Abstract
The adherence of 58 nontypeable Haemophilus influenzae isolates obtained from patients with otitis media or chronic obstructive pulmonary disease (COPD) and obtained from the throats of healthy individuals to Chang and NCI-H292 epithelial cells was compared. Otitis media isolates, but not COPD isolates, adhered significantly more to both cell lines than did throat isolates. Since high-molecular-weight (HMW) proteins are major adhesins of nontypeable H. influenzae, the isolates were screened for HMW protein expression by Western blotting with two polyclonal sera and PCR with hmw-specific primers. Twenty-three of the 32 adhering isolates (72%) and only 1 of the 26 nonadherent strains were HMW protein or hmw gene positive. Among the 32 isolates adhering to either cell line, 5 different adherence patterns were distinguished based on the inhibiting effect of dextran sulfate. Using H. influenzae strain 12 expressing two well-defined HMW proteins (HMW1 and HMW2) and its isogenic mutants as a reference, we observed HMW1-like adherence to both cell lines for 16 of the 32 adherent isolates. Four others showed HMW2-like adherence to NCI-H292. Of the three other patterns of adherence, one probably also involved HMW protein. Screening of the isolates with six HMW-specific monoclonal antibodies in a whole-cell enzyme-linked immunosorbent assay showed that the HMW proteins of COPD isolates and carrier isolates were more distinct from the HMW proteins from H. influenzae strain 12 than those from otitis media isolates. Characterization of the HMW protein of a COPD isolate by adherence and DNA sequence analysis showed that despite large sequence diversity in the hmwA gene, probably resulting in the antigenic differences, the HMW protein mediated the HMW2-like adherence of this strain.
The gram-negative bacterium Haemophilus influenzae is a commensal of the human upper respiratory tract. Encapsulated strains, in particular those with a serotype b polysaccharide, are important pathogens causing systemic disease, such as meningitis, epiglottitis, cellulitis, arthritis, sepsis, and pneumonia. Nonencapsulated (nontypeable) H. influenzae is a frequent cause of respiratory tract infections, including otitis media, sinusitis, and lower respiratory tract infections in patients with chronic obstructive pulmonary disease (COPD) (20, 34, 35).
Adherence of H. influenzae to the respiratory epithelial cells is considered an important step in the colonization of the respiratory mucosa. Several adhesins of H. influenzae have been determined, each with different adherence specificities (23, 30, 39). Adherence of H. influenzae by a fimbria-mediated as well as a fimbria-independent mechanism has been described. Fimbria-mediated adherence seems to be especially relevant for the adherence of encapsulated H. influenzae isolates to different cells (8, 17, 24, 29), since this type of adherence is not hampered by capsule expression (28, 30). Only a minority of nontypeable H. influenzae isolates from different sources contained a fimbria gene cluster (9, 10, 15). Attachment of nontypeable H. influenzae to different cell lines is mediated by various nonfimbrial proteins (3, 25, 31). The most common of these are two immunogenic high-molecular-weight (HMW) proteins designated HMW1 and HMW2 (2, 25), which are detected in 75 to 80% of unrelated nontypeable H. influenzae strains (4, 15, 28). Despite the significant sequence similarity of the predicted amino acid sequences of HMW1 and HMW2, these proteins mediate binding to distinct human epithelial cells, indicating different receptor specificity (14, 26–28). HMW1 recognizes a sialylated glycoprotein, and HMW1-mediated adherence is inhibited in the presence of heparin or dextran sulfate (21, 32). The receptor for HMW2 is currently unknown.
We were interested in the adherence characteristics of nontypeable H. influenzae isolates from carriers compared to those isolated from COPD patients and from otitis media patients. Adherence of 58 nontypeable H. influenzae isolates was determined with two human epithelial cell lines, the Chang conjunctiva epithelial cell line and the lung epithelial cell line NCI-H292. The association between the presence of HMW protein and adherence was analyzed by PCR with hmw primers and whole-cell enzyme-linked immunosorbent assay (ELISA) and Western blotting with polyclonal sera. Among the H. influenzae isolates, five different adherence patterns were present, including the patterns for HMW1- and HMW2-mediated adherence. The HMW proteins showed very different reactivity patterns with six HMW monoclonal antibodies (MAbs), irrespective of the adherence patterns of the isolates. The hmw gene of a COPD isolate was cloned and sequenced. It appeared that the HMW2 type of adherence of this isolate was associated with the HMW protein encoded by this gene and that this protein differed antigenically from the HMW protein of the prototype H. influenzae strain 12 due to sequence diversity of the hmwA gene.
MATERIALS AND METHODS
Bacterial isolates and plasmids.
A total of 58 nonencapsulated (nontypeable) H. influenzae isolates were used. Nineteen isolates were isolated from sputum samples of 17 patients with COPD ranging from 30 to 85 years of age, 9 were from middle ear fluid samples of 9 children with otitis media, and 30 were from throat swabs of healthy carriers. Of these throat isolates, 21 isolates were from 20 healthy children visiting the health care center for routine checks, 6 isolates were from 2 children (18), and 3 were from 2 students at our department. All isolates were determined as nontypeable based on the absence of agglutination with antisera for H. influenzae capsule types a to f. COPD isolates that were derived from the same patient have been characterized genotypically as different strains by random amplified polymeric DNA analysis (19). Nontypeable H. influenzae strain 12, originally obtained from a child with acute otitis media, is the prototype isolate of which the genes encoding the HMW1 and HMW2 proteins were originally isolated and sequenced (2). Strain 12 mutants expressing HMW1 but not HMW2 (strain 12-2), HMW2 but not HMW1 (12-10), or neither HMW1 nor HMW2 (strain 12-4) have been described previously (25) and were kindly provided by S. J. Barenkamp. Plasmid pT1-17 (25) contains the hmw1 gene cluster with an insertion of a 1.3-kb kanamycin resistance gene (hmw::kan) and was also provided by S. J. Barenkamp. Plasmid pGJB103 is a shuttle vector that replicates in both H. influenzae and Escherichia coli (33). It was obtained from the recombinant plasmid pEJH39-1, which was provided by E. J. Hansen (12).
Culture conditions.
All H. influenzae isolates were grown overnight on chocolate agar plates at 37°C in 5% CO2. E. coli DH5α was grown on Luria-Bertani agar plates at 37°C. H. influenzae transformants expressing the kanamycin marker were grown on chocolate agar plates supplemented with 20 μg of kanamycin per ml. Transformants expressing pGJB103 were grown in the presence of tetracycline at a concentration of 5 μg/ml for H. influenzae or 12.5 μg/ml for E. coli DH5α. All isolates were stored at −70°C in broth containing 20% glycerol.
Adherence assays.
NCI-H292 epithelial cells, derived from a human lung mucoepidermoid carcinoma (ATTC CRL1848) (1, 38), and Chang epithelial cells, originating from human conjunctiva (ATTC CCL20.2), were grown to near confluency or confluency on 12-mm-diameter glass coverslips (Menzel-gläser, Braunschweitz, Germany) in 24-well tissue culture plates (Falcon, Becton Dickinson, Franklin Lakes N.J.) in 1 ml of RPMI (Gibco, Life Technologies, Breda, The Netherlands) plus 10% fetal calf serum (FCS). Bacterial isolates were cultured overnight on chocolate agar plates and suspended in Dulbecco's phosphate-buffered saline (DPBS; Gibco) to an optical density at 600 nm (OD600) of 1.0 (equivalent to 109 CFU/ml). Epithelial cells were incubated with a 50-μl bacterial suspension added to 450 μl of fresh RPMI, supplemented with 25 mM HEPES buffer (Gibco) and 10% FCS for 6 h. Inhibition of HMW1-mediated adherence was performed by addition of dextran sulfate to a final concentration of 0.1 mg/ml. The end volume of each well was kept constant at 500 μl. After incubation, the unbound bacteria were removed from the cells by being washed three times with DPBS. Cells were fixed to the coverslips by addition of 1 ml of fixative (1% glutaraldehyde, 4% paraformaldehyde; Merck, Darmstadt, Germany) and stained with 0.007% crystal violet solution. Bacterial adherence was determined by counting the number of bacteria per cell on 10 cells of at least three experiments performed in duplicate, as described before (38).
Detection of HMW proteins in whole-cell ELISA.
Expression of HMW proteins was determined by whole-cell ELISA as described before (37), using 3D6, 1D5, 2G3, 4G4, AD6 and 10C5 mouse immunoglobulin G MAbs in a 1:250 dilution, or the polyclonal antiserum 25D, E. coli absorbed against the recombinant HMW1 protein (2), in a 1:50 dilution. The MAbs and antiserum were provided by S. J. Barenkamp. Rabbit anti-mouse-horseradish peroxidase was used as a conjugate. The reactivity of the antibodies was determined by measuring the OD405 with an ELISA reader. The reactivity was expressed as follows: −, OD of <0.5; +, OD of 0.5 to 1.0; ++, OD of 1.0 to 1.5; +++, OD of >1.5. All isolates were tested two times.
Detection of HMW by Western blotting.
Whole-cell lysates were prepared from bacteria grown overnight on chocolate agar plates. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose filters followed by Western blot analysis. Rabbit polyclonal serum 25D (E. coli absorbed) was used in a 1:50 dilution, and rabbit polyclonal serum K1-050, raised against nontypeable H. influenzae strain 1482, was used in a 1:75 dilution.
Detection of hmw genes by PCR.
On the basis of the sequence analysis of the hmw gene clusters (2), two primers were designed, recognizing sequences in the part of the hmwA gene that are identical in both hmw1 and hmw2. The first primer, HMWP1HI (5′-GCGTCGACGAGGGAGCTGAACGAACG-3′), recognizes nucleotides 288 to 304, and the second primer, HMWP3RHI (5′-GCCCCACACAATAGCGCG-3′), recognizes nucleotides 1515 to 1532. This primer combination gives rise to an expected PCR product of 1.24 kb. Nonadhering isolates were screened by PCR with this combination one time, and most adhering isolates were screened at least two times. PCR was performed with chromosomal DNA isolated by the phenol-chloroform method (16) or with bacterial lysates obtained by heating an H. influenzae colony suspended in 1 ml of H2O for 5 min at 100°C. PCR was performed in a TRIO-thermoblock (Biometra), with an initial denaturation step of 5 min at 95°C followed by 30 cycles of 1 min at 95°C, 1 min at 55°C, and 3 min at 72°C. The program was finished with 10 min at 72°C. The reaction products were determined by agarose gel electrophoresis. Strain 12 was included as a positive control.
Molecular cloning of the hmw gene.
Chromosomal DNA was isolated by the phenol-chloroform method (16). Sau3A partial restriction digests of chromosomal DNA were separated by agarose gel electrophoresis, and DNA fragments in the 8- to 12-kb range were used to construct a library by ligation into the unique BglII site of pGJB103. The ligation mixture was transformed to E. coli DH5α by electroporation. Approximately 1,000 clones were obtained and selected for hmw-positive clones by two subsequent adherence assays on NCI-H292 cells.
Construction of hmw knockout mutants of H. influenzae isolates.
Knockout mutants of H. influenzae isolates were constructed by insertion of a kanamycin resistance gene into the hmw genes by homologous recombination. H. influenzae isolates were made competent and transformed according to the method of Herriott et al. (13). Competent cells were used fresh or stored at −70°C after addition of 15% glycerol. Plasmid pT1-17 was linearized with XbaI, and 0.5 μg was used per transformation.
DNA sequence analysis.
DNA sequence analysis was performed by using the big dye terminator cycle sequencing ready reaction kit (Applied Biosystems) as suggested by the manufacturer. DNA analysis was performed with an automated fluorescent DNA sequencer, model 310 (Applied Biosystems). Data were analyzed with Auto assembler 2.0 (ABI Prism) and aligned with known hmw sequences by using Clone Manager 4.1. Two primers near the BglII site of pGJB103 were designed to sequence the fragment in the pGJB103 clone (PGJB-P1, CCGCTCATGAGACAATAACCCTGAT; PGJB-P2, GGGAATAAGGGCGACACGGAAATGTT). The sequences of several subclones in pUC19 were determined by using the M13 and M13 reversed primers. Several oligonucleotide primers were generated as necessary to complete the sequences.
Statistics.
Data were evaluated with Fisher's exact test or the chi-square test with Yate's corrections for sample sizes greater than 50. P values of <0.05 were considered statistically significant.
Nucleotide sequence accession number.
The sequences of the complete hmwA and hmwC genes have been submitted to GenBank under accession no. AF180944 and AF180945, respectively.
RESULTS
Adherence of H. influenzae isolates to two human epithelial cell lines.
The abilities of the 30 nontypeable H. influenzae isolates from the throats of healthy individuals, 9 otitis media isolates, and 19 COPD isolates to adhere to the Chang and NCI-H292 epithelial cell lines were compared (Table 1). In total, 32 isolates (55%) adhered to either the Chang or NCI-H292 cells. Significantly more otitis media isolates adhered to Chang cells (89%) than did isolates from healthy individuals (33%) (P < 0.01) and isolates from COPD patients (42%) (P < 0.05). Also, significantly more isolates from patients with otitis media (78%) than from healthy carriers (33%) adhered to NCI-H292 (P < 0.05). Although more COPD isolates adhered to the NCI-H292 cells than did isolates of healthy individuals, the difference was not significant.
TABLE 1.
Adherence of 58 H. influenzae isolates from different sources to Chang or NCI-H292 epithelial cells
| Isolate source (n) | No. (%) of isolates adherent to cell type:
|
No. (%) of nonadherent cells | |||
|---|---|---|---|---|---|
| Chang | NCI-H292 | Chang and NCI-H292 | Chang or NCI-H292 | ||
| Carriers (30) | 10 (33) | 10 (33) | 8 (26) | 12 (40) | 18 (60) |
| Otitis media (9) | 8 (89)a | 7 (78)b | 7 (78) | 8 (89) | 1 (11) |
| COPD (19) | 8 (42) | 11 (58) | 7 (36) | 12 (63) | 7 (37) |
| Total (58) | 27 (45) | 28 (47) | 22 (38) | 32 (55) | 26 (47) |
P < 0.01 compared to carrier isolates and P < 0.05 compared to COPD isolates.
P < 0.05 compared to carrier isolates.
Since the HMW proteins are major adhesins, a PCR assay was designed to determine the presence of the hmwA gene cluster among the isolates, using primers annealing to a conserved part of the hmw1A and hmw2A genes. With chromosomal DNA of strain 12 as a template, an expected PCR product of 1.24 kb was amplified. Of the 58 nontypeable H. influenzae isolates, 23 (40%) gave a PCR product of the expected length, represented by 22 of the 32 adherent isolates and 1 nonadherent isolate (Table 2). Whole-cell ELISA and Western blotting with the polyclonal rabbit serum 25D, performed to identify HMW expression, showed the presence of HMW proteins in 21 (36%) isolates. There was a large variation in the molecular masses of the HMW proteins, ranging from 100 to 150 kDa, confirming data described before for other isolates (2, 27). Of the total of 23 hmw PCR-positive isolates, 3 isolates did not react with the 25D polyclonal serum. Since HMW proteins vary strongly, we reasoned that this negative result may be due to lack of cross-reactivity of the antiserum 25D. Therefore, another rabbit serum (K1-050) was used. Western blotting with this serum showed a strong positive reaction with both the HMW1 and the HMW2 proteins of the prototype H. influenzae strain 12. Also, HMW proteins of two of the three 25D-negative isolates were recognized. So, of the 23 hmw PCR-positive strains, 22 showed an HMW reaction in Western blots (Table 2). In addition, 1 of the 35 PCR-negative isolates expressed HMW proteins.
TABLE 2.
Association of hmw or HMW with adherence of 58 H. influenzae isolates from different sources
| Isolate source | No. of isolates
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Adherent
|
Nonadherent
|
|||||||
| Total | hmwa | HMWb | HMW MAbsc | Total | hmw | HMW | HMW MAbs | |
| Carriers | 12 | 7 | 6 | 3 | 18 | 0 | 0 | 0 |
| Otitis media | 8 | 4 | 5 | 5 | 1 | 1 | 1 | 1 |
| COPD | 12 | 11 | 11 | 6 | 7 | 0 | 0 | 0 |
| Total | 32 | 22 | 22 | 14 | 26 | 1 | 1 | 1 |
hmw was detected by PCR.
Expression of HMW protein(s) recognized by at least one of the two polyclonal sera in a Western blot.
Expression of HMW protein(s) recognized by at least one of the six MAbs against HMW proteins in the whole-cell ELISA.
Taking these results together, we found that detection of hmw with PCR as well as HMW with Western blotting gave false-negative results, which were probably due to sequence diversity and antigenic diversity, respectively. We therefore considered isolates that were positive in either the PCR or the Western blotting with the polyclonal sera as hmw and HMW positive. Thus, a total of 24 (41%) of the 58 isolates were hmw and HMW positive. Of the 32 adhering H. influenzae isolates, 23 isolates (72%) were hmw and HMW positive. All 26 nonadherent isolates were hmw or HMW negative, except for 1 otitis media isolate (Table 2). These data show that there is a strong correlation between hmw and HMW and adherence to the two cell lines used. There was a significant difference in the total number of hmw- or HMW-positive otitis media isolates (67%) (P < 0.05) and COPD isolates (57%) (P < 0.05) compared to throat isolates from healthy individuals (23%) (Table 2).
Using whole-cell ELISA with a panel of six HMW-specific MAbs to detect HMW expression by these isolates, we found only 15 isolates positive by at least one of the MAbs, indicating lack of cross-reactivity of the Mabs (Table 2). The 6 hmw- and HMW-positive otitis media isolates were MAb positive, but only 3 (43%) of the 7 hmw- and HMW-positive isolates obtained from healthy carriers and 6 (55%) of the 11 hmw- and HMW-positive isolates from COPD patients were positive with one of the MAbs (Table 2). These results showed that the HMW proteins expressed by isolates obtained from otitis media patients differed less from the prototype HMW proteins than the HMW proteins expressed by isolates from healthy carriers or COPD patients.
Adherence patterns of the nontypeable H. influenzae isolates.
For H. influenzae up to now, two HMW proteins have been characterized that display distinct cellular binding specificities (26). It has previously been shown that HMW1 mediates a high level of adherence to Chang epithelial cells and is inhibited by dextran sulfate, and HMW2 mediates only a low level of adherence to Chang epithelial cells (27). To establish the specificity of HMW1- and HMW2-mediated adherence to the NCI-H292 cell line, the adherence patterns of the three isogenic mutants of strain 12, expressing HMW1, HMW2, or neither of these HMW proteins, to both epithelial cell lines were determined. The mutant strain 12-4, deficient in both HMW proteins, did not adhere to either cell line, indicating that strain 12 did not express adhesins for these cell lines other than HMW1 and HMW2. The mutant strain 12-2 (HMW1 positive) adhered efficiently to both cell lines. This HMW1-mediated adherence to both cell lines was inhibited in the presence of dextran sulfate, as expected. Using the mutant strain 12-10 (HMW2 positive), less than five bacteria per cell bound to Chang cells, but this strain adhered efficiently to NCI-H292 cells. This HMW2-mediated adherence was not inhibited in the presence of dextran sulfate.
To discriminate between the HMW1 and HMW2 types of adherence of the isolates, we determined the adherence of the 32 adhering isolates to the two cell lines in the presence and absence of dextran sulfate (Table 3). Of the 32 adhering isolates, 16 isolates adhered to both cell lines in the absence of dextran sulfate, while in the presence of dextran sulfate, no adherence occurred, indicating an HMW1-mediated adherence (group I, HMW1). Four isolates adhered only to NCI-H292 cells, irrespective of the presence of dextran sulfate, which is indicative of HMW2-mediated adherence (group II, HMW2). Twelve isolates adhering to Chang epithelial cells showed different adherence patterns to NCI-H292. Four isolates did not adhere to NCI-H292 cells (group III); four isolates adhered to NCI-H292 cells, but not in the presence of dextran sulfate (group IV); and four isolates adhered to NCI-H292 in the presence as well as the absence of dextran sulfate (group V). The distribution of the 32 adhering isolates across the five adherence patterns seemed not to be associated with the source of the isolates. However, the numbers of isolates per group are too low to make a statistically valid statement.
TABLE 3.
Grouping of 32 adherent H. influenzae isolates based on adherence properties
| Adherence toa:
|
No. of isolates | Adherence pattern | No. of isolates
|
|||||
|---|---|---|---|---|---|---|---|---|
| Chang cells
|
NCI-H292 cells
|
hmw | HMW | HMW MAbs | ||||
| Without dextran sulfate | With dextran sulfate | Without dextran sulfate | With dextran sulfate | |||||
| ++ | − | +++ | − | 16 | Ib (HMW1)c | 13d | 13d | 9 |
| − | − | +++ | +++ | 4 | II (HMW2)c | 4 | 4 | 0 |
| ++ | ++ | − | − | 4 | III | 0 | 0 | 0 |
| ++ | ++ | +++ | − | 4 | IV | 4 | 4 | 4 |
| ++ | ++ | ++ | ++ | 4 | V | 1 | 1 | 1 |
| Total | 32 | 22 | 22 | 15 | ||||
−, <5 bacteria per cell; ++, 10 to 50 bacteria per cell; +++, >50 bacteria per cell.
Two isolates did not adhere to Chang epithelial cells.
This adherence pattern correlated with HMW1- or HMW2-mediated adherence of strain 12, as indicated.
One isolate was hmw positive and HMW negative, another was hmw negative and HMW positive.
HMW expression was associated with three adherence patterns: 14 of the 16 group I (HMW1) isolates (88%) and the 4 isolates of group II (HMW2) were hmw and HMW positive, as were the 4 isolates of group IV (Table 3). The differences in adherence patterns of the four group IV strains may be explained by expression of an HMW adhesin with specificity other than HMW1 and HMW2 or an HMW protein involved in the HMW1-like adherence pattern to NCI-H292 cells, together with another adhesin.
The HMW proteins of the isolates were further studied by using six HMW-specific MAbs in a whole-cell ELISA. Using the prototype strain 12 and its HMW1 and HMW2 isogenic mutants, it was observed that MAb 3D6 recognized both HMW1 and HMW2, MAb 2G3 and MAb 10C5 were specific for HMW1, and MAb AD6 was specific for HMW2. Our results with MAbs 10C5 and AD6 were similar, as obtained earlier by St. Geme and Grass (26), who used Western blotting. The MAbs 1D5 and 4G4 did not react with the reference strains in whole-cell ELISA, although they reacted in the Western blot. Apparently, these MAbs recognized only the denatured proteins. Of the 16 isolates of group I exhibiting an HMW1 type of adherence, 7 did not react with any MAb. The other nine isolates reacted with MAb 3D6: one of them reacted in addition with MAb 2G3, one reacted with MAb AD6, and four reacted with both MAbs 2G3 and AD6. None of the four isolates of group II correlating with the strain 12 HMW2-mediated adherence pattern showed reactivity with MAb AD6 or with any other MAb (Table 3). The 4 hmw- and HMW-positive isolates of group IV all reacted with MAbs 3D6 and AD6: one reacted in addition with 2G3, and another reacted with 10C5. The hmw- and HMW-positive strain of group V showed reactivity with MAbs 2G3 and 3D6, and the nonadherent hmw- and HMW-positive isolate reacted with MAb AD6. These results indicate that the HMW1- and HMW2-like adherence patterns of the isolates are not expressed together with the epitopes for the HMW1- or HMW2-specific MAbs.
Cloning and sequencing of the hmw gene of a COPD isolate.
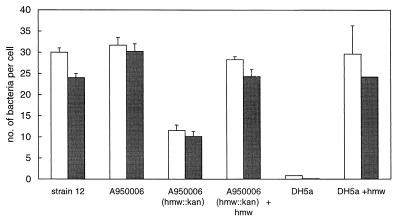
The 11 COPD isolates adhering to NCI-H292 cells expressed HMW proteins. However, the HMW proteins of COPD isolates differed antigenically from HMW proteins expressed by strain 12. Only six reacted with the HMW-specific MAbs. Of the four isolates of group II (HMW2), none was recognized by the MAbs in the ELISA. Among these four isolates, the COPD isolate A950006 was also negative with the 25D polyclonal serum in whole-cell ELISA and Western blotting. An HMW knockout mutant of this isolate, constructed by kanamycin box insertion in the hmw gene, showed reduced adherence compared to the parent isolate, indicating that the HMW protein was the major adhesin of this isolate (Fig. 1). To characterize the HMW adhesin of this strain, A950006, a chromosomal library was made with 8- to 12-kb Sau3A-digested chromosomal fragments of A950006. The fragments were ligated into the BglII site of plasmid pGJB103, and this was transformed to E. coli DH5α. After selection for clones adhering to NCI-H292 cells, eight different clones were obtained that adhered to NCI-H292. All of these clones were positive for hmw by PCR, indicating that the hmw gene product of A950006 was likely mediating the adherence to NCI-H292 cells. The plasmid of clone 6 containing a 10-kb chromosomal fragment was transformed to the hmw mutant of strain A950006, resulting in a wild-type adherence pattern of this clone (Fig. 1). Transformation of this plasmid to H. influenzae strain Rd also resulted in adherence of the Rd clone to NCI-H292 cells (data not shown).
FIG. 1.
Adherence of strain A950006 and clones to NCI-H292 cells shown as the number of bacteria per cell. Open bars, adherence without dextran sulfate; solid bars, adherence in the presence of dextran sulfate.
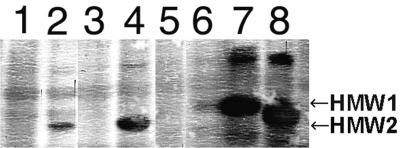
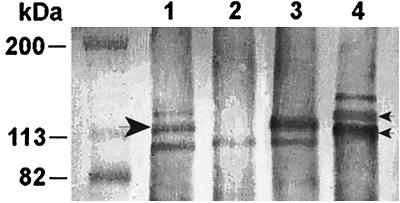
The results of the analysis of the clones with the polyclonal rabbit serum 25D in the Western blot are summarized in Fig. 2. The serum did not react with whole-cell lysates of strain A950006, suggesting a low affinity of this polyclonal serum for the A950006 HMW protein. In contrast, the E. coli and Rd clones containing the hmw gene on a plasmid did react with the 25D serum. Also when the expression of an HMW protein was restored in the A950006 hmw knockout mutant by complementation of the cloned gene, a protein was recognized (not shown). When Western blotting was performed with the polyclonal rabbit serum K1-050, an HMW protein of A950006 was recognized which disappeared after hmw::kan insertion (Fig. 3). The expression was restored in this A950006 hmw knockout mutant by complementation with the plasmid of clone 6. Combination of the results of adherence assays and the immunoblotting with the polyclonal sera suggests that strain A950006 expressed an HMW protein with HMW2-type adherence specificity, although this protein was not detected by serum 25D.
FIG. 2.
Western blot of whole-cell lysates of strain A950006 and clones. The lysates were probed with E. coli-absorbed rabbit serum 25D. Lanes: 1, E. coli DH5α containing pGJB103; 2, E. coli DH5α clone 6 containing hmw; 3, H. influenzae Rdrec1 containing pGJB103; 4, H. influenzae Rdrec1 clone 6 containing hmw; 5, strain A950006; 6, A950006 (hmw::kan); 7, strain 12-2 (HMW1 positive and HMW2 negative) 8, strain 12-10 (HMW1 negative and HMW2 positive).
FIG. 3.
Western blot of whole-cell lysates of strain A950006 and clones. The lysates were probed with rabbit serum K1-050. Small arrowheads, HMW1 and HMW2 of strain 12; large arrowhead, HMW protein of A950006. Lanes: 1, strain A950006; 2, mutant A950006 (hmw::kan); 3, mutant A950006 (hmw::kan) containing hmw; 4, strain 12.
Sequencing analysis of the hmw gene of strain A950006.
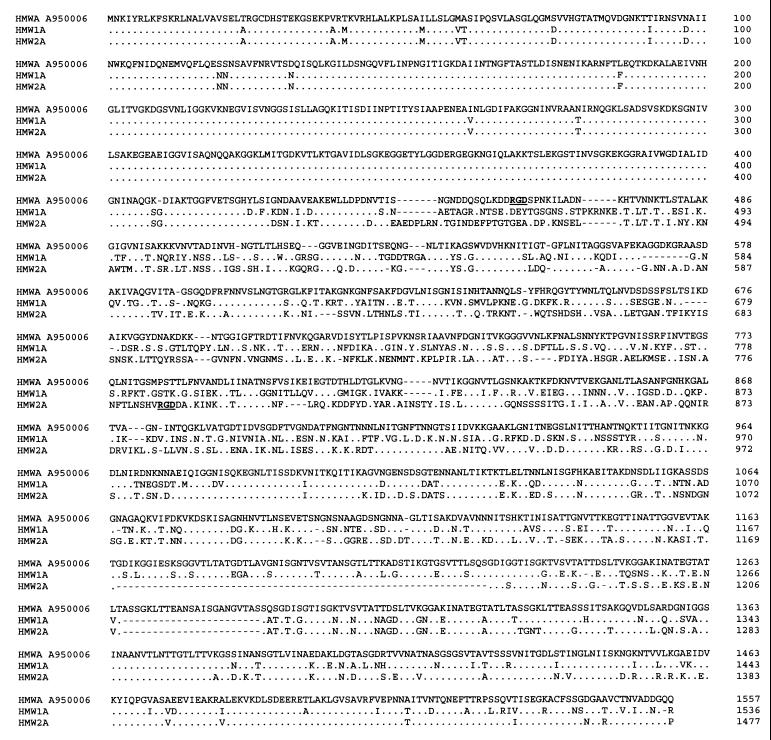
The hmw gene of strain A950006 was further characterized by DNA sequencing analysis of the cloned fragment. The fragment contained a complete hmwABC gene cluster, as judged from the length of the chromosomal insert. This was confirmed by aligning sequences obtained from subclones, which aligned to parts of all three open reading frames (ORFs) of the gene cluster (not shown). An additional ORF in front of the hmwA gene showed homology to ORF HI1598 of H. influenzae Rd. Sequence analysis of the complete hmwC gene revealed that the nucleotide sequences of the hmwC gene of strain A950006 are 97 and 96% identical to those of the hmw1C and hmw2C genes of strain 12, respectively, and the deduced amino acid sequences are 98 and 96% identical to those of HMW1C and HMW2C. Similar to the upstream region of the hmw1C gene, the 5′-flanking region of the A950006 hmwC gene contains a series of direct tandem repeats with a 9-bp sequence repeated multiple times. However, the hmwC gene of strain A950006 contained 19 AAAACTAAG repeats, which differed from the repeated sequence from hmw1C, which is CAAACCAAG. The sequence of the hmwA gene of A950006 consisted of a 4,671-bp ORF with 75% homology to hmw1A and 76% homology to hmw2A of strain 12, while the deduced amino acid sequence was 70% homologous to HMW1A and 68% homologous to HMW2A (Fig. 4). The first 1,255 bp of the hmwA gene are more conserved, showing 92% homology to both hmw1A and hmw2A. The upstream region of the hmwA gene contained 22 copies of the 7-bp repeat ATCTTTC, which was also apparent in the hmw1A and hmw2A flanking regions.
FIG. 4.
Complete amino acid sequence comparison of the predicted HMWA protein of H. influenzae strain A950006 compared to the derived amino acid sequences of HMW1 and HMW2 of H. influenzae strain 12 (2). Sequences were aligned and compared by Align Plus 3.0. Identical amino acids are indicated with dots; gaps are indicated with dashes. The RGD sequences of A950006 HMW protein and HMW2 are indicated.
In conclusion, the hmwA gene of H. influenzae A950006, encoding an HMW protein mediating HMW2-like adherence, differed considerably from hmw1A and hmw2A.
DISCUSSION
In this study, we analyzed the adherence patterns to two human epithelial cell lines of nontypeable H. influenzae from 9 otitis media patients and 19 COPD patients in comparison with those of 30 isolates from the throat of healthy individuals. Of the 58 isolates tested, 32 (55%) adhered to one or both of the epithelial cell lines used. The majority (72%) of the adherent isolates expressed HMW proteins, as determined by PCR with hmw-specific primers and Western blotting with two polyclonal sera, suggesting that these adhesins were involved in the adherence.
Different adherence patterns of the isolates to the two human epithelial cell types were observed. We showed that 20 isolates had HMW1-like or HMW2-like adherence. In addition, three other patterns of adherence were detected among the other 12 adherent isolates. The presence of the hmw gene or HMW proteins correlated with the HMW-like adherence patterns in most cases. Only two isolates with an HMW-like adherence pattern were hmw and HMW negative. All 12 strains with adherence patterns different from HMW-like adherence (groups III, IV, and V), adhered to Chang epithelial cells, irrespective of the presence of dextran sulfate. The four isolates from group III, and three of the four isolates within group V were hmw and HMW negative. Although the involvement of HMW in adherence of these isolates to Chang or NCI-H292 cells cannot be ruled out, adherence to these cells due to another adhesin is more likely. Since the Hia protein mediates efficient in vitro adherence of nontypeable H. influenzae to Chang epithelial cells (3), the Hia protein or other unknown adhesins may play a role in the adherence patterns of these isolates. Five of the 12 isolates, the 4 isolates of group IV and 1 isolate of group V, expressed HMW proteins. Therefore, these five isolates may express HMW proteins that give rise to an adherence pattern different from HMW1- and HMW2-type adherence, or they may express HMW adhesins in combination with Hia or an unknown adhesin. The combined presence of hia and hmw was reported earlier for an otitis media isolate (15), but among 59 nontypeable H. influenzae strains in another study, none harbored both hmw and hia (27).
One isolate expressing an HMW protein did not adhere, indicating that HMW proteins may not always function in adherence. In addition, the hmw knockout mutant of strain A950006 showed residual adherence, indicating that besides the HMW protein, another adhesin was involved in the adherence of this strain. However, loss of adherence by knockout mutation of the hmw of three representative isolates from groups I, II, and IV confirmed that the HMW proteins were the major adhesins of these strains (data not shown). Although HMW-expressing strains may also adhere through other adhesins, the strong correlation of adherence with HMW and the inhibitory effect of dextran sulfate in most HMW-positive isolates indicate that HMW proteins were relevant adhesins for these isolates.
Since significantly more otitis media isolates and COPD isolates were hmw and HMW positive than isolates from healthy carriers, HMW expression may be important for the onset of infections such as otitis media as well as for COPD. A similar high frequency of HMW-positive nontypeable H. influenzae isolates from otitis media has been reported in other studies, which showed that 75 to 80% of these isolates expressed HMW proteins (4, 15, 27). Otitis media isolates also adhered significantly more to the two cell lines than carrier isolates. In contrast, the proportions of COPD isolates and carrier isolates adhering to the cell lines were similar, suggesting that, in our in vitro assay, the carrier isolates adhered more often by adhesins other than HMW. It may be that nonadhering isolates express adherence factors for which no receptors are available on these two cell lines, as shown for fimbria-mediated adherence of H. influenzae (30, 38). For nonadherent isolates, an alternative adherence mechanism may be important in COPD patients. In the lower respiratory tract of these patients, neutrophil defensins are present continuously due to the low-level inflammation of the bronchial tree, and it has been shown that nonadherent H. influenzae cells are able to adhere to epithelial cells in the presence of neutrophil defensins (11).
Screening of the isolates with a panel of six HMW protein-specific MAbs in whole-cell ELISA showed that the HMW proteins of COPD isolates and carrier isolates were more distinct from the HMW proteins from strain 12 than those from otitis media isolates. In another study using electrophoretic typing of H. influenzae isolates from different sources, it was found that otitis media isolates were more clonal than isolates from COPD isolates (36). Since infections in COPD are chronic and antibody-mediated defense mechanisms are active in these patients, the antigenic heterogeneity may be the consequence of accumulation of mutations, as observed in other immunogenic outer membrane proteins during persistent infections of these patients (6, 7).
Adherence characteristics and the results of Western blotting of strain A950006 and its hmw knockout mutants showed that this strain expressed an HMW adhesin that was not recognized by serum 25D nor by any of the MAbs. Sequence analysis of the hmwA gene of this strain showed that it was 75% homologous to hmw1A and 76% homologous to hmw2A, as published by Barenkamp and Leininger (2). Also, 22 copies of a 7-bp repeat were found in front of the hmwA gene of strain A950006. Since it was shown that the presence of 17 copies or more of this sequence leads to a low level of expression of the HMW proteins (5), the high number of these copies in strain A950006 may lead to a low level of HMW expression in this strain, and the diversity in the hmwA gene may lead to antigenic differences. A low expression level in conjunction with antigenic differences can explain the lack of the reactivity of the anti-HMW polyclonal serum 25D with strain A950006. Reactivity of this polyclonal serum with the A950006 HMW expressed by the E. coli and H. influenzae clones may be due to overexpression of the HMW from the plasmid by these clones.
We found no association between the HMW1- and HMW2-like adherence patterns of the isolates and reactivity with the HMW1-specific MAbs or the HMW2-specific MAb, respectively, indicating that antigenic sites and adherence sites are different. The predicted amino acid sequence of the HMWA of strain A950006 contained the sequence RGD from amino acids 460 to 462. The RGD sequence of the Bordetella pertussis filamentous hemagglutinin, which is related to the HMW proteins, has been implicated in adherence to the integrin CR3 (22). HMW2A also contains the RGD sequence, but at a different position, namely from amino acid 785 to amino acid 787. Since the HMW protein of strain A950006 mediated adherence similar to HMW2-mediated adherence, this RGD sequence may be involved in adherence of group II isolates.
In conclusion, HMW is strongly related to the ability of nontypeable H. influenzae isolates to adhere to the Chang and NCI-H292 epithelial cell lines. Probably as a result of the variability in hmwA sequence, HMW proteins show a large antigenic diversity, although their HMW1- and HMW2-like adherence patterns are conserved.
ACKNOWLEDGMENTS
We thank Wim van der Steeg and Forien Geluk for assistance with adherence assays and cloning, Wim van Est and Wilma Witkamp for photographic assistance, and Arie van der Ende for help with statistical analysis. We are very grateful to S. J. Barenkamp for supplying plasmids, strains, and HMW-specific serum and MAbs and E. J. Hansen for supplying plasmid pEJH39-1.
Peter van Ulsen was supported by Netherlands Asthma Foundation grant NAF 96.50.
REFERENCES
- 1.Banks-Schlegel S P, Gazdar A F, Harris C C. Intermediate filament and cross-linked envelope expression in human lung tumor cell lines. Cancer Res. 1985;45:1187–1197. [PubMed] [Google Scholar]
- 2.Barenkamp S J, Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992;60:1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barenkamp S J, St. Geme J W., III Identification of a second family of high-molecular-weight adhesion proteins expressed by non-typeable Haemophilus influenzae. Mol Microbiol. 1996;19:1215–1223. doi: 10.1111/j.1365-2958.1996.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 4.Barenkamp S J, St. Geme J W., III Identification of surface-exposed B-cell epitopes on high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae. Infect Immun. 1996;64:3032–3037. doi: 10.1128/iai.64.8.3032-3037.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawid S, Barenkamp S J, St. Geme J W., III Variation in expression of the Haemophilus influenzae HMW adhesins: a prokaryotic system reminiscent of eukaryotes. Proc Natl Acad Sci USA. 1999;96:1077–1082. doi: 10.1073/pnas.96.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duim B, Bowler L D, Eijk P P, Jansen H M, Dankert J, van Alphen L. Molecular variation in the major outer membrane protein P5 gene of nonencapsulated Haemophilus influenzae during chronic infections. Infect Immun. 1997;65:1351–1356. doi: 10.1128/iai.65.4.1351-1356.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duim B, van Alphen L, Eijk P, Jansen H M, Dankert J. Antigenic drift of non-encapsulated Haemophilus influenzae major outer membrane protein P2 in patients with chronic bronchitis is caused by point mutations. Mol Microbiol. 1994;11:1181–1189. doi: 10.1111/j.1365-2958.1994.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 8.Farley M M, Stephens D S, Kaplan S L, Mason E O., Jr Pilus and non-pilus-mediated interactions of Haemophilus influenzae type b with human erythrocytes and human nasopharyngeal mucosa. J Infect Dis. 1990;161:274–280. doi: 10.1093/infdis/161.2.274. [DOI] [PubMed] [Google Scholar]
- 9.Geluk F, Eijk P P, van Ham S M, Jansen H M, van Alphen L. The fimbria gene cluster of nonencapsulated Haemophilus influenzae. Infect Immun. 1998;66:406–417. doi: 10.1128/iai.66.2.406-417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilsdorf J R, Chang H Y, McCrea K W, Bakaletz L O. Comparison of hemagglutinating pili of Haemophilus influenzae type b with similar structures of nontypeable H. influenzae. Infect Immun. 1992;60:374–399. doi: 10.1128/iai.60.2.374-379.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorter A, Eijk P P, van Wetering S, Hiemstra P S, Dankert J, van Alphen L. Stimulation of adherence of Haemophilus influenzae to human lung epithelial cells by antimicrobial neutrophil defensins. J Infect Dis. 1998;178:1067–1074. doi: 10.1086/515667. [DOI] [PubMed] [Google Scholar]
- 12.Hansen E J, Gonzales F R, Chamberlain N R, Norgard M V, Miller E E, Cope L D, Pelzel S E, Gaddy B, Clausell A. Cloning of the gene encoding the major outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1988;56:2709–2716. doi: 10.1128/iai.56.10.2709-2716.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herriott R M, Meyer E M, Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970;101:517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hultgren S J, Abraham S, Caparon M, Falk P, St. Geme III J W, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- 15.Krasan G P, Cutter D, Block S L, St. Geme J W., III Adhesin expression in matched nasopharyngeal and middle ear isolates of nontypeable Haemophilus influenzae from children with acute otitis media. Infect Immun. 1999;67:449–454. doi: 10.1128/iai.67.1.449-454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langenberg W, Rauws E A J, Widjojokusumo A, Tytgat G N J, Zanen H C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986;24:414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loeb M R, Connor E, Penney D. A comparison of the adherence of fimbriated and nonfimbriated Haemophilus influenzae type b to human adenoids in organ culture. Infect Immun. 1988;56:484–489. doi: 10.1128/iai.56.2.484-489.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lomholt H, van Alphen L, Kilian M. Antigenic variation of immunoglobulin A1 proteases among sequential isolates of Haemophilus influenzae from healthy children and patients with chronic obstructive pulmonary disease. Infect Immun. 1993;61:4575–4581. doi: 10.1128/iai.61.11.4575-4581.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moller L V, Regelink A G, Grasselier H, Dankert-Roelse J E, Dankert J, van Alphen L. Multiple Haemophilus influenzae strains and strain variants coexist in the respiratory tract of patients with cystic fibrosis. J Infect Dis. 1995;172:1388–1392. doi: 10.1093/infdis/172.5.1388. [DOI] [PubMed] [Google Scholar]
- 20.Moxon E R, Wilson R. The role of Haemophilus influenzae in the pathogenesis of pneumonia. Rev Infect Dis. 1991;13:S518–S527. doi: 10.1093/clinids/13.supplement_6.s518. [DOI] [PubMed] [Google Scholar]
- 21.Noel G J, Love D C, Mosser D M. High-molecular-weight proteins of nontypeable Haemophilus influenzae mediate bacterial adhesion to cellular proteoglycans. Infect Immun. 1994;62:4028–4033. doi: 10.1128/iai.62.9.4028-4033.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Relman D, Tuomanen E, Falkow S, Golenbock D T, Saukkonen K, Wright S D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 23.Stephens D S, Farley M. Pathogenic events during infection of the human nasopharynx with Neisseria meningitidis and Haemophilus influenzae. Rev Infect Dis. 1991;13:22–33. doi: 10.1093/clinids/13.1.22. [DOI] [PubMed] [Google Scholar]
- 24.Sterk L M, van Alphen L, Geelen-van den Broek L, Houthoff H J, Dankert J. Differential binding of Haemophilus influenzae to human tissues by fimbriae. J Med Microbiol. 1991;35:129–138. doi: 10.1099/00222615-35-3-129. [DOI] [PubMed] [Google Scholar]
- 25.St. Geme J W, III, Falkow S, Barenkamp S J. High-molecular-weight proteins of nontypeable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci USA. 1993;90:2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St. Geme J W, III, Grass S. Secretion of the Haemophilus influenzae HMW1 and HMW2 adhesins involves a periplasmic intermediate and requires the HMWB and HMWC proteins. Mol Microbiol. 1998;27:617–630. doi: 10.1046/j.1365-2958.1998.00711.x. [DOI] [PubMed] [Google Scholar]
- 27.St. Geme J W, III, Kumar V V, Cutter D, Barenkamp S J. Prevalence and distribution of the hmw and hia genes and the HMW and Hia adhesins among genetically diverse strains of nontypeable Haemophilus influenzae. Infect Immun. 1998;66:364–368. doi: 10.1128/iai.66.1.364-368.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St. Geme J W., III Molecular determinants of the interaction between Haemophilus influenzae and human cells. Am J Respir Crit Care Med. 1996;154:S192–S196. doi: 10.1164/ajrccm/154.4_Pt_2.S192. [DOI] [PubMed] [Google Scholar]
- 29.St. Geme J W, III, Cutter D. Evidence that surface fibrils expressed by Haemophilus influenzae type b promote attachment to human epithelial cells. Mol Microbiol. 1995;15:77–85. doi: 10.1111/j.1365-2958.1995.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 30.St. Geme J W, III, Cutter D. Influence of pili, fibrils, and capsule on in vitro adherence by Haemophilus influenzae type b. Mol Microbiol. 1996;21:21–31. doi: 10.1046/j.1365-2958.1996.6241331.x. [DOI] [PubMed] [Google Scholar]
- 31.St. Geme J W, III, de la Morena M L, Falkow S. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol Microbiol. 1994;14:217–233. doi: 10.1111/j.1365-2958.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 32.St. Geme J W., III The HMW1 adhesin of nontypeable Haemophilus influenzae recognizes sialylated glycoprotein receptors on cultured human epithelial cells. Infect Immun. 1994;62:3881–3889. doi: 10.1128/iai.62.9.3881-3889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomb J-F, Barcak G J, Chandler M S, Redfield R J, Smith H O. Transposon mutagenesis, characterization, and cloning of transformation genes of Haemophilus influenzae Rd. J Bacteriol. 1989;171:3796–3802. doi: 10.1128/jb.171.7.3796-3802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turk D C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984;18:1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- 35.van Alphen L. Epidemiology and prevention of respiratory tract infections due to nonencapsulated Haemophilus influenzae. J Infect Dis. 1992;165:S177–S180. doi: 10.1093/infdis/165-supplement_1-s177. [DOI] [PubMed] [Google Scholar]
- 36.van Alphen L, Caugant D A, Duim B, O'Rourke M, Bowler L D. Differences in genetic diversity of nonencapsulated Haemophilus influenzae from various diseases. Microbiology. 1997;143:1423–1431. doi: 10.1099/00221287-143-4-1423. [DOI] [PubMed] [Google Scholar]
- 37.van Alphen L, Eijk P, Geelen-van den Broek L, Dankert J. Immunochemical characterization of variable epitopes of outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1991;59:247–252. doi: 10.1128/iai.59.1.247-252.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Schilfgaarde M, van Alphen L, Eijk P, Everts V, Dankert J. Paracytosis of Haemophilus influenzae through cell layers of NCI-H292 lung epithelial cells. Infect Immun. 1995;63:4729–4737. doi: 10.1128/iai.63.12.4729-4737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson R, Read R, Cole P. Interaction of Haemophilus influenzae with mucus, cilia, and respiratory epithelium. J Infect Dis. 1992;165:S100–S102. doi: 10.1093/infdis/165-supplement_1-s100. [DOI] [PubMed] [Google Scholar]