Abstract
The Mycoplasma arthritidis mitogen (MAM) superantigen (SAg) is a potent activator of human and murine cells and is produced by an organism that is a cause of acute and chronic arthritis of rodents. It is phylogenetically unrelated to other bacterial SAgs and exhibits a number of unique features. We recently demonstrated that MAM differentially regulates the cytokine responses of different mouse strains following in vivo administration. Here we show that the presence in inbred C3H/HeJ mice of the mutant Lpsd gene, which is associated with a defect in Toll-like receptor 4 (TLR4), influences MAM regulation of cytokine profiles in vivo. Whereas the levels of type 1 cytokines (interleukin-2 [IL-2], gamma interferon, IL-12, and tumor necrosis factor alpha) were depressed in cells from MAM-injected wild-type C3H/HeSnJ mice, they were elevated in cells from C3H/HeJ mice. Furthermore, the levels of type 2 cytokines (IL-4, IL-6, and IL-10) were elevated in Lpsn C3H/HeSnJ mice but depressed in Lpsd C3H/HeJ mice. The transcript for IL-12 p40 was highly expressed in C3H/HeJ but not C3H/HeSnJ mice. F1 mice exhibited the same cytokine profile as C3H/HeJ mice, indicating that the mutant gene exhibited dominant-negative inheritance. In addition, C3H/HeJ mice were highly susceptible to toxic death in comparison with C3H/HeSnJ mice after injection with live M. arthritidis organisms. Our results suggest that MAM interacts with the lipopolysaccharide signaling pathway, possibly involving TLR4 or a combinatorial Toll complex.
Mycoplasma arthritidis induces in rodents an acute to chronic arthritis that can be associated with lethal toxicity following systemic injection or with tissue necrosis resembling necrotizing fasciitis following subcutaneous injection. Toxicity and necrosis are markedly more pronounced in strains that are strongly reactive to M. arthritidis mitogen (MAM) (9, 11). In many respects, MAM is a typical superantigen (SAg); however, it has a number of properties that distinguish it from other bacterial SAgs (8). It has a strong preference for presentation to T cells by H-2E and HLA-DR major histocompatibility complex (MHC) molecules but can also use H-2A and HLA-DQ for presentation (7). In addition, although the T-cell receptor (TCR) Vβ chain has been shown to be predominantly involved in the activation of T cells in response to the stimulatory effects of SAgs, in the case of MAM-induced T-cell activation, Jβ segments as well as the contact points within the CDR3 region of the TCR also influence the reactivity of T cells to MAM (19). MAM can also directly induce proinflammatory cytokines in macrophage cultures and cell lines in the absence of T cells (6), probably by cross-linking the α and β chains of surface MHC molecules (25). In addition, MAM possesses legume lectin motif β, which is involved in lymphocyte activation by lectin mitogens (8). The responsiveness of lymphocytes to the stimulatory effects of MAM depends on the expression of specific MHC class II alleles as well as on the expression of specific TCRs borne on the Vβ-chain segments of the α/β TCR (17, 20, 23).
We recently demonstrated that splenocytes from mice injected with the MAM SAg elicited different cytokine profiles following in vitro challenge with MAM in different strains of mice (28). Whereas cells from BALB/c and C3H/HeJ mice induced similar strong proliferative responses and high cytokine levels in vitro in response to MAM, the cytokine responses in vivo were quite different. In the arthritis-resistant BALB/c mice, the cytokine profile was shifted toward a type 2 pattern (dominated by interleukin-4 [IL-4], IL-6, and IL-10), whereas the profile for the arthritis-susceptible C3H/HeJ mice was shifted toward a type 1 pattern (dominated by IL-2, gamma interferon [IFN-γ], IL-12, and tumor necrosis factor alpha [TNF-α]).
The C3H/HeJ mouse is known to be highly sensitive to infection with gram-negative bacteria due to the failure of lipopolysaccharide (LPS; endotoxin) recognition. LPS, a very potent activator of host leukocytes, stimulates the synthesis and release of TNF-α and other proinflammatory cytokines. Timely recognition of LPS by cells of the innate immune system allows effective clearance of a gram-negative bacterial infection before it becomes widely disseminated. Lack of responsiveness to LPS is thought to be associated with impaired host responses to infection with gram-negative bacteria. The C3H/HeJ mouse carries a spontaneous mutation, Lpsd, that confers resistance to the inflammatory properties of LPS and toxic shock (1, 26, 34, 35, 37). Recent studies have shown that the Lpsd allele is associated with a defect in Toll-like receptor (TLR) 4 (TLR4) which, along with CD14 and LPS binding protein, determines responsiveness to LPS (31, 32). Defects in TLR4 expression are also seen in C57BL/10ScNCr mice, which are also resistant to LPS (31, 33). TLRs, which belong to the IL-1 receptor family, are believed to play a major role in host responses to bacterial products. Whereas LPS uses predominantly TLR4, many biologically active lipoproteins from gram-positive bacteria (43), such as OspA from Borrelia burgdorferi (18) and mycoplasma lipopeptide MALP-2 from Mycoplasma fermentans, use another TLR family member, TLR2 (38).
In view of the unusual properties of MAM and the differential cytokine responses of C3H/HeJ and BALB/c mice to MAM, we investigated whether the Lpsd mutant gene of C3H/HeJ mice might influence the interaction of MAM with the immune system. The data obtained demonstrate that there are profound differences in the in vivo immune responses of wild-type C3H/HeSnJ (Lpsn) and mutant C3H/HeJ (Lpsd) mice to MAM. Furthermore, these differences are associated with an enhanced rate of death in C3H/HeJ mice following injection of live M. arthritidis organisms.
MATERIALS AND METHODS
Mice.
Female C3H/HeJ (H-2k Eα+ Lpsd) and C3H/HeSnJ (H-2k Eα+ Lpsn) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). F1 mice were bred in the Animal Resource Center (ARC) of the University of Utah Health Science Center. C3H/HeN (H-2k Eα+ Lpsn) and C57BL/10ScNCr (H-2b Eα− LPS hyporesponsive) mice were obtained from Clarence Reeder (National Institutes of Health). All mice were maintained in specific-pathogen-free conditions at the ARC and were used at 8 to 12 weeks of age. The ARC guarantees strict compliance with regulations established by the Animal Welfare Act.
MAM and LPS.
LPS-free, homogenous native MAM was prepared as described previously (2) and was stored in aliquots at −70°C. LPS from Escherichia coli O111:B4 was purchased from Difco Laboratories, Detroit, Mich.
Cell culturing for cytokine analysis and enzyme-linked immunosorbent assay (ELISA).
Cells obtained from the spleens of mice were cultured under serum-free conditions. Briefly, mice were anesthetized and sacrificed by cervical dislocation. Single-cell suspensions were prepared from the spleens of these animals. The collected splenocytes were washed three times and cultured at 107 cells/ml in freshly prepared serum-free medium consisting of RPMI 1640, 1% Nutridoma-NS (Boehringer Mannheim Biochemicals, Indianapolis, Ind.), 200 mM l-glutamine, antibiotics, and 5 × 10−5 M 2-mercaptoethanol.
For in vitro induction of cytokines, triplicate suspensions of splenocytes were activated by the addition of 0.1 to 100 ng of MAM/ml, and supernatants were collected after 24 h of incubation at 37°C in a 5% CO2-humidified incubator. Supernatants were harvested, clarified by centrifugation, and stored at 4°C until assayed for specific cytokine content.
For in vivo priming studies, MAM at doses of 0.1 to 100 ng/mouse was injected intravenously (i.v.) into mice. Control mice were given phosphate-buffered saline (PBS) injections. After 90 min, mice were bled by cardiac puncture, and the sera were collected for analysis of cytokines and nitric oxide (NO) production. The splenic tissues were collected at various times after injection for reverse transcription (RT)-PCR, or the splenocytes were cultured in vitro in the presence or absence of MAM (1 ng/ml) 24 or 72 h after injection.
Cell culture supernatants were assayed for quantitative evaluation of cytokines by an ELISA as previously described (28). Monoclonal rat anti-murine cytokine antibodies and murine recombinant cytokine standards were purchased from PharMingen, San Diego, Calif.
RT-PCR for the expression of IL-12 p40 mRNA.
RNA was prepared by the method of Chomczynski and Sacchi (5), and RT-PCR was performed as described by Mu and Sewell (27). PCR was carried out with a model 480 DNA thermal cycler (Perkin-Elmer Cetus, Emeryville, Calif.). PCR conditions were as follows: denaturation at 94°C for 1 min, annealing at 59°C for 30 s, and elongation at 72°C for 30 s. Sixteen cycles were performed for β-actin; 28 cycles were performed for IL-12 p40. Gene-specific sequences were derived from GenBank submissions. Oligonucleotides used for these analyses have been published previously (36).
Preparation of macrophages and analysis of NO production.
Resident peritoneal exudate cells, a major source of peritoneal macrophages (PM) of mice, were harvested and prepared as described elsewhere (29). Adherent cells from spleens (splenic macrophages [SM]) were prepared as described by Ayala et al. (3, 4). Macrophages (2 × 106/ml) were stimulated by the addition of 10 ng of MAM/ml with or without recombinant murine IFN-γ (5 ng/ml; PharMingen). Macrophages stimulated by LPS (100 ng/ml) were also included as a control. Cells were incubated for 48 h at 37°C in 5% CO2. Cell culture supernatants were then collected for quantitative evaluation of NO. For in vivo priming studies, sera collected from mice receiving 10 ng of MAM/mouse at different times were assayed for nitrite (NO2−) plus nitrate (NO3−) contents.
Measurement of levels of NO (NO2− plus NO3−) in cell supernatants or in serum samples was performed as described previously (29). Briefly, culture supernatants were diluted 1:2, and serum was diluted 1:10. Sera were incubated with 4 μl of a solution containing nitrate reductase from Aspergillus species (Boehringer) and NADPH at final concentrations of 20 mU/100-μl test sample and 100 μM/100-μl test sample, respectively, for 45 min at room temperature. Following centrifugation for 10 min at 1,000 × g, nitrate reductase- and NADPH-treated supernatants or sera were placed in a 96-well plate, and a modified Griess assay (Sigma Chemical Co., St. Louis, Mo.) was performed.
Induction and evaluation of pathogenic effects of M. arthritidis in mice.
M. arthritidis strain 14124 P10 (14) was grown in modified Edward medium as previously described (11, 28). Cells were harvested by centrifugation at 27,000 × g, washed once in serum-free medium, suspended in PBS–5% sucrose, and frozen in aliquots at −70°C. Female mice 8 to 12 weeks of age were injected i.v. in groups of 6 to 10 with 5 × 108 CFU of M. arthritidis. Mice were examined for toxicity at days 1, 2, 3, 5, 7, 10, 14, and 28 after injection. Mice were assigned an arbitrary score of 0 to 2 for each of the following characteristics: ruffling of fur, lethargy, and ocular discharge; a maximum score of 8 was assigned upon death. Severity of arthritis was scored as described previously (29). The results were expressed as the mean and standard error of the mean (SEM).
Statistical analysis.
Cytokine concentrations were reported as the mean and SEM. Two-tailed Student's t test values were calculated using the Statview program (Abacus Concepts, Inc., Berkeley, Calif.). A P value of less than 0.05 was considered significant.
RESULTS
Induction of cytokines by MAM in splenic cell cultures from Lpsn (C3H/HeSnJ) and Lpsd (C3H/HeJ) mice.
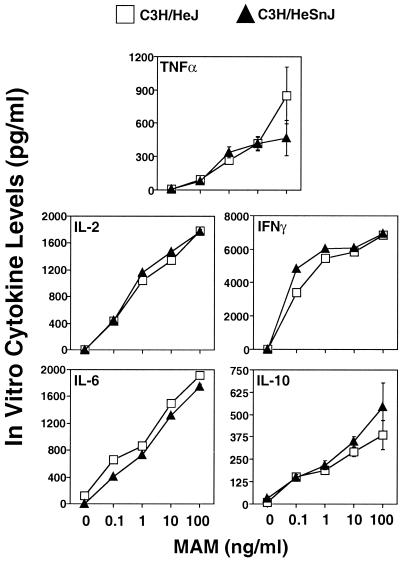
We considered the possibility that the different cytokine responses observed previously (28) for the C3H/HeJ and BALB/c mouse strains with MAM may relate in some way to the fact that C3H/HeJ mice carry the mutant Lpsd gene, which confers hyporesponsiveness to LPS. As far as is known, this mutation and a second mutation, RAN, which also interrupts the LPS signaling pathway (41), probably represent the major difference(s) having an impact on the immune systems of these mice. To this end, we examined the cytokine profiles of splenocytes from naive C3H/HeJ (Lpsd) and C3H/HeSnJ (Lpsn) mice for their ability to produce cytokines upon challenge with MAM in vitro. As shown in Fig. 1, the levels of all cytokines were markedly increased in a dose-dependent manner in cells from both mouse strains. As little as 0.1 ng of MAM per ml elicited detectable amounts of these cytokines. In addition, both strains induced similar cytokine levels and profiles, unlike the responses of cells from these strains to LPS: culture supernatants obtained from C3H/HeJ splenic cells developed little TNF-α and IL-6 in comparison with those obtained from C3H/HeSnJ mice (data not shown).
FIG. 1.
Cytokine responses to MAM in vitro in splenocyte cultures from C3H/HeSnJ and C3H/HeJ mice. Splenocytes (107 cells/ml) were stimulated with or without various concentrations of MAM. Twenty-four hours later, culture supernatants were collected and analyzed by a capture ELISA for TNF-α, IL-2, IFN-γ, IL-6, and IL-10. Splenocytes from three or four mice were included in each experiment for each specific dose point, and results are expressed as the mean and SEM. The data shown are representative of two experiments.
Early cytokine responses induced in vivo by MAM in Lpsn (C3H/HeSnJ) and Lpsd (C3H/HeJ) mice.
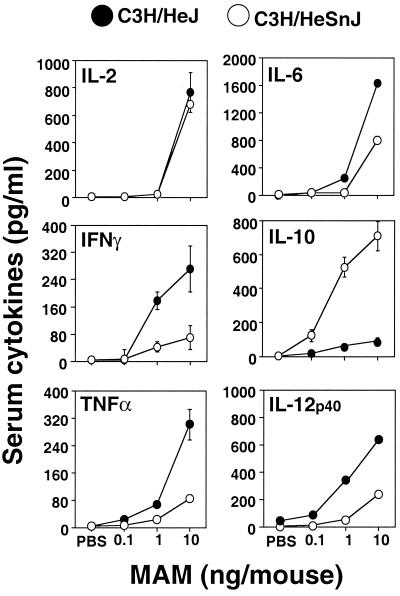
We next determined whether there might be in vivo differences in the cytokine profiles induced in the two strains of mice. The serum cytokine responses of mice 90 min after in vivo administration of MAM at doses of 0.1 to 10 ng or PBS are shown in Fig. 2. As expected, mice injected with PBS failed to elicit serum cytokines (Fig. 2). Ninety minutes after injection of MAM, levels in serum of all cytokines were increased, depending upon the MAM dose given. Cytokines were detectable with as little as 1 ng/mouse. In C3H/HeSnJ mice, injection of 10 ng/mouse elicited significantly elevated serum IL-10 levels (P < 0.05), whereas this cytokine was barely detectable in C3H/HeJ mice. Serum IFN-γ, TNF-α, and IL-12 p40 levels were significantly higher in C3H/HeJ mice than in C3H/HeSnJ mice (Fig. 2). In contrast to the in vitro induction of IL-6 by MAM, serum IL-6 levels were somewhat higher in C3H/HeJ mice than in C3H/HeSnJ mice after injection of 10 ng/mouse (P < 0.05) (Fig. 2). By 3, 6, and 9 h, there were clear increases in the levels of IFN-γ, TNF-α, and IL-12 p40 in the sera of C3H/HeJ mice (data not shown). However, the levels of all of these serum cytokines had largely abated by 24 h postinjection (data not shown).
FIG. 2.
Effect of different doses of MAM on serum cytokine profiles induced in C3H/HeSnJ and C3H/HeJ mice. Mice were injected i.v. with diluent PBS or MAM at a dose of 0.1, 1, or 10 ng/mouse. After 90 min, mice were exsanguinated under anesthesia, and sera were collected for cytokine assays for IL-2, IL-6, IFN-γ, IL-10, TNF-α, and IL-12 p40. Sera from three to five mice were assayed in each experiment for each specific dose point. Similar results were seen in three repeat experiments.
Differential modulation of cytokine responses in vivo by MAM in C3H/HeSnJ and C3H/HeJ mice upon rechallenge with MAM in vitro.
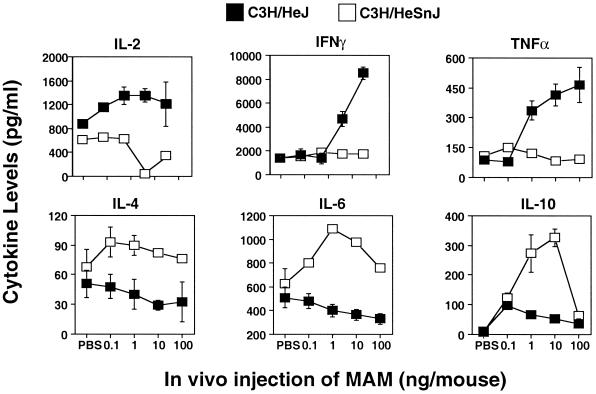
Studies were next conducted to determine whether differences could be seen in the responses of splenocytes to MAM taken from mice after exposure to MAM in vivo for 24 and 72 h prior to rechallenge with 1 ng of MAM/ml in vitro. There were striking differences in the inducible cytokine profiles between C3H/HeJ and C3H/HeSnJ mice at both 24 h (data not shown) and 72 h. The most striking divergence in the ability of C3H/HeJ and C3H/HeSnJ splenocytes to respond to MAM upon in vitro challenge occurred when the splenocytes were harvested following 72 h (Fig. 3) of exposure to MAM in vivo. Thus, with increasing in vivo MAM doses, IL-2, IFN-γ and TNF-α levels in the supernatants of C3H/HeJ splenocytes were all markedly elevated, whereas they were depressed or showed no increase in C3H/HeSnJ supernatants (P was <0.05 for IL-2; P was <0.01 for IFN-γ and TNF-α). In contrast, levels of IL-4, IL-6, and IL-10 were all markedly increased in C3H/HeSnJ cell culture supernatants (P was <0.05) but were decreased or remained low in C3H/HeJ supernatants. Doses as low as 1 ng and, for some cytokines, 0.1 ng of MAM/mouse were usually sufficient to induce a profound change in inducible cytokine profiles, but these changes were mostly optimal with the higher doses of MAM. The results indicate that MAM induces the cytokine profile to a type 1-like response in C3H/HeJ mice but to a type 2-like response in C3H/HeSnJ mice. To confirm that the MAM-induced type 2 cytokine profile was not a peculiarity of the C3H/HeSnJ mice, we tested another C3H Lpsn substrain, C3H/HeN, for responses to MAM. A typical type 2 response was seen (data not shown).
FIG. 3.
Inducible cytokines in cells from MAM-injected mice 3 days postinjection. Splenocytes (107 cells/ml) from C3H/HeJ and C3H/HeSnJ mice injected 72 h previously with MAM (0.1 to 100 ng/mouse) were rechallenged in vitro with a second dose of MAM (1 ng/ml) for an additional 24 h. Inducible cytokines (IL-2, IFN-γ, TNF-α, IL-4, IL-6, and IL-10) were analyzed by an ELISA. Splenocytes from three to five mice were included in each experiment for each specific dose point. The data shown are representative of three experiments.
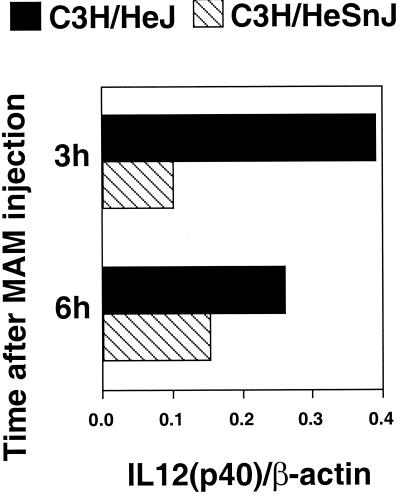
Since it has been documented that IL-12 plays a key role in the induction of type 1 immune responses, the expression of IL-12 p40 mRNA in spleen tissues at various times was examined using RT-PCR. IL-12 p40 expression was more markedly elevated in C3H/HeJ splenic tissue than in C3H/HeSnJ spleens (Fig. 4), confirming the IL-12 p40 results obtained with sera (Fig. 2). Similar results were obtained for C3H/HeN mice (data not shown).
FIG. 4.
In vivo mRNA IL-12 expression induced by MAM in C3H/HeSnJ and C3H/HeJ mice. RT-PCR was performed on mRNA isolated from homogenized spleens obtained from mice 3 and 6 h after injection with 10 ng of MAM/mouse or with PBS (not shown). Ratios of IL-12 p40 to β-actin densitometric values are illustrated. Similar results were seen in three repeat experiments.
Cytokine responses of F1 mice expressing Lpsn/Lpsd.
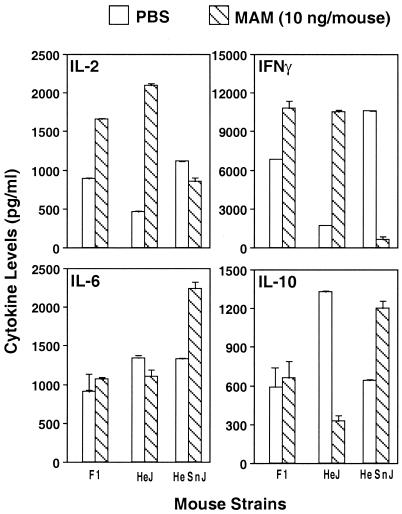
Previous work established that cells from (C3H/HeSnJ × C3H/HeJ) F1 mice, expressing Lpsn/d, behaved similarly to cells from mutant C3H/HeJ mice in that they failed to respond to LPS; this result indicated that the trait encoded by the mutation in TLR4 is inherited as a dominant-negative trait. We therefore tested the responses of cells from these F1 mice to MAM to determine whether MAM regulation of cytokine production was similarly inherited in these mice. The results presented in Fig. 5 show that, as in Lpsd C3H/HeJ mice, the levels of type 1 cytokines were also increased in the F1 progeny, in contrast to the decreased levels of type 1 cytokines in wild-type Lpsn C3H/HeSnJ mice. Hence, cytokine profiles in these mouse strains are also inherited in a dominant-negative fashion.
FIG. 5.
In vivo cytokine profiles exhibited by mouse strains C3H/HeJ and C3H/HeSnJ and their F1 progeny in response to MAM. Splenocytes (107 cells/ml) from mice injected 24 h previously with 10 ng of MAM/mouse were rechallenged in vitro with a second dose of MAM (1 ng/ml) for an additional 24 h. Inducible cytokines (IL-2, IFN-γ, IL-6, and IL-10) were analyzed by an ELISA. Splenocytes from three mice were included in each experiment. The data shown are representative of three experiments.
Differential induction of NO in macrophages from Lpsn and Lpsd mice in response to MAM and LPS.
To begin to identify the cell type(s) that might be responsible for the observed differences in cytokines elicited in splenocyte cultures from Lpsn and Lpsd mice, we examined the macrophage, since it is a major source of inflammatory cytokines. We first confirmed that naive C3H/HeSnJ and C3H/HeJ mice differ in their responses to the effects of LPS by showing that resident PM from C3H/HeSnJ mice produced elevated levels of TNF-α, whereas those from C3H/HeJ mice secreted much lower levels, in response to LPS at 100 ng/ml for 24 h (data not shown). As previously established, C3H/HeSnJ mice were susceptible to toxic death by injection of 10 μg of LPS/mouse (five of five mice dead by 18 h), whereas C3H/HeJ mice were resistant (zero of five mice dead at 72 h).
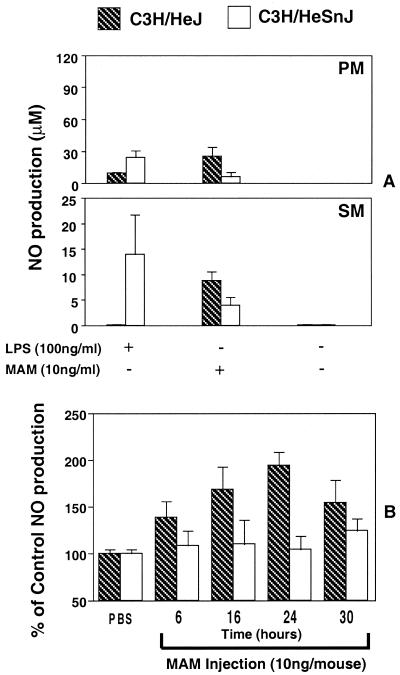
In the next experiment, unstimulated resident PM and SM from naive C3H/HeSnJ and C3H/HeJ mice were prepared. The cells were exposed for 48 h in vitro to LPS (100 ng/ml) in the presence or absence of IFN-γ and to MAM (10 ng/ml), and NO levels in culture supernatants were measured. As expected, LPS induced much higher levels of NO in PM and SM cultures from the LPS-responsive C3H/HeSnJ mice than in the LPS-hyporesponsive C3H/HeJ mice, which showed a low or negative response (Fig. 6A). In contrast, the pattern was reversed when MAM was used, in that levels of NO were significantly elevated in both PM and SM cultures from C3H/HeJ mice compared to those in C3H/HeSnJ cell cultures.
FIG. 6.
(A) In vitro induction of nitric oxide by naive resident macrophages (PM or SM) stimulated by MAM. Macrophages from C3H/HeSnJ and C3H/HeJ mice were prepared, and 2 × 106 cells/ml were stimulated with or without MAM (10 ng/ml) or with LPS (100 ng/ml). Forty-eighty hours later, culture supernatants were collected and analyzed by the Griess assay for NO values. Data were pooled from two experiments. (B) Time course of serum NO production in C3H/HeJ and C3H/HeSnJ mice following injection of MAM. Mice were injected i.v. with MAM at 10 ng or with diluent PBS. After 6, 16, 24, and 30 h, mice were bled and sera were collected for NO analysis. Sera from three mice receiving MAM or PBS were assayed in each experiment for each time point. The results are expressed as the percent change in serum NO levels in mice receiving in vivo MAM versus mice receiving diluent PBS. Thus, control levels (PBS) were set as 100%. The data shown are representative of two experiments and are expressed as the mean and SEM.
Next, we measured NO levels after conversion of NO3− to NO2− in sera at various times following i.v. injection of 10 ng of MAM into C3H/HeJ or C3H/HeSnJ mice. The results are summarized in Fig. 6B and are expressed as a percentage of the levels seen in mice injected with PBS. In sera from C3H/HeSnJ mice, MAM had no significant effect on NO levels. In contrast, levels of NO were substantially elevated at all times (up to 200% at 24 h) in sera from C3H/HeJ mice. Dose-response experiments showed that as little as 0.1 ng of MAM/ml in vitro and 0.1 ng/mouse in vivo were sufficient to induce significant levels of NO in PM in vitro as well as in sera of injected C3H/HeJ mice (data not shown).
Thus, as for the cytokine studies in vivo, macrophages from the LPS-hyporesponsive C3H/HeJ mouse strain gave a proinflammatory cytokine profile following exposure to MAM in vitro.
Pathogenic effects of M. arthritidis in Lpsn and Lpsd mice.
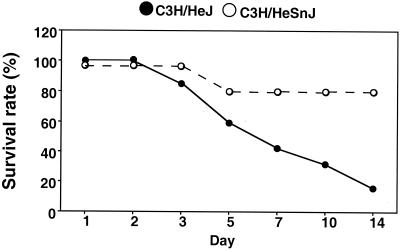
Although MAM itself fails to induce significant toxic effects when injected alone, some mouse strains develop severe toxicity when injected with live M. arthritidis organisms; this result is dependent, in part, upon the degree of the response of lymphocytes from these strains to MAM and the dose of organisms given (10). As shown in Fig. 7, C3H/HeSnJ and C3H/HeJ mice were injected i.v. in groups of 9 or 10 with 5 × 108 CFU of M. arthritidis and examined at regular intervals for up to 28 days for clinical disease. Mice of both strains exhibited some toxic effects as early as 1 to 2 days postinjection, as characterized by ruffling of fur, lethargy, and rapid breathing. However, the effects were only transient in C3H/HeSnJ mice at the dose given but became more severe in C3H/HeJ mice. The rate of survival was also significantly higher in the wild-type Lpsn mice, at 80%, than in the mutant Lpsd mice, which had a survival rate of 16% (P < 0.05). There was no clear prediction of susceptibility to arthritis between these mouse strains, since the high incidence of death in the C3H/HeJ mice confused the situation, as the longer-surviving C3H/HeSnJ mice developed overall more severe arthritis (data not shown). The results strongly suggest that the different responses seen with MAM-induced cytokine profiles in C3H/HeJ and C3H/HeSnJ mice in vivo correlate with enhanced toxicity induced by live organisms in these same mouse strains.
FIG. 7.
Susceptibility of C3H/HeSnJ and C3H/HeJ mice to M. arthritidis-induced toxic death. Mice were injected i.v. with 5 × 108 CFU of M. arthritidis and scored for systemic toxic effects through 28 days. Shown are the data obtained for the first 15 days, since they did not change after this time. C3H/HeJ mice were significantly more susceptible at all times (P < 0.005).
DISCUSSION
A number of novel findings have emerged from these studies. First, we have shown that C3H/HeJ mice carrying the Lpsd mutation exhibit a cytokine profile in vivo different from that of wild-type C3H/HeSnJ mice (Lpsn) following exposure to MAM. Second, evidence was obtained that macrophages might play an important role in the differential responses of these mice to MAM. Third, the presence of the Lpsd mutation appeared to predispose mice to a severe lethal toxicity syndrome following injection of M. arthritidis organisms.
In an earlier report (28), we observed major differences in the responses to MAM administered i.v. with regard to cytokine profiles in C3H/HeJ mice and BALB/c mice. In C3H/HeJ mice, a type 1 cytokine profile that was associated with increased susceptibility to arthritis induced by live M. arthritidis was seen; in contrast, a protective type 2 cytokine profile was induced by MAM in arthritis-resistant BALB/c mice. The reason for these differences was not immediately apparent, although the increasing sensitivity of lymphoid cells to the influence of circulating glucorticoids during lymphocyte activation and the IL-12 hyporesponsiveness of CD4+ T cells in BALB/c mice are known to favor a type 2 cytokine response (12, 15, 16).
In the present study, naive splenocytes from either C3H/HeSnJ mice (Lpsn) or C3H/HeJ mice (Lpsd) produced similarly high levels of type 1 and type 2 cytokines when exposed to various doses of MAM in vitro. However, the in vivo administration of MAM to these mice resulted in markedly different cytokine profiles when splenocytes were harvested after 1 and 3 days and challenged with MAM in vitro. The first sign of changes in cytokine levels after i.v. injection of MAM was evident in serum 90 min postinjection. Although the levels of most cytokines were elevated in both strains of mice, the IL-10 level was notably increased in C3H/HeSnJ mice but remained low in C3H/HeJ mice. The changes in serum cytokine profiles were more marked 3, 6, and 9 h after injection of MAM, with proinflammatory cytokines predominating in the C3H/HeJ mice (data not shown). The most striking differences in cytokine profiles were seen when mice were exposed to MAM for 1 and 3 days and the splenocytes were harvested and challenged with MAM in vitro. In these cases, there was a clear shift in profiles to a type 2 response in C3H/HeSnJ mice and a type 1 response in C3H/HeJ mice. A type 2 response was also seen in another Lpsn mouse strain, C3H/HeN.
The molecular mechanism(s) underlying the presently observed differences in cytokine profiles between mice of the tested strains is not yet defined, although both mouse strains are nearly identical genetically. The major known difference is that the C3H/HeJ mouse strain is characterized by hyporesponsiveness to LPS, which is due to a single mutation in the TLR4 molecule that is not present in wild-type mice (Lpsn) (31, 32). Evidence was obtained that the Lpsd mutation is likely linked to the differential cytokines elicited in Lpsd and Lpsn mice, since Lpsn/d F1 progeny showed a strong type 1 cytokine profile like that seen for C3H/HeJ mice (Lpsd), indicating that the inheritance of the response to MAM is similar to that for LPS. Although our studies suggested that the Lpsd mutation influences cytokine responses to MAM as well as responses to LPS, the results are nevertheless paradoxical, since the cytokine profile seen for MAM in Lpsd and Lpsn mice is the reverse of that seen for LPS. To confirm these findings, additional studies were undertaken using C57BL/10ScNCr mice, which also carry a defect in the LPS signaling pathway resulting from a total lack of TLR4. The data, however, were not conclusive, in part because of the low response of the wild-type C57BL/10J strain to MAM due to the absence of a functional H-2E molecule (7, 10). Our recent observations suggest that an MHC class II molecule, such as H-2E, that is strongly reactive with MAM is in fact required for a type 1 cytokine response (unpublished observations).
Since MAM interacts with multiple cell types, including T, B, and NK cells as well as macrophages, studies were begun to identify the cell subpopulations responsible for the observations. Initial studies were conducted to compare the responses of PM and SM challenged in vitro with LPS or MAM. As expected, macrophages from C3H/HeJ mice produced little or no NO in response to LPS, but substantial amounts were produced by cells from wild-type C3H/HeSnJ mice. In contrast, the levels of NO induced by MAM were reversed in macrophages from these two mouse strains, with C3H/HeJ cells producing higher levels. Also, MAM injected in vivo gave high levels of NO in the sera of C3H/HeJ mice but low or undetectable levels in the sera of C3H/HeSnJ mice.
Based upon the kinetics of the cytokine responses elicited by MAM in the two strains of mice tested, we suggest that IL-12, IFN-γ, TNF-α, and IL-10 are key players in the determination of the resulting cytokine profiles. Thus, IL-12, IFN-γ, and TNF-α were dramatically augmented whereas IL-10 was depressed in C3H/HeJ mice within 90 min of injection with MAM. The activation of macrophages as described above could have been responsible for the differences in the in vivo profiles. Their rapid activation suggests that innate immune mechanisms are most likely responsible. In C3H/HeSnJ and C3H/HeN mice, early IL-10 production by macrophages would suppress IL-12-mediated effects on type 1 cell differentiation, thus leading to a type 2 profile. We further propose that this type 2 profile might represent a normal “default” response, since it is also seen in BALB/c mice (28) and CBA (H-2k) and DBA/2 (H-2d) mice (H.-H. Mu et al., unpublished observations). This mechanism may be somewhat analogous to the LPS situation in which the immune response to LPS protects the host against infection with gram-negative organisms. However, as discussed previously (28), other factors may influence cytokine responses to MAM.
A preliminary intriguing finding in the present study was that the difference in responses between Lpsd and Lpsn mice appeared to influence disease expression induced by live M. arthritidis. Whereas both C3H/HeJ and C3H/HeSnJ mice exhibited an early toxic effect likely induced by the early production of cytokines in serum, as seen previously for MAM, the severity of symptoms progressed in C3H/HeJ mice, leading to a survival rate of only 16%. In contrast, C3H/HeSnJ mice gradually improved, with a final 80% survival rate. Although all mice were susceptible to arthritis, the early death or moribund state induced by the organisms in Lpsd mice, which required euthanasia, preempted meaningful comparative data within the design of the experiments conducted. Thus, once again the in vivo data seen with MAM and the toxic effects of the organisms seen in mutant Lpsd mice are in contrast to the effects of LPS and are consistent with the induction by M. arthritidis of a more inflammatory profile in these mice. We cannot conclude that MAM alone is responsible for the toxic death seen in Lpsd mice, since in the absence of organisms MAM is not toxic.
It has been proposed that cross-linking of MHC molecules on macrophage cell surfaces by MAM and some other SAgs, such as staphylococcal enterotoxin A, can lead to macrophage activation, with the resultant release of proinflammatory cytokines. It has certainly been demonstrated in various laboratories (10, 22) that MAM can use multiple class II MHC molecules, including murine H-2E and selected H-2A molecules, as well as human HLA-DR and selected HLA-DQ molecules. However, the observed difference in the responses of C3H/HeJ and C3H/HeSnJ macrophages to MAM cannot be explained on this basis, since these cells exhibit identical MHC expression.
Our observation that the MAM-induced secretion of cytokines and NO by macrophages differs reciprocally from that of LPS suggests that the TLR signaling pathway may play a fundamental role in initiating the differentiation of cytokine profiles in response to MAM. Although TLR4 and TLR2 have both been thought to contribute to the LPS responses of human cells (21, 31, 32, 42), recent studies have shown that highly purified LPS requires only TLR4 (39, 40). A number of other microbial products, such as gram-positive bacterial cell wall components (43), Listeria monocytogenes (13), Mycobacterium avium (24), and OspA from B. burgdorferi (18), stimulate monocytes exclusively via the TLR2 molecule. Furthermore, combinatorial signaling requiring both TLR2 and TLR6 has been described for gram-positive organisms and for yeast products (30). It remains to be definitively established which TLRs are used by MAM or whether other receptors or mechanisms also contribute to the results presented here. Studies are in progress to further define these issues.
In conclusion, this is the first report to indicate that a SAg, MAM, may be able to interact with the TLR signaling pathway and that this interaction may alter disease expression induced by M. arthritidis. In preliminary studies using the bacterial SAgs SEA and SEB, no differences were demonstrated in the in vitro or in vivo cytokine responses of splenocytes from naive or injected C3H/HeJ or C3H/HeSnJ mice (Mu et al., unpublished observations). Our findings lend additional credence to the growing awareness that there is considerable diversity in the interaction of different SAgs with the various arms of the immune system.
ACKNOWLEDGMENTS
This research was supported by NIH grants AI 12103 from NIAID and AR 02255 from NIAMS and by a grant from the Nora Eccles Treadwell Foundation. We also acknowledge the support of the DNA Synthesis Facility, University of Utah Health Sciences Center, by grant CA42014 from the National Cancer Institute. B.C.C. is the recipient of the Nora Eccles Harrison Chair in Rheumatology.
We thank Tamara Knappenburger and Joseph Merrill for excellent technical assistance.
REFERENCES
- 1.Apte R N, Ascher O, Pluznik D H. Genetic analysis of generation of serum interferon by bacterial lipopolysaccharide. J Immunol. 1977;119:1898–1902. [PubMed] [Google Scholar]
- 2.Atkin C L, Wei S, Cole B C. The Mycoplasma arthritidis superantigen MAM: purification and identification of an active peptide. Infect Immun. 1994;62:5367–5375. doi: 10.1128/iai.62.12.5367-5375.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala A, O'Neill P J, Uebele S A, Herdon C D, Chaudry I H. Mechanism of splenic immunosuppression during sepsis: key role of Kupffer cell mediators. J Trauma. 1997;42:882–888. doi: 10.1097/00005373-199705000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Ayala A, Perrin M M, Wang P, Ertel W, Chaudry I H. Hemorrhage induces enhanced Kupffer cell cytotoxicity while decreasing peritoneal or splenic macrophage capacity. Involvement of cell-associated tumor necrosis factor and reactive nitrogen. J Immunol. 1991;147:4147–4154. [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate phenol chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Cole B, Sawitzke A, Mu H-H. Mycoplasma arthritidis and its superantigen M. arthritidis mitogen as a model for inflammatory and autoimmune disease. In: Cunningham M W, Fujinami R S, editors. Effects of microbes on the immune system. Philadelphia, Pa: Lippincott, Williams and Wilkins; 1999. pp. 93–107. [Google Scholar]
- 7.Cole B C, David C S, Lynch D H, Kartchner D R. The use of transfected fibroblasts and transgenic mice establishes that stimulation of T cells by the Mycoplasma arthritidis mitogen is mediated by Eα. J Immunol. 1990;144:420–424. [PubMed] [Google Scholar]
- 8.Cole B C, Knudtson K L, Oliphant A, Sawitzke A D, Pole A, Manohar M, Benson L S, Ahmed E, Atkin C L. The sequence of the Mycoplasma arthritidis superantigen, MAM: identification of functional domains and comparison with microbial superantigens and plant lectin mitogens. J Exp Med. 1996;183:1105–1110. doi: 10.1084/jem.183.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole B C, Piepkorn M W, Wright E C. Influence of genes of the major histocompatibility complex on ulcerative dermal necrosis induced in mice by Mycoplasma arthritidis. J Investig Dermatol. 1985;85:357–361. doi: 10.1111/1523-1747.ep12276973. [DOI] [PubMed] [Google Scholar]
- 10.Cole B C, Sawitzke A D, Ahmed E A, Atkin C L, David C S. Allelic polymorphisms at the H-2A and HLA-DQ loci influence the response of murine lymphocytes to the Mycoplasma arthritidis superantigen MAM. Infect Immun. 1997;65:4190–4198. doi: 10.1128/iai.65.10.4190-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole B C, Thorpe R N, Hassell L A, Ward J R. Toxicity but not arthritogenicity of Mycoplasma arthritidis for mice associates with the haplotype expressed at the major histocompatibility complex. Infect Immun. 1983;41:1010–1015. doi: 10.1128/iai.41.3.1010-1015.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daynes R, Araneo B, Hennebold J, Enioutina E, Mu H. Steroids as essential regulators of the mammalian immune response. J Investig Dermatol. 1995;105:14S–19S. doi: 10.1111/1523-1747.ep12315187. [DOI] [PubMed] [Google Scholar]
- 13.Flo T H, Halaas O, Lien E, Ryan L, Teti G, Golenbock D T, Sundan A, Espevik T. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 14.Golightly-Rowland L, Cole B C, Ward J R, Wiley B B. Effect of animal passage on arthritogenic and biological properties of Mycoplasma arthritidis. Infect Immun. 1970;1:538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorham J D, Guler M L, Murphy K M. Genetic control of interleukin 12 responsiveness: implications for disease pathogenesis. J Mol Med. 1997;75:502–511. doi: 10.1007/s001090050135. [DOI] [PubMed] [Google Scholar]
- 16.Guler L M, Gorham J D, Hsieh C S, Mackey A J, Steen R G, Dietrich W F, Murphy K M. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science. 1996;271:984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 17.Herman A, Kappler J W, Marrack P, Pullen A M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 18.Hirschfeld M, Kirschning C J, Schwandner R, Wesche H, Weis J H, Wooten R M, Weis J J. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 19.Hodtsev A S, Choi Y, Spanopoulou E, Posnett D. Mycoplasma superantigen is a CDR3-dependent ligand for the T cell antigen receptor. J Exp Med. 1998;187:319–327. doi: 10.1084/jem.187.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janeway C A, Yagi J, Conrad P J, Katz M E, Jones B, Vroegop S, Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 21.Kirschning C J, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langlois A M, Etongue-Mayer P, Ouellette M, Mourad W. Binding of Mycoplasma arthritidis-derived mitogen to human MHC class II molecules via its N terminus is modulated by invariant chain expression and its C terminus is required for T cell activation. Eur J Immunol. 2000;30:1748–1756. doi: 10.1002/1521-4141(200006)30:6<1748::AID-IMMU1748>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 24.Means T K, Wang S, Lien E, Yoshimura A, Golenbock D T, Fenton M J. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 25.Mehindate K, al-Daccak R, Rink L, Mecheri S, Hebert J, Mourad W. Modulation of Mycoplasma arthritidis-derived superantigen-induced cytokine gene expression by dexamethasone and interleukin-4. Infect Immun. 1994;62:4716–4721. doi: 10.1128/iai.62.11.4716-4721.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michalek S M, Moore R N, McGhee J R, Rosenstreich D L, Mergenhagen S E. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxin. J Infect Dis. 1980;141:55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- 27.Mu H, Sewell W. Enhancement of interleukin-4 production of pertussis toxin. Infect Immun. 1993;61:2834–2840. doi: 10.1128/iai.61.7.2834-2840.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu H H, Sawitzke A D, Cole B C. Modulation of cytokine profiles by the Mycoplasma superantigen Mycoplasma arthritidis mitogen parallels susceptibility to arthritis induced by M. arthritidis. Infect Immun. 2000;68:1142–1149. doi: 10.1128/iai.68.3.1142-1149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhlradt P F, Frisch M. Purification and partial biochemical characterization of a Mycoplasma fermentans-derived substance that activates macrophages to release nitric oxide, tumor necrosis factor, and interleukin-6. Infect Immun. 1994;62:3801–3807. doi: 10.1128/iai.62.9.3801-3807.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozinsky A, Underhill D M, Fontenot J D, Hajjar A M, Smith K D, Wilson C B, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 32.Poltorak A, Smirnova I, He X, Liu M Y, Van Huffel C, McNally O, Birdwell D, Alejos E, Silva M, Du X, Thompson P, Chan E K, Ledesma J, Roe B, Clifton S, Vogel S N, Beutler B. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis. 1998;24:340–355. doi: 10.1006/bcmd.1998.0201. . (Erratum, 25:78, 1999.) [DOI] [PubMed] [Google Scholar]
- 33.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenstreich D L, Glode L M, Mergenhagen S E. Action of endotoxin on lymphoid cells. J Infect Dis. 1977;136(Suppl.):S239–S245. doi: 10.1093/infdis/136.supplement.s239. [DOI] [PubMed] [Google Scholar]
- 35.Rosenstreich D L, Vogel S N, Jacques A R, Wahl L M, Oppenheim J J. Macrophage sensitivity to endotoxin: genetic control by a single codominant gene. J Immunol. 1978;121:1664–1670. [PubMed] [Google Scholar]
- 36.Spencer N F, Daynes R A. IL-12 directly stimulates expression of IL-10 by CD5+ B cells and IL-6 by both CD5+ and CD5− B cells: possible involvement in age-associated cytokine dysregulation. Int Immunol. 1997;9:745–754. doi: 10.1093/intimm/9.5.745. [DOI] [PubMed] [Google Scholar]
- 37.Sultzer B M. Genetic control of leucocyte responses to endotoxin. Nature. 1968;219:1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Muhlradt P F, Akira S. Cutting edge: preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 39.Tapping R I, Akashi S, Miyake K, Godowski P J, Tobias P S. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for escherichia and salmonella lipopolysaccharides. J Immunol. 2000;165:5780–5787. doi: 10.4049/jimmunol.165.10.5780. [DOI] [PubMed] [Google Scholar]
- 40.Tapping R I, Tobias P S. Soluble CD14-mediated cellular responses to lipopolysaccharide. Chem Immunol. 2000;74:108–121. doi: 10.1159/000058751. [DOI] [PubMed] [Google Scholar]
- 41.Wong P M, Kang A, Chen H, Yuan Q, Fan P, Sultzer B M, Kan Y W, Chung S W. Lps(d)/Ran of endotoxin-resistant C3H/HeJ mice is defective in mediating lipopolysaccharide endotoxin responses. Proc Natl Acad Sci USA. 1999;96:11543–11548. doi: 10.1073/pnas.96.20.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang R B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura A, Lien E, Ingalls R R, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]