Abstract
Background and Purpose:
The circuitry underlying heterogenous cognitive profiles in Parkinson’s disease (PD) remains unclear. The purpose of this study is to investigate whether structural changes in frontostriatal and limbic pathways contribute to different cognitive trajectories in PD.
Methods:
We obtained clinical and multimodal MRI data from 120 control and 122 PD subjects without dementia or severe motor disability. T1/T2-weighted images estimated volume, and diffusion imaging evaluated fractional anisotropy (FA) of frontostriatal (striatum and frontostriatal white matter [FSWM]) and limbic (hippocampus and fornix) structures. Montreal Cognitive Assessment (MoCA) gauged total and domain-specific (attention/executive and memory) cognitive function. Linear mixed-effects models were used to compare MRI and cognitive progression over 4.5 years between controls and PD and evaluate associations between baseline MRI and cognitive changes in PD.
Results:
At baseline, control and PD groups were comparable, except PD participants had smaller striatal volume (p < 0.001). Longitudinally, PD showed faster decline in hippocampal volume, FSWM FA, and fornix FA (ps < .016), but not striatal volume (p = .218). Total and domain-specific MoCA scores declined faster in PD (ps < .030). In PD, lower baseline hippocampal volume (p = .005) and fornix FA (p = .032), but not striatal volume (p = .662) or FSWM FA (p = .143), were associated with faster total MoCA decline. Baseline frontostriatal metrics of striatal volume and FSWM FA were associated with faster attention/executive decline (p < .038), whereas lower baseline hippocampal volume was associated with faster memory decline (p = .005).
Conclusion:
In PD, frontostriatal structural metrics are associated with attention/executive tasks, whereas limbic changes correlated with faster global cognitive decline, particularly in memory tasks.
Keywords: cognition, fornix, hippocampus, longitudinal, MRI, Parkinson’s disease
INTRODUCTION
Although Parkinson’s disease (PD) is defined clinically by motor dysfunction, up to 80% of patients develop dementia.1 Cognitive dysfunction in PD is associated with lower quality of life,2 increased caregiver burden,3 and higher medical costs.4 The rate of cognitive decline, however, is variable and the mechanisms underlying this heterogeneity are poorly understood. Since nigrostriatal dopaminergic loss is a key pathology in PD,5 prior studies of PD-related cognitive decline have focused mainly on structures and functions related to frontostriatal circuitry.6,7 The results from this line of investigation suggest that attention and executive impairments represent predominantly the early cognitive profile in PD.8,9
Recent literature, however, indicates that a subset of PD patients who experience rapid progression to dementia display a profile of memory-based dysfunction and limbic (eg, hippocampal) changes.10–12 Longitudinal studies of both frontostriatal and limbic changes related to PD cognitive trajectory remain limited. Also, despite identification of widespread gray and white matter alterations in PD dementia,13 it remains unknown how the frontostriatal and limbic structures are involved in PD prior to severe cognitive impairment.
Structural MRI has improved in vivo investigation of neurodegenerative processes due to its high spatial resolution, lack of radiation exposure, and wide availability. Volume of brain structures, calculated from T1- and T2-weighted MRI, may capture gray matter alterations such as cell loss and proteinopathy.14 Therefore, gray matter volume is becoming a promising biomarker in PD15 and other neurodegenerative diseases.16 Subcortical changes in structures such as the striatum (ie, the caudate and putamen) and hippocampus have been reported in PD.17,18 More recently, diffusion imaging, which measures the magnitude and direction of water molecule flow, has advanced microstructural investigation. Since diffusion in white matter is normally highly anisotropic (ie, not equal in all directions), loss of anisotropy (ie, lower fractional anisotropy [FA]) is a promising biomarker of demyelination and breakdown of axonal cystoskeleton.19–21 In PD, lower FA has been found in widespread networks, including frontal white matter22 and the fornix.23 Together, these imaging metrics allow us to investigate the contribution of specific brain structures and its critical white matter connections to cognitive profiles in PD.
This study aimed to delineate frontostriatal and limbic structures and their longitudinal MRI changes in PD and its relationship with cognitive decline. We hypothesized that in PD, frontostriatal deterioration would relate with changes in attention/executive function, whereas limbic deterioration would associate with faster global cognitive decline, particularly in memory tasks. We first compared imaging and cognitive metrics between PD and control participants at baseline and longitudinally. We then determined whether baseline imaging metrics of circuit structure were associated with global and domain-specific (ie, attention/executive vs. memory) cognitive changes. The findings from this investigation will untangle the heterogeneity of PD-related cognitive decline and identify whether structural differences at baseline within PD patients can predict cognitive decline.
METHODS
Standard protocol approvals, registrations, and participant consents
Study approval was received from the Penn State Hershey Institutional Review Board (IRB)/Human Subjects Protection Office. Written informed consent was obtained from all participants.
Participants
Penn State enrolled a total of 150 control and 177 PD participants over two National Institute of Health-funded studies since 2009.24,25 PD participants were recruited from a tertiary movement disorders clinic, and control participants from the spousal population or local community using IRB-approved materials. PD diagnosis was confirmed by movement disorders specialists according to previously published criteria.26 From this cohort, we identified 120 control and 122 PD participants who met the following inclusion criteria: MRI data available, mild/intermediate motor disability (Hoehn-Yahr [HY] stage ≤3), and no clear signs of dementia (Montreal Cognitive Assessment [MoCA] ≥21). These inclusion criteria helped ensure that a few severely impaired participants did not skew results. Figure 1 outlines the selection process.
FIGURE 1.
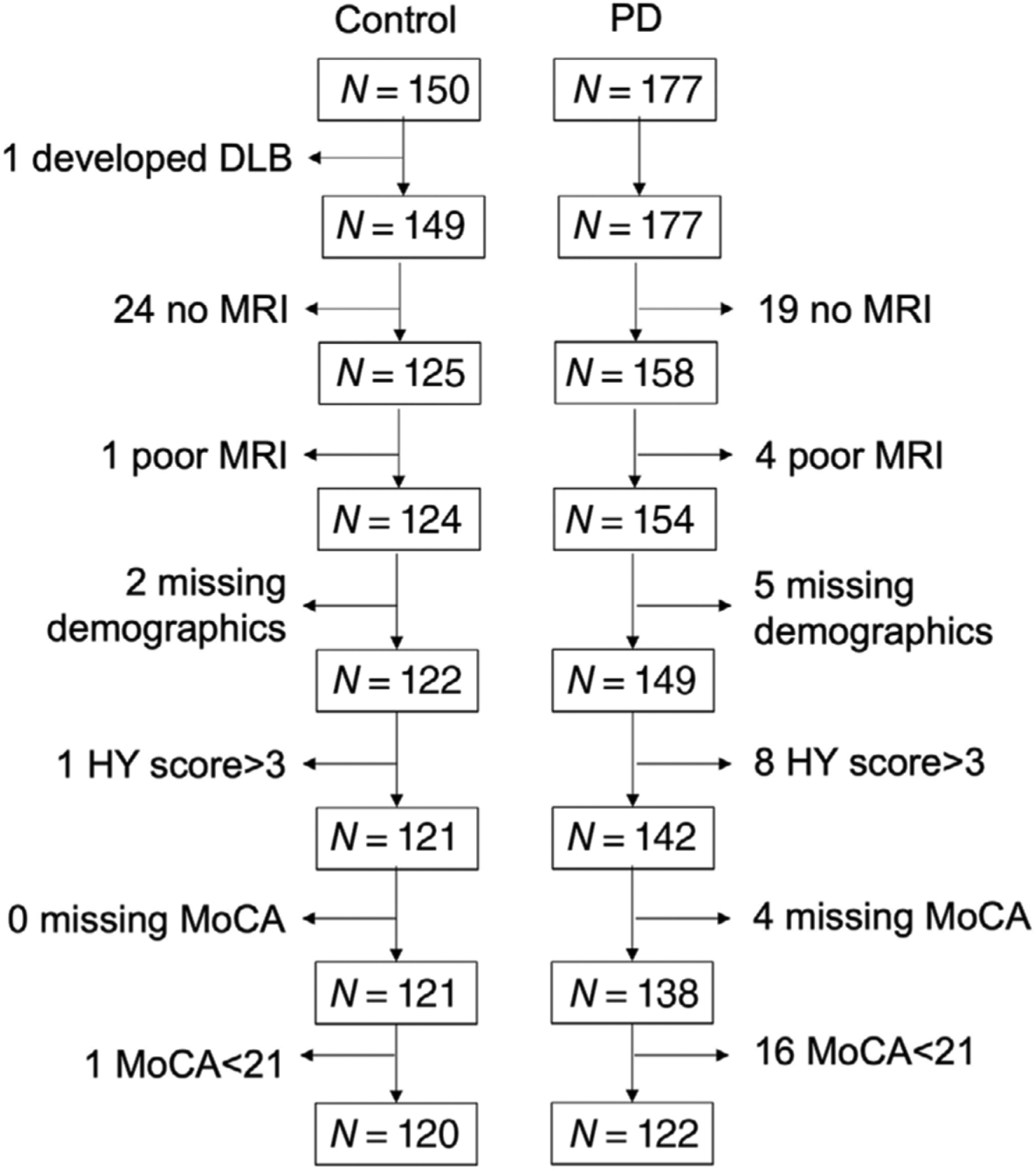
Flowchart of patient selection. Penn State enrolled 150 control and 177 Parkinson’s (PD) participants as part of two National Institutes of Health-funded MRI biomarker studies since 2009. Participants were then sequentially excluded based on (1) unavailable/unusable structural MRI data, (2) severe motor disability (Hoehn and Yahr [HY] score greater than 3 or missing), and (3) dementia (Montreal Cognitive Assessment [MoCA] score less than 21 or missing). A total of 120 control and 122 PD were then selected for the study. N, number of patients; DLB, Dementia with Lewy Bodies The study duration was defined as a maximum of three visits or 4.5 years.
Demographic and clinical measures
Age, sex, and education information were obtained for all participants at baseline. Clinical measures for PD participants included disease duration (date since first diagnosis by a physician) and disease severity defined by HY staging27 and the Movement Disorders Society Unified PD Rating Scale II and III scores. All clinical measures (along with cognitive metrics, see below) were obtained in the “on” medication state. Levodopa equivalent daily dosage (LEDD) was calculated according to published criteria.28
MRI acquisition
All subjects were scanned using a 3.0 Tesla MR scanner (Trio, Siemens Magnetom, Erlangen, Germany, with an eight-channel phased array head coil) at baseline. T1-weighted images were acquired using a magnetization-prepared rapid gradient echo sequence with the following parameters: repetition time (TR)/echo time (TE) = 1540/2.34 milliseconds, field of view = 256 × 256 mm, matrix = 256 × 256, slice thickness = 1 mm (no gap), and slice number = 176. T2-weighted images were acquired using a fast spin-echo sequence with TR/TE = 2500/316 milliseconds and the same spatial resolution parameters as the T1-weighted images. To obtain diffusion-weighted images, acquisition parameters were as follows: TR/TE = 8300/82 milliseconds, b-value = 1000 seconds/mm2, diffusion gradient directions = 42 and 7 b = 0 scans, field of view = 256 × 256 mm, matrix = 128 × 128, slice thickness = 2 mm (with no gap), slice number = 65. Imaging was acquired at each subject visit.
MRI image processing
Gray matter volume
Volume segmentation was performed using the “recon-all” command from the FreeSurfer image analysis suite (version 6.0.0),29 which is open-source and freely available. T1-weighted images were processed using the standard processing pipeline. The T2-weighted images were included using the “T2-” flag and registered to the T1-weighted images using a symmetric registration with a half-way space prior to segmentation. The extra contrast flag improves pial surface reconstruction. The image processing pipeline includes tissue classification, brain extraction, registration, and volumetric segmentation of gray matter structures. The technical details are explained in other manuscripts.30–32 FreeSurfer documentation also provides specific details on the segmentation procedures and parameters.
Striatal (defined by the sum of caudate and putamen volumes) and hippocampal volumes then were extracted from the left and right hemi-sphere and averaged. As a reference structure, thalamic volume also was obtained in a similar manner. To control for intersubject variability of brain structures, volumes were normalized using estimated total intracranial volume.33
White matter FA
White matter tracts were selected based on their association with the gray matter regions of interest (ie, striatum and hippocampus). It is well established that the striatum connects to the prefrontal cortex and this pathway contributes to PD-related executive function.6,7,34 Thus, these fibers were selected as representative of frontostriatal white matter (FSWM). Since the fornix has been associated with hippocampal volume during aging,35 this region (including both precommissural and postcommissural branches) was selected as representative of limbic white matter. Finally, the corticospinal tract (CST) is not expected to be altered in PD36 or aging.37 Thus, the CST was chosen as a control white matter tract.
Since there were no widely accepted FSWM connection templates available, whole brain tractography was used to determine this tract in a subject-specific manner. The following steps were used: first, tensor fitting and deterministic whole brain tractography was performed in a single step using a Slicer unscented Kalman filter tractography module38 with 5 seeds per voxel, two tensors, FA cutoff of 0.2, and an unscented Kalman filter to reduce noise while tracking. An FA cutoff of 0.2 is a conventional partial-voluming threshold to estimate white matter and exclude gray matter based on previous work.39 Second, a brain parcellation atlas that was optimized for structural connectivity40 was registered nonlinearly to each subject using Advanced Normalization Tools software (ANTs; http://stnava.github.io/ANTs). Lastly, fibers ending within 1 cm of the prefrontal cortex (ie, Brodmann areas 8, 9, 10, and 46) and striatum were selected, and a binary fiber map was generated.
For the fornix and CST, we used established templates.41,42 The fornix and CST templates were registered nonlinearly to subject space using ANTs. The three binary templates containing the bilateral tracts of interest (ie, FSWM, fornix, or CST) in subject space were applied to the diffusion metric maps to calculate the mean FA in each region. A binarized mask using a 0.2 threshold on the FA map was used to ensure the measurements were from white matter, which may have included lesions since these were not segmented and removed. Figure 2 shows a representation of the delineated regions.
FIGURE 2.
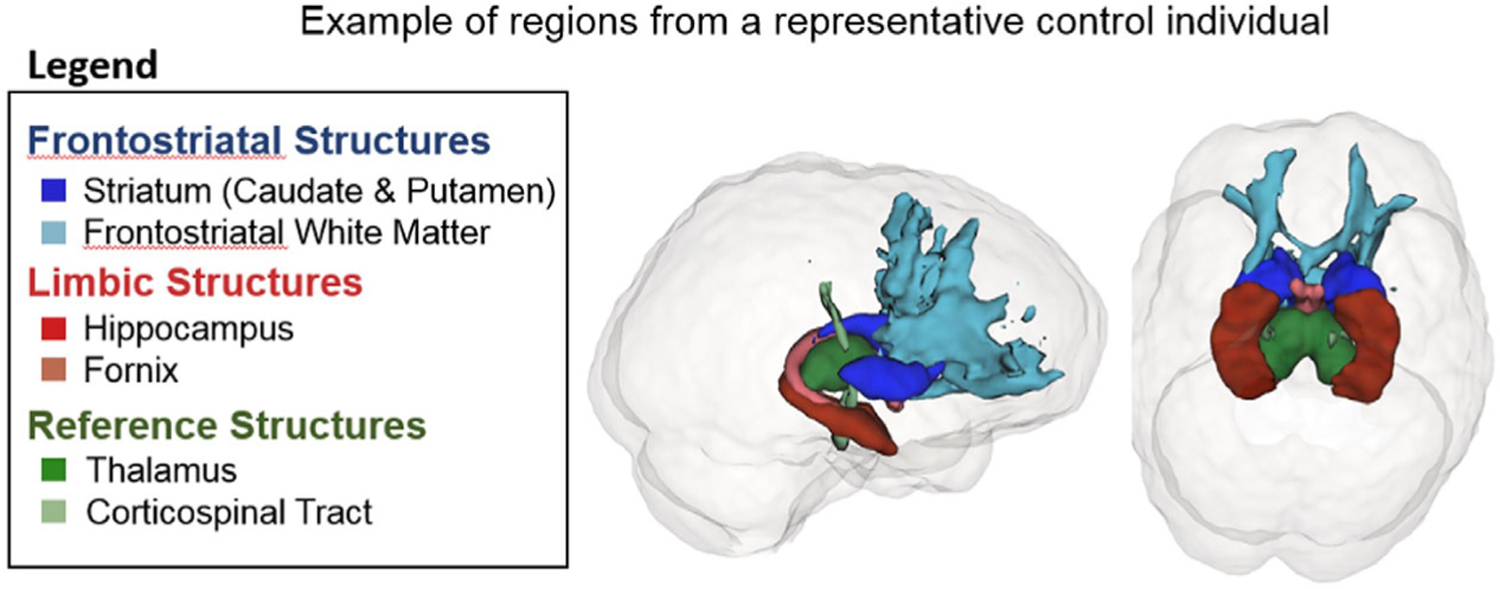
Representation of frontostriatal, limbic, and reference structures. Representative image of regions studied: striatum (dark blue), frontostriatal white matter (light blue), hippocampus (dark red), fornix (light red), thalamus (dark green), and corticospinal tract (green)
To obtain FA maps, diffusion tensor images (DTI) were preprocessed using DTIPrep (NIRAL, UNC-Chapel Hill, North Carolina), where intersection and intervolume correlation analysis, eddy currents, and motion artifact correction were performed for quality control. A brain mask was obtained using the FSL brain extraction tool on the b0 image, and then FA maps were estimated using the Slicer diffusion MRI extension (http://slicerdmri.github.io/) and smoothed using a 2-mm gaussian kernel.
Cognitive evaluation
MoCA scores were obtained from all participants at each visit and used as the primary cognitive assessment for this study. Total MoCA score reflected the sum of all tasks. For the attention/executive subscore, trails, clock drawing, digit span, letter tapping, and serial 7s scores were summed. For the memory subscore, delayed recall and orientation scores were summed.
At baseline, 74 control and 73 PD participants also had comprehensive cognitive testing that was used as an internal validation of the MoCA subscores defined for this study. Attention/executive function tests included the Dementia Rating Scale 2-attention subscore, Stroop color-word test, and a Letter-Number sequence task. Memory tests included the Brief Visuospatial Memory Test, Hopkins Verbal Learning Test, and a visual-verbal memory task. A z-score for each cognitive test was calculated based on the mean and standard deviation of baseline control participants, who were similar to PD participants in age, sex, and education. This method ensured scores were standardized against scores obtained with identical assessment techniques and means were representative of the population studied. Composite z-scores were created for attention/executive and memory domains by averaging the individual test z-scores in those domains. Cognitive domain z-scores from full cognitive testing then were correlated with MoCA subscores. Both MoCA subscores correlated stronger with their respective domain z-score than the other domain (Figure 3).
FIGURE 3.
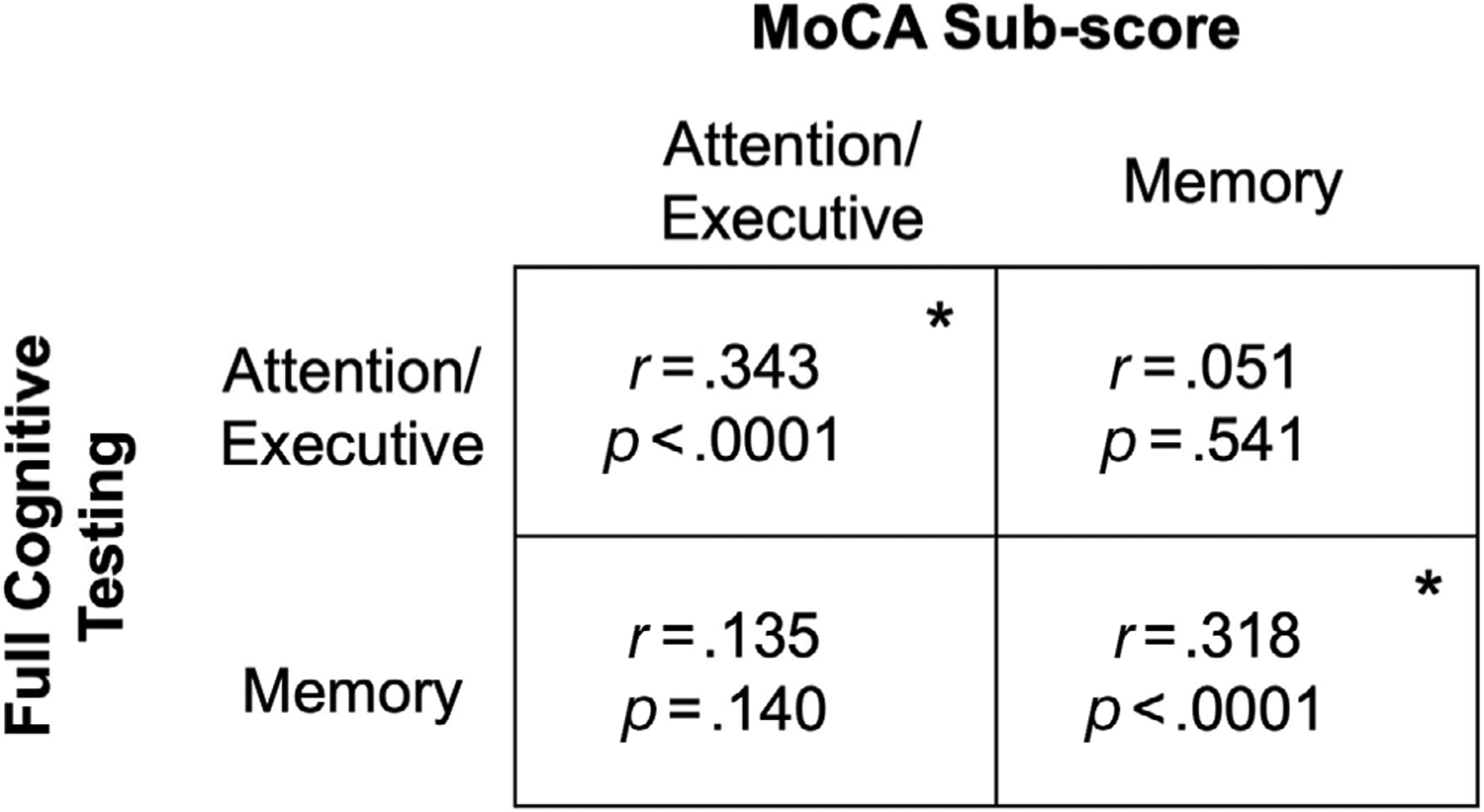
Validation of cognitive metrics. Each box represents Pearson’s correlation coefficient of baseline Montreal Cognitive Assessment (MoCA) subscores and domain Z-scores using full cognitive testing. Significance (*) indicates p-value < .05. Attention/executive function tests included Dementia Rating Scale 2-attention subscore, Stroop color-word test, and a Letter-Number sequence task. Memory tests included the Brief Visuospatial Memory Test, Hopkins Verbal Memory Test, and a visual-verbal memory task. The MoCA subscores are made up of the sum of trails, clock drawing, digit span, letter tapping, and serial 7s scores for attention/executive subscore and the sum of delayed recall and orientation scores for memory subscore.
Statistical analysis
Demographic data were compared using Fischer’s exact test for sex and two sample t-tests for all other metrics. Linear mixed-effects models were used to compare MRI, total MoCA, and MoCA subscore trajectories between control and PD participants. Baseline age, sex, study time in years (±1 month), group, and study time × group interaction were the fixed effects. Intercept and study time were the random effects for each participant. Within PD participants, linear mixed-effects models were used to determine the association between baseline MRI metrics and MoCA total and subscore trajectories among PD participants. MoCA scores (ie, total MoCA or MoCA subscore) over time were the outcome variables. Baseline age, baseline disease duration, sex, baseline MRI metric, study time, and the study time × baseline MRI interaction terms were the fixed effects. Intercept and study time were the random effects for each PD participant. Visualization of baseline MRI marker interaction effects was created using predicted trajectories at ±1 standard deviation of the sample average for a 65-year-old female with a disease duration of 4 years. Raw p-values are reported for all analyses since including the thalamus and CST as negative controls prevents false positives. All data analyses were performed in Matlab or R.
RESULTS
Participant characteristics at baseline
Baseline participant characteristics are shown in Table 1. Control and PD participants were similar in age (p = .483), sex (p = .248), and education (p = .062). At baseline, PD participants had an average disease duration of 4.1 years, HY score of 1.7, and LEDD of 554 mg.
TABLE 1.
Characteristics of participants at beginning of study.
| Controls | PD | p-value | |
|---|---|---|---|
| Demographics | |||
| Participants, n | 120 | 122 | |
| Age, years | 63.3 ± 10.3 | 64.2 ± 9.3 | .483 |
| Sex(M/F) | 60/60 | 70/52 | .250 |
| Education, years | 15.6 ± 2.7 | 15.0 ± 2.8 | .074 |
| Clinical information | |||
| Disease duration, years | – | 4.1 ± 4.6 | – |
| HY Score | – | 1.7 ± 0.8 | – |
| UPDRSII | – | 9.4 ± 7.3 | – |
| UPDRS III | – | 20.8 ± 12.2 | – |
| LEDD, mg | – | 554 ± 439 | – |
| Structural MRI metrics | |||
| Frontostriatal structures | |||
| Striatal volume, mm3 | 7697 ± 597 | 7367 ± 683 | <.001* |
| FSWM FA | 0.41 ± 0.02 | 0.41 ± 0.02 | .766 |
| Limbic structures | |||
| Hippocampal volume, mm3 | 4096 ± 623 | 4021 ± 336 | .090 |
| Fornix FA | 0.28 ± 0.02 | 0.27 ± 0.02 | .113 |
| Reference structures | |||
| Thalamicvolume, mm3 | 7160 578 | 7202 ± 542 | .558 |
| CSTFA | 0.48 ± 0.03 | 0.48 ± 0.03 | .929 |
| Cognitive metrics | |||
| MoCA | 25.9 ± 2.4 | 25.3 ± 2.6 | .093 |
| Attention/Executive subscore | 9.2 ± 0.9 | 9.0 ± 0.9 | .270 |
| Memory subscore | 8.6 ± 1.6 | 8.5 ± 1.7 | .536 |
Note: Data are presented as number (n) or mean ± standard deviation. p-value is calculated from Fischer’s exact test for sex, and from two sample t-tests for all other metrics. Significance (*) represents p-value < .05.
Abbreviations: CST, corticospinal tract; FA, fractional anisotropy; F, female; HY, Hoehn and Yahr; LEDD, levodopa equivalent daily dosage; M, Male; UPDRS, Unified Parkinson’s Disease Rating Scale; FSWM, frontostriatal white matter.
At baseline, striatal volume was smaller in PD compared to control participants (p < .001). FSWM FA and limbic structure metrics (ie, hippocampal volume, fornix FA) were similar between PD and controls (ps > .090). Cognitively, there was no difference between PD and control participants at baseline in total MoCA (p = .093) or attention/executive (p = .270) and memory (p = .536) subscores.
The average interval between visits was 1.7 years and the mean time in the study was 3.2 years. Sixty-seven percent of control and 58% of PD participants completed all three visits. In both the PD and control groups, participants who dropped out tended to be younger. In addition, PD participants who dropped out tended to have higher LEDD. All other demographic, clinical, cognitive, and imaging measures were similar among participants with one, two, or three visits after correcting for age and sex (Table 2).
TABLE 2.
Baseline characteristics of individuals who completed one, two, or three visits of the study.
| One visit | Two visits | Three visits | p-value | p-value (age/sex corrected) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Participants, n | 43 | 47 | 152 | ||
| Control/PD | 22/21 | 17/30 | 81/71 | .120 | .163 |
| Age, years | 65.8 ± 11.3 | 65.9 ± 10.2 | 62.5 ± 9.0 | .035 | n/a |
| Sex(M/F) | 16/27 | 18/29 | 78/74 | .125 | n/a |
| Education, years | 15.3 ± 2.5 | 15.1 ± 2.7 | 15.3 ± 2.9 | .885 | .781 |
| Disease duration, years | 3.7 ± 4.5 | 5.1 ± 5.3 | 3.7 ± 4.4 | .370 | .433 |
| HY Score | 1.1 ± 1.1 | 1.2 ± 1 | 0.8 ± 1 | .012 | .086 |
| UPDRSII | 3.9 ± 5.3 | 6.9 ± 8 | 4.4 ± 6.6 | .071 | .107 |
| UPDRSIII | 16.6 ± 15.8 | 15.3 ± 12.4 | 9.7 ± 11.7 | .002 | .024* |
| LEDD, mg | 344.1 ± 387.8 | 713.4 ± 482.6 | 522 ± 410.1 | .010 | .012* |
| Structural MRI metrics | |||||
| Frontostriatal structures | |||||
| Striatal volume, mm3 | 7430 ± 735 | 7372 ± 683 | 7610 ± 645 | .059 | .231 |
| FSWM FA | 0.4 ± 0.0 | 0.4 ± 0 | 0.4 ± 0 | .074 | .444 |
| Limbic structures | |||||
| Hippocampal volume, mm3 | 4012 ± 405 | 4010 ± 331 | 4086 ± 326 | .260 | .480 |
| Fornix FA | 0.3 ± 0.0 | 0.3 ± 0 | 0.3 ± 0 | 0.031 | .098 |
| Reference structure | |||||
| Thalamic volume, mm3 | 7091 ± 679 | 7170 ± 620 | 7212 ± 500 | .451 | .454 |
| CST FA | 0.5 ± 0.0 | 0.5 ± 0 | 0.5 ± 0 | .236 | .324 |
| Cognitive metrics (MoCA) | |||||
| Total | 25.7 ± 2.5 | 25.4 ± 2.8 | 25.6 ± 2.4 | .789 | .065 |
| Attention/Executive score | 9.2 ± 0.8 | 8.8 ± 1.2 | 9.2 ± 0.9 | .045 | .249 |
| Memory score | 8.5 ± 1.8 | 8.6 ± 1.7 | 8.5 ± 1.6 | .884 | .834 |
Note: Data are presented as number (n) or mean ± standard deviation. p-value compares each visit type using analysis of variance. Significance (*) represents p-value < .05.
Abbreviations: CST, corticospinal tract; FA, fractional anisotropy; F, female; HY, Hoehn and Yahr; LEDD, levodopa equivalent daily dosage; FSWM, frontostriatal white matter; M, male.
Comparison of MRI metrics between PD and control participants
Striatal volume was the only MRI structural metric lower at baseline in PD participants compared to controls (ps < .001; Figure 2A). Longitudinally, striatal volume and FSWM FA decreased in both control and PD participants over time (ps < .036). There was no significant group difference in the rate of striatal volume decline (p = .218; Figure 4A), whereas FSWM FA declined faster over time in PD participants (p = .010; Figure 4B). In limbic structures, hippocampal volume and fornix FA decreased in both control and PD participants over time (ps < .007). Both hippocampal volume (Figure 4C) and fornix FA (Figure 4D) declined faster over time in PD compared to control participants (ps < .016). Thalamic volume declined significantly over the study in PD participants (p = .008) but not control participants (p = .117), while CST FA declined significantly in both PD and control participants over the study (ps < .038). There was no significant difference in the rate of change between control and PD participants for thalamic volume (p = .458; Figure 4E) and CST FA (p = .316; Figure 4F).
FIGURE 4.
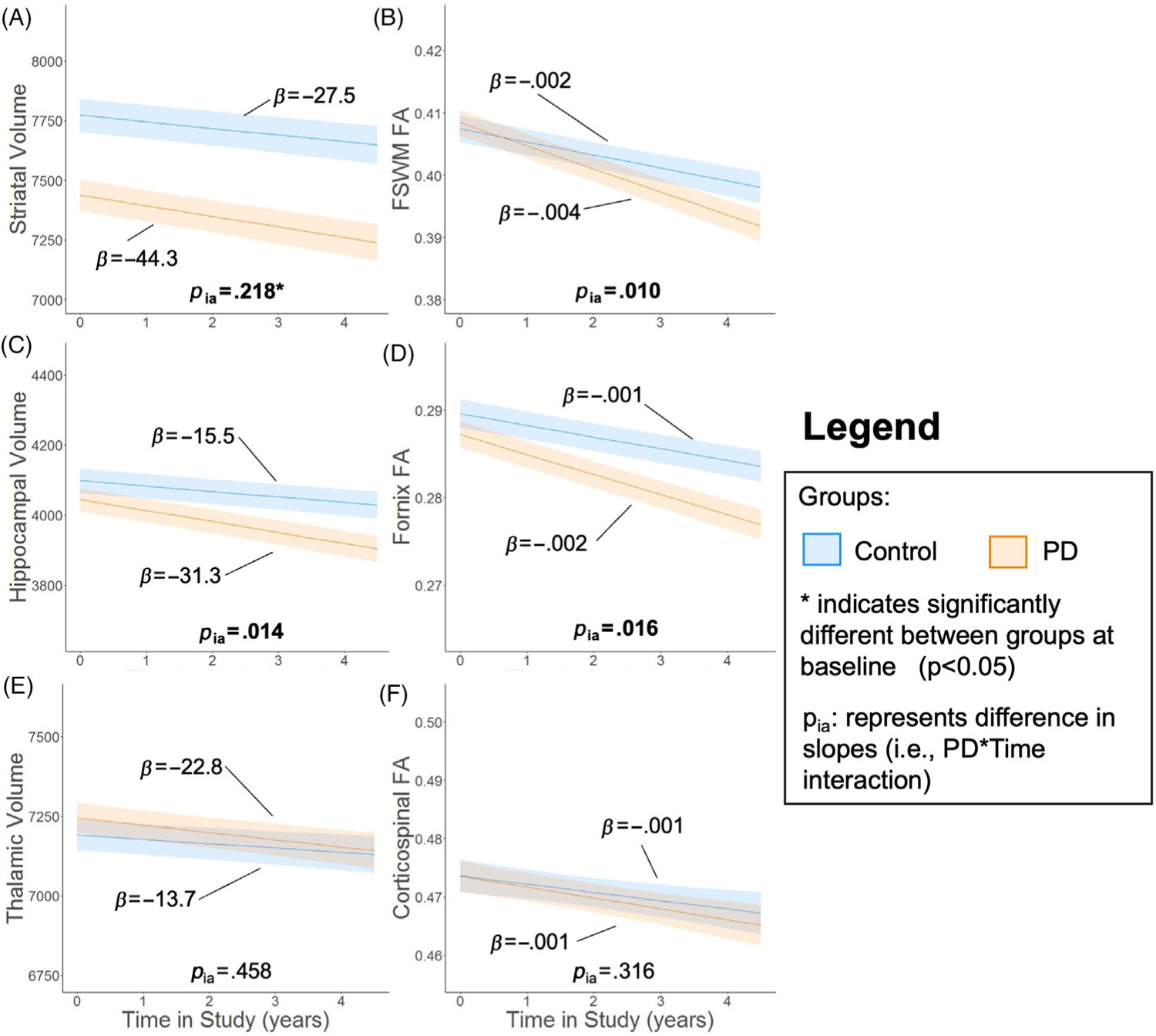
Frontostriatal and limbic pathways are affected by PD. Trajectory of (A) striatum volume, (B) frontostriatal white matter (FSWM) anisotropy (FA), (C) hippocampal volume, (D) fornix FA, (E) thalamic volume, and (F) corticospinal tract FA. The metric of interest (y-axis) was the outcome variable. Baseline age, sex, study time, Parkinson’s disease (PD) status, and study time × PD status interaction were the fixed effects. Intercept and study time were the random effects for each participant. The bold lines and shading represent mean and standard error of the mean for the two groups with covariates (ie, baseline age and sex) set at sample means. Asterisk (*) indicates significantly different MRI metric between PD and control groups at baseline (p-value < .05). pia represents comparison of slopes between PD and control participants (ie, PD × time interaction).
Comparison of cognitive progression between PD and control participants
There was no significant change in total MoCA scores among control participants over time (p = .056), whereas they declined significantly in PD participants (p < .001; Figure 5A). The rate of decline in total MoCA scores was significantly faster in PD than control participants (p < .001). MoCA subscores remained stable in control participants (p > .199) and declined in PD participants (p < .001; Figure 5B,C). Both subscores declined significantly faster over time in PD than control participants (p < .030).
FIGURE 5.
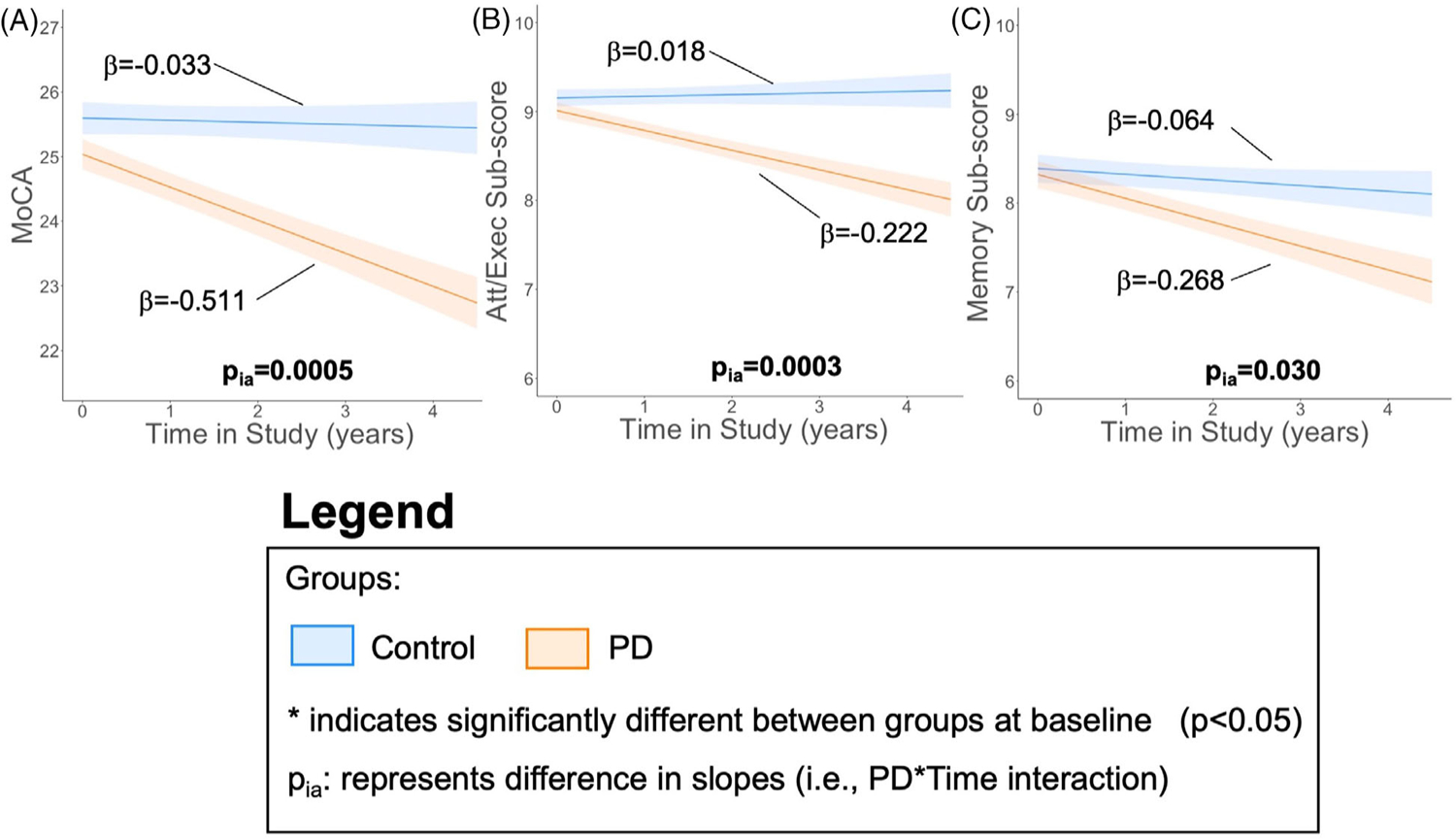
MoCA declines faster in PD participants. Cognitive trajectory measured with (A) total Montreal Cognitive Assessment (MoCA), (B) Attention and executive (att/exec) subscore (trails, clock, digits, vigilance, and serial 7s), and (C) memory subscore (delayed recall and orientation). The metric of interest (y-axis) was the outcome variable. Baseline age, sex, study time, Parkinson’s disease (PD) status, and study time × PD status interaction were the fixed effects. Intercept and slope were the random effects for each participant. The bold lines and shading represent mean and standard error of the mean, respectively, for the two groups with covariates (ie, baseline age and sex) set at sample means. Asterisk (*) indicates significantly different cognitive scores between PD and control groups at baseline (p-value < .05). pia represents comparison of slopes between PD and control participants (ie, PD × time interaction).
Associations of MRI metrics with total MoCA scores in PD participants
Baseline striatal volume (Figure 6A) and FSWM FA (Figure 6B) were not associated with either baseline total MoCA scores (ps > .087) or rate of change in total MoCA scores (ps > .143). Baseline hippocampal volume and fornix FA also were not associated with baseline total MoCA (ps > .349). Lower hippocampal volume (p = .005; Figure 6C) and fornix FA (p = .032; Figure 6D) at baseline, however, were correlated with faster longitudinal decline in total MoCA. Baseline thalamic volume and CST FA were not associated with either baseline values or progression of total MoCA scores (ps > .238; Figure 7A,B).
FIGURE 6.
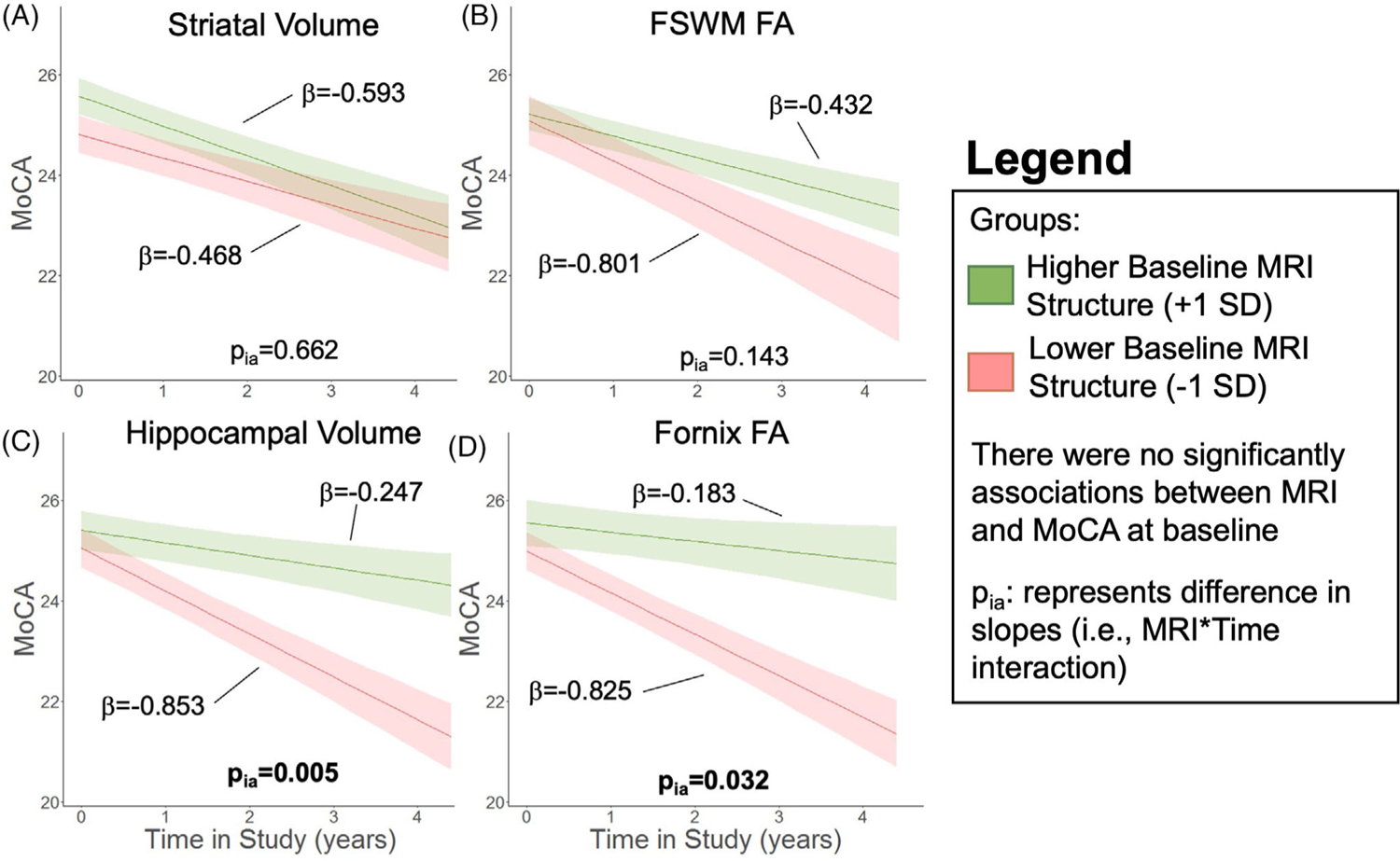
Relation of baseline alterations with total MoCA trajectory. Cognitive trajectory in Parkinson’s disease (PD) participants measured with total Montreal Cognitive Assessment (MoCA) based on baseline MRI metrics of (A) striatal volume, (B) frontostriatal white matter (FSWM) fractional anisotropy (FA), (C) hippocampal volume, and (D) fornix FA. The bold lines represent the expected trajectory for a 65-year-old woman at 4 years disease duration with a baseline metric 1 standard deviation (SD) above the sample mean (green) and 1 SD below the sample mean (red). The low/high value for each metric is 6748/8098 mm2 for striatum, 0.37/0.42 for FSWM FA, 3692/4370 mm3 for hippocampal volume, and 0.26/0.29 for fornix FA. Shading represents the standard error of the mean of the outcome variable. Total MoCA was the outcome variable. Baseline age, baseline disease duration, sex, baseline MRI metric, study time, and the study time × baseline MRI interaction term were the fixed effects. Intercept and slope were the random effects for each PD participant. There were no associations between the MRI metric and MoCA at baseline. pia represents effect of baseline metric on slope of cognitive metric.
FIGURE 7.
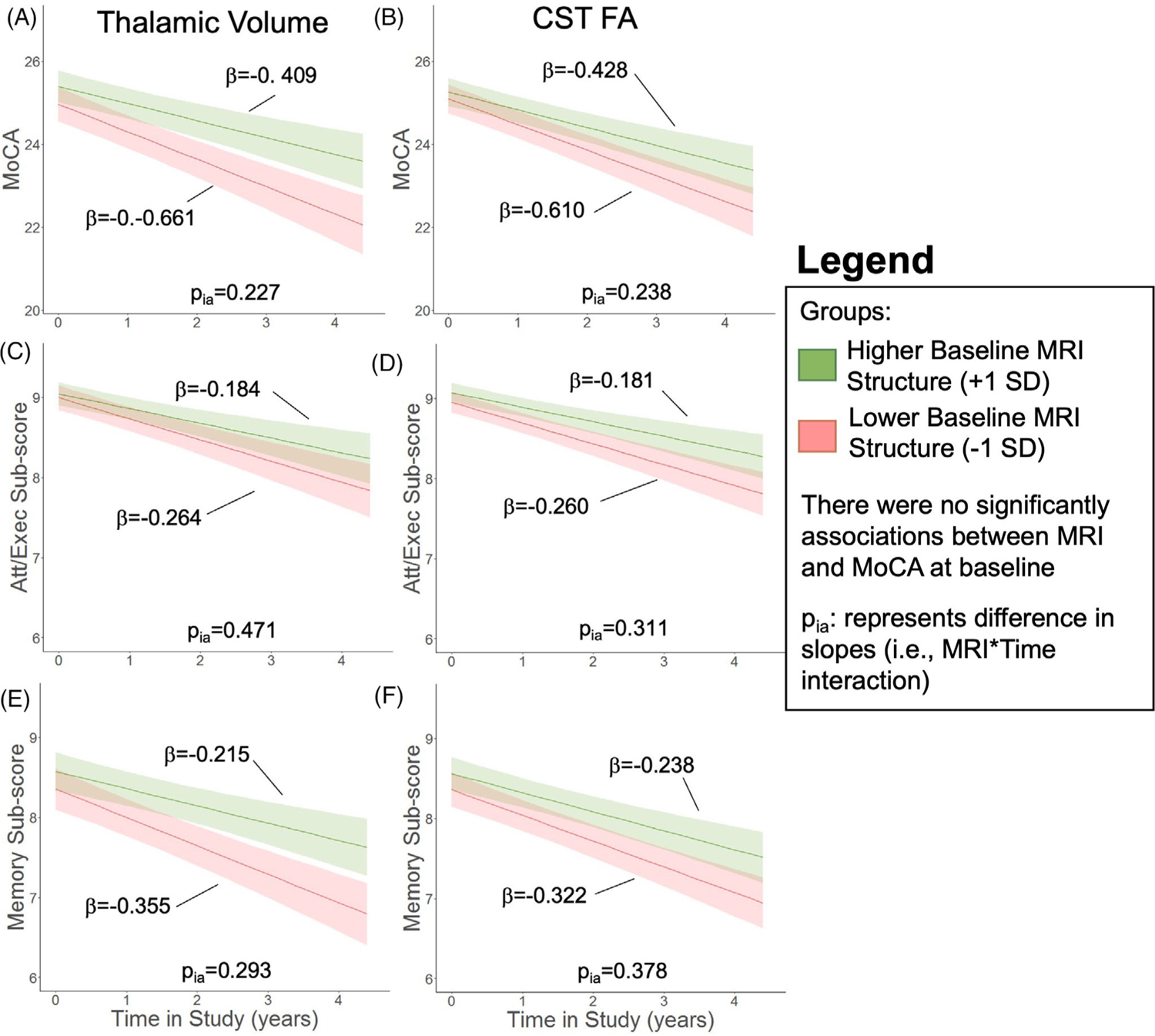
Relation of baseline reference structures on MoCA total and subscore trajectory. In Parkinson’s disease (PD) patients, expected total Montreal cognitive assessment (MoCA) trajectory based on (A) baseline thalamic volume and (B) corticospinal tract (CST) fractional anisotropy (FA). Expected attention/executive (att/exec) subscore trajectory based on (C) baseline thalamic volume and (D) CST FA. Expected memory subscore trajectory based on (E) baseline thalamic volume and (F) CST FA. The bold lines represent the expected trajectory for a 65-year-old woman at 4 years disease duration with a baseline metric 1 standard deviation (SD) above the sample mean (green) and 1 SD below the sample mean (red). The low/high value is 6683/7742 mm3 for thalamic volume and 0.36/0.38 for CST FA. The cognitive metric (total MoCA or MoCA subscore, y-axis) was the outcome variable. Shading represents the standard error of the mean of the outcome variable. Baseline age, baseline disease duration, sex, baseline MRI metric, study time, and the study time × baseline MRI interaction term were the fixed effects. Intercept and slope were the random effects for each PD participant. pia represents effect of baseline metric on slope of cognitive metric. Attention/executive subscore is the sum of trails, clock, digits, vigilance, and serial 7s, and memory subscore is the sum of delayed recall and orientation.
Associations of MRI metrics with MoCA subscores in PD participants
Lower striatal volume was associated with lower attention/executive subscores in PD participants (p = .021; Figure 8A) but not memory subscores (p = .407; Figure 8B) at baseline. Baseline FSWM FA (Figure 8C,D) was not associated with either domain of MoCA subscores at baseline (ps > .394). Higher baseline striatal volume (p = .022, Figure 8A) and lower baseline FSWM FA (p = .038) were associated with faster decline in attention/executive subscores. Baseline striatal volume and FSWM FA did not associate with change in memory subscores (ps > .055).
FIGURE 8.
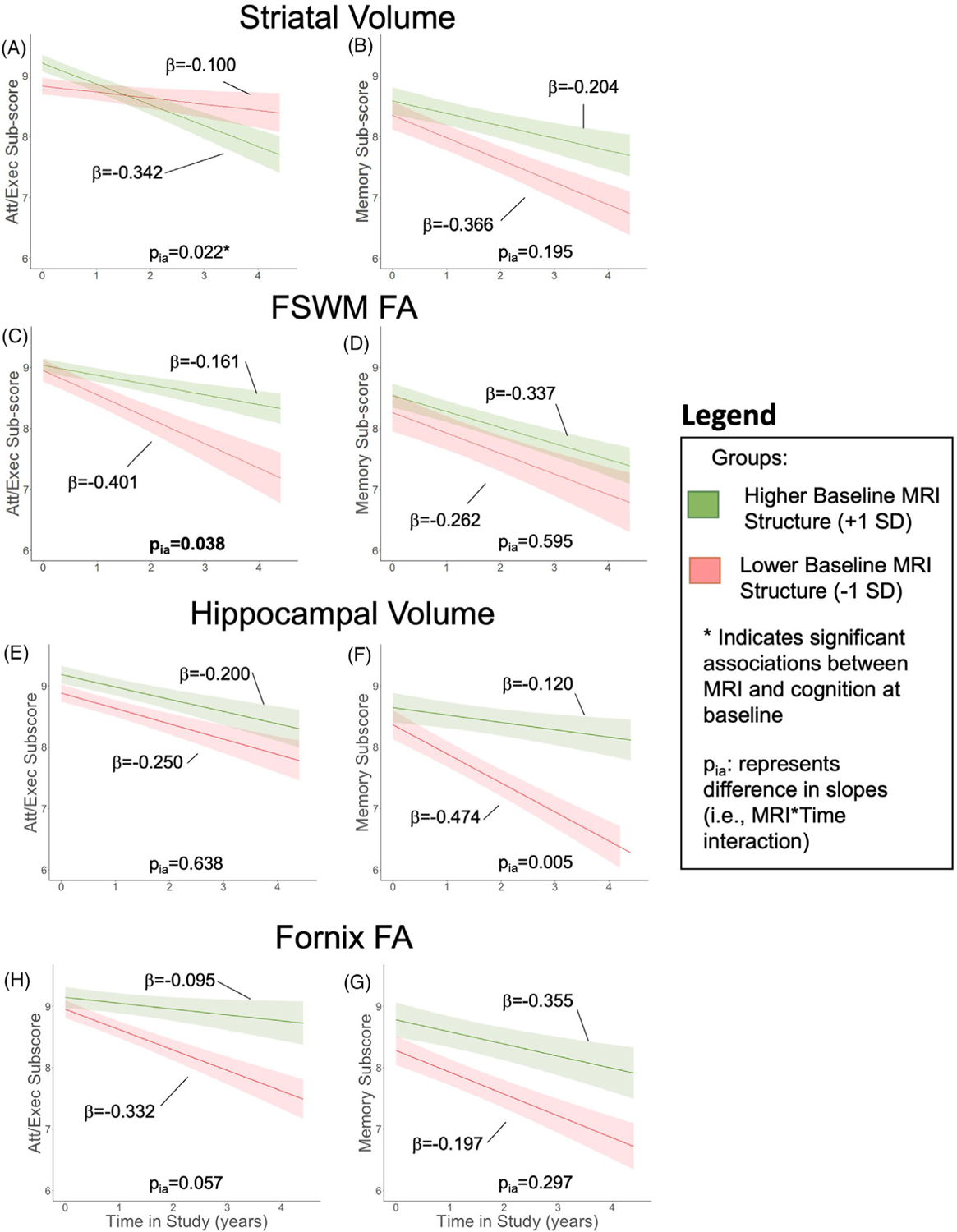
Relation of baseline alterations with MoCA subscore trajectory. In Parkinson’s disease (PD) patients, relation between baseline striatal volume with (A) attention/executive (att/exec) subscore trajectories and (B) memory subscore trajectories, baseline frontostriatal white matter (FSWM) fractional anisotropy (FA) with (C) att/exec subscore trajectories and (D) memory subscore trajectories, baseline hippocampal volume with (E) attention/executive (att/exec) subscore trajectories and (F) memory subscore trajectories, and baseline fornix FA with (G) attention/executive (att/exec) subscore trajectories and (H) memory subscore trajectories. The bold lines represent the expected trajectory for a 65-year-old woman at 4.1 years disease duration with a baseline metric 1 standard deviation (SD) above the sample mean (green) and 1 SD below the sample mean (red). The low/high value for each metric is 6748/8098 mm2 for striatum, 0.37/0.42 for FSWM FA, 3692/4370 mm3 for hippocampal volume, and 0.26/0.29 for fornix FA. Shading represents the standard error of the mean of the outcome variable. MoCA subscore was the outcome variable. Baseline age, baseline disease duration, sex, baseline MRI metric, study time, and the study time × baseline MRI interaction term were the fixed effects. Intercept and slope were the random effects for each PD participant. Asterisk (*) indicates a significant (p < .05) association between the MRI metric and MoCA subscore at baseline. pia represents effect of baseline metric on slope of cognitive metric. Attention/executive subscore is the sum of trails, clock, digits, vigilance, and serial 7s, and memory subscore is the sum of delayed recall and orientation.
Hippocampal volume did not associate with either attention/executive or memory subscores at baseline (Figure 8E,F). Baseline hippocampal volume also was not associated with progression of attention/executive subscores (p = .638; Figure 8E), but lower hippocampal volume was associated with faster memory subscore decline (p = .005; Figure 8F). Baseline fornix FA, thalamic volume, and CST FA were not associated with baseline scores or change in scores for either the attention/executive or memory domain (ps > .182; Figures 7C–F and 8G,H).
DISCUSSION
Utilizing longitudinal T1/T2-weighted and diffusion MRI, we delineated the contribution of frontostriatal and limbic gray and white matter structures in a cohort of PD patients without severe motor disability (HY ≤ 3) or significant cognitive impairments (MoCA ≥ 21). Consistent with prior findings,17,43,44 striatal gray matter was lower in PD participants at baseline. Moreover, both frontostriatal structures were associated with attention/executive dysfunction. In contrast, limbic structures were similar in control and PD participants at baseline, but hippocampal volume and fornix FA changes in PD became more apparent with disease progression. In addition, limbic deterioration was associated with faster decline in total MoCA and memory scores. This information adds to our understanding of PD-related cognitive decline and the underlying structures involved. It also emphasizes the importance of future studies focused on limbic system structures.
Frontostriatal structure in PD associates with attention/executive deficits
Using both severe motor and cognitive dysfunction as exclusion criteria, our study captured PD participants prior to major impairments and monitored disease progression for up to 4.5 years. Studies by Hornykiewicz and colleagues were the first to show striatal dopamine concentration was reduced in PD,5 and motor symptoms manifested only after a substantial loss of striatal dopamine concentration occured.45 Previous studies by our group17 and others44 report similar findings that the striatum is affected in early PD. Literature investigating longitudinal changes in both white and gray matter in frontostriatal pathways is limited, so the timing of white and gray matter changes in this system remains largely unexplored. In our study, FA in FSWM changes occurred after striatal atrophy already was evident in PD.46 Previous studies have shown that reduced dopamine signaling in the striatum may lead to maladaptive compensation and axonal disorganization in FSWM,45 which is consistent with our findings.
We only investigated FA in this study due to concerns that single-tensor mapping of component diffusivities may not relate directly to underlying microstructure.39 Previous studies investigating component diffusivities in PD have indicated both axial and radial diffusivities are increased in subcortical circuitry47 and associate with worse motor48 and cognitive performance.49,50 Some studies suggest radial diffusivity is more sensitive than axial diffusivity.48,51 Biological interpretation of axial and radial diffusivities from single-tensor models, however, has been discouraged.52 Advanced diffusion modeling may help to elucidate potential microstructural underpinnings of white matter alterations. Indeed, recent multishell diffusion imaging has reported that white matter alterations in PD are associated with decreased axonal cross section rather than decreased axonal density. Similar to the hypothesis that decreased striatal dopamine signaling leads to maladaptive dendritic disorganization in frontostriatal fibers, advanced diffusion metrics also support axonal disorganization and not axonal death may be the primary mechanism of white mater alteration in PD-related cognitive decline. Exploring additional multi-tensor modeling of white mater alterations in these circuits is an enticing direction for future investigation.
The role of frontostriatal structures in attention/executive functions in PD is well known.53 Furthermore, the association with both dopamine deficiency6 and motor symptoms34 suggests that attention/executive dysfunction could be a principle feature of PD. Frontostriatal structural changes and attention/executive dysfunction appear to occur early in the disease and then stabilize with disease progression.54 Indeed, we found that baseline striatal atrophy was associated with baseline attention/executive deficits. Intriguingly, participants with lower baseline striatal volume displayed less longitudinal decline in attention/executive functions. This observation may suggest that striatal atrophy and attention/executive dysfunction have already occurred and that further progression may not be measurable within the timeframe of this study (ie, 4.5 years).
Neuropsychological evidence has shown that attention/executive dysfunction is not associated with faster conversion to dementia in PD.55,56 Consistent with this idea, we found baseline frontostriatal structural MRI metrics did not predict rapid decline in global cognition, as measured by total MoCA score. Therefore, although frontostriatal structural changes and associated attention/executive dysfunction may be a core feature of early PD pathophysiology, these processes may not be related to rapid progression to dementia in PD. Future studies with longer follow-up of patients may help test this hypothesis more rigorously.
Limbic system changes in PD are associated with faster cognitive decline, particularly in memory tasks
Hippocampal volume and fornix FA alterations have been suggested to reflect a single structural unit of the limbic system where pathological processes may lead to deterioration in both structures.57 Although both the hippocampus and fornix have been found to be altered in PD,58 the biological mechanisms underlying the degenerative process are not clearly understood. Previous studies have suggested PD-specific changes (eg, α-synuclein positive Lewy-bodies), age, or other dementing processes (eg, AD co-pathology) may drive limbic changes in PD.59,60 Our current longitudinal data provide additional support that both gray (ie, hippocampal) and white matter (ie, fornix) limbic structures deteriorate faster in PD than in normal aging alone. Furthermore, we found that limbic differences between PD and controls were not evident at baseline. This is not surprising since others also have reported a lack of hippocampal alterations in early PD.61 However, our data support the hypothesis that limbic system alterations do occur, and there is likely heterogeneity in when limbic deteriorations present in PD patients.
Within PD patients, some patients may develop structural limbic deterioration early in the disease course. Our data suggest that more hippocampal atrophy and disorganized fornix microstructure at baseline are associated with faster global cognitive decline. Previous literature has established the involvement of the hippocampus and fornix in memory function in PD.18,23 Our longitudinal data build upon growing evidence that early hippocampal atrophy predicts cognitive decline in PD10,11 and are the first to suggest the fornix also may be involved in rapid PD-related cognitive decline. Furthermore, our data suggest that decreased hippocampal volume may be related specifically to memory deficits, and once pathology extends to the fornix, more global decline becomes imminent. Follow-up with these participants and their caregivers is needed to determine whether early limbic deterioration predicts faster conversion to clinically significant functional impairments (ie, dementia).
Limitations and future directions
Although the current study provided intriguing insights into frontostriatal and limbic contributions to PD cognitive decline, it has several limitations. First, we only included participants with mild/moderate disease severity. Forthcoming studies should include various disease stages, particularly those in later stages with a higher risk of converting to dementia. This will allow a more rigorous investigation of frontostriatal and limbic changes across the disease course. In addition, longitudinal follow-up studies should include metrics to ascertain a clinical diagnosis of dementia that will provide a better understanding of the conversion to severe morbidity in this cohort. Second, there are technical limitations to our work. No effort was made to identify and exclude white matter lesions using the T2-weighted images. Therefore, areas with high levels of destruction may have an FA value less than 0.2 and been removed based on our white matter threshold mask. Furthermore, more advanced multi-tensor diffusion imaging may provide greater understanding of axon microstructure. Third, we included only four structures in our hypothesis-focused study: one gray matter (volume) and one white matter (FA) metric from our two systems of interest (ie, frontostriatal and limbic). Gray matter volume and white matter FA are promising biomarkers in PD15,47 and have been validated for biological interpretability.52,62 It is possible, however, that other important changes outside of these regions/metrics of focus were missed. Also, including other MRI metrics (eg, susceptibility, diffusion, and/or functional MRI) and more brain regions (eg, cingulum and medial temporal lobe) and networks may help to elucidate structural changes in PD-related cognitive decline. Fourth, our attention/executive and memory subscores calculated from MoCA reflected PD-related cognitive changes that were associated with comprehensive neurological testing. More validation of MoCA subscores, however, is needed to employ them for clinical utility. Finally, our study does not indicate the mechanisms underlying frontostriatal and limbic changes. Future studies should investigate potential mechanisms, which may provide an opportunity for intervention.
Conclusions
The current study demonstrated that both frontostriatal and limbic structures were affected in PD as reflected by gray matter atrophy and reduced white matter integrity. Decreased striatal volume was present at baseline in PD and associated with attention/executive function. Most importantly, monitoring limbic structure metrics in PD patients may identify those at risk for faster global cognitive decline and progressive memory changes. This study provides valuable longitudinal insight that indicates distinct contributions of frontostriatal and limbic circuitry in PD-related cognitive decline.
ACKNOWLEDGMENTS AND DISCLOSURE
We express gratitude to all the study participants who volunteered for this study and to the study personnel who contributed to its success.
The authors declare no conflict of interest.
Funding information
National Institute of Neurological Disorders and Stroke, Grant/Award Numbers: NS060722, NS082151, NS112008; National Institute of Aging, Grant/Award Numbers: F30 AG067651, R00 AG056670; Hershey Medical Center Clinical Research Center, Grant/Award Numbers: UL1 RR033184, UL1 TR000127; National Center for Advancing Translational Sciences, Grant/Award Number: UL1 TR002014; PA Department of Health Tobacco CURE Funds; Michael J. Fox Foundation for Parkinson’s Research; Alzheimer’s Association; Alzheimer’s Research UK; Weston Brain Institute Neuroimaging
REFERENCES
- 1.Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 2008;23:837–44. [DOI] [PubMed] [Google Scholar]
- 2.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry 2000;69:308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarsland D, Larsen JP, Karlsen K, et al. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 1999;14:866–74. [PubMed] [Google Scholar]
- 4.Vossius C, Larsen JP, Janvin C, et al. The economic impact of cognitive impairment in Parkinson’s disease. Mov Disord 2011;26:1541–4. [DOI] [PubMed] [Google Scholar]
- 5.Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Parkinsonism Relat Disord 1998;4:53–7. [DOI] [PubMed] [Google Scholar]
- 6.Owen AM, Sahakian BJ, Hodges JR, et al. Dopamine-dependent frontostriatal planning deficits in early Parkinson’s disease. Neuropsychology 1995;9:126–40. [Google Scholar]
- 7.Jokinen P, Karrasch M, Bruck A, et al. Cognitive slowing in Parkinson’s disease is related to frontostriatal dopaminergic dysfunction. J Neurol Sci 2013;329:23–8. [DOI] [PubMed] [Google Scholar]
- 8.Litvan I, Aarsland D, Adler CH, et al. Mds task force on mild cognitive impairment in Parkinson’s disease: critical review of pd-mci. Mov Disord 2011;26:1814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roussel M, Lhommee E, Narme P, et al. Dysexecutive syndrome in Parkinson’s disease: the GREFEX study. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2017;24:496–507. [DOI] [PubMed] [Google Scholar]
- 10.Weintraub D, Dietz N, Duda JE, et al. Alzheimer’s disease pattern of brain atrophy predicts cognitive decline in Parkinson’s disease. Brain 2012;135:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandiah N, Zainal NH, Narasimhalu K, et al. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson’s disease. Parkinsonism Relat Disord 2014;20:1203–8. [DOI] [PubMed] [Google Scholar]
- 12.Aarsland D, Creese B, Politis M, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol 2017;13:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prell T. Structural and functional brain patterns of non-motor syndromes in Parkinson’s disease. Front Neurol 2018;9:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung CW, Guo J, Fu H, et al. Atrophy associated with tau pathology precedes overt cell death in a mouse model of progressive tauopathy. Sci Adv 2020;6:eabc8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foo H, Mak E, Yong TT, et al. Progression of subcortical atrophy in mild Parkinson’s disease and its impact on cognition. Eur J Neurol 2017;24:341–8. [DOI] [PubMed] [Google Scholar]
- 16.Marquez F, Yassa MA. Neuroimaging biomarkers for Alzheimer’s disease. Mol Neurodegener 2019;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterling NW, Du G, Lewis MM, et al. Striatal shape in Parkinson’s disease. Neurobiol Aging 2013;34:2510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyer MK, Bronnick KS, Hwang KS, et al. Verbal memory is associated with structural hippocampal changes in newly diagnosed Parkinson’s disease. J Neurol Neurosurg Psychiatry 2013;84:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier-Hein KH, Westin CF, Shenton ME, et al. Widespread white matter degeneration preceding the onset of dementia. Alzheimers Dement 2015;11:485–93.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen B, Fan GG, Liu H, et al. Changes in anatomical and functional connectivity of Parkinson’s disease patients according to cognitive status. Eur J Radiol 2015;84:1318–24. [DOI] [PubMed] [Google Scholar]
- 21.Deng B, Zhang Y, Wang L, et al. Diffusion tensor imaging reveals white matter changes associated with cognitive status in patients with Parkinson’s disease. Am J Alzheimers Dis Other Demen 2013;28:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theilmann RJ, Reed JD, Song DD, et al. White-matter changes correlate with cognitive functioning in Parkinson’s disease. Front Neurol 2013;4:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Z, Shemmassian S, Wijekoon C, et al. DTI correlates of distinct cognitive impairments in Parkinson’s disease. Hum Brain Mapp 2014;35:1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenthal LS, Drake D, Alcalay RN, et al. The NINDS Parkinson’s disease biomarkers program. Mov Disord 2016;31:915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis MM, Harkins E, Lee EY, et al. Clinical progression of Parkinson’s disease: insights from the NINDS common data elements. J Parkinsons Dis 2020;10:1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30:1591–601. [DOI] [PubMed] [Google Scholar]
- 27.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–42. [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 2010;25:2649–53. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B. Freesurfer. Neuroimage 2012;62:774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9: 179–94. [DOI] [PubMed] [Google Scholar]
- 31.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207. [DOI] [PubMed] [Google Scholar]
- 32.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–55. [DOI] [PubMed] [Google Scholar]
- 33.Voevodskaya O, Simmons A, Nordenskjold R, et al. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer’s disease. Front Aging Neurosci 2014;6:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owen AM, James M, Leigh PN, et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain 1992;115:1727–51. [DOI] [PubMed] [Google Scholar]
- 35.Pelletier A, Periot O, Dilharreguy B, et al. Structural hippocampal network alterations during healthy aging: a multi-modal MRI study. Front Aging Neurosci 2013;5:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gattellaro G, Minati L, Grisoli M, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. AJNR Am J Neuroradiol 2009;30:1222–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slater DA, Melie-Garcia L, Preisig M, et al. Evolution of white matter tract microstructure across the life span. Hum Brain Mapp 2019;40:2252–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao R, Ning L, Chen Z, et al. Performance of unscented Kalman filter tractography in edema: analysis of the two-tensor model. Neuroimage Clin 2017;15:819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Figley CR, Uddin MN, Wong K, et al. Potential pitfalls of using fractional anisotropy, axial diffusivity, and radial diffusivity as biomarkers of cerebral white matter microstructure. Front Neurosci 2021;15:799576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan L, Li H, Zhuo J, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex 2016;26:3508–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown CA, Johnson NF, Anderson-Mooney AJ, et al. Development, validation and application of a new fornix template for studies of aging and preclinical Alzheimer’s disease. Neuroimage Clin 2017;13:106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori S, Crain BJ, Chacko VP, et al. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–9. [DOI] [PubMed] [Google Scholar]
- 43.Lewis MM, Du G, Lee EY, et al. The pattern of gray matter atrophy in Parkinson’s disease differs in cortical and subcortical regions. J Neurol 2016;263:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SH, Kim SS, Tae WS, et al. Regional volume analysis of the Parkinson disease brain in early disease stage: gray matter, white matter, striatum, and thalamus. AJNR Am J Neuroradiol 2011;32:682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernheimer H, Birkmayer W, Hornykiewicz O, et al. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 1973;20:415–55. [DOI] [PubMed] [Google Scholar]
- 46.Blesa J, Trigo-Damas I, Dileone M, et al. Compensatory mechanisms in Parkinson’s disease: circuits adaptations and role in disease modification. Exp Neurol 2017;298:148–61. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Burock MA. Diffusion tensor imaging in Parkinson’s disease and Parkinsonian syndrome: a systematic review. Front Neurol 2020;11:531993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Wu IW, Buckley S, et al. Diffusion tensor imaging of the nigrostriatal fibers in Parkinson’s disease. Mov Disord 2015;30:1229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Auning E, Kjaervik VK, Selnes P, et al. White matter integrity and cognition in Parkinson’s disease: a cross-sectional study BMJ Open 2014;4:e003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gargouri F, Gallea C, Mongin M, et al. Multimodal magnetic resonance imaging investigation of basal forebrain damage and cognitive deficits in Parkinson’s disease. Mov Disord 2019;34:516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boska MD, Hasan KM, Kibuule D, et al. Quantitative diffusion tensor imaging detects dopaminergic neuronal degeneration in a murine model of Parkinson’s disease. Neurobiol Dis 2007;26:590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med 2009;61:1255–60. [DOI] [PubMed] [Google Scholar]
- 53.Arnaldi D, Campus C, Ferrara M, et al. What predicts cognitive decline in de novo Parkinson’s disease? Neurobiol Aging 2012;33:1127. [DOI] [PubMed] [Google Scholar]
- 54.Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. J Neuropsychol 2013;7:193–224. [DOI] [PubMed] [Google Scholar]
- 55.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol 2010;9:1200–13. [DOI] [PubMed] [Google Scholar]
- 56.Green J, McDonald WM, Vitek JL, et al. Cognitive impairments in advanced PD without dementia. Neurology 2002;59:1320–4. [DOI] [PubMed] [Google Scholar]
- 57.Lee DY, Fletcher E, Carmichael OT, et al. Sub-regional hippocampal injury is associated with fornix degeneration in Alzheimer’s disease. Front Aging Neurosci 2012;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HJ, Kim SJ, Kim HS, et al. Alterations of mean diffusivity in brain white matter and deep gray matter in Parkinson’s disease. Neurosci Lett 2013;550:64–8. [DOI] [PubMed] [Google Scholar]
- 59.Hurtig HI, Trojanowski JQ, Galvin J, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson’s disease. Neurology 2000;54:1916–21. [DOI] [PubMed] [Google Scholar]
- 60.Bancher C, Braak H, Fischer P, et al. Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer’s and Parkinson’s disease patients. Neurosci Lett 1993;162:179–82. [DOI] [PubMed] [Google Scholar]
- 61.Tinaz S, Courtney MG, Stern CE. Focal cortical and subcortical atrophy in early Parkinson’s disease. Mov Disord 2011;26:436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 2002;15:435–55. [DOI] [PubMed] [Google Scholar]


