Abstract
Fibroblast migration is an important aspect of wound healing. Different factors can influence migration and as such proper wound healing. In vitro scratch wound assays are used to examine cellular migration. However, the wide array of techniques available reduces reproducibility of findings. In this paper, we compare two techniques for wound creation; i.e. the exclusion method or scratching of cell monolayers. Furthermore, we investigate if analysis software influences experimental outcome by comparing both commercially and freely available analysis software. Besides, we examine the effect of cortisol on migration behavior of fibroblasts and identify possible caveats in experimental design. Results show a significantly reduced migration of fibroblasts when wounds are created using a cell exclusion method. Furthermore, addition of cortisol to the cell culture media only reduced migration of fibroblast monolayers that had been scratched but not in those where wounds were created using the exclusion method. A possible explanation related to cytokine expression is discussed.
Keywords: In vitro migration, Wound healing, Fibroblast, Stress hormones
Highlights
-
•
Migration of fibroblasts is slower with exclusion method for wounding compared to scratched cell monolayers.
-
•
Cortisol induced delay in cell migration was only observed in scratched cell monolayers.
-
•
Live cell imaging allows for precise analyses of wound closure over time; reducing statistical uncertainties.
-
•
Analysis software did not affect experimental outcome.
1. Introduction
The skin protects the body from environmental hazards and, as such, acts as a protective barrier for the underlying tissue [1]. To fulfill this vital function, the skin is able to heal wounds through a complex and multi-phase process [2]. During the hemostasis phase, formation of a blood clot happens immediately following wounding. After blood clot formation, different immune cells are recruited to clean the wound site from tissue debris and pathogens, marking the inflammation phase. During this phase, immune and endothelial cells produce signaling molecules which act as chemoattractant for mast cells, fibroblasts, endothelial cells and macrophages [3]. Fibroblasts and keratinocytes migrate to the site of the wound and start proliferating, marking the next phase of the wound healing process. As the major cellular component of the dermis, fibroblasts play an important role in the wound healing process of the skin [4]. During the proliferation phase, stimulation of fibroblasts by transforming growth factor-beta (TGF-β) induces their differentiation into myofibroblasts. These contractile cells express alpha-smooth muscle actin (α-SMA) needed for migration [5]. Fibroblasts further contribute to the remodeling of the extracellular matrix (ECM) by secreting ECM proteins and matrix metalloproteinases (MMPs) [4]. This final remodeling phase of wound healing ensures wound tensile strength and can last for many weeks after injury [2].
As described, the wound healing process is a complex multiphase process with a delicate interaction between cellular and molecular components. Interference of the wound healing process during any phase, usually leads to a defective repair. This is exemplified in studies where chronic psychological stress has been linked to slower wound healing, presumably as result of the suppressive effects of stress hormones on the inflammatory process of wound healing [6]. Activation of the hypothalamic pituitary adrenocortical (HPA) axis leads to a release glucocorticoids in the blood. During sustained stress, a dysregulation of the HPA-axis leads to a chronic exposure to high levels of circulating glucocorticoids [7]. In the skin, this upregulation of cortisol has previously been linked to a decreased keratinocyte and fibroblast proliferation, which potentially contributes to delayed wound healing during the proliferation phase [8]. Furthermore, in patients suffering from Cushing's syndrome, a disorder hallmarked by excess production of endogenous glucocorticoids, skin atrophy and delayed wound healing are observed as well [9].
A wide array of both in vivo and in vitro research models are available to study the wound healing process. In vivo models of rat, mouse, rabbit, and guinea pig can be used, depending on the phase of wound healing that researchers are interested in. For a comprehensive overview of these models readers are referred to Gottrup et al. [10]. Wound healing processes such as migration, proliferation, protein syntheses, wound contraction, cell-cell, and cell-matrix interactions are often studied with the use of in vitro models.
Fibroblast migration to the wound site is an essential feature of proper wound healing. To study this migration process, closure of an open wound area, which was created in a cell monolayer, is often investigated. To create this open wound area, two different types of model can be used. Direct manipulation (i.e., cell depletion) creates a gap by damaging the cell layer (i.e. mechanically with a pipette tip or needle, electrically, chemically or thermally). Secondly, standardized cell-free zones can be created by exclusion of cells by means of a physical barrier such as a silicon inserts. Proliferation of cells can affect closure of the wound area. Therefore, strategies to reduce proliferation such as reduced serum concentrations in culture media are often used [10].
The in vitro scratch wound assay is a reliable method to investigate cell migration capacity [[10], [11], [12]]. Nevertheless, given the wide variety of techniques available for wound creation, and many factors in experimental design that can influence the outcome, difficulties in direct comparison between different studies remain. To overcome this issue efforts have been made to introduce standardization and enhance reproducibility of wound healing assays [11]. Nevertheless, it remains unclear to which extend the described techniques of cell depletion and cell exclusion can affect experimental outcome. In this paper, we compare two different techniques of the wound healing assay related to creating of open wound area. We chose two techniques that are most commonly described in the literature and easily available or obtainable in a standard cell culture laboratory. That is, either cell monolayers were scratched (using a pipette tip, or the IncuCyte Wound Maker tool) or cells were excluded from growing in a defined area by means of placing a silicon insert. Furthermore, we compare live cell imaging at multiple time points after creating the open wound area, to that of cells that had been fixed at one specific time point after creating the open wound area. Besides, with a specific focus on cortisol-induced delayed wound healing, we exposed cells to the stress hormone cortisol in order to test the effect of this stress hormone on the migration capacity of the fibroblasts. In addition, we compare outcome of cell migration using the different in vitro wound healing techniques described above.
2. Methodology
2.1. Fibroblast culture
Primary normal human dermal fibroblasts (NHDF, PromoCell ®, C-12302) obtained from one donor (female, 33 year old Caucasian) were cultured in 10% fetal bovine serum (FBS, GIBCO 10500064) in Dulbecco's Modified Eagle Medium containing GlutaMAX™ (GIBCO, 10566016) supplemented with 0.25% Penicillin-Streptomycin (Pen-Strep, Sigma-Aldrich, P4333). Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2. Cells were passaged at 80–90% confluence using 0.05% Trypsin-EDTA (GIBCO, 25300062). Experiments were performed with passage numbers ranging from 4 to 6.
2.1.1. Cortisol exposure
Hydrocortisone (HC) was used to investigate psychological stress effects. Firstly, a 1 mg/mL stock solution was made by dissolving hydrocortisone BioReagent (Sigma-Aldrich, H0888) in 96% Ethanol. This was further diluted in phosphate-buffered saline (PBS) to obtain a concentration of 100 μmol/L HC. The control stock solution was made by dissolving equal amounts of 96% Ethanol in PBS. These solutions were further diluted in the cell culture medium to obtain a final concentration of 1 μmol/L hydrocortisone or equal amount of control vehicle. Stress medium was added to cell cultures one day after seeding followed by an incubation period of 48 h before wounding the cells.
2.2. Migration assays
2.2.1. Live cell migration
NHDF cells were seeded in 96-well Imagelock (for IncuCyte) at density of 7000 cells per well (70,000 cells/mL) and incubated overnight in 10% fetal bovine serum (FBS) medium. After 48 h of incubation with stress hormones or control vehicle, an open wound area was created in the cell monolayer using the IncuCyte ® Wound Maker tool, washed with PBS and subsequently incubated with DMEM-GlutaMAX stress medium or control containing 1% FBS to reduce the effects of proliferation on wound closure.
2.2.2. Fixation of migrating cells
For the exclusion method, NHDF cells were seeded in Ibidi insert inside Ibidi μ-Slides (Ibidi GmbH, Gräfelfing, Germany) at a density of 5000 cells per chamber (0.22 cm2, 70,000 cells/mL). Cells for the scratch method were seeded directly inside Ibidi μ-Slides at a density of 21,000 (1 cm2, 70,000 cells/mL) per well. After seeding, cells were incubated overnight in medium with 10% FBS. After attachment, cells were washed with PBS and incubated with medium containing cortisol or control vehicle. After 48 h of incubation with stress hormones, Ibidi inserts were removed to create an open wound area in the cell monolayer (exclusion method) or cell monolayers were scratched with a bended 1000 μL pipette tip (Rainin). Cells were washed with PBS and subsequently incubated with 1% FBS (to reduce the effect of proliferation on wound closure) DMEM-GlutaMAX containing stress hormones or control vehicle. After 24 h of incubation, wells were washed with PBS and fixed in a 10% Formalin solution (Sigma-Aldrich, HT5014).
2.3. Image acquisition and processing
Images at baseline were taken immediately after creating the open wound area. IncuCyte ZOOM™ (10X), and Leica Microscope (5X) images were analyzed for wound width at the center of the scratch using Fiji ImageJ [13].
2.3.1. IncuCyte live cell imaging
Cells were imaged after wounding every 4 h for a total duration of 72 h using the IncuCyte live cell imaging system at 10X magnification. For each time point, relative wound closure was calculated using the Scratch Wound analyses pipeline of the IncucCyte ZOOM™ software.
2.3.2. Microscopy with fixed cells
Fixed cells were imaged using the Leica Application Suite microscope software using a 5X magnification. Contrast between cell and background was maximized by using bright field, maximum light intensity and nearly closed aperture. Brightness gradients were avoided to optimize a homogeneous light distribution and as such prevent image artifacts.
2.3.3. Data processing software
Different open source software tools were used to determine the open scratch wound area. The TScratch tool is a freely available program designed for the analysis of wound healing assays. The image analysis algorithm runs in Matlab (Mathworks, Natick, MA, USA) and comes with a graphical user interface (GUI). As output, percentage of open image area is calculated [14]. A more recent automated algorithm for wound gap segmentation is described in Kauanova et al. [15]. The High-Throughput Microscopy HTM Wound Healing Tool is a freely available Matlab run script. Relative wound closure was calculated by means of the following equation and compared between the different groups:
| Eq. 1 |
T0 represents the open wound area at baseline immediately after creating the wound and T24 represents the open wound area as measured after 24 h.
2.4. Statistical analysis
Statistical differences in wound closure between experimental groups were determined using Beta regression in R [16]. Main- and interaction effects of methodology for creating open wound area and stress hormones were included in the regression model. In addition, wound width directly after wound creation was included as a confounding factor. Post-hoc pairs-wise comparison with Bonferroni correction for multiple testing was then carried out to identify any statistical significant differences between each experimental condition. Differences in wound closure over time of live cells incubated with cortisol or control were evaluated using a two-way ANOVA with time and stress hormones as independent variables. Post-hoc comparison with Bonferroni correction for multiple testing was carried out to identify statistical significant differences between cells incubated with cortisol or control vehicle at each time point. For each endpoint, mean and standard deviation values (σ) were calculated.
3. Results
In this paper, we have compared different methods of the IncuCyte Wound Maker tool and pipette tip for scratching cell monolayers to silicon inserts for the exclusion technique to create an open wound area for in vitro wound healing assays. Here we describe how these techniques may influence migration capacity of primary human dermal fibroblasts. Besides, we investigated how these techniques can affect experimental outcome by investigating the effect of cortisol on the migration of cells. Furthermore, we compare different experimental set-ups by using both fixed cells as live cell imaging and we compare as well different software for analyzing migration outcome. Table 1 shows an overview of the observed effects of methodology on open wound area at baseline and average closure after 24 h.
Table 1.
Overview of effect of using the exclusion (insert) versus scratching (pipette or IncyCyte) techniques on experimental parameters.
| insert | pipette scratch | IncuCyte Wound Maker | |
|---|---|---|---|
| average open area 0 h (μm) | 528 | 736 | 995 |
| SD open area 0 h (μm) | 27 | 164 | 251 |
| average closure at 24 h (%) | |||
| control | 24 | 74 | 68 |
| cortisol | 25 | 50 | 35 |
SD: standard deviation, hrs: hours.
3.1. Scratch method vs. exclusion method
3.1.1. Wound width
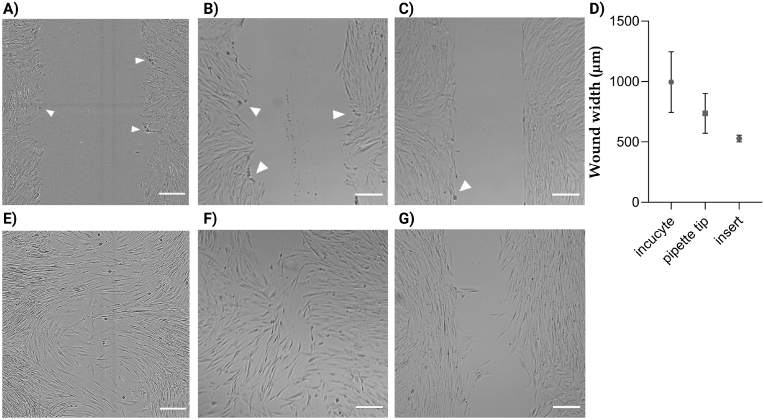
Scratches created using the IncuCyte Wound Maker Tool had the largest open area with a mean of 995 μm (n = 43, σ = 251), as compared to the pipette scratches with an average width of 736 μm (n = 49, σ = 164) and the Ibidi inserts (mean = 528 μm, n = 45, σ = 27). Open wound area created with the latter method showed the least variation in width compared with both scratching techniques (Fig. 1). Visual inspection of baseline images showed alignment of fibroblasts with Ibidi insert walls and few damaged cells at wound edges (see white arrows Fig. 1). Contrary, scratched cells (WoundMaker and pipette tip) showed a higher variety of orientation at the wound edge and more damaged cells. Furthermore, invasion of cells below Ibidi insert walls was observed in several cases. Scratched monolayers in turn could show damage where the cell monolayer detached from the substrate or an irregular open wound area due to cell detachment.
Fig. 1.
Comparison of wound after gap creation (A–C) and 24 h after (E–G). A, E: scratch created using IncuCyte Wound Maker Tool. B, F: Scratched cell monolayer by means of pipette tip. C, G: Open wound area created with Ibidi insert. D: comparison of wound width at baseline shows largest gap created using IncuCyte Wound Maker Tool followed by pipette scratch. Gaps created using Ibidi inserts had smallest distance and showed less variation. Scale bar: 200 μm. Graph shows mean values and standard deviation, IncuCyte: n = 43, pipette tip: n = 49, insert: n = 45. Arrow heads show examples of damaged cells at the wound edge.
3.1.2. Relative wound closure after 24 h
Pipette scratched monolayers showed a significant increased relative wound closure (average closure = 74%, n = 26, σ = 30) compared with open wound areas created with Ibidi inserts (average closure = 24%, n = 19, σ = 28) after 24 h (Fig. 2 A).
Fig. 2.
A) Reduced migration after 24 h of fibroblasts using exclusion method compared to scratching the monolayer. B&C) Cortisol delays migration capacity of scratched fibroblasts (pipette tip) but not for open wound areas created using the Ibidi insert (insert). *p < 0.05, ***p < 0.001 ***p < 0.001.
Next, we investigated whether the difference in method used (exclusion vs. scratching) would influence experimental outcome when administering cortisol to the culture media. In scratched monolayers, migration was significantly reduced in cortisol-exposed fibroblast compared to controls. After 24 h, average relative wound closure was 50% in scratched fibroblasts incubated with cortisol (Fig. 2 B). However, this effect did not show in fibroblast cultures using the exclusion method for open wound area creation (Fig. 2C).
3.2. Live cell imaging
In this assay, live cell imaging using the IncyCyte system was used to evaluate closure of open wound area every 4 h over a period of in total 72 h. Fibroblasts in the control group reached 50% wound closure at approximately 20 h after scratching. At 40 h after wound creation, most wounds had reached 100% confluence. In addition, a negative effect of stress hormone cortisol on relative wound closure was found (p < 0.001) (Fig. 3). Cells exposed to cortisol reached 50% of wound closure only after 32 h of scratch creation.
Fig. 3.
Effect of cortisol (1 μmol/L) exposure on wound closure by NHDF cells evaluated over time as measured with the InuCyte sytem. Between 12 and 60 h a significant difference was observed between the two groups p < 0.001. n = 8 per group, repeated three times.
3.3. Effect of software on data output
To investigate potential differences in experimental outcome induced by different image analysis software, a total of 21 images of cells scratched using the WoudMaker tool and imaged with the IncuCyte system, were analyzed using the IncuCyte ZOOM™, the TScratch tool [14], or the HTM tool Matlab script [15]. Relative wound closure was calculated for each image as described previously. All analyses software showed similar closure of the used image set (Fig. 4). IncuCyte ZOOM™ software showed an average closure of 69% (σ = 12) after 24 h in this data set. TScratch showed a slightly lower average closure of 64% (σ = 13) whereas the HTM tool showed an average closure of 67% (σ = 15). Group differences were not statistically significant.
Fig. 4.
Effect of software on data output. No significant differences were found between the groups. Graph shows mean values and standard deviation, n = 21.
4. Discussion
In this paper, we have used dermal fibroblast to compare different in vitro scratch wound techniques (exclusion vs. scratched cell monolayers). In addition, several analysis techniques were compared as well. Table 2 shows an overview of the (dis-)advantages of the described techniques. Measures of width of the open wound area immediately after scratching, relative closure after 24 h, and the effect of cortisol exposure on wound closure were evaluated. Wounds created with the exclusion method showed a significantly reduced closure after 24 h compared with scratched cell monolayers (Fig. 2A). Furthermore, addition of the stress hormone cortisol to the cell culture media significantly delayed fibroblast migration in scratched monolayers (Fig. 2B). However, this effect of cortisol exposure was not observed using the exclusion method (Fig. 2C). Nevertheless, wounds created using the exclusion method were more uniform and reproducible than those of scratched cell monolayers (Fig. 1D). In the latter, non-uniform wound edges are likely to be created as cells can detach during the process of scratching. Furthermore, a comparison with different types of software programs to evaluate open wound area did not yield any significant differences (Fig. 4).
Table 2.
Overview of pros and cons for using the exclusion (insert) versus scratching (pipette or IncyCyte) techniques.
| technique | pros | cons |
|---|---|---|
| insert | reproducible open area | detachment of insert or invasion of cells below silicon wall |
| useful for measuring spontaneous cell migration | less desirable to test experimental effect of migration suppressor reagents | |
| longer time interval needed for migration measurements | ||
| pipette scratch | useful for measuring migration upon damage/injury | less reproducible wounds |
| shorter time period needed for migration measurements | irregular open wound area due to detachment of cells from the substrate | |
| IncuCyte Wound Maker | suitable for use with 96-well plates | less freedom in experimental set-up |
| standardized methods for scratching and analysis which improve reproducibility | irregular open wound area due to detachment of cells from the substrate | |
| automated live cell imaging |
4.1. Exclusion vs. scratching
Remarkably, we showed a reduced migration of fibroblasts when the exclusion method for open wound area was used in comparison to scratched monolayers (Fig. 2A). This indicates that while the exclusion method creates the most reproducible open wound areas, it is a less desirable technique to measure fibroblast migration related to wound healing during a short time-interval. Contrary, for studies aiming to investigate spontaneous migration of cells, rather than induced migration after wound creation, researchers may find it more desirable to use the exclusion method in combination with measurements taken over a longer time interval.
During wound healing, damaged cells at the wound site produce a variety of cytokines and chemokines which induce cell proliferation and attract inflammatory cells [17]. In this experiment, cell layer disruption in scratched cells, could possibly induce the wound healing cascade more effectively by stimulating cytokine expression during the inflammatory phase crucial for inducing proliferation and migration behavior in fibroblasts. The finding of delayed wound closure observed in fibroblasts exposed to cortisol further supports this notion as this effect was only observed in scratched cell monolayer. Cortisol is well known to suppress the cellular expression of inflammation markers including cytokines and growth factors and has been linked to a delayed wound healing in humans and mice (as reviewed in Ref. [6]). Given the possibility that Ibidi inserts may not induce cytokine expression needed to stimulate cell migration, a reducing effect of cortisol on cytokine expression and thus cell migration will therefore not be observed.
To better understand the role of different cytokines and growth factors on cell migration, future studies could repeat these experiments and measure the difference in expression of these migration stimulants between the two techniques. In addition, to counteract the delayed migration as observed in these experiments, future studies could investigate the effect of reagents that may stimulate fibroblast migration. Such studies have previously been performed in cell cultures that had been exposed to simulated microgravity, which impaired the migration capacity of dermal fibroblasts. Addition of platelet-rich plasma (PRP) to the cell culture media successfully prevented a simulated microgravity induced delayed migration [18]. Finally, in this paper, fibroblasts obtained from one single donor have been used. To better understand possible interindividual differences in sensitivity to cortisol exposure, these experiments should be repeated with fibroblasts obtained from multiple donors.
4.2. Data analysis software
To assess the possible effects of software usage on image segmentation, direct comparison of IncuCyte ZOOM™, Tscratch [14] and HTM tool Matlab script [15] was performed. Slight differences, though not statistically significant, were found between the different software tools (Fig. 4). This indicates that analyses performed with freely available software reproduce segmentation to the same level of commercially available tools. However, we would like to emphasize that image quality is an important factor determining image segmentation. The high-quality dataset we have used for this comparison was obtained using the IncuCyte system which helped in optimal segmentation of the images for all software tools.
While not significant, we observed slight differences in wound closure when analyzing the same image set using different available software. These differences may be contributed to differences in image segmentation. To correctly measure closure of open wound area and obtain reliable data, optimal segmentation is a crucial step in the data processing pipeline. Thresholding an image is one of the factors that defines the quality of segmentation [11]. For the IncuCyte ZOOM software, an image set is used to train the program to distinguish cells from the background and define analysis parameters which will then be applied to other experiments using the same set-up. When using the Tscratch GUI, thresholding of each individual image can be manually adjusted. This is a straightforward way of defining the open wound area. However, a disadvantage of this method is the sensitivity to user-specific differences between images of the same image set in defining the threshold. This issue does not occur with the HTM Tool Matlab script. Using this tool, users have to determine the optimal threshold for their image set which is then identical for all images that are being analyzed [15].
4.3. Live cell imaging
The IncuCyte system for in vitro scratch wound creation offers a standardized method which is one of its main advantages. These methods include standardized wound making, as well as live cell imaging where images are taken at a regular time interval at precise locations. Cell migration kinetics is non-linear with high variability between replicates, especially during the first 12 h, and shows a non-uniform closure velocity [15]. Because of this, measuring wound closure after fixation of cells at a specific time point can yield data with high variability. Frequent sampling of wound closure helps to overcome these issues and improve analysis sensitivity. The IncuCyte setup allows for precise analyses of wound closure over time and therefore reduces statistical uncertainties (Fig. 3).
The standardized methodology of the IncuCyte does however come with its own limitations as researchers are bound to the methodology that is being offered (i.e. well-plate and Wound Maker Tool have to be used). This reduces freedom in experimental set-up and can negatively affect experiments that do not allow for the usage of aforementioned methods. Besides, even though the IncuCyte ZOOM™ software offers a user-friendly GUI and many options for analyses, it is bound to analyze images taken with the IncuCyte which poses another limitation. It would therefore be highly desirable to allow researchers to upload images taken with other systems to be analyzed with the IncuCyte ZOOM™ masking algorithm.
To conclude, a variety of techniques is available for in vitro wound healing assays. In this paper we have shown that the two most commonly used techniques for creating an open wound area (exclusion vs. scratching) differently affect fibroblast migration and can influence experimental outcome. Therefore, it is important for researchers to consider the proper method for their experiment depending on cell type and research question to be answered. Showing advantages and disadvantages of the methods used for creation of open wound areas as well as software available for analysis, we hope to provide a comprehensive overview for researchers aiming to use this assay in their future work. Furthermore, we found a significant delay in migration of scratched fibroblasts that had been exposed to cortisol. This further indicates the harmful effects of this stress hormone on proper skin functioning.
Funding
This work was funded by BELSPO/Prodex, grant number CO-90-11-2801-04 (IMPULSE).
Declaration of competing interest
The authors report no conflict of interest.
Data availability
Data will be made available on request.
References
- 1.Burge S., Matin R., Wallis D. Oxford Handb Med Dermatology. Oxford University Press, 2016. 2016. Structure and function of the skin; pp. 1–16. [Google Scholar]
- 2.Sanon S., Hart D.A., Tredget E.E. In: Ski Tissue Eng Regen Med [Internet] Albanna M.Z., Holmes I.V.J.H., editors. Academic Press; Boston: 2016. Chapter 2 - molecular and cellular biology of wound healing and skin regeneration; pp. 19–47.https://www.sciencedirect.com/science/article/pii/B9780128016541000024 Available from: [Google Scholar]
- 3.Werner S., Grose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 4.Bainbridge P. Wound healing and the role of fibroblasts. J. Wound Care. 2013;22:407–412. doi: 10.12968/jowc.2013.22.8.407. [DOI] [PubMed] [Google Scholar]
- 5.Darby I.A., Laverdet B., Bonté F. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Invest. Dermatol. 2014;7:301–311. doi: 10.2147/CCID.S50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christian L.M., Graham J.E., Padgett D.A., et al. Stress and wound healing. Neuroimmunomodulation. 2007;13:337–346. doi: 10.1159/000104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fulford A.J., Harbuz M.S. An introduction to the HPA axis. Handb Stress Brain. 2005:43–65. [Google Scholar]
- 8.Terao M., Murota H., Kimura A., et al. 11Β-Hydroxysteroid dehydrogenase-1 is a novel regulator of skin homeostasis and a candidate target for promoting tissue repair. PLoS One. 2011;6:1–11. doi: 10.1371/journal.pone.0025039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibli-Rahhal A., Van Beek M., Schlechte J.A. Cushing's syndrome. Clin. Dermatol. 2006;24:260–265. doi: 10.1016/j.clindermatol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Gottrup F., Ågren M.S., Karlsmark T. Models for use in wound healing research: a survey focusing on in vitro and in vivo adult soft tissue. Wound Repair Regen. 2000;8:83–96. doi: 10.1046/j.1524-475x.2000.00083.x. [DOI] [PubMed] [Google Scholar]
- 11.Jen Jonkman, Cathcart J.A., Xu F., et al. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migrat. 2014;8:440–451. doi: 10.4161/cam.36224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Justus C.R., Leffler N., Ruiz-Echevarria M., et al. In vitro cell migration and invasion assays. JoVE. 2014;1–8 doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schindelin J., Arganda-Carreras I., Frise E., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebäck T., Schulz M.M.P., Koumoutsakos P., et al. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques. 2009;46:265–274. doi: 10.2144/000113083. [DOI] [PubMed] [Google Scholar]
- 15.Kauanova S., Urazbayev A., Vorobjev I. The frequent sampling of wound scratch assay reveals the “opportunity” window for quantitative evaluation of cell motility-impeding drugs. Front. Cell Dev. Biol. 2021;9:1–14. doi: 10.3389/fcell.2021.640972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team. R . R Foundation for Statistical Computing; Vienna, Austria: 2019. A Language and Environment for Statistical Computing.https://www.r-project.org/ [Internet] Available from: [Google Scholar]
- 17.Wu Y. vols. 558–566. 2014. (Pathology of Tissue Regeneration Repair : Skin Regeneration). [Google Scholar]
- 18.Cialdai F., Colciago A., Pantalone D., et al. Effect of unloading condition on the healing process and effectiveness of platelet rich plasma as a countermeasure: study on in vivo and in vitro wound healing models. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.