Abstract
Although a novel hemostatic agent has been used for endoscopic mucosal resection in submucosal dissection, there are few case reports of its use in pancreato‐biliary endoscopic procedures. We describe a case of post‐endoscopic papillectomy bleeding in which endoscopic hemostasis was achieved using a novel hemostatic agent.
Keywords: bleeding, endoscopic sphincterotomy, endoscopic retrograde cholangiopancreatography, hemostasis, PuraStat
Although a novel hemostatic agent has been used for endoscopic mucosal resection in submucosal dissection, there are few case reports of its use in pancreato‐biliary endoscopic procedures. We describe a case of post‐endoscopic papillectomy bleeding in which endoscopic hemostasis was achieved using a novel hemostatic agent.
Endoscopic papillectomy (EP) has recently become the accepted procedure for early ampullary carcinoma as well as for ampullary adenoma. A recent large study, however, has reported post‐EP bleeding occurring with a frequency of 11%. 1 To obtain hemostasis, methods such as coagulation are usually attempted, but these carry concerns such as perforation or post‐endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. A novel hemostatic agent (PuraStat gel, 3D Matrix Europe SAS, Caluire‐et‐Cuire, France) has recently become available. Although this gel has been used for endoscopic mucosal resection in submucosal dissection, 2 there are few case reports of its use in pancreato‐biliary endoscopic procedures. 3 We describe here a case of post‐EP bleeding in which endoscopic hemostasis was achieved using PuraStat.
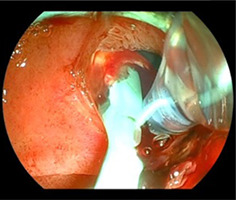
A 79‐year‐old female was admitted for treatment of ampullary adenoma. An initial EP for this lesion was performed successfully, and a 7 Fr plastic stent was deployed for bile and pancreatic duct stenting without any adverse events, including bleeding. However, melena was observed 3 days later, and endoscopic examination was attempted. After introduction of the duodenoscope into the duodenum, blood clots and oozing were observed at the ampulla of Vater. As an ulcer had been observed during the papillectomy, we considered that using a traditional coagulation method might risk perforation, and so we selected the PuraStat injection technique accordingly. To prevent migration of the PuraStat into the third part of the duodenum, the duodenoscope was adjusted until the ampulla of Vater was at 3–6 O'clock position on the endoscopic image (Fig. 1). If PuraStat was applied under any other position, PuraStat itself may migrate into third part of the duodenum because of the gravity. The dedicated delivery system was then affixed around the bleeding point, PuraStat was applied, and hemostasis was achieved without any adverse events (Video S1). After 1 day, complete hemostasis was obtained.
Figure 1.
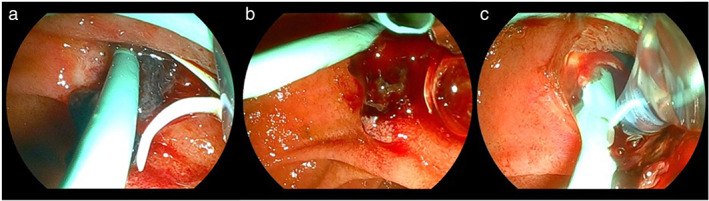
Ulcer formation is observed after papillectomy (a), and blood clots and oozing are observed at the ampulla of Vater (b and c). To prevent migration of PuraStat into the third part of the duodenum, the duodenoscope is adjusted until the ampulla of Vater is at 3–6 O'clock position in the endoscopic image.
In conclusion, PuraStat injection has no risk of perforation or pancreatitis and should thus be considered as a first‐line endoscopic hemostasis technique following post‐EP bleeding.
Supporting information
Video S1. Blood clots and oozing are observed. To prevent migration of PuraStat dislocation into the third part of the duodenum, the duodenoscope is adjusted until the ampulla of Vater is at 3 to 6 O'clock position in the endoscopic image. PuraStat injection is performed, and endoscopic hemostasis is achieved.
Declaration of conflict of interest: The authors declare no conflicts of interest.
References
- 1. Gondran H, Musquer N, Perez‐Cuadrado‐Robles E et al. Efficacy and safety of endoscopic papillectomy: a multicenter, retrospective, cohort study on 227 patients. Therap. Adv. Gastroenterol. 2022; 15: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Subramaniam S, Kandiah K, Chedgy F et al. A novel self‐assembling peptide for hemostasis during endoscopic submucosal dissection: a randomized controlled trial. Endoscopy. 2021; 53: 27–35. [DOI] [PubMed] [Google Scholar]
- 3. Gagliardi M, Oliviero G, Fusco M et al. Novel hemostatic gel as rescue therapy for postsphincterotomy bleeding refractory to self‐expanding metallic stent placement. ACG Case Rep J. 2022; 9: e0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Blood clots and oozing are observed. To prevent migration of PuraStat dislocation into the third part of the duodenum, the duodenoscope is adjusted until the ampulla of Vater is at 3 to 6 O'clock position in the endoscopic image. PuraStat injection is performed, and endoscopic hemostasis is achieved.


