Abstract
Purpose
Fluorescence-guided surgery applying 5-aminolevulinic acid (5-ALA) in high-grade gliomas is an established method in adults. In children, results have so far been ambiguous. The aim of this study was to investigate 5-ALA-induced fluorescence in pediatric brain tumors by using the surgical microscope and a spectroscopic hand-held probe.
Methods
Fourteen randomly selected children (age 4–17) with newly MRI-verified brain tumors were included. No selection was based on the suspected diagnosis prior to surgery. All patients received 5-ALA (20 mg /kg) either orally or via a gastric tube prior to surgery. Intratumoral fluorescence was detected with the microscope and the probe. Moreover, fluorescence in the skin of the forearm was measured. Histopathology samples revealed seven low-grade gliomas, four medulloblastomas, one diffuse intrinsic pontine glioma, one glioblastoma and one atypical meningioma. Blood samples were analyzed, and potential clinical side effects were monitored.
Results
Microscopically, vague fluorescence was visible in two patients. Intratumoral fluorescence could be detected in five patients with the probe, including the two patients with vague microscopic fluorescence. Three of the oldest children had PpIX fluorescence in the skin. Nine children did not show any fluorescence in the tumor or in the skin. No clinical side effects or laboratory adverse events were observed.
Conclusion
Fluorescence could not be used to guide surgery in this study, neither with the surgical microscope nor with the hand-held probe. In nine children, no fluorescence was discerned and children with noticeable fluorescence were all older than nine years. 5-ALA was considered safe to apply in children.
Keywords: 5-ALA, Brain tumor, Children, Spectroscopy
Introduction
The extent of resection (EOR) in low- and high-grade gliomas is pivotal for the progression free and overall survival in both children [3, 45] and adults [21, 25, 37], recently also demonstrated irrespective of the molecular subgroup [28]. However, there is a delicate balance between achieving maximal EOR and the risk for postoperative neurological deficits, ranging between 24–44% in some studies in children, thus leading to long-term neurological and cognitive deficits [4, 13]. Great care must be taken to attain maximal resection with preservation of neurological function. Determining the tumor border zone, delineating between tumor and healthy brain tissue is thus a vital challenge for neurosurgeons. In adults, the frequency of gross total resection (GTR) is moderate, approximately 30% in high-grade gliomas (HGG) when applying standard microsurgical techniques [2]. To optimize resection, several intraoperative techniques have been developed, such as neuronavigation, intraoperative magnetic resonance imaging (MRI), intraoperative ultrasound and optical techniques using fluorescing agents [20]. Fluorescence-guided surgery implies oral or intravenous administration of a dye for visualization of fluorescence intraoperatively [46].
During the last decades, fluorescence-guided surgery using five-aminolevulinic acid (5-ALA) has been established as an intraoperative tool in HGG surgery in adults, significantly enhancing GTR [42]. Briefly, the mechanism of action is believed to be mediated through conversion of 5-ALA to protoporphyrin IX (PpIX) but not to heme, tentatively due to a downregulation of the enzyme ferrochelatase, and accumulation of PpIX in malignant tumor cells with a disrupted blood brain barrier (BBB) [12, 19, 41, 44]. Being extensively used in adults, 5-ALA is still considered an off-label product in the pediatric population. Lately, numerous studies and case reports have been published examining the role of 5-ALA in pediatric brain tumors with somewhat conflicting results, not showing a clear intraoperative benefit [1, 8–10, 14, 22–24, 32, 35, 36, 38–40, 43, 47]. Stummer et al. have previously suggested that 5-ALA should mainly be administered to children with supratentorial, strongly contrast-enhancing tumors [43]. Given the different biology of pediatric brain tumors in comparison with adults [40], these equivocal results may be conceivable. However, one also must consider the possibility of age-dependent differences in the pharmacodynamics and pharmacokinetics of 5-ALA in neonates, infants, adolescents, and adults [5, 6, 15, 26, 27].
To improve the intraoperative diagnostic accuracy of PpIX fluorescence, our group has previously developed a spectroscopic system using a hand-held probe [16], enabling detection of augmented fluorescence indicative of tumor tissue, outside the tumor margins, as identified by the fluorescence in the microscope [33]. PpIX fluorescence could also be detected in the forearm skin in adults as an indicator of potential light sensitivity [17].
The aim of this study was to examine the intraoperative PpIX fluorescence in brain tumors in children with blue-light surgical microscope and the hand-held probe system, analyzing whether it could accurately depict tumor margins. The spectroscopy system was mainly used to investigate whether the fluorescence invisible in the microscope could be detected with the probe. Another aim was to carefully monitor 5-ALA´s safety profile in children by analyzing blood tests, registering adverse events and investigating whether 5-ALA has similar light sensitivity profile in the skin as seen in adults.
Material and methods
Patients
Inclusion criteria were newly verified brain tumors on MR images; age 4–17 years according to decision in the ethical review board and informed, written consent by patient (when possible) and both parents. All patients referred to the center within the study time frame that fulfilled the inclusion criteria were considered for participation in the study. No selection was done based on the suspected tumor diagnosis or grade. In total 14 patients (male N = 7, female N = 7, range 4 -17 years, median age 9 years) were included in the study. Patients with recurrent tumors or planned secondary surgery were not included. All study participants had no other disease or current medications, except levetiracetam in one case of epilepsy.
Three additional patients were excluded before surgery due to blood value abnormalities. Exclusion criteria were hepatic or renal disease, known skin hypersensitivity to 5-ALA, known or first degree relative with acute or chronic porphyria, pregnancy, breast-feeding or more than 10% deviation from the normal standard lab values for liver (bilirubin, alanine aminotransferase—ALT, aspartate aminotransferase—AST, Alkaline phosphatase -ALP), kidney (Cystatin C) and hematology (C reactive protein—CRP, Reticulocytes, Leukocytes, Thrombocytes, Erythrocytes, hemoglobin – Hb, mean corpuscular hemoglobin—MCV and mean corpuscular hemoglobin concentration—MCHC) enzymes prior to surgery.
Study protocol
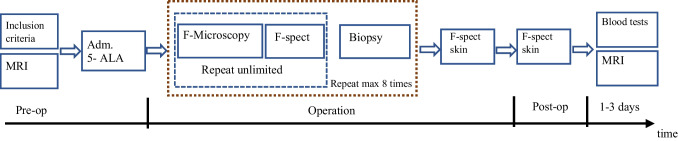
The original study protocol is described in Fig. 1. Data was collected between September 2014 and September 2019, documented in a Case Report Form (CRF) according to Good Clinical Practice (GCP) standards. The study was monitored by a research coordinator from the regional clinical research center (Forum Östergötland). Drug approval for the clinical study was granted by the Swedish Medical Product Agency MPA (Läkemedelsverket, EudraCT: 2013–005565-40). Ethical approval was obtained from the Regional Ethical Review Board in Linköping, Sweden (Dnr 2014/350–32).
Fig. 1.
Original study design. F-spect: fluorescence spectroscopy, MRI: magnetic resonance imaging
Patients were given a preoperative dose of 20 mg/kg 5-ALA, Gliolan® (Medac GmbH, Wedel, Germany) dissolved in 50–100 mL of tap water about three to four hours before induction of general anesthesia. Depending on age and cooperation, 5-ALA was administered orally or via a gastric tube in the operation room with the patient sedated. Patients were operated under general anesthesia with a combination of propofol, fentanyl and sevoflurane and placed in either prone or supine position depending on the location of the tumor. Tumor resection was performed using standardized microneurosurgical techniques together with neuronavigation (StealthStation S8, Medtronic Inc., USA) and ultrasonic aspiration (Söring GmbH, Quickborn, Germany). A surgical microscope (M720 OH5, Leica GmbH, Germany) was used during resection with the FL 400 filter option, enabling the surgeon to switch filters to detect fluorescing tissue. Spectroscopic measurements using the hand-held probe were performed in vivo under the FL 400 microscope, and ex vivo on tissue samples after resection. All operations except one were performed by the authors JH and PM. A postoperative MRI scan was performed within 48 h after surgery. Blood tests (liver, kidney and hematology) were repeated on day one and day three post-operatively (Fig. 1). Fluorescence was measured on the skin of the forearm during and after the operation.
Fluorescence spectroscopy and the hand-held probe
A custom-made fluorescence spectroscopy system with excitation laser wavelength of 405 nm was used for measuring the fluorescence emission spectra within optical range of 450—850 nm. The laser pulse length and the spectrometer’s integration time were electronically synchronized and set to 400 ms. For skin measurements, the integration time was additionally set to 1 s to confirm spectra measured with shorter integration times. The excitation output power (10 mW) and the light collection through the fiber were calibrated before probe sterilization prior to each surgical session. The system was used together with a fiber optic hand-held probe with an outer diameter of 2 mm, a shaft length of 12 cm and a cable longer than 4 m that could extend to outside of the sterile zone in the operating room. The hand-held probe could be used as a standalone system or under the surgical microscope in the FL400 mode. The system has previously been described in detail [16, 33].
Intraoperative fluorescence measurement procedure
Fluorescence in brain tumors was observed during surgery using both the surgical microscope and the fluorescence spectroscopy system with the fiber optic probe, separately and simultaneously with the microscope set in the FL 400 mode [33]. Three spectra were captured within 2 s for each measurement spot. One to three tissue samples from the tumor were removed and remeasured with the probe for a second time under a more controlled setup. Multiple sites on the samples were measured on and the maximum signal detected was included in the results to represent the PpIX uptake in the tumor. All observations were documented. The samples were then sent for the routine clinical histopathology examination. The overall diagnosis is included in this study, and no histopathology analysis was performed on the exact fluorescence measurement site. Since the resection was not based on fluorescence guidance, the effect of measurements on the length of the surgery was considered minimal. Measurements on the tissue samples did not affect the time of the surgery.
Fluorescence measurements on skin
Fluorescence was measured once intra- and once postoperatively in the skin of the inner side of the forearm or on the foot or leg (when the arm could not be accessed). The postoperative measurement was performed in the postoperative ward within 24 h after 5-ALA administration. A different but similar hand-held probe was used for skin measurements with the same spectroscopy system as described above.
Data Analysis
The fluorescence spectra were quantified by a ratiometric analysis motivated by the prior knowledge that the autofluorescence is lower in tumor and that division of PpIX fluorescence by autofluorescence would increase the contrast between tumor and non-tumor tissue in adult brain tumors [7, 18]. Moreover, this approach would reduce the variability in signals caused by the probe positioning. The Ratio was calculated by dividing the PpIX fluorescence intensity at wavelength of 635 nm in the spectrum by the maximum autofluorescence intensity (at approximately 510 nm). The unit of the Ratio is arbitrary [a.u.]. Any signal below the system’s average noise was set to zero. Data was analyzed in MATLAB® 2019–2020 (The MathWorks, Inc., Natick, MA, USA). Details of the calculation have previously been described [5]. The fluorescence intensity viewed under the surgical microscope was categorized as “none”, “vague” and “strong” based on the visual perception of the surgeon. Blood samples were analyzed with standard equipment provided by the Clinical Chemistry Department at Linköping University Hospital, Sweden.
Statistical analysis was performed using median value and non-parametric significant test (Wilcoxon signed rank test, two-tailed) in MATLAB® 2020b. The Wilcoxon signed rank test is a nonparametric test for populations with paired observations. No power calculations were performed regarding the hypothesis significance testing due to the low number of patients; therefore, the calculated p-values are only indicative of changes in the measured values.
Results
Patient characteristics and tumor features
Among the fourteen children undergoing primary surgery according to the study protocol, the most common initial symptoms were headache, nausea and vomiting. All patients had a preoperative Lansky score > 70. Thirteen of the tumors displayed contrast enhancement in MR images (negative, moderate or strong). Tumors were located infratentorial in nine and supratentorial in five patients. The pathology report showed medulloblastoma grade 4 (N = ), pilocytic astrocytoma grade 1 (N = 3), pilomyxoid astrocytoma grade 2 (N = 2), glioblastoma (GB) grade 4 (N = 1), oligodendroglioma grade 2 (N = 1), atypical meningioma grade 2 (N = 1), diffuse intrinsic pontine glioma (DIPG) (N = 1), desmoplastic infantile ganglioglioma grade 1 (N = 1). Ten patients underwent GTR, five patients subtotal resection (STR). Three patients developed neurological deficits post-operatively (cerebellar mutism, left leg paresis, right hemiparesis), all transient within three months. These adverse events were considered to have been caused by the surgical resection and unrelated to the administration of 5-ALA. Interestingly, these patients were all operated with STR. One patient underwent awake surgery. One patient with GB was re-operated but excluded in the study for the second operation and survived approximately two years after diagnosis. All other patients were alive in June 2020. Clinical characteristics of symptoms, tumor pathology, MRI features, location and EOR are summarized in Table 1.
Table 1.
Clinical characteristics of patients, MRI features, tumor pathology and grade, location and EOR: Extent of Resection
| PAT. NO | AGE | GENDER | WEIGHT (KG) | CONTRAST ENHANCEMENT | PATHOLOGY | LOCATION | LANSKY | EOR | |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 4Y 2 M | F | 16 | moderate | Medulloblastoma, gr 4 | Infratentorial | vermis | 70 | Partial |
| #2 | 4Y 3 M | M | 20 | strong | Pilocytic astrocytoma gr 1 | Infratentorial | vermis | 70 | GTR |
| #3 | 4Y 7 M | M | 20 | strong | Glioblastoma, gr 4 | Supratentorial | temporal | 80 | Partial |
| #4 | 4Y 8 M | M | 20 | moderate | Pilomyxoid astrocytoma gr 2 | Infratentorial | vermis | 70 | GTR |
| #5 | 4Y 10 M | M | 19 | strong | Pilocytic astrocytoma gr 1 | Infratentorial | vermis | 70 | GTR |
| #6 | 5Y 10 M | M | 22 | strong | Desmoplastic infantile astrocytoma/ganglioglioma gr 1 | Supratentorial | occipital | 90 | GTR |
| #7 | 5Y 11 M | F | 19 | strong | Medulloblastoma, gr 4 | Infratentorial | vermis | 80 | GTR |
| #8 | 9Y 2 M | F | 25 | moderate | Pilocytic astrocytoma gr 1 | Infratentorial | vermis | 90 | GTR |
| #9 | 9Y 8 M | M | 31 | strong | Medulloblastoma, gr 4 | Infratentorial | vermis | 80 | GTR |
| #10 | 10Y 11 M | F | 38 | moderate | Pilomyxoid astrocytoma gr 2 | Supratentorial | parietal | 80 | GTR |
| #11 | 10Y 11 M | F | 36 | strong | Medulloblastoma, gr 4 | Infratentorial | vermis | 80 | GTR |
| #12 | 11Y | F | 49 | moderate | Diffuse intrinsic pontine glioma (DIPG) | Infratentorial | brain stem | 70 | Partial |
| #13 | 13Y 2 M | M | 47 | strong | Atypical meningioma gr 2 | Supratentorial | ventricle | 80 | GTR |
| #14 | 17Y 1 M | M | 75 | negative | Oligodendroglioma gr 2 | Supratentorial | frontal | 90 | Partial |
5-ALA administration
5-ALA dissolved in tap water was given via a gastric tube with the patient sedated at the induction of general anesthesia to the seven youngest children (< 6 years, #1 to #7), approximately three hours before tumor resection. The 5-ALA administered via the gastric tube was flushed with 9 ml of water (the equivalent inner volume of the tube). The gastric tube was used since taste of 5-ALA is quite bitter and difficult to give to a small child. Seven children (#8 to #14) received oral 5-ALA according to standardized procedure about 3–4 h before surgery. The median surgery time was 4–5 h, well within the described peak 5-ALA fluorescence time in adults.
Intraoperative fluorescence measurement in the microscope and with the probe
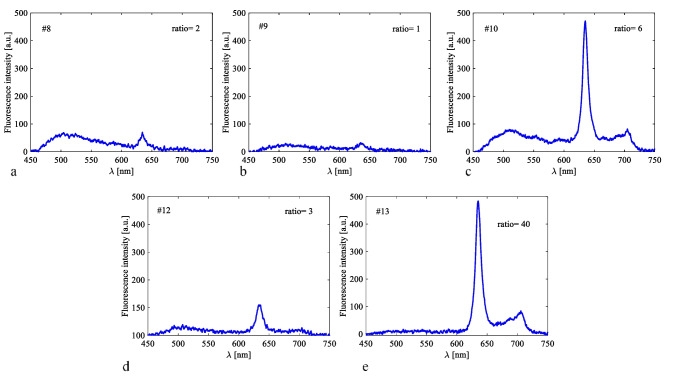
Microscopically, there was “vague” fluorescence in two tumors (# 10 – pilomyxoid astrocytoma grade 2, #13 – atypical meningioma grade 2). The other twelve brain tumors did not reveal any visible fluorescence in the microscope. With the fiber optic hand-held probe fluorescence spectra could be detected in five tumors (# 8 – Pilocytic astrocytoma grade 1, # 9 – Medulloblastoma grade 4, # 10 – pilomyxoid astrocytoma grade 2I, #12 – diffuse intrinsic pontine glioma grade 3 (DIPG), # 13 – Atypical meningioma grade 2) (Table 2). There was no detectable fluorescence with the probe in the other nine brain tumors. Intraoperatively, neither the “vague” fluorescence in the microscope nor the spectroscopic signals provided useful guidance for the surgeon. Fluorescence spectroscopic peaks in brain tumors are shown in Fig. 2. The autofluorescence measured in pediatric brain was generally weaker than in adults making the calculated Ratios not directly comparable.
Table 2.
ALA-administration and fluorescence measurement results represented by the fluorescence ratio. NA: not available. a.u.: arbitrary units
| PATIENT NO | ADMIN | PROBE-TUMOR [a.u.] | MICROSCOPE- TUMOR | PROBE-SKIN INTRAOP [a.u.] | PROBE-SKIN POST OP [a.u.] |
|---|---|---|---|---|---|
| #1 | tube | 0 | none | 0 | 0 |
| #2 | tube | 0 | none | 0 | 0 |
| #3 | tube | 0 | none | 0 | 0 |
| #4 | tube | 0 | none | 0 | 0 |
| #5 | tube | 0 | none | 0 | 0 |
| #6 | tube | 0 | none | 0 | 0 |
| #7 | tube | 0 | none | 0 | 0 |
| #8 | oral | 2 | NA | 0 | 0 |
| #9 | oral | 1 | none | 0 | 0 |
| #10 | oral | 6 | vague | NA | 0 |
| #11 | oral | 0 | none | 0 | 0 |
| #12 | oral | 3 | none | 0.02 | 0.01 |
| #13 | oral | 40 | vague | 0.19 | 0.14 |
| #14 | oral | 0 | none | 0.41 | 0.10 |
Fig. 2.
Fluorescence spectra with the highest fluorescence ratio measured in the ex vivo brain tumor tissue in five patients directly after tumor removal. The corresponding fluorescence Ratios are given in the graphs
Fluorescence measurement on the skin
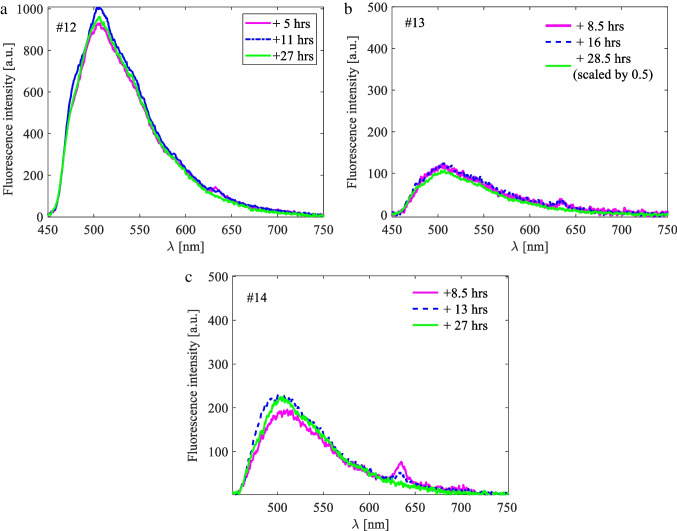
Spectroscopic measurements on the skin showed PpIX fluorescence in three patients both intra- and post-operatively (# 12, #13 and #14). No fluorescence was found in ten patients during and after the operation. In one additional patient (#10) measurement was performed only after the operation since the forearm could not be accessed intraoperatively. No PpIX fluorescence could be detected in the skin of this patient. The youngest child with detectable skin fluorescence was 11 years old. The PpIX in the skin was not measurable after 24 h. Fluorescence Ratios calculated for skin measurements are small due to the low PpIX value in skin and the high skin autofluorescence (Table 2, Fig. 3).
Fig. 3.
Examples of skin measurements for cases #12, #13 and #14, during operation (pink solid line), post-operatively within 24 h (dashed blue line) and 24 h after ALA administration (solid green line). The exact measurement time is stated in the graphs with reference to the time of ALA administration
Blood test analysis and adverse events
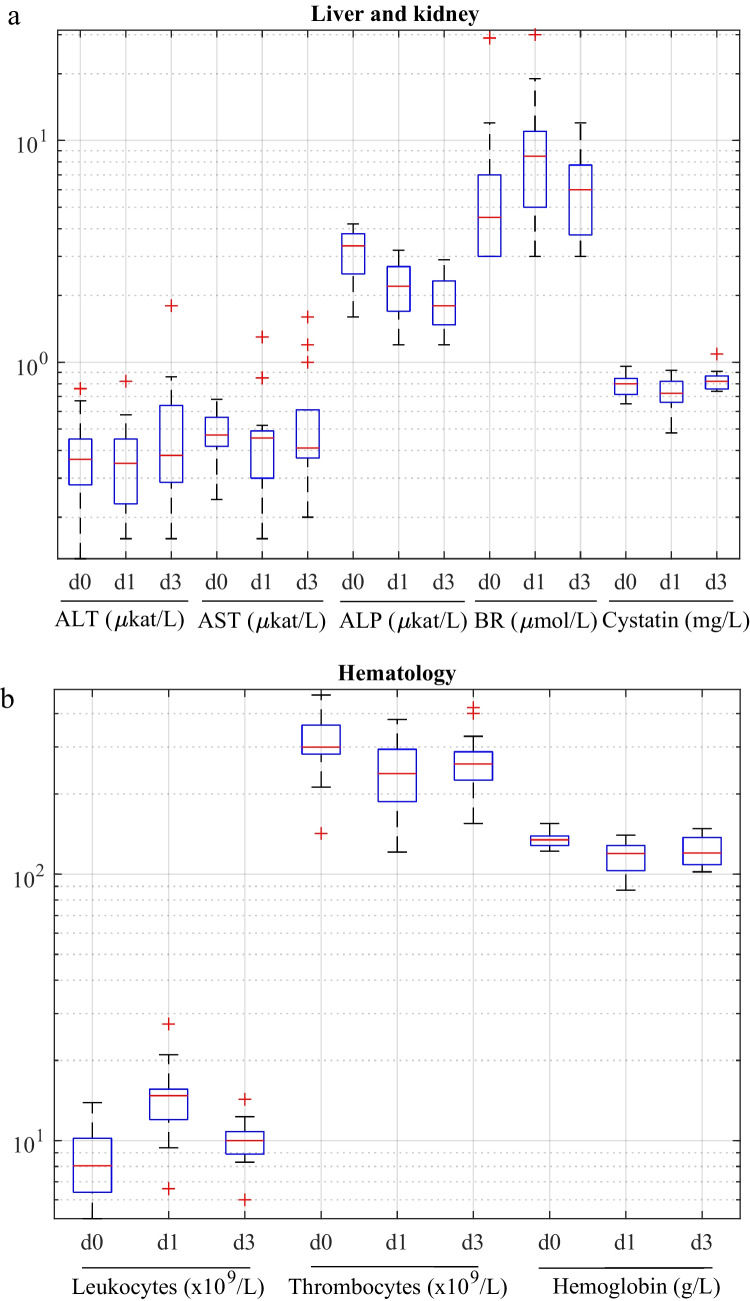
Blood test results did not reveal any significant impact of 5-ALA on liver, kidney and hematology enzymes when comparing the whole group of children (Table 3, Fig. 4). However, values for bilirubin increased significantly (p < 0.05) on day 1, values for leukocytes increased significantly (p < 0.05) on day 1 and 3 and values for ALP, thrombocytes and Hb decreased on day 1 and 3 (p < 0.05). ALT, AST, cystatin values were statistically unchanged together with C reactive protein, reticulocytes, erythrocytes, MCV and MCHC (data not shown). All aberrant blood values in patients were eventually normalized when re-examined after approximately three weeks. No adverse or severe adverse events were observed in any patient during the study.
Table 3.
The median and range of the blood test values prior to the operation (preop), on day 1 and day 3. P-values depict significant difference between preoperative and day 1 values, and preoperative and day 3 values. ALT: alanine aminotransferase, AST: aspartate aminotransferase, ALP: Alkaline phosphatase, BR: Bilirubin, LC: Leukocytes, TC: Thrombocytes, Hb: Hemoglobin
| ALT (µkat/L) | AST (µkat/L) | ALP (µkat/L) | BR (µmol/L) | Cystatin (mg/L) | LC (× 109/ L) | TC (× 109/ L) | Hb (g/L) | ||
|---|---|---|---|---|---|---|---|---|---|
| Median [min, max] | preop | 0.37 [0.13, 0.76] | 0.47 [0.24, 0.68] | 3.35 [1.60, 4.20] | 4.5 [3.0, 29.0] | 0.8 [0.65, 0.96] | 8 [5.1, 13.9] | 299 [142, 470] | 134 [122, 155] |
| day 1 | 0.35 [0.16, 0.82] | 0.46 [0.16, 1.30] | 2.20 [1.20, 3.20] | 8.5 [3.0, 30.0] | 0.73 [0.48, 0.92] | 14 [6.6, 27.4] | 238 [121, 381] | 119 [87, 140] | |
| day 3 | 0.38 [0.16, 1.8] | 0.41 [0.2, 1.6] | 1.8 [1.20, 2.90] | 6 [3.0, 12.0] | 0.82 [0.74, 1.09] | 10 [6, 14.3] | 259 [155, 421] | 120 [102, 148] | |
| p-value | preop-day 1 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | |||
| preop-day 3 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 |
Fig. 4.
Box plots for a) the liver and kidney function, and b) hematology values. d0: prior to operation, d1: one day after the operation, d3: three days after the operation. The boxplots show the median value and the 25th-75th percentile range. ALT: alanine aminotransferase, AST: aspartate aminotransferase, ALP: Alkaline phosphatase, BR: Bilirubin, LC: Leukocytes, TC: Thrombocytes
Discussion
Of fourteen patients, only two (# 10, # 13) displayed “vague” fluorescence in the surgical microscope intraoperatively, not sufficiently useful to guide resection. These tumors were a pilomyxoid astrocytoma grade 2 and an atypical meningioma grade 2, both located in the supratentorial region. In five patients (#8, #9, #10, #12, #13), spectroscopic fluorescence could be detected in the tumor. These included the ones with “vague” microscopic fluorescence. Two of these tumors (#9, #12) could be considered as high grade (medulloblastoma grade 4, pontine glioma grade 3), whereas the other three were low-grade tumors and meningioma (pilocytic astrocytoma grade 1, pilomyxoid astrocytoma grade 2, atypical meningioma grade 2). The three tumors with only spectroscopic fluorescence were located in the infratentorial region. Observation of fluorescence in the surgical microscope is a subjective finding depending on the experience of the surgeon. Measurements with the spectroscopic system in vivo and on multiple tissue samples provide an objective and, in our view, accurate assessment of true fluorescence [16, 18, 33, 34] as the fluorescence intensity can be quantified.
Furthermore, fluorescence in the skin was observed only in the three oldest patients in contrast to our previous findings in adults where approximately 95% had PpIX fluorescence on the forearm after receiving 5-ALA 20 mg/kg [17]. Fluorescence detection in the skin may be interpreted as an indirect sign of adequate intestinal 5-ALA uptake. However, as the autofluorescence of the skin increased with age in adults [41], it cannot be excluded that the dermal and epidermal structures could have influenced the PpIX synthesis. Nonetheless, it is remarkable that the youngest children in our study did not show any signs of fluorescence, neither in the tumor nor in the skin. Seven of the youngest children (# 1 to # 7) had been given the 5-ALA suspension through a nasogastric tube when sedated, which to our knowledge is the first time this administration of 5-ALA in children has been described in the literature. Propofol and fentanyl, also used for anesthesia in our study, have been reported to delay gastric emptying, intestinal motility and drug absorption in the small intestine [29] which may have influenced the intestinal absorption and pharmacokinetics of 5-ALA in these children. Our results are in a fair agreement with the findings in other publications where reported PpIX fluorescence in tumor seems to be more common in adolescents than in infants and toddlers [1, 8, 10, 14, 32, 35, 36, 40]. Given the lack of spectroscopic tumor and skin PpIX fluorescence in most children other tumor intrinsic or age specific factors may have contributed to the differences in PpIX fluorescence patterns in children, in comparison with adults [5, 6, 11, 15, 26, 27].
No intratumoral fluorescence was seen in the four-year-old child with a supratentorial GB. Fluorescence has previously been reported as useful in 78% of resected GBs (both primary and recurrent) in children whereas it was unhelpful or non-existent in 22% [28]. Although reported as being useful in resection of recurrent tumors [24, 39] we chose not to include recurrent GBs in our study since unspecific PpIX fluorescence may be seen in recurrent tumors originating from gliosis and reactive astrocytes and not only from tumor cells [12, 19, 30, 41, 44].
Usefulness of 5-ALA fluorescence in children has hitherto been described in a minority of cases in larger series; 47%, 44% and 43%, respectively [38, 43, 47] with predominantly high-grade histology (GB, Anaplastic Astrocytoma, Ependymoma grade II and III, Oligodendroglioma grade III, Germinoma) and supratentorial location (60% versus 40% infratentorial). However, when fluorescence was deemed helpful GTR was considerably higher than in non-fluorescing tumors (58% vs 22%) [39]. Stummer et al. have formerly advocated that 5-ALA should mainly be given to contrast enhanced, supratentorial tumors [43]. In contrast, Labuschagne recently reported two series of 23 fluorescing infratentorial (cerebellar and brain stem) tumors out of 27 [23, 24]. Among these 16 displayed “strong” fluorescence and fluorescence was considered helpful in 15 of the 27 operations (56%). The histology of tumors with “strong” fluorescence was Ependymoma grade II and III in 9 of 16 cases. Interestingly, fluorescence was not considered helpful in six tumors with “strong” fluorescence and was regarded useful in six tumors with “vague” fluorescence.
In this study thirteen tumors displayed contrast enhancement on the initial MRI scan. Only six of these where eventually diagnosed as HGG: one supratentorial (GB) and five infratentorial tumors (4 medulloblastomas, one pontine glioma grade 3), the rest were categorized as low-grade tumors and meningioma. Considering the final pathology diagnosis in all tumors, the scarcity of tumor fluorescence in our cases is conceivable. However, from a clinical viewpoint a potential HGG tumor can only be presumed preoperatively from the contrast enhancement, edema and mode of infiltration and tumor invasiveness on the MRI. The decision to use 5-ALA in adults is mainly based on these criteria [12, 19, 41, 44]. However, the larger tumor diversity in pediatric brain tumors, MRI contrast enhancement in the many LGGs but lack of contrast enhancement in some HGGs [31] makes it more difficult to adapt a clear preoperative algorithm for the administration of 5-ALA in pediatric patients. From the initial, diagnostic MRI scans we had expected more cases of HGGs and detection of tumor fluorescence, especially with the spectroscopic probe system.
No clinical side effects or adverse effects from 5-ALA were noted during the study. Transient elevations of blood samples were observed in some patients but eventually normalized and did not warrant any medical measures.
This study comprises a small number of patients with different tumor histopathology, mainly low-grade tumors, making it difficult to properly discern the potential usefulness of 5-ALA in a series consisting of more HGGs. However, thirteen out of fourteen tumors were contrast enhancing on preoperative MRI, mimicking a possible high-grade glioma, and thus justifying inclusion in the study.
Conclusions
Five of fourteen tumors showed PpIX fluorescence. Microscopic fluorescence was “vague” in two patients and not useful to guide tumor resection in our study. The hand-held probe revealed fluorescence in additional three tumors and in the skin in the three oldest children, resembling our previous results in adults. Children displaying fluorescence were all older than nine years. 5-ALA appear to be safe for use in children older than four years and in adolescents.
Acknowledgements
The authors would like to thank the staff at the Dept. of Neurosurgery, Dept. of Anesthesiology and Intensive care, Dept. of Clinical Pathology, Linköping University Hospital for invaluable assistance and, Forum Östergötland for monitoring the study.
Funding
Open access funding provided by Linköping University. The study was funded by the Swedish Childhood Cancer Foundation Grant No. MT 2013–0043 and MT2016-0013. LiU Cancer Project Grant and ALF Grant Region Östergötland, Sweden (Grant No. LIO-599651).
Declarations
Disclosure of potential conflict of interest
Authors Neda Haj-Hosseini, and Karin Wårdell are inventors of a related patent [48, 49]. Author Karin Wårdell has shares in FluoLink AB, Linköping, Sweden. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Research involving human participants
All procedures performed in this study involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study and by all of their caregivers.
Footnotes
This article is part of the Topical Collection on Pediatric Neurosurgery
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Comments:
Small study where the efficacy of 5-ALA was examined both with the hand-held spectroscopy in tumor and skin as well as the general fluorescens intraoperatively were judged. Based on the findings in this cohort routine use is not supported for pediatric brain tumors but again based on the small sample size in cannot be discouraged either.
Jane Skjoth-Rasmussen.
Copenhagen,Denmark.
Peter Milos and Neda Haj-Hosseini have contributed equally to the work.
References
- 1.Agawa Y, Wataya T. The use of 5-aminolevulinic acid to assist gross total resection of pediatric astroblastoma. Childs Nerv Syst. 2018 doi: 10.1007/s00381-017-3714-5. [DOI] [PubMed] [Google Scholar]
- 2.Albert FK, Forsting M, Sartor K, Adams HP, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34:45–60. doi: 10.1097/00006123-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, et al. Effects of medulloblastoma resections on outcome in children: a report from the Children's Cancer Group. Neurosurgery. 1996;38:265–271. doi: 10.1097/00006123-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Albright AL, Sposto R, Holmes E, Zeltzer PM, Finlay JL, et al. Correlation of neurosurgical subspecialization with outcomes in children with malignant brain tumors. Neurosurgery. 2000;47:879–885. doi: 10.1097/00006123-200010000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55:667–686. doi: 10.1016/s0169-409x(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr. 2006;165:819–829. doi: 10.1007/s00431-006-0189-x. [DOI] [PubMed] [Google Scholar]
- 7.Andersson-Engels S, Elner Å, Johansson J, Karlsson SE, Salford LG, et al. Clinical recording of laser-induced fluorescence spectra for evaluation of tumour demarcation feasibility in selected clinical specialities. Lasers Med Sci. 1991;6:415–424. doi: 10.1007/BF02042464. [DOI] [Google Scholar]
- 8.Barbagallo GM, Certo F, Heiss K, Albanese V. 5-ALA fluorescence-assisted surgery in pediatric brain tumors: report of three cases and review of the literature. Br J Neurosurg. 2014;28:750–754. doi: 10.3109/02688697.2014.913779. [DOI] [PubMed] [Google Scholar]
- 9.Beez T, Sarikaya-Seiwert S, Steiger HJ, Hänggi D. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of brain tumors in children–a technical report. Acta Neurochir (Wien) 2014;156:597–604. doi: 10.1007/s00701-014-1997-9. [DOI] [PubMed] [Google Scholar]
- 10.Bernal García LM, Cabezudo Artero JM, Royano Sánchez M, Marcelo Zamorano MB, López Macías M. Fluorescence-guided resection with 5-aminolevulinic acid of meningeal sarcoma in a child. Childs Nerv Syst. 2015;31:1177–1180. doi: 10.1007/s00381-015-2703-9. [DOI] [PubMed] [Google Scholar]
- 11.Braunstein S, Raleigh D, Bindra R, Mueller S, Haas-Kogan D. Pediatric high-grade glioma: current molecular landscape and therapeutic approaches. J Neurooncol. 2017;134:541–549. doi: 10.1007/s11060-017-2393-0. [DOI] [PubMed] [Google Scholar]
- 12.Coburger J, Wirtz CR. Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: a systematic review. J Neurooncol. 2019;141:533–546. doi: 10.1007/s11060-018-03052-4. [DOI] [PubMed] [Google Scholar]
- 13.Cochrane DD, Gustavsson B, Poskitt KP, Steinbok P, Kestle JR. The surgical and natural morbidity of aggressive resection for posterior fossa tumors in childhood. Pediatr Neurosurg. 1994;20:19–29. doi: 10.1159/000120761. [DOI] [PubMed] [Google Scholar]
- 14.Eicker S, Sarikaya-Seiwert S, Borkhardt A, Gierga K, Turowski B, et al. ALA-induced Porphyrin Accumulation in Medulloblastoma and its use for Fluorescence-Guided Surgery. Cen Eur Neurosurg. 2011;72:101–104. doi: 10.1055/s-0030-1252010. [DOI] [PubMed] [Google Scholar]
- 15.Ginsberg G, Hattis D, Sonawane B, Russ A, Banati P, et al. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci. 2002;66:185–200. doi: 10.1093/toxsci/66.2.185. [DOI] [PubMed] [Google Scholar]
- 16.Haj-Hosseini N, Richter J, Andersson-Engels S, Wårdell K. Optical touch pointer for fluorescence guided glioblastoma resection using 5-aminolevulinic acid. Lasers Surg Med. 2010;42:9–14. doi: 10.1002/lsm.20868. [DOI] [PubMed] [Google Scholar]
- 17.Haj-Hosseini N, Richter JCO, Hallbeck M, Wårdell K. Low dose 5-aminolevulinic acid: Implications in spectroscopic measurements during brain tumor surgery. Photodiagn Photodyn Ther. 2015;12:209–214. doi: 10.1016/j.pdpdt.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Haj-Hosseini N, Richter JCO, Milos P, Hallbeck M, Wårdell K. 5-ALA fluorescence and laser Doppler flowmetry for guidance in a stereotactic brain tumor biopsy. Biomed Opt Express. 2018;9:2284–2296. doi: 10.1364/BOE.9.002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollon T, Stummer W, Orringer D, Suero Molina E. Surgical Adjuncts to Increase the Extent of Resection: Intraoperative MRI, Fluorescence, and Raman Histology. Neurosurg Clin N Am. 2019;30:65–74. doi: 10.1016/j.nec.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Jenkinson MD, Barone DG, Bryant A, Vale L, Bulbeck H, et al. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD012788.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang B, Chaichana K, Veeravagu A, Chang SD, Black KL, et al. Biopsy versus resection for the management of low-grade gliomas. Cochrane Database Syst Rev. 2017;4:CD009319–CD009319. doi: 10.1002/14651858.CD009319.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labuschagne JJ. 5-Aminolevulinic Acid-Guided Surgery for Recurrent Supratentorial Pediatric Neoplasms. World Neurosurg. 2020;141:e763–e769. doi: 10.1016/j.wneu.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Labuschagne J. The Use of 5-Aminolevulinic Acid to Assist Gross Total Resection of Paediatric Posterior Fossa Tumours. Pediatr Neurosurg. 2020;55:268–279. doi: 10.1159/000511289. [DOI] [PubMed] [Google Scholar]
- 24.Labuschagne J. 5-aminolevulinic acid-guided surgery for focal pediatric brainstem gliomas: A preliminary study. Surg Neurol Int. 2020;11:334. doi: 10.25259/sni_246_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 26.Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther. 2014;19:262–276. doi: 10.5863/1551-6776-19.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madabushi R, Cox DS, Hossain M, Boyle DA, Patel BR, et al. Pharmacokinetic and pharmacodynamic basis for effective argatroban dosing in pediatrics. J Clin Pharmacol. 2011;51:19–28. doi: 10.1177/0091270010365550. [DOI] [PubMed] [Google Scholar]
- 28.Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020;6:495–503. doi: 10.1001/jamaoncol.2019.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mushambi MC, Rowbotham DJ, Bailey SM. Gastric emptying after minor gynaecological surgery The effect of anaesthetic technique. Anaesthesia. 1992;47:297–299. doi: 10.1111/j.1365-2044.1992.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 30.Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, et al. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery. 2009;65:1070–1076. doi: 10.1227/01.Neu.0000360128.03597.C7. [DOI] [PubMed] [Google Scholar]
- 31.Porto L, Jurcoane A, Schwabe D, Hattingen E. Conventional magnetic resonance imaging in the differentiation between high and low-grade brain tumours in paediatric patients. Eur J Paediatr Neurol. 2014;18:25–29. doi: 10.1016/j.ejpn.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Preuß M, Renner C, Krupp W, Christiansen H, Fischer L, et al. The use of 5-aminolevulinic acid fluorescence guidance in resection of pediatric brain tumors. Childs Nerv Syst. 2013;29:1263–1267. doi: 10.1007/s00381-013-2159-8. [DOI] [PubMed] [Google Scholar]
- 33.Richter J, Haj-Hosseini N, Hallbeck M, Wårdell K. Combination of Hand-Held Probe and Microscopy for Fluorescence Guided Surgery in the Brain Tumor Marginal Zone. Photodiagn Photodyn Ther. 2017;18:185–192. doi: 10.1016/j.pdpdt.2017.01.188. [DOI] [PubMed] [Google Scholar]
- 34.Richter J, Haj-Hosseini N, Milos P, Hallbeck M, Wårdell K. Optical Brain Biopsy with a Fluorescence and Vessel Tracing Probe. Oper Neurosurg (Hagerstown) 2021;21:217–224. doi: 10.1093/ons/opab216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth J, Constantini S. 5ALA in pediatric brain tumors is not routinely beneficial. Childs Nerv Syst. 2017;33:787–792. doi: 10.1007/s00381-017-3371-8. [DOI] [PubMed] [Google Scholar]
- 36.Ruge JR, Liu J. Use of 5-aminolevulinic acid for visualization and resection of a benign pediatric brain tumor. J Neurosurg Pediatr. 2009;4:484–486. doi: 10.3171/2009.6.PEDS08428. [DOI] [PubMed] [Google Scholar]
- 37.Sanai N, Polley M-Y, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas: Clinical article. Journal of Neurosurgery JNS. 2011;115:3–8. doi: 10.3171/2011.2.Jns10998. [DOI] [PubMed] [Google Scholar]
- 38.Schwake M, Schipmann S, Müther M, Köchling M, Brentrup A, et al. 5-ALA fluorescence-guided surgery in pediatric brain tumors-a systematic review. Acta Neurochir (Wien) 2019;161:1099–1108. doi: 10.1007/s00701-019-03898-1. [DOI] [PubMed] [Google Scholar]
- 39.Schwake M, Kaneko S, Suero Molina E, Müther M, Schipmann S, et al. Spectroscopic measurement of 5-ALA-induced intracellular protoporphyrin IX in pediatric brain tumors. Acta Neurochir. 2019 doi: 10.1007/s00701-019-04039-4. [DOI] [PubMed] [Google Scholar]
- 40.Skjøth-Rasmussen J, Bøgeskov L, Sehested A, Klausen C, Broholm H, et al. The use of 5-ALA to assist complete removal of residual non-enhancing part of childhood medulloblastoma: a case report. Childs Nerv Syst. 2015;31:2173–2177. doi: 10.1007/s00381-015-2762-y. [DOI] [PubMed] [Google Scholar]
- 41.Stepp H, Stummer W. 5-ALA in the management of malignant glioma. Lasers Surg Med. 2018;50:399–419. doi: 10.1002/lsm.22933. [DOI] [PubMed] [Google Scholar]
- 42.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 43.Stummer W, Rodrigues F, Schucht P, Preuss M, Wiewrodt D, et al. Predicting the "usefulness" of 5-ALA-derived tumor fluorescence for fluorescence-guided resections in pediatric brain tumors: a European survey. Acta Neurochir (Wien) 2014;156:2315–2324. doi: 10.1007/s00701-014-2234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suero Molina E, Schipmann S, Stummer W. Maximizing safe resections: the roles of 5-aminolevulinic acid and intraoperative MR imaging in glioma surgery-review of the literature. Neurosurg Rev. 2019;42:197–208. doi: 10.1007/s10143-017-0907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson EM, Hielscher T, Bouffet E, Remke M, Luu B, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17:484–495. doi: 10.1016/s1470-2045(15)00581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang DY, Singhal S, Lee JYK. Optical Principles of Fluorescence-Guided Brain Tumor Surgery: A Practical Primer for the Neurosurgeon. Neurosurgery. 2019;85:312–324. doi: 10.1093/neuros/nyy315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Boop FA, Ruge J. The use of 5-aminolevulinic acid in resection of pediatric brain tumors: a critical review. J Neurooncol. 2019;141:567–573. doi: 10.1007/s11060-018-03004-y. [DOI] [PubMed] [Google Scholar]
- 48.Haj-Hosseini N, Wårdell K, Richter J (2017) Probe with multipurpose features for stereotactic optical biopsy, SE542683.
- 49.Haj-Hosseini N, Wårdell K, Richter J (2017) Optical probe for localizing and identifying a target tissue prior to harvesting a biopsy, WO2017135873A1.