Abstract
Artificial tears are the mainstay of dry eye disease management, but also have a role in corneal abrasion and wound healing, pain and inflammation management, conjunctivitis, keratitis, contact lens rewetting and removal, and foreign body removal. A systematic review of randomized controlled trials (PROSPERO registration CRD42022369619) comparing the efficacy of artificial tears in patients with dry eye to inform prescribing choices using Web of Science, PubMed and Medline databases identified 64 relevant articles. There is good evidence that artificial tears improve symptoms of dry eye disease within a month of regular use, applied about four times a day, but signs generally take several months to improve. Not all patients with dry eye disease benefit from artificial tears, so if there is no benefit over a month, alternative management should be considered. Combination formulations are more effective than single active ingredient artificial tears. Artificial tears containing polyethylene glycol are more effective than those containing carboxymethylcellulose/carmellose sodium and hydroxypropyl methylcellulose. Those classified as having evaporative dry eye disease, benefit from artificial tears with liposomes, especially of higher concentration. The data available is limited by the definition of dry eye disease applied in published studies being variable, as well as the disease severity examined and compliance with artificial tears being rarely quantified.
Keywords: artificial tears, dry eye, comfort, contact lenses
Artificial tear drops are most commonly associated with the management of dry eye disease (DED). Artificial tears are typically included in first-line management options for dry eye, as they are easy to use, accessible in a wide range of formulations, and have a low risk-profile.1 Most artificial tear preparations have been found to be effective in reducing the symptoms and signs of DED, however the Tear Film and Ocular Surface Society (TFOS) dry eye workshop in 2017 (DEWS II) concluded there had been relatively few high quality randomized controlled trials comparing different formulations with each other.1,2 Furthermore, few clinical trials have compared the efficacy of different artificial tear products, and attempted to correlate this with patient characteristics, in order to aid management decisions for an individual.3,4 The issue with this is that both practitioners and patients are faced with a bewildering array of different products with varying ingredients, and little or no clear way of knowing which is the most effective. Practitioners will often be asked “which is the best drop for dry eye”, but with no scientific evidence to base their answer on. In addition, other aspects that influence practitioner and patient choices are:
storage bottle design.7–10
Patients may therefore face a trial-and-error approach to product selection, incurring mounting costs and frustration in the process. This will be felt even more keenly by patients who are highly price sensitive, since over-the-counter products are no longer easily available via National Health Service (NHS) subsidised prescriptions11 in the UK. A recent study12 on the reported experience of dry eye management across four continents identified that on average, DED still caused a moderate impact on an individual’s quality of life (median impact 3/10); less than half of the individuals in any country had undergone a consultation with an eye or health-care practitioner about their dry eye; about half had tried dry eye treatment, with artificial tears being the most common treatment, followed by warm compresses, and both therapies were rated as reasonably effective (median 5–7/10).
Formulation
The majority of artificial tear products are aqueous-based and contain viscosity-enhancing agents, such as carbomer 940, carboxymethyl cellulose (CMC), dextran, hyaluronic acid, sodium hyaluronate (which has a smaller molecular size), hydroxypropyl guar (HP-guar), hydroxypropyl methylcellulose (HPMC hypromellose), polyvinyl alcohol, polyvinylpyrrolidone and polyethylene glycol, which aid lubrication and increase on-eye retention time.1 Other ingredients may include osmotic agents, osmoprotectants, antioxidants, preservatives and inactives such as pH buffers, excipients and electrolytes.1 Aqueous-based artificial tears target principally the muco-aqueous phase of the tear film, but have been shown to improve dry eye symptoms related to all subtypes of DED.2
In recent years, there has been an increase in the popularity and availability of lipid-based drops, which target the superficial tear lipid layer13,14 as the emphasis on meibomian gland dysfunction and its role in evaporative dry eye continues to increase.1 It has been demonstrated in randomised controlled trials that lipid-based drops are more effective at managing DED classified as evaporative.3,4 These can take the form of nano-emulsion drops or liposomal sprays, which are applied to the closed eye and may be easier for those who struggle to instil drops, for example those with reduced manual dexterity or hand tremor. A completely water-free drop comprised of 100% lipid (perfluorohexyloctane) is available now, with the added benefit of being preservative-free.15
Preservatives
Multidose eye drops, including artificial and medicated topical ocular drops, commonly contain preservatives to maintain sterility and prolong shelf life, however, these are also known to produce toxicity. Benzalkonium chloride, commonly found in multidose drops, can produce toxic, proinflammatory and detergent effects, which may actually lead to or exacerbate DED.16 For this reason, there has been a move towards preservative-free and unit dose formulations, due to the risk of toxic and allergic reactions, especially when frequent instillation is required. Newer preparations may contain less damaging preservatives such as polyquaternium, or “vanishing” preservatives such as sodium perborate and purite, or feature specially designed bottles, which prevent the entry of microorganisms.17 Preservative-free formulations are recommended for all types of dry eye, however this is even more important for severe dry eye or sensitive individuals, and more details can be found in the TFOS DEWS II iatrogenic report.6
Ideal Properties
It is important that artificial tear drops behave in a similar way to natural tears. One aspect of this is the physical property of rheology, which refers to the way fluids and soft solids flow. The viscosity of human tears is high between blinks, but reduces during each blink cycle in order to protect the ocular surface from damage due to fluid turbulence.1 Hence, they do not display Newtonian behaviours and are referred to as having non-Newtonian properties. Hyaluronic acid has been the subject of a significant amount of research and has been shown to exhibit these non-Newtonian shear-thinning properties,18 making it more like the tear film and hence suitable for use in artificial tears.19 Hyaluronic acid, a common constituent of artificial tears, is a naturally occurring glycosaminoglycan, which is found in and around body cells and tissues, for example in synovial fluid, and vitreous and aqueous humour.20 Its use in ophthalmology was pioneered by Andre Balazs in the late 1960s,21 with Polack and McNiece22 being the first to report its use in dry eye. Hyaluronic acid is water soluble and is capable of binding large quantities of water, compared to its own weight, but its physical properties vary depending upon its molecular weight.23 There is evidence to suggest that high molecular weight hyaluronic acid (HMWHA) is clinically superior in the treatment of DED compared to its low molecular weight counterpart.24 Furthermore, HMWHA has been found to be protective against corneal cell apoptosis due to benzalkonium chloride toxicity, ultraviolet light radiation and chemical burns,25–27 as well as being anti-inflammatory and having a role in reducing pain sensation.24,28
Artificial Tears for Dry Eye Disease
There have been several systematic reviews2,29–31 conducted over the past decade, concluding that artificial tears are a safe and effective way of treating DED. A meta-analysis concluded that the effectiveness of sodium hyaluronate did not differ based on its preparation30 and another32 suggested that CMC appeared to be better than hyaluronic acid in treating DED, but the results were not statistically significant. Two recent reviews5,33 both identified that while hyaluronic acid was effective in reducing the symptoms of DED, the ideal drop frequency and formulation (both concentration and molecular weight) for different ages and severities were yet to be investigated.
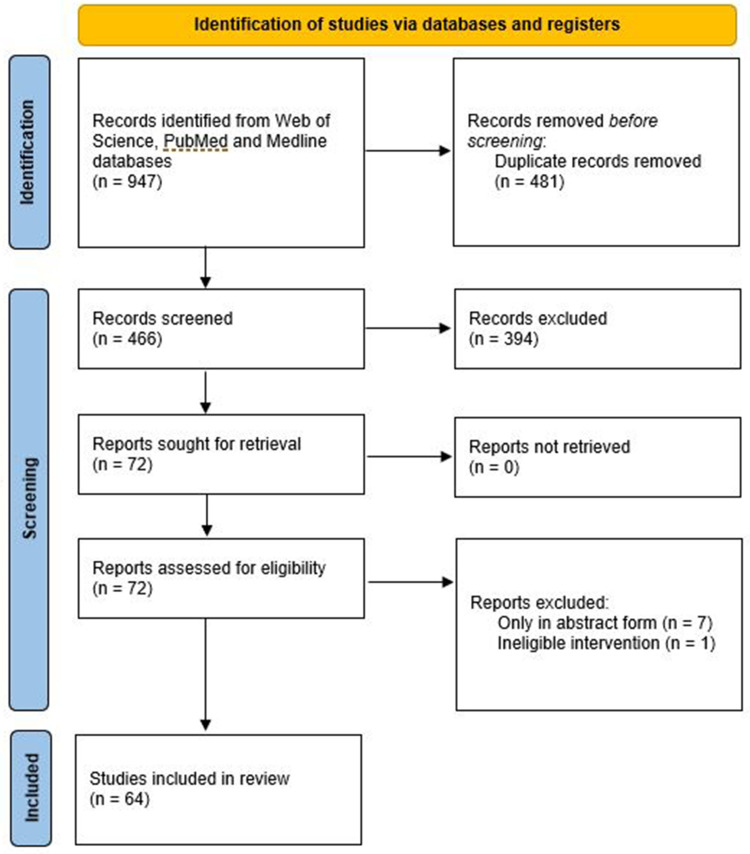
To date, there has been no review of studies which compared different artificial tears to identify whether certain formulations are more effective. Hence, with the objective to better understand the evidence for the effect of different artificial tears in managing dry eye, a search was made of the Web of Sciences databases (Clarivate Analytics, Philadelphia, USA) which includes the Science Citation Index Expanded covering over 9200 of the world’s most impactful journals from 1900 to the present day along with PubMed (including MEDLINE) from its inception. The systematic review was prospectively registered on PROSPERO (CRD42022369619) and was conducted in the format prescribed by PRISMA (2020).34 A search for “artificial tear*” AND “randomi?ed” identified 481 unique results which were screened independently by two researchers (DB and DS) and verified by a third (JSW). Studies were eligible to be accepted if they were in full paper form (not abstracts or book chapters), compared two or more artificial tears against each other (not just with a placebo) and involved randomisation to avoid bias. This resulted in 64 papers being accepted (Figure 1) and the full text scrutinized for the key factors, which were tabulated in a spreadsheet and are summarised in Table 1. The study design, artificial tears compared, number and age profile of participants completing the trial, duration of use and dosing, tests conducted which showed a significant difference/did not differentiate between the products or change from baseline and general comments (dyes used for ocular surface staining, adverse events when reported and subanalyses) were extracted. Missing information is highlighted in the table and risk of bias analysis performed with the Cochrane Tool reported.35 No data synthesis was attempted due to heterogeneity particularly in drop duration.
Figure 1.
PRISMA 2020 flow diagram of the systematic review search results.
Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.127
Table 1.
Randomised Clinical Trials That Have Used Artificial Tears for the Treatment of Dry Eye Disease
| Paper | Design | Comparators | Participants Completing | Age (Years) | Duration (Dosing) | Tests Showing Significant Difference | Tests Not Differentiating Products | Tests Showing Significant Difference | Tests Showing no Change | General Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Cross Comparator | Compared to Baseline | |||||||||
| Amran et al 201469 | Randomized, open-label, multi-center study | Cationic Emulsion - Cationorm PVA-Povidone - Refresh |
N = 44 N = 35 |
61.3 ± 15.4 61.9 ± 12.5 |
4 weeks (4x/day) | Symptoms, TBUT, eyelid erythema, Conjunctival staining with Cationic | Schirmer’s Corneal staining |
Sub-analysis with MGD participants CLINICAL TRIAL NOT REGISTERED |
||
| Aragona et al 202048 | Randomized, double-masked, multi-center study | CMC + HA - Optive Fusion UD CMC - Refresh Optive Sensitive/Optive UD |
N = 180 N = 184 |
59.4 ± 13.8 57.5 ± 13.7 |
90 days (2x/day) | Lower ocular pain/discomfort CMC-HA | OSDI TBUT Ocular surface staining Schirmer’s II |
OSDI Symptoms (VAS) TBUT Ocular surface staining |
Schirmer’s II | 10% minor AE CLINICAL TRIAL NOT REGISTERED |
| Baeyens et al 201274 | Randomized, double-masked, multi-center study | SH 0.18% - Vismed Carbomer 0.3% NaCL |
N = 100 N = 96 N = 96 |
59.3 ± 15.0 (across groups) | 84 days | Symptoms & Corneal staining with SH vs saline | Symptoms, corneal and conjunctival staining, Schirmer’s, TBUT SH vs carbomer | Symptoms Corneal staining |
Conjunctival staining, Schirmer’s, TBUT | CLINICAL TRIAL NOT REGISTERED |
| Barabino et al 2014104 | Randomized, double-masked, multi-center study | CMC 0.5% / glycerin 0.9% - Optive HA 0.2% / tamarind seed polysaccharide 0.2% - Xiloial |
N = 25 N = 23 |
57.1 ± 17.4 52.2 ± 14.9 |
3 months (4x/day) | Symptoms with HA+TS | TBUT Ocular surface staining Schirmer’s |
OSDI TBUT Ocular surface staining |
Schirmer’s | CLINICAL TRIAL NOT REGISTERED |
| Baudouin et al 201257 | Randomized, investigator-masked, multi-center study | CMC 0.5% and Osmoprotectant – Optive SH 0.18% - Vismed Multi |
N = 37 N = 29 |
58.1 ± 14.2 55.4 ± 13.4 |
3 months | None | Osmolarity, Schirmer’s-I, OSDI, staining | Osmolarity, Schirmer’s-I, OSDI, staining | None | Clinical Trial NCT00987727 - only symptom primary and secondary outcomes and day 35 data missing Cochrane Risk of Bias R?C?M-O?I-S-B? |
| Benelli et al 201062 | Randomized, investigator-masked, single-center study | CMC 0.5% - Cellufresh PEG 400 2.5% - Blink Intensive HP‐guar 0.18%/PEG 400/PG - Systane |
N = 20 N = 20 N = 20 |
Not stated | 30 days (up to 4x/day) | Osmolarity with PEG400 | VA Aberrometry Staining TBUT Schirmer’s |
Aberrometry | Osmolarity VA Staining TBUT Schirmer’s |
CLINICAL TRIAL NOT REGISTERED |
| Brignole et al 200540 | Randomized, masked-observer, single-center study | CMC 1% - Celluvisc SH 0.18% - Vismed |
N = 11 N = 10 |
69 ± 2 57 ± 2 |
2 months (3x/day) | CD44, comfort (only at day 7), keratitis recovery with SH | All other inflammatory markers, cornea and conjunctival staining, TBUT, corneal topography, tear meniscus height | Symptoms and ocular surface staining | None | Moderate dry eye and keratitis patients CLINICAL TRIAL NOT REGISTERED |
| Brodwall et al 1997105 | Randomized, investigator-masked, single-center study | Polyacrylic acid 0.2% - Visco Tears PVA 1.4% |
N = 38 N = 41 |
60.2 61.8 |
4 weeks (Drops/day a study variable; avg 3–5) | Symptoms (16/27 study days), hyperaemia, Rose Bengal staining, compliance with polyacrylic acid | TBUT Schirmer’s |
Symptoms & signs (unspecified) | TBUT Schirmer’s |
CLINICAL TRIAL NOT REGISTERED |
| Bron et al 1998106 | Randomized, double masked, multi-center study | Carbomer 940 0.2% - Lacrinorm/GelTears, Laboratoire Chauvin Carbomer 940 0.2% - Viscotears/Vidisic/Lacrigel |
N = 92 N = 87 |
58.6 ± 16.2 64.0 ± 14.0 |
4 weeks (4x/day) | None | Symptoms TBUT, Schirmer’s, corneal and conjunctival staining |
Symptoms TBUT, Schirmer’s, fluorescein/ lissamine green staining |
None | AEs in n=21 Lacrinorm group and 17 Viscotears group CLINICAL TRIAL NOT REGISTERED |
| Calvao-Santos et al 201141 | Randomized, open-label, single-center study | Tears Again [lipidic] Opticol [aqueous] Optive [mucin] No treatment |
N = 7 N = 6 N = 7 N = 7 |
24 to 53 years | 30 days (not stated) | None | OSDI TBUT Schirmer’s |
Symptoms, Schirmer’s for tears again |
TBUT | Patients with digital eye strain. Compared drops primarily acting on one tear layer CLINICAL TRIAL NOT REGISTERED |
| Chiambaretta et al 201750 | Randomized, investigator-masked, multi-center study | HA-trehalose HA |
N = 52 N = 49 |
60.0 ± 12.2 58.5 ± 13.4 |
84 days (3–6x/day; average 4) | Symptoms with HA-trehalose | Cornea & conjunctival staining | OSDI [Schirmer’s, TBUT, staining, hyperaemia, no statistics presented] | None | AEs: 3 with HA-trehalose vs 24 with HA Clinical Trial NCT02023268 - only staining as primary outcome and day 35 data missing Cochrane Risk of Bias R?C-M-O?I?S-B? |
| Christensen et al 200461 | Randomized, double-masked, multi-center study | PEG 400 0.4% / PG/HP‐guar 0.3% - Systane CMC 0.5% - Refresh Tears |
N = 42 N = 45 |
58.5 59.5 |
6 weeks (4x/day) | Lissamine green staining, dryness, refreshed and FB symptoms with 0.5% PEG | Fluorescein staining, use ratings, ocular signs or symptom frequency | Corneal & conjunctival staining only with 0.4% PEG | Conjunctival staining with CMC | CLINICAL TRIAL NOT REGISTERED |
| Cohen et al 201463 | Randomized, double-masked, multi-center study | CMC 1% - Refresh LiquiGel PEG 400 0.4%/ PG/HP‐Guar 0.3% - Systane Gel |
N = 70 N = 67 |
57.5±16.6 56.5±15.0 |
6 weeks (4x/day) | Corneal staining with PEG | Conjunctival staining, TBUT, symptoms | Corneal staining | Lissamine green staining, TBUT, symptoms | CLINICAL TRIAL NOT REGISTERED |
| Comez et al 2013107 | Randomized, patient-masked, 2 group contralateral, single-center study | PG 0.3% and PEG 0.4% - Systane SH 15% - Eyestil HPMC - Tears Naturale CMC 0.5% - Refresh Tears |
N = 17 N = 13 |
47.4±14.5 46.3±15.5 |
12 weeks (5x/day) | None | OSDI, Osmolarity, Schirmer’s, TBUT | OSDI, osmolarity, Schirmer’s, TBUT | None | ~30% drop-out CLINICAL TRIAL NOT REGISTERED |
| Craig et al 20214 | Randomized, double-masked, multi-center study | Aminomethylpropanol, HP-guar - Systane Ultra Dimyristoyl phosphatidylglycerol, HP-guar, mineral oil, polyoxl 40 stearate - Systane Complete |
N = 49 N = 50 |
43 ± 17 45 ± 16 |
6 months (4x/day +) |
Lipid thickness | Symptoms, TMH, lipid, osmolarity, hyperaemia, expressibility, blinking | Symptoms (OSDI, DEQ-5, SANDE) NIBUT, LWE Cornea & conjunctival staining |
TMH, osmolarity, hyperaemia, expressibility, blinking | Symptoms improved @1+ month, LWE @ 2+ months, lipid @ 3+ months staining @ 4+ months. 1 in 3 had no benefit in signs or symptoms. Those with lipid layer grade ≤ 3 benefit more from lipid-based drop Clinical Trial ACTRN12619000390189 - additional questionnaire, acuity and lid data presented Cochrane Risk of Bias R+C+M+O+I+S-B+ |
| Dausch et al 200676 | Randomized, investigator-masked, cross-over, multi-center study | Liposomes - Tears Again Carbomer triglycerides - Liposic |
N = 74 with deficient lipid layer |
n=1 <25 years n=9 25–45 years n=16 46–60 years n=49 >60 years |
6 weeks (3x/day) | Symptoms, LIPCOF, TBUT, Schirmer’s, lid margin inflammation with Tears Again | Symptoms, LIPCOF, TBUT, Schirmer’s, lid margin inflammation | - | Photo sequence of phospholipid liposomes sprayed on eyelid reaching ocular surface CLINICAL TRIAL NOT REGISTERED |
|
| Davitt et al 201064 | Randomized, double-masked, single-center study | PEG 400/PG/HP‐guar CMC 0.5% - Optive |
N = 52 N = 53 |
33 x 18–64 years, 19 x ≥65 years 41x 18–64 years, 12 x ≥65 years |
6 weeks (4x/day) | Cornea & conjunctival staining with PEG 400/PG/HP‐guar group | Symptoms, TBUT | Symptoms | TBUT | CLINICAL TRIAL NOT REGISTERED |
| Diaz-Llopis et al 2019108 | Randomized, investigator-masked, multi-center study | Seawater spray - Quinton CMC 0.5% -Viscofresh |
N = 60 N = 60 |
68.1 ± 6.3 66.8 ± 8.4 |
12 weeks (5x/day) | OSDI, IL-1 β and IL-6 with seawater spray |
Cornea & conjunctival staining, Schirmer I, osmolarity, TBUT, TMH | OSDI, Cornea & conjunctival staining | Schirmer I, osmolarity, TBUT, TMH | CLINICAL TRIAL NOT REGISTERED |
| Downie et al 2020109 | Randomized, double-masked, multi-center study | CMC, glycerin, flaxseed oil and castor oil and osmoprotectants (levocarnitine, Erythritol & trehalose) (OM3) Refresh Optive Advanced |
N = 120 N = 122 |
54.3 ± 17.3 52.8 ± 16.7 |
90 days (2x/day +) | Combined corneal / conjunctival staining with OM3 | OSDI TBUT |
OSDI TBUT Combined corneal / conjunctival staining |
None | AEs (OM3 0% vs ROA 4.1%) Clinical Trial NCT02553772 Cochrane Risk of Bias R+C+M+O+I+S+B? |
| Dumbleton et al 2009110 | Randomized, double-masked, single-center study | PEG 400 0.25% - Blink gel tears CMC 1% - Refresh Liquigel |
N = 56 N = 54 |
46.3 ± 19.3 47.2 ± 19.1 |
30 days (3x/day) | Symptoms with PEG | Phenol red test, TMH, NIBUT, hyperaemia, corneal and conjunctival staining | Hyperaemia, corneal and conjunctival staining | No notable AE’s CLINICAL TRIAL NOT REGISTERED |
|
| Essa et al 20183 | Randomized, investigator- masked, crossover, single-center study | SH 0.4% - Clinitas Soothe SH 0.15% - Hyabak Phospholipid liposomes -Tears Again CMC - TheraTears |
N = 50 (for all treatments) | 60.8 ± 14.2 | 4 weeks (drops/day a study variable; average 2–3) | None | OSDI NIBUT FBUT TMH Phenol Red LIPCOF Ocular surface staining Lipid layer grading Osmolarity (baseline visit only) |
OSDI LIPCOF Conjunctival staining |
NIBUT FBUT TMH Phenol Red Lipid layer grading |
Artificial tears performed similarly. However, osmolarity balanced preferred in those with low baseline tear volume and lipisomal spray for those with lipid layer deficiency. Clinical Trial NCT02420834 Cochrane Risk of Bias R?C?M-O?I+S+B? |
| Fogt et al 2019111 | Randomized, observer- masked, crossover, non-dispensing, single-center study | Omega 3 - Refresh Optive MEGA-3 Refresh Optive |
N = 19 with thin lipid |
46.5 ± 8.7 | 60 minutes (Single application) | Lipid layer thickness (overall), Symptoms with MEGA-3 | None | Lipid layer thickness Symptoms |
Symptoms Schirmer’s |
Clinical Trial NCT03380624 - 15 min data missing Cochrane Risk of Bias R?C?M-O?I+S-B? |
| Fondi et al 2018112 | Randomized, patient-masked, crossover single-center study | SH and trehalose - Thealoz Duo HA, trehalose and carbomer - Thealoz Duo Gel |
N = 40 (for both treatment) | 43.7 ± 12.3 | 1 week (actual 3.2 ± 2.6x/day HT & 1.9 ± 2.2x/day HTC-gel) | None | Corneal / conjunctival staining TBUT Sleep quality |
Corneal / conjunctival staining TBUT Sleep quality |
None | Clinical Trial NCT02980913 Cochrane Risk of Bias R?C?M-O?I?S+B? |
| Garcia-Lazaro et al 201167 | Randomized, investigator masked, cross‐over, single-center study | PEG 400 2.5% - Blink Intensive Tears HPMC 0.3% - Artific Tears |
N = 20 | 57.5 ± 8.4 | 1 month (3x/day) | Tear meniscus volume with PEG | None | Tear meniscus volume | None | CLINICAL TRIAL NOT REGISTERED |
| Gensheimer et al 2012113 | Randomized, double-masked, contralateral, non-dispensing, single-center study | Glycerin 1% with PLL-g-PEG - Eyeon PG 0.3% and PEG 0.4% - Systane |
N = 16 | 44.5 | 120 mins (single application) | NIBUT, TBUT with glycerine | None | NIBUT with glycerine | TBUT | CLINICAL TRIAL NOT REGISTERED |
| Gokul et al 201854 | Randomized, double-masked, contralateral, non-dispensing, single-center study | Systane Balance Systane Ultra |
N = 30 | 27 ± 9 | 30 mins (following 2.5 mins in adverse conditions) | Lipid thickness with liposomal Systane Balance | NIBUT | Lipid thickness, NIBUT | Glare acuity, temperature variation, TMH | CLINICAL TRIAL NOT REGISTERED |
| Grene et al 199266 | Randomized, double-masked, single-center study | CMC 1.0% - Celluvisc Lubricant HPMC 0.3% - Tears Naturale 2 |
N=28? N=28? severe |
?? | 2 months (8x/day) | Symptoms, corneal erosions and impression cytology grades with CMC | Schirmer’s Corneal & conjunctival staining Lid & conjunctival swelling |
Corneal staining, Symptoms, impression cytology grade (CMC only) | Schirmer’s | CLINICAL TRIAL NOT REGISTERED |
| Iester et al 200070 | Randomized, open-label?, multi-center, study | HPMC 0.3% HA 0.4% |
N = 55 N = 58 |
56.4 ± 12.8 52.2 ± 10.6 |
2–3 months (6x/day) | Symptoms, Tear ferning Osmolarity, impression cytology With HA |
TBUT Staining Schirmer’s I |
TBUT, staining, Schirmer’s I, symptoms Impression cytology |
- | Ferning, osmolarity and impression cytology only measured in ~33% of sample each CLINICAL TRIAL NOT REGISTERED |
| Jacobi et al 2012114 | Randomized, open-label? Single-center study |
HP-Guar - Systane UD Tamarindus indica seed polysaccharide 1% - VISINE INTENSIV |
N=14 N=14 |
44 ± 8 overall | 3 months (5x/day) | TBUT with HP-Guar | OSDI Schirmer’s II LIPCOF Corneal & conjunctival (rose Bengal) |
TBUT LIPCOF OSDI with HP-Guar |
Schirmer’s II LIPCOF Corneal & conjunctival (rose Bengal) |
CLINICAL TRIAL NOT REGISTERED |
| Jerkins et al 2020115 | Randomized, double-masked, multi-center study | Systane Balance Refresh Optive advanced |
N = 117 N = 114 |
56.7 ± 14.7 55.6 ± 16.4 |
35 days (4x/day) | TBUT with Systane | Symptoms | Symptoms TBUT |
None | 2 lipid based drops Clinical Trial NCT02776670 - exploratory lid wiper epitheliopathy and questionnaire additionally reported Cochrane Risk of Bias R+C+M?O?I-S+B? |
| Johnson et al 200660 | Randomized, double-masked, contralateral, single-center study | SH 0.1% SH 0.3% NaCL 0.9% |
N = 13 (for all treatments) | Range 21–34 | 6 hours (single application) | NIBUT (0.3% SH performed better than 0.1% SH) | Symptoms | Symptoms NIBUT | None | CLINICAL TRIAL NOT REGISTERED |
| Johnson et al 200856 | Randomized, double-masked study, single-center study | Carbomer 934 0.3% - Lacryvisc SH 0.18% - Vismed |
N = 33 N = 32 |
Median 36 Median 39 Range 21–64 |
1 month (drops/day a study variable; median 2.1–2.3) | Corneal & conjunctival staining with SH | Symptoms NIBUT TBUT |
Symptoms Corneal & conjunctival staining |
NIBUT TBUT |
CLINICAL TRIAL NOT REGISTERED |
| Khaireddin and Schmidt, 2010116 | Randomized, multi-center study | HA - Vismed light Phospholipid - Tears Again |
N = 103 N=113 Evaporative |
n=9 <25 years, n=26 25–45 years, n=42 46–60 years, n=139 >60 years |
3 months 3x/day + |
LIPCOF, lid inflammation NIBUT with Tears Again | Schirmer’s | LIPCOF, lid Inflammation NIBUT |
Schirmer’s | CLINICAL TRIAL NOT REGISTERED |
| Khanal et al 2007117 | Randomized, investigator-masked, single-center study | Castor oil 0.1.25% HPMC 0.32% - Artelac Single Dose Unit |
N = 27 N = 26 |
Unclear from text | 1 month (3x/day) |
Tear evaporation with HPMC | Schirmer’s, osmolarity | Tear evaporation; Lipid layer with caster oil |
Schirmer’s, osmolality | CLINICAL TRIAL NOT REGISTERED |
| Labetoulle et al 2018118 | Randomized, double-masked, multi-center study | HP-Guar - HA dual-polymer – Systane Hydration SH 0.15% - Hyabak |
N = 50 N = 49 |
61.7 ± 12.3 56.7 ± 14.3 |
6 weeks (4x/day) | None | Symptoms, TBUT, ocular surface staining | Ocular surface staining | Symptoms, TBUT | Fluorescein dye only Clinical Trial NCT02470429 - exploratory end points additionally reported in n=30 Cochrane Risk of Bias R+C+M?O?I+S+B? |
| Lahia et al 2020119 | Randomized, double-masked, single-center study | Sacha inchi microemulsion (SIME) HA 0.2% |
N = 26 N = 26 |
53.3 ± 12.6 overall |
1 month (3x/day) | Ocular protection index with SIME | Symptoms, Corneal & conjunctival staining, TBUT | Symptoms, osmolarity in hyperosmolar subgroup. Corneal and conjunctival (nasal) staining, TBUT & lid redness only with SIME |
Osmolarity, Conjunctival temporal staining | Fluorescein dye only Clinical Trial NCT03569202 Cochrane Risk of Bias R+C+M+O+I+S+B+ |
| Lee et al 201158 | Randomized, observer-masked, single-center study | CMC 0.5% - Refresh Plus SH 0.1% - Hynex |
N = 33 N = 32 |
39 ± 14.6 37 ± 13.4 |
2 months (6x/day) | None | Corneal & conjunctival staining TBUT Symptoms |
Cornea & conjunctival staining TBUT Symptoms |
None | Fluorescein staining only CLINICAL TRIAL NOT REGISTERED |
| Lievens et al 201952 | Randomized, double-masked, multi-center study | CMC 1.0% and glycerin 0.9% CMC 1.0% |
N = 94 N = 94 |
≥ 18 years of age | 1 month (2x/day +) | Symptoms Corneal staining TBUT With CMC-GLY at day 7 only |
Symptoms Corneal staining TBUT at all other time points |
Symptoms, corneal staining, and TBUT | None | Clinical Trial NCT02280473 Cochrane Risk of Bias R+C+M+O+I+S+B+ |
| Marner et al 199671 | Randomized, open-label, crossover, multi-center study | Carbomer gel - Lubrithal PVA 1.4% - Lacril/Liquifilm |
N=54 (for all treatment) | 64.3, range 38–89 | 2 weeks (drops/day a study variable (carbomer 3.9 vs PVA 4.6x) | Symptoms, TBUT. Instillation frequency with carbomer | Schirmer’s I Ocular surface staining, Corneal sensitivity |
Schirmer’s I, TBUT, ocular surface staining, symptoms | None | Rose Bengal only used AEs 33% with carbomer, 8% with PVA CLINICAL TRIAL NOT REGISTERED |
| Mihaltz et al 201843 | Randomized, investigator-masked, single-center study | Carbomer, triglycerides - Artelac Lipids UD SH - Artelac Splash Edo UD |
N=10 N=13 |
55.5 ± 11.3 53.8 ± 17.9 |
3 months (4x/day +) | None | Schirmer’s, TBUT, Ocular surface staining Symptoms MG dropout aberrations |
Schirmer’s, TBUT, Ocular surface staining | None | Lipid drops better for those with >50% MG dropout improving Schirmer’s & aberrations CLINICAL TRIAL NOT REGISTERED |
| Muntz et l 202055 | Randomized, double-masked, contralateral crossover, single-center study | Lipid, PG, HP-guar and mineral oil - Systane Complete PEG 400, PG and HP-guar - Systane Ultra |
N = 28 (for all treatments) | 29 ± 9 | Single application – adverse environment | Symptoms, lipid layer quality, NIBUT with Systane complete | TMH Hyperaemia |
Symptoms, NIBUT, Lipid layer quality only with Systane Complete | TMH Hyperaemia |
Clinical Trial ACTRN12619000361101 Cochrane Risk of Bias R+C+M+O+I+S+B? |
| Nelson and Farris, 1998120 | Randomized, double-masked, multi-center study | PVA 1.4% - Liquifilm SH 0.1% |
N = 16 N = 20 |
52.3 ± 16.4 64.8 ± 10.8 |
8 weeks 8x/day + |
- | Symptoms, Osmolality, TBUT, rose bengal staining, Schirmer’s I, impression cytology | Symptoms, Osmolality, TBUT, rose bengal staining, Schirmer’s I | Impression cytology | CLINICAL TRIAL NOT REGISTERED |
| Ousler et al 200765 | Randomized, double-masked crossover, single-center study | PEG & HP-Guar - Systane CMC - Refresh Tears CMC - Refresh Endura |
N = 50 | 62.7 | Single application | TBUT, Ocular protection index with Systane | Blink rate | No comparison presented | No difference between CMC products CLINICAL TRIAL NOT REGISTERED |
|
| Park et al 201759 | Randomized, investigator-masked, multi-center study | SH 0.1% SH 0.15% SH 0.3% Cyclosporine 0.05% |
N = 43 N = 41 N = 47 N = 45 |
44.1 ± 13.9 46.2 ± 14.0 44.8 ± 16.2 45.2 ± 15.4 | 12 weeks (5–6x/day) | Schirmer’s (0.15% SH group) | Corneal & conjunctival staining TBUT |
Corneal & conjunctival staining TBUT |
Schirmer’s | AEs 13% 0.1% SH. 20% 0.15% SH, 13% 0.3% SH, 31% 0.05% CS group. Clinical Trial KCT0001796 Cochrane Risk of Bias R+C?M-O?I+S+B? |
| Perez-Balbuena et al 201647 | Randomized, double-masked, multi-center study | Xanthan gum 0.09% and chondroitin sulfate 0.1% PEG 400 0.4% and PG 0.3% |
N = 76 N = 72 |
49.9 ± 16.0 45.5 ± 12.7 |
2 months (4x/day) | None | Schirmer’s, TBUT, Symptoms, Corneal & conjunctival staining | Schirmer’s, TBUT, Symptoms | Corneal & conjunctival staining | Clinical Trial NCT01657253 Cochrane Risk of Bias R+C?M+O?I+S+B+ |
| Pinto-Bonilla et 201542 | Randomized, open-label, crossover, single-center study | Trehalose and SH 1.5mg/mL -Thealoz Duo PEG & HP-guar - Systane |
N = 9 N = 8 |
45.3 ± 11.8 53.8 ± 14.6 |
1 week (5x/day) (Actual 3.7±0.9 / 3.5±0.9) | None | Symptoms, Corneal & conjunctival staining, Schirmer’s, TBUT | Symptoms | Schirmer’s TBUT, Corneal & conjunctival staining | CLINICAL TRIAL NOT REGISTERED |
| Postorino et al 201853 | Randomized, investigator-masked, single-center study | HA crosslinked + CoQ10 HA 0.15% crosslinked |
N = 20 N = 20 |
60.2 ± 13.6 60.9 ± 12.5 |
3 months (4x/day) | Symptoms, MGD assessment, corneal / conjunctival staining, epithelial hyperreflectivity and keratocytes with HA + CoQ10 | Symptoms, corneal aesthesiometry TBUT | OSDI MGD assessment, corneal / conjunctival staining, epithelial hyperreflectivity and keratocytes with HA + CoQ10 only |
Corneal aesthesiometry TBUT | Fluorescein staining only Clinical Trial NCT03074344 - meibomian gland assessment and confocal additionally reported Cochrane Risk of Bias R+C+M-O?I?S-B? |
| Pult et al 202177 | Randomized, double-masked, crossover, multi-center study | Phospholipid 0.98% - Tears Again Phospholipid 0.12% - Ocuvers |
N=30 (all treatments) | 33.2±1.8 | Single application | Symptoms, NIBUT with high concentration lipid | None | Symptoms, NIBUT with high concentration lipid only | None | CLINICAL TRIAL NOT REGISTERED |
| Robert et al 201668 | Randomized, investigator masked, multi-center study | Cationic Emulsion (Cation Norm) SH 0.18% - Vismed |
N = 37 N = 37 Moderate to severe |
60.0 ± 14.6 65.3 ± 11.1 |
3 months (4x/day) | Symptoms at 1 month with SH | TBUT, Schirmer’s, Corneal & conjunctival staining, Osmolarity, Impression cytology | Symptoms, Corneal & conjunctival staining | Schirmer’s, TBUT, Osmolarity, Impression cytology | AE’s 18% CE, 27% HS >10% drop-out Clinical Trial EudraCT 2011-A00955-36 Cochrane Risk of Bias R+C?M-O?I+S+B? |
| Safarzadeh et al 2017121 | Randomized patient-masked, single-center study | Dextran 70, 1 mg/mL and HPMC – Tears Naturale Dextran 70, 0.1 mg/mL and 0.3 g HPMC – Tearlose |
N = 41 N = 47 |
44.1 ± 6.3 45.8 ± 8.4 |
4 weeks (2x/day) | None | Symptoms, TBUT, Schirmer’s Corneal & conjunctival staining |
Symptoms, TBUT, Corneal & conjunctival staining | Schirmer’s | Fluorescein staining only CLINICAL TRIAL NOT REGISTERED |
| Sanchez et al 201739 | Randomized, investigator-masked, single-center study | CMC 0.5% (Viscofresh) HA 0.15% (Lubristil) |
N = 7 N = 8 |
51.8 ± 14.1 71.8 ± 12.2 |
1 month (4x/day) | TBUT, corneal staining, and HLA-DR with CMC | Schirmer’s Other inflammatory markers |
HLA-DR, TBUT & corneal staining with CMC | Schirmer’s, Tear clearance, | No Aes CLINICAL TRIAL NOT REGISTERED |
| Schmidl et al 201551 | Randomized, double-masked, single-center study | Trehalose and SH 1.5mg/mL -Thealoz Duo SH, 0.15% - Hyabak NaCL 0.9% - Hydrabak |
N = 20 N = 20 N = 20 |
43.6 ± 13.3 42.9 ± 12.0 41.8 ± 9.9 |
240 minutes Single application |
Tear film thickness (SH+trehalose to 240min and SH to 40min only) | TBUT, Schirmer’s | Tear film thickness (both SH products) | TBUT, Schirmer’s | CLINICAL TRIAL NOT REGISTERED |
| Simmons and Vehige, 200778 | Randomized, double-masked, crossover and parallel groups, multi-center studies | CMC 1.0% CMC 0.5% (Refresh Tears) |
N = 43 single application Parallel N = 53 N = 50 |
Mean 62 Not stated |
60 minute (single-application) 1 month (4x/day) |
Ocular protection index (low viscosity to 20min, high viscosity to 30min). Corneal & conjunctival staining with higher viscosity |
Symptoms | Symptoms, Corneal and conjunctival staining | None | Fluorescein staining only. More AEs with high viscosity – visual disturbance 23vs4%; discharge 13vs2% CLINICAL TRIAL NOT REGISTERED |
| Simmons et al 201579 | Randomized, investigator-masked, multi-center study | CMC (Refresh Optive Advanced Sensitive), unit dose CMC (Refresh Optive Sensitive), unit dose CMC (Refresh Optive Advanced Sensitive), multi-dose CMC (Refresh Optive Sensitive), multi-dose |
N = 105 N = 103 N = 51 N = 56 |
54.4 ± 14.8 55.8 ± 14.1 55.2 ± 14.5 53.5 ± 13.9 |
30 days (2x/day +) | None | Symptoms, TBUT, Corneal & conjunctival staining Schirmer’s |
OSDI TBUT |
Corneal & conjunctival staining, Schirmer’s | No clinically significant differences in safety, effectiveness, and acceptability between lipid and aqueous artificial tears Clinical Trial NCT01459588 Cochrane Risk of Bias R+C?M-O?I+S+B? |
| Simmons et al 201549 | Randomized, double-masked, multi-center study | CMC 0.5% + 0.1% HA (Optive Fusion) CMC 0.5% + 0.15HA CMC 0.5% (Refresh Tears) |
N = 87 N = 87 N = 90 |
59.6 ± 14.5 59.2 ± 16.3 60.0 ± 13.3 |
3 months (2x/day +) (actual 4.3, 3.9, 3.8x/day) |
Some symptoms with Fusion Corneal staining with Fusion vs Refresh |
Conjunctival staining | Symptoms, Corneal & Conjunctival staining | None | Investigational formulations Clinical Trial NCT01294384 - visual disturbance questionnaire additionally reported Cochrane Risk of Bias R+C+M+O?I+S-B? |
| Szegedi et al 2018122 | Randomized, patient-masked, single-center study | SH 0.18% + triglycerides, and phospholipids SH 0.18% - Vismed sodium chloride 0.9% - Hydrabak |
N = 20? N = 20? N = 20? |
34.6 ± 11.7 40.5 ± 9.9 39.2 ± 12.8 |
40 minutes Single-application |
Tear film thickness 40min vs 20min vs 0min with phospholipids | TBUT, Corneal staining, Lipid thickness | Tear film thickness, TBUT, Corneal staining, Lipid thickness | None | Clinical Trial NCT03161080 Cochrane Risk of Bias R?C?M-O-I+S+B? |
| Tomlison et al 2013123 | Randomized, double-masked, crossover, single-center study | CMC 0.5% - Refresh Tears CMC 0.5%/castor oil - Optive Plus Glycerin 1%/castor oil - Refresh Ultra |
N = 18 with dry eye N = 19 controls For all treatments |
41 ± 14 30 ± 12 |
2 weeks 3x/day |
Evaporation for both CMCs | Symptoms, TBUT, NIBUT (except for controls), osmolarity | Symptoms, evaporation, TBUT, NIBUT (except for controls), osmolarity |
Lipid thickness | Measures taken after adaptation to environmental centre CLINICAL TRIAL NOT REGISTERED |
| Troiano and Monaco, 200846 | Randomized, patient-masked, crossover, single-center study | HA 0.4% 300mOsm/L HA 0.4% 150mOsm/L |
N = 28 For all treatments |
55.5 ± 7.3 | 7 days 4x/day |
Foreign body and dryness symptoms and ocular surface staining with 150mOsm/L | None | Symptoms, hyperaemia, ocular surface staining | None | Reducing osmolarity effective Rose Bengal staining only CLINICAL TRIAL NOT REGISTERED |
| van Setten et al 2020124 | Substitution, open-label, multi-center study | High molecular weight HA 0.15% - Comfort Shield Over habitual controls |
N = 44 N = 40 |
57.7 ± 14.4 59.5 ± 12.5 |
8 weeks Actual 8.2 vs 6.5 |
Symptoms, Visual acuity, nerve fibre length with high molecular weight HA |
Corneal staining, TBUT, Schirmer’s, Lid wiper epitheliopathy, mucotaneous junction, osmolarity | Change from habitual optimal artificial tears. No change with controls CLINICAL TRIAL NOT REGISTERED |
||
| Waduthantri et al 2012125 | Randomized, double-masked, single-center study | CMC 0.5% - Refresh Tears PEG 400 0.4% / PG/HP-guar 0.3% - Systane Ultra |
N = 15 N = 15 |
54.7 ± 5.8 55.9 ± 5.3 |
6 weeks 4x/day |
None | Symptoms Schirmer’s TBUT, Corneal staining |
Symptoms | Schirmer’s TBUT, Corneal staining |
Clinical Trial NCT00796926 - meibography, osmolarity and tear meniscus height not reported Cochrane Risk of Bias R+C?M+O+I+S-B+ |
| Wang et al 200773 | Randomized, open label, single-center study | Carbomer - Vidisic Ophthalmic Gel Cellulose - Artelac Ophthalmic Solution Mineral oil (lanolin) -Duratears Ointment |
N = 22 N = 23 N = 22 |
55.9 ± 15.7 50.1 ± 14.3 60.3 ± 11.2 |
4 weeks (4x/day for Carbomer and Cellulose) (1x/day before sleep for mineral oil) |
Schirmer’s with Carbomer and Cellulose & TBUT with Carbomer | Schirmer’s | Symptoms, TBUT, Schirmer’s | Fluorescein staining only, but not reported in results CLINICAL TRIAL NOT REGISTERED |
|
| Wang et al 2010126 | Randomized, open label, single-center study | Carbomer + lipid gel - Liposic Ophthalmic Liquid Gel HP-guar gel - Systane Lubricant Eye Drops |
N = 15 N = 15 |
40.4 ± 15.0 49.5 ± 12.2 |
2 months (4x/day) | Symptoms & Schirmer’s with Carbomer + lipid | TBUT | Symptoms Schirmer’s TBUT |
None | Fluorescein staining only, but not analysed in results CLINICAL TRIAL NOT REGISTERED |
| Xiao et al 200872 | Randomized, investigator-masked, single-center study | Carbomer‐based 0.4% gel CMC 1.0% |
N = 30 N = 30 |
46.7 ± 2.3 46.6 ± 2.1 |
3 months 3x/day + |
Symptoms, TBUT, Schirmer’s, corneal staining, ocular residence time with carbomer gel | None | Symptoms, TBUT, Schirmer’s corneal staining (but no statistics presented) | None | Method relating to precorneal residence time missing. Fluorescein staining only. CLINICAL TRIAL NOT REGISTERED |
Notes: Grey box = no statistical comparison made; ? = not certain from paper. Cochrane Risk of Bias Tool Rating:35 “+”: low risk; “-”: high risk; “?”: unclear risk; for Random sequence generation (selection bias), allocation Concealment (selection bias); Masking of participants/researchers (performance bias), masking of Outcome assessment (detection bias), Incomplete outcome data (attrition bias), Selective reporting (reporting bias), other Biases (respectively). CLINICAL TRIAL NOT REGISTERED - based on a search of the main registries and paper. © Aston University.
Abbreviations: CoQ10, coenzyme Q10; CMC, carboxymethylcellulose/ carmellose sodium; HA, hyaluronic acid/ hyaluronan; HPMC, Hydroxypropyl methylcellulose; HP-Guar, Hydroxypropyl guar; NaCL, sodium chloride; NIBUT, non-invasive tear breakup time; PEG, polyethylene glycol; PG, propylene glycol; PVA, polyvinyl alcohol; SH, sodium hyaluronate; TBUT, fluorescein tear breakup time; TMH, tear meniscus height; LIPCOF, Lid parallel conjunctival folds.
All studies are prospective (as expected) and involve parallel groups (unless stated otherwise) of dry eye patients (diagnosed using National Eye Institute, arbitrary or recently TFOS DEWS II criteria). However, less than half (20 out of 42) are registered with a clinical trials database and even those that are have high risk of bias characteristics,35 hence the certainty of the result is generally low. The lack of a definitive severity classification has been identified as a factor in differentiating the effectiveness of the available artificial tears,31 but previous attempts at a severity matrix table in TFOS DEWS I36 led to patients being graded at different levels of severity by different tests and was abandoned in TFOS DEWS II;37 severity to a dry eye patient is based on symptoms whereas it is more likely to be based on signs on the ocular surface to a cataract surgeon for example. While the intention of many of the analysed studies is to demonstrate non-inferiority compared to an established treatment, some are underpowered (see TFOS sample size recommendations)37 and/or both eyes included without accounting for the correlation between the two eyes38 of an individual.39–43 In most studies, fluorescein sodium is used for assessing corneal staining (although an appropriate blue light with a peak around 395nm [not cobalt blue whose peak is ~450nm] and yellow filter with a cut off around 500nm is often not stated).44 Most studies use lissamine green for conjunctival staining (unless otherwise stated in Table 1) which is the recommended practice,37 but few state the brand which can dramatically affect the staining observed.45 Some studies46,47 report differences even when they do not meet the standard criteria of p < 0.05 and therefore any “difference” should be considered as noise in the data. While many trials comparing artificial tears are manufacturer initiated or sponsored, unless the research was conducted by the company or not conducted by a reputable research organisation, this should not lead to concerns regarding bias.
From the studies summarised to date (with the caveat that the effects might be affected by dry eye severity and full artificial tear formulation as well as the patient demographic) it would appear from direct comparisons between artificial tears that:
- Combination formulations are more effective than single active ingredient artificial tears.
Some studies suggest sodium hyaluronate could be more effective than CMC40 and carbomers,56 while others find no difference,57,58 the optimal percentage is not clear.59,60
PEG containing artificial tears are more effective than those containing CMC61–65 and HPMC.66,67
Cationic formulations are more effective than sodium hyaluronate (for objective signs)68 and polyvinyl alcohol.69
Hyaluronic acid containing artificial tears might be better than those with HPMC,70 but worse than those with CMC.39
Carbomer containing artificial tears might be more effective than those based on PVA71 or CMC72 or cellulose/mineral oils,73 but less56,74 or as effective43 as sodium hyaluronate.
Most studies recommend 4x/day use, but reported/measured use is generally less than that advised.42
Long-term compliance is needed to improve ocular surface signs rather than just symptoms4 and symptoms benefit from 4x/day compared to “as needed” dosing.75
Higher liposomal concentration increases effectiveness.76,77
Lower osmolarity drops increase the effectiveness of an artificial tear drop.46
Higher concentration (viscosity) CMC is more effective in reducing corneal and conjunctival staining, but caused more reports of visual disturbance.78
While drops targeting individual layers of the tear film seem equally effective,41,79 studies have shown that the most effective drop for an individual can be predicted from their baseline classification; drops containing phospholipids are more effective in those with evaporative dry eye3,4 and osmoprotectants benefit those with high tear film osmolarity.3
Artificial tears may not be effective for as much as one-third of patients, but this can be predicted by one month of compliant use.4
These findings can inform clinical dry eye practice; in summary: non-preserved or soft preserved artificial tears being appropriate to prescribe to patients, regardless of the severity of their DED; patients with evaporative dry eye should be prescribed artificial tears containing a high concentration of liposomes; one month’s compliant use 4x/day is recommended to determine whether an artificial tear can manage the patients’ symptoms in the longer-term; signs of ocular surface disease typically take up to 4 months to start improving so patience is needed; artificial tears with multiple active ingredients (especially with PEG) seem to outperform more basic previous generation drops; ability to use different types of artificial tear bottles/sprays varies9 and should be part of the prescribing consideration. While the efficacy of artificial tears is well established for managing DED, its use in ocular surface disease without symptoms to improve post-surgical symptomology and to reduce refractive ‘surprises’ from poor ocular biometry80 is less well established. The data available as reviewed in this study is limited by the definition of dry eye disease applied in published studies being variable as well as the disease severity examined and compliance with artificial tears being rarely quantified.
Other Therapeutic Functions of Artificial Tears
As well as being a management option for dry eye disease and the ocular surface, artificial tears can also be utilised for a wide range of therapeutic functions such as in the treatment of anterior eye trauma, infection, inflammation and disease as well as contact lens management.
Corneal Abrasion and Wound Healing
Corneal abrasions can be caused by foreign bodies, trauma, and trichiasis, and may result in pain, redness, lacrimation, and photophobia. Artificial tears improve epithelial healing.81 Ideally, preservative free drops are used as they tend to be associated with better ocular surface health and tolerability.82 The most common treatment for perioperative corneal abrasions is artificial tears followed by a combination of artificial tears and antibiotic ointment.83 Most artificial tears contain hydrogels; these are known to activate the epidermal growth factor (EGF) receptor which promotes the healing of corneal epithelial wounds.84
Pain and Inflammation Management
Artificial tears are commonly used in the management of ocular pain and inflammation. In the treatment of episcleritis, the combination of artificial tears and cold compresses provide symptomatic relief.85 No significant differences have been observed in the signs or symptoms of idiopathic episcleritis when either artificial tears or topical ketorolac (NSAID) is used.86 Following photorefractive keratectomy (PRK) surgery, the application of preservative-free artificial tears reduces postoperative ocular discomfort and increases visual recovery.87 Cooled artificial tears have been shown to reduce corneal and conjunctival sensation, with 4°C being the most comfortable temperature.88 In contrast to this, Bitton et al found no improvement in perceived patient comfort when refrigerated Systane Ultra artificial tears were used for mild to moderate dry eye sufferers.89 It is also worth noting that pain complaints can be associated with contrasting subjective responses,90 and in some patients artificial tears are not effective in relieving uncomfortable symptoms.91
Conjunctivitis
Allergic conjunctivitis causes ocular itching, watery discharge, lid oedema and conjunctival chemosis. Bilkhu et al exposed 18 participants (who had a known allergy to grass pollen) to grass pollen, and found that artificial tears and cold compresses improved the signs of allergic conjunctivitis and provided symptomatic relief.92 However, if symptoms are persistent, short-term use of topical antihistamines and mast cell stabiliser drops is recommended.93
Viral (non-herpetic) conjunctivitis causes redness, discomfort, and watering. Follicles on the palpebral conjunctiva and punctate epithelial lesions on the cornea may also be observed. It has been shown that 0.5% topical ketorolac,94 0.45% ketorolac tromethamine,95 and 1% prednisolone acetate96 are no better in relieving signs or symptoms of viral conjunctivitis compared to artificial tears.
Bacterial conjunctivitis causes redness, discomfort, and produces a sticky discharge with crusting of the eyelids. Bacterial conjunctivitis usually self-resolves, but the application of artificial tears and eye bathing aids ocular comfort and hygiene. If bacterial conjunctivitis persists after 3–4 days, the application of topical antibiotics is usually recommended.97
Keratitis
Keratitis is an inflammation of the cornea and has several different aetiologies including viral (Herpes Simplex), bacterial (marginal keratitis), fungal, contact-lens associated and unprotected exposure to ultraviolet radiation (photokeratitis). In dry eye and photokeratitis,98 the application of artificial tears has been recommended. In herpetic keratitis, marginal keratitis, fungal keratitis, and contact-lens associated keratitis, artificial tears are advised (for lubrication and symptomatic relief) alongside additional treatment such as topical antivirals, topical and/or oral antibiotics, and antifungals.
Contact Lens Rewetting and Removal
Contact lens wearers commonly use preservative free artificial tears for ocular lubrication, comfort and contact lens rehydration.99–101 Towards the end of wear, contact lenses become drier and fit tighter. The application of artificial tears reduces friction against the cornea and can facilitate safe lens removal.
Foreign Body Removal
Corneal foreign bodies can cause irritation, lacrimation, blurred vision, and redness. Loose foreign bodies can be irrigated away with normal saline or artificial tears. Upon successful removal of a foreign body, prophylactic antibiotics,102 analgesia and artificial tears are advised.103
Summary
Artificial tears are the mainstay of DED management, but also have a role in corneal abrasion and wound healing, pain and inflammation management, conjunctivitis, keratitis, contact lens rewetting and removal, and foreign body removal. A review of randomized controlled trials comparing artificial tears identified 64 papers. There is good evidence that artificial tears improve symptoms of DED within a month of regular use, applied ~4x a day, but signs generally take several months. Not all patients with DED benefit from artificial tears, so if there is no benefit over a month, alternative management should be considered. Combination formulations are more effective than single active ingredient artificial tears. PEG containing artificial tears are more effective than those containing CMC and HPMC. Those classified as having evaporative DED, benefit from artificial tears with liposomes, especially of higher concentration.
Disclosure
JSW is on the executive of the Tear Film and Ocular Surface Society and the Aston University Optometry Research Group have received research funding from Alcon, the Eye Doctor, Scope Ophthalmic and Thea Pharmaceuticals. No funding was received to conduct this review. The authors report no other conflicts of interest in this work.
References
- 1.Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocular Surface. 2017;15(3):575–628. doi: 10.1016/j.jtos.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2016(2):Cd009729. doi: 10.1002/14651858.CD009729.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essa L, Laughton D, Wolffsohn JS. Can the optimum artificial tear treatment for dry eye disease be predicted from presenting signs and symptoms? Contact Lens Anterior Eye. 2018;41(1):60–68. doi: 10.1016/j.clae.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 4.Craig JP, Muntz A, Wang MTM, et al. Developing evidence-based guidance for the treatment of dry eye disease with artificial tear supplements: a six-month multicentre, double-masked randomised controlled trial. Ocular Surface. 2021;20:62–69. doi: 10.1016/j.jtos.2020.12.006 [DOI] [PubMed] [Google Scholar]
- 5.Hynnekleiv L, Magno M, Vernhardsdottir RR, et al. Hyaluronic acid in the treatment of dry eye disease. Acta Ophthalmol. 2022;100:844–860. doi: 10.1111/aos.15159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocular Surface. 2017;15(3):511–538. doi: 10.1016/j.jtos.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 7.Dietlein TS, Jordan JF, Lüke C, Schild A, Dinslage S, Krieglstein GK. Self‐application of single‐use eyedrop containers in an elderly population: comparisons with standard eyedrop bottle and with younger patients. Acta Ophthalmologica. 2008;86(8):856–859. doi: 10.1111/j.1755-3768.2007.01155.x [DOI] [PubMed] [Google Scholar]
- 8.Connor A, Severn P. Force requirements in topical medicine use—the squeezability factor. Eye. 2011;25(4):466–469. doi: 10.1038/eye.2011.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drew T, Wolffsohn JS. Usability of prostaglandin monotherapy eye droppers. Br J Ophthalmol. 2015;99(9):1251–1254. doi: 10.1136/bjophthalmol-2014-306291 [DOI] [PubMed] [Google Scholar]
- 10.Kashiwagi K. Wide variation of squeezing force and dispensing time interval among eyedropper bottles. J Ophthalmol. 2019:2019:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NHS. Why can’t I get a prescription for an over-the-counter medicine? NHS. Available from: https://www.nhs.uk/common-health-questions/medicines/why-cant-i-get-prescription-over-counter-medicine/. Accessed September 6, 2022. [Google Scholar]
- 12.Bilkhu P, Sivardeen Z, Chen C, et al. Patient-reported experience of dry eye management: an international multicentre survey. Cont Lens Anterior Eye. 2022;45(1):101450. doi: 10.1016/j.clae.2021.101450 [DOI] [PubMed] [Google Scholar]
- 13.Lee S-Y, Tong L. Lipid-containing lubricants for dry eye: a systematic review. Optomet Vision Sci. 2012;89(11):1654–1661. doi: 10.1097/OPX.0b013e31826f32e0 [DOI] [PubMed] [Google Scholar]
- 14.Moshirfar M, Pierson K, Hanamaikai K, Santiago-Caban L, Muthappan V, Passi SF. Artificial tears potpourri: a literature review. Clin Ophthalmol. 2014;8:1419. doi: 10.2147/OPTH.S65263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal P, Khun D, Krösser S, et al. Preclinical studies evaluating the effect of semifluorinated alkanes on ocular surface and tear fluid dynamics. Ocul Surf. 2019;17(2):241–249. doi: 10.1016/j.jtos.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 16.Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retinal Eye Res. 2010;29(4):312–334. doi: 10.1016/j.preteyeres.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 17.Kathuria A, Shamloo K, Jhanji V, Sharma A. Categorization of marketed artificial tear formulations based on their ingredients: a rational approach for their use. J Clin Med. 2021;10(6):1289. doi: 10.3390/jcm10061289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisárčik M, Bakoš D, Čeppan MJC, et al. Non-Newtonian properties of hyaluronic acid aqueous solution. Am J Phys Anthropol. 1995;97(3):197–202. doi: 10.1002/ajpa.1330970209 [DOI] [PubMed] [Google Scholar]
- 19.Arshinoff SA, Hofmann I, Nae H, Surgery R. Role of rheology in tears and artificial tears. J Cataract Refract Surg. 2021;47(5):655–661. [DOI] [PubMed] [Google Scholar]
- 20.Rah MJ. A review of hyaluronan and its ophthalmic applications. Optomet-J Am Optometr Assoc. 2011;82(1):38–43. doi: 10.1016/j.optm.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 21.Balazs E, Freeman M, Klöti R, Meyer-Schwickerath G, Regnault F, Sweeney D. Hyaluronic acid and replacement of vitreous and aqueous humor. Mod Probl Ophthalmol. 1972;10:3–21. [PubMed] [Google Scholar]
- 22.Polack FM, McNiece M. The treatment of dry eyes with Na hyaluronate (Healon®). Cornea. 1982;1(2):133–136. doi: 10.1097/00003226-198201020-00007 [DOI] [Google Scholar]
- 23.Müller-Lierheim WG. Why chain length of hyaluronan in eye drops matters. Diagnostics. 2020;10(8):511. doi: 10.3390/diagnostics10080511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojima T, Nagata T, Kudo H, et al. The effects of high molecular weight hyaluronic acid eye drop application in environmental dry eye stress model mice. Int J Mol Sci. 2020;21(10):3516. doi: 10.3390/ijms21103516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauloin T, Dutot M, Warnet J-M, Rat P. In vitro modulation of preservative toxicity: high molecular weight hyaluronan decreases apoptosis and oxidative stress induced by benzalkonium chloride. Eur J Pharma Sci. 2008;34(4–5):263–273. doi: 10.1016/j.ejps.2008.04.006 [DOI] [PubMed] [Google Scholar]
- 26.Pauloin T, Dutot M, Joly F, Warnet J-M, Rat P. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Molecular Vision. 2009;15:577. [PMC free article] [PubMed] [Google Scholar]
- 27.Wu CL, Chou HC, Li JM, et al. Hyaluronic acid‐dependent protection against alkali‐burned human corneal cells. Electrophoresis. 2013;34(3):388–396. doi: 10.1002/elps.201200342 [DOI] [PubMed] [Google Scholar]
- 28.Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte CJA. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthrit Rheumat. 2004;50(1):314–326. doi: 10.1002/art.11421 [DOI] [PubMed] [Google Scholar]
- 29.Song JK, Lee K, Park HY, et al. Efficacy of carboxymethylcellulose and hyaluronate in dry eye disease: a systematic review and meta-analysis. Korean J Fam Med. 2017;38(1):2–7. doi: 10.4082/kjfm.2017.38.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ang BCH, Sng JJ, Wang PXH, Htoon HM, Tong LHT. Sodium hyaluronate in the treatment of dry eye syndrome: a systematic review and meta-analysis. Sci Rep. 2017;79013. doi: 10.1038/s41598-017-08534-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alves M, Fonseca EC, Alves MF, et al. Dry eye disease treatment: a systematic review of published trials and a critical appraisal of therapeutic strategies. Ocul Surf. 2013;11(3):181–192. doi: 10.1016/j.jtos.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 32.Liu R, Rong B, Tu P, et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. Am J Ophthalmol. 2017;183:81–90. doi: 10.1016/j.ajo.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 33.Yang YJ, Lee WY, Kim YJ, Hong YP. A meta-analysis of the efficacy of hyaluronic acid eye drops for the treatment of dry eye syndrome. Int J Environ Res Public Health. 2021;18(5). doi: 10.3390/ijerph18052383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol. 2021;74(9):790–799. doi: 10.1016/j.rec.2021.07.010 [DOI] [PubMed] [Google Scholar]
- 35.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemp MA, Foulks GN. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2 [DOI] [PubMed] [Google Scholar]
- 37.Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15(3):539–574. doi: 10.1016/j.jtos.2017.05.001 [DOI] [PubMed] [Google Scholar]
- 38.Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt. 2013;33(1):7–14. doi: 10.1111/opo.12009 [DOI] [PubMed] [Google Scholar]
- 39.Sanchez MA, Torralbo-Jimenez P, Giron N, et al. Comparative analysis of carmellose 0.5% versus hyaluronate 0.15% in dry eye: a flow cytometric study. Cornea. 2010;29(2):167–171. doi: 10.1097/ICO.0b013e3181b11648 [DOI] [PubMed] [Google Scholar]
- 40.Brignole F, Pisella PJ, Dupas B, Baeyens V, Baudouin C. Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch Clin Exp Ophthalmol. 2005;243(6):531–538. doi: 10.1007/s00417-004-1040-6 [DOI] [PubMed] [Google Scholar]
- 41.Calvao-Santos G, Borges C, Nunes S, Salgado-Borges J, Duarte L. Efficacy of 3 different artificial tears for the treatment of dry eye in frequent computer users and/or contact lens users. Eur J Ophthalmol. 2011;21(5):538–544. doi: 10.5301/ejo.2011.6324 [DOI] [PubMed] [Google Scholar]
- 42.Pinto-Bonilla JC, Del Olmo-Jimeno A, Llovet-Osuna F, Hernandez-Galilea E. A randomized crossover study comparing trehalose/hyaluronate eyedrops and standard treatment: patient satisfaction in the treatment of dry eye syndrome. Ther Clin Risk Manag. 2015;11:595–603. doi: 10.2147/TCRM.S77091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mihaltz K, Faschinger EM, Vecsei-Marlovits PV. Effects of lipid- versus sodium hyaluronate-containing eye drops on optical quality and ocular surface parameters as a function of the meibomian gland dropout rate. Cornea. 2018;37(7):886–892. doi: 10.1097/ico.0000000000001523 [DOI] [PubMed] [Google Scholar]
- 44.Peterson RC, Wolffsohn JS, Fowler CW. Optimization of anterior eye fluorescein viewing. Am J Ophthalmol. 2006;142(4):572–575. doi: 10.1016/j.ajo.2006.04.062 [DOI] [PubMed] [Google Scholar]
- 45.Delaveris A, Stahl U, Madigan M, Jalbert I. Comparative performance of lissamine green stains. Cont Lens Anterior Eye. 2018;41(1):23–27. doi: 10.1016/j.clae.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 46.Troiano P, Monaco G. Effect of hypotonic 0.4% hyaluronic acid drops in dry eye patients: a cross-over study. Cornea. 2008;27(10):1126–1130. doi: 10.1097/ICO.0b013e318180e55c [DOI] [PubMed] [Google Scholar]
- 47.Perez-Balbuena AL, Ochoa-Tabares JC, Belalcazar-Rey S, et al. Efficacy of a fixed combination of 0.09 % xanthan gum/0.1 % chondroitin sulfate preservative free vs polyethylene glycol/propylene glycol in subjects with dry eye disease: a multicenter randomized controlled trial. BMC Ophthalmol. 2016;16164. doi: 10.1186/s12886-016-0343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aragona P, Benitez-del-Castillo JM, Coroneo MT, et al. Safety and efficacy of a preservative-free artificial tear containing carboxymethylcellulose and hyaluronic acid for dry eye disease: a randomized, controlled, multicenter 3-month study. Clin Ophthalmol. 2020;14:2951–2963. doi: 10.2147/opth.S256480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simmons PA, Liu H, Carlisle-Wilcox C, Vehige JG. Efficacy and safety of two new formulations of artificial tears in subjects with dry eye disease: a 3-month, multicenter, active-controlled, randomized trial. Clin Ophthalmol. 2015;9:665–675. doi: 10.2147/opth.S78184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiambaretta F, Doan S, Labetoulle M, et al. A randomized, controlled study of the efficacy and safety of a new eyedrop formulation for moderate to severe dry eye syndrome. Eur J Ophthalmol. 2017;27(1):1–9. doi: 10.5301/ejo.5000836 [DOI] [PubMed] [Google Scholar]
- 51.Schmidl D, Schmetterer L, Witkowska KJ, et al. Tear film thickness after treatment with artificial tears in patients with moderate dry eye disease. Cornea. 2015;34(4):421–426. doi: 10.1097/ICO.0000000000000358 [DOI] [PubMed] [Google Scholar]
- 52.Lievens C, Berdy G, Douglass D, et al. Evaluation of an enhanced viscosity artificial tear for moderate to severe dry eye disease: a multicenter, double-masked, randomized 30-day study. Contact Lens Anterior Eye. 2019;42(4):443–449. doi: 10.1016/j.clae.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 53.Postorino EI, Rania L, Aragona E, et al. Efficacy of eyedrops containing cross-linked hyaluronic acid and coenzyme Q10 in treating patients with mild to moderate dry eye. Eur J Ophthalmol. 2018;28(1):25–31. doi: 10.5301/ejo.5001011 [DOI] [PubMed] [Google Scholar]
- 54.Gokul A, Wang MTM, Craig JP. Tear lipid supplement prophylaxis against dry eye in adverse environments. Contact Lens Anterior Eye. 2018;41(1):97–100. doi: 10.1016/j.clae.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 55.Muntz A, Marasini S, Wang MTM, Craig JP. Prophylactic action of lipid and non-lipid tear supplements in adverse environmental conditions: a randomised crossover trial. Ocular Surface. 2020;18(4):920–925. doi: 10.1016/j.jtos.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 56.Johnson ME, Murphy PJ, Boulton M. Carbomer and sodium hyaluronate eyedrops for moderate dry eye treatment. Optomet Vision Sci. 2008;85(8):750–757. doi: 10.1097/OPX.0b013e318182476c [DOI] [PubMed] [Google Scholar]
- 57.Baudouin C, Cochener B, Pisella PJ, et al. Randomized, Phase III study comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye disease. Eur J Ophthalmol. 2012;22(5):751–761. doi: 10.5301/ejo.5000117 [DOI] [PubMed] [Google Scholar]
- 58.Lee JH, Ahn HS, Kim EK, Kim T-I. Efficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye disease. Cornea. 2011;30(2):175–179. doi: 10.1097/ICO.0b013e3181e9adcc [DOI] [PubMed] [Google Scholar]
- 59.Park Y, Song JS, Choi CY, et al. A randomized multicenter study comparing 0.1%, 0.15%, and 0.3% sodium hyaluronate with 0.05% cyclosporine in the treatment of dry eye. J Ocular Pharmacol Therap. 2017;33(2):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson ME, Murphy PJ, Boulton M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin Exp Ophthalmol. 2006;244(1):109–112. doi: 10.1007/s00417-005-0028-1 [DOI] [PubMed] [Google Scholar]
- 61.Christensen MT, Cohen S, Rinehart J, et al. Clinical evaluation of an HP-guar gellable lubricant eye drop for the relief of dryness of the eye. Curr Eye Res. 2004;28(1):55–62. doi: 10.1076/ceyr.28.1.55.23495 [DOI] [PubMed] [Google Scholar]
- 62.Benelli U, Nardi M, Posarelli C, Albert TG. Tear osmolarity measurement using the TearLab™ Osmolarity System in the assessment of dry eye treatment effectiveness. Contact Lens Anterior Eye. 2010;33(2):61–67. doi: 10.1016/j.clae.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 63.Cohen S, Martin A, Sall K. Evaluation of clinical outcomes in patients with dry eye disease using lubricant eye drops containing polyethylene glycol or carboxymethylcellulose. Clin Ophthalmol. 2014;8:157–164. doi: 10.2147/opth.S53822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davitt WF, Bloomenstein M, Christensen M, Martin AE. Efficacy in patients with dry eye after treatment with a new lubricant eye drop formulation. J Ocular Pharmacol Therap. 2010;26(4):347–353. doi: 10.1089/jop.2010.0025 [DOI] [PubMed] [Google Scholar]
- 65.Ousler GW, Michaelson C, Christensen MT. An evaluation of tear film breakup time extension and ocular protection index scores among three marketed lubricant eye drops. Cornea. 2007;26(8):949–952. doi: 10.1097/ICO.0b013e3180de1c38 [DOI] [PubMed] [Google Scholar]
- 66.Grene RB, Lankston P, Mordaunt J, Harrold M, Gwon A, Jones R. Unpreserved carboxymethylcellulose artificial tears evaluated in patients with keratoconjunctivities sicca. Cornea. 1992;11(4):294–301. doi: 10.1097/00003226-199207000-00004 [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Lazaro S, Belda-Salmeron L, Ferrer-Blasco T, Cervino A, Montes-Mico R. Comparison of two artificial tear formulations for dry eye through high-resolution optical coherence tomography. Clin Exp Optomet. 2011;94(6):549–556. doi: 10.1111/j.1444-0938.2011.00632.x [DOI] [PubMed] [Google Scholar]
- 68.Robert PY, Cochener B, Amrane M, et al. Efficacy and safety of a cationic emulsion in the treatment of moderate to severe dry eye disease: a randomized controlled study. Eur J Ophthalmol. 2016;26(6):546–555. doi: 10.5301/ejo.5000830 [DOI] [PubMed] [Google Scholar]
- 69.Amrane M, Creuzot-Garcher C, Robert PY, et al. Ocular tolerability and efficacy of a cationic emulsion in patients with mild to moderate dry eye disease - A randomised comparative study. J Francais D Ophtalmologie. 2014;37(8):589–598. doi: 10.1016/j.jfo.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 70.Iester M, Orsoni GJ, Gamba G, et al. Improvement of the ocular surface using hypotonic 0.4% hyaluronic acid drops in keratoconjunctivitis sicca. Eye. 2000;14(6):892–898. doi: 10.1038/eye.2000.244 [DOI] [PubMed] [Google Scholar]
- 71.Marner K, Moller PM, Dillon M, RaskPedersen E. Viscous carbomer eye drops in patients with dry eyes - Efficacy and safety. A randomized, open, cross-over, multicentre study. Acta Ophthalmologica Scandinavica. 1996;74(3):249–252. doi: 10.1111/j.1600-0420.1996.tb00086.x [DOI] [PubMed] [Google Scholar]
- 72.Xiao Q, Hu Y, Chen F, Chen X. A comparative assessment of the efficacy of carbomer gel and carboxymethyl cellulose containing artificial tears in dry eyes. J Huazhong Univ Sci Technol. 2008;28(5):592–595. doi: 10.1007/s11596-008-0523-9 [DOI] [PubMed] [Google Scholar]
- 73.Wang IJ, Lin IC, Hou YC, Hu FR. A comparison of the effect of carbomer-, cellulose-, and mineral oil-based artificial tear formulations. Eur J Ophthalmol. 2007;17(2):151–159. doi: 10.1177/112067210701700202 [DOI] [PubMed] [Google Scholar]
- 74.Baeyens V, Bron A, Baudouin C; Vismed Hylovis Study Group. Efficacy of 0.18% hypotonic sodium hyaluronate ophthalmic solution in the treatment of signs and symptoms of dry eye disease. J Francais D Ophtalmologie. 2012;35(6):412–419. doi: 10.1016/j.jfo.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 75.Asbell P, Vingrys AJ, Tan J, et al. Clinical outcomes of fixed versus as-needed use of artificial tears in dry eye disease: a 6-week, observer-masked phase 4 clinical trial. Invest Ophthalmol Vis Sci. 2018;59(6):2275–2280. doi: 10.1167/iovs.17-23733 [DOI] [PubMed] [Google Scholar]
- 76.Dausch D, Lee S, Dausch S, Kim JC, Schwert G, Michelson W. Comparative study of treatment of the dry eye syndrome due to disturbances of the tear film lipid layer with lipid-containing tear substitutes - efficacy of lipid-containing tear substitutes. Klin Monbl Augenheilkd. 2006;223(12):974–983. doi: 10.1055/s-2006-927266 [DOI] [PubMed] [Google Scholar]
- 77.Pult H, Khatum FS, Trave-Huarte S, Wolffsohn JS. Effect of eye spray phospholipid concentration on the tear film and ocular comfort. Eye Contact Lens-Sci Clin Pract. 2021;47(8):445–448. doi: 10.1097/icl.0000000000000788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simmons PA, Vehige JG. Clinical performance of a mid-viscosity artificial tear for dry eye treatment. Cornea. 2007;26(3):294–302. doi: 10.1097/ICO.0b013e31802e1e04 [DOI] [PubMed] [Google Scholar]
- 79.Simmons PA, Carlisle-Wilcox C, Vehige JG. Comparison of novel lipid-based eye drops with aqueous eye drops for dry eye: a multicenter, randomized controlled trial. Clin Ophthalmol. 2015;9:657–664. doi: 10.2147/opth.S74849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Röggla V, Leydolt C, Schartmüller D, et al. Influence of artificial tears on keratometric measurements in cataract patients. Am J Ophthalmol. 2021;221:1–8. doi: 10.1016/j.ajo.2020.08.024 [DOI] [PubMed] [Google Scholar]
- 81.Prinz J, Mehta JS, Walter P, Fuest M. Simple limbal epithelial transplantation (SLET) A simple technique for the treatment of unilateral complete limbal stem cell deficiency. Video article. Ophthalmologe. 2021;118(4):404–412. doi: 10.1007/s00347-021-01346-z [DOI] [PubMed] [Google Scholar]
- 82.Walsh K, Jones L. The use of preservatives in dry eye drops. Clin Ophthalmol. 2019;13:1409–1425. doi: 10.2147/opth.S211611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Segal KL, Fleischut P, Kim C, et al. Evaluation and treatment of perioperative corneal abrasions. J Ophthalmol. 2014;2014:901901. doi: 10.1155/2014/901901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lozano JS, Chay EY, Healey J, Sullenberger R, Klarlund JK. Activation of the epidermal growth factor receptor by hydrogels in artificial tears. Exp Eye Res. 2008;86(3):500–505. doi: 10.1016/j.exer.2007.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salama A, Elsheikh A, Alweis R. Is this a worrisome red eye? Episcleritis in the primary care setting. J Commun Hosp Intern Med Perspect. 2018;8(1):46–48. doi: 10.1080/20009666.2017.1418110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams CPR, Browning AC, Sleep TJ, Webber SK, McGill JI. A randomised, double-blind trial of topical ketorolac vs artificial tears for the treatment of episcleritis. EYE. 2005;19(7):739–742. doi: 10.1038/sj.eye.6701632 [DOI] [PubMed] [Google Scholar]
- 87.Mohammadpour M, Khorrami-Nejad M, Shakoor D. Role of artificial tears with and without hyaluronic acid in controlling ocular discomfort following PRK: a randomized clinical trial. Int J Ophthalmol. 2021;14(8):1225–1230. doi: 10.18240/ijo.2021.08.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fujishima H, Yagi Y, Shimazaki J, Tsubota K. Effects of artificial tear temperature on corneal sensation and subjective comfort. Cornea. 1997;16(6):630–634. doi: 10.1097/00003226-199711000-00005 [DOI] [PubMed] [Google Scholar]
- 89.Bitton E, Crncich V, Brunet N. Does the temperature of an artificial tear affect its comfort? Clin Exp Optomet. 2018;101(5):641–647. doi: 10.1111/cxo.12664 [DOI] [PubMed] [Google Scholar]
- 90.Galor A, Batawi H, Felix ER, et al. Incomplete response to artificial tears is associated with features of neuropathic ocular pain. Br J Ophthalmol. 2016;100(6):745–749. doi: 10.1136/bjophthalmol-2015-307094 [DOI] [PubMed] [Google Scholar]
- 91.Kim M, Lee Y, Mehra D, Sabater AL, Galor A. Dry eye: why artificial tears are not always the answer. BMJ Open Ophthalmol. 2021;6(1):e000697. doi: 10.1136/bmjophth-2020-000697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bilkhu PS, Wolffsohn JS, Naroo SA, Robertson L, Kennedy R. Effectiveness of nonpharmacologic treatments for acute seasonal allergic conjunctivitis. Ophthalmology. 2014;121(1):72–78. doi: 10.1016/j.ophtha.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 93.Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A. Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis. Cochrane Database Syst Rev. 2015;(6):Cd009566. doi: 10.1002/14651858.CD009566.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shiuey Y, Ambati BK, Adamis AP; Viral Conjunctivitis Study Group. A randomized, double-masked trial of topical ketorolac versus artificial tears for treatment of viral conjunctivitis. Ophthalmology. 2000;107(8):1512–1517. doi: 10.1016/S0161-6420(00)00177-9 [DOI] [PubMed] [Google Scholar]
- 95.Lyra AFV, Bastos LC, Lima RCD, Maranhao LDL, Arantes TE. Artificial tears alone versus 0.45% ketorolac tromethamine with artificial tears for the treatment of acute viral conjunctivitis. Arq Bras Oftalmol. 2014;77(2):99–102. doi: 10.5935/0004-2749.20140025 [DOI] [PubMed] [Google Scholar]
- 96.Santiago LA, da Silva JMR, de Azevedo OGR, de Vasconcelos PRL. Comparative study on the efficacy of non-steroidal, steroid and non-use of anti-inflammatory in the treatment of acute epidemic conjunctivitis. Acta Cirurgica Brasileira. 2019;34(12):e201901206. doi: 10.1590/s0102-865020190120000006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Messmer EM. [Bacterial conjunctivitis--diagnosis and therapy update] Bakterielle Konjunktivitis--Update zu Diagnose und Therapie. Klin Monbl Augenheilkd. 2012;229(5):529–533. doi: 10.1055/s-0031-1299523 [DOI] [PubMed] [Google Scholar]
- 98.Sengillo JD, Kunkler AL, Medert C, et al. UV-photokeratitis associated with germicidal lamps purchased during the COVID-19 pandemic. Ocul Immunol Inflamm. 2021;29(1):76–80. doi: 10.1080/09273948.2020.1834587 [DOI] [PubMed] [Google Scholar]
- 99.Choy CKM, Cho P, Boost MV. Cytotoxicity of rigid gas-permeable lens care solutions. Clin Exp Optomet. 2013;96(5):467–471. doi: 10.1111/cxo.12039 [DOI] [PubMed] [Google Scholar]
- 100.Pucker AD. A review of the compatibility of topical artificial tears and rewetting drops with contact lenses. Contact Lens Anterior Eye. 2020;43(5):426–432. doi: 10.1016/j.clae.2020.04.013 [DOI] [PubMed] [Google Scholar]
- 101.Pucker AD, McGwin G, Franklin QX, Dubey J, Nattis A, Lievens C. Application of systane complete for the treatment of contact lens discomfort. Contact Lens Anterior Eye. 2021;44(4):101399. doi: 10.1016/j.clae.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 102.Chou H-D, Chen K-J, Kang EY-C, et al. Eye irrigation as a first-line treatment and diagnostic method for emergency department patients who complain of ocular foreign bodies. Sci Rep. 2021;11(1):23386. doi: 10.1038/s41598-021-02989-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.NICE. Corneal superficial injury. Available from: https://cks.nice.org.uk/topics/corneal-superficial-injury/. Accessed July 20, 2022.
- 104.Barabino S, Rolando M, Nardi M, Bonini S, Aragona P, Traverso CE. The effect of an artificial tear combining hyaluronic acid and tamarind seeds polysaccharide in patients with moderate dry eye syndrome: a new treatment for dry eye. Eur J Ophthalmol. 2014;24(2):173–178. doi: 10.5301/ejo.5000355 [DOI] [PubMed] [Google Scholar]
- 105.Brodwall J, Alme G, Gedde‐Dahl S, et al. A comparative study of polyacrylic acid (Viscotears®) liquid gel versus polyvinylalcohol in the treatment of dry eyes. Acta Ophthalmologica Scandinavica. 1997;75(4):457–461. doi: 10.1111/j.1600-0420.1997.tb00413.x [DOI] [PubMed] [Google Scholar]
- 106.Bron A, Daubas P, Siou-Mermet R, Trinquand C. Comparison of the efficacy and safety of two eye gels in the treatment of dry eyes: lacrinorm and viscotears. Eye. 1998;12(5):839–847. doi: 10.1038/eye.1998.215 [DOI] [PubMed] [Google Scholar]
- 107.Comez AT, Tufan HA, Kocabiyik O, Gencer B. Effects of lubricating agents with different osmolalities on tear osmolarity and other tear function tests in patients with dry eye. Curr Eye Res. 2013;38(11):1095–1103. doi: 10.3109/02713683.2013.806670 [DOI] [PubMed] [Google Scholar]
- 108.Diaz-Llopis M, Dolores Pinazo-Duran M, Diaz-Guinon L, et al. A randomized multicenter study comparing seawater washes and carmellose artificial tears eyedrops in the treatment of dry eye syndrome. Clin Ophthalmol. 2019;13:483–490. doi: 10.2147/opth.S185409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Downie LE, Hom MM, Berdy GJ, et al. An artificial tear containing flaxseed oil for treating dry eye disease: a randomized controlled trial. Ocul Surf. 2020;18(1):148–157. doi: 10.1016/j.jtos.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 110.Dumbleton K, Woods C, Fonn D. An investigation of the efficacy of a novel ocular lubricant. Eye Contact Lens-Sci Clin Pract. 2009;35(3):149–155. doi: 10.1097/ICL.0b013e3181a2c986 [DOI] [PubMed] [Google Scholar]
- 111.Fogt JS, Fogt N, King-Smith PE, Liu HX, Barr JT. Changes in tear lipid layer thickness and symptoms following the use of artificial tears with and without omega-3 fatty acids: a randomized, double-masked, crossover study. Clin Ophthalmol. 2019;13:2553–2561. doi: 10.2147/OPTH.S228261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fondi K, Wozniak PA, Schmidl D, et al. Effect of hyaluronic acid/trehalose in two different formulations on signs and symptoms in patients with moderate to severe dry eye disease. J Ophthalmol. 2018;2018:20184691417. doi: 10.1155/2018/4691417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gensheimer WG, Kleinman DM, Gonzalez MO, et al. Novel formulation of glycerin 1% artificial tears extends tear film break-up time compared with systane lubricant eye drops. J Ocular Pharmacol Therap. 2012;28(5):473–478. doi: 10.1089/jop.2011.0053 [DOI] [PubMed] [Google Scholar]
- 114.Jacobi C, Kruse FE, Cursiefen C. Prospective, randomized, controlled comparison of SYSTANE UD eye drops versus VISINE INTENSIV 1% EDO eye drops for the treatment of moderate dry eye. J Ocular Pharmacol Therap. 2012;28(6):598–603. doi: 10.1089/jop.2012.0066 [DOI] [PubMed] [Google Scholar]
- 115.Jerkins G, Greiner JV, Tong L, et al. A comparison of efficacy and safety of two lipid-based lubricant eye drops for the management of evaporative dry eye disease. Clin Ophthalmol. 2020;14:1665–1673. doi: 10.2147/opth.S256351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khaireddin R, Schmidt KG. Comparative investigation of treatments for evaporative dry eye. Klin Monbl Augenheilkd. 2010;227(2):128–134. doi: 10.1055/s-0028-1109686 [DOI] [PubMed] [Google Scholar]
- 117.Khanal S, Tomlinson A, Pearce EI, Simmons PA. Effect of an oil-in-water emulsion on the tear physiology of patients with mild to moderate dry eye. Cornea. 2007;26(2):175–181. doi: 10.1097/ICO.0b013e31802b492d [DOI] [PubMed] [Google Scholar]
- 118.Labetoulle M, Schmickler S, Galarreta D, et al. Efficacy and safety of dual-polymer hydroxypropyl guar- and hyaluronic acid-containing lubricant eyedrops for the management of dry-eye disease: a randomized double-masked clinical study. Clin Ophthalmol. 2018;12:2499–2508. doi: 10.2147/opth.S177176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Laihia J, Jarvinen R, Wylegala E, Kaarniranta K. Disease aetiology-based design of multifunctional microemulsion eye drops for moderate or severe dry eye: a randomized, quadruple-masked and active-controlled clinical trial. Acta Ophthalmol. 2020;98(3):244–254. doi: 10.1111/aos.14252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nelson JD, Farris RL. Sodium hyaluronate and polyvinyl-alcohol artificial tear preparations - a comparison in patients with keratoconjunctivitis sicca. Arch Ophthalmol. 1988;106(4):484–487. doi: 10.1001/archopht.1988.01060130530029 [DOI] [PubMed] [Google Scholar]
- 121.Safarzadeh M, Azizzadeh P, Akbarshahi P. Comparison of the clinical efficacy of preserved and preservative-free hydroxypropyl methylcellulose-dextran-containing eyedrops. J Optom. 2017;10(4):258–264. doi: 10.1016/j.optom.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Szegedi S, Scheschy U, Schmidl D, et al. Effect of single instillation of two hyaluronic acid-based topical lubricants on tear film thickness in patients with dry eye syndrome. J Ocular Pharmacol Therap. 2018;34(9):605–611. doi: 10.1089/jop.2018.0069 [DOI] [PubMed] [Google Scholar]
- 123.Tomlinson A, Madden LC, Simmons PA. Effectiveness of dry eye therapy under conditions of environmental stress. Curr Eye Res. 2013;38(2):229–236. doi: 10.3109/02713683.2012.757323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Setten GB, Baudouin C, Horwath-Winter J, et al. The HYLAN M study: efficacy of 0.15% high molecular weight hyaluronan fluid in the treatment of severe dry eye disease in a multicenter randomized trial. J Clin Med. 2020;9(11):3536. doi: 10.3390/jcm9113536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Waduthantri S, Yong SS, Tan CH, Htoon HM, Tong L. Lubricant with gelling agent in treating dry eye in adult Chinese patients. Optomet Vision Sci. 2012;89(11):1647–1653. doi: 10.1097/OPX.0b013e31826cfc41 [DOI] [PubMed] [Google Scholar]
- 126.Wang TJ, Wang IJ, Ho JD, Chou HC, Lin SY, Huang MC. Comparison of the clinical effects of carbomer-based lipid-containing gel and hydroxypropyl-guar gel artificial tear formulations in patients with dry eye syndrome: a 4-week, prospective, open-label, randomized, parallel-group, noninferiority study. Clin Ther. 2010;32(1):44–52. doi: 10.1016/j.clinthera.2010.01.024 [DOI] [PubMed] [Google Scholar]
- 127.Liberati A, Altman DG and Tetzlaff J, et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ, 339(jul21 1), b2700–b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]