Abstract
Background
The decrease of vitamin D plays a critical role in diabetes mellitus (DM)-induced oxidative stress and vascular endothelial injury. Therefore, we investigated the effect and mechanism of 25-hydroxyvitamin D3 (25 (OH) D3) on oxidative stress and ferroptosis induced by high glucose in human retinal microvascular endothelial cells (hRMVECs). And the objective of this paper was to propose a new strategy for the prevention and treatment of diabetic retinopathy (DR).
Methods
First, hRMVECs were transfected with mimics NC or miR-93. After that, cells were treated with 100 nM / 500 nM 25 (OH) D3 and then cultured in a high glucose (30 mM) environment. Subsequently, qRT-PCR was employed to detect the expression level of miR-93; CCK-8 for the proliferation of cells in each group; biochemical tests for the level of intracellular reactive oxygen species (ROS), malondialdehyde (MDA), reduced glutathione (GSH) and ferrous ion (Fe2+); and Western blot for the expression of ferroptosis-related proteins glutathione peroxidase 4 (GPX4) and SLC7A11).
Results
Under a high glucose environment, 25 (OH) D3 at 100 nM/500 nM could significantly promote the proliferation of hRMVECs, remarkably decrease the level of intracellular ROS/MDA, and up-regulate the level of GSH. Besides, 25 (OH) D3 greatly reduced Fe2+ level in the cells while increased protein level of GPX4 and SLC7A11. Subsequently, we found that high glucose induced miR-93 expression, while 25 (OH) D3 markedly decreased high glucose-induced miR-93 overexpression. Furthermore, overexpression of miR-93 inhibited the functions of 25 (OH) D3 by activating ROS (ROS and MDA were up-regulated while GSH was down-regulated) and inducing Fe2+ (Fe2+ level was up-regulated while GPX4 and SLC7A11 level was down-regulated) in cells.
Conclusion
25 (OH) D3 may inhibit oxidative stress and ferroptosis in hRMVECs induced by high glucose via down-regulation of miR-93.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12886-022-02762-8.
Keywords: Human retinal microvascular endothelial cells (hRMVECs), Oxidative stress, Ferroptosis, 25-hydroxyvitamin D3(25 (OH) D3), MiR-93
Background
Diabetic retinopathy (DR) is not only a common microvascular complication of diabetes mellitus (DM) but also the main cause of blindness and visual loss in the working age group [1]. According to the International Diabetes Federation report, about 1/3 of the 425 million patients with DM worldwide are affected by DR [2]. In clinical practice, the treatment for DR is principally focused on prevention, including systematic management and control of the hyperglycemia, hypertension and hyperlipidemia in patients as well as vision protection by reducing vascular leakage [3]. However, current treatments are not satisfactory because they can only reduce the downstream outcomes of DR and are limited by the side effects [4–6]. Therefore, new strategies are needed to address the threat to human health posed by DR.
MicroRNA (miRNA) is a novel non-coding small RNA with about 22 nucleotides in length, which plays a key role in the pathogenesis of DM [7]. MiR-93, a newly discovered miRNA, exerts important functions in various diseases or injuries [8–13]. Several reports have revealed that miR-93 is associated with DR damage. For example, Hirota et al. observed evidently increased level of miR-93 in patients with proliferative DR [14], and a clinical study by Zou et al. also indicated that high plasma level of miR-93 was linked to a high risk of developing type 2 DR [15]. Subsequently, Ahmed et al. discovered that highly expressed miR-93 could induce inflammatory and oxidative stress responses in retinal pigment epithelium and promote the development of DR, suggesting the vital role of miR-93 in DR development [16].
Vitamin D (Vit D) is an important vitamin in the human body, and 25-hydroxyvitamin D3 (25 (OH) D3) is the main active substance of Vit D. Recently, many research has shown that Vit D also participates in cell proliferation and differentiation, and has immunomodulatory and regulatory properties [17]. A study by Codo Er-Franch et al. demonstrated notably lowered 25 (OH) D3 level in obese children with increased markers of oxidative stress, inflammation, and endothelial activation [18]. A clinical trial by Anandabaskar et al. revealed that continuous 8-week oral 25 (OH) D3 could improve vascular function and reduce oxidative stress in VD-deficient type 2 DM patients [19]. From the above, 25 (OH) D3 supplementation may be an effective strategy to alleviate DM-induced oxidative stress and angiogenesis. Nevertheless, there are no reports displaying the relationship between 25 (OH) D3 and DR currently. It’s reported that Vit D can regulate the transcription of miRNA genes through Vit D receptor binding to its sequence motif located in the promoter of target miRNA genes, miRNA maturation through regulating genes involved in miRNA processing or miRNA stability [20, 21]. Therefore, miRNAs could serve as biomarkers and molecular targets of Vit D which could be modulated by nutritional interventions in health and disease. In this study, we explored the effects of Vit D targets miR-93 on high glucose induction on the activity, oxidative stress and ferroptosis of human retinal microvascular endothelial cells (hRMVECs) in vitro.
Materials and methods
Cell culture and grouping treatment
HRMVECs were obtained from Cell Systems (Kirkland, USA) and cultured in a DMEM containing 10% fetal bovine serum (FBS, Gibco, USA) and 100 × penicillin streptomycin, and the cells were maintained in an incubator at 37 °C and 5% CO2.
Twenty-Five (OH) D3 was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. And the grouping of hRMVECs was performed as follows. In the NG group and HG group, hRMVECs were treated at normal glucose (5 mM) and high glucose (30 mM) concentration for 48 h, respectively [22]. In 25 (OH) D3 100 nM and 25 (OH) D3 500 nM groups, hRMVECs were pretreated with 100 nM and 500 nM of 25 (OH) D3 for 3 h, respectively [23], and then the cells were induced by high glucose for 48 h.
Both miR-93 mimics and their control NC mimics were provided by Shanghai RiboBio Co., Ltd. According to the steps of lipo 2000 transfection, NC mimics or miR-93 mimics were transfected into hRMVECs. Afterwards, the cells were pretreated with 500 nM 25 (OH) D3 for 3 h, induced by high glucose for 48 h, and then were named as 25 (OH) D3 + NC group or 25 (OH) D3 + miR-93 group, respectively.
qRT-PCR
Total RNA was extracted from cells using Trizol reagent (Sigma, USA), and then the quality and concentration of the extracted RNA were tested by standard denaturing agarose gel electrophoresis and NanoDrop spectrophotometer ND-8000. Next, RNA was reverse transcribed to synthesize cDNA based on the PrimeScript ™ RT Master Mix kit (Takara, Japan) instructions. Besides, miR-93 expression level was checked according to the instructions of SYBR Premix Ex TaqTM II kit (Takara, Japan). U6 served as an internal control. And the 2−ΔΔCt method was adopted to calculate the relative expression of the target gene based on the experimental data obtained by qRT-PCR. The relevant primer sequences were displayed in Table 1.
Table 1.
Quantitative Primer Sequences
| Gene | Sequences (5’ to 3’) |
|---|---|
| miR-93 | F: GCAGCAAACTTCTGAGACAC |
| R: GTGCAGGGTCCGAGGTATTC | |
| U6 | F: CTCGCTTCGGCAGCACA |
| R: AACGCTTCACGAATTTGCGT |
Cell viability detection
The treated hRMVECs were seeded in a 96-well plate at 5 × 103 cells/well and detected after adherence at 0 and 24 h according to the instructions of CCK-8 reagent (Solarbio, China). To be specific, 10 µL CCK-8 reagent was added to each well, then the cells were incubated at 37 ℃ for 1–4 h. Later, the absorbance at 450 nm was detected utilizing a microplate reader and the proliferation rate was calculated.
Biochemical tests
The treated hRMVECs were collected and the supernatant was removed. After being washed with PBS 3 times, the cells were collected using a cell scraper. Next, 2 mL PBS was added for the preparation of cell suspension. Subsequently, the suspension was centrifuged at 1000 × g at 4 ℃ for 10 min, and the supernatant was absorbed. Later, 300–500 µL homogenization medium was added and adequate mechanical crushing was performed. After that, the suspension was centrifuged at 12,000 r/min at 4 ℃ for 30 min, and the supernatant was placed on ice for testing. Finally, the level of ROS, malondialdehyde (MDA), reduced glutathione (GSH) and ferrous ion (Fe2+) in the cells of each group was measured by an automatic biochemical analyzer (Olympus, Japan) with the corresponding biochemical kits (Nanjing Jiancheng Bioengineering Institute, China).
Western blot
Total cellular protein was extracted via RIPA lysate (Solarbio, China), and the concentration of extracted protein was determined with BCA kit (Beyotime, China). Later, 20 µg of treated proteins were separated by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), and then the separated proteins were transferred to polyvinylidene fluoride (PVDF) membrane. After blocking with 5% non-fat dry milk for 1–3 h, the diluted primary antibodies (GPX4, ab125066; SLC7A11, ab175186; Abcam, UK) were added into the membrane for incubation at 4 ℃ overnight. The membrane was washed 3 times, and the secondary antibodies (bs-0295G-HRP, Bioworld, USA) were added for incubation at room temperature for 1 h. Afterwards, the membrane was washed 3 times again, and then electrogenerated chemiluminescence (ECL) reagent (Beyotime, China) was added. The proteins were developed and the image collection was performed in the gel imaging system; and the gray level of protein bands was analyzed using Image J software. Besides, GAPDH (ab9485) acted as an internal control to calculate the relative protein expression.
Statistical analysis
The results were expressed as mean ± standard deviation (SD). SPSS 26.0 was employed to conduct one-way analysis of variance among multiple groups and independent samples T-test between two groups. And P < 0.05 was considered as a significant difference.
Results
25-hydroxyvitamin D3 (25 (OH) D3) reduces the inhibitory effect of high glucose on the activity of human retinal microvascular endothelial cells (hRMVECs)
The examination outcomes presented that the cell proliferation rate in the HG group was much lower than that in the NG group (P < 0.01); compared with the HG group, the cell proliferation rates in the 25 (OH) D3 100 nM group and 25 (OH) D3 500 nM group were increased in a concentration-dependent manner (Fig. 1). The above indicated that high glucose could significantly inhibit the activity of hRMVECs, while 25 (OH) D3 could reduce the inhibitory effect of high glucose on the cell activity of hRMVECs.
Fig. 1.
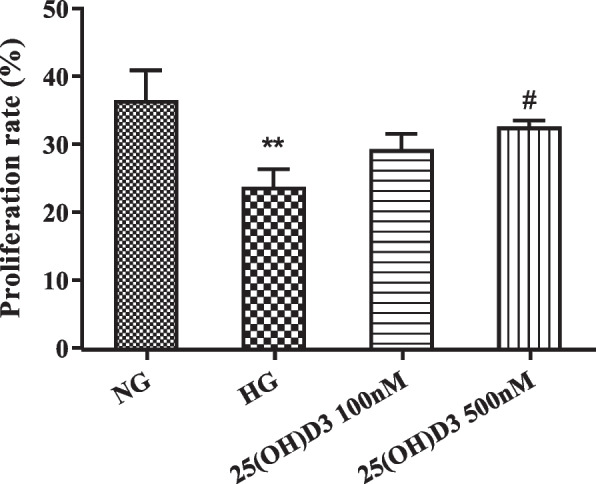
25-hydroxyvitamin D3 (25 (OH) D3) reduces the inhibitory effect of high glucose on the activity of human retinal microvascular endothelial cells (hRMVECs). The effect of different concentrations (100 nM and 500 nM) of 25 (OH) D3 on the cell viability of hRMVECs under high glucose environment determined by CCK-8, **P < 0.01 vs. NG group, #P < 0.05 vs. HG group
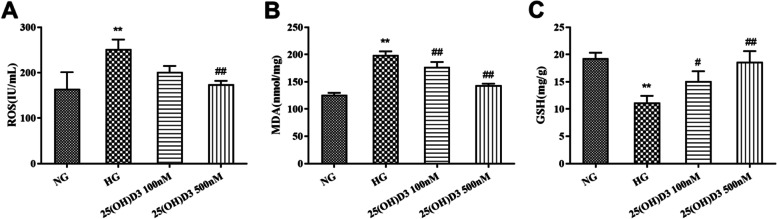
25-hydroxyvitamin D3 alleviates the oxidative stress response induced by high glucose in human retinal microvascular endothelial cells
Compared with the NG group, the HG group exhibited a noticeable increase in the ROS and MDA level while a marked decrease in the GSH level (Fig. 2 A-C, P < 0.01). Besides, compared with the HG group, the ROS and MDA level in 25 (OH) D3 100 nM group and 25 (OH) D3 500 nM group declined, while the GSH level was raised greatly in a concentration-dependent manner (Fig. 2 A-C, P < 0.05). From the above, 25 (OH) D3 could inhibit the oxidative stress response induced by high glucose in hRMVECs.
Fig. 2.
25-hydroxyvitamin D3 alleviates the oxidative stress response induced by high glucose in human retinal microvascular endothelial cells. A-C: Biochemical tests for the effect of different concentrations (100 nM and 500 nM) of 25 (OH) D3 on the level of oxidative stress substances (reactive oxygen species (ROS), malondialdehyde (MDA), reduced glutathione (GSH)) in hRMVECs induced by high glucose. **P < 0.01 vs. NG group, #P < 0.05 vs. HG group, ##P < 0.01 vs. HG group
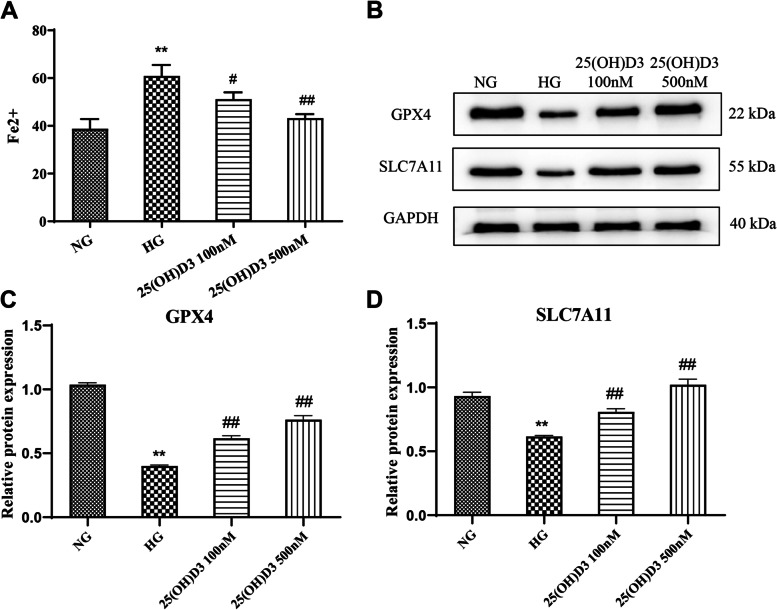
25-hydroxyvitamin D3 inhibits high glucose-induced ferroptosis in human retinal microvascular endothelial cells
The results revealed that the Fe2+ level in hRMVECs in the HG group was remarkably elevated compared with the NG group (P < 0.01). In addition, 25 (OH) D3 at 100 nM and 500 nM significantly inhibited the increase of intracellular Fe2+ level induced by high glucose in a concentration-dependent manner (Fig. 3 A, P < 0.05). In addition, Western blot results displayed an evident decrease in the protein level of GPX4 and SLC7A11 in the HG group in comparison with that in the NG group. Also, the protein level of GPX4 and SLC7A11 in the 25 (OH) D3 100 nM group and 25 (OH) D3 500 nM group was a lot higher than that in the HG group in a concentration-dependent manner (Fig. 3B-D, P < 0.01). All in all, high glucose induced ferroptosis in hRMVECs, and 25 (OH) D3 significantly inhibited ferroptosis in hRMVECs induced by high glucose.
Fig. 3.
25-hydroxyvitamin D3 inhibits high glucose-induced ferroptosis in human retinal microvascular endothelial cells. A: Biochemical tests for the effect of different concentrations (100 nM and 500 nM) of 25 (OH) D3 on Fe2+ level in hRMVECs induced by high glucose; B/C: Western blot for detecting the effect of different concentrations (100 nM and 500 nM) of 25 (OH) D3 on the expression of ferroptosis-related proteins (GPX4 and SLC7A11) in hRMVECs induced by high glucose, **P < 0.01 vs. NG group, #P < 0.05 vs. HG group, ##P < 0.01 vs. HG group
25-hydroxyvitamin D3 can down-regulate miR-93 expression in human retinal microvascular endothelial cells induced by high glucose
The results manifested that miR-93 expression level in hRMVECs in the HG group was obviously increased compared with the NG group (P < 0.01); while pretreatment with 100 nM and 500 nM 25 (OH) D3 could evidently reduce miR-93 expression level in cells in a concentration-dependent manner (Fig. 4, P < 0.01).
Fig. 4.
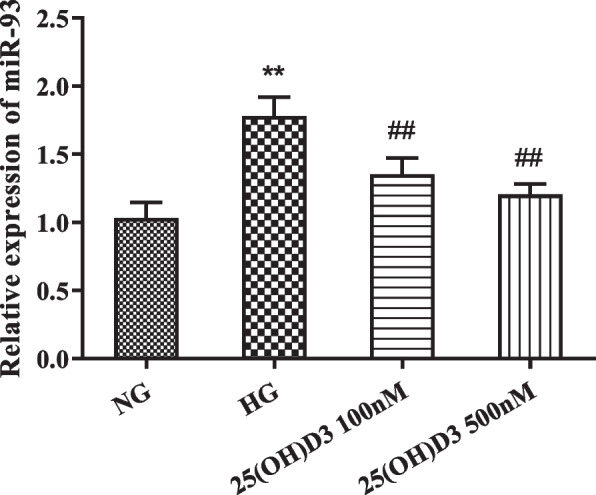
25-hydroxyvitamin D3 can down-regulate miR-93 expression in human retinal microvascular endothelial cells induced by high glucose. QRT-PCR was performed to detect the effect of different concentrations (100 nM and 500 nM) of 25 (OH) D3 on miR-93 expression in hRMVECs induced by high glucose, **P < 0.01 vs. NG group, ##P < 0.01 vs. HG group
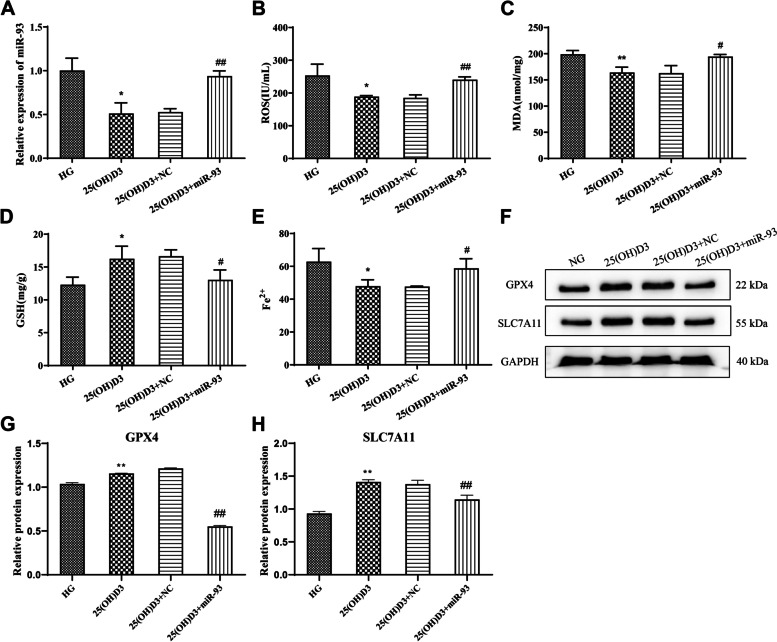
MiR-93 overexpression reverses the effect of 25-hydroxyvitamin D3 on oxidative stress and ferroptosis induced by high glucose in human retinal microvascular endothelial cells
QRT-PCR results demonstrated that miR-93 was significantly down-regulated in cells treated with 25 (OH) D3, while miR-93 mimics transfection significantly reversed the inhibitory effect of 25 (OH) D3 on miR-93 expression in cells (Fig. 5 A, P < 0.01). Moreover, we examined the oxidative stress-related substances and ferroptosis-related parameters in each group of cells. And the examination outcomes showed that miR-93 mimics could reverse the inhibitory effect of 25 (OH) D3 on high glucose-induced oxidative stress (ROS and MDA level was up-regulated, while GSH level was down-regulated) (Fig. 5B-D, P < 0.05) and ferroptosis in hRMVECs (Fe2+ level and protein level of GPX4 and SLC7A11 was greatly lowered) (Fig. 5E-H, P < 0.05). In summary, miR-93 mimics could reverse the inhibitory effect of 25 (OH) D3 on high glucose-induced oxidative stress and ferroptosis in hRMVECs.
Fig. 5.
miR-93 overexpression reverses the effect of 25-hydroxyvitamin D3 on high glucose-induced oxidative stress and ferroptosis in human retinal microvascular endothelial cells. A: qRT-PCR for testing the expression of miR-93 in hRMVECs induced by high glucose in each group; B-E: Biochemical assay for detecting the level of ROS (B), MDA (C), GSH (D) and Fe2+ (E) in hRMVECs induced by high glucose in each group. F-H: Western blot for checking the protein expression level of ferroptosis-related proteins (GPX4 and SLC7A11) in hRMVECs induced by high glucose in each group, *P < 0.05 and **P < 0.01 vs. HG group, #P < 0.05 and ##P < 0.01 vs. 25 (OH) D3 + NC group
Discussion
DR has become a worldwide concern, which can gradually develop into the proliferative vitreoretinopathy (PVR), leading to significant vision loss, and even blindness [24]. Therefore, early intervention and diagnosis can help delay the progression of the disease. The mechanism of DR is complex, including pericyte loss, neovascularization (NV), glycation end products, oxidative stress and ROS, inflammatory response and so on [25]. Currently, anti-vascular endothelial-derived growth factor (VEGF) therapy is widely used in clinical practice, carried out by means of different agents, including aflibercept (Eylea1), bevacizumab (Avastin1) and ranibizumab (Lucentis1) [26]. The VEGF family consists of six proteins: VEGF-A,-B,-C,-D,-E, and placental growth factor (PGF) [27]. Giurdanella et al. revealed that intravitreally injected anti-VEGF may exert relevant effect on retinal pericytes [28]. Usui-Ouchi et al. showed that intra-vitreal injection of the CITED2 peptide inhibited retinal NV by down-regulation of HIF-mediated transcription in retinal cells [29]. However, the adverse effects after the administration of high-dose anti-VEGF drugs can not be ignored [30]. It’s necessary to find alternative drugs without adverse effects to treat DR.
Recently, in vitro studies have shown that the active form of vit D has an effect of inhibiting neovascularization by HIF-1α/VEGF pathway [31, 32]. A large number of data have reported that Vit D level in the blood of DM patients is noticeably declined, and VD supplementation can greatly reduce insulin resistance in patients [33]. The study by Khumaedi et al. pointed out that the DM-induced ROS accumulation not only was the key to causing the oxidative response of retinaldehyde but also resulted in retinal vascular endothelial tissue damage and loss of endothelial cells and then promoted the process of DR [34]. In this study, 25 (OH) D3 remarkably alleviated the proliferation capacity reduction and oxidative stress state of hRMVECs caused by high glucose. Some previous research also have reported good antioxidant effect of Vit D. For instance, Tohari et al. stated that Vit D was able to protect retinal pigment epithelial cells from high glucose-induced oxidative damage and inflammatory damage through high glucose-treated ARPE-19 cells and streptozotocin-induced diabetic mouse models [35]. Zhu et al. claimed that Vit D could inhibit high glucose-induced oxidative stress in tubular cells via the AKT/UCP2 signaling pathway [36]. And it can be known from previous studies that the antioxidant functions of 25 (OH) D3 may be related to the reduction of inflammatory level [37].
Ferroptosis is a novel mode of non-apoptotic cell death, and its main features include intracellular iron overload, iron-dependent lipid peroxide accumulation, and oxidoreductase deficiency (especially GPX4) [38]. Several studies have displayed that ferroptosis is involved in DM pathogenesis. Additionally, ferroptosis also participates in the pathogenesis of diabetic complications such as myocardial ischemia and diabetic cardiomyopathy [39]. Many iron-containing proteins are involved in the phototransduction cascade in the retina [40]. Despite being an essential micronutrient for many protein functions, excessive irons are potentially harmful pro-oxidants [41]. Excessive irons result in the occurrence of Fenton reaction, catalyze the conversion of H2O2 to hydroxyl radicals, induce lipid peroxidation, DNA strand breaks and degradation of cellular components, and ultimately cause tissue damage [42]. As negative regulators of the ferroptosis, both GPX4 and SLC7A11 can greatly reduce the level of peroxidation in cells [43]. Also, we found that high glucose resulted in the accumulation of Fe2+ and the decrease of GPX4 and SLC7A11 protein level in hRMVECs, indicating that high glucose induced ferroptosis in hRMVECs. And our findings were consistent with the research of Singh and Zhu et al. that high glucose could induce ferroptosis in retinal pigment epithelial cells [44, 45]. Furthermore, we discovered that 25 (OH) D3 could reduce the high glucose-induced intracellular iron content, and notably increase the protein level of GPX4 and SLC7A11. The above results indicated that 25 (OH) D3 could effectively hinder the occurrence of ferroptosis induced by high glucose in hRMVECs. However, the specific mechanism of 25 (OH) D3 remains unknown and needs further exploration. There are no studies on the relationship between 25 (OH) D3 and ferroptosis, but 25 (OH) D3, another active component of Vit D, was found to be able to inhibit ferroptosis in zebrafish hepatocytes.
Liu et al. identified potential key genes of ferroptosis in the pathogenesis of intracerebral hemorrhage by bioinformatics analysis, and genes such as miR-93 and SNHG16 were screened and obtained in their report [46]. Related research has suggested that changes in the miR-93 level are evidently associated with high risk of DM [47]. And some studies manifested the correlation of the down-regulation of miR-93 expression with the imbalance of oxidative stress. For example, Su et al. revealed that up-regulation of miR-93 promoted oxidative stress and inflammatory response by activating RhoA/ROCK signaling pathway [48]. As demonstrated in a previous study, an increase of miR-93 expression is a leading cause of the occurrence of polycystic ovary syndrome (PCOS) and insulin resistance by targeting at GLUT4, an important protein in regulating glucose homeostasis [49]. Also, another experiment discovered that miR-93 expression is distinctly high in proliferative DR eyes with angiogenesis and proliferative responses, indicating that miR-93 may be associated with angiogenesis and fibrosis [50]. As mentioned above, miR-93 is a key signaling molecule that promotes the oxidative stress responses in cells and has been indicated as a significant regulator in DR. In addition, miR-93 is relevant to ferroptosis. In our study, high glucose could induce miR-93 expression in hRMVECs, while 25 (OH) D3 treatment effectively inhibited miR-93 expression in cells induced by high glucose; besides, overexpression of miR-93 could reverse the protective effect of 25 (OH) D3 on hRMVECs by promoting oxidative stress and ferroptosis. All the above indicated that 25 (OH) D3 inhibits the damages such as cell viability reduction, oxidative stress and ferroptosis in high glucose-induced hRMVECs. And the protective effects of 25 (OH) D3 on cells may be correlated with its down-regulation on miR-93 expression.
However, the function of vit D in DR is complex, it is a potent inhibitor of retinal neovascularization, and related to vessel related biomarkers [51]. In the process of DR, the epithelial-mesenchymal transition (EMT) develops in retinal pigment epithelium (RPE) cells, which transforms epithelial cells into stromal cells, enhancing their proliferation, migration and anti-apoptotic capacity, playing a key role in PVR formation [52, 53]. Bonventre and his colleagues reported the synthetic ligands of the vit D receptor can target the TGF-β-SMAD signaling pathway and inhibit the EMT progression [54]. Lai et al. showed that vit D can increase the protein expression of CD31, and reduce the protein expressions of alpha-smooth muscle actin (α-SMA) and fibronectin in the TGF-β1-induced fibrosis model. Additionally, the protein expression of VE-cadherin was increased and fibroblast-specific protein-1 (FSP1) was decreased after vit D treatment in the isoproterenol-induced fibrosis rat [55]. Vila et al. reported that active vit D rescued the uraemic medium-induced loss of cell-cell adhesion by increasing VE-cadherin and F-actin [56]. Collectively, Vit D is conducive to maintaining insulin secretion and improving insulin resistance, which can resist oxidative stress and ferroptosis, inhibit the growth of vascular smooth muscle cells, inhibit the EMT progression and retinal vascular proliferation, so then slow down the occurrence and development of DR. The specific molecular mechanisms of 25 (OH) D3 have not been revealed yet. Therefore, in order to lay the foundation for the treatment of DR, more in vitro and in vivo experiments are needed for further exploration.
Conclusion
To sum up, 25 (OH) D3 can effectively alleviate the impaired viability, oxidative stress, and ferroptosis of high glucose-induced hRMVECs, and its mechanism of action may be achieved by down-regulating the expression of miR-93.
Supplementary Information
Additional file 1. Western Blot's original impression.
Acknowledgements
Not applicable.
Abbreviations
- 25 (OH) D3
25-hydroxyvitamin D3
- hRMVECs
Human retinal microvascular endothelial cells
- DR
Diabetic retinopathy
- ROS
Reactive oxygen species
- MDA
Malondialdehyde
- GSH
Reduced glutathione
- Fe2+
Ferrous ion
- GPX4
Glutathione peroxidase 4
- DM
Diabetes mellitus
- PRP
Panretinal photocoagulation
- Vit D
Vitamin D
Author’s contributions
Dongmei Zhan and Juan Lou designed the study. Juan Zhao and Qin Shi collated the data, carried out data analyses and produced the initial draft of the manuscript. Dongmei Zhan and Weiling Wang contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
Funding
This work was supported by Natural Science Foundation of Ningxia, China (No. 2021AAC03376).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14):e93751. doi: 10.1172/jci.insight.93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bavinger JC, Dunbar GE, Stem MS, Blachley TS, et al. The Effects of Diabetic Retinopathy and Pan-Retinal Photocoagulation on photoreceptor cell function as assessed by Dark Adaptometry. Invest Ophthalmol Vis Sci. 2016;57(1):208–17. doi: 10.1167/iovs.15-17281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung N, Wong IY, Wong TY. Ocular anti-VEGF therapy for diabetic retinopathy: overview of clinical efficacy and evolving applications. Diabetes Care. 2014;37(4):900–5. doi: 10.2337/dc13-1990. [DOI] [PubMed] [Google Scholar]
- 4.Durham JT, Dulmovits BM, Cronk SM, Sheets AR, Herman IM. Pericyte chemomechanics and the angiogenic switch: insights into the pathogenesis of proliferative diabetic retinopathy? Invest Ophthalmol Vis Sci. 2015;56(6):3441–59. doi: 10.1167/iovs.14-13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44(2):129–46. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 6.Singh LP. Thioredoxin Interacting Protein (TXNIP) and pathogenesis of diabetic retinopathy. J Clin Exp Ophthalmol. 2013;4:10.4172/2155-9570.1000287. 10.4172/2155-9570.1000287. [DOI] [PMC free article] [PubMed]
- 7.Mastropasqua R, Toto L, Cipollone F, Santovito D, et al. Role of microRNAs in the modulation of diabetic retinopathy. Prog Retin Eye Res. 2014;43:92–107. doi: 10.1016/j.preteyeres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Tanzer A, Stadler PF. Molecular evolution of a microRNA cluster. J Mol Biol. 2004;339(2):327–35. doi: 10.1016/j.jmb.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 9.Xu F, Zha G, Wu Y, Cai W, Ao J. Overexpressing lncRNA SNHG16 inhibited HCC proliferation and chemoresistance by functionally sponging hsa-miR-93. Onco Targets Ther. 2018;11:8855–63. doi: 10.2147/OTT.S182005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu M, Jiang Z, Li H, Peng J, et al. MiR-93/HMGB3 regulatory axis exerts tumor suppressive effects in colorectal carcinoma cells. Exp Mol Pathol. 2021;120:104635. doi: 10.1016/j.yexmp.2021.104635. [DOI] [PubMed] [Google Scholar]
- 11.Ding Y, Wang L, Zhao Q, Wu Z, Kong L. MicroRNA93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NFkappaB signaling pathway. Int J Mol Med. 2019;43(2):779–90. doi: 10.3892/ijmm.2018.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ke ZP, Xu P, Shi Y, Gao AM. MicroRNA-93 inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by targeting PTEN. Oncotarget. 2016;7(20):28796–805. doi: 10.18632/oncotarget.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Yang H, Zhou X, Zhang L, Lu X. MiR-93 targeting EphA4 promotes Neurite Outgrowth from spinal cord neurons. J Mol Neurosci. 2016;58(4):517–24. doi: 10.1007/s12031-015-0709-0. [DOI] [PubMed] [Google Scholar]
- 14.Hirota K, Keino H, Inoue M, Ishida H, Hirakata A. Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):335–42. doi: 10.1007/s00417-014-2692-5. [DOI] [PubMed] [Google Scholar]
- 15.Zou HL, Wang Y, Gang Q, Zhang Y, Sun Y. Plasma level of miR-93 is associated with higher risk to develop type 2 diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1159–66. doi: 10.1007/s00417-017-3638-5. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed LHM, Butler AE, Dargham SR, Latif A, et al. Association of vitamin D2 and D3 with type 2 diabetes complications. BMC Endocr Disord. 2020;20(1):65. doi: 10.1186/s12902-020-00549-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitri J, Pittas AG. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2014;43(1):205–32. doi: 10.1016/j.ecl.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Codoner-Franch P, Tavarez-Alonso S, Simo-Jorda R, Laporta-Martin P, et al. Vitamin D status is linked to biomarkers of oxidative stress, inflammation, and endothelial activation in obese children. J Pediatr. 2012;161(5):848–54. doi: 10.1016/j.jpeds.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 19.Anandabaskar N, Selvarajan S, Dkhar SA, Kamalanathan SK, et al. Effect of vitamin D supplementation on vascular functions and oxidative stress in type 2 Diabetic patients with vitamin D Deficiency. Indian J Endocrinol Metab. 2017;21(4):555–63. doi: 10.4103/ijem.IJEM_140_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giangreco AA, Nonn L. The sum of many small changes: microRNAs are specifically and potentially globally altered by vitamin D3 metabolites. J Steroid Biochem Mol Biol. 2013;36:86–93. doi: 10.1016/j.jsbmb.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giangreco AA, Vaishnav A, Wagner D, Finelli A, Fleshner N, Van der Kwast T, Vieth R, Nonn L. Tumor suppressor microRNAs, miR-100 and – 125b, are regulated by 1,25-dihydroxyvitamin D in primary prostate cells and in patient tissue. Cancer Prev Res (Phila) 2013;6:483–94. doi: 10.1158/1940-6207.CAPR-12-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manasson J, Tien T, Moore C, Kumar NM, Roy S. High glucose-induced downregulation of connexin 30.2 promotes retinal vascular lesions: implications for diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(3):2361–6. doi: 10.1167/iovs.12-10815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phadnis R, Nemere I. Direct, rapid effects of 25-hydroxyvitamin D3 on isolated intestinal cells. J Cell Biochem. 2003;90(2):287–93. doi: 10.1002/jcb.10639. [DOI] [PubMed] [Google Scholar]
- 24.Lin KY, Hsih WH, Lin YB, Wen CY, Chang TJ. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig. 2021;12(8):1322–5. doi: 10.1111/jdi.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filippov VM, Petrachkov DV, Budzinskaya MV, Sidamonidze AL. Sovremennye kontseptsii patogeneza diabeticheskoi retinopatii [Modern concepts of pathogenesis of diabetic retinopathy]. Vestn Oftalmol. 2021;137(5. Vyp. 2):306–13. Russian. 10.17116/oftalma2021137052306. [DOI] [PubMed]
- 26.Kollias AN, Ulbig MW. Diabetic retinopathy: early diagnosis and effective treatment. Dtsch Arztebl Int. 2010;107(5):75–83. doi: 10.3238/arztebl.2010.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chekhonin ES, Fayzrakhmanov RR, Sukhanova AV, Bosov ED. Anti-VEGF preparaty v lechenii diabeticheskoi retinopatii [Anti-VEGF therapy for diabetic retinopathy] Vestn Oftalmol. 2021;137(4):136–42. doi: 10.17116/oftalma2021137041136. [DOI] [PubMed] [Google Scholar]
- 28.Giurdanella G, Anfuso CD, Olivieri M, Lupo G, Caporarello N, Eandi CM, Drago F, Bucolo C, Salomone S. Aflibercept, bevacizumab and ranibizumab prevent glucose-induced damage in human retinal pericytes in vitro, through a PLA2/COX-2/VEGF-A pathway. Biochem Pharmacol. 2015;96(3):278–87. doi: 10.1016/j.bcp.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Usui-Ouchi A, Aguilar E, Murinello S, Prins M, Gantner ML, Wright PE, Berlow RB, Friedlander M. An allosteric peptide inhibitor of HIF-1α regulates hypoxia-induced retinal neovascularization. Proc Natl Acad Sci U S A. 2020;117(45):28297–306. doi: 10.1073/pnas.2017234117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma P, Pan X, Liu R, Qu Y, Xie L, Xie J, Cao L, Chen Y. Ocular adverse events associated with anti-VEGF therapy: a pharmacovigilance study of the FDA adverse event reporting system (FAERS) Front Pharmacol. 2022;13:1017889. doi: 10.3389/fphar.2022.1017889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxena S. Vitamin D supplementation in diabetic retinopathy in the era of COVID-19. Indian J Ophthalmol. 2021;69(3):483–4. doi: 10.4103/ijo.IJO_3798_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. 1alpha,25-dihydroxyvitamin D3 (calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6(4):1433–9. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 33.Aludwan M, Kobyliak N, Abenavoli L, Kyriienko D, Fagoonee S, Pellicano R, Komisarenko I. Vitamin D3 deficiency is associated with more severe insulin resistance and metformin use in patients with type 2 diabetes. Minerva Endocrinol. 2020;45(3):172–80. doi: 10.23736/S0391-1977.20.03161-2. [DOI] [PubMed] [Google Scholar]
- 34.Khumaedi AI, Purnamasari D, Wijaya IP, Soeroso Y. The relationship of diabetes, periodontitis and cardiovascular disease. Diabetes Metab Syndr. 2019;13(2):1675–8. doi: 10.1016/j.dsx.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Tohari AM, Almarhoun M, Alhasani RH, Biswas L, et al. Protection by vitamin D against high-glucose-induced damage in retinal pigment epithelial cells. Exp Cell Res. 2020;392(1):112023. doi: 10.1016/j.yexcr.2020.112023. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Wu S, Guo H, Active Vitamin D, Vitamin D. Receptor help prevent high glucose Induced oxidative stress of renal tubular cells via AKT/UCP2 signaling pathway. Biomed Res Int. 2019;2019:9013904. doi: 10.1155/2019/9013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lan N, Luo G, Yang X, Cheng Y, et al. 25-Hydroxyvitamin D3-deficiency enhances oxidative stress and corticosteroid resistance in severe asthma exacerbation. PLoS ONE. 2014;9(11):e111599. doi: 10.1371/journal.pone.0111599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei P, Bai T, Sun Y. Mechanisms of ferroptosis and relations with regulated cell death: a review. Front Physiol. 2019;10:139. doi: 10.3389/fphys.2019.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sha W, Hu F, Xi Y, Chu Y, Bu S. Mechanism of ferroptosis and its role in type 2 diabetes Mellitus. J Diabetes Res. 2021;2021:9999612. doi: 10.1155/2021/9999612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gnana-Prakasam JP, Ananth S, Prasad PD, Zhang M, et al. Expression and iron-dependent regulation of succinate receptor GPR91 in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011;52(6):3751–8. doi: 10.1167/iovs.10-6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arjunan P, Gnanaprakasam JP, Ananth S, Romej MA, et al. Increased retinal expression of the pro-angiogenic receptor GPR91 via BMP6 in a mouse model of Juvenile Hemochromatosis. Invest Ophthalmol Vis Sci. 2016;57(4):1612–9. doi: 10.1167/iovs.15-17437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73(11–12):2195–209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xie Y, Hou W, Song X, Yu Y, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–79. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh LP, Yumnamcha T, Devi TS, Mitophagy Ferritinophagy and Ferroptosis in Retinal Pigment epithelial cells under high glucose conditions: implications for Diabetic Retinopathy and Age-Related retinal Diseases. JOJ Ophthalmol. 2021;8(5):77–85. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Z, Duan P, Song H, Zhou R, Chen T. Downregulation of circular RNA PSEN1 ameliorates ferroptosis of the high glucose treated retinal pigment epithelial cells via miR-200b-3p/cofilin-2 axis. Bioengineered. 2021;12(2):12555–67. doi: 10.1080/21655979.2021.2010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Li X, Cui Y, Meng P, et al. Bioinformatics Analysis identifies potential ferroptosis key genes in the pathogenesis of Intracerebral Hemorrhage. Front Neurosci. 2021;15:661663. doi: 10.3389/fnins.2021.661663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou M, Hou Y, Wu J, Li G, et al. Mir-93-5p promotes insulin resistance to regulate type 2 diabetes progression in HepG2 cells by targeting HGF. Mol Med Rep. 2021;23(5):1–2. doi: 10.3892/mmr.2021.11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su Q, Zhang P, Yu D, Wu Z, et al. Upregulation of miR-93 and inhibition of LIMK1 improve ventricular remodeling and alleviate cardiac dysfunction in rats with chronic heart failure by inhibiting RhoA/ROCK signaling pathway activation. Aging. 2019;11(18):7570–86. doi: 10.18632/aging.102272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, Chen BS, Chazenbalk G, Azziz R. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes. 2013;62:2278–86. doi: 10.2337/db12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirota K, Keino H, Inoue M, Ishida H, Hirakata A. Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253:335–42. doi: 10.1007/s00417-014-2692-5. [DOI] [PubMed] [Google Scholar]
- 51.Jamali N, Wang S, Darjatmoko SR, Sorenson CM, Sheibani N. Vitamin D receptor expression is essential during retinal vascular development and attenuation of neovascularization by 1, 25(OH)2D3. PLoS ONE. 2017;12(12):e0190131. doi: 10.1371/journal.pone.0190131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tamiya S, Kaplan HJ. Role of epithelial-mesenchymal transition in proliferative vitreoretinopathy. Exp Eye Res. 2016;142:26–31. doi: 10.1016/j.exer.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Chen Z, Shao Y, Li X. The roles of signaling pathways in epithelial-to-mesenchymal transition of PVR. Mol Vis. 2015;21:706–10. [PMC free article] [PubMed] [Google Scholar]
- 54.Bonventre JV. Antifibrotic vitamin D analogs. J Clin Invest. 2013;123(11):4570–3. doi: 10.1172/JCI72748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai CC, Juang WC, Sun GC, Tseng YK, Jhong RC, Tseng CJ, Wong TY, Cheng PW. Vitamin D attenuates loss of endothelial biomarker expression in Cardio-Endothelial cells. Int J Mol Sci. 2020;21(6):2196. doi: 10.3390/ijms21062196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vila Cuenca M, van Bezu J, Beelen RHJ, Vervloet MG, Hordijk PL. Stabilization of cell-cell junctions by active vitamin D ameliorates uraemia-induced loss of human endothelial barrier function. Nephrol Dial Transplant. 2019;34(2):252–64. doi: 10.1093/ndt/gfy111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Western Blot's original impression.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.