Abstract
Context:
Children with severe neurological impairment and polypharmacy are exposed to anticholinergic (AC) medications that may have anticholinergic side effects, but this is understudied. Anticholinergic Cognitive Burden (ACB) scores measure total anticholinergic burden for a medication regimen, and scores ≥3 have been associated with increased morbidity and mortality in adults.
Objective:
We assessed the relationship between ACB scores and parent-reported anticholinergic symptoms in children.
Methods:
Cross-sectional study of patients 1–18 years-old with ICD-defined severe neurological impairment and polypharmacy. At routine clinical visits, total ACB scores were computed for all medications. Parent-reported AC symptoms (constipation, drowsiness, difficulty concentrating, dry mouth, or urinary problems) were assessed. Multivariable logistic regression was used to test the association between total ACB scores ≥3 for scheduled medications and the presence of AC symptoms, adjusted for age and recent acute healthcare utilization.
Results:
Among 123 unique patients, 87% were prescribed AC medications. Common AC medication classes included: systemic antihistamines (64%), anxiolytics (53%), antidepressants (30%), H2 blockers (22%), and muscle relaxants (20%). Total ACB scores ≥3 were observed in 44% for scheduled medications and in 63% of patients for scheduled plus PRN medications. Total ACB scores ≥3 were significantly associated with an increased odds of ≥1 anticholinergic symptoms for scheduled medications (OR: 3.1; 95% CI: 1.4, 6.7) and for scheduled plus PRN medications (OR: 2.9; 95% CI: 1.3, 6.4).
Conclusion:
If replicated in larger populations, the association between elevated total ACB scores and anticholinergic side effects in children should prompt clinicians to consider deprescribing potentially unneeded anticholinergic medications.
Keywords: Children with severe neurological impairment, Polypharmacy, Medication safety, Anticholinergic burden, Symptom burden, Deprescribing
INTRODUCTION
Many children with medical complexity have severe neurological impairment (SNI), such as intractable epilepsy or degenerative neurologic disease, and rely on multiple chronic medications to sustain their lives and treat substantial symptom burdens.1–7 As part of their complex medication regiments, children with SNI and polypharmacy are exposed to anticholinergic (AC) medications that may have additive adverse anticholinergic side effects (e.g., constipation). However, it is unknown whether children with SNI who take multiple anticholinergic medications are at higher risk for developing these bothersome side effects. If pediatric clinicians had a simple clinical tool to assess this relationship among children with polypharmacy, potentially avoidable anticholinergic symptoms could be recognized and addressed earlier, ultimately leading to improved patient outcomes and reduced healthcare utilization.2,3
Notably, key clinical and research tools necessary to study this relationship exist. Multiple adult anticholinergic burden scales are available that can quantify anticholinergic burden for clinical and research purposes. Like adults, children express the same cholinergic receptors that are susceptible to anticholinergic medications; anticholinergic burden scales could thus be extended for use in pediatrics.8,9 The widely used, validated, and freely available Anticholinergic Cognitive Burden (ACB) score measures additive total anticholinergic burden for a medication regimen using a simple online calculator (www.acbcalc.com).10 Among adult and geriatric patients with polypharmacy, scores ≥3 have been associated with increased morbidity and mortality.10 Furthermore, for children with SNI who cannot self-report symptoms, parent-reported symptom burdens can be measured using the comprehensive Parent-Reported Outcomes of Symptoms (PRO-Sx) symptom inventory.4 The PRO-Sx inventory includes 5 anticholinergic symptoms, including constipation, drowsiness, concentration issues, dry mouth, and urinary problems.4
Using these tools in a population of children with SNI and polypharmacy, we assessed the relationship between a child’s anticholinergic burden, as measured by ACB scores, and parent-reported anticholinergic symptoms, as measured using the PRO-Sx inventory. We hypothesized that children with scores at or above the published ACB cutoff of 3 would be more likely to experience anticholinergic symptoms, when compared to those below the cutoff.
METHODS
Study Design, Data Source, and Study Population
This was a cross-sectional analysis of children with SNI and polypharmacy at the time of a non-acute routine primary care visit, as described in prior publications.3,4 Between 4/1/2019 and 12/31/2020, we obtained parental written consent, enrolled, and assessed English- and Spanish-speaking patients between the ages of 1–18 years with SNI and polypharmacy (≥5 medications) who received primary care in a large, hospital-based special healthcare needs clinic. Patients with SNI were defined as having neurological diagnoses expected to last ≥12 months and resulting in systemic or multi-system physiologic impairment requiring pediatric subspecialty care; they were identified using published classification systems based on International Classification of Disease 10th Revision Clinical Modification diagnosis codes.11–13 We analyzed patients’ parent-reviewed medication information captured in the electronic health record, as well as parent-reported symptoms using the PRO-Sx inventory (available in English and Spanish). We followed the STROBE checklist when writing this report.14 The parent study was approved by the Colorado Multiple Institutional Review Board and registered with ClinicalTrials.gov (NCT03849066).3,4
Primary Exposure Variable: ACB Scores
ACB scores were computed using the publicly available ACB calculator.10 We used the previously published adult ACB score threshold of 3 as the primary exposure variable.10 To best represent current medication use, we calculated ACB scores based on parent-reviewed pre-visit medication lists, before any new medications or changes were made. Prescribing information was available for all prescription and over-the-counter medications, including both scheduled and “as needed” pro re nata (PRN) medications. To represent consistent daily medication use, we first calculated ACB scores based on scheduled medications only. We then calculated ACB scores for both scheduled and PRN medications to represent maximal possible exposure to anticholinergic medications.
Primary Outcome Variable: AC Symptoms
The main outcome variable was the presence of any of the 5 parent-reported anticholinergic symptoms contained in the PRO-Sx inventory.4 Parents were asked to report whether their child experienced each symptom in the past 7 days.4
Statistical Analysis
Descriptive statistics were used to characterize the study population, including patients’ prescribed medications and parent-reported symptoms. Bivariate associations between patient characteristics and parent-reported AC symptoms were assessed using chi square and t-tests. Multivariable logistic regression was used to test the hypothesis that ACB scores ≥3 were associated with the presence of any AC symptom(s), adjusting a priori for variables known to be associated with increased symptom burdens (age and acute healthcare utilization in the prior 30-days).15 Analyses were conducted using Stata 17.0, and significance was set at a 2-tailed P value <0.05.
RESULTS
We enrolled and analyzed the data from a total of 123 unique patients with SNI and polypharmacy (Table 1). The mean patient age was 9.1 years (standard deviation: +/− 4.8) and 59% were male. Patients were prescribed a median of 14 medications (interquartile range: 10–18) and had a median of 1 acute visit (IQR: 1–2), such as an emergency department visit, in the prior 30 days.
Table 1.
Demographics and clinical characteristics of the study population.
| Demographics and Clinical Characteristics | % of Patients (N=123) |
|---|---|
| Patient Age at Visit (years) | |
| 1–4 | 20.3 |
| 5–8 | 27.6 |
| 9–12 | 28.5 |
| 13–17 | 23.6 |
| Patient Sex | |
| Male | 59.3 |
| Female | 40.7 |
| Acute Visits in Past 30 Days | |
| 1 | 70.7 |
| 2 | 18.7 |
| 3+ | 10.6 |
| Pre-Visit Medication Count (Parent-Reviewed) | |
| 5–9 | 20.3 |
| 10–14 | 35.8 |
| 15+ | 43.9 |
| Anticholinergic (AC) Medication Exposure | |
| Any AC Medication | 86.9 |
| ≥2 AC Medications | 64.2 |
| Positive ACB Score (≥3) | |
| For Scheduled Medications Only | 43.9 |
| For Scheduled and PRN Medications | 63.4 |
| Parent-Reported Anticholinergic Symptoms | |
| Any AC symptom | 58.5 |
AC medications were prescribed to 87% of patients and multiple AC medications were prescribed to 64% of our sample. Patients used a median of 2 AC medications (IQR: 1–4; range: 0–8), comprised of 1 scheduled AC medication (IQR: 0–3; range: 0–6) and 1 as needed medication (IQR: 0–2; range: 0–6). Common AC medication classes included: systemic antihistamines (64%), anxiolytics (53%), antidepressants (30%), H2 blockers (22%), and muscle relaxants (20%). Cyproheptadine was the most common medication with high anticholinergic risk, cetirizine was the most common with medium anticholinergic risk, and diazepam was the most common medication with low anticholinergic risk. Table 2 lists the top 5 most common specific AC medications by their individual ACB scores. Total ACB scores ≥3 were observed in 44% of patients for scheduled medications only (mean ACB score: 4.9 +/− 1.7) and in 63% of patients for scheduled plus PRN medications (mean ACB score: 6.3 +/− 3.0).
Table 2.
Top 5 specific anticholinergic (AC) medications by component Anticholinergic Cognitive Burden (ACB) scores.
| Component ACB Score | Generic Name | % of Patients (N=123) |
|---|---|---|
| High (3) | ||
| Cyproheptadine | 11.4 | |
| Diphenhydramine | 10.6 | |
| Hydroxyzine | 8.1 | |
| Amitriptyline | 4.1 | |
| Scopolamine | 4.1 | |
| Medium (2) | ||
| Cetirizine | 32.5 | |
| Baclofen | 16.3 | |
| Loratadine | 5.7 | |
| Sertraline | 4.1 | |
| Oxcarbazepine | 4.1 | |
| Low (1) | ||
| Diazepam | 44.7 | |
| Ranitidine | 22.0 | |
| Hydrocortisone | 21.1 | |
| Trazodone | 12.2 | |
| Prednisolone | 5.7 | |
Anticholinergic symptoms were reported in 59% of patients. The presence of anticholinergic symptoms was not significantly associated with mean patient age (p=0.08), number of medications (p=0.88), or number of acute visits in the prior 30 days (p=0.79) but was significantly associated with an ACB score ≥3 for both the scheduled (p=0.002) and scheduled plus PRN groups (p=0.002). As displayed in Figure 1A & 1B, after adjusting for age and acute healthcare visits, total ACB scores ≥3 were significantly associated with an increased odds of ≥1 anticholinergic symptoms for scheduled medications only (odds ratio: 3.1; 95% confidence interval: 1.4, 6.7) and for scheduled plus PRN medications (OR: 2.9; 95% CI: 1.3, 6.4). Similar trends were noted for individual AC symptoms (Figure 1A & 1B).
Figure 1.
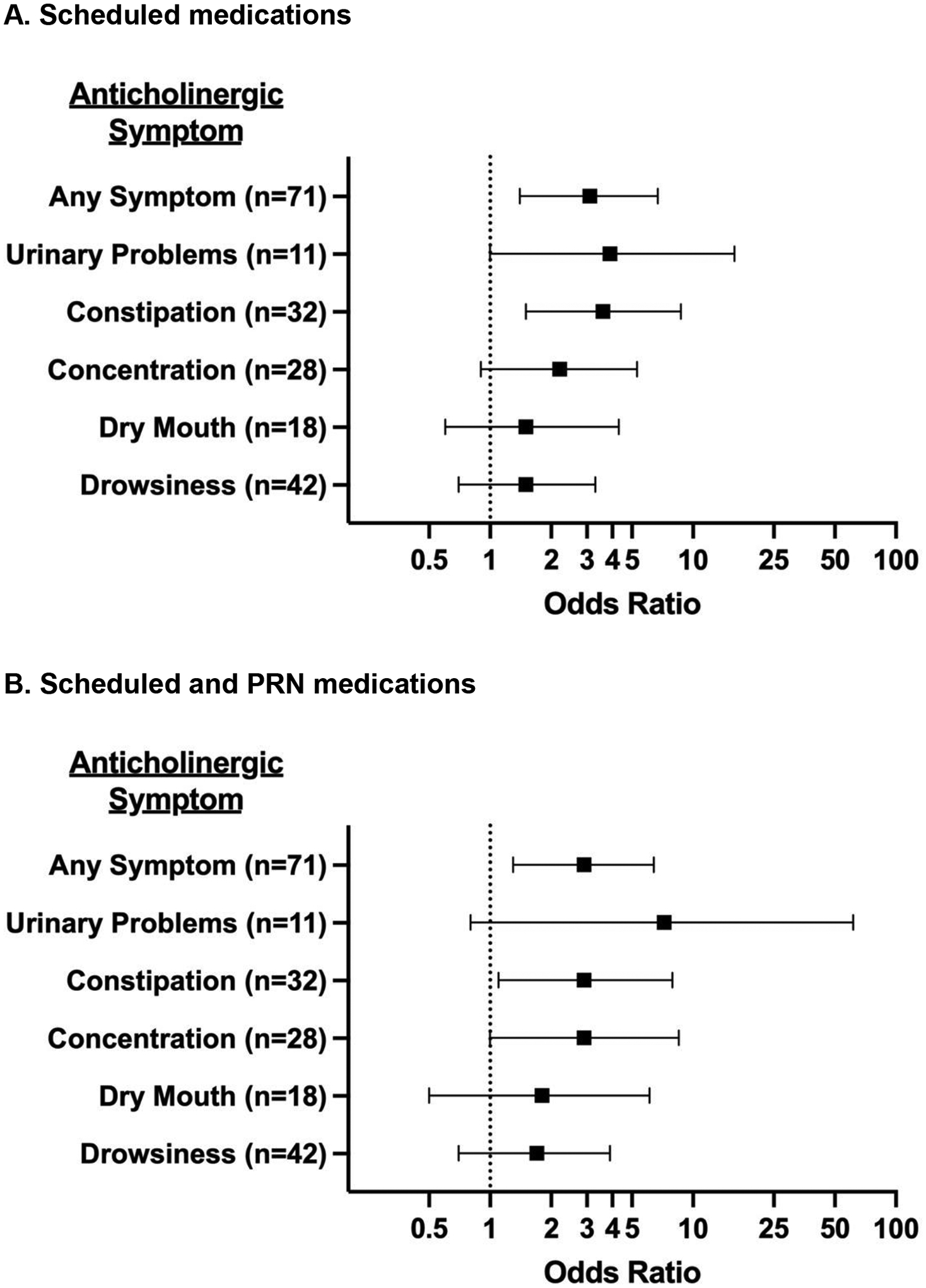
Association between total Anticholinergic Cognitive Burden (ACB) score ≥3 and parent-reported anticholinergic (AC) symptoms, adjusted for age and recent acute healthcare utilization.
Panel A displays the odds ratios and 95% confidence intervals for the associations between positive ACB scores and parent-reported AC symptoms for scheduled medications only. Panel B displays the odds ratios and 95% confidence intervals for the associations between positive ACB scores and parent-reported AC symptoms for scheduled plus PRN medications.
DISCUSSION
In our study, 87% of patients were prescribed AC medications and 64% were prescribed multiple AC medications. The most common AC medication classes included systemic antihistamines, anxiolytics, antidepressants, H2 blockers, and muscle relaxants. Forty-four percent of all children had positive ACB scores ≥3. Positive ACB scores were associated with a 3-fold increase in parent-reported anticholinergic symptoms, even after controlling for age and recent acute healthcare utilization. Two aspects of these results warrant further discussion.
First, the strong association between AC medications and parent-reported AC symptoms highlights the potential need to routinely assess both 1) a child’s anticholinergic burden and 2) any unwanted or concerning anticholinergic symptoms. Currently, no standardized system exists to streamline clinicians’ assessment of pediatric anticholinergic burden. In this study, ACB scores were simple to calculate using readily available basic medication data, PRO-Sx symptom data were easily collected from parents, and summary data could help flag patients warranting further review. During already busy complex care visits, a simple scoring tool may increase the actionable information available to clinicians and improve the identification of potentially avoidable AC medication side effects.
Second, in partnership with families, opportunities may exist to thoughtfully deprescribe an unneeded anticholinergic medication.16 Not all AC medications can or should be discontinued, for example, important and commonly prescribed tone or antiepileptic medications like diazepam. Our study identified, however, a variety of common AC medications that may be more amenable to a trial of weaning or discontinuation, for example, a legacy cyproheptadine, cetirizine, or ranitidine prescription that may no longer be indicated. Although the solitary use of these medications does not carry a substantial risk of anticholinergic side effects, their additive effects might. Highlighting those medications that commonly contribute to a patient’s total anticholinergic burden may enhance clinicians’ ability to identify potential medications for deprescribing.
Our study must be considered in the context of several limitations. First, the ACB threshold of 3 is based on standard adult medication dosing, but the risk threshold may differ for by a child’s age or weight.8 Subsequent studies should examine the linear relationship between ACB scores and parent-reported symptoms. Additionally, other adult scoring systems, like the Drug Burden Index, account for the total anticholinergic dose relative to the minimum effective daily dose, but calculations are more complex because the minimum daily effective pediatric dose of a medication can vary by age and/or weight and the required clinical data elements (e.g. dose, accurate patient weight, etc.) may not be readily available.8 Second, PRN medications present a challenge when included in the ACB score calculation, because we cannot determine how frequently they are administered without detailed home administration data. Third, we measured parent-reported AC symptoms, but we did not assess whether these symptoms impacted subsequent patient quality of life, clinical outcomes, or healthcare utilization; these will be important outcomes to quantify in future work. Finally, the small sample size and the inclusion of only English and Spanish speaking participants may limit the generalizability of this single-center study.
These limitations notwithstanding, the observed association between elevated total ACB scores and anticholinergic side effects, if replicated in larger populations and linked to adverse outcomes, should prompt clinicians to periodically monitor total anticholinergic burdens and to consider deprescribing potentially unneeded anticholinergic medications.
KEY MESSAGE.
Among 123 children with severe neurological impairment, we used the Anticholinergic Cognitive Burden (ACB) score to measure total anticholinergic burden. Many children had positive ACB scores ≥3, which were associated with a 3-fold increase in parent-reported anticholinergic symptoms. This association should prompt clinicians to consider deprescribing potentially unneeded anticholinergic medications.
Funding/Support:
Dr. Feinstein was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD091295.
Role of the Funder/Sponsor:
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions:
We acknowledge the clinical staff in the Children’s Hospital Colorado Special Care Clinic, who facilitated the conduct of this study.
Abbreviations:
- AC
anticholinergic
- ACB
Anticholinergic Cognitive Burden
- PRN
pro re nata (as needed)
- PRO-Sx
Parent-Reported Outcomes of Symptoms
- SNI
severe neurological impairment
Footnotes
Prior Presentations: Presented as a platform presentation at Pediatric Academic Societies 2022 in Denver, CO
Conflict of Interest Disclosures: There are no conflicts of interest relevant to this article to disclose.
Disclaimer: The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or the U.S. government.
Data Access, Responsibility, and Analysis: Dr. Feinstein had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Originality of Content: All presented information and materials are original.
Data Sharing Statement:
All data collected as part of this study will be released in accordance with standard NIH data sharing policies and procedures after completion of the larger parent study described on ClinicalTrials.gov (NCT03849066).
REFERENCES
- 1.Feinstein JA, Hall M, Antoon JW, Thomson J, Flores JC, Goodman DM, Cohen E, Azuine R, Agrawal R, Houtrow AJ, DeCourcey DD, Kuo DZ, Coller R, Gaur DS, Berry JG. Chronic Medication Use in Children Insured by Medicaid: A Multistate Retrospective Cohort Study. Pediatrics. 2019;143(4). Epub 2019/03/28. doi: 10.1542/peds.2018-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feinstein JA, Friedman H, Orth LE, Feudtner C, Kempe A, Samay S, Blackmer AB. Complexity of Medication Regimens for Children With Neurological Impairment. JAMA Netw Open. 2021;4(8):e2122818. Epub 20210802. doi: 10.1001/jamanetworkopen.2021.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feinstein JA, Feudtner C, Blackmer AB, Valuck RJ, Fairclough DL, Holstein J, Gregoire L, Samay S, Kempe A. Parent-Reported Symptoms and Medications Used Among Children With Severe Neurological Impairment. JAMA Netw Open. 2020;3(12):e2029082. Epub 20201201. doi: 10.1001/jamanetworkopen.2020.29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feinstein JA, Feudtner C, Valuck RJ, Fairclough DL, Holstein JA, Samay S, Kempe A. Identifying Important Clinical Symptoms in Children With Severe Neurological Impairment Using Parent-Reported Outcomes of Symptoms. JAMA Pediatr. 2020;174(11):1114–7. Epub 2020/10/13. doi: 10.1001/jamapediatrics.2020.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackmer AB, Fox D, Arendt D, Phillips K, Feinstein JA. Perceived Versus Demonstrated Understanding of the Complex Medications of Medically Complex Children. J Pediatr Pharmacol Ther. 2021;26(1):62–72. Epub 20210104. doi: 10.5863/1551-6776-26.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinstein J, Dai D, Zhong W, Freedman J, Feudtner C. Potential drug-drug interactions in infant, child, and adolescent patients in children’s hospitals. Pediatrics. 2015;135(1):e99–108. Epub 20141215. doi: 10.1542/peds.2014-2015. [DOI] [PubMed] [Google Scholar]
- 7.Feinstein JA, Feudtner C, Valuck RJ, Kempe A. The depth, duration, and degree of outpatient pediatric polypharmacy in Colorado fee-for-service Medicaid patients. Pharmacoepidemiol Drug Saf. 2015;24(10):1049–57. Epub 20150807. doi: 10.1002/pds.3843. [DOI] [PubMed] [Google Scholar]
- 8.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. Epub 20150325. doi: 10.1186/s12877-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutelstetter M, Livolsi A, Greney H, Helms P, Schmidt-Mutter C, De Melo C, Roul G, Zores F, Bolle A, Dali-Youcef N, Beaugey M, Simon A, Niederhoffer N, Regnard J, Bouhaddi M, Adamopoulos C, Schaeffer M, Sauleau E, Bousquet P. Increased expression of blood muscarinic receptors in patients with reflex syncope. PLoS One. 2019;14(7):e0219598. Epub 20190718. doi: 10.1371/journal.pone.0219598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, Harris TB, Hanlon JT, Rubin SM, Shorr RI, Bauer DC, Abernethy DR. A drug burden index to define the functional burden of medications in older people. Archives of internal medicine. 2007;167(8):781–7. Epub 2007/04/25. doi: 10.1001/archinte.167.8.781. [DOI] [PubMed] [Google Scholar]
- 11.Feinstein JA, Russell S, DeWitt PE, Feudtner C, Dai D, Bennett TD. R Package for Pediatric Complex Chronic Condition Classification. JAMA Pediatr. 2018;172(6):596–8. Epub 2018/05/02. doi: 10.1001/jamapediatrics.2018.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson JE, Feinstein JA, Hall M, Gay JC, Butts B, Berry JG. Identification of Children With High-Intensity Neurological Impairment. JAMA Pediatr. 2019;173(10):989–91. Epub 2019/08/20. doi: 10.1001/jamapediatrics.2019.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson J, Hall M, Nelson K, Flores JC, Garrity B, DeCourcey DD, Agrawal R, Goodman DM, Feinstein JA, Coller RJ, Cohen E, Kuo DZ, Antoon JW, Houtrow AJ, Bastianelli L, Berry JG. Timing of Co-occurring Chronic Conditions in Children With Neurologic Impairment. Pediatrics. 2021;147(2). Epub 20210107. doi: 10.1542/peds.2020-009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. Epub 2007/12/08. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Roberts PA, Dhaliwal SS, Della PR. Risk factors associated with paediatric unplanned hospital readmissions: a systematic review. BMJ Open. 2019;9(1):e020554. Epub 2019/01/31. doi: 10.1136/bmjopen-2017-020554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogler O, Roth D, Feinstein J, Strzelecki M, Seto W, Cohen E. Choosing medications wisely: Is it time to address paediatric polypharmacy? Paediatr Child Health. 2019;24(5):303–5. Epub 20190105. doi: 10.1093/pch/pxy188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data collected as part of this study will be released in accordance with standard NIH data sharing policies and procedures after completion of the larger parent study described on ClinicalTrials.gov (NCT03849066).


