Abstract
Helicobacter pylori infection exhibits a wide disease spectrum ranging from asymptomatic gastritis, peptic ulcer disease, to gastric cancer. H. pylori can induce dysbiosis of gastric microbiota in the pathway of carcinogenesis and successful eradication can restore gastric homeostasis. Diagnostic testing and treatment for H. pylori infection is recommended in patients with active or past history of peptic ulcer, chronic dyspepsia, chronic non‐steroidal anti‐inflammatory drugs (NSAID) or aspirin use, precancerous gastric lesions, gastric cancer, mucosa‐associated lymphoid tissue (MALT) lymphoma, family history of gastric cancer, family history of peptic ulcers, household family member having active H. pylori infection, iron deficiency anemia, idiopathic thrombocytopenic purpura, or vitamin B12 deficiency. Recommended first‐line regimens for H. pylori eradication are classified according to clarithromycin resistance. In areas of high clarithromycin resistance (≥15%), we recommend 14‐day concomitant therapy or 14‐day bismuth quadruple therapy (BQT) as first‐line regimen. In areas of low clarithromycin resistance (<15%), we recommend 14‐day triple therapy or 14‐day BQT as first‐line treatment. Second‐line regimens are 14‐day levofloxacin triple therapy or 14‐day BQT if BQT is not previously used. For patients with multiple treatment failure, antimicrobial susceptibility testing (AST) should be performed. If AST is not available, we recommend using antibiotics not previously used or for which resistance is unlikely, such as amoxicillin, tetracycline, bismuth, or furazolidone. High‐dose potent proton pump inhibitor or vonoprazan is recommended to achieve adequate acid suppression. Probiotics can be used as an adjuvant treatment to reduce the side effects of antibiotics and enhance eradication rate.
Keywords: Helicobacter pylori, management, treatment
Antibiotic‐resistant H. pylori strains have been increasing in most regions. Recommended first‐line regimens for H. pylori eradication are classified according to clarithromycin resistance. For patients with multiple treatment failure, antimicrobial susceptibility testing (AST) should be performed.
Introduction
Helicobacter pylori (H. pylori), a spiral‐shaped gram‐negative bacterium, is one of the most common causes of serious chronic bacterial infections worldwide. H. pylori infection has been proven to be etiologically associated with chronic gastritis, peptic ulcers, gastric mucosa‐associated lymphoid tissue (MALT) lymphoma, and gastric adenocarcinoma. 1 Over 1 million new cases of gastric cancer and nearly 800 000 deaths occurred in 2020 making H. pylori‐related disease the third leading cause of global cancer deaths. 2 H. pylori infection is designated as an infectious disease and it is recommended that it should be treated regardless of symptoms to prevent serious complications and transmission. 3
The rapid emergence of antibiotic‐resistant H. pylori has become the greatest global threat influencing treatment outcomes. For example, almost all World Health Organization (WHO) regions now experience resistance rates to clarithromycin, metronidazole, and levofloxacin of over 15%. 4 Clinical guidelines recommend different first‐line treatments depending on antimicrobial resistance patterns in each region. 5 , 6 , 7 Appropriate antibiotic selection, adequate acid suppression, and adherence to therapy all affect successful eradication. Host CYP2C19 genetic polymorphisms are associated with decreasing effectiveness of some proton pump inhibitors (PPIs). 8 Second‐generation PPIs (rabeprazole and esomeprazole) are less affected by CYP2C19 and are recommended. Furthermore, novel potent acid blockers such as vonoprazan have also been introduced and are not affected by CYP2C19. 9
This review aimed to provide an overview of the prevalence, disease spectrum, diagnostic tests, and treatment options for H. pylori infection. Regimens for first‐line, second‐line therapy, and multiple treatment failures were recommended regarding the latest global antibiotic resistance patterns.
The prevalence of H. pylori infection
The global prevalence of H. pylori infection ranged widely from 18.9% to 87.7%, with an estimated 4.4 billion infected people worldwide in 2015. Regions with the highest prevalence of H. pylori are Africa (70.1%) and South America (69.4%), while Oceania (24.4%) and Western Europe (34.3%) have the lowest prevalence. 10 The country with the highest prevalence of H. pylori infection was Nigeria (87.7%), whereas the lowest was Switzerland (18.9%). 10 H. pylori is known as a strong risk factor for gastric cancer. There were more than 2 billion H. pylori‐positive patients and three‐quarters of global gastric cancer cases residing in Asia. 10 , 11 Mongolia had the highest age‐standardized incidence rate (ASIR) of gastric cancer, followed by Japan (ASIR of 32.5 and 31.6 per 100 000 persons, respectively). 11 Africa had high H. pylori prevalence but low incidence of gastric cancer. 11 This “African enigma” is likely related to the limited care available in many parts of Africa. Vietnam (70.3%), Myanmar (69%), Laos (68.7%), and Thailand (28.2%) were among the Association of Southeast Asian Nations (ASEAN) with varying prevalence of H. pylori infection. 12 Countries in ASEAN with high H. pylori prevalence also have high incidence of gastric cancer (ASIR > 10). The global prevalence of H. pylori infection and ASIR of gastric cancer in ASEAN are demonstrated in Figure 1.
Figure 1.
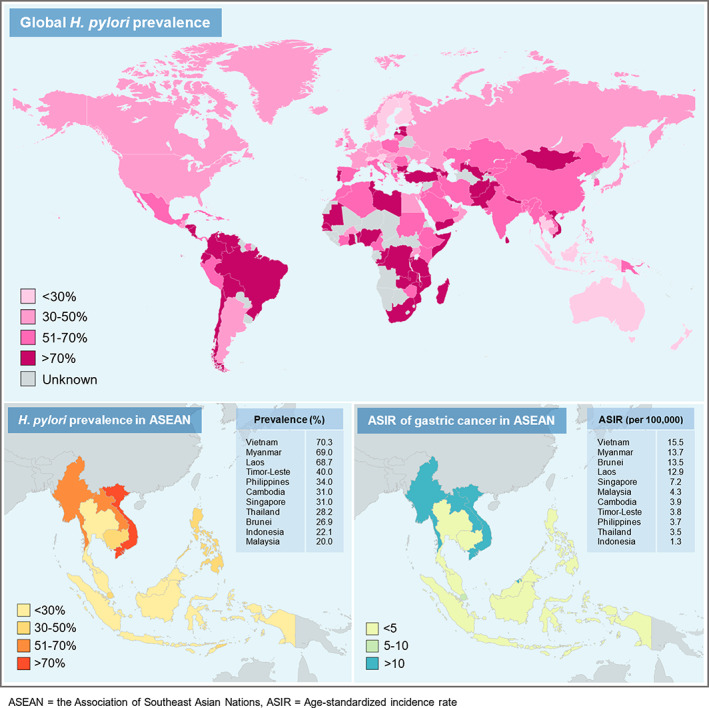
Global prevalence (upper panel), regional prevalence of H. pylori infection (lower left panel), and age‐standardized incidence rate of gastric cancer in ASEAN (lower right panel).
Factors affecting the prevalence of H. pylori infection are age, ethnicity, and socioeconomic status. 13 The possible routes of transmission are fecal–oral, oral–oral, and gastric–oral routes. 14 Children became infected with H. pylori at a very young age (mean age of 32.78 months). 15 Intrafamilial clustering of H. pylori infection implied person‐to‐person transmission or common exposure to same contaminated source. 16 H. pylori prevalence gradually increased with age from adolescents (10%–30%) to the elderly (40%–60%) 17 and also varied between ethnic groups. In the United States, non‐Hispanic Whites had H. pylori prevalence of 26.9%, while non‐Hispanic Blacks and Mexican Americans had a prevalence of 51.1% and 57.9%, respectively. 18 Moreover, low socioeconomic class during childhood and crowded living conditions were related to H. pylori infection. 19 Higher prevalence of H. pylori infection in Asian countries than in Western countries might be explained by lower socioeconomic status in most countries in Asia. 10 Despite being in the same region, developing Asian countries also had higher prevalence than the developed ones. 20
H. pylori and gastric microbiome
The human stomach harbors much less microbial density [101–103 colony forming units (CFUs)/g] than the colon (1010–1012 CFUs/g) due to highly acidic environment. 21 The gastric microbiota is generally composed of dominant phyla including Actinobacteria (genus Bifidobacterium), Bacteroidetes (genus Prevotella), Firmicutes (genera Lactobacillus, Streptococcus, Clostridium, Veillonella), Fusobacteria, and Proteobacteria. 22 The most abundant phylum is Firmicutes, while the most common genera are Streptococcus, Prevotella, Neisseria, Hemophilus, Fusobacterium, and Veillonella. 22 The recent study reported that H. pylori had strong coexcluding interactions with Fusobacterium, Neisseria, Prevotella, Veillonella, and Rothia only in patients with advanced gastric lesions. 23 H. pylori can induce gastric dysbiosis resulting in decreased microbial diversity. Successful eradication can restore gastric microbiota resembling the status of H. pylori‐negative controls, increase the abundance of Bifidobacterium, and hypothetical downregulation of drug‐resistance mechanisms. 23 The dysbiotic gastric microbiota profile, decreased H. pylori abundance, and enriched oral or intestinal bacteria were found in patients with gastric cancer. 24 Interplays between H. pylori infection and gastric microbiome are demonstrated in Figure 2.
Figure 2.
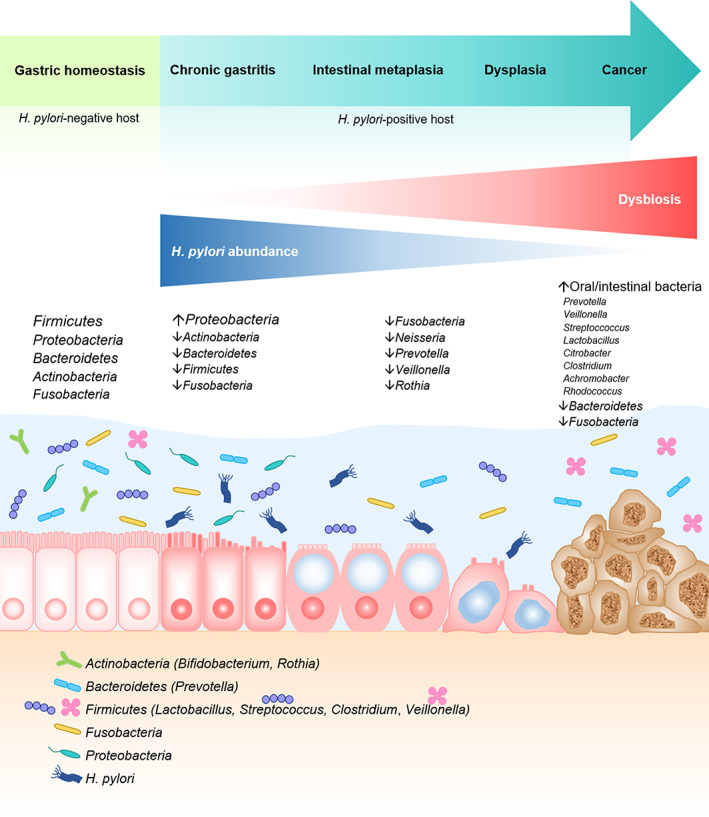
Interplays between H. pylori infection and gastric microbiome. In normal condition, the mucus layer over gastric epithelial cells is the habitat of highly diverse bacteria phyla. The most abundant phylum is Firmicutes, followed by Proteobacteria, and Bacteroidetes. In H. pylori‐associated chronic gastritis, dysbiosis develops with decreased microbial diversity. There is higher abundance of Proteobacteria, but lower number of other phyla. As carcinogenesis continues, reduced H. pylori abundance and higher degree of dysbiosis are observed in intestinal metaplasia and dysplasia. In gastric cancer, oropharyngeal or intestinal commensals (Streptococcus, Lactobacillus, Veillonella, and Prevotella) are enriched.
The disease spectrum of H. pylori infection
H. pylori infection exhibits a wide spectrum of clinical manifestations. H. pylori‐related gastrointestinal diseases and extragastric manifestations are described below.
Gastrointestinal diseases
Dyspepsia
Young dyspeptic patients without alarm symptoms and no risk for gastric cancer are recommended to be tested for H. pylori infection by noninvasive testing, whereas older patients should undergo upper gastrointestinal (GI) endoscopy due to increased risk of gastric cancer. 7 The age cutoffs for performing upper GI endoscopy are different between countries according to the prevalence of gastric cancer. 25 The optimal cutoffs for endoscopy are 40 years for high‐prevalence countries (e.g., China, Korea, Japan, Taiwan 26 ), and 45 or 50 years for intermediate or low‐prevalence countries (e.g., India, Malaysia, Singapore, Thailand, Africa, North America, Western Europe). 25 The age cutoffs for endoscopy in Vietnam are lower than most guidelines as 30 years for women and 35 years for men to detect 98.2% of upper GI malignancy. 27 Since H. pylori can be detected in visibly normal gastric mucosa, gastric biopsies should be obtained in dyspeptic patients undergoing gastroscopy. H. pylori eradication is recommended to provide symptomatic relief for dyspepsia. 28
Gastritis
Persistent H. pylori infection causes chronic gastritis of varying severity. H. pylori typically causes antral‐predominant gastritis. When inflammation continues, parietal cell destruction results in decreased acid secretion. Atrophic gastritis and intestinal metaplasia, which are precancerous lesions, subsequently develop.
Peptic ulcer disease
H. pylori and nonsteroidal anti‐inflammatory drug (NSAID) are common causes of peptic ulcers. The guideline recommends testing for H. pylori infection for patients with active or a past history of peptic ulcer disease (PUD). 5 The prevalence of H. pylori infection was 90%–100% in duodenal ulcers and 60%–100% in gastric ulcers. 29 Recently, there has been a decreasing trend of H. pylori infection in patients with PUD in the United States. 30
Gastric cancer
H. pylori can turn chronic gastritis into atrophic gastritis, intestinal metaplasia, dysplasia, and eventually adenocarcinoma via Correa's precancerous cascade. 31 H. pylori‐induced chronic gastritis generates increased epithelial cell turnover and reactive oxygen species, resulting in DNA damage along with microRNA dysregulation. 32 Furthermore, H. pylori can induce DNA methylation changes, causing epigenetic aberrations in gastric epithelial cells. Promoter hypermethylation of E‐cadherin, a tumor suppressor gene, is related to the development of gastric cancer. 32 Men, non‐White racial and ethnic minority groups, and smokers were associated with higher risk of gastric cancer in H. pylori‐infected patients. 33 Family history of gastric cancer in first‐degree relatives was associated with approximately three‐fold higher risk of gastric cancer. 34 H. pylori eradication not only decreases the risk of gastric cancer development, 35 but also reduces histologic progression to gastric dysplasia. 36 H. pylori testing is recommended in patients with a history of endoscopic resection of early gastric cancer (EGC). There were lower rates of metachronous gastric cancer in patients with EGC receiving H. pylori eradication than the placebo group during a median follow‐up of 5.9 years [7.2% vs. 13.4% (HR 0.5; 95% CI 0.26–0.94, P = 0.03)]. 37
Mucosa‐associated lymphoid tissue lymphoma
H. pylori infection is associated with over 90% of mucosa‐associated lymphoid tissue (MALT) lymphomas. 38 The pathogenesis of MALT lymphoma might be related to direct antigenic stimulation of B‐cell proliferation by H. pylori. 38 Previous studies revealed that majority of patients (80%) with low‐grade gastric MALT lymphomas receiving H. pylori eradication could achieve complete remission with an annual recurrence rate of 5%. 39 H. pylori eradication is also recommended in patients with H. pylori‐negative gastric MALT lymphoma as this can induce complete remission in 29.3% of patients. 40
Extragastric diseases
Iron deficiency anemia
H. pylori infection can induce depletion of iron stores by several mechanisms including chronic occult gastrointestinal blood loss, impaired iron absorption due to decreased intragastric acidity and ascorbic acid concentration, 41 and enhanced iron uptake for bacterial growth. 42 The previous study reported that the prevalence of H. pylori‐related chronic gastritis was 60% in patients with unexplained iron deficiency anemia (IDA) compared with 43% of the control group without IDA (P < 0.01). 43 H. pylori infection was associated with IDA with pooled OR of 2.22 (95% CI 1.52–3.24, P < 0.0001). 44 The meta‐analysis demonstrated significant improvement in IDA after combination of H. pylori eradication and oral iron compared with oral iron alone. 45
Idiopathic thrombocytopenic purpura
Potential mechanisms of H. pylori‐related idiopathic thrombocytopenic purpura (ITP) might be caused by molecular mimicry between H. pylori‐induced antibodies and platelet glycoprotein antigens. 46 Moreover, antibodies to cytotoxin‐associated gene A protein (CagA), one of H. pylori virulence factors, can cross‐react with specific peptides expressed by platelets of ITP patients. 46 The prevalence of H. pylori infection was significantly higher in patients with ITP than in controls (90.6% vs. 43.8%, P = 0.00006) in the Colombian study. 47 The systematic review reported that among 696 ITP patients receiving H. pylori eradication, 42.7% achieved complete response (platelet count ≥100 × 109/L). 48 Higher response rate was observed in patients with milder degree of thrombocytopenia and in countries with high prevalence of H. pylori infection. 48 The American Society of Hematology recommends H. pylori eradication for infected patients with ITP (grade 1B) and also suggests H. pylori screening in patients with ITP (grade 2C). 49
Diagnostic tests for H. pylori infection
Clinical guidelines recommend diagnostic testing for H. pylori infection in patients with conditions as follows: (1) active or past history of peptic ulcer, (2) chronic dyspepsia, (3) chronic NSAID or aspirin use, (4) precancerous gastric lesions, (5) gastric cancer, (6) MALT lymphoma, (7) family history of gastric cancer in a first‐degree relative, (8) family history of peptic ulcer, (9) having household family member with active H. pylori infection, (10) unexplained IDA, (11) ITP, and (12) vitamin B12 deficiency. 5 , 6 , 7 , 50 As gastric cancer prevalence is relatively high in Asia, the Bangkok consensus recommends community screening and treatment for H. pylori infection to prevent gastric cancer, especially in the area with high cancer burden. 6
Current diagnostic tests for H. pylori infection are classified into two groups, which are noninvasive and invasive methods. Invasive diagnostic tests are endoscopy‐based tests including histology, rapid urease test, culture, and polymerase chain reaction. Noninvasive tests are urea breath test, stool antigen test, and antibody tests in serum, urine, and saliva. 51
A patient should discontinue PPI for at least 2 weeks, antibiotics and bismuth compounds for at least 4 weeks before testing by histology, culture, rapid urease test, urea breath test, or stool antigen test to avoid a false negative result.
Invasive tests
Histology
Histologic examination is considered to be the gold standard in H. pylori detection with high sensitivity and specificity of 95% and 99%, respectively. 52 Histology can provide information about associated lesions such as atrophic gastritis, intestinal metaplasia, dysplasia, and MALT lymphoma.
Rapid urease test
Gastric biopsy specimens are placed in a medium containing urea and pH indicator. Urease from H. pylori converts urea to ammonia, which increases the pH resulting in color change of the pH indicator. Sensitivity and specificity are approximately 85%–95% and 95%–100%, respectively. 51
Culture
Culture has lower sensitivity (85%–95%) than rapid urease test or histology, but the specificity is as high as almost 100%. 51 This test provides essential information about antimicrobial susceptibility.
Polymerase chain reaction
H. pylori can be detected by polymerase chain reaction (PCR) in several types of specimens such as gastric juice, gastric biopsies, stool, and saliva. PCR had high sensitivity of 100% and 98% for gastric biopsy and stool specimen respectively, while specificity was 98% for both specimens. 53 Antibiotic resistance can also be determined by amplification of resistance‐associated genes using real‐time PCR.
Non‐invasive tests
Urea breath test
13C labeled urea is administered orally and hydrolysed by H. pylori's urease producing ammonia and labeled CO2, which is subsequently exhaled and collected as breath sample. Until now, urea breath test (UBT) has been the most popular and accurate noninvasive test for diagnosis of H. pylori infection with high sensitivity (96%) and specificity (93%). 54 In patients with upper gastrointestinal bleeding, the diagnostic accuracy of UBT is still high with sensitivity and specificity of 93% and 92%, respectively. 55 UBT can also be used after treatment to confirm H. pylori eradication.
Stool antigen test
There are two types of stool antigen tests that detect H. pylori antigens by using monoclonal or polyclonal antibodies. The monoclonal stool antigen test had higher pooled sensitivity (94% vs. 83%) than polyclonal stool antigen test but comparable specificity (97% vs. 96%). 56 Therefore, monoclonal stool antigen test should be used for diagnosis or confirmation of eradication. 56 PPI can decrease the sensitivity of stool antigen test from 95.2% to 88.9% and should be discontinued at least 2 weeks before the test. 57
Antibody tests in serum, urine, and saliva
Serologic tests are inexpensive and noninvasive. The serological assay detects anti‐H. pylori IgG in serum, which generally becomes positive 3 weeks after H. pylori infection and persists up to 6–12 months after eradication. 55 Serologic test had lower sensitivity and specificity of 85% and 79%, respectively. 58 Most serologic tests cannot differentiate past infection from current infection except for rapid test with current infection marker (CIM) test providing sensitivity and specificity of 93.2% and 96.2%, respectively compared with UBT. 59 Upper gastrointestinal bleeding did not affect the accuracy of serologic tests. There are less commonly used urine and saliva antibody tests. Urine‐based tests had fair sensitivity (84.7%), specificity (89.9%), and accuracy (87%). 60 Salivary tests provided unsatisfactory results with fair sensitivity (81%) and specificity (73%). 61 Urine and saliva antibody tests still have high variability of diagnostic accuracy; therefore test usage is found only in research field.
Confirmation of cure
Confirmation of successful eradication should be performed to avoid further complications of H. pylori infection and prevent transmission to other family members. Posttreatment tests are UBT, stool antigen test, or biopsy‐based test. UBT is recommended as the best option for confirmation of cure. Suitable timing for testing should be at least 4 weeks after treatment completion.
Antibiotic resistance patterns
Antibiotic resistance patterns are determined by H. pylori culture and antimicrobial susceptibility testing (AST). This test requires proper gastric biopsy handling, efficient specimen transportation, and microbiological expertise. 62 Moreover, traditional H. pylori culture is time‐consuming and unavailable in some areas. Next‐generation sequencing (NGS) is a new molecular‐based test, which determines antibiotic resistance by identifying mutations or variances of the H. pylori DNA. 62 Fresh or formalin‐fixed paraffin‐embedded gastric biopsies, gastric juice, or feces can be used for NGS. Targeted genes associated with antibiotic resistance are as follows: 23 S rRNA for clarithromycin, 16 S rRNA for tetracycline, gyrA for fluoroquinolones, rdxA for metronidazole, pbp1 for amoxicillin, and rpoB for rifabutin. 62 NGS reliably determined clarithromycin and levofloxacin resistance from culture isolates and formalin‐fixed gastric tissue compared with agar dilution. 62
Most of WHO regions had high pooled prevalence of primary and secondary resistance to metronidazole, clarithromycin, and levofloxacin (>15%). 4 The most common antibiotic resistance in all regions was metronidazole. 12 , 63 , 64 , 65 , 66 The ASEAN, the United States, and Europe had almost the same antibiotic resistance patterns, while extremely high resistance rates to all antibiotics were demonstrated in Africa. 66 Amoxicillin and tetracycline resistance rates were low in all regions except Africa. Antibiotic resistance rates stratified by regions are demonstrated in Table 1.
Table 1.
Helicobacter pylori antibiotic resistance stratified by regions
| Antibiotic resistance | ASEAN 12 , 63 | The United States 64 2011–2021 N = 2660 | Europe 65 2008–2017, N = 1211 | Africa 66 1986–2017, N = 2085 |
|---|---|---|---|---|
| Metronidazole | 30%–73% | 42.1% | 38.9% | 75.8% |
| Clarithromycin | 17%–43% | 31.5% | 21.4% | 29.2% |
| Levofloxacin | 13%–34% | 37.6% | 15.8% | 17.4% |
| Amoxicillin | 0%–5% | 2.6% | 0.2% | 72.6% |
| Tetracycline | 0% | 0.87% | 0% | 48.7% |
| Others | ‐ | Rifabutin 0.17%, CLR‐MNZ 11.7% | Rifampicin 0.9% | ‐ |
Abbreviations: ASEAN, the Association of Southeast Asian Nations; CLR, Clarithromycin; MNZ, Metronidazole.
Treatment
Treatment goal for H. pylori infection is an intention‐to‐treat eradication rate of at least 90%. 67 Choosing the suitable first‐line regimen depends on known or anticipated pattern of regional antibiotic resistance. 5 , 6 , 7 Treatment regimen comprises antisecretory drug and antibiotics. The choice of antisecretory drugs is either PPIs or potassium‐competitive acid blockers (P‐CABs). At least two antibiotics are generally chosen in regimens. Treatment guidelines for H. pylori infection recommend different first‐line regimens regarding local antibiotic resistance as demonstrated in Table 2.
Table 2.
Treatment regimens for Helicobacter pylori infection according to different guidelines
| Regimen | ACG Clinical Guideline, 5 2017 | ASEAN Consensus, 6 2018 | Maastricht VI/Florence Consensus Report, 7 2022 |
|---|---|---|---|
| First‐line therapy |
Recommend
Triple therapy (14 days) Suggest
|
Depends on antibiotic resistance
Triple therapy (14 days)
|
Recommend routine AST before first‐line therapy Empirical regimens if AST is not available
|
| Penicillin allergy |
|
|
first‐line: BQT (14 days) second‐line: FQ regimen or BQT (if BQT is not previously used) (14 days) |
| Second‐line therapy |
BQT or LVX salvage regimens
CLR or LVX salvage regimens |
|
|
| Salvage therapy |
Recommend
Suggest
|
|
|
| Antimicrobial susceptibility testing | No recommendation | After failure of second‐line therapy | Recommend AST even before first‐line treatment (if available) for implementation of antibiotic stewardship |
Note: AST = Antimicrobial susceptibility testing, Hybrid therapy = PPI + AMX (7 days) then PPI + AMX + CLR + MNZ (7 days), FQ sequential therapy = PPI‐AMX (5–7 days) then PPI‐FQ‐MNZ (5–7 days), FRZ quadruple therapy (14 days) = PPI + FRZ 100 mg tid + Bismuth + (AMX or TET), Rifabutin triple therapy (12–14 days) = PPI + rifabutin (150 mg daily) + AMX (1.5 g tid).
Abbreviations: AMX, Amoxicillin; BQT, Bismuth quadruple therapy; CLR, Clarithromycin; FQ, Fluoroquinolone; FRZ, Furazolidone; LVX, Levofloxacin; MNZ, Metronidazole; TET, Tetracycline.
Antisecretory drugs
Proton pump inhibitors
Proton pump inhibitors (PPIs) irreversibly bind and inhibit H+, K+‐ATPase on parietal cells causing effective gastric acid secretion blockage. PPIs are mainly metabolized by CYP2C19 enzyme. The meta‐analysis reported that using high‐dose PPI was more effective in H. pylori eradication than using standard‐dose PPI (82% vs. 74%, RR 1.09; 95% CI 1.01–1.17). 68 High‐dose PPI is defined as a double dose of 40 mg of omeprazole or equivalent. 7 , 69 Potency of PPI is determined based on omeprazole equivalent (OE). Equivalent doses of each PPI are as follows: pantoprazole 40 mg = 9 mg OE; lansoprazole 30 mg = 27 mg OE; esomeprazole 20 mg = 32 mg OE; rabeprazole 20 mg = 36 mg OE; dexlansoprazole 30 mg = 50–60 mg OE. This review suggests using twice‐daily dosing of PPI because of its ability to maintain intragastric pH at 4 or higher for 15.6–20.4 h. 70 Potent PPI (e.g., esomeprazole, rabeprazole) is recommended and pantoprazole should be avoided due to its relatively lower potency than other PPIs. 69 The recommended dose of esomeprazole or rabeprazole is 20–40 mg twice daily. 69
Potassium‐competitive acid blockers
Potassium‐competitive acid blockers (P‐CABs) reversibly bind to K+ ions and consequently block H+, K+‐ATPase inhibiting acid secretion. These drugs have a rapid onset of action and are capable of achieving therapeutic levels after the first dose. At present, P‐CABs released to the market are revaprazan, vonoprazan, and tegoprazan. Vonoprazan is mainly metabolized by CYP3A4/5. Exactly 10 mg of vonoprazan is equivalent to 60 mg of omeprazole. 70
Treatment regimens
Triple therapy
Standard triple therapy (STT) should not be used if the local clarithromycin resistance is >15%. 5 , 7 One study demonstrated that 14‐day STT could provide 87.8% eradication in clarithromycin resistance rate of 14.8%. 71 The OPTRICON study demonstrated the eradication rate for 14‐day STT of 82.3% without antimicrobial susceptibility data. 72 Another study revealed excellent eradication rate of 100% when using 14‐day STT with high‐dose PPI (lansoprazole 60 mg twice daily) in low clarithromycin resistance area. 73 Treatment duration of 14 days is recommended for H. pylori eradication. Although empiric STT yielded suboptimal eradication rates in some studies, 74 it could still be used in area with low clarithromycin resistance or available AST.
Concomitant therapy
Previous studies demonstrated high eradication rates (93.8%–96.4%) of 10‐day concomitant regimen. 72 , 75 The European registry (Hp‐EuReg) with 21 533 patients also reported a high eradication rate (92.2%) of 14‐day concomitant regimens. 76 One Korean study reported that concomitant therapy had provided the best eradication rate (94.4%) compared with triple therapy and sequential therapy. 77 However, adverse effects, for example, nausea, vomiting, and diarrhea, from concomitant regimen were significantly more common than from STT. 72 Although the concomitant regimen achieves high eradication rate, it can cause unnecessary antibiotic use accelerating antimicrobial resistance.
Sequential therapy
The meta‐analysis including 13 532 patients reported an overall eradication rate for sequential regimen of 84.3% without superiority over STT, concomitant, and bismuth quadruple therapy (BQT). 78 In Thailand, 10‐day sequential therapy has also demonstrated decreasing eradication rate to 59.7% in recent years. 63 This might be caused by low medication adherence associated with regimen complexity. Therefore, sequential therapy is not recommended for first‐line treatment in this review.
Bismuth quadruple therapy
Bismuth results in rapid antimicrobial effect on H. pylori and also produces synergistic effect with metronidazole. 79 Bismuth quadruple therapy (BQT) can be used as first‐line or second‐line therapy. As first‐line treatment, one study compared 14‐day and 10‐day BQT, revealing high efficacy (96% vs. 95%). 80 BQT provided high eradication rates of >90% in Taiwan and Europe. 74 , 76 The Thai study that evaluated 7‐day BQT as first‐line treatment using amoxicillin instead of tetracycline provided the eradication rate of 82.5% despite high metronidazole resistance rate (50%). 81 It is noted that metronidazole resistance can be overcome by increasing the dosage of metronidazole to at least 1500 mg/day and extending the duration of treatment to 14 days. 8 As second‐line therapy, eradication rates were 76%–94.5%. 82 , 83
Levofloxacin triple therapy
Levofloxacin triple therapy is primarily recommended for second‐line therapy by most guidelines. The recent US study demonstrated high levofloxacin resistance (37.6%), making levofloxacin triple therapy not suitable to be a first‐line regimen. 64 This regimen demonstrated fair eradication rates as first‐line treatment (79.1%–85.2%). 76 , 84 As second‐line therapy, eradication rates were 78%–92.9%. 82 , 85
Vonoprazan‐containing regimens
Recently, vonoprazan‐containing regimens have been more popularly used due to P‐CABs' potent acid inhibitory effect. Vonoprazan has been used in H. pylori treatment regimens since its first approval in Japan in 2015. Previous studies in Japan reported higher eradication rate of vonoprazan triple therapy (88.9%–95.7%) than PPI‐based triple therapy (71.4%–86.7%) in a population with high clarithromycin resistance rate. 86 , 87 , 88 In Japan, the cure rates with PPI and vonoprazan in clarithromycin susceptible infection were high and similar. Other studies demonstrated no difference in eradication rates of approximately 90% between vonoprazan dual and vonoprazan triple therapy. 89 , 90 In the latest trial conducted in the United States and Europe, 1046 patients were randomized 1:1:1 to receive vonoprazan dual, vonoprazan triple, or lansoprazole triple therapy. This study demonstrated comparable eradication rates of vonoprazan triple (84.7%), vonoprazan dual (78.5%), and lansoprazole triple therapy (78.8%) in population with high clarithromycin resistance rates in each group (19.1%–23.7%). 91 The study also reported that both vonoprazan‐based regimens provided significantly higher eradication rates than lansoprazole triple therapy in patients with clarithromycin‐resistant strains (vonoprazan dual therapy 69.6%, vonoprazan triple therapy 65.8% vs. lansoprazole triple therapy 31.9%, P < 0.001). 91 Vonoprazan dual and triple therapies have been recently approved for H. pylori treatment by the US Food and Drug Administration since May 2022. For other vonoprazan‐based regimens, pilot studies in Thailand revealed high efficacy of 7‐day high‐dose vonoprazan triple therapy (92.3%) and 14‐day vonoprazan triple therapy plus bismuth (96.2%). 92 Another prospective study demonstrated high efficacy (95%) of 14‐day vonoprazan bismuth quadruple therapy. 93
The current trend in H. pylori eradication is adopting the principles of antibiotic stewardship to improve treatment outcome, minimize antibiotic resistance, and reduce healthcare costs. Since AST is not available in some areas, recent guidelines have also provided guidance on effective empirical regimens. Vonoprazan is increasingly used because of superior eradication rate for antibiotic‐resistant strains and non‐inferiority to PPI‐based therapy. Recommended first‐line regimens for H. pylori eradication are classified according to clarithromycin resistance. In areas of high clarithromycin resistance (≥15%), we recommend 14‐day concomitant therapy or 14‐day BQT as first‐line regimen. In areas of low clarithromycin resistance (<15%), we recommend 14‐day triple therapy or 14‐day BQT as first‐line treatment. Second‐line regimens are 14‐day levofloxacin triple therapy or 14‐day BQT if BQT is not previously used. Recommended first‐ and second‐line regimens are demonstrated in Figure 3. Eradication rates and composition of each first‐ and second‐line regimen are summarized in Table 3.
Figure 3.
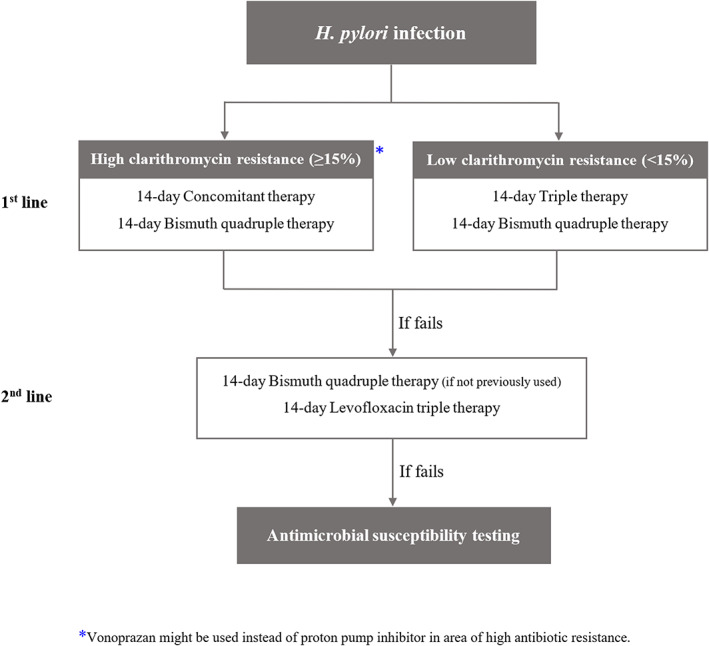
Algorithm for H. pylori management.
Table 3.
Eradication rates and composition of each first‐line and second‐line regimen
| Regimen and duration | Regimen composition | Eradication rates | Reference number |
|---|---|---|---|
| First‐line regimens | |||
| Concomitant therapy (10 days) | PPI + AMX (1 g bid) + MNZ (500 mg bid) + CLR (500 mg bid) | 93.8%–96.4% | 72, 75, 76, 77 |
| Triple therapy (14 days) | PPI + AMX (1 g bid) + CLR (1 g/day) | 82.3%–87.8% | 71, 72, 76 |
| Bismuth quadruple therapy (14 days) | PPI + Bismuth subsalicylate (524 mg qid or 1048 mg bid) + MNZ (500 mg tid) + TET (500 mg qid) | 88.2%–96.0% | 74, 76, 80 |
| LVX triple therapy (10–14 days) | PPI + AMX (1 g bid) + LVX (500 mg/day) | 79.1%–85.2% | 76, 84 |
| Sequential therapy (10 days) | PPI + AMX (1 g bid) (5 days) followed by PPI + CLR (1 g/day) + MNZ (500 mg bid) (5 days) | 59.7%–84.3% | 63, 76, 78 |
| Vonoprazan triple therapy | VPZ (20 mg bid) + AMX (750 mg bid) + CLR (200 or 400 mg bid) (7 days) | 88.9%–95.7% | 86, 87, 88 |
| VPZ (20 mg bid) + AMX (1 mg bid) + CLR (500 mg bid) (14 days) | 84.7% | 91 | |
| Vonoprazan dual therapy | VPZ (20 mg bid) + AMX (750 mg bid or 500 mg tid) (7 days) | 87.1%–94.4% | 89, 90 |
| VPZ (20 mg bid) + AMX (1 g tid) (14 days) | 78.5% | 91 | |
| Second‐line regimens | |||
| LVX triple therapy (14 days) | PPI + AMX (1 g bid) + LVX (500 mg/day) | 78.0%–92.9% | 82, 85 |
| Bismuth quadruple therapy (14 days) | PPI + Bismuth subsalicylate (524 mg qid or 1048 mg bid) + MNZ (500 mg tid) + TET (500 mg qid) | 76.0%–94.5% | 82, 83 |
Abbreviations: AMX, Amoxicillin; bid, twice a day; CLR, Clarithromycin; LVX, Levofloxacin; MNZ, Metronidazole; PPI, Proton pump inhibitor; qid, four times a day; TET, Tetracycline; tid, three times a day; VPZ, Vonoprazan.
Multiple H. pylori treatment failure
Multiple H. pylori treatment failure is an unsuccessful eradication after completing two or more courses of standard H. pylori treatment regimens. 8 Factors associated with successful eradication are interactions among host, bacteria, and systems. The most common causes of eradication failure are poor adherence to medical therapy and antibiotic resistance. 8 Barriers to adherence, for example, regimen complexity, adverse effects, should be explored and addressed. Antibiotic resistance has been increasing in the past decade except for low tetracycline and amoxicillin resistance. After second‐line treatment failure, it is recommended to perform antimicrobial susceptibility testing (AST). If AST is available, we recommend using tailored treatment based on AST. If AST is not available, we recommend using antibiotic with low resistance potential such as amoxicillin, tetracycline, rifabutin, or furazolidone in combination with antibiotic, which was not used in previously failed regimens. CYP2C19 polymorphisms also have an impact on PPI metabolism contributing to change in intragastric pH, and eventually affecting H. pylori eradication. It is recommended to use high‐dose potent PPI or switch to use P‐CAB to achieve adequate acid suppression. Bismuth has bactericidal activity on H. pylori and also produces synergistic effect with antibiotics. 79 , 94 The addition of bismuth to H. pylori treatment regimen can increase the eradication rate by an additional 30%–40% despite resistant infection. 79 Lastly, treatment duration of 14 days provided higher eradication rates than shorter duration. 8 Hence, we recommend treatment duration of at least 14 days for H. pylori treatment failure. The proposed algorithm for management of H. pylori treatment failure is demonstrated in Figure 4.
Figure 4.
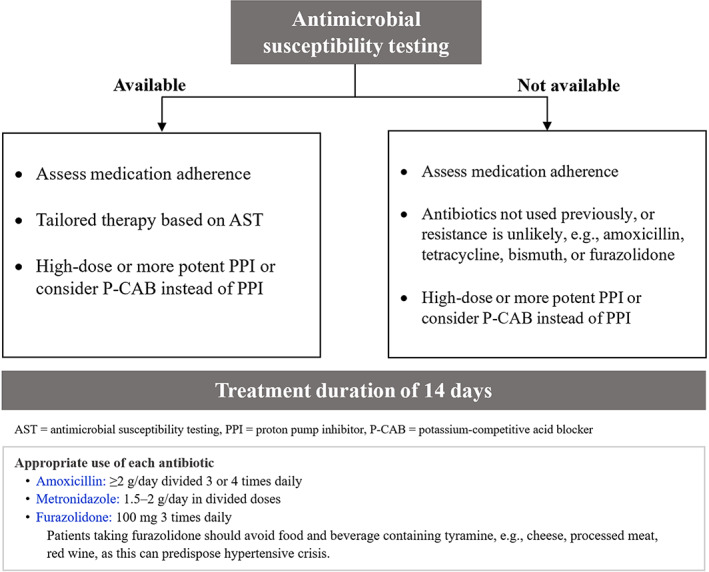
Algorithm for management of multiple H. pylori treatment failure.
Adjuvant therapy for H. pylori infection
Probiotics are used as an adjuvant treatment to reduce the side effects of antibiotics, which may further increase tolerability and enhance the eradication rate. In vitro and in vivo studies demonstrated that Lactobacillus, Bifidobacterium species, and Saccharomyces boulardii could exert an inhibitory effect on H. pylori. 95 , 96 Shi et al. conducted the network meta‐analysis including 40 studies (N = 8924) reporting that using probiotics could provide higher eradication rates and lower side effects. Lactobacillus and multiple strains contributed to better subgroup eradication rates. However, there were high heterogeneity, small sample size, and different strains of probiotics used in each study. 97 As H. pylori‐associated gastric atrophy can cause reduced acid secretion, it was previously suspected that eradication therapy might aggravate gastroesophageal reflux disease (GERD). 98 Nevertheless, prior studies reported that H. pylori eradication was not associated with development of GERD 98 and did not increase risk of Barrett's esophagus and esophageal adenocarcinoma. 99 In any case, if patients develop reflux symptoms after eradication, probiotics might be used to improve heartburn or regurgitation. 100 More trials are still needed to evaluate the association of probiotics and GERD.
Statins can inhibit H. pylori‐induced inflammation by inducing autophagy in macrophages. One randomized trial reported that patients receiving anti‐H. pylori therapy with statin had significantly higher eradication rate than the group without statin. 101 However, this study did not provide data about antimicrobial susceptibility test. Another large prospective cohort revealed slightly higher eradication rates in statin users than non‐statin group using empirical treatment (89% vs. 86%, OR 1.3; 95% CI 1.1–1.5, P = 0.002). 102 Larger clinical trials are needed to confirm the efficacy of adding statin in treatment regimen.
Vaccine against H. pylori
H. pylori vaccine has been developed for a decade. The largest phase 3 trial conducted in China (Chinese children, N = 4464) concluded that an efficacy of oral recombinant H. pylori vaccine was 71.8% in the first year and decreased to 55% in the second year. 103 Protection against H. pylori infection was maintained for 3 years after vaccination. Mild adverse reactions such as vomiting, fever, and headache were detected in 7% of both vaccine and placebo groups. 103 In contrast, another study reported that parenteral vaccine was not superior to placebo in prevention of H. pylori infection in adult patients. 104 Future trials are required to study the safety and efficacy of vaccine against H. pylori.
Conclusions
H. pylori infection is a major risk factor for gastric cancer. Diagnostic testing and treatment for H. pylori infection are recommended. High‐dose potent PPI or vonoprazan should be used to achieve adequate acid suppression. Fourteen‐day concomitant therapy or 14‐day BQT is recommended as first‐line eradication regimen in high clarithromycin resistance area, while 14‐day triple therapy or 14‐day BQT is recommended in low clarithromycin resistance area. AST should be performed in patients with multiple treatment failures to provide best‐tailored therapy and further increase eradication success.
Acknowledgments
This study was supported by Thailand Science Research and Innovation Fundamental Fund, Bualuang ASEAN Chair Professorship at Thammasat University, and Center of Excellence in Digestive Diseases, Thammasat University, Thailand.
Declaration of conflict of interest: None of the authors has any conflict of interests.
References
- 1. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984; 1: 1311–15. [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021; 71: 209–49. [DOI] [PubMed] [Google Scholar]
- 3. Sugano K, Tack J, Kuipers EJ et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015; 64: 1353–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta‐analysis in World Health Organization regions. Gastroenterology. 2018; 155: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment Of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017; 112: 212–39. [DOI] [PubMed] [Google Scholar]
- 6. Mahachai V, Vilaichone RK, Pittayanon R et al. Helicobacter pylori management in ASEAN: The Bangkok consensus report. J. Gastroenterol. Hepatol. 2018; 33: 37–56. [DOI] [PubMed] [Google Scholar]
- 7. Malfertheiner P, Megraud F, Rokkas T et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022; 71: 1724–62. [Google Scholar]
- 8. Shah SC, Iyer PG, Moss SF. AGA Clinical practice update on the management of refractory Helicobacter pylori Infection: expert review. Gastroenterology. 2021; 160: 1831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rokkas T, Gisbert JP, Malfertheiner P et al. Comparative effectiveness of multiple different first‐line treatment regimens for Helicobacter pylori Infection: a network meta‐analysis. Gastroenterology. 2021; 161: e4. [DOI] [PubMed] [Google Scholar]
- 10. Hooi JKY, Lai WY, Ng WK et al. Global prevalence of Helicobacter pylori infection: systematic review and meta‐analysis. Gastroenterology. 2017; 153: 420–9. [DOI] [PubMed] [Google Scholar]
- 11. Ferlay J, Ervik M, Lam F et al. Global cancer observatory: cancer today. Lyon, France: International agency for research on cancer. Available from: https://gco.iarc.fr/today, accessed [28 Jul 2022].
- 12. Vilaichone RK, Quach DT, Yamaoka Y, Sugano K, Mahachai V. Prevalence and pattern of antibiotic resistant strains of Helicobacter pylori infection in ASEAN. Asian Pac. J. Cancer Prev. 2018; 19: 1411–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vilaichone RK, Mahachai V, Graham DY. Helicobacter pylori diagnosis and management. Gastroenterol. Clin. North Am. 2006; 35: 229–47. [DOI] [PubMed] [Google Scholar]
- 14. Brown LM. Helicobacter pylori: Epidemiology and routes of transmission. Epidemiol. Rev. 2000; 22: 283–97. [DOI] [PubMed] [Google Scholar]
- 15. Rowland M, Daly L, Vaughan M, Higgins A, Bourke B, Drumm B. Age‐specific incidence of Helicobacter pylori . Gastroenterology. 2006; 130: 65–72 quiz 211. [DOI] [PubMed] [Google Scholar]
- 16. Malaty HM, Graham DY, Klein PD, Evans DG, Adam E, Evans DJ. Transmission of Helicobacter pylori infection: Studies in families of healthy individuals. Scand. J. Gastroenterol. 1991; 26: 927–32. [DOI] [PubMed] [Google Scholar]
- 17. Breckan RK, Paulssen EJ, Asfeldt AM, Kvamme JM, Straume B, Florholmen J. The all‐age prevalence of Helicobacter pylori infection and potential transmission routes. a population‐based study. Helicobacter. 2016; 21: 586–95. [DOI] [PubMed] [Google Scholar]
- 18. Everhart JE, Kruszon‐Moran D, Perez‐Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. 2000; 181: 1359–63. [DOI] [PubMed] [Google Scholar]
- 19. Malaty HM, Graham DY. Importance of childhood socioeconomic status on the current prevalence of Helicobacter pylori infection. Gut. 1994; 35: 742–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J. Gastroenterol. Hepatol. 2010; 25: 479–86. [DOI] [PubMed] [Google Scholar]
- 21. Sheh A, Fox JG. The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes. 2013; 4: 505–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen CC, Liou JM, Lee YC, Hong TC, el‐Omar EM, Wu MS. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes. 2021; 13: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo Y, Zhang Y, Gerhard M et al. Effect of Helicobacter pylori on gastrointestinal microbiota: a population‐based study in Linqu, a high‐risk area of gastric cancer. Gut. 2020; 69: 1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferreira RM, Pereira‐Marques J, Pinto‐Ribeiro I et al. Gastric microbial community profiling reveals a dysbiotic cancer‐associated microbiota. Gut. 2018; 67: 226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miwa H, Ghoshal UC, Fock KM et al. Asian consensus report on functional dyspepsia. J. Gastroenterol. Hepatol. 2012; 27: 626–41. [DOI] [PubMed] [Google Scholar]
- 26. Liou JM, Lin JT, Wang HP et al. The optimal age threshold for screening upper endoscopy for uninvestigated dyspepsia in Taiwan, an area with a higher prevalence of gastric cancer in young adults. Gastrointest. Endosc. 2005; 61: 819–25. [DOI] [PubMed] [Google Scholar]
- 27. Quach DT, Tran LT, Tran TL et al. Age cutoff and yield of prompt esophagogastroduodenoscopy to detect malignancy in Vietnamese with upper gastrointestinal symptoms: an endoscopic database review of 472,744 patients from 2014 to 2019. Can. J. Gastroenterol. Hepatol. 2021; 2021: 1184848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moayyedi P, Lacy BE, Andrews CN et al. ACG and CAG Clinical guideline: Management of dyspepsia. Am. J. Gastroenterol. 2017; 112: 988–1013. [DOI] [PubMed] [Google Scholar]
- 29. Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment. Pharmacol. Ther. 1995; 9: 59–69. [PubMed] [Google Scholar]
- 30. Sonnenberg A, Turner KO, Genta RM. Low prevalence of Helicobacter pylori‐positive peptic ulcers in private outpatient endoscopy centers in the United States. Am. J. Gastroenterol. 2020; 115: 244–50. [DOI] [PubMed] [Google Scholar]
- 31. Correa P, Houghton J. Carcinogenesis of Helicobacter pylori . Gastroenterology. 2007; 133: 659–72. [DOI] [PubMed] [Google Scholar]
- 32. Graham DY. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology. 2015; 148: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar S, Metz DC, Ellenberg S, Kaplan DE, Goldberg DS. Risk factors and incidence of gastric cancer after detection of Helicobacter pylori infection: a large cohort study. Gastroenterology. 2020; 158: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shin CM, Kim N, Yang HJ et al. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J. Clin. Gastroenterol. 2010; 44: e34–9. [DOI] [PubMed] [Google Scholar]
- 35. Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta‐analysis. Gut. 2020; 69: 2113–21. [DOI] [PubMed] [Google Scholar]
- 36. Aumpan N, Vilaichone RK, Pornthisarn B et al. Predictors for regression and progression of intestinal metaplasia (IM): A large population‐based study from low prevalence area of gastric cancer (IM‐predictor trial). PLoS One. 2021; 16: e0255601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi IJ, Kook MC, Kim YI et al. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N. Engl. J. Med. 2018; 378: 1085–95. [DOI] [PubMed] [Google Scholar]
- 38. Parsonnet J, Hansen S, Rodriguez L et al. Helicobacter pylori infection and gastric lymphoma. N. Engl. J. Med. 1994; 330: 1267–71. [DOI] [PubMed] [Google Scholar]
- 39. Stolte M, Bayerdörffer E, Morgner A et al. Helicobacter and gastric MALT lymphoma. Gut. 2002; 50 Suppl 3: III19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jung K, Kim DH, Seo HI, Gong EJ, Bang CS. Efficacy of eradication therapy in Helicobacter pylori‐negative gastric mucosa‐associated lymphoid tissue lymphoma: A meta‐analysis. Helicobacter. 2021; 26: e12774. [DOI] [PubMed] [Google Scholar]
- 41. Annibale B, Capurso G, Lahner E et al. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut. 2003; 52: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systematic review and meta‐analysis. Helicobacter. 2008; 13: 323–40. [DOI] [PubMed] [Google Scholar]
- 43. Nahon S, Lahmek P, Massard J et al. Helicobacter pylori‐associated chronic gastritis and unexplained iron deficiency anemia: a reliable association? Helicobacter. 2003; 8: 573–7. [DOI] [PubMed] [Google Scholar]
- 44. Qu XH, Huang XL, Xiong P et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? a meta‐analysis. World J. Gastroenterol. 2010; 16: 886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yuan W, Li Y, Yang K et al. Iron deficiency anemia in Helicobacter pylori infection: meta‐analysis of randomized controlled trials. Scand. J. Gastroenterol. 2010; 45: 665–76. [DOI] [PubMed] [Google Scholar]
- 46. Stasi R, Provan D. Helicobacter pylori and chronic ITP. Hematology Am. Soc. Hematol. Educ. Program. 2008; 2008: 206–11. [DOI] [PubMed] [Google Scholar]
- 47. Campuzano‐Maya G. Proof of an association between Helicobacter pylori and idiopathic thrombocytopenic purpura in Latin America. Helicobacter. 2007; 12: 265–73. [DOI] [PubMed] [Google Scholar]
- 48. Stasi R, Sarpatwari A, Segal JB et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009; 113: 1231–40. [DOI] [PubMed] [Google Scholar]
- 49. Neunert C, Terrell DR, Arnold DM et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019; 3: 3829–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. El‐Serag HB, Kao JY, Kanwal F et al. Houston Consensus Conference on testing for Helicobacter pylori Infection in the United States. Clin. Gastroenterol. Hepatol. 2018; 16: 992–1002 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang YK, Kuo FC, Liu CJ et al. Diagnosis of Helicobacter pylori infection: Current options and developments. World J. Gastroenterol. 2015; 21: 11221–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee JY, Kim N. Diagnosis of Helicobacter pylori by invasive test: histology. Ann Transl Med. 2015; 3: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schabereiter‐Gurtner C, Hirschl AM, Dragosics B et al. Novel real‐time PCR assay for detection of Helicobacter pylori infection and simultaneous clarithromycin susceptibility testing of stool and biopsy specimens. J. Clin. Microbiol. 2004; 42: 4512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ferwana M, Abdulmajeed I, Alhajiahmed A et al. Accuracy of urea breath test in Helicobacter pylori infection: meta‐analysis. World J. Gastroenterol. 2015; 21: 1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gisbert JP, Abraira V. Accuracy of Helicobacter pylori diagnostic tests in patients with bleeding peptic ulcer: a systematic review and meta‐analysis. Am. J. Gastroenterol. 2006; 101: 848–63. [DOI] [PubMed] [Google Scholar]
- 56. Gisbert JP, de la Morena F, Abraira V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: a systematic review and meta‐analysis. Am. J. Gastroenterol. 2006; 101: 1921–30. [DOI] [PubMed] [Google Scholar]
- 57. Kodama M, Murakami K, Okimoto T et al. Influence of proton pump inhibitor treatment on Helicobacter pylori stool antigen test. World J. Gastroenterol. 2012; 18: 44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Loy CT, Irwig LM, Katelaris PH, Talley NJ. Do commercial serological kits for Helicobacter pylori infection differ in accuracy? A meta‐analysis. Am. J. Gastroenterol. 1996; 91: 1138–44. [PubMed] [Google Scholar]
- 59. Wang XY, Yang Y, Shi RH, Ho B, Wang HD, Zhang GX. An evaluation of a serologic test with a current infection marker of Helicobacter pylori before and after eradication therapy in Chinese. Helicobacter. 2008; 13: 49–55. [DOI] [PubMed] [Google Scholar]
- 60. Quach DT, Hiyama T, Shimamoto F et al. Value of a new stick‐type rapid urine test for the diagnosis of Helicobacter pylori infection in the Vietnamese population. World J. Gastroenterol. 2014; 20: 5087–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Luzza F, Imeneo M, Marasco A et al. Evaluation of a commercial serological kit for detection of salivary immunoglobulin G to Helicobacter pylori: a multicentre study. Eur. J. Gastroenterol. Hepatol. 2000; 12: 1117–20. [DOI] [PubMed] [Google Scholar]
- 62. Hulten KG, Genta RM, Kalfus IN et al. Comparison of culture with antibiogram to next‐generation sequencing using bacterial isolates and formalin‐fixed, paraffin‐embedded gastric biopsies. Gastroenterology. 2021; 161: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aumpan N, Pornthisarn B, Chonprasertsuk S et al. Predictive factors for successful eradication in patients with Helicobacter pylori treatment failures: A large population‐based study. Gastroenterology. 2022; 162: S‐874. [Google Scholar]
- 64. Ho JJC, Navarro M, Sawyer K, Elfanagely Y, Moss SF. Helicobacter pylori antibiotic resistance in the United States between 2011‐2021: A systematic review and meta‐analysis. Am. J. Gastroenterol. 2022; 117: 1221–30. [DOI] [PubMed] [Google Scholar]
- 65. Megraud F, Bruyndonckx R, Coenen S et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut. 2021; 70: 1815–22. [DOI] [PubMed] [Google Scholar]
- 66. Jaka H, Rhee JA, Ostlundh L et al. The magnitude of antibiotic resistance to Helicobacter pylori in Africa and identified mutations which confer resistance to antibiotics: systematic review and meta‐analysis. BMC Infect. Dis. 2018; 18: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007; 12: 275–8. [DOI] [PubMed] [Google Scholar]
- 68. Villoria A, Garcia P, Calvet X, Gisbert JP, Vergara M. Meta‐analysis: high‐dose proton pump inhibitors vs. standard dose in triple therapy for Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 2008; 28: 868–77. [DOI] [PubMed] [Google Scholar]
- 69. Lee YC, Dore MP, Graham DY. Diagnosis and treatment of Helicobacter pylori infection. Annu. Rev. Med. 2022; 73: 183–95. [DOI] [PubMed] [Google Scholar]
- 70. Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin. Gastroenterol. Hepatol. 2018; 16: e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Phiphatpatthamaamphan K, Vilaichone RK, Siramolpiwat S et al. Effect of IL‐1 polymorphisms, CYP2C19 genotype and antibiotic resistance on Helicobacter pylori eradication comparing between 10‐day sequential therapy and 14‐day standard triple therapy with four‐times‐daily‐dosing of amoxicillin in Thailand: a prospective randomized study. Asian Pac. J. Cancer Prev. 2016; 17: 1903–7. [DOI] [PubMed] [Google Scholar]
- 72. Molina‐Infante J, Lucendo AJ, Angueira T et al. Optimised empiric triple and concomitant therapy for Helicobacter pylori eradication in clinical practice: the OPTRICON study. Aliment. Pharmacol. Ther. 2015; 41: 581–9. [DOI] [PubMed] [Google Scholar]
- 73. Prasertpetmanee S, Mahachai V, Vilaichone RK. Improved efficacy of proton pump inhibitor ‐ amoxicillin ‐ clarithromycin triple therapy for Helicobacter pylori eradication in low clarithromycin resistance areas or for tailored therapy. Helicobacter. 2013; 18: 270–3. [DOI] [PubMed] [Google Scholar]
- 74. Liou JM, Fang YJ, Chen CC et al. Concomitant, bismuth quadruple, and 14‐day triple therapy in the first‐line treatment of Helicobacter pylori: a multicentre, open‐label, randomised trial. Lancet. 2016; 388: 2355–65. [DOI] [PubMed] [Google Scholar]
- 75. Kongchayanun C, Vilaichone RK, Pornthisarn B, Amornsawadwattana S, Mahachai V. Pilot studies to identify the optimum duration of concomitant Helicobacter pylori eradication therapy in Thailand. Helicobacter. 2012; 17: 282–5. [DOI] [PubMed] [Google Scholar]
- 76. Nyssen OP, Bordin D, Tepes B et al. European registry on Helicobacter pylori management (Hp‐EuReg): patterns and trends in first‐line empirical eradication prescription and outcomes of 5 years and 21,533 patients. Gut. 2021; 70: 40–54. [DOI] [PubMed] [Google Scholar]
- 77. Lee HJ, Kim JI, Lee JS et al. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J. Gastroenterol. 2015; 21: 351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gatta L, Vakil N, Vaira D, Scarpignato C. Global eradication rates for Helicobacter pylori infection: systematic review and meta‐analysis of sequential therapy. BMJ. 2013; 347: f4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016; 65: 870–8. [DOI] [PubMed] [Google Scholar]
- 80. Dore MP, Farina V, Cuccu M, Mameli L, Massarelli G, Graham DY. Twice‐a‐day bismuth‐containing quadruple therapy for Helicobacter pylori eradication: a randomized trial of 10 and 14 days. Helicobacter. 2011; 16: 295–300. [DOI] [PubMed] [Google Scholar]
- 81. Vilaichone RK, Prapitpaiboon H, Gamnarai P et al. Seven‐day bismuth‐based quadruple therapy as an initial treatment for Helicobacter pylori infection in a high metronidazole resistant area. Asian Pac. J. Cancer Prev. 2015; 16: 6089–92. [DOI] [PubMed] [Google Scholar]
- 82. Lin TF, Hsu PI. Second‐line rescue treatment of Helicobacter pylori infection: Where are we now? World J. Gastroenterol. 2018; 24: 4548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kim SE, Park MI, Park SJ et al. Second‐line bismuth‐containing quadruple therapy for Helicobacter pylori eradication and impact of diabetes. World J. Gastroenterol. 2017; 23: 1059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Peedikayil MC, Alsohaibani FI, Alkhenizan AH. Levofloxacin‐based first‐line therapy versus standard first‐line therapy for Helicobacter pylori eradication: meta‐analysis of randomized controlled trials. PLoS One. 2014; 9: e85620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Antos D, Schneider‐Brachert W, Bästlein E et al. 7‐day triple therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and high‐dose esomeprazole in patients with known antimicrobial sensitivity. Helicobacter. 2006; 11: 39–45. [DOI] [PubMed] [Google Scholar]
- 86. Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium‐competitive acid blocker, as a component of first‐line and second‐line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double‐blind study. Gut. 2016; 65: 1439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Maruyama M, Tanaka N, Kubota D et al. Vonoprazan‐based regimen is more useful than PPI‐based one as a first‐line Helicobacter pylori eradication: A randomized controlled trial. Can. J. Gastroenterol. Hepatol. 2017; 2017: 4385161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sue S, Ogushi M, Arima I et al. Vonoprazan‐ vs proton‐pump inhibitor‐based first‐line 7‐day triple therapy for clarithromycin‐susceptible Helicobacter pylori: A multicenter, prospective, randomized trial. Helicobacter. 2018; 23: e12456. [DOI] [PubMed] [Google Scholar]
- 89. Suzuki S, Gotoda T, Kusano C et al. Seven‐day vonoprazan and low‐dose amoxicillin dual therapy as first‐line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020; 69: 1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Furuta T, Yamade M, Kagami T et al. Dual therapy with vonoprazan and amoxicillin is as effective as triple therapy with vonoprazan, amoxicillin and clarithromycin for eradication of Helicobacter pylori . Digestion. 2020; 101: 743–51. [DOI] [PubMed] [Google Scholar]
- 91. Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: Randomized clinical trial. Gastroenterology. 2022; 163: 608–19. [DOI] [PubMed] [Google Scholar]
- 92. Ratana‐Amornpin S, Vilaichone RK, Sanglutong L et al. Pilot studies of vonoprazan‐containing Helicobacter pylori eradication therapy suggest Thailand may be more similar to the US than Japan. Gastroenterology. 2022; 162: S‐872‐S‐873. [DOI] [PubMed] [Google Scholar]
- 93. Tungtrongchitr N, Pornthisarn B, Chonprasertsuk S et al. High efficacy of 14‐day vonoprazan‐based quadruple therapy for Helicobacter pylori eradication in areas with high clarithromycin resistance: A prospective randomized study (VQ‐HP trial). Gastroenterology. 2022; 162: S‐871. [Google Scholar]
- 94. McNicholl AG, Bordin DS, Lucendo A et al. Combination of bismuth and standard triple therapy eradicates Helicobacter pylori infection in more than 90% of patients. Clin. Gastroenterol. Hepatol. 2020; 18: 89–98. [DOI] [PubMed] [Google Scholar]
- 95. Zhang L, Su P, Henriksson A, O'Rourke J, Mitchell H. Investigation of the immunomodulatory effects of Lactobacillus casei and Bifidobacterium lactis on Helicobacter pylori infection. Helicobacter. 2008; 13: 183–90. [DOI] [PubMed] [Google Scholar]
- 96. Szajewska H, Horvath A, Piwowarczyk A. Meta‐analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment. Pharmacol. Ther. 2010; 32: 1069–79. [DOI] [PubMed] [Google Scholar]
- 97. Shi X, Zhang J, Mo L, Shi J, Qin M, Huang X. Efficacy and safety of probiotics in eradicating Helicobacter pylori: A network meta‐analysis. Medicine (Baltimore). 2019; 98: e15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Qian B, Ma S, Shang L, Qian J, Zhang G. Effects of Helicobacter pylori eradication on gastroesophageal reflux disease. Helicobacter. 2011; 16: 255–65. [DOI] [PubMed] [Google Scholar]
- 99. Doorakkers E, Lagergren J, Santoni G, Engstrand L, Brusselaers N. Helicobacter pylori eradication treatment and the risk of Barrett's esophagus and esophageal adenocarcinoma. Helicobacter. 2020; 25: e12688. [DOI] [PubMed] [Google Scholar]
- 100. Cheng J, Ouwehand AC. Gastroesophageal reflux disease and probiotics: A systematic review. Nutrients. 2020; 12: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sarkeshikian SS, Ghadir MR, Alemi F, Jalali SM, Hormati A, Mohammadbeigi A. Atorvastatin in combination with conventional antimicrobial treatment of Helicobacter pylori eradication: A randomized controlled clinical trial. J. Gastroenterol. Hepatol. 2020; 35: 71–5. [DOI] [PubMed] [Google Scholar]
- 102. Caldas M, Perez‐Aisa A, Tepes B et al. The role of statins on Helicobacter pylori eradication: Results from the European registry on the management of H. pylori (Hp‐EuReg). Antibiotics (Basel). 2021; 10: 965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zeng M, Mao XH, Li JX et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2015; 386: 1457–64. [DOI] [PubMed] [Google Scholar]
- 104. Malfertheiner P, Selgrad M, Wex T et al. Efficacy, immunogenicity, and safety of a parenteral vaccine against Helicobacter pylori in healthy volunteers challenged with a Cag‐positive strain: a randomised, placebo‐controlled phase 1/2 study. Lancet Gastroenterol. Hepatol. 2018; 3: 698–707. [DOI] [PubMed] [Google Scholar]


