Abstract
Background:
Laparoscopic Roux-en-Y gastric bypass (LRYGB) has been established as a leading treatment of obesity. Surgical site infections (SSIs) remain the most common complication.
Objective:
To compare the incidence of SSIs before and after the implementation of our technique.
Methods:
Our intraoperative technique limits enteric contact with the abdominal wall through a wound protector at the end-to-end anastomosis stapler port site, with enteric retrieval with a specimen bag followed by betadine irrigation. We analyzed our SSIs outcomes before and after implementation of our technique in all RYGB and laparoscopic sleeve-to-bypass conversions at our institution performed by two providers between January 1, 2009 to December 31, 2011 and January 1, 2019 to December 31, 2021. We compared patient age, sex, body mass index, American Society of Anesthesiologists class; and comorbidities including hypertension, diabetes, and hyperlipidemia. The χ2, Fischer exact, Wilcoxon Rank Sum tests, and multivariate analysis were performed.
Results:
Four hundred twenty-nine patients underwent LRYGB and sleeve-to-bypass conversion during the two study periods. Group 1 (162 patients, 37.76%) all underwent RYGB. Group 2 (267 patients, 62.24%) of whom 199 underwent RYGB and 68 underwent a laparoscopic sleeve-to-bypass conversion. The SSI rate was 9.26% in Group 1 and 2.62% in Group 2 (p = 0.002514). Statistical significance was also noted for operating room time (137 min vs 123 min, p = 0.02) and hospital length of stay (2 – 3 interquartile range vs 1 – 2 interquartile range, p = 0.04).
Conclusion:
We propose a safe, reproducible technique that significantly reduces SSI rates during LRYGB.
Keywords: Circular stapler, EEA, Gastric bypass, Surgical site infection
INTRODUCTION
The obesity epidemic continues to bring significant challenges to the overall health of our population and to the infrastructure of our healthcare system. In the last 20 years, the prevalence of obesity and severe obesity has steadily increased from 30.5% to 41.9% and from 4.7% to 9.2%, respectively. In fact, approximately 9.2% of adults now qualify as severely obese, making obesity the second leading cause of preventable death in the United States.1,2
The obesity epidemic has led to an increase in the number of bariatric surgeries performed worldwide. Among the available procedures, the laparoscopic Roux-en-Y gastric bypass (LRYGB) has long been established as the gold standard in the treatment of obesity due to its significant effect on weight loss, quality of life, and obesity-related medical conditions such as Type 2 diabetes, hypertension, and coronary artery disease.3,4 The safety and efficacy of the LRYGB is well established and has been improving over time as surgical technique evolves and perioperative care improves. Nonetheless, perioperative complications are still reported to occur in about 11.5% of cases. Among these, surgical site infection (SSI) is the most commonly reported perioperative complication occurring about 1.07% of the time among all techniques of LRYGB.5
Patients with severe obesity are particularly susceptible to SSI due to a higher rate of obesity-related comorbidities such as Type 2 diabetes. Moreover, a baseline pro-inflammatory state and a large soft tissue burden may further predispose them to developing an SSI.6 These unwanted infections are associated with increased morbidity stemming from readmissions, repeated imaging, invasive procedures, and the use of antibiotics. Additionally, the psychosocial morbidity of these infections is often overlooked but plays an important role and can lead to an overall reduction in quality of life after LRYGB. Implementing standardized methods that decrease postoperative infections may help to decrease their associated morbidity and help thwart the overall cost to our healthcare system.4 Herein we describe a set of reproducible intraoperative techniques to reduce SSI when using a circular stapled technique during LRYGB. Additionally, we evaluate the effects of these techniques in our high-volume bariatric center.
METHODS
a) Population
This is a retrospective review from a single institution of all patients undergoing LRYGB or a conversion from gastric sleeve to LRYGB performed by two surgeons (D.R.C & J.V) at our institution in two discrete time periods, January 1, 2009 – December 31, 2011 and January 1, 2019 – December 31, 2021. The first time period was before implementation of several intraoperative steps to reduce SSIs, while the later time period constituted a time where these measures were fully implemented. The electronic medical record (EMR) was reviewed to collect demographic, clinical, and perioperative information. This study was approved by the Institutional Review Board.
b) Primary Outcome
The primary outcome of our study was the rate of SSI; which per the Center for Disease Control guidelines is defined as an infection involving the incisional skin and/or subcutaneous tissues of a surgical wound in the 30-days following a surgical procedure.6 In all the cases where signs of SSI were encountered, the surgeon proceeded with measures to diagnose and treat the infection, including drainage and antibiotic therapy. A diagnosis of SSI was made on a clinical basis by an experienced surgical provider when one or more of the following were present:
Purulent drainage from the surgical incision.
Identification of an organism by an aseptically obtained surgical site culture.
An incision with significant and worsening erythema, tenderness, warmth and/or swelling that is deliberately opened by a physician.
Diagnosis of SSI by a physician.
c) Characteristics
The patients were divided into two groups based on the year of their surgery. Group 1 (G1) were patients who had surgery before implementation of our SSI reduction techniques; Group 2 (G2) were patients who had surgery after implementation of these techniques. Baseline and intraoperative patient characteristics were compared between the groups and based on the primary outcome. Variables compared included sex, age, body mass index (BMI), American Society of Anesthesiologists (ASA) class, smoking status, pre-operative use of steroids; and obesity-associated medical conditions such as Type 2 diabetes mellitus, hypertension, and hyperlipidemia. In cases where an SSI was diagnosed, data collected included number of days to detection, hospital length of stay, imaging studies obtained, whether of incision and drainage (I&D) was performed and if cultures were collected, blood and/or urine cultures, administration of intravenous antibiotics, and discharge with allocation of visiting nursing services (VNS).
d) Technique
The technique used by the surgeons included in this study consists of constructing a 150 cm roux limb in the antecolic and antegastric position and a biliopancreatic limb with a length of 30 cm from the ligament of Treitz. The gastrojejunostomy is created with the transoral passage of an anvil and an end-to-end anastomosis (EEA) stapler inserted through an abdominal wound. A leak test of the anastomosis is performed and the EEA stapler along with the anvil are removed from the abdomen through the abdominal wound.
Our modified intraoperative technique focuses on limiting or inhibiting enteric contact with the abdominal wall. In order to achieve this a laparoscopic wound protector with (Alexis O Wound Protector with KII Fios First Entry; Applied Medical, Rancho Santa Margarita, CA), is introduced during the initial port-placement in the left lower abdomen at the midclavicular line (Figures 1A and 1B). This port is then used for the introduction and retrieval of the EEA stapler device (DST Series EEA Xl 25 mm Stapler, 30.5 mm, Covidien, New Haven, CT), which is also covered in a sterile plastic protector (Steri-Drape Isolation Bag 1003, 3M, St. Paul, MN) (Figures 1C and 1D). After protruding through the sterile cover (Figure 1E) and mating with the anvil, the stapler is pulled back into the cover prior to its removal from the abdomen (Figure 1F) to ensure lack of contact with the abdominal wall. Following the EEA, the redundant small bowel at the gastrojejunostomy site is transected and inserted into a specimen bag (Endo Catch Gold 10 mm Specimen Pouch, Covidien, Mansfield, MA) (Figure 1G) and removed through this same port, thus ensuring lack of contact with the subcutaneous tissue and skin edges. During closure, the wound protector is removed and all the members in the team undergo a change of gloves prior to fascial closure, which is accomplished with three interrupted sutures of 0 VICRYL (Ethicon, Somerville, NJ) using a laparoscopic suture passer. The port site is then irrigated with an iodine based solution (APLICARE Povidone-Iodine Paint Sponge Sticks; Aplicare Products, Meriden, CT) (Figure 1H). All port skin sites are reapproximated with 4-0 absorbable sutures in a running subcuticular fashion. Prior to skin incision all patients receive a weight based dose of antibiotics with coverage of enteric organisms (i.e. cefoxitin, clindamycin), followed by two postoperative doses. All patients underwent active warming to maintain perioperative normothermia.
Figure 1.

(A) Dual ring wound protector. (B) Dual ring wound protector with laparoscopic entry device.
Figure 1.

(C) EEA stapler with sterile plastic cover. (D) Introduction of covered EEA stapler through dual ring wound protector site.
Figure 1.
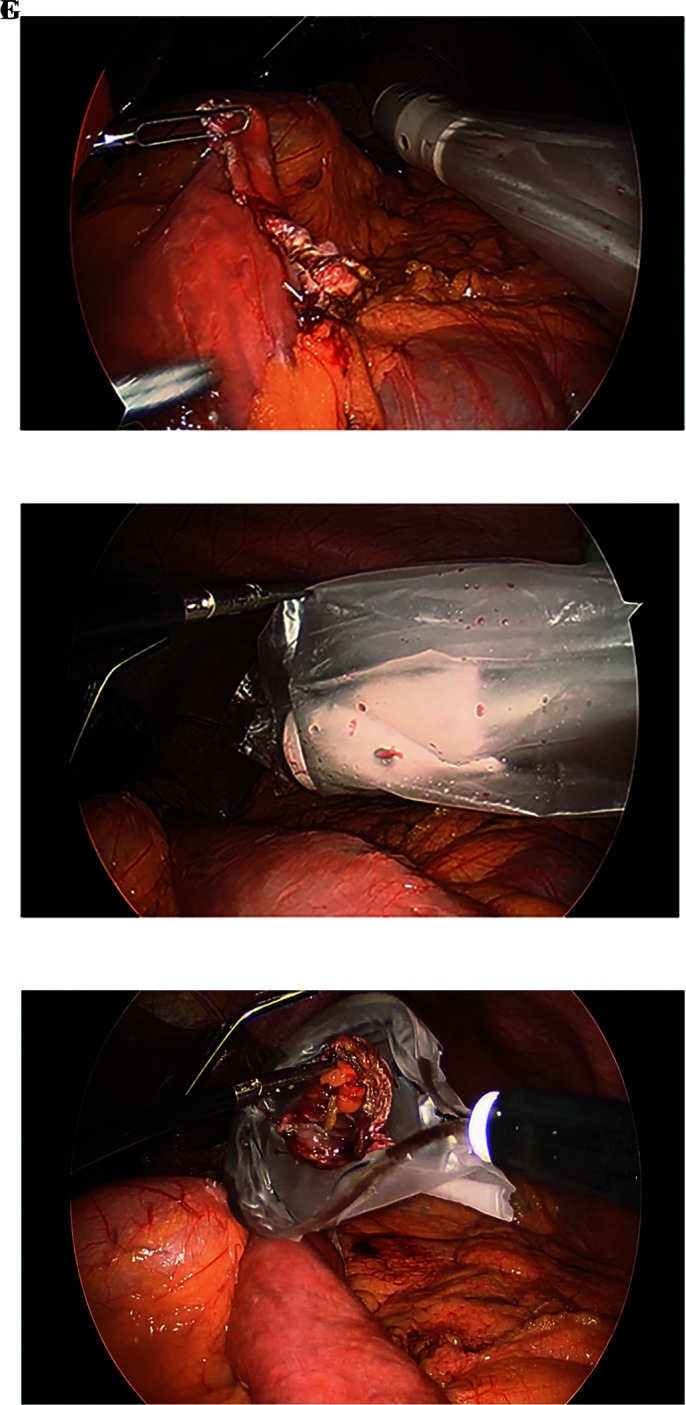
(E) Protrusion of EEA stapler through plastic cover. (F) Retraction of EEA stapler back into plastic cover. (G) Resected small bowel insertion into specimen bag.
Figure 1.
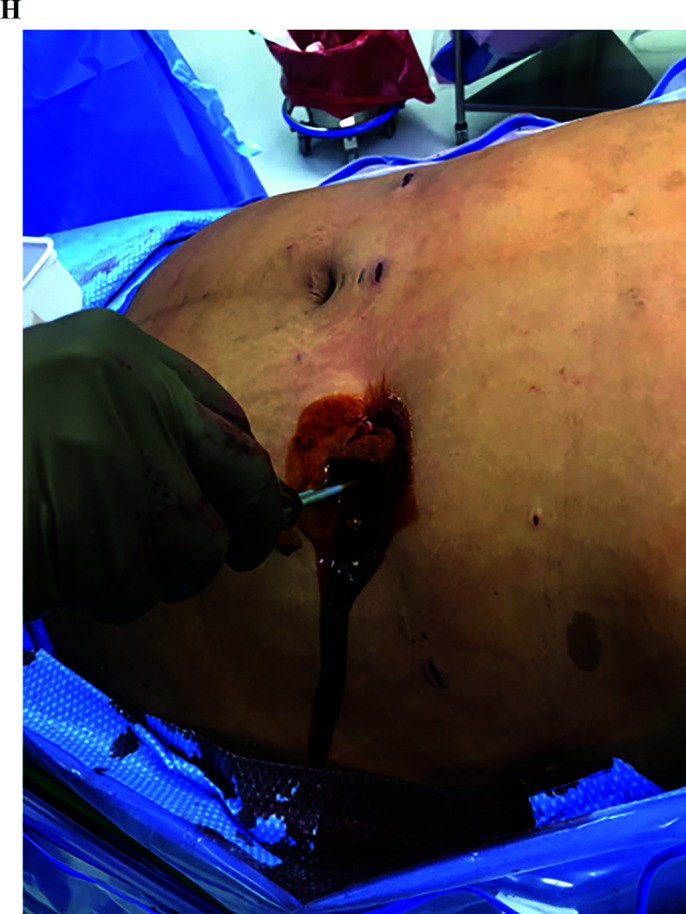
(H) Irrigation of midclavicular port-site with iodine based solution.
e) Statistical Analysis
All statistical analyses were performed with SAS version 90.4 (SAS, Cary, NC, USA). The χ2 test was used to compare categorical variables. The Fischer exact test was used for categorical variables when an observed value was < 5. Continuous variables had nonnormal distribution and were compared using the Wilcoxon Rank Sum test. Statistical significance was considered at P < .05. Variables with clinical and statistical significance in the univariate analysis were included in a binary logistic regression model to generate a multivariate analysis. Unless otherwise indicated, results are reported as counts and percentages for categorical variables, medians, and interquartile ranges (IQR) for continuous variables, odds ratio (OR), and 95% confidence intervals (CI).
RESULTS
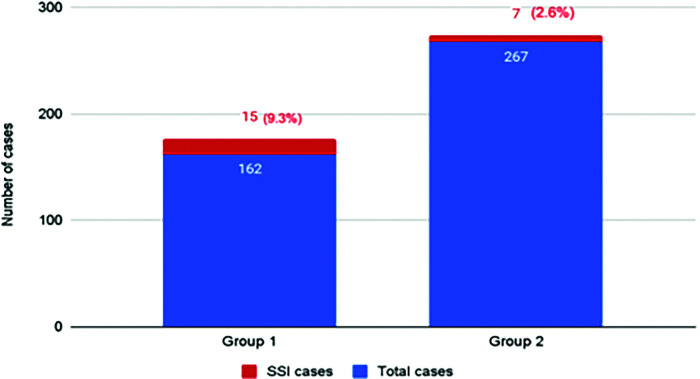
A total of 429 patients underwent LRYGB within the two study periods. G1 included a total of 162 patients (37.8%) who underwent LRYGB. G2 included 267 patients (620.2%) of whom 199 underwent LRYGB and 68 underwent a laparoscopic sleeve-to-RYGB conversion. Patient characteristics are listed in Table 1. The groups demonstrated statistically significant differences in age, BMI, ASA class, smoking status, prevalence of diabetes mellitus and hypertension as well as procedure type, operating room time (ORT), length of stay (LOS), and incidence of SSI. Smoking status differed with G1 having a total of 13 positive smoker subjects (8.0%), contrary to zero smoking subjects in G2 (0%, P = .001). G1 exhibited a higher BMI at 47 compared to G2 with a median BMI of 42 (P = .001) and longer ORT of 140 minutes for G1 vs 113 minutes for G2 (P = .001). LOS for G1 averaged two days while G2 averaged one days (P = .001). A total of 22 patients presented with a superficial SSIs within the 30-day postoperative period, 15 in G1 and 7 in G2 (P = .002). The superficial SSI rate was significantly lower with our procedure change from 9.3% in G1 to 2.6% in G2 (P = .002) (Figure 2).
Table 1.
Patient Characteristics by Group
| Group 1 (n = 162) | Group 2 (n = 267) | p | |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 139 (86%) | 236 (88%) | 0.43 |
| Male | 23 (14%) | 31 (12%) | |
| Age (years); median (IQR) | 41 (31 – 50) | 43 (34 – 53) | 0.02 |
| BMI (kg/m2); median (IQR) | 47 (43 – 52) | 42 (38 – 46) | 0.001 |
| ASA Class; n (%) | |||
| 1 and 2 | 78 (48%) | 76 (28%) | 0.001 |
| 3 and 4 | 84 (52%) | 191 (72%) | |
| Current smoker; n (%) | 13 (8%) | 0 (0%) | 0.001 |
| Steroid use; n (%) | 0 (0%) | 3 (1.1%) | 0.29 |
| Diabetes Mellitus; n (%) | 37 (23%) | 84 (31%) | 0.05 |
| Hypertension; n (%) | 60 (37%) | 133 (50%) | 0.01 |
| Hyperlipidemia; n (%) | 34 (21%) | 64 (24%) | 0.48 |
| Procedure | |||
| LRYGB | 162 (100%) | 199 (75%) | 0.001 |
| Conversion | 0 (0%) | 68 (25%) | |
| OR time (min), median (IQR) | 140 (124 – 162) | 113 (93 – 133) | 0.001 |
| LOS (days); median (IQR) | 2 (2 – 3) | 1 (1 – 2) | 0.001 |
| Surgical Site Infection; n (%) | 15 (9.3%) | 7 (2.6%) | 0.002 |
Abbreviations: IQR, interquartile range; BMI, body mass index; ASA, American Society of Anesthesiologists; LRYGB, laparoscopic Roux-en-Y Gastric Bypass; OR, operating room; LOS, length of stay.
Figure 2.
Surgical site infection prevalence.
The comparison of patient characteristics based on presence of SSI is shown in Table 2 and this demonstrated there was difference between the groups in the allocated patient group (G1 9.3% vs G2 2.6%, P = .003), ORT (137 min vs 123 min, P = .02), and LOS. In the multivariate analysis, the group allocation remained statistically significant with the G2 being preventive for the development of SSI (odds ratio [OR] 00.3 95%, confidence interval [CI] 00.1 – 0.77) but with no significant association to ORT (OR 1.00 95%, CI 0.99 – 1.01), or LOS (OR 0.85 95%, CI 0.54 – 1.34). (Table 3).
Table 2.
Patient Characteristics Based on Presence of Surgical Site Infection
| SSI (n = 22) | No SSI (n = 407) | p | |
|---|---|---|---|
| Group | |||
| Group 1 | 15 (9.3%) | 147 (90.7%) | 0.003 |
| Group 2 | 7 (2.6%) | 260 (97.4%) | |
| Procedure | |||
| LRYGB | 20 (5.5%) | 341 (94.5%) | 0.55 |
| Conversion | 2 (2.9%) | 66 (97.1%) | |
| Gender, n (%) | |||
| Female | 17 (4.5%) | 358 (95.5%) | 0.18 |
| Male | 5 (9.3%) | 49 (90.7%) | |
| Age (years); median (IQR) | 44 (33 – 55) | 42 (33 – 52) | 0.62 |
| BMI (kg/m2); median (IQR) | 45 (39 – 53) | 44 (40 – 49) | 0.25 |
| ASA Class 1 and 2 | 7 (4.5%) | 147 (95.5%) | 0.68 |
| ASA Class 3 and 4 | 15 (5.4%) | 260 (94.5%) | |
| Current smoker; n (%) | 1 (4.5%) | 12 (2.9%) | 0.50 |
| Steroid use; n (%) | 0 (0%) | 3 (0.7%) | 0.99 |
| Diabetes Mellitus; n (%) | 9 (41%) | 112 (27.5%) | 0.17 |
| Hypertension; n (%) | 12 (55%) | 181 (45%) | 0.35 |
| Hyperlipidemia; n (%) | 6 (27%) | 92 (23%) | 0.61 |
| OR time (min), median (IQR) | 137 (126 – 161) | 123 (101 – 148) | 0.02 |
| LOS (days); median (IQR) | 2 (2 – 3) | 2 (1 – 2) | 0.04 |
Abbreviations: SSI, surgical site infection; LRYGB, laparoscopic Roux-en-Y Gastric Bypass; IQR, interquartile range; BMI, body mass index; ASA, American Society of Anesthesiologists; OR, operating room; LOS, length of stay.
Table 3.
Multivariate Analysis for Surgical Site Infection
| Variable | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Group: G2 vs G1 | 0.3 | 0.1 – 0.77 |
| Body mass index | 1.03 | 0.97 – 1.09 |
| OR Time | 1.00 | 0.99 – 1.01 |
| Smoker: No vs Yes | 1.24 | 0.15 – 10.50 |
| Diabetes Mellitus: No vs Yes | 0.45 | 0.18 – 1.12 |
| LOS | 0.85 | 0.54 – 1.34 |
Abbreviations: SSI, surgical site infection; OR, operating room; LOS, length of stay.
The subanalysis of patients with SSI demonstrated that in G1 the patients presented at an average of seven days while patients in G2 presented at an average of 12 days. All SSI presentations belonged to the left midclavicular line port site, which is where the circular stapler is introduced in our technique. Upon readmission the median LOS was three days for G1 and one day for G2. Incision and drainage was performed on 13 patients from G1 (87%), and seven patients from G2 (100%). Wound cultures were obtained in 11 patients for G1 (73%) and give patients for G2 (71%). All wound culture specimens obtained grew bacterial organisms. While G1 had a varied range of microorganisms in said positive cultures, all patients in G2 were noted to grow Streptococcus constellatus, all other organisms isolated can be seen in Table 4. No patients in either group had positive blood cultures. Among G1 patients, 93% required intravenous antibiotics vs only 71% of G2 patients. All patients were discharged with oral antibiotics courses, most commonly oral Sulfamethoxazole/Trimethoprim or Amoxicillin/Potassium Clavulanate.
Table 4.
Characteristics of Patients with Surgical Site Infections
| Group 1 (n = 15) | Group 2 (n = 7) | p | |
|---|---|---|---|
| Days to detection (days); median (IQR) | 7 (6–13) | 12 (8–26) | 0.16 |
| LOS for readmission (days); median (IQR) | 3 (1–4) | 1 (0–1) | 0.007 |
| Imaging studies; n (%) | 7 (47%) | 4 (57%) | 0.99 |
| Abdominal CT | 6 (40%) | 4 (57%) | 0.65 |
| Abdominal US | 1 (7%) | 1 (14%) | 0.99 |
| I&D; n (%) | 13 (87%) | 7 (100%) | 0.99 |
| Wound culture; n (%) | 11 (73%) | 5 (71%) | 0.99 |
| Eikenella corrodens | 1 | 0 | – |
| Enterobacter cloacae | 1 | 0 | – |
| Escherichia coli | 1 | 1 | – |
| Group B streptococcus | 2 | 0 | – |
| Haemophilus parainfluenzae | 1 | 0 | – |
| Serratia marcescens | 1 | 0 | – |
| Staphylococcus aureus | 3 | 0 | – |
| Streptococcus anginosus | 0 | 1 | – |
| Streptococcus constellatus | 0 | 5 | – |
| Streptococcus viridans | 3 | 0 | – |
| Blood culture; n (%) | 11 (73%) | 3 (43%) | 0.34 |
| Positive blood culture | 0 | 0 | 0.99 |
| Urine culture; n (%) | 2 (13%) | 2 (29%) | 0.56 |
| Positive urine culture | 2 (13%) | 0 (0%) | 0.17 |
| IV antibiotics; n (%) | 14 (93%) | 5 (71%) | 0.23 |
| VNS; n (%) | 6 (40.0%) | 4 (71%) | 0.36 |
Abbreviations: SSI, surgical site infection; IQR, interquartile range; LOS, length of stay; CT, computerized tomography; US, ultrasound; I&D, incision and drainage; IV, intravenous; VNS, visiting nurse services.
DISCUSSION
The LRYGB is the gold standard for the treatment of obesity and its related complications. Reports have shown that the LRYGB, particularly if a circular stapled technique is used to construct the gastrojejunostomy, has an increased rate of SSI when compared to other bariatric procedures.7 In this retrospective review, we evaluated all LRYGB cases performed using the circular stapled technique at our institution during two discrete time periods and we compared the incidence of SSIs before and after the implementation of various infection reduction techniques. With an easily proposed surgical technique we noted a significant decrease in the SSI rate from 9.3% to 2.6%.
SSI is not an issue specific to bariatric surgery or obese patients. In fact, SSIs occur in 2% to 5% of any patient undergoing surgery in the United States.8 Nonetheless, direct entry into the gastrointestinal tract in higher than average risk individuals undergoing LRYGB confers an elevated rate of SSIs. This is particularly true with the use of circular staplers and there are multiple explanations that may explain this finding. First, given the design and size of the stapler head the surgeon is unable to use standard laparoscopic ports to introduce and withdraw the instrument from the abdomen. Consequently, surgeons must make an approximately 3 cm incision over the entry site and place the stapler directly into the abdomen, which directly exposes the tissue to enteric contents. Additionally, the circular stapled technique results in a circumferential tissue excision or “donuts” that must be withdrawn from the body as a specimen, again directly exposing the tissue to enteric contents. Due to these technical differences, the use of circular staplers in LRYGB results in elevated SSI rates. This specific technique has been shown to have an SSI rate as high as 5.2%, which is significantly higher than that the 00.4% seen with linear staplers.7 Despite these shortcomings there are many advantages to the circular stapler in the construction of the gastrojejunostomy during a LRYGB. The technique is easily reproducible, the size of the anastomosis is predetermined and reliably accurate, and the anastomosis can be fashioned quickly with a single fire of the stapler.
The use of sterile barriers has recently shown to help reduce postoperative infection rates.9,10 Similar to our study, the University of Wisconsin noted a reduction in SSI rate from 15% to 3.8% over a four year period by implementing a chlorhexidine based skin preparation, a focus on maintaining normothermia during the operation, deploying a sterile stapler cover, wound irrigation, and primary wound closure with overlying antibiotic cream.9 In a study performed at the University of Utah evaluating the use of a dual ring wound protector in conjunction with a conical EEA introducer on 158 patients undergoing RYGB over a two-year period, an 86% relative risk reduction was noted in SSI, dropping from a 9.5% SSI rate prior to intervention to a 1.35% SSI rate post intervention.10 The authors above were all successful in developing steps that decreased SSIs from circular staplers during RYGB. When considering the specific interventions to implement in our technique, we incorporated steps that could limit enteric exposure in a safe, reliable, and easily reproducible manner and that were cost effective and readily available at our institution. Although there are some notable differences among the techniques described and those presented in our own study, all were able to decrease the SSI rate significantly. This “Swiss Cheese” model approach is an ideal risk reduction strategy for this particular problem. In our case, different interventions used sequentially can provide layers of safety and ultimately decrease the rate of SSI occurrence.
In our study there are differences in patient demographic between cohorts. Most notably, G1 had a higher incidence of smokers at 8% compared to 0 smokers on G2; however, within G1 only 1 out of the 15 patients who developed SSI was a positive smoker (4.5%) with no noted statistical significance between positive smoking status and development of SSI. The decrease in smoking incidence between groups can be credited to the implementation of routine pre-operative nicotine testing prior to undergoing RYGB at our institution. The decrease in LOS for G2 might be attributable to changes in routine postoperative care of bariatric patients after implementation of new enhanced recovery after surgery protocols at our institution since 2018. The difference in baseline BMI can be explained due to guideline changes in approval criteria for bariatric surgery from a BMI ≥ 40, which was lowered to BMI ≥ 35 in 2013.11 Given the temporal distribution of our patient populations, increased surgeon experience may be accountable for the decrease in overall ORT in G2 when compared to G1 as studies have shown that an individual surgeon’s ORT for LRYGB can continue to decrease with time as the number of performed procedures increases.12,13 Throughout these years, the technical steps of the procedure between both groups remained exactly the same, so as not to add further confounding bias.
LIMITATIONS
The retrospective nature of our study makes it susceptible to selection bias and limits the availability of data due to patients being lost to follow-up. This limited data diminishes the capacity to control for confounding variables and to attribute for risk factors such as perioperative glycemic control. The single institution and inclusion of two providers in our study accounts for its small sample size and may also further contribute to selection bias and temporal changes such as the providers’ learning curves that may contribute to our improved results.
CONCLUSION
Frequent examination of patient outcomes and databases should alert surgeons to re-examine and modify surgical techniques as deemed necessary. Evaluation and optimization of surgical techniques can reduce the complications associated with a particular procedure.14 We propose a safe and reproducible technique that significantly reduces SSI rates during LRYGB. Ultimately, this has the potential to improve postoperative outcomes, hospital costs, and the overall burden on the healthcare system.
Footnotes
Conflict of interests: none.
Disclosure: none.
Funding sources: none.
Informed consent: Dr. Ana T. Garcia Cabrera declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Ana T. Garcia Cabrera, Department of Surgery. Montefiore Medical Center, Bronx, NY..
Gustavo Romero-Velez, Department of Endocrine Surgery. Cleveland Clinic, Cleveland, OH..
Xavier Pereira, Department of Surgery. Montefiore Medical Center, Bronx, NY..
Joseph T. Vazzana, Department of Surgery. Montefiore Medical Center, Bronx, NY..
Diego R. Camacho, Department of Surgery. Montefiore Medical Center, Bronx, NY..
References:
- 1.Matarasso A, Roslin MS, Kurian M. Bariatric surgery: an overview of obesity surgery. Plast Reconstr Surg. 2007;119(4):1357–1362. [DOI] [PubMed] [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, no 360. 2020. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- 3.Kassir R, Debs T, Blanc P, et al. Complications of bariatric surgery: presentation and emergency management. Int J Surg. 2016;27:77–81. [DOI] [PubMed] [Google Scholar]
- 4.Contival N, Menahem B, Gautier T, Le Roux Y, Alves A. Guiding the non-bariatric surgeon through complications of bariatric surgery. J Visc Surg. 2018;155(1):27–40. [DOI] [PubMed] [Google Scholar]
- 5.Falvo A, Vacharathit V, Kuhn JE, et al. Comparison of short-term outcomes following Roux-en-Y gastric bypass in male and female patients using the MBSAQIP database. Surg Obes Relat Dis. 2020;16(9):1236–1241. [DOI] [PubMed] [Google Scholar]
- 6.Anaya DA, Dellinger EP. The obese surgical patient: a susceptible host for infection. Surg Infect (Larchmt). 2006;7(5):473–480.Oct [DOI] [PubMed] [Google Scholar]
- 7.Sima E, Hedberg J, Ehrenborg A, Sundbom M. Differences in early complications between circular and linear stapled gastrojejunostomy in laparoscopic gastric bypass. Obes Surg. 2014;24(4):599–603. [DOI] [PubMed] [Google Scholar]
- 8.Ferraz ÁAB, Vasconcelos CFM, Santa-Cruz F, Aquino MAR, Buenos-Aires VG, Siqueira LT. Surgical site infection in bariatric surgery: results of a care bundle. Rev Col Bras Cir. 2019;46(4):e2252. [DOI] [PubMed] [Google Scholar]
- 9.Shabino PJ, Khoraki J, Elegbede AF, et al. Reduction of surgical site infections after laparoscopic gastric bypass with circular stapled gastrojejunostomy. Surg Obes Relat Dis. 2016;12(1):4–9. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Miller M, Ibele A, Morrow E, Glasgow R, Volckmann E. Dual ring wound protector reduces circular stapler related surgical site infections in patients undergoing laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2018;28(10):3352–3359. [DOI] [PubMed] [Google Scholar]
- 11.Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures – 2019 update. Endocr Pract. 2019;25(12):1346–1359. [DOI] [PubMed] [Google Scholar]
- 12.Dworak J, Wysocki M, Rzepa A, et al. Learning curve for laparoscopic Roux-en-Y gastric bypass based on the experience of a newly created bariatric center. Pol Przegl Chir. 2020;92(4):23–30. [DOI] [PubMed] [Google Scholar]
- 13.Ballantyne GH, Ewing D, Capella RF, et al. The learning curve measured by operating times for laparoscopic and open gastric bypass: roles of surgeon's experience, institutional experience, body mass index and fellowship training. Obes Surg. 2005;15(2):172–182. [DOI] [PubMed] [Google Scholar]
- 14.Beitner M, Luo Y, Kurian M. Procedural changes to decrease complications in laparoscopic gastric bypass. JSLS. 2015;19(1):e2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]