related liver disease: a population-based nationwide cohort study of 3453 patients. Gut. 2021;70:170–179. doi: 10.1136/gutjnl-2019-320446. [DOI] [PubMed] [Google Scholar]
- 18.Altamirano J., Miquel R., Katoonizadeh A., et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231–1239.e1-6. doi: 10.1053/j.gastro.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michelena J., Altamirano J., Abraldes J.G., et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology. 2015;62:762–772. doi: 10.1002/hep.27779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddrey W.C., Boitnott J.K., Bedine M.S., Weber F.L., Jr., Mezey E., White R.I., Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. [PubMed] [Google Scholar]
- 21.Dunn W., Jamil L.H., Brown L.S., et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005;41:353–358. doi: 10.1002/hep.20503. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez M., Rincón D., Abraldes J.G., et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. 2008;103:2747–2756. doi: 10.1111/j.1572-0241.2008.02104.x. [DOI] [PubMed] [Google Scholar]
- 23.Forrest E.H., Evans C.D., Stewart S., et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut. 2005;54:1174–1179. doi: 10.1136/gut.2004.050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales-Arráez D., Ventura-Cots M., Altamirano J., et al. The MELD score is superior to the Maddrey discriminant function score to predict short-term mortality in alcohol-associated hepatitis: a global study. Am J Gastroenterol. 2022;117:301–310. doi: 10.14309/ajg.0000000000001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seitz H.K., Bataller R., Cortez-Pinto H., et al. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16. doi: 10.1038/s41572-018-0014-7. [DOI] [PubMed] [Google Scholar]
- 26.Clemente-Sánchez A., Oliveira-Mello A., Bataller R. Moderate alcoholic hepatitis. Clin Liver Dis. 2021;25:537–555. doi: 10.1016/j.cld.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thursz M.R., Forrest E.H., Ryder S. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;373:282–283. doi: 10.1056/NEJMc1506342. [DOI] [PubMed] [Google Scholar]
- 28.Louvet A., Thursz M.R., Kim D.J., et al. Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo-a meta-analysis of individual data from controlled trials. Gastroenterology. 2018;155:458–468.e8. doi: 10.1053/j.gastro.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Altamirano J., López-Pelayo H., Michelena J., et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: prediction and impact on long-term survival. Hepatology. 2017;66:1842–1853. doi: 10.1002/hep.29338. [DOI] [PubMed] [Google Scholar]
- 30.Degré D., Stauber R.E., Englebert G., et al. Long-term outcomes in patients with decompensated alcohol-related liver disease, steatohepatitis and Maddrey's discriminant function <32. J Hepatol. 2020;72:636–642. doi: 10.1016/j.jhep.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Mathurin P., Moreno C., Samuel D., et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–1800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 32.Mathurin P. Early liver transplantation for acute alcoholic hepatitis: we can't say no. J Hepatol. 2021;75:718–722. doi: 10.1016/j.jhep.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Golabi P., Fazel S., Otgonsuren M., Sayiner M., Locklear C.T., Younossi Z.M. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine. 2017;96 doi: 10.1097/MD.0000000000005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bucci L., Garuti F., Camelli V., et al. Comparison between alcohol- and hepatitis C virus-related hepatocellular carcinoma: clinical presentation, treatment and outcome. Aliment Pharmacol Ther. 2016;43:385–399. doi: 10.1111/apt.13485. [DOI] [PubMed] [Google Scholar]
- 35.Ganne-Carrié N., Chaffaut C., Bourcier V., et al. Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J Hepatol. 2018;69:1274–1283. doi: 10.1016/j.jhep.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Stickel F., Buch S., Nischalke H.D., et al. Genetic variants in PNPLA3 and TM6SF2 predispose to the development of hepatocellular carcinoma in individuals with alcohol-related cirrhosis. Am J Gastroenterol. 2018;113:1475–1483. doi: 10.1038/s41395-018-0041-8. [DOI] [PubMed] [Google Scholar]
- 37.Hassan M.M., Hwang L.Y., Hatten C.J., et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 38.Altamirano J., Bataller R. Cigarette smoking and chronic liver diseases. Gut. 2010;59:1159–1162. doi: 10.1136/gut.2008.162453. [DOI] [PubMed] [Google Scholar]
- 39.D'Amico G., Bataller R. Need for surveillance of hepatocellular carcinoma in patients with alcoholic cirrhosis. J Hepatol. 2018;69:1219–1220. doi: 10.1016/j.jhep.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 40.Savolainen V.T., Liesto K., Männikkö A., Penttilä A., Karhunen P.J. Alcohol consumption and alcoholic liver disease: evidence of a threshold level of effects of ethanol. Alcohol Clin Exp Res. 1993;17:1112–1117. doi: 10.1111/j.1530-0277.1993.tb05673.x. [DOI] [PubMed] [Google Scholar]
- 41.Ntandja Wandji L.C., Gnemmi V., Mathurin P., Louvet A. Combined alcoholic and non-alcoholic steatohepatitis. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiang D.J., McCullough A.J. The impact of obesity and metabolic syndrome on alcoholic liver disease. Clin Liver Dis. 2014;18:157–163. doi: 10.1016/j.cld.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vilar-Gomez E., Calzadilla-Bertot L., Wai-Sun Wong V., et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a Multi-National Cohort Study. Gastroenterology. 2018;155:443–457.e17. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 44.Whitfield J.B., Schwantes-An T.H., Darlay R., et al. A genetic risk score and diabetes predict development of alcohol-related cirrhosis in drinkers. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anstee Q.M., Daly A.K., Day C.P. Genetics of alcoholic liver disease. Semin Liver Dis. 2015;35:361–374. doi: 10.1055/s-0035-1567832. [DOI] [PubMed] [Google Scholar]
- 46.Stickel F., Buch S., Lau K., et al. Genetic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2011;53:86–95. doi: 10.1002/hep.24017. [DOI] [PubMed] [Google Scholar]
- 47.Trépo E., Gustot T., Degré D., et al. Common polymorphism in the PNPLA3/adiponutrin gene confers higher risk of cirrhosis and liver damage in alcoholic liver disease. J Hepatol. 2011;55:906–912. doi: 10.1016/j.jhep.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 48.Sato N., Lindros K.O., Baraona E., et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res. 2001;25:40s–45s. doi: 10.1097/00000374-200105051-00007. [DOI] [PubMed] [Google Scholar]
- 49.Levy R., Catana A.M., Durbin-Johnson B., Halsted C.H., Medici V. Ethnic differences in presentation and severity of alcoholic liver disease. Alcohol Clin Exp Res. 2015;39:566–574. doi: 10.1111/acer.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lackner C., Spindelboeck W., Haybaeck J., et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66:610–618. doi: 10.1016/j.jhep.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 51.Muntaner L., Altamirano J.T., Augustin S., et al. High doses of beta-blockers and alcohol abstinence improve long-term rebleeding and mortality in cirrhotic patients after an acute variceal bleeding. Liver Int. 2010;30:1123–1130. doi: 10.1111/j.1478-3231.2010.02287.x. [DOI] [PubMed] [Google Scholar]
- 52.EASL Clinical Practice Guidelines Management of alcohol-related liver disease. J Hepatol. 2018;69:154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 53.Delacôte C., Bauvin P., Louvet A., et al. A model to identify heavy drinkers at high risk for liver disease progression. Clin Gastroenterol Hepatol. 2020;18:2315–2323.e6. doi: 10.1016/j.cgh.2019.12.041. [DOI] [PubMed] [Google Scholar]
- 54.Serra-Burriel M., Graupera I., Torán P., et al. Transient elastography for screening of liver fibrosis: cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J Hepatol. 2019;71:1141–1151. doi: 10.1016/j.jhep.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 55.Ginès P., Castera L., Lammert F., et al. Population screening for liver fibrosis: toward early diagnosis and intervention for chronic liver diseases. Hepatology. 2022;75:219–228. doi: 10.1002/hep.32163. [DOI] [PubMed] [Google Scholar]
- 56.Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association Publishing; 2013. [Google Scholar]
- 57.Fleming M.F., Smith M.J., Oslakovic E., et al. Phosphatidylethanol detects moderate-to-heavy alcohol use in liver transplant recipients. Alcohol Clin Exp Res. 2017;41:857–862. doi: 10.1111/acer.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cabezas J., Lucey M.R., Bataller R. Biomarkers for monitoring alcohol use. Clin Liver Dis. 2016;8:59–63. doi: 10.1002/cld.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saunders J.B., Aasland O.G., Babor T.F., de la Fuente J.R., Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 60.Johnson J.A., Lee A., Vinson D., Seale J.P. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res. 2013;37(suppl 1):E253–E259. doi: 10.1111/j.1530-0277.2012.01898.x. [DOI] [PubMed] [Google Scholar]
- 61.Bush K., Kivlahan D.R., McDonell M.B., Fihn S.D., Bradley K.A. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 62.Ewing J.A. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 63.Aertgeerts B., Buntinx F., Kester A. The value of the CAGE in screening for alcohol abuse and alcohol dependence in general clinical populations: a diagnostic meta-analysis. J Clin Epidemiol. 2004;57:30–39. doi: 10.1016/S0895-4356(03)00254-3. [DOI] [PubMed] [Google Scholar]
- 64.Hock B., Schwarz M., Domke I., et al. Validity of carbohydrate-deficient transferrin (%CDT), gamma-glutamyltransferase (gamma-GT) and mean corpuscular erythrocyte volume (MCV) as biomarkers for chronic alcohol abuse: a study in patients with alcohol dependence and liver disorders of non-alcoholic and alcoholic origin. Addiction. 2005;100:1477–1486. doi: 10.1111/j.1360-0443.2005.01216.x. [DOI] [PubMed] [Google Scholar]
- 65.Nanau R.M., Neuman M.G. Biomolecules and biomarkers used in diagnosis of alcohol drinking and in monitoring therapeutic interventions. Biomolecules. 2015;5:1339–1385. doi: 10.3390/biom5031339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wurst F.M., Thon N., Yegles M., Schrück A., Preuss U.W., Weinmann W. Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res. 2015;39:2060–2072. doi: 10.1111/acer.12851. [DOI] [PubMed] [Google Scholar]
- 67.Allen J.P., Wurst F.M., Thon N., Litten R.Z. Assessing the drinking status of liver transplant patients with alcoholic liver disease. Liver Transpl. 2013;19:369–376. doi: 10.1002/lt.23596. [DOI] [PubMed] [Google Scholar]
- 68.Lee B.P., Terrault N.A. Return to alcohol use after liver transplant: patterns and surveillance. Clin Liver Dis. 2018;12:160–164. doi: 10.1002/cld.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simons J.S., Wills T.A., Emery N.N., Marks R.M. Quantifying alcohol consumption: self-report, transdermal assessment, and prediction of dependence symptoms. Addict Behav. 2015;50:205–212. doi: 10.1016/j.addbeh.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arab J.P., Atkinson S.R., Bataller R. 7th ed. Wiley; 2022. Alcohol-Related Liver Disease; pp. 1966–1978. (Yamada’s Textbook of Gastroenterology). Print ISBN: 9781119600169 | Online ISBN: 9781119600206. [Google Scholar]
- 71.Vergis N., Atkinson S.R., Knapp S., et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology. 2017;152:1068–1077.e4. doi: 10.1053/j.gastro.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Altamirano J., Fagundes C., Dominguez M., et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:65–71.e3. doi: 10.1016/j.cgh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Sujan R., Cruz-Lemini M., Altamirano J., et al. A validated score predicts acute kidney injury and survival in patients with alcoholic hepatitis. Liver Transpl. 2018;24:1655–1664. doi: 10.1002/lt.25328. [DOI] [PubMed] [Google Scholar]
- 74.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rusyn I., Bataller R. Alcohol and toxicity. J Hepatol. 2013;59:387–388. doi: 10.1016/j.jhep.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez-Reimers E., Alonso-Socas M., Santolaria-Fernandez F., Hernandez-Peña J., Conde-Martel A., Rodriguez-Moreno F. Autonomic and peripheral neuropathy in chronic alcoholic liver disease. Drug Alcohol Depend. 1991;27:219–222. doi: 10.1016/0376-8716(91)90004-i. [DOI] [PubMed] [Google Scholar]
- 77.Alatalo P., Koivisto H., Puukka K., et al. Biomarkers of liver status in heavy drinkers, moderate drinkers and abstainers. Alcohol Alcohol. 2009;44:199–203. doi: 10.1093/alcalc/agn099. [DOI] [PubMed] [Google Scholar]
- 78.Sorbi D., Boynton J., Lindor K.D. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94:1018–1022. doi: 10.1111/j.1572-0241.1999.01006.x. [DOI] [PubMed] [Google Scholar]
- 79.Dunn W., Angulo P., Sanderson S., et al. Utility of a new model to diagnose an alcohol basis for steatohepatitis. Gastroenterology. 2006;131:1057–1063. doi: 10.1053/j.gastro.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eslam M., Newsome P.N., Sarin S.K., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 81.Crabb D.W., Bataller R., Chalasani N.P., et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology. 2016;150:785–790. doi: 10.1053/j.gastro.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bataller R., Gao B. Liver fibrosis in alcoholic liver disease. Semin Liver Dis. 2015;35:146–156. doi: 10.1055/s-0035-1550054. [DOI] [PubMed] [Google Scholar]
- 83.Imbert-Bismut F., Ratziu V., Pieroni L., Charlotte F., Benhamou Y., Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 84.Chrostek L., Panasiuk A. Liver fibrosis markers in alcoholic liver disease. World J Gastroenterol. 2014;20:8018–8023. doi: 10.3748/wjg.v20.i25.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thiele M., Detlefsen S., Sevelsted Møller L., et al. Transient and 2-dimensional shear-wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology. 2016;150:123–133. doi: 10.1053/j.gastro.2015.09.040. [DOI] [PubMed] [Google Scholar]
- 86.Thiele M., Madsen B.S., Hansen J.F., Detlefsen S., Antonsen S., Krag A. Accuracy of the enhanced liver fibrosis test vs FibroTest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology. 2018;154:1369–1379. doi: 10.1053/j.gastro.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 87.Mueller S., Englert S., Seitz H.K., et al. Inflammation-adapted liver stiffness values for improved fibrosis staging in patients with hepatitis C virus and alcoholic liver disease. Liver Int. 2015;35:2514–2521. doi: 10.1111/liv.12904. [DOI] [PubMed] [Google Scholar]
- 88.Voican C.S., Louvet A., Trabut J.B., et al. Transient elastography alone and in combination with FibroTest(®) for the diagnosis of hepatic fibrosis in alcoholic liver disease. Liver Int. 2017;37:1697–1705. doi: 10.1111/liv.13440. [DOI] [PubMed] [Google Scholar]
- 89.Pavlov C.S., Casazza G., Nikolova D., et al. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst Rev. 2015;1:Cd010542. doi: 10.1002/14651858.CD010542.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nguyen-Khac E., Thiele M., Voican C., et al. Non-invasive diagnosis of liver fibrosis in patients with alcohol-related liver disease by transient elastography: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:614–625. doi: 10.1016/S2468-1253(18)30124-9. [DOI] [PubMed] [Google Scholar]
- 91.Pavlov C.S., Casazza G., Nikolova D., Tsochatzis E., Gluud C. Systematic review with meta-analysis: diagnostic accuracy of transient elastography for staging of fibrosis in people with alcoholic liver disease. Aliment Pharmacol Ther. 2016;43:575–585. doi: 10.1111/apt.13524. [DOI] [PubMed] [Google Scholar]
- 92.Papatheodoridi M., Hiriart J.B., Lupsor-Platon M., et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J Hepatol. 2021;74:1109–1116. doi: 10.1016/j.jhep.2020.11.050. [DOI] [PubMed] [Google Scholar]
- 93.EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol. 2021;75:659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 94.Mueller S., Nahon P., Rausch V., et al. Caspase-cleaved keratin-18 fragments increase during alcohol withdrawal and predict liver-related death in patients with alcoholic liver disease. Hepatology. 2017;66:96–107. doi: 10.1002/hep.29099. [DOI] [PubMed] [Google Scholar]
- 95.Bissonnette J., Altamirano J., Devue C., et al. A prospective study of the utility of plasma biomarkers to diagnose alcoholic hepatitis. Hepatology. 2017;66:555–563. doi: 10.1002/hep.29080. [DOI] [PubMed] [Google Scholar]
- 96.Singh S., Venkatesh S.K., Wang Z., et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440–451.e6. doi: 10.1016/j.cgh.2014.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 98.Lucey M.R., Mathurin P., Morgan T.R. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 99.Lackner C., Stauber R.E., Davies S., et al. Development and prognostic relevance of a histologic grading and staging system for alcohol-related liver disease. J Hepatol. 2021;75:810–819. doi: 10.1016/j.jhep.2021.05.029. [DOI] [PubMed] [Google Scholar]
- 100.Tsochatzis E., Bruno S., Isgro G., et al. Collagen proportionate area is superior to other histological methods for sub-classifying cirrhosis and determining prognosis. J Hepatol. 2014;60:948–954. doi: 10.1016/j.jhep.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 101.Kim S.U., Oh H.J., Wanless I.R., Lee S., Han K.H., Park Y.N. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol. 2012;57:556–563. doi: 10.1016/j.jhep.2012.04.029. [DOI] [PubMed] [Google Scholar]