Dear Editor,
We read with interest the recent meta-analysis assessing the clinical efficacy and safety of nirmatrelvir plus ritonavir (NMV-r) in the treatment of patients with COVID-19.1 Based on the analysis of 13 studies involving 186,306 patients, the authors concluded that NMV-r was effective in reducing the mortality (odds ratio [OR], 0.12; 95% CI, 0.04-0.36) and the risk of hospitalization (OR, 0.32; 95% CI, 0.13-0.75) for patients with COVID-19.1 These findings indicated that NMV-r could be a useful antiviral treatment for COVID-19. However, it was also noted that a significant proportion of individuals who have recovered from acute COVID-19 may experience long-term complications, a phenomenon referred to as long COVID.2 , 3 Moreover, long COVID has been shown to have a negative impact on both physical and mental well-being.2 , 3 Therefore, how to prevent the development of long COVID has become a serious concern. In fact, one study showed the promising role of NMV-r in reducing the risks of post-acute COVID-19 sequelae, such as dysrhythmia and ischemic heart disease, deep vein thrombosis, pulmonary embolism, fatigue, liver disease, acute kidney disease, muscle pain, neurocognitive impairment, and shortness of breath.4 Study from Taquet et al. showed that compared to a matched cohort with influenza, COVID-19 patients had a higher incidence of new seizures or epilepsy diagnoses in the six months following their illness, particularly among those who were not hospitalized.5 However, the preventive effect of NMV-r on the risk of long-term complications – epilepsy and seizure remained unknown. Therefore, we conducted this retrospective cohort study to evaluate the impact of NMV-r on the long-term risks of epilepsy and seizure.
This study utilized the database from the TriNetX Research Network - a global health-collaborative clinical-research platform, which provided real-time multi-healthcare organization (HCO) and multinational healthcare-associated information.6 The search and data curation was conducted on January 7, 2023. Initially, we created a cohort of non-hospitalized patients with COVID-19 from 74 HCOs, as previously described.7 The inclusion criteria were (a) they had at least two times of medical encounters with healthcare organizations from March 1, 2020, to January 1, 2022; (b) people who were older than 18 years old; (c) they had a new diagnosis of COVID-19. Exclusion criteria included (a) the patients who had a prior history of epilepsy or seizure, (b) patients who ever received remdesivir, molnupiravir, monoclonal antibody or convalescent plasma, and (c) COVID-19 patients requiring hospitalization. Thereafter, we divided this population into two cohorts based on the use of NMV-r – a study group receiving NMV-r and a control group without NMV-r. To adjust for the difference in baseline characteristics between the groups, two matched cohorts were created by propensity score with a 1:1 matching method. A standard difference of less than 0.1 indicates good matching. The primary outcome was the one-year incidence of the composite endpoint of epilepsy (ICD-10 code G40) or seizures (ICD-10 code R56). The secondary outcomes included either code separately.5 The hazard ratio (HR) with 95% confidence interval (95% CI) of incident epilepsy and seizure was calculated for the NMV-r control groups. All statistical analyses were conducted using the built-in function of TriNetX network.
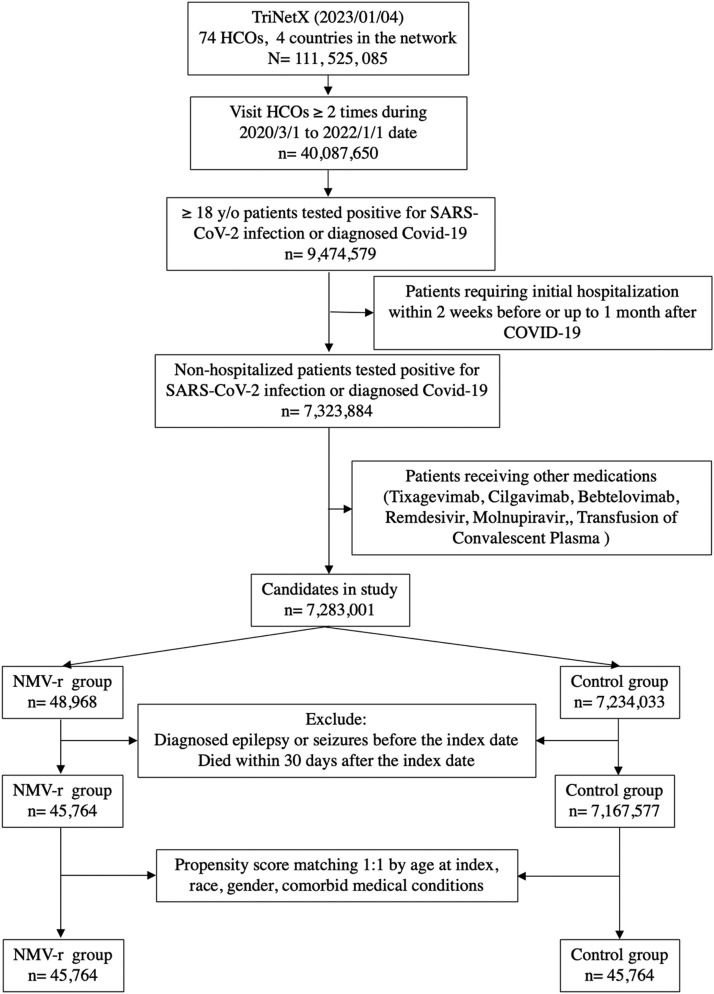
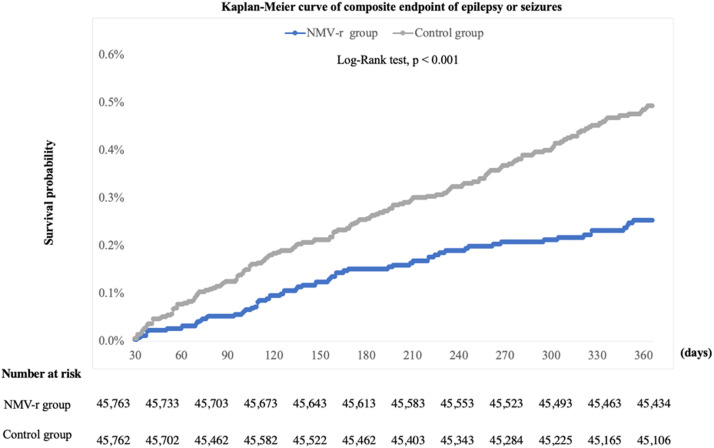
Initially, 45,764 patients receiving NMV-r and 7,167,604 COVID-19 patients without NMV-r were identified (Fig. 1 ). Through propensity score matching, equal numbers of 45,764 cases were retained in both cohorts (Table 1 ). Compared to the control cohort. NMV-r cohort had a lower risk of epilepsy and seizure (HR = 0.516; 95% CI = 0.389-0.685) within one year. Specifically, the NMV-r group also had a lower risk of epilepsy (HR, 0.584; 95% CI, 0.362-0.941) and seizure (HR, 0.463; 95% CI, 0.331-0.647) than the control group. Fig. 2 displays the Kaplan-Meier curve of the survival probability of epilepsy and seizure. The curve shows that the NMV-r cohort had a lower risk of epilepsy and seizure during the one-year follow-up period (Log rank p < 0.001).
Fig. 1.
The algorithm of patient selection and cohort construction.
Table 1.
Comparison of characteristics of patients receiving nirmatrelvir plus ritonavir (NMV-r) and not receiving NMV-r before and after matching.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| NMV-r group (n=45,764) | Control group (n=7,167,604) | Std diff | NMV-r group (n=45,764) | Control group (n=45,764) | Std diff | |
| Age at index, Mean ± SD | 56.3 ± 16.2 | 47.2 ±18.5 | 0.527 | 56.3 ±16.2 | 56.3 ±16.2 | 0.001 |
| Gender | ||||||
| Female | 27,747 | 4,003,071 | 0.097 | 27,747 | 27,235 | 0.023 |
| Male | 17,742 | 2,878,324 | 0.028 | 17,742 | 17,737 | <0.001 |
| Race, n(%) | ||||||
| White | 37,154 | 3,926,626 | 0.590 | 37,154 | 37,167 | 0.001 |
| Black or African American | 3,811 | 977,306 | 0.170 | 3,811 | 4,092 | 0.022 |
| Asian | 897 | 150,955 | 0.010 | 897 | 581 | 0.055 |
| Unknown Race | 3,767 | 2,082,102 | 0.555 | 3,767 | 3,799 | 0.003 |
| Problems related to housing and economic circumstances | 380 | 37,082 | 0.038 | 380 | 335 | 0.011 |
| Comorbidities | ||||||
| Hypertensive diseases | 18,367 | 1,316,121 | 0.493 | 18,367 | 18,462 | 0.004 |
| Ischemic heart diseases | 3,992 | 345,818 | 0.156 | 3,992 | 3,953 | 0.003 |
| Overweight, obesity and other hyperalimentation | 9,606 | 715,262 | 0.308 | 9,606 | 9,593 | 0.001 |
| Diabetes mellitus | 7,173 | 570,229 | 0.241 | 7,173 | 6,672 | 0.031 |
| Neoplasms | 13,049 | 961,176 | 0.378 | 13,049 | 13,065 | 0.001 |
| Asthma | 6,168 | 421,274 | 0.259 | 6,168 | 6,176 | 0.001 |
| Chronic obstructive pulmonary disease | 1,871 | 158,466 | 0.108 | 1,871 | 1,887 | 0.002 |
| Bronchitis, not specified as acute or chronic | 2,196 | 121,842 | 0.175 | 2,196 | 2,139 | 0.006 |
| Emphysema | 850 | 58,803 | 0.090 | 850 | 730 | 0.020 |
| Bronchiectasis | 252 | 18,386 | 0.046 | 252 | 233 | 0.006 |
| Chronic bronchitis | 201 | 12,685 | 0.047 | 201 | 158 | 0.015 |
| Chronic kidney disease | 2,158 | 247,519 | 0.064 | 2,158 | 2,182 | 0.002 |
| Alcoholic liver disease | 63 | 13,200 | 0.012 | 63 | 136 | 0.034 |
| Hepatic failure | 39 | 10,393 | 0.018 | 39 | 88 | 0.029 |
| Chronic hepatitis | 29 | 3,483 | 0.006 | 29 | 40 | 0.009 |
| Fibrosis and cirrhosis of liver | 305 | 42,257 | 0.010 | 305 | 454 | 0.036 |
| Other diseases of liver | 3,062 | 187,974 | 0.194 | 3,062 | 2,375 | 0.064 |
| Rheumatoid arthritis with rheumatoid factor | 413 | 21,073 | 0.079 | 413 | 264 | 0.038 |
| Other rheumatoid arthritis | 1,023 | 61,423 | 0.112 | 1,023 | 785 | 0.037 |
| Nicotine dependence | 3,785 | 432,429 | 0.087 | 3,785 | 3,962 | 0.014 |
| Mental and behavioral disorders due to psychoactive substance use | 4,840 | 595,588 | 0.078 | 4,840 | 5,345 | 0.035 |
| Schizophrenia, schizotypal, delusional, psychotic disorders | 257 | 57,479 | 0.029 | 257 | 502 | 0.059 |
| Cerebral infarction | 706 | 103,535 | 0.008 | 706 | 1,053 | 0.055 |
| Systemic lupus erythematosus | 291 | 21,633 | 0.049 | 291 | 196 | 0.029 |
| Psoriasis | 930 | 54,575 | 0.108 | 930 | 667 | 0.044 |
| Certain disorders involving the immune mechanism | 1,181 | 77,716 | 0.112 | 1,181 | 825 | 0.053 |
| Renal transplantation procedures | 10 | 1,391 | 0.002 | 10 | 10 | <0.001 |
| Liver transplantation procedures | 10 | 496 | 0.012 | 10 | 10 | <0.001 |
Fig. 2.
Kaplan-Meier curves of the primary outcome.
In summary, the results of this large retrospective cohort study suggest that non-hospitalized COVID-19 patients receiving NMV-r may have a lower long-term risk of epilepsy and seizure compared to those who did not receive anti-viral agents. This finding suggests that NMV-r may be effective in reducing the risk of post-acute COVID-19 sequelae, including epilepsy and seizure. Our findings were based on analyzing a large database involving multination, multi-institution, and multi-races. To minimize the potential effects of possible confounding factors, we exclude the patients with prior history of epilepsy or seizure, and the baseline characteristics were well-matched between groups. Therefore, our findings are generalizable, and the level of evidence is robust.
Our findings, in combination with those from a previous observational study,4 demonstrating the preventive effect of NMV-r on ten post-acute sequelae, suggest a potential role for NMV-r in preventing long COVID. These findings are extremely important because no effective measure can prevent the development of long COVID during this pandemic. If NMV-r can provide clinical benefits for acute COVID-19 and reduce the risk of post-acute sequelae, it may be worth considering changing clinical practice to encourage the use of NMV-r for patients with SARS-CoV-2 infection.
This study had several limitations. First, although we matched the baseline characteristics of NMV-r and control cohorts using the propensity score method, some residual confounding factors, such as the disease severity, SARS-CoV-2 variant, and the vaccine effect, could exist. Second, like other studies using claims databases, the mechanism of NMV-r in preventing long COVID remains unknown. Further study is warranted.
In conclusion, this study demonstrated that COVID-19 patients receiving NMV-r would be associated with a lower risk of epilepsy and seizure and suggested the potential role of NMV-r in preventing long COVID.
Edited by K. Neal
References
- 1.Zheng Q., Ma P., Wang M., Cheng Y., Zhou M., Ye L., et al. Efficacy and safety of Paxlovid for COVID-19:a meta-analysis. J Infect. 2023;86(1):66–117. doi: 10.1016/j.jinf.2022.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Righi E., Mirandola M., Mazzaferri F., Dossi G., Razzaboni E., Zaffagnini A., et al. Determinants of persistence of symptoms and impact on physical and mental wellbeing in Long COVID: a prospective cohort study. J Infect. 2022;84(4):566–572. doi: 10.1016/j.jinf.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernández-de-Las-Peñas C., Martín-Guerrero J.D., Cancela-Cilleruelo I., Moro-López-Menchero P., Rodríguez-Jiménez J., Navarro-Pardo E., et al. Exploring the recovery curves for long-term post-COVID functional limitations on daily living activities: the LONG-COVID-EXP-CM multicenter study. J Infect. 2022;84(5):722–746. doi: 10.1016/j.jinf.2022.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y., Choi T., Al-Aly Z.. Nirmatrelvir and the risk of post-acute sequelae of COVID-19. medRxiv 2022:2022.11.03.22281783.
- 5.Taquet M., Devinsky O., Cross J.H., Harrison P.J., Sen A. Incidence of epilepsy and seizures over the first 6 months after a COVID-19 diagnosis: A retrospective cohort study. Neurology. 2022 doi: 10.1212/WNL.0000000000201595. Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://trinetx.com/Accessed on January 7, 2023.
- 7.Ganatra S., Dani S.S., Ahmad J., Kumar A., Shah J., Abraham G.M., et al. Oral nirmatrelvir and ritonavir in non-hospitalized vaccinated patients with Covid-19. Clin Infect Dis. 2022:ciac673. doi: 10.1093/cid/ciac673. Aug 20. [DOI] [PMC free article] [PubMed] [Google Scholar]