Abstract
Background and Objectives:
To estimate the average treatment effect on the treated (ATT) and to assess the clinical outcomes in two different types of mesh in robotic Rives-Stoppa (rRS) ventral hernia repair (VHR).
Methods:
A retrospective analysis of a robotic VHR database between February 1, 2013 and May 31, 2022. Patients who underwent a rRS VHR were included in this study and separated into two groups depending on the mesh used: SynecorTM Preperitoneal Biomaterial (SynecorTM Pre) and Bard™ Soft. Through propensity score and inverse-probability-treatment-weighting, the ATT was estimated for two scenarios; the first with the treated target having used the SynecorTM Pre, the second having used the Bard™ Soft mesh. Adjusted linear regression models, including lingering imbalanced variables, were used for both the primary outcome of the Comprehensive Complication Index (CCI®), and the secondary outcome of the hospital cost.
Results:
A total of 186 patients who underwent rRS were separated into the two groups (SynecorTM Pre mesh, n = 85; Bard™ Soft mesh, n = 101). Adjusted linear regression models for the CCI showed no statistical difference between both groups (p > 0.05), whereas ATT on hospital cost was significantly higher (p < 0.001) in the SynecorTM Pre group in both scenarios [(95% confidence interval) = 3882 (2352, 5413) and −5185 (−8213, −2157), respectively].
Conclusion:
Both mesh materials provided excellent outcomes with no difference in complications or recurrence rates. However, hospital cost was found to be higher in the hybrid mesh group. Long-term follow-up is needed to fully assess the performance of both mesh types in rRS.
Keywords: Biosynthetic, Rives-Stoppa, Robotic, Synthetic, Ventral hernia repair
INTRODUCTION
Ventral hernia remains as one of the most prevalent diseases worldwide, with more than average of 350,000 ventral hernia repairs (VHR) performed in the United States each year.1 High recurrence rates have pushed surgeons to opt for mesh-based repairs, as the evidence favors it versus simple suturing, at the expense of increased of surgical site events such as infections and seromas.2 To remediate the latter issues, companies have continuously improved upon their mesh technologies. Thus, several mesh products are currently available, each with a preferred anatomical position and clinical setting.3
Although successful in reducing recurrence rates,4 earlier publications associated synthetic mesh usage with serious complications such as prosthesis infection.5 An example of this category of products is the Bard™ Soft mesh, a flexible polypropylene mesh with a large pore knit structure.
Recently, biosynthetic or hybrid meshes have been designed to combine features of both biologic and synthetic material.6 Enhanced tissue ingrowth, which mimics a biologic mesh, and a permanent backbone, a synthetic mesh characteristic, are two of their most prominent features.7 The SynecorTM Preperitoneal Biomaterial (W.L. Gore & Associates Inc., Newark, DE, USA) (SynecorTM Pre), a biosynthetic mesh, has been suggested to provide favorable midterm outcomes in high-risk patients.8,9 It is composed of three layers: a monofilament polytetrafluoroethylene (PTFE) layer inserted between two bioabsorbable layers.
Postoperative outcomes also depend on the type of the VHR and anatomical position of the mesh.10 Across the different techniques, a Rives-Stoppa repair offers optimal outcomes with lower recurrence rates, even in complex hernia repairs with synthetic mesh use.11,12 Previous studies have already established synthetic material as a major risk factor for increased infection risk. Moreover, literature is still scarce on comparisons of biosynthetic meshes, a relatively new technology, with other types of mesh material. Hence, the aim of this study is to compare the postoperative clinical outcomes of SynecorTM Pre with those of the Bard™ Soft mesh in robotic Rives-Stoppa (rRS) VHR.
MATERIALS AND METHODS
Study Design
This is a retrospective analysis of a prospectively collected database that includes patients who underwent robotic ventral hernia repair between February 1, 2013 and May 31, 2022. The objective of this study is to compare the clinical outcomes of the two mesh materials in robotic Rives-Stoppa VHR, with consideration of the treatment effects of both mesh types. The primary outcome is the Comprehensive Complication Index (CCI®), a scoring system based on the Clavien-Dindo classification for postoperative complications. The secondary outcome is to evaluate the hospital cost. The study was approved by the Institutional Review Board, and an informed consent was obtained from patients.
Variables and Outcomes
Collected data included patients’ demographics (age, sex, body mass index [BMI]), comorbidities and risk factors, hernia characteristics, operative variables (operating time, estimated blood loss [EBL]), and intraoperative complications, postoperative variables (pain score [verbally assessed on a numeric scale graded from 0 to 10, with 10 being the worst], total amount of narcotic-analgesics received as morphine milligram equivalent before postanesthesia care unit discharge, hospital length of stay (LOS), and postoperative outcomes including surgical site infections (SSIs), surgical site occurrences, and surgical site occurrences requiring procedural intervention, other complications, and hernia recurrence. The Clavien-Dindo classification system was used to categorize postoperative complications.13 Morbidity scores were measured using the CCI® (University of Zurich, Zurich, Switzerland).14
Hospital Cost Analysis
Hospital charges for each procedure were obtained through the electronic medical record (EMR) and included all expenditures during the hospital stay (operating room allocated time, medications and material used, intrahospital complications, transfer to the intensive care unit, etc.). Afterwards, charges were corrected to reflect the 2022 value of the United States Dollar (USD), according to the specific inflation rate of each fiscal year from 2012 till 2021 by means of a web-based calculator.15
To calculate costs, charges were multiplied by the specific cost-to-charge ratio for each type of admission (inpatient vs. outpatient), provided by the hospital for each financial year.
Surgical Technique (Robotic Rives-Stoppa)
Upon accessing the retrorectus plane, dissection was carried out medially, followed by an incision of the medial end of the ipsilateral rectus sheath. Dissection of the preperitoneal plane was performed at the posterior side of linea alba, along with an incision of the medial edge of the contralateral posterior fascia, giving access to the contralateral rectus space. After the completion of the dissection, the anterior fascial defect was closed using a long-lasting absorbable barbed suture. In case of transabdominal access retromuscular repair, barbed absorbable suture was used to approximate the opening of the posterior rectus sheaths in a running fashion. Deployment of the mesh was performed, followed by a release of the pneumoperitoneum under direct vision. Skin incisions were closed using absorbable sutures.
Statistical Analysis
All analyses were performed using SPSS software (Statistical Package for Social Sciences for Windows Version 28), the R statistical software package (V0.4.20.0, the R Foundation for Statistical Computing), and a menu-driven web application based on the Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG) R package.16 A p < 0.05 was considered statistically significant.
In order to account for the differences between both groups, an inverse probability treatment weighting method was opted for. Propensity score in the estimation of the average treatment effect on the treated (ATT) for the SynecorTM Pre group and for the Bard™ Soft mesh group was used. In the first scenario, ATT on the SynecorTM Pre group answers the question: What would have been the outcomes if all patients of the SynecorTM Pre group had received the Bard™ Soft mesh instead, with the covariate distribution of the SynecorTM Pre group? Conversely, the question for the ATT on the Bard™ Soft mesh was: What would have been the outcomes if all patients of the Bard™ Soft mesh group had received the SynecorTM Pre mesh instead, with the covariate distribution of the Bard™ Soft mesh group? Pretreatment variables, such as patient demographics and hernia characteristics, were included in the propensity score model as covariates. The TWANG Shiny app, which uses generalized boosted regression models (GBM) to estimate propensity score weights, is a form of machine learning that uses a nonparametric approach, checks all the potential confounders and interactions observed and entered in the model, handles missing data, and determines the optimal iteration based on balance.17 The GBM iteration was set as 1000, interaction depths at 2, shrinkage at 0.01, and stop method as maximum absolute effect size. The quality of the propensity score weights was evaluated in terms of standardized differences and visual assessments, such as convergence plot, balance plot, and overlap boxplot. Then, the outcome analyses were run in the weighted sample. In case of lingering imbalance, multiple regression analyses with additional covariate adjustment were run (doubly robust method).
Categorical variables were analyzed using Pearson χ-Square or Fisher’s Exact Test and were expressed as the frequency with percentage [n (%)]. Continuous variables were compared with the Independent-Sample t test or Mann-Whitney U test depending on the distributions and were reported as mean ± standard deviation or median (interquartile range), as appropriate.
RESULTS
A total of 186 patients who underwent rRS were included in this study. Patients were separated into two groups depending on the type of mesh used in the procedure (SynecorTM Pre mesh, n = 85; Bard™ Soft mesh, n = 101). Propensity score model summary table and graphical assessments are provided in Table 1 and Figures 1–4, respectively.
Table 1.
The Comparison of Pre-, Intraoperative Characteristics between the Study Groups (Average Treatment Effect on the Treated Weighted, Target = SynecorTM Pre)
| Unweighted Study Groups |
Weighted Study Groups |
|||||||
|---|---|---|---|---|---|---|---|---|
| SynecorTM Pre | BardTM Soft | p | ASD* | SynecorTM Pre | BardTM Mesh | p | ASD* | |
| Age (years), mean ± SD | 54.4 ± 13.6 | 53.8 ± 13.9 | 0.757 | 0.046 | 54.4 ± 13.6 | 54.1 ± 11.6 | 0.907 | 0.019 |
| Sex, male, n (%) | 56 (65.9) | 72 (71.3) | 0.428 | 0.114 | 56 (65.9) | 40 (70.2) | 0.592 | 0.098 |
| BMI (kg/m2), mean ± SD | 36.6 ± 5.9 | 28.6 ± 4.8 | <0.001 | 1.343 | 36.6 ± 5.9 | 34.6 ± 4.8 | 0.045 | 0.323 |
| ASA Score, median (IQR) | 3 (2 – 3) | 2 (2–3) | 0.001 | 0.546 | 3 (2–3) | 2 (2–3) | 0.528 | 0.162 |
| HT, yes, n (%) | 44 (51.8) | 42 (41.6) | 0.165 | 0.204 | 44 (51.8) | 28 (49.1) | 0.758 | 0.059 |
| MI, yes, n (%) | 2 (2.4) | 1 (1) | 0.593 | 0.090 | 2 (2.4) | 1 (1.8) | 1.000 | 0.043 |
| CAD, yes, n (%) | 8 (9.4) | 6 (5.9) | 0.371 | 0.119 | 8 (9.4) | 2 (3.5) | 0.316 | 0.222 |
| COPD, yes, n (%) | 7 (8.2) | 5 (5) | 0.364 | 0.119 | 7 (8.2) | 5 (8.9) | 1.000 | 0.025 |
| Smoking, yes, n (%) | 20 (23.5) | 19 (18.8) | 0.431 | 0.111 | 20 (23.5) | 16 (28.1) | 0.542 | 0.095 |
| DM, yes, n (%) | 18 (21.2) | 10 (9.9) | 0.032 | 0.276 | 18 (21.2) | 6 (10.7) | 0.106 | 0.245 |
| History of wound infection, yes, n (%) | 5 (5.9) | 4 (4) | 0.734 | 0.082 | 5 (5.9) | 6 (10.5) | 0.349 | 0.189 |
| Hernia etiology | 0.017 | 0321 | 0.071 | |||||
| Primary ventral, n (%) | 58 (68.2) | 84 (83.2) | 58 (68.2) | 40 (71.4) | 0.687 | |||
| Incisional, n (%) | 27 (31.8) | 17 (16.8) | 27 (31.8) | 13 (28.6) | ||||
| Recurrent hernia, yes, n (%) | 10 (11.8) | 8 (7.9) | 0.377 | - | 10 (11.8) | 4 (7.1) | 0.369 | - |
| Procedure setting | 0.190 | 0.192 | 0.146 | 0.223 | ||||
| Elective, n (%) | 78 (91.8) | 98 (97) | 78 (91.8) | 55 (98.2) | ||||
| Emergency, n (%) | 7 (8.2) | 3 (3) | 7 (8.2) | 1 (1.8) | ||||
| Concomitant procedure, yes, n (%) | 4 (4.7) | 18 (17.8) | 0.006 | 0.619 | 4 (4.7) | 43 (5.4) | 1.000 | 0.040 |
| Defect size, cm2, median (IQR) | 15.7 (15.7 – 25.1) | 15.7 (9.4 – 15.7) | <0.001 | - | 15.7 (15.7 – 25.1) | 15.7 (9.4 – 15.7) | <0.001 | - |
| Defect class | 0.611 | - | 0.026 | - | ||||
| Small, n (%) | 2 (2.4) | 5 (5) | 2 (2.4) | 8 (14.3) | ||||
| Medium, n (%) | 29 (34.1) | 36 (25.6) | 29 (34.1) | 17 (30.4) | ||||
| Large, n (%) | 54 (63.5) | 60 (59.4) | 54 (63.5) | 31 (55.4) | ||||
| Mesh size, cm2, median (IQR) | 300 (300 – 300) | 225 (225 – 225) | <0.001 | - | 300 (300-300) | 225 (225 – 287) | <0.001 | - |
| Operating time, min., median (IQR) | 88 (72 – 108) | 81 (57 – 104) | 0.079 | - | 88 (72 – 108) | 77 (53 – 95) | 0.011 | - |
| EBL, mL, median (IQR) | 5 (5 – 5) | 5 (5 – 5) | 0.670 | - | 5 (5 – 5) | 5 (5 – 5) | 0.142 | - |
Abbreviations: ASD, absolute standardized difference; ATT, average treatment for treated; BMI, body mass index; ASA, American Society of Anesthesiologists, HT, hypertension; CAD, coronary artery disease; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; SD, standard deviation; IQR, interquartile range.
Absolute standardized difference is calculated as the absolute value in the difference in means of a covariate across the treatment groups, divided by the standard deviation in the treated group. Variables with ASD values were used in the propensity score model.
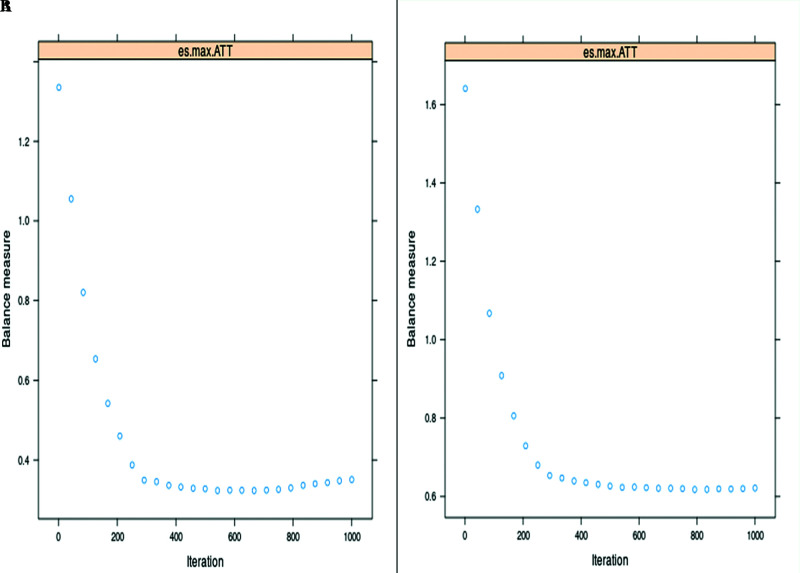
Figure 1.
Convergence plots for; (A) ATT – SynecorTM Pre group, (B) ATT – BardTM Soft mesh group. The convergence plot shows the balance measures [maximum effect size (es.max) in this setting] as a function of the number of iterations in the generalized boosted method (GBM) algorithm. The specified value of GBM iterations (it was set as 1000) allowed the GBM to explore sufficiently complicated models. In our propensity score (PS) models, optimal iterations [it was 577 for ATT-SynecorTM Pre and 794 for ATT- BardTM Soft mesh (See Table 1) were achieved before the specified value of 1000 GBM iterations.
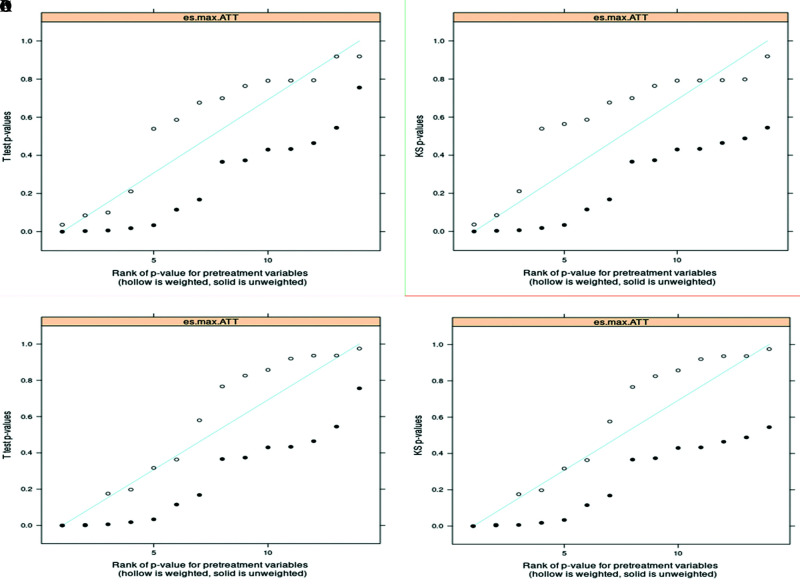
Figure 4.
Quantile-quantile (QQ) plots (a and b for ATT- SynecorTM Pre, plots c and d for ATT- BardTM Soft mesh) for examining the p-values for effect size (ES) and Kolmogorov-Smirnov (KS) statistics for each pretreatment covariate. Closed circles for before weighting, open circles for after weighting. Severe deviation of the p-values below the 45-degree line suggests lack of balance, and p-values running at or above the diagonal suggests balance might have been achieved. The inspected p-values of the t-tests and the p-values from the KS test indicated that the balance was improved for some covariates, while there are still a few for which imbalances exist.
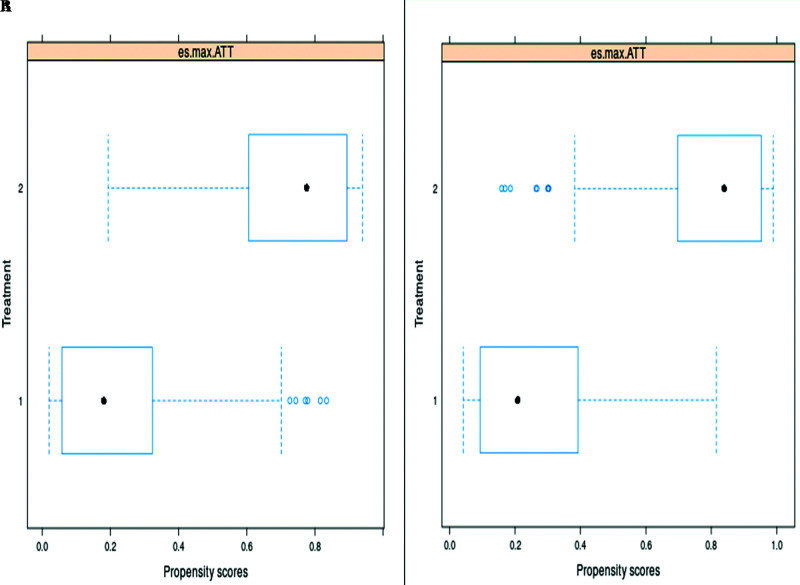
Figure 2.
The comparisons of the spread of the estimated propensity scores (PS) in the studied groups by boxplots for a) ATT - SynecorTM Pre group and b) ATT – BardTM Soft mesh group indicated that there is sufficient overlap in the studied groups.
Figure 3.
Balance plots [a) ATT - SynecorTM Pre group and b) ATT – BardTM Soft mesh group] illustrates the effect of the propensity score weights on the magnitude of differences, as a standardized effect size (ES), between groups on each pretreatment covariates. There are substantial reductions in between the ESs of unweighted and weighted samples for most variables (blue lines). However, inevitably increased ESs observed for three variables in the ATT- SynecorTM Pre (a) balance plot and five variables in the ATT- BardTM Soft mesh plot (red lines). For both plots, the ESs are still pretty large after propensity score weights due to difficulty in getting high-quality ATT weights on the data set of the studied groups.
The comparisons of patient demographics and intraoperative variables before and after weighting are summarized in Tables 1 and 2, as ATT for SynecorTM Pre group and ATT for Bard™ Soft mesh group, respectively. Accordingly, imbalances in pre-operative variables were relatively balanced in ATT weighted groups, except for BMI, concomitant procedure, defect size. These lingering imbalances were probably due to the criteria applied in the mesh selection, and therefore used in the doubly robust regression analysis.
Direct comparisons of postoperative variables in the weighted samples showed hospital costs to be statistically higher in the SynecorTM Pre group, in addition to a longer hospital LOS observed in the ATT weighted SynecorTM Pre sample. However, neither the overall postoperative complication rates nor CCI® scores differed between groups in both weighted samples (Table 3). There were no hernia recurrences recorded in either group during a follow-up period of 25 (range, 4.6 – 59.3) months. The comparison of postoperative complications in the weighted samples is given in Table 2.
Table 3.
Comparison of Postoperative Outcomes
| ATT Weighted for Synecor Pre |
ATT Weighted for Soft Mesh |
|||||
|---|---|---|---|---|---|---|
| SynecorTM Pre | Soft Mesh | p | SynecorTM Pre | Soft Mesh | p | |
| Last pain score, 0 – 10, median (IQR) | 5 (4 – 6) | 4 (4 – 5) | 0.272 | 4 (3 – 6) | 5 (3 – 6) | 0.745 |
| MME, median (IQR) | 10 (2.5 – 16) | 7.5 (2.5 – 14) | 0.413 | 6.9 (2.5 – 13.8) | 7.5 (0 – 12.5) | 0.481 |
| LOS, day, median (IQR) | 0 (0 0) | 0 (0 – 0) | 0.003 | 0 (0 – 0) | 0 (0 – 0) | 0.005 |
| Same day discharge, n (%) | 70 (82.4) | 55 (96.5) | 0.016 | 39 (79.6) | 93 (92.1) | 0.027 |
| Postoperative complications, n (%) | 9 (10.6) | 4 (7) | 0.563 | 3 (6.1) | 6 (5.9) | 1.000 |
| CCI® score, median (max) | 0 (42.4) | 0 (42.4) | 0.487 | 0 (42.4) | 0 (42.4) | 0.846 |
| Hospital costs, USD, median (IQR) | 6,416 (5,928 – 7,857) | 4,283 (3,642 – 5,242) | <0.001 | 6,530 (5,964 – 7,357) | 4,205 (3,579 – 5,382) | <0.001 |
Abbreviations: MME, morphine milligram equivalent; LOS, length of stay; CCI®, comprehensive complication index; IQR interquartile range.
Table 2.
The Comparison of Pre-, Intraoperative Characteristics between the Study Groups (Average Treatment Effect on the Treated Weighted, Target = BardTM Soft Mesh)
| Unweighted Study Groups |
Weighted Study Groups |
|||||||
|---|---|---|---|---|---|---|---|---|
| SynecorTM Pre | BardTM Soft | p | ASD* | SynecorTM Pre | BardTM Soft | p | ASD* | |
| Age (years), mean ± SD | 54.4 ± 13.6 | 53.8 ± 13.9 | 0.757 | 0.045 | 52.3 ± 12.6 | 53.8 ± 13.9 | 0.529 | 0.107 |
| Sex, male, n (%) | 56 (65.9) | 72 (71.3) | 0.428 | 0.119 | 35 (71.4) | 72 (71.3) | 0.986 | 0.007 |
| BMI (kg/m2), mean ± SD | 36.6 ± 5.9 | 28.6 ± 4.8 | <0.001 | 1.647 | 31.6 ± 4.2 | 28.6 ± 4.8 | <0.001 | 0.618 |
| ASA Score, median (IQR) | 3 (2 – 3) | 2 (2 – 3) | <0.001 | 0.491 | 2 (2 – 3) | 2 (2 – 3) | 0.244 | 0.002 |
| HT, yes, n (%) | 44 (51.8) | 42 (41.6) | 0.165 | 0.207 | 21 (42.9) | 42 (41.6) | 0.882 | 0.039 |
| MI, yes, n (%) | 2 (2.4) | 1 (1) | 0.593 | 0.138 | 2 (2) | 1 (1) | 1.000 | 0.031 |
| CAD, yes, n (%) | 8 (9.4) | 6 (5.9) | 0.371 | 0.147 | 3 (6.1) | 6 (5.9) | 1.000 | 0.013 |
| COPD, yes, n (%) | 7 (8.2) | 5 (5) | 0.364 | 0.151 | 2 (4.1) | 5 (5) | 1.000 | 0.046 |
| Smoking, yes, n (%) | 20 (23.5) | 19 (18.8) | 0.431 | 0.121 | 14 (28) | 19 (18.8) | 0.199 | 0.228 |
| DM, yes, n (%) | 18 (21.2) | 10 (9.9) | 0.032 | 0.378 | 5 (10.2) | 10 (9.9) | 1.000 | 0.012 |
| History of wound infection, yes, n (%) | 5 (5.9) | 4 (4) | 0.734 | 0.099 | 5 (10) | 4 (4) | 0.158 | 0.292 |
| Hernia etiology | 0.017 | 0.399 | 0.305 | |||||
| Primary ventral, n (%) | 58 (68.2) | 84 (83.2) | 35 (71.4) | 84 (83.2) | 0.096 | |||
| Incisional, n (%) | 27 (31.8) | 17 (16.8) | 14 (28.6) | 17 (16.8) | ||||
| Recurrent hernia, yes, n (%) | 10 (11.8) | 8 (7.9) | 0.377 | - | 6 (8.5) | 8 (7.9) | 0.901 | - |
| Procedure setting | 0.190 | 0.310 | 0.216 | 0.330 | ||||
| Elective, n (%) | 78 (91.8) | 98 (97) | 45 (91.84) | 98 (97) | ||||
| Emergency, n (%) | 7 (8.2) | 3 (3) | 4 (8.2) | 3 (3) | ||||
| Concomitant procedure, yes, n (%) | 4 (4.7) | 18 (17.8) | 0.006 | 0.343 | 1 (2) | 18 (17.8) | 0.006 | 0.399 |
| Defect size, cm2, median (IQR) | 15.7 (15.7 – 25.1) | 15.7 (9.4 – 15.7) | <0.001 | - | 16.3 (15.7 – 27.7) | 15.7 (9.4 – 15.7) | <0.001 | - |
| Defect class | 0.611 | - | 0.275 | - | ||||
| Small, n (%) | 2 (2.4) | 5 (5) | 1 (2) | 5 (5) | ||||
| Medium, n (%) | 29 (34.1) | 36 (25.6) | 15 (30.6) | 36 (25.6) | ||||
| Large, n (%) | 54 (63.5) | 60 (59.4) | 33 (67.3) | 60 (59.4) | ||||
| Mesh size, cm2, median (IQR) | 300 (300 – 300) | 225 (225 – 225) | <0.001 | - | 300 (300 – 300) | 225 (225 – 225) | <0.001 | - |
| Operating time, min., median (IQR) | 88 (72 – 108) | 81 (57 – 104) | 0.079 | - | 88 (72 – 97) | 81 (57 – 104) | 0.064 | - |
| EBL, mL, median (IQR) | 5 (5 – 5) | 5 (5 – 5) | 0.670 | - | 5 (5 – 5) | 5 (5 – 5) | 0.256 | - |
Abbreviations: ASD, absolute standardized difference; ATT, average treatment for treated; BMI, body mass index; ASA, American Society of Anesthesiologists; HT, hypertension; CAD, coronary artery disease; MI, myocardial infarction; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; SD, standard deviation; IQR, interquartile range.
Absolute standardized difference is calculated as the absolute value in the difference in means or proportions of a covariate across the treatment groups, divided by the standard deviation in the treated group. Variables with ASD values were used in the propensity score model.
In regard to study objectives, there were no ATT differences in terms of CCI® scores. However, ATT for the hospital costs was statistically higher for the SynecorTM Pre group in both scenarios. Since there were remaining imbalances between groups (effect sizes > 00.2), linear models with doubly robust estimations were applied. Adjusted linear models showed that ATT of the SynecorTM Pre group on the hospital cost remained higher and ATT of Bard™ Soft mesh on hospital cost remained lower (Table 4).
Table 4.
Average Treatment Effects of the Treated Groups
| CCI ® |
Hospital Cost |
|||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | ATT | Standard Error | p-Value | 95% CI | ATT | Standard Error | p-Value | 95% CI |
| SynecorTM Prea | 0.311 | 1.290 | 0.811 | (−2.24, 2.86) | 5436 | 935 | <0.001 | (3592, 7280) |
| SynecorTM Preb | −0.348 | 1.07 | 0.746 | (−2.46, 1.77) | 3882 | 776 | <0.001 | (2352, 5413) |
| BardTM Softa | 0.077 | 0.940 | 0.934 | (−1.93, 1.78) | −4780 | 1724 | <0.001 | (−8180, −1379) |
| BardTM Softc | 0.159 | 0.844 | 0.851 | (−1.51, 1.82) | −5185 | 1534 | <0.001 | (−8213, −2157) |
aUnadjusted.
bAdjusted for the variables with absolute standardized difference >00.2 in the ATT weighted comparisons (Body mass index, procedure setting, coronary artery disease, diabetes mellitus).
cAdjusted for the variables with absolute standardized difference >00.2 in the ATT weighted comparisons (Body mass index, procedure setting, hernia etiology, smoking, history of wound infection, concomitant procedure).
Abbreviations: CCI®, comprehensive complication index; ATT, average treatment effect of the treated; CI, confidence interval.
DISCUSSION
Although not considered as a clinical challenge, ventral hernia incidence is on the rise due to the aging of the population and increased obesity.18 With considerable impact on healthcare costs, its financial burden is mainly driven by the recurrence rates, reaching as high as 43%, even with mesh use.19 A reduction of 1% in the recurrence rates could save around US $32 million.1 Currently, several options to improve the outcomes are at hand. Among all VHR techniques, retromuscular repairs seem to have an edge in their track record, especially with the robotic approach.20 Synthetic meshes are known for their excellent clinical outcomes but with a concern for infection risk. On the other hand, hybrid meshes have offered low recurrence rates with an excellent safety profile in high-risk patients.8,9,21 Therefore, this study focused on evaluating the clinical outcomes of hybrid material in contrast to synthetic meshes in patients undergoing rRS VHR.
The results of this single center retrospective study did not favor the SynecorTM Pre mesh over the Bard™ Soft mesh, as data did not show any significant differences in terms of postoperative complications, with both mesh materials offering excellent outcomes and no recurrences. However, hospital costs of rRS were significantly higher in all scenarios in the SynecorTM Pre group. In a study by Parker et al., biosynthetic mesh performed similarly to a synthetic one in terms of postoperative outcomes, even in terms of recurrence. One possible explanation for their result was the use of the mesh in higher risk patients. However, reduced risk of recurrence was witnessed in patients who developed a surgical site occurence.21 Keogh et al. found the hybrid mesh to provide superior outcomes in contaminated hernia repairs, but to perform as equally well as the synthetic mesh in clean wounds.22
Optimal management of ventral hernia should revolve around patients’ comorbidities, with the safety profile of each mesh being compatible with the hernia’s characteristics. Biological meshes are typically used in septic and contaminated setting, where synthetic products are not an option.23 However, their benefits come at higher costs and significantly increased recurrence rate in comparison to synthetic meshes.24 On the other hand, wound contamination is a contraindication for synthetic material placement, as it increases surgical site infection risk.25 Biosynthetic meshes are composed of absorbable and nonabsorbable materials. The nonabsorbable element of the SynecorTM Pre is a macroporous knit of PTFE monofilament fiber with benefits in strength and resistance to bacterial adherence. Its absorbable constituent is made of polyglycolide:trimethylene carbonate copolymer bioabsorbable web scaffold surrounding the PTFE component throughout, and is intended to hasten cell infiltration, vascularization, and tissue regeneration. There are few studies that have explored the clinical outcomes of VHR using the SynecorTM Pre mesh. With a median follow-up period of two years, Rios-Diaz et al. did not record any hernia recurrence in patients undergoing incisional hernia repair.9 This finding was consolidated by a study we have previously published, where no recurrence, nor surgical site infections were noted.8
In terms of costs, biologic mesh remains the most expensive option of the three types of materials, with one mesh averaging at $10,000.26 In our study, we found biosynthetic mesh procedures to be more costly than synthetic mesh repairs. However, several factors, such as the LOS, operative time, and mesh size, may increase the procedure’s expenses. Thus, the higher costs found in the SynecorTM Pre group could not be attributed only to the price of the technology.
Lastly, retromuscular hernia repair was deemed as the gold standard for open VHR, due to its superior clinical outcomes, with a low recurrence rate of 3% to 6%.27,28 Combined with the improved outcomes of minimally invasive surgery, rRS has an estimated five-year free-from-recurrence rate of 98.2%.20 With these numbers taken into consideration, the comparability of clinical outcomes between our two study groups, especially in terms of recurrence rate, could partially be attributed to the surgical technique itself.
This study has several limitations. It is a retrospective study and thus potential selection bias may occur and hinder our results. Moreover, our data is from a single institution, thus, our findings may not be applicable for other practices and would not reflect the reality of other institutions. Additionally, our study is limited by its short-term follow-up. Hernia recurrence is a long-term complication, irrespective of the type of mesh used and therefore long-term results are needed to evaluate the efficacy of either meshes in preventing hernia recurrence. Also, our paper conveys the outcomes of a relatively small population. To support our results, a multicenter trial, with a longer follow-up period and larger number of patients is needed. Furthermore, our cost analysis may not be reproducible at other institutions as charges tend to change between hospitals, and therefore readers should not make their choice based on cost data alone. Lingering imbalances after weighting both groups remained and could affect the results obtained through the ATT analysis. However, we aimed to reduce the model misspecification and consequent potential bias by opting for a doubly robust regression analysis.
CONCLUSION
In conclusion, both meshes provided excellent outcomes. No differences in complications or recurrence rate were found between both groups. Hospital cost was found to be higher in the hybrid mesh group but could not be completely attributed to the technology itself. Long-term follow-up is needed to fully assess the performance of both mesh types.
Footnotes
Acknowledgements: none.
Disclosure: none.
Funding sources: none.
Ethical approval: The database used for this study was approved by the Institutional Review Board (IRB #HW202).
Conflict of interests: O.Y.K. has received teaching course, grant funding, and/or consultancy fees from Intuitive Surgical, Bard, and W.L. Gore outside the submitted work.
Informed consent: Dr. Omar Yusef Kudsi declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Omar Yusef Kudsi, Department of Surgery, Good Samaritan Medical Center, Brockton, MA.; Department of Surgery, Tufts University School of Medicine, Boston, MA.
Georges Kaoukabani, Department of Surgery, Good Samaritan Medical Center, Brockton, MA..
Naseem Bou-Ayash, Department of Surgery, Tufts University School of Medicine, Boston, MA..
Fahri Gokcal, Department of Surgery, Good Samaritan Medical Center, Brockton, MA..
References:
- 1.Poulose BK, Shelton J, Phillips S, et al. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia. 2012;16(2):179–183. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen MT, Berger RL, Hicks SC, et al. Comparison of outcomes of synthetic mesh vs suture repair of elective primary ventral herniorrhaphy: a systematic review and meta-analysis. JAMA Surg. 2014;149(5):415–421. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery A. The battle between biological and synthetic meshes in ventral hernia repair. Hernia. 2013;17(1):3–11. [DOI] [PubMed] [Google Scholar]
- 4.Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240(4):578–583. discussion 583-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voyles CR, Richardson JD, Bland KI, Tobin GR, Flint LM, Polk HC., Jr.Emergency abdominal wall reconstruction with polypropylene mesh: short-term benefits versus long-term complications. Ann Surg. 1981;194(2):219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bittner JGt, El-Hayek K, Strong AT, et al. First human use of hybrid synthetic/biologic mesh in ventral hernia repair: a multicenter trial. Surg Endosc. 2018;32(3):1123–1130. [DOI] [PubMed] [Google Scholar]
- 7.Reid CM, Jacobsen GR. A current review of hybrid meshes in abdominal wall reconstruction. Plast Reconstr Surg. 2018;142(3 Suppl):92S–96S. [DOI] [PubMed] [Google Scholar]
- 8.Kudsi OY, Kaoukabani G, Bou-Ayash N, et al. Quality of life and surgical outcomes of robotic retromuscular ventral hernia repair using a new hybrid mesh reinforcement. Hernia. 2022;26(3):881–888. [DOI] [PubMed] [Google Scholar]
- 9.Rios-Diaz AJ, Hitchner M, Christopher AN, Broach R, Cunning JR, Fischer JP. Early clinical and patient-reported outcomes of a new hybrid mesh for incisional hernia repair. J Surg Res. 2021;265:49–59. [DOI] [PubMed] [Google Scholar]
- 10.Rosen MJ, Bauer JJ, Harmaty M, et al. Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: The COBRA Study. Ann Surg. 2017;265(1):205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartog F, Sneiders D, Darwish EF, et al. Favorable outcomes after retro-rectus (Rives-Stoppa) mesh repair as treatment for noncomplex ventral abdominal wall hernia, a systematic review and meta-analysis. Ann Surg. 2022;276(1):55–65. [DOI] [PubMed] [Google Scholar]
- 12.Iqbal CW, Pham TH, Joseph A, Mai J, Thompson GB, Sarr MG. Long-term outcome of 254 complex incisional hernia repairs using the modified Rives-Stoppa technique. World J Surg. 2007;31(12):2398–2404. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. [DOI] [PubMed] [Google Scholar]
- 15.US Inflation Calculator. Available at: https://www.usinflationcalculator.com. Accessed December 10, 2021.
- 16.Griffin BA, Sanchez R, Cefalu M, et al. Toolkit for Weighting and Analysis of Nonequivalent Groups: A Tutorial on the TWANG Shiny App for Two Treatments. RAND Corporation. 2022. Available at: https://www.rand.org/pubs/tools/TLA570-2-v2.html.
- 17.Moons P. Propensity weighting: how to minimise comparative bias in non-randomised studies? Eur J Cardiovasc Nurs. 2020;19(1):83–88. Jan [DOI] [PubMed] [Google Scholar]
- 18.UK ventral hernia data. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity/2017-18#. Accessed September 4, 2022.
- 19.Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med. 2000;343(6):392–398. [DOI] [PubMed] [Google Scholar]
- 20.Kudsi OY, Gokcal F, Bou-Ayash N, et al. Robotic ventral hernia repair: lessons learned from a 7-year experience. Ann Surg. 2022;275(1):9–16. [DOI] [PubMed] [Google Scholar]
- 21.Parker MJ, Kim RC, Barrio M, et al. A novel biosynthetic scaffold mesh reinforcement affords the lowest hernia recurrence in the highest-risk patients. Surg Endosc. 2021;35(9):5173–5178. [DOI] [PubMed] [Google Scholar]
- 22.Keogh K, Slater K. Comparison of biosynthetic versus synthetic mesh in clean and contaminated ventral hernia repairs. ANZ J Surg. 2020;90(4):542–546. [DOI] [PubMed] [Google Scholar]
- 23.Kamarajah SK, Chapman SJ, Glasbey J, et al. Systematic review of the stage of innovation of biological mesh for complex or contaminated abdominal wall closure. BJS Open. 2018;2(6):371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosen MJ, Krpata DM, Petro CC, et al. Biologic vs synthetic mesh for single-stage repair of contaminated ventral hernias: a randomized clinical trial. JAMA Surg. 2022;157(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breuing K, Butler CE, Ferzoco S, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148(3):544–558. [DOI] [PubMed] [Google Scholar]
- 26.Carbonell AM, Criss CN, Cobb WS, Novitsky YW, Rosen MJ. Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg. 2013;217(6):991–998. [DOI] [PubMed] [Google Scholar]
- 27.Jin J, Rosen MJ. Laparoscopic versus open ventral hernia repair. Surg Clin North Am. 2008;88(5):1083–1100. [DOI] [PubMed] [Google Scholar]
- 28.Pauli EM, Rosen MJ. Open ventral hernia repair with component separation. Surg Clin North Am. 2013;93(5):1111–1133. [DOI] [PubMed] [Google Scholar]