Abstract
The multivalent pneumococcal conjugate vaccine is effective against both systemic disease and otitis media caused by serotypes contained in the vaccine. However, serotypes not covered by the current conjugate vaccine may still cause pneumococcal disease. To address these serotypes and the remaining otitis media due to Streptococcus pneumoniae, we have been evaluating antigenically conserved proteins from S. pneumoniae as vaccine candidates. A previous report identified a 20-kDa protein with putative human complement C3-proteolytic activity. By utilizing the publicly released pneumococcal genomic sequences, we found the gene encoding the 20-kDa protein to be part of a putative open reading frame of approximately 2,400 bp. We recombinantly expressed a 79-kDa fragment (rPhpA-79) that contains a repeated HxxHxH motif and evaluated it for vaccine potential. The antibodies elicited by the purified rPhpA-79 protein were cross-reactive to proteins from multiple strains of S. pneumoniae and were against surface-exposed epitopes. Immunization with rPhpA-79 protein adjuvanted with monophosphoryl lipid A (for subcutaneous immunization) or a mutant cholera toxin, CT-E29H (for intranasal immunization), protected CBA/N mice against death and bacteremia, as well as reduced nasopharyngeal colonization, following intranasal challenge with a heterologous pneumococcal strain. In contrast, immunization with the 20-kDa portion of the PhpA protein did not protect mice. These results suggest that rPhpA-79 is a potential candidate for use as a vaccine against pneumococcal systemic disease and otitis media.
Infections with Streptococcus pneumoniae are a major cause of human diseases such as otitis media, bacteremia, meningitis, and fatal pneumonia worldwide (5). The rapid emergence of multidrug-resistant pneumococcal strains throughout the world has led to increased emphasis on the prevention of pneumococcal infections by vaccination (10). The currently available 23-valent pneumococcal capsular polysaccharide vaccine is not effective in children younger than 2 years of age or in immunocompromised patients, two of the major populations at risk for pneumococcal infection (9). A seven-valent pneumococcal polysaccharide-protein conjugate vaccine recently licensed in the United States was shown to be highly effective in protecting infants and children against systemic pneumococcal disease caused by the vaccine serotypes and against cross-reactive capsular serotypes (26). The seven capsular types cover greater than 80% of the invasive disease isolates in children in the United States but only 57 to 60% of the disease isolates in other areas of the world (11). Therefore, there is an immediate need for a cost-effective vaccine that covers most or all of the disease-causing serotypes of pneumococci. While this can be achieved by adding conjugates covering additional serotypes, some investigators have raised concerns over possible replacement of vaccine serotypes with nonvaccine serotypes. Thus, efforts continue to find noncapsular vaccine antigens that are conserved among all pneumococcal serotypes and effective against pneumococcal disease.
Protein antigens of S. pneumoniae have been evaluated for protective efficacy in animal models of pneumococcal infection. Some of the most commonly studied vaccine candidates include the PspA proteins, PsaA lipoprotein, and the CbpA protein. Numerous studies have shown that PspA protein is a virulence factor (6, 20), but it is antigenically variable among pneumococcal strains. A recent study has indicated that some antigenically conserved regions of a recombinant PspA variant may elicit cross-reactive antibodies in human adults (22). PsaA, a 37-kDa lipoprotein with similarity to other gram-positive adhesins, is involved in Mn2+ transport in pneumococci (8, 25) and has also been shown to be protective in mouse models of systemic disease (29). The surface-exposed choline binding protein CbpA is antigenically conserved and protective in mouse models of pneumococcal disease (24). Since nasopharyngeal colonization is a prerequisite for otic disease, intranasal (i.n.) immunization of mice with pneumococcal proteins and appropriate mucosal adjuvants has been used to enhance the mucosal antibody response and thus the effectiveness of protein vaccine candidates (4, 32). While these protein antigens appear promising, it is possible that no one protein antigen will be effective against all pneumococcal serotypes. Thus, laboratories continue to search for additional candidates that are antigenically conserved and elicit antibodies that reduce colonization (important for otitis media), are protective against systemic disease, or both.
Pneumococci have evolved other factors to evade the innate immune system of the host. Interactions between human C3 and virulent pneumococci may serve to mediate attachment to pulmonary epithelial type II cells, decrease opsonophagocytosis, and provide camouflage from the immune system (1, 14, 18, 28). In a recent paper describing the interactions of human complement component C3 with the pneumococcal surface, Hostetter reported that two proteins of approximately 29 and 20 kDa appear to have proteolytic activity against human C3 (13). Since surface-exposed or secreted components potentially involved in virulence are logical targets for vaccine development, we decided to further investigate the ability of one of these putative proteases, the 20-kDa protein, to elicit biologically active antibodies against pneumococci. We identified a putative ≈2,400-bp open reading frame (ORF) (phpA) and recombinantly expressed an ≈79-kDa fragment (rPhpA-79). Results presented here show that the rPhpA-79 protein appears to be a viable candidate for inclusion in a protein-based vaccine against pneumococcal disease.
MATERIALS AND METHODS
Bacteria.
The S. pneumoniae strains utilized in this work were of serotype 3, originally obtained from Robert Austrian, University of Pennsylvania; serotypes 4, 5, 6B, and 7, originally obtained from Gerald Schiffman, State University of New York, Brooklyn; and serotype 14, originally obtained from the American Type Culture Collection (no. 6314). S. pneumoniae CP1200, a nonencapsulated, highly transformable derivative of R36A, a rough variant of D39, a virulent type 2 strain (21), was also used. S. pneumoniae was grown to log phase in Todd-Hewitt medium (Difco Laboratories, Detroit, Mich.) with 0.5% yeast extract (Difco) at 37°C with aeration or on tryptic soy (Difco) blood agar plates. Streptomycin-resistant mutants of S. pneumoniae serotypes 3 and 14 were used for challenge. The human isolate of S. pneumoniae type 3 (WU2) or 14 was grown to an optical density at 550 nm (OD550) of 1.0 and plated on 20 tryptic soy blood agar plates (5% sheep blood with streptomycin at 100 μg/ml and gentamicin at 5 μg/ml) and incubated at 37°C for 3 days. A single colony of each isolate was selected and grown in Todd-Hewitt broth with streptomycin at 100 μg/ml. Small aliquots of streptomycin-resistant type 3 or 14 pneumococci in mid-log growth phase were stored frozen at −70°C, ready for challenge experiments after thawing and appropriate culturing. The Escherichia coli strains used were BL21(DE3) (Novagen, Madison, Wis.) and Top10F′ (Invitrogen, San Diego, Calif.); they were grown in SOB medium (19) containing appropriate antibiotics at 37°C with aeration. Chloramphenicol was used at 20 μg/ml, ampicillin was used at 100 μg/ml, streptomycin was used at 100 μg/ml, and kanamycin was used at 25 μg/ml.
Cloning and expression of phpA-79 and phpA-20 gene fragments.
The DNA sequence of the cloned S. pneumoniae 20-kDa protein previously described (13) was aligned with the early release of the S. pneumoniae genome (serotype 4) sequence, generously made available by The Institute for Genomic Research (TIGR; www.tigr.org), by using the MacVector DNA analysis package (Oxford Molecular Group, Campbell, Calif.). Two primers flanking an approximately 2,100-bp ORF (phpA-79) were designed and subsequently synthesized using the ABI 380A DNA synthesizer. The 5′ primer included an NcoI site and had a glutamic acid residue added after the ATG start codon to maintain the correct reading frame. The 3′ primer included a HindIII site. The sequences were 5′ AGA GCT CCC ATG GAA GAT CCG AAT TAT CAG and 3′ GGG AAG CTT AGG AGT TAG AAA ATG CTG CTA CCT TTA, respectively.
To amplify a 20-kDa truncated form of the phpA gene, two primers were designed and synthesized. The 5′ primer included an NcoI site and had an alanine residue added after the ATG start codon to maintain the correct reading frame. The 3′ primer included a BamHI site and no stop codon. This construct added a six-His tag at the carboxy-terminal end of the 20-kDa fragment. The sequences were 5′ CCA TGG CCT CAA GCC TTT TAC GTG AAT TG and 3′ GGA TCC CTA GCT ATA TGA GAT AAA CTT TCC TGC T, respectively.
PCR fragments of the expected sizes were generated from S. pneumoniae serotype 7 and strain CP1200, and each fragment was ligated into the pCR2.1 vector and used to transform OneShot Top10F′ cells. Ampicillin-resistant transformants were screened, and recombinant plasmids containing the serotype 7 phpA-79 and CP1200 phpA-20 products were identified and confirmed by DNA sequencing. The inserts were excised with either NcoI and HindIII or NcoI and BamHI, purified from low-melting-point agarose, and subsequently ligated into the corresponding sites of T7-promoted expression vector pET28a (Novagen).
The plasmids were transformed into E. coli Top10F′ cells, and the kanamycin-resistant transformants were screened by restriction digestion of plasmid DNA prepared by alkaline lysis (3). Recombinant plasmids containing the phpA-79 gene fragment (pLP515) and the phpA-20 gene fragment (pLP505) were subsequently transformed into BL21(DE3) cells and grown in SOB medium supplemented with kanamycin at 30 μg/ml. Cells were grown to an OD600 of 0.6 and subsequently induced with 0.4 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Boehringer Mannheim, Indianapolis, Ind.) for 2 to 4 h.
Purification of PhpA-79 and PhpA-20 protein fragments.
Recombinant PhpA-79 was purified from E. coli strain BL21(DE3)(pLP515), which expresses large amounts of the protein under the control of the T7 promoter. Preliminary experiments showed that the Php-79 protein was expressed as inclusion bodies in BL21(DE3)(pLP515). Bacterial cells were grown to mid-log phase in SOB medium containing kanamycin at 30 μg/ml to select for the plasmid. Expression of PhpA-79 was induced by the addition of IPTG to 0.4 mM and continuing incubation for 3 h. Induced bacteria were harvested by centrifugation at 7,700 × g for 15 min at 4°C and then stored at −20°C. The cell pellet was resuspended in 1/50 of the initial volume of distilled H2O and then frozen at −70°C. After thawing, the cells were lysed by three passages through a French pressure cell (S.L.M. Aminco, Urbana, Ill.). Cell debris was removed by low-speed centrifugation (7,000 × g at 4°C) for 10 min. The insoluble pellet was resuspended in 20 ml of 50 mM NaPO4 buffer (pH 7.5)–1 mM EDTA containing 1% Triton X-100 (Sigma) and allowed to mix for 1 to 3 h at 4°C. This treatment effectively solubilized the rPhpA-79 protein. The solubilized fraction was dialyzed against 50 mM NaPO4 (pH 7.5)–1 mM EDTA overnight at 4°C, and insoluble material was removed by centrifugation at 150,000 × g for 30 min at 4°C in a 60Ti rotor (Beckman Instruments). The soluble fraction was applied to a 25-ml bed volume CM Sepharose fast-flow (Pharmacia, Piscataway, N.J.) column pre-equilibrated with 50 mM NaPO4 (pH 7.5)–1 mM EDTA. The rPhpA-79 protein remained in the flowthrough and was then applied to a 25-ml bed volume Q Sepharose fast-flow (Pharmacia) column equilibrated with the above-described buffer. The recombinant protein bound to the column and was eluted at approximately 300 mM NaCl by using a step gradient of 50 to 500 mM NaCl in the equilibration buffer. The protein was then concentrated by using a 10,000 molecular weight cutoff Centricon concentrator (Amicon, Beverly, Mass.) in accordance with the manufacturer's directions. The protein was stored at 4°C.
Cells expressing the recombinant PhpA-20 protein were prepared as described above. Upon thawing, cells were resuspended in 50 mM NaPO4 (pH 8.0)–1 mM EDTA–1%Triton X-100, and inclusion bodies were obtained as described above. The pellet was solubilized with 6 M urea, and insoluble material was removed by ultracentrifugation at 5,000 × g for 60 min at 4°C. The 6 M soluble material was diluted to 1 M urea with 50 mM NaPO4, pH 8.0, and applied to a 1-ml Qiagen (Valencia, Calif.) nickel column pre-equilibrated with the above-described buffer. The column was washed in steps with 3 column volumes of the above-described buffer at pHs 8.0, 7.5, 7.0, 6.5, and 5.5. The protein was eluted with the above-described buffer at pH 4.3. Following elution, the pH of the solution was adjusted to 7.0. The protein was analyzed for homogeneity by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and stored at 4°C.
Polyclonal antiserum for Western blot analysis.
rPhpA-79 protein was used to generate polyclonal antiserum in mice. Briefly, 10 μg of rPhpA-79 protein was adjuvanted for each dose with 20 μg of QS21 (Aquila Biopharmaceuticals, Framingham, Mass.) and injected subcutaneously (s.c.) into 6- to 8-week-old Swiss Webster mice (10 per group). The mice were bled and vaccinated at week 0, boosted at week 4, and then exsanguinated at week 6. The sera from all of the mice in a group were pooled and used for further analysis.
IEM of CP1200.
The reactivity of mouse polyclonal anti-rPhpA-79 serum to the outer surface of S. pneumoniae strain CP1200 was examined by immunoelectron microscopy (IEM) by a modified form of the method of Slot and Geuze (27). Pneumococci were grown to log phase and centrifuged to pellet the cells. Bacteria were resuspended in phosphate-buffered saline (PBS) to an OD600 of 1 and placed in droplet form on Parafilm. Four hundred mesh Formvar carbon gold grids (Ted Pella, Redding, Calif.) were inverted over the droplets for 5 min. The grids were removed and floated face down on 15-μl droplets of 10 mM PBS, pH 7.2, containing 1% bovine serum albumin (BSA; Sigma type V) (PBS-BSA) for 5 min and then transferred to a new 15-μl droplet of PBS containing 1% cold water fish gelatin for 10 min. The grids were then incubated with anti-rPhpA-79 serum diluted 1:500 in PBS for 30 min and washed five times for 1 min each time in PBS-BSA. The grids were subsequently incubated with 18-nm colloidal gold-labeled anti-mouse antibody (Jackson Immunoresearch Laboratories, West Grove, Pa.) diluted 1:5 in PBS for 30 min and washed as described above. The grids were fixed with 1% glutaraldehyde in PBS at room temperature for 3 min and then rinsed five times for 1 min each time in distilled water. The cells were then stained with 1% vanadium, pH 6.8 (Nanoprobes, Stony Brook, N.Y.), by a modification of Beesley's negative staining technique (2). The cells were examined on a Zeiss 10C transmission electron microscope operating at 100 kV and photographed at a magnification of ×50,000.
Intranasal and parenteral immunization of mice prior to challenge.
Six-week-old, pathogen-free, male CBA/CaHN xid/J (CBA/N) mice were purchased from the Jackson Laboratory (Bar Harbor, Maine) and housed in cages under standard temperature, humidity, and lighting conditions. CBA/N mice, at 10 animals per group, were immunized with 5 μg of either rPhpA-79 or rPhpA-20. For parenteral immunization, rPhpA-79 or rPhpA-20 protein was mixed with 100 μg of monophosphoryl lipid A (MPL; Corixa, Hamilton, Mont.) per dose to a final volume of 200 μl in saline and then injected s.c. into mice. All groups received a booster with the same dose and by the same route 3 and 5 weeks after the primary immunization. Control mice were injected with MPL alone. All mice were bled 2 weeks after the last boosting; sera were then isolated and stored at −20°C. For i.n. immunization, mice received three immunizations 1 week apart. On each occasion, 5 μg of rPhp-79 or rPhp-20 formulated with 0.1 μg of CT-E29H, a genetically modified cholera toxin (CT) with reduced enzymatic activity and toxicity (30), was slowly instilled into a nostril of each mouse in a 10-μl volume. Mice immunized with CT-E29H alone were used as controls. Serum samples were collected 1 week after the last immunization.
LD50 determination.
Six- or twelve-week-old CBA/N mice (10 per group) were challenged i.n. with 10 μl of a suspension of type 3 S. pneumoniae diluted to 5 × 109 CFU/ml in PBS. The actual doses of bacteria administrated were determined by plating dilutions of the inoculum on streptomycin-containing tryptic soy agar plates. The 50% lethal dose (LD50) was calculated by the Reed-Muench method as discussed by Lennette (16). The LD50 for 13-week-old CBA/N mice was 1 × 105 CFU, while the LD50 for 6-week-old CBA/N mice was 1 × 104 CFU.
Mouse i.n. challenge model.
Mice were challenged with either serotype 3 or serotype 14 streptomycin-resistant S. pneumoniae. Pneumococci were grown as previously described until mid-log phase and then diluted to the desired concentration with Todd-Hewitt broth and stored on ice until use. Each mouse was anesthetized with 1.2 mg of ketamine HCl (Fort Dodge Laboratory, Ft. Dodge, Iowa) by intraperitoneal injection. The bacterial suspension was inoculated into the nostrils of anesthetized mice (10 μl per mouse). The actual dose of bacteria administered was confirmed by plate count. At 2 or 3 days after challenge, mice were sacrificed and the noses were removed and homogenized in 3 ml of sterile saline with a tissue homogenizer (Ultra-Turax T25; Janke & Kunkel Ika-Labortechnik, Staufen, Germany). The homogenate was serially diluted 10-fold in saline and plated on streptomycin-containing tryptic soy agar plates. Fifty microliters of blood collected 2 days postchallenge from each mouse was also plated. Plates were incubated overnight at 37°C, and then colonies were counted. CBA/N mice were observed daily after challenge, and mortality was recorded for 14 days.
ELISAs for rPhpA-79 and rPhpA-20 proteins.
Titers of antibodies against the rPhpA-79 and rPhpA-20 proteins were determined by enzyme-linked immunosorbent assay (ELISA). ELISAs were performed by using either rPhpA-79 protein (a 5-μg/ml stock in PBS, pH 7.1, at 100 μl per well) to coat Nunc-Immuno PolySorp plates (Nunc, Roskilde, Denmark) or rPhpA-20 (a 1-μg/ml stock in PBS, pH 7.1, at 100 μl per well) to coat Nunc-Immuno MaxiSorp plates. Plates were coated and then incubated overnight at 4°C. After blocking with 200 μl of PBS containing 5% nonfat dry milk (blocking buffer) for 1 h at room temperature, the plates were incubated with serial dilutions of test sera in blocking buffer for 1.5 h at room temperature. The plates were then washed five times with PBS containing 0.1% Tween (PBS-T) and incubated with biotinylated goat anti-mouse immunoglobulin G (IgG) or IgA (1:8,000 or 1:4,000 in PBS; Brookwood Biomedical, Birmingham, Ala.) for 1 h at room temperature. After five additional washes with PBS-T, the plates were incubated with streptavidin-conjugated horseradish peroxidase (1:10,000 in PBS; Zymed Laboratories, South San Francisco, Calif.) for 1 h at room temperature. The plates were then washed five times with PBS-T and incubated for 20 min with 100 μl of 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate (Kirkegaard & Perry Laboratories [KPL], Gaithersburg, Md.); this was followed by addition of 100 μl of stopping solution (1% SDS). A405 values were read by using a VERSAmax microplate reader (Molecular Devices Corp., Sunnyvale, Calif.). The endpoint titer of each test serum was the reciprocal of the highest mean dilution that resulted in an OD405 reading of 0.1. The mean background titers of test sera were quantified by determining the A405 values of the wells that had all of the reagents except sera. These background values were between 0.01 and 0.04.
Pneumococcal whole-cell ELISAs.
Titers of antibodies against whole cells of S. pneumoniae serotype 3 were determined by ELISA. S. pneumoniae serotype 3 was grown in Todd-Hewitt broth with streptomycin at 100 μg/ml and harvested in late log phase by centrifugation, and the cell pellet was resuspended in PBS to an OD550 of 1. Fifty microliters of this suspension was then added to each well of 96-well plates (Nunc). The plates were air dried at room temperature and blocked with 200 μl of PBS containing 5% (wt/vol) dry milk (blocking buffer) for 1 h. After the plates had been washed five times with PBS, 100 μl of mouse serum diluted in blocking buffer was added to each well and incubated at room temperature for 1.5 h. The plates were then washed with PBS-T and incubated with 100 μl of peroxidase-labeled goat anti-mouse IgG or IgA (1:1,000 dilution of 1 mg/ml in PBS; KPL) for 1.5 h at room temperature. Finally, the plates were washed five times with PBS-T, developed with ABTS (100 μl/well; KPL) for 20 min, and subjected to stopping reactions with 1% SDS (100 μl/well). A405 values were read with a VERSAmax microplate reader, and the endpoint titers of test sera were determined as described above.
DNA sequencing.
All sequencing reactions were performed with the Applied Biosystems Prism Dye Terminator cycle sequencing core kit and based on the Prism protocol supplied by the vendor. Approximately 1 mg of template DNA and 100 ng of primer were used for each cycling reaction. The reaction mixtures were cycled on a GeneAmp PCR Systems 2400 unit, purified using the Prism method, and analyzed on an ABI 373A DNA sequencer (Applied Biosystems).
Protein determination.
The concentration of protein during purification was determined by the method of Lowry et al. (17). Protein concentration prior to immunization was determined by using a BCA kit obtained from Pierce Chemicals (Northbrook, Ill.) in accordance with the manufacturer's directions. BSA was used as the protein standard.
SDS-PAGE and Western blotting.
Whole-cell lysates and concentrated culture supernatant samples were prepared by centrifuging equivalent numbers of pneumococcal cells, based on the OD600, in a microcentrifuge at 13,000 × g for 30 s. The supernatant was removed, and proteins were precipitated with 72% trichloroacetic acid. Pneumococcal cell pellets were resuspended in an appropriate volume of loading buffer, and precipitated proteins were resuspended in 10 μl of SDS-PAGE loading buffer. All samples were boiled for 5 min and separated by SDS–10% PAGE as described by Laemmli (15). The samples were transferred to nitrocellulose (Bio-Rad, Hercules, Calif.) by using a Bio-Rad Mini Transblot cell, and the blots were blocked at room temperature for 30 min in PBS containing 5% nonfat milk (BLOTTO). Membranes were probed with pooled mouse antisera diluted 1:1,000 in BLOTTO for 60 min and then washed for 25 min in PBS–0.2% Tween 80. Goat anti-mouse IgG plus IgM conjugated to alkaline phosphatase (Biosource International, Camarillo, Calif.) was used to detect bound antibodies at a 1:1,000 dilution in BLOTTO. The blots were washed as previously described and detected with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (BCIP) from Bio-Rad in accordance with the manufacturer's directions.
Statistical analysis.
Comparison of nasal colonization and ELISA titers among groups was performed using the Student t test. Results were considered significant at P < 0.05.
Nucleotide sequence accession numbers.
The GenBank/EMBL accession numbers for the nucleotide sequences of phpA, phpA-79, and phpA-20 are as follows: phpA, AF340221; phpA-79, AF340222; and phpA-20, AF340223.
RESULTS
Cloning and expression of phpA gene fragments.
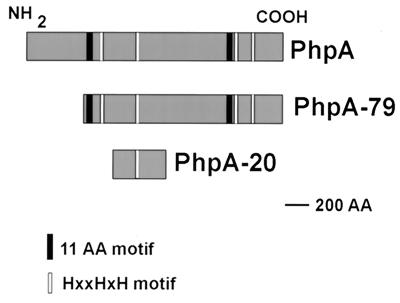
The sequence of the putative C3 peptidase described by Hostetter was aligned to an early release (August 1998) of the S. pneumoniae genome made available by TIGR (www.tigr.org). The sequence had homology to three separate but similar ORFs of approximately 2,100 to 2,400 bp each in the S. pneumoniae genomic sequence, encoding proteins of ≈77 to 88 kDa. Several interesting features that the deduced amino acid sequences of these ORFs had in common were observed. A histidine triad, composed of HXXHXH, is present four to five times in each of the sequences (Fig. 1); thus, the genes were named for S. pneumoniae histidine proteins A, B, and C (phpA, -B, and -C). Another amino acid repeat, composed of GXYTTXDGYIF, is present twice in each of the sequences, flanking a central proline-rich region. However, the COOH-terminal regions of each of the sequences are very different and are characteristic for each protein. The sequence of the cloned putative C3 protease (13) most closely aligned with the 2,163 bp of the phpA gene sequence, and consequently, this ORF was chosen for further study.
FIG. 1.
Relationship of PhpA to rPhpA-79 and rPhpA-20. AA, amino acid(s).
A later release of the genomic sequence by TIGR (October 1998) revealed another potential start site in frame with the phpA ORF, 105 amino acids upstream of the methionine originally thought to be the first amino acid of the protein. While the larger gene sequence was successfully cloned, efforts to express this larger protein were unsuccessful (data not shown), probably because the message produced was unstable or the protein expressed was lethal to E. coli. Thus, the cloned phpA gene fragments that expressed a protein were used for all further studies.
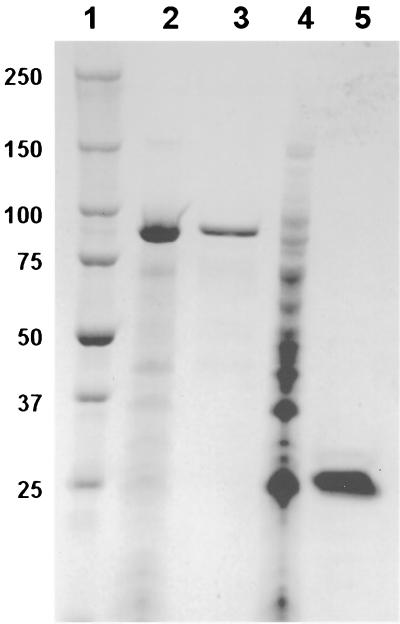
The purified rPhpA-79 protein was expressed in inclusion bodies (Fig. 2, lane 2) and could be slowly refolded in phosphate buffer without detergent after being resolubilized in a chaotropic agent. This is not surprising, given the overall hydrophilic nature of the protein sequence (data not shown). After the final chromatographic elution step, the rPhpA-79 protein was >90% homogeneous (Fig. 2, lane 3), and it was concentrated to approximately 1 mg/ml.
FIG. 2.
SDS-PAGE of purified rPhpA-79 and rPhpA-20. Whole-cell lysates of induced pLP515 and pLP505 cultures and 5 μg of purified rPhpA-79 and rPhpA-20 were subjected to SDS–4 to 20% PAGE. Lanes: 1, Bio-Rad Precision prestained molecular weight markers; 2, whole-cell lysate of pLP515; 3, rPhpA-79 protein; 4, whole-cell lysate of pLP505; 5, rPhpA-20 protein. The values on the left are molecular sizes in kilodaltons.
Since a smaller fragment of the PhpA protein has been implicated in the degradation of human complement component C3 (13) and the sequence of the gene thought to encode this fragment aligned with an internal portion of the phpA gene, primers were designed to amplify and express this fragment. The relationships of the putative full-length PhpA protein, the PhpA-79 protein fragment, and the PhpA-20 protein fragment are shown in Fig. 1. As expected, pLP505 expressed a protein of approximately 20 kDa, designated PhpA-20, that was present in whole-cell lysates (Fig. 2, lane 4). rPhpA-20 was present in inclusion bodies and required solubilization with 8 M urea. The final purified rPhpA-20 protein was soluble without detergent and did not require imidazole for purification. The purified protein was analyzed by SDS-PAGE and shown to be >80% homogeneous (Fig. 2, lane 5).
Reactivity of anti-rPhpA-79 serum.
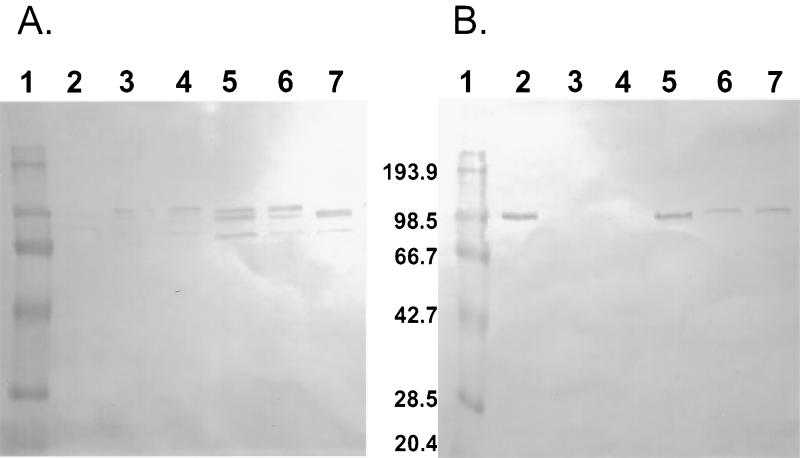
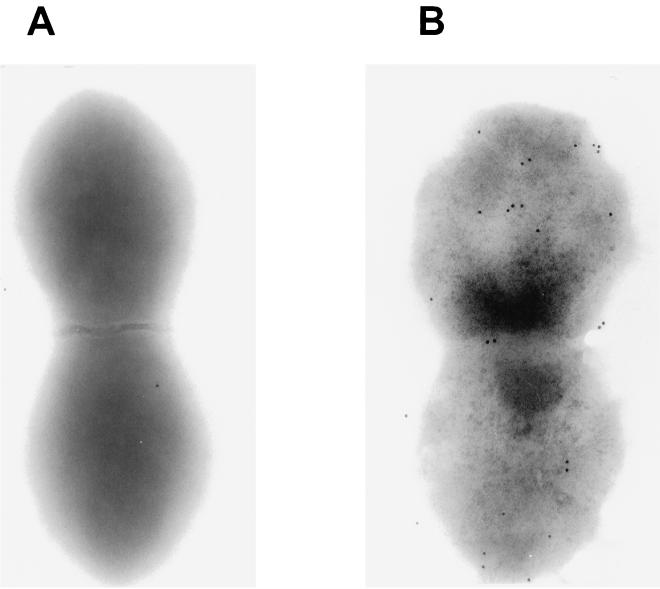
Polyclonal antiserum to rPhpA-9 was generated in Swiss Webster mice to determine if the rPhpA protein was expressed by S. pneumoniae and to evaluate antigenic conservation of the protein between strains. Antiserum to the rPhpA-79 protein reacted with proteins of approximately 80 to 97 kDa in six of seven culture supernatants (Fig. 3A) and in five of seven whole-cell lysates (Fig. 3B). Every pneumococcal strain tested produced at least one reactive band in either the culture supernatants or whole-cell lysates in this size range. Some strains had no detectable reactive bands in whole-cell lysates. This may have been due to various amounts of Php proteins released from the whole cells or active secretion of the proteins. These antibody-reactive bands corresponded to the predicted sizes of the PhpA, -B, and -C proteins based on the later release (October 1998) of the S. pneumoniae genome by TIGR. Because the serum reacted with proteins with sizes between 80 and 94 kDa in concentrated culture supernatants (Fig. 3A), it is likely that anti-rPhpA-79 serum is able to react with the PhpB and PhpC proteins in these samples. The same polyclonal serum was used in IEM to determine the presence of the protein on the surface of whole S. pneumoniae strain CP1200 cells. The micrograph in Fig. 4 shows that while it is not highly expressed, the protein appears to be distributed over the entire surface of the bacterium. IEM evaluation of other pneumococcal strains has not been done.
FIG. 3.
Western blot analysis of whole-cell lysates and culture supernatants of S. pneumoniae serotypes. Concentrated culture supernatants (A) or whole-cell lysates (B) were subjected to SDS–4 to 20% PAGE and blotted onto nitrocellulose for analysis. Polyclonal mouse antiserum to rPhpA-79 was used as the primary antibody. Lanes: 1, Bio-Rad prestained low-molecular-weight markers; 2, serotype 3; 3, serotype 5B; 4, serotype 6B; 5, serotype 14; 6, serotype 18C; 7, strain CP1200. The values between the panels are molecular sizes in kilodaltons.
FIG. 4.
Transmission IEM of S. pneumoniae CP1200. Polyclonal mouse antiserum to rPhpA-79 was used as the primary antibody. Eighteen-nanometer gold particle-labeled anti-mouse IgG and IgM were used as the secondary antibodies. Cells were photographed at a magnification of ×50,000. Panels: A, primary serum from a preimmune mouse; B, anti-rPhpA-79 primary serum.
Immune response to s.c. immunization with rPhpA-79 and rPhpA-20.
Mice were immunized three times s.c. with 5 μg of either rPhpA-79 or rPhpA-20 formulated with MPL (100 μg/dose). Serotype 3 pneumococcal polysaccharide conjugated to CRM197 protein (PNC-3) was used as a positive control (12). As shown in Table 1, mice administered rPhpA-79–MPL exhibited significant titers of IgG toward whole cells of S. pneumoniae serotype 3. No serum antibody was detected after immunization with MPL alone. rPhpA-79 elicited a level of IgG antibody reactive toward whole cells of S. pneumoniae type 3 equivalent to that of 1 μg of PNC-3 adjuvanted with AlPO4 (Table 1).
TABLE 1.
Serum antibody titers against serotype 3 S. pneumoniae whole cells and purified rPhpA-79 or rPhpA-20 after s.c. immunization
| Immunogena | Type 3 S. pneumoniae whole-cell ELISAb
|
rPhpA-79-specific ELISAc
|
rPhpA-20-specific ELISAc
|
|||
|---|---|---|---|---|---|---|
| IgG | IgA | IgG | IgA | IgG | IgA | |
| rPhpA-79–MPL | 4.59 ± 0.24 | <1.7 | 5.10 ± 0.27 | <1.7 | 5.67 ± 0.46 | <1.7 |
| rPhpA-20–MPL | 3.48 ± 0.39 | <1.7 | 4.68 ± 0.41 | <1.7 | 6.37 ± 0.39 | <1.7 |
| PNC-3–AlPO4 | 4.93 ± 0.31 | <1.7 | <1.7 | <1.7 | <1.7 | <1.7 |
| MPL | <1.7 | NDd | <1.7 | <1.7 | <1.7 | <1.7 |
CBA/N mice were vaccinated s.c. at weeks 0, 3, and 5. The doses were as follows: rPhpA-79 or rPhpA-20, 5 μg/mouse; PNC-3, 1 μg/mouse; and MPL or AlPO4, 100 μg/mouse.
The S. pneumoniae serotype 3 whole-cell ELISA assessed sera from 20 individual mice at week 7, and results are expressed as the mean log10 titer ± the standard deviation.
Endpoint anti-rPhpA-79 or anti-rPhpA-20 antibody titers were determined by ELISA of sera collected from 20 individual mice at week 7, and results are expressed as the mean log10 titer ± the standard deviation.
ND, not done.
The immune sera to the rPhpA-79 and rPhpA-20 proteins were also assessed in protein-specific ELISAs. At 2 weeks after the last booster immunization, strong, antigen-specific IgG antibody responses were generated in mice immunized with rPhpA-79–MPL or rPhpA-20–MPL (Table 1). Furthermore, the immune sera from mice immunized by either rPhpA-79–MPL or rPhpA-20–MPL were highly cross-reactive to the rPhpA-79 and rPhpA-20 proteins, which was not unexpected since rPhpA-20 is an internal region of rPhpA-79. Although s.c. immunization with rPhpA-79–MPL or rPhpA-20–MPL induced a strong serum IgG antibody response to whole cells of S. pneumoniae type 3 and the rPhpA-79 and rPhpA-20 proteins, it did not induce any detectable serum IgA antibody. Immunization of BALB/c mice using a similar s.c. vaccination protocol elicited responses essentially the same as those obtained with CBA/N mice (data not shown).
Protection against invasive disease caused by S. pneumoniae serotype 3.
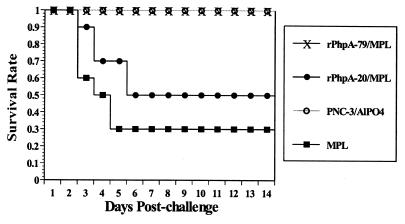
To test whether s.c. immunization with rPhpA-79 is able to protect mice against pneumococcal infection, CBA/N mice immunized with various antigens were challenged i.n. with S. pneumoniae serotype 3 2 weeks after the last immunization. This pneumococcal strain is virulent for CBA/N mice, with an i.n. inoculum of 106 CFU corresponding to 10 times the LD50 (data not shown). This challenge resulted in a rapid systemic infection ending in death. Mice that received MPL alone started to die on day 3 after challenge (40%); deaths continued to increase to 70% by day 5, and no further change in mortality was noted to the end of the 14-day observation time (Fig. 5). This is not surprising, since 13- to 15-week-old mice had a shallow mortality curve due to S. pneumoniae serotype 3 infection. Importantly, immunization with either rPhpA-79–MPL or PNC-3–AlPO4 provided 100% protection against death caused by type 3 S. pneumoniae infection. In contrast to the results obtained after immunization with rPhpA-79, only 50% of the mice immunized with rPhpA-20–MPL survived for up to 14 days postchallenge. Those mice immunized with rPhpA-79–MPL did not exhibit pathological changes such as ruffled fur, lethargy, degeneration of organs, inflammation of the lungs, or enlargement of the spleen and were indistinguishable from surviving challenged mice upon gross necropsy (data not shown).
FIG. 5.
Survival of mice after s.c. vaccination with rPhpA-79–MPL and i.n. challenge with 10 LD50s of S. pneumoniae serotype 3. Mice were immunized with rPhpA-79 (5 μg) or rPhpA-20 (5 μg) plus MPL (100 μg). Groups immunized with PNC-3 (1 μg) plus AlPO4 (100 μg) or MPL alone were used as controls.
Mice immunized with rPhpA-79–MPL were also protected against bacteremia. Two days after i.n. challenge, all rPhpA-79–MPL-immunized mice had <10 CFU/ml (limit of detection) in their blood whereas 40% of both the MPL control mice and rPhpA-20–MPL mice had >3 × 103 CFU/ml (data not shown). Thus, mice immunized with rPhpA-20–MPL were not protected against bacteremia compared to control mice. In all of the CBA/N i.n. challenge experiments performed, bacteremia always preceded death. Therefore, protection against death may have been due primarily, if not exclusively, to protection against bacteremia.
Protection against nasopharyngeal colonization by S. pneumoniae serotype 3.
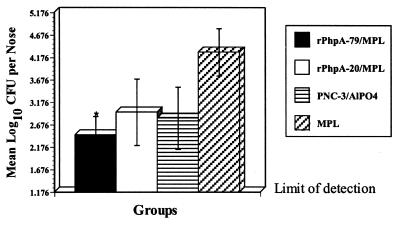
To test whether parenteral immunization with rPhpA-79 could elicit an effective immune response at the nasopharyngeal mucosa, we examined nasopharyngeal colonization in CBA/N mice immunized with rPhpA-79 and challenged i.n. with S. pneumoniae. Groups of mice were immunized s.c. with rPhpA-79–MPL or rPhpA-20–MPL at weeks 0, 3, and 5. Two weeks after the last immunization, mice were challenged i.n. with 106 CFU of type 3 S. pneumoniae as described above. Mice immunized with MPL alone and challenged at the same time served as controls. Bacterial colonization was determined from the number of bacteria recovered from homogenized nasal tissue 48 h after challenge. Compared to the MPL control group, s.c. immunization with rPhpA-79–MPL significantly reduced nasopharyngeal colonization by type 3 S. pneumoniae (P < 0.05) (Fig. 6). Although fewer bacteria were recovered from the noses of mice immunized with rPhpA-20–MPL or PNC-3–AlPO4 than from those of the adjuvant control group, these differences were not statistically different as determined by Student's t test.
FIG. 6.
Nasopharyngeal colonization of mice vaccinated s.c. with rPhpA-79–MPL and challenged i.n. with 106 CFU of S. pneumoniae serotype 3. Mice were immunized with rPhpA-79 (5 μg) or rPhpA-20 (5 μg) plus MPL. Groups immunized with PNC-3 (1 μg) plus AlPO4 or MPL alone were controls. Numbers of pneumococci recovered from nasal tissues 2 days postchallenge are expressed as the mean log10 CFU per nose ± the standard error of the mean. The asterisk represents significant reduction compared to the MPL control as determined by Student's t test (P < 0.05).
Immune response to i.n. immunization with rPhpA-79.
To determine whether i.n. immunization with rPhpA-79 can induce serum immune responses, CBA/N mice were administered 5 μg of rPhpA-79 or rPhpA-20 three times at weekly intervals by using CT-E29H (0.1 μg/dose) as a mucosal adjuvant. Immune sera collected 1 week after administration of the last booster immunization were tested by S. pneumoniae serotype 3 whole-cell and antigen-specific ELISAs. The IgG antibody titer produced in mice against whole cells of S. pneumoniae type 3 was significantly higher in the mice immunized with rPhpA-79 than in the mice given rPhpA-20 (P < 0.05) (Table 2). However, serum IgA to whole pneumococcal cells was below detectable limits in all of the sera tested. Importantly, rPhpA-79 induced very strong IgA and IgG responses against both the rPhpA-79 and rPhpA-20 proteins while rPhpA-20 elicited rPhpA-20-specific IgA responses and considerably lower titers of antibodies against both of the proteins tested. It is unclear why the antigen specificity of the IgG produced was different between s.c. immunization and i.n. immunization (Tables 1 and 2).
TABLE 2.
Serum antibody titers against serotype 3 S. pneumoniae whole cells and purified rPhpA-79 or rPhpA-20 after i.n. immunization
| Immunogena | Type 3 S. pneumoniae whole-cell ELISAb
|
ELISA titer vs:
|
||||
|---|---|---|---|---|---|---|
| rPhpA-79c
|
rPhpA-20c
|
|||||
| IgG | IgA | IgG | IgA | IgG | IgA | |
| rPhpA-79–CT-E29H | 3.22 ± 0.18 | <1.7 | 4.09 ± 0.20 | 2.45 ± 0.38 | 5.15 ± 0.40 | 2.96 ± 0.28 |
| rPhpA-20–CT-E29H | 1.75 ± 0.10 | <1.7 | 2.48 ± 0.66 | <1.7 | 4.63 ± 0.49 | 1.94 ± 0.50 |
| CT-E29H | <1.7 | <1.7 | <1.7 | NDd | <1.7 | ND |
CBA/N mice were vaccinated i.n. at weeks 0, 1, and 2. The doses were as follows: rPhpA-79 or rPhpA-20, 5 μg/mouse; and CT-E29H, 0.1 μg/mouse.
The S. pneumoniae type 3 whole-cell ELISA assessed sera from 20 individual mice at week 3, and results are expressed as the mean log10 titer ± the standard deviation.
Endpoint anti-rPhpA-79 or anti-rPhpA-20 antibody titers were determined by ELISA of sera collected from 20 individual mice at week 3, and results are expressed as the mean log10 titer ± the standard deviation.
ND, not done.
Protection against invasive disease by i.n. immunization with rPhpA-79.
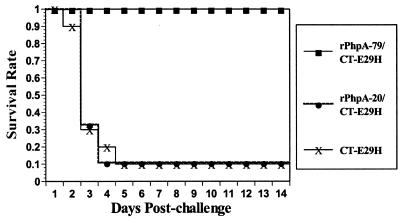
Since i.n. immunization elicited high serum IgG antibody titers, the ability of i.n. immunization with rPhpA-79 to protect against i.n. challenge was examined. CBA/N mice immunized with rPhpA-79–CT-E29H, rPhpA-20–CT-E29H, or CT-E29H alone were challenged i.n. with 106 CFU of S. pneumoniae serotype 3 1 week after the third immunization. By day 5 after challenge, all of the mice immunized with rPhpA-79–CT-E29H were still alive while only 10% of the mice that had received CT-E29H alone were alive. There was no difference in survival between the CT-E29H control mice and mice that had received rPhpA-20–CT-E29H (Fig. 7). No further change in mortality occurred through the 14-day observation period. At the end of the observation period, the number of surviving mice in the rPhpA-79-immunized group was statistically significantly greater than in the rPhpA-20 or adjuvant control group (P < 0.001). Furthermore, the lungs of surviving mice immunized with rPhpA-79–CT-E29H did not show any pathological changes compared to those of placebo control mice. Thus, immunization with rPhpA-79–CT-E29H not only protected against death but also prevented damage to the lungs.
FIG. 7.
Survival of mice i.n. vaccinated with rPhpA-79–CT-E29H and challenged i.n. with 106 CFU of S. pneumoniae serotype 3. Mice were immunized with rPhpA-79 (5 μg) or rPhpA-20 (5 μg) plus CT-E29H (0.1 μg). Mice immunized with CT-E29H (0.1 μg) were used as a negative control for i.n. immunization.
To determine the effects of immunization on bacteremia, additional groups of CBA/N mice were vaccinated i.n. and challenged as described above. Two days after an i.n. challenge, all of the rPhpA-79–CT-E29H-immunized mice had <10 CFU/ml (limit of detection) in their blood whereas 80% of the mice immunized with rPhpA-20–CT-E29H had >3 × 103 CFU/ml (data not shown). Therefore, i.n. immunization with rPhpA-79–CT-E29H also conferred protection against bacteremia.
Protection against nasopharyngeal colonization by S. pneumoniae serotypes 3 and 14 after i.n. immunization.
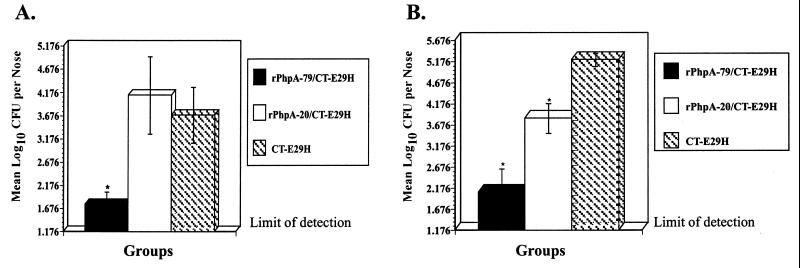
Since parenteral immunization with rPhpA-79 reduced nasopharyngeal colonization and i.n. immunization with rPhpA-79 protected against death, the effect of i.n. immunization with rPhpA-79 on colonization was examined. CBA/N mice were immunized i.n. with 5 μg of rPhpA-79 or rPhpA-20 and 0.1 μg of CT-E29H. Mice were immunized three times at 1-week intervals. One week after the last immunization, mice were challenged i.n. with either 106 CFU of type 3 S. pneumoniae or 105 CFU of a nonlethal type 14 S. pneumoniae strain. This strain of type 14 S. pneumoniae can colonize the nasopharynges of mice but is unable to enter the bloodstream or kill the animals. Mice immunized with CT-E29H alone and challenged at the same time served as controls. Bacterial colonization was determined as previously described 2 days postchallenge with type 3 S. pneumoniae or 3 days postchallenge with type 14 S. pneumoniae. Compared to the CT-E29H control group, i.n. immunization with rPhpA-79–CT-E29H significantly reduced nasopharyngeal colonization of both type 3 and type 14 S. pneumoniae (P < 0.05) (Fig. 8). Interestingly, a reduction in the nasal colonization of mice immunized with rPhpA-20–CT-E29H was observed after challenge with serotype 14, but not with serotype 3, S. pneumoniae.
FIG. 8.
Nasopharyngeal colonization of mice vaccinated i.n. with rPhpA-79–CT-E29H and challenged i.n. with S. pneumoniae serotype 3 or 14. Mice were immunized with rPhpA-79 (5 μg) or rPhpA-20 (5 μg) plus CT-E29H (0.1 μg) at weeks 0, 1, and 2. Mice immunized with CT-E29H alone were used as controls. One week after the third immunization, mice were challenged i.n. with either 106 CFU of serotype 3 (A) or 105 CFU of serotype 14 (B). The numbers of pneumococci recovered from nasal tissues 2 days postchallenge with serotype 3 (A) or 3 days postchallenge with serotype 14 (B) are expressed as the mean log10 CFU per nose ± the standard error of the mean. The asterisks represent significant reduction compared to the CT-E29H control as determined by Student's t test (P < 0.05).
DISCUSSION
Initial attempts to clone and express the gene encoding the putative C3-degrading proteinase originally described by Hostetter (13) resulted in the identification of a much larger ORF capable of encoding an up to 97-kDa protein. This ORF has been designated the phpA gene. Genomic analysis indicates that phpA is one of three similar but distinct ORFs in S. pneumoniae (phpA, -B, and -C). These ORFs are similar in primary amino acid sequence over the first two-thirds of the protein and share conserved histidine triad motifs, as well as a larger 11-amino-acid motif. There are five histidine motifs in the PhpA protein that occur in hydrophilic areas of the molecule and may be involved in its biological function. Histidine motifs have been shown to be important in divalent cation binding and specifically in the binding of zinc ions (7, 23). The two 11-amino-acid motifs appear in areas of intermediate hydrophilicity and do not resemble any motifs with known functions. Experiments are in progress to evaluate the metal binding capabilities of the rPhpA-79 protein.
The exact transcriptional start site of the PhpA protein is unknown, as are the size and location of the mature protein in S. pneumoniae. Polyclonal antisera generated in mice selectively bound to a single protein of the largest predicted size (i.e., 94 kDa) in four of the six whole-cell lysates examined, while several bands in the 80- to 95-kDa size range in culture supernatants of all six of the serotypes examined reacted with the antisera (Fig. 3). Perhaps the phpA gene is either not present or not transcribed or the protein is secreted in serotypes 5 and 6B.
Given the degree of sequence homology among PhpA, -B, and -C, a polyclonal antiserum should cross-react with the three proteins if they are all expressed. Reactive species corresponding to the predicted sizes of PhpB and -C were seen in the culture supernatants from all of the serotypes examined but not in the whole-cell lysates (Fig. 3). The cross-reactive epitopes in PhpB and -C may not be exposed when the protein is still attached to the cell wall, PhpB and -C may just be secreted proteins, or possibly the smaller reactive species may just be clipped versions of the PhpA protein. Monoclonal antibodies specific for the unique carboxy-terminal third of the PhpA, PhpB, and PhpC proteins are being developed to further elaborate the expression of the individual proteins by S. pneumoniae and the cellular location of each expressed protein.
From the data presented in Fig. 3, it is unclear whether the PhpA protein is a secreted or cell wall-associated protein. IEM (Fig. 4) indicated that at least some of the PhpA protein remains cell associated in late-log S. pneumoniae strain CP1200. Since either whole-cell lysates or culture supernatants from every strain examined reacted with antisera to either rPhpA-79 or rPhpA-20 and the PhpA protein appeared to be surface exposed, both truncates were selected for further evaluation as potential vaccine candidates.
Since it is likely that the pneumococci found in the nasopharynges of xid mice 2 or 3 days after i.n. challenge represent true colonization, not just inoculated bacteria in the process of being cleared, a reduction in the level of colonization at this time point by a vaccine candidate should be important for disease prevention. In these studies, immunization with rPhpA-79 significantly reduced nasopharyngeal colonization by S. pneumoniae serotype 3 (Fig. 6 and 8A), as well as protected mice against death (Fig. 5 and 7). These results support the hypothesis that prevention or reduction of pneumococcal colonization of the nasopharynx could be an effective way to prevent systemic disease.
The best immunization route by which to elicit protective immunity to pneumococcal colonization is still unclear. Theoretically, protection against colonization should result from local rather than systemic immunity. Previously published studies have shown that i.n. immunization with PspA elicits better protection against colonization than does s.c. immunization, while PsaA can elicit some immunity to carriage after parenteral immunization (4, 31). In contrast, rPhpA-79 reduced nasopharyngeal colonization by S. pneumoniae when administered by either the i.n. or s.c. immunization route, which could make rPhpA-79 a more useful vaccine candidate than either of these proteins. It has been noted that although higher serum IgG titers were elicited by s.c. than by i.n. immunization, only i.n. immunization elicited rPhpA-79-specific serum IgA (Tables 1 and 2). Additionally, i.n. immunization of BALB/c mice with rPhpA-79–CT-E29H elicited detectable IgA and IgG responses in mucosal secretions (data not shown). Based on the data produced by these studies, we speculate that optimal immunization against colonization should be carried out i.n. Furthermore, the observation that i.n. immunization with rPhpA-79–CT-E29H can cross-protect against colonization with two strains (serotypes 3 and 14) enhances the prospects for the use of rPhpA-79 as a mucosal vaccine, especially for the strains not covered by the current polysaccharide conjugate vaccine.
The rPhpA-20 protein corresponds to the region of the PhpA ORF originally identified by Hostetter (13). Subcutaneous immunization with rPhpA-20 gave only partial protection from death (Fig. 5), compared to the complete protection afforded by s.c. immunization with rPhpA-79. Also, s.c. immunization with the rPhpA-20 protein did not significantly reduce colonization after challenge (Fig. 6). The i.n. immunization with the rPhpA-20 protein failed to elicit an immune response capable of either reducing colonization or protecting against death after challenge with S. pneumoniae serotype 3 (Fig. 7 and 8A). These results indicate that the rPhpA-20 protein truncate represents a suboptimal vaccine candidate. Although the rPhpA-79 truncate confers significant protection against death, the exact location of the epitope(s) required for protection on PhpA is not known, and other truncates could be as effective.
The i.n. administration of antigens requires adjuvants, such as CT and mutant forms of E. coli heat-labile toxin (LT). In this study, we used the mucosal adjuvant CT-E29H, which is a genetically detoxified mutant form of CT (30). The data from this study indicate that CT-E29H enhanced the protective systemic and mucosal immune responses to rPhpA-79 in animals. While the greatly reduced toxicity and enzymatic activity of the CT-E29H protein make it a good potential human mucosal adjuvant, some recent publications have raised concerns over the safety of i.n. administration of wild-type and mutant CTs in humans. Thus, we also plan to study other mucosal adjuvants for effectiveness in animal models of S. pneumoniae infection.
To summarize, our present study indicates that rPhpA-79 could be a promising vaccine candidate for human pneumococcal disease. This recombinant protein elicits antibodies against surface-exposed epitopes on S. pneumoniae and has the advantage of inducing cross-reactive antibodies against multiple strains of S. pneumoniae. The i.n. immunization with rPhpA-79 and CT-E29H significantly reduced nasopharyngeal colonization in mice challenged with S. pneumoniae type 3 or 14. Both s.c. and i.n. immunizations with rPhpA-79 protected mice against bacteremia and death after i.n. challenge. Since colonization of the nasopharynx is a strong indicator of otitis media, we speculate that a reduction of pneumococcal nasopharyngeal colonization may contribute to initial protection against not only systemic disease but also otitis media. These data warrant further investigations into the vaccine potential of the rPhpA-79 protein, along with the other members of the family, PhpB and PhpC.
ACKNOWLEDGMENTS
Electron microscopy was performed by Rob Smith, and his assistance is greatly appreciated. We thank Kathryn Mason for expert technical assistance with the mouse models, Leslie Croy for molecular biology work, Duzhang Zhu for advice on various aspects of this research, and John Eldridge and Sub Pillai for critical review of the manuscript.
M.K.H. was supported by NIH grant AI 24162.
REFERENCES
- 1.Angel C S, Ruzek M, Hostetter M K. Degradation of C3 by Streptococcus pneumoniae. J Infect Dis. 1994;170:600–608. doi: 10.1093/infdis/170.3.600. [DOI] [PubMed] [Google Scholar]
- 2.Beesley J, Day S, Betts M, Thorley C. Immunocytochemical labelling of Bacteroides nodosus pili using an immunogold technique. J Gen Microbiol. 1984;130:1481–1487. doi: 10.1099/00221287-130-6-1481. [DOI] [PubMed] [Google Scholar]
- 3.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briles D E, Ades E, Paton J C, Sampson J S, Carlone G M, Huebner R C, Virolainen A, Swiatlo E, Hollingshead S K. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect Immun. 2000;68:796–800. doi: 10.1128/iai.68.2.796-800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler J C, Shapiro E D, Carlone G M. Pneumococcal vaccines: history, current status, and future directions. Am J Med. 1999;107:69S–76S. doi: 10.1016/s0002-9343(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 6.Crain J M, Waltman W D, Turner J S, Yother J, Talkington D F, McDaniel L S, Gray B M, Briles D E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels J M, Turner-Cavet J S, Selkirk R, Sun H, Parkinson J A, Sadler P, Robinson N J. Coordination of Zn2+ (and Cd2+) by prokaryotic metallothionein. J Biol Chem. 1998;273:22957–22961. doi: 10.1074/jbc.273.36.22957. [DOI] [PubMed] [Google Scholar]
- 8.Dintilhac A, Alloing G, Granadel C, Claverys J-P. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25:727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- 9.Douglas R M, Paton J C, Duncan S J, Hansman D J. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983;148:131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein F W, Garau J. 30 years of penicillin-resistant S. pneumoniae: myth or reality? Lancet. 1997;350:233–234. doi: 10.1016/s0140-6736(05)62222-2. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorff W P, Bryant J, Paradiso P R, Siber G R. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000;30:100–121. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 12.Henriksen J L, Preheim L C, Gentry M J. Vaccination with protein-conjugated and native type 3 capsular polysaccharide in an ethanol-fed rat model of pneumococcal pneumonia. Alcohol Clin Exp Res. 1997;21:1630–1637. [PubMed] [Google Scholar]
- 13.Hostetter M K. Opsonic and nonopsonic interactions of C3 with Streptococcus pneumoniae. Microb Drug Resist. 1999;5:85–89. doi: 10.1089/mdr.1999.5.85. [DOI] [PubMed] [Google Scholar]
- 14.Hostetter M K. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986;153:682–693. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lennette D A. General principles for laboratory diagnosis of viral, rickettsial, and chlamydial infections. In: Lennette E H, Lennette D A, Lennette E T, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. 7th ed. Washington, D.C.: American Public Health Association; 1995. pp. 17–18. [Google Scholar]
- 17.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Madsen M, Lebenthal Y, Cheng Q, Smith B L, Hostetter M K. A pneumococcal protein that elicits interleukin-8 from pulmonary epithelial cells. J Infect Dis. 2000;181:1330–1336. doi: 10.1086/315388. [DOI] [PubMed] [Google Scholar]
- 19.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 20.McDaniel L S, Scott G, Kearney J F, Briles D E. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. J Exp Med. 1984;160:386–397. doi: 10.1084/jem.160.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison D A, Lacks S A, Guild W R, Hageman J R. Isolation and characterization of three new classes of transformation-deficient mutants of Streptococcus pneumoniae that are defective in DNA transport and genetic recombination. J Bacteriol. 1983;156:281–290. doi: 10.1128/jb.156.1.281-290.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nabors G S, Braun P A, Hermann D J, Heise M L, Pyle D J, Gravenstein S, Schilling M, Ferguson L M, Hollingshead S K, Briles D E, Becker R S. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine. 2000;18:1743–1754. doi: 10.1016/s0264-410x(99)00530-7. [DOI] [PubMed] [Google Scholar]
- 23.Omburo G A, Jacobitz S, Torphy T J, Colman R W. Critical role of conserved histidine pairs HNXXH and HDXXH in recombinant human phosphodiesterase 4A. Cell Signal. 1998;10:491–497. doi: 10.1016/s0898-6568(97)00175-7. [DOI] [PubMed] [Google Scholar]
- 24.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure H R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 25.Sampson J S, O'Connor S P, Stinson A R, Tharpe J A, Russell H. Cloning and nucleotide sequence analysis of psaA, the Streptococcus pneumoniae gene encoding a 37-kilodalton protein homologous to previously reported Streptococcus sp. adhesins. Infect Immun. 1994;62:319–324. doi: 10.1128/iai.62.1.319-324.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinefield H R, Black S. Efficacy of pneumococcal conjugate vaccines in large scale field trials. Pediatr Infect Dis J. 2000;19:394–397. doi: 10.1097/00006454-200004000-00036. [DOI] [PubMed] [Google Scholar]
- 27.Slot J W, Geuze H J. Immunoblotting for electron microscopy. Amsterdam, The Netherlands: Elsevier Science Publishers; 1984. [Google Scholar]
- 28.Smith B L, Hostetter M K. C3 as substrate for adhesion of Streptococcus pneumoniae. J Infect Dis. 2000;182:497–508. doi: 10.1086/315722. [DOI] [PubMed] [Google Scholar]
- 29.Talkington D F, Brown B G, Tharpe J A, Koenig A, Russell H. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesin A (PsaA) Microb Pathog. 1996;21:17–22. doi: 10.1006/mpat.1996.0038. [DOI] [PubMed] [Google Scholar]
- 30.Tebbey P W, Unczur C A, Peek J A, Zhu D, LaPierre N A, Phillips E D, Ibraghimov A R, Green B A, Eldridge J H, Hancock G E. Effective mucosal immunization against respiratory syncytial virus using a genetically detoxified cholera holotoxin, CT-E29H. Vaccine. 2000;18:2723–2734. doi: 10.1016/s0264-410x(00)00058-x. [DOI] [PubMed] [Google Scholar]
- 31.Wu H Y, Nahm M H, Guo Y, Russell M W, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto M, Briles D E, Yamamoto S, Ohmura M, Kiyono H, McGhee J R. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J Immunol. 1998;161:4115–4121. [PubMed] [Google Scholar]