The mammalian brain is organized in overlapping, intercalated circuits, and an extensive body of information has focused on the maturation of sensory (visual, auditory) and motor circuits (1–3). Yet, much less is known about the maturation principles of “emotional” brain circuits, including those governing reward-, stress-, and fear-related behaviors. Evidence suggests that sensory inputs from the environment during a sensitive period in early postnatal life have important effects on emotional circuit development, just as adverse or positive images, odors, and sounds influence feelings and actions in adulthood. Disrupted operation of emotional circuits underlies mental illnesses and substance use disorders. Therefore, enhanced recognition of the principles guiding the development of these circuits is important for understanding human health.
The establishment of sensory circuits throughout development involves an initial phase of genetically and molecularly driven events, including neuronal migration and the construction of synapses. The subsequent strengthening or pruning of synapses is a network activity–dependent process that sculpts mature circuits (4). The network activity crucial to this process is, in turn, driven by circuit-specific sensory inputs (e.g., sequences of tone, light, or touch). In addition, the sensory signal–driven network activity must take place during a critical or sensitive period (1–3).
However, the execution of complex behaviors in humans and other mammals—and the computations, decisions, and emotions that contribute to such behaviors—requires additional brain circuits. These receive converging information from networks encoding and processing environmental signals, and from nerve projections that convey the internal state of the body (see the figure). These high-order circuits, considered “emotional” or “cognitive” according to their primary involvement in human behavior (e.g., memory may be cognitive whereas “instinct” may be emotional), adjudicate numerous streams of information to drive complex behaviors. Whereas discoveries about the structure and function of emotional circuits are increasing, their development, and specifically the influence of environmental signals on their maturation, remains poorly understood. Focusing on the influence of sensory signals early in postnatal life on emotional circuit maturation, it is proposed that unpredictable sequences of environmental signals influence emotional circuit development and refinement, promoting vulnerabilities to emotional illnesses.
Learning from sensory and memory circuits.
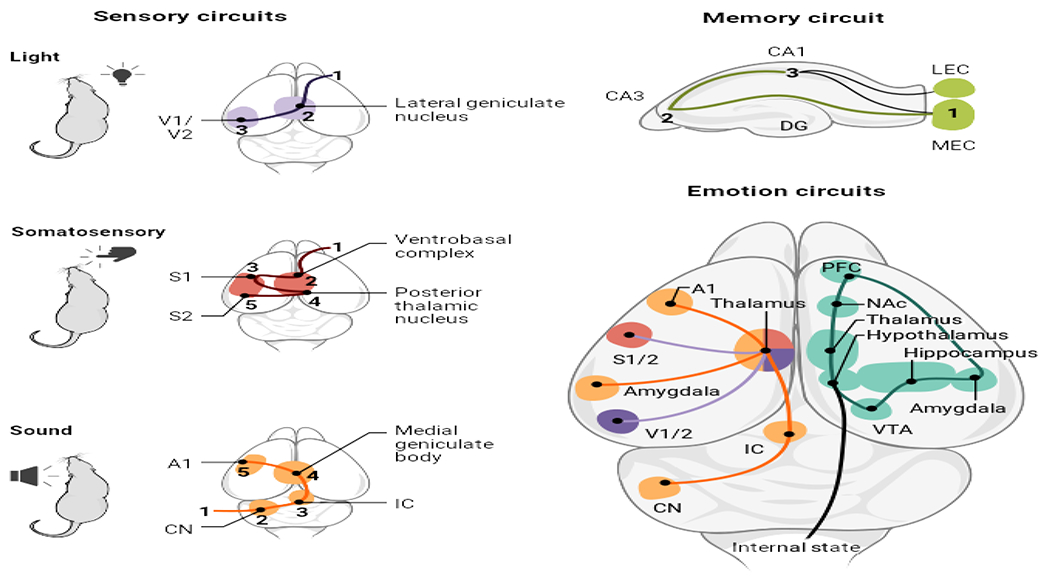
Maturation principles of sensory (e.g., visual, somatosensory, and auditory) and memory circuits are instructive for how environmental signals influence emotion circuit development. The building blocks and organization of emotion circuits include components of sensory and memory circuits, and of signals providing information about internal body states (hunger, fatigue, cold). Cortical and subcortical components process these inputs in emotion-related circuits (teal).
A1, auditory cortex; CN, cochlear nucleus; DG, dentate gyrus; IC, inferior colliculus; LEC, lateral entorhinal cortex; MEC, medial entorhinal cortex; NAc, nucleus accumbens; PFC, prefrontal cortex; S1/S2, somatosensory 1/2 cortex; V1/V2, visual 1/2 cortex; VTA, ventral tegmental area.
Emotional circuits comprise prefrontal cortical areas, thalamic nuclei, hippocampus, amygdala, and hypothalamic nuclei, as well as additional subcortical regions. The coordinated activities of these circuits require the maturation of their components and further refinement of their integrative connections. Whereas many questions about the nature of emotional circuit maturation are not fully resolved, information from both sensory and memory circuit development is instructive. Common to both processes is the concept of hierarchy: In the visual, sensory-motor, and auditory circuits, development proceeds from peripheral signal–receiving neurons to first-order thalamic nuclei to cortex, followed by second-order thalamic nuclei and cortical regions which, in turn, participate in high-order emotional and cognitive circuits. Notably, the appropriate environmental signal for each sensory circuit specifies gene expression and cell identity of the first-order neurons, and the activities of these neurons specify the identity and function of their cortical targets.
Neurons within emotional (e.g., reward) circuits function as target cells for the sensory circuit output, and thus their identities and activities may be driven by input from intercalating sensory circuits. In support of this idea, deprivation of sensory input perturbs the synaptic connections of both the primary sensory relay neurons and the high-order neurons that belong to emotional integrative circuitry (5). Once the basic circuitry is established, additional sculpting of emotional circuits involves quantitative changes in the numbers and/or strength of synapses and changes in the relative contributions of cell type–specific neuronal projections to the synaptic complement of neurons in key brain regions (hub nodes) of the circuit. In this model, hierarchical development of integrative emotional circuits commences with the environmental signal–dependent maturation of sensory networks, coupled with that of relay neurons conveying internal body states.
A similar hierarchy of circuit development, influenced by sensory environmental signals, takes place in the learning and memory hippocampal circuit. Here, sensory signals from the environment are conveyed through association regions in the cortex to the superficial-layer neurons in the medial entorhinal cortex, the first stage in the hierarchical spatiotemporal maturation of this network. Their sequences of synaptic signals (activity) in turn drive subsequent stages of maturation of the circuit, including hippocampal neurons along the trisynaptic pathway followed by deep-layer lateral entorhinal cortex cells (6). In support of this stepwise activity–dependent progression of learning and memory-circuit development, silencing excitatory activity at any stage of the network in mice impairs maturation of downstream neurons but not of those upstream.
Thus, information from both sensory- and memory-circuit development suggests that sensory signals of several types, occurring during sensitive periods, are required to establish synaptic connections of first- and higher-order components of nascent emotional circuits (2, 3, 5, 7). Yet, whether the subsequent refinement of functional neuronal connections involved in executing the complex behavioral output of emotional networks depends on sensory signals, and the source and characteristics of such signals, remain unclear.
Human studies support a strong influence of early-life sensory signals from the environment on the development and function of emotional circuits (8). The potential sources and characteristics of these inputs have remained unclear, but foundational studies, buttressed by emerging evidence, indicate that salient sensory inputs to the maturation of emotional circuits arise from the proximate environment of a developing human (or rodent). During the sensitive period in which these emotional circuits develop—shown by a randomized controlled study in Romanian orphans adopted at different ages (9) and recent work across humans and rodents (8) to encompass the first 2 years and 2 weeks of life, respectively—the principal proximate environment consists of the parents. Therefore, sensory inputs from parents may be a salient parameter that influences maturation of emotions and their underlying circuits.
The nature of parental and other environmental sensory signals that either promote or disrupt the maturation of emotional brain circuits has attracted a rich set of observational and mechanistic studies (9–13). Most attention in human studies has centered on the presence, quantity, and quality of parental signals (e.g., sensitivity, responsiveness) in relation to the needs of the infant, with particular focus on maternal, rather than paternal, behaviors (8). However, studies inspired by the maturation of the auditory network support a prime role not only of the positive or negative valence of parental signals but also of their patterns or sequences in the maturation of emotional circuits (1, 12). In humans, unpredictable (high entropy) sequences of maternal sensory signals to the infant predict enduring adverse emotional outcomes, including poorer control of emotions and behaviors (effortful control) (13), an established predictor of mental vulnerabilities and risk of posttraumatic stress disorder later in life. Notably, in controlled mouse and rat studies, unpredictable sequences of dam behaviors directly led to aberrant emotional circuit maturation and consequent disrupted pleasure-like behaviors in the pups (11, 12, 14).
The mechanisms by which predictable or unpredictable sequences of parental-derived sensory signals modulate the maturation of specific brain circuits are only now emerging. For example, unpredictable sequences of mouse maternal care behaviors influence synaptic connectivity in key brain nodes that contribute to stress and other emotional circuits. Specifically, mice reared by dams displaying unpredictable sequences of care (but with the same amount of care overall) during the sensitive early postnatal period have augmented density of functional excitatory synapses on stress-sensitive and regulatory corticotropin-releasing hormone (CRH)–expressing hypothalamic neurons (14). This aberrant synaptic connectivity leads to disrupted behavioral and hormonal responses to acute and chronic stresses later in life. The mechanisms for the exuberant persistence of excitatory synapses on the CRH cells involve attenuation of the normal developmental pruning of these excitatory synapses by the adjacent microglial brain cells. Specifically, both the expression and the function of the phagocytic (synapse engulfing) microglial Mer tyrosine kinase receptor (MERTK) are reduced. It is not yet known whether this results from direct effects of unpredictable signals on microglia or if neuronal signaling to microglia is perturbed.
Studies in humans suggest that unpredictable sensory-signal sequences and their potential impact on brain-circuit maturation in infants and children may explain a significant portion of the variance in emotional outcomes (13). Prospective studies in the United States and Finland found that unpredictable sequences of maternal behaviors portended deficits in effortful control, and these effects persisted despite correction for other important early-life variables, including maternal sensitivity to the infant’s needs (a common measure of the quality of maternal care behaviors), socioeconomic status, and maternal depressive symptoms (13). The findings of an enduring influence of unpredictable sequences of early-life signals on the functional maturation of emotional circuits reveal avenues for future research. For example, sequences of sensory signals might drive neuronal activity within an already developing emotional network. It is also unknown whether there is hierarchical progression of synaptic refinement and maturation within specific emotional circuits, analogous to sensory and memory circuits. Further investigation of the cell populations (such as hypothalamic CRH cells) that are most susceptible to unpredictable sequences of sensory signals is needed. Additionally, can the enduring deficits in the operations of emotional circuits resulting from unpredictable early-life signals be prevented or ameliorated?
New technologies, including noninvasive optogenetics (15), would allow delivery of predictable and/or unpredictable sequences of signals that activate specific cell populations at different time points during sensitive periods or later. Such experiments in animal models could test whether administration of predictable signal sequences overcomes the deficits in emotional-like behaviors resulting from rearing in unpredictable environments and may inform behavioral interventions in children. Indeed, the conceptual framework described here carries substantial potential benefit: If unpredictable patterns of early-life sensory signals disrupt the normal maturation of emotional circuits, leading to vulnerabilities to mental illness, then these aberrant patterns may be mitigated by preventive or interventional behavioral approaches (8).
ACKNOWLEDGMENTS
We thank C. M. Gall, G. Lynch, and T. Hensch for valuable discussions. The authors are supported by the National Institutes of Health (grants P50 MH096889, MH73136, and NS108296), the Bren Foundation, and the Hewitt Foundation for Biomedical Research.
REFERENCES AND NOTES
- 1.Takesian AE, Bogart LJ, Lichtman JW, Hensch TK, Nat. Neurosci 21, 218 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng S, et al. Cell 185, 311 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khazipov R, et al. Nature 432, 758 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Faust TE, Gunner G, Schafer DP, Nat. Rev. Neurosci 22, 657 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frangeul L et al. Nature 538, 96 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Donato F, Jacobsen RI, Moser M-B, Moser EI, Science 355, eaai8178 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Kloc ML, Velasquez F, Niedecker RW, Barry JM, Holmes GL, Brain Stimul. 13, 1535 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luby JL, Baram TZ, Rogers CE, Barch DM, Trends Neurosci. 43, 744 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson CA 3rd et al. Science 318, 1937 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Goodwill HL et al. Cell Rep. 25, 2299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis D, Diorio J, Liu D, Meaney MJ, Science 286, 1155 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Molet J et al. Transl. Psychiatry 6, e702 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis EP et al. EBioMedicine 46, 256 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolton JL et al. Cell Rep. 38, 110600 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R et al. Nat. Biotechnol 39, 161 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


