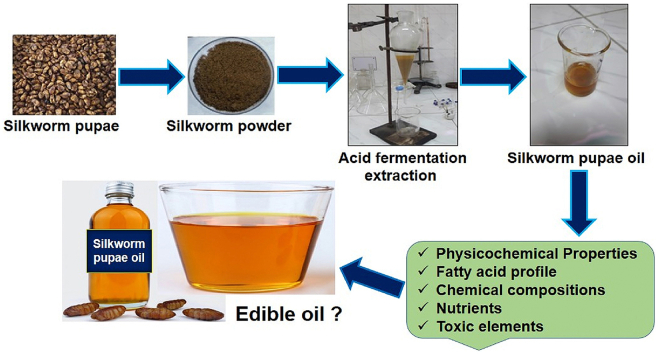
Abstract
Considering the increasing demand for edible oil in recent times, their price in the world market is becoming skyrocketing. In this research, we produced cost-effective edible oil from desilked silkworm pupae (Bombyx mori) applying a facile acid fermentation process, for the first time. The extraction was performed using two different types of organic acids, 3% of each acetic and citric acid. The yield of the extracted oil was 3.52 ± 0.23% from fresh silkworm pupae. The produced oil was then characterized physically and chemically to know its suitability to be used as edible oil. The oil was found with a low peroxide and acid value of 4.82 meq/kg and 1.35 mg KOH/g oil, respectively, and comprised of different fatty acids, in which palmitic acid (32.04%) and oleic acid (34.62%) were in large portions among the total fatty acids. Additionally, the extracted oil included linoleic, α-linolenic, and dihomo-gamma-linolenic acid which have health benefits. The oil was rich with minerals such as Iron, Sodium, Potassium, Calcium, Magnesium, Zinc, and Phosphorus with a negligible concentration of toxic elements such as Manganese, Cobalt, Nickel, Copper, Lead, Cadmium, Chromium, Arsenic, and Silver, indicating a good nutritive value of the extracted oil. Overall, the outcomes of all the characterizations showed that the extracted oil could be used as good edible oil and the corresponding acid fermentation extraction process has the potential to be used as an effective oil extraction method for silkworm pupae.
Keywords: Insects, Proximate analyses, Bio-oil production, Fatty acids, Peroxide and acid values, Nutritive oil
Graphical abstract
Highlights
-
•
Applied acid fermentation method for the first time to produce silkworm pupae oil.
-
•
Oil contained low peroxide and acid values, and a high % of palmitic and oleic acid.
-
•
Oil included health beneficiary linoleic, α-linolenic, and dihomo-γ-linolenic acid.
-
•
Achieved mineral-rich, toxic elements free, and organic molecules-rich edible oil.
-
•
Oil extracted by this fermentation process could be a good edible oil candidate.
1. Introduction
Natural silk is renowned for its applications in the counting, textile, parachute, tire, electrical, medical, and cosmetics industries. Silk thread results from the bioconversion of plant materials and highly nutritive biomass through silkworms. In sericulture, the most common silkworm species adopted over the globe are the mulberry silkworm (Bombyx mori L.), oak silkworm (Antheraea pernyi), and eri silkworm (Samia cynthia ricina). Most silkworm pupae are a highly potential by-product of the agro‐textile industrial supply exclusively devoted to silk production. After silk extraction, the desilked silkworm pupae consisting of 60% of dry cocoon weight are considered waste in the silk industry and are commonly dumped in local places or on agricultural lands to be utilized as a bio-fertilizer [1]. Dumping a massive amount of silkworm pupae in open areas can lead to environmental damage due to its ablative properties [2]. However, exploiting these valuable biological resources containing protein, oil, and fatty acids (α-linolenic acid) [3,4] in the feed and food industry may create a path to abate the environmental crisis of silk production.
In recent years, the demand for edible oils (e. g., soybean oil) is increased greatly and their price in the local markets is also increasing frequently [5]. For instance, the price of soybean and palm oil has increased considerably in the last few years [5]. From May 2021 to May 2022, the bottled soybean oil price in Bangladesh increased by around 47% per liter [6,7] and in June 2022, the price increased further by 3.5% per liter [8]. Other edible/plant oils such as peanut, sesame, and mustard oil are also available in the country, however, their prices are much higher than the commonly consumed soybean and palm oil. The total annual demand for edible oil and fat in Bangladesh is around 3 million tonnes [5]. The country imports around 90% of the total annual demand for edible oil from the countries such as Brazil, Argentina, Malaysia, Indonesia, and so on which is very costly [5]. Further, in addition to the recent Covid-19 pandemic, the Ukraine-Russia war exacerbates the worse of the global economy and assists the price increase of essential edible oil. Now, what should do developing countries like Bangladesh in this worse backdrop? The probable answer to this crucial question may be the turning of Bangladesh into a self-depended edible oil production country utilizing the existing natural resources. Bangladesh being an agricultural country has the ability to grow many oil-extracting crops or seeds such as mustard, sesame, coconut, soybean, olive, almond, and so on. In addition, most importantly, some insect species such as mulberry silkworm, oak silkworm, and eri silkworm may also be an important alternative for oil extraction.
Extracts of silkworm pupae are a source of high protein content, which lies between 45 and 80% on a dry matter (DM) basis [3]. In addition, it contains a high level of good lipids for human health, while omega-3 ranges from 35 to 40% of its total fatty acids (FAs) [4]. It has been studied that silkworm pupae oil is completely secure and nutritionally similar to some regularly consumed vegetable oils, for example, sunflower oil [9]. It is enriched with unsaturated fatty acids (60–70% of the total fatty acid content), especially α-linolenic and oleic acids [10]. A couple of α-linolenic and oleic acids are recognized for their health benefits and are utilized in the production of food, supplements, and feed [11,12,13,14]. However, the extraction of oil from silkworm pupae is challenging since the approach should be easy, cost-effective, and secure.
Formerly, the utilization of silkworm pupae for extracting oil was performed with differential techniques, for example, mechanical pressing extraction [15], solvent and supercritical fluid extraction [1,16,17,18], microwave-assisted gravimetric extraction [11], and aqueous saline oil extraction [19]. Some of the mentioned methods involve a lot of labor, time, and solvents, making the extraction process expensive. Initially, these procedures need to dry the silkworm pupae before the extraction and thus the protein by-product's characteristics may also be disrupted due to the extraction conditions. Additionally, the physicochemical parameters of oil can be changed by the consequential lipid oxidation due to sun or oven drying [20]. However, the yield of solvent extraction and enzymatic extraction was high with an expensive extraction cost, while the mechanical pressing extraction reached a low yield [20]. Furthermore, supercritical fluid extraction is rarely used for complex operating methods and is expensive for apparatus [21]. The yield of microwave-assisted extraction is high, but the apparatus price and the used solvent make the extraction expensive. On the other hand, the aqueous saline oil extraction method requires a frozen sample and repeated centrifugation for a long time [19]. In this regard, acid fermentation with organic acid can be used as a new feasible method with low cost and comparatively high yield for the wide utilization of available silkworm pupae. In acid fermentation, generally, raw samples were used to extract the protein concentrated with spoiled yogurt; it took a long time in the primary stage, while supernatant oil was a byproduct [22]. Next, inorganic and organic acids were added in a minimal amount to provoke the fermentation in a short time [22]. Recently, a low concentration of organic acids was also used to extract oil from fish waste [23].
However, there is no effective work on oil extraction from insects by acid fermentation. Consequently, the primary aim of this work was to develop a facile acid fermentation extraction process for separating oil from silkworm pupae, followed by its physical and chemical characterization to appraise its suitability to be used as edible oil. The outcome of this research work will introduce a new dimension in the oil extraction process from insects, and the extracted oil could find a potential application as an edible oil in the food industry based on the characterization results.
2. Materials and methods
2.1. Raw materials collection, processing, and storing
Fresh desilked silkworm pupae (Bombyx mori) (Fig. 1) were collected from the Bangladesh Sericulture Research and Training Institute (BSRTI), Rajshahi, Bangladesh. After collection, the silkworm pupae were processed to remove visible impurities and unsuitable pupae for utilization. These were then stored at −20 °C in the refrigerator for further analysis at several laboratories of the Bangladesh Council of Scientific and Industrial Research (BCSIR), Rajshahi, Bangladesh.
Fig. 1.
Collected fresh desilked silkworm pupae (Bombyx mori).
2.2. Proximate composition analysis
The fresh desilked pupae were used to estimate the contents of moisture, crude protein, crude fat, crude fiber, total carbohydrate, and ash in the silkworm pupae following standard procedures [24,25,26]. The moisture content of the silkworm was determined by drying it in an oven at 105 °C, and data were obtained until a constant weight loss was achieved. The crude protein content was estimated by the measurement of the total nitrogen content of the samples using the Kjeldahl method (DKL 42/26 automatic digestion and UDK 129 distillation unit, Velp Scientifica, Italy) followed by the multiplication of the result of total nitrogen with a conversion factor of 6.25. Fat content was measured by a Soxhlet apparatus. The ash content was determined by incineration of precisely 5 g of samples (in the ceramic crucible) in a muffle furnace at 600 °C for 6 h. For the estimation of crude fiber content, approximately 5 g of the moisture- and fat-free samples were heated with a mixture of dilute H2SO4 (1.25%) and NaOH (1.25%) solutions. After heating, the samples were placed on the porcelain crucible and dehydrated overnight at 80 °C. The crucible with the sample was heated in the furnace at 600 °C for 1 h. Finally, the crude fiber content of samples was counted by the weight difference of the samples of post-drying (80 °C) and ashing (600 °C). The carbohydrate fraction was estimated by deducting the sum of the other contents from 100.
2.3. Extraction of oil from silkworm pupae
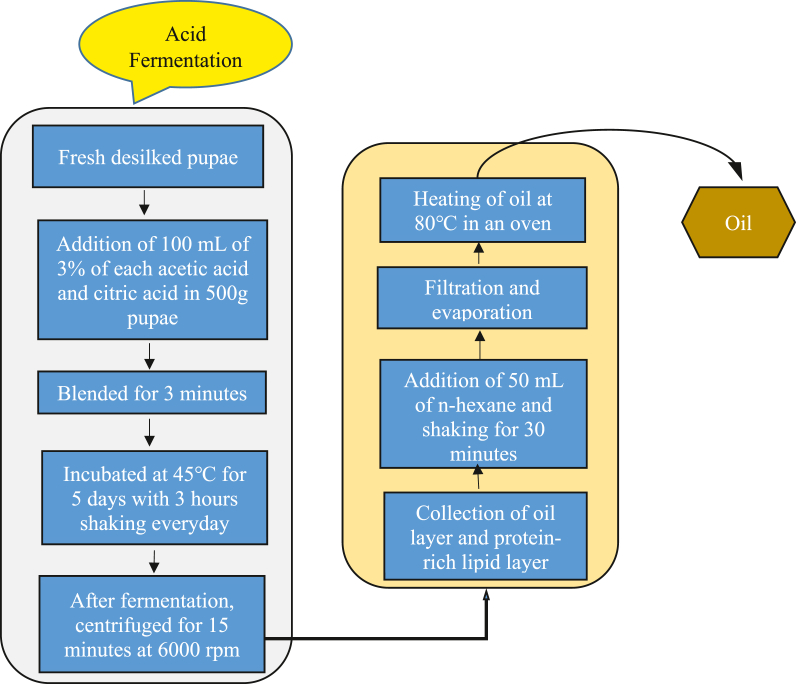
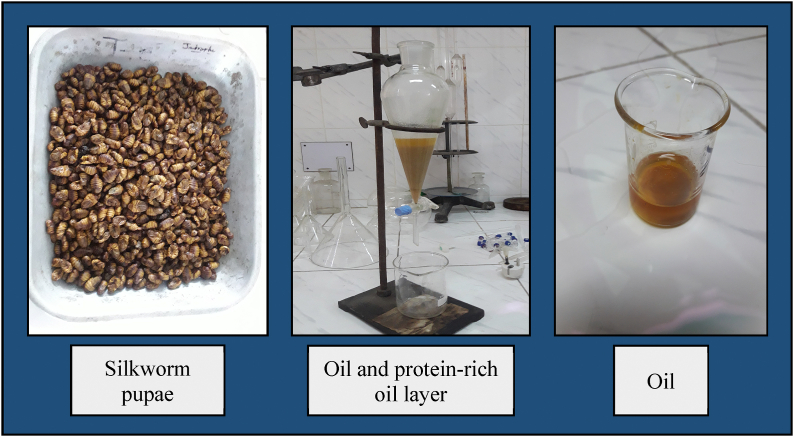
Silkworm pupae oil was extracted by acid fermentation following Salih et al. [23] with slight modification and the entire process is shown as a flow diagram in Fig. 2. First, 100 mL of 3% acetic acid and citric acid was added to a weight of 500 g of desilked fresh pupae. After that, these samples were blended with a grinder (Jaipan, India) and placed in a 1000 mL glass container sealed tightly with a lid for fermentation. Then, this glass container containing the sample was sonicated in an ultrasound cleaner (Skymen Cleaning Equipment Shenzhen Co., Ltd, China) for 1 h, shaken in an orbital shaker for 3 h, and kept at 40–45 °C in an incubator for 5 days. After complete fermentation, the mixture was centrifuged for 15 min at 6000 rpm and obtained as three fractions, including the top (lipid layer), middle (protein-rich lipid layer), and bottom (aqueous residues) layers. The oil or lipid layer and protein-rich lipid layers were pipetted out and mixed with 50 mL of n-hexane. This n-hexane mixture was then shaken for 30 min and filtered with Whatman filter paper. After filtration, the residue solvent was volatilized by a rotary evaporator, and clear oil was heated at 80 °C in an oven. The different stages of oil extraction from silkworm pupae are visualized in Fig. 3. Finally, oil was collected, and the percentage of oil yield was determined by the following equation (1).
| (1) |
Fig. 2.
Acid fermentation of oil extraction from silkworm pupae.
Fig. 3.
Different stages of oil extraction from silkworm pupae.
2.3.1. Analysis of physicochemical properties
Physical properties such as moisture content, refractive index, density, specific gravity, and viscosity of the silkworm oil were investigated using standard methods [27,28,29]. Acid value, saponification value, iodine value, peroxide value, unsaponifiable matter, and free fatty acid were determined following the AOAC [30] and official methods of the American Oil Chemists Society (AOCS) [31]. The viscosity of oil was estimated by the Fungilab digital rotational viscometer.
2.3.2. Fatty acid profiling
The fatty acid content of the silkworm oil was determined by following Ferdousi et al. [26]. In brief, 3.5 mL of 0.5 M Sodium methoxide was added to 200 mg of oil sample in a 10 mL test tube. After heating on a burner to remove the bubbles, 1.5 mL of n-hexane was mixed with the mixture and homogenized well using a vortex. Then, 5 mL of deionized water was slowly flowed into the test tube and waited for precipitation. After precipitation, the top organic layer was collected for fatty acid analysis.
The fatty acid profiling of the oil was performed by GC-MS (GC-2010, Shimadzu, Japan). The GC was assembled with an auto-sampler (AOC-20s), auto-injector (AOC-20i), and SH Rxi 5MS Sill capillary column with 30 m × 0.25mm × 0.25 μm film. The flow rate of helium gas was 2.0 mL/min. The initial oven temperature of GC was 40 °C for 10 min, then hiked up at 70 °C/min and reached 250 °C to continue for 10 min. The injector was injected at a 1.0 μL volume with a 75:1 split ratio at 250 °C. The total running time was 35.71 min while the solvent cutting time was 3.40 min. The detector (Shimadzu GCMS-QP-2020, Japan) was operated at 25–50 °C. Identification and quantification of fatty acids in the samples were performed by differentiating retention times for the samples with authenticated standards.
2.3.3. Fourier transform infrared (FTIR) spectroscopic analysis
FTIR is an essential analytical tool that can be used to identify the functional groups of the organic compounds present in substances. In this work, the functional groups of chemical biomolecules in the oil sample were determined by performing FTIR analysis with a PerkinElmer Spectrum IR Version 10.6.2 (UK). The absorption frequency spectra were organized as transmittance versus wavenumber in a plot.
2.4. Analysis of chemical elements in the fresh silkworm pupae and extracted oil
To determine the elemental content in the fresh silkworm pupae and extracted oil, the samples were prepared according to our published works [32,33]. Briefly, about 6 g of the fresh silkworm pupae were incinerated in an electrical muffle furnace at 600 °C for 6 h to make an ash of the sample. The obtained ash was then treated with a mixture of concentrated nitric acid and perchloric acid in a ratio of 2:1 and heated on a hot plate for complete digestion of the sample. For the preparation of the oil sample, around 6 g of the sample was digested with a mixture of nitric acid, perchloric acid, and hydrogen peroxide in the ratio of (2:1:0.5) in a Kjeldahl flask under reflux. The final volume of both silkworm pupae and oil samples was made to 100 mL with deionized water. The samples were prepared in triplicates and blank samples were also prepared following the same approaches.
The concentrations of 16 chemical elements viz., Fe, Mn, Na, K, Ca, Mg, Zn, P, Co, Ni, Cu, Pb, Cd, Cr, As, and Ag in the samples were determined using spectrophotometric techniques at the ISO/IEC 17025:2017 accredited laboratory of the Institute of National Analytical Research and Service (INARS), Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka Bangladesh. All elements were measured using an Atomic Absorption Spectrophotometer (Model: AA240FS, Varian, Australia), except for P, which was analyzed using an Ultraviolet–Visible Spectrophotometer (Model: UV-1650PC, Shimadzu, Japan) in the colorimetric method. The details of the experimental parameters are provided in Supplementary Table S1. To produce highly reliable data, the scheme of quality assurance and quality control during the analysis of samples was almost the same as those of previous works [33,34,35]. All samples were measured in triplicates (relative standard deviation was less than 8%) and the mean results were reported for each chemical element.
3. Results and discussion
3.1. Proximate composition of silkworm pupae
The proximate composition of the silkworm pupae is displayed in Table 1. In this work, the protein content of fresh silkworm pupae was found to be 10.77%, which is slightly lower than the protein content (11.70%) of the previously published report for silkworm pupae [19], though it was noticed that the protein percent (10.30%) of the edible insect Acheta domesticus was a little bit lower than our finding [36]. However, it was investigated that the protein content of the eri silkworm was 16 and 24% in pupae cultured on two different types of leaves tapioca and castor, respectively [37]. In general, most of the edible insect's pupae and adults are enriched with high protein (more than 10 g proteins/100 g edible portion of fresh weight basis) [38]. On a dry weight basis, the protein content (49.43%), in this work, was slightly less than previous findings (approximate range: 51–53%) [12,19,37].
Table 1.
Proximate compositions of silkworm pupae, yield of oil by acid fermentation, and physicochemical characteristics of silkworm pupae oil.
| Proximate compositions |
Physicochemical characteristics |
|||
|---|---|---|---|---|
| Parameters | Content (g per 100 g) |
Characteristics |
Results |
|
| Fresh Pupae | Dry Basis | Moisture content (%) | 0.06 ± 0.00 | |
| Moisture | 78.26 ± 0.21 | – | Density (g/ml) at 25 °C | 0.912 ± 0.03 |
| Protein | 10.77 ± 0.08 | 49.43 ± 0.75 | Specific gravity (g/ml) at 25 °C | 0.919 ± 0.05 |
| Crude fat | 6.25 ± 0.21 | 27.82 ± 0.85 | Refractive index at 25 °C | 1.43 ± 0.01 |
| Total carbohydrate | 3.96 ± 0.07 | 16.75 ± 0.32 | Viscosity, cst | 30.34 ± 1.25 |
| Crude fiber | 1.05 ± 0.03 | 4.85 ± 0.81 | Free Fatty Acid (as oleic %) | 2.14 ± 0.13 |
| Ash | 0.89 ± 0.02 | 3.96 ± 0.15 | Acid value (mg KOH/g) | 1.35 ± 0.01 |
| Peroxide value (meq/kg) | 4.82 ± 0.21 | |||
| Yield of oil (acid fermentation) | 3.52 ± 0.23% | – | Iodine value (g/100 g) | 129.23 ± 1.53 |
| Unsaponifiable matter (%) | 3.1 ± 0.45 | |||
Note: Values are means ± standard deviations of triplicate analyses.
In this study, the fat content of the fresh silkworm pupae was found to be around 6.25%, which is the same as the precedent study [19], on the other hand, lower than the fat content (8.0–8.6%) in eri silkworm pupae mentioned by Longvah et al. [37]. Studies observed that the different sexes of the silkworm Bombyx mori pupae are responsible for a diverse range of fat content [15]. On a dry weight basis, the fat content (27.82%) in this work was found to be higher than in the former investigations [12,19,37]. The total carbohydrate (3.95%) and crude fiber (1.05%) contents of this work were largely consistent with the findings of the earlier research [19,37]. However, these values greatly vary on the insect's diet, species, and life stage [15]. According to the former research, silkworm pupae contain 30–55% protein, 10–15% carbohydrates, and 25–30% oil on a dry weight basis, which is enriched with several saturated and unsaturated fatty acids [39,40,41].
3.2. Oil yield of silkworm pupae by the acid fermentation process
In our work, the acid fermentation process was replicated three times and the oil yield was 3.52 ± 0.23% on fresh pupae, slightly higher than the extracted oil by saline aqueous process [19]. Earlier studies reported that the yield of the extracted oil from silkworm pupae significantly depends on the applied methods, time, and species [19]. Hence, the few diversifications in the overall findings of oil yield of silkworm pupae are observed in this work compared to the former studies, which may be accomplished by utilizing the varieties of the applied methodology, nourished food sources, and geographical locations.
3.3. Physicochemical properties of silkworm pupae oil
The physicochemical properties of silkworm pupae oil are summarized in Table 1. In this research, the moisture content is found to be 0.06% which is lower than the value of moisture content (0.2%) in edible oils [42]. The higher moisture content (more than 0.2%) is responsible for the growth of the fungus species including, Aspergillus niger and Mucor species on the material surface [43]. In this regard, our extracted silkworm pupae oil seems good. In our investigation, the density (0.91 g/mL at 25 °C) of silkworm oil was similar to the density value of eri silkworm pupae oil reported by Ravinder et al. [44]. Furthermore, the specific gravity (0.92 g/mL) and refractive index (1.43) of the oil extracted by this acid fermentation process were largely consistent with the previous studies [11,45]. The oil's viscosity refers to a property that resists oil flow. In the current work, the viscosity of the extracted oil (Table 1) was slightly less than the eri silkworm pupae oil noticed by earlier research [44].
Free fatty acid (FFA) is considered edible oil's most important quality parameter. In this study, the FFA value (Table 1) was similar to the value of eri silkworm pupae oil reported in the literature [44], but higher than avocado oil (0.29–0.38 as oleic %) [46]. The acid value (AV) (1.35 mg KOH/g) and peroxide value (PV) (4.82 meq/kg) of oil extracted by the acid fermentation process in the present investigation were slightly lower than oil extracted by the microwave-assisted extraction method reported by Hu et al. [11] but higher than oil extracted by saline aqueous process [19]. From this result, it can be summarized that the silkworm pupae oil with low AV and PV could be considered a good quality oil according to Codex Standards [47]. Additionally, the iodine value (IV) of oil (129.2 g I/100 g oil) in this work was comparably higher than oil extracted by microwave-assisted extraction and Soxhlet extraction reported by previous research [11]. Comparison with the previous works on plant oils of almost similar profiles shows that our extracted silkworm pupae oil has higher IV than the avocado (80–85 g I/100 g) [11] and papaya (66–69 g I/100 g) [48] seed oils, but almost similar IV with sunflower (120–127 g I/100 g) [49] and Kalahari melon (125–141 g I/100 g) [50] seed oils. Furthermore, it was found that the unsaponifiable matter for oil was 3.1% which is mostly consistent with the documented result for palm oil [51]. Hence, the overall results of physicochemical parameters for the silkworm pupae oil revealed that the oil obtained by our acid fermentation process fulfilled almost all qualities of oils and could be used as a good source of edible oil.
3.4. Fatty acid composition of silkworm pupae oil
Table 2 presented the fatty acid composition of the silkworm pupae oil extracted from the acid fermentation process. In this experiment, the vital fatty acid constituents were myristic acid (C14:0), palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), and linoleic acid (18:2). The saturated fatty acids (SFA) were approximately 54.82% among total fatty acids which dominated among the total fatty acids, but this result was significantly higher than previous studies [11,19]. Palmitic (32.04%) and stearic (C18:0, 10.95%) acids were the main acids found in SFA which corresponded with previously published data [12,19], though in avocado oil, palmitic acid (17%) was the main SFA like this extracted oil [46]. The total unsaturated fatty acids (USFA) were about 45% including, polyunsaturated fatty acids (PUFA) and monounsaturated fatty acids (MUFA) which were significantly less than the previously reported data [11,17,19]. However, PUFA in extracted oil was lower than PUFA in avocado oil [46]. Furthermore, oleic acid (18:1) in MUFA and linoleic acid (C18:2, n-6) in PUFA were the main components in the extracted oil, which are mostly in agreement with the findings of Tangsanthatkun et al. [19] and Tan [52]. Furthermore, a literature comparison exhibits the oleic acid (18:1) of the extracted oil is in agreement with avocado oil's oleic acid (30–61%) [46,52] but, lower than papaya seed oil (70–78%) [53]. However, the different ratios of the fatty acid composition may be due to the variations in extraction processes and factors, such as the origin of species, maturation stage, sex, season, and geographical regions, as observed in previous studies [11,14].
Table 2.
Fatty acid compositions the silkworm pupae oil and concentrations of chemical elements in the fresh silkworm pupae and silkworm pupae oil obtained from acid fermentation extraction.
| Fatty acid compositions |
Concentrations (mg/kg) of chemical elements |
|||
|---|---|---|---|---|
| Fatty acids | (% Total fatty acids) | Elements | Fresh silkworm pupae | Silkworm pupae oil |
| Saturated fatty acids (SFA) | 54.82 ± 1.98 | Fe | 29.2 ± 0.4 | 9.42 ± 1.6 |
| Lauric acid (C12:0) | 2.84 ± 0.12 | Mn | 9.15 ± 0.4 | 0.72 ± 5.7 |
| Myristic acid (C14:0) | 7.26 ± 0.09 | Na | 218.1 ± 1.5 | 106.9 ± 0.7 |
| Palmitic acid (C16:0) | 32.04 ± 1.44 | K | 15589.2 ± 0.4 | 479.5 ± 0.3 |
| Behenic acid (C22:0) | 1.73 ± 0.07 | Ca | 2176.7 ± 1.2 | 159.0 ± 0.2 |
| Stearic acid (C18:0) | 10.95 ± 0.53 | Mg | 3856.7 ± 1.5 | 71.1 ± 0.3 |
| Zn | 247.7 ± 0.2 | 2.91 ± 0.2 | ||
| Monounsaturated fatty acids (MUFA) | 35.16 ± 1.06 | P | 9520.8 ± 0.1 | 979.2 ± 0.1 |
| Palmitoleic acid (C16:1) | 0.34 ± 0.01 | Co | 0.35 ± 2.6 | 0.32 ± 6.5 |
| Oleic acid (C18:1) | 34.82 ± 1.01 | Ni | 1.13 ± 0.6 | 1.00 ± 6.8 |
| Cu | 1.35 ± 2.3 | 0.20 ± 5.6 | ||
| Polyunsaturated fatty acids (PUFA) | 10.41 ± 0.34 | Pb | 0.37 ± 0.8 | 0.17 ± 1.5 |
| Linoleic acid (C18:2, n-6) | 6.70 ± 0.29 | Cd | 0.18 ± 3.9 | 0.14 ± 1.3 |
| Linolenic acid (C18:3, n-3) | 1.14 ± 0.12 | Cr | 0.38 ± 7.8 | 0.22 ± 6.1 |
| Dihomo-Gamma-linolenic acid (C20:3) | 2.57 ± 0.08 | As | 0.09 ± 0.9 | 0.03 ± 4.9 |
| Ag | 0.38 ± 7.3 | 0.27 ± 0.2 | ||
Note: Values are means ± standard deviations of triplicate analyses.
3.5. Chemical analysis of the extracted silkworm pupae oil using FTIR spectroscopy
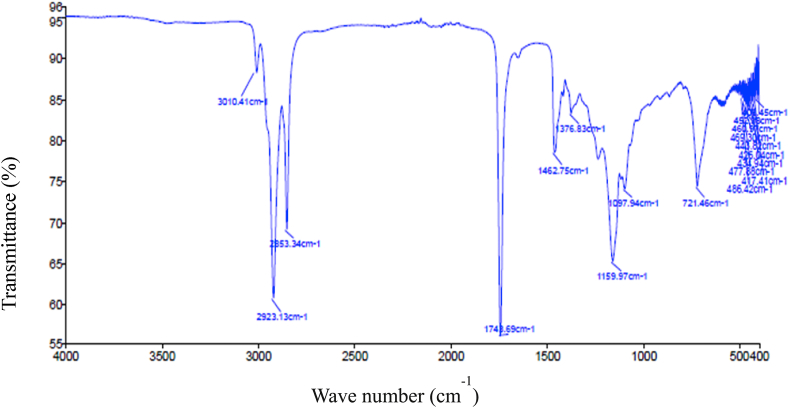
Fig. 4 presents the FTIR spectra of the produced oil by acid fermentation extraction. From Fig. 4, the band at 3010.41 cm−1 is ascribed to the = C–H stretching vibration of the alkene group [54]. The robust absorbance between 2853.3 and 2923.1 cm−1 was similar to the aliphatic C–H stretching vibration and displayed a high quantity of methyl and methylene groups [55,56]. The absorption peak near 1743.7 cm−1 showed the C O group stretching vibration in ketones or carboxylic acids, which was in agreement with a large number of ketones in the oil. The low-intensity peak lining at∼1600 cm−1 was consistent with the presence of alkenes (-C C- stretch) groups. The absorbance peaks between 1376.8 and 1462.8 cm−1 represented the X–H stretching vibrations (X = C, N). These peaks proved the presence of triglyceride functional groups in the silkworm oil. The presence of aromatic amine (C–N stretch) was confirmed by the absorption peak at 1160 cm−1. The band at 1097.9 cm−1 indicated the ester group (C–O stretch or C–H bend). The FTIR bands reported in this investigation are mostly similar to the previously published works for the extraction of fat content from other feedstocks and insects [56,57,58,59]. The band at 721.46 cm−1 was referred to as the rocking vibration of the –CH2– group.
Fig. 4.
FTIR spectra of the extracted oil by acid fermentation.
3.6. Elemental contents in the fresh silkworm pupae and extracted oil
Table 2 showed the concentrations (mg/kg) of the analyzed chemical elements in the fresh silkworm pupae and silkworm pupae oil samples. In these findings, the concentration of K (15589.2 mg/kg) was the highest among other mineral contents in silkworm pupae powder which is, however, two times less than the K content (34000 mg/kg) of silkworm Antheraea pernyi [60]. Though P content (979.2 mg/kg) among other elements was highest in silkworm pupae oil, this was similar to the range of olive oil (900–1145 mg/kg) [61]. In the present study, the ratio of Na/K is significantly lower (0.01) in silkworm pupae than the Na/K ratio (0.08) of silkworm Antheraea pernyi, which is good for health because of reducing the risk of stroke, hypertension, and cardiovascular disease, etc. [62,63], but higher in silkworm pupae oil (0.22) which is still in the safe limit (<0.5) [62]. Other minerals such as Mg, Zn, P, and Ca concentrations in silkworm pupae are at a good level but slightly lower than previously observed data [64]. In addition, Fe and Na concentrations in the extracted oil are found higher than in sunflower oil [65]. Furthermore, Mg, Zn, P, and Ca concentrations in extracted silkworm pupae oil were lower than earlier mentioned mineral concentrations in olive [61] but higher than in rice bran and sunflower oils [65,66]. Nevertheless, the concentrations of elements, Pb and As in both silkworm pupae and extracted oil were less than the acceptable level (Pb: 0.5 mg/kg, As: 0.1 mg/kg) for food and feed according to FAO [67] and Codex Standards [47].
The overall results of the elemental contents in the fresh silkworm pupae and silkworm pupae oil revealed that the concentrations of all the measured elements are lower in the extracted oil than in the fresh silkworm pupae. This is plausible since most of the elements will be removed after treating silkworm pupae with acetic acid and citric acid in the oil extraction process, forming the soluble acetate and citrate salts. However, it is interesting to note that even after acid treatments of silkworm pupae, the extracted oil still contains a significant amount of some minerals such as Fe, Na, K, Ca, Mg, Zn, and P, with a negligible concentration of toxic elements such as Mn, Co, Ni, Cu, Pb, Cd, Cr, As, and Ag. The sources of the minerals in the extracted oil may be attributed to the strong bindings of the metals within the core structure of the biomolecules present in the silkworm pupae or to the presence of some inorganic materials, which are not removable by the simple acid treatment of the silkworm pupae with the weak organic acids (acetic acid and citric acid). The considerable presence of the minerals in the extracted silkworm pupae oil enhanced the nutritive value of the oil extracted from the silkworm pupae through the adopted acid fermentation process.
4. Conclusions
For the first time in this work, an acid fermentation process was applied for the extraction of oil from the fresh desilked silkworm pupae (Bombyx mori). The yield of the oil was 3.52 ± 0.23% in fresh pupae. The produced oil has a low peroxide value (4.82 meq/kg) and an acid value (1.35 mg KOH/g) indicating its good quality. The proximate composition of the silkworm pupae demonstrated that it is a nutrient resource, including protein, fat, and carbohydrates. The extracted oil contains negligible moisture content, considerably less viscosity, and comprises a wide variety of fatty acids, covering a large percentage of palmitic acid (32.04%) and oleic acid (34.62%). In addition, the oil is comprised of health beneficiary α-linolenic acid, linoleic acid, and dihomo-gamma-linolenic acid. FTIR analysis of the oil revealed the presence of several functional groups of organic molecules such as ketones, carboxylic acids, alkenes, triglycerides, aromatic amines, and esters. The oil contains considerable amounts of minerals such as Fe, Na, K, Ca, Mg, Zn, and P but with only a shallow content of toxic elements such as Mn, Co, Ni, Cu, Pb, Cd, Cr, As, and Ag, indicating its good nutritive value. Overall, the tested findings of several physical and chemical parameters suggested that the silkworm oil attained from this facile extraction process could be a potential candidate to be utilized as edible oil. However, the percentage of oil yield is still not at a very high level restricting its use to a small scale only. Further, the method also takes a long time for oil extraction. Thus, to be utilized this method extensively on a large or industrial scale, further fruitful research should be conducted to increase the percentage of oil yield in a short time through the probable apt modifications in the extraction steps as well as adopting suitable chemicals and reagents.
Acknowledgment
The authors are grateful to the authority of the BCSIR Laboratories Rajshahi, and the Institute of National Analytical Research and Service (INARS), BCSIR, Bangladesh for providing analytical, technical, and other logistic support for conducting this research work.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e12815.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Wei Z.J., Lia A.M., Zhang H.X., Liu J., Jiang S.T. Optimization of supercritical carbon dioxide extraction of silkworm pupal oil applying the response surface methodology. Bioresour. Technol. 2009;100:4214–4219. doi: 10.1016/j.biortech.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Wu F.A., Liang Y., Wang M. Process optimization for the enrichment of α- linolenic acid from silkworm pupal oil using response surface methodology. Afr. J. Biotechnol. 2010;9:2956–2964. [Google Scholar]
- 3.Zotte A.D., Singh Y., Squartini A., Stevanato P., Cappellozza S., Kovitvadhi A., Subaneg S., Bertelli D., Cullere M. Effect of a dietary inclusion of full-fat or defatted silkworm pupa meal on the nutrient digestibility and faecal microbiome of fattening quails. Animal. 2021;15(2) doi: 10.1016/j.animal.2020.100112. [DOI] [PubMed] [Google Scholar]
- 4.Makkar H.P., Tran G., Heuzé V., Ankers P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014;197:1–33. [Google Scholar]
- 5.Hassan S. Increased mustard oil production: a solution to edible oil crisis. The Daily Star, Economy section. 2022. https://www.thedailystar.net/business/economy/news/mustard-oil-solution-edible-oil-crisis-3027596 Published online on.
- 6.TDS (The Daily Star) Edible oil prices: what goes up, never comes down. The Daily Star, Editorial Section. 2022. https://www.thedailystar.net/views/editorial/news/edible-oil-prices-what-goes-never-comes-down-3017816 Published online on.
- 7.Halder S., Suman M., Mirdha R.U. Record hike in soybean oil prices. Section: price of essentials. 2022. https://www.thedailystar.net/health/food/price- essentials/news/record-hike-soybean-oil-prices-3017321 Published online on.
- 8.TFE (The Financial Express) The Financial Express; 2022. Soybean Oil Price Goes up Again.https://thefinancialexpress.com.bd/national/soybean-oil-price-goes-up-again-1654792019 National section, Published online on. [Google Scholar]
- 9.Longvah T., Manghtya K., Qadri S.S. Eri silkworm: a source of edible oil with a high content of α-linolenic acid and of significant nutritional value. J. Sci. Food Agric. 2012;92:1988–1993. doi: 10.1002/jsfa.5572. [DOI] [PubMed] [Google Scholar]
- 10.Usub T., Lertsatitthanakorn C., Poomsa-ad N., Wiset L., Yang L., Siriamornpun S. Experimental performance of a solar tunnel dryer for drying silkworm pupae. Biosyst. Eng. 2008;101:209–216. [Google Scholar]
- 11.Hu B., Li C., Zhang Z., Zhao Q., Zhu Y., Su Z., Chen Y. Microwave-assisted extraction of silkworm pupal oil and evaluation of its fatty acid composition, physicochemical properties and antioxidant activities. Food Chem. 2017;231:348–355. doi: 10.1016/j.foodchem.2017.03.152. [DOI] [PubMed] [Google Scholar]
- 12.Pereira N.R., Ferrarese-Filho O., Matsushita M., de Souza N.E. Proximate composition and fatty acid profile of Bombyx mori L. chrysalis toast. J. Food Compos. Anal. 2003;16:451–457. [Google Scholar]
- 13.Rao P.U. Chemical composition and nutritional evaluation of spent silkworm pupae. J. Agric. Food Chem. 1994;42:2201–2203. [Google Scholar]
- 14.Ray M., Gangopadhyay D. Effect of maturation stage and sex on proximate, fatty acid and mineral composition of eri silkworm (Samia ricini) from India. J. Food Compos. Anal. 2021;100 [Google Scholar]
- 15.Saviane A., Tassoni L., Naviglio D., Lupi D., Savoldelli S., Bianchi G., Cortellino G., Bondioli P., Folegatti L., Casartelli M. Mechanical processing of Hermetia illucens larvae and Bombyx mori pupae produces oils with antimicrobial activity. Animals. 2021;11:783. doi: 10.3390/ani11030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotake-Nara E., Yamamoto K., Nozawa M., Miyashita K., Murakami T. Lipid profiles and oxidative stability of silkworm pupal oil. J. Oleo Sci. 2002;51:681–690. [Google Scholar]
- 17.Pan W.J., Liao A.M., Zhang J.G., Dong Z., Wei Z.J. Supercritical carbon dioxide extraction of the oak silkworm (Antheraea pernyi) pupal oil: process optimization and composition determination. Int. J. Mol. Sci. 2012;13:2354–2367. doi: 10.3390/ijms13022354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivas G., Nidoni U., Ramachandra C.T., Ramappa K.T., Ashoka J. Supercritical fluid extraction of pupae oil from mulberry silkworm (Bombyx mori L.) J. Pharmacogn. Phytochem. 2019;8:4507–4513. [Google Scholar]
- 19.Tangsanthatkun J., Peanparkdee M., Katekhong W., Harnsilawat T., Tan C.P., Klinkesorn U. Application of aqueous saline process to extract silkworm pupae oil (Bombyx mori): process optimization and composition analysis. Foods. 2022;11:291. doi: 10.3390/foods11030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu M., Qu Q., Yang X.Y., Zhang X.H. Effect of intermittent oven drying on lipid oxidation, fatty acids composition and antioxidant activities of walnut. LWT--Food Sci. Technol. 2016;65:1126–1132. [Google Scholar]
- 21.Chen F.L., Du X., Zu Y.G., Yang L., Wang F. Microwave-assisted method for distillation and dual extraction in obtaining essential oil, proanthocyanidins and polysaccharides by one- pot process from Cinnamomi Cortex. Separ. Purif. Technol. 2016;164:1–11. [Google Scholar]
- 22.Vidotti R.M., Pacheco M.T.B., Gonçalves G.S. Characterization of the oils present in acid and fermented silages produced from tilapia filleting residue. Rev. Bras. Zootec. 2011;40(2):240–244. [Google Scholar]
- 23.Salih A.W., Najim S.M., Al-Noor J.M. Some physical, chemical and sensory properties of fish oil extracted from fish wastes by physical and chemical method. Biological and applied environmental research. 2021;5(1):152–162. [Google Scholar]
- 24.AOAC . seventeenth ed. Association of Official Analytical Chemists; Gaithersburg, MD: 2000. Official Methods of Analysis. [Google Scholar]
- 25.Helrich K. Association of official analytical; 1990. Official Methods of Analysis of the Association of Official Analytical Chemists. [Google Scholar]
- 26.Ferdousi L., Sultana N., Bithi U.H., Lisa S.A., Hasan M.R., Siddique M.A.B. Nutrient profile of wild black soldier fly (Hermetia illucens) prepupae reared on municipal dustbin's organic waste substrate. Proc. Natl. Acad. Sci. India B Biol. Sci. 2022;92(2):351–357. doi: 10.1007/s40011-021-01340-0. [DOI] [Google Scholar]
- 27.Garba A.A., Medugu D.W., Gwaski P.A., Amusat R.O. Extraction and characterization of moringa oleifera seed oil. Appl. Res. J. 2015;1(9):473–477. [Google Scholar]
- 28.AOAC . eighteenth ed. vol. 500. AOAC International, Suite; USA: 2005. Association of Official Analytical Chemist, Official Methods of Analysis; p. 481. North Frederick Avenue, Gaithersburg, Maryland 20877-2417. [Google Scholar]
- 29.Yeasmin M.S., Rana G.M., Chowdhury T.A., Rahman M., Ferdousi L. Physico-chemical properties and GCMS analyses of indigenous rice bran and mustard seed oils and their blends. Biomed. J. Sci. Tech. Res. 2021;34(5):27167–27172. [Google Scholar]
- 30.AOAC . sixteenth ed. AOAC International; Gaithersburg: 1999. Official Methods of Analysis. [Google Scholar]
- 31.Mehlenbacher V.C., Hopper T.H., Sallee E.M., Walker R.O., Walker R.C., et al. sixth ed. American Oil Chemists Society; Champaign: 2009. Official Methods and Recommended Practices of the American Oil Chemists Society. [Google Scholar]
- 32.Nasrin S., Islam M.N., Tayab M.A., Nasrin M.S., Siddique M.A.B., Emran T.B., Reza A.S.M.A. Chemical profiles and pharmacological insights of Anisomeles indica Kuntze: an experimental chemico-biological interaction. Biomed. Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112842. [DOI] [PubMed] [Google Scholar]
- 33.Hasan A.B., Reza A.H.M.S., Kabir S., Siddique M.A.B., Ahsan M.A., Akbor M.A. Accumulation and distribution of heavy metals in soil and food crops around the ship breaking area in southern Bangladesh and associated health risk assessment. SN Appl. Sci. 2020;2(2):155. doi: 10.1007/s42452-019-1933-y. [DOI] [Google Scholar]
- 34.Ahsan M.A., Satter F., Siddique M.A.B., Akbor M.A., Ahmed S., Shajahan M., Khan R. Chemical and physicochemical characterization of effluents from the tanning and textile industries in Bangladesh with multivariate statistical approach. Environ. Monit. Assess. 2019;191:575. doi: 10.1007/s10661-019-7654-2. [DOI] [PubMed] [Google Scholar]
- 35.Siddique M.A.B., Alam M.K., Islam S., Diganta M.T.M., Akbor M.A., Bithi U.H., Chowdhury A.I., Ullah A.K.M.A. Apportionment of some chemical elements in soils around the coal mining area in northern Bangladesh and associated health risk assessment. Environ. Nanotechnol. Monit. Manag. 2020;14 doi: 10.1016/j.enmm.2020.100366. [DOI] [Google Scholar]
- 36.Pennino M., Dierenfeld E., Behler J. Retinol, α-tocopherol and proximate nutrient composition of invertebrates used as feed. Int. Zoo Yearbk. 2007;30:143–149. [Google Scholar]
- 37.Longvah T., Mangthya K., Ramulu P. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chem. 2011;128:400–403. doi: 10.1016/j.foodchem.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 38.FAO, WHO . FAO; Rome, Italy: 2007. Food Labelling. 2007. [Google Scholar]
- 39.Wang W., Wang N., Zhou Y., Zhang Y., Xu L., Xu J., Feng F., He G. Isolation of a novel peptide from silkworm pupae protein components and interaction characteristics to angiotensin I-converting enzyme. Eur. Food Res. Technol. 2011;232:29–38. [Google Scholar]
- 40.Xu X.-H., Liu Z.-X., Shi X.-Y., Miao C., Sheng S., Xu Y., Wu F.-A., Wang J. Fed-batch fermentation of Yarrowia Lipolytica using defatted silkworm pupae hydrolysate: a dynamic model-based approach for high yield of lipid production. Waste Biomass Valoriz. 2018;9:2399–2411. [Google Scholar]
- 41.Zhang R., Rao Z., Li Y., Li H., Fei L., Lei S., Wang Y. Silkworm excrement derived in-situ co-doped nanoporous carbon as confining sulfur host for lithium sulfur batteries. Chem. Select. 2019;4:5678–5685. [Google Scholar]
- 42.Federation OF . Australian Oilseeds Federation; Australia Square: 2011. Section 1: Quality Standards, Technical Information & Typical Analysis; pp. 40–45. [Google Scholar]
- 43.Commission C.A. 2001. Codex Standard for Named Vegetable Oils Codex Standard 210-1999. Report of the 17 the Session of the Codex Committee on Fats and Oils; pp. 19–23. London. [Google Scholar]
- 44.Ravinder T., Kaki S.S., Kunduru K.R., Kanjila S., Rao B.V.S.K., Swain S.K., Prasad R.B.N. Physico-chemical characterization and oxidative stability studies of eri silkworm oils. Int. J. Mod. Chem. Appl. Sci. 2016;3(1):293–300. [Google Scholar]
- 45.Yap J.W.-L., Lee Y.-Y., Tang T.-K., Chong L.-C., Kuan C.-H., Lai O.-M., Phuah E.-T. Fatty acid profile, minor bioactive constituents and physicochemical properties of insect-based oils: a comprehensive review. Crit. Rev. Food Sci. Nutr. 2021 doi: 10.1080/10408398.2021.2015681. [DOI] [PubMed] [Google Scholar]
- 46.Tan C.X., Chong G.H., Hamzah H., Ghazali H.M. Comparison of subcritical CO2 and ultrasound-assisted aqueous methods with the conventional solvent method in the extraction of avocado oil. J. Supercrit. Fluids. 2018;135:45–51. [Google Scholar]
- 47.Codex Standards for Edible Fats and Oils Not Covered by Individual Standards. Codex Stan 19-1981. Amendment 2015. [Google Scholar]
- 48.Puangsri T., Abdulkarim S.M., Ghazali H.M. Properties of Carica papaya L. (papaya) seed oil following extractions using solvent and aqueous enzymatic methods. J. Food Lipids. 2005;12(1):62–76. [Google Scholar]
- 49.Latif S., Anwar F. Effect of aqueous enzymatic processes on sunflower oil quality. J. Am. Oil Chem. Soc. 2009;86(4):393–400. [Google Scholar]
- 50.Nyam K.L., Tan C.P., Che Man Y.B., Lai O.M., Long K. Physicochemical properties of Kalahari melon seed oil following extractions using solvent and aqueous enzymatic methods. Int. J. Food Sci. Technol. 2009;44(4):694–701. [Google Scholar]
- 51.Tan C., Ghazali H.M., Kuntom A., Tan C., Ariffin A.A. Extraction and physicochemical properties of low free fatty acid crude palm oil. Food Chem. 2009;113(2):645–650. [Google Scholar]
- 52.Tan C.X. Virgin avocado oil: an emerging source of functional fruit oil. J. Funct.Foods. 2019;54:381–392. [Google Scholar]
- 53.Tan C.X., Tan S.T., Tan S.S. An overview of papaya seed oil extraction methods. Int. J. Food Sci. Technol. 2020;55(4):1506–1514. [Google Scholar]
- 54.Mecozzi M., Sturchio E. Computer assisted examination of infrared and near infrared spectra to assess structural and molecular changes in biological samples exposed to pollutants: a case of study. J. Imaging. 2017;3(1):11. doi: 10.3390/jimaging3010011. [DOI] [Google Scholar]
- 55.Silverstein R.M., Webster F.X., Kiele D.J. seventh ed. John Wiley & Sons; New York (NY, USA): 2005. Spectrometric Identification of Organic Compounds; pp. 88–90. [Google Scholar]
- 56.Wang C., Qian L., Wang W., Wang T., Deng Z., Yang F., Xiong J., Feng W. Exploring the potential of lipids from black soldier fly: new paradigm for biodiesel production (I) Renew. Energy. 2017;111:749–756. [Google Scholar]
- 57.Dutta R., Sarkar U., Mukherjee A. Extraction of oil from Crotalaria Juncea seeds in a modified Soxhlet apparatus: physical and chemical characterization of a prospective bio-fuel. Fuel. 2014;116(2014):794–802. [Google Scholar]
- 58.Reshad A.S., Tiwari P., Goud V.V. Extraction of oil from rubber seeds for biodiesel application: optimization of parameters. Fuel. 2015;150:636–644. [Google Scholar]
- 59.Yan W.H., Duan P.G., Wang F., Xu Y.P. Composition of the bio-oil from the hydrothermal liquefaction of duckweed and the influence of the extraction solvents. Fuel. 2016;185:229–235. [Google Scholar]
- 60.Zhou J., Han D. Proximate, amino acid and mineral composition of pupae of the silkworm Antheraea pernyi in China. J. Food Compos. Anal. 2006;19(8):850–853. [Google Scholar]
- 61.Tanilgan K., Özcanb M.M., Ünverb A. Physical and chemical characteristics of five Turkish olive (Olea europea L.) varieties and their oils. Grasas Aceites. 2007;58(2):142–147. [Google Scholar]
- 62.Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., AlMazroa M.A., Amann M., Anderson H.R., Andrews K.G., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2021;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodrigues S.L., Baldo M.P., Machado R.C., Forechi L., Molina Mdel C., Mill J.G. High potassium intake blunts the effect of elevated sodium intake on blood pressure levels. J. Am. Soc. Hypertens. 2014;8:232–238. doi: 10.1016/j.jash.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y., Zhou S., Duan H., Wang J., Yan W. Silkworm pupae: a functional food with health benefits for humans. Foods. 2022;11:1594. doi: 10.3390/foods11111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petraru A., Ursachi F., Amariei S. Nutritional characteristics assessment of sunflower seeds, oil and cake perspective of using sunflower oilcakes as a functional ingredient. Plants. 2021;10(11):2487. doi: 10.3390/plants10112487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olabanji I.O., Ajayi O.S., Adekunle A.S. Fatty acids, metal composition and physico- chemical parameters of Igbemo Ekiti rice bran oil. J. Environ. Chem. Ecotoxicol. 2013;5(3):39–46. [Google Scholar]
- 67.Codex Alimentarius International Food Standards . second ed. Joint FAO/WHO Food Standard Programme; 1995. General Standard for Contaminants and Toxins in Food and Feed. Revised 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.