Abstract
Nitric oxide (NO) is an important effector molecule of the immune system in eliminating numerous pathogens. Peritoneal macrophages from Trypanosoma brucei brucei-infected mice express type II NO synthase (NOS-II), produce NO, and kill parasites in the presence of l-arginine in vitro. Nevertheless, parasites proliferate in the vicinity of these macrophages in vivo. The present study shows that l-arginine availability modulates NO production. Trypanosomes use l-arginine for polyamine synthesis, required for DNA and trypanothione synthesis. Moreover, arginase activity is up-regulated in macrophages from infected mice from the first days of infection. Arginase competes with NOS-II for their common substrate, l-arginine. In vitro, arginase inhibitors decreased urea production, increased macrophage nitrite production, and restored trypanosome killing. In vivo, a dramatic decrease in l-arginine concentration was observed in plasma from infected mice. In situ restoration of NO production and trypanosome killing were observed when excess l-arginine, but not d-arginine or l-arginine plus Nω-nitro-l-arginine (a NOS inhibitor), was injected into the peritoneum of infected mice. These data indicate the role of l-arginine depletion, induced by arginase and parasites, in modulating the l-arginine–NO pathway under pathophysiological conditions.
Activated macrophages have been shown previously to express arginase isoforms and nitric oxide (NO) synthase type II (NOS-II) (14, 22, 38). The common substrate used by these enzymes is l-arginine, which can be hydrolyzed by arginase to ornithine and urea or oxidized by NOS-II to l-citrulline and NO. Arginase, a suppressor of macrophage-derived cytotoxicity (8, 19), has also been shown in vitro to modulate NO production by macrophages through competition for intracellular l-arginine (6, 12, 14, 44). Arginase and NOS-II activities are induced in rodent macrophages by lipopolysaccharides (6, 20, 35, 44). In elicited macrophages, substrate availability relies mainly on exogenous concentration, since recycling of l-arginine from l-citrulline or l-ornithine occurs at a low rate (15). The NOS-II–arginase balance is competitively regulated by the Th1-Th2 cytokine balance (27).
NO synthesis must be accurately regulated, as NO is implicated in the pathophysiology of inflammatory diseases, septic shock, and immunosuppression (24, 36, 41). NOS-II and arginase pathways have opposite biological effects (13). However, it has been proposed that their successive induction plays an important role in wound healing (37). The expression of NOS may be important in favoring microbiostasis-microbial killing and vasodilatation in the early stages, whereas the expression of arginase, favoring polyamine biosynthesis (5), fibroblast replication, and collagen induction (39), may be important in the later stages. These data suggest that a modification in the intensity, location, and chronology of these events may have negative effects during infectious diseases, by favoring microbial growth and reducing host effector mechanisms.
Trypanosomes of the brucei group, the causative agents of human and animal African trypanosomiasis, are extracellular parasites highly susceptible to NO (43). A large expansion of macrophages in the liver, spleen, and bone marrow has been observed for Trypanosoma brucei brucei-infected mice (29). The presence of NOS-II was demonstrated in these cells (36). The antitrypanosome effects of NO synthesized by macrophages from infected mice have been analyzed in vitro (10, 25). Nevertheless, in infected mice, parasites proliferate in the vicinity of macrophages in the peritoneal cavity, suggesting a reduced efficiency of NO-dependent cytotoxicity in vivo. NO production is dependent on l-arginine concentration (15). Complex, strong interactions between the l-arginine-metabolizing enzymes have been demonstrated elsewhere (3, 4). These interactions are even more complex in infected mice, as trypanosomes use l-arginine for the polyamine synthesis required for the synthesis of DNA and trypanothione, the equivalent of glutathione in mammals (9). Arginase activity may be involved in NOS activity impairment (6). In this study, an increase in arginase activity was observed in peritoneal macrophages from the first days of infection. An induction of NOS-II in macrophages and a decrease in plasma l-arginine concentration were observed later. Intraperitoneal NO production and NO-dependent parasite killing were restored by injection of l-arginine.
MATERIALS AND METHODS
Reagents.
l-Arginine, d-arginine, Nω-hydroxy-l-arginine (NOHA), and Nω-nitro-l-arginine (l-NA) were obtained from Alexis (San Diego, Calif.). Nω-Hydroxynor-l-arginine (nor-NOHA) was prepared as previously described (26). l-[Guanido-14C]arginine (l-[14C]arginine) was purchased from NEN (Cambridge, Mass.). All other chemicals were from Sigma (St. Louis, Mo.).
Parasites and mice.
The Antat 1.1 E clone of T. b. brucei was obtained from the Institute of Tropical Medicine in Antwerp, Belgium. Trypanosomes were kept frozen in liquid nitrogen as stabilates (10). Female Swiss mice (Iffa Credo, Saint-Germain-sur-l'Arbresle, France) were subcutaneously infected with 104 parasites per mouse. All animals were housed under conventional conditions, given water and chow ad libitum, and infected at 10 to 15 weeks of age. The use of the animals conformed to institutional guidelines.
Cells.
Peritoneal cells from control or infected ether-anesthetized mice were collected in Hanks' buffered salt solution (HBSS) free of Ca2+, Mg2+, and phenol red (Life Technologies, Paisley, Scotland), supplemented with 20 mM HEPES, 2 mM sodium pyruvate, and 10 μg of gentamicin per ml. Cells (8 × 105/well) were layered on 24-well plates (Nunc Inc., Naperville, Ill.), and macrophages were enriched by washing off nonadherent cells after a 1-h incubation and were counted. l-Arginine or l-[14C]arginine was used as a substrate for NOS-II and arginase.
Due to the high concentration of l-arginine in minimal essential medium culture medium (0.6 mM), cocultures of macrophages (4 × 105/well) and T. b. brucei 1.1 E (5 × 104/well) were performed in 24-well plates with a minimal essential medium Select-Amine kit (Life Technologies) containing 10% fetal calf serum, 20 mM HEPES, 2 mM sodium pyruvate, 10 μg of gentamicin per ml, and variable concentrations of l-arginine (500 μl/well). nor-NOHA, an arginase inhibitor that is not a substrate for NOS (26), was added when required. Parasites were counted after 3 days of culture.
Determination of arginase activity.
Two methods were used. (i) Arginase activity was evaluated in ex vivo macrophages from uninfected mice or from mice at days 2, 4, 6, 8, 10, 12, and 14 postinfection using the micromethod described by Corraliza et al. (7). Briefly, 105 cells were treated with 0.1% Triton X-100–0.01% pepstatin–0.01% aprotinin–0.01% antipain. MnCl2 (10 mM) and l-arginine (0.5 M) were successively added to the supernatants. The reaction was stopped by the addition of an acid solution, and the urea formed by the arginase was analyzed by adding α-isonitrosopropiophenone. The colored product was quantified by absorption at 540 nm. (ii) Arginase activity was measured in cultures by the conversion of l-[14C]arginine to [14C]urea (34). Briefly, macrophages (4 × 105/well) were cultured in supplemented HBSS containing 2 mM l-arginine and 0.1 μM l-[14C]arginine (specific activity, 51.5 mCi/mmol), with or without l-norvaline (10 mM), nor-NOHA (0.1 mM), or NOHA (1 mM). The same media were incubated without cells as a negative control. At the indicated times, 150 μl of supernatant was removed and added to 800 μl of 250 mM acetic acid solution, pH 4.5, containing 100 mM urea and 10 mM l-arginine. After the addition of Dowex resin (HCR-W2; Sigma), the tubes were centrifuged at 120 × g for 5 min. Supernatants (500 μl) containing [14C]urea were removed and added to 3 ml of scintillation fluid in counting vials. The percentage of l-[14C]arginine converted to [14C]urea was calculated as previously described (34).
l-Arginine-dependent killing of intraperitoneal trypanosomes.
HBSS (0.5 ml) alone or containing 0.1 M l-arginine, 0.1 M d-arginine, or 0.1 M l-arginine plus 0.1 M l-NA was intraperitoneally injected into 10-day-infected mice (i.e., the quantity injected per mouse was 8.7 mg of l-arginine or d-arginine or 10.9 mg of l-NA).
The injection of the arginase inhibitor nor-NOHA was not used because it was available in only limited quantities, insufficient for in vivo experiments. l-NA was chosen as an NOS inhibitor as it does not modify trypanosome and cell cultures (10). The peritoneal content of each mouse was collected by an injection of HBSS (4 ml) 24 h later to measure nitrite concentration and number of parasites.
Measurement of NO2− concentration.
The induction of NOS-II activity was assessed by measuring NO2− concentration in supernatants of macrophages cultured for 24 h in HBSS supplemented with an excess of l-arginine (1 mM).
After reaction with the Griess reagent (11), the concentration of NO2−, a stable oxidized derivative of NO in cell cultures (23), was determined spectrophotometrically at 540 nm in each cell culture supernatant or in peritoneal liquid, as previously described.
Measurement of plasma l-arginine concentration.
Plasma l-arginine was quantified on an automatic amino acid analyzer (Beckman 6300; Beckman, London, England) using a high-resolution method for amino acid analysis involving the use of lithium buffers and a high-performance chromatography lithium ion-exchange column (16).
RESULTS
Time course of NOS-II and arginase activities in macrophages from T. b. brucei-infected mice.
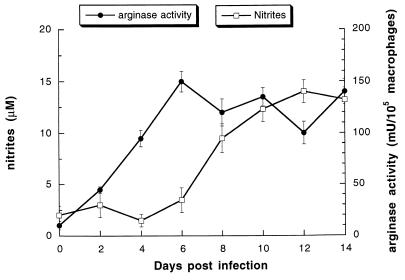
In the peritoneal fluid from 8-day-infected mice, macrophages had the morphological characteristics of activated cells (enlargement and numerous cytoplasmic vacuoles) and trypanosomes were also observed (ranging from 107 to 108 per mouse). Arginase and NOS activities were measured in macrophages from mice at days 2, 4, 6, 8, 10, 12, and 14 postinfection. Day 0 represents arginase and nitrite production of macrophages from uninfected mice (control macrophages). An increase in macrophage arginase activity was observed after 2 days of infection, whereas significant NO2− production occurred after 6 days (Fig. 1). Arginase and nitrite production remained high during the disease until the animals died (mean survival time, 14 days).
FIG. 1.
NOS-II and arginase activities in macrophages from infected mice. Mice were infected with 104 parasites. Peritoneal macrophages were collected at indicated times. Arginase activity was determined on cell lysates (●). NO2− concentration was measured in the supernatant of macrophages cultured for 24 h in HBSS containing 1 mM l-arginine (□). Data are the means ± standard errors from 10 mice for each day.
l-Arginine degradation by macrophage arginase from infected mice.
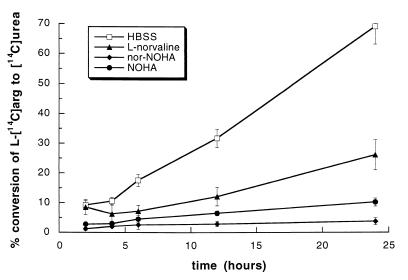
The increase in arginase activity in macrophages from infected mice was confirmed by the generation of [14C]urea from l-[14C]arginine in culture. A time-dependent consumption of l-[14C]arginine by macrophages from 10-day-infected mice was observed (Fig. 2). After 12 and 24 h of culture, 32 and 65% of the l-arginine had been metabolized into urea, respectively. Urea synthesis by macrophages from infected animals was inhibited by using NOHA or nor-NOHA in cultures. l-Norvaline was a less effective arginase inhibitor. Basal arginase activity was detected in supernatants from control macrophages (5% of conversion after a 24-h incubation).
FIG. 2.
Conversion of l-[14C]arginine to [14C]urea by macrophages from T. b. brucei-infected mice. Macrophages from 10-day-infected mice were cultured in HBSS supplemented with 2 mM l-arginine and 0.1 μM l-[14C]arginine (□), with or without 10 mM l-norvaline (▴), 1 mM NOHA (●), or 100 μM nor-NOHA (⧫). The conversion of l-[14C]arginine to [14C]urea was determined at indicated times. Data represent means ± standard errors of four experiments.
The effect of arginase inhibition on NO2− production and trypanosome growth in vitro.
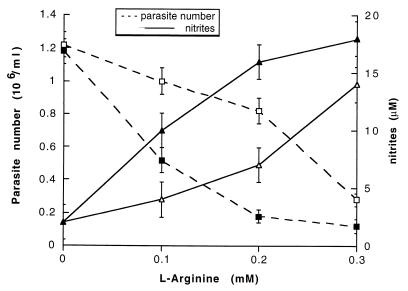
The inhibition of macrophage arginase on parasite growth and nitrite production was assessed in vitro. nor-NOHA, the most efficient arginase inhibitor, was used for in vitro experiments. Macrophages from 10-day-infected mice were cocultured with parasites in a medium containing increasing concentrations of l-arginine, with or without 500 μM nor-NOHA. Nitrite production and trypanosome counts were assessed 3 days later (Fig. 3). Parasite killing and nitrite production were maximal at l-arginine concentrations greater than 0.3 mM. At physiological concentrations of l-arginine (0.1 to 0.2 mM), the presence of 500 μM nor-NOHA enhanced nitrite production and trypanosome killing (Fig. 3). A direct toxic effect of nor-NOHA on trypanosomes, independent of NO generation, was ruled out. In cocultures of control macrophages and trypanosomes, parasite growth was not affected by the presence of nor-NOHA (data not shown).
FIG. 3.
Effect of arginase inhibition by nor-NOHA on NO production and trypanosome growth in vitro. T. b. brucei (5 × 104/well) was cocultured with macrophages (4 × 105/well) from infected mice, with (solid symbols) or without (open symbols) 500 μM nor-NOHA, in a medium containing l-arginine at concentrations ranging from 0 to 0.3 mM. After 3 days, NO2− concentration (triangles) was measured and parasites (squares) were counted in each supernatant. Data represent means ± standard errors of four experiments.
Measuring plasma l-arginine concentration.
The l-arginine concentration in the plasma of control mice was 189 ± 20 μM (mean ± standard error from eight animals). A decrease in l-arginine concentration was observed in the plasma of 6-, 10-, and 13-day-infected mice (96 ± 15, 42 ± 13, and 39 ± 16 μM, respectively; mean ± standard error from eight animals).
l-Arginine-dependent killing of intraperitoneal trypanosomes.
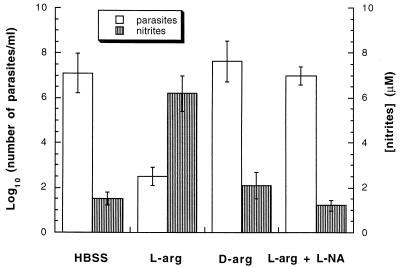
HBSS (0.5 ml) alone or containing 0.1 M l-arginine, 0.1 M d-arginine, or 0.1 M l-arginine plus 0.1 M l-NA was intraperitoneally injected into 10-day-infected mice. The arginase inhibitor nor-NOHA was not used as it was available in only limited quantities, insufficient for in vivo experiments. Peritoneal content was collected by washing with 4 ml of HBSS 24 h later. When l-arginine was injected, an increase in [NO2−] and a dramatic decrease in the number of peritoneal parasites were observed (Fig. 4). Compared to the injection of HBSS alone, the injection of l-arginine plus l-NA or d-arginine had no effect on NO2− concentrations and parasite counts (Fig. 4). No significant differences in peritoneal macrophage numbers were observed in any of the mice. The number of macrophages and the parasite/macrophage ratio were (9.2 ± 0.4) × 106 and 7.8 ± 3.5 (HBSS-injected mice), (9.4 ± 0.5) × 106 and (5.6 ± 3.9) × 10−4(l-arginine-injected mice), (9.6 ± 0.3) × 106 and 12.1 ± 4.2 (d-arginine-injected mice), and (9.4 ± 0.5) × 106 and 9.1 ± 3.1 (l-arginine-plus-l-NA-injected mice), respectively. Trypanosome growth in culture was not affected by using l-arginine, d-arginine, l-NA, or l-arginine plus l-NA, as previously indicated (10).
FIG. 4.
Effect of l-arginine injection on peritoneal T. b. brucei count and NO2− concentration. HBSS (0.5 ml), alone or containing l-arginine (0.1 M), d-arginine (0.1 M), or l-arginine (0.1 M) plus l-NA (0.1 M), was intraperitoneally injected into 10-day-infected mice (i.e., the quantity injected per mouse was 8.7 mg of l-arginine or d-arginine or 10.9 mg of l-NA). After 24 h, peritoneal content was gathered by injecting 4 ml of HBSS. Parasites were then counted (open bars). After centrifugation at 1,500 × g, NO2− concentrations were assayed in each supernatant (gray bars). Each result represents the mean ± standard error from six animals.
DISCUSSION
This study highlights the major role of l-arginine availability in NO production and parasite growth control by macrophages in vivo. Arginase activity increased in macrophages from T. b. brucei-infected mice. The use of l-[14C]arginine showed that, in these macrophages, the majority of l-arginine was metabolized by arginase. Moreover, parasites use l-arginine in their polyamine metabolism. All these elements contribute to a decrease in plasma l-arginine in T. b. brucei-infected mice. This decrease in l-arginine, previously reported for Trypanosoma brucei gambiense-infected voles (30), may reduce NO production and alter parasite killing. The restoration of NO production and trypanosome killing were observed in vitro when an arginase inhibitor was added to a culture medium containing physiological concentrations of l-arginine and in vivo in the peritoneum of infected mice when an excess of l-arginine was intraperitoneally injected. These data suggest an in situ regulation of NO production through substrate depletion.
In T. b. brucei-infected mice, an increase in serum nitrate is followed by a decrease (21). Although the expression of NOS-II in the presence of sufficient l-arginine is able to exert a trypanocidal activity, reduced availability of l-arginine may reduce nitrite production and trypanosome killing. Besides consumption by parasites and insufficient NO production for parasite killing, l-arginine availability may be reduced by the activity of arginase isoforms, the hepatic type (arginase I) and extrahepatic type (arginase II). The genes for the two arginase isoforms are regulated differentially. Both arginase I and arginase II are induced in activated mouse peritoneal macrophages (20, 35). Arginase I is induced by Th2 cytokines in bone marrow-derived macrophages (28). Endotoxin and circulating Th1 and Th2 cytokine levels are increased in trypanosomiasis (1, 32, 33).
Trypanosomes release a T-lymphocyte-triggering factor that induces the secretion of gamma interferon (IFN-γ) (31). This production of IFN-γ during experimental trypanosomiasis is probably responsible for NOS-II induction in macrophages. However, for cattle infected with Trypanosoma congolense, reduced secretion of NO by IFN-γ-activated monocytes and increased transcription of interleukin-10 have been reported previously (40). T-lymphocyte-triggering factor may also be involved in the production of interleukin-4 and transforming growth factor β, a potent stimulator of arginase activity, by CD8+ T cells (1, 5). All these data suggest that the induction of NOS-II and arginase may reflect the marked dysregulation of the cytokine network in trypanosomiasis and/or be involved in several distinct phenomena at various stages in the disease. Induction of arginases I and II in various tissues in infected mice may contribute to the decreased availability of l-arginine and impair NO-dependent mechanisms. As Th1 and Th2 cytokines regulate the NOS-II–arginase balance in macrophages, the production and time course of these cytokines in trypanosomiasis deserve further investigation.
The production of NO, assessed by measuring plasma nitrate concentrations, has been evidenced in response to T. b. brucei infection in mice (21). At different times and locations in trypanosome infections, NO-dependent mechanisms may have opposite effects on the host-parasite relationship. As NO and S-nitroso proteins are involved in the cytostatic-cytotoxic activity of macrophages (10, 25), an impairment of NO production may be correlated with trypanosome growth and disease severity at the onset of infection, as trypanoresistant mice produce more nitrites than do susceptible ones (18). It has also been speculated that antitrypanosome antibodies mediate the attachment of trypanosomes to activated macrophages and that NO-derived reactive species may affect the juxtaposed parasites (18). However, as NO is cytotoxic for several cell types, such as neurons or lymphocytes, arginase activity may be the result of a regulation mechanism limiting NO synthesis in infected mice. Inappropriate, persistent NO production may also be involved in immunosuppressive mechanisms (36) and the pathophysiology of the meningoencephalitis observed during trypanosomiasis. In vivo, the systemic inhibition of NOS attenuates immunosuppressive phenomena in T. brucei-infected mice (39). The conditions required for NO synthesis, the quantities produced, and the timing of these phenomena may be critical for this molecule's role in trypanosomiasis. NO may enhance trypanosome resistance in certain tissues and trypanosome susceptibility in others, as has been suggested for experimental malaria (17). The local restoration of NO production by administration of l-arginine to infected mice has been correlated with a decrease in parasite burden in the peritoneum. The restoration of NO production is likely to be ineffective in other locations. In blood, due to the excess of NO scavengers, the role of other elements, such as antibodies or tumor necrosis factor alpha, may be predominant.
Trypanosomes have developed several strategies for bypassing the immune system, including evasion of the specific immune response via the antigenic variation phenomenon (2). Arginase also promotes the synthesis of l-ornithine, a precursor of the polyamines involved in DNA and trypanothione synthesis (9). Difluoromethyl ornithine, an inhibitor of ornithine decarboxylase, a key enzyme in polyamine synthesis, is now used in the treatment of human sleeping sickness (42). The early increase in arginase production in trypanosomiasis may represent a way for parasites to avoid the antimicrobial effect of reactive nitrogen intermediates and benefit from larger quantities of l-ornithine. Arginase inhibitors are currently being developed and may represent good candidates for controlling microbial proliferation.
ACKNOWLEDGMENT
This research was supported by grants from the Conseil Régional d'Aquitaine.
REFERENCES
- 1.Bakhiet M, Olsson T, Ljungdahl A, Hojeberg B, Van Der Meide P, Kristenson K. Induction of interferon-gamma, transforming growth factor-beta, and interleukin-4 in mouse strains with different susceptibilities to Trypanosoma brucei brucei. J Interferon Cytokine Res. 1996;16:427–433. doi: 10.1089/jir.1996.16.427. [DOI] [PubMed] [Google Scholar]
- 2.Barry J D, Turner C M R. The dynamics of antigenic variation and growth of African trypanosomes. Parasitol Today. 1991;7:207–211. doi: 10.1016/0169-4758(91)90143-c. [DOI] [PubMed] [Google Scholar]
- 3.Beaumier L, Castillo L, Yu Y M, Ajami A M, Young V R. Arginine: new and exciting developments for an “old” amino acid. Biomed Environ Sci. 1996;9:296–315. [PubMed] [Google Scholar]
- 4.Boucher J L, Moali C, Tenu J P. Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cell Mol Life Sci. 1999;55:1015–1028. doi: 10.1007/s000180050352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutard V, Havouis R, Fouqueray B, Philippe C, Moulinoux J P, Baud L. Transforming growth factor-β stimulates arginase activity in macrophages. J Immunol. 1995;155:2077–2084. [PubMed] [Google Scholar]
- 6.Chang C I, Liao J C, Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am J Physiol. 1998;274:H342–H348. doi: 10.1152/ajpheart.1998.274.1.H342. [DOI] [PubMed] [Google Scholar]
- 7.Corraliza I M, Campo M L, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231–235. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 8.Currie G A, Gyure L, Cifuentes L. Microenvironmental arginine depletion by macrophages in vivo. Br J Cancer. 1979;39:613–620. doi: 10.1038/bjc.1979.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fairlamb A H, Cerami A. Metabolism and functions of trypanothione in the kinetoplastida. Annu Rev Microbiol. 1992;46:695–729. doi: 10.1146/annurev.mi.46.100192.003403. [DOI] [PubMed] [Google Scholar]
- 10.Gobert A P, Semballa S, Daulouede S, Lesthelle S, Taxile M, Veyret B, Vincendeau P. Murine macrophages use oxygen- and nitric oxide-dependent mechanisms to synthesize S-nitroso-albumin and to kill extracellular trypanosomes. Infect Immun. 1998;66:4068–4072. doi: 10.1128/iai.66.9.4068-4072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hageman R H, Reed A J. Nitrate reductase from higher plants. Methods Enzymol. 1980;40:427–429. [Google Scholar]
- 12.Hibbs J B, Taintor R R, Vavrin Z. l-Arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition of target cells. J Immunol. 1987;138:550–565. [PubMed] [Google Scholar]
- 13.Hibbs J B, Taintor R R, Vavrin Z, Granger D L, Drapier J C, Amber I J, Lancaster J R. Synthesis of nitric oxide from a terminal guanidino nitrogen atom of l-arginine. In: Moncada S, Higgs E A, editors. Nitric oxide from l-arginine. A bioregulatory system. New York, N.Y: Elsevier; 1990. pp. 189–223. [Google Scholar]
- 14.Hrabak A, Antoni F, Csuka I. Differences in the arginase activity produced by resident and stimulated murine and rat peritoneal macrophages. Int J Biochem. 1991;23:997–1003. doi: 10.1016/0020-711x(91)90136-b. [DOI] [PubMed] [Google Scholar]
- 15.Hrabak A, Idei M, Temesi A. Arginine supply for nitric oxide synthesis and arginase is mainly exogenous in elicited murine and rat macrophages. Life Sci. 1994;55:797–805. doi: 10.1016/0024-3205(94)00563-x. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard R W, Chambers J G, Sanchez A. Amino acid analysis of plasma: studies in sample preparation. J Chromatogr. 1988;431:163–169. doi: 10.1016/s0378-4347(00)83080-7. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs P, Radzioch D, Stevenson M M. Nitric oxide expression in the spleen, but not in the liver, correlates with resistance to blood-stage malaria in mice. J Immunol. 1995;155:5306–5311. [PubMed] [Google Scholar]
- 18.Kaushik R S, Uzonna J E, Gordon J R, Tabel H. Innate resistance to Trypanosoma congolense infections: differential production of nitric oxide by macrophages from susceptible BALB/c and resistant C57Bl/6 mice. Exp Parasitol. 1999;92:131–143. doi: 10.1006/expr.1999.4408. [DOI] [PubMed] [Google Scholar]
- 19.Kung J T, Brooks S B, Jakway J P, Leonard L L, Talmage D W. Suppression of in vitro cytotoxic response by macrophages due to induced arginase. J Exp Med. 1977;146:665–672. doi: 10.1084/jem.146.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis C A, Reichner J S, Mastrofrancesco W L B, Gotoh T, Mori M, Albina J E. Distinct arginase isoforms expressed in primary and transformed macrophages: regulation by oxygen tension. Am J Physiol. 1998;274:R775–R782. doi: 10.1152/ajpregu.1998.274.3.R775. [DOI] [PubMed] [Google Scholar]
- 21.Mabbot N A, Coulson P S, Smythies L E, Wilson R A, Sternberg J M. African trypanosome infections in mice that lack the interferon-γ receptor gene: nitric oxide-dependent and -independent suppression of T-cell proliferative responses and the development of anemia. Immunology. 1998;94:476–480. doi: 10.1046/j.1365-2567.1998.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mac Micking J, Xie Q W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 23.Marletta M A, Yoon P S, Iyengar R, Leaf C D, Wishnok J. Macrophage oxidation of l-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988;27:8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- 24.McInnes I B, Leung B P, Field M, Wei X Q, Huang F P, Sturrock R G, Kinninmonth A, Weidner J, Munford R, Liew F Y. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996;184:1519–1524. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mnaimneh S, Geffard M, Veyret B, Vincendeau P. Albumin nitrosylated by activated macrophages possesses antiparasitic effects neutralized by anti-NO-acetylated-cysteine antibodies. J Immunol. 1997;158:308–314. [PubMed] [Google Scholar]
- 26.Moali C, Boucher J L, Sari M A, Stuehr D J, Mansuy D. Substrate specificity of NO synthases: detailed comparison of L-arginine, homo-L-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-L-arginine. Biochemistry. 1998;29:10453–10460. doi: 10.1021/bi980742t. [DOI] [PubMed] [Google Scholar]
- 27.Modolell M, Corraliza I M, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginine balance in mouse bone marrow-derived macrophages by Th1 and Th2 cytokines. Eur J Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- 28.Munder M, Eichman K, Moran J M, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dentritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 29.Murray P K, Jennings W, Murray M, Urquhart G M. The nature of immunosuppression in Trypanosoma brucei infections in mice. The role of the macrophage. Immunology. 1974;27:815–824. [PMC free article] [PubMed] [Google Scholar]
- 30.Newport G R, Page C R, Ashman P U, Stibbs H H, Seed J R. Alteration of free serum amino acids in voles infected with Trypanosoma brucei gambiense. J Parasitol. 1977;63:15–24. [PubMed] [Google Scholar]
- 31.Olson T, Bakhiet M, Edlund C, Höjeberg B, Van Der Meide P, Kristensson K. Bidirectional activating signals between Trypanosoma brucei and CD8+ T cells: a trypanosome-released factor triggers interferon-γ production that stimulates parasite growth. Eur J Immunol. 1991;21:2447–2454. doi: 10.1002/eji.1830211022. [DOI] [PubMed] [Google Scholar]
- 32.Pentreath V W, Alafiatayo R A, Crawley B, Doua F B, Oppenheim B A. Endotoxins in the blood and cerebrospinal fluid of patients with African sleeping sickness. Parasitology. 1996;112(Pt. 1):67–73. doi: 10.1017/s0031182000065082. [DOI] [PubMed] [Google Scholar]
- 33.Rhind S G, Sabiston B H, Shek P N, Buguet A, Muanga G, Stanghellini A, Dumas M, Radomski M W. Effect of melarsoprol treatment on circulating IL-10 and TNF-alpha levels in human African trypanosomiasis. Clin Immunol Immunopathol. 1997;83:185–189. doi: 10.1006/clin.1997.4350. [DOI] [PubMed] [Google Scholar]
- 34.Russel A S, Ruegg U T. Arginase production by peritoneal macrophages: a new assay. J Immunol Methods. 1980;32:375–382. doi: 10.1016/0022-1759(80)90029-0. [DOI] [PubMed] [Google Scholar]
- 35.Salimuddin, Nagasaki A, Gotoh T, Isobe H, Mori M. Regulation of the genes for arginase isoforms and related enzymes in mouse macrophages by lipopolysaccharide. Am J Physiol. 1999;277(1 Pt. 1):E110–E117. doi: 10.1152/ajpendo.1999.277.1.E110. [DOI] [PubMed] [Google Scholar]
- 36.Schleifer K W, Mansfield J M. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993;151:5492–5503. [PubMed] [Google Scholar]
- 37.Shearer J D, Richards J R, Mills C D, Caldwell M D. Differential regulation of macrophage arginine metabolism: a proposed role in wound healing. Am J Physiol. 1997;272:E181–E190. doi: 10.1152/ajpendo.1997.272.2.E181. [DOI] [PubMed] [Google Scholar]
- 38.Sonoki T A, Nagazaki T, Gotoh T, Takiguchi M, Takeya M, Matsuzaki H, Mori M. Coinduction of nitric-oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysaccharide. J Biol Chem. 1997;272:3689–3693. doi: 10.1074/jbc.272.6.3689. [DOI] [PubMed] [Google Scholar]
- 39.Sternberg J M, Mabbott N, Sutherland I, Liew F Y. Inhibition of nitric oxide synthesis leads to reduced parasitemia in murine Trypanosoma brucei infection. Infect Immun. 1994;62:2135–2137. doi: 10.1128/iai.62.5.2135-2137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor K, Mertens B, Lutje V, Saya R. Trypanosoma congolense infection of trypanotolerant N'Dama (Bos taurus) cattle is associated with decreased secretion of nitric oxide by interferon-gamma-activated monocytes and increased transcription of interleukin-10. Parasite Immunol. 1998;20:421–429. doi: 10.1046/j.1365-3024.1998.00165.x. [DOI] [PubMed] [Google Scholar]
- 41.Thiemermann C. Nitric oxide and septic shock. Gen Pharmacol. 1997;29:159–166. doi: 10.1016/s0306-3623(96)00410-7. [DOI] [PubMed] [Google Scholar]
- 42.Van Nieuwenhove S. Present strategies in the treatment of human African trypanosomiasis. In: Dumas M, Bouteille B, Buguet A, editors. Progress in human African trypanosomiasis. Berlin, Germany: Springer-Verlag; 1999. pp. 253–280. [Google Scholar]
- 43.Vincendeau P, Daulouède S, Veyret B, Dardé M L, Bouteille B, Lemesre J L. Nitric oxide-mediated cytostatic activity on Trypanosoma brucei gambiense and Trypanosoma brucei brucei. Exp Parasitol. 1992;75:353–360. doi: 10.1016/0014-4894(92)90220-5. [DOI] [PubMed] [Google Scholar]
- 44.Wang W W, Jenkinson C P, Griscavage J M, Kern R M, Arabolos N S, Byrns R E, Cederbaum S D, Ignarro L J. Co-induction of arginase and nitric oxide synthase in murine macrophages activated by lipopolysaccharide. Biochem Biophys Res Commun. 1995;210:1009–1016. doi: 10.1006/bbrc.1995.1757. [DOI] [PubMed] [Google Scholar]