Abstract
The Plasmodium falciparum liver-stage antigen 3 (LSA3), a recently identified preerythrocytic antigen, induces protection against malaria in chimpanzees. Using antibodies from individuals with hyperimmunity to malaria affinity purified on recombinant or synthetic polypeptides of LSA3, we identified four non-cross-reactive B-cell epitopes in Plasmodium yoelii preerythrocytic stages. On sporozoites the P. yoelii protein detected has a molecular mass similar to that of LSA3. T-cell epitopes cross-reacting with P. yoelii were also demonstrated using peripheral blood lymphocytes from LSA3-immunized chimpanzees. In contrast, no cross-reactive epitopes were found in Plasmodium berghei. LSA3-specific human antibodies exerted up to 100% inhibition of in vitro invasion of P. yoelii sporozoites into mouse hepatocytes. This strong in vitro activity was reproduced in vivo by passive transfer of LSA3 antibodies. These results indicate that the homologous epitopes may be biologically functional and suggest that P. yoelii could be used as a model to assess the antisporozoite activity of anti-LSA3 antibodies.
The development of a malaria preerythrocytic vaccine has been greatly influenced by the observation that sterile immunity could be experimentally induced in humans by immunization with Plasmodium falciparum radiation-attenuated sporozoites (9). The critical role of liver-stage trophozoites (reviewed in reference 16) led researchers to initiate a detailed study of the antigens expressed in P. falciparum preerythrocytic stages (22). To date, scientists have identified a series of new molecules expressed at sporozoite and/or liver stage (14). Liver-stage antigen 3 (LSA3), expressed on both the sporozoite surface and in the liver forms, was found to be of particular interest. LSA3 was identified by differential screening of immune responses from protected versus nonprotected volunteers (11). A number of dominant B- and T-cell epitopes to which a high prevalence of responses is detected in individuals exposed to malaria or in LSA3-immunized animals were identified (1, 2). The vaccine potential of this molecule has been recently demonstrated in the chimpanzee model, an animal susceptible and fully receptive to P. falciparum preerythrocytic stages and whose immune system is closest to that of humans. In this model, protection against successive challenges with P. falciparum sporozoites was obtained (11). However, the use of this primate for research purposes is severely hampered by cost and ethical constraints.
Several homologues of P. falciparum antigens have been identified, through a variety of immunological and molecular cross-reactivity assays, in other Plasmodium species and particularly in those infecting rodents (5, 10, 12, 13, 27, 28). The identification of structurally and functionally conserved homologues of P. falciparum proteins in rodent malaria species might help to better understand the role of these molecules in the P. falciparum parasite life cycle. This is particularly true for preerythrocytic stages, where the relative ease of obtaining sporozoites and mouse primary hepatocytes would allow more extensive investigations to be carried out.
In the process of identification of LSA3, a preerythrocytic subset of P. falciparum genomic fragments (22) was used to affinity purify antibodies (Abs) from sera of individuals with hyperimmunity to malaria. These Abs were consequently checked for immunofluorescence antibody test (IFAT) cross-reactivity with rodent malaria sporozoites, and in this assay Abs against all clones coding for various fragments of LSA3 proved to be strongly reactive with Plasmodium yoelii sporozoites but not with those from Plasmodium berghei.
The present study was undertaken to investigate the extent of antigenic cross-reactivity between the LSA3 molecule and its putative homologue in P. yoelii and to determine whether specific human Abs are of biological significance in the mouse model. We show that such cross-reactivity exists for human anti-LSA3 Abs, which encompass four distinct LSA3 epitopes, including both nonrepeated and repeated domains, and extend to both sporozoite and liver stages of P. yoelii. A similar result was obtained using Abs from chimpanzees immunized with LSA3. Moreover, P. yoelii, but not P. berghei, sporozoites were able to induce dose-dependent proliferation of T cells collected from one of these animals. Finally, we have established that these homologous epitopes are targets for immune responses which have a biological consequence, since LSA3-specific human Abs inhibit the invasion of P. yoelii sporozoites into mouse hepatocytes both in vitro and in vivo.
MATERIALS AND METHODS
Recombinant proteins and peptides.
Three recombinant proteins corresponding to the 5′ end (β-Gal-DG729, GST-729 [β-Gal, β-galactosidase; GST, glutathione S-transferase]), central region (GST-NN), and 3′ end (GST-PC) of the LSA3 gene product (Fig. 1) were produced as previously described (11). β-Gal-DG671 and GST-671 derived from the P. falciparum sporozoite and liver-stage antigen (SALSA) molecule were prepared as previously described (3) and used as controls (1, 3). The peptides LSA3-RE (VESVAPSVEESVAPSVEESVAENVEESV). LSA3-NRI (DELFNELLNSVDVNGENILEESQ), and LSA3-NRII (LEESQVNDDIFNSLVKSVQQEQQHNV) correspond to the sequences of the gene in P. falciparum T9-96 clone (11), and the SALSA2 peptide (NGKDDVKEEKKTNEKKDDGKTDQEKVLEKSPKEF) was also derived from the gene of T9-96 (3). All the peptides used in this study were prepared by solid-phase synthesis (25).
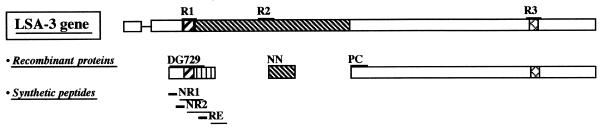
FIG. 1.
Location of the various LSA3 peptides and recombinant proteins. R1, R2, and R3 represent repeat regions. DG729, NN, and PC sequences were expressed as either GST-fused or β-Gal-fused recombinant proteins (see Materials and Methods).
Abs. (i) Human Abs.
Sera were collected from African adults living in the Ivory Coast (hyperimmune sera), an area where malaria is hyperendemic. These adults were more than 20 years old and no longer show signs of clinical malaria, even though they are exposed to malaria infections, suggesting that they have acquired antiparasite immunity and are clinically protected (22). Human Abs were affinity purified on the recombinant proteins β-Gal-DG729 and β-Gal-DG671 by successive absorption of Abs from seven hyperimmune patients' sera that had been depleted of Abs reactive with β-galactosidase (22). Briefly, the recombinant proteins induced by and adsorbed on isopropylthiogalactoside-impregnated nitrocellulose filters (BA 85; Schleicher & Schuell, Dassel, Germany) were incubated successively with each hyperimmune individual's serum and washed extensively. Abs were eluted using 0.2 M glycine (pH 2.5) and were immediately neutralized by addition of 2 M Tris, pH 11. Eluted Abs were dialyzed first against phosphate-buffered saline (PBS), pH 7.4, and then against RPMI medium in both cases over 24 h at 4°C. Samples were concentrated using a Centricon 30 membrane (Amicon; Millipore, Bedford, Mass.) to a volume corresponding to a 1/20 dilution of the original serum.
Human Abs to GST-NN and GST-PC recombinant proteins as well as Abs to the synthetic peptides LSA3-RE and LSA3-NRII were immunopurified on enzyme-linked immunosorbent assay (ELISA) plates using a previously described method (4). A single serum sample chosen as having the highest ELISA titer against each of these molecules was used for this immunopurification. Eluted Abs were concentrated to a volume corresponding to a 1/10 dilution of the serum employed for the affinity purification. The specificity of each Ab preparation was ascertained by ELISA using the corresponding peptides or recombinant proteins, together with control peptides and recombinants from SALSA (3), as well as by IFAT on P. falciparum sporozoites.
(ii) Chimpanzee sera.
Anti-LSA3 sera were obtained from animals immunized with β-Gal-DG729 (chimpanzee Dirk) or with the lipopeptide NRII (chimpanzee Gerda) (1). Anti-SALSA serum was obtained from chimpanzee Bart immunized with β-Gal-DG671 (3). The sera used in this study were collected 15 days after the third immunization and stored frozen at −80°C until use.
(iii) Anti-P. yoelii sera.
BALB/c and C3H/Hej mice were infected intravenously (i.v.) with 150 live sporozoites or intraperitoneally with 104 parasitized red blood cells of P. yoelii 17XNL. Peripheral blood parasitemia was detectable from day 5 in the mice infected with sporozoites and at day 3 in the mice infected with parasitized red blood cells. Sera were collected from mice bled 6 days after sporozoite infection and 14 days after blood infection.
Parasites.
P. falciparum (NF54), P. yoelii (either 17XNL, clone 1.1 derived from 17XNL, or 265BY), and P. berghei (ANKA strain) sporozoites were prepared aseptically, resuspended in RPMI medium, and were either stored at −70°C for future T-cell assays or were fixed with 0.01% glutaraldehyde (15) and stored at 4°C before use for IFAT. P. yoelii liver-stage schizonts were obtained by i.v. injection of 106 sporozoites into the tail vein of C3H/HeJ mice. Infected livers were removed 44 h later and embedded in Tissue-Tek O.C.T. compound (Tissue-Tek; SAKURA, Zoeterwoude, The Netherlands), and frozen sections were prepared for use in IFAT. Blood stages of the two rodent malaria species were obtained by infecting BALB/c mice with 104 infected red blood cells. Thin blood smears were made when parasitemia reached 20 to 25%.
IFAT.
The reactivity of human and animal Abs with either sporozoites, infected hepatocytes, or infected erythrocytes was assessed by incubating antibodies for 30 min at 37°C with (i) glutaraldehyde-fixed sporozoites, (ii) cryosections of infected liver tissue fixed 10 min in cold acetone (−20°C), and (iii) air-dried, acetone-fixed, blood-stage parasites. The slides were then washed three times with PBS, and the contents were incubated for a further 30 min with fluorescein isothiocyanate-labeled goat anti-human immunoglobulin G (IgG), IgA, and IgM (Diagnostic Pasteur, Rarnes-la-Coquette, France) or anti-mouse IgG, IgA, and IgM (Cappel; Organon Teknika, West Chester, Pa.). In all cases, they were diluted 1/200 in PBS supplemented with 1/2,000 Evans blue.
Western blot analysis.
Proteins from 106 P. yoelii sporozoites were solubilized in sodium dodecyl sulfate-containing sample buffer, subjected to electrophoresis under reducing conditions on sodium dodecyl sulfide–5% polyacrylamide gels, electroblotted onto nitrocellulose membranes, and incubated with Abs as described previously (17).
ELISA.
The ELISA was performed essentially as described in reference 4. Prior to conducting the ELISA, for each recombinant protein and each synthetic peptide, preliminary experiments were made to determine the optimal conditions for coating and for subsequent incubations with the same sera from hyperimmune individuals used in this study (SHI1 to SHI8) and 10 serum samples from healthy French blood donors with no history of malaria exposure. Hence, optimal antigen concentrations employed differ from one molecule to the other and particularly between recombinants and synthetic peptides. The Ab ratio was calculated by dividing the mean optical density at 492 nm (OD492) of the test serum by the mean plus 3 standard deviations of the 10 control serum samples from the same plates. Briefly, 96-well ELISA plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 50 μl of recombinant proteins at 0.5 μg/ml (GST-729 and GST-NN) or 10 μg/ml (GST-PC and GST-DG671) or with 50 μl of synthetic peptide solution at 10 μg/ml (LSA3-RE), 3 μg/ml (LSA3-NRI and LSA3-NRII) in PBS (pH 7.4), or 5 μg/ml (SALSA2) in Tris, pH 7.4. ELISA was performed in duplicate as previously described (4). Human affinity-purified Abs were tested undiluted, and sera from mice infected with P. yoelii were tested at 1/100 dilution. Peroxidase anti-human IgG (heavy plus light chains) and anti-mouse IgG (heavy plus light chains) (Biosys, Compiègne, France) were used as the second Ab. The results are expressed as the mean OD for human affinity-purified Abs and for mouse sera as a ratio of the mean OD of the sera from immunized mice to the mean OD of sera from preimmune mice.
T-cell assays.
Heparinized venous blood, collected from chimpanzee Gerda (immunized by LSA3 lipopeptide NRII), was used to isolate peripheral blood mononuclear cells (PBMC) by centrifugation on Ficoll-Hypaque density gradient, which were then used for the T-cell proliferation assay (1). Cells (2 × 105/well) were cultured in a 96 flat-bottomed-well plate (Costar, Cambridge, Mass.) in the presence of freeze-thawed P. yoelii or P. berghei sporozoites (at concentrations equivalent to 50, 150, 500, 1,500, or 5,000 sporozoites per well) for 5 days in RPMI 1640 medium supplemented with 10% human AB serum. One microcurie of [3H]thymidine ([3H]TdR) (Amersham, Les Ulis, France) was added to each well for the final 16 h of culture. The results were expressed as stimulation indices, which were calculated by dividing the mean counts per minute of [3H]TdR incorporated into stimulated culture cells by the mean counts per minute of [3H]TdR incorporated into unstimulated cell cultures (Δcpm; average of triplicates).
Inhibition-of-liver-stage-development assay.
Human Abs affinity purified upon the recombinant protein DG729 (LSA3) were tested for their inhibitory effect on P. yoelii invasion into hepatocytes as previously described (24). Human anti-DG671 (SALSA) antibodies were used as controls. Briefly, mouse hepatocytes suspended in complete medium were seeded in eight-chamber Lab-Tek plastic slides (Nunc Inc., Naperville, Ill.) at a ratio of 105 cells/chamber. After a 24-h incubation at 37°C in a 5% CO2 atmosphere, the medium was removed and both anti-DG729 Abs (or anti-SALSA Abs) and 6 × 104 P. yoelii or P. berghei sporozoites (clone 1.1 or ANKA strain, respectively) suspended in culture medium were simultaneously added to hepatocyte cultures. After 3 h at 37°C, the medium containing Abs and noninvaded sporozoites was discarded and replaced by fresh medium. After 48 h of culture, hepatocytes were fixed for 10 min in cold methanol and developing P. yoelii liver stages were identified by IFAT with the monoclonal Ab (MAb) NYLS3 as described in reference 8 by epifluorescence, using an Olympus UV microscope. The human Abs were tested at a concentration corresponding to their IFAT end point titer (1/2), at which the reactivity on the surface of the sporozoites was still detectable.
Parallel experiments were performed with P. berghei to evaluate interspecies inhibition. The MAb directed against the circumsporozoite protein (CS) of P. berghei was used as control of inhibition of P. berghei sporozoite invasion and to detect P. berghei liver schizonts by IFAT staining, as described in reference 8. The anti-CS MAb of P. berghei was used at a dilution of 5 × 10−4 (IFAT end point titer of 10−6).
The total number of liver schizonts in each culture well was counted and used to calculate the mean number of the liver schizonts in duplicate culture wells. Results were expressed as the percentage of inhibition: (number of liver schizonts in control − number of liver schizonts in test/number of liver schizonts in control) × 100.
In vivo passive protection by Abs.
To assess the in vivo effects of human affinity-purified Abs on the development of P. yoelii, 100 μg of either anti-DG729-specific Ab, control human anti-DG671 Abs, or RPMI medium per ml was added to 150 sporozoites of P. yoelii. The two components were mixed in a 1-ml syringe immediately before i.v. inoculation into the tail vein of BALB/c mice. Parasitemia was monitored from day 4 to day 21 by microscopic examination of Giemsa-stained thin smears of tail blood. The binding of the Abs to the sporozoites was tested by a direct fluorescence assay using the mixture remaining in the syringe after inoculation. A drop of this mixture was spread on a glass slide, air dried, and fixed for 10 min in acetone. Fluorescein isothiocyanate-labeled goat anti-human IgG, IgA, or IgM (Diagnostic Pasteur) was then added at 1/200 dilution for 30 min at 37°C. The slides were then washed, mounted in 30% glycerol in PBS, and examined under UV light.
RESULTS
Human anti-P. falciparum LSA3 Abs react with P. yoelii preerythrocytic stages.
Abs from hyperimmune individuals were affinity purified on three recombinant fusion proteins previously reported as β-Gal-DG729, GST-NN, and GST-PC (11), which correspond to the N-terminal region, the R2 repeats, and the C-terminal region of LSA3, respectively (Fig. 1). Anti-DG729 and -NN (containing Glu-rich repeat regions) and anti-PC Abs proved to define distinct groups of non-cross-reactive epitopes (Table 1). The three isolated Ab fractions were tested by IFAT upon sporozoites from P. falciparum and from the two rodent malaria species P. yoelii and P. berghei. Strong surface labeling of the P. falciparum and P. yoelii sporozoites, but not of those of P. berghei, was observed for all three Ab fractions (Table 2). Epitopes contained within the DG729 recombinant protein were further analyzed using human Abs immunopurified on synthetic peptides corresponding to the repeat (LSA3-RE) or the nonrepeat (LSA3-NRII) regions (Fig. 1). These two peptides define non-cross-reactive epitopes (Table 1), and Abs specifically directed to each peptide also showed strong cross-reactivity with P. yoelii sporozoites (Table 2). No IFAT labeling of P. yoelii or P. berghei sporozoites was obtained using human Abs affinity purified on recombinant protein DG671 or the synthetic peptide SALSA2 from the P. falciparum preerythrocytic SALSA molecule (3).
TABLE 1.
Specificity of human affinity-purified Absa
| Human Ab | Specifity result for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Recombinant protein
|
Synthetic peptide
|
|||||||
| GST-729 | GST-NN | GST-PC | GST-671 | LSA3-RE | LSA3-NRI | LSA3-NRI1 | SALSA2 | |
| LSA3 | ||||||||
| β-Gal-DG729 | 1,100 | 1,110 | 60 | 68 | 1,010 | 71 | 368 | 75 |
| CST-NN | ND | 1,200 | 81 | 78 | 1,119 | 52.5 | 32 | 79 |
| GST-PC | 36.5 | 86 | 1,025 | 89 | 32 | 38.5 | 26 | 83.5 |
| LSA3-RE | 998 | 1,053 | 32 | 30 | 906 | 34 | 30.5 | 29 |
| LSA3-NRII | 325 | 26 | 28 | 26 | 23 | 25.5 | 420 | 27 |
| SALSA | ||||||||
| β-Gal-DG671 | 65 | 33 | 75 | 836 | 35.5 | 30.5 | 33 | 580 |
| SALSA2 | 22 | 25 | 28 | 710 | 32 | 26 | 25 | 608 |
Each Ab preparation was tested in duplicate on the corresponding recombinant protein or synthetic peptide in ELISAS. Results are expressed as mean OD values obtained by the ELISA reader and multiplied by 1,000. Results that were ≥100 were considered positive. ND, not determined.
TABLE 2.
IFAT on sporozoitesa
| Ab source | Antigen | Positivity of result from:
|
||
|---|---|---|---|---|
| P. falciparum | P. yoelii | P. berghei | ||
| Human | β-Gal-DG729 | ++++ | ++++ | − |
| GST-NN | ++ | ++ | − | |
| GST-PC | +++ | +++ | − | |
| LSA3-RE | +++ | +++ | − | |
| LSA3-NRII | ++ | ++ | − | |
| β-Gal-DG671 | +++ | − | − | |
| SALSA2 | +++ | − | − | |
| Chimpanzee | β-Gal-DG729 | 1/800 | 1/400 | <100 |
| LSA3-NRII | 1/400 | 1/200 | <100 | |
| β-DG671 | 1/800 | <100 | <100 | |
Results from IFAT using human affinity-purified Abs or sera from immunized chimpanzees and sporozoites from the three species: P. falciparum (NF54), P. yoelii (17XNL), and P. berghei (ANKA). Abs were affinity purified on LSA3 recombinant proteins (β-Gal-DG729, GST-NN, and GST-PC) and on LSA3 synthetic peptides (LSA3-RE and LSA3-NRII). Control Abs were purified on β-Gal-DG671 (SALSA) and on the synthetic peptide SALSA2. For chimpanzee sera, the titer was determined; a titer of ≥1/100 was considered positive. Affinity-purified Abs were used undiluted: therefore, results are expressed as negative or positive (a positivity score of +++ usually corresponds to a titer of >1:50).
Having established that naturally occurring human anti-LSA3 Abs cross-reacted with P. yoelii sporozoites, we next investigated the reactivity of antibodies induced in LSA3-immunized chimpanzees. Sera from two chimpanzees, Gerda and Dirk, immunized with the lipopeptide NRII and the recombinant protein DG729, respectively (1, 11), were tested. These Abs also reacted at high titers with P. falciparum and P. yoelii sporozoites, and again no reactivity was observed with P. berghei sporozoites (Table 2). The serum from Bart, a chimpanzee immunized with the recombinant SALSA molecule (3), used as control, reacted only with P. falciparum sporozoites (Table 2).
By using P. falciparum and P. yoelii sporozoites, a similar pattern with an even distribution over the entire sporozoite surface was obtained with anti-LSA3 Abs in IFAT (Fig. 2B). In both cases all the sporozoites were labeled. The intensity and pattern of the fluorescence were similar when sporozoites from different P. yoelii lines (265BY, 17XNL, or clone 1.1) were used (data not shown), indicating that the cross-reactive P. yoelii epitopes display little or no polymorphism.
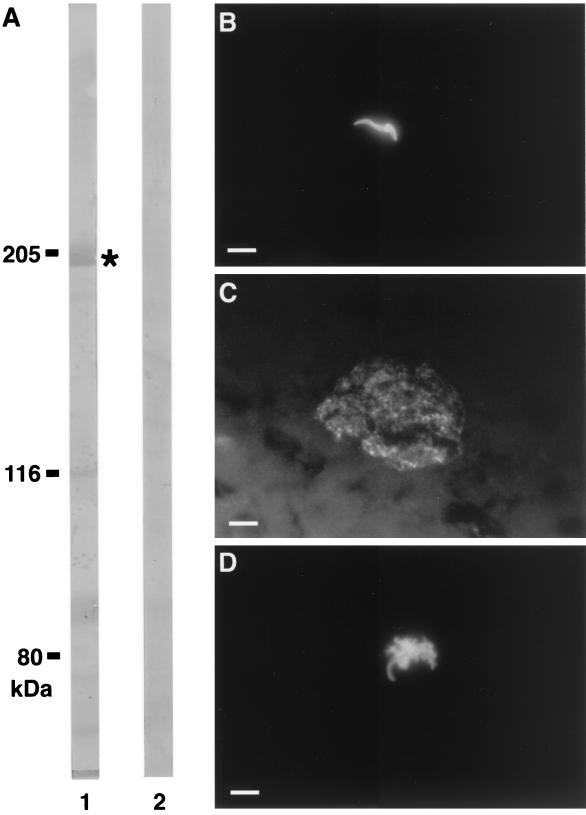
FIG. 2.
Ab reactivity on sporozoite and liver stages. (A) Western blot analysis of 17XNL sporozoites extracts with human Abs immunopurified on the recombinant protein GST-PC (LSA3-Cterm) (lane 1) or human Abs immunopurified on the recombinant protein β-Gal-DG671 (SALSA antigen) used as control (lane 2). Masses are shown in kilodaltons. ∗, reactivity with a 205-kDa polypeptide. IFAT was performed with human anti-β-Gal-DG729 (LSA3-Nterm) immunopurified Abs on P. yoelii sporozoites isolated from salivary glands of infected Anopheles stephensi mosquitoes (B) and on a cryosection of P. yoelii liver schizont (44-h development) obtained in C3H/Hej mice (C). (D) A direct immunofluorescence assay was performed on the suspension injected into mice (Table 3), showing the detachment of the sporozoite membrane induced by anti-LSA3 antibodies upon live P. yoelii sporozoites. White bars, 10 μm.
Western-blotted P. yoelii sporozoite extracts, probed with human affinity-purified Abs specific to either the N-terminal (DG729) or the C-terminal (PC) (Fig. 2A) regions of LSA3, showed reactivity with a 205-kDa polypeptide, whereas control Abs purified on the SALSA protein were negative.
The cross-species reactivity was also investigated for liver-stage parasites using human anti-DG729 Abs (Fig. 2C), human anti-PC Abs, and chimpanzee anti-LSA3-NRII Abs. All proved equally and strongly reactive with P. yoelii mature liver schizonts. The distribution of the antigen in the P. yoelii liver forms was similar to that found in P. falciparum. In both species it was localized to the flocculent material present in the parasitophorous vacuole, as well as around the pseudocytomeres and mature liver merozoites (Fig. 2C). When tested on asexual and sexual blood stages of both P. yoelii and P. berghei, the anti-LSA3 Abs were negative, suggesting that the putative cross-reactive P. yoelii homologue is not expressed in erythrocytic stages.
LSA3 shares T-cell epitopes with P. yoelii sporozoite stage.
In a previous study, it was reported that T cells from chimpanzee Gerda immunized with the LSA3-NRII lipopeptide could specifically respond to in vitro stimulation by native LSA3, whereas control nonimmunized chimpanzees did not (1, 2). We therefore investigated the presence of cross-reactive T-cell epitopes in the rodent parasite molecule. A strong proliferative response of T cells derived from an LSA3-immunized chimpanzee was observed in a dose-dependent manner when a P. yoelii sporozoite extract was used as antigen (Fig. 3). These cells were not stimulated when P. berghei sporozoite extracts were used (Fig. 3), demonstrating the specificity of the T-cell responses induced by P. yoelii sporozoite extracts.
FIG. 3.
T-cell responses to P. yoelii sporozoite extract. Antisporozoite proliferative responses of lymphocytes from chimpanzee Gerda sampled 4 weeks after the third immunizing dose with lipopeptide LSA3-NRII. PBMC from Gerda were prepared and were incubated with freeze-thawed P. yoelii or P. berghei sporozoites at concentrations ranging from 50 to 5,000 sporozoites per 2 × 105 cells (given as x axis). Experimental and control wells were run in triplicate. The results are presented as stimulation indices calculated as the ratio of geometric mean counts per minute from Ag-stimulated cultures to the value from cultures without Ag. The mean background counts per minute of responses in control cultures without Ag was 2,659 for Gerda.
Anti-P. yoelii Abs induced in mice cross-react with P. falciparum LSA3.
Conversely, infection of mice with live P. yoelii sporozoites also induced IgG Abs that reacted with the three synthetic peptides LSA3-RE, LSA3-NRI, and LSA3-NRII, with mean ELISA ratios of 7.1, 10, and 7.2, respectively. This cross-reactivity was detectable on day 6 after the infection induced by sporozoites, when the blood parasitemia was only 0.01%. In contrast, in mice infected with parasitized red blood cells, serum taken at day 14 (anti-blood-stage IFAT Ab titer of 5,400) contained no cross-reactive Abs (mean ELISA ratio of <1.5, using each of the three peptides). No cross-reactive Abs were found in sera from mice infected with P. berghei sporozoites (data not shown).
Invasion of P. yoelii sporozoites into mouse hepatocytes is strongly reduced by naturally acquired human anti-LSA3 Abs.
Having demonstrated the existence of shared B-cell epitopes between LSA3 and P. yoelii preerythrocytic stages, we examined the effect of anti-LSA3 Abs on in vitro invasion of murine hepatocytes by P. yoelii sporozoites. P. berghei sporozoites were used as control.
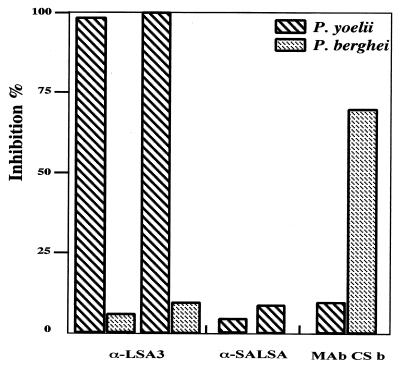
Experiments using the two rodent species were conducted in parallel, i.e., performed using a single hepatocyte preparation (Fig. 4). We found 99 to 100% inhibition of P. yoelii sporozoite invasion, when a preparation of human Ab affinity purified against DG729 at an IFAT end point titer as low as 1/2, was used. Inversely, Abs from the non-cross-reactive antigen (SALSA) had little or no effect (<10% inhibition). The inhibitory effect was strain independent, since similar results were obtained when sporozoites from the 265BY strain of the parasite were used (data not shown), but was species specific, since no inhibition was observed with P. berghei sporozoites.
FIG. 4.
In vitro inhibition of hepatocyte invasion by P. yoelii sporozoites. The inhibitory effect of human anti-LSA3 Abs was evaluated upon P. yoelii or P. berghei sporozoite invasion into mice hepatocytes. Abs were affinity purified on the recombinant protein β-Gal-DG729 (LSA3-Nterm) or β-Gal-DG671 (SALSA). Anti-SALSA Abs (α-SALSA) were used as control for human Abs (α-LSA3). The anti-CS MAb of P. berghei was used as control for the P. berghei sporozoite invasion. The human Abs were tested at a concentration corresponding to their IFAT end point titer (1/2). The anti-CS MAb of P. berghei was used at a dilution of 5 × 10−4 (IFAT end point titer of 10−6). All samples were tested in duplicate. The number of liver schizonts was counted in each well, and the percentage of inhibition was calculated compared to that for control wells to which no Abs were added. The numbers of P. yoelii and P. berghei liver schizonts in control wells without Abs were 101.5 ± 15.45 and 110.5 ± 21.70, respectively.
Human anti-LSA3 Abs protect mice against an infection with P. yoelii sporozoites.
The human affinity-purified anti-DG729 (LSA3) or anti-DG671 (SALSA) Abs were further tested in passive transfer experiments. Groups of six mice were used in duplicate experiments. In each group two mice were injected with sporozoites mixed with anti-DG729 Abs, two were injected with sporozoites mixed with anti-DG671 Abs, and the last two mice served as infectivity controls. We found that all mice receiving the anti-DG729 Abs were completely protected, with no parasites detectable in the blood from day 4 to day 21 postinoculation. Conversely, control mice who received sporozoites preincubated with anti-DG671 Abs or untreated sporozoites had a patent parasitemia from day 5, and the infection followed a normal course (Table 3).
TABLE 3.
In vivo protective effect in passive transfer experimentsa
| Ab used | Sporozoite IFAT result for:
|
No. infected/no. tested:
|
|||
|---|---|---|---|---|---|
| P. falciparum | P. yoelii | Expt 1 | Expt 2 | Total | |
| Anti-β-Gal-DG729 | + | + | 0/2 | 0/2 | 0/4 |
| Anti-β-Gal-DG671 | + | − | 2/2 | 2/2 | 4/4 |
| None | 2/2 | 2/2 | 4/4 | ||
One hundred micrograms of human anti-β-Gal-DG729 (LSA3) or anti-β-Gal-DG671 (SALSA) Ab per ml was added to 150 P. yoelii sporozoites, in a final volume of 200 μl/mouse, and injected into the tail vein of BALB/c mice. Parasitemia was recorded from day 4 to day 21 after challenge.
Direct immunofluorescence assays, performed using an aliquot of the injected preparation containing live sporozoites and human anti-DG729 Abs, showed that Abs labeled the whole surface of the P. yoelii sporozoite and induced a reaction (Fig. 2D) similar to that previously described with anti-CS protein Abs, termed the precipitin reaction (30). The sporozoites mixed with human Abs specific to SALSA were not labeled, and no change in their morphology was observed.
DISCUSSION
Research on the development of vaccines against P. falciparum malaria has been severely hampered by the narrow host specificity of the human parasite (19), which precludes the use of common laboratory animals. This limitation is particularly marked when the preerythrocytic stage antigens are targeted. In this context, homologous antigens of rodent parasite species that are recognized by naturally acquired human Abs would allow one to carry out functional investigations in the more amenable laboratory models. In this article we present evidence that the preerythrocytic LSA3 antigen of P. falciparum, a new and very promising vaccine candidate, has an antigenic homologue in P. yoelii.
The cross-reactivity of human anti-LSA3 Abs, affinity purified against the N terminus of the molecule, was first observed in IFAT assays using P. yoelii sporozoites. This cross-reactivity was further characterized using recombinant proteins and synthetic peptides representative of the whole molecule. Our results suggest that there is an LSA3 analogue in P. yoelii. All the LSA3-specific Abs tested cross-reacted with the three different lines of P. yoelii used in this study. The pattern of expression of this putative analogue was found to be similar to that described for the human parasite P. falciparum (11), namely, strong specific labeling of sporozoite surface and liver schizonts but not of erythrocytic forms. The protein in the rodent parasite also has a molecular mass close to the 200-kDa mass of the K1 strain LSA3 (11). It should be pointed out that LSA3 has a repeated region which is polymorphic in length between different strains; thus, LSA3 of the NF54 strain is 175 kDa.
Our results indicate the presence of shared B- and T-cell epitopes between the P. falciparum LSA3 and a molecule of P. yoelii. At least four distinct B-cell epitopes were found to be shared by LSA3 and its putative homologue in P. yoelii, with one found in the repeat region and three occurring in the nonrepeated domains of the protein. Cross-reactivity at the T-cell level, which has been previously suggested (2), has been confirmed. PBMC from a chimpanzee immunized with the LSA3-NRII peptide, which contains Th and cytotoxic T-lymphocyte epitopes (1), proliferated in a dose-dependent manner in the presence of P. yoelii sporozoites. To our knowledge this is the first molecule described as containing a cross-reactive T-cell epitope between a human and a rodent malaria preerythrocytic antigen. Further, immunoepidemiological and immunization studies have indicated that LSA3 is highly antigenic and immunogenic (1, 2). The same seems to hold true for the P. yoelii homologue, since as few as 150 sporozoites proved sufficient to induce IgG antibodies to several distinct B-cell epitopes.
The most striking feature of the cross-reactivity observed with P. yoelii lies in the biological effect. Thus, naturally acquired human anti-LSA3 Abs inhibit the invasion of hepatocytes by rodent malaria sporozoites. This inhibition is specific and is observed both in vitro and in vivo. Similar in vitro results were found using P. falciparum sporozoites in the inhibition-of-liver-stage-development human hepatocyte assay (V. Pasquetto, unpublished data). The inhibitory activity obtained in vitro is particularly notable for its unusually high level and the low Ab concentrations at which it was obtained. Indeed, these inhibitory levels are among the highest observed in primary hepatocyte cultures (20, 21, 23, 24, 26) and are higher than those obtained so far with human Abs directed to other sporozoite surface antigens (26).
The in vitro invasion inhibition was confirmed by in vivo studies, which are obviously untenable for P. falciparum in humans, and hereby stresses the value of the P. yoelii-LSA3 model. In our experiments anti-LSA3 Abs purified from the sera of clinically protected individuals, with Ab levels as low as 20 μg, were sufficient to provide complete protection to mice against P. yoelii sporozoite challenge. Passive transfer of 1 mg of MAb NYS1 anti-CS protein was employed to obtain 100% protection of mice against a challenge with P. yoelii, but at a lower dose of 125 μg, only 67% protection was obtained (7). In other animal models, protection was achieved in Saimiri monkeys after passive transfer of 2 mg of anti-CS MAb NVS3 directed to the CS protein from Plasmodium vivax (6). Recently Gantt et al. (18) showed that the MAb F3B5 directed against thrombospondin-related anonymous protein repeat did not inhibit the in vivo infectivity of P. yoelii sporozoites even when incubated at concentrations as high as 500 μg/ml. Moreover, polyclonal antisera directed against thrombospondin-related anonymous protein N10, a region that is highly conserved among Plasmodium species that infect mammals (33), also did not reduce the infectivity of sporozoites in vivo (18). Furthermore, it has been shown that the precipitin reaction (CS precipitation) can be induced by Abs directed to the surface of sporozoites (30). This reaction, which immobilizes the sporozoite, inhibits the invasion of the hepatocytes by acting on parasite gliding motility (32). In the present study, we found that human anti-LSA3 Abs induce the same phenomenon on the surface of P. yoelii sporozoites.
Our knowledge of the critical mechanisms involved in protection against the preerythrocytic stages of either human or rodent malaria remains insufficient. The roles of Abs and various T-cell subsets have been indicated in both situations, but the more critical importance of one over the other remains controversial. It is today generally agreed, however, that Abs most likely play a role, but that their effect is incomplete (16, 29). The potential of Abs was reinforced in recent clinical trials of a hybrid CS recombinant protein, termed RTS,S, which have clearly demonstrated that CS subunit vaccines can elicit protective immunity that correlates with high levels of anti-CS antibody and CD4+ T cells in the absence of detectable CD8+ T cells (31). Most of this knowledge is based on anti-CS Abs and to a lesser extent on anti-TRAP-SSP2 or anti-STARP. Few data are available for the LSA3 antigen, which was characterized more recently and which, in contrast to other preerythrocytic malaria molecules, is highly conserved. The underlying mechanism(s) of the protection induced in a reproducible manner in chimpanzees also remains to be elucidated. Results presented here with the P. yoelii LSA3 homologue would suggest that the role of anti-LSA3 Abs against P. falciparum preerythrocytic stages in vivo now deserves to be addressed. In conclusion, this mouse model may be of potential value in the study of the biological activity of immune responses induced by P. falciparum LSA3, where it might be very profitably exploited to assess the actual efficacy elicited by LSA3-based vaccination.
ACKNOWLEDGMENTS
We thank Sylvie Mellouk for her contribution to the in vitro studies, Steve Hoffman and Yupin Charoenvit for their generous gift of the anti-CS MAbs employed in this study, and Wijnand Eling for supplying of NF54 P. falciparum sporozoites.
REFERENCES
- 1.BenMohamed L, Gras-Masse H, Tartar A, Daubersies P, Brahimi K, Bossus M, Thomas A, Druilhe P. Lipopeptide immunization without adjuvant induces potent and long-lasting B, T helper, and cytotoxic T lymphocyte responses against a malaria liver stage antigen in mice and chimpanzees. Eur J Immunol. 1997;27:1242–1253. doi: 10.1002/eji.1830270528. [DOI] [PubMed] [Google Scholar]
- 2.BenMohamed L, Thomas A, Bossus M, Brahimi K, Wubben J, Gras-Masse H, Druilhe P. High immunogenicity in chimpanzees of peptides and lipopeptides derived from four new Plasmodium falciparum pre-erythrocytic molecules. Vaccine. 2000;18:2843–2855. doi: 10.1016/s0264-410x(00)00068-2. [DOI] [PubMed] [Google Scholar]
- 3.Bottius E, BenMohamed L, Brahimi K, Gras-Masse H, Lepers J P, Aikawa M, Meis J, Slierendregt B, Tartar A, Thomas A, Druilhe P. A novel Plasmodium falciparum sporozoite and liver stage antigen (SALSA) defines major B, Th and CTL epitopes. J Immunol. 1996;156:2874–2884. [PubMed] [Google Scholar]
- 4.Brahimi K, Pérignon J L, Bossus M, Gras H, Tartar A, Druilhe P. Fast immunopurification of small amounts of specific antibodies on peptides bound to ELISA plates. J Immunol Methods. 1993;162:69–75. doi: 10.1016/0022-1759(93)90408-y. [DOI] [PubMed] [Google Scholar]
- 5.Burns J M, Parke L A, Daly T M, Cavacini L A, Weidanz W P, Long C A. A protective monoclonal antibody recognizes a variant-specific epitope in the precursor of the major merozoite surface antigen of the rodent malarial parasite Plasmodium yoelii. J Immunol. 1989;142:2835–2840. [PubMed] [Google Scholar]
- 6.Charoenvit Y, Collins W E, Jones T R, Millet P, Yuan L, Campbell G H, Beaudoin R L, Broderson J R, Hoffman S L. Inability of malaria vaccine to induce antibodies to a protective epitope within its sequence. Science. 1991;251:668–671. doi: 10.1126/science.1704150. [DOI] [PubMed] [Google Scholar]
- 7.Charoenvit Y, Mellouk S, Cole C, Bechara R, Leef M F, Sedegah M, Yuan L F, Robey F A, Beaudoin R L, Hoffman S L. Monoclonal, but not polyclonal, antibodies protect against Plasmodium yoelii sporozoites. J Immunol. 1991;146:1020–1025. [PubMed] [Google Scholar]
- 8.Charoenvit Y, Mellouk S, Sedegah M, Toyoshima T, Leef M F, De La Vega P, Beaudoin R L, Aikawa M, Fallarme V, Hoffman S L. Plasmodium yoelii: 17-kDa hepatic and erythrocytic stage protein is the target of an inhibitory monoclonal antibody. Exp Parasitol. 1995;80:419–429. doi: 10.1006/expr.1995.1054. [DOI] [PubMed] [Google Scholar]
- 9.Clyde D F, Most H, McCarthy V, Vanderberg J P. Immunization of man against sporozoite-induced falciparum malaria. Am J Med Sci. 1973;266:169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Da Silveira F J, Sibilli L, Brunet E, Mercereau O. Cloning of cDNAs that code for Plasmodium chabaudi antigens of the erythrocytic forms and crosshybridize with P. falciparum. Mol Biochem Parasitol. 1984;8:45–51. doi: 10.1016/0166-6851(84)90060-4. [DOI] [PubMed] [Google Scholar]
- 11.Daubersies P, Thomas A W, Millet P, Brahimi K, Langermans J A M, Ollomo B, BenMohamed L, Slierendregt B, Eling W, Van Belkum A, Dubreuil G, Meis J F G M, Guérin-Marchand C, Cayphas S, Cohen J, Gras-Masse H, Druilhe P. Protection against Plasmodium falciparum malaria in chimpanzees by immunization with the conserved pre-erythrocytic liver-stage antigen 3. Nat Med. 2000;6:1258–1263. doi: 10.1038/81366. [DOI] [PubMed] [Google Scholar]
- 12.Del Portillo H A, Longacre S, Khouri E, David P H. Primary structure of the merozoite surface antigen 1 of Plasmodium vivax reveals sequences conserved between different Plasmodium species. Proc Natl Acad Sci USA. 1991;88:4030–4034. doi: 10.1073/pnas.88.9.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doolan D L, Hedstrom R C, Rogers O W, Charoenvit Y, Rogers M, de la Vega P, Hoffman S L. Identification and characterisation of the protective hepatocyte erythrocyte protein 17 kDa gene of Plasmodium yoelii, homolog of Plasmodium falciparum exported protein 1. J Biol Chem. 1996;271:17861–17868. doi: 10.1074/jbc.271.30.17861. [DOI] [PubMed] [Google Scholar]
- 14.Druilhe P, Marchand C. From sporozoite to liver stages: the saga of the irradiated sporozoite vaccine. In: McAdam K, editor. New strategies in parasitology. Edinburgh, United Kingdom: Churchill Livingstone; 1989. pp. 39–48. [Google Scholar]
- 15.Druilhe P, Pradier O, Marc J P, Miltgen F, Mazier D, Parent D. Levels of antibodies to Plasmodium falciparum sporozoite surface antigens reflect malaria transmission rates and are persistent in the absence of reinfections. Infect Immun. 1986;53:393–397. doi: 10.1128/iai.53.2.393-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Druilhe P, Renia L, Fidock D A. Immunity to liver stages. In: Sherman I W, editor. Malaria: parasite biology, pathogenesis, and protection. Washington, D.C.: ASM Press; 1998. pp. 513–543. [Google Scholar]
- 17.Fidock D A, Bottius E, Brahimi K, Moelans I I M D, Aikawa M, Konings R N H, Certa U, Olafsson P, Kaidoh T, Asavanich A, Guerin-Marchand C, Druilhe P. Cloning and characterization of a novel Plasmodium falciparum sporozoite surface antigen, STARP. Mol Biochem Parasitol. 1994;64:219–232. doi: 10.1016/0166-6851(94)00012-3. [DOI] [PubMed] [Google Scholar]
- 18.Gantt S, Persson C, Rose K, Birkett A J, Abagyan R, Nussenzweig V. Antibodies against thrombospondin-related anonymous protein do not inhibit Plasmodium sporozoite infectivity in vivo. Infect Immun. 2000;68:3667–3673. doi: 10.1128/iai.68.6.3667-3673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garnham P C C. Malaria parasites and other haemosporidia. Oxford, United Kingdom: Blackwell Scientific; 1966. [Google Scholar]
- 20.Hollingdale M R, Appiah A, Leland P, do Rosario V E, Mazier D, Pied S, Herrington D A, Chulay J D, Ballou W R, Derks T, Yap S H, Beaudoin R L, Verhave J P. Activity of human volunteer sera to candidate Plasmodium falciparum circumsporozoite protein vaccines in the inhibition of sporozoite invasion assay of human hepatoma cells and hepatocytes. Trans R Soc Trop Med Hyg. 1990;84:325–329. doi: 10.1016/0035-9203(90)90303-v. [DOI] [PubMed] [Google Scholar]
- 21.Hollingdale M R, Nardin E H, Tharavanij S, Schwartz A L, Nussenzweig R S. Inhibition of entry of Plasmodium falciparum and P. vivax sporozoites into cultured cells: an in vitro assay of protective antibodies. J Immunol. 1984;132:909–913. [PubMed] [Google Scholar]
- 22.Marchand C, Druilhe P. How to select P. falciparum pre-erythrocytic antigens in an expression library without defined probe. Bull W H O. 1990;68:158–164. [PMC free article] [PubMed] [Google Scholar]
- 23.Mazier D, Mellouk S, Beaudoin R, Texier B, Druilhe P, Heckmeyer W, Trosper J, Paul C, Young J, Miltgen F, Galley B, Brandicourt O, Charoenvit Y, Chedid G, Chigot J P, Gentilini M. Effect of antibodies to recombinant and synthetic peptides on development of Plasmodium falciparum sporozoites in vitro. Science. 1986;231:156–159. doi: 10.1126/science.3510455. [DOI] [PubMed] [Google Scholar]
- 24.Mellouk S, Berbiguier N, Druilhe P, Sedegah M, Galey B, Yuan L, Leef M, Charoenvit Y, Paul C, Hoffman S, Beaudoin R. Evaluation of an in vitro assay aimed at measuring protective antibodies against sporozoites. Bull W H O. 1990;68:52–59. [PMC free article] [PubMed] [Google Scholar]
- 25.Merrifield R B. Solid-phase peptide synthesis. The synthesis of a tetrapeptide. J Am Chem Soc. 1963;85:2149–2152. [Google Scholar]
- 26.Pasqueto V, Fidock D, Gras H, Badell E, Eling W, Ballou W R, Belghiti J, Tartar A, Druilhe P. Plasmodium falciparum sporozoite invasion is inhibited by naturally acquired or experimentally induced polyclonal antibodies to the STARP antigen. Eur J Immunol. 1997;27:2502–2513. doi: 10.1002/eji.1830271007. [DOI] [PubMed] [Google Scholar]
- 27.Ray P, Sahoo N, Singh B, Kironde F A S. Serum antibody immunoglobulin G of mice convalescent from Plasmodium yoelii infection inhibits growth of Plasmodium falciparum in vitro: blood stage antigens of P. falciparum involved in interspecies cross-reactive inhibition of parasite growth. Infect Immun. 1994;62:2354–2361. doi: 10.1128/iai.62.6.2354-2361.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers W O, Malik A, Mellouk S, Nakamura K, Rogers M D, Szarfman A, Gordon D M, Nussler A K, Aikawa M, Hoffman S L. Characterization of Plasmodium falciparum sporozoite surface protein 2. Proc Natl Acad Sci USA. 1992;89:9176–9180. doi: 10.1073/pnas.89.19.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinnis P, Nussenzweig V. Preventing sporozoite invasion of hepatocytes. Infect Agents Dis. 1996;5:182–189. [PubMed] [Google Scholar]
- 30.Stewart M, Nawrot R J, Schulman S, Vanderberg J P. Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect Immun. 1986;51:859–864. doi: 10.1128/iai.51.3.859-864.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stoute J A, Kester K E, Krzych U, Wellde B T, Hall T, White K, Glenn G, Ockenhouse C F, Garcon N, Schwenk R, Lanar D E, Sun P, Momin P, Wirtz R A, Golenda C, Saloui M, Wortmann G, Holland C, Dowler M, Cohen J, Ballou W R. Long-term efficacy and immune responses following immunization with the RTS,S malaria vaccine. J Infect Dis. 1998;178:1139–1144. doi: 10.1086/515657. [DOI] [PubMed] [Google Scholar]
- 32.Sultan A, Thathy V, Frevert U, Robson K, Crisanti A, Nussenzweig V, Nussenzweig R, Menard R. TRAP is necessary for gliding motility and infectivity of plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 33.Templeton T J, Kaslow D C. Cloning and cross-species comparison of the thrombospondin-related anonymous protein (TRAP) gene from Plasmodium knowlesi, Plasmodium vivax and Plasmodium gallinaceum. Mol Biochem Parasitol. 1997;84:13–24. doi: 10.1016/s0166-6851(96)02775-2. [DOI] [PubMed] [Google Scholar]