ABSTRACT
The tissue engineering triad comprises the combination of cells, scaffolds and biological factors. Therefore, we prepared cell- and drug-loaded hydrogels using in situ silk fibroin (SF) hydrogels induced by dimyristoyl glycerophosphoglycerol (DMPG). DMPG is reported to induce rapid hydrogel formation by SF, facilitating cell encapsulation in the hydrogel matrix while maintaining high cell viability and proliferative capacity. In addition, DMPG can be used for liposome formulations in entrapping drug molecules. Dexamethasone (Dex) was loaded into the DMPG-induced SF hydrogels together with human osteoblast-like SaOS-2 cells, then the osteogenic differentiation of the entrapped cells was evaluated in vitro and compared to cells cultured under standard conditions. Calcium production by cells cultured in DMPG/Dex-SF hydrogels with Dex-depleted osteogenic medium was equivalent to that of cells cultured in conventional osteogenic medium containing Dex. The extended-release of the entrapped Dex by the hydrogels was able to provide a sufficient drug amount for osteogenic induction. The controlled release of Dex was also advantageous for cell viability even though its dose in the hydrogels was far higher than that in osteogenic medium. The results confirmed the possibility of using DMPG-induced SF hydrogels to enable dual cell and drug encapsulation to fulfil the practical applications of tissue-engineered constructs.
Keywords: DMPG, osteogenic differentiation, silk fibroin, three-dimensional cell culture, tissue engineering
Introduction
Tissue engineering is a novel approach for the treatment of physiologically or functionally damaged tissues, and employs three major components: cells, scaffolds and biomolecules.1, 2 Autologous or allogeneic cells can be used as well as off-the-shelf cells with advanced reprogramming or engineering.3 Scaffolds serve as supporting substrates for cell growth and possess biodegradability to allow tissue in-growth or substitution. Biological factors are required for recruiting surrounding cells or inducing and supporting cellular functions for specific purposes. Nevertheless, it is not critical that a tissue-engineered construct is composed of all these three components. For example, the rational selection of the materials and design of scaffolds could provide biophysical and/or biochemical cues that are sufficient to direct cell behaviours. Additionally, drug-loaded scaffolds could recruit surrounding cells as well as control their biological functions to avoid an invasive cell collection process from the patients.3 However, to create these constructs requires logical design, starting from materials selection, processing and fabrication as well as the utilisation of specific cells or biomolecules, which could narrow down practical uses. Therefore, in this study, we produced a tissue engineering platform that is capable of loading cells and biomolecules based on the in situ gelation of silk fibroin (SF).
SF is a fibrous protein derived from various arthropods, serving as a structural molecule. Indeed, it possesses outstanding mechanical properties in comparison to other naturally-derived materials. The mechanical strength of SF results from repetitive sequences in the primary structure, leading to the formation of highly ordered secondary structures, such as β-sheet. For example, SF derived from Bombyx mori silkworms contains considerable amounts of Gly-Ala sequences, which leads to β-sheet formation.4 Therefore, SF has been considered a base material for engineering tissues requiring high stiffness or biophysical cues in directing specific cell functions, such as bone, cartilage, or blood vessels.5, 6
For cell encapsulation, a rapid but controllable sol-to-gel transition of the SF solution is necessary. This process allows for fine dispersion of the cells in a three-dimensional microenvironment upon gelation.7 Typically, gelation of the regenerated SF solution takes approximately one week.8 However, the gelation of SF solution can be accelerated by inducing the self-assembly process, with which the gelation time of SF can be controlled ranging from a few minutes to hours, using physical interventions, such as ultrasonication9 or vortexing,10 the addition of chemicals, namely anionic surfactants11, 12 or metal salts,13, 14 or polymer blending, e.g., with polyethylene glycol or SF from non-mulberry silkworms15 or spiders.16 SF hydrogels can also be formed using a photo-crosslinking reaction17 or enzymatic crosslinking,18, 19 which are compatible with physiological conditions. In addition, we have found that a negatively-charged phospholipid, dimyristoyl glycerophosphoglycerol or DMPG, can accelerate SF gelation to within one hour, and the encapsulated cells exhibit high viability and proliferation potential.20 DMPG can also be used to produce liposomes encapsulating different molecules. In this way, the developed liposomes can induce SF gelation while controlling the release of the loaded molecules.21
Dexamethasone (Dex) possesses the ability to enhance the expression and activation of Runx2, a transcription factor involved in osteogenesis, and in the presence of ascorbic acid and β-glycerophosphate, induces collagen 1 secretion and hydroxyapatite formation, respectively.22 However, its poor water solubility requires an organic solvent for dissolution which might not be suitable for clinical use. Lipid- or polymeric-based delivery systems, therefore, can improve the Dex content in an aqueous environment as well as enable sustained release.23
In this work, a dual drug- and cell-loaded DMPG-SF hydrogel was fabricated, and the in vitro osteogenic differentiation of the entrapped cells was evaluated and compared to their culture under general conditions. Dex was loaded into the SF hydrogels, and their gelation was accelerated using DMPG phospholipid, together with encapsulation of osteoblastic SaOS-2 cells. The continuous release of Dex from the hydrogels was proposed, and the osteogenesis of the entrapped cells was expected without a requirement for extra Dex in the culture medium. The developed hydrogel system would thus form a three-dimensional cell encapsulation matrix conforming to the tissue-engineering concept with the loading of biological factors, directing the biological activities of the entrapped cells.
Methods
Preparation of cell- and dexamethasone-loaded silk fibroin hydrogel
Silk cocoons of Thai domestic Bombyx mori silkworms (Nangnoi Srisaket 1 breed) were kindly provided by the Queen Sirikit sericulture centre, Nakhon Ratchasima province, Thailand. SF fibres were isolated by boiling silk cocoons in 0.02 M Na2CO3 (Sigma-Aldrich, St. Louis, MO, USA) for 20 minutes twice to eliminate silk sericin and other soluble components. Dry SF fibres were then dissolved in 9.3 M LiBr (Sigma-Aldrich) for 4 hours at 70°C before dialysis against deionised water for 48 hours to remove the salt.24 Finally, the obtained SF solution was steam-sterilised at 121°C for 20 minutes.
To prepare Dex-loaded SF hydrogels, DMPG (Lipoid GmbH, Ludwigshafen/Rhine, Germany) was used to induce a rapid sol-to-gel transition of the protein, and the hydrogels were prepared according to the protocol provided in our previous studies.20, 21 To do so, an ethanolic solution of DMPG and Dex (Sigma-Aldrich) at a molar ratio of 2 to 0.2525 was prepared. Then, the organic solvent was evaporated using a rotary evaporator (RV 3, IKA, Staufen, Germany) before hydrating the lipid film with water. The obtained suspension of multilamellar liposomes was subjected to bath sonication for 1 hour. The resulting liposomes were sterile-filtered through 0.22-µm membranes. Subsequently, they were mixed with the SF solution to achieve a final concentration of 3% (w/v) SF and 5, 10 or 15 mM DMPG (corresponding to 0.625, 1.25 and 1.875 mM Dex, respectively). In addition, dextrose (Sigma-Aldrich) and HEPES (Invitrogen, Waltham, MA, USA) buffer were added at final concentrations of 5% (w/v) and 10 mM to control the physiological osmolarity (∼250 mOsm/L)26 and pH (pH 7.4), respectively. The mixtures were allowed to gel in a silicone mould and the hydrogels were punched into a disc shape of 5 mm diameter and 2 mm thickness for further experiments.
For cell-loaded hydrogels, the SaOS-2 (RRID: CVCL_0548) cell suspension was added to pH- and osmolarity-controlled SF solution prior to mixing with the DMPG or DMPG/Dex mixture. The final cell density encapsulated in the hydrogels was 1 × 106 cells/mL. The samples were divided into four groups as follows:
Group 1: cell-loaded DMPG-SF hydrogels cultured in proliferation medium;
Group 2: cell-loaded DMPG-SF hydrogels cultured in osteogenic medium;
Group 3: cell-loaded DMPG/Dex-SF hydrogels cultured in proliferation medium;
Group 4: cell-loaded DMPG/Dex-SF hydrogels cultured in Dex-depleted osteogenic medium
where the proliferation medium consisted of high-glucose Dulbecco’s modified Eagle medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% foetal bovine serum (FBS) (Thermo Fisher Scientific) and 1% penicillin/streptomycin (Gibco). The osteogenic medium was the above medium supplemented with 50 µM L-ascorbic acid 2-phosphate (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich) and 0.1 µM Dex. All hydrogels were cultured in a 24-well non-cell culture treated multiwell plate (1 hydrogel disc/well; Corning Life Sciences, Corning, NY, USA) with a medium change three times per week for 28 days. The culture plates were incubated at 37°C in a 5% CO2 biological incubator.
Characterisation of the hydrogel
Mechanical properties of the cell-free hydrogels were evaluated in an unconfined compressive mode using a universal testing machine (Instron 5540, Instron, Norwood, MA, USA) equipped with 1 kN load. The compressive load was automatically terminated when the samples collapsed. Prior to the tests, the hydrogels were immersed in 0.01 M phosphate buffered saline (PBS; Sigma-Aldrich) solution overnight to ensure full hydration and mimic cell culture conditions. A preload of 0.01 N and crosshead movement of 2 mm/min were applied. At least 10 samples were tested, and the compressive modulus was determined from the slope of a stress-strain curve in an elastic region.
Protein diffusivity of the hydrogels was evaluated using FBS as a model soluble protein, and the procedure was conducted according to a previous study.27 The samples were immersed in 10% (v/v) FBS in PBS for between 10 minutes and 5 hours at 37°C, before rinsing briefly with deionised water three times to remove released proteins. Subsequently, 1 mL PBS was added, and the samples were incubated at 4°C overnight with gentle shaking. The amount of proteins in the supernatants was determined using the Bradford protein assay (Bio-Rad Laboratories, Hercules, CA, USA), according to the manufacturer’s protocol.
To investigate the release profile of Dex, DMPG/Dex-SF hydrogels were immersed in 1 mL of 0.01 M PBS with 0.01% sodium azide. At each time point, 100 µL of sample were collected and replaced with the same amount of buffer. Then, the absorbance of the supernatants was measured at 244 nm, using a Microtiter¯ UV-transparent 96-well plate (Corning). The slope of the standard curve was used to calculate the amount of Dex released. DMPG-SF hydrogels were used as a blank since the absorbance of protein (∼260 nm) could interfere with the absorbance of Dex.
At the final timepoint, the buffers were discarded and replaced with 6 N HCl. Then, they were incubated at 60°C until the samples were completely dissolved. The absorbance of supernatants was measured as described above. These values were used to calculate the percentage of the cumulative release of Dex from the hydrogels.
Proliferation and osteogenic differentiation of encapsulated SaOS-2 cells
The number of encapsulated SaOS-2 cells in the hydrogels was calculated from the DNA content determined by Hoechst 33258 (Thermo Fisher Scientific) assay.28 At designated time points (days 1, 7, 14, 21 and 28), cell-loaded hydrogels were collected, washed with PBS, and immersed in a lysis buffer containing 0.02% sodium dodecyl sulphate in saline citrate buffer. Three freeze-thaw cycles (frozen at -80°C overnight and thawed at room temperature for 6 hours) were applied to ensure complete cell lysis. After that, the cell supernatants were mixed with Hoechst 33258 working solution and the fluorescence emission values at 460 nm were measured under an excitation wavelength of 355 nm using a fluorescence microplate reader (FLUOstar Omega, BMG Labtech, Ortenberg, Germany).
Osteogenic differentiation of encapsulated SaOS-2 cells was analysed based on changes in the activity of alkaline phosphatase (ALP) and the deposition of calcium. To determine ALP activity, the supernatant from cell lysates obtained previously was mixed with 4-nitrophenyl phosphate substrate (Sigma-Aldrich) and incubated for 15 minutes at 37°C. After terminating the reaction with 0.02 N NaOH, the absorbance values at 405 nm were measured using a microplate reader. ALP activity was calculated based on the standard curve of the reference nitrophenol. Calcium production by the osteogenic-differentiated SaOS-2 cells was analysed using the orthocresolphthalein complexone assay.29 Calcium was extracted from the cell lysates by incubation with 1 N HCl at 4°C overnight. The calcium-extracted cell lysates were then mixed with the orthocresolphthalein complexone substrate (Sigma-Aldrich) in 0.8 M ethanolamine buffer (pH 11), and the absorbance values at 570 nm were determined.
In addition, mineralisation in the hydrogels was visualised using alizarin red staining of thin sections. Briefly, on day 7, the samples were collected, washed with PBS, cut into thin sections using a razor blade and fixed with 2.5% (v/v) glutaraldehyde (Sigma-Aldrich) in PBS for 30 minutes at room temperature. The samples were then stained with 2% (w/v) alizarin red (HiMedia Laboratories, Mumbai, India) solution (pH 4.1) for 2 minutes, before rinsing with deionised water. The mineralisation pattern of the hydrogels was microscopically observed using a phase-contrast microscope (Nikon Eclipse 80i, Nikon, Tokyo, Japan).
Statistical analysiss
All experiments were conducted in triplicate unless otherwise stated. IBM¯ SPSS¯ Statistics software (version 22; IBM, Armonk, NY, USA) was used for statistical analysis using one-way analysis of variance with Bonferroni post-hoc tests at P-value ≤ 0.05.
Results and Discussion
Besides the biocompatibility, biodegradability, and versatility of SF-based materials, the mechanical properties of this protein, which outperforms other natural-derived biopolymers, make it suitable for tissue-engineered constructs.30 SF has been widely studied for use in the regeneration of bones, where material stiffness is necessary for osteogenesis and to support bone homeostasis.31 The concept of tissue engineering raised by Langer and Vacanti2 in 1993, involves three main components, namely cells, scaffolds, and bioactive molecules. With recent approaches, cells can be advance-engineered or reprogrammed, or their functions can be directed by specific biological factors for different applications.3 In this work, SF hydrogels with a rapid sol-to-gel conversion induced by DMPG were prepared to encapsulate cells together with the loading of a drug, Dex, in liposomes. Dex was used to induce osteogenic differentiation of the entrapped cells since it is involved in the induction and activation of Runx2.22
Physical properties of the hydrogel
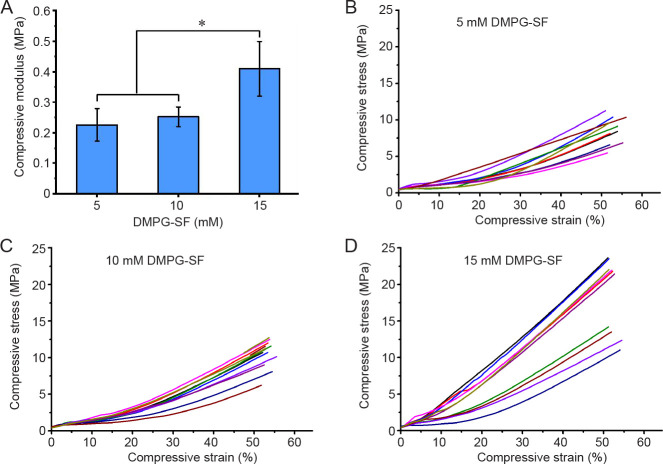
The mechanical characteristics of the hydrogels were investigated using compressive testing to evaluate their potential as bone scaffolds. With a fixed amount of 3% (w/v) SF and a varied DMPG concentration, the elastic modulus of the hydrogels showed no significant differences between 5 and 10 mM DMPG-SF hydrogels, while that of 15 mM DMPG-SF samples was significantly greater than others (P ≤ 0.05; Figure 1). These findings are in accordance with our previous result,20 which showed that the mechanical properties of the hydrogels were enhanced with increases in the added amounts of DMPG.
Figure 1. (A) Elastic modulus of the 5, 10 and 15 mM DMPG-3% (w/v) SF hydrogels determined by unconfined compression (mean ± SD, n = 10). *P ≤ 0.05 (one-way analysis of variance followed by Bonferroni post-hoc tests). (B-D) Compressive stress-strain plots of 5 (B), 10 (C), and 15 (D) mM DMPG-3% (w/v) SF hydrogels. DMPG: dimyristoyl glycerophosphoglycerol; SF: silk fibroin.
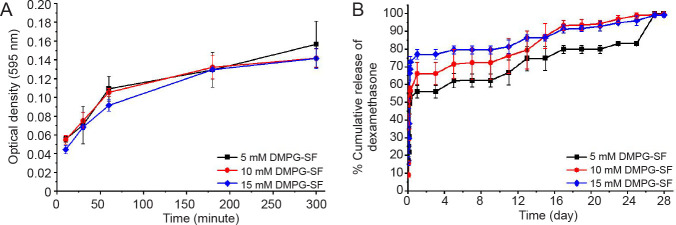
The diffusivity of small molecules, proteins and gases through the hydrogels is an important factor in keeping entrapped cells viable. Indeed, it allows for mass transfer of nutrients, biological factors, and waste products.7 FBS was used as a model since it is composed of a mixture of proteins and bioactive molecules with different diffusion coefficients, and thus acted as a model of the cell culture conditions.16, 32 For all samples, rapid diffusion of FBS from the hydrogels was noticeable in the first 60 minutes before gradually slowing and then reaching a plateau after 180 to 300 minutes (3 to 5 hours) (Figure 2A). These results could be related to the release of Dex (Figure 2B) which exhibits a burst release within the first 6 hours. Such rapid mass transfer in the first few hours could reflect the release of Dex that was not entrapped in the liposomes or physically associated with the hydrogels. After that, remaining Dex entrapped within the hydrogels was gradually released to the medium over at least 28 days. The results indicated that the DMPG-SF hydrogels can entrap small molecules and enable prolonged release. This is in accordance with our previous studies using DMPG-based SF hydrogels to entrap curcumin.21
Figure 2. (A) Diffusivity of fetal bovine serum from the DMPG-SF hydrogels, determined from the protein released into the supernatants using Bradford protein assay. (B) Release profile of dexamethasone from 5, 10 and 15 mM DMPG/dexamethasone-SF hydrogels (containing 0.625, 1.25 and 1.875 mM dexamethasone, respectively) in 0.01 M phosphate-buffered saline (pH 7.4). Data are expressed as mean ± SD, and were analysed by one-way analysis of variance followed by Bonferroni post-hoc tests. The experiment was repeated three times. DMPG: dimyristoyl glycerophosphoglycerol; SF: silk fibroin.
Proliferation of encapsulated SaOS-2 cells in the hydrogel
The cytocompatibility of the DMPG-induced SF hydrogels was proven in our previous reports. Cell viability after exposure to extracts of DMPG-SF hydrogels was higher than 70%, thus the materials were considered non-toxic according to the ISO 10993 standard.20 Furthermore, cells cultured either on the hydrogel or encapsulated within the hydrogel network were viable and able to proliferate in long-term culture.20, 21
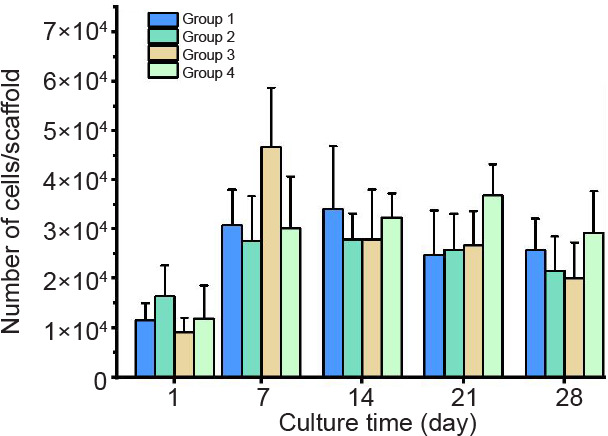
In this work, to evaluate the osteogenic potential of the entrapped cells in Dex-loaded SF hydrogels, the human osteoblast-like cell line, SaOS-2 was chosen since this cell line is well known for its biological activities in bone production, and is widely used for studies relating to bone tissue engineering.33 SaOS-2 cells were encapsulated in the DMPG-SF hydrogels and cultured under different conditions, as mentioned earlier. The proliferation profile of the entrapped SaOS-2 cells, as shown in Figure 3, revealed no significant difference among groups (P ≤ 0.05). The number of cells increased within the first week and then plateaued after this time point up to 28 days. This finding was discussed in our previous study20 since cells cultured in a three-dimensional environment could remain in a dormant state rather than proliferating rapidly. Additionally, SF has been considered a biologically inert material. Indeed, it does not support cell adhesion and proliferation well, or enhance cellular activities but also does not cause deterioration of the cells.34, 35 Our recent data also confirmed that the majority of cells grown on SF-based materials were in S phase, leading to cell cycle arrest and growth inhibition.36
Figure 3. The number of SaOS-2 cells entrapped within DMPG-SF hydrogels cultured under different conditions. Group 1: cell-loaded DMPG-SF hydrogels cultured in proliferation medium; Group 2: cell-loaded DMPG-SF hydrogels cultured in osteogenic medium; Group 3: cell-loaded DMPG/Dex-SF hydrogels cultured in proliferation medium; Group 4: cell-loaded DMPG/Dex-SF hydrogels cultured in Dex-depleted osteogenic medium. Cell number was determined using Hoechst 33258 DNA quantification assay. Data are expressed as mean ± SD, and were analysed by one-way analysis of variance followed by Bonferroni post-hoc tests. The experiment was repeated three times. Dex: dexamethasone; DMPG: dimyristoyl glycerophosphoglycerol; SF: silk fibroin.
Alkaline phosphatase activity of the encapsulated SaOS-2 cells and calcium deposition of the hydrogel
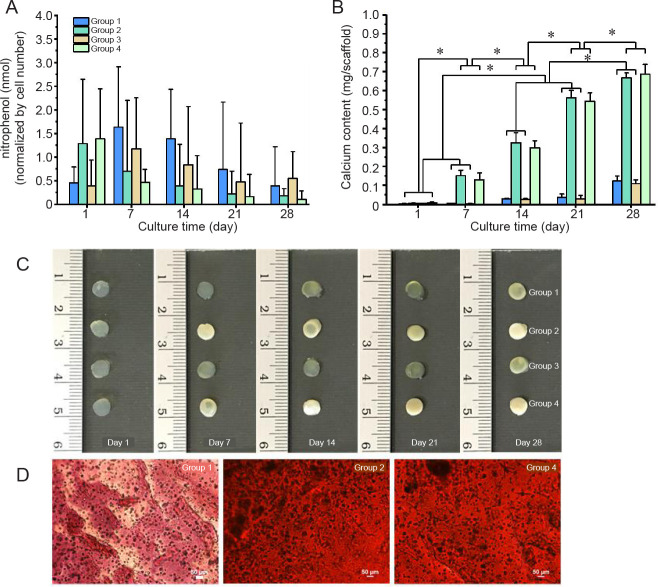
Osteogenic differentiation of the entrapped SaOS-2 cells was evaluated from ALP activity (Figure 4A) and calcium deposition (Figure 4B). Considering the ALP activity, an increase was observed as expected in Group 2, the DMPG-SF hydrogels cultured in complete osteogenic medium and in Group 4, the DMPG/Dex-SF hydrogels in Dex-depleted osteogenic medium (Figure 4A). However, the ALP activity was higher only on the first day of culture before declining at later time points (after day 7). Conversely, for Groups 1 and 3 (hydrogels in proliferation medium), ALP activity was higher at the later time points. This could be because ALP is recognised as an early marker of differentiation and is gradually down-regulated during osteoblastic differentiation.37 Therefore, calcium deposition was determined, and, as expected, the hydrogels in osteogenic induction medium (Groups 2 and 4) showed a substantial increase in mineralisation, with a marked increase at every time point (Figure 4B). For Groups 1 and 3, which were the cells cultured in proliferation medium, without and with Dex loading, respectively, the deposition of calcium after 7 days of culture was significantly lower than that of the groups cultured in osteogenic medium (P ≤ 0.05). Since the cells were cultured in the absence of osteogenic induction condition, and, for Group 3, the lack of β-glycerophosphate, which is the phosphate source supporting cell mineralisation,22 calcium deposition was low in the hydrogels. As shown in Figure 4C, the calcium production by the entrapped SaOS-2 cells within the hydrogels can be visualised in the highly opaque colored samples of Groups 2 and 4, which were the cell-loaded hydrogels cultured in complete osteogenic medium and the Dex hydrogels cultured in the osteogenic medium without the supplementation of Dex, respectively. In addition, microscopic evaluation of the samples after alizarin red staining also confirmed the high calcium production of Groups 2 and 4, while that of Group 1, cultured in proliferation medium, showed no calcium deposition (Figure 4D). Thus it can be implied that osteogenesis of the cells cultured in the Dex-loaded hydrogels was almost equivalent to that of the cells cultured in typical osteogenic conditions, confirming that Dex can be entrapped and released in a controllable manner in the DMPG-SF hydrogels.
Figure 4. Osteogenic differentiation of encapsulated SaOS-2 cells in DMPG- or DMPG/Dex-SF hydrogels. Group 1: cell-loaded DMPG-SF hydrogels cultured in proliferation medium; Group 2: cell-loaded DMPG-SF hydrogels cultured in osteogenic medium; Group 3: cell-loaded DMPG/Dex-SF hydrogels cultured in proliferation medium; Group 4: cell-loaded DMPG/Dex-SF hydrogels cultured in Dex-depleted osteogenic medium. (A) Alkaline phosphatase activity of the encapsulated cells, represented as the concentration of nitrophenol standard normalised to the number of cells. (B) Calcium content deposited in the hydrogels. Data are expressed as mean ± SD. The experiment was repeated three times. *P ≤ 0.05 (one-way analysis of variance followed by Bonferroni post-hoc tests). (C) Appearance of the SaOS-2 cells encapsulated in hydrogels and cultured under different conditions at each time point. The opacity of Groups 2 and 4 hydrogels can be seen, implying high calcium deposition in the samples. (D) Microscopic analysis of alizarin red staining of calcium deposition within the cell-entrapped hydrogels cultured for 7 days. The orange-red stain indicates calcium mineralisation. Scale bars: 50 μm. DMPG: dimyristoyl glycerophosphoglycerol; Dex: dexamethasone; SF: silk fibroin.
Typically, the concentration of Dex used for osteogenic induction is 100 nM, so in this study, the loaded Dex in the DMPG-SF hydrogels was approximately 5 to 20 times higher than the recommended concentration. However, the results showed no negative effect on the viability of the entrapped cells (Figure 3). This is likely the result of the gradual release of Dex from the hydrogels over the entire experimental period, after burst release in the first 6 hours (Figure 2B). The effect on calcium production implied that the osteogenesis of the loaded SaOS-2 cells in DMPG/Dex-SF hydrogels cultured in Dex-depleted osteogenic medium was equivalent to that of cells cultured in standard osteogenic medium. Indeed, the Dex-loaded DMPG-SF hydrogels were capable of controlling the release of Dex in sufficient concentrations to induce osteogenic differentiation without the addition of extra Dex in the osteogenic medium.
Certainly, our study demonstrated the loading of a single drug molecule, Dex in this context, in a hydrogel scaffold. The rational design of the DMPG-based liposomes to entrap both hydrophilic and lipophilic molecules could enable loading of more chemicals providing suitable conditions for supporting or enhancing cellular activities. Therefore, such cell encapsulation systems, in which the biological functions of the entrapped cells can be directed without the addition of extra factors, can be simply fabricated, providing an on-the-use application for tissue regeneration in practice.
Conclusion
This work demonstrated the utilisation of tissue-engineered constructs composed of the three major components required for the concept of tissue engineering, namely cells, scaffolds, and biological factors for directing cell behaviours towards specific applications. DMPG, a phospholipid used for liposome formulations, is capable of accelerating SF gelation. Consequently, DMPG-based liposomes can be used not only for encapsulating bioactive molecules, but also to induce in situ gel formation of SF, as previously proposed for curcumin-loaded liposome-SF hydrogels.21 This study revealed that the DMPG-SF hydrogels can load both cells and biological factors, giving them the potential to regenerate the target tissue.
Footnotes
Author contributions: Conceptualisation: NMN, SD; methodology: CL, HF; formal analysis, investigation, visualisation and manuscript draft: CL; validation: HF; manuscript resivion: HF, SK, JAL, NMN, SD; resources: SK, JAL, SD; supervision: SK, JAL, NMN, SD; funding acquisition: SK, NMN, SD; project administration: SD. All authors approved the final version of the manuscript.
Financial support: This research was partially supported by the Asahi Glass Foundation (grant number: RES_65_530_33_026). CL would like to express gratitude for the Second Century Fund (C2F), Chulalongkorn University, for the support of a post-doctoral fellowship.
Acknowledgement: The human osteoblast-like SaOS-2 cell line was a gift from Dr. Supansa Yodmuang, Faculty of Medicine, Chulalongkorn University.
Conflicts of interest statement: The authors declare no conflict of interest.
Reference
- 1.Murphy C. M., O’Brien F. J., Little D. G., Schindeler A. Cell-scaffold interactions in the bone tissue engineering triad. Eur Cell Mater. 2013;26:120–132. doi: 10.22203/ecm.v026a09. [DOI] [PubMed] [Google Scholar]
- 2.Langer R., Vacanti J. P. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Gaharwar A. K., Singh I., Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat Rev Mater. 2020;5:686–705. [Google Scholar]
- 4.Murphy A. R., Romero I. S. 8 - Biochemical and biophysical properties of native Bombyx mori silk for tissue engineering applications. In: Kundu S. C., editor. Silk Biomaterials for Tissue Engineering and Regenerative Medicine. Woodhead Publishing; 2014. pp. 219–238. [Google Scholar]
- 5.Kundu B., Rajkhowa R., Kundu S. C., Wang X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65:457–470. doi: 10.1016/j.addr.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharjee P., Kundu B., Naskar D., Kim H. W., Maiti T. K., Bhattacharya D., Kundu S. C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017;63:1–17. doi: 10.1016/j.actbio.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Nicodemus G. D., Bryant S. J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev. 2008;14:149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumoto A., Chen J., Collette A. L., Kim U. J., Altman G. H., Cebe P., Kaplan D. L. Mechanisms of silk fibroin sol-gel transitions. J Phys Chem B. 2006;110:21630–21638. doi: 10.1021/jp056350v. [DOI] [PubMed] [Google Scholar]
- 9.Wang X., Kluge J. A., Leisk G. G., Kaplan D. L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29:1054–1064. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yucel T., Cebe P., Kaplan D. L. Vortex-induced injectable silk fibroin hydrogels. Biophys J. 2009;97:2044–2050. doi: 10.1016/j.bpj.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X., Hou J., Li M., Wang J., Kaplan D. L., Lu S. Sodium dodecyl sulfate-induced rapid gelation of silk fibroin. Acta Biomater. 2012;8:2185–2192. doi: 10.1016/j.actbio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F., Li J., Zhu T., Zhang S., Kundu S. C., Lu S. Potential of biocompatible regenerated silk fibroin/sodium N-lauroyl sarcosinate hydrogels. J Biomater Sci Polym Ed. 2015;26:780–795. doi: 10.1080/09205063.2015.1058576. [DOI] [PubMed] [Google Scholar]
- 13.Choi J., McGill M., Raia N. R., Hasturk O., Kaplan D. L. Silk hydrogels crosslinked by the Fenton reaction. Adv Healthc Mater. 2019;8:e1900644. doi: 10.1002/adhm.201900644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laomeephol C., Ferreira H., Yodmuang S., Reis R. L., Damrongsakkul S., Neves N. M. Exploring the gelation mechanisms and cytocompatibility of gold (III)-mediated regenerated and thiolated silk fibroin hydrogels. Biomolecules. 2020;10:466. doi: 10.3390/biom10030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhunia B. K., Mandal B. B. Exploring gelation and physicochemical behavior of in situ bioresponsive silk hydrogels for disc degeneration therapy. ACS Biomater Sci Eng. 2019;5:870–886. doi: 10.1021/acsbiomaterials.8b01099. [DOI] [PubMed] [Google Scholar]
- 16.Laomeephol C., Vasuratna A., Ratanavaraporn J., Kanokpanont S., Luckanagul J. A., Humenik M., Scheibel T., Damrongsakkul S. Impacts of blended bombyx mori silk fibroin and recombinant spider silk fibroin hydrogels on cell growth. Polymers. 2021;13:4182. doi: 10.3390/polym13234182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui X., Soliman B. G., Alcala-Orozco C. R., Li J., Vis M. A. M., Santos M., Wise S. G., Levato R., Malda J., Woodfield T. B. F., Rnjak-Kovacina J., Lim K. S. Rapid photocrosslinking of silk hydrogels with high cell density and enhanced shape fidelity. Adv Healthc Mater. 2020;9:e1901667. doi: 10.1002/adhm.201901667. [DOI] [PubMed] [Google Scholar]
- 18.Das S., Pati F., Choi Y. J., Rijal G., Shim J. H., Kim S. W., Ray A. R., Cho D. W., Ghosh S. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015;11:233–246. doi: 10.1016/j.actbio.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Partlow B. P., Hanna C. W., Rnjak-Kovacina J., Moreau J. E., Applegate M. B., Burke K. A., Marelli B., Mitropoulos A. N., Omenetto F. G., Kaplan D. L. Highly tunable elastomeric silk biomaterials. Adv Funct Mater. 2014;24:4615–4624. doi: 10.1002/adfm.201400526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laomeephol C., Guedes M., Ferreira H., Reis R. L., Kanokpanont S., Damrongsakkul S., Neves N. M. Phospholipid-induced silk fibroin hydrogels and their potential as cell carriers for tissue regeneration. J Tissue Eng Regen Med. 2020;14:160–172. doi: 10.1002/term.2982. [DOI] [PubMed] [Google Scholar]
- 21.Laomeephol C., Ferreira H., Kanokpanont S., Neves N. M., Kobayashi H., Damrongsakkul S. Dual-functional liposomes for curcumin delivery and accelerating silk fibroin hydrogel formation. Int J Pharm. 2020;589:119844. doi: 10.1016/j.ijpharm.2020.119844. [DOI] [PubMed] [Google Scholar]
- 22.Langenbach F., Handschel J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther. 2013;4:117. doi: 10.1186/scrt328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madamsetty V. S., Mohammadinejad R., Uzieliene I., Nabavi N., Dehshahri A., García-Couce J., Tavakol S., Moghassemi S., Dadashzadeh A., Makvandi P., Pardakhty A., Aghaei Afshar A., Seyfoddin A. Dexamethasone: insights into pharmacological aspects, therapeutic mechanisms, and delivery systems. ACS Biomater Sci Eng. 2022;8:1763–1790. doi: 10.1021/acsbiomaterials.2c00026. [DOI] [PubMed] [Google Scholar]
- 24.Rockwood D. N., Preda R. C., Yücel T., Wang X., Lovett M. L., Kaplan D. L. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monteiro N., Martins A., Ribeiro D., Faria S., Fonseca N. A., Moreira J. N., Reis R. L., Neves N. M. On the use of dexamethasone-loaded liposomes to induce the osteogenic differentiation of human mesenchymal stem cells. J Tissue Eng Regen Med. 2015;9:1056–1066. doi: 10.1002/term.1817. [DOI] [PubMed] [Google Scholar]
- 26.Carvalho A. F., Gasperini L., Ribeiro R. S., Marques A. P., Reis R. L. Control of osmotic pressure to improve cell viability in cell-laden tissue engineering constructs. J Tissue Eng Regen Med. 2018;12:e1063–e1067. doi: 10.1002/term.2432. [DOI] [PubMed] [Google Scholar]
- 27.Ding X., Yang G., Zhang W., Li G., Lin S., Kaplan D. L., Jiang X. Increased stem cells delivered using a silk gel/scaffold complex for enhanced bone regeneration. Sci Rep. 2017;7:2175. doi: 10.1038/s41598-017-02053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 102;:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 29.Connerty H. V., Briggs A. R. Determination of serum calcium by means of orthocresolphthalein complexone. Am J Clin Pathol. 1966;45:290–296. doi: 10.1093/ajcp/45.3.290. [DOI] [PubMed] [Google Scholar]
- 30.Bucciarelli A., Motta A. Use of Bombyx mori silk fibroin in tissue engineering: From cocoons to medical devices, challenges, and future perspectives. Biomater Adv. 2022;139:212982. doi: 10.1016/j.bioadv.2022.212982. [DOI] [PubMed] [Google Scholar]
- 31.Melke J., Midha S., Ghosh S., Ito K., Hofmann S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016;31:1–16. doi: 10.1016/j.actbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Leong M. F., Chian K. S., Mhaisalkar P. S., Ong W. F., Ratner B. D. Effect of electrospun poly(D,L-lactide) fibrous scaffold with nanoporous surface on attachment of porcine esophageal epithelial cells and protein adsorption. J Biomed Mater Res A. 2009;89:1040–1048. doi: 10.1002/jbm.a.32061. [DOI] [PubMed] [Google Scholar]
- 33.Anderson H. C., Hsu H. H., Raval P., Hunt T. R., Schwappach J. R., Morris D. C., Schneider D. J. The mechanism of bone induction and bone healing by human osteosarcoma cell extracts. Clin Orthop Relat Res. 1995:129–134. [PubMed] [Google Scholar]
- 34.Acharya C., Ghosh S. K., Kundu S. C. Silk fibroin protein from mulberry and non-mulberry silkworms: cytotoxicity, biocompatibility and kinetics of L929 murine fibroblast adhesion. J Mater Sci Mater Med. 2008;19:2827–2836. doi: 10.1007/s10856-008-3408-3. [DOI] [PubMed] [Google Scholar]
- 35.Madden P. W., Lai J. N., George K. A., Giovenco T., Harkin D. G., Chirila T. V. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials. 2011;32:4076–4084. doi: 10.1016/j.biomaterials.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 36.Panchamanee J., Laomeephol C., Luckanagul J. A., Wang Q., Damrongsakkul S. In vitro biological activities of the flexible and virus nanoparticle-decorated silk fibroin-based films. Int J Biol Macromol. 2022;216:437–445. doi: 10.1016/j.ijbiomac.2022.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Long M. W. Osteogenesis and bone-marrow-derived cells. Blood Cells Mol Dis. 2001;27:677–690. doi: 10.1006/bcmd.2001.0431. [DOI] [PubMed] [Google Scholar]