Abstract
Red pepper (Capsicum annum L.) is an increasingly important economic crop in the world. Thus, this study aimed to investigate the growth, physiological, and biochemical responses of red pepper cultivars under drought stress conditions. A pot culture experiment was conducted in a completely randomized design with three replications, four treatments, and three cultivars. Totally, 36 pots and six seeds per pot were used to grow the seeds. After five weeks, the cultivars were exposed to different drought stress conditions (100% FC or control, 80% FC or low stress, 60% FC or moderate stress, and 40% FC or severe stress). All the collected data were subjected to an analysis of variance (ANOVA). Shoot length was reduced significantly (p < 0.05) in the Hagerew cultivar under severe drought stress. The photosynthesis rate was reduced by 21.11% (p < 0.05) in the Mitmita cultivar under severe drought stress. The highest percentage reduction of chlorophyll content (77.28%) was recorded in the Hagerew cultivar. Both Markofana and Mitmita responded to drought stress by increasing the accumulation of proline and phenolic compounds. The root-to-shoot ratio was increased significantly in both Markofana and Mitmita cultivars (27.91% and 50.92%), respectively, under drought-stress conditions. This study depicted that the cultivar Mitmita was the most drought-tolerant cultivar among the three cultivars.
1. Introduction
Red pepper (Capsicum annum L.) belongs to the family Solanaceae and originated in the world's tropics and subtropics over 2000 years ago [1]. The crop is grown widely under various environmental and climatic conditions. Hot pepper is the world's third most important vegetable, next to potatoes and tomatoes [2, 3], which produced approximately 40 in 2020 (World) and 7.70 (Africa) million tons of green fruit [4]. Red pepper cultivars are widely grown in various parts of Ethiopia, particularly in the Amhara, Oromia, and Southern Nations Nationalities, and Peoples Regional States regions [5]. According to MoARD [6]; the total pepper production was 0.25 million tons from 118, 987 hectares in Ethiopia. The production in the green form was 220, 791 tons from 97, 712 ha, and 118, 514 tons in the dry form from an area of 300,000 ha [7]. Peppers are important cash crops for smallholder farmers in countries like Ethiopia [8] and are good sources of nutrients in the human diet [9, 10]. However, their productivities are less since they are susceptible to horticultural plant drought stress [11–13].
The economies of most developing countries are based on agriculture, which is fully dependent on nature [14]. However, this agriculture has been affected by different biotic and abiotic factors such as drought, salinity, heat, and waterlogging stress [15, 16]. Among these, drought stress is an important issue that greatly affects agriculture productivity [17]. The insufficiency of water caused by erratic and poorly distributed rainfall causes tremendous global losses in agriculture [18]. Drought decreases the productivity and quality of crops [19] and also limits the successful utilization of land throughout the world [20]. The metabolism activities such as physiological processes become highly disturbed in plants when exposed to drought stress [21, 22]. According to Alqudah et al. [23] and Lamaoui et al. [24], drought stress is considered the most important abiotic factor that adversely affects the yield and quality of many field crops by altering the growth, physiology, and metabolic activities of plants. In particular, it reduces plant growth by affecting various physiological and biochemical processes, such as osmotic adjustments, water relations [25, 26], and photosynthetic activity [27, 28], and consequently causes a reduction in flower production [29–31].
The best approach to cope with the adverse effects of drought stresses is screening (testing) the available red pepper cultivars for water scarcity [32]. The adaptive responses to water deficit include morphological, physiological, and biochemical changes such as changes in growth rate, stomatal conductance, photosynthesis rate, chlorophyll content, and the root-to-shoot ratio [33]. Morphological and some leaf-related characteristics were also used as indicators for the detection of drought stress in chili pepper [34]. However, drought stress on the parameters of growth, physiological, and biochemical was not conducted comprehensively on the response of red pepper cultivars in the studied area to get the red pepper cultivars tolerant to drought stress. Therefore, the objective of this research was to study the effect of drought stress on selected morphological, physiological, and biochemical parameters of red pepper cultivars so that the responses of the cultivars were evaluated against drought stress.
2. Materials and Methods
The research was performed with three red pepper (Capsicum annum L.) cultivars in response to drought stress, namely, Hagerew, Markofana, and Mitmita. The seeds of these cultivars were obtained from the Bahir Dar Agricultural Research Centre. The experiment was carried out at the Botany Laboratory of the University of Gondar (12°35′ 11.7″ N and 37°26′ 27″ E, 2148m above sea level). The annual average of the maximum and minimum temperature lies approximately 27°C and 16°C, respectively, while mean relative humidity and precipitation are approximately 56% and 1161 mm, respectively. The annual wind speed and pressure for the area were found to be 7.1 km/h and 1023 mbar, respectively. During the experimental period (March to April), the relative humidity was 50.5%, the maximum and minimum daily temperatures were found to be 29.1°C and 18°C, respectively, and no rainfall occurred. The red pepper crops have a growing period of 120–210 days [35], and these three cultivars have approximately the same development periods.
Thereafter, the seeds of the three red pepper cultivars (Capsicum annum L.) were sterilized with ethanol (80%) for around 15 min, bathed with distilled water, and then sown in plastic pots (25 cm wide × 26 cm height) containing 6 kg of farmyard manure and soil in a 1 : 3 ratio (25% FYM and 75% soil). The soil texture was determined using the hydrometer method and it was identified as sandy loam soil (Table 1). The physical and chemical properties of the soil sample used to grow the pepper plants were analyzed as follows.
Table 1.
Physical and chemical properties of the experimental soil.
| Properties examined | Units | Values |
|---|---|---|
| Sand | % | 62.57 |
| Silt | % | 22.68 |
| Clay | % | 14.75 |
| Moisture saturation | % | 30.4 |
| Wilting point value | % | 10.1 |
| Field capacity moisture | % | 24.5 |
|
| ||
| Textural classification | Sandy loam soil | |
| Electrical conductivity | ms/cm | 0.68 |
| pH | 7.21 | |
| OM | % | 0.22 |
Soil moisture content was determined using an instrument (IMKO, Trime-Pico TDR) in each pot under study up to the end of the experiment. Then, the moisture readings were taken directly by inserting the instrument deep into the soil after the instrument was calibrated [36]. The moisture data were recorded at regular intervals up to the end of the experiment for each treatment (Figure 1) and then irrigation was performed carefully.
Figure 1.
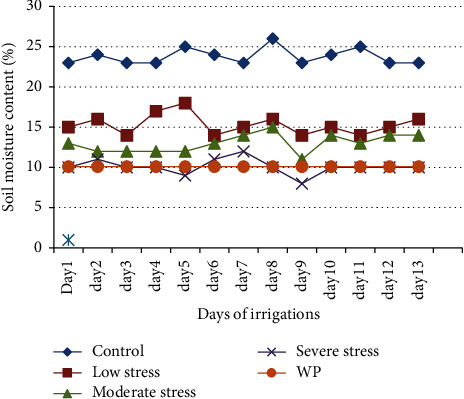
Soil moisture content depletion on different days before irrigation (WP = permanent wilting point of the soil).
After 2 weeks of germination, uniform-sized seedlings of the cultivar were allowed to continue in plastic pots. Therefore, each plastic pot had three seedlings up to the end of the experiment to observe the response of the cultivars against drought stress (Figures 2(a)–2(c)). The potted seedlings were watered with tap water daily at a field capacity of 100% (FC) for up to 2 weeks, which was considered the accommodation period. After 4 weeks, a completely randomized design (CRD) with three replications and four treatments was adopted for the three cultivars.
Figure 2.

Seedlings of potted plants for the three cultivars: (a) Hagerew; (b) Markofana; (c) Mitmita.
2.1. Drought Stress Condition
Drought condition is a feature of climate that appear when the rainfall is deficient compared to the statistical multiyear average for a region, over an extended period of a season or year, or even more [37]. To realize the drought stress, plants were subjected to the following three drought levels along with the control following the method used by Al-Maskri et al. [38]. These were low stress, or 80% FC, moderate stress, or 60% FC, severe stress, or 40% FC, and the control group, or 100% FC. For the experiment, there were 40 days of drought exposure on the seedlings for all the treatments.
2.2. Irrigation Water Application
The irrigation water application was done following the method used by Kazgöz Candemi̇r et al. [39]. The pots used to grow the seedlings have pores underneath for water leakage. Three pots were used where irrigation water was applied with a specific beaker at certain intervals until water began to leak from underneath the pots to determine the irrigation water amount before each irrigation. As soon as leakage was seen from underneath the pot, water application was stopped to determine the volume of water (in ml). This amount determined corresponded to the 100% FC, while 80% FC, 60% FC, and 40% FC of this amount were applied to the other pots. Accordingly, the amount of water applied per irrigation to maintain the soil field capacity was 600 ml for 100% FC, 480 ml for 80% FC, 360 ml for 60% FC, and 240 ml for 40% FC, with a scheduled irrigation interval of 3 days.
The tap water was irrigated within the three days interval up to the end of the experiment. The electrical conductivity of the tap water used in the experiment was on an average of 0.0066 dS/m, and the average pH was found to be 6.99. According to Ayers and Westcot [40]; the quality of water used in the experiment was within the permissible levels required for irrigation water.
2.3. Data Collection
2.3.1. Growth Measurements
Shoot length, root length, number of leaves, stem thickness, number of branches, leaf area, leaf width, leaf length, and canopy diameter were measured after beginning the water treatments. Changes in the shoot and root growth of the cultivars within 10 days intervals till the end of the experiment were recorded. All the leaves and branches were counted individually in each replica at the end of the stress period. The stem thickness of each plant per pot was measured by using a Digital Vernier hand caliper meter (AP-961) and the average stem diameter of the individual plants in the replicate was taken as the stem thickness at the end of the stress period. A leaf area meter (AM 300, ADC Bio Scientific Limited, UK) was used to measure leaf area and leaf width at the end of the experiment. The canopy diameter was measured within each pot following the method of Delelegn et al. [41]. Then, the mean values of the North-South and East-West measurements were taken as canopy diameters.
2.3.2. Physiological Measurements
The physiological parameters such as leaf relative water content (LRWC), chlorophyll fluorescence (CF), net photosynthesis rate (A), transpiration rate (E), and stomatal conductance (gs) were measured.
LRWC was determined from the leaves collected from the cultivars in all treatments following Kirnak et al. [42]. Similarly, sample fresh leaves were taken following the method of Moradi et al. [43] and immediately weighed using a digital electronic balance to get a fresh weight (FW). Then, the leaves were immersed in large Petri dishes containing distilled water for twenty-four (24) hours. After 24 hours, the turgid weight (TW) was determined. The leaves were then placed in a preheated oven at 72°C for 24 hours and dried to obtain their dry weight (DW). Then, the LRWC was calculated using the formula given by Kirnak et al. [42] as follows:
| (1) |
where F.W = fresh weight; D.W = dry weight; and T.W = turgidity weight of leaves.
Chlorophyll fluorescence was measured using a portable multimode OS5P Chlorophyll Fluorometer (Opti-Sciences, Inc., USA) from 10:00 to 11:00 AM using the methods of Husen et al. [44]. Before it was recorded, the upper surface of the leaf was predarkened for 30 minutes by using leaf clips to secure a complete relaxation of all the reaction centers, as recommended by Kauser et al. [45]. PSII efficiency (F0/Fm) readings were taken from the whole plants per pot (9 leaves were taken from each plant in the pots) as recommended by Almeselmani [46]. Therefore, the maximum quantum yield of PSII efficiency (Fv/Fm) was directly taken from the instrument reading.
The net photosynthesis rate, transpiration rate, and stomatal conductance were measured at the final using a gas analyzer (LC Pro + Photometer, ADC Bio Scientific Ltd., Hoddesdon, United Kingdom). Fully expanded attached leaves were taken and all these measurements were performed on 10 leaves from each plant as recommended by Husen et al. [44].
2.3.3. Biochemical Measurements
Biochemical parameters such as chlorophyll content, internal proline content, and total phenolic compounds were determined. To determine the chlorophyll “a,” “b,” and total chlorophyll, leaf samples were taken from each plant per replica. Fresh samples and homogenization were done as shown in Figures 3(a) and 3(b). In the end, the concentration of chlorophyll “a” and “b” total chlorophyll content was determined using the formula given in Arnon [47] as follows:
Chl. a (mgg-1FW) = 12.7 × (A663) − 2.69 × (A645)
Chl. b (mgg-1FW) = 2.9 × (A663) − 4.68 × (A645)
Figure 3.

(a) Extraction and (b) determination of chlorophyll content from sample leaves.
Total chlorophyll concentration (mg/g FW) = 20.2 × A663 + 8.02A645.
Here (A663) and (A645) represent absorbance values read at 663 and 645 nm wavelengths. This was collected at the end of a drought stress period to compare the chlorophyll content between stressed and nonstressed red pepper plants.
Proline content was determined following the method used by Bates et al. [48] based on the reaction of proline with ninhydrin. Then, the absorbance at 520 nm was determined using a microprocessor UV-Visdouble-beam spectrophotometer. The total phenolic content of the extracts was determined following the method of Rispail et al. [49] using the following formula at the end of the experiment.
Total phenolic content = gallic acid equivalent (mg/L) × total volume of the methanol extract x sample weight (kg/g)/Dilution factor (L/mL).
2.3.4. Biomass Estimation
In the end, plants were harvested carefully. Then, the shoot and root fresh weights (SFW and RFW) of each replica of the treatments for the three cultivars were measured using a digital electronic balance (CY510, Citizen Scale, Poland), and the mean values were taken as the shoot and root fresh weights of the red pepper cultivars. Shoot and root dry weights (SDW and RDW) for the cultivars were also measured using a digital electronic balance.
To get the root-to-shoot ratio of biomass, the whole plants were uprooted, rinsed, separated into shoot and root, and oven-dried for 24 hours at 72°C. Then, the root-to-shoot ratio was computed using the formula given by Luvaha et al. [50] as follows:
| (2) |
2.4. Data Analysis
All collected data were subjected to analysis of variance (ANOVA), mean comparisons were performed using LSD, and graphical comparisons were presented using the SPSS version 20 software. The significance level of the data was accepted at p < 0.05 and rejected when p > 0.05 confidence interval level. A one-way ANOVA was used to determine statistically significant differences between the means of the parameters of the three red pepper cultivars under different drought levels. The parameters of each of the cultivars were measured on the same independent variable after having undergone the same condition. On the other hand, a two-way ANOVA was used to analyze the interaction effect of both the cultivar type and watering regime. A correlation was also made to determine the direction of the relationship and to measure the strength of the association between two continuous variables.
3. Results and Discussion
3.1. Shoot Length
Changes in the shoot growth of the cultivars within 10 days intervals till the end of the experiment were recorded. The results showed that drought stress decreased the shoot length by 19.75%, 21.87%, and 31.02% at 80% FC, 60% FC, and 40% FC, respectively, in the Hagerew cultivar in the first 10 days of drought exposure compared to the control (Figure 4(a)). Similarly, the shoot length declined by 23.83%, 35.94%, and 45.19% at 80% FC, 60% FC, and 40% FC on the 20th day of drought exposure in the Hagerew cultivar. At prolonged drought exposure (40th day), shoot length declined by 21.44%, 43.46%, and 52.91% in similar treatments, and the variation was statistically significant (F = 39.89, p < 0.05).
Figure 4.
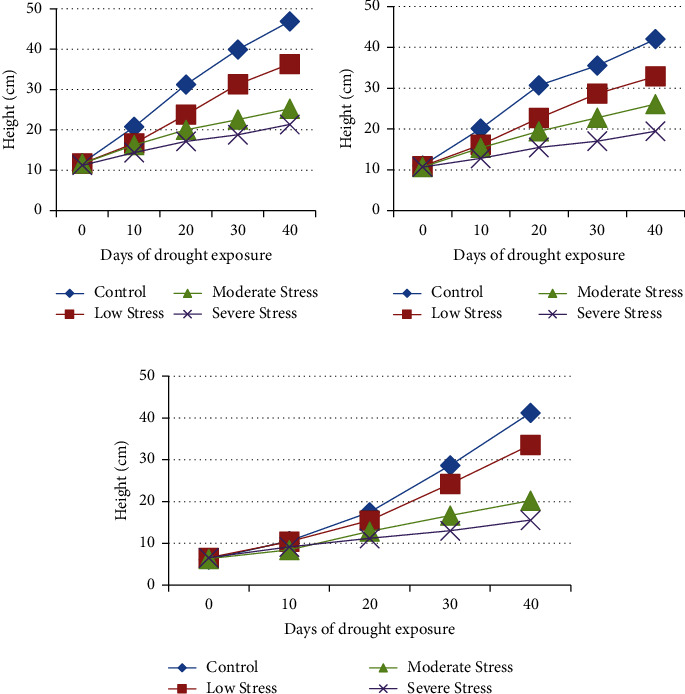
Shoot variations in (a) Hagerew, (b) Markofana, and (c) Mitmita cultivars after 40 days of drought exposure.
The Markofana shoot length (SL) was also reduced by 19.91%, 23.25%, and 36.44% on the 10th day of drought exposure and by 26.08%, 36.62%, and 49.66% on the 20th day of drought exposure at 80% FC, 60% FC, and 40% FC, respectively (Figure 4(b)). At prolonged stress (40th day), the effect was statistically significant (F = 37.27, p < 0.05). Similarly, shoot length was reduced in the Mitmita crop by 1.23%, 19.90%, and 13.74% on the 10th day of drought exposure and by 11.47%, 26.09%, and 35.67% on the 20th day of drought exposure (Figure 4(c)) at 80% FC, 60% FC, and 40% FC, respectively, compared to the control. At severe drought stress (40th day), the effect was statistically significant (F = 45.01, p < 0.05). The finding in shoot reduction is in agreement with the reports on soybean [51, 52], rice [53], common bean [54], maize [55], and turfgrass [56]. Shoot length is reduced by up to 25% in citrus seedlings under drought stress conditions [57]. This is related to the reduction in cell turgor, which decreases the rate of cell division and cell expansion due to the inhibiting effect of water shortage on growth-promoting hormones [58, 59]. Another reason [60] is diminished cell expansion and a higher leaf senescence rate. According to Farooq et al. [61], the imposition of plants to drought stress disrupts water flow from the xylem to the surrounding elongating cells and causes a reduction in shoot lengths as well.
3.1.1. Number of Leaves
It was found that the number of leaves decreased by 52.28% in the Hagerew cultivar (Figure 5(a)), by 52.15% in the Markofana cultivar (Figure 5(b)), and by 47.08% in the Mitmita cultivar (Figure 5(c)). In this study, the number of leaves decreased significantly in the Markofana cultivar at p < 0.05 level. This finding is similar to the previous reports by Anjum et al. [62] on plants and maize [55]. Others [63–65] also found that a water deficit could limit the growth of the plant, which can be seen by the reduced leaf number. This might be due to a reduction in leaf formation and the abscission of lower leaves. It may also be the result of leaf senescence caused by increased carbon remobilization from the leaves and their redistribution to stems and roots [66].
Figure 5.
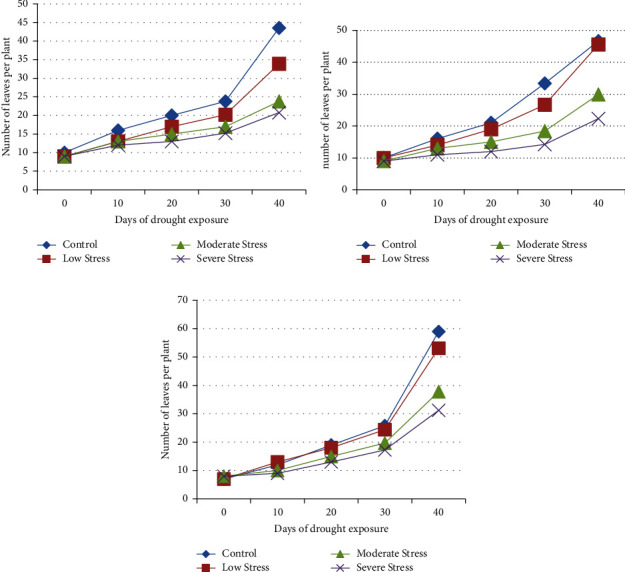
Variations in the number of leaves in the (a) Hagerew, (b) Markofana, and (c) Mitmita cultivars after 40 days of drought exposure.
3.2. Root Length and Canopy Diameter
The root length (RL) of the three red pepper cultivars that were measured after 40 days of drought exposure is displayed in Figure 6(a). As the results showed, root length was reduced by 20.99% at 40% FC (the 40th day of drought exposure) in the Hagerew cultivar. The inhibitory effect was also significantly increased (F = 11.46; p < 0.05) in the Markofana cultivar at 40% FC as compared to the control group. The root length was reduced from 18.83 cm in the control to 10.00 cm in the 40% FC in the cultivar. On the other hand, root length declined by 27.64% in the Mitmita cultivar under severe drought stress, or 40% FC compared to the control group. Similar findings were found in marigolds [56] and mung bean plants [67]. Water deficit stress is initially sensed by the root and root growth becomes affected [68]. Similarly, different scholars reported that root length becomes impeded by drought at the early stages in alfalfa plants [69] and sunflowers [70, 71] and became shrinking in lengthened drought stress [72].
Figure 6.
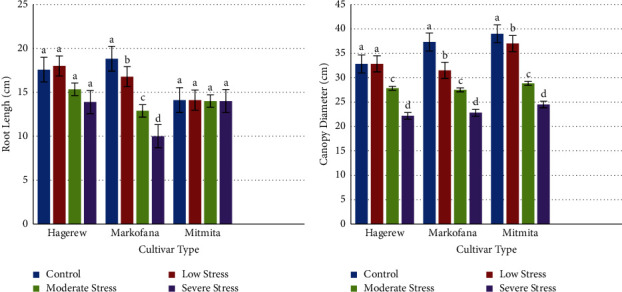
Effect of drought stress on (a) root length and (b) canopy diameter of the three cultivars. Columns with different letters are mean values ± SE of the three replicates. Bars with different letters show significant differences at p < 0.05 (LSD).
The average canopy diameter of the three red pepper cultivars was recorded from the growing plants (Figure 6(b)). Severe drought stress significantly reduced canopy diameter by 32.47%, 38.84%, and 37.18% in the Hagerew, Markofana, and Mitmita cultivars, respectively, as compared to the control. The decrease in canopy diameter was significant under severe drought stress irrespective of the control (F = 8.70; p < 0.05). This finding is in line with the work of Al-Mahmud et al. [73]; which showed that canopy diameter was significantly decreased under severe drought stress in potato plants. These might be due to the traits they inherited, and they may determine the yielding potential of the crop. The water deficits reduced the canopy growth of strawberries [65].
3.3. Stem Thickness and Leaf Data
The effects of drought stress on stem thickness, number of leaves, branches, leaf area, leaf width, and length of the three cultivars were recorded and presented in Table 2. The results revealed that severe drought stress or 40% FC reduced the stem thickness by 30.93% in the Hagerew cultivars compared to the control group. The stem thickness of the Markofana cultivar was also reduced by 29.33% under severe drought stress as compared to the control. On the other hand, severe drought stress decreased the stem thickness by 30.43% in the Mitmita cultivar compared to the control. The decline in stem diameter was significant in severe drought stress compared to the control. This result is in agreement with the report of Luvaha et al. [50] on mango seedlings and maize crops [55]. The result was also consistent with the previous report by Alordzinu et al. [36] on tomato plants.
Table 2.
Morphological responses of the cultivars against drought stress conditions.
| Cultivars | Treatments | Stem thickness (mm) | Number of branches (per plant) | Leaf area (mm2) | Leaf width (mm) | Leaf length (mm) |
|---|---|---|---|---|---|---|
| Hagerew | Control | 5.27 ± 0.38a | 3.22 ± 0.48a | 9688.67 ± 232.54a | 56.2 ± 17.38a | 156.57 ± 8.69a |
| Low stress | 4.89 ± 0.20a (7.21) |
2.33 ± 0.00a (27.64) |
9425.33 ± 458.83a (2.72) |
55.13 ± 5.95a (1.90) |
199.70 ± 10.72b (27.55) |
|
| Moderate stress | 3.96 ± 0.22b (30.93) |
0.67 ± 0.38b (79.19) |
7011.67 ± 185.93b (27.63) |
46.97 ± 2.43a (16.42) |
157.5 ± 2.06a (0.59) |
|
| Severe stress | 3.64 ± 0.23c (30.93) |
0.22 ± 0.22c (93.17) |
4235.67 ± 479.79c (56.28) |
36.33 ± 4.08a (35.36) |
118.53 ± 7.39c (24.29) |
|
|
| ||||||
| Markofana | Control | 4.91 ± 0.21a | 4.67 ± 1.35a | 10645.67 ± 1473.06a | 102.10 ± 29.85a | 226.30 ± 37.79a |
| Low stress | 4.73 ± 0.13a (3.67) |
5.33 ± 0.58a (14.13) |
7601.33 ± 201.85b (28.59) |
51.06 ± 2.32a (49.99) |
163.63 ± 2.67a (27.69) |
|
| Moderate stress | 3.70 ± 0.11b (21.77) |
2.44 ± 0.56a (47.75) |
5319.00 ± 672.50c (50.04) |
40.20 ± 2.55a (60.63) |
139.20 ± 11.68b (38.49) |
|
| Severe stress | 3.47 ± 0.24c (29.33) |
1.34 ± 0.22b (76.23) |
4151.00 ± 215.71d (61.01) |
33.53 ± 6.23a (67.16) |
121.10 ± 4.97c (46.49) |
|
|
| ||||||
| Mitmita | Control | 4.83 ± 1.01a | 7.22 ± 0.45a | 8242.67 ± 380.37a | 47.43 ± 5.25a | 181.67 ± 15.33a |
| Low stress | 4.69 ± 0.32a (2.89) |
6.55 ± 0.22a (9.28) |
5479.67 ± 217.29b (33.52) |
29.03 ± 5.95a (39.43) |
152.33 ± 1.59b (16.15) |
|
| Moderate stress | 3.66 ± 0.06a (24.22) |
3.00 ± 0.51b (58.45) |
4150.33 ± 277.49c (49.65) |
31.43 ± 2.18a (33.73) |
130.50 ± 1.53c (28.17) |
|
| Severe stress | 36 ± 0.03a (30.43) |
3.11 ± 0.80c (56.93) |
2853.33 ± 203.98d (65.38) |
29.97 ± 1.35a (36.81) |
107.07 ± 4.10d (41.06) |
|
The values represent the mean ± SE of the three replicates. Numbers followed by different letters in the columns indicate significant differences (p < 0.05) according to the LSD test values within parenthesis are percent variations as obtained from the control plants of respective cultivars.
The number of branches in the Hagerew cultivar was significantly affected by severe drought stress (F = 18.25, p < 0.05). Similarly, the number of branches was significantly affected under severe drought stress in both the Markofana and Mitmita cultivars (F = 6.11, p < 0.05; F = 17.23, p < 0.05), respectively. A similar finding was reported by Ichwan et al. [74] in red chili peppers under water deficit conditions. The number of branches decreased under severe drought stress in sweet peppers [75]. However, leaf width was insignificantly affected by severe drought stress in the Hagerew, Markofana, and Mitmita red pepper cultivars. The leaf length was also significantly reduced by 24.29%, 46.49%, and 41.06% under severe drought stress in the Hagerew, Markofana, and Mitmita cultivars.
The leaf area was also affected by severe drought stress in all three cultivars. As the result showed, the effect of severe drought stress on the leaf area was statistically significant (F = 48.76, p < 0.05) in the Hagerew cultivar as compared to the control. The leaf area in the Markofana cultivar was also significantly reduced (F = 12.13, p < 0.05). Similarly, the leaf area was significantly affected (F = 68.46, p < 0.05) in the Mitmita cultivar under severe drought stress. The result was consistent with the study done by Zhang et al. [76] on soybean. The reduction of leaf area under water deficit stress was also previously reported in many plants such as wheat cultivars [77], marigold plants [56], and strawberries [65, 78]. Drought stress reduced leaf area by 51.6% during the vegetative stage of cowpea due to the inhibition of cell growth [79]. According to Manandhar et al. [80], limited water availability decreases leaf area, thereby reducing plant yield. The reason for leaf growth reduction might be turgor loss and increased synthesis of abscission acid under stress [81, 82]. This is to achieve a balance between the water status of plant tissues and the water absorbed by the plant roots [83]. According to Blum [84]; a small leaf area is beneficial under drought stress to avoid hydration.
3.4. The Physiological Responses of Red Pepper Cultivars to Drought Stress
3.4.1. Leaf Relative Water Content
Drought stress greatly reduced the physiological efficiency of leaves in the three cultivars in comparison with the controls. The degree of reduction of LRWC was high in the Hagerew cultivar (20.26%), followed by the lowest reduction of LRWC for the Mitmita cultivar (15.92%). LRWC also declined by 17.33% in the Markofana cultivar (Table 3). LRWC was significantly reduced as drought stress increased compared to control in the Hagerew cultivar. In line with this finding DaCosta and Huang [85] also found that a water deficit reduces LRWC in wheat cultivars. This was also confirmed in the previous reports [86–90]. The LRWC decreased as a result of high water stress levels and increasing resistance to water flow in the stems and leaves of plants [77, 91, 92]. However, the LRWC between the control and severe drought stress in the Markofana and Mitmita cultivars was statistically insignificant. This may be due to the variation in the ability of red pepper cultivars to avoid stress by maintaining tissue turgor osmotically. Tezera et al. [82] also found that reduced water availability in soil lowers leaf water content, causing guard cells to lose turgor, and hence the size of stomatal pores is reduced.
Table 3.
Physiological responses of the three red pepper cultivars against drought stress.
| Cultivar | Treatments | LRWC (%) | CF (Fv/Fm) | A (μ mol CO2 m−2s−1) | E (m mol m−2s−1) | Gs (mol m−2 s−1) |
|---|---|---|---|---|---|---|
| Hagerew | Control | 83.86 ± 1.81a | 0.7533 ± 0.02a | 16.87 ± 0.74a | 5.11 ± 0.19a | 0.199 ± 0.007a |
| Low stress | 82.26 ± 3.37a (1.92) |
0.6823 ± 0.02a (1.59) |
15.71 ± 0.66b (20.70) |
5.27 ± 0.20b (3.33) |
0.188 ± 0.004b (1.11) |
|
| Moderate stress | 77.77 ± 3.38a (7.26) |
0.6690 ± 0.003a (3.50) |
14.27 ± 0.34bc (23.56) |
4.67 ± 0.13c (4.35) |
0.182 ± 0.002c (2.55) |
|
| Severe stress | 66.87 ± 6.19b (20.26) |
0.6702 ± 0.009b (3.33) |
12.78 ± 0.53d (26.78) |
3.23 ± 0.11d (5.45) |
0.111 ± 0.001d (11.45) |
|
|
| ||||||
| Markofana | Control | 86.44 ± 3.01a | 0.7630 ± 0.03a | 15.79 ± 0.84a | 5.81 ± 0.31a | 0.185 ± 0.005a |
| Low stress | 85.14 ± 3.45a (1.50) |
0.6533 ± 0.03a (4.35) |
15.32 ± 0.54b (21.45) |
5.23 ± 0.29a (3.35) |
0.188 ± 0.006b (1.08) |
|
| Moderate stress | 78.08 ± 2.36a (9.67) |
0.6423 ± 0.02a (5.96) |
14.35 ± 0.44c (22.21) |
4.97 ± 0.25a (4.11) |
0.167 ± 0.004c (3.83) |
|
| Severe stress | 71.46 ± 14.21a (17.33) |
0.6577 ± 0.006a (3.70) |
13.56 ± 0.47d (24.35) |
4.11 ± 0.16a (5.00) |
0.134 ± 0.003d (10.97) |
|
|
| ||||||
| Mitmita | Control | 87.41 ± 1.67a | 0.7713 ± 0.008a | 17.12 ± 0.89a | 6.76 ± 0.45a | 0.198 ± 0.007a |
| Low stress | 86.57 ± 3.01a (0.96) |
0.7030 ± 0.007a (0.24) |
17.00 ± 0.79a (10.11) |
6.45 ± 0.40a (3.23) |
0.199 ± 0.007a (1.02) |
|
| Moderate stress | 82.44 ± 0.54a (5.69) |
0.6707 ± 0.02a (4.36) |
16.34 ± 0.47a (19.99) |
5.67 ± 0.32a (3.56) |
0.178 ± 0.005a (2.66) |
|
| Severe stress | 73.49 ± 0.003a (15.92) |
0.6810 ± 0.01a (2.89) |
15.00 ± 0.74a (21.11) |
4.99 ± 0.22a (3.00) |
0.166 ± 0.003a (4.33) |
|
The values represent the mean ± SE of the three replicates. Numbers followed by different letters in the columns indicate significant differences (p < 0.05) according to the LSD test values within parenthesis are percent variations as obtained from the control plants of respective cultivars.
3.4.2. Chlorophyll Fluorescence
Under severe drought stress, the percentage reduction of chlorophyll fluorescence (Fv/Fm) was 3.33% in the Hagerew, 3.70% in the Markofana, and 2.89% in the Mitmita cultivars (Table 3). In the present study, drought stress imposed for 40 days had a statistically significant effect on the PSII photochemical efficiency (Fv/Fm) of red pepper cultivars. This is in agreement with the earlier reports by Liu et al. [93] on plants, Wang et al. [94] on apple tree leaves, and Widuri et al. [34] on chili pepper plants. A significant reduction was also perceived in barley, young wheat, and pepper crop plants under drought stress [95–97]. This may be because of the suppression of PSII by decreasing electron transport and the release of magnesium and calcium ions from their binding [98, 99]. The changes in PSII activity under water deficit stress are related to photoinhibition rather than to direct damage to PSII [100]. Moreover, the reduction might be due to the development of slowly relaxing quenching processes [101].
3.4.3. Photosynthetic Rate and Stomatal Conductance
The analysis of variance in the photosynthetic rate of both Hagerew and Markofana cultivars showed significant variation at p < 0.05 (Table 3) under severe drought conditions. However, there was an insignificant variation in the photosynthesis rate of the Mitmita cultivar. The Mitmita cultivar had the highest assimilation rate of 15.00 μmolem−2 S−1 followed by Markofana at 13.56 μmolem−2 S−1, and the lowest was noted in the Hagerew cultivar with a mean value of 12.78 μmolem−2 S−1. Stomatal conductance was also reduced by 4.33%, 10.97%, and 11.45% in the Mitmita, Markofana, and Hagerew cultivars, respectively, under severe drought stress conditions. In the present study, the photosynthetic rate was significantly affected under severe drought stress conditions in the Hagerew cultivar, which was consistent with the reports on sorghum [102], rice [103], and chickpea cultivars [104]. Zhang et al. [105] also proved that water stress inhibited the process of photosynthesis in maize. Drought stress decreases guard cells and causes the stomata to close, which in turn inhibits the uptake of CO2 needed for photosynthesis [106–108]. The stomatal conductance also decreased with increasing drought stress levels. This determines plant tolerance to drought [109]. This is because the closing of stomata to restrict gas exchange between the atmosphere and the leaf is one of the first responses of plants to drought. Mafakheri et al. [102] also reported that the decrease in photosynthesis rate can be due to both stomatal and nonstomatal factors. Related to this, Berahim et al. [110] described how stomatal movement is a critical attribute in monitoring water transpiration and CO2 absorption under drought stress. Stomata activity causes a change in the photosynthetic rate under drought stress conditions [111]. Chaves and Oliviera [112] also presented that stomatal conductance only affects the photosynthesis rate under severe drought stress. In this study, however, moderate drought stress also decreased the photosynthesis rate. In line with this finding, Flexas and Medrano [113] found that mild and moderate drought stresses decreased photosynthesis due to the stomatal closure and the resulting CO2 deficit in the chloroplasts.
3.4.4. Transpiration Rate
The cultivars showed a significant difference in transpiration rates at p < 0.05 (Table 3). The Mitmita red pepper cultivar had a significantly higher transpiration rate than the other cultivars. The highest transpiration rate was observed in the Mitmita cultivar with a mean value of 4.99 mmol m−2 S−1 under severe drought conditions. Relatively, the Hagerew cultivar showed the lowest transpiration rate (3.23 mmol m−2 S−1) followed by the Markofana cultivar (4.11 mmol m−2 S−1). Table 3 shows that the higher the drought stress level (40% FC) is, the lower the transpiration rate of all red pepper cultivars. This finding agrees with the previous reports on wheat crops [114]. A significant reduction in the transpiration rate was also observed under drought stress conditions in crops such as wheat, rice, and maize [115, 116].
3.5. The Biochemical Responses of Red Pepper Cultivars to Drought Stress
3.5.1. Chlorophyll Content
The leaf pigments (chlorophylls “a,” “b”, and total chlorophyll) were quantified and the results indicated that the chlorophyll content declined with an increase in the drought stress level (Table 4). Under severe drought stress, chlorophyll “a” was reduced by 72.14%, chlorophyll “b” by 28.49%, and total chlorophyll by 77.28% in the Hagerew cultivar. In the Markofana cultivar, chlorophyll “a” was reduced by 57%, chlorophyll “b” by 23.33%, and total chlorophyll by 45.19% at 40% FC. Similarly, in the Mitmita cultivar chlorophyll “a” was reduced by 52.35%, chlorophyll “b” by 18%, and total chlorophyll by 45.45% under severe drought stress or at 40% FC compared to the control group (Table 4). This reduction in pigment content was previously reported in several crop plants, including bread wheat [117], sorghum [3], millet [118], red chili varieties [119], and different sunflower varieties [70]. Others [65, 120, 121] also found that lower water field capacity tends to decrease total chlorophyll in chamomile, strawberries, and peanut plants, respectively. The reduction may be because of the low activity of the photosynthetic elements under stress. It may also be because of the reduced synthesis of the main chlorophyll pigment complexes encoded by the Cab gene family due to drought stress [122]. Chloroplasts are destructed by the reactive oxygen species under drought stress [123–125]. Chiral macroaggregates of light-harvesting chlorophyll “a” or “b” pigment-protein complexes may also be destructed [126]. On the other hand, there were insignificant differences in the treatments of both the Markofana and the Mitmita cultivars compared to the control. This may be due to the production of osmolytes during stress in the cultivars. Similarly, Ichwan et al. [74] reported that red chili pepper increases osmolytes as the mechanism of drought stress tolerance.
Table 4.
Effects of drought stress on chlorophyll content.
| Treatments | Chlorophyll “a” (mgg-1FW) | Chlorophyll “b” (mgg-1FW) | Total chlorophyll (mgg-1FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hagerew | Markofana | Mitmita | Hagerew | Markofana | Mitmita | Hagerew | Markofana | Mitmita | |
| Control | 23.80 ± 3.05a | 16.21 ± 3.19a | 17.61 ± 1.69a | 1.79 ± 0.63a | 1.80 ± 0.83a | 3.00 ± 0.18a | 49.61 ± 8.30a | 33.92 ± 5.57a | 31.09 ± 3.85a |
| Low stress | 16.59 ± 6.23a (52.66) |
7.60 ± 1.91a (48.89) |
10.62 ± 3.65a (39.69) |
4.85 ± 3.43b (1.71) |
1.85 ± 0.56a (2.78) |
2.61 ± 0.79a (0.13) |
30.78 ± 13.59a (37.96) |
20.49 ± 2.92b (39.59) |
18.89 ± 6.37a (39.24) |
| Moderate stress | 10.78 ± 5.07a (54.71) |
7.71 ± 4.58a (52.44) |
11.01 ± 4.43a (37.48) |
1.61 ± 0.82c (10.06) |
2.16 ± 1.05a (20.00) |
2.74 ± 0.63b (8.67) |
19.74 ± 9.60a (60.21) |
19.18 ± 7.35a (43.45) |
22.44 ± 10.11a (27.82) |
| Severe stress | 6.63 ± 5.47a (72.14) |
6.97 ± 1.91a (57.00) |
8.39 ± 2.92a (52.35) |
1.28 ± 0.75d (28.49) |
2.22 ± 0.66a (23.33) |
2.46 ± 0.73a (18.00) |
11.27 ± 4.96b (77.28) |
18.59 ± 2.47a (45.19) |
16.96 ± 5.98a (45.45) |
The values represent the mean ± SE of the three replicates. Numbers followed by different letters in the columns indicate significant differences (p < 0.05) according to the LSD test values within parenthesis are percent variations as obtained from the control plants of respective cultivars.
3.6. Proline Content and Total Phenolic Compound
Proline content was significantly increased under water deficit stress in the Markofana cultivar (Table 5). However, the increase in proline content was not significantly affected by the stress in the Hagerew cultivar. Proline content increased by 37%, and by 22.14% in the Hagerew and Markofana cultivars, respectively. Similarly, drought stress also influenced the total leaf phenolic content (p < 0.05) in the cultivars (Table 5). Under severe drought stress, total phenolic content increased by 22.57%, 44.12%, and 47.11% in the Hagerew, Markofana, and Mitmita cultivars, respectively. Crops respond to drought stress by accumulating osmolytes in response to stress [127–129]. In the present study, there was a significant increase in the proline content in the Markofana and Mitmita cultivars under severe drought stress. This agrees with the previous reports on pepper plants [130]. Similar findings were also previously reported on pea cultivars [131], Petunia hybrida [132], and soybean genotypes [133–135] under drought stress. Ghorbanli et al. [136] and Choudhary et al. [137] also reported that an increase in proline content keeps cell water levels under drought stress. Increased proline provides osmoprotective functions during drought stress [93, 138, 139]. The higher proline content under water stress signals multiple responses as part of the adaptation process in plants [140, 141]. There was a great effect of drought stress on the total phenolic content of the three cultivars (p < 0.05). In the present study, the total phenolic content in the sample leaves increased significantly with increasing drought stress levels in the Markofana cultivar. This agrees with the previous reports on capsicum species [96]. The synthesis of phenolic compounds due to drought stress has also been reported in many studies [131, 142–144]. This and other phenolic compounds are highly involved in protection against drought stress [145]. According to Heim et al. [146]; phenolic compounds protect against oxidative damage to cells and increment the stability of cell membranes.
Table 5.
Effects of drought stress on proline and total phenolic content after 40 days of drought exposure.
| Treatments | Proline content (μmol/g) | Total phenolic (mg/100 g) | ||||
|---|---|---|---|---|---|---|
| Hagerew | Markofana | Mitmita | Hagerew | Markofana | Mitmita | |
| Control | 3.80 ± 0.25a | 4.21 ± 0.69a | 4.89 ± 0.78a | 19.79 ± 1.63a | 21.61 ± 1.83a | 22.34 ± 1.98a |
| Low stress | 4.59 ± 0.26a (12.66) |
5.60 ± 1.91b (18.89) |
4.99 ± 0.80b (6.9) |
27.85 ± 4.01a (14.89) |
29.85 ± 1.56b (13.59) |
30.45 ± 1.83b (15.67) |
| Moderate stress | 5.78 ± 0.07a (14.71) |
6.71 ± 4.58c (19.44) |
7.45 ± 0.81c (21.78) |
36.51 ± 0.82a (19.56) |
31.26 ± 0.05c (28.00) |
32.33 ± 0.83c (29.0) |
| Severe stress | 9.63 ± 0.47a (22.14) |
10.97 ± 1.91d (37.00) |
11.23 ± 1.92d (40.00) |
45.28 ± 0.75a (22.57) |
36.32 ± 0.66d (44.12) |
38.11 ± 1.83d (47.11) |
The data represent the mean ± SE of the three replicates in the experiment. Means followed by the different letters in a column are significantly different at p < 0.05 level according to the LSD test values within parenthesis are percent variation as obtained from the control plants of respective cultivars.
3.7. Biomass Estimation
3.7.1. Shoot and Root Fresh Weight
At the end of the experiment, all plants were collected for biomass analysis. As the results revealed, shoot fresh weight declined by 83.83%, 77.99%, and 77.50% in the Hagerew, Markofana, and Mitmita cultivars, respectively, under severe drought stress compared to the control group (Figure 7(a)). The result of this study also revealed that the highest shoot fresh weight reduction was observed in the Hagerew cultivar. Similarly, the fresh root weight of the Hagerew, Markofana, and Mitmita cultivars was reduced by 80.93%, 69.95%, and 73.09%, respectively, under severe drought stress conditions (Figure 7(b)). There was a significant reduction in dry matter (biomass) of red pepper cultivars (p < 0.05) at severe drought stress compared to the control group. This is in agreement with the previous reports by [42, 147–149]. This may be because of the reduction in the leaf area index that resulted in reduced photosynthesis. Another possible reason might be the close of leaves openings by signals of roots, which leads to a reduction in leaf gas exchange and ultimately lead to decreased biomass [150]. Nam et al. [151] also described that inhibition of dry matter production is largely due to the inhibitory effects of drought on leaf expansion, leaf development, and consequently reduced light interception.
Figure 7.
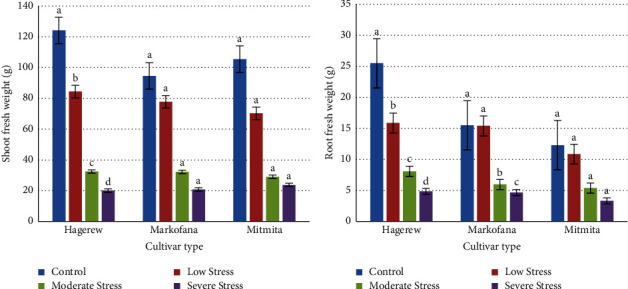
(a) Effect of drought stress on the shoot. (b) Root fresh weight of the three cultivars. Columns with different letters are the mean values ± SE of the three replicates. Bars with different letters show significant differences at p < 0.05 (LSD).
3.8. Shoot and Root Dry Weight
Drought stress significantly affected both the shoot and root dry weights of the cultivars (Table 6). Under severe drought stress, the shoot dry weight declined by 75.29%, 83.23%, and 81.97% in the Markofana, Hagerew, and Mitmita cultivars, respectively, compared to the control. In this stress condition, both shoot and root dry biomass were significantly (p < 0.05) increased compared to the control in the Markofana and Mitmita cultivars. This is in agreement with the reports of Kerepesi and Galiba [152] on wheat plants. The finding also confirmed the work of Westgate and Boyer [153] and Wu and Cosgrove [154]. Leport et al. [155] and Liu et al. [93] also reported that drought stress decreases shoot and root dry weight with more influences on shoots than on roots, which increased the root-to-shoot ratio. This may be the result of increased root length rather than inhibited shoot growth under severe drought stress. It may also be due to the more extensive growth of adventitious and tap roots in plants exposed to severe water deficits than the control ones [50]. This in turn may be due to an increase in phytohormones under stress conditions than in the normal period. Chaves and Oliveria [112] also reason that higher root growth under water deficit conditions can increase drought tolerance in plants.
Table 6.
Effects of drought stress on the biomass formation of red pepper cultivars.
| Cultivars | Treatments | RDW (g) | SDW (g) | Root: shoot ratio |
|---|---|---|---|---|
| Hagerew | Control | 3.26 ± 0.51a | 26.72 ± 2.11a | 12.07 ± 1.18a |
| Low stress | 2.31 ± 0.09a (29.14) |
15.27 ± 0.85b (11.45) |
15.25 ± 1.35a (26.35) |
|
| Moderate stress | 1.14 ± 0.06b (65.03) |
6.59 ± 0.49c (75.34) |
17.64 ± 2.28a (46.15) |
|
| Severe stress | 0.91 ± 0.29c (72.09) |
4.48 ± 0.38d (83.23) |
19.97 ± 5.57a (65.45) |
|
|
| ||||
| Markofana | Control | 2.08 ± 0.13a | 16.55 ± 0.35a | 12.61 ± 0.91a |
| Low stress | 1.98 ± 0.12a (4.81) |
12.99 ± 1.83b (21.51) |
15.74 ± 2.16b (24.82) |
|
| Moderate stress | 0.89 ± 0.09b (57.21) |
6.16 ± 0.28c (62.78) |
14.65 ± 0.77c (16.18) |
|
| Severe stress | 0.66 ± 0.00c (68.27) |
4.04 ± 0.06d (75.29) |
16.13 ± 0.24d (27.91) |
|
|
| ||||
| Mitmita | Control | 2.44 ± 0.32a | 18.03 ± 0.46a | 13.51 ± 1.74a |
| Low stress | 1.84 ± 0.24a (24.59) |
11.38 ± 0.75b (36.88) |
16.09 ± 1.59b (19.09) |
|
| Moderate stress | 0.91 ± 0.12b (62.70) |
5.12 ± 0.58c (71.60) |
17.87 ± 1.46c (32.27) |
|
| Severe stress | 0.51 ± 0.08c (79.89) |
3.25 ± 0.97d (81.97) |
20.39 ± 0.06d (50.92) |
|
The values represent the mean ± SE of the three replicates. Numbers followed by different letters in the columns indicate significant differences (p < 0.05) according to the LSD test values within parenthesis are percent variations as obtained from the control plants of respective cultivars.
3.9. ANOVA Results
The interaction effect of watering level and cultivar type on the shoot length, leaf area, shoot fresh weight, root fresh weight, root dry weight, and total chlorophyll is presented in Table 7. As the results showed, there was a statistically significant interaction between the effects of watering level and cultivar type on the root fresh weight of the cultivars (p=0.003). However, there was a statistically insignificant interaction between the effects of the cultivar type and watering level on shoot length, leaf area, shoot fresh weight, total chlorophyll, and root dry weight among the three cultivars. The results also showed there was no statistically significant difference in shoot fresh weight (p=0.055) and total chlorophyll (p=0.415) between the cultivars. On the other hand, there were statistically significant differences between shoot length, leaf area, and shoot fresh weight (p < 0.05).
Table 7.
ANOVA results of the effect of drought stress on some parameters of the three red pepper cultivars.
| Mean squares | |||||||
|---|---|---|---|---|---|---|---|
| Sources of variation | Df | SL | LA | SFW | RFW | RDW | Total chl |
| Watering level (W) | 3 | 1083.796∗∗ | 56266014.028∗∗ | 14770.054∗∗ | 360.722∗∗ | 7.188∗∗ | 879.703∗∗ |
| Cultivar type (C) | 2 | 70.919∗∗ | 18690886.861∗∗ | 292.381ns | 95.109∗∗ | 0.936∗∗ | 148.565ns |
| WXC | 6 | 8.373ns | 1867180.194ns | 182.825ns | 25.969∗∗ | 0.167 ns | 152.930ns |
| Error | 21 | 3.45 | 4.67 | 4.55 | 2.78 | 0.36 | 3.97 |
ns-not significant and ∗∗-significant at p < 0.05.
3.10. Correlation Results in the Three Cultivars
Correlations between various morphological and physiological parameters were made and summarized as follows presented in Tables 8–10 for Hagerew, Markofana, and Mitmita cultivars (p < 0.01, or p < 0.05 level). As the result revealed the most correlated parameters were shoot fresh weight and shoot dry weight in Hagerew (r = 0.985, p < 0.01), in Markofana (r = 0.997, p < 0.01), and in Mitmita (r = 0.989, p < 0.05). This indicates that an increase in shoot fresh weight increases the shoot dry weight in the cultivars. On the other hand, the least correlated parameters were root length with shoot fresh weight (r = 0.754) at the 0.01 level in the Hagerew cultivar. Root fresh weight with shoot length is the least correlated parameter in both Markofana (r = 0.873) and Mitmita (r = 0.753) at a 0.01 level.
Table 8.
Correlation between different morphological and physiological parameters in the Hagerew cultivar.
| Parameters | SL | RL | SFW | RFW | SDW | RDW | Total chl |
|---|---|---|---|---|---|---|---|
| SL | 1 | 0.677∗ | 0.925∗∗ | 0.942∗∗ | 0.926∗∗ | 0.886∗∗ | 0.722∗∗ |
| RL | 1 | 0.754∗∗ | 0.651∗ | 0.690∗ | 0.598∗ | 0.359 | |
| SFW | 1 | 0.977∗∗ | 0.985∗∗ | 0.955∗∗ | 0.615∗ | ||
| RFW | 1 | 0.978∗∗ | 0.962∗∗ | 0.670∗ | |||
| SDW | 1 | 0.943∗∗ | 0.655∗ | ||||
| RDW | 1 | 0.547 | |||||
| Total chl | 1 |
∗ Correlation is significant at the 0.05 level (2-tailed). ∗∗ Correlation is significant at the 0.01 level (2-tailed).
Table 9.
Correlation between different morphological and physiological parameters in the Markofana cultivar.
| Parameters | SL | RL | SFW | RFW | SDW | RDW | Total chl |
|---|---|---|---|---|---|---|---|
| SL | 1 | 0.889∗∗ | 0.922∗∗ | 0.873∗∗ | 0.932∗∗ | 0.895∗∗ | 0.895∗∗ |
| RL | 1 | 0.913∗∗ | 0.896∗∗ | 0.915∗∗ | 0.922∗∗ | 0.313 | |
| SFW | 1 | 0.952∗∗ | 0.997∗∗ | 0.957∗∗ | 0.280 | ||
| RFW | 1 | 0.935∗∗ | 0.990∗∗ | 0.199 | |||
| SDW | 1 | 0.942∗∗ | 0.329 | ||||
| RDW | 1 | 0.247 | |||||
| Total chl | 1 |
∗∗ Correlation is significant at the 0.01 level (2-tailed).
Table 10.
Correlation between different morphological and physiological parameters in the Mitmita cultivar.
| Parameters | SL | RL | SFW | RFW | SDW | RDW | Total chl. |
|---|---|---|---|---|---|---|---|
| SL | 1 | 0.885∗∗ | 0.942∗∗ | 0.753∗∗ | 0.944∗∗ | 0.883∗∗ | 0.322 |
| RL | 1 | 0.830∗∗ | 0.761∗∗ | 0.846∗∗ | 0.842∗∗ | 0.447 | |
| SFW | 1 | 0.806∗∗ | 0.989∗∗ | 0.914∗∗ | 0.346 | ||
| RFW | 1 | 0.817∗∗ | 0.966∗∗ | 0.238 | |||
| SDW | 1 | 0.926∗∗ | 0.398 | ||||
| RDW | 1 | 0.321 | |||||
| Total chl | 1 |
∗∗ Correlation is significant at the 0.01 level (2-tailed).
4. Conclusion
Based on the results, it can be concluded that cultivar Mitmita was the most drought tolerant among the three cultivars. This was manifested by insignificant reduction values of root length, shoot length, stem thickness, leaf width, leaf relative water content, chlorophyll fluorescence, photosynthesis rate, transpiration rate, chlorophylls “a,” “b”, and total chlorophylls, shoot fresh weight, and root fresh weight. The cultivar also had a higher accumulation of biochemical metabolites, mainly proline and total phenolic compounds, against drought stress. To fully utilize the potential of the studied cultivar Mitmita, further studies on molecular and biochemical drought response mechanisms of the cultivars and agronomic and nutritional evaluation experiments are recommended.
Acknowledgments
The authors would like to thank Bahir Dar Agricultural center for providing seeds for the three red pepper cultivars.
Symbols and Abbreviations
- Fo:
Minimum fluorescence
- F M :
Maximum fluorescence
- Fv:
Variable fluorescence
- LSD:
List significance difference
- Chl. a:
Chlorophyll a
- Chl. b:
Chlorophyll b
- Total chl:
Total chlorophyll
- SDW:
Shoot dry weight
- RDW:
Root dry weight
- df:
Degree of freedom
- SL:
Shoot length
- LA:
Leaf area
- RFW:
Root fresh weight
- SFW:
Shoot fresh weight
- W:
Watering level
- C:
Cultivar type
- RL:
Root length
- FAO:
Food and agriculture organization of the united nations
- MoARD:
Ministry of agriculture and rural development
- FYM:
Farmyard manure
- FC:
Field capacity
- ppm:
Parts per million
- LRWC:
Leaf relative water content
- CF:
Chlorophyll fluorescence
- A:
Photosynthetic rate
- E:
Transpiration rate
- gs:
Stomatal conductance
- TW:
Turgid weight
- DW:
Dry weight
- FW:
Fresh weight.
Data Availability
The dataset that supports the findings in the study is available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Abiyu E. Mola conceptualized and designed the study; Animut M. Andualem and Zelalem G. Tarekegn carried out the methodology; Abiyu E. Mola, Animut M. Andualem, Wubetie A. Wassie, Zelalem G. Tarekegn, Misganaw T. Ayana, and Mersha W. Aragaw developed the software, validated the study, and performed the formal analysis; Abiyu E. Mola, Animut M. Andualem, Misganaw T. Ayana, Wubetie A. Wassie, and Zelalem G. Tarekegn curated the data and wrote and prepared the original draft; Animut M. Andualem, Abiyu E. Mola, Wubetie A. Wassie, Zelalem G. Tarekegn, Misganaw T. Ayana, and Mersha W. Aragaw reviewed and edited the manuscript.
References
- 1.Rodriguez Y., Depestre T., Gomez O. Efficiency of selection in pepper lines (Capsicum annuum) from four subpopulations in characters of productive interest. Ciencia e Investigacian Agraria . 2008;35(1):29–40. [Google Scholar]
- 2.Ochoa-Alejo N., Ramirez-Malagon R. Invited Review: in vitro chilli pepper biotechnology. In Vitro Cell. Dev. Biol. Plant . 2001;37:701–729. [Google Scholar]
- 3.Qadir M., Bibi A., Tahir M., Saleem M., Sadaqat A. H. Screening of Sorghum (Sorghum Bicolor L) Genotypes under Various Levels of Drought Stress . Maydica; 2015. pp. 1–4. [Google Scholar]
- 4.FAO. FAOSTAT Statistics Database. 2021. https://www.fao.org/faostat/en/#data/QCL .
- 5.Roukens O., Tadele W., Tamrat K. Export Potential of Ethiopian Oleoresins . Addis Ababa, Ethiopia: Ethiopian Export Promotion Department; 2005. pp. 7–14. [Google Scholar]
- 6.MoARD. Animal and Plant Health Regulatory Directorate: Crop VarietyR. Issue No. 12 . Addis Abeba, Ethiopia: Ministry of Agriculture and Rural Development; 2009. [Google Scholar]
- 7.FAO. FAOSTAT online statistical service. Food and agriculture organization of the united Nations (FAO), Ethiopia. 2017. https://www.fao.org/faostat/en/#country/38 Available via URL.
- 8.Lin S. W. L., Chou Yu, Ching S. H., et al. Pepper (capsicum spp.) germplasm dissemination by AVRDC-the world vegetable center: an overview and introspection. Chronica Horticulturae . 2013;53(3):21–27. [Google Scholar]
- 9.Perez-Galvez A., Martin H. D., Sies H., Stahl W. Incorporation of carotenoids from paprika oleoresin into human chylomicrons. British Journal of Nutrition . 2003;89(6):787–793. doi: 10.1079/bjn2003842. [DOI] [PubMed] [Google Scholar]
- 10.Shetty A. A., Magadum S., Managanvi K. Vegetables as sources of antioxidants. Journal of Food and Nutritional Disorders . 2013;2(1):1–5. [Google Scholar]
- 11.Bae Y., Lim C. W., Lee S. C. Differential functions of pepper stress-associated proteins in response to abiotic stresses. Frontiers of Plant Science . 2021;12:756068–756114. doi: 10.3389/fpls.2021.756068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fekadu M. Related Traits in Hot Pepper . Ethiopia: Adama; 2008. Genetic components and heritability of yield and yield; p. p. 25. [Google Scholar]
- 13.Kang S., Zhang L., Hu X., Li Z., Jerie P. An improved water use efficiency for hot pepper grown under controlled alternate drip irrigation on partial roots. Scientia Horticulturae . 2001;89(4):257–267. doi: 10.1016/s0304-4238(00)00245-4. [DOI] [Google Scholar]
- 14.Mendelsohn R. The impact of climate change on agriculture in Asia. Journal of Integrative Agriculture . 2014;13(4):660–665. doi: 10.1016/s2095-3119(13)60701-7. [DOI] [Google Scholar]
- 15.Hossain A., Sarker M. A. Z., Saifuzzaman M., Teixeira da Silva J. A., Lozovskaya M. V., Akhter M. M. Evaluation of growth, yield, relative performance and heat susceptibility of eight wheat (Triticum aestivum L.) genotypes grown under heat stress. International Journal of Plant Production . 2013;7:615–636. [Google Scholar]
- 16.Jahan M. A. H. S., Hossain A., Teixeira da Silva J. A., El Sabagh A., Rashid M. H., Barutçular C. Effect of naphthalene acetic acid on root and plant growth and yield of ten irrigated wheat genotypes. Pakistan Journal of Botany . 2019;51(2):451–459. doi: 10.30848/pjb2019-2(11). [DOI] [Google Scholar]
- 17.Li G. L., Wu H. X., Sun Y. Q., Zhang S. Y. Response of chlorophyll fluorescence parameters to drought stress in sugar beet seedlings. Russian Journal of Plant Physiology . 2013;60(3):337–342. doi: 10.1134/s1021443713020155. [DOI] [Google Scholar]
- 18.Ahmad Z., Waraich E. A., Akhtar S., et al. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiologiae Plantarum . 2018;40(4):80–13. doi: 10.1007/s11738-018-2651-6. [DOI] [Google Scholar]
- 19.Waraich E. A., Ahmad R., Ashraf M. Y. Role of mineral nutrition in alleviation of drought stress in plants. Australian Journal of Crop Science . 2011;5:764–777. [Google Scholar]
- 20.Liu Y., Liang H., Lv X., Liu D., Wen X., Liao Y. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiology and Biochemistry . 2016;100:113–129. doi: 10.1016/j.plaphy.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Gargallo-Garriga A., Sardans J., Perez-Trujillo M., et al. Opposite metabolic responses of shoots and roots to drought. Scientific Reports . 2014;4:6829–6837. doi: 10.1038/srep06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A., Rico-Medina A., Cano-Delgado A. I. The physiology of plant responses to drought. Science . 2020;368(6488):266–269. doi: 10.1126/science.aaz7614. [DOI] [PubMed] [Google Scholar]
- 23.Alqudah A. M., Samarah N. H., Mullen R. E. Drought stress effect on crop pollination, seed set, yield and quality. In: Lichtfouse E., editor. Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilization . Berlin, Germany: Springer; 2010. pp. 193–213. [Google Scholar]
- 24.Lamaoui M., Jemo M., Datla R., Bekkaoui F. Heat and drought stress in crops and approaches for their mitigation. Frontiers of Chemistry . 2018;6:1–14. doi: 10.3389/fchem.2018.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchese J. A., Ferreira J. F. S., Rehder V. L. G., Rodrigues O. Water deficit effect on the accumulation of biomass and artemisinin in annual wormwood(Artemisia annua L., Asteraceae) Brazilian Journal of Plant Physiology . 2010;22(1):1–9. doi: 10.1590/s1677-04202010000100001. [DOI] [Google Scholar]
- 26.Mohammadi M., Ghassemi-Golezani K., Zehtab-Salmasi S., Nasrollahzade S. Assessment of some physiological traits in spring safflower (Carthamus tinctorius L.) cultivars under water stress. International Journal of Life Sciences . 2016;10(1):58–64. doi: 10.3126/ijls.v10i1.14512. [DOI] [Google Scholar]
- 27.Ma D. Y., Sun D. X., Wang C. Y., et al. Silicon application alleviates drought stress in wheat through transcriptional regulation of multiple antioxidant defense pathways. Journal of Plant Growth Regulation . 2016;35:1–10. doi: 10.1007/s00344-015-9500-2. [DOI] [Google Scholar]
- 28.Praba M. L., Cairns J. E., Babu R. C., Lafitte H. R. Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. Journal of Agronomy and Crop Science . 2009;195(1):30–46. doi: 10.1111/j.1439-037x.2008.00341.x. [DOI] [Google Scholar]
- 29.Caser M., Chitarra W., D’Angiolillo F., et al. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Industrial Crops and Products . 2019;129:85–96. doi: 10.1016/j.indcrop.2018.11.068. [DOI] [Google Scholar]
- 30.Khan F., Upreti P., Singh R., Shukla P. K., Shirke P. A. Physiological performance of two contrasting rice varieties under water stress. Physiology and Molecular Biology of Plants . 2017;23(1):85–97. doi: 10.1007/s12298-016-0399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tombesi S., Frioni T., Poni S., Palliotti A. Effect of water stress “memory” on plant behavior during subsequent drought stress. Environmental and Experimental Botany . 2018;150:106–114. doi: 10.1016/j.envexpbot.2018.03.009. [DOI] [Google Scholar]
- 32.Mahmood T., Rana R. M., Ahmar S., et al. Effect of drought stress on capsaicin and antioxidant contents in pepper genotypes at reproductive stage. Plants . 2021;10(7):1286–1313. doi: 10.3390/plants10071286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan B., Yang Y., Lu Y., Korpelainen H., Berninger F., Li C. Interactions between water deficit, ABA, and provenances in Picea asperata. Journal of Experimental Botany . 2007;58(11):3025–3036. doi: 10.1093/jxb/erm160. [DOI] [PubMed] [Google Scholar]
- 34.Widuri L. I., Lakitan B., Hasmeda M., et al. Relative leaf expansion rate and other leaf-related indicators for detection of drought stress in chili pepper (Capsicum annuum L.) Australian Journal of Crop Science . 2017;11(12):1617–1625. doi: 10.21475/ajcs.17.11.12.pne800. [DOI] [Google Scholar]
- 35.Abdelmonem Y. Plant Water Consumption . Egypt: Irrigation and Drainage Engineering Faculty of Engineering, Ain Shams University; 2020. pp. 8–9. [Google Scholar]
- 36.Alordzinu E. K., Jiuhao L., Appiah A. S., Aasmi L. A. A., Blege K. P., Afful A. E. Water stress affects the physio-morphological development of tomato growth. African Journal of Agricultural Research . 2021;17(5):733–742. doi: 10.5897/ajar2021.15450. [DOI] [Google Scholar]
- 37.Kumar S., Sehgal K. V., Mall K. R. Manual for Drought Management . New Dlehi, India: Department of Agriculture and Cooperation Ministry of Agriculture, Government of India; 2009. p. p. 9. [Google Scholar]
- 38.Al-Maskri A., Al-Busaidi W., Al-Nadabi H., Al-Fahdi A., Khan M. M. Effects of drought stress on wheat (Triticum aestivum L.) cultivar coolly. Proceedings of the International Conference on Agricultural, Food, Biological and Health Sciences (AFBHS-16); August 2016; Kuala Lumpur, Malaysia. [Google Scholar]
- 39.Kazgöz Candemi̇r D., Ödemi̇ş B., Evrendilek F. Responses to drought stress levels of strawberry grown in greenhouse conditions. Horticultural Studies . 2020;37(2):113–122. doi: 10.16882/hortis.805196. [DOI] [Google Scholar]
- 40.Ayers R. S., Westcot D. W. Water Quality for Agriculture . Italy: Food and Agriculture Organization of the United Nations Rome; 1985. [Google Scholar]
- 41.Delelegne S., Belew D., Mohammed A., Getachew Y. Evaluation of elite hot pepper varieties (capsicum spp.) for growth, dry pod yield and quality under jimma condition, south west Ethiopia. International Journal of Agricultural Research . 2014;9(7):364–374. doi: 10.3923/ijar.2014.364.374. [DOI] [Google Scholar]
- 42.Kirnak H., Cengiz K., David H., Sinan G. A long-term experiment to study the role of mulches in physiology and macro-nutrition of strawberries grown under water stress. Australian Journal of Agricultural Research . 2001;52(9):1307–1316. [Google Scholar]
- 43.Moradi A., Ahmadi A. H. M. D., Hossain Z. A. The effects of different timings and severity of drought stress on gas exchange parameters of mung bean. Desert . 2008;13(1):59–66. [Google Scholar]
- 44.Husen A., Iqbal M., Sohrab S. S., Ansari M. K. A. Salicylic acid alleviates salinity-caused damage to foliar functions, plant growth and antioxidant system in Ethiopian mustard (Brassica carinata A. Br.) Agriculture & Food Security . 2018;7(1):44–14. doi: 10.1186/s40066-018-0194-0. [DOI] [Google Scholar]
- 45.Kauser R., Athar H. U. R., Ashraf M. U. Chlorophyll fluorescence: a potential indicator for rapid assessment of water stress tolerance in canola (Brassica napus L.) Pakistan Journal of Botany . 2006;38(5):1501–1509. [Google Scholar]
- 46.Almeselmani M., Abdullah F., Hareri F., Naaesan M., Ammar M. A., ZuherKanbar O. Effect of drought on different physiological characters and yield components in different varieties of Syrian durum wheat. Journal of Agricultural Science . 2011;3(3):127–133. [Google Scholar]
- 47.Arnon D. I. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiology . 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bates L. S., Waldren R. P., Teare I. D. Rapid determination of free proline for water-stress studies. Plant and Soil . 1973;39(1):205–207. doi: 10.1007/bf00018060. [DOI] [Google Scholar]
- 49.Rispail N., Moris P., Webb K. J. Phenolic compounds-extraction and analysis. In: Márquez A. J., Stougaard J., Udvardi M. K., et al., editors. Lotus Japonicus Handbook . Dordrecht, The Netherlands: Springer; 2005. [Google Scholar]
- 50.Luvaha E., Netondo G. W., Ouma G. Effect of water deficit on the physiological and morphological characteristics of mango (mangifera indica) rootstock seedlings. American Journal of Plant Physiology . 2009;5(1):7–21. doi: 10.3923/ajpp.2010.7.21. [DOI] [Google Scholar]
- 51.Specht J. E., Chase K., Macrander M., et al. Soybean response to water. A QTL analysis of drought tolerance. Crop Science . 2001;41(2):493–509. doi: 10.2135/cropsci2001.412493x. [DOI] [Google Scholar]
- 52.Zhang Z., Lu A., D’arcy G. W. Capsicum annuum linnaeus, special plant. Flora of China . 2002;17:313–320. [Google Scholar]
- 53.Chutipaijit S., Cha-um S., Sompornpailin K. An evaluation of water deficit tolerance screening in pigmented indica rice genotypes. Pakistan Journal of Botany . 2012;44:65–72. [Google Scholar]
- 54.EmamY, Shekoofa A., Salehi F., Jalali A. H. Water stress effects on two common bean cultivars with contrasting growth habits. Am. Eur. J. Agric. Environ. Sci. . 2010;9(5):495–499. [Google Scholar]
- 55.Kamara A. Y., Menkir A., Badu-Apraku B., Ibikunle O. The influence of drought stress on growth, yield and yield components of selected maize genotypes. The Journal of Agricultural Science . 2003;141(1):43–50. doi: 10.1017/s0021859603003423. [DOI] [Google Scholar]
- 56.Riaz A., Younis A., Taj A. R., et al. Effect of drought stress on growth and flowering of marigold (Tagetes erecta L.) Pakistan Journal of Botany . 2013;45(51):123–131. [Google Scholar]
- 57.Wu Q. S., Xia R. X., Zou Y. N. Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. European Journal of Soil Biology . 2008;44(1):122–128. doi: 10.1016/j.ejsobi.2007.10.001. [DOI] [Google Scholar]
- 58.Hussain M., Malik M. A., Farooq M., Ashraf M. Y., Cheema M. A. Improving drought tolerance by exogenous application of glycine betaine and salicylic acid in sunflower. Journal of Agronomy and Crop Science . 2008;194(3):193–199. doi: 10.1111/j.1439-037x.2008.00305.x. [DOI] [Google Scholar]
- 59.Pandey S., Chakraborty D., Ror S. Analysis of biochemical responses in Vigna mungo varieties subjected to drought stress and possible amelioration. International Journal of Scientific Research in Agricultural Sciences . 2014;1(1):6–15. doi: 10.12983/ijsras-2014-p0006-0015. [DOI] [Google Scholar]
- 60.Bhatt R. M., Rao N. K. Influence of pod load response of okra to water stress. Indian Journal of Plant Physiology . 2005;10(1):54–59. [Google Scholar]
- 61.Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S. M. A. Plant drought stress: effects, mechanisms and management. Agronomy for Sustainable Development . 2009;29(1):185–212. doi: 10.1051/agro:2008021. [DOI] [Google Scholar]
- 62.Anjum S. A., Wang L., Farooq M., Xue L., Ali S. Fulvic acid application improves the maize performance under well‐watered and drought conditions. Journal of Agronomy and Crop Science . 2011;197(6):409–417. doi: 10.1111/j.1439-037x.2011.00483.x. [DOI] [Google Scholar]
- 63.Nezhadahmadi A., Faruq G., Rashid K. The impact of drought stress on morphological and physiological parameters of three strawberry varieties in different growing conditions. Pakistan Journal of Agricultural Sciences . 2015;52:79–92. [Google Scholar]
- 64.Rizza F., Badeck F. W., Cattivelli L., Lidestri O., Di Fonzo N., Stanca A. M. Use of a water stress index to identify barley genotypes adapted to rain fed and irrigated conditions. Crop Science . 2004;44(6):2127–2137. doi: 10.2135/cropsci2004.2127. [DOI] [Google Scholar]
- 65.Yenni M., Ibrahim M. H., Nulit R., Sakimin S. Z. Influence of drought stress on growth, biochemical changes and leaf gas exchange of strawberry (Fragaria × ananassa</i> Duch.) in Indonesia. AIMS Agriculture and Food . 2022;7(1):37–60. doi: 10.3934/agrfood.2022003. [DOI] [Google Scholar]
- 66.Arndt A. K., Clifford S., Wanek W., Jones G. H., Popp M. Physiological and morphological adaptations of the fruit tree Ziziphus ritundifolia in response to progressive drought. Tree Physiology . 2001;21:1–11. doi: 10.1093/treephys/21.11.705. [DOI] [PubMed] [Google Scholar]
- 67.Afzal A., Gulzar I., Shahbaz M., Ashraf M. Water deficit-induced regulation of growth, gas exchange, chlorophyll fluorescence, inorganic nutrient accumulation and antioxidative defense mechanism in mungbean (Vigna radiata L) Journal of Applied Botany and Food Quality . 2014;87:147–156. [Google Scholar]
- 68.Salazar C., Hernández C., Teresa P. M. A review on Plant water stress: associations between ethylene and abscisic acid response. Chilean Journal of Agriculture . 2015;75(1):1–4. [Google Scholar]
- 69.Zeid I. M., Shedeed Z. A. Response of alfalfa to putrescine treatment under drought stress. Biologia Plantarum . 2006;50(4):635–640. doi: 10.1007/s10535-006-0099-9. [DOI] [Google Scholar]
- 70.Manivannan P., Jaleel C. A., Sankar B., et al. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids and Surfaces B: Biointerfaces . 2007;59(2):141–149. doi: 10.1016/j.colsurfb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 71.Tahir M. H. N., Mehid S. S. Evaluation of open pollinated sunflower (Helianthus annuus L.) populations under water stress and normal conditions. International Journal of Agriculture and Biology . 2001;3:236–238. [Google Scholar]
- 72.Temesgen B. Effects of drought stress on crop production and productivity. International Journal of Research Studies in Agricultural Sciences . 2020;6(9):34–43. [Google Scholar]
- 73.Al-Mahmud A., Hossain A. M., Mamun A. A., et al. Plant canopy, tuber yield and growth analysis of potato under moderate and severe drought condition. Journal of Plant Sciences . 2014;2(5):201–208. doi: 10.11648/j.jps.20140205.18. [DOI] [Google Scholar]
- 74.Ichwan B., Suwignyo A. R., Susilawat H. R. Response of red chilli varieties under drought stress. RJOAS . 2017;6(66):1–9. [Google Scholar]
- 75.Showemimo F., Olarewaju J. Drought tolerance indices in sweet pepper (capsicum annuum L.) International Journal of Plant Breeding and Genetics . 2006;1(1):29–33. doi: 10.3923/ijpbg.2007.29.33. [DOI] [Google Scholar]
- 76.Zhang J. Z., Creelman R. A., Zhu J. K. From laboratory to field. Using information from Arabidopsis to engineer salt, cold, and drought tolerance in crops. Plant Physiology . 2004;135(2):615–621. doi: 10.1104/pp.104.040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boutraa T., Akhkha A., Al-Shoaibi A. A., Alhejeli A. M. Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. Journal of Taibah University for Science . 2010;3(1):39–48. doi: 10.1016/s1658-3655(12)60019-3. [DOI] [Google Scholar]
- 78.Klamkowski K., Treder W. Response to drought stress of three strawberry cultivars grown under greenhouse conditions. Journal of Fruit and Ornamental Plant Research . 2008;16:179–188. [Google Scholar]
- 79.Hayatu M., Muhammad S. Y., Habibu U. A. Effect of water stress on the leaf relative water content and yield of some cowpea (Vigna Unguiculata L.) Walp.) genotype. International Journal of Scientific and Technology Research . 2014;3(7):148–152. [Google Scholar]
- 80.Manandhar A., Sinclair T. R., Rufty T. W., Ghanem M. E. Leaf emergence (phyllochron index) and leaf expansion response to soil drying in cowpea genotypes. Physiologia Plantarum . 2017;160(2):201–208. doi: 10.1111/ppl.12544. [DOI] [PubMed] [Google Scholar]
- 81.Chartzoulakis K., Noitsakis B., Therios I. Photosynthesis, plant growth and dry matterdistribution in kiwifruit as influenced by water deficits. Irrigation Science . 1993;14:1–5. doi: 10.1007/bf00194999. [DOI] [Google Scholar]
- 82.Tezara W., Mitchell V., Dviscoll S. P., Lawlor D. W. Effects of water deficit and its interaction with Co2 supply on the biochemistry and physiology of photosynthesis in sun flower. Journal of Experimental Botany . 2002;53(375):1781–1791. doi: 10.1093/jxb/erf021. [DOI] [PubMed] [Google Scholar]
- 83.Lonbani M., Arzani A. Morpho-physiological traits associated with terminal drought stress tolerance in triticale and wheat. Agricultural Research . 2011;9:315–329. [Google Scholar]
- 84.Blum A. Mitigation of drought stress by crop management. 2005. Available at: 2005 www.Plant Stress.com.
- 85.DaCosta M., Huang B. Changes in antioxidant enzyme activities and lipid peroxidation for bentgrass species in response to drought stress. Journal of the American Society for Horticultural Science . 2007;132(3):319–326. doi: 10.21273/jashs.132.3.319. [DOI] [Google Scholar]
- 86.Cornic G. Drought stress inhibits photosynthesis by decreasing stomatal aperture—not by affecting ATP synthesis. Trends in Plant Science . 2000;5:187–188. doi: 10.1016/s1360-1385(00)01625-3. [DOI] [Google Scholar]
- 87.Nayyar H., Gupta D. Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Environmental and Experimental Botany . 2006;58(1-3):106–113. doi: 10.1016/j.envexpbot.2005.06.021. [DOI] [Google Scholar]
- 88.Saeidi M., Ardalani S., Jalali-Honarmand S., Ghobadi M. E., Abdoli M. Evaluation of drought stress at vegetative growth stage on the grain yield formation and some physiological traits as well as fluorescence parameters of different bread wheat cultivars. Acta Biologica Szegediensis . 2015;59(1):535–544. [Google Scholar]
- 89.Siddique M. R., Hamid A., Islam M. S. Drought stress effects on water relations of wheat. Botanical Bulletin of Academia Sinica . 2001;41:35–39. [Google Scholar]
- 90.Singh R., Pandey N., Naskar J., Shirke P. A. Physiological performance and differential expression profiling of genes associated with drought tolerance in contrasting varieties of two Gossypium species. Protoplasma . 2015;252(2):423–438. doi: 10.1007/s00709-014-0686-0. [DOI] [PubMed] [Google Scholar]
- 91.Chen S., Zhou Z. j, Andersen M. N., Hu T. t. Tomato yield and water use efficiency-coupling effects between growth stage specific soil water deficits. Acta Agriculturae Scandinavica Section B Soil and Plant Science . 2015;65(5):460–469. doi: 10.1080/09064710.2015.1024279. [DOI] [Google Scholar]
- 92.El Jaafari S. Durum wheat breeding for abiotic stresses resistance: defining physiological traits and criteria. Options Mediterraneennes . 2000;40(31):251–256. [Google Scholar]
- 93.Liu C., Guo K., Liu Y., et al. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environmental and Experimental Botany . 2011;71(2):174–183. doi: 10.1016/j.envexpbot.2010.11.012. [DOI] [Google Scholar]
- 94.Wang Z., Li G., Sun H., et al. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biology open . 2018;7(11):27. doi: 10.1242/bio.035279.035279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mamnouie E., FotouhiGhazvini R., Esfahany M., Nakhoda B. The effects of water deficit on crop yield and the physiological characteristics of Barley (Hordeum vulgare L.) Varieties. Journal of Agricultural Science and Technology A . 2006;8:211–219. [Google Scholar]
- 96.Masoumi Z., Haghighi M., Jalali S. A. H. Flooding ordrought which one is more offensive on pepper physiology and growth? Molecular Biology Reports . 2021;48(5):4233–4245. doi: 10.1007/s11033-021-06437-3. [DOI] [PubMed] [Google Scholar]
- 97.Zlatev Z. Drought-Induced changes in chlorophyll fluorescence of young wheat plants. Biotechnology & Biotechnological Equipment . 2009;23(1):438–441. doi: 10.1080/13102818.2009.10818458. [DOI] [Google Scholar]
- 98.Barta C., Dunkle A. M., Wachter R. M., Salvucci M. E. Structural changes associated with the acute thermal instability of Rubisco activase. Archives of Biochemistry and Biophysics . 2010;499(1-2):17–25. doi: 10.1016/j.abb.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 99.Lidon Z., Lidon F. C. An overview on drought induced changes in plant growth, water relations<br>and photosynthesis. Emirates Journal of Food and Agriculture . 2012;24(1):57–72. doi: 10.9755/ejfa.v24i1.10599. [DOI] [Google Scholar]
- 100.Baker N. R., Bowyer J. R. Photoinhibition of Photosynthesis from Molecular Mechanisms to the Field . Oxford, UK: Bios Scientific Publishers; 1994. pp. 1–24. [Google Scholar]
- 101.Baker N. R., Rosenqvist E. Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. Journal of Experimental Botany . 2004;55(403):1607–1621. doi: 10.1093/jxb/erh196. [DOI] [PubMed] [Google Scholar]
- 102.Mafakheri A., Siosemardeh A., Bahramnejad B., Struik P. C., Sohrabi Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Australian Journal of Crop Science . 2010;4(8):580–585. [Google Scholar]
- 103.Yang C., Liu X., Chen Z., Lin Y., Wang S. Comparison of oil content and fatty acid profile of ten new Camellia oleifera cultivars. Journal of Lipids . 2016;2016:6. doi: 10.1155/2016/3982486.3982486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang F., Zhu K., Wang Y., et al. Changes in photosynthetic and chlorophyll fluorescence characteristics of sorghum under drought and waterlogging stress. Photosynthetica . 2019;57(4):1156–1164. doi: 10.32615/ps.2019.136. [DOI] [Google Scholar]
- 105.Zhang X., Lei L., Lai J., Zhao H., Song W. Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biology . 2018;18:68–16. doi: 10.1186/s12870-018-1281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bloch D., Hoffmann C. M., Marlander B. Impact of water supply on photosynthesis, water use and carbon isotope discrimination of sugar beet genotypes. European Journal of Agronomy . 2006;24(3):218–225. doi: 10.1016/j.eja.2005.08.004. [DOI] [Google Scholar]
- 107.Klamkowski K., Treder W., Wojcik K. Effects of long-term water stress on leaf gas exchange, growth and yield of three strawberry cultivars. Acta Sci Pol Hortorum Cultus . 2015;14:55–65. [Google Scholar]
- 108.Saeidi M., Abdoli M. Effect of drought stress during grain filling on yield and its components, gas exchange variables, and some physiological traits of wheat cultivars. Journal of Agriculture, Science and Technology . 2015;17:885–898. [Google Scholar]
- 109.Lauteri M., Haworth M., Serraj R., Monteverdi M. C., Centritto M. Photosynthetic diffusional constraints affect yield in drought-stressed rice cultivars during flowering. PLoS One . 2014;9(10):1090544–e109113. doi: 10.1371/journal.pone.0109054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Berahim Z., Dorairaj D., Omar M. H., Saud H. M., Ismail M. R. Spermine mediated improvements on stomatal features, growth, grain filling and yield of rice under differing water availability. Scientific Reports . 2021;11(1) doi: 10.1038/s41598-021-89812-1.10669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garcia-Sanchez F., Syvertsen J. P., Gimeno V., Botia P., Perez-Perez J. G. Responses to flooding and drought stress by two citrus rootstock seedlings with different water use efficiency. Physiologia Plantarum . 2007;130(4):532–542. doi: 10.1111/j.1399-3054.2007.00925.x. [DOI] [Google Scholar]
- 112.Chaves M. M., Oliveira M. M. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany . 2004;55(407):2365–2384. doi: 10.1093/jxb/erh269. [DOI] [PubMed] [Google Scholar]
- 113.Flexas J., Medrano H. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Annals of Botany . 2002;89(2):183–189. doi: 10.1093/aob/mcf027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhan H. X., Chang Z. J., Wei A. L., Zhang X. J., Li X. Impact of drought on wheat physiological index. J Shanxi Agric Sci . 2011;39:1049–1051. [Google Scholar]
- 115.Farooq M., Wahid A., Lee D. J. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiologiae Plantarum . 2009;31(5):937–945. doi: 10.1007/s11738-009-0307-2. [DOI] [Google Scholar]
- 116.Jaleel C. A., Gopi R., Sankar B., Gomathinayagam M., Panneerselvam R. Differential responses in water use efficiency in two varieties of Catharanthus roseus under drought stress. Comptes Rendus Biologies . 2008;331(1):42–47. doi: 10.1016/j.crvi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 117.Saeidi M., Moradi F., Ahmadi A., Spehri R., Naja G., Shabani A. The effects of terminal water stress on physiological characteristics and sink-source relations in two bread wheat (Triticum aestivum L.) cultivars. Iran J Crop Sci . 2010;12(4):392–408. [Google Scholar]
- 118.Wang Y., Li L., Tang S., et al. Combined small RNA and degradome sequencing to identify miRNAs and their targets in response to drought in foxtail millet. BMC Genetics . 2016;17(1):57–65. doi: 10.1186/s12863-016-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sikuku P. A., Netondo J. C., Musyimi M. D. Chlorophyll fluorescence, protein and chlorophyll content of three nerica rain fed rice varieties undervarying irrigation regimes. ARPN J. Agric. and Biol. Sci. . 2010;5:19–25. [Google Scholar]
- 120.Meher A., Shivakrishna P., Ashok Reddy K., Manohar Rao D. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi Journal of Biological Sciences . 2018;25(2):285–289. doi: 10.1016/j.sjbs.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pirzad A., Shakiba R. M., Zehtab-Salmasi S., Mohammadi A. S., Darvishzadeh R., Samadi A. Effect of water stress on leaf relative water content, chlorophyll, proline and soluble carbohydrates in Matricaria chamomilla L. Journal of Medicinal Plants Research . 2011;5:2483–2488. [Google Scholar]
- 122.Allakhverdiev S. I., Sakamoto A., Nishiyama Y., Murata N. Inactivation of photosystems I and II in response to osmotic stress in Synechococcus. Contribution of water channels. Plant Physiology . 2000;122(4):1201–1208. doi: 10.1104/pp.122.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Anjum A. S., Xie X., Wang L., Saleem F. M., Man C., Lei W. Morphological, physiological and biochemical responses of plants to drought stress. African Journal of Agricultural Research . 2011;6(9):2026–2032. [Google Scholar]
- 124.Ashraf M., Harris P. J. C. Photosynthesis under stressful environments: an overview. Photosynthetica . 2013;51(2):163–190. doi: 10.1007/s11099-013-0021-6. [DOI] [Google Scholar]
- 125.Kannan N. D., Kulandaivelu G. Drought induced changes in physiological, biochemical and phytochemical properties of Withania somnifera Dun. Journal of Medicinal Plants Research . 2011;5:3929–3935. [Google Scholar]
- 126.Gussakovsky E. E., Salakhutdinov B. A., Shahak Y. Chiral macro-aggregates of LHCII detected by circularly polarized luminescence in intact pea leaves are sensitive to drought stress. Functional Plant Biology . 2002;29(8):955–963. doi: 10.1071/pp01224. [DOI] [PubMed] [Google Scholar]
- 127.Anjum S. A., Ashraf U., Tanveer M., et al. Drought induced changes in growth, osmolytes accumulation and antioxidant metabolism of three maize hybrids. Frontiers of Plant Science . 2017;8(69):1–12. doi: 10.3389/fpls.2017.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Serraj R., Sinclair T. R. Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant, Cell and Environment . 2002;25(2):333–341. doi: 10.1046/j.1365-3040.2002.00754.x. [DOI] [PubMed] [Google Scholar]
- 129.Tanveer M., Shahzad B., Sharma A., Khan E. A. 24-Epibrassinolide application in plants: an implication for improving drought stress tolerance in plants. Plant Physiology and Biochemistry . 2019;135:295–303. doi: 10.1016/j.plaphy.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 130.Khazaei Z., Esmaielpour B., Estaji A. Ameliorative effects of ascorbic acid on tolerance to drought stress on pepper (Capsicum annuum L) plants. Physiology and Molecular Biology of Plants . 2020;26(8):1649–1662. doi: 10.1007/s12298-020-00846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alexieva V., Sergiev I., Mapelli S., Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell and Environment . 2001;24(12):1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- 132.Yamada M., Morishita H., Urano K., et al. Effects of free proline accumulation in petunias under drought stress. Journal of Experimental Botany . 2005;56(417):1975–1981. doi: 10.1093/jxb/eri195. [DOI] [PubMed] [Google Scholar]
- 133.Kachare S., Tiwari S., Tripathi N., Thakur V. V. Assessment of genetic diversity of soybean (Glycine max) genotypes using qualitative traits and microsatellite markers. Agricultural Research . 2019;9(1):23–34. doi: 10.1007/s40003-019-00412-y. [DOI] [Google Scholar]
- 134.Khan S. A., Karim M. A., Mahmud A., Sarveen S., Bazzaz M. M., Hossain M. A. Plant water relations and proline accumulations in soybean under salt and water stress environment. Journal of Plant Sciences . 2015;3:272–278. [Google Scholar]
- 135.Sharma A., Tripathi M. K., Tiwari S., Gupta N., Tripathi N., Mishra N. Evaluation of soybean (Glycine max L.) genotypes on the basis of biochemical contents and anti-oxidant enzyme activities. Legume Research an International Journal . 2021;44:1–11. doi: 10.18805/lr-4678. [DOI] [Google Scholar]
- 136.Ghorbanli M., Gafarabad M., Amirkian T., Mamaghani B. A. Investigation of praline, total protein, chlorophyll, ascorbate and dehydro-ascorbate changes under drought stress in Akria and Mobil tomato cultivars. Irnian Journal of Plant Physiology . 2012;3:651–658. [Google Scholar]
- 137.Choudhary M. L., Tripathi M. K., Tiwari S., et al. Screening of pearl millet [Pennisetum glaucum (L.) R. Br.] germplam lines for drought tolerance based on morpho-physiological traits and SSR markers. Current Journal of Applied Science and Technology . 2021;40:46–63. doi: 10.9734/cjast/2021/v40i531303. [DOI] [Google Scholar]
- 138.Ahmed C. B., Rouina B. B., Sensoy S., Boukhris M., Abdallah F. B. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environmental and Experimental Botany . 2009;67(2):345–352. doi: 10.1016/j.envexpbot.2009.07.006. [DOI] [Google Scholar]
- 139.George S., Minhas N. M., Jatoi S. A., Siddiqui S. U., Ghafoor A. Impact of polyethylene glycol on proline and membrane stability index for water stress regime in tomato (solarium lycopersicum) Pakistan Journal of Botany . 2015;47:835–844. [Google Scholar]
- 140.Claussen W. Proline as a measure of stress in tomato plants. Plant Science . 2005;168(1):241–248. doi: 10.1016/j.plantsci.2004.07.039. [DOI] [Google Scholar]
- 141.Maggio A., Miyazaki S., Veronese P., et al. Does proline accumulation play an active role in stress-induced growth reduction? The Plant Journal . 2002;31(6):699–712. doi: 10.1046/j.1365-313x.2002.01389.x. [DOI] [PubMed] [Google Scholar]
- 142.Mandal S., Yadav S., Yadav S., Nema R. K. Antioxidants: a review. J Chem Pharmacol Res . 2009;1(1):102–104. [Google Scholar]
- 143.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science . 2002;7(9):405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 144.Sanchez-Rodriguez E., Rubio-Wilhelmi M., Cervilla L. M., et al. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Science . 2010;178(1):30–40. doi: 10.1016/j.plantsci.2009.10.001. [DOI] [Google Scholar]
- 145.Ayaz F. A., Kadioglu A., Turgut R. Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthe setosa (Rosc.) Eichler. Canadian Journal of Plant Science . 2000;80(2):373–378. doi: 10.4141/p99-005. [DOI] [Google Scholar]
- 146.Heim K. E., Tagliaferro A. R., Bobilya D. J. Flavonoid antioxidants: chemistry, metabolism, and structure-activity relationships. The Journal of Nutritional Biochemistry . 2002;13(10):572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 147.Muñoz‐Perea C. G., Teran H., Allen R. G., Wright J. L., Westermann D. T., Singh S. P. Selection for drought resistance in dry bean landraces and cultivars. Crop Science . 2006;46(5):2111–2120. doi: 10.2135/cropsci2006.01.0029. [DOI] [Google Scholar]
- 148.Kumar N., Nandwal A. S., Waldia R. S., et al. Drought tolerance in chickpea as evaluated by root characteristics, plant water status, membrane integrity and chlorophyll fluorescence techniques. Experimental Agriculture . 2012;48(3):378–387. doi: 10.1017/s0014479712000063. [DOI] [Google Scholar]
- 149.Rostampour F. M., Yarnia M., Khoee R. F. Effect of polymer and irrigation regimes on dry matter yield and several physiological traits of forage sorghum. African Journal of Biotechnology . 2012;11(48):10834–10840. [Google Scholar]
- 150.Saradadevi R., Bramley H., Siddique K. H., Edwards E., Palta J. A. Contrasting stomatal regulation and leaf ABA concentrations in wheat genotypes when split root systems were exposed to terminal drought. Field Crops Research . 2014;162:77–86. doi: 10.1016/j.fcr.2014.02.004. [DOI] [Google Scholar]
- 151.Nam N. H., Subbarao G. V., Chauhan Y. S., Johansen C. Importance of canopy attributes in determining dry matter accumulation of pigeon pea under contrasting moisture regimes. Crop Science . 1998;38(4):955–961. doi: 10.2135/cropsci1998.0011183x003800040013x. [DOI] [Google Scholar]
- 152.Kerepesi I., Galiba G. Osmotic and salt stressinduced alteration in soluble carbohydrate content in wheat seedlings. Crop Science . 2000;40(2):482–487. doi: 10.2135/cropsci2000.402482x. [DOI] [Google Scholar]
- 153.Westgate M. E., Boyer J. S. Osmotic adjustment and the inhibition of leaf, root, stem and silk growth at low water potentials in maize. Planta . 1985;164(4):540–549. doi: 10.1007/bf00395973. [DOI] [PubMed] [Google Scholar]
- 154.Wu Y., Cosgrove D. J. Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. Journal of Experimental Botany . 2000;51(350):1543–1553. doi: 10.1093/jexbot/51.350.1543. [DOI] [PubMed] [Google Scholar]
- 155.Leport L., Turner N. C., Davies S. L., Siddique K. H. M. Variation in pod production and abortion among chickpea cultivars under terminal drought. European Journal of Agronomy . 2006;24(3):236–246. doi: 10.1016/j.eja.2005.08.005. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset that supports the findings in the study is available from the corresponding author upon request.


