Abstract
Systemic juvenile idiopathic arthritis associated with interstitial lung disease (SJIA-LD) represents a highly morbid subset of SJIA for which effective therapies are lacking. We report the case of a patient with refractory SJIA-LD who underwent treatment with MAS-825, an investigational bispecific monoclonal antibody targeting IL-1β and IL-18. MAS-825 treatment was associated with a marked reduction in total IL-18 and free IL-18 in both serum and bronchoalveolar lavage fluid (BAL). Baseline oxygen saturation, exercise tolerance, and quality of life metrics improved after treatment with MAS-825, while pulmonary function testing remained stable. Following treatment, the BAL showed no evidence of pulmonary alveolar proteinosis and inflammatory infiltrates were markedly reduced, reflected by decreased numbers of CD4 T cells, CD8 T cells, and macrophages. The patient was able to wean entirely off systemic corticosteroids and other biologics after 10 months of treatment with MAS-825 and experienced no side effects of the drug. This case demonstrates improvement in pulmonary symptoms, lung inflammation, and burden of immunomodulatory therapy after treatment with MAS-825 and suggests that simultaneous targeting of both IL-1β and IL-18 may be a safe and effective treatment strategy in SJIA-LD.
Introduction
Systemic Juvenile Idiopathic Arthritis (SJIA) is an autoinflammatory disorder characterized by daily recurrent fever, arthritis, and systemic inflammation resulting in variable presentation of lymphadenopathy, organomegaly, rash, and serositis. Although only about 10% of patients develop a fulminant hemophagocytic syndrome referred to as Macrophage Activation Syndrome (MAS), many SJIA patients display MAS features including hyperferritinemia, hemophagocytosis, cytopenias, and fibrinogen consumption(1, 2)]. MAS is thought to be in large part driven by excessive Interferon gamma (IFNγ) activity, as evidenced by elevated levels of the IFNγ inducible protein CXCL9 in SJIA patients with MAS [(3)]. Elevated levels of IL-18, a cytokine that promotes IFNγ production by T- and NK-cells, are found in SJIA patients predisposed to MAS (4). Thus, an IL-18/IFNγ axis has been implicated in disease pathogenesis.
More recently, an additional endotype within the IL-18 high SJIA subpopulation has been described, featuring life-threatening interstitial lung disease (ILD) and recurrent MAS (5–7). These SJIA-LD patients present with erythematous digital clubbing, protein in the bronchoalveolar lavage fluid (BAL) consistent with pulmonary alveolar proteinosis (PAP), and biopsy evidence of endogenous lipoid pneumonia. Though our ability to diagnose and treat this entity is evolving, the prognosis of SJIA-LD is grim, with morbidity and mortality associated with both unfettered MAS-like inflammation as well as progression to life-threatening pulmonary insufficiency. There is not a clear therapeutic pathway for children suffering from this complication. Furthermore, it has been suggested that exposure to the IL-1- or IL-6-blocking biologic medications that have been so effective in treating SJIA (and/or components common to these medicines), coupled with a strong HLA-DRB1*15 association (5, 8), may be contributing to this ILD presentation. However, SJIA-LD has occurred in patients with no exposure to such medications. Notably, a recent serum biomarker analysis of SJIA-LD showed a strong correlation between elevated IL-18 and SJIA-LD, even after controlling for MAS disease activity (2).
Herein, we describe a patient with refractory SJIA-LD who received therapy with an investigational bispecific antibody that blocks both IL-1β and IL-18. We report improvements in multiple measures of lung inflammation, symptomatic relief of work of breathing, and significant weaning of immunomodulatory treatment. These results have important implications both for IL-18 as a therapeutic target in SJIA-LD and the role of biologic medication administration in ILD progression.
Methods
Following informed consent and assent, clinical and biomarker data were collected as part of a natural history study approved by the Institutional Review Board of Children’s Hospital of Philadelphia. All data were obtained as part of routine clinical care except as indicated. MAS-825 was administered under a single patient Investigational New Drug application #155958 with the US Food and Drug Administration, and with informed consent and assent under a protocol approved by the Institutional Review Board of Children’s Hospital of Philadelphia.
Serum total IL-18 and CXCL9 were measured clinically. Serum and BAL IL-18BP and BAL total IL-18 were measured by bead-based assay as in Weiss et al.(4). Free IL-18 was measured by ELISA as in Girard et al. [(9), but recombinant human IL-18BP-Fc (R&D systems) was substituted as capture reagent. Serum ICAM5 was measured by ELISA as in Chen et al.(2).
Remnant BAL was centrifuged to collect the cells. Paired BAL cells and whole blood cells were subsequently stained with two panels of fluorophore-conjugated antibodies: one focused on T cells and their subsets, and the other focused on myeloid cells and their subsets (Table S1, Figures S1–S2). Cells were analyzed on an 18-color LSR flow cytometer (BD Biosciences) and analyzed using FlowJo software. The same standard gating strategy was applied to all specimens.
Results
Description of case
A male child was diagnosed at 22 months of age with SJIA on the basis of daily fever, rash, and arthritis. He had MAS associated with varicella viremia at an early age. He was initially treated with canakinumab, methotrexate, and prednisone, with varying degrees of efficacy for his fevers and arthritis. Temporary withdrawal of canakinumab resulted in immediate return of fevers and systemic inflammation, so he has been persistently maintained on IL-1β blockade. With the exception of a 4-month period when anakinra was administered to treat MAS, he has been continuously on canakinumab since 6 years of age. He first presented to our clinic at age 8, after the development of erythematous clubbing of finger- and toe-nails as well as clinical and radiographic lung disease consistent with SJIA-LD (Figure 1A–B). Transbronchial biopsy of his lungs was consistent with endogenous lipoid pneumonia (Figure 1C). Clinical trio whole exome sequencing did not identify a genetic explanation for his phenotype and reported only a heterozygous, paternally-inherited STK4 variant of uncertain significance. HLA-DRB1 typing demonstrated *11:04:01 and *15:06:01 haplotypes. His IL-18 levels were consistently high throughout his course, averaging ~83,000 pg/mL (normal: <540 pg/mL). He was subsequently treated with prednisone, cyclophosphamide, and azathioprine in conjunction with canakinumab. Due to increase in cough, shortness of breath, and decreased diffusion capacity (DLCO), as well as a marked increase in his serum CXCL9 to 1700 pg/mL (normal: <647 pg/mL), his azathioprine was replaced with tofacitinib after two years. Despite these efforts, his pulmonary symptoms and DLCO continued to worsen, and alternative approaches were sought. Treatment with the IL-1β/IL-18 bispecific antibody MAS-825 was initiated under an expanded access program with concomitant cessation of canakinumab. Two months after initiation of MAS-825, tofacitinib was discontinued without issue. By the following month, he reported subjective resolution of his cough and dyspnea and improved ability to keep up with peers when exercising. Within ten months, his prednisone was weaned completely off (Table 1), representing the first time that he has been stably sustained off glucocorticoids. No adverse events attributable to drug have been reported.
Figure 1: Physical exam, radiographic, and histologic markers of SJIA-LD.
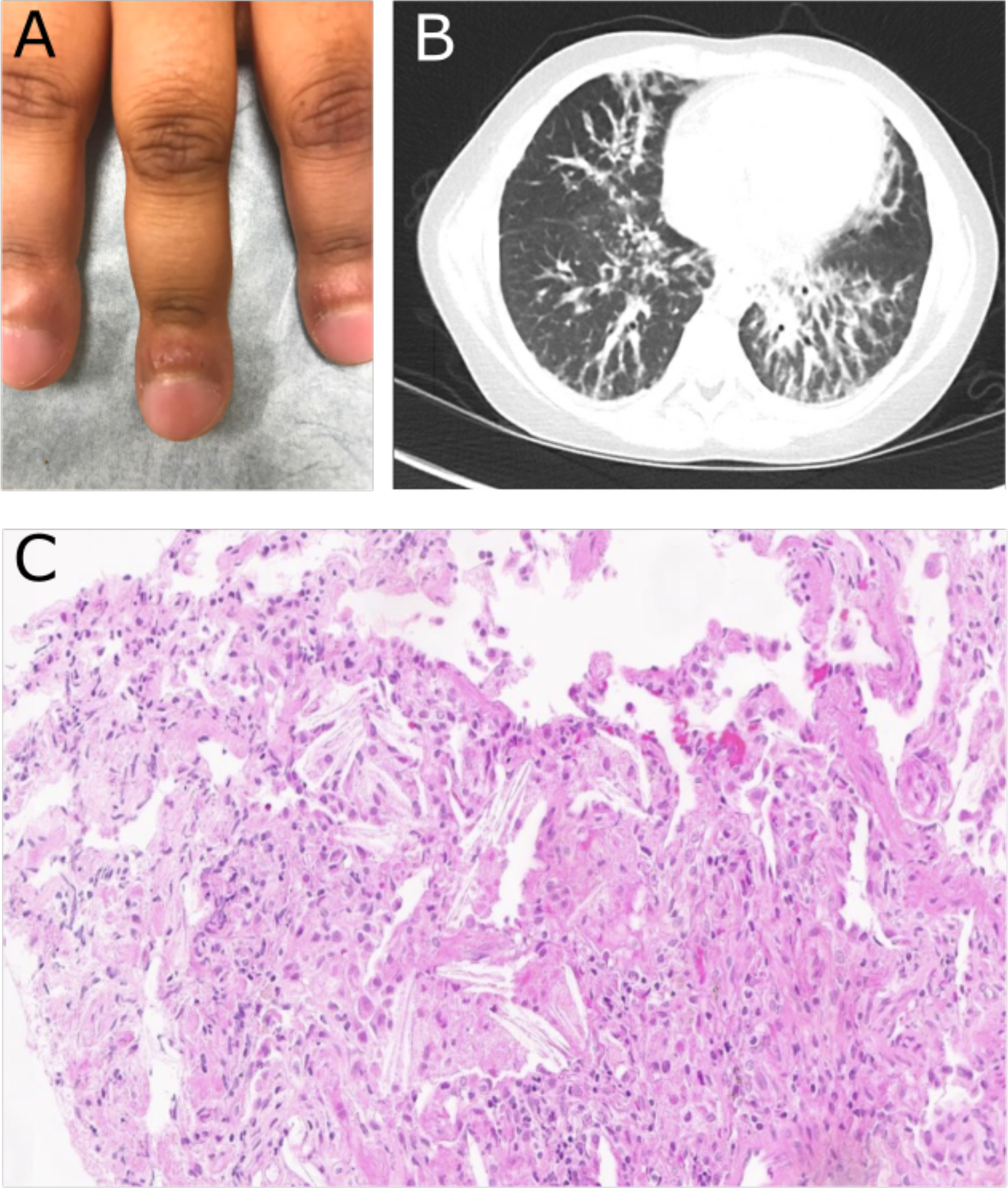
(A) Photograph of fingers prior to MAS-825 showing significant clubbing and erythematous, inflamed periungual tissue. (B) Representative image of lung CT prior to MAS-825 therapy demonstrating peribronchovascular interstitial thickening. (C) Photomicrograph of transbronchial biopsy of lesion demonstrating interalveolar cholesterol clefts (lower right) and foamy macrophages (upper left) consistent with the endogenous lipoid pneumonia pattern of SJIA-LD.
Table 1:
Patient medications and clinical evaluations before and after MAS-825 treatment
| Pre MAS-825 | Post MAS-825 | |
|---|---|---|
|
| ||
| Medicationsa | Canakinumab 300 mg monthly Tofacitinib 5 mg twice daily Prednisone 5 mg daily Celecoxib as needed Pentamidine monthly |
MAS-825 every 2 weeks |
|
| ||
| Daytime resting SpO2 | 95% | 97% |
|
| ||
| Post-exercise SpO2 nadir | 91% | 97% |
|
| ||
| 6-minute walk | ||
| Pre SpO2 | 93% | 99% |
| Post SpO2 | 93% | 97% |
| Distance (m) | 350 | 390 |
|
| ||
| Pulmonary Function Testsb | ||
| FVC % Ref | 61 | 66 |
| FEV1 % Ref | 56 | 67 |
| FEV1/FVC | 82 | 90 |
| DLCO [Hb] % Ref | 59 | 59 |
| DLCO/VA % Ref | 108 | 115 |
Doses of azithromycin and inhaled fluticasone/salmeterol did not change.
Test results from 1 week prior to initiation of MAS-825 and 40 weeks post are shown.
SpO2: peripheral oxygen saturation. FVC: Forced Vital Capacity. FEV: Forced Expiratory Volume. DLCO [Hb]: diffusion of carbon monoxide corrected for hemoglobin. DLCO/VA: diffusion of carbon monoxide corrected for alveolar volume. % Ref: percentage of age/height/sex-appropriate reference value
Laboratory/clinical value trends on MAS-825
Spirometry, DLCO, and chest CT findings were stable during therapy (Table 1 and data not shown). However, his oxygenation saturation at baseline and after exercise tolerance testing improved following treatment with MAS-825 (Figure 2A, Table 1). The distance of his 6 minute walk test also improved by 40 meters (Table 1). Subjective measures of quality of life, including PROMIS Physical Function Mobility score and provider global assessment (PGA) score improved after initiation of MAS-825 therapy (Figures 2B–C).
Figure 2: Functional outcomes and patient- and physician-reported measures.
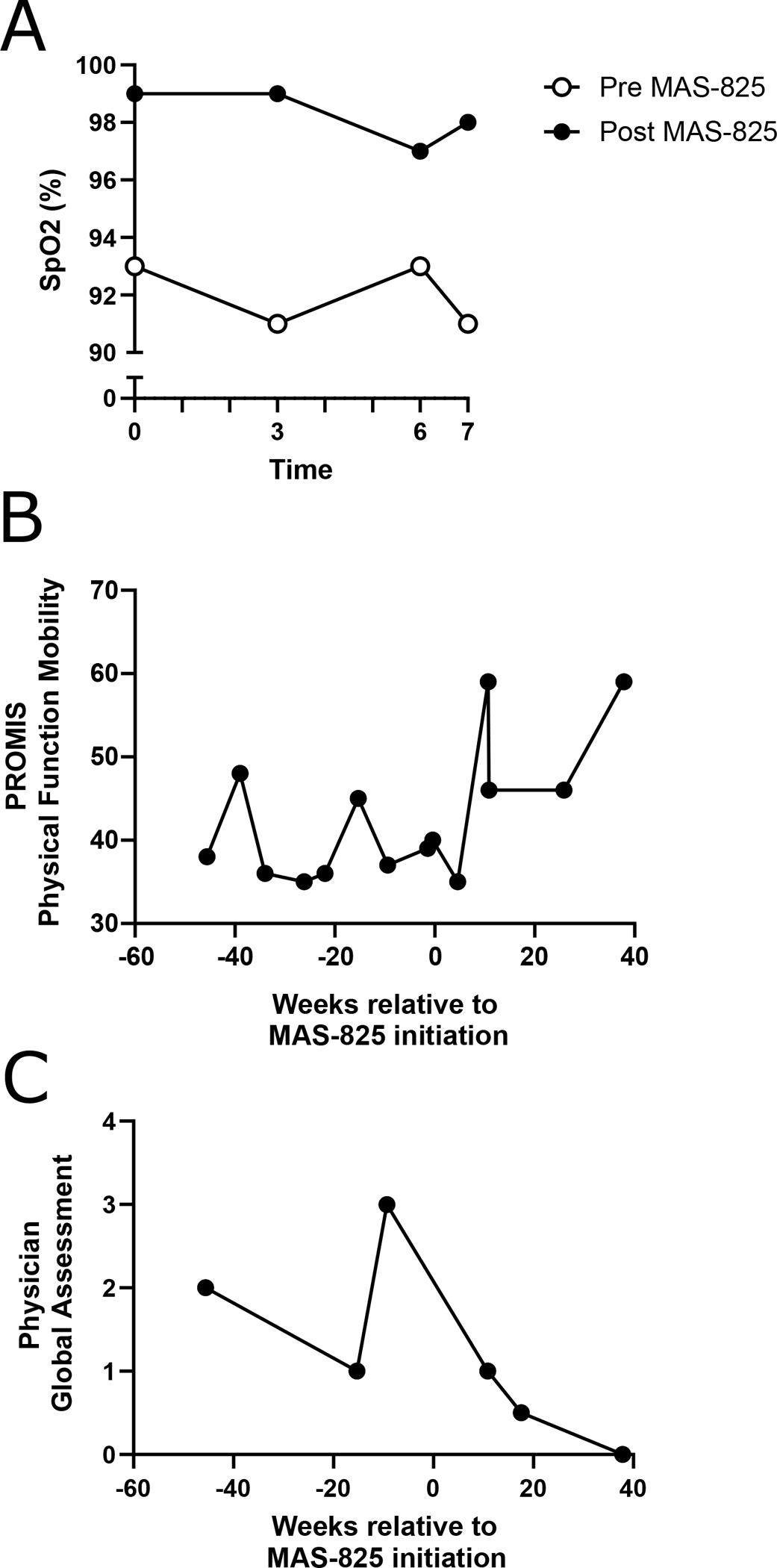
(A) Oxygen saturation (SpO2) during 6-minute walk test and 1 minute post-test, performed 3 days prior to and 7.5 weeks after initiation of MAS-825. (B) Patient-reported scores on the PROMIS Pediatric Short Form V1.0 Physical Function Mobility Questionnaire for all available timepoints 45 weeks prior to and 45 weeks after initiation of MAS-825, with higher scores indicating better mobility. (C) Physician global assessment as reported by the treating rheumatologist for all available timepoints 45 weeks prior to and 45 weeks after initiation of MAS-825, with lower scores indicating lower disease activity.
When first measured many years after disease onset, his total serum IL-18 was >75,000 pg/mL, a level typical of SJIA-LD (Figure 3A). His total IL-18 remained elevated over several years and was associated with detectable free IL-18. Upon treatment with MAS-825, his total serum IL-18 dropped to near normal, and free IL-18 became undetectable. This same pattern was observed in his BAL fluid pre- and post-treatment (Figure 3B). It is not known whether MAS-825 interferes with the ability to detect IL-18 by either assay. Levels of IL-18 binding protein (IL-18BP), the endogenous inhibitor of IL-18, did not appreciably change over the course of treatment (Figures 3A–B). Biomarkers of MAS, including ferritin, LDH, and CXCL9, showed a brief spike during influenza infection that improved prior to treatment, and they remained stable throughout MAS-825 therapy (Figure 3C). LDH and ICAM5 may be biomarkers of lung disease in SJIA-LD (2), and they behaved similarly throughout the course of treatment. Overall, these biomarkers identify excess IL-18 prior to treatment, with normalization of free IL-18 and no significant increase in serologic activity of MAS while under combined IL-1β/IL-18 neutralization.
Figure 3: Longitudinal assessment of serum and BAL biomarkers.
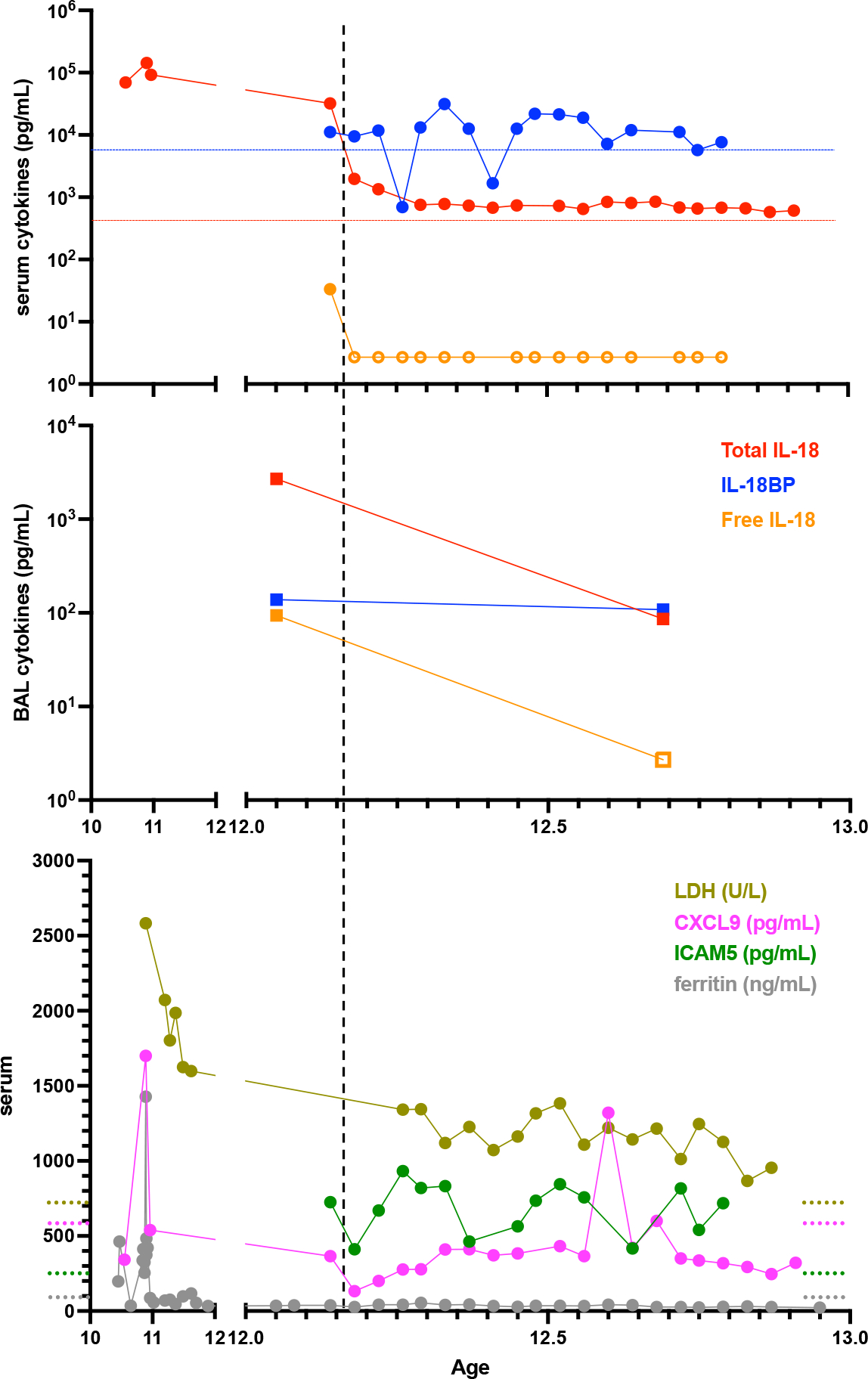
Patient serum (A) and BAL fluid (B) cytokines were assessed for total IL-18, IL-18BP, and Free IL-18. (C) Serum CXCL9, LDH, ICAM5, and ferritin. Dashed horizonal lines represent upper limits of normal except blue and green lines, which represent the highest values among six healthy control patients for IL-18BP and ICAM5, respectively (data not shown). Open shapes indicate below limit of detection. Vertical line indicates initiation of MAS-825.
BAL findings
BAL from prior to MAS-825 contained significant amounts of proteinaceous globules and foamy macrophages, consistent with the PAP associated with SJIA-LD (Figure 4). The BAL 6 months post therapy showed no such protein globules. The immediate pre-treatment BAL had 181,818 cells per mL of BAL. This was reduced to 30,000 cells per mL in the 6 month post-treatment BAL. Post-treatment changes in multiple immune cell populations were observed in BAL and matching whole blood samples, each measured at a single timepoint (Figure 5A–E, Tables S2–S3). Absolute numbers of T-cells decreased after 6 months of therapy in both blood and BAL. Although there was an increase in blood macrophage/monocyte populations, these cells dramatically decreased in the BAL (Figure 5A). Major CD4 T cell subsets, including Th1, Th17, Th17.1, and Treg cells, decreased in number in the blood post-therapy. This was also true in the BAL, with the notable exception of Th17.1 CD4 T-cells, which remained stable in number (Figure 5B). Correspondingly, the frequency of Th1, Th17, Treg, and other non-Treg memory CD4 T cells in the BAL decreased, while there was an increase in the percentage of Th17.1 cells (Figure 5C). Other non-Treg memory cells that were not phenotyped, likely including Th2 cells, also decreased in frequency after MAS-825. Naïve CD8 T-cells dropped in number in the blood, with a concomitant increase in effector memory and TEMRA cells. In the BAL, all CD8 T cell populations decreased in number (Figure 5D). In the blood, the increase in monocyte/macrophage numbers was mostly explained by an increase in classical CD14+CD16− monocytes. However, in the BAL both classical and non-classical inflammatory monocytes (CD14+CD16+) decreased after therapy (Figure 5E).There was a very small decrease in HLA-DR expression on both blood and BAL macrophages post-therapy, possibly consistent with a decrease in IFNγ activity (Figure 5F).
Figure 4: Photomicrograph of cytospin from BAL prior to MAS-825 therapy.
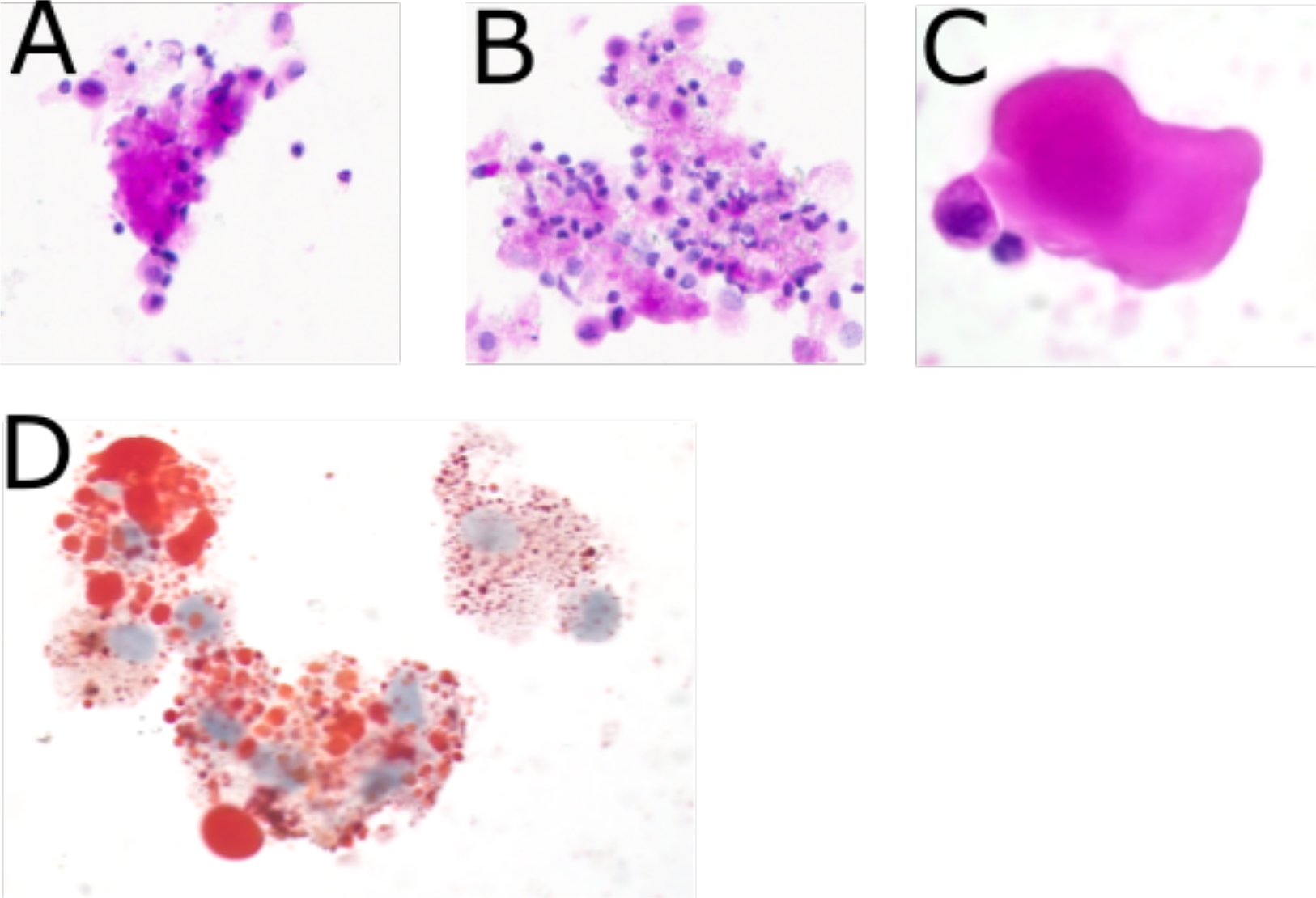
(A) Acellular protein globule consistent with PAP. (PAS stain, 40X) (B) Numerous foamy macrophages present within the fluid. (PAS stain 40X) (C) High power view of acellular protein globule. (H&E stain 100X) (D) Lipid laden foamy macrophages. (Oil Red O stain 100X)
Figure 5: Flow cytometric analysis of immune populations from blood and BAL before and after MAS-825 therapy.
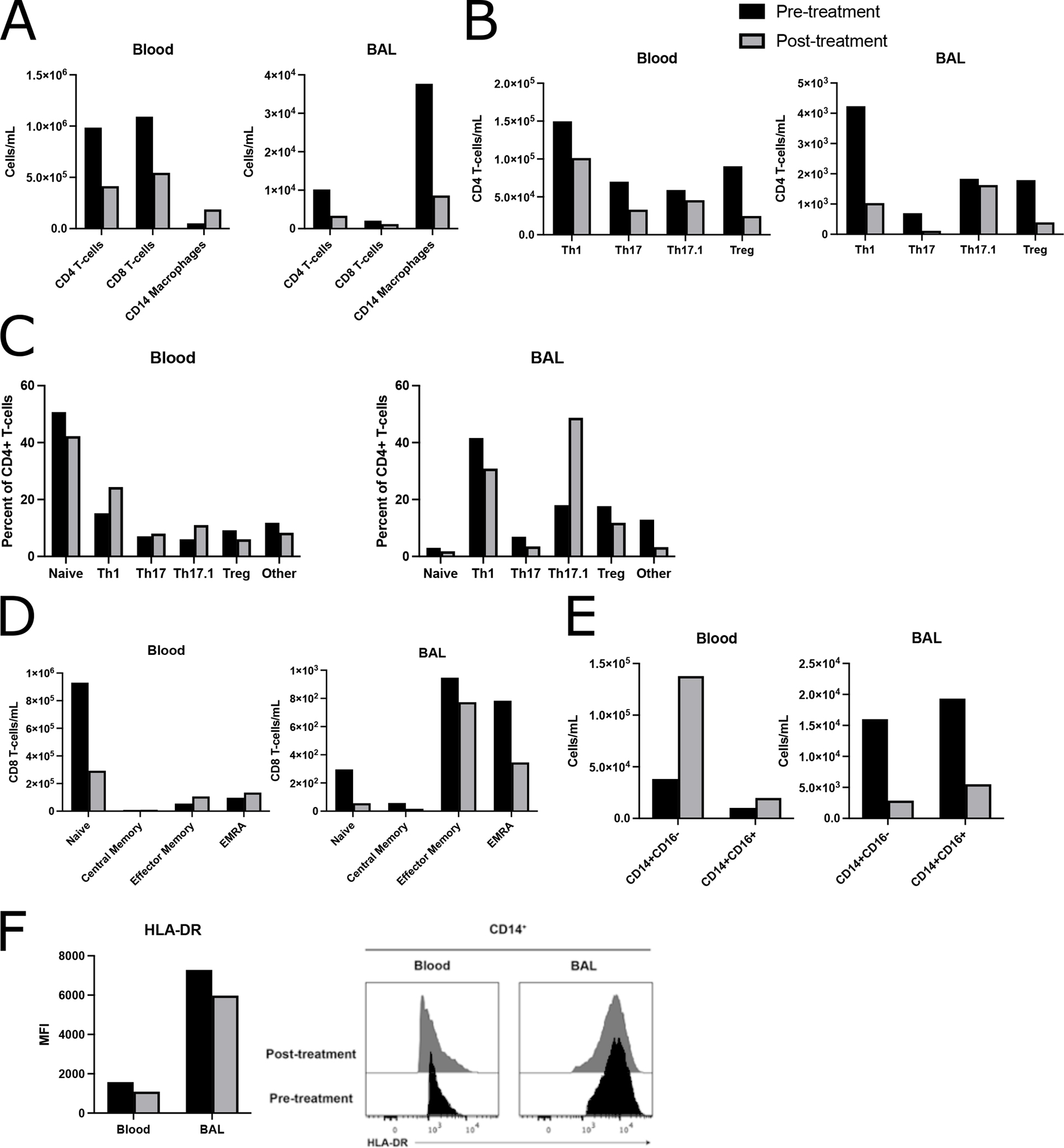
(A, B, D, E) Absolute counts and (C) frequencies of immune cell subsets in blood and BAL at a single timepoint pre- and post-MAS-825. (A) Major immune cell populations: CD8+ T-cells (CD45+CD3+CD8+CD4−), CD4+ T-cells (CD45+CD3+CD8−CD4+), CD14 macrophages (CD45+CD20−HLA−DR+CD14+) (B) Memory CD4+ T-cell (defined as all CD4+ T-cells not in the naïve CCR7+CD45RO− gate) populations: Th1 (CD25loCD127+CXCR3+CCR6−), Th17 (CD25loCD127+CXCR3−CCR6+), Th17.1 (CD25loCD127+CXCR3+CCR6+), and total Treg (CD25hiCD127lo). (C) Naïve, Th1, Th17, Th17.1, Treg, and other non-Treg memory CD4+ T cells not included among the preceding populations. (D) CD8+ T-cell populations: Naïve (CCR7+CD45RO−), Central Memory (CCR7+CD45RO+), Effector memory (CCR7−CD45RO+), EMRA (CCR7−CD45RO−). (E) CD14+ macrophage absolute counts dichotomized by CD16 positivity. (F) HLA-DR median florescence intensity and corresponding histograms showing HLA-DR expression on CD14+ macrophages.
Discussion
SJIA-LD remains a clinical challenge with limited therapeutic options. We report stabilization of clinical measures of lung fibrosis and damage, accompanied by reduction in lung inflammation and significant improvement in functional and pulmonary symptoms in an SJIA-LD patient treated with a novel IL-1β/IL-18 neutralizing bispecific antibody. Initiation of therapy allowed for discontinuation of glucocorticoids and other immune modulatory medications.
Extremely elevated levels of serum IL-18 have remarkable specificity for SJIA/MAS, may indicate MAS susceptibility, and are being explored as a therapeutic target in this population. In the patient reported herein, blockade of both IL-1β and IL-18 led to significant improvement in the symptoms of SJIA-LD, whereas IL-1 blockade alone had been insufficient to control disease. This suggests a central role for IL-18 in promoting inflammation in SJIA-LD. A number of laboratory and clinical parameters in our patient improved on therapy, but it should be noted that neither his lung CT nor LDH improved. LDH is a biomarker of lung disease, but also of MAS and cellular injury in general. The persistence of slightly elevated ICAM5, and its temporal association with LDH levels, suggest similar regulation. ICAM5 is expressed in lung interstitium, but it is unclear if its serum levels reflect tissue injury or compensatory wound healing responses(2).
The striking association of elevated IL-18 with SJIA-LD might suggest a possible causative link and therefore support the use of IL-18 blocking therapies. Notably, recombinant IL-18-binding protein (tadekinig-alfa), showed promise in an open-label Phase II study of patients with adult-onset Still’s disease (AOSD, the adult corollary to SJIA)(10). Several investigational IL-18 blocking drugs are currently in clinical trials for monogenic autoinflammatory diseases, AOSD, sarcoidosis, and multiple myeloma. However, further studies will be needed to firmly establish whether such drugs are effective in treating SJIA. This report reflects an important first step in this process.
There is considerable controversy in the field regarding the etiology of SJIA-LD, and our findings may offer insight into the various pathogenic mechanisms that have been proposed. Drug hypersensitivity reaction to several IL-1 or IL-6 biologic medication preparations (including those manufactured by the maker of MAS-825), has been proposed as a potential causative factor in SJIA-LD (8). The full list of excipients in MAS-825 is proprietary but overall is similar to other monoclonal antibodies (Novartis, Inc., personal communication). However, HLA-associated drug reactions typically occur via more complex mechanisms (e.g. hapten-mediated, pharmacological interaction, or altered peptide repertoire mechanisms) (11). Thus, symptom improvement via removal of an ongoing drug reaction remains formally possible.
Another hypothesis aimed at explaining the phenomenon of SJIA-LD, the “Cytokine Plasticity Hypothesis” suggests that IL-1 and/or IL-6 blockade causes HLA-DRB1*15-restricted T-cells to adopt a Th1 program (12). As such, one might expect effective treatment of SJIA-LD to demonstrate a decrease in BAL Th1 and Th17.1 cells, with concomitant increase in Treg or Th17 populations. In this patient, most inflammatory cells decreased in the BAL after 6 months of therapy with MAS-825. In addition to improvement in lung disease, we noted a decrease in BAL Th1 cells by frequency and number, consistent with the idea that these cells may contribute to pathogenesis. However, numbers of Th17.1 cells, the cell type which may most closely approximate active Th1/Th17 plasticity, did not change with therapy. Although the frequency of Th17.1 cells increased after MAS-825, the number of such cells did not appreciably change, despite decreases in both number and frequency of other activated T cell populations (e.g. Th1 and Th17). The change in Th17.1 frequency therefore reflects the loss of activated T cells of other lineages, rather than a true change in the numbers of these plastic cells themselves. Furthermore, under this model, normal lung Th17 cells are proposed to adopt alternate fates and therefore be lower in number in active SJIA-LD; conversely, these cells would be expected to increase with any return to homeostasis. However, numbers of Th17 cells actually decreased as the patient improved. Taken together, this patient’s decrease in Th1 cells fits this hypothesis and is also consistent with the known biologic effects of blocking IL-18, a Th1 promoting cytokine. However, the absence of an increase in Th17 and Treg populations (which were in fact decreased in our patient), and the stability of his Th17.1 population, may not be consistent with this hypothesis.
Neither drug hypersensitivity nor cytokine plasticity appear to have complete explanatory capacity based on the observations in this single patient experience, and both hypotheses currently await additional experimental and observational data. Notably, excess IL-18 in the lung features in both: promoting abnormal drug reactions in the former and supporting the inflammatory milieu that permits/propagates “cytokine plasticity” in the latter. In this single patient, combined IL-1β/IL-18 blockade represented a novel therapeutic approach to SJIA-LD and preliminarily appears safe and effective. Given the significant morbidity and mortality of this condition and the massive unmet need for effective therapies, we believe further trials of IL-18 targeted therapies are of urgent importance.
Supplementary Material
Acknowledgments
This study would not have been possible without Lindsay Waqar, Whitney Petrosa, the Children’s Hospital of Philadelphia infusion center staff, and most importantly the patient and his family.
Funding:
EMB is supported by The Children’s Hospital of Philadelphia Immune Dysregulation Frontiers Program. SWC is supported by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD098428).
Footnotes
Statements and Declarations:
Disclosure of Conflicts of Interest
EMB has received research support for an ongoing clinical trial from AB2Bio, Ltd. SWC has performed consulting for Simcha Therapeutics, Novartis, and SOBI. All other authors have no financial relationships relevant to this article to disclose.
Ethics Approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of The Children’s Hospital of Philadelphia.
Consent to Participate: Freely-given, written informed consent to participate in the study was obtained according the above-referenced protocol.
Consent to Publish: Consent to publish is included in the informed consent to participate in the above-referenced protocol.
Data Availability:
All data generated or analyzed during this study are included in this submission.
References
- 1.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34(5):1133–8. [PubMed] [Google Scholar]
- 2.Chen G, Deutsch GH, Schulert G, Zheng H, Jang S, Trapnell B, et al. Serum proteome analysis of systemic JIA and related lung disease identifies distinct inflammatory programs and biomarkers. Arthritis & rheumatology. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bracaglia C, de Graaf K, Pires Marafon D, Guilhot F, Ferlin W, Prencipe G, et al. Elevated circulating levels of interferon-gamma and interferon-gamma-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2017;76(1):166–72. [DOI] [PubMed] [Google Scholar]
- 4.Weiss ES, Girard-Guyonvarc’h C, Holzinger D, de Jesus AA, Tariq Z, Picarsic J, et al. Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood. 2018;131(13):1442–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saper VE, Chen G, Deutsch GH, Guillerman RP, Birgmeier J, Jagadeesh K, et al. Emergent high fatality lung disease in systemic juvenile arthritis. Ann Rheum Dis. 2019;78(12):1722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulert GS, Yasin S, Carey B, Chalk C, Do T, Schapiro AH, et al. Systemic Juvenile Idiopathic Arthritis-Associated Lung Disease: Characterization and Risk Factors. Arthritis & rheumatology. 2019;71(11):1943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimura Y, Weiss JE, Haroldson KL, Lee T, Punaro M, Oliveira S, et al. Pulmonary hypertension and other potentially fatal pulmonary complications in systemic juvenile idiopathic arthritis. Arthritis care & research. 2013;65(5):745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saper VE, Ombrello MJ, Tremoulet AH, Montero-Martin G, Prahalad S, Canna S, et al. Severe delayed hypersensitivity reactions to IL-1 and IL-6 inhibitors link to common HLA-DRB1*15 alleles. Ann Rheum Dis. 2022;81(3):406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard C, Rech J, Brown M, Allali D, Roux-Lombard P, Spertini F, et al. Elevated serum levels of free interleukin-18 in adult-onset Still’s disease. Rheumatology (Oxford). 2016;55(12):2237–47. [DOI] [PubMed] [Google Scholar]
- 10.Gabay C, Fautrel B, Rech J, Spertini F, Feist E, Kotter I, et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still’s disease. Ann Rheum Dis. 2018;77(6):840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hama N, Abe R, Gibson A, Phillips EJ. Drug-Induced Hypersensitivity Syndrome (DIHS)/Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): Clinical Features and Pathogenesis. J Allergy Clin Immunol Pract. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binstadt BA, Nigrovic PA. The Conundrum of Lung Disease and Drug Hypersensitivity-like Reactions in Systemic Juvenile Idiopathic Arthritis. Arthritis & rheumatology. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this submission.


