Highlights
-
•
The role of the bull in infectious cattle infertility seems to be underestimate.
-
•
There are pathogens barely studied in bulls and their venereal transmission.
-
•
The bacterium most frequently described was Campylobacter fetus.
-
•
The main parasite described was Tritrichomonas foetus.
-
•
The main virus described were BoHV1 and BVDV.
Keywords: Cattle infertility, Breeding bulls, Systematic review, Infectious infertility
Abstract
Numerous pathogens affect cow fertility. Nevertheless, little information has been published about microorganisms associated with cattle infertility focusing on bulls. The present review offers a current analysis and highlights potential key aspects on the relevance of bulls in the emergence of infertility problems of infectious origin within herds that are still not completely determined. The present systematic review was conducted using the PubMed, Web of Science, and Scopus databases on December 9, 2022. In total, 2,224 bibliographic records were reviewed and, according to strict inclusion criteria, 38 articles were selected from 1966 to 2022, from which we ranked more than 27 different microorganisms (fungi were not identified). The most cited pathogens were BoHV (described by 26.3% of the papers), Campylobacter fetus (23.7%), Tritrichomonas foetus (18.4%), and BVDV, Ureaplasma spp., and Mycoplasma spp. (10.5% each). Despite the general trend towards an increasing number of publications about bull-infertility problems, a number of pathogens potentially transmitted through both natural breeding and seminal doses given to females and associated with infertility within herds were not ranked in the study (e.g., Chlamydia spp.). This work highlights i) the need to clearly establish the role of certain microorganisms not traditionally associated with reproductive problems in bull infertility (e.g., Staphylococcus spp. or BoHV-4) and ii) the need to perform additional studies on breeding bulls to clarify their role in infertility problems within herds. This would allow monitoring for pathogens that have gone unnoticed and those that are fastidious to diagnose and/or potentially transmitted to females.
1. Introduction
The global population is rapidly increasing. The United Nations and FAO estimate that the world population will reach 9.8 billion people in 2050, necessitating an increase in global food production of 70% (Le Mouël & Forslund, 2017). Such an increase in food production will require an improvement in livestock management, which is inherently accompanied by a multitude of factors, such as better fodder manufacturing, decreasing gas emissions and waste, and enhanced longevity, health, and fertility within herds (European Union, 2013). Indeed, cattle infertility is an important economic factor in cattle farming (Bellows, Ott & Bellows, 2002; European Union, 2013; Kastelic, 2013), which is interpreted in the livestock sector as a decreased number of calves weaned per year per cow (Titterington et al., 2017) and infectious infertility understood as those infectious that cause a decrease in the number of pregnancies and successful births in a herd (Givens, 2006).
Numerous infectious agents can affect cattle reproduction at different levels with different symptoms (Yoo, 2010), negatively affecting the fertility of females due to their inability to become pregnant and successfully carry the pregnancy to term (Adnane & Chapwanya, 2022; Moore et al., 2021). In addition, several pathogens may have a negative impact on the fertility of bulls (Givens & Marley, 2008). Furthermore, there are microorganisms that apparently do not affect the fertility of bulls but that can be transmitted to females, in which they cause reproductive problems (Givens, 2018). For example, Campylobacter fetus subsp. venerealis causes a bacterial infection in bulls traditionally considered to be asymptomatic that can be transmitted to females, where it translates into a diversity of reproductive problems, such as irregular estrus and early embryonic and fetal mortality (Michi et al., 2016). Similarly, opportunistic pathogens ubiquitously present in the environment or as part of the animal microbiota can be found associated with bovine infertility, e.g., fungal species within the genera Aspergillus, Mucor, or Candida (Perumal et al., 2013) or bacteria such as Ureaplasma diversum (Buzinhani et al., 2011). In addition, several pathogens linked to cattle infertility are zoonotic and may pose a risk to public health (Yoo, 2010).
Males play an important role in infectious cattle infertility within the herd in two ways: directly (by which pathogens affect the reproductive potential of bulls or sperm quality (Carli et al., 2022; Givens, 2018)) or indirectly (by which microorganisms are transmitted from males to females through natural breeding and artificial insemination but are potentially asymptomatic for bulls (Carli et al., 2022; Givens, 2018; Michi et al., 2016)). Consequently, the impact of certain microorganisms potentially present in bulls on fertility must be considered to avoid problems such as delayed conception in females, which prolongs the calving season and increases the number of culled animals, resulting in a reduction in the efficiency of production and, consequently, economic losses (Carli et al., 2022; Kastelic, 2013). Indeed, certain authors have suggested that low fertility in extensive herds is probably due to the fact that the importance of the bull has been overlooked, for example, in terms of the importance that infectious diseases, such as bovine diarrhea (caused by BVDV) and bovine rhinotracheitis (caused by BoHV-1), may play in the low fertility rates of female herds, in which these etiological agents could be transmitted from males (Montoya-Monsalve et al., 2021). For these reasons, it is necessary to clarify the role of bulls in infertility problems within a herd, particularly concerning pathogens that directly affect the reproductive potential of bulls. In this context, the detection and monitoring of infectious agents in bulls associated with cattle infertility has become a key element in establishing accurate diagnoses and, consequently, appropriate disease prevention management. There are various techniques for the detection of microorganisms, from cultures to advanced molecular analysis based on massive sequencing. Techniques based on massive sequencing may provide an overall picture of all known microorganism, e.g., bacterial populations (Cojkic et al., 2021; Polo et al., 2022) present in clinical samples, providing extensive knowledge of the infectious agents potentially associated with cattle infertility.
Despite the information published in journals and scientific databases about bull infertility, for example the work of Givens and Marley (2008), no systematic analysis has been published about infectious agents associated with cattle infertility focusing on the role of the bull. In this context, this study provides a detailed bibliographical analysis of the available information on this subject and establishes a powerful tool for analyzing the relevance of various potential etiological agents of infertility, ranking the main pathogens that cause fertility problems in bulls. In addition, certain pathogens that may be transmitted to females (traditionally considered asymptomatic in males) that cause reproductive problems were also included in the ranking. Finally, evaluation of the information about coinfections and the techniques used for diagnosis in bulls over time are also evaluated.
2. Materials and methods
2.1. Information collection
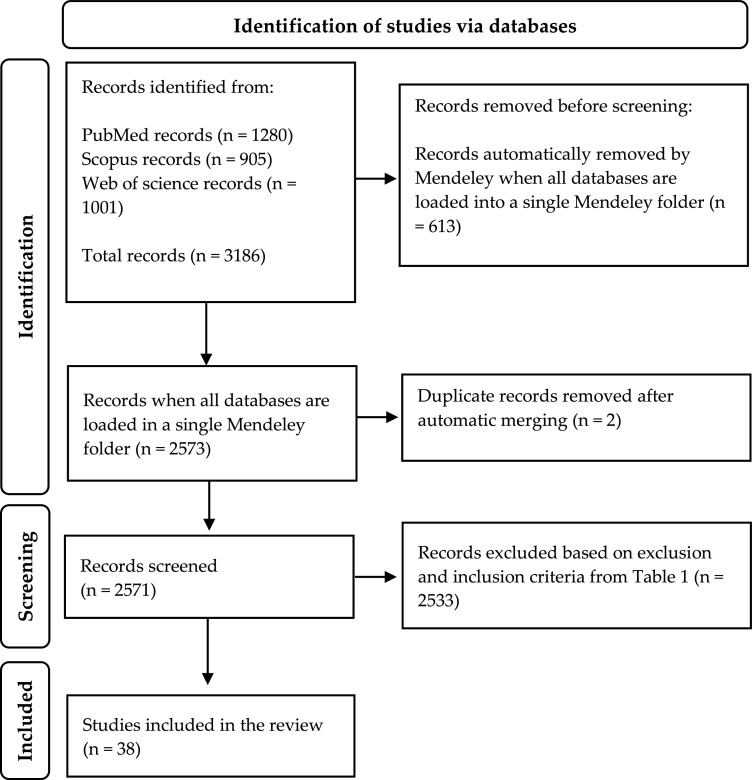
We conducted a systematic review of the literature using the PubMed, Web of Science (WOS), and Scopus databases on December 9, 2022. The search strings used for each database were: i) for the PubMed database ‘((“Infection”[Mesh]) OR “Reproductive Tract Infections”[Mesh]) AND “Fertility”[Mesh]) OR “Infertility”[Mesh]) AND “Cattle”[Mesh]’; ii) for the Scopus database ‘TITLE-ABS-KEY ((“Infection” AND (“Fertility” OR “Infertility”) AND “Cattle”))’; and iii) for the Web Of Science database ‘TS=((“Infection” AND (“Fertility” OR “Infertility”) AND “Cattle”))’. Using these strings, a total of 3186 bibliographic records were imported into the bibliographic manager Mendeley Desktop 1.19.8 software in such a way that 613 records were automatically removed (Fig. 1). In total, 2224 records were independently screened using strict inclusion and exclusion criteria (Table 1) to select articles focusing on bull fertility (or those including mixed herds in which bulls were assessed). According to these criteria, studies carried out exclusively in female herds or those carried out on both males and females, but for which the results were not sex-defined, were excluded (Table 1). Finally, 38 articles were included in the present study.
Fig. 1.
PRISMA flowchart of literature for the selection of papers included in the current study about bull infertility due to infectious causes.
Table 1.
Publication criteria selection.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Original work | Reviews, letters, editorials, conference abstracts, and vaccination trials |
| Field/experimental studies: diagnosis, prevalence, and epidemiology | Lab/bench studies (basic research) |
| Studies performed on samples from farms or slaughterhouses | All other locations |
| Direct samples from animals | Others, such as surfaces, food, water, etc. |
| Infectious causes related to infertility | Other causes related to infertility (e.g., nutritional deficiencies (Kenny & Byrne, 2018)) |
| Studies performed in herds of males or both males and females (in which bulls were analyzed) | Study performed exclusively in female herds or mixed herds in which the results were not sex-defined were excluded |
| English language | Other languages |
2.2. Information processing
The extracted information was collected in an Excel spreadsheet to standardize the process with a description of the following variables: reference; country in which the study took place and year of data publication, pathogens identified, and diagnostic tests used to evaluate the presence of microorganisms. The following collected variables were not further evaluated due to the low number of papers that detailed these points: production type, breed, feeding, age, type of sample, fertility ratio, and statistical tests.
2.3. Database management
The data search in the Excel spreadsheet resulting from the bibliographic information processing was carried out using tools integrated in Microsoft Excel 2016 and the R programming language (R studio 4.1.2 software).
3. Results and discussion
3.1. Ranking of identified microorganisms
Overall, information about more than 3000 bulls from 38 publications from 1966 to 2022 were included in this analysis. It is important to consider that the present review was focused on infectious causes of bull infertility, although several studies derived from studies carried out on mixed herds (cows + bulls, in which bulls were analyzed) with fertility problems were also included. The highly strict search parameters (through the search strings indicated in section ‘2.1. Information collection’ focused on infertility), and the selection criteria (indicated in Table 1) may have excluded certain studies, for example, those exclusively focused on the study of pathogens that cause infertility problems in females that can be transmitted by bulls. All the pathogens associated with infertility in bulls are summarized in Table 2, which also summarizes the impact of these microorganisms on bull infertility. Eighteen publications (47.4% of all papers) identified bacteria as the causative agent of infertility, 15 (39.5%) identified viruses, 11 (29%) identified parasites, and none identified a fungus. The set of 38 selected papers described 18 species and six genera of bacteria, five species of viruses, and four species of parasites. Our review identified microorganism not traditionally associated with cattle infertility, e.g. BoHV-5 (Marin et al., 2020) (Table 2). Thus, it is necessary to investigate the true impact of these microorganisms, which may act as primary or secondary agents, on bull infertility and the potential role of bulls as carriers in certain cases.
Table 2.
Pathogens described within cattle herds with reproduction failure by the 38 papers included in the current study. Indicated from left to right: Pathogen; the effect of the pathogen infection in bull fertility and whether it is able to be transmitted to females (T) through both natural breeding (NB) and/or seminal doses (AI) in which it is associated with reproductive problems in cows;%, percentage of total papers in which the pathogen has been described; Zoonotic; Classification: for bacteria, whether they are gram-positive/negative or without a cell wall is indicated; for parasites, the kingdom or infrakingdom is indicated; and for viruses, the Baltimore classification (Baltimore, 1971) is indicated.
| Pathogen | Effect in bull fertility | T | % | Zoonotic | Classification | |
|---|---|---|---|---|---|---|
| Bovine herpesvirus (BoHV) | 26.3 | No | virus (Group I: dsDNA) | |||
| BoHV serovar 1 | Affects sperm quality due to the weakness of the infected bull (Givens, 2018). Nevertheless, the presence of the virus in bull semen appears to decrease sperm concentration, viability, and motility and increase sperm abnormalities (El-Mohamady et al., 2020). | NB, AI (Givens, 2018) | 21.1 | No | virus (Group I: dsDNA) | |
| BoHV serovar 4 | * | * | 5.3 | No | virus (Group I: dsDNA) | |
| BoHV serovar 5 | * | * | 2.6 | No | virus (Group I: dsDNA) | |
| Campylobacter spp. | 23.7 | Yes | bacteria (gram-negative) | |||
| Campylobacter fetus | 23.7 | Yes** | bacteria (gram-negative) | |||
| Campylobacter fetus subp. fetus | In experimental work, the bacteria is able to irreversibly bind to bull spermatozoa and negatively affect sperm quality, altering the structure and functionality of the sperm plasma membrane (Cagnoli et al., 2020). | * | 10.5 | Yes | bacteria (gram-negative) | |
| Campylobacter fetus subp. veneralis | In experimental work, the bacteria is able to irreversibly bind to bull spermatozoa and negatively affect sperm quality, altering the structure and functionality of the sperm plasma membrane (Cagnoli et al., 2020). | NB, AI (Givens, 2018) | 10.5 | Yes | bacteria (gram-negative) | |
| Campylobacter jejuni | * | * | 2.6 | Yes | bacteria (gram-negative) | |
| Campylobacter cryaerophilus | * | * | 2.6 | Yes | bacteria (gram-negative) | |
| Tritrichomonas foetus | Appears to be able to adhere to sperm, decreasing sperm quality, damaging the sperm cell, and causing its death (Benchimol et al., 2008). | NB, AI (Givens, 2018) | 18.4 | Yes | parasite (Protista) | |
| Bovine viral diarrhea virus | The virus can infect the testicles, where it negatively affects sperm quality, decreasing sperm concentration, viability, and motility and increasing sperm abnormalities (El-Mohamady et al., 2020). | NB, AI (Givens, 2018) | 10.5 | No | virus (Group IV: +ssRNA) | |
| Mycoplasma spp. | 10.5 | No | bacteria (no cell wall) | |||
| Mycoplasma bovigenitalium | In experimental work, the bacteria is able to infect the upper genital tract of bulls where it appears to decrease sperm motility. (Panangala et al., 1981).* | * | 2.6 | No | bacteria (no cell wall) | |
| Mycoplasma suis | * | * | 2.6 | No | bacteria (no cell wall) | |
| Mycoplasma wenyonii | * | * | 2.6 | No | bacteria (no cell wall) | |
| Mycoplasma bovis | * | NB, AI* (Dudek et al., 2020) | 2.6 | No | bacteria (no cell wall) | |
| Ureaplasma spp. | 10.5 | Yes** | bacteria (no cell wall) | |||
| Ureaplasma diversum | The bacteria can infect the testicles where it negatively affects sperm quality through morphological and functional changes of the sperm (Santos Junior et al., 2021). | NB, AI (Givens, 2018) | 7.9 | No | bacteria (no cell wall) | |
| Besnoitia besnoiti | The parasite can produce severe alterations in the reproductive tract, causing permanent infertility (Cortes et al., 2005). | * | 5.3 | No | parasite (Alveolata) | |
| Coxiella burnetii | * | NB, AI (Givens, 2018) | 2.6 | Yes | bacteria (gram-negative) | |
| Neospora caninum | Appears to not affect sperm quality (Kemel et al., 2022; van Velsen, 2021). | NB* (Givens, 2018; van Velsen, 2021) | 2.6 | Potential | parasite (Alveolata) | |
| Bacillus spp. | * | * | 2.6 | No | bacteria (gram-positive) | |
| Mycobacterium avium subp. paratuberculosis | * | NB, AI (Givens, 2018) | 2.6 | bacteria (gram-positive) | ||
| Staphylococcus spp. | Appears to decrease sperm motility (Ďuračka et al., 2021). | NB, AI* (Cojkic et al., 2021) | 2.6 | Yes** | bacteria (gram-positive) | |
| Bluetongue virus | Serotype 8 decreases the motility of sperm and increases the percentage of sperm with morphological abnormalities (De Clercq et al., 2021). | NB, AI (Givens, 2018) | 2.6 | No | virus (Group III: dsRNA) | |
| Bovine enterovirus serotype I | * | * | 2.6 | Potential | virus (Group IV: +ssRNA) | |
| Corynebacterium pyogenes | * | * | 2.6 | Yes | bacteria (gram-positive) | |
| Fasciola spp. | * | * | 2.6 | Yes** | parasite (Animalia) | |
| Helicobacter cinaedi | * | * | 2.6 | Potential | bacteria (gram-negative) | |
| Parainfluenza III Virus | * | * | 2.6 | Potential | virus (Group V: -ssRNA) | |
| Streptococcus spp. | * | NB, AI* (Cojkic et al., 2021) | 2.6 | Yes** | bacteria (gram-positive) | |
| Trueperella pyogenes | * | * | 2.6 | Yes | bacteria (gram-positive) | |
* Not clarified. ** Some species within the genus are zoonotic pathogens.
3.1.1. Bacteria
Campylobacter spp. was identified in 23.7% of the papers. All papers that described Campylobacter spp. identified the species C. fetus, although other species were also described: C. jejuni and the related bacteria Arcobacter cryaerophilus (McFadden et al., 2004). Concerning C. fetus species, two subspecies, i) C. fetus subsp. venerealis and ii) C. fetus subsp. fetus, were identified in the same proportion of papers (10.5%). C. fetus subsp. venerealis is responsible for bovine genital campylobacteriosis, a venereal disease of cattle that is frequently asymptomatic for males and that can be infective for humans (Wagenaar et al., 2014). It is considered to be one of the relevant causes of reproductive failure in cows in many countries, especially when natural breeding is practiced (Balzan et al., 2020; OIE, 2021; Pena-Fernández et al., 2021). On the contrary, C. fetus subsp. fetus is described as a sporadic pathogen in the reproductive tract of cattle, where it may cause infertility (OIE, 2021). It can also be isolated from humans (Holst et al., 1987). According to our results (Table 2), the potential role of C. fetus subsp. fetus as an etiological agent of infertility within herds may be underestimated, as previously suggested (Polo et al., 2021).
Finally, both subspecies are clearly related to reproductive failure in females (Michi et al., 2016; Mshelia et al., 2010) and the recent study of Cagnoli et al. observed how both C. fetus subspecies can negatively affect the sperm quality of bulls in experimental studies (Cagnoli et al., 2020), which would mean that C. fetus may also affect the fertility of the bull.
In addition, it is important to highlight that the identification of C. fetus at the subspecies level by techniques commonly used in diagnostic laboratories may be hampered by the limitations of certain techniques, such as culture or PCR (Chaban et al., 2013; Polo et al., 2021), making it difficult to establish the true role of C. fetus subsp. fetus and C. fetus subsp. venerealis in relation to low fertility rates in bulls.
Other bacteria identified in a lower percentage of publications included Mycoplasma spp. (identified by 10.5%), in which the species M. bovigenitalium, M. suis, M. wenyonii, and M. bovis were described (in our ranking, Table 2). Mycoplasma spp. are already known to be associated with potential reproductive problems in bulls (Parker et al., 2018), whereas M. bovigenitalium and M. bovis appear to be carried by breeding bulls (Carli et al., 2022; Dudek et al., 2020). In females, infections by Mycoplasma spp. translates onto reproductive disorders, such as vulvovaginitis, endometritis, dystocia (Parker et al., 2018), earlier return to estrus, and sporadic abortion (Carli et al., 2022), whereas the pathogenesis in bulls has been less studied (Carli et al., 2022) and the absence of symptoms could be common (Parker et al., 2018). Ureaplasma spp. were also identified (10.5% of the publications), in which U. diversum was the only described species (identified in 7.9% of the publications) able to adversely affect the fertility of males (Buzinhani et al., 2011; Hobson et al., 2013). Infections in bulls can induce seminal vesiculitis, balanoposthitis, epididymitis, and other pathologies caused by morphological and functional changes in sperm (Santos Junior et al., 2021). Other bacteria are also identified in our ranking list in a lower percentage of papers (summarized in Table 2).
3.1.2. Viruses
Two predominant viruses were noted: i) bovine herpesvirus (BoHV) (identified in 26.3% of the publications) and ii) bovine viral diarrhea virus (BVDV) (identified in 10.5% of the publications). BoHV and BVDV are viruses that cause a wide variety of reproductive syndromes (Newcomer & Givens, 2016). Three serovars were identified in the current analysis: BoHV serovars 1 (BoHV-1), 4 (BoHV-4), and 5 (BoHV-5). According to our review, BoHV-1 was identified in 21.1% of the papers that described BoHV, BoHV-4 by 5.3%, and BoHV-5 by 2.6%.
BoHV-1 is a ubiquitous microorganism and can remain latent for long periods of time in cattle populations, in which infections appear to affect the sperm quality of bulls, decreasing the sperm concentration, viability, and motility and increasing sperm abnormalities (El-Mohamady et al., 2020) (Table 2). In females, BoHV-1 induces abortions at the end of embryonic development or newborn death during the first week of life (Newcomer & Givens, 2016). The virus can be transmitted by males, including through artificial insemination (Givens, 2018). For BoHV-4, there is a strong correlation of virus infection with postpartum metritis and abortion (Kruger et al., 2015), whereas BoHV-5 is responsible for meningoencephalitis in calves (Marin et al., 2020). There is a lack of information concerning the exact role of BoHV-4 and BoHV-5 in bull infertility or their transmission to females by natural breeding or artificial insemination (Table 2), despite the fact that they were described as the cause of infertility in certain papers (Aslan et al., 2015). Similarly to BoHV-1, BVDV infections result in a reduction in sperm density and motility and an increase in sperm abnormalities in males (El-Mohamady et al., 2020) (Table 2). In females, these infections cause abortions (Grooms, 2004). The virus can be transmitted by males, including through artificial insemination (Givens, 2018).
3.1.3. Parasites
There is a remarkable predominance of the species Tritrichomonas foetus (identified in 18.4% of the publications). T. foetus causes trichomoniasis in cattle and is a traditionally considered agent of asymptomatic long-term infections in bulls. Nevertheless, Benchimol et al. showed that T. foetus is able to adhere to and damage sperm cells in vitro, causing their death (Benchimol et al., 2008), which suggests that this parasite could be a potential etiological agent of fertility problems in bulls. In females, this protozoan is responsible for genital inflammation and embryonic death (Michi et al., 2016). The major ranked microorganisms (Table 2) are present in most continents (data not shown) and are therefore of worldwide relevance. Other parasites are described in a smaller proportion of papers, such as Neospora caninum (2.6%) (Table 2). In bulls, infections by N. caninum appear to affect sperm viability and motility, but this is still debated and the negative affect on fertility is not clear due to some naturally infected bulls showing high conception rates (van Velsen, 2021).
3.1.4. Fungi
We found no fungi associated with infertility in bulls in the publications included in this study, despite fungi sometimes being present in bull semen (Joya et al., 2011). It is known that fungi can cause fertility disturbances in cattle (Mingoas et al., 2009). For example, Aspergillus fumigatus and Candida spp. cause abortions and other reproduction problems in females (Foley & Schlafer, 1987; Henker et al., 2022; Yoo, 2010). Although knowledge concerning the pathogenesis of bacteria, parasites, and viruses in cattle is generally extensive, data related to the pathogenesis of fungal infections are limited. A low number of fungi are sufficiently virulent to be considered primary pathogens in immunocompetent individuals. Nonetheless, opportunistic pathogenic infections are mainly due to periods of immune deficiency of the animal or because the protective barriers of the skin and mucous membranes have been altered (Dixon et al., 1996; Seyedmousavi et al., 2018). Fungal infections are indeed rare events that can easily go unnoticed or be under-diagnosed and thus they are not present in the current review.
3.2. Time, techniques, and co-infections
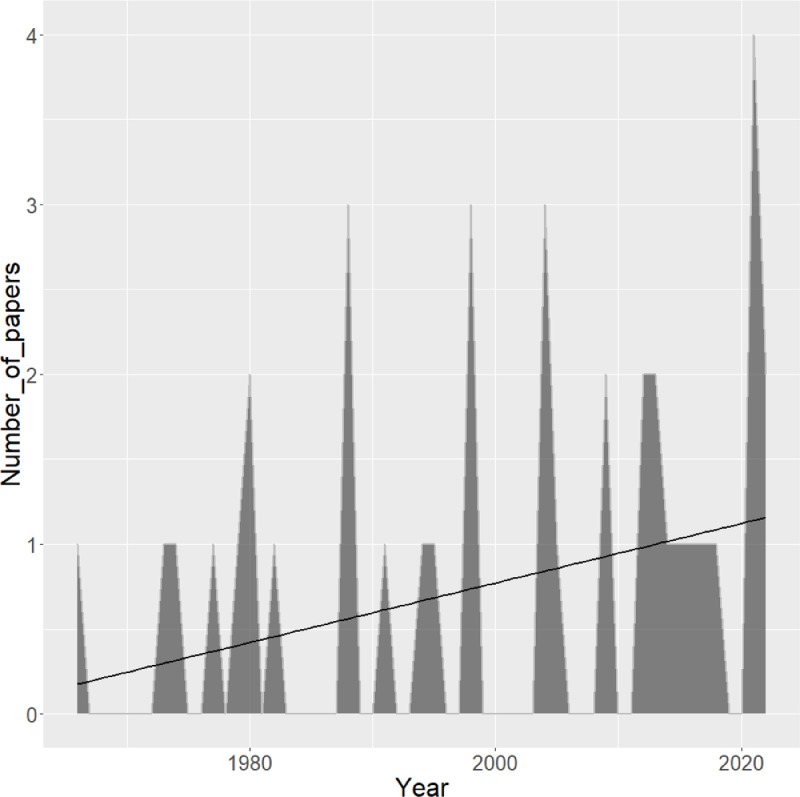
Over time, there has been a general trend towards an increasing number of publications (Fig. 2), indicating a growing concern about the relevance of considering males for the diagnosis, management, and control of infertility problems of infectious origin within bulls and herds composed of both males and females.
Fig. 2.
Temporal representation of the number of papers (Y axis) published per year (X axis). The continuous black line represents the line of regression.
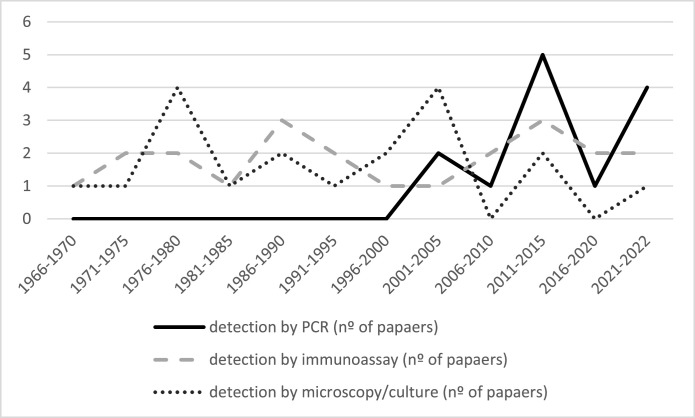
According to our results, the main microorganism detection techniques used have been immunoassays (22 of the 38 selected publications used them) and culture and identification by microscopy as the direct detection technique (19/38) (Fig. 3). Detection by PCR has also been widely used as a direct molecular detection method (13/38) (Fig. 3). There are still situations in which establishing the cause of a reproductive disease or set of symptoms in cows is challenging using conventional techniques. For example, a study by Petit et al. conducted on cervical swabs from cows with reproductive problems indicated that a potential pathogen could be identified through conventional techniques in less than 30% of cases (Petit et al., 2009).
Fig. 3.
Commonly used techniques for microorganism identification. The continuous black line represents the number of papers (X axis) over time (Y axis) that used PCR as the diagnostic tool, the discontinuous gray line refers to indirect methods (different immunoassays), and the dotted black line refers to cultures and direct detection by microscopy.
Massive sequencing allows the simultaneous identification and characterization of known organisms in a sample at a genomic level. Through techniques such as the metagenomic analysis of populations of microorganisms, it is possible to obtain information about the relative abundance of taxa, as well as relate the composition of these populations with certain conditions (Humières et al., 2021). With the implementation of new techniques and increasingly sophisticated data management in the coming years, the number of microorganism known to be associated with infectious cattle infertility will likely increase, as well as information about the composition of the microbiome and the association between microorganisms, including in seminal and preputial (Cojkic et al., 2021; Polo et al., 2022; Wickware et al., 2020) samples from bulls.
As mentioned above, there are many microorganisms of varying nature (from primary pathogens, such as C. fetus, to opportunistic pathogens, such as U. diversum) associated with cattle infertility (Table 2) shown by studies performed in bulls and mixed herds. However, only a small proportion of the selected papers (10/38, 26.3%) identified more than one microorganism as an etiological agent of infertility problems. For example, Carli et al. showed the possible effect of coinfections on fertility, e.g., U. diversum and M. bovis present in 19.5% of bulls with low fertility rates (Carli et al., 2022). However, coinfections and synergy between microorganisms could be more common than expected, as suggested by recent studies, which have characterized microbial populations by massive sequencing. For example, Koziol et al. observed a potential synergy between Campylobacter spp. and Fusobacterium spp. with other microorganisms in bulls with low sperm quality (Koziol et al., 2022). These data highlight the need to carry out more studies about the true infectious causes of bovine infertility focusing on males, given that more than one etiological agent could be involved.
3.3. Key aspects about breeding bulls
One of the most striking aspects of our results (Table 2) is the number of microorganisms identified in bulls with infertility problems by the selected papers but not traditionally considered to be etiological agents in the differential diagnosis of infectious infertility in bulls (e.g., BoHV-4 or Mycoplasma spp.).
Another of our important key findings is the absence of relevant pathogens that cause reproductive failure in females that can be transmitted by males (e.g. Chlamydia spp. (Givens, 2018; Teankum et al., 2007)) (Table 2). This may be due to the string search and inclusion criteria used in the present study, which focused on pathogens that affect bull fertility. The limited information about fertility problems caused by infectious agents in bulls could be associated with the low number of studies focusing on males and the limited information about the epidemiological data on the presence of these microorganisms in bulls, e.g., Chlamydiae (Kauffold et al., 2007). Indeed, there are approximately twice as many studies published in the literature that associate the presence of various microorganisms with fertility problems in females than males (data not shown), likely due to the higher number of females bred than males.
The presence of pathogens related to cattle infertility in one bull may have a more relevant impact on fertility rates at the herd level than pathogens in one cow, as one bull can be used to breed with several females by natural servicing or artificial insemination. Thus, sub-fertile bulls or those carrying a pathogen associated with cow infertility could result in economic losses (Kastelic, 2013) and increase the ecological footprint of cattle production (European Union, 2013). In this scenario, undetected microorganisms in bulls and their seminal doses, especially those that cause asymptomatic infections in bulls (e.g., T. foetus (Yoo, 2010)), have been an important obstacle to the eradication of pathogens that cause a reduction in the number of calves per year from cattle herds.
With this study, we intended to highlight the fact that studies carried out in bull populations from herds with low fertility ratios are scarce, even those concerning recognized microorganisms associated with infertility in males as primary pathogens and/or those transmitted to females (although traditionally considered asymptomatic in bulls). For example, Chlamydia spp. can be transmitted from males to females through semen (Givens, 2018) and cause reproductive problems in females and males (Yoo, 2010). Nevertheless, Chlamydia spp. are missing from our results (Table 2). Other pathogens associated with cattle infertility described in females are also missing in our ranking (Table 2), e.g., H. somni, as well as Enterococcus spp., in which experimental infections in bulls have demonstrated decreased fertility in bulls through the deterioration of sperm quality (Ďuračka et al., 2021). Other microorganisms ranked in the present study are unknown as etiological agents of bull infertility because studies are lacking about their role in bull fertility, such as for BoHV-4 (Table 2). Thus, further studies are required to establish a real-life view of the etiological causes of infertility in bull populations, the economic impact of infected bulls in the livestock sector, and as a fundamental tool to establish the true role of bulls in cattle infertility for the implementation of prevention and control measures that contribute to improve the fertility ratio of herds. In addition, the implementation of new techniques, e.g. metagenomic studies (Cojkic et al., 2021; Polo et al., 2022; Wickware et al., 2020) opens the possibility of studying the importance of pathogens that have been neglected or that are not routinely identified by diagnostic laboratories through conventional techniques such as PCR or culture.
4. Conclusions
Infections that negatively affect the fertility of bulls and, consequently, the fertility of herds and their impact on production, as reflected by the increasing number of publications, appear to be have been of increasing concern over the last several years. Fertility, and the presence of pathogens related to cattle infertility, may be more important in an individual bull than a cow, as one bull can be used to breed with several females. Thus, fertility problems of bulls and their role as pathogen carriers related to cattle infertility translates onto potential economic losses and an increase in the ecological footprint of cattle production. According to our study, a total of six bacterial genera (13 different species), five virus species, and four parasite species have been shown to be associated with infertility in bulls and mixed herds (where bulls were analyzed), in which the most frequently described pathogens were BoHV1, C. fetus, T. foetus, BVDV, Ureaplasma spp., and Mycoplasma spp. These major pathogens associated with infertility have been widely identified throughout the world. There are many species that were described in a lower proportion of papers that have been barely studied in relation to bull infertility and potential transmission to females, e.g., Staphylococcus spp. or Bacillus spp. A number of pathogens known to be associated with infertility in females are missing in our ranking, despite their having been experimentally demonstrated to induce decreased fertility in bulls through the deterioration of sperm quality (e.g., Enterococcus faecium) and/or that they can be transmitted from males to females through both natural breeding and seminal doses (e.g., Candida spp.). This is likely due to the strict selection of publications (focusing on bull infertility problems), the low number of studies focusing on bulls, and the limited information about the epidemiological data on the presence of certain microorganism in bulls (e.g., Chlamydiae), In this scenario, further studies carried out on breeding bulls using next generation techniques for microorganism detection may provide data about the importance of potentially neglected pathogens or those that are not routinely identified by diagnostic laboratories using conventional techniques of relevance for bull fertility and, thus, bovine herd fertility.
Ethical statement
In the present study, only data from previous publications from the Pubmed, Scopus and Web of Science databases were used. No animals were used.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was partially funded by the Region of Madrid (Spain) (IND2018/BIO-9246).
References
- Adnane M., Chapwanya A. A review of the diversity of the genital tract microbiome and implications for fertility of cattle. Animals. 2022;12(4):1–14. doi: 10.3390/ani12040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan M.E., Azkur A.K., Gazyagci S. Epidemiology and genetic characterization of BVDV, BHV-1, BHV-4, BHV-5 and Brucella spp. infections in cattle in turkey. Journal of Veterinary Medical Science. 2015;77(11):1371–1377. doi: 10.1292/jvms.14-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Expression of animal virus genomes. Bacteriological Reviews. 1971;35:235–241. doi: 10.1128/mmbr.35.3.235-241.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzan C., Ziech R.E., Gressler L.T., de Vargas A.P.C. Bovine genital campylobacteriosis: Main features and perspectives for diagnosis and control. Ciencia Rural. 2020;(3):50. doi: 10.1590/0103-8478cr20190272. [DOI] [Google Scholar]
- Bellows D.S., Ott S.L., Bellows R.A. Review: Cost of reproductive diseases and conditions in cattle. The Professional Animal Scientist. 2002;18(1):26–32. doi: 10.15232/S1080-7446(15)31480-7. [DOI] [Google Scholar]
- Benchimol M., De Andrade Rosa I., Da Silva Fontes R., Burla Dias Â.J. Trichomonas adhere and phagocytose sperm cells: Adhesion seems to be a prominent stage during interaction. Parasitology Research. 2008;102(4):597–604. doi: 10.1007/s00436-007-0793-3. [DOI] [PubMed] [Google Scholar]
- Buzinhani M., Yamaguti M., Oliveira R.C., Cortez B.A., Marques L., MacHado-Santelli G.M., Assumpção M.E., Timenetsky J. Invasion of Ureaplasma diversum in bovine spermatozoids. BMC Research Notes. 2011;4:0–7. doi: 10.1186/1756-0500-4-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnoli C.I., Chiapparrone M.L., Cacciato C.S., Rodríguez M.G., Aller J.F., Catena M.del, C. Effects of Campylobacter fetus on bull sperm quality. Microbial Pathogenesis. 2020;149 doi: 10.1016/j.micpath.2020.104486. [DOI] [PubMed] [Google Scholar]
- Carli S.D., Dias M.E., da Silva M.E.R.J., Breyer G.M., Siqueira F.M. Survey of beef bulls in Brazil to assess their role as source of infectious agents related to cow infertility. Journal of Veterinary Diagnostic Investigation. 2022;34(1):54–60. doi: 10.1177/10406387211050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban B., Garcia Guerra A., Hendrick S.H., Waldner C.L., Hill J.E. Isolation rates of Campylobacter fetus subsp venerealis from bovine preputial samples via passive filtration on nonselective medium versus selective medium, with and without transport medium. American Journal of Veterinary Research. 2013;74(8):1066–1069. doi: 10.2460/ajvr.74.8.1066. [DOI] [PubMed] [Google Scholar]
- Cojkic A., Niazi A., Guo Y., Hallap T., Padrik P., Morrell J.M. Article identification of bull semen microbiome by 16S sequencing and possible relationships with fertility. Microorganisms. 2021;9(12):1–12. doi: 10.3390/microorganisms9122431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes H., Leitão A., Vidal R., Vila-Viçosa M.J., Ferreira M.L., Caeiro V., Hjerpe C.A. Besnoitiosis in bulls in Portugal. Veterinary Record. 2005;157(9):262–264. doi: 10.1136/vr.157.9.262. [DOI] [PubMed] [Google Scholar]
- De Clercq K., Vandaele L., Vanbinst T., Riou M., Deblauwe I., Wesselingh W.…De Leeuw I. Transmission of bluetongue virus serotype 8 by artificial insemination with frozen–thawed semen from naturally infected bulls. Viruses. 2021;13(4):652. doi: 10.3390/v13040652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D.M., McNeil M.M., Cohen M.L., Gellin B.G., La Montagne J.R. Fungal infections: A growing threat. Public Health Reports (Washington, D.C.: 1974. 1996;111(3):226–235. [PMC free article] [PubMed] [Google Scholar]
- Dudek K., Nicholas R.A.J., Szacawa E., Bednarek D. Mycoplasma bovis infections—Occurrence, diagnosis and control. Pathogens (Basel, Switzerland) 2020;9(8):1–21. doi: 10.3390/pathogens9080640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ďuračka M., Belić L., Tokárová K., Žiarovská J., Kačániová M., Lukáč N., Tvrdá E. Bacterial communities in bovine ejaculates and their impact on the semen quality. Systems Biology in Reproductive Medicine. 2021;67(6):438–449. doi: 10.1080/19396368.2021.1958028. [DOI] [PubMed] [Google Scholar]
- Ďuračka M., Galovičová L., Kačániová M., Lukáč N., Tvrdá E. The effect of Enterococcus faecium -Induced in vitro infection in bovine semen. Animal Science and Biotechnologies. 2021;54(1):80–85. [Google Scholar]
- El-Mohamady R.S., Behour T.S., Rawash Z.M. Concurrent detection of bovine viral diarrhoea virus and bovine herpesvirus-1 in bulls’ semen and their effect on semen quality. International Journal of Veterinary Science and Medicine. 2020;8(1):106–114. doi: 10.1080/23144599.2020.1850197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Union . European commission; 2013. Animal production: A decade of production research. [Google Scholar]
- Foley G.L., Schlafer D.H. Candida abortion in cattle. Veterinary Pathology. 1987;24(6):532–536. doi: 10.1177/030098588702400610. [DOI] [PubMed] [Google Scholar]
- Givens M.D. A clinical, evidence-based approach to infectious causes of infertility in beef cattle. Theriogenology. 2006;66(3):648–654. doi: 10.1016/j.theriogenology.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Givens M.D. Review: Risks of disease transmission through semen in cattle. Animal: An International Journal of Animal Bioscience. 2018;12(s1):s165–s171. doi: 10.1017/S1751731118000708. [DOI] [PubMed] [Google Scholar]
- Givens M.D., Marley M.S.D. Pathogens that cause infertility of bulls or transmission via semen. Theriogenology. 2008;70(3):504–507. doi: 10.1016/j.theriogenology.2008.05.033. [DOI] [PubMed] [Google Scholar]
- Grooms D.L. Reproductive consequences of infection with bovine viral diarrhea virus. The Veterinary Clinics of North America. Food Animal Practice. 2004;20(1):5–19. doi: 10.1016/j.cvfa.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Henker L.C., Lorenzett M.P., Lopes B.C., Dos Santos I.R., Bandinelli M.B., Bassuino D.M., Juffo G.D., Antoniassi N.A.B., Pescador C.A., Sonne L., Driemeier D., Pavarini S.P. Pathological and etiological characterization of cases of bovine abortion due to sporadic bacterial and mycotic infections. Brazilian Journal of Microbiology : [Publication of the Brazilian Society for Microbiology] 2022;53(4):2251–2262. doi: 10.1007/s42770-022-00853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson N., Chousalkar K.K., Chenoweth P.J. Ureaplasma diversum in bull semen in Australia: Its detection and potential effects. Australian Veterinary Journal. 2013;91(11):469–473. doi: 10.1111/avj.12113. [DOI] [PubMed] [Google Scholar]
- Holst E., Wathne B., Hovelius B., Mårdh P.A. Bacterial vaginosis: Microbiological and clinical findings. European Journal of Clinical Microbiology. 1987;6(5):536–541. doi: 10.1007/BF02014242. [DOI] [PubMed] [Google Scholar]
- Humières C., Salmona M., Dellière S., Leo S., Rodriguez C., Angebault C., Alanio A., Fourati S., Lazarevic V., Woerther P.L. The potential role of clinical metagenomics in infectious diseases : Therapeutic perspectives. Drugs. 2021;81(13):1453–1466. doi: 10.1007/s40265-021-01572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joya M.A.P., Góngora A., Jiménez C. Infectious agents affecting fertility of bulls, and transmission risk through semen. Retrospective analysis of their sanitary status in Colombia. Revista Colombiana de Ciencias Pecuarias. 2011;24(4):634–646. [Google Scholar]
- Kastelic J.P. Male involvement in fertility and factors affecting semen quality in bulls. Animal Frontiers. 2013;3(4):20–25. doi: 10.2527/af.2013-0029. [DOI] [Google Scholar]
- Kauffold J., Henning K., Bachmann R., Hotzel H., Melzer F. The prevalence of Chlamydiae of bulls from six bull studs in Germany. Animal Reproduction Science. 2007;102(1–2):111–121. doi: 10.1016/j.anireprosci.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Kemel C., Salamone M., Van Loo H., Latour C., Vandeputte S., Callens J., Hostens M., Opsomer G. Unaffected semen quality parameters in Neospora caninum seropositive Belgian Blue bulls. Theriogenology. 2022;191:10–15. doi: 10.1016/j.theriogenology.2022.07.013. [DOI] [PubMed] [Google Scholar]
- Kenny D.A., Byrne C.J. Review: The effect of nutrition on timing of pubertal onset and subsequent fertility in the bull. Animal: An International Journal of Animal Bioscience. 2018;12(s1):s36–s44. doi: 10.1017/S1751731118000514. [DOI] [PubMed] [Google Scholar]
- Koziol J.H., Sheets T., Wickware C.L., Johnson T.A. Composition and diversity of the seminal microbiota in bulls and its association with semen parameters. Theriogenology. 2022;182:17–25. doi: 10.1016/j.theriogenology.2022.01.029. [DOI] [PubMed] [Google Scholar]
- Kruger E.R., Penha T.R., Regina D., Stoffelo E., Roehe P.M., Ribeiro M.C., Soccol V.T., Diagnóstico C.De, Enrietti M. Bovine herpesvirus 4 in Parana State, Brazil : Case report, viral isolation, and molecular identification. Brazilian Journal of Microbiology. 2015;283:279–283. doi: 10.1590/S1517-838246120130949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Mouël C., Forslund A. How can we feed the world in 2050? A review of the responses from global scenario studies. European Review of Agricultural Economics. 2017;44(4):541–591. doi: 10.1093/erae/jbx006. [DOI] [Google Scholar]
- Marin M., Burucúa M., Rensetti D., José J., Odeón A., Pérez S. Distinctive features of bovine alphaherpesvirus types 1 and 5 and the virus ‑ host interactions that might influence clinical outcomes. Archives of Virology. 2020;165(2):285–301. doi: 10.1007/s00705-019-04494-5. [DOI] [PubMed] [Google Scholar]
- McFadden A.M., Heuer C., Jackson R., West D.M., Parkinson T.J. Investigation of bovine venereal campylobacteriosis in beef cow herds in New Zealand. New Zealand Veterinary Journal. 2004;53(1):45–52. doi: 10.1080/00480169.2005.36468. [DOI] [PubMed] [Google Scholar]
- Michi A.N., Favetto P.H., Kastelic J., Cobo E.R. A review of sexually transmitted bovine trichomoniasis and campylobacteriosis affecting cattle reproductive health. Theriogenology. 2016;85(5):781–791. doi: 10.1016/j.theriogenology.2015.10.037. [DOI] [PubMed] [Google Scholar]
- Mingoas J., Tchoumboue J., Awah-Ndukum J. An abattoir survey of bacterial and fungal infections of cattle reproductive tract in Cameroon highlands. Cameroon Journal of Experimental Biology. 2009;4(2):75–79. doi: 10.4314/cajeb.v4i2.37978. [DOI] [Google Scholar]
- Montoya-Monsalve G., Sánchez-Calabuig M.-.J., Blanco-Murcia J., Elvira L., Gutiérrez-Adán A., Ramos-Ibeas P. Impact of overuse and sexually transmitted infections on seminal parameters of extensively managed bulls. Animals. 2021;11(3):1–13. doi: 10.3390/ani11030827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P D., Cantón J G., Louge Uriarte L., .E.. Editorial: Infectious diseases affecting reproduction and the neonatal period in cattle. Frontiers in Veterinary Science. 2021;8 doi: 10.3389/fvets.2021.679007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mshelia G.D., Amin J.D., Woldehiwet Z., Murray R.D., Egwu G.O. Epidemiology of bovine venereal campylobacteriosis: Geographic distribution and recent advances in molecular diagnostic techniques. Reproduction in Domestic Animals. 2010;(5):45. doi: 10.1111/j.1439-0531.2009.01546.x. [DOI] [PubMed] [Google Scholar]
- Newcomer B.W., Givens D. Diagnosis and control of viral diseases of reproductive importance infectious bovine rhinotracheitis and bovine viral diarrhea. The Veterinary clinics of North America. Food Animal Practice. 2016;32(2):425–441. doi: 10.1016/j.cvfa.2016.01.011. [DOI] [PubMed] [Google Scholar]
- OIE. (2021). Bovine genital campylobacteriosis. Chapter 3.4.4., 1–12. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.04.04_BGC.pdf Accessed December 23, 2022.
- Panangala V.S., Winter A.J., Wijesinha A., Foote R.H. Decreased motility of bull spermatozoa caused by Mycoplasma bovigenitalium. American Journal of Veterinary Research. 1981;42(12):2090–2093. [PubMed] [Google Scholar]
- Parker A.M., House J.K., Sheehy P.A., Hazelton M.S., Bosward K.L. A review of mycoplasma diagnostics in cattle. Journal of Veterinary Internal Medicine. 2018:1241–1252. doi: 10.1111/jvim.15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Fernández N., Cano-Terriza D., García-Bocanegra I., Horcajo P., Vázquez-Arbaizar P., Cleofé-Resta D., Pérez-Arroyo B., Ortega-Mora L.M., Collantes-Fernández E. Prevalence of Bovine genital campylobacteriosis, associated risk factors and spatial distribution in Spanish beef cattle based on veterinary laboratory database records. Frontiers in Veterinary Science. 2021;8:1–10. doi: 10.3389/fvets.2021.750183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal P., Kiran Kumar T., Srivastava S.K. Infectious causes of infertility in buffalo bull (Bubalus bubalis) Buffalo Bulletin. 2013;32(2):71–82. [Google Scholar]
- Petit T., Spergser J., Rosengarten R., Aurich J. Prevalence of potentially pathogenic bacteria as genital pathogens in dairy cattle. Reproduction in Domestic Animals. 2009;44(1):88–91. doi: 10.1111/j.1439-0531.2007.01002.x. [DOI] [PubMed] [Google Scholar]
- Polo C., García-Seco T., Hernández M., Fernández V., Rodríguez-Lázaro D., Goyache J., Domínguez L., Pérez-Sancho M. Evaluation of PCR assays for Campylobacter fetus detection and discrimination between C. fetus subspecies in bovine preputial wash samples. Theriogenology. 2021;172:300–306. doi: 10.1016/j.theriogenology.2021.06.020. [DOI] [PubMed] [Google Scholar]
- Polo C., Hernández M., García-Seco T., Fernández V., Briones V., Diez-Guerrier A., Abad D., Rodríguez-Lázaro D., Domínguez L., Pérez-Sancho M. Exploiting 16S rRNA-based metagenomics to reveal neglected microorganisms associated with infertility in breeding bulls in Spanish extensive herds. Research in Veterinary Science. 2022;150:52–57. doi: 10.1016/j.rvsc.2022.04.019. [DOI] [PubMed] [Google Scholar]
- Santos Junior M.N., Neres Macêdo, de N.S., Campos G.B., Bastos B.L., Timenetsky J., Marques L.M. A Review of Ureaplasma diversum: A representative of the Mollicute class associated with reproductive and respiratory disorders in cattle. Frontiers in Veterinary Science. 2021;8:1–19. doi: 10.3389/fvets.2021.572171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedmousavi S., Bosco S., De Hoog S., Ebel F., Elad D., Gomes R.R., Jacobsen I.D., Martel A., Mignon B., Pasmans F., Piecková E., Rodrigues A.M., Singh K., Vicente V.A., Wibbelt G., Wiederhold N.P., Guillot J. Fungal infections in animals: A patchwork of different situations. Medical Mycology. 2018;56:S165–S187. doi: 10.1093/mmy/myx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teankum K., Pospischil A., Janett F., Brugnera E., Hoelzle L.E., Hoelzle K., Weilenmann R., Zimmermann D.R., Gerber A., Polkinghorne A., Borel N. Prevalence of Chlamydiae in semen and genital tracts of bulls, rams and bucks. Theriogenology. 2007;67(2):303–310. doi: 10.1016/j.theriogenology.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Titterington F., Lively F., Dawson S., Gordon A., Morrison S. The effects of breed, month of parturition and sex of progeny on beef cow fertility using calving interval as a measure. Advances in Animal Biosciences, v. 2017;8(s1):s67. doi: 10.1017/S2040470017001741. [DOI] [Google Scholar]
- van Velsen C.M. Neosporosis in bulls: Potential for venereal transmission, and effect on semen quality and production. New Zealand Veterinary Journal. 2021;69(4):193–200. doi: 10.1080/00480169.2020.1854883. [DOI] [PubMed] [Google Scholar]
- Wagenaar J.A., Van Bergen M.A.P., Blaser M.J., Tauxe R.V., Newell D.G., Van Putten J.P.M. Campylobacter fetus infections in humans: Exposure and disease. Clinical Infectious Diseases. 2014;58(11):1579–1586. doi: 10.1093/cid/ciu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickware C.L., Johnson T.A., Koziol J.H. Composition and diversity of the preputial microbiota in healthy bulls. Theriogenology. 2020;145:231–237. doi: 10.1016/j.theriogenology.2019.11.002. [DOI] [PubMed] [Google Scholar]
- Yoo H.S. Infectious causes of reproductive disorders in cattle. Journal of Reproduction and Development. 2010;56 doi: 10.1262/jrd.1056S53. SUPPL. [DOI] [PubMed] [Google Scholar]