ABSTRACT
Microorganisms with innate and artificial advantages have been regarded as intelligent drug delivery systems for cancer therapy with the help of engineering technology. Although numerous studies have confirmed the promising prospects of microorganisms in cancer, several problems such as immunogenicity and toxicity should be addressed before further clinical applications. This review aims to investigate the development of engineered microorganism-based delivery systems for targeted cancer therapy. The main types of microorganisms such as bacteria, viruses, fungi, microalgae, and their components and characteristics are introduced in detail. Moreover, the engineering strategies and biomaterials design of microorganisms are further discussed. Most importantly, we discuss the innovative attempts and therapeutic effects of engineered microorganisms in cancer. Taken together, engineered microorganism-based delivery systems hold tremendous promise for biomedical applications in targeted cancer therapy.
Keywords: drug delivery systems, engineering strategies, microorganisms, targeted cancer therapy
Introduction
Microorganism-based cancer therapies have been rapidly developed and their use has been reported in numerous studies.1, 2 The application of living microorganisms to treat cancers is attracting growing interest owing to their great anti-cancer advantages compared with conventional therapies.3 As is well known, microorganisms have evolved special bio-functionalities to adapt to changing environments,4 such as targeting ability, immunogenic effects, and metabolic behaviours, and these can be utilised for anti-cancer therapies.5, 6 Furthermore, microorganisms have also reportedly been genetically engineered to confer advantageous anti-cancer properties, including reduced toxicity, activated metabolic behaviours, and generation of anti-cancer drugs or proteins.2 Several types of microorganisms have been observed to have significant probiotic effects such as Clostridium butyricum, Lactobacillus, and Bifidobacterium, and could be utilised to treat disordered microbiota and other diseases.7-9 Accordingly, microorganisms with the above innate and artificial advantages have been regarded as potential intelligent drug delivery systems for cancer therapy with the help of bio-engineering technology.5, 10
Although numerous studies have confirmed the promising prospects of microorganism-based cancer therapy, several problems need to be addressed before this can be realised. For example, microbial immunogenicity might lead to rapid clearance from the blood circulation as well as side effects in normal tissues.11 Even though a few types of microorganisms might be appropriate for cancer therapy if their toxicity was reduced, their therapeutic effects might be weakened at the same time.12 Accordingly, engineering technologies are warranted to produce artificial microorganisms. Such an approach could not only improve the anti-cancer efficacy but also overcome such intrinsic drawbacks. With the rapid development of nanotechnology, microorganism-based biohybrid systems have been constructed to optimise the bio-functionalities of microorganisms in cancer therapy.13 Numerous studies have found that functional biomaterials can be successfully integrated with microorganisms via techniques such as chemical conjugation, physical adsorption, biological assembly, and gene engineering.14-20 To sum up, engineering of microorganism-based delivery systems may lead to good anti-cancer effects and overcome existing limitations, which could greatly expand the applications of functional materials-combined microorganisms in anti-cancer therapies.
For this review, articles describing strategies involving engineered microorganisms were retrieved using the search terms: (Microorganism (MeSH Terms) OR Microbe (MeSH Terms)) AND (Engineered OR Engineering OR Integration OR Biomaterials (MeSH Terms)). Then, articles related to various anti-cancer therapies mediated by engineered microorganisms were retrieved using the search terms: (Microorganism (MeSH Terms) OR Microbe (MeSH Terms) OR Bacteria (MeSH Terms) OR Virus (MeSH Terms) OR Fungi (MeSH Terms) OR Microalgae (MeSH Terms)) AND (Cancer (MeSH Terms) OR Tumor (MeSH Terms)) AND (Chemotherapy (MeSH Terms) OR Immunotherapy (MeSH Terms) OR Phototherapy (MeSH Terms) OR Radiotherapy (MeSH Terms) OR Oncolytic virotherapy (MeSH Terms)). All these searches were performed on PubMed, Embase, Web of Science, and CNKI databases up to August 2022. The results were further screened by title and abstract. Irrelevant articles were excluded.
This review aims to investigate developments of engineered microorganism-based delivery systems for targeted cancer therapy (Figure 1). The main types and characteristics of microorganisms and relevant components are introduced in detail. Moreover, the engineering strategies and biomaterials design of microorganisms are further explained. Most importantly, we discuss the innovative attempts and therapeutic effects of engineered microorganisms in cancer. The concluded limitations and future research directions in this study might help enhance the application of microorganisms in cancer treatment.
Figure 1. Schematic diagram of microorganism-based delivery systems for targeted cancer therapy.
The Advantages and Functions of Microorganisms in Anti-Cancer Therapy
The advantages of microorganism-based delivery systems in cancer therapy have been demonstrated in relation to specific characteristics including active mobility or swimming capability,21 precise tumour targeting and penetrating behaviours,6 stable biological structures,22, 23 flexible survival and proliferation in tumour microenvironments,24 induction of innate immunity,25 and easy engineering and functionalisation.26-28
Tumour targeting and penetration abilities
The major mechanisms allowing the targeted accumulation of microorganisms in cancer are as follows: (a) active targeting and movement toward tumour tissues in response to the hypoxic nature of cancers by some obligate or facultative anaerobic microorganisms because of their hypoxic environment tropism; (b) chemotaxis of microorganisms toward compound gradients produced by cancer cells; (c) trapping of microorganisms in the blood circulation system due to the tumour’s vasculature, contributing to their passive accumulation in cancers; (d) escape from immune clearance by microorganisms which migrate into cancer tissues, because cancers are commonly regarded as immunologically-exempt compartments. Thus both active and passive targeting abilities of microorganisms play a vital role in enhanced anti-cancer efficacy.29
Intrinsic stability
Owing to the relatively intact and stable structure, most microorganisms are able to live within the normal physiology of the human body, instead of being influenced by changes in the environment. More importantly, this structural stability indicates that microorganism-based drug delivery systems might remain stable and target delivery of drugs to cancers.30
Regulation of tumour metabolism
Microorganisms could stay and survive in cancer tissues by taking up nutrients derived from necrotic cancer cells. Therefore, only a one-dose treatment would be enough to enable long-term anti-cancer efficacy. Microorganisms could also play a role in tumour metabolism and further affect tumour growth. In detail, microorganisms might compete for nutritional resources, remodel the tumour microenvironment and even dysregulate tumour metabolic activities.31 Moreover, microbial metabolites or components could function as tumour vaccines or anti-cancer therapeutic agents.32 Microorganisms can be genetically engineered to produce proteins, enzymes, or drugs for cancer therapy.10
Regulation of the disordered microbiota
It has been shown that a disordered microbiota participates in the development and progression of various types of cancer.33, 34 For instance, the overgrowth of certain microorganisms within a disordered microbiota might contribute to tumorigenesis accompanied by DNA damage, immune dysfunction, and excess carcinogenic metabolites.35, 36 However, several probiotic microorganisms might play an anti-cancer role by regulating the disordered microbiota, which could be applied in intelligent and effective anti-cancer therapies.37
The Engineering Strategies of Microorganisms
With regard to living microorganisms, their essential advantages are mainly based on their active characteristics such as directed tropism, metabolic activity, swimming capability, easy modification, and natural anti-cancer capability,1 which equip them with powerful anti-cancer effects.
However, exogenous microorganisms are deemed to be quickly inactivated and cleared by the immune system in the human body, thereby weakening any therapeutic effects and leading to unexpected adverse effects.38 Moreover, the human body is also lacking in several indispensable elements for maintaining the metabolic activity of microorganisms.2 Accordingly, it is necessary to combine the merits of microorganisms with complementary merits of biomaterials. Several engineering strategies employing microorganisms and materials are designed for functionalisation, which include physical, chemical, and biological integrations. In this way, enhanced anti-cancer effects can be achieved by various strategies such as improving microbial activity and avoiding immune clearance. The three main integration methods are introduced below.
Physical integration strategies via electrostatic interactions
It is well known that the surfaces of microorganisms are often negatively charged. Based on this, microorganisms might be physically modified by attaching positively-charged materials onto the surface via electrostatic absorption.2, 39 One study indicated that poly-lactic-co-glycolic acid-encapsulated nanoparticles could be attached to bacteria by coating the positive glycol chitosan on the surface of the bacteria40 (Figure 2A). In another study, metal cations such as calcium ions appeared to assist the assembly of extra lipid membranes and reverse microbial surface charges.41 The charged functional groups enable the further modification of microorganisms by charge-reversal layer-by-layer encapsulation42 and charge-guided membrane intercalation.43 For instance, alternating layers on the surface of probiotic organisms were achieved by sequentially depositing the cationic polysaccharide chitosan and the anionic polysaccharide alginate, which then protected the activity of probiotics and further promoted their oral delivery.28
Figure 2. Representative examples of (A) physical integration strategies via electrostatic interactions, (B) chemical engineering strategies via covalent conjugation, (C) biological engineering strategies via genetic editing, and (D) cell membrane coating strategy. Scale bars: 1 μm. Ce6: chlorin e6; DOX: doxorubicin; E. Coli: Escherichia coli; E. Coli(p): Escherichia coli with a plasmid expressing the catalase; E. faecalis: Enterococcus faecalis; Ec-PR848: PR848 nanoparticle-load E. Coli; LP: liposome; MTB: magnetotactic bacteria; pDA: polydopamine; PDOX: DOX-loaded PLGA nanoparticles; PLGA: poly(lactic-co-glycolic acid); S. aureus: Staphylococcus aureus. A was reprinted with permission from Wei et al.40 Copyright 2021 American Chemical Society. B was reprinted with permission from Taherkhani et al.51 Copyright 2014 American Chemical Society. C was reprinted from Deng et al.59 Copyright 2021, with permission from Elsevier Ltd. D was reprinted from Cao et al.45.
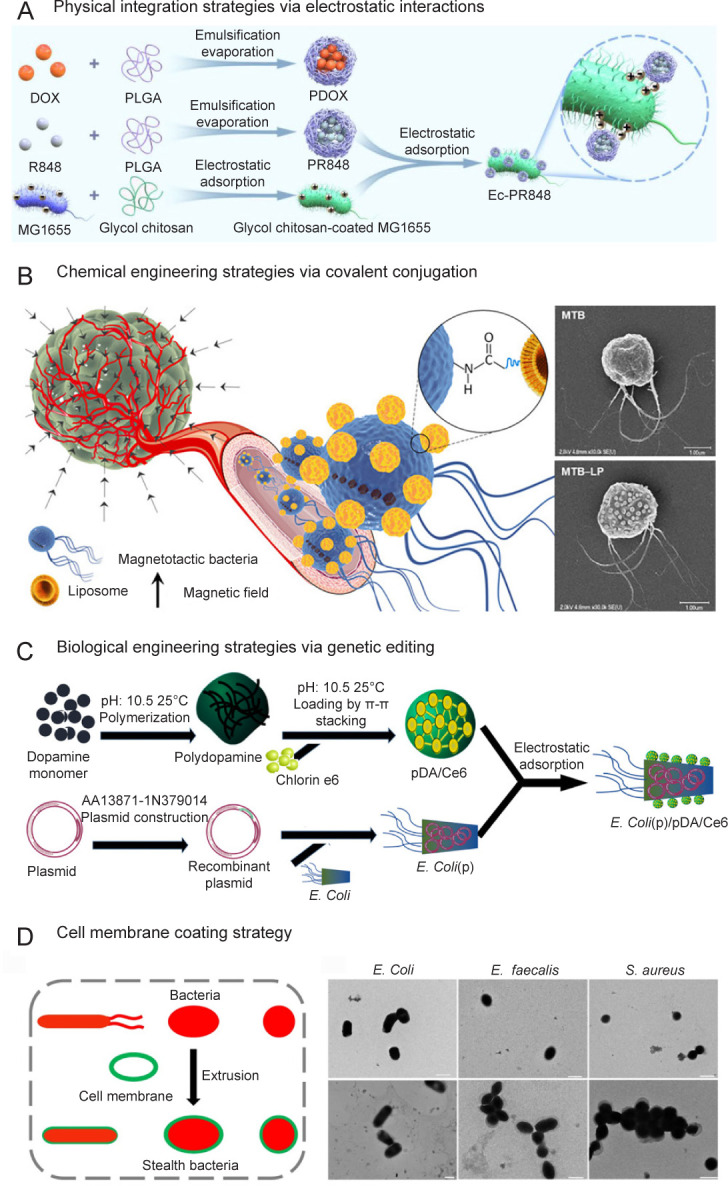
Moreover, the membrane-intercalating method by preparing the side chains with specific functional groups was also utilised to modify microorganisms.43 One study constructed cationic side chains containing organic semiconductors to enhance the light-harvesting ability of bacteria. In this study, with the help of cationic side chains, perylene diimide derivative and poly (fluorene-co-phenylene) could be absorbed on the bacterial surface by electrostatic interactions, and then intercalated into cell membranes via hydrophobic interactions.44
To avoid the immune response caused by microorganisms, cell membrane-coated nanotechnology has been widely utilised for camouflaging the available microorganisms.45 The microorganism-delivered cell membrane nanovesicles are often constructed by the physical extrusion method via polycarbonate membranes.46
The present physical integration strategies are mainly based on electrostatic interactions, which are relatively convenient to integrate microorganisms with functional materials. However, more positively-charged materials are needed to assist their structural stability, which could be harmful to microorganisms by perturbing their membrane stability. Accordingly, structural stability and microorganism activity are two major problems which need to be completely solved.
Chemical engineering strategies via covalent conjugation
The chemical components of the microbial cell wall are mainly peptidoglycans, polysaccharides, proteins, lipopolysaccharides, and so on.47 Therefore, it might be possible to modify the microbial cell surface owing to the presence of various functional groups such as amino, carboxyl, thiol, and hydroxyl groups.48, 49 Additionally, the high surface-to-volume ratios of microorganisms largely reduce the adverse effects of surface chemical modification against the host cells.50 With the primary amines on bacterial surfaces, the carboxyl-carrying materials could be chemically conjugated onto bacteria via carbodiimide-induced amido bond formation.51, 52 Similarly, the carbodiimide-induced conjugation between primary amine-carrying materials and bacteria is achieved because the bacterial cytoderm is rich in N-acetylmuramic acid and carboxyl groups.53 Owing to the thiol-based crosslinking, the thiol on the microbial surface is deemed to be a common method of covalently modifying microorganisms.54
Chemical groups including carboxyl, amine, and thiol groups play a vital role in the chemical surface conjugation of microorganisms.55 Conjugating functional materials to microorganisms has the potential to greatly enhance therapeutic efficacy when compared with microorganisms alone (Figure 2B). More importantly, biodegradable materials and environmentally-responsive linkers are considered to minimise toxicity and promote spatiotemporal delivery.
Biological engineering strategies employing gene editing
Biological engineering of microorganism-based delivery systems is a rapidly-growing and well-developed technology. For instance, siRNA or CRISPR-Cas9 techniques are widely utilised to edit cell genes.56 When compared with chemical engineering strategies, biological engineering strategies are much more biocompatible and host cell-friendly because fewer chemical molecules are involved. More importantly, unlike the temporary modification of chemical engineering, the functionality of microorganisms can be permanently changed by biological engineering (Figure 2C).
As for microorganism-based delivery systems, genetic engineering is widely utilised to construct safe microorganisms by deleting virulence genes and customising microorganisms with specific functions. Based on this, various bacterial functionalities including targeting capability and synthesis of metabolites might be enhanced to treat different diseases.57 Furthermore, engineered microorganisms could serve as the “therapeutic factory” to produce desired therapeutic agents in tumour tissues for improved anti-cancer efficacy and reduced adverse effects. Another study constructed a genetic circuit via computational modelling, allowing engineered bacteria to activate anti-tumour immunity, which might resolve the current clinical obstacles in anti-tumour immunotherapy.58 It is also reported that non-invasive Escherichia coli (E. coli) could be genetically modified through plasmid transfection to endow E. Coli(p) with overexpressed human catalase for catalysing H2O2 into O2 at the tumour site.59 Genetically engineered microorganisms have been investigated in clinical trials to treat various diseases including cancer.60 However, the following problems need to be resolved before further application in the clinic: (a) it is of great importance to protect microorganisms from harsh environments and sustain their viability and ability to secrete therapeutic agents. The combination of surface coatings and engineered microorganisms might be useful to resolve this issue; (b) safety is the main concern during the application of both living microorganisms and the genetic engineering approach, which should be thoroughly investigated before clinical applications.
Cell membrane coating strategy
Microorganisms play a vital role in the development of soft microrobots because of their active targeting, robust self-propulsion, and sensing abilities.61 However, their pathogenicity and potential side effects greatly limit their safety and efficacy. Cell membranes are characterised by superior biocompatibility and “self-recognition” in the human body, and so are used for coating nanoparticles to protect them from the immune system.62 Based on cell membrane-coating nanotechnology, engineering microorganisms with cell membranes might overcome the obstacles above. One study coated bacteria with red blood cell membranes to prepare cell membrane-coated bacteria by mechanical extrusion.45 With the help of a low immunogenic red blood cell membrane, cell membrane-coated bacteria might preserve the inherent bioactivity of bacteria while providing superior safety. Moreover, cell membranes could also be attached to microorganisms via biotin–avidin affinity (Figure 2D). One study designed a biohybrid microswimmer by binding bacteria to red blood cells via the biotin–avidin interaction.22 In this way, this microswimmer could load doxorubicin and superparamagnetic iron oxide nanoparticles with immune evasion properties. In addition to the motility of bacteria, sperparamagnetic iron oxide nanoparticle-containing microswimmers might also move in response to magnetic fields.
The above cell membrane coating or binding strategies have expanded clinical use of microorganism-based delivery systems. Additionally, hybrid membranes derived from different cells can be applied in microorganism engineering to provide multifunctionality,63 which might further promote the potential clinical translation of microorganisms. Owing to the advantages and disadvantages of each strategy, it is necessary to combine the complementary merits of two or more strategies according to the desired functions of the microorganisms.
Engineered Microorganisms for Various Anti-Cancer Therapies
Chemotherapy
Bacteria-mediated chemotherapy
Although it is widely known that some bacterial infections can result in malignancy or increase the risk of cancer, some bacteria have long been regarded as potential agents for chemotherapy. On the one hand, bacteria could exert a direct anti-tumour effect via toxins, peptides, and bacteriocins. For instance, Bovicin HC5 from Streptococcus devastates tumour cells by drilling pores in the cell membrane which induce potassium efflux,12 while Pyocin S2 destroys the DNA sequence of multiple lines of cancer cells to induce cell death.64 On the other hand, they have been widely used as engineered vectors of peptides, therapeutic drugs, or genes30 (Figure 3). When the engineered bacteria are administered systematically, bacteria accumulate in the tumour sites rather than healthy tissues as a result of several mechanisms. Generally, the bacteria disseminated in the healthy tissues would be quickly cleared by the immune system, while in tumour sites, the immunosuppressive microenvironment protects the bacteria from immune clearance. The infiltrating levels of T cells are decreased and the functions of T cells are reduced in the local sites, a phenomenon which is also termed T cell exhaustion. In addition, the macrophage phenotypes in tumour sites are different compared with normal tissues, with lower expression of M1 macrophages, leading to tolerance to infection. These mechanisms would lead to the continuous proliferation of bacteria. Several bacteria species such as Salmonella spp., E. coli, or Listeria spp. have been attenuated via genetic deletion of their key virulence genes and they were then used in phase I or phase II clinical trials as nanocarriers.2, 65-68 Besides, the hypoxic environment provides shelter for anaerobic bacteria.69 In addition, a recent investigation revealed that microbiota from the gut may influence the body responses to chemotherapies.70 These mechanisms endowed the bacteria with tumour-targeting ability and satisfactory anti-tumour efficacy, which make bacteria a potential choice for chemotherapy12, 64, 71-75 (Table 1).
Figure 3. Two applications of bacteria in cancer chemotherapy. Reprinted from Cao and Liu.30 Copyright 2020 Elsevier B.V.
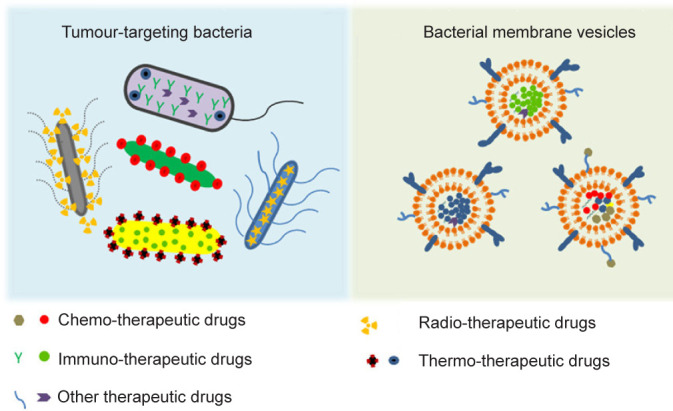
Table 1. The origin and anti-cancer mechanisms or effects of microorganism-based anti-cancer therapy.
| Therapy | Microorganisms | Substance | Mechanisms/effects | References |
|---|---|---|---|---|
| Chemotherapy | Streptococcus | Bovicin HC5 | Drilled pores in cell membranes | 12 |
| Pseudomonas aeruginosa | Pyocin S2 | Destroyed the DNA sequence | 64 | |
| Escherichia coli | Colicin E1/A | Induced necrosis | 71 | |
| Brevibacillus sp. | Laterosporulin 10 | Induced apoptosis or necrosis | 72 | |
| Lactococcus lactis | Nisin A/ZP | Inhibited cell proliferation and angiogenesis | 73, 74 | |
| Pediococcus acidilactici | Pediocin CP2/K2a2 | Induced apoptosis | 75 | |
| Mushroom | Polysaccharide-peptide | Prolonged the 5-year survival rate in oesophageal cancer | 78 | |
| Chlorella ellipsoidea | Carotenoid | Devastated colon or colorectal cancer | 81 | |
| Synedra acus | Chrysolaminaran | Devastated colon or colorectal cancer | 82 | |
| Cocconeis scutellum | Eicosatetraenoic acid | Exerted anti-cancer effect in breast cancer | 83 | |
| Immunotherapy | Gram-negative bacteria | Peptidoglycan, LPS | Triggered the immune responses toward malignancy | 84, 85 |
| Gram-positive bacteria | Peptidoglycan, LTA | |||
| Phototherapy | E. Coli(p) | Membrane | Coated pDA@Ce6 to trigger phototherapy | 59 |
| Radiotherapy | Gut microbiome | Adjusted the responses to radiotherapy | 91, 92 | |
| OV therapy | HSV | T-VEC | Targeted and killed the tumour cell | 95, 96 |
Note: E. Coli(p): Escherichia coli with a plasmid expressing the catalase; HSV: herpes simplex virus; LPS: lipopolysaccharide; LTA: lipoteichoic acid; OV: oncolytic virus; pDA@Ce6: chlorin e6 conjugated poly(dopamine) nanosphere; T-VEC: talimogene laherparepvec.
Fungi-mediated chemotherapy
Fungi are widespread in the world and several chemicals extracted from several species of fungi have exhibited anti-tumour efficacy in vitro or in vivo (Table 1). All these fungi are confirmed non-toxic and are well-tolerated. For example, an active hexose-correlated compound has shown its potential in clinical application.76 Fungal β-glucan modulates the immune system, promoting the incretion of anti-tumour cytokines and increasing the concentration of ROS in the tumour microenvironment to kill the tumour cells77 (Figure 4). In double-blind trials, a polysaccharide-peptide extracted from mushrooms prolonged the 5-year survival rate in oesophageal cancer,78 significantly relieved pain, and improved patients’ quality of life. The significant efficacy, as well as the good compatibility, make fungus a suitable choice for cancer management.79
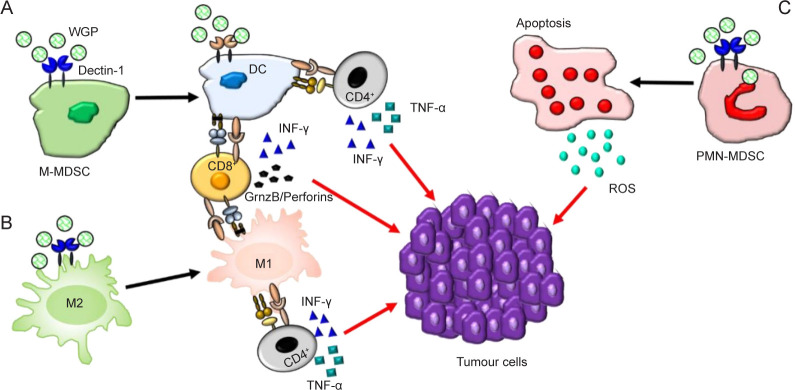
Figure 4. The modulatory mechanisms of the immune cells in the tumour microenvironment by fungal β-glucan. DC: dendritic cell; GrnzB: granzyme B; INF-γ: interferon-γ; M-MDSC: monocytic myeloid-derived suppressor cell; PMNMDSC: polymorphonuclear myeloid-derived suppressor cell; ROS: reactive oxygen species; TNF-α: tumor necrosis factor-α; WGP: whole-glucan particles. Reprinted from Geller et al.77.
Microalgae-mediated chemotherapy
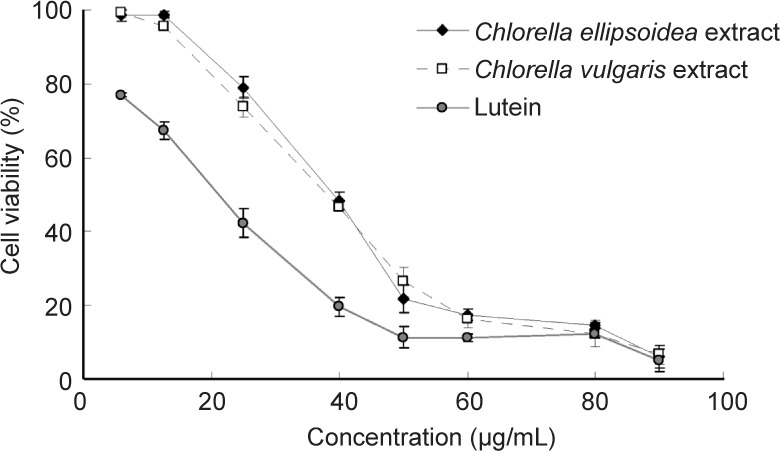
Microalgae are a type of eukaryotic unicellular plant and some of them are reported to have anti-tumour effects.80 Studies reported that carotenoid extracted from Chlorella ellipsoidea and chrysolaminaran from Synedra acus effectively devastated colon or colorectal cancer80-82 (Figure 5). In addition, eicosatetraenoic acid from Cocconeis scutellum was confirmed to have anti-cancer effects in breast cancer.83 These fractions or compounds generated from the microalgae could exhibit specific cytotoxic effects in cancer cells (Table 1).
Figure 5. Anti-tumour effects of chlorella extracts and lutein on human colon cancer cells. Reprinted with permission from Cha et al.81 Copyright 2008 American Chemical Society.
Immunotherapy
The hypoxia targeting-ability of bacteria, as well as their immunogenicity, makes them ideal candidates for immunotherapy. The hypoxic environment and the nutrients released from the dead tumour cells facilitate the growth and proliferation of bacteria. Moreover, the immunosuppressive microenvironment prevents the immune system from clearing the injected bacteria in the tumour. These multiplied bacteria finally activate the immune system, causing large numbers of immune cells to infiltrate the tumours.84 Bacteria-based cancer immunotherapy exhibits anti-tumour effects in several ways, including ways in which naïve-living bacteria, engineered bacteria, or components of bacteria regulate tumour-associated immune systems.85 Bacterial components, such as peptidoglycan, lipopolysaccharide, and lipoteichoic acid, can be recognised by pattern recognition receptors expressed on immune cells, which trigger an interaction between host and bacteria and enhance the immune responses toward malignancy. Among all the components, lipopolysaccharide from the outer membrane of Gram-negative bacteria is a strongly immunogenic microbial-associated molecular pattern that can combine with Toll-like receptors on immune cell membranes and up-regulate the expression of interleukin-6 in immune cells. Subsequently, interleukin-6 activates the nuclear factor-κB signalling pathway, which promotes the maturation and proliferation of immune cells, such as DCs, thereby exerting anti-tumour immunity.86
Fungi are rarely investigated in tumour immunotherapies, but nevertheless some investigators have reported that certain fungi exhibit special tumour-specific effects, i.e., the fungus promotes tumour proliferation via fungal activation of the host’s C3 complement cascade in pancreatic cancer87, 88 (Table 1).
Phototherapy
Phototherapy consists of several kinds of light-triggered killing strategies, especially photothermal therapy (PTT) and photodynamic therapy (PDT). PTT and PDT are effective and non-invasive therapy methods, due to their capabilities of selective tumour ablation and satisfactory efficacy. The subsequent immunological death of cancer cells after phototherapy may lead to enduring antitumor immunological reactions.89, 90
Either live attenuated bacteria or bacterial membrane-coated nanoparticles can be used as targeting vehicles for phototherapy of malignant carcinoma. These engineered bacteria or membrane vehicles efficiently and safely express phototherapy agents at the local tumour areas, thereby triggering a strong anti-tumour effect within the near-infrared radiated position. Deng et al. 59 integrated genetically-modified bacteria with polydopamine (pDA) and chlorin e6 (Ce6), a photosensitiser. Modified E. Coli(p) was coated with pDA@Ce6 to achieve the final engineered bacterial vehicle, E. Coli(p)/pDA@Ce6. The obtained system was administered and selectively accumulated in the hypoxic tumour area. Near-infrared irradiation was introduced to trigger PTT and PDT. The engineered bacteria exhibited efficient anti-tumour effects in vitro and in vivo, which suggested a promising application potential for precise tumour inhibition59 (Figure 2C and Table 1). In the future, bacterial membranes could also be noted as potential nano-carriers for photosensitisers, which may promote the targeting ability and efficacy of PDT and PTT while lowering the possible side effects of live bacteria.
Radiotherapy
Radiotherapy is an effective way to treat tumours, but large variations exist among cancer patients in their response to tumour radiotherapy and radiotherapy-induced side effects, which limit its use as a treatment.91 The gut microbiota is considered an important factor in modulating the tumour microenvironment, ultimately affecting the efficacy of radiotherapy treatment. The interaction of microorganisms and radiotherapy is bidirectional. Generally, radiotherapy interferes with the microbiome and these disruptions in turn influence the effectiveness of the treatment in a feedback loop. However, limited data have shown positive interactions between microorganisms and radiotherapy. On the other hand, the gut microbiome influences radiation-induced gastrointestinal mucositis through translocation and dysbiosis. Therefore, radiation exposure can lead to radiation enteropathy in cancer patients91, 92 (Table 1). In that case, extrinsic microorganisms can be introduced to modify the gut microbiome, which may optimise the responses to radiotherapy and minimise adverse effects.92 The most used approach to investigate the distribution and diversity of gut microorganisms is 16S ribosomal RNA-sequencing. However, many challenges remain unknown, including the mechanisms by which the gut microbiome affects radiosensitivity, interactions between other combination treatments, and the gut microbiome. In future, the gut microbiome may be designed to provide predictive and prognostic biomarkers of radiotherapy based on advanced understanding of these questions.
Oncolytic virotherapy
Oncolytic virus (OV) therapy is an advanced biological therapy with more physiological effects and is perfectly tolerated. Most available OVs are genetically attenuated and modified to enhance tumour tropism, which lowers the virulence of the healthy host cells.93 Compared with phototherapy and radiotherapy, OV therapy could target tumour cells more precisely and pose few risks to human health, since it relies on specific tumour cells and is naturally present in many organisms. Besides, OV therapy would be a sustainable and cascade strategy because oncolytic viruses can self-replicate and spread to reach more tumour cells. Therefore, transfection of oncolytic virus into tumour sites induces infiltration of immune cells and results in apoptosis, direct cell lysis, niche disruption, and phagocytosis85 (Figure 6). Moreover, they stimulate a pro-inflammatory environment by enhancing antigen release and immune activation to counteract the immune evasiveness of cancer cells. OVs also take advantage of the tolerogenic microenvironment, which facilitates the viral infection of tumour cells that are not protected by the immune system. Theoretically, these processes lead to a domino effect including chained viral transfection and further immune activation. In addition, OV therapy can escape the adverse effects of chemotherapy and maximise the anti-tumour efficacy of OVs via the most optimal niche. Apart from the anti-tumour effect of the virus itself, the immune system also exerts important effects in OV therapy through OV-induced immunogenic tumour cell death. The principle of OV cancer therapy aims at achieving a balance between anti-tumour immunity, autoimmunity, and antiviral immunity.93, 94 The first approved OV was T-VEC, which targets tumour cells via recombinant herpes simplex viruses with the transgene granulocyte-macrophage colony-stimulating factor95, 96 (Table 1). OV is also used in breast cancer therapy, changing the tumour microenvironment from “cold” to “hot”.97 The limitation of OV monotherapy could be solved by the combination of radiotherapy or immunotherapy. Wang et al. designed an oncolytic vaccinia virus co-expressing a mouse PD-L1 inhibitor and granulocyte-macrophage colony-stimulating factor and exhibited and demonstrated it in breast cancer therapy. This model provided a potent and individual therapy that was able to activate tumour neoantigen-specific T cell responses.98
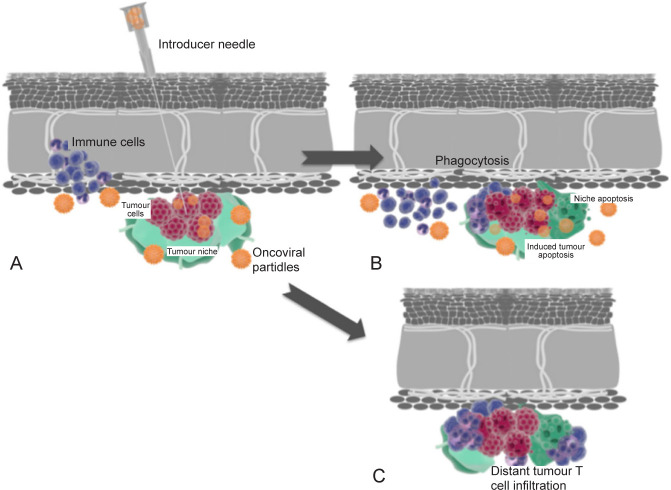
Figure 6. (A) Intratumoral inoculation of OV with transfection and immune cell recruitment. (B) Advanced transfection of an oncolytic virus into the tumour and niche cells with induction of immune cells resulting in apoptosis, direct cell lysis, niche disruption, and phagocytosis. (C) Distant tumour immune infiltration is induced by local immune conditioning. Blue: immune cells; red: tumour; orange: OV particles; green: tumour niche. OV: oncolytic virus. Reprinted from Raja et al.85.
Conclusions and Future Outlooks
This review thoroughly explores the engineered microorganism-based delivery systems for targeted cancer therapy. First, the main types and characteristics of microorganisms such as bacteria, viruses, fungi, microalgae, and their components are highlighted. Moreover, several engineering strategies for creating microorganisms and functional materials have been introduced, which include physical, chemical, and biological integrations based on the methods of functionalisation. Most importantly, we discuss the innovative attempts and therapeutic effects of engineered microorganisms in cancer. In summary, engineered microorganism-based delivery systems hold tremendous prospects for biomedical applications in targeted cancer therapy.
There are still several limitations and challenges before the further application of engineered microorganism-based delivery systems can be realised in targeted cancer therapy. (a) The dynamic activities and final fates of microorganisms and functional materials in the human body should be further investigated, which is of great importance in clinical applications. (b) Microorganisms could function as vaccines for immune stimulation in anti-cancer immunotherapy. However, it is important to balance the double-edged sword between an appropriate immune response and a serious inflammatory storm. (c) Unlike microorganisms, the accompanying functional materials cannot proliferate and duplicate, which limits the therapeutic efficacy of customised material-assisted microorganisms. To overcome this obstacle, bioengineering technology could contribute to the synchronous proliferation of functional materials with microorganisms by transfecting genes encoding functional materials into the target microorganisms. (d) The indiscriminate clearance of beneficial or pathogenic microorganisms might lead to unsatisfactory anti-cancer effects and even cancer progression. Accordingly, more precise and directed regulation of microorganisms is critical in anti-cancer therapies.
Footnotes
Author contributions: Conceptualization, investigation, methodology, and writing-original draft: XH and HYG; funding acquisition: ZWS; software, and supervision: HYG and LTW; writing-review & editing: ZWS, HYG and LTW. All authors have given final approval for this version of the manuscript to be published.
Financial support: This work was supported by the National Key Research and Development Program of China (No. 2016YFC1100100), and the Major Research Plan of the National Natural Science Foundation of China (No. 91649204).
Acknowledgement: None.
Conflicts of interest statement: The authors have declared that no competing interest exists.
Editor note: Zengwu Shao is an Editorial Board member of Biomaterials Translational. He was blinded from reviewing or making decisions on the manuscript. The article was subject to the journal’s standard procedures, with peer review handled independently of his Editorial Board member and his research group.
References
- 1.Hosseinidoust Z., Mostaghaci B., Yasa O., Park B. W., Singh A. V., Sitti M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev. 2016;106:27–44. doi: 10.1016/j.addr.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Zhou S., Gravekamp C., Bermudes D., Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18:727–743. doi: 10.1038/s41568-018-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolgin E. Fighting cancer with microbes. Nature. 2020;577:S16–S18. doi: 10.1038/d41586-020-00199-x. [DOI] [PubMed] [Google Scholar]
- 4.McAdams H. H., Srinivasan B., Arkin A. P. The evolution of genetic regulatory systems in bacteria. Nat Rev Genet. 2004;5:169–178. doi: 10.1038/nrg1292. [DOI] [PubMed] [Google Scholar]
- 5.Zu C., Wang J. Tumor-colonizing bacteria: a potential tumor targeting therapy. Crit Rev Microbiol. 2014;40:225–235. doi: 10.3109/1040841X.2013.776511. [DOI] [PubMed] [Google Scholar]
- 6.Suh S., Jo A., Traore M. A., Zhan Y., Coutermarsh-Ott S. L., Ringel-Scaia V. M., Allen I. C., Davis R. M., Behkam B. Nanoscale bacteria-enabled autonomous drug delivery system (NanoBEADS) enhances intratumoral transport of nanomedicine. Adv Sci (Weinh) 2019;6:1801309. doi: 10.1002/advs.201801309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zitvogel L., Daillère R., Roberti M. P., Routy B., Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol. 2017;15:465–478. doi: 10.1038/nrmicro.2017.44. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Sung C. Y., Lee N., Ni Y., Pihlajamäki J., Panagiotou G., El-Nezami H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc Natl Acad Sci U S A. 2016;113:E1306–1315. doi: 10.1073/pnas.1518189113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy S., Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q. W., Qiao J. Y., Liu X. H., Zhang C., Zhang X. Z. Customized materials-assisted microorganisms in tumor therapeutics. Chem Soc Rev. 2021;50:12576–12615. doi: 10.1039/d0cs01571g. [DOI] [PubMed] [Google Scholar]
- 11.Chen W., Wang J., Du L., Chen J., Zheng Q., Li P., Du B., Fang X., Liao Z. Kefir microbiota and metabolites stimulate intestinal mucosal immunity and its early development. Crit Rev Food Sci Nutr. 2022 doi: 10.1080/10408398.2022.2115975. [DOI] [PubMed] [Google Scholar]
- 12.Paiva A. D., de Oliveira M. D., de Paula S. O., Baracat-Pereira M. C., Breukink E., Mantovani H. C. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology (Reading) 2012;158:2851–2858. doi: 10.1099/mic.0.062190-0. [DOI] [PubMed] [Google Scholar]
- 13.Cao J., Zaremba O. T., Lei Q., Ploetz E., Wuttke S., Zhu W. Artificial bioaugmentation of biomacromolecules and living organisms for biomedical applications. ACS Nano. 2021;15:3900–3926. doi: 10.1021/acsnano.0c10144. [DOI] [PubMed] [Google Scholar]
- 14.Guo Z., Richardson J. J., Kong B., Liang K. Nanobiohybrids: materials approaches for bioaugmentation. Sci Adv. 2020;6:eaaz0330. doi: 10.1126/sciadv.aaz0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao S., Jin B., Liu Z., Shao C., Zhao R., Wang X., Tang R. Biomineralization: from material tactics to biological strategy. Adv Mater. 2017;29:1605903. doi: 10.1002/adma.201605903. [DOI] [PubMed] [Google Scholar]
- 16.Magennis E. P., Fernandez-Trillo F., Sui C., Spain S. G., Bradshaw D. J., Churchley D., Mantovani G., Winzer K., Alexander C. Bacteria-instructed synthesis of polymers for self-selective microbial binding and labelling. Nat Mater. 2014;13:748–755. doi: 10.1038/nmat3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y., Beitelshees M., Fang L., Hill A., Ahmadi M. K., Chen M., Davidson B. A., Knight P., 3rd, Smith R. J., Jr., Andreadis S. T., Hakansson A. P., Jones C. H., Pfeifer B. A. In situ pneumococcal vaccine production and delivery through a hybrid biological-biomaterial vector. Sci Adv. 2016;2:e1600264. doi: 10.1126/sciadv.1600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y., Zeng H., Xie L., Gao R., Zhou S., Liang Q., Zhang X., Liang K., Jiang L., Kong B. Super-assembled chiral mesostructured heteromembranes for smart and sensitive couple-accelerated enantioseparation. J Am Chem Soc. 2022;144:13794–13805. doi: 10.1021/jacs.2c04862. [DOI] [PubMed] [Google Scholar]
- 19.Yan M., Liu T., Li X., Zhou S., Zeng H., Liang Q., Liang K., Wei X., Wang J., Gu Z., Jiang L., Zhao D., Kong B. Soft patch interface-oriented superassembly of complex hollow nanoarchitectures for smart dual-responsive nanospacecrafts. J Am Chem Soc. 2022;144:7778–7789. doi: 10.1021/jacs.2c01096. [DOI] [PubMed] [Google Scholar]
- 20.Yan M., Xie L., Tang J., Liang K., Mei Y., Kong B. Recent advances in heterosilica-based micro/nanomotors: designs, biomedical applications, and future perspectives. Chem Mater. 2021;33:3022–3046. [Google Scholar]
- 21.Mostaghaci B., Yasa O., Zhuang J., Sitti M. Bioadhesive bacterial microswimmers for targeted drug delivery in the urinary and gastrointestinal tracts. Adv Sci (Weinh) 2017;4:1700058. doi: 10.1002/advs.201700058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alapan Y., Yasa O., Schauer O., Giltinan J., Tabak A. F., Sourjik V., Sitti M. Soft erythrocyte-based bacterial microswimmers for cargo delivery. Sci Robot. 2018;3:eaar4423. doi: 10.1126/scirobotics.aar4423. [DOI] [PubMed] [Google Scholar]
- 23.Vargason A. M., Santhosh S., Anselmo A. C. Surface modifications for improved delivery and function of therapeutic bacteria. Small. 2020;16:e2001705. doi: 10.1002/smll.202001705. [DOI] [PubMed] [Google Scholar]
- 24.Fan J. X., Li Z. H., Liu X. H., Zheng D. W., Chen Y., Zhang X. Z. Bacteria-mediated tumor therapy utilizing photothermally-controlled TNF-α expression via oral administration. Nano Lett. 2018;18:2373–2380. doi: 10.1021/acs.nanolett.7b05323. [DOI] [PubMed] [Google Scholar]
- 25.Zheng J. H., Nguyen V. H., Jiang S. N., Park S. H., Tan W., Hong S. H., Shin M. G., Chung I. J., Hong Y., Bom H. S., Choy H. E., Lee S. E., Rhee J. H., Min J. J. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017;9:eaak9537. doi: 10.1126/scitranslmed.aak9537. [DOI] [PubMed] [Google Scholar]
- 26.Johnston T. G., Yuan S. F., Wagner J. M., Yi X., Saha A., Smith P., Nelson A., Alper H. S. Compartmentalized microbes and co-cultures in hydrogels for on-demand bioproduction and preservation. Nat Commun. 2020;11:563. doi: 10.1038/s41467-020-14371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai Z., Lee A. J., Roberts S., Sysoeva T. A., Huang S., Dzuricky M., Yang X., Zhang X., Liu Z., Chilkoti A., You L. Versatile biomanufacturing through stimulus-responsive cell-material feedback. Nat Chem Biol. 2019;15:1017–1024. doi: 10.1038/s41589-019-0357-8. [DOI] [PubMed] [Google Scholar]
- 28.Anselmo A. C., McHugh K. J., Webster J., Langer R., Jaklenec A. Layer-by-layer encapsulation of probiotics for delivery to the microbiome. Adv Mater. 2016;28:9486–9490. doi: 10.1002/adma.201603270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lou X., Chen Z., He Z., Sun M., Sun J. Bacteria-mediated synergistic cancer therapy: small microbiome has a big hope. Nanomicro Lett. 2021;13:37. doi: 10.1007/s40820-020-00560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Z., Liu J. Bacteria and bacterial derivatives as drug carriers for cancer therapy. J Control Release. 2020;326:396–407. doi: 10.1016/j.jconrel.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q. W., Wang J. W., Wang X. N., Fan J. X., Liu X. H., Li B., Han Z. Y., Cheng S. X., Zhang X. Z. Inhibition of tumor progression through the coupling of bacterial respiration with tumor metabolism. Angew Chem Int Ed Engl. 2020;59:21562–21570. doi: 10.1002/anie.202002649. [DOI] [PubMed] [Google Scholar]
- 32.Huang X., Pan J., Xu F., Shao B., Wang Y., Guo X., Zhou S. Bacteria-based cancer immunotherapy. Adv Sci (Weinh) 2021;8:2003572. doi: 10.1002/advs.202003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helmink B. A., Khan M. A. W., Hermann A., Gopalakrishnan V., Wargo J. A. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25:377–388. doi: 10.1038/s41591-019-0377-7. [DOI] [PubMed] [Google Scholar]
- 34.Schwabe R. F., Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arthur J. C., Perez-Chanona E., Mühlbauer M., Tomkovich S., Uronis J. M., Fan T. J., Campbell B. J., Abujamel T., Dogan B., Rogers A. B., Rhodes J. M., Stintzi A., Simpson K. W., Hansen J. J., Keku T. O., Fodor A. A., Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu T., Guo F., Yu Y., Sun T., Ma D., Han J., Qian Y., Kryczek I., Sun D., Nagarsheth N., Chen Y., Chen H., Hong J., Zou W., Fang J. Y. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170:548–563.e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zagato E., Pozzi C., Bertocchi A., Schioppa T., Saccheri F., Guglietta S., Fosso B., Melocchi L., Nizzoli G., Troisi J., Marzano M., Oresta B., Spadoni I., Atarashi K., Carloni S., Arioli S., Fornasa G., Asnicar F., Segata N., Guglielmetti S., Honda K., Pesole G., Vermi W., Penna G., Rescigno M. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat Microbiol. 2020;5:511–524. doi: 10.1038/s41564-019-0649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atasheva S., Emerson C. C., Yao J., Young C., Stewart P. L., Shayakhmetov D. M. Systemic cancer therapy with engineered adenovirus that evades innate immunity. Sci Transl Med. 2020;12:eabc6659. doi: 10.1126/scitranslmed.abc6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lui L. T., Xue X., Sui C., Brown A., Pritchard D. I., Halliday N., Winzer K., Howdle S. M., Fernandez-Trillo F., Krasnogor N., Alexander C. Bacteria clustering by polymers induces the expression of quorum-sensing-controlled phenotypes. Nat Chem. 2013;5:1058–1065. doi: 10.1038/nchem.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei B., Pan J., Yuan R., Shao B., Wang Y., Guo X., Zhou S. Polarization of tumor-associated macrophages by nanoparticle-loaded escherichia coli combined with immunogenic cell death for cancer immunotherapy. Nano Lett. 2021;21:4231–4240. doi: 10.1021/acs.nanolett.1c00209. [DOI] [PubMed] [Google Scholar]
- 41.Cao Z., Wang X., Pang Y., Cheng S., Liu J. Biointerfacial self-assembly generates lipid membrane coated bacteria for enhanced oral delivery and treatment. Nat Commun. 2019;10:5783. doi: 10.1038/s41467-019-13727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drachuk I., Calabrese R., Harbaugh S., Kelley-Loughnane N., Kaplan D. L., Stone M., Tsukruk V. V. Silk macromolecules with amino acid-poly(ethylene glycol) grafts for controlling layer-by-layer encapsulation and aggregation of recombinant bacterial cells. ACS Nano. 2015;9:1219–1235. doi: 10.1021/nn504890z. [DOI] [PubMed] [Google Scholar]
- 43.Zhou X., Zeng Y., Tang Y., Huang Y., Lv F., Liu L., Wang S. Artificial regulation of state transition for augmenting plant photosynthesis using synthetic light-harvesting polymer materials. Sci Adv. 2020;6:eabc5237. doi: 10.1126/sciadv.abc5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gai P., Yu W., Zhao H., Qi R., Li F., Liu L., Lv F., Wang S. Solar-powered organic semiconductor-bacteria biohybrids for CO(2) reduction into acetic acid. Angew Chem Int Ed Engl. 2020;59:7224–7229. doi: 10.1002/anie.202001047. [DOI] [PubMed] [Google Scholar]
- 45.Cao Z., Cheng S., Wang X., Pang Y., Liu J. Camouflaging bacteria by wrapping with cell membranes. Nat Commun. 2019;10:3452. doi: 10.1038/s41467-019-11390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lv P., Liu X., Chen X., Liu C., Zhang Y., Chu C., Wang J., Wang X., Chen X., Liu G. Genetically engineered cell membrane nanovesicles for oncolytic adenovirus delivery: a versatile platform for cancer virotherapy. Nano Lett. 2019;19:2993–3001. doi: 10.1021/acs.nanolett.9b00145. [DOI] [PubMed] [Google Scholar]
- 47.Chodisetti P. K., Reddy M. Peptidoglycan hydrolase of an unusual cross-link cleavage specificity contributes to bacterial cell wall synthesis. Proc Natl Acad Sci U S A. 2019;116:7825–7830. doi: 10.1073/pnas.1816893116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo G. F., Chen W. H., Zeng X., Zhang X. Z. Cell primitive-based biomimetic functional materials for enhanced cancer therapy. Chem Soc Rev. 2021;50:945–985. doi: 10.1039/d0cs00152j. [DOI] [PubMed] [Google Scholar]
- 49.Spicer C. D., Pashuck E. T., Stevens M. M. Achieving controlled biomolecule-biomaterial conjugation. Chem Rev. 2018;118:7702–7743. doi: 10.1021/acs.chemrev.8b00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ojkic N., Serbanescu D., Banerjee S. Surface-to-volume scaling and aspect ratio preservation in rod-shaped bacteria. eLife. 2019;8:e47033. doi: 10.7554/eLife.47033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taherkhani S., Mohammadi M., Daoud J., Martel S., Tabrizian M. Covalent binding of nanoliposomes to the surface of magnetotactic bacteria for the synthesis of self-propelled therapeutic agents. ACS Nano. 2014;8:5049–5060. doi: 10.1021/nn5011304. [DOI] [PubMed] [Google Scholar]
- 52.Felfoul O., Mohammadi M., Taherkhani S., de Lanauze D., Zhong Xu Y., Loghin D., Essa S., Jancik S., Houle D., Lafleur M., Gaboury L., Tabrizian M., Kaou N., Atkin M., Vuong T., Batist G., Beauchemin N., Radzioch D., Martel S. Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat Nanotechnol. 2016;11:941–947. doi: 10.1038/nnano.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan J. X., Peng M. Y., Wang H., Zheng H. R., Liu Z. L., Li C. X., Wang X. N., Liu X. H., Cheng S. X., Zhang X. Z. Engineered bacterial bioreactor for tumor therapy via fenton-like reaction with localized H(2) O(2) generation. Adv Mater. 2019;31:e1808278. doi: 10.1002/adma.201808278. [DOI] [PubMed] [Google Scholar]
- 54.Kim H., Shin K., Park O. K., Choi D., Kim H. D., Baik S., Lee S. H., Kwon S. H., Yarema K. J., Hong J., Hyeon T., Hwang N. S. General and facile coating of single cells via mild reduction. J Am Chem Soc. 2018;140:1199–1202. doi: 10.1021/jacs.7b08440. [DOI] [PubMed] [Google Scholar]
- 55.Chen F., Li X., Hihath J., Huang Z., Tao N. Effect of anchoring groups on single-molecule conductance: comparative study of thiol-, amine-, and carboxylic-acid-terminated molecules. J Am Chem Soc. 2006;128:15874–15881. doi: 10.1021/ja065864k. [DOI] [PubMed] [Google Scholar]
- 56.Yin H., Kauffman K. J., Anderson D. G. Delivery technologies for genome editing. Nat Rev Drug Discov. 2017;16:387–399. doi: 10.1038/nrd.2016.280. [DOI] [PubMed] [Google Scholar]
- 57.Riglar D. T., Giessen T. W., Baym M., Kerns S. J., Niederhuber M. J., Bronson R. T., Kotula J. W., Gerber G. K., Way J. C., Silver P. A. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat Biotechnol. 2017;35:653–658. doi: 10.1038/nbt.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gurbatri C. R., Lia I., Vincent R., Coker C., Castro S., Treuting P. M., Hinchliffe T. E., Arpaia N., Danino T. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci Transl Med. 2020;12:eaax0876. doi: 10.1126/scitranslmed.aax0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deng X., Yang W., Shao Z., Zhao Y. Genetically modified bacteria for targeted phototherapy of tumor. Biomaterials. 2021;272:120809. doi: 10.1016/j.biomaterials.2021.120809. [DOI] [PubMed] [Google Scholar]
- 60.Vargason A. M., Anselmo A. C. Clinical translation of microbe-based therapies: Current clinical landscape and preclinical outlook. Bioeng Transl Med. 2018;3:124–137. doi: 10.1002/btm2.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh A. V., Hosseinidoust Z., Park B. W., Yasa O., Sitti M. Microemulsion-based soft bacteria-driven microswimmers for active cargo delivery. ACS Nano. 2017;11:9759–9769. doi: 10.1021/acsnano.7b02082. [DOI] [PubMed] [Google Scholar]
- 62.Hu Q., Sun W., Qian C., Wang C., Bomba H. N., Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27:7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu W. L., Zou M. Z., Liu T., Zeng J. Y., Li X., Yu W. Y., Li C. X., Ye J. J., Song W., Feng J., Zhang X. Z. Expandable immunotherapeutic nanoplatforms engineered from cytomembranes of hybrid cells derived from cancer and dendritic cells. Adv Mater. 2019;31:e1900499. doi: 10.1002/adma.201900499. [DOI] [PubMed] [Google Scholar]
- 64.Abdi-Ali A., Worobec E. A., Deezagi A., Malekzadeh F. Cytotoxic effects of pyocin S2 produced by Pseudomonas aeruginosa on the growth of three human cell lines. Can J Microbiol. 2004;50:375–381. doi: 10.1139/w04-019. [DOI] [PubMed] [Google Scholar]
- 65.Mercado-Lubo R., Zhang Y., Zhao L., Rossi K., Wu X., Zou Y., Castillo A., Leonard J., Bortell R., Greiner D. L., Shultz L. D., Han G., McCormick B. A. A Salmonella nanoparticle mimic overcomes multidrug resistance in tumours. Nat Commun. 2016;7:12225. doi: 10.1038/ncomms12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Felgner S., Kocijancic D., Frahm M., Heise U., Rohde M., Zimmermann K., Falk C., Erhardt M., Weiss S. Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. Oncoimmunology. 2018;7:e1382791. doi: 10.1080/2162402X.2017.1382791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fritz S. E., Henson M. S., Greengard E., Winter A. L., Stuebner K. M., Yoon U., Wilk V. L., Borgatti A., Augustin L. B., Modiano J. F., Saltzman D. A. A phase I clinical study to evaluate safety of orally administered, genetically engineered Salmonella enterica serovar Typhimurium for canine osteosarcoma. Vet Med Sci. 2016;2:179–190. doi: 10.1002/vms3.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chowdhury S., Castro S., Coker C., Hinchliffe T. E., Arpaia N., Danino T. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat Med. 2019;25:1057–1063. doi: 10.1038/s41591-019-0498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kucerova P., Cervinkova M. Spontaneous regression of tumour and the role of microbial infection--possibilities for cancer treatment. Anticancer Drugs. 2016;27:269–277. doi: 10.1097/CAD.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Viaud S., Saccheri F., Mignot G., Yamazaki T., Daillère R., Hannani D., Enot D. P., Pfirschke C., Engblom C., Pittet M. J., Schlitzer A., Ginhoux F., Apetoh L., Chachaty E., Woerther P. L., Eberl G., Bérard M., Ecobichon C., Clermont D., Bizet C., GaboriauRouthiau V., Cerf-Bensussan N., Opolon P., Yessaad N., Vivier E., Ryffel B., Elson C. O., Doré J., Kroemer G., Lepage P., Boneca I. G., Ghiringhelli F., Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chumchalová J., Smarda J. Human tumor cells are selectively inhibited by colicins. Folia Microbiol (Praha) 2003;48:111–115. doi: 10.1007/BF02931286. [DOI] [PubMed] [Google Scholar]
- 72.Baindara P., Gautam A., Raghava G. P. S., Korpole S. Anticancer properties of a defensin like class IId bacteriocin Laterosporulin10. Sci Rep. 2017;7:46541. doi: 10.1038/srep46541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Begde D., Bundale S., Mashitha P., Rudra J., Nashikkar N., Upadhyay A. Immunomodulatory efficacy of nisin--a bacterial lantibiotic peptide. J Pept Sci. 2011;17:438–444. doi: 10.1002/psc.1341. [DOI] [PubMed] [Google Scholar]
- 74.Kamarajan P., Hayami T., Matte B., Liu Y., Danciu T., Ramamoorthy A., Worden F., Kapila S., Kapila Y. Nisin ZP, a bacteriocin and food preservative, inhibits head and neck cancer tumorigenesis and prolongs survival. PLoS One. 2015;10:e0131008. doi: 10.1371/journal.pone.0131008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lim Y. C., Kim C. H., Kim Y. B., Joo J. Y., Shin Y. S., Chung J. Incidence and risk factors for rebleeding during cerebral angiography for ruptured intracranial aneurysms. Yonsei Med J. 2015;56:403–409. doi: 10.3349/ymj.2015.56.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ritz B. W. Supplementation with active hexose correlated compound increases survival following infectious challenge in mice. Nutr Rev. 2008;66:526–531. doi: 10.1111/j.1753-4887.2008.00085.x. [DOI] [PubMed] [Google Scholar]
- 77.Geller A., Shrestha R., Yan J. Yeast-derived β-glucan in cancer: novel uses of a traditional therapeutic. Int J Mol Sci. 2019;20:3618. doi: 10.3390/ijms20153618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kidd P. M. The use of mushroom glucans and proteoglycans in cancer treatment. Altern Med Rev. 2000;5:4–27. [PubMed] [Google Scholar]
- 79.Wong J. H., Sze S. C. W., Ng T. B., Cheung R. C. F., Tam C., Zhang K. Y., Dan X., Chan Y. S., Cho W. C., Ng C. C. W., Waye M. M. Y., Liang W., Zhang J., Yang J., Ye X., Lin J., Ye X., Wang H., Liu F., Chan D. W., Ngan H. Y. S., Sha O., Li G., Tse R., Tse T. F., Chan H. Apoptosis and anti-cancer drug discovery: the power of medicinal fungi and plants. Curr Med Chem. 2018;25:5613–5630. doi: 10.2174/0929867324666170720165005. [DOI] [PubMed] [Google Scholar]
- 80.Martínez Andrade K. A., Lauritano C., Romano G., Ianora A. Marine microalgae with anti-cancer properties. Mar Drugs. 2018;16:165. doi: 10.3390/md16050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cha K. H., Koo S. Y., Lee D. U. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J Agric Food Chem. 2008;56:10521–10526. doi: 10.1021/jf802111x. [DOI] [PubMed] [Google Scholar]
- 82.Shishlyannikov S. M., Klimenkov I. V., Bedoshvili Y. D., Mikhailov I. S., Gorshkov A. G. Effect of mixotrophic growth on the ultrastructure and fatty acid composition of the diatom Synedra acus from Lake Baikal. J Biol Res (Thessalon) 2014;21:15. doi: 10.1186/2241-5793-21-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nappo M., Berkov S., Massucco C., Di Maria V., Bastida J., Codina C., Avila C., Messina P., Zupo V., Zupo S. Apoptotic activity of the marine diatom Cocconeis scutellum and eicosapentaenoic acid in BT20 cells. Pharm Biol. 2012;50:529–535. doi: 10.3109/13880209.2011.611811. [DOI] [PubMed] [Google Scholar]
- 84.Duong M. T., Qin Y., You S. H., Min J. J. Bacteria-cancer interactions: bacteria-based cancer therapy. Exp Mol Med. 2019;51:1–15. doi: 10.1038/s12276-019-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raja J., Ludwig J. M., Gettinger S. N., Schalper K. A., Kim H. S. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. 2018;6:140. doi: 10.1186/s40425-018-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X., Yin S., Chen Y., Wu Y., Zheng W., Dong H., Bai Y., Qin Y., Li J., Feng S., Zhao P. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol Med Rep. 2018;17:5484–5491. doi: 10.3892/mmr.2018.8542. [DOI] [PubMed] [Google Scholar]
- 87.Aykut B., Pushalkar S., Chen R., Li Q., Abengozar R., Kim J. I., Shadaloey S. A., Wu D., Preiss P., Verma N., Guo Y., Saxena A., Vardhan M., Diskin B., Wang W., Leinwand J., Kurz E., Kochen Rossi J. A., Hundeyin M., Zambrinis C., Li X., Saxena D., Miller G. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 2019;574:264–267. doi: 10.1038/s41586-019-1608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sepich-Poore G. D., Zitvogel L., Straussman R., Hasty J., Wargo J. A., Knight R. The microbiome and human cancer. Science. 2021;371:eabc4552. doi: 10.1126/science.abc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y., Zhang F., Wang Q., Tong R., Lin H., Qu F. Near-infrared light-mediated LA-UCNPs@SiO(2)-C/HA@mSiO(2)-DOX@NB nanocomposite for chemotherapy/PDT/PTT and imaging. Dalton Trans. 2017;46:14293–14300. doi: 10.1039/c7dt02529g. [DOI] [PubMed] [Google Scholar]
- 90.Gulzar A., Xu J., Yang D., Xu L., He F., Gai S., Yang P. Nanographene oxide-UCNP-Ce6 covalently constructed nanocomposites for NIR-mediated bioimaging and PTT/PDT combinatorial therapy. Dalton Trans. 2018;47:3931–3939. doi: 10.1039/c7dt04141a. [DOI] [PubMed] [Google Scholar]
- 91.Doppalapudi R., Vundavalli S., Prabhat M. P. Effect of probiotic bacteria on oral Candida in head- and neck-radiotherapy patients: A randomized clinical trial. J Cancer Res Ther. 2020;16:470–477. doi: 10.4103/jcrt.JCRT_334_18. [DOI] [PubMed] [Google Scholar]
- 92.Liu J., Liu C., Yue J. Radiotherapy and the gut microbiome: facts and fiction. Radiat Oncol. 2021;16:9. doi: 10.1186/s13014-020-01735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mondal M., Guo J., He P., Zhou D. Recent advances of oncolytic virus in cancer therapy. Hum Vaccin Immunother. 2020;16:2389–2402. doi: 10.1080/21645515.2020.1723363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harrington K., Freeman D. J., Kelly B., Harper J., Soria J. C. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov. 2019;18:689–706. doi: 10.1038/s41573-019-0029-0. [DOI] [PubMed] [Google Scholar]
- 95.Watanabe D., Goshima F. Oncolytic virotherapy by HSV. Adv Exp Med Biol. 2018;1045:63–84. doi: 10.1007/978-981-10-7230-7_4. [DOI] [PubMed] [Google Scholar]
- 96.Kanai R., Wakimoto H., Cheema T., Rabkin S. D. Oncolytic herpes simplex virus vectors and chemotherapy: are combinatorial strategies more effective for cancer? Future Oncol. 2010;6:619–634. doi: 10.2217/fon.10.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kwan A., Winder N., Muthana M. Oncolytic virotherapy treatment of breast cancer: barriers and recent advances. Viruses. 2021;13:1128. doi: 10.3390/v13061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang G., Kang X., Chen K. S., Jehng T., Jones L., Chen J., Huang X. F., Chen S. Y. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses. Nat Commun. 2020;11:1395. doi: 10.1038/s41467-020-15229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]