To the Editor:
Remestemcel-L is comprised of culture-expanded mesenchymal stromal cells derived from the bone marrow of an unrelated donor and under investigation as treatment for graft-vs-host disease (GVHD). A phase 3 multicenter single arm study (NCT02336230) of 54 children with steroid-refractory (SR) GVHD treated with remestemcel-L as second-line therapy has recently been reported (1). Here we report the analyses of the Mount Sinai Acute GVHD International Consortium (MAGIC) Algorithm Probability of 25 of the 54 pediatric study patients with serum samples taken at the beginning of treatment and compare them to 27 closely matched pediatric patients with SR acute GVHD who participated in a prospective GVHD natural history study. Control patients were included if they met the remestemcel-L study criteria for GVHD severity at the time they received second line therapy and had serum samples available when second line therapy was initiated. All biomarker assays were conducted by investigators blinded to clinical outcomes.
All 52 of these patients in both groups had GVHD that had either worsened within 3 days, not responded within 7 days, or progressed after an initial response to treatment with systemic steroids and was Grade B-D GVHD according to the IBMTR severity scale (2) at the time second line treatment was initiated. In order to enrich for patients with more severe disease, patients with steroid-refractory grade B skin-only disease (i.e., stage 2 skin rash only) were not eligible for the remestemcel-L study and such patients were also excluded from the control group. Figure 1A shows a comparison of baseline characteristics at the time of second treatment. There were no significant differences between the two groups for age, time to second line treatment, Minnesota high risk or multi organ involvement. There was a significantly higher percentage of clinically severe (grade III/IV) acute GVHD in the remestemcel-L group (20/25 (85%) v 16/27 (59%), p=0.02). Thus, a very large percentage of these patients had severe, high risk clinical disease at the initiation of remestemcel-L therapy. Second line treatments in the control group were determined by the treating physician and included extracorporeal photopheresis, etanercept, infliximab, ruxolitinib, anti-thymocyte globulin, mycophenolate, alemtuzumab, basiliximab and tocilizumab. The clinical response after 28 days in the remestemcel-L group (18/25, 72%) was higher than in the control group (13/27, 48%) although the difference did not mark statistical significance (p=0.08).
Figure 1. Baseline characteristics and 6 month survival of SR acute GVHD patients according to treatment.
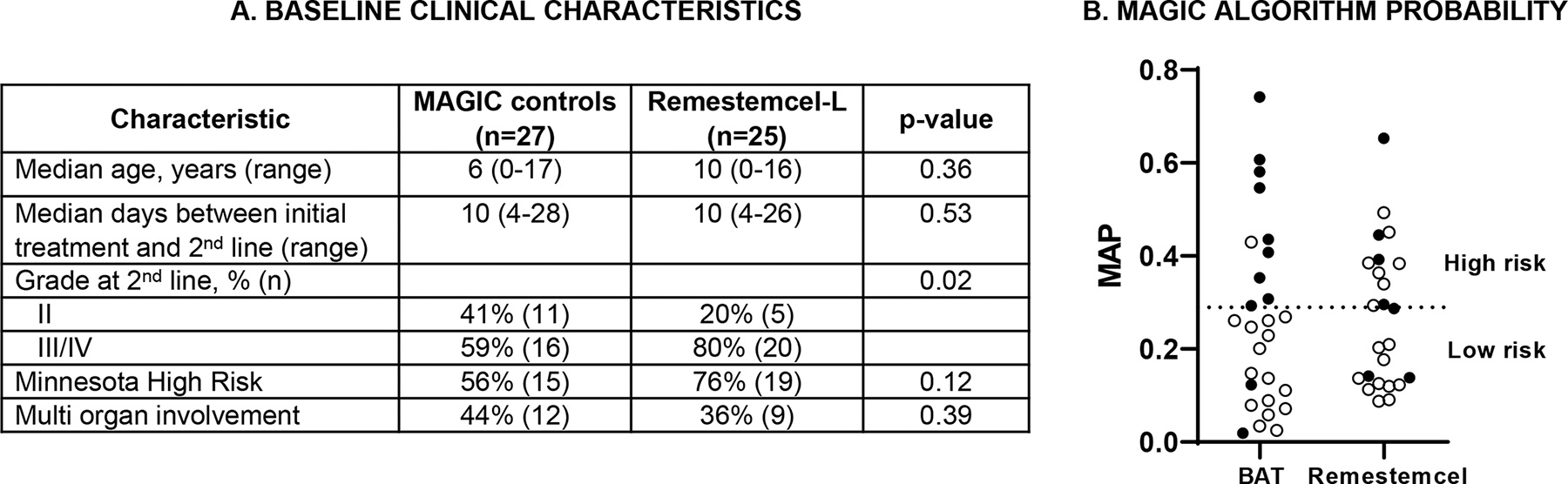
(A) Baseline clinical characteristics of patients receiving second line systemic therapy for steroid resistant acute GVHD. (B) Patients alive (open circles) and dead (solid circles) at day +180 according to MAP at baseline. 1/10 high risk patients treated with BAT were alive compared to 7/11 receiving remestemcel-L (64% vs 10%, p=0.01). The high risk threshold of 0.291 is indicated by the dotted line.
The MAGIC Algorithm Probability (MAP) combines the serum concentrations of two biomarkers, Reg3α and ST2, into a single value that predicts long term outcomes such as response to therapy after 28 days and 6 month non-relapse mortality (NRM) (3). The MAP is an accurate monitoring biomarker of GVHD in the GI tract and is considered a “liquid biopsy” of crypt damage (4). MAPs ≥0.291 predict high NRM and poor responses to therapy in SR acute GVHD (5). We measured the serum concentrations of Reg3α and ST2 by ELISA and calculated the MAP for all 52 patients. At baseline the mean MAPs were similar between the study patients treated with remestemcel-L vs MAGIC controls (0.283±0.17 vs 0.262±0.20, p=0.67). 48% (12/25) remestemcel-L study patients and 37% (10/27) of MAGIC control patients were high risk by baseline MAP ≥0.291. One remestemcel-L patient was lost to follow-up and excluded from the survival analysis. As shown in Figure 1B, survival at day +180 was significantly better for patients with a high risk MAP treated with remestemcel-L compared to high risk control patients who received best available therapy (7/11, 64% vs 1/10, 10%, p = 0.01). Consistent with these survival results, 8/12 study patients treated with remestemcel-L, who were high risk by biomarkers, achieved a clinical response by day 28 of remestemcel-L therapy, compared to 1/10 MAGIC patients that received the best available treatment (67% vs 10%, p = 0.01).
We have previously shown that MAPs segregate patients with steroid resistant GVHD into two groups with strikingly different outcomes based on the threshold of 0.291 (6). The MAP has been shown to more accurately predict GVHD outcomes than clinical response throughout the first month of systemic therapy, a timeframe that includes all of the patients in both the control and the remestemcel-L groups (6). The predictive accuracy of the MAP can be attributed to the fact that it better estimates the damage to GI crypts, a key biologic metric of GI tract health, than does the volume of diarrhea. Patients with high MAPs can be considered true non-responders who have significant GI crypt damage and a very low probability of achieving a clinical response to additional therapy within 28 days; these patients have a low overall survival rate at six months on the order of 20% (5). Patients who have SR acute GVHD and low MAPs have less extensive GI crypt damage and are very likely to be slow responders, and may in fact not need additional treatment; the overall survival after six months of treatment for these patients is approximately 80%. The control patients from the MAGIC database who received best available therapy exhibited this pattern: 15 of 17 (88%) patients with low MAPs survived six months, whereas only 1 of 10 (10%) with high MAPs survived. But study patients who received remestemcel-L therapy showed a much different pattern: survival in patients with a low MAP (11/13, 85%) was high as expected, but survival in patients with a high MAP (7/11, 64%), was also high and significantly better compared to the controls with high MAPs (1/10, 10%). As noted above, this pattern was also seen in the response rate of patients 28 days after therapy. The fact that nearly two thirds of study patients who were categorized as high risk by biomarkers responded to remestemcel-L therapy and survived six months is clinically important.
The mechanism of action of mesenchymal stromal cells (MSCs) in reducing GVHD is not entirely clear and may be multifactorial. In vitro, MSCs have several immunomodulatory properties and reduce the secretion of inflammatory cytokines such as IFNγ and TNFα by alloreactive T cells (7). In vivo, infusion of MSCs reduced the expansion of donor T cells and reduced damage in target organs in experimental GVHD models (8). It is possible that remestemcel-L exerts its beneficial effect through a number of mechanisms, including wound healing properties in the GI tract, since the analysis of MAPs show that GI crypt damage was quite extensive at the initiation of remestemcel-L therapy and that the large majority of these study patients not only experienced clinical responses within twenty eight days but 87% were able to discontinue immunosuppressive therapy within six months (1). In summary, nearly one half of pediatric patients with SR-acute GVHD at the initiation of remestemcel-L therapy were diagnosed with severe lower GI disease as measured by MAP, and 64% of these patients survived, compared to 10% of patients with SR-acute GVHD and high MAPs from the MAGIC database. These findings support and extend the recently published results that showed that children with severe, SR acute GVHD benefit from remestemcel-L therapy.
Acknowledgments
FUNDING:
National Cancer Institute (P01CA039542 and P30CA196521), the Pediatric Cancer Foundation and Mesoblast
Footnotes
Competing interests: Mesoblast provided funding for this study. FS, JH, FG, and EB are employees of Mesoblast, JEL and JLMF have received consulting fees from Mesoblast and royalties from a GVHD biomarker patent
References
- 1.Kurtzberg J, Abdel-Azim H, Carpenter P, Chaudhury S, Horn B, Mahadeo K, et al. A phase 3, single-arm, prospective study of remestemcel-L, ex vivo culture-expanded adult human mesenchymal stromal cells for the treatment of pediatric patients who failed to respond to steroid treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2020;26(5):845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahn JY, Klein JP, Lee SJ, Milpied N, Blaise D, Antin JH, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Société Française de Greffe de Moëlle et Thérapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106(4):1495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartwell MJ, Özbek U, Holler E, Renteria AS, Major-Monfried H, Reddy P, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017;2(3):e89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrara JLM, Chaudhry MS. GVHD: biology matters. Blood Adv. 2018;2(22):3411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Major-Monfried H, Renteria AS, Pawarode A, Reddy P, Ayuk F, Holler E, et al. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood. 2018;131(25):2846–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinagesh HK, Özbek U, Kapoor U, Ayuk F, Aziz M, Ben-David K, et al. The MAGIC algorithm probability is a validated response biomarker of treatment of acute graft-versus-host disease. Blood Adv. 2019;3(23):4034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22. [DOI] [PubMed] [Google Scholar]
- 8.Auletta JJ, Eid SK, Wuttisarnwattana P, Silva I, Metheny L, Keller MD, et al. Human mesenchymal stromal cells attenuate graft-versus-host disease and maintain graft-versus-leukemia activity following experimental allogeneic bone marrow transplantation. Stem Cells. 2015;33(2):601–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


