Abstract
Krabbe disease (KD) is a lysosomal storage disease (LSD) caused by mutations in the galc gene. There are over 50 monogenetic LSDs, which largely impede the normal development of children and often lead to premature death. At present, there are no cures for LSDs and the available treatments are generally insufficient, short acting, and not without co-morbidities or long-term side effects. The last 30 years have seen significant advances in our understanding of LSD pathology as well as treatment options. Two gene therapy-based clinical trials, NCT04693598 and NCT04771416, for KD were recently started based on those advances. This review will discuss how our knowledge of KD got to where it is today, focusing on preclinical investigations, and how what was discovered may prove beneficial for the treatment of other LSDs.
Keywords: monogenetic diseases, Krabbe disease, lysosomal storage disease, adeno-associated viral vectors, substrate reduction therapies, demyelination, psychosine
Graphical abstract
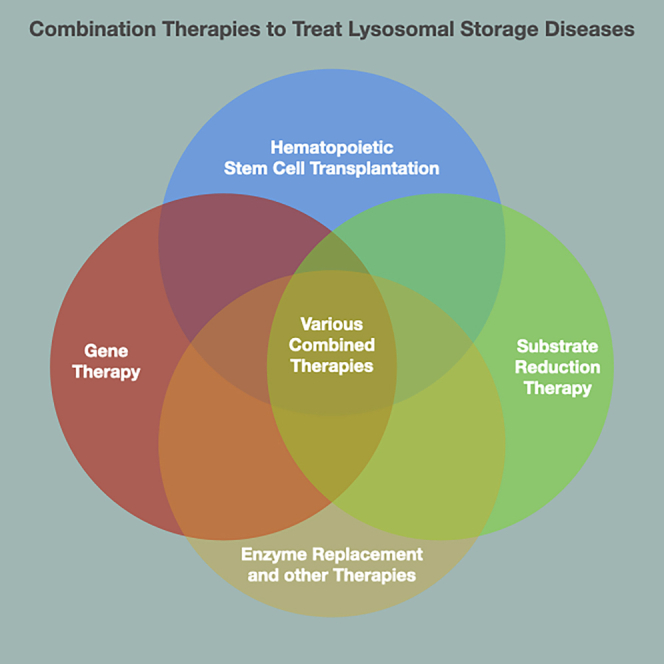
The current standard-of-care treatment of Krabbe disease (hematopoietic stem cell transplantation) only moderately increases life expectancy and partially protects against symptoms. Adeno-associated virus-based gene therapy is the most effective single-modality treatment of Krabbe disease. Combination therapies are the overall most effective treatments to increase life expectancy and protect against disease signs.
Krabbe disease: Introduction and pathogenesis
Lysosomal storage diseases (LSDs) are family of over 50 diseases caused by genetic defects in lysosomal genes. While each individual LSD is rare, together they are relatively common with a combined estimated incidence rate of 1 in 2,300–7,700 live births.1 These genetic defects, usually leading to expression of defective lysosomal enzymes, typically result in accumulation of the defective enzyme’s substrate because it cannot be catabolized. The symptoms exhibited in LSDs vary significantly depending on the specific genetic defect but there are some commonalities such as developmental delay/regression, vision loss, hepatosplenomegaly, and premature death.1 At present, there are no cures for any LSD and the existing treatments mostly only delay symptom development. This is particularly true for LSDs that affect the central nervous system (CNS). Additionally, many of the current treatments, such as hematopoietic stem cell transplantation (HSCT), for LSDs pose risks of their own.1 This review will focus on Krabbe disease (KD) as an introduction to potential therapeutics for other LSDs.
KD is a leukodystrophy and LSD first described by Knud H. Krabbe in 1916.2 In the last century there have been countless breakthroughs in our understanding of the underlying pathology as well as treatment, but there is still no cure. KD is an autosomal recessive disease caused by mutations in the galc gene that result in reduced activity of the lysosomal hydrolase, galactosylceramidase (GALC).
KD is typically divided into three subtypes based on age of onset and severity of symptoms: (1) infantile (symptom onset before 3 years of age; some physicians and investigators further divide it into early infantile and late infantile), (2) juvenile (onset after 3 years of age), and (3) adult onset (onset after 18 years of age).3 A retrospective analysis of KD cases in the United States estimates an incidence of 1 in 310,000 live births.4 Newborn screening (NBS) data from New York State estimate an incidence of 1 in 394,000 live births.5 It should be noted that these estimates are from hospitalization and NBS data, respectively, and both of these data collection methods are biased toward identifying infantile KD. Given that, the actual incidence may be higher because juvenile- and adult-onset patients may not have been detected in these studies. Additionally, there is some disparity between different ethnic groups; for example, the Druze population in the Middle East has an estimated incidence of 1 in 100 live births.6
GALC is responsible for the catabolic removal of galactose from sphingolipids containing galactose, such as galactosylceramides.7,8 GALC deficiency renders the body unable to metabolize several galactosphingolipids, which leads to their subsequent accumulation. This process is illustrated in Figure 1.7,9 Elevated levels of galactosylceramides cause the characteristic globoid cell reaction in macrophages that gives rise to KD’s formal name, globoid cell leukodystrophy (GLD).10 However, it should be mentioned that KD is different from many other LSDs in that the main substrate of the GALC enzyme, galactosylceramides, does not reach the levels of accumulated substrate seen in other LSDs.9,11
Figure 1.
Main metabolic pathways involving GALC
This figure outlines some of the relevant molecules and enzymes in KD. It is important to note that most of these reactions are normally reversible using differing enzymes; for example, ceremide galactosyltransferase (CGT) catalyzes addition of galactose to ceramides to form galactosylceramides, and GALC normally catalyzes the removal of galactose from galactosylceramides. Additionally, many of these enzymes catalyze reactions with other substrates, such as sphingosine and psychosine. One exception is the irreversible catalytic removal of the fatty acid group from galactosylceramides by acid ceramidase (ACD).
Galactosylsphingosine (commonly referred to as psychosine) is a sphingolipid that is catabolized by GALC, such that it is nearly undetectable in unaffected people and animals.11,12,13,14,15 High concentrations of psychosine are cytotoxic and trigger a cascade of pathogenic mechanisms, primarily by disrupting cell membranes as well as activating apoptotic pathways.16,17,18,19,20 Psychosine accumulation has been confirmed in KD patients, the Twitcher (TWI) mouse model of KD, new gene edited mouse KD models, and the canine model of KD.11,14,15,21 Miyatake and Suzuki first proposed what is now known as the psychosine hypothesis in 1972 to address the relatively low levels of galactosylceramides and high levels of psychosine. This hypothesis suggests that, because of the metabolic block preventing the normal breakdown of galactosylceramides into galactose and ceramides, galactosylceramides are instead deacylated into psychosine, which cannot be catabolized due to GALC deficiency.13 Most of the psychosine hypothesis has been confirmed, the one exception being that psychosine was originally thought to be generated through aberrant sphingolipid synthesis.12 However, it was recently shown that the main source of psychosine is through catabolic de-acylation of galactosylceramides by acid ceramidase with the anabolic pathway possibly playing a minor role.22
Myelinating cells, oligodendrocytes, and Schwann cells generate the aforementioned sphingolipids in significantly higher quantities than other cell types, thus they are particularly vulnerable to the metabolic blockade caused by the lack of GALC.11,23 The main pathological characteristics of KD are global diffuse demyelination (both CNS and peripheral nervous system [PNS]) and the appearance of multinucleated globoid cells.2,10,24 There are, however, many other pathological processes occurring in KD, such as disordered cell membrane dynamics (particularly within lipid rafts), disrupted signal transduction pathways, axonopathy, synaptic complications, overactivation of the proteosome degradation pathway, and problems regulating apoptosis.16,17,18,25,26,27,28,29,30 Together, these pathologic processes give rise to the clinical signs observed in KD patients.
All of the research into KD pathology, NBS, treatments, and more by numerous independent investigators has provided the foundation of two recently started phase 1/2 clinical trials investigating adeno-associated virus (AAV)-based gene therapy for KD: NCT04771416 and NCT04693598.
KD: Clinical presentation
Untreated infantile KD is typically divided into an asymptomatic stage, followed by three progressively worsening symptomatic stages. Affected infants typically have a short asymptomatic period where they seemingly develop normally. Stage 1 is characterized by mostly nonspecific symptoms such as difficulty feeding, vomiting, hypersensitivity, hyperirritability, and fever. Stiffness, developmental delay or regression, and seizures are less common but possible in this stage. The main laboratory finding at this stage is increased cerebrospinal fluid protein levels. Stage 2 symptoms include severe motor and mental deterioration with hypertonicity and hyperactive reflexes. Optic atrophy and tonic/clonic seizures are less common symptoms at this stage. At stage 3, the infant has very limited mobility, is decerebrate, and is blind. Untreated infants rarely live beyond 2–3 years.3,31
Later-onset subtypes of KD, including juvenile and adult onset, have more variable presentations. Often, the patients are clinically normal prior to symptom onset. Common presenting symptoms include visual deficits, weakness, balance problems, and cognitive impairment. Not only are symptoms variable but the age of onset and disease course are also variable, even between siblings with the same mutation.32,33
KD: Genetics and animal models
The galc gene was localized to chromosome 14 by linkage analysis and was subsequently sequenced and cloned.34,35,36 Full sequencing of the galc gene led to identification of at least 147 mutations and many single-nucleotide polymorphisms.37,38 Given the relatively large number of variants of unknown significance (VUSs) and the variable nature of the disease, it has been difficult to establish a clear genotype-phenotype correlation. A notable exception is a 30-kb deletion that encompasses exons 11 through 17, which correlates to a loss of the entirety of the smaller 30-kDa subunit and part of the larger 50- to 52-kDa subunit of the GALC protein, leading to an infantile presentation in individuals homozygous for this mutation.38,39,40,41 Patients with juvenile- and adult-onset KD are typically compound heterozygotes, usually possessing one copy of the galc gene containing the 30-kb deletion, and one copy with mutations that retain greater GALC functionality than mutations that more frequently lead to infantile KD.33,42,43,44
There are many animal models of KD, such as mouse, dog, and rhesus macaque.45,46,47,48 These models have allowed research into its pathogenesis as well as possible treatment options.49,50,51,52,53 The most commonly used animal models include the TWI mouse and canine KD models, both of which closely model infantile KD.49,51,52,54,55,56,57,58 TWI are a naturally occurring KD model possessing a spontaneous nonsense mutation in the galc gene leading to nonsense-mediated mRNA decay and a complete lack of functional GALC enzyme.45,59,60,61 TWI exhibit psychosine accumulation analogous to KD patients. Similar to KD patients, TWI show no signs of disease for the first weeks of life. TWI reach their maximum mass of 8–12 g around postnatal day (PND) 30, then continuously decline until their death at ∼PND40, analogous to failure to thrive in KD infants. Around PND20, muscle atrophy and weakness begin to develop, particularly in the hind limbs, ultimately leading to paralysis at approximately PND35–40. TWI begin to display the eponymous tremor or twitch around PND30. The histological features of disease in TWI include diffuse, global demyelination (both CNS and PNS), globoid cell formation, axonopathy, and neuroinflammation characterized by astrogliosis and microgliosis.45,61,62,63,64
The trs mouse is another KD model; they harbor a H168C missense mutation leading to 10%–20% wildtype (WT) mouse GALC activity. trs mice accumulate psychosine slightly slower than TWI. The median survival of trs mice is approximately 50 days, and they exhibit remarkably similar disease signs to TWI but delayed by about 10 days. Finally, the trs mice display similar levels of demyelination to TWI but have fewer infiltrating macrophages.65 An inducible galc knockout (KO) mouse was developed using a galc gene flanked by loxP sites and the Cre/enzyme replacement therapy (ERT) system to examine the effect of galc loss at different time points. It was found that very early (PND0) galc KO led to disease progression essentially identical to TWI, but, as galc KO was introduced later, disease onset was delayed and disease severity was reduced.66,67 A lysosomal membrane tethered GALC mouse, GALCLAMP1, was created to investigate cell-autonomous expression of GALC. GALCLAMP1 mice can also be induced to delete galc to recapitulate the TWI phenotype because the galclamp1 gene construct is flanked by loxP sites. Furthermore, GALCLAMP1 can be crossed with mouse lines that express Cre only in certain cell types to selectively delete galclamp1 expression in specific cells and determine the unique role of galc in only those cells.68 CRISPR-based gene editing has also been used to generate a severe infantile-like murine line carrying the T513M mutation and an adult-onset model murine line carrying the G41S mutation. The T513M mice exhibit disease progression almost identically to TWI as assessed by median survival, weight, tremor, and a multitude of behavioral tests. Pathologically, T513M mice are also extremely similar to TWI in that GALC activity is severely reduced and psychosine is elevated compared with WT. Since the G41S mice model adult-onset KD, they do not present pathological abnormalities until ∼PND200 and, even then, display much milder disease signs and pathology than TWI.21
The canine model of KD is another naturally occurring model that was first reported in 1966 in the West Highland terriers and Cairn terrier breeds.46 This model has reduced GALC activity due to a spontaneous missense mutation, Y158S, leading to elevated psychosine levels throughout the nervous system.52,58,69,70,71 The KD canine phenotype typically manifests at 4–6 weeks of age, with the first disease signs being hind limb weakness, forelimb dysmetria, and tremor. The signs progressively worsen, such that, by 12 weeks of age, they display hind limb ataxia and reach humane endpoint by 16 weeks of age due to disease burden.52,72,73 Histologically, KD canines display myelin loss, astrogliosis, microgliosis, and globoid cell accumulation similar to the human disease.52,58,72,74
KD: Treatments and screening
Currently, the standard-of-care treatment for pre-symptomatic infants with KD is HSCT.75 HSCT can utilize either bone marrow transplantation (BMT) or umbilical cord blood transplantation (UCBT). BMT was first found to increase survival in TWI from PND40 to PND80 days in 1984 but it resulted in only a minor increase in GALC activity and a small decrease in psychosine accumulation within the brain.49,76,77 In addition to providing a source of GALC enzyme, it is thought that BMT may also play a role in reducing neuroinflammation, as shown by a significant reduction in astrogliosis and microgliosis.78 BMT was first attempted in infantile- and juvenile-onset patients in 1998.79 BMT attenuated KD pathology in the juvenile-onset patients and significantly slowed the development of symptoms in infantile KD. BMT increased leukocyte GALC activity from a nearly undetectable level to approximately 3 nmol/h/mg protein (normal: >0.8 nmol/h/mg protein) within a few months of the treatment.31,79 This effect was sustained for at least 3 years. Additionally, BMT reduced abnormal signal intensity on magnetic resonance imaging (MRI), indicating improved myelination.79
A subsequent study of UCBT for infantile KD examined 11 pre-symptomatic patients and 14 symptomatic patients. All pre-symptomatic patients and six of the 14 symptomatic patients were alive at the end of a 3-year follow-up period with a median survival of 30 months for symptomatic patients, compared with untreated early infantile KD patients with a median survival of 13–18 months.80,81 UCBT had little effect on symptomatic patients. UCBT delayed the development of many standard KD symptoms in the pre-symptomatic treatment group, such that they had improved vision, cognitive skills, and peripheral nerve conduction, compared with untreated KD children. Most of the pre-symptomatic treatment group patients displayed improved language skills and gross motor skills, and ∼50% showed normal hearing.82 Long-term follow-up of 16 UCBT and two BMT early infantile KD patients treated prior to symptom onset showed that 13 were alive after a 15-year follow-up period with a median survival of 11 years. All patients had normalized leukocyte GALC activity ranging from 1.1–4.4 nmol/h/mg (pre-transplant levels ranged from 0–0.12 nmol/h/mg). The majority showed stable MRI findings, approximately average language skills, could walk with assistance, and slightly below-average cognitive development. However, many exhibited symptoms, including spasticity, eating difficulties, vision impairment, as well as bone and joint involvement.75 These studies show that, while HSCT provides a significant benefit to infants compared with untreated KD patients, it has limitations in preventing disease progression.
While HSCT provides significant benefit for KD patients, it is associated with both acute and chronic complications that pose a risk to patients. Patients must complete an immunosuppression regime for graft-versus-host disease (GVHD) prophylaxis prior to the procedure. Acute and chronic GVHD are associated with potential life-threatening complications. HSCT graft failure leads to minimal effectiveness and symptoms comparable with those of untreated KD.75,79,82 Altogether, while HSCT is not a cure for KD, it increases survival and improves quality of life for patients, but it has the potential for procedure-related complications such as GVHD.
HSCT has also been performed in other LSDs with varied levels of success. One of the most successful cases, in addition to KD, is metachromatic leukodystrophy (MLD). MLD is a demyelinating disorder similar to KD caused by mutations in arylsulfatase A (ARSA). Similar to the therapeutic effect observed in KD, HSCT attenuates clinical symptoms and extends median lifespan of treated MLD patients into teenage years.83 Additionally, HSCT is the standard-of-care treatment for mucopolysaccharidosis-1 (MPS1). MPS1 is an LSD caused by mutations in the IDUA gene, which is responsible for making the α-L-iduronidase enzyme. HSCT slows MPS1 progression and significantly extends life expectancy beyond untreated patients.84,85 Given the therapeutic efficacy of HSCT for KD and other LSDs, combined with the minimal number of currently available safe and effective treatments, HSCT will likely continue to be the standard of care for many of these diseases.
As mentioned previously, HSCT is most successful when performed prior to development of symptoms.82 Furthermore, HSCT has the highest chances of ameliorating KD symptoms when performed as early as possible.86 These time constraints make treatment more complicated because they necessitate the ability to identify KD patients as early as possible. Historically, patients were diagnosed with KD either through symptom development, and thus would not benefit from HSCT, or family history.75,79,82 One potential way of solving this issue is through NBS programs. New York State was the first state to start testing newborns for KD in 2006; since then, seven more states have added KD to their NBS testing panel. Additionally, KD is currently undergoing evidence review to be added to the Recommended Uniform Screening Panel (RUSP), which would recommend every state add KD to their NBS testing panel.5,87 NBS programs take dried blood spot (DBS) samples from infants in the first days of life and then analyze them for indications of a given disease. In the case of KD, the exact tests performed on the samples vary by state, but the most common first-tier test examines GALC activity. Babies that display low GALC activity are then moved onto second-tier testing, which again varies by state but is usually galc genotyping or psychosine quantification. The families are notified at this point and the diagnosis is then confirmed with additional testing, such as MRI and nerve conduction tests.4,5,87
It should be noted that the outcomes of the first KD patients identified through NBS in New York were mixed due to a multitude of factors, such as second-tier testing taking too long and patients not being promptly referred to specialty transplant centers. In the first 9 years, five infants were diagnosed with KD and four underwent HSCT. Of these four patients, one is overall doing well with some motor deficits, one has severe disabilities (received HSCT at 2 months), and two died due to transplant-related complications.5,86 Given these results, new protocols have been put in place to expedite second-tier testing and have confirmed cases transferred to transplant centers as fast as possible. The current goal of NBS programs is to have the results back by the seventh day of life. The infant should then be moved to a specialty center for confirmation of the KD diagnosis. Finally, the infant is referred to a transplant center for final evaluations and to have the procedure performed. The goal is to have the transplant completed by the 30th day of life to ensure the highest probability of success.86
KD: Preclinical investigative therapies
Many different therapies have been attempted for KD over the years. A notable, but not exhaustive, list includes stem cell transplantation, substrate reduction therapy (SRT), ERT, anti-inflammatory therapy, chaperone therapy, gene therapy, and various combination investigative therapies.49,50,51,53,54,57,62,78,88,89,90,91,92,93,94,95,96,97,98,99,100 The effects of these investigative therapies on TWI survival, GALC activity, psychosine levels, phenotypic changes, and histologic changes are outlined in Table 1 (single-modality treatment) and Table 2 (combination treatments).
Table 1.
Single-modality investigative therapies in TWI
| Therapy | Median survival increase (days) | Brain G and P | Phenotypic change | Pathology |
|---|---|---|---|---|
| 1 × 107 HSC i.p. on PND10 49 | 40 | NA | slight delay in motor problems | no change |
| 4 × 105 HSC i.p. on PND10 77 | 10 | increased G to 15% WT | improved weight gain | NA |
| 75 mg/kg LCS SQ every other day starting PND5 50 | 13 | NA | delayed weight loss 2 weeks | decreased GC and AC no change in M |
| 1.9 × 107 vg Adenovirus-GALC i.c.v. on PND0 97 | 3 | increased G to 15% WT decreased P by 45% |
delayed Twitching 3 days. improved weight gain 30% |
no change |
| 6 mg/kg GALC i.p. 51every other day starting PND10 | 7 | increased G to 7% WT decreased P by 20% |
improved weight gain and mobility | no change |
| 2.6 × 109 vg AAV2-GALC i.c. or 1.4 × 1010 vg AAV5-GALC i.c. on PND3 98 | AAV2: 10 AAV5: 14 |
NA | improved weight gain and rotarod | decreased MP and improved M |
| 1.2 × 1011 vg AAV1-GALC i.c.v. on PND1 101 | 15 | increased G to 5× WT | NA | normalized M, reduced MP, and reduced AC |
| 1 × 108 vg lentivirus-GALC i.v. or i.p. on PND7. 102 | 0 | increased G to 10% WT | no change | no change |
| Single GALC injection i.c.v. on PND20 103 | 10 | increased G to 5× WT decreased P but 17% |
NA | NA |
| Anti-inflammatory therapy 92∗ | 5–15 | NA | NA | reduced inflammatory markers 30–50% |
| GALC-expressing neural progenitors i.c. on PND2 88 | 7 | qualitatively increased G | delayed tremor and hindlimb paralysis 5 days Improved weight |
reduced GC and AC Improved M |
| 2.4 × 109 vg AAV5-GALC i.c. on PND3 104 | 22 | increased G to 4× WT Decreased P by 50% |
delayed muscle atrophy 2 weeks Improved weight gain |
improved M |
| 7.5 × 105 NSC i.c.v. on PND2 89 | 5 | increased G to 33% WT | improved gait | reduced GC, MP, MG, and AC. Improved myelination |
| 4 × 105 MSC i.c.v. on PND3.5 90 | 5 | no change in G | increased weight gain | decreased MP and MG |
| 4 × 105 MSC i.c. on PND3.5 105 | 0 | NA | improved rotarod performance | NA |
| 2 × 1010 vg i.t. + 1.6 × 1010 vg i.c. AAV5-GALC on PND2.5 106 | 30 | increased G to 5× WT | improved rotarod performance, moderately improved weight gain, reduced tremor, and no effect on wire hang | increased CD4 and CD8 T cells and decreased AC |
| 4.5 × 109 vg i.c. + 7.6e9 vg i.v. AAVrh10-GALC on PND299 | 64 | increased G to 4× WT | normalized motor ability | normal M reduced GC |
| 0.15 mg/kg S202 i.p. 3×/week 91 | 20 | decreased P by 85% | no effect on weight gain | normalized M. nonsignificant decrease in MP and AC |
| 1 × 1011 vg AAVhu68-GALC i.c.v. on PND0 107 | 90 | increased G to 2,1× WT | improved rotarod performance | NA |
| ∼2 × 1012 vg AAVrh10 -GALC i.v. on PND10 (4 × 1014vg/kg) 108 | 240 | increased G to ∼3.4× WT | normalized weight gain, wire hang, and gait | normalized MP and AC |
HSC, hematopoetic stem cells; i.p., intraperitoneal; LCS, L-cycloserine; SQ, subcutaneous; vg, viral genomes; i.c.v, intracerebroventricular; i.c., intracerebral; MSC, mesenchymal stem cells; i.t., intrathecal; GC, globoid cells; AC, astrocytes; M, myelin; MP, macrophages; MG, microglia; NSC, neural stem cell; G, GALC activity; P, psychosine; NA, not available. ∗Study done on trs mice, not TWI.
Table 2.
Combination investigative therapies in TWI.
| Therapy | Median survival increase (days) | Effect on brain G and P | Phenotypic improvement | Pathology |
|---|---|---|---|---|
| HSCT PND10-20 and SQ 50 mg/kg LCS 54 | 85 | NA | improved weight gain | decreased GC and AC |
| 1.4 × 1010 vg AAV5-GALC i.c. and BMT on PND3 100 | 65 | increased G to 4× WT | delayed motor problems increased weight |
reduced MP, AC, and GC normalized M |
| BMT and 1 × 107 vg lentivirus-GALC i.v. on PND1.5 109 | 35 | increased G to 33% of WT reduced P |
NA | reduced GC and improved M |
| BMT on PND3.5 and 2 × 1010 vg i.t. + 1.6 × 1010 vg i.c. AAV5-GALC on PND2.5 106 | 82 | increased G to 1.5× WT reduced P by 66% |
mostly normalized motor problems increased weight |
Decreased CD4, CD8, and MG |
| 5.33 ug i.t. + 1.6 ug i.c.v. GALC on PND2 and BMT i.v. on PND3 78 | 19 | G increased to 2× WT at 24 h but decreased over time reduced P by 90% |
delayed motor problems 10 days | moderate normalization of M |
| 2 × 1010 vg i.t. +1.5 × 109 vg i.c. AAV5-GALC on PND2 BMT on PND3 25–50 mg/kg LCS 3/week starting PND5 110 |
250 | increased G to 6× WT but decreased to 2× over time reduced P to WT level |
delayed motor problems 15 weeks | initially normalized M but multifocal demyelination later GC, AC, and MG reduced at p35 but elevated later |
| BMT on PND 9.5 and 2 × 1011 vg AAV9-GALC i.t. on PND10.5 56 | 39 | NA | NA | near normalization of M |
| BMT on p8/9 and 2 × 1011 vg AAVrh10-GALC i.v. on PND10-12 111 | 300 | increased G to WT but declined over time | normalized gait increased weight |
normalized M decreased AC and MG |
| 2 × 106 vg lentivirus-GALC i.c. or 4 × 105 NSC i.c.v. on p2 and 5 × 106BMT on PND7 112 | LV + BMT: 102 NSC + BMT: 173 |
increased G to 30% WT reduced P |
increased weight | partially restored M AC initially reduced by increased over time |
| 9 × 109 vg i.c. + 8.25 × 1010 vg i.t. + 3.3 × 1011 vg i.v. AAV9-GALC on PND0 and BMT on PND1 53 | 241 | increased G to 14× WT but decline over time reduced P to WT level but increased over time |
delayed motor problems 20 weeks increased weight |
M normalized but focal demyelination later reduced AC and MG |
| 1.5 × 1011 vg i.t. + 6 × 1010 vg i.c. AAV9-GALC on PND0 BMT on PND1 25–50 mg/kg LCS 3×/week 57 |
363 | increased G to WT normalized P |
normalized motor ability increased weight |
decreased MG but increased over time |
| 2 × 1011 vg AAVrh10-GALC i.v. on PND10 BMT on PND10 108 |
311 | increased G to WT level | NA | NA |
LCS, L-cycloserine; SQ, subcutaneous; vg, viral genomes; i.c., intracerebral; i.v., intraventricular; i.t., intrathecal; i.c.v., intracerebroventricular; NSC, neural stem cells; GC, globoid cells; AC, astrocytes; M, myelin; MG, microglia; G, GALC activity; P, psychosine; NA, not available.
One of the most important early findings from these investigations was that GALC overexpression can lead to secretion and uptake of GALC by neighboring cells; this phenomenon is known as cross-correction. Cross-correction was first demonstrated in 1968 when cultured fibroblasts from Hurler and Hunter disease patients were shown to attenuate the biochemical defect in the other cells when mixed together. The same held true when either Hurler or Hunter disease fibroblasts were mixed with fibroblasts from healthy patients.113 This phenomenon was then demonstrated specifically for KD after TWI nerves were grafted into healthy animals; this led to a reduction in pathology over time.114 It was then shown that, when GALC-overexpressing cells secreted GALC into media, neighboring cells endocytosed the secreted enzyme.115,116 Finally, TWI oligodendrocyte precursor cells (OPCs) were transplanted into myelin basic protein (MBP)-deficient shiverer mice, where they migrated away from the injection site, matured into oligodendrocytes, and synthesized nearly normal levels of myelin. These transplanted TWI oligodendrocytes were shown to have functional GALC, likely endocytosed from their neighboring GALC-expressing cells.117 This was an important finding because it reinforced the concept that therapies do not need to reach every cell in the body to be effective; GALC could naturally redistribute to many cells not directly targeted by the therapy.
SRT typically utilizes a small-molecule drug to inhibit the enzyme responsible for synthesis of the accumulating substrate, thus slowing the accumulation of that substrate. Up until recently, SRT for KD has primarily focused on the use of L-cycloserine, an inhibitor of serine palmitoyltransferase (previously called 3-ketodyhydrosphingosine synthetase/synthase), the first enzyme in the synthetic pathway of many ceramides and sphingolipids.118,119,120 Repeated subcutaneous injections of L-cycloserine increased median survival in TWI by 13 days, increased body weight, reduced the number of globoid cells, and decreased astrogliosis.50,54 Unfortunately, L-cycloserine has a narrow therapeutic window since it leads to a reduction of many other critical lipids in addition to psychosine.118,119 Recently developed SRT drugs target enzymes that are more proximal to psychosine synthesis, such as ceramide galactosyltransferase (CGT) or acid ceramidase, in an attempt to limit off-target effects.91,121 Intraperitoneal (i.p.) injections of the CGT inhibitor S202 in TWI increased median survival ∼20 days and improved body weight. Furthermore, S202 reduced psychosine by >70% and normalized myelination when used at >0.15mg/kg. However, long-term treatment with such doses of S202 led to worsened nerve conduction velocity, poor performance on wire hang, and vacuolation in the CNS of WT mice. These side effects, which may be caused by collateral effects of CGT inhibition on the production of sulfatides and accumulation of ceramides, appear to be dose dependent.91 Another new SRT drug, 22m, inhibits acid ceramidase and has shown potential in reducing psychosine both in vitro and in vivo.121 An important observation from these studies is that the level of toxicity appears to correlate with the inhibitor dose. This suggests that an appropriate dose might provide treatment conditions where side effects are minimal or negligent, while still exerting therapeutic effects on psychosine production.
SRT has been investigated for a number of other LSDs.122 As with KD, finding drugs that are effective but have minimal side effects has been difficult because many of the drugs target early steps within synthetic pathways. Additionally, drug discovery for CNS disorders is further complicated by finding drugs that will cross the blood-brain barrier (BBB).123 Miglustat was the first SRT drug approved in humans for type 1 Gaucher disease (GD). Miglustat improved bone density, increased hemoglobin levels, and reduced liver and spleen volume.124,125,126,127,128,129 Miglustat’s success in treating GD led to investigators using it to treat other LSDs with defects in common biochemical pathways, such as Niemann-Pick C (NPC). Miglustat treatment of NPC patients led to an improvement in multiple symptoms of NPC, such as supranuclear gaze palsy and dysphagia.123,130 Eliglustat was later found to be superior to miglustat in treating GD and is now a first-line therapy.131 Two new drugs, venglustat and lucerastat, are currently being investigated predominately for the treatment of GD and Fabry disease.132,133 Looking forward, SRT has the potential to treat many LSDs, especially as new, more specific drugs are discovered.91,121
ERT involves supplementing the defective enzyme using externally synthesized replacement enzyme. ERT using recombinant GALC delivered via i.p. injections every other day increased median survival in TWI by ∼10 days. There was a minimal reduction of psychosine within the brain, suggesting that GALC cannot cross the BBB.51 Although a negative result, it was still an important finding since it showed that any successful therapy will need to be administered directly to the CNS or utilize technology to cross the BBB. Single dose intracerebroventricular (i.c.v.) administration of GALC enzyme increased TWI median survival by ∼10 days. The minimal effectiveness of ERT is likely due to rapid enzyme turnover as shown by there being no detectable enzyme at a terminal time point.103 KD patients would almost certainly require continuous GALC injections directly into the CNS for the duration of their life for ERT to work effectively. This severely limits the applicability of CNS-directed, ERT-based treatment for KD.
ERT has been attempted with varying levels of success in many other LSDs. Historically, as with KD, the replacement enzyme has not been able to sufficiently enter the CNS to significantly alter disease pathology or symptom development.1,125,131,134 ERT has been recommended mostly for non-neuropathic LSDs such as type 1 GD and Pompe disease; however, repeated i.c.v. administration of tripeptidyl peptidase 1 (TPP1) was recently approved for the treatment of CLN2 disease.125,135,136,137 For type 1 GD, long-term ERT normalized height, RBC counts, and bone density as well as reducing hepatosplenomegaly.138 One method that has been attempted to circumvent the BBB is administering the replacement enzyme intrathecally (i.t.) or i.c.v. In the past, this treatment route has exhibited limited efficacy. For example, a clinical trial (NCT01510028) using repeated i.t. administered ARSA to treat MLD only showed slightly reduced disease symptoms and sulfatide accumulation.139 More recently, there have been a few developments to improve ERT for CNS disorders. One system fuses the enzyme to antibodies targeting endogenous proteins (e.g., leptin, insulin, and transferrin) expressed in the lumen of brain capillary endothelial cells. Then, the enzyme-antibody-protein construct is taken into the endothelial cell via endocytosis. Finally, the enzyme-antibody-protein construct is exocytosed on the brain parenchyma side of the capillary. This process is known as transcytosis.140,141,142,143,144,145 An antibody-enzyme fusion protein, JR-141, containing the transferrin receptor antibody and iduronate-2-sulftase was developed to treat MPS2. Following intravenous (i.v.) administration of JR-141 in a MPS2 mouse model, JR-141 was detected within the brain and was shown to decrease the accumulation of glycosaminoglycans.146 Additionally, following the success of implantable CNS injection devices for ERT delivery in the canine model of neuronal ceroid lipofuscinosis (CLN2), a clinical trial indicated it significantly delayed symptom development, which led to its US Food and Drug Administration (FDA) approval.136,137,147 Implantable CNS injection devices have existed for some time, but using them for ERT has been limited due to the frequent injections, as often as every 2 weeks, and long injection time required for ERT. The frequent injections and long injection time significantly increase the risks of adverse events such as infection. However, a recent study developed guidelines that significantly reduced the risk of these adverse events.137,148 Given these recent advances in ERT for CNS LSDs, ERT could be an important component of their treatment in the future.
As a monogenic loss-of-function disorder, KD is an ideal candidate for gene therapy. A variety of viruses have been investigated for use in treating KD, including adenovirus, lentivirus, and AAV.53,55,57,58,97,98,102,108 To date, AAV appears to be the most promising viral vector for KD gene therapy.53,57,58,108
The AAV capsid determines its cellular tropism and is, therefore, an important factor in therapy efficacy.149,150,151,152,153 Naturally occurring AAVs generally have a broad tropism but often still have a bias toward one specific cell type, with neuronal bias being the most common within the CNS.154,155,156 Each AAV’s tropism varies depending on administration route, target species, age of infusion, and purification methods.154,157,158 CNS-directed, AAV-mediated gene therapy using vectors pseudotyped with either the AAV5 or AAV1 capsids carrying a galc transgene, initiated during the neonatal period, increased the median survival of TWI by 10–15 days.98,101 Addition of i.t. injections in conjunction with the intracerebral (i.c.) route further increased median survival by another ∼15 days.106 The greatest increase in lifespan, median survival of 280 days of age, in TWI attributed solely to AAV-mediated gene therapy was observed following high-dose i.v. AAVrh10. This approach significantly increased GALC activity in both the brain and sciatic nerve, reduced neuroinflammation, improved weight gain, increased strength, and normalized gait.108
A study investigating gene therapy in the canine model of KD administered AAVrh10-GALC i.v. and i.c.v. at 3 days and 6 weeks, respectively. The therapy had a clear dose response and resulted in increased GALC activity, reduced psychosine, and improved myelination. However, correction was not complete, likely due to the treatment dosage being too low.52 A subsequent report from the same laboratory completed a dose-response study of AAV9-GALC delivered by intracisternal injection at pre-symptomatic (2 weeks) and post-symptomatic (6 weeks) time points. The second study found that delivery of high-dose AAV9-GALC extended survival over seven times that of untreated canines and corrected neurological dysfunction as shown by attenuated loss of myelin and significantly reduced psychosine.58
Gene therapy has also been investigated in many other LSDs; see Ohashi et al. 2019 for a review focusing on this topic.159 Like KD, many LSDs have shown significant improvements in median survival and disease pathology in animal models following gene therapy and are now moving into clinical trials. For example, a mouse model of GM1 gangliosidosis, displayed near-complete correction of the β-galactosidase (βgal) deficiency following therapy with an AAV2 vector administered i.c.v.160 Treating the GM1 gangliosidosis mouse model with an AAV9 vector administered i.v. increased βgal, significantly reduced GM1, improved behavioral performance on multiple assays, and increased median survival by 150 days.161 A similar therapeutic approach of i.v. AAV9 was used to treat a feline model of GM1 gangliosidosis. This treatment decreased GM1, nearly normalized βgal, reduced pathology, partially normalized myelination, and increased survival by almost 3 years.162 These results led to a phase 1/2 clinical trial using an AAV9 vector to treat GM1 gangliosidosis (NCT03952637). Any information that is gained from this and other clinical trials investigating AAV therapy for LSDs, such as which AAV serotype, treatment route, or dosing strategies are most efficacious, will be immensely useful for future LSD studies and clinical trials. For a more complete list of AAV-based gene therapy trials for LSDs currently underway, see clinicaltrials.gov.
Although each single-modality therapy has different targets and provides moderate benefit, they also each have their own limitations. The most promising therapies are those that employed combinations of previously discussed treatments.53,57,106,108,110 The first combination therapy attempted in TWI utilized BMT and L-cycloserine-mediated SRT. These therapies had a synergistic effect such that they increased median survival to 110 days, more than either achieved in isolation.54 Similarly, combining BMT with i.c. AAV5-GALC increased median survival to 100 days and improved motor function.100 Addition of an i.t. injection of AAV5-GALC further increased median survival an additional 25 days. This treatment protocol also increased GALC activity to five times WT levels in the brain and reduced psychosine to nearly WT levels in the brain and spinal cord. However, there were limited improvements in the wire hang assay, which may indicate this combination therapy did not adequately treat the PNS.100,106 Combining neonatal administrations of i.v., i.t., and i.c. AAV9-GALC with BMT increased median survival to 285 days. Additionally, the mice showed decreased signs of disease: increased body weight, improved locomotion, and improved performance on neurobehavioral tests. The mice exhibited improved myelination and supraphysiological GALC activity, and decreased psychosine accumulation.53 Another AAV + BMT study administered the treatments later, BMT at PND8.5 and i.v. AAVrh10-GALC at PND11. This study found that the later treatment schedule led to better outcomes, including increasing median survival to 340 days, no tremor, normalized gait, improved weight, and near-normalized myelination.111 The later dosing schedule may also better model the age of KD children when they should receive AAV therapy; they are currently treated with HSCT around 1 month old and potentially would be treated with AAV around the same time.86 A triple therapy combining i.c. and i.t. AAV5, BMT, and L-cycloserine SRT increased the median survival to 290 days. This approach increased GALC activity to 3.5 times WT, reduced psychosine to nearly WT levels, reduced neuroinflammation, and effectively preserved myelin. To highlight the synergistic efficacy of SRT, this study was able to achieve a similar median survival increase as an AAV + BMT-only study using 100-fold less AAV.110,111 A follow-up study of the triple therapy that changed the AAV capsid from AAV5 to AAV9 further increased median survival to 404 days.57 Looking forward, future combination therapies utilizing newer SRT drugs, more effective AAV vectors, and improved HSCT protocols may further increase survival and reduce pathology due to improved specificity.91,121
As with KD, combination therapies for other LSDs have been shown to be synergistic in reducing disease pathology and increasing median survival.163,164,165,166 Most of the combination therapies investigated for KD have also been studied in other LSDs, such as ERT + BMT, BMT + gene therapy, and SRT + BMT.163,165,166 These therapies had varying levels of success, but, like combination therapies for KD, they invariably were additive, and most showed some level of synergy. One previously discussed example, Eliglustat-based SRT, has already been shown to synergize with other treatment modalities in preclinical investigations.54,57,110,167 This indicates that SRT can be used in combination with other treatment modalities, such as HSCT and gene therapy, to treat LSD patients. Combination therapies allow for each treatment to address the weaknesses of the other treatment to ensure more complete therapeutic coverage.164
AAV cellular dynamics and potential shortfalls
While AAV-based gene therapy both alone and in combination with other therapies appears to have enormous potential for the treatment of KD, there are still concerns that may limit its efficacy in clinic. Chief among these limitations is the fact that the most successful therapies still have a median survival of only 400 days in TWI, which is significantly lower than the WT mouse median survival of 700–900 days.57,168 Furthermore, there is evidence from multiple laboratories that the efficacy of the gene therapy treatment wanes over time. This was observed using several criteria, including decreasing myelination, worsening inflammation, declining GALC activity, and increasing psychosine.53,56,57,110 Additionally, many animals showed no signs of disease during the months immediately after treatment initiation, such that they were indistinguishable from WT animals, but began to exhibit progressively debilitating disease signs later in life.53,56,57,58,110,169 At present, the mechanism behind this age-related decline in therapeutic efficacy is unknown, although some clarity is emerging.
The majority of transcriptionally active recombinant AAV genomes exist as circular, non-replicating extrachromosomal episomes.170,171,172,173 We hypothesize that therapeutic efficacy declines over time because AAV episomes are not replicating in tandem when the host cell is proliferating. The Bongarzone lab recently presented evidence of this occurrence both in vitro and in vivo.169 Myelinating oligodendrocytes and neurons are terminally differentiated and, therefore, should be permanently corrected by AAV. In contrast, other CNS cell types such as OPCs and neuronal precursors produced in the subventricular and hippocampal germinal zones and also residing throughout the CNS replicate throughout life to modify myelination in response to motor learning and contribute to neuronal regeneration.174,175,176,177,178 Based on this, we proposed that, since OPCs can replicate in the adult brain, eventually a cell may not inherit copies of the therapeutic episome during cellular division. This would revert its phenotype to an untreated state. In this scenario, the “uncorrected” cell may initially show little or no signs of distress as it may endocytose GALC through cross-correction from neighboring treated cells. However, over time and with more cell divisions, there would be fewer OPCs that harbor the therapeutic transgene to provide sufficient GALC to effectively eliminate psychosine. At this point, areas containing an increasing number of uncorrected cells may activate repair mechanisms due to cellular damage, including recruitment of additional uncorrected repair/inflammatory cells, which would likely worsen the developing lesion. This hypothesized pathway is outlined in Figure 2 and supported by evidence including loss of galc expression and GALC activity as well as the presence of non-corrected OPCs within late-onset demyelinating lesions in the brain of long-surviving AAV-treated TWI. Notably, these late-onset demyelinating lesions have only been observed within the brain; none have been found in the spinal cord or peripheral nerves.53,110,169 However, this does not mean the spinal cord and peripheral nerves do not contain any pathology. The spine and sciatic nerves of long-term AAV9-GALC-treated animals exhibited significant decreases in GALC activity and significant increases psychosine over time.53 Additionally, the lack of demyelinating lesions in the peripheral nerves may simply mean that similar processes are occurring but develop at slower rates. There is evidence of a similar decline in AAV episome copy number and therapeutic efficacy over time for other AAV-treated LSDs.179,180,181,182,183 There are still many unanswered questions regarding this proposed pathway, including determining the timing and specific mechanisms of each step. Furthermore, a more complete understanding of the relationship between the dose of AAV and the duration of protection against disease in the human brain is needed.
Figure 2.
Proposed mechanism of episomal AAV dilution during myelinating cell proliferation
This figure proposes a theoretical mechanism of replication-induced dilution of episomal AAV DNA in proliferating myelinating cells starting from initial treatment in (A). (B) How replication leads to some proliferating myelinating cells not inheriting a copy of the therapeutic AAV DNA, but they initially receive GALC from neighboring treated cells. At some point, the ratio of untreated to treated cells becomes high enough that cross-correction can no longer supply sufficient GALC to all of the untreated cells, and the cells that lost the AAV DNA begin to die. Finally, uncorrected immune cells are recruited, culminating in the development of disease signs.
The observed decline in efficacy in animal models does not preclude the use of AAV-based therapies for the treatment of KD or other LSDs. There is a long history in preclinical AAV-based gene therapy research in KD showing a strong dose response from increasing survival by 10 days in the earliest experiments with 2.5 × 109 viral genomes (vg)/mouse to increasing survival by over 200 days with 6 × 1011 vg/mouse, as shown in Figure 3.52,53,56,58,98,99,108 We performed regression analysis of these experimental outcomes, which show the R2 of AAV-only therapies is 0.64, indicating a reasonable correlation and dose response. The applicability of the R2 is limited by the varying serotypes, injection routes, and other differences between investigators. The R2 of AAV + BMT therapies is only 0.23, the lower R2 for AAV + BMT is likely, at least partially, due to differing amounts of stem cells delivered to the animals and immune suppression protocols in different studies. An example from a single laboratory, Bradbury et al, demonstrated a clear dose response in their consecutive papers, indicating higher doses leads to reduced signs of disease and improved median survival in the canine KD model.52,58 Figure 3 also highlights the importance of AAV serotype and injection route. AAV9 and AAVrh10 had the largest increases in survival. Injection route also influenced treatment efficacy: i.c. and i.t. alone were generally the least efficacious, while i.v. alone, i.c. + i.t., and i.c. + i.t. + i.v. were generally the most efficacious.
Figure 3.
Preclinical AAV therapy efficacy in KD
This figure shows all preclinical AAV studies completed to date as dose (viral genomes) versus the increase in survival (days) from the treatment. The graph is organized by both route of administration (color: bronze, i.c.; blue, i.t./i.c.v.; red, i.v.; green, i.c. + i.t.; orange, i.c. + i.v.; and black, i.c. + i.t. + i.v.) and serotype (shape: X, AAV1; circle, AAV5; diamond, AAV9; square, AAVrh10; and hexagon, AAVOlig001). ∗All therapies centered around 2e11 viral genomes (vg) were given 2 × 1011 vg but were separated in the graph for clarification.
Taking all of this into consideration, it may simply mean that AAV gene therapy needs further improvements, such as higher doses, multiple treatments, or new vectors with improved targeting of intended cell populations. Determining the relationship between the amount of AAV administered and expected median survival is critical. While it is very difficult to directly compare the dosage and survival from the different laboratories over the years because of differing AAV constructs, AAV serotypes, administration routes, timing of administration, use of immunomodulation, and other differences, the relationship does not appear to be linear.52,53,56,58,98,99,108 One alternative to simply increasing the dosage of the neonatal treatment is the possibility of redosing. While there is a concern about an immune reaction against the AAV in the second treatment, this could be mitigated by using a different AAV serotype for the second dose or potentially immune modulation prior to dosing. Certain AAV serotypes have been shown to have minimal cross reactivity, and serotype switching has already been shown to be possible.184,185,186,187,188,189,190,191 Corticosteroids are probably the most commonly used immunomodulatory treatments, but other modalities, such as B cell inhibition with rituximab, rapamycin (also known as sirolimus) inactivation of B and T cells, or plasmapheresis to remove anti-AAV neutralizing antibodies, are possible to minimize an immune response against the AAV capsid or transgene.192,193
While there does not appear to be a direct linear relationship between AAV dose and survival, it generally appears that higher AAV doses lead to greater survival. This begs the question: why not continuously increase AAV dose to increase survival to that of WT animals? The answer to this is that high-dose systemic AAV therapy poses its own risks in the form of acute liver injury as well as pathology such as neuronal degeneration in the dorsal root ganglia.194,195,196 Another potential risk of high-dose AAV therapy is insertional mutagenesis leading to hepatocellular carcinoma.57,197,198 While the vast majority of AAV genomes exist as extrachromosomal episomes, some may insert into the genomic DNA.170,171,172,173 The murine Rian locus appears to be particularly vulnerable to AAV insertion; at present it is unknown if the human Rian locus is similarly a target for AAV insertion.57,197 It appears that the Knudson hypothesis stating multiple genomic “hits” are required to develop cancer applies here.199 One hit appears to be AAV insertion, with other hits possibly occurring from ionizing radiation used to condition animals prior to BMT, L-cycloserine-induced reduction of ceramides, or chronic liver injury from co-morbidities.57,198,200,201,202
AAV future advances and clinical trials
As mentioned previously, each AAV serotype has a unique tropism based on the capsid.149,150,151,152,153,154,155,156 There has been a significant amount of research into engineering AAV capsids for specific purposes, whether that be targeting specific cell types, detargeting off-target cell types, or increasing penetration through the BBB as reviewed in von Jonquieres et al. 2021.154 One particularly relevant novel AAV capsid for KD and other LSDs that predominately affect oligodendrocytes is Olig001, which preferentially targets oligodendrocytes.203 This could potentially allow utilization of a lower total dose of AAV since a higher proportion of the virus will transduce oligodendrocytes. Alternatively, a neuron-specific AAV could be used to increase transduction of neurons in LSDs that primarily affect neurons.149,150,151,152,153,154,155,156,158 Additionally, transgene expression can be controlled using cell-specific promoters.154 For example, the MBP promoter has been used to limit transgene expression to oligodendrocytes.204 Using engineered cell-specific capsids in conjunction with cell-specific promoters allows for precise targeting of transgene expression with minimal off-target effects.
All of the information acquired on KD genetics, pathology, and investigative therapies in the past 35 years have provided the foundation of two current trials. NCT04771416 is examining the efficacy of AAVhu68 (modified AAV9) administered into the cisterna magna and has already treated the first patient. This trial is based on work showing that the i.c.v. AAVhu68 increased median survival in TWI 3-fold, increased brain GALC activity to 200% WT, and improved rotarod latency to fall. Additionally, AAVhu68 was tested in the KD canine model; treated animals did not display any of the classical signs of disease throughout their lives. Furthermore, the canines displayed partially normalized myelination, normal auditory evoked potential, and reduced neuroinflammation. Finally, safety and tolerability of AAVhu68 was tested in non-human primates and found to be well tolerated.107 NCT04693598 is studying HSCT combined with AAVrh10 administered i.v. based on preclinical studies discussed earlier.108,111 Since these clinical trials were only recently initiated, there are no data regarding efficacy yet. Whatever information is acquired from these trials will help not only KD patients but also those suffering from other LSDs as well by guiding the design of future clinical trials. In addition to these KD studies, there are currently ongoing AAV-based clinical trials for numerous LSDs such as GD, MLD, and GM1 gangliosidosis, as seen on clinicaltrials.gov.
Conclusions
Combination therapies using AAV gene therapy plus HSCT or SRT may provide an alternative to high-dose AAV therapies.53,57,100,106,110,112,205 Furthermore, the latest SRT drugs target more proximal enzymes to psychosine synthesis than L-cycloserine to limit off-target effects.91,121 Combination therapies could achieve similar efficacy to high-dose AAV therapies while using less AAV, thus avoiding the potential perils of high-dose AAV therapy. In tandem with combination therapies, engineered AAV serotypes allow for improved targeting and potentially further reduction of the required dose for effective treatment. In summary, investigative therapeutics for KD have significantly improved since the development of HSCT 35 years ago, particularly combination therapies. Looking to the future, the likely treatment for KD will be a combination of the current standard-of-care treatment, HSCT, and AAV, as well as possibly a third treatment modality such as ERT or SRT. This specific treatment combination used may even change depending on the subtype of KD (infantile, juvenile, or adult onset) or the specific mutation a patient may have, and this type of personalized medicine may further improve treatment outcomes. For example, an infantile KD patient homozygous for the 30-kb deletion may receive HSCT, gene therapy, and SRT, while a juvenile-onset patient may benefit from receiving only gene therapy, and an adult-onset KD patient may be managed with just ERT or SRT. This customized treatment plan could minimize the impact of KD for all patients, minimize treatment costs, and improve access to post-therapy follow-up.
The preclinical experiments and clinical trials using single and combination therapies for KD may also aid the development of more effective therapeutic strategies for other LSDs. Given that MLD is potentially the most similar disorder to KD with regard to pathology, symptoms, and treatment, we will use it as an example. At present, like KD, MLD is treated with HSCT, but this is not very effective in stopping disease pathology or symptom development. However, recently the European Medicine Agency approved a new gene-cell therapy using lentiviral transduced stem cells to treat MLD. This therapy significantly increased ARSA activity as well as improving motor function, verbal skills, and cognitive ability.206 Many of both the preclinical and clinical therapies being investigated for KD have a similar counterpart being investigated for MLD.207 For example, an ERT trial (NCT01510028) was completed recently and an AAV gene therapy trial (NCT01801709) is currently ongoing. Similarly, these same treatment modalities are currently being or will be investigated for other LSDs. The future of LSD treatment is advancing every day through earlier diagnosis, improved HSCT protocols, new SRT drugs, novel ERT protein constructs, and innovative AAV construct design.
Acknowledgments
Funding: pre-doctoral NRSA fellowship from the NIH (F30HD103447) to G.H.; NIH (R01 NS065808, R01NS127403), Legacy of Angels Foundation, and the European Leukodystrophy Association to E.R.B.; NIH (NICHD) R00HD096115 to A.M.B.; NIH (RO1NS100779) to M.S.
Author contributions
G.H. wrote the manuscript. G.H., A.M.B., M.S.S., and E.R.B. contributed equally to reviewing, editing, and final formatting. All authors read and approved the manuscript.
Declaration of interests
E.R.B. is a consultant for Lysosomal Therapeutics Inc., and Gain Therapeutics. Neither entity provided support in the form of salaries for any listed author nor played additional roles in the design, data collection and analysis, decision to publish, or preparation of this manuscript.
A.M.B. is a beneficiary of a licensing agreement with Axovant Gene Therapies (royalties), has received income from Neurogene (consulting and honorarium), and is an inventor on a patent application (Gray SJ, Lykken E, Vite CH, Bradbury AM. Optimized GALC Genes and Expression Cassettes and Their Use. PCT/US2019/067727).
G.H. and M.S.S. report no conflicts of interest.
Contributor Information
Gregory Heller, Email: ghelle2@uic.edu.
Allison M. Bradbury, Email: allison.bradbury@nationwidechildrens.org.
Mark S. Sands, Email: mssands@wustl.edu.
Ernesto R. Bongarzone, Email: ebongarz@uic.edu.
References
- 1.Edelmann M.J., Maegawa G.H.B. CNS-targeting therapies for lysosomal storage diseases: current advances and challenges. Front. Mol. Biosci. 2020;7:559804. doi: 10.3389/fmolb.2020.559804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krabbe K., FAMILIAL A.N.E.W. Infantile form of diffuse brain-sclerosis. Brain. 1916;39:74–114. [Google Scholar]
- 3.Hagberg B., Sourander P., Svennerholm L. Diagnosis of Krabbe's infantile leucodystrophy. J. Neurol. Neurosurg. Psychiatry. 1963;26:195–198. doi: 10.1136/jnnp.26.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghabash G., Wilkes J., Barney B.J., Bonkowsky J.L. Hospitalization burden and incidence of krabbe disease. J. Child Neurol. 2022;37:12–19. doi: 10.1177/08830738211027717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orsini J.J., Kay D.M., Saavedra-Matiz C.A., Wenger D.A., Duffner P.K., Erbe R.W., Biski C., Martin M., Krein L.M., Nichols M., et al. Newborn screening for Krabbe disease in New York State: the first eight years' experience. Genet. Med. 2016;18:239–248. doi: 10.1038/gim.2015.211. [DOI] [PubMed] [Google Scholar]
- 6.Rafi M.A., Luzi P., Zlotogora J., Wenger D.A. Two different mutations are responsible for Krabbe disease in the Druze and Moslem Arab populations in Israel. Hum. Genet. 1996;97:304–308. doi: 10.1007/BF02185759. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K., Suzuki Y. Globoid cell leucodystrophy (Krabbe's disease): deficiency of galactocerebroside beta-galactosidase. Proc. Natl. Acad. Sci. USA. 1970;66:302–309. doi: 10.1073/pnas.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki Y., Austin J., Armstrong D., Suzuki K., Schlenker J., Fletcher T. Studies in globoid leukodystrophy: enzymatic and lipid findings in the canine form. Exp. Neurol. 1970;29:65–75. doi: 10.1016/0014-4886(70)90037-3. [DOI] [PubMed] [Google Scholar]
- 9.Eto Y., Suzuki K., Suzuki K. Globoid cell leukodystrophy (Krabbe's disease): isolation of myelin with normal glycolipid composition. J. Lipid Res. 1970;11:473–479. [PubMed] [Google Scholar]
- 10.Austin J.H., Lehfeldt D. Studies in globoid (krabbe) leukodystrophy. 3. Significance of experimentally-produced globoid-like elements in rat white matter and spleen. J. Neuropathol. Exp. Neurol. 1965;24:265–289. [PubMed] [Google Scholar]
- 11.Svennerholm L., Vanier M.T., Månsson J.E. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J. Lipid Res. 1980;21:53–64. [PubMed] [Google Scholar]
- 12.Cleland W.W., Kennedy E.P. The enzymatic synthesis of psychosine. J. Biol. Chem. 1960;235:45–51. [PubMed] [Google Scholar]
- 13.Miyatake T., Suzuki K. Globoid cell leukodystrophy: additional deficiency of psychosine galactosidase. Biochem. Biophys. Res. Commun. 1972;48:539–543. doi: 10.1016/0006-291x(72)90381-6. [DOI] [PubMed] [Google Scholar]
- 14.Igisu H., Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984;224:753–755. doi: 10.1126/science.6719111. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi T., Shinoda H., Goto I., Yamanaka T., Suzuki Y. Globoid cell leukodystrophy is a generalized galactosylsphingosine (psychosine) storage disease. Biochem. Biophys. Res. Commun. 1987;144:41–46. doi: 10.1016/s0006-291x(87)80472-2. [DOI] [PubMed] [Google Scholar]
- 16.White A.B., Givogri M.I., Lopez-Rosas A., Cao H., van Breemen R., Thinakaran G., Bongarzone E.R. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J. Neurosci. 2009;29:6068–6077. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins-Salsbury J.A., Parameswar A.R., Jiang X., Schlesinger P.H., Bongarzone E., Ory D.S., Demchenko A.V., Sands M.S. Psychosine, the cytotoxic sphingolipid that accumulates in globoid cell leukodystrophy, alters membrane architecture. J. Lipid Res. 2013;54:3303–3311. doi: 10.1194/jlr.M039610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taniike M., Mohri I., Eguchi N., Irikura D., Urade Y., Okada S., Suzuki K. An apoptotic depletion of oligodendrocytes in the twitcher, a murine model of globoid cell leukodystrophy. J. Neuropathol. Exp. Neurol. 1999;58:644–653. doi: 10.1097/00005072-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Tohyama J., Matsuda J., Suzuki K. Psychosine is as potent an inducer of cell death as C6-ceramide in cultured fibroblasts and in MOCH-1 cells. Neurochem. Res. 2001;26:667–671. doi: 10.1023/a:1010991420942. [DOI] [PubMed] [Google Scholar]
- 20.Jatana M., Giri S., Singh A.K. Apoptotic positive cells in Krabbe brain and induction of apoptosis in rat C6 glial cells by psychosine. Neurosci. Lett. 2002;330:183–187. doi: 10.1016/s0304-3940(02)00655-9. [DOI] [PubMed] [Google Scholar]
- 21.Rebiai R., Rue E., Zaldua S., Nguyen D., Scesa G., Jastrzebski M., Foster R., Wang B., Jiang X., Tai L., et al. CRISPR-Cas9 knock-in of T513M and G41S mutations in the murine beta-galactosyl-ceramidase gene Re-capitulates early-onset and adult-onset forms of krabbe disease. Front. Mol. Neurosci. 2022;15:896314. doi: 10.3389/fnmol.2022.896314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y., Xu Y., Benitez B.A., Nagree M.S., Dearborn J.T., Jiang X., Guzman M.A., Woloszynek J.C., Giaramita A., Yip B.K., et al. Genetic ablation of acid ceramidase in Krabbe disease confirms the psychosine hypothesis and identifies a new therapeutic target. Proc. Natl. Acad. Sci. USA. 2019;116:20097–20103. doi: 10.1073/pnas.1912108116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deshmukh D.S., Flynn T.J., Pieringer R.A. The biosynthesis and concentration of galactosyl diglyceride in glial and neuronal enriched fractions of actively myelinating rat brain. J. Neurochem. 1974;22:479–485. doi: 10.1111/j.1471-4159.1974.tb06882.x. [DOI] [PubMed] [Google Scholar]
- 24.Collier J., Greenfield J.G. The encephalitis periaxialis of Schilder: a clinical and pathological study with an account of two cases, one of which was diagnosed during life. Brain. 1924;47:489–519. [Google Scholar]
- 25.D'Auria L., Reiter C., Ward E., Moyano A.L., Marshall M.S., Nguyen D., Scesa G., Hauck Z., van Breemen R., Givogri M.I., Bongarzone E.R. Psychosine enhances the shedding of membrane microvesicles: implications in demyelination in Krabbe's disease. PLoS One. 2017;12:e0178103. doi: 10.1371/journal.pone.0178103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantuti-Castelvetri L., Maravilla E., Marshall M., Tamayo T., D'auria L., Monge J., Jeffries J., Sural-Fehr T., Lopez-Rosas A., Li G., et al. Mechanism of neuromuscular dysfunction in Krabbe disease. J. Neurosci. 2015;35:1606–1616. doi: 10.1523/JNEUROSCI.2431-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castelvetri L.C., Givogri M.I., Zhu H., Smith B., Lopez-Rosas A., Qiu X., van Breemen R., Bongarzone E.R. Axonopathy is a compounding factor in the pathogenesis of Krabbe disease. Acta Neuropathol. 2011;122:35–48. doi: 10.1007/s00401-011-0814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantuti-Castelvetri L., Zhu H., Givogri M.I., Chidavaenzi R.L., Lopez-Rosas A., Bongarzone E.R. Psychosine induces the dephosphorylation of neurofilaments by deregulation of PP1 and PP2A phosphatases. Neurobiol. Dis. 2012;46:325–335. doi: 10.1016/j.nbd.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cantuti Castelvetri L., Givogri M.I., Hebert A., Smith B., Song Y., Kaminska A., Lopez-Rosas A., Morfini G., Pigino G., Sands M., et al. The sphingolipid psychosine inhibits fast axonal transport in Krabbe disease by activation of GSK3beta and deregulation of molecular motors. J. Neurosci. 2013;33:10048–10056. doi: 10.1523/JNEUROSCI.0217-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sural-Fehr T., Singh H., Cantuti-Catelvetri L., Zhu H., Marshall M.S., Rebiai R., Jastrzebski M.J., Givogri M.I., Rasenick M.M., Bongarzone E.R. Inhibition of the IGF-1-PI3K-Akt-mTORC2 pathway in lipid rafts increases neuronal vulnerability in a genetic lysosomal glycosphingolipidosis. Dis. Model. Mech. 2019;12:dmm036590. doi: 10.1242/dmm.036590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bascou N., DeRenzo A., Poe M.D., Escolar M.L. A prospective natural history study of Krabbe disease in a patient cohort with onset between 6 months and 3 years of life. Orphanet J. Rare Dis. 2018;13:126. doi: 10.1186/s13023-018-0872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenger D.A., Rafi M.A., Luzi P. Krabbe disease: one Hundred years from the bedside to the bench to the bedside. J. Neurosci. Res. 2016;94:982–989. doi: 10.1002/jnr.23743. [DOI] [PubMed] [Google Scholar]
- 33.Debs R., Froissart R., Aubourg P., Papeix C., Douillard C., Degos B., Fontaine B., Audoin B., Lacour A., Said G., et al. Krabbe disease in adults: phenotypic and genotypic update from a series of 11 cases and a review. J. Inherit. Metab. Dis. 2013;36:859–868. doi: 10.1007/s10545-012-9560-4. [DOI] [PubMed] [Google Scholar]
- 34.Zlotogora J., Chakraborty S., Knowlton R.G., Wenger D.A. Krabbe disease locus mapped to chromosome 14 by genetic linkage. Am. J. Hum. Genet. 1990;47:37–44. [PMC free article] [PubMed] [Google Scholar]
- 35.Oehlmann R., Zlotogora J., Wenger D.A., Knowlton R.G. Localization of the Krabbe disease gene (GALC) on chromosome 14 by multipoint linkage analysis. Am. J. Hum. Genet. 1993;53:1250–1255. [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y.Q., Rafi M.A., de Gala G., Wenger D.A. Cloning and expression of cDNA encoding human galactocerebrosidase, the enzyme deficient in globoid cell leukodystrophy. Hum. Mol. Genet. 1993;2:1841–1845. doi: 10.1093/hmg/2.11.1841. [DOI] [PubMed] [Google Scholar]
- 37.Wenger, D.A., Escolar, M.L., Luzi, P., Rafi, M.A. Krabbe Disease (Globoid Cell Leukodystrophy). In The Online Metabolic and Molecular Bases of Inherited Disease. Chapter 8, 2013. McGraw Hill Ed. N.Y.
- 38.Graziano A.C.E., Cardile V. History, genetic, and recent advances on Krabbe disease. Gene. 2015;555:2–13. doi: 10.1016/j.gene.2014.09.046. [DOI] [PubMed] [Google Scholar]
- 39.Kleijer W.J., Keulemans J.L., van der Kraan M., Geilen G.G., van der Helm R.M., Rafi M.A., Luzi P., Wenger D.A., Halley D.J., van Diggelen O.P. Prevalent mutations in the GALC gene of patients with Krabbe disease of Dutch and other European origin. J. Inherit. Metab. Dis. 1997;20:587–594. doi: 10.1023/a:1005315311165. [DOI] [PubMed] [Google Scholar]
- 40.Wenger D.A., Rafi M.A., Luzi P. Molecular genetics of Krabbe disease (globoid cell leukodystrophy): diagnostic and clinical implications. Hum. Mutat. 1997;10:268–279. doi: 10.1002/(SICI)1098-1004(1997)10:4<268::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 41.Rafi M.A., Luzi P., Chen Y.Q., Wenger D.A. A large deletion together with a point mutation in the GALC gene is a common mutant allele in patients with infantile Krabbe disease. Hum. Mol. Genet. 1995;4:1285–1289. doi: 10.1093/hmg/4.8.1285. [DOI] [PubMed] [Google Scholar]
- 42.De Gasperi R., Gama Sosa M.A., Sartorato E.L., Battistini S., MacFarlane H., Gusella J.F., Krivit W., Kolodny E.H. Molecular heterogeneity of late-onset forms of globoid-cell leukodystrophy. Am. J. Hum. Genet. 1996;59:1233–1242. [PMC free article] [PubMed] [Google Scholar]
- 43.De Gasperi R., Gama Sosa M.A., Sartorato E., Battistini S., Raghavan S., Kolodny E.H. Molecular basis of late-life globoid cell leukodystrophy. Hum. Mutat. 1999;14:256–262. doi: 10.1002/(SICI)1098-1004(1999)14:3<256::AID-HUMU9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Luzi P., Rafi M.A., Wenger D.A. Multiple mutations in the GALC gene in a patient with adult-onset Krabbe disease. Ann. Neurol. 1996;40:116–119. doi: 10.1002/ana.410400119. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi T., Yamanaka T., Jacobs J.M., Teixeira F., Suzuki K. The Twitcher mouse: an enzymatically authentic model of human globoid cell leukodystrophy (Krabbe disease) Brain Res. 1980;202:479–483. doi: 10.1016/0006-8993(80)90159-6. [DOI] [PubMed] [Google Scholar]
- 46.Fletcher T.F., Kurtz H.J., Low D.G. Globoid cell leukodystrophy (Krabbe type) in the dog. J. Am. Vet. Med. Assoc. 1966;149:165–172. [PubMed] [Google Scholar]
- 47.Luzi P., Rafi M.A., Victoria T., Baskin G.B., Wenger D.A. Characterization of the rhesus monkey galactocerebrosidase (GALC) cDNA and gene and identification of the mutation causing globoid cell leukodystrophy (Krabbe disease) in this primate. Genomics. 1997;42:319–324. doi: 10.1006/geno.1997.4744. [DOI] [PubMed] [Google Scholar]
- 48.Baskin G.B., Ratterree M., Davison B.B., Falkenstein K.P., Clarke M.R., England J.D., Vanier M.T., Luzi P., Rafi M.A., Wenger D.A. Genetic galactocerebrosidase deficiency (globoid cell leukodystrophy, Krabbe disease) in rhesus monkeys (Macaca mulatta) Lab. Anim. Sci. 1998;48:476–482. [PubMed] [Google Scholar]
- 49.Yeager A.M., Brennan S., Tiffany C., Moser H.W., Santos G.W. Prolonged survival and remyelination after hematopoietic cell transplantation in the twitcher mouse. Science. 1984;225:1052–1054. doi: 10.1126/science.6382609. [DOI] [PubMed] [Google Scholar]
- 50.LeVine S.M., Pedchenko T.V., Bronshteyn I.G., Pinson D.M. L-cycloserine slows the clinical and pathological course in mice with globoid cell leukodystrophy (twitcher mice) J. Neurosci. Res. 2000;60:231–236. doi: 10.1002/(SICI)1097-4547(20000415)60:2<231::AID-JNR12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 51.Lee W.C., Courtenay A., Troendle F.J., Stallings-Mann M.L., Dickey C.A., DeLucia M.W., Dickson D.W., Eckman C.B. Enzyme replacement therapy results in substantial improvements in early clinical phenotype in a mouse model of globoid cell leukodystrophy. FASEB J. 2005;19:1549–1551. doi: 10.1096/fj.05-3826fje. [DOI] [PubMed] [Google Scholar]
- 52.Bradbury A.M., Rafi M.A., Bagel J.H., Brisson B.K., Marshall M.S., Pesayco Salvador J., Jiang X., Swain G.P., Prociuk M.L., ODonnell P.A., et al. AAVrh10 gene therapy ameliorates central and peripheral nervous system disease in canine globoid cell leukodystrophy (krabbe disease) Hum. Gene Ther. 2018;29:785–801. doi: 10.1089/hum.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marshall M.S., Issa Y., Jakubauskas B., Stoskute M., Elackattu V., Marshall J.N., Bogue W., Nguyen D., Hauck Z., Rue E., et al. Long-term improvement of neurological signs and metabolic dysfunction in a mouse model of krabbe's disease after global gene therapy. Mol. Ther. 2018;26:874–889. doi: 10.1016/j.ymthe.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biswas S., LeVine S.M. Substrate-reduction therapy enhances the benefits of bone marrow transplantation in young mice with globoid cell leukodystrophy. Pediatr. Res. 2002;51:40–47. doi: 10.1203/00006450-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Lattanzi A., Neri M., Maderna C., di Girolamo I., Martino S., Orlacchio A., Amendola M., Naldini L., Gritti A. Widespread enzymatic correction of CNS tissues by a single intracerebral injection of therapeutic lentiviral vector in leukodystrophy mouse models. Hum. Mol. Genet. 2010;19:2208–2227. doi: 10.1093/hmg/ddq099. [DOI] [PubMed] [Google Scholar]
- 56.Karumuthil-Melethil S., Marshall M.S., Heindel C., Jakubauskas B., Bongarzone E.R., Gray S.J. Intrathecal administration of AAV/GALC vectors in 10-11-day-old twitcher mice improves survival and is enhanced by bone marrow transplant. J. Neurosci. Res. 2016;94:1138–1151. doi: 10.1002/jnr.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Miller C.A., Shea L.K., Jiang X., Guzman M.A., Chandler R.J., Ramakrishnan S.M., Smith S.N., Venditti C.P., Vogler C.A., et al. Enhanced efficacy and increased long-term toxicity of CNS-directed, AAV-based combination therapy for krabbe disease. Mol. Ther. 2021;29:691–701. doi: 10.1016/j.ymthe.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bradbury A.M., Bagel J.H., Nguyen D., Lykken E.A., Pesayco Salvador J., Jiang X., Swain G.P., Assenmacher C.A., Hendricks I.J., Miyadera K., et al. Krabbe disease successfully treated via monotherapy of intrathecal gene therapy. J. Clin. Invest. 2020;130:4906–4920. doi: 10.1172/JCI133953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakai N., Inui K., Tatsumi N., Fukushima H., Nishigaki T., Taniike M., Nishimoto J., Tsukamoto H., Yanagihara I., Ozono K., Okada S. Molecular cloning and expression of cDNA for murine galactocerebrosidase and mutation analysis of the twitcher mouse, a model of Krabbe's disease. J. Neurochem. 1996;66:1118–1124. doi: 10.1046/j.1471-4159.1996.66031118.x. [DOI] [PubMed] [Google Scholar]
- 60.Lee W.C., Tsoi Y.K., Dickey C.A., Delucia M.W., Dickson D.W., Eckman C.B. Suppression of galactosylceramidase (GALC) expression in the twitcher mouse model of globoid cell leukodystrophy (GLD) is caused by nonsense-mediated mRNA decay (NMD) Neurobiol. Dis. 2006;23:273–280. doi: 10.1016/j.nbd.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Duchen L.W., Eicher E.M., Jacobs J.M., Scaravilli F., Teixeira F. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain. 1980;103:695–710. doi: 10.1093/brain/103.3.695. [DOI] [PubMed] [Google Scholar]
- 62.Kagitani-Shimono K., Mohri I., Fujitani Y., Suzuki K., Ozono K., Urade Y., Taniike M. Anti-inflammatory therapy by ibudilast, a phosphodiesterase inhibitor, in demyelination of twitcher, a genetic demyelination model. J. Neuroinflammation. 2005;2:10. doi: 10.1186/1742-2094-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.LeVine S.M., Brown D.C. IL-6 and TNFalpha expression in brains of twitcher, quaking and normal mice. J. Neuroimmunol. 1997;73:47–56. doi: 10.1016/s0165-5728(96)00166-x. [DOI] [PubMed] [Google Scholar]
- 64.Ohno M., Komiyama A., Martin P.M., Suzuki K. Proliferation of microglia/macrophages in the demyelinating CNS and PNS of twitcher mouse. Brain Res. 1993;602:268–274. doi: 10.1016/0006-8993(93)90692-g. [DOI] [PubMed] [Google Scholar]
- 65.Luzi P., Rafi M.A., Zaka M., Curtis M., Vanier M.T., Wenger D.A. Generation of a mouse with low galactocerebrosidase activity by gene targeting: a new model of globoid cell leukodystrophy (Krabbe disease) Mol. Genet. Metab. 2001;73:211–223. doi: 10.1006/mgme.2001.3194. [DOI] [PubMed] [Google Scholar]
- 66.Weinstock N.I., Kreher C., Favret J., Nguyen D., Bongarzone E.R., Wrabetz L., Feltri M.L., Shin D. Brainstem development requires galactosylceramidase and is critical for pathogenesis in a model of Krabbe disease. Nat. Commun. 2020;11:5356. doi: 10.1038/s41467-020-19179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayashi S., McMahon A.P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 68.Mikulka C.R., Dearborn J.T., Benitez B.A., Strickland A., Liu L., Milbrandt J., Sands M.S. Cell-autonomous expression of the acid hydrolase galactocerebrosidase. Proc. Natl. Acad. Sci. USA. 2020;117:9032–9041. doi: 10.1073/pnas.1917675117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurtz H.J., Fletcher T.F. The peripheral neuropathy of canine globoid-cell leukodystrophy (krabbe-type) Acta Neuropathol. 1970;16:226–232. doi: 10.1007/BF00687362. [DOI] [PubMed] [Google Scholar]
- 70.Fletcher T.F., Jessen C.R., Bender A.P. Quantitative evaluation of spinal cord lesions in canine globoid leukodystrophy. J. Neuropathol. Exp. Neurol. 1977;36:84–99. doi: 10.1097/00005072-197701000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Wenger D.A., Victoria T., Rafi M.A., Luzi P., Vanier M.T., Vite C., Patterson D.F., Haskins M.H. Globoid cell leukodystrophy in cairn and West Highland white terriers. J. Hered. 1999;90:138–142. doi: 10.1093/jhered/90.1.138. [DOI] [PubMed] [Google Scholar]
- 72.Fletcher T.F., Kurtz H.J. Animal model: globoid cell leukodystrophy in the dog. Am. J. Pathol. 1972;66:375–378. [PMC free article] [PubMed] [Google Scholar]
- 73.Bradbury A.M., Bagel J.H., Jiang X., Swain G.P., Prociuk M.L., Fitzgerald C.A., O'Donnell P.A., Braund K.G., Ory D.S., Vite C.H. Clinical, electrophysiological, and biochemical markers of peripheral and central nervous system disease in canine globoid cell leukodystrophy (Krabbe's disease) J. Neurosci. Res. 2016;94:1007–1017. doi: 10.1002/jnr.23838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bradbury A., Peterson D., Vite C., Chen S., Ellinwood N.M., Provenzale J. Diffusion tensor imaging analysis of the brain in the canine model of Krabbe disease. Neuroradiol. J. 2016;29:417–424. doi: 10.1177/1971400916665378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright M.D., Poe M.D., DeRenzo A., Haldal S., Escolar M.L. Developmental outcomes of cord blood transplantation for Krabbe disease: a 15-year study. Neurology. 2017;89:1365–1372. doi: 10.1212/WNL.0000000000004418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ichioka T., Kishimoto Y., Brennan S., Santos G.W., Yeager A.M. Hematopoietic cell transplantation in murine globoid cell leukodystrophy (the twitcher mouse): effects on levels of galactosylceramidase, psychosine, and galactocerebrosides. Proc. Natl. Acad. Sci. USA. 1987;84:4259–4263. doi: 10.1073/pnas.84.12.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoogerbrugge P.M., Poorthuis B.J., Romme A.E., van de Kamp J.J., Wagemaker G., van Bekkum D.W. Effect of bone marrow transplantation on enzyme levels and clinical course in the neurologically affected twitcher mouse. J. Clin. Invest. 1988;81:1790–1794. doi: 10.1172/JCI113521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin E.Y., Hawkins-Salsbury J.A., Jiang X., Reddy A.S., Farber N.B., Ory D.S., Sands M.S. Bone marrow transplantation increases efficacy of central nervous system-directed enzyme replacement therapy in the murine model of globoid cell leukodystrophy. Mol. Genet. Metab. 2012;107:186–196. doi: 10.1016/j.ymgme.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krivit W., Shapiro E.G., Peters C., Wagner J.E., Cornu G., Kurtzberg J., Wenger D.A., Kolodny E.H., Vanier M.T., Loes D.J., et al. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N. Engl. J. Med. 1998;338:1119–1126. doi: 10.1056/NEJM199804163381605. [DOI] [PubMed] [Google Scholar]
- 80.Krieg S.I., Krägeloh-Mann I., Groeschel S., Beck-Wödl S., Husain R.A., Schöls L., Kehrer C. Natural history of Krabbe disease - a nationwide study in Germany using clinical and MRI data. Orphanet J. Rare Dis. 2020;15:243. doi: 10.1186/s13023-020-01489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Komatsuzaki S., Zielonka M., Mountford W.K., Kölker S., Hoffmann G.F., Garbade S.F., Ries M. Clinical characteristics of 248 patients with Krabbe disease: quantitative natural history modeling based on published cases. Genet. Med. 2019;21:2208–2215. doi: 10.1038/s41436-019-0480-7. [DOI] [PubMed] [Google Scholar]
- 82.Escolar M.L., Poe M.D., Provenzale J.M., Richards K.C., Allison J., Wood S., Wenger D.A., Pietryga D., Wall D., Champagne M., et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe's disease. N. Engl. J. Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 83.Boucher A.A., Miller W., Shanley R., Ziegler R., Lund T., Raymond G., Orchard P.J. Long-term outcomes after allogeneic hematopoietic stem cell transplantation for metachromatic leukodystrophy: the largest single-institution cohort report. Orphanet J. Rare Dis. 2015;10:94. doi: 10.1186/s13023-015-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van den Broek B.T.A., van Doorn J., Hegeman C.V., Nierkens S., Lindemans C.A., Verhoeven-Duif N., Boelens J.J., van Hasselt P.M. Hurdles in treating Hurler disease: potential routes to achieve a "real" cure. Blood Adv. 2020;4:2837–2849. doi: 10.1182/bloodadvances.2020001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aldenhoven M., Jones S.A., Bonney D., Borrill R.E., Coussons M., Mercer J., Bierings M.B., Versluys B., van Hasselt P.M., Wijburg F.A., et al. Hematopoietic cell transplantation for mucopolysaccharidosis patients is safe and effective: results after implementation of international guidelines. Biol. Blood Marrow Transpl. 2015;21:1106–1109. doi: 10.1016/j.bbmt.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 86.Kwon J.M., Matern D., Kurtzberg J., Wrabetz L., Gelb M.H., Wenger D.A., Ficicioglu C., Waldman A.T., Burton B.K., Hopkins P.V., Orsini J.J. Consensus guidelines for newborn screening, diagnosis and treatment of infantile Krabbe disease. Orphanet J. Rare Dis. 2018;13:30. doi: 10.1186/s13023-018-0766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Page K.M., Ream M.A., Rangarajan H.G., Galindo R., Mian A.Y., Ho M.L., Provenzale J., Gustafson K.E., Rubin J., Shenoy S., Kurtzberg J. Benefits of newborn screening and hematopoietic cell transplant in infantile Krabbe disease. Blood Adv. 2022;6:2947–2956. doi: 10.1182/bloodadvances.2021006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strazza M., Luddi A., Carbone M., Rafi M.A., Costantino-Ceccarini E., Wenger D.A. Significant correction of pathology in brains of twitcher mice following injection of genetically modified mouse neural progenitor cells. Mol. Genet. Metab. 2009;97:27–34. doi: 10.1016/j.ymgme.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 89.Neri M., Ricca A., di Girolamo I., Alcala'-Franco B., Cavazzin C., Orlacchio A., Martino S., Naldini L., Gritti A. Neural stem cell gene therapy ameliorates pathology and function in a mouse model of globoid cell leukodystrophy. Stem Cells. 2011;29:1559–1571. doi: 10.1002/stem.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ripoll C.B., Flaat M., Klopf-Eiermann J., Fisher-Perkins J.M., Trygg C.B., Scruggs B.A., McCants M.L., Leonard H.P., Lin A.F., Zhang S., et al. Mesenchymal lineage stem cells have pronounced anti-inflammatory effects in the twitcher mouse model of Krabbe's disease. Stem Cells. 2011;29:67–77. doi: 10.1002/stem.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Babcock M.C., Mikulka C.R., Wang B., Chandriani S., Chandra S., Xu Y., Webster K., Feng Y., Nelvagal H.R., Giaramita A., et al. Substrate reduction therapy for Krabbe disease and metachromatic leukodystrophy using a novel ceramide galactosyltransferase inhibitor. Sci. Rep. 2021;11:14486. doi: 10.1038/s41598-021-93601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luzi P., Abraham R.M., Rafi M.A., Curtis M., Hooper D.C., Wenger D.A. Effects of treatments on inflammatory and apoptotic markers in the CNS of mice with globoid cell leukodystrophy. Brain Res. 2009;1300:146–158. doi: 10.1016/j.brainres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berardi A.S., Pannuzzo G., Graziano A., Costantino-Ceccarini E., Piomboni P., Luddi A. Pharmacological chaperones increase residual beta-galactocerebrosidase activity in fibroblasts from Krabbe patients. Mol. Genet. Metab. 2014;112:294–301. doi: 10.1016/j.ymgme.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 94.Lee W.C., Kang D., Causevic E., Herdt A.R., Eckman E.A., Eckman C.B. Molecular characterization of mutations that cause globoid cell leukodystrophy and pharmacological rescue using small molecule chemical chaperones. J. Neurosci. 2010;30:5489–5497. doi: 10.1523/JNEUROSCI.6383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hossain M.A., Higaki K., Saito S., Ohno K., Sakuraba H., Nanba E., Suzuki Y., Ozono K., Sakai N. Chaperone therapy for Krabbe disease: potential for late-onset GALC mutations. J. Hum. Genet. 2015;60:539–545. doi: 10.1038/jhg.2015.61. [DOI] [PubMed] [Google Scholar]
- 96.Hill C.H., Viuff A.H., Spratley S.J., Salamone S., Christensen S.H., Read R.J., Moriarty N.W., Jensen H.H., Deane J.E. Azasugar inhibitors as pharmacological chaperones for Krabbe disease. Chem. Sci. 2015;6:3075–3086. doi: 10.1039/c5sc00754b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shen J.S., Watabe K., Ohashi T., Eto Y. Intraventricular administration of recombinant adenovirus to neonatal twitcher mouse leads to clinicopathological improvements. Gene Ther. 2001;8:1081–1087. doi: 10.1038/sj.gt.3301495. [DOI] [PubMed] [Google Scholar]
- 98.Lin D., Fantz C.R., Levy B., Rafi M.A., Vogler C., Wenger D.A., Sands M.S. AAV2/5 vector expressing galactocerebrosidase ameliorates CNS disease in the murine model of globoid-cell leukodystrophy more efficiently than AAV2. Mol. Ther. 2005;12:422–430. doi: 10.1016/j.ymthe.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 99.Rafi M.A., Rao H.Z., Luzi P., Curtis M.T., Wenger D.A. Extended normal life after AAVrh10-mediated gene therapy in the mouse model of Krabbe disease. Mol. Ther. 2012;20:2031–2042. doi: 10.1038/mt.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin D., Donsante A., Macauley S., Levy B., Vogler C., Sands M.S. Central nervous system-directed AAV2/5-mediated gene therapy synergizes with bone marrow transplantation in the murine model of globoid-cell leukodystrophy. Mol. Ther. 2007;15:44–52. doi: 10.1038/sj.mt.6300026. [DOI] [PubMed] [Google Scholar]
- 101.Rafi M.A., Zhi Rao H., Passini M.A., Curtis M., Vanier M.T., Zaka M., Luzi P., Wolfe J.H., Wenger D.A. AAV-mediated expression of galactocerebrosidase in brain results in attenuated symptoms and extended life span in murine models of globoid cell leukodystrophy. Mol. Ther. 2005;11:734–744. doi: 10.1016/j.ymthe.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 102.Dolcetta D., Perani L., Givogri M.I., Galbiati F., Amadio S., Del Carro U., Finocchiaro G., Fanzani A., Marchesini S., Naldini L., et al. Design and optimization of lentiviral vectors for transfer of GALC expression in Twitcher brain. J. Gene Med. 2006;8:962–971. doi: 10.1002/jgm.924. [DOI] [PubMed] [Google Scholar]
- 103.Lee W.C., Tsoi Y.K., Troendle F.J., DeLucia M.W., Ahmed Z., Dicky C.A., Dickson D.W., Eckman C.B. Single-dose intracerebroventricular administration of galactocerebrosidase improves survival in a mouse model of globoid cell leukodystrophy. FASEB J. 2007;21:2520–2527. doi: 10.1096/fj.06-6169com. [DOI] [PubMed] [Google Scholar]
- 104.Lin D.S., Hsiao C.D., Liau I., Lin S.P., Chiang M.F., Chuang C.K., Wang T.J., Wu T.Y., Jian Y.R., Huang S.F., Liu H.L. CNS-targeted AAV5 gene transfer results in global dispersal of vector and prevention of morphological and function deterioration in CNS of globoid cell leukodystrophy mouse model. Mol. Genet. Metab. 2011;103:367–377. doi: 10.1016/j.ymgme.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 105.Wicks S.E., Londot H., Zhang B., Dowden J., Klopf-Eiermann J., Fisher-Perkins J.M., Trygg C.B., Scruggs B.A., Zhang X., Gimble J.M., et al. Effect of intrastriatal mesenchymal stromal cell injection on progression of a murine model of Krabbe disease. Behav. Brain Res. 2011;225:415–425. doi: 10.1016/j.bbr.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reddy A.S., Kim J.H., Hawkins-Salsbury J.A., Macauley S.L., Tracy E.T., Vogler C.A., Han X., Song S.K., Wozniak D.F., Fowler S.C., et al. Bone marrow transplantation augments the effect of brain- and spinal cord-directed adeno-associated virus 2/5 gene therapy by altering inflammation in the murine model of globoid-cell leukodystrophy. J. Neurosci. 2011;31:9945–9957. doi: 10.1523/JNEUROSCI.1802-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hordeaux J., Jeffrey B.A., Jian J., Choudhury G.R., Michalson K., Mitchell T.W., Buza E.L., Chichester J., Dyer C., Bagel J., et al. Efficacy and safety of a krabbe disease gene therapy. Hum. Gene Ther. 2022;33:499–517. doi: 10.1089/hum.2021.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rafi M.A., Luzi P., Wenger D.A. Can early treatment of twitcher mice with high dose AAVrh10-GALC eliminate the need for BMT? Bioimpacts. 2021;11:135–146. doi: 10.34172/bi.2021.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Galbiati F., Givogri M.I., Cantuti L., Rosas A.L., Cao H., van Breemen R., Bongarzone E.R. Combined hematopoietic and lentiviral gene-transfer therapies in newborn Twitcher mice reveal contemporaneous neurodegeneration and demyelination in Krabbe disease. J. Neurosci. Res. 2009;87:1748–1759. doi: 10.1002/jnr.22006. [DOI] [PubMed] [Google Scholar]
- 110.Hawkins-Salsbury J.A., Shea L., Jiang X., Hunter D.A., Guzman A.M., Reddy A.S., Qin E.Y., Li Y., Gray S.J., Ory D.S., Sands M.S. Mechanism-based combination treatment dramatically increases therapeutic efficacy in murine globoid cell leukodystrophy. J. Neurosci. 2015;35:6495–6505. doi: 10.1523/JNEUROSCI.4199-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rafi M.A., Rao H.Z., Luzi P., Wenger D.A. Long-term improvements in lifespan and pathology in CNS and PNS after BMT plus one intravenous injection of AAVrh10-GALC in twitcher mice. Mol. Ther. 2015;23:1681–1690. doi: 10.1038/mt.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ricca A., Rufo N., Ungari S., Morena F., Martino S., Kulik W., Alberizzi V., Bolino A., Bianchi F., Del Carro U., et al. Combined gene/cell therapies provide long-term and pervasive rescue of multiple pathological symptoms in a murine model of globoid cell leukodystrophy. Hum. Mol. Genet. 2015;24:3372–3389. doi: 10.1093/hmg/ddv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fratantoni J.C., Hall C.W., Neufeld E.F. Hurler and Hunter syndromes: mutual correction of the defect in cultured fibroblasts. Science. 1968;162:570–572. doi: 10.1126/science.162.3853.570. [DOI] [PubMed] [Google Scholar]
- 114.Scaravilli F., Suzuki K. Enzyme replacement in grafted nerve of twitcher mouse. Nature. 1983;305:713–715. doi: 10.1038/305713a0. [DOI] [PubMed] [Google Scholar]
- 115.Rafi M.A., Fugaro J., Amini S., Luzi P., de Gala G., Victoria T., Dubell C., Shahinfar M., Wenger D.A. Retroviral vector-mediated transfer of the galactocerebrosidase (GALC) cDNA leads to overexpression and transfer of GALC activity to neighboring cells. Biochem. Mol. Med. 1996;58:142–150. doi: 10.1006/bmme.1996.0042. [DOI] [PubMed] [Google Scholar]
- 116.Luddi A., Volterrani M., Strazza M., Smorlesi A., Rafi M.A., Datto J., Wenger D.A., Costantino-Ceccarini E. Retrovirus-mediated gene transfer and galactocerebrosidase uptake into twitcher glial cells results in appropriate localization and phenotype correction. Neurobiol. Dis. 2001;8:600–610. doi: 10.1006/nbdi.2001.0407. [DOI] [PubMed] [Google Scholar]
- 117.Kondo Y., Wenger D.A., Gallo V., Duncan I.D. Galactocerebrosidase-deficient oligodendrocytes maintain stable central myelin by exogenous replacement of the missing enzyme in mice. Proc. Natl. Acad. Sci. USA. 2005;102:18670–18675. doi: 10.1073/pnas.0506473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brady R.O., Koval G.J. The enzymatic synthesis of sphingosine. J. Biol. Chem. 1958;233:26–31. [PubMed] [Google Scholar]
- 119.Sundaram K.S., Lev M. Comparative inhibition of bacterial and microsomal 3-ketodihydrosphingosine synthetases by L-cycloserine and other inhibitors. Antimicrob. Agents Chemother. 1984;26:211–213. doi: 10.1128/aac.26.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miller S.L., Denisova L. Cycloserine-induced decrease of cerebroside in myelin. Lipids. 1998;33:441–443. doi: 10.1007/s11745-998-0226-6. [DOI] [PubMed] [Google Scholar]
- 121.Di Martino S., Tardia P., Cilibrasi V., Caputo S., Mazzonna M., Russo D., Penna I., Realini N., Margaroli N., Migliore M., et al. Lead optimization of benzoxazolone carboxamides as orally bioavailable and CNS penetrant acid ceramidase inhibitors. J. Med. Chem. 2020;63:3634–3664. doi: 10.1021/acs.jmedchem.9b02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marshall J., Sun Y., Bangari D.S., Budman E., Park H., Nietupski J.B., Allaire A., Cromwell M.A., Wang B., Grabowski G.A., et al. CNS-Accessible inhibitor of glucosylceramide synthase for substrate reduction therapy of neuronopathic gaucher disease. Mol. Ther. 2016;24:1019–1029. doi: 10.1038/mt.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coutinho M.F., Santos J.I., Alves S. Less is more: substrate reduction therapy for lysosomal storage disorders. Int. J. Mol. Sci. 2017;18:E178. doi: 10.3390/ijms17071065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Platt F.M., Jeyakumar M. Substrate reduction therapy. Acta Paediatr. 2008;97:88–93. doi: 10.1111/j.1651-2227.2008.00656.x. [DOI] [PubMed] [Google Scholar]
- 125.Gary S.E., Ryan E., Steward A.M., Sidransky E. Recent advances in the diagnosis and management of Gaucher disease. Expert Rev. Endocrinol. Metab. 2018;13:107–118. doi: 10.1080/17446651.2018.1445524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stirnemann J., Belmatoug N., Camou F., Serratrice C., Froissart R., Caillaud C., Levade T., Astudillo L., Serratrice J., Brassier A., et al. A review of gaucher disease pathophysiology, clinical presentation and treatments. Int. J. Mol. Sci. 2017;18:E441. doi: 10.3390/ijms18020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Elstein D., Hollak C., Aerts J.M.F.G., van Weely S., Maas M., Cox T.M., Lachmann R.H., Hrebicek M., Platt F.M., Butters T.D., et al. Sustained therapeutic effects of oral miglustat (Zavesca, N-butyldeoxynojirimycin, OGT 918) in type I Gaucher disease. J. Inherit. Metab. Dis. 2004;27:757–766. doi: 10.1023/B:BOLI.0000045756.54006.17. [DOI] [PubMed] [Google Scholar]
- 128.Pastores G.M., Barnett N.L., Kolodny E.H. An open-label, noncomparative study of miglustat in type I Gaucher disease: efficacy and tolerability over 24 months of treatment. Clin. Ther. 2005;27:1215–1227. doi: 10.1016/j.clinthera.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 129.Cox T., Lachmann R., Hollak C., Aerts J., van Weely S., Hrebícek M., Platt F., Butters T., Dwek R., Moyses C., et al. Novel oral treatment of Gaucher's disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 2000;355:1481–1485. doi: 10.1016/S0140-6736(00)02161-9. [DOI] [PubMed] [Google Scholar]
- 130.Patterson M.C., Vecchio D., Prady H., Abel L., Wraith J.E. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6:765–772. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- 131.Parenti G., Medina D.L., Ballabio A. The rapidly evolving view of lysosomal storage diseases. EMBO Mol. Med. 2021;13:e12836. doi: 10.15252/emmm.202012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peterschmitt M.J., Crawford N.P.S., Gaemers S.J.M., Ji A.J., Sharma J., Pham T.T. Pharmacokinetics, pharmacodynamics, safety, and tolerability of oral Venglustat in healthy volunteers. Clin. Pharmacol. Drug Dev. 2021;10:86–98. doi: 10.1002/cpdd.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Felis A., Whitlow M., Kraus A., Warnock D.G., Wallace E. Current and investigational therapeutics for Fabry disease. Kidney Int. Rep. 2020;5:407–413. doi: 10.1016/j.ekir.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Migita M., Hamada H., Fujimura J., Watanabe A., Shimada T., Fukunaga Y. Glucocerebrosidase level in the cerebrospinal fluid during enzyme replacement therapy--unsuccessful treatment of the neurological abnormality in type 2 Gaucher disease. Eur. J. Pediatr. 2003;162:524–525. doi: 10.1007/s00431-001-0859-7. [DOI] [PubMed] [Google Scholar]
- 135.Meena N.K., Ralston E., Raben N., Puertollano R. Enzyme replacement therapy can reverse pathogenic cascade in Pompe disease. Mol. Ther. Methods Clin. Dev. 2020;18:199–214. doi: 10.1016/j.omtm.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schulz A., Ajayi T., Specchio N., de Los Reyes E., Gissen P., Ballon D., Dyke J.P., Cahan H., Slasor P., Jacoby D., et al. Study of intraventricular cerliponase alfa for CLN2 disease. N. Engl. J. Med. 2018;378:1898–1907. doi: 10.1056/NEJMoa1712649. [DOI] [PubMed] [Google Scholar]
- 137.Schwering C., Kammler G., Wibbeler E., Christner M., Knobloch J.K.M., Nickel M., Denecke J., Baehr M., Schulz A. Development of the "hamburg best practice guidelines for ICV-enzyme replacement therapy (ERT) in CLN2 disease" based on 6 Years treatment experience in 48 patients. J. Child Neurol. 2021;36:635–641. doi: 10.1177/0883073821989154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Andersson H., Kaplan P., Kacena K., Yee J. Eight-year clinical outcomes of long-term enzyme replacement therapy for 884 children with Gaucher disease type 1. Pediatrics. 2008;122:1182–1190. doi: 10.1542/peds.2007-2144. [DOI] [PubMed] [Google Scholar]
- 139.í Dali C., Sevin C., Krägeloh-Mann I., Giugliani R., Sakai N., Wu J., Wasilewski M. Safety of intrathecal delivery of recombinant human arylsulfatase A in children with metachromatic leukodystrophy: results from a phase 1/2 clinical trial. Mol. Genet. Metab. 2020;131:235–244. doi: 10.1016/j.ymgme.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 140.Sato Y., Okuyama T. Novel enzyme replacement therapies for neuropathic mucopolysaccharidoses. Int. J. Mol. Sci. 2020;21:E400. doi: 10.3390/ijms21020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pardridge W.M., Eisenberg J., Yang J. Human blood-brain barrier insulin receptor. J. Neurochem. 1985;44:1771–1778. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- 142.Golden P.L., Maccagnan T.J., Pardridge W.M. Human blood-brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J. Clin. Invest. 1997;99:14–18. doi: 10.1172/JCI119125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fishman J.B., Rubin J.B., Handrahan J.V., Connor J.R., Fine R.E. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res. 1987;18:299–304. doi: 10.1002/jnr.490180206. [DOI] [PubMed] [Google Scholar]
- 144.Jefferies W.A., Brandon M.R., Hunt S.V., Williams A.F., Gatter K.C., Mason D.Y. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312:162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 145.Pardridge W.M., Eisenberg J., Yang J. Human blood-brain barrier transferrin receptor. Metabolism. 1987;36:892–895. doi: 10.1016/0026-0495(87)90099-0. [DOI] [PubMed] [Google Scholar]
- 146.Sonoda H., Morimoto H., Yoden E., Koshimura Y., Kinoshita M., Golovina G., Takagi H., Yamamoto R., Minami K., Mizoguchi A., et al. A blood-brain-barrier-penetrating anti-human transferrin receptor antibody fusion protein for neuronopathic mucopolysaccharidosis II. Mol. Ther. 2018;26:1366–1374. doi: 10.1016/j.ymthe.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vuillemenot B.R., Kennedy D., Cooper J.D., Wong A.M.S., Sri S., Doeleman T., Katz M.L., Coates J.R., Johnson G.C., Reed R.P., et al. Nonclinical evaluation of CNS-administered TPP1 enzyme replacement in canine CLN2 neuronal ceroid lipofuscinosis. Mol. Genet. Metab. 2015;114:281–293. doi: 10.1016/j.ymgme.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 148.Iwan K., Patel N., Heslegrave A., Borisova M., Lee L., Bower R., Mole S.E., Mills P.B., Zetterberg H., Mills K., et al. Cerebrospinal fluid neurofilament light chain levels in CLN2 disease patients treated with enzyme replacement therapy normalise after two years on treatment. F1000Res. 2021;10:614. doi: 10.12688/f1000research.54556.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Burger C., Gorbatyuk O.S., Velardo M.J., Peden C.S., Williams P., Zolotukhin S., Reier P.J., Mandel R.J., Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol. Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 150.Cearley C.N., Wolfe J.H. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 151.Taymans J.M., Vandenberghe L.H., Haute C.V.D., Thiry I., Deroose C.M., Mortelmans L., Wilson J.M., Debyser Z., Baekelandt V. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum. Gene Ther. 2007;18:195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- 152.Cearley C.N., Vandenberghe L.H., Parente M.K., Carnish E.R., Wilson J.M., Wolfe J.H. Expanded repertoire of AAV vector serotypes mediate unique patterns of transduction in mouse brain. Mol. Ther. 2008;16:1710–1718. doi: 10.1038/mt.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Klein R.L., Dayton R.D., Tatom J.B., Henderson K.M., Henning P.P. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol. Ther. 2008;16:89–96. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.von Jonquieres G., Rae C.D., Housley G.D. Emerging concepts in vector development for glial gene therapy: implications for leukodystrophies. Front. Cell. Neurosci. 2021;15:661857. doi: 10.3389/fncel.2021.661857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Passini M.A., Watson D.J., Vite C.H., Landsburg D.J., Feigenbaum A.L., Wolfe J.H. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J. Virol. 2003;77:7034–7040. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bartlett J.S., Samulski R.J., McCown T.J. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum. Gene Ther. 1998;9:1181–1186. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- 157.von Jonquieres G., Mersmann N., Klugmann C.B., Harasta A.E., Lutz B., Teahan O., Housley G.D., Fröhlich D., Krämer-Albers E.M., Klugmann M. Glial promoter selectivity following AAV-delivery to the immature brain. PLoS One. 2013;8:e65646. doi: 10.1371/journal.pone.0065646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Watakabe A., Ohtsuka M., Kinoshita M., Takaji M., Isa K., Mizukami H., Ozawa K., Isa T., Yamamori T. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci. Res. 2015;93:144–157. doi: 10.1016/j.neures.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 159.Ohashi T. Gene therapy for lysosomal storage diseases and peroxisomal diseases. J. Hum. Genet. 2019;64:139–143. doi: 10.1038/s10038-018-0537-5. [DOI] [PubMed] [Google Scholar]
- 160.Broekman M.L.D., Baek R.C., Comer L.A., Fernandez J.L., Seyfried T.N., Sena-Esteves M. Complete correction of enzymatic deficiency and neurochemistry in the GM1-gangliosidosis mouse brain by neonatal adeno-associated virus-mediated gene delivery. Mol. Ther. 2007;15:30–37. doi: 10.1038/sj.mt.6300004. [DOI] [PubMed] [Google Scholar]
- 161.Weismann C.M., Ferreira J., Keeler A.M., Su Q., Qui L., Shaffer S.A., Xu Z., Gao G., Sena-Esteves M. Systemic AAV9 gene transfer in adult GM1 gangliosidosis mice reduces lysosomal storage in CNS and extends lifespan. Hum. Mol. Genet. 2015;24:4353–4364. doi: 10.1093/hmg/ddv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gross A.L., Gray-Edwards H.L., Bebout C.N., Ta N.L., Nielsen K., Brunson B.L., Lopez Mercado K.R., Osterhoudt D.E., Batista A.R., Maitland S., et al. Intravenous delivery of adeno-associated viral gene therapy in feline GM1 gangliosidosis. Brain. 2022;145:655–669. doi: 10.1093/brain/awab309. [DOI] [PubMed] [Google Scholar]
- 163.Sands M.S., Vogler C., Torrey A., Levy B., Gwynn B., Grubb J., Sly W.S., Birkenmeier E.H. Murine mucopolysaccharidosis type VII: long term therapeutic effects of enzyme replacement and enzyme replacement followed by bone marrow transplantation. J. Clin. Invest. 1997;99:1596–1605. doi: 10.1172/JCI119322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hawkins-Salsbury J.A., Reddy A.S., Sands M.S. Combination therapies for lysosomal storage disease: is the whole greater than the sum of its parts? Hum. Mol. Genet. 2011;20:R54–R60. doi: 10.1093/hmg/ddr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Macauley S.L., Roberts M.S., Wong A.M., McSloy F., Reddy A.S., Cooper J.D., Sands M.S. Synergistic effects of central nervous system-directed gene therapy and bone marrow transplantation in the murine model of infantile neuronal ceroid lipofuscinosis. Ann. Neurol. 2012;71:797–804. doi: 10.1002/ana.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jeyakumar M., Norflus F., Tifft C.J., Cortina-Borja M., Butters T.D., Proia R.L., Perry V.H., Dwek R.A., Platt F.M. Enhanced survival in Sandhoff disease mice receiving a combination of substrate deprivation therapy and bone marrow transplantation. Blood. 2001;97:327–329. doi: 10.1182/blood.v97.1.327. [DOI] [PubMed] [Google Scholar]
- 167.Marshall J., McEachern K.A., Chuang W.L., Hutto E., Siegel C.S., Shayman J.A., Grabowski G.A., Scheule R.K., Copeland D.P., Cheng S.H. Improved management of lysosomal glucosylceramide levels in a mouse model of type 1 Gaucher disease using enzyme and substrate reduction therapy. J. Inherit. Metab. Dis. 2010;33:281–289. doi: 10.1007/s10545-010-9072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Turturro A., Duffy P., Hass B., Kodell R., Hart R. Survival characteristics and age-adjusted disease incidences in C57BL/6 mice fed a commonly used cereal-based diet modulated by dietary restriction. J. Gerontol. A. Biol. Sci. Med. Sci. 2002;57:B379–B389. doi: 10.1093/gerona/57.11.b379. [DOI] [PubMed] [Google Scholar]
- 169.Heller G.J., Marshall M.S., Issa Y., Marshall J.N., Nguyen D., Rue E., Pathmasiri K.C., Domowicz M.S., van Breemen R.B., Tai L.M., et al. Waning efficacy in a long-term AAV-mediated gene therapy study in the murine model of Krabbe disease. Mol. Ther. 2021;29:1883–1902. doi: 10.1016/j.ymthe.2021.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Afione S.A., Conrad C.K., Kearns W.G., Chunduru S., Adams R., Reynolds T.C., Guggino W.B., Cutting G.R., Carter B.J., Flotte T.R. In vivo model of adeno-associated virus vector persistence and rescue. J. Virol. 1996;70:3235–3241. doi: 10.1128/jvi.70.5.3235-3241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Duan D., Sharma P., Yang J., Yue Y., Dudus L., Zhang Y., Fisher K.J., Engelhardt J.F. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long-term episomal persistence in muscle tissue. J. Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nakai H., Yant S.R., Storm T.A., Fuess S., Meuse L., Kay M.A. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McCarty D.M., Young S.M., Jr., Samulski R.J. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu. Rev. Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- 174.Tatsumi N., Inui K., Sakai N., Fukushima H., Nishimoto J., Yanagihara I., Nishigaki T., Tsukamoto H., Fu L., Taniike M. Molecular defects in Krabbe disease. Hum. Mol. Genet. 1995;4:1865–1868. doi: 10.1093/hmg/4.10.1865. [DOI] [PubMed] [Google Scholar]
- 175.Keirstead H.S., Levine J.M., Blakemore W.F. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- 176.Levine J.M., Reynolds R., Fawcett J.W. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. doi: 10.1016/s0166-2236(00)01691-x. [DOI] [PubMed] [Google Scholar]
- 177.Reynolds R., Cenci di Bello I., Dawson M., Levine J. The response of adult oligodendrocyte progenitors to demyelination in EAE. Prog. Brain Res. 2001;132:165–174. doi: 10.1016/s0079-6123(01)32073-3. [DOI] [PubMed] [Google Scholar]
- 178.Crawford A.H., Stockley J.H., Tripathi R.B., Richardson W.D., Franklin R.J.M. Oligodendrocyte progenitors: adult stem cells of the central nervous system? Exp. Neurol. 2014;260:50–55. doi: 10.1016/j.expneurol.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 179.Takaura N., Yagi T., Maeda M., Nanba E., Oshima A., Suzuki Y., Yamano T., Tanaka A. Attenuation of ganglioside GM1 accumulation in the brain of GM1 gangliosidosis mice by neonatal intravenous gene transfer. Gene Ther. 2003;10:1487–1493. doi: 10.1038/sj.gt.3302033. [DOI] [PubMed] [Google Scholar]
- 180.Gray-Edwards H.L., Maguire A.S., Salibi N., Ellis L.E., Voss T.L., Diffie E.B., Koehler J., Randle A.N., Taylor A.R., Brunson B.L., et al. 7T MRI predicts amelioration of neurodegeneration in the brain after AAV gene therapy. Mol. Ther. Methods Clin. Dev. 2020;17:258–270. doi: 10.1016/j.omtm.2019.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Miyake N., Miyake K., Sakai A., Yamamoto M., Suzuki H., Shimada T. Treatment of adult metachromatic leukodystrophy model mice using intrathecal administration of type 9 AAV vector encoding arylsulfatase A. Sci. Rep. 2021;11:20513. doi: 10.1038/s41598-021-99979-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Johnson T.B., White K.A., Brudvig J.J., Cain J.T., Langin L., Pratt M.A., Booth C.D., Timm D.J., Davis S.S., Meyerink B., et al. AAV9 gene therapy increases lifespan and treats pathological and behavioral abnormalities in a mouse model of CLN8-batten disease. Mol. Ther. 2021;29:162–175. doi: 10.1016/j.ymthe.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hu H., Mosca R., Gomero E., van de Vlekkert D., Campos Y., Fremuth L.E., Brown S.A., Weesner J.A., Annunziata I., d'Azzo A. AAV-mediated gene therapy for galactosialidosis: a long-term safety and efficacy study. Mol. Ther. Methods Clin. Dev. 2021;23:644–658. doi: 10.1016/j.omtm.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Harbison C.E., Weichert W.S., Gurda B.L., Chiorini J.A., Agbandje-McKenna M., Parrish C.R. Examining the cross-reactivity and neutralization mechanisms of a panel of mAbs against adeno-associated virus serotypes 1 and 5. J. Gen. Virol. 2012;93(Pt 2):347–355. doi: 10.1099/vir.0.035113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Thwaite R., Pagès G., Chillón M., Bosch A. AAVrh.10 immunogenicity in mice and humans. Relevance of antibody cross-reactivity in human gene therapy. Gene Ther. 2015;22:196–201. doi: 10.1038/gt.2014.103. [DOI] [PubMed] [Google Scholar]
- 186.Xiao W., Chirmule N., Berta S.C., McCullough B., Gao G., Wilson J.M. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Halbert C.L., Rutledge E.A., Allen J.M., Russell D.W., Miller A.D. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rivière C., Danos O., Douar A.M. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13:1300–1308. doi: 10.1038/sj.gt.3302766. [DOI] [PubMed] [Google Scholar]
- 189.Nathwani A.C., Gray J.T., McIntosh J., Ng C.Y.C., Zhou J., Spence Y., Cochrane M., Gray E., Tuddenham E.G.D., Davidoff A.M. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Majowicz A., Salas D., Zabaleta N., Rodríguez-Garcia E., González-Aseguinolaza G., Petry H., Ferreira V. Successful repeated hepatic gene delivery in mice and non-human primates achieved by sequential administration of AAV5(ch) and AAV1. Mol. Ther. 2017;25:1831–1842. doi: 10.1016/j.ymthe.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sun J., Hua B., Chen X., Samulski R.J., Li C. Gene delivery of activated factor VII using alternative adeno-associated virus serotype improves hemostasis in hemophiliac mice with FVIII inhibitors and adeno-associated virus neutralizing antibodies. Hum. Gene Ther. 2017;28:654–666. doi: 10.1089/hum.2017.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Corti M., Elder M., Falk D., Lawson L., Smith B., Nayak S., Conlon T., Clément N., Erger K., Lavassani E., et al. B-cell depletion is protective against anti-AAV capsid immune response: a human subject case study. Mol. Ther. Methods Clin. Dev. 2014;1:14033. doi: 10.1038/mtm.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Monteilhet V., Saheb S., Boutin S., Leborgne C., Veron P., Montus M.F., Moullier P., Benveniste O., Masurier C. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol. Ther. 2011;19:2084–2091. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Greig J.A., Wang Q., Reicherter A.L., Chen S.J., Hanlon A.L., Tipper C.H., Clark K.R., Wadsworth S., Wang L., Wilson J.M. Characterization of adeno-associated viral vector-mediated human factor VIII gene therapy in hemophilia A mice. Hum. Gene Ther. 2017;28:392–402. doi: 10.1089/hum.2016.128. [DOI] [PubMed] [Google Scholar]
- 195.Perez B.A., Shutterly A., Chan Y.K., Byrne B.J., Corti M. Management of neuroinflammatory responses to AAV-mediated gene therapies for neurodegenerative diseases. Brain Sci. 2020;10:E119. doi: 10.3390/brainsci10020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hordeaux J., Buza E.L., Dyer C., Goode T., Mitchell T.W., Richman L., Denton N., Hinderer C., Katz N., Schmid R., et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum. Gene Ther. 2020;31:808–818. doi: 10.1089/hum.2020.167. [DOI] [PubMed] [Google Scholar]
- 197.Donsante A., Miller D.G., Li Y., Vogler C., Brunt E.M., Russell D.W., Sands M.S. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 198.Dalwadi D.A., Torrens L., Abril-Fornaguera J., Pinyol R., Willoughby C., Posey J., Llovet J.M., Lanciault C., Russell D.W., Grompe M., Naugler W.E. Liver injury increases the incidence of HCC following AAV gene therapy in mice. Mol. Ther. 2021;29:680–690. doi: 10.1016/j.ymthe.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Knudson A.G., Jr. Mutation and cancer: statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Adewoye A.B., Lindsay S.J., Dubrova Y.E., Hurles M.E. The genome-wide effects of ionizing radiation on mutation induction in the mammalian germline. Nat. Commun. 2015;6:6684. doi: 10.1038/ncomms7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Koybasi S., Senkal C.E., Sundararaj K., Spassieva S., Bielawski J., Osta W., Day T.A., Jiang J.C., Jazwinski S.M., Hannun Y.A., et al. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J. Biol. Chem. 2004;279:44311–44319. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- 202.Yang J., Tian Y., Zheng R., Li L., Qiu F. Endocannabinoid system and the expression of endogenous ceramides in human hepatocellular carcinoma. Oncol. Lett. 2019;18:1530–1538. doi: 10.3892/ol.2019.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Powell S.K., Khan N., Parker C.L., Samulski R.J., Matsushima G., Gray S.J., McCown T.J. Characterization of a novel adeno-associated viral vector with preferential oligodendrocyte tropism. Gene Ther. 2016;23:807–814. doi: 10.1038/gt.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen H., McCarty D.M., Bruce A.T., Suzuki K. Oligodendrocyte-specific gene expression in mouse brain: use of a myelin-forming cell type-specific promoter in an adeno-associated virus. J. Neurosci. Res. 1999;55:504–513. doi: 10.1002/(SICI)1097-4547(19990215)55:4<504::AID-JNR10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 205.Rafi M.A., Rao H.Z., Luzi P., Luddi A., Curtis M.T., Wenger D.A. Intravenous injection of AAVrh10-GALC after the neonatal period in twitcher mice results in significant expression in the central and peripheral nervous systems and improvement of clinical features. Mol. Genet. Metab. 2015;114:459–466. doi: 10.1016/j.ymgme.2014.12.300. [DOI] [PubMed] [Google Scholar]
- 206.Faverio P., Fumagalli A., Conti S., Madotto F., Bini F., Harari S., Mondoni M., Oggionni T., Barisione E., Ceruti P., et al. Nutritional assessment in idiopathic pulmonary fibrosis: a prospective multicentre study. ERJ Open Res. 2022;8 doi: 10.1183/23120541.00443-2021. 00443-02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shaimardanova A.A., Chulpanova D.S., Solovyeva V.V., Mullagulova A.I., Kitaeva K.V., Allegrucci C., Rizvanov A.A. Metachromatic leukodystrophy: diagnosis, modeling, and treatment approaches. Front. Med. 2020;7:576221. doi: 10.3389/fmed.2020.576221. [DOI] [PMC free article] [PubMed] [Google Scholar]





