Abstract
Hematopoietic cell transplantation (HCT) has been successfully utilized as treatment for many malignant and non-malignant conditions. As supportive care, donor selection, and treatment modalities evolve, documenting HCT trends and outcomes is critical. This report from the Center for International Blood and Marrow Transplant Research (CIBMTR) provides an update to current transplantation activity and survival rates in the United States. Additional data on the use and outcomes of HCT in the adolescent and young adult (AYA) population are included. AYA patients more frequently receive peripheral blood stem cell grafts than pediatric patients, which may reflect differences in practice in pediatric vs adult treatment centers. The proportions of donor types also differ from adult and pediatric populations. Outcomes for patients in the AYA age range are similar to pediatric patients for acute myeloid leukemia (AML), but worse than pediatric patients for acute lymphoblastic leukemia (ALL). Outcomes for both leukemias are better in AYA patients than in older adults. When comparing the time period of 2000–2009 to 2010–2019, improvements in overall survival were significant across the age spectrum, but greatest in the AYA age group.
Keywords: Activity, Hematopoietic cell transplantation, Summary slides, Adolescent and young adult
Introduction
Hematopoietic cell transplantation (HCT) has been used since the 1960s as treatment for a variety of non-malignant and malignant conditions.1 Patterns of use and subsequent outcomes of HCT evolve over time. The Center for International Blood and Marrow Transplant Research (CIBMTR) collects longitudinal outcome data on United States (US) and international patients who receive cellular therapies. These data were submitted voluntarily by centers starting in the early 1970s. CIBMTR was subsequently charged with prospectively collecting data for all allogeneic HCTs performed in the US and holds the contract for the national Stem Cell Therapeutic Outcomes Database (SCTOD) as part of the Stem Cell Therapeutic and Research Act (Stem Cell Act). This act, established in 2005 and renewed in 2010, 2015, and 2021, made submission of data for allogeneic transplants mandatory in the US. CIBMTR also collects data for most (>85%) autologous transplants performed in the US, although data submission is not required. CIBMTR has also been collecting data on alternative cell therapies such as chimeric antigen receptor T-cells (CAR-T cells) and gene therapies. Three hundred sixty-four centers in the US have reported data for 156,248 allogeneic transplants (including 77,540 related, 66,167 unrelated, and 12,541 cord blood), as well as 217,651 autologous transplants from the registry’s inception through 2019. The number of US centers reporting data in the year 2019 is 198.
This report updates HCT trends from the report published in 2020 by D’Souza, et al.2 Overall activity and trends in the US are the primary focus; however, this is the first CIBMTR summary report that includes additional analyses focused on the adolescent and young adults (AYA) population, defined as those treated between the ages of 15 and 39 years.3,4 It is a population of growing interest, due to several unique attributes. There is greater variability in care practices and cancer outcomes, which may relate to differences in treating institutions (majority pediatric vs adult-based), as well as greater problems with loss to follow-up care, changes in insurance coverage, and compliance with prescribed therapies.5,6
Methods
Data Collection:
Transplantation centers submit data electronically to the CIBMTR into a web-based electronic data collection system, FormsNet. The CIBMTR Coordinating Centers assigns patients to either a Transplant Essential Data (TED) track, which collects core (“essential”) data, or a Comprehensive Report Form (CRF) track that captures detailed disease- and treatment-related data. Assignment to each track is done on submission of the initial pre-transplantation TED form and uses a weighted randomization algorithm designed to produce a cohort with detailed data for in-depth analysis that is representative of current practice but with adequate numbers of patients who underwent transplantation for rare conditions or with emerging transplantation strategies. Data are collected at specific timepoints, including pre-transplantation and 100 days, 6 months, 1 year, and then annually for 6 years post-transplantation, and then biannually until death or loss to follow-up. Repeat cellular infusions from the same donor and second transplantations are also captured. The FormsNet system enforces allowable data and performs simple logic checking. Further quality checks are performed after data receipt using both computerized and manual inspection. Centers are audited on-site once within a 4-year audit cycle, in which data submitted to the CIBMTR are compared with source documents. Discrepancies are reviewed, and centers may be required to submit a corrective action plan following the audit. A slide set presenting the data summarized in this article and additional details of HCT use and outcomes is available online at https://www.cibmtr.org, along with the accompanying citation to be referenced when these slides are utilized publicly.
Statistics:
Total transplantation numbers are estimated based on data reported to the CIBMTR. Estimates of allogeneic HCT activity assume 95% capture of data. Estimates of autologous HCT activity are based on an assumption of 85% capture of autologous HCT data, considering data collected by the Foundation for the Accreditation of Cellular Therapy (FACT) for centers who do not report to the CIBMTR. Overall survival probabilities are presented according to disease, disease status, donor type, recipient age, and conditioning regimen intensity. Estimates of survival and comparisons across survival curves are univariate and are not adjusted for potentially important contributing factors. Causes of death are reported by centers. Trends in the total numbers of transplantations performed in the United States up to 2019 are analyzed. Survival analyses using Kaplan-Meier estimators are reported at a 3-year time point with 95% confidence intervals (CIs).
Results
Transplantation Activity in the United States in 2019
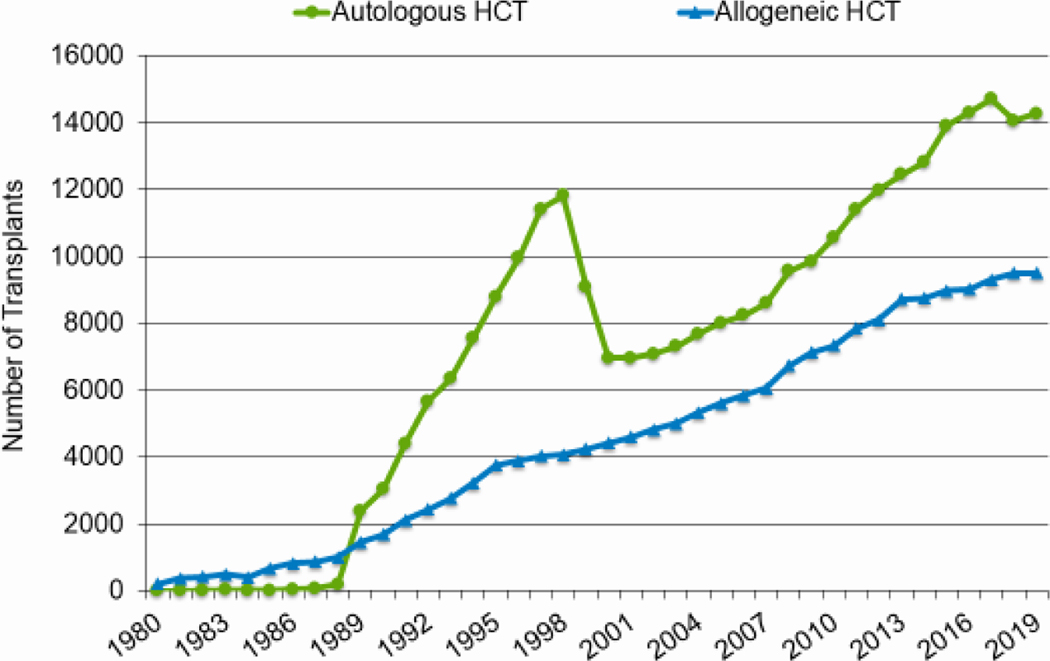
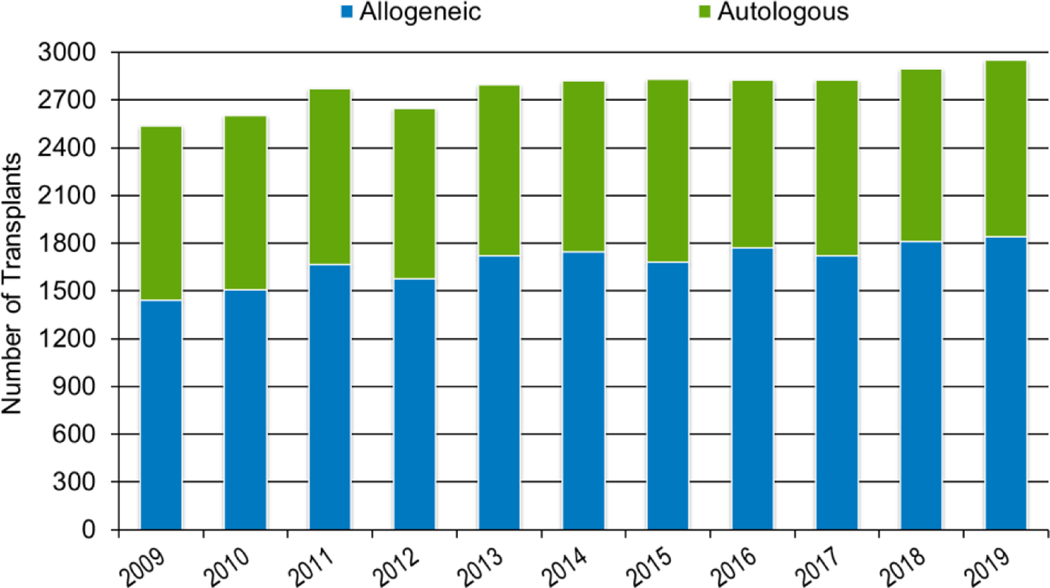
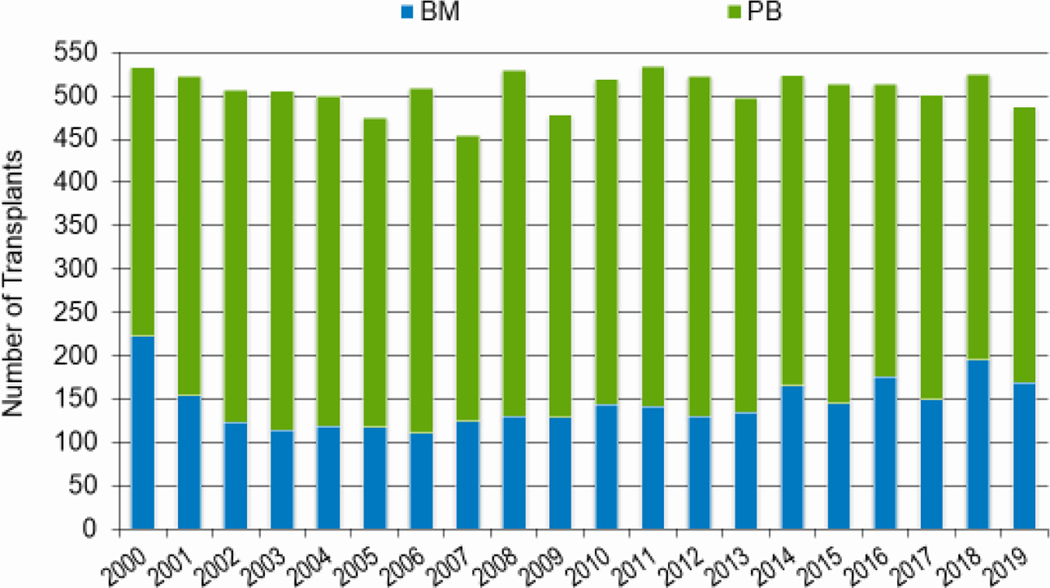
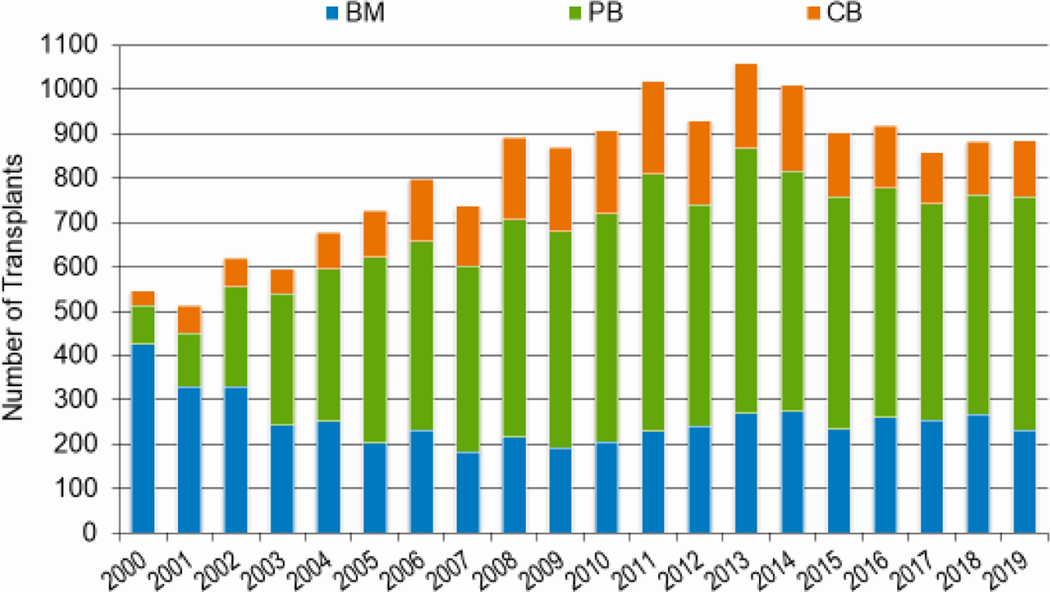
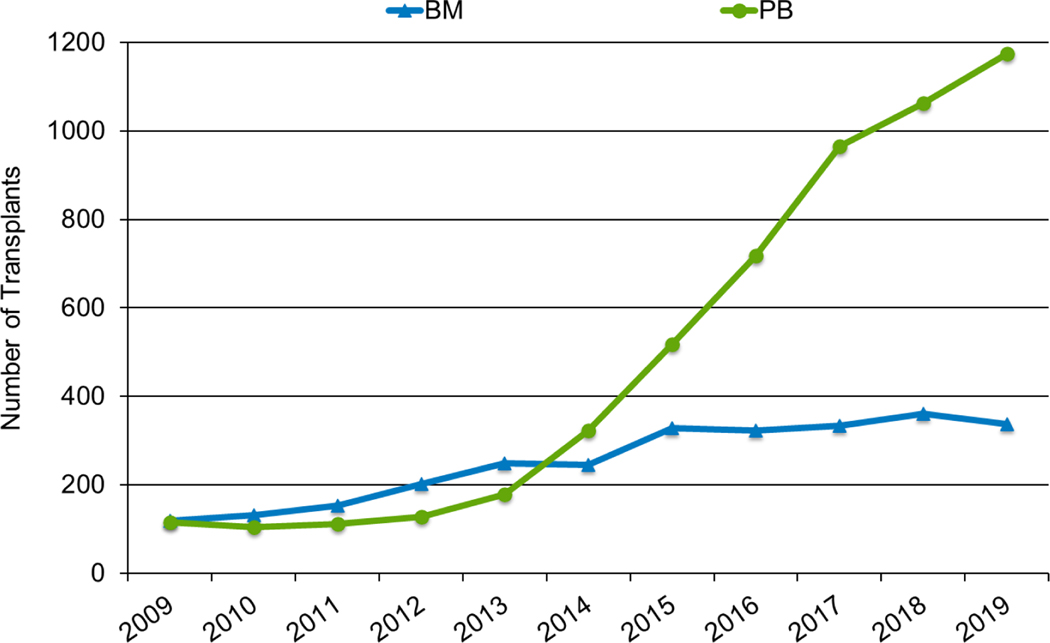
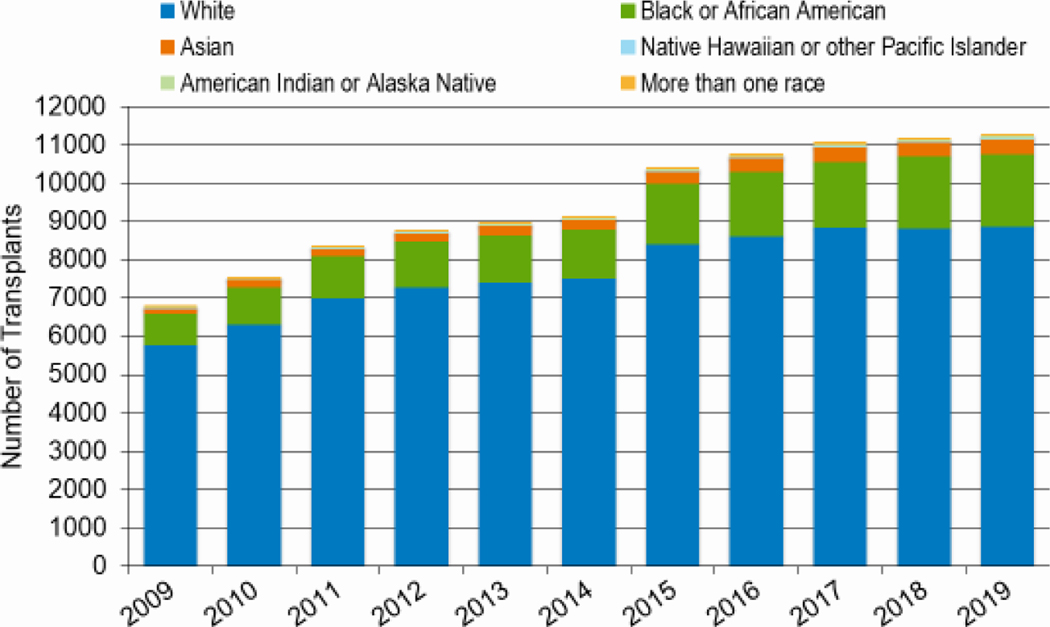
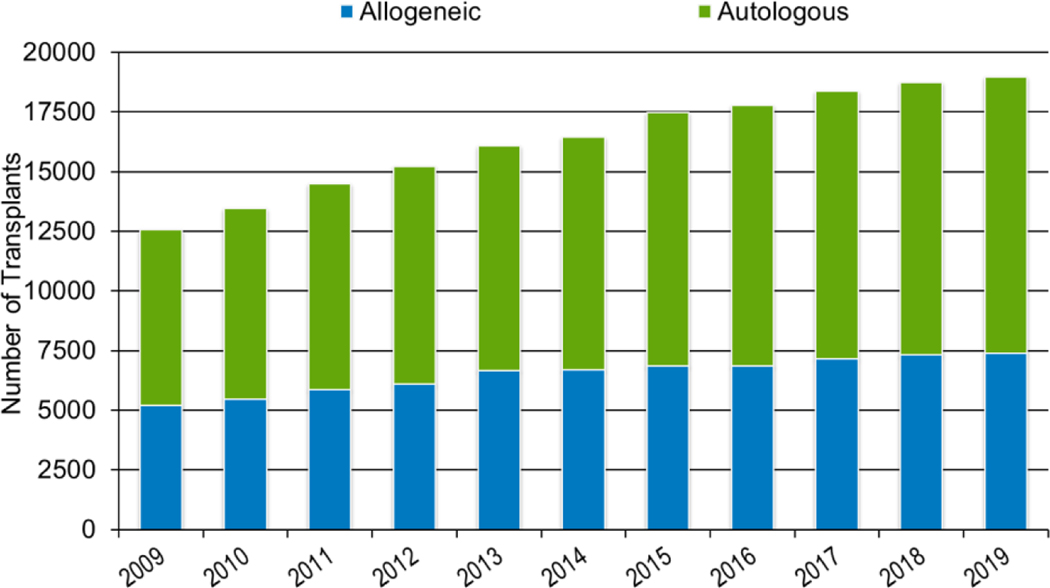
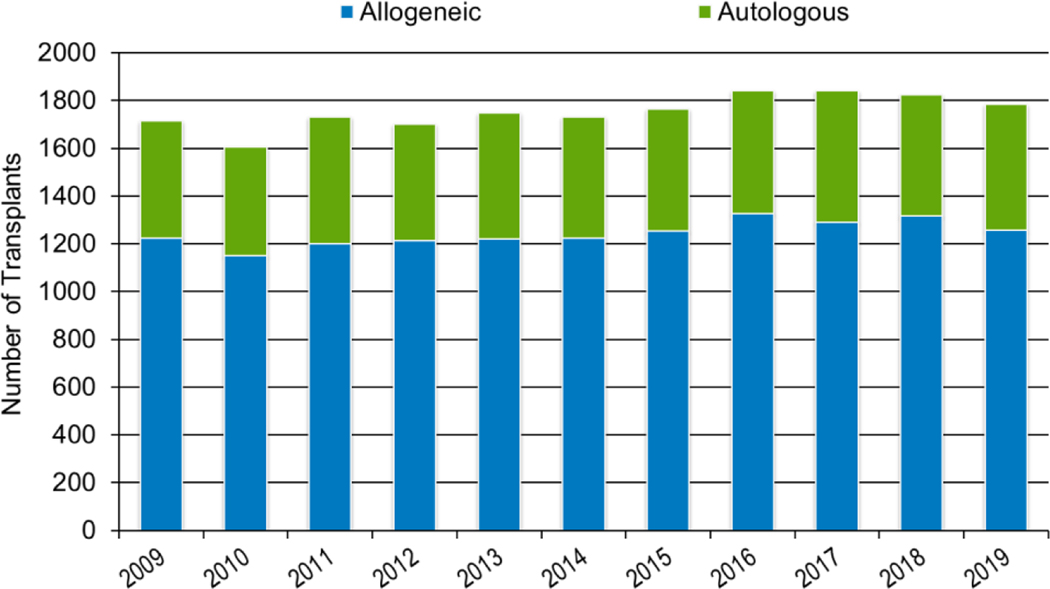
In 2019, a total of 23,535 transplantations were reported to the CIBMTR. Of these, 14,236 were autologous. Among the 9,299 allogeneic HCTs performed, 4,123 (44%) were from a related donor, 4,511 (49%) from an unrelated donor, and 589 (6%) from banked umbilical cord blood (UCB). Figures 1a–d display the number of annual HCT recipients by year, separated by autologous and allogeneic transplantation types. The number of pediatric and AYA HCTs performed each year remained relatively stable over recent years, while those in the adult population showed a steady increase.
Figure 1a: Estimated annual number of HCT recipients in the United States by transplant type, overall.
Figure 1d: Estimated annual number of HCT recipients in the United States by transplant type, AYA recipients (15-≤39 years) in the United States.
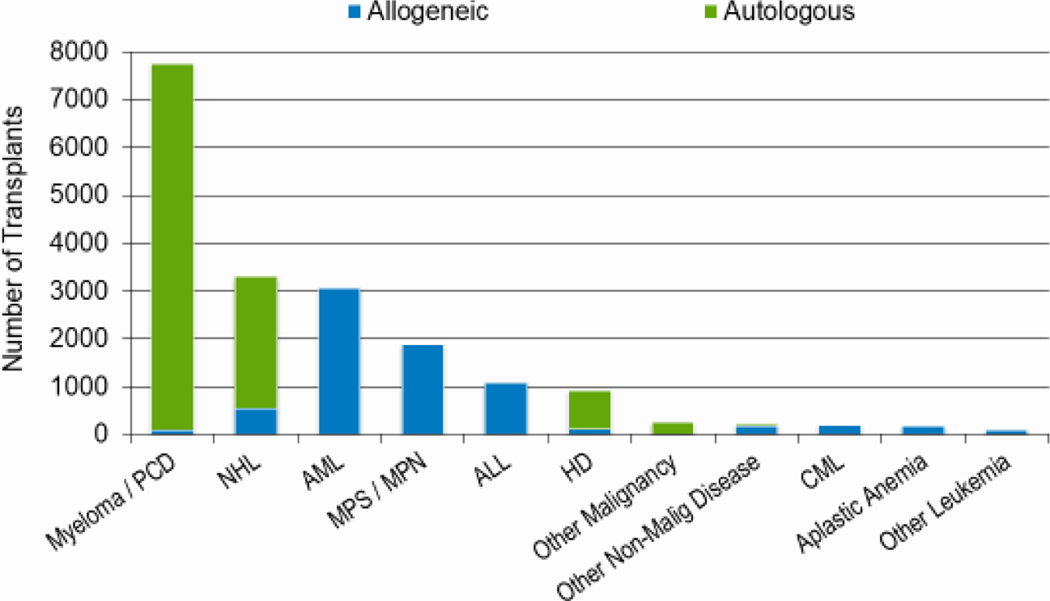
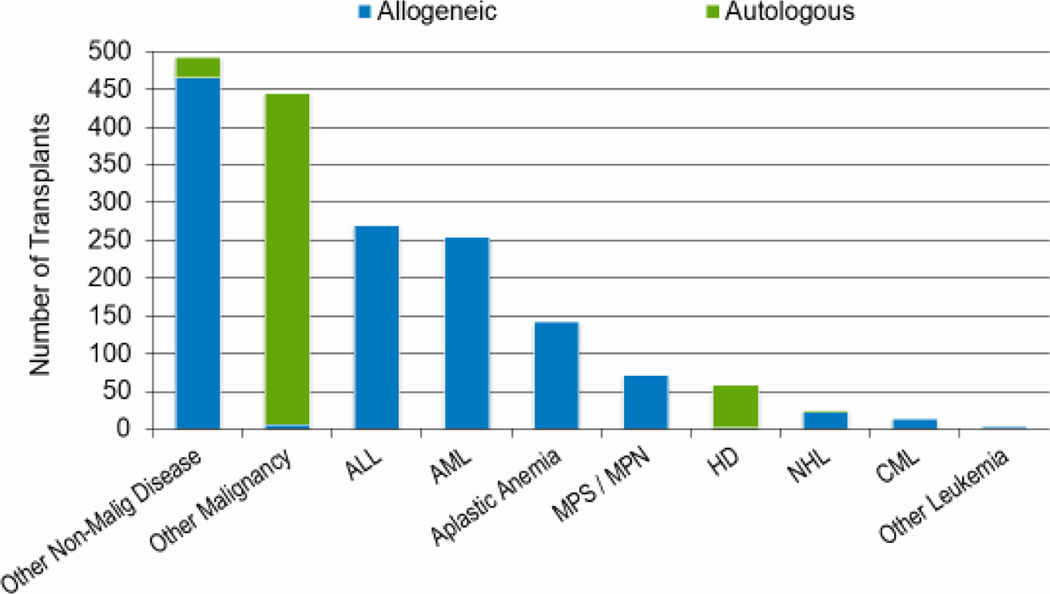
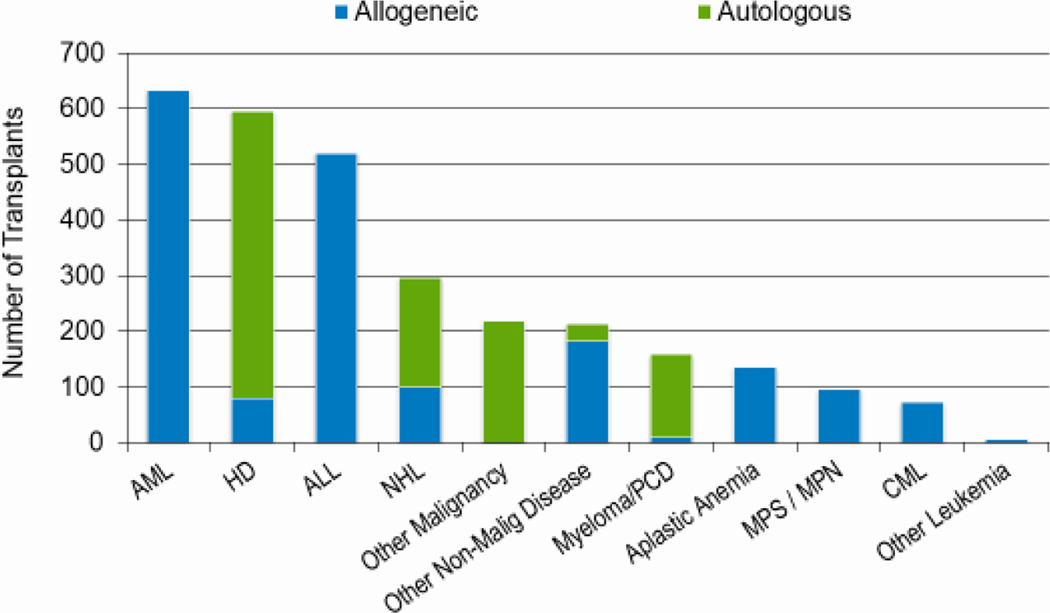
Figures 2a, 2b, and 2c show the indications for transplantation, separated by adult (≥18 years), pediatric (<18 years), and AYA (15–39 years) age groups, respectively. The total number of adult HCTs (18,948), pediatric HCTs (1,773), and AYA HCTs (2,949) performed in 2019 was similar to that in 2018 (18,743, 1,824, and 2,897). Disease indications were also largely unchanged, with most HCTs in the adult population being autologous HCTs for multiple myeloma, and most HCTs in the pediatric and AYA populations being allogenic transplantations performed for leukemia (acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), combined).
Figure 2a: Indications for HCT for adult recipients (≥ 18 years) in the United States in 2019.
Figure 2b: Indications for HCT for pediatric recipients (<18 years) in the United States in 2019.
Figure 2c: Indications for HCT for AYA recipients (15-≤39 years) in the United States in 2019.
Trends in HCT Utilization
Age
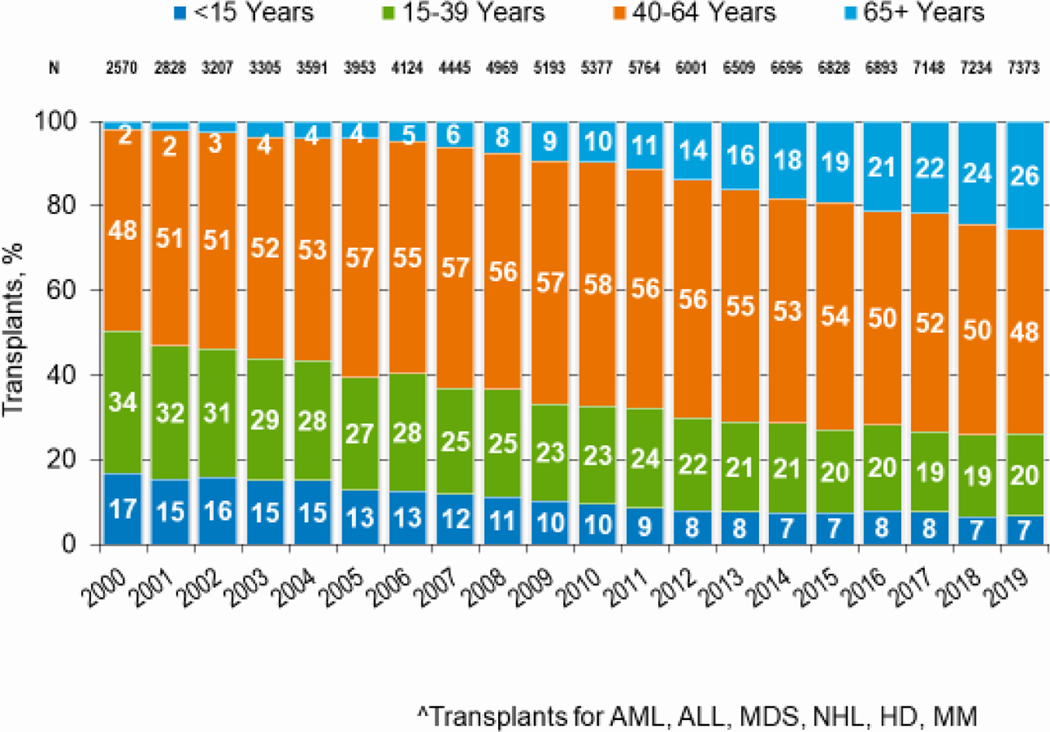
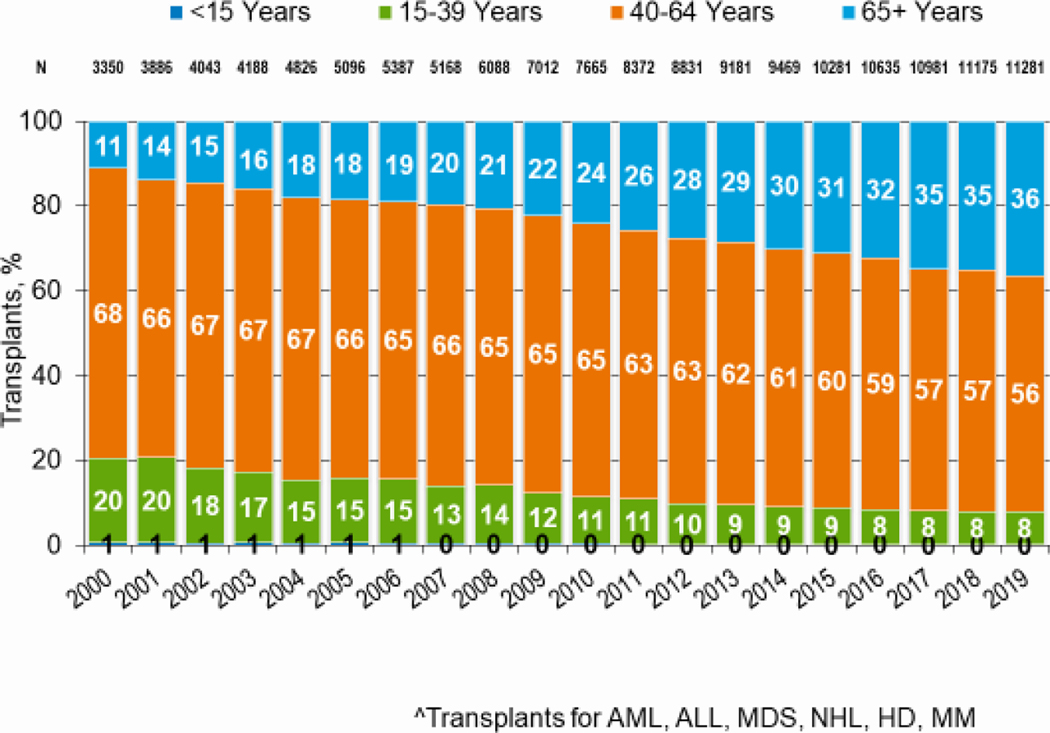
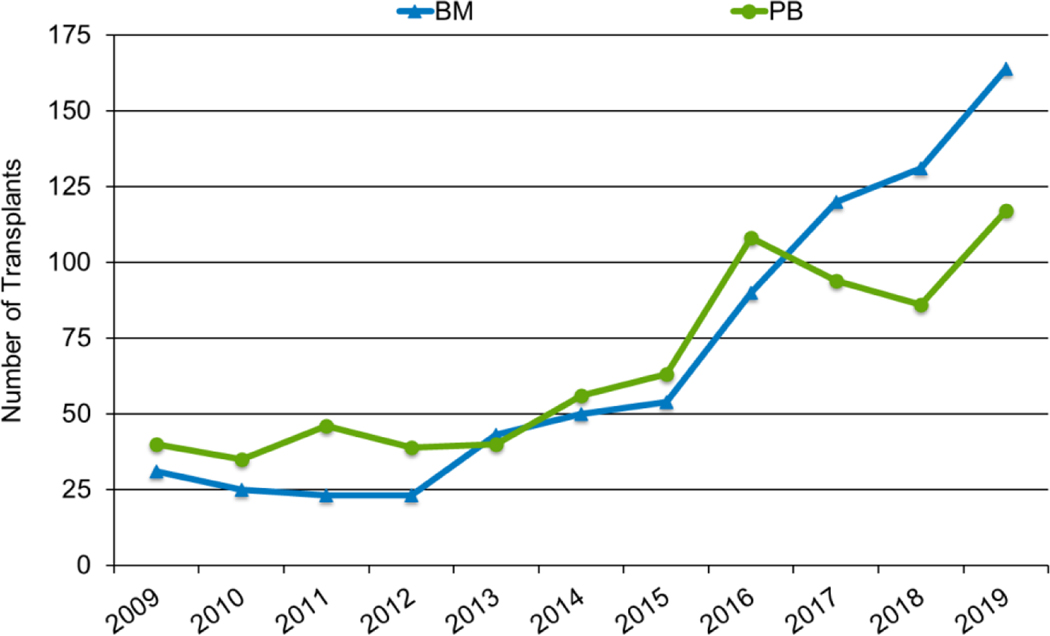
Figures 3a and 3b, demonstrate the dramatic increase in numbers of patients 65 years of age or older receiving HCT in both the allogeneic and autologous settings over the last two decades. In the year 2000, 48 patients (2% of total alloHCT recipients) receiving an alloHCT reported to the CIBMTR were ≥65 in comparison to 1888 patients (26% of total alloHCT recipients) in 2019. Similar trends in autoHCT were observed, with 372 patients ≥65 receiving an autoHCT in 2000 (11% of total autoHCT recipients) versus 4,103 patients (36% of total autoHCT recipients) in 2019. In comparison, overall pediatric and AYA transplantation numbers had more gradual increases over this period. Four hundred thirty patients under 15 years of age in 2000 underwent alloHCT versus 496 in 2019. Eight hundred sixty-nine AYA patients received alloHCT in 2000 versus 1429 in 2019. Very few autologous HCTs are performed in the pediatric and AYA age groups.
Figure 3a: Trends in allogeneic HCT by recipient age in the United States.
Figure 3b: Trends in autologous HCT by recipient age in the United States.
Donor type
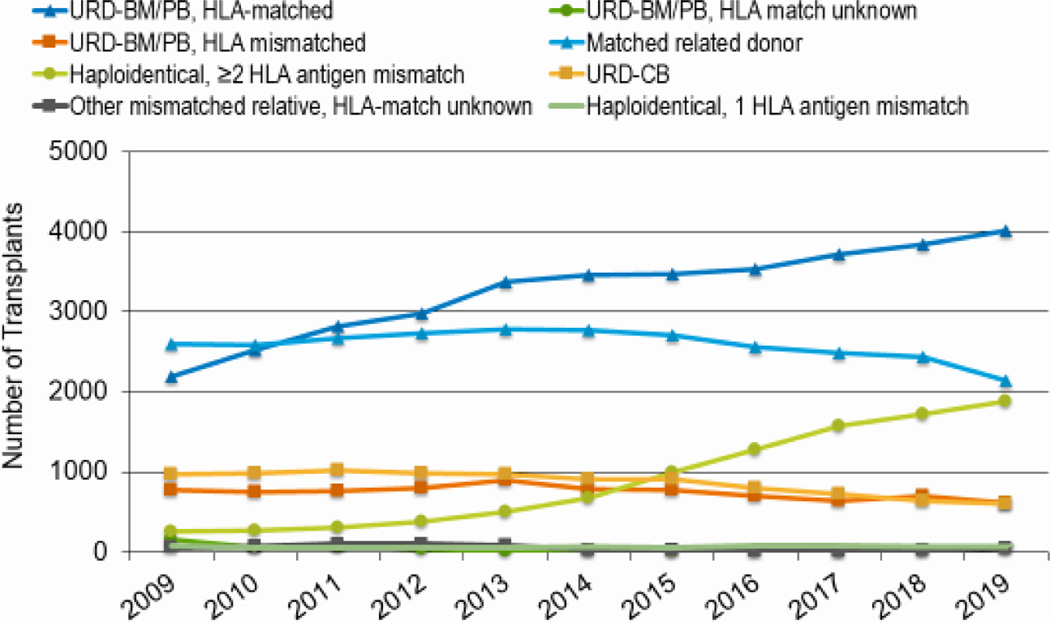
The overall number of alloHCTs by donor type is presented in Figure 4. Trends show a continued decrease in the use of HLA-matched related donors (MRD) with a 17% decrease from 2010 (n=2378) to 2019 (n=1970). The use of HLA-mismatched (haploidentical) donors increased by 506% during his time from 296 in 2010 to 1,793 in 2019. The use of HLA-matched unrelated donors (MUD) continues to rise (59% increase (2010, n=2314; 2019, n=3680)), while the use of HLA-mismatched unrelated donors (MMUD) decreased slightly (17% decrease (2010, n=683; 2019, n=568)) and umbilical cord blood (UCB) more substantially (40% decrease (2010, n=788; 2019, n=476)) over the past decade.
Figure 4: Allogeneic HCT in the United States by donor type.
The distribution of allogeneic donor type is overall similar by age group, with the most variation in UCB and MUD use. Among adults in 2019, 22% of patients had a MRD, 21% a haploidentical donor, 46% MUD, 6% MMUD and 4% UCB. Among children in 2019, 26% had an MRD, 23% a haploidentical donor, 27% MUD, 9% MMUD and 15% UCB. Among AYAs in 2019, 27% had an MRD, 24% a haploidentical donor, 34% MUD, 7% MMUD and 7% UCB.
Graft Sources
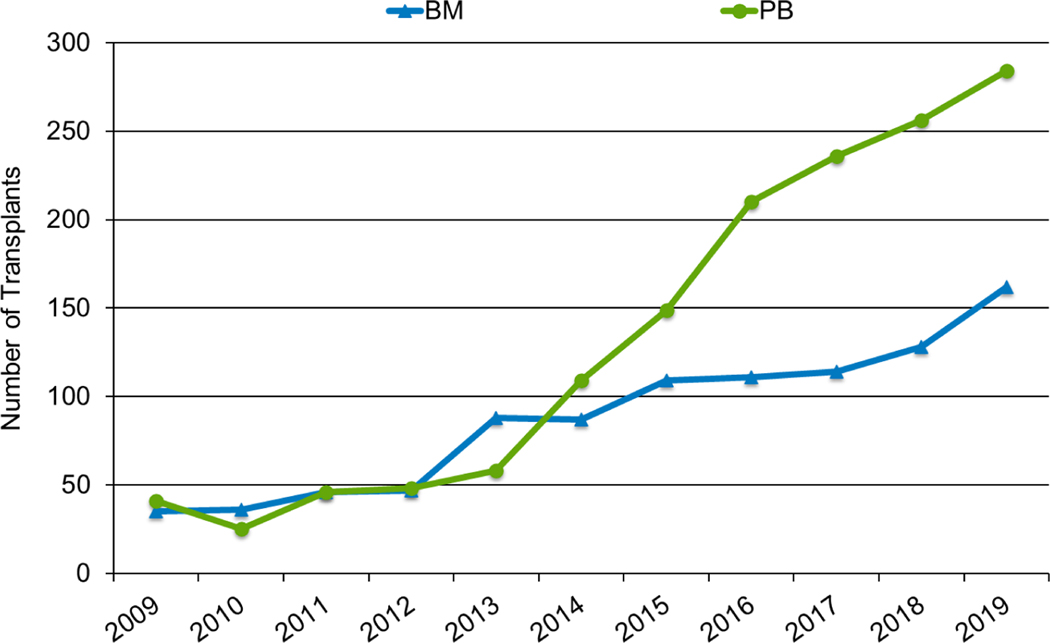
Graft sources differed among the adult, pediatric, and AYA populations. Most HCTs performed in adults in 2019 utilized peripheral blood stem cells, in both the MRD (89%) and MUD (79%) settings, similar to the proportions in 2010 (91% of MRD and 72% of MUD). UCB use is low in adults (14% of HCTs in 2010 and 7% in 2019). In contrast, bone marrow remains the predominant graft source in children (91% of MRD and 40% of MUD HCTs in 2010; 87% of MRD and 52% of MUD HCTs in 2019). The proportion of children receiving UCB decreased over time (49% in 2010 and 29% in 2019). AYA patients (Figure 5a and 5b) received more peripheral blood grafts in 2019 than children (65% of MRD and 59% of MUD HCTs), but more bone marrow (35% of MRD HCTs and 26% of MUD) and UCB HCTs (14%) than adults. Consistent with other age groups, UCB use in AYA patients decreased compared to 2010 (21% of HCTs).
Figure 5a: Matched related donor allogeneic HCT in the United States in patients 15-≤39 years by graft source.
Figure 5b: Unrelated donor allogeneic HCT in the United States in patients 15-≤39 years by graft source.
As noted above, the use of haploidentical donors increased dramatically over the past 10 years and an increasing proportion utilize peripheral blood (Figures 6a and 6b), although a higher proportion of these HCTs use bone marrow in children (58% in 2019) than in adults (22% in 2019). Among AYA recipients of haploidentical HCTs, the pattern was more similar to the adult population with only 36% of HCTs in 2019 using bone marrow (Figure 6c).
Figure 6a: Haploidentical donor allogeneic HCT activity in the United States, by graft type, adults (>18 years).
Figure 6b: Haploidentical donor allogeneic HCT activity in the United States, by graft type, pediatric (<18 years).
Figure 6c: Haploidentical donor allogeneic HCT activity in the United States, by graft type, AYA (15–39 years).
Disease Status
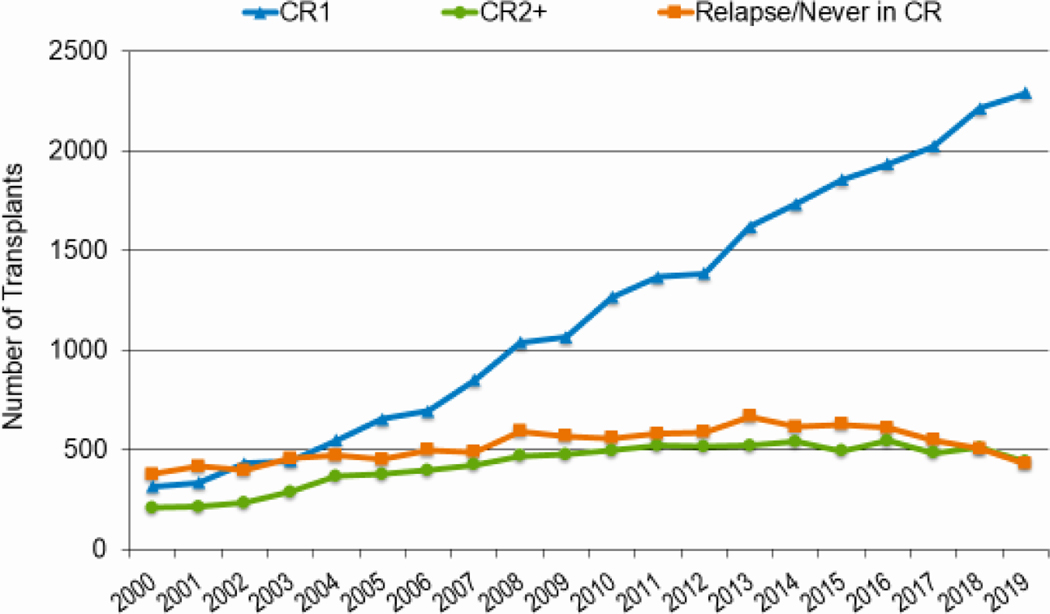
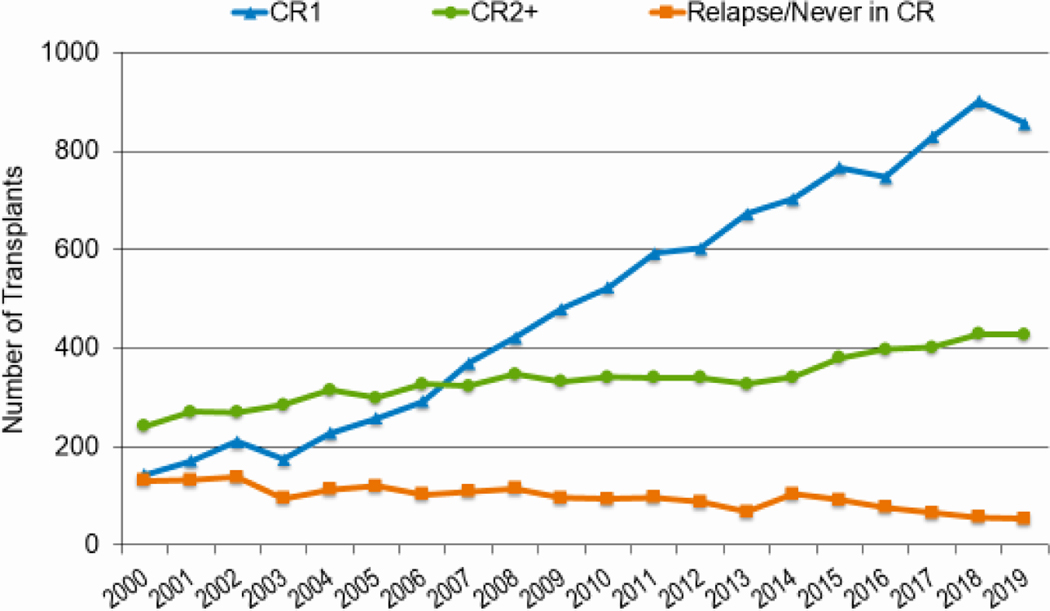
As knowledge regarding disease risk classification evolves, upfront therapies improve, donor options increase, and transplant-related mortality (TRM) decreases, more patients receive transplantation in complete remission (CR). Figures 7a and 7b highlight the increasing likelihood of performing HCT in CR, particularly CR1, rather than with active disease for both AML and ALL. In 2019, 73% of AML and 64% of ALL patients were in CR1 at the time of transplantation compared to 55% for both AML and ALL in 2010; almost all of the increase in HCT activity in these diseases was in patients in CR1. This change was most notable across all ages for AML. In 2010, 55% adults underwent HCT for AML in CR1, compared to 73% in 2019. The figures were similar for pediatric and AYA patients (55% versus 72% and 52% versus 71%, respectively). The proportion of adult and AYA patients with ALL transplanted in CR1 also increased from 2010 to 2019 (62% versus 71% and 47% to 57%, respectively). In contrast, the proportion of children patients with ALL undergoing HCT in CR1 (36%) and CR2+ (60–61%) remained consistent across this timespan.
Figure 7a: Trends in allogeneic HCT for AML by disease status in the United States.
Figure 7b: Trends in allogeneic HCT for ALL by disease status in the United States.
Race and Ethnicity
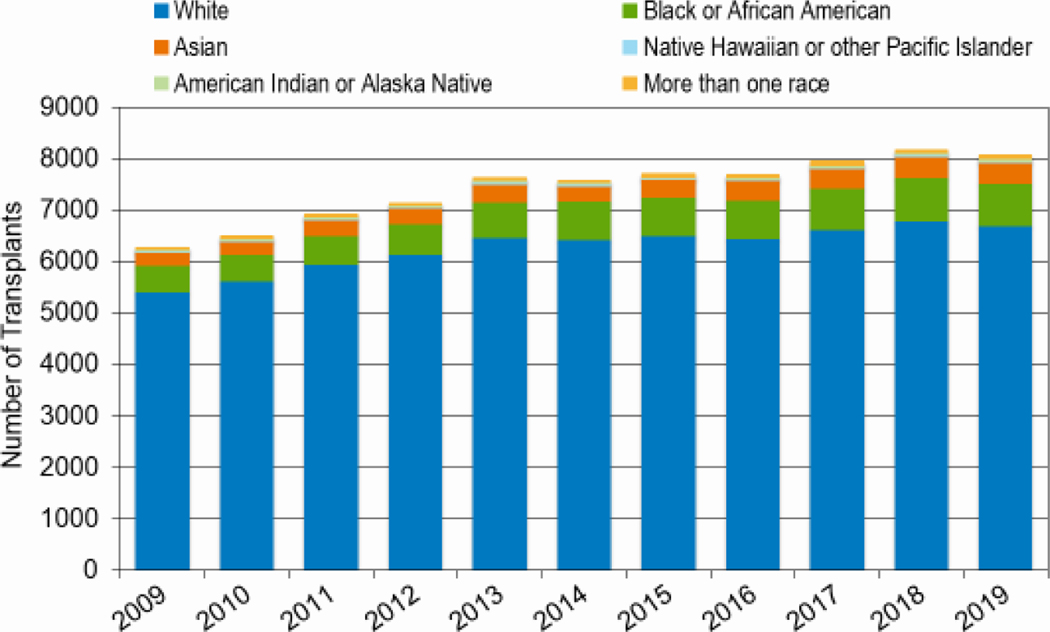
Figures 8a and 8b highlight the increasing number of African American patients undergoing both autologous and allogeneic HCT since 2009. In 2010, 521 African Americans underwent allogeneic HCT, compared to 828 in 2019 (59% increase); 980 African Americans underwent autologous HCT 2010 and 1,904 patients in 2019 (51% increase). The number of allogeneic HCTs in Hispanic patients also increased (819 in 2010 versus 1,249 in 2019, a 53% increase). The relative increase in numbers of transplantations for white patients during this same period was 19%, with 5,618 non-Hispanic white patients undergoing an allogeneic HCT in 2010 compared to 6,695 in 2019.
Figure 8a: Allogeneic HCT for different races over time in the United States.
Figure 8b: Autologous HCT for different races over time in the United States.
Survival Rates
Allogeneic HCT
Adults who underwent allogeneic HCT during this period (2010–2019), for all indications, had 100 day and 3-year OS rates of 90% and 53%, respectively. This also demonstrates statistically significant improvement (p<0.001) over OS rates of adults transplanted between 2000 and 2009, which was 81% at 100 days and 42% at 3 years. All 95% CI were less than 2%. Among adults transplanted in 2017–2018, the primary cause of death in the first 100 days after an MRD transplantation was disease relapse followed by organ failure. Early (first 100 days) deaths attributed to acute GVHD were more frequent in adults than in children (13% with MRD, 8% with haploidentical donor, and 16% with an unrelated donor). Among adults receiving an HCT from a haploidentical donor, organ failure accounted for most deaths in the first 100 days, followed by infection and relapse; among those receiving HCT from an unrelated donor, the most common cause of early death was disease relapse. For adults dying more than 100 days posttransplant, 56% died from disease relapse. The percentage of deaths after 100 days that were related to chronic GVHD was low (8–13%, depending on donor type).
For children undergoing allogeneic transplantation, for all indications, between 2010 and 2019, the 100 day and 3-year overall survival (OS) rates was 93% and 74%, respectively. In comparison, the OS rates for children undergoing transplantation between 2000 and 2009, were 86% at 100 days and 60% at 3 years. This reflects statistically significant improvement at both timepoints (100 days and 3 years, p<0.001). All 95% CI were less than 2%. Among children transplanted in 2017–2018, the primary cause of death in the first 100 days after an MRD HCT was disease relapse. For children receiving an HCT from a haploidentical or unrelated donor, organ failure accounted for most deaths in the first 100 days, followed by infection and relapse. Deaths from acute graft-versus-host disease (GVHD) were rare. For children dying more than 100 days posttransplant, most died from relapse (54%) and with only a minority from complications related to chronic GVHD (5%).
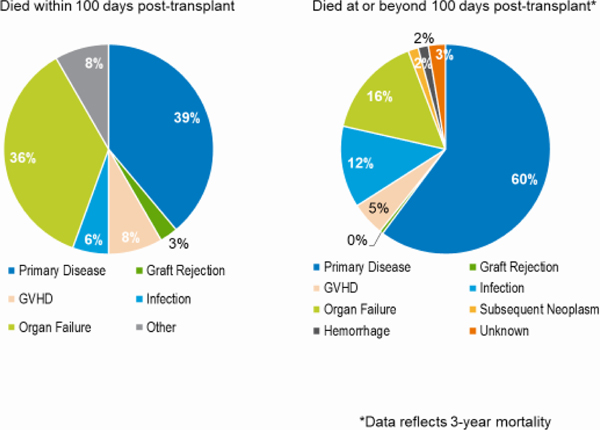
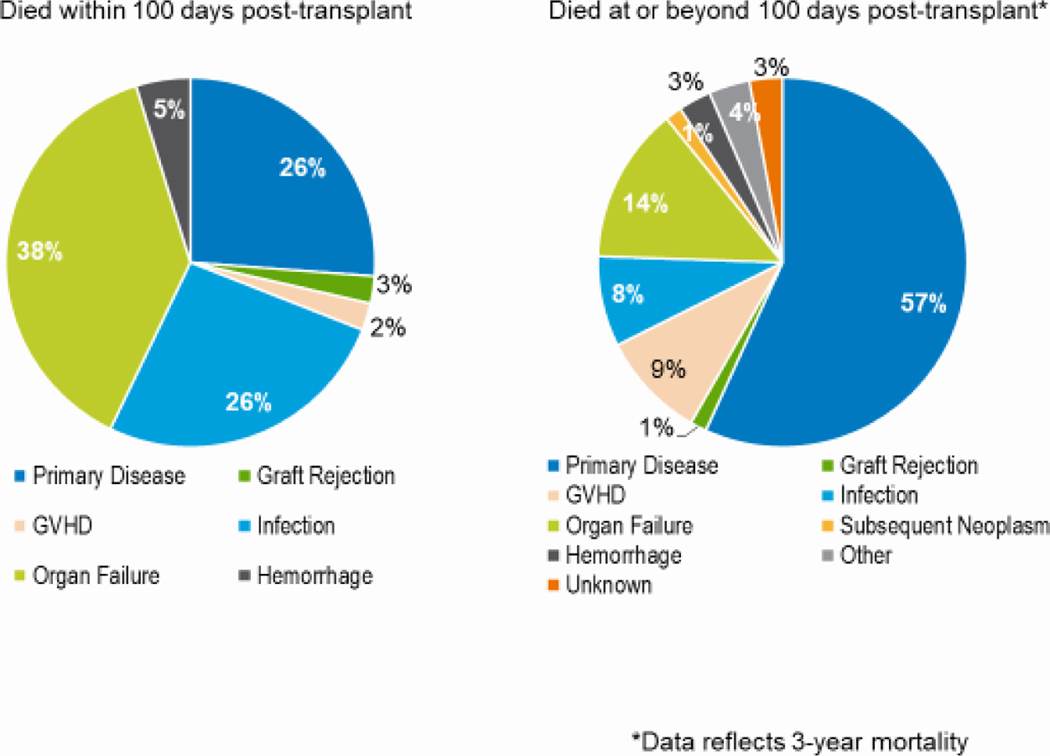
AYA patients had survival outcomes that were worse than children but better than adults. Between 2010 and 2019, this age group had OS rates at day 100 and 3 years of 92% and 64%, respectively. These were significantly higher than OS rates of AYA patients transplanted between 2000 and 2009 (82% at 100 days and 47% at 3 years, p<0.001). All 95% CI were less than 2%. The gains in OS at 100 days and 3 years between these 2 time periods were higher in the AYA than in the pediatric or adult groups. Among AYAs transplanted in 2017–2018, the primary cause of both early and later deaths was disease relapse. Among those receiving haploidentical or unrelated donor HCT, the primary cause of early death was organ failure; disease relapse was the primary cause of deaths occurring more than 100 days after HCT (Figures 9a–c). Table 1 demonstrates worse 3-year survival probabilities after HCT for ALL in the AYA population compared to children in comparison to the pediatric population for ALL, but similar survival for AYAs and children after HCT for AML.
Figure 9a: Causes of death after AYA (15–39 years) matched related HCT in the United States, 2017–2018.
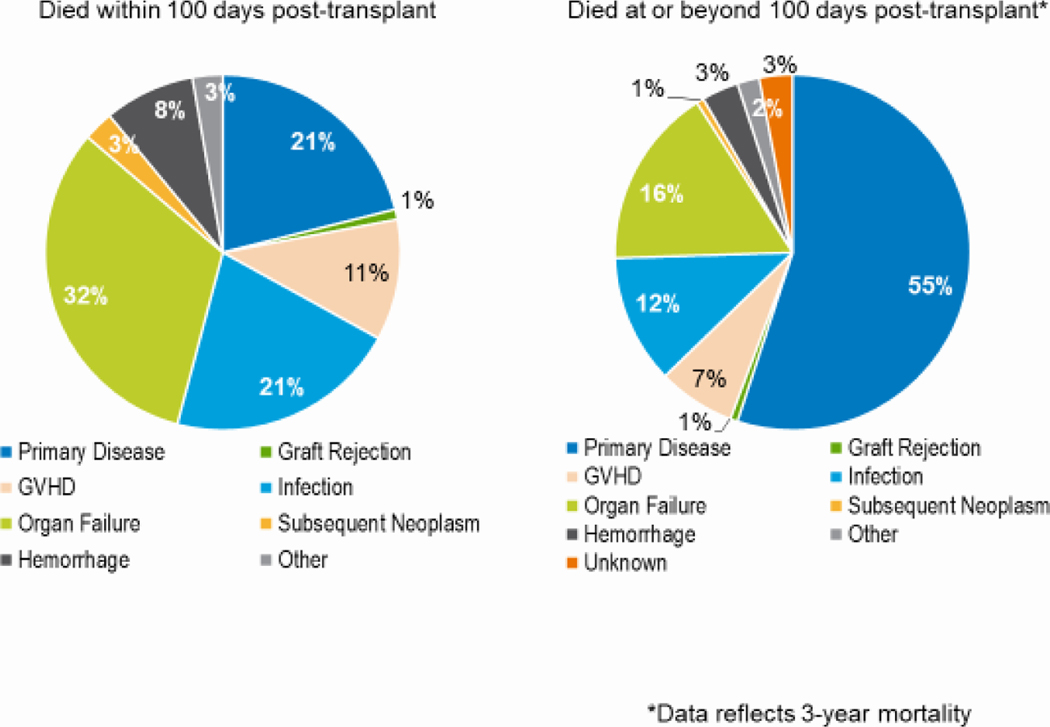
Figure 10: Causes of death after AYA (15–39 years) autologous HCT in the United States, 2017–2018.
Table 1:
Three-Year Survival Probabilities with 95% CIs for Various Malignant and Non-Malignant Disorders, 2010–2019
| Disorder | Matched Related Donor (MRD), % | Unrelated Donor (URD), % | |||||
|---|---|---|---|---|---|---|---|
| CR1 | CR2+ | Relapse/Never in CR | CR1 | CR2 | Relapse/Never in CR | ||
| AML | Pediatric | 69 (63–74) | 66 (57–74) | 29 (18–41) | 60 (56–65) | 63 (57–69) | 34 (26–43) |
| AYA | 69 (65–72) | 69 (63–75) | 41 (35–47) | 65 (63–68) | 59 (55–63) | 32 (28–37) | |
| Adult | 58 (57–60) | 53 (50–56) | 32 (30–34) | 55 (54–57) | 52 (50–54) | 31 (29–32) | |
| ALL | Pediatric | 79 (73–83) | 71 (66–75) | 59 (42–75) | 75 (70–79) | 63 (59–67) | 62 (48–75) |
| AYA | 71(68–74) | 55 (49–60) | 37 (28–47) | 69 (66–72) | 51 (47–55) | 38 (30–46) | |
| Adult | 63 (61–66) | 45 (41–50) | 36 (30–43) | 63 (61–65) | 44 (40–47) | 36 (31–42) | |
| Early | Advanced | Early | Advanced | ||||
| MDS | 54 (50–58) | 46 (44–49) | 50 (47–52) | 44 (43–46) | |||
| Chronic | Accelerated | Blast | Chronic | Accelerated | Blast | ||
| CML | 66 (60–71) | 47 (35–59) | 34 (21–48) | 62 (58–66) | 54 (45–64) | 35 (24–47) | |
| CLL | 64 (59–68) | 58 (55–61) | |||||
| Myelofibrosis | 65 (61–69) | 56 (53–59) | |||||
| Other MPN | 57 (52–62) | 57 (53–61) | |||||
| SAA | Pediatric | 98 (96–99) | 86 (83–89) | ||||
| Adult | 84 (81–87) | 75 (71–78) | |||||
AML indicates acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; CLL, chronic lymphoblastic leukemia; CML, chronic myelogenous leukemia; SAA, severe aplastic anemia
Autologous HCT
OS rates after autologous HCT between 2010 and 2019 were fairly consistent across the age spectrum. Adults had OS rates at 100 days and three years after HCT of 98% and 80%. Corresponding rates for children were 97% and 75%, respectively. Corresponding rates for AYAs were 98% and 82%. All 95% CI were less than 3%. These OS rates, across all ages, were significantly higher (p<0.001) than the rates for patients transplanted in 2000–2009. Adults transplanted in 2000–2009 had OS rates at 100 days and three years after HCT of 96% and 69%. Corresponding rates for children were 96% and 65%, respectively. Corresponding rates for AYAs were 96% and 71%. All 95% CI were less than 3%.
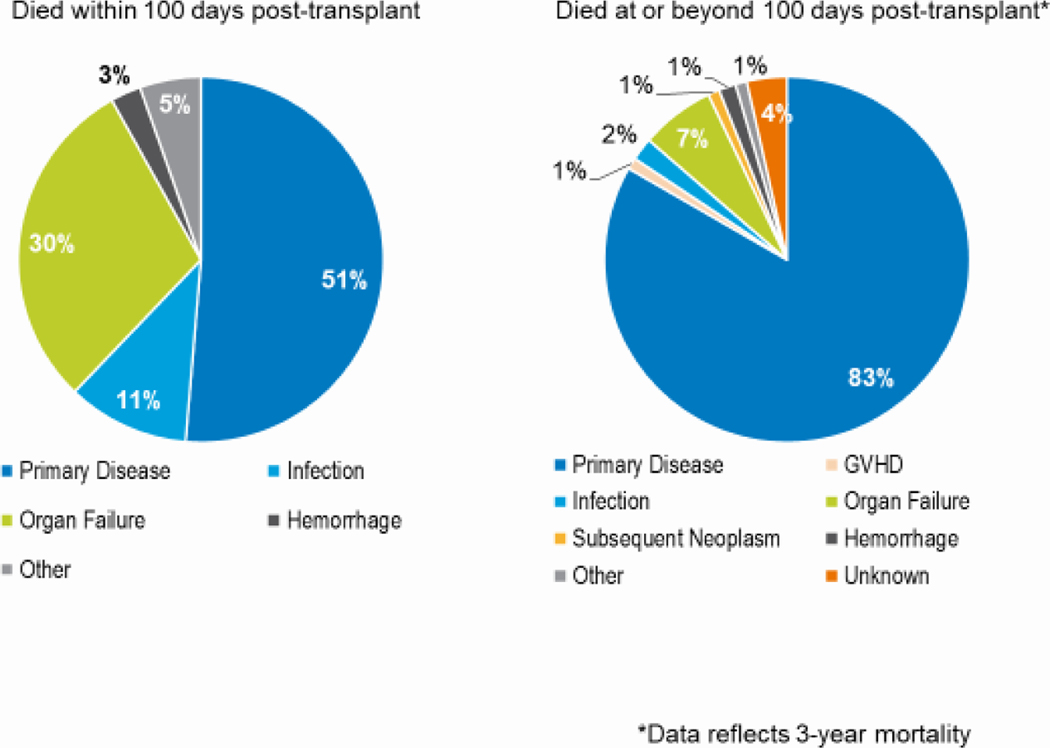
Relapse or persistence of the primary disease was the primary cause of early and late deaths after autologous HCT in all age groups (Figure 10). Among patients dying in the first 100 days after, disease relapse accounted for 38%, 44%, and 51% of deaths in the adult, pediatric, and AYA groups, respectively. Among those dying more than 100 days after HCT, relapse accounted for 76%, 86%, and 83% of deaths in the adult, pediatric, and AYA groups, respectively.
Table 1 and Table 2 show estimated 3-year survival rates in children and adults with various diseases treated with allogeneic and autologous HCT in 2010–2019.
Table 2:
Three-Year Survival Probabilities with 95% CIs for Various Lymphomas, 2010–2019
| Autologous HCT, % | Allogeneic HCT, % | |||||
|---|---|---|---|---|---|---|
| Chemosensitive | Chemoresistant | Chemosensitive | Chemoresistant | |||
| MRD | URD | MRD | URD | |||
| HL | 90 (88–90) | 78 (75–82) | 71 (66–75) | 64 (60–69) | 47 (35–59) | 58 (49–67) |
| FL | 83 (81–85) | 67 (58–76) | 78 (74–83) | 71 (66–75) | 63 (50–76) | 55 (44–66) |
| DLBCL | 70 (69–71) | 52 (48–56) | 58 (54–62) | 52 (48–56) | 34 (27–42) | 30 (23–37) |
HL indicates Hodgkin lymphoma; FL, follicular lymphoma; DLBCL, diffuse large B cell lymphoma
Discussion:
This annual activity report from the CIBMTR demonstrates increasing numbers of total HCTs performed each year and improving survival over time. The expanded use of HCT for patients of older ages is evident in both the autologous and allogeneic setting and may be related to improved supportive care and the effective use of reduced intensity conditioning regimens for patients ineligible for myeloablative therapy.7–13 Recurrence of disease following HCT remains the major cause of death, highlighting the need for improved upfront and maintenance strategies for malignancies. Most patients with leukemia are being treated in CR, which likely reflects advancements in early identification of high-risk patients and improved pretransplant therapies leading to subsequent disease control prior to HCT.
Notably, the use of MRDs decreased over this decade with an increase in haploidentical donors in recent years, improving access to those with limited traditional donor options.14–16 The previously unacceptable risk of GVHD associated with the use of haploidentical donors has been largely overcome with new prophylactic strategies, such as incorporating post-transplant cyclophosphamide (PTCy) or ex-vivo T-cell depletion.17–23 The former strategy is increasingly being used in other settings, including MMUDs, MUDs and MRDs.24,25 Respondents to a recent survey of practicing HCT physicians asking them to predict near-future trends in practice showed that most anticipate increases in haploidentical and MMUD use, with a decrease in the use of UCB.26 Increased numbers of alloHCTs in Black and African American patients reflects, in part, the expanded use of haploidentical donors in this specific patient population who are unlikely to identify a MUD in a timely manner. Effective use of HLA-mismatched donors, both related and unrelated address an important gap in access to allogeneic HCT for patients from racial and ethnic groups other an non-Hispanic white.27–29 The ACCESS trial (ClinicalTrials.gov Identifier: NCT04904588) is a Phase II, prospective, multicenter trial sponsored by NMDP that seeks to further understand the safety and efficacy of the use of MMUD peripheral blood HCT with PTCy as treatment for hematologic malignancies in both the pediatric and adult setting. This trial will expand upon the encouraging results seen in a prior prospective phase II study which enrolled patients with hematologic malignancies (age 15–71), who were eligible for bone marrow HCT with PTCy and had no suitable matched-sibling or MUD. The primary outcome of OS was successfully met, and it was noted that 48% of those who enrolled on the trial were from a racial or ethnic minority group.25
Cancer patients classified as AYA have been previously identified as a population requiring further focus and investigation to improve outcomes.4 Outcomes for various cancers are worse for AYA patients than their pediatric counterparts, which may reflect several factors, including biology of the disease, lower clinical trial enrollment and availability, and compliance with therapies. 30–32 Like pediatric patients, most HCTs performed in the AYA population are allogeneic and for malignant diseases. However, differing upfront treatment modalities in pediatric vs adult centers may lead to significantly different outcomes, specifically in the AYA population undergoing treatment for ALL.33–35 Unfortunately, we do not have robust data on whether AYAs were transplanted in primarily adult or pediatric centers to be able to examine this factor as a potential explanatory variable. A combined CIBMTR and Alliance for Clinical Trials in Oncology (CALGB 10403) analysis comparing outcomes of AYA patients with Ph-negative ALL in CR1, highlighted the importance of investigating differing approaches between pediatric and adult-based centers. The study demonstrated improved outcomes with pediatric-style chemotherapy regimens alone in select AYA patients in comparison to proceeding straight to allogeneic HCT in CR1.33 The data summarized here indicate that outcomes for the AYA population who are eligible and who do go on to receive allogeneic HCT are comparable to pediatric patients for AML and comparable to adult patients for ALL. The data also indicate that more AYA patients undergo HCT in CR1 than pediatric patients, which may indicate continued treatment on adult-based protocols where patients are more often taken to HCT in first remission, rather than continuing on chemotherapy-based protocols. These findings are consistent with those reported previously, and highlight the need for even further study to summarize practice patterns and improve ALL outcomes in this population.36–39 The current data also show that graft sources used in AYA patients are a mix of that seen with pediatric and adult patients, likely also reflecting treating institution practice differences (pediatric vs adult). Similar mixed trends are seen in donor type. MUD use is most prevalent in the adult setting, followed by AYA, then pediatrics. More UCB donors are used for AYA than adult patients, but fewer than for pediatric patients. Encouragingly, when comparing overall survival for all indications over time (2000–2009 vs 2010–2019), these data demonstrate significant improvements in all age groups, but more so in the AYA group.
Prior studies, including those using CIBMTR data, show loss to follow-up is an issue for AYA patients, who are often in a period of their lives where numerous life changes are occurring. This may be one contributor to poorer 3-year OS in comparison to the pediatric cohort. A focus on transition and survivorship care for AYA patients is critical to prevent attrition and provide the ability to screen for ongoing or late-occurring side-effects of prior therapies.40,41 Prior studies conducted by the CIBMTR show that occurrence of late effects in this population is frequent and that other aspects of life, including return to work or school, are highly impacted by the HCT process.42,43
Conclusion:
CIBMTR has been collecting longitudinal patient outcome data for almost 50 years. As HCT indications, conditioning regimens, donor types/access, supportive cares, and novel upfront therapies emerge, it is useful to continually re-evaluate the HCT landscape. Such evaluations identify the most important areas for continued improvement (e.g. prevention of relapse), identify therapies that have changed practice (e.g. post-transplant cyclophosphamide to allow transplantation across HLA barriers) and reveal disparities in access (e.g. under-representation of individuals of a specific race or ethnicity). Although CIBMTR initially collected data only for HCT recipients, this was recently expanded to include recipients of non-HCT cellular therapies, where rapidly evolving technologies will necessitate the same kind of population-level evaluations.
The CIBMTR is committed to collecting and disseminating high-quality and accessible data to improve outcomes in patients undergoing HCT and other cellular therapies. This annual report of the current state of HCT activity in the United States, along with the annual summary slides posted at https://www.cibmtr.org, are intended to give investigators, clinicians, patients, and payors access to accurate and timely data. Future registry-based studies and clinical trials concentrating on the AYA population will be critical for better understanding the trends in HCT and subsequently identifying ways to continue improvements in various short and long-term outcomes.
Supplementary Material
Figure 1b: Estimated annual number of HCT recipients in the United States by transplant type, adult recipients (≥ 18 years) in the United States.
Figure 1c: Estimated annual number of HCT recipients in the United States by transplant type, pediatric recipients (<18 years) in the United States.
Figure 9b: Causes of death after AYA (15–39 years) haploidentical HCT in the United States, 2017–2018.
Figure 9c: Causes of death after AYA (15–39 years) unrelated donor HCT in the United States, 2017–2018.
Highlights.
As supportive cares, donor selection, and treatment modalities evolve, documenting HCT trends and outcomes is critical.
The expanded use of HCT for patients of older ages is evident in both the autologous and allogeneic setting.
There has been an increase in haploidentical donor use in recent years, as well as studies evaluating the used of mismatched unrelated donors, both potentially improving access to HCT for those with limited traditional donor options.
Recurrence of disease following HCT remains the major cause of death, highlighting the need for improved upfront and maintenance strategies for malignancies.
When comparing the time period of 2000–2009 to 2010–2019, improvements in overall survival were significant across the age spectrum, but greatest in the adolescent and young adult (AYA) age group.
Financial Disclosure Statement:
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014–20-1–2705 and N00014–20-1–2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Accenture; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allovir, Inc.; Amgen, Inc.; Astellas Pharma US; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Fate Therapeutics; Gamida-Cell, Ltd.; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Kadmon; Karius; Karyopharm Therapeutics; Kiadis Pharma; Kite Pharma Inc; Kite, a Gilead Company; Kyowa Kirin International plc; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Medac GmbH; Medexus; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OncoImmune, Inc.; Oncopeptides, Inc.; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc; Pfizer, Inc.; Pharmacyclics, LLC; Priothera; Sanofi Genzyme; Seagen, Inc.; Stemcyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Tscan; Vertex; Vor Biopharma; Xenikos BV.
Data Use Statement:
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
Conflicts of Interest:
Dr. Phelan reports Advisory Board from bluebird Bio; Research support from Amgen.
Dr. D’Souza reports Institutional research funding from Sanofi, TeneoBio and Takeda; consulting fees from Janssen; advisory board from Imbrium, Akcea and Pfizer; and payments: Janssen: member of Independent Review Committee for a multinational phase 3 clinical trial, 2/2020-current.
Dr. Hamadani reports compensation from Incyte Corporation, ADC Therapeutics, Magenta Therapeutics, Omeros, AbGenomics, MorphoSys, Kite, Novartis, GenMab, Seattle Genetics, Sanofi Genzyme, Astrazeneca, BeiGene, Gamida cell, Legend Biotech, Kadmon.
Dr. Lee reports Spouse Consultant: 4SC, EMD Serono, Genzyme, Merck Sharpe Dohme, Phizer, Regeneron, Sanofi and Spouse Honoraria: Wolters Kluwer; research funding from Amgen, AstraZeneca, Incyte, Kadmon, Novartis, Pfizer, Syndax, Takeda; consulting from Mallinckrodt, Equillium, Kadmon; Steering committee (Incyte) Spouse: Research funding: BMS, EMD Serono; proprietary interests: Spouse: IP for immunotherapy for Merkel Cell carcinoma.
Dr. Pasquini reports CIBMTR related conflict of interest (research support to MCW by an industry sponsored in which I am listed as the PI): Novartis (active), Kite (active), BMS (active), Amgen (completed 2019), Consultancy (listed me as the professional providing insite), BMS (CAR T cell Steering Committee – active until June 2021).
Dr. Riches reports payments from Jazz Pharmaceuticals, ATARA Biotherapeutics; Employee- IQVIA Biotech.
Dr. Weisdorf reports research support from Incyte and Fate Therapeutics, and compensation, paid to my institution for my role as Senior Research Advisor and Scientific Director of CIBMTR.
Dr. Shaw reports consulting fees from Orca bio and mallinkrodt.
Dr. Arora reports compensation from Fate Therapeutics and research funding from Pharmacyclics, Kadmon and Syndax.
Dr. Devine reports research support paid to NMDP from Magenta, Vor Bio, Orca Bio; Advisor to Janssen, BMS, Sanofi, Orca and Vor Bio.
Dr. Chhabra reports Institutional research funding from Amgen, Janssen, Sanofi, and Syndax; honoraria for advisory board from GSK and Sanofi.
Dr. Stefanski reports Advisory Board from Novartis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kanate AS, Majhail NS, Savani BN, et al. : Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant 26:1247–1256, 2020 [DOI] [PubMed] [Google Scholar]
- 2.D’Souza A, Fretham C, Lee SJ, et al. : Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant 26:e177–e182, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geiger AM, Castellino SM: Delineating the age ranges used to define adolescents and young adults. J Clin Oncol 29:e492–3, 2011 [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Adolescent and young adult oncology version 1.2022. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 1 September 2021.
- 5.Mehta PA, Rotz SJ, Majhail NS: Unique Challenges of Hematopoietic Cell Transplantation in Adolescent and Young Adults with Hematologic Malignancies. Biol Blood Marrow Transplant 24:e11–e19, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Friend BD, Tang K, Markovic D, et al. : Identifying risk factors associated with worse outcomes in adolescents and young adults undergoing hematopoietic stem cell transplantation. Pediatr Blood Cancer 66:e27940, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Munshi PN, Vesole DH, St Martin A, et al. : Outcomes of upfront autologous hematopoietic cell transplantation in patients with multiple myeloma who are 75 years old or older. Cancer, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munshi PN, Vesole D, Jurczyszyn A, et al. : Age no bar: A CIBMTR analysis of elderly patients undergoing autologous hematopoietic cell transplantation for multiple myeloma. Cancer 126:5077–5087, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra A, Preussler JM, Bhatt VR, et al. : Breaking the Age Barrier: Physicians’ Perceptions of Candidacy for Allogeneic Hematopoietic Cell Transplantation in Older Adults. Transplant Cell Ther 27:617 e1–617 e7, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muffly L, Pasquini MC, Martens M, et al. : Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood 130:1156–1164, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devine SM, Owzar K, Blum W, et al. : Phase II Study of Allogeneic Transplantation for Older Patients With Acute Myeloid Leukemia in First Complete Remission Using a Reduced-Intensity Conditioning Regimen: Results From Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. J Clin Oncol 33:4167–75, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oran B, Ahn KW, Fretham C, et al. : Fludarabine and Melphalan Compared with Reduced Doses of Busulfan and Fludarabine Improve Transplantation Outcomes in Older Patients with Myelodysplastic Syndromes. Transplant Cell Ther 27:921 e1–921 e10, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malagola M, Polverelli N, Rubini V, et al. : Gitmo Registry Study on Allogeneic Transplantation in Patients Aged over 60 from 2000 to 2017. Improvements and Criticisms. Transplant Cell Ther, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Ciurea SO, Al Malki MM, Kongtim P, et al. : The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant 55:12–24, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Aljurf M, Weisdorf D, Alfraih F, et al. : “Worldwide Network for Blood & Marrow Transplantation (WBMT) special article, challenges facing emerging alternate donor registries”. Bone Marrow Transplant 54:1179–1188, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisdorf D: Can haploidentical transplantation meet all patients’ needs? Best Pract Res Clin Haematol 31:410–413, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Bashey A, Solomon SR: T-cell replete haploidentical donor transplantation using post-transplant CY: an emerging standard-of-care option for patients who lack an HLA-identical sibling donor. Bone Marrow Transplant 49:999–1008, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Bashey A, Zhang X, Jackson K, et al. : Comparison of Outcomes of Hematopoietic Cell Transplants from T-Replete Haploidentical Donors Using Post-Transplantation Cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 Allele-Matched Unrelated Donors and HLA-Identical Sibling Donors: A Multivariable Analysis Including Disease Risk Index. Biol Blood Marrow Transplant 22:125–33, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Wieduwilt MJ, Metheny L, Zhang MJ, et al. : Haploidentical vs. sibling, unrelated, or cord blood hematopoietic cell transplantation for acute lymphoblastic leukemia. Blood Adv, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahebi F, Eikema DJ, Koster L, et al. : Post-Transplant Cyclophosphamide for Graft vs Host Disease Prophylaxis in Multiple Myeloma Patients Who Underwent Allogeneic Hematopoietic Cell Transplantation: First Comparison by Donor Type: A Study from the Chronic Malignancies Working Party of the EBMT. Transplant Cell Ther, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Aydin M, Dovern E, Leeflang MMG, et al. : Haploidentical Allogeneic Stem Cell Transplantation in Sickle Cell Disease: a Systematic Review and Meta-analysis. Transplant Cell Ther, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Prezioso L, Manfra I, Bonomini S, et al. : Haploidentical hematopoietic stem cell transplantation in adults using the alphabetaTCR/CD19-based depletion of G-CSF-mobilized peripheral blood progenitor cells. Bone Marrow Transplant 54:698–702, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Sahasrabudhe K, Otto M, Hematti P, et al. : TCR alphabeta+/CD19+ cell depletion in haploidentical hematopoietic allogeneic stem cell transplantation: a review of current data. Leuk Lymphoma 60:598–609, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw BE: Related haploidentical donors are a better choice than matched unrelated donors: Counterpoint. Blood Adv 1:401–406, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw BE, Jimenez-Jimenez AM, Burns LJ, et al. : National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide. J Clin Oncol 39:1971–1982, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farhadfar N, Burns LJ, Mupfudze T, et al. : Hematopoietic Cell Transplantation: Practice Predictions for the Year 2023. Transplant Cell Ther 27:183 e1–183 e7, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dehn J, Chitphakdithai P, Shaw BE, et al. : Likelihood of Proceeding to Allogeneic Hematopoietic Cell Transplantation in the United States after Search Activation in the National Registry: Impact of Patient Age, Disease, and Search Prognosis. Transplant Cell Ther 27:184 e1–184 e13, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshua TV, Rizzo JD, Zhang MJ, et al. : Access to hematopoietic stem cell transplantation: effect of race and sex. Cancer 116:3469–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niederwieser D, Baldomero H, Bazuaye N, et al. : One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari A, Stark D, Peccatori FA, et al. : Adolescents and young adults (AYA) with cancer: a position paper from the AYA Working Group of the European Society for Medical Oncology (ESMO) and the European Society for Paediatric Oncology (SIOPE). ESMO Open 6:100096, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw PH, Reed DR, Yeager N, et al. : Adolescent and Young Adult (AYA) Oncology in the United States: A Specialty in Its Late Adolescence. J Pediatr Hematol Oncol 37:161–9, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Smith AW, Keegan T, Hamilton A, et al. : Understanding care and outcomes in adolescents and young adult with Cancer: A review of the AYA HOPE study. Pediatr Blood Cancer 66:e27486, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wieduwilt MJ, Stock W, Advani A, et al. : Superior survival with pediatric-style chemotherapy compared to myeloablative allogeneic hematopoietic cell transplantation in older adolescents and young adults with Ph-negative acute lymphoblastic leukemia in first complete remission: analysis from CALGB 10403 and the CIBMTR. Leukemia 35:2076–2085, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S, Pole JD, Baxter NN, et al. : The effect of adopting pediatric protocols in adolescents and young adults with acute lymphoblastic leukemia in pediatric vs adult centers: An IMPACT Cohort study. Cancer Med 8:2095–2103, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muffly L, Alvarez E, Lichtensztajn D, et al. : Patterns of care and outcomes in adolescent and young adult acute lymphoblastic leukemia: a population-based study. Blood Adv 2:895–903, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta PA, Zhang MJ, Eapen M, et al. : Transplantation Outcomes for Children with Hypodiploid Acute Lymphoblastic Leukemia. Biol Blood Marrow Transplant 21:1273–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke MJ, Verneris MR, Le Rademacher J, et al. : Transplant Outcomes for Children with T Cell Acute Lymphoblastic Leukemia in Second Remission: A Report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 21:2154–2159, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burke MJ, Gossai N, Cao Q, et al. : Similar outcomes between adolescent/young adults and children with AML following allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 49:174–8, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Majhail NS, Brazauskas R, Hassebroek A, et al. : Outcomes of allogeneic hematopoietic cell transplantation for adolescent and young adults compared with children and older adults with acute myeloid leukemia. Biol Blood Marrow Transplant 18:861–73, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyamura K, Yamashita T, Atsuta Y, et al. : High probability of follow-up termination among AYA survivors after allogeneic hematopoietic cell transplantation. Blood Adv 3:397–405, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buchbinder D, Brazauskas R, Bo-Subait K, et al. : Predictors of Loss to Follow-Up Among Pediatric and Adult Hematopoietic Cell Transplantation Survivors: A Report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 26:553–561, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatt NS, Brazauskas R, Tecca HR, et al. : Post-transplantation employment status of adult survivors of childhood allogeneic hematopoietic cell transplant: A report from the Center for International Blood and Marrow Transplant Research (CIBMTR). Cancer 125:144–152, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kornblit B, Enevold C, Wang T, et al. : Toll-like receptor polymorphisms in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 21:259–65, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.