Abstract
Sinonasal malignancies make up less than 5% of all head and neck neoplasms, with an incidence of 0.5–1.0 per 100,000. The outcome of these rare malignancies has been poor while significant progress is being made in management of other cancers. This review aims to describe the incidence, causes, presentation, diagnosis, treatment and recent developments of malignancies of the sinonasal tract. The diagnoses that we cover in this review include sinonasal undifferentiated carcinoma, sinonasal adenocarcinoma, sinonasal squamous cell carcinoma, and esthesioneuroblastoma, which are exclusive to the sinonasal tract. In addition, we will also cover malignances that are likely to be encountered in the sinonasal tract - primary mucosal melanoma, NUT carcinoma, and extranodal NK/T Cell lymphoma. For the purpose of keeping this review as concise and focused as possible, we have excluded sarcomas or malignancies that can be classified as salivary gland neoplasms.
Keywords: rare diseases, head and neck neoplasms, paranasal sinus neoplasms, sinonasal carcinoma
Introduction
Sinonasal malignancies make up less than 5% of all head and neck neoplasms, with an incidence of 0.556 per 100,000 individuals per year. (1) While outcomes of other cancers of the head and neck have significantly improved over the past decade, the outcomes of sinonasal malignancies have been relatively stable. (2) This is likely related to, at least in part, the exclusion of sinonasal malignancies from most clinical studies testing therapeutic agents for head and neck cancer patients. (3)The scope of this review is to cover incidence, mortality, survival, causes, presentation, diagnosis, treatment, and ongoing developments in malignancies that occur in the sinonasal tract. We include malignancies that are exclusive to the sinonasal tract such as sinonasal undifferentiated carcinoma, sinonasal adenocarcinoma, sinonasal squamous cell carcinoma, and esthesioneuroblastoma. Additionally, we include other malignancies that can occur in the sinonasal tract but are not classified as sarcomas or salivary gland neoplasms. This includes tumors that are seen most often in the sinonasal region such as primary mucosal melanoma, NUT carcinoma, and extranodal NK/T Cell lymphoma. We have specifically emphasized the management of these tumors due to the lack of specific protocols due to paucity of data. Nasopharyngeal carcinoma is not included in this review because of its distinct anatomical presentation.
The histology of the sinonasal tract is diverse. The nasal vestibule at the entrance of each nostril is composed of keratinized squamous epithelium, and like the skin, has sweat glands, hair follicles, and sebaceous glands. Approximately one to two centimeters into the nose at the limen nasi this epithelium transitions to a ciliated pseudostratified columnar epithelium, also called the Schneiderian epithelium, which lines most of the nasal cavity and paranasal sinuses. This lining is like that of the lower respiratory tract and contains submucosal seromucous glands. The mucus membrane of the paranasal sinuses is thinner than that of the nasal cavity and has fewer seromucous glands. The olfactory mucosa is located over the cribriform plate and the superior third of the nasal septum. This mucosa consists of specialized olfactory neuronal cells that protrude from the mucosa alongside columnar sustentacular epithelial cells. (4,5)
Presentation and evaluation of a sinonasal malignancy
Clinical presentation
Early symptoms are non-specific and include nasal obstruction most commonly, along with epistaxis, facial pain, or persistent rhinorrhea. (6,7) Locally advanced disease has more localizing symptoms such as proptosis, diplopia or cranial neuropathy, chronic rhinosinusitis, or headache. (6) Symptoms of advanced disease correlate to location and extent of disease. Tumors advancing towards the anterior cranial fossa via the cribriform plate or the orbit cause anosmia or proptosis, whereas tumors extending to the lateral bony wall into the cavernous sinuses cause neuropathy of cranial nerves III, IV, VI, V1, and V2, leading to diplopia and paresthesias of the face. When the tumor invades the middle cranial fossa, patients experience paresthesias of the lower face or trismus due to involvement of cranial nerve V3 or invasion of the pterygoid muscles. Because many of the early symptoms are associated with common benign states such as allergies, most patients with these tumors tends to be diagnosed when it is locally advanced, or even metastatic. (8)
Initial evaluation
Clinical examination of patients with the above symptoms should include, complete head and neck exam including all mucosal surfaces and assessment of cranial nerves. An endoscopic exam is necessary to visualize the extent of the tumor in the sinonasal area, as well as obtain tissue for histopathological examination. This is usually followed by imaging. Computed tomography (CT) and magnetic resonance imaging (MRI) can be used alone or in combination depending on the clinical context. CT is helpful for examining bony landmarks and erosion and is important for the use of image guidance during surgery. MRI is helpful for soft tissue differentiation, specifically about invasion of the orbit, intracranial structures (dura, brain, cavernous sinus), perineural spread, infratemporal fossa and facial soft tissues. MRI can also provide higher resolution of the tumor itself. Relevant nuclear imaging for a subset of sinonasal malignancies will be discussed in the context of individual disease entities in the respective sections below.
Staging of sinonasal malignancies is as per the American Joint Committee on Cancer (AJCC) tumor/node/metastasis (TNM) classifications for cancer of nasal cavity and paranasal sinuses. (9) T-staging varies based on the location of the tumor, with separate T staging for maxillary sinus and nasal cavity/ethmoid sinus. It is based on the depth of invasion and degree of destruction of surrounding structures. N-staging is based on regional nodal status, clinically or pathologically. While most tumors share common staging, certain diseases like ONB and mucosal melanoma have unique staging systems, as discussed in their respective sections of this article. Table 1 summarizes the TNM staging of sinonasal malignancies outside ONB and mucosal melanoma.
Table 1:
TNM staging of sinonasal malignancies as per AJCC 8th edition
| TNM Staging | Nasal cavity/Ethmoid Sinus | Maxillary Sinus |
|---|---|---|
| T-stage | ||
| Tis | Carcinoma in situ | |
| T1 | tumor limited to one subsite (septum, floor, lateral wall, or vestibule (edge of naris to mucocutaneous junction of nasal cavity); left or right ethmoid sinus) | tumor limited to maxillary sinus mucosa (no bone erosion/destruction) |
| T2 | tumor involving two subsites in one region or extending to involve an adjacent region in the nasoethmoidal complex | tumor with bone erosion/destruction, including extension into hard palate and/or middle meatus, excluding structures in a higher T category |
| T3 | Tumor invades any of the following: • medial wall or floor of orbit • maxillary sinus • palate • cribriform plate |
Tumor invades any of the following: • bone of posterior wall of maxillary sinus • subcutaneous tissues • floor or medial wall of orbit • pterygoid fossa • ethmoid sinuses |
| T4a | Tumor invades any of the following: • anterior orbital contents • skin of nose or cheek • minimal extension to anterior cranial fossa • pterygoid plates • Sphenoid sinus • Frontal sinus |
Tumor invades any of the following: • anterior orbital contents • skin of cheek • pterygoid plates • infratemporal fossa • cribriform plate • sphenoid sinus • frontal sinus |
| T4b | Tumor invades any of the following: • orbital apex • middle cranial fossa • dura • brain • cranial nerves other than maxillary division of trigeminal nerve (V2) • nasopharynx • clivus |
|
| N-stage | ||
| N0 | no regional node metastases | |
| N1 | metastasis in single ipsilateral node, ≤3 cm, and no extranodal extension (ENE(−)) | |
| N2a | metastasis in single ipsilateral node, >3 cm and ≤6 cm, and ENE(−); or metastasis in single ipsilateral node, ≤3 cm, and ENE(+) | |
| N2b | metastasis in multiple ipsilateral nodes, all ≤6 cm, and ENE(−) | |
| N2c | metastasis in bilateral or contralateral nodes, all ≤6 cm, and ENE(−) | |
| N3a | metastasis in a node, >6 cm, and ENE(−) | |
| N3b | metastasis in single ipsilateral node, >3 cm, and ENE(+); or multiple ipsilateral, contralateral, or bilateral nodes any with ENE(+); or single contralateral node of any size and ENE(+) | |
Sinonasal Squamous Cell Carcinoma
Incidence and patient demographics
Sinonasal squamous cell cancers (SNSCC) are the most common sinonasal malignancy, making up about 3% of head and neck cancers. (10) Within sinonasal malignancies, sinonasal squamous cell carcinoma is the most common, making up 61% of cases. (11) A recent study examined 4,994 cases of SNSCC between 1973 and 2009, as documented by the National Cancer Institute’s Surveillance Epidemiology and End Results (SEER) Program, which revealed that the overall incidence rate was 0.36 cases per 100,000 population per year. There was a male-to-female incidence ratio of 2.26:1 and almost 80% of patients were older than 55 years old. The incidence rate has steadily declined over the last 30+ years. Trend analysis showed annual percentage change (APC), which is the percentage change in incidence rate over time, of −2.63% for male SNSCC incidence and −1.69% for female SNSCC incidence during the same period. (8)
Pathophysiology
SNSCC is a complex disease, and its pathogenesis is poorly understood. Occupational exposure to several industry compounds, such as wood dust, leather dust, glue, chrome, nickel, formaldehyde, arsenic, welding fumes, and multiple compounds in the textile industry, has been reported to increase the risk of SNSCC. (12) A case-control study by Hayes (13) revealed a strong association between SNSCC and tobacco use (relative risk of 3.1) with a dose-response relationship, especially for recent tobacco users. Fukuda et al (14) revealed similar evidence to support tobacco smoke exposure as a risk factor. In addition to environmental exposures, a meta-analysis also showed a strong prevalence of HPV in SNSCC. (15)
Squamous cell cancers in several anatomic locations, including the oropharyngeal region, are well known to be linked to human papillomavirus (HPV) and recent studies support a potential causative role for the virus in SNSCC as well. Recent studies have revealed that around 31% of SNSCC patients had HPV-positive tumors from 770 cases of SNSCC with known HPV status. (16,17) These patients with HPV-positive SNSCC were significantly younger than HPV-negative patients. HPV-positivity was associated with a higher 5-year OS, like squamous cell carcinoma of the oropharynx. Another observation that is often discussed is the association of SNSCC with inverted sinonasal papilloma (ISP). Lawson et al. (18) reviewed all studies evaluating the association between HPV and ISP and hypothesized that low-risk HPV may induce ISP formation which then disappears as infected cells die while high-risk HPV causes dysplastic ISP and squamous cell carcinoma. The College of American Pathologists do not recommend routine HPV testing in non-oropharyngeal primary tumors of the head and neck due to undetermined prognostic significance. (19)
In 2017, the WHO included human papillomavirus-related multi-phenotypic sinonasal carcinoma (HMSC) as a new subtype of non-keratinizing squamous cell carcinoma of the sinonasal tract. This entity, first described in 2013, has favorable clinical outcome despite aggressive histomorphology. (20) HPV status is commonly defined by p16 overexpression of tumor and morphologically is described as basaloid proliferation with focal areas of squamous differentiation. A systematic review by Zupancic et al. (21) reported 79 cases of HMSC, of which 77 (97.5%) occurred in the sinonasal cavity and 2 cases in the breast and tonsils respectively. Of the tumors originating in the sinonasal cavity, none had regional metastasis.
The contribution of genetic abnormalities to the pathogenesis of SNSCC is poorly understood. TP53 mutations in the tumor have been described in 80% of SNSCCs. (22) Patients with TP53 mutations in their SNSCC tended to have worse 3 years OS (43.8% vs 84.1%). (23) KRAS mutations are rarely detected in SNSCCs, however they are seen in almost all cases associated with Oncocytic Sinonasal Papilloma (OSP). (24) 50% of ISP converting into malignancy have an EGFR 20 exon mutation, and overall EGFR mutated SNSCC has worse prognosis (25,26) Amplification of FGFR1 has been reported in approximately 20% SNSCC, SOX2 amplification has been reported in 37% of SNSCCs and was associated with a higher incidence of disease recurrence. (27) Also, VEGFR gene overexpression has been detected in ~50% of SNSCCs. (28)
Presentation
The most common site of SNSCC is the maxillary sinus (~60%), followed by the nasal cavity (25%) and ethmoidal complex (15%). (29,30) Like most sinonasal malignancies, diagnosis of SNSCC most often occurs at an advanced stage due to nonspecific symptoms early in the disease, with >80% of patients reportedly presenting with at least stage T3 disease. (31)
Pathology
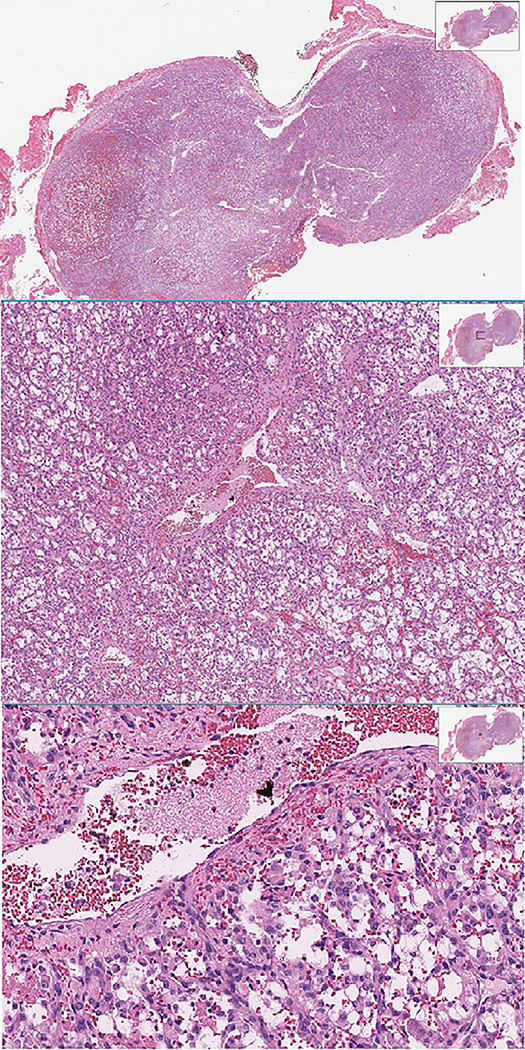
Histologic diagnosis of SNSCCs can be challenging due to relative rarity, a large miscellany of different possible histology, and overlapping appearance of other malignancy or inflammatory disease. Both keratinizing and nonkeratinizing types of squamous cell carcinoma are encountered. (32) In 1 to 7 percent of cases, SCC is seen embedded within ISP, and are classified as IP-associated SCC (IP-SCC). A recent retrospective review that was performed on 52 sinonasal tumors showed 21% of these patients had discrepancies between preliminary pathology and post-surgical diagnosis, with alteration in management in all cases with a change in histologic diagnosis. (33,34) These findings support the general recommendation to provide the pathologist with abundant material to emhasize the importance of second-opinion review by subspecialized pathologists to minimize diagnostic error. Figure 1 shows an H&E slide of a representative SNSCC originating from an ISP.
Figure 1:
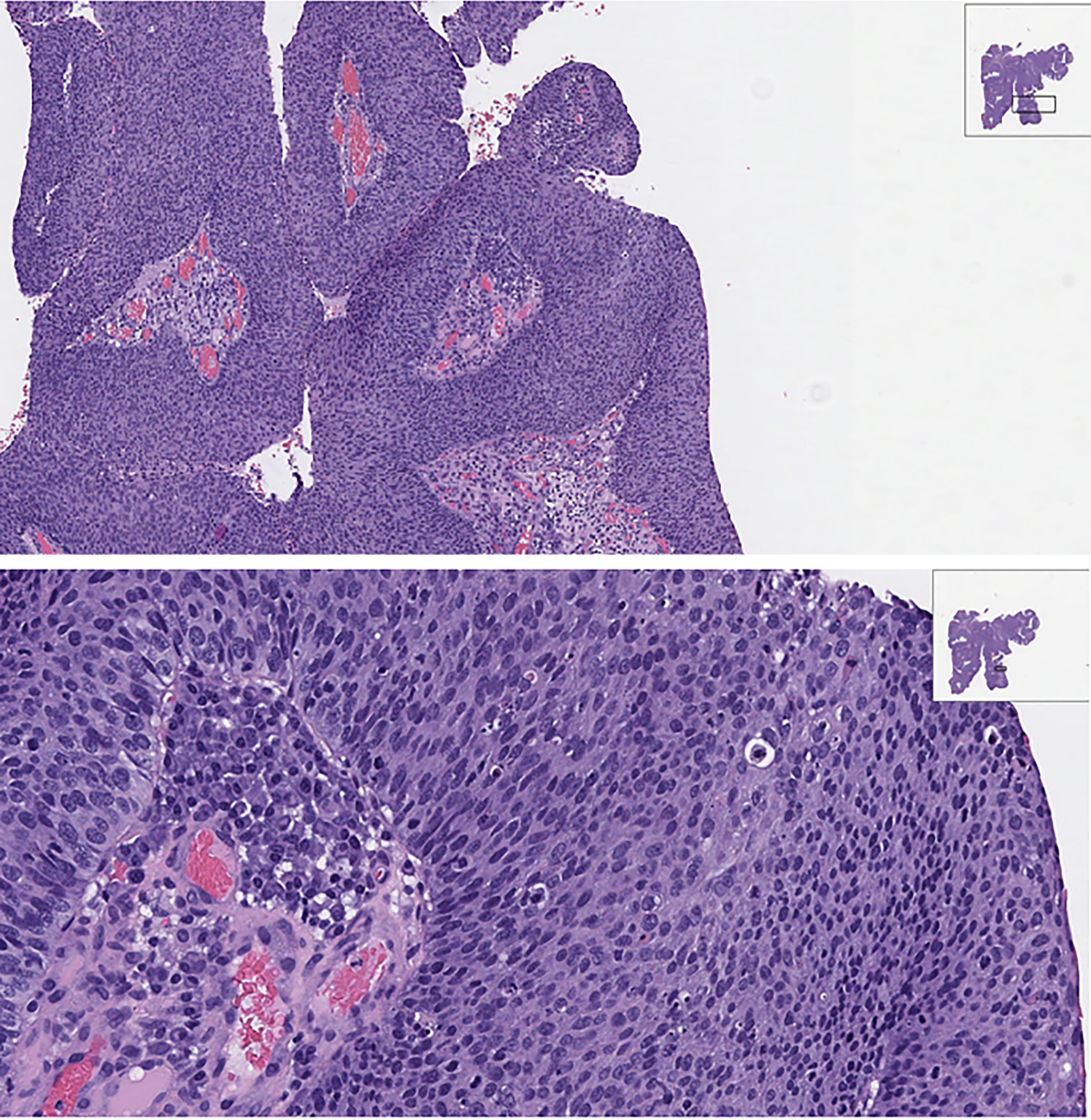
H&E of Sinonasal Squamous Cell Carcinoma originating from an inverted papilloma
Imaging
SNSCCs are characterized by aggressive bony destruction of the adjacent sinus walls. Invasion of the contralateral sinonasal area, orbital wall, infratemporal fossa, and skull base is sometimes observed. Therefore, a combination of CT and MRI provides the most accurate assessment of tumor extent. Due to its high spatial resolution, CT is required to visualize the structural changes and reabsorption of thin bone structures and should be utilized when malignancy is suspected. Gadolinium-enhanced MRI is valuable to assess soft tissue involvement, which can otherwise resemble other conditions such as lymphoma, necrosis, inflammatory, mucus retention, and fibrosis. (29,35,36). Evaluation of apparent diffusion coefficient (ADC) values on MRI is a promising way to differentiate benign ISPs from IP-SCCs which are associated with a loss of convoluted cerebriform pattern and lower ADC values. (37) ADC values are also useful in differentiating sinonasal lymphoma as the ADC values of maxillary SNSCCs are typically higher than those for lymphoma.(35) PET-CT is also widely used as an alternative to MRI since there appears to be a correlation between different histopathologic types of sinonasal tumors and SUV values. PET-CT can be used as “metabolic biopsy” to characterize sinonasal malignancy. Figure 2 shows PET-CT of SNSCC arising from ISP. Not surprisingly, the authors found overlap in fluorodeoxyglucose (FDG) uptake values among some histologic subgroups. Therefore, a surgical biopsy is necessary. (38) PET-CT has also been used as a prognostic tool. A retrospective study described that absence of pathologic FDG uptake at the first post-treatment PET-CT was associated with better OS. (39)
Figure 2:
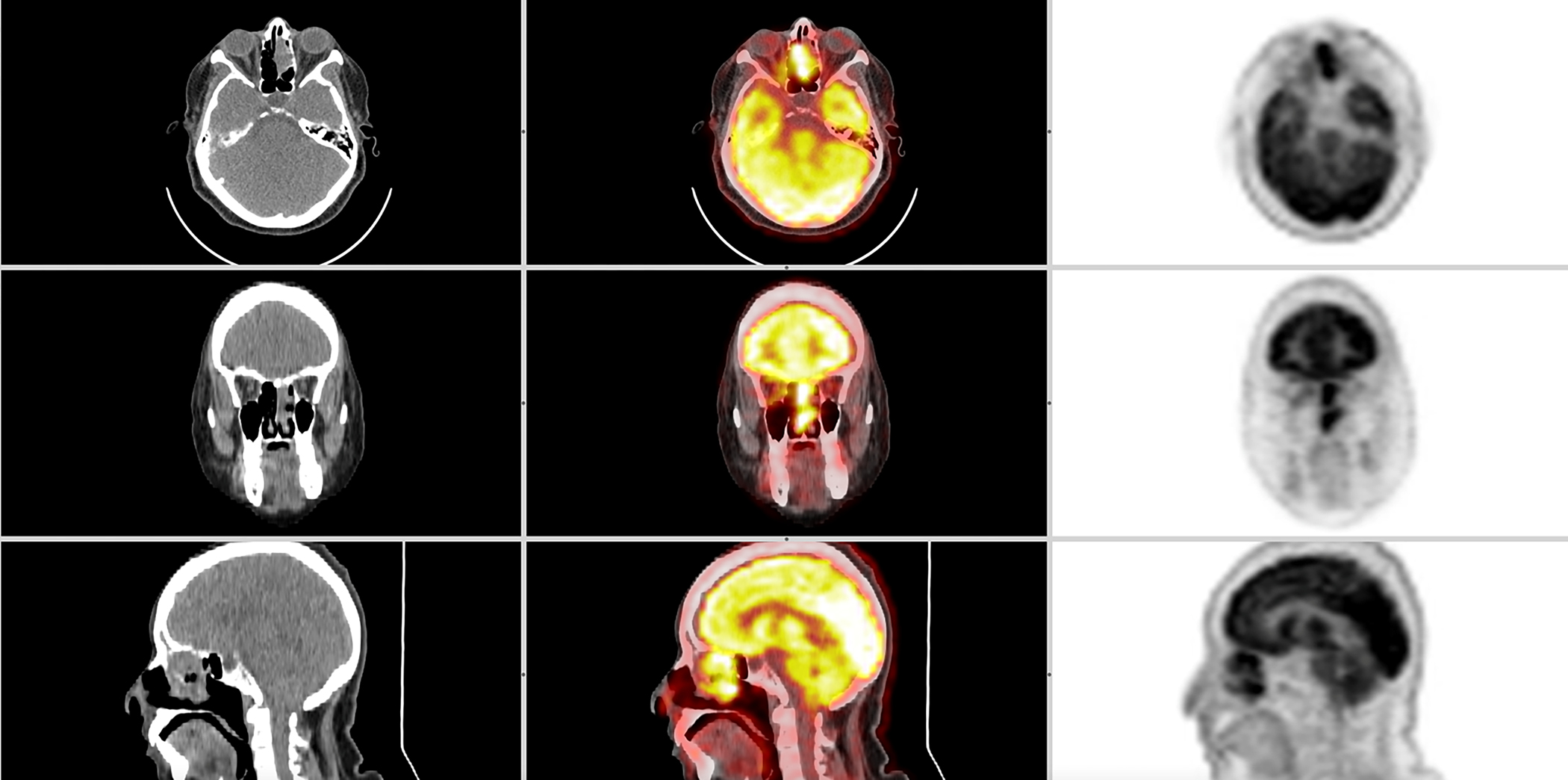
PET/CT of Sinonasal Squamous Cell Carcinoma originating from an inverted papilloma
Current treatment
Surgery is a potentially curative treatment for resectable SNSCCs with the goal of complete resection and achieving negative margins. Due to the proximity of the nasal cavity and paranasal sinuses to critical structures, complete resection can be prohibitively morbid. For example, radical surgeries such as craniofacial resection, ethmoidectomy, sphenotomy, or total maxillectomy are associated with high morbidity, adverse effect on function, and disfigurement. The surgical approach, including endoscopic or open surgery, should be selected based on tumor location and local extension.
A large retrospective study by Jafari et al (40) identified 7,808 patients with SNSCC who were treated with different approaches and highlights the importance of negative surgical margins. 31.1% of patients who underwent surgery had positive surgical margins (PSM) and of those cases, around half had microscopic PSM and the rest had macroscopic PSM. Compared to nonsurgical treatment, negative surgical margins (NSM) and microscopic PSM were associated with improved survival. The benefit of surgical treatment was not seen in the macroscopic PSM group, particularly in advanced stages, including nodal metastasis. The median OS was 90.5 months, 56.7 months, 38.4 months, and 36.4 months for patients with NSM, microscopic PSM, macroscopic PSM, and nonsurgical therapy, respectively. Risks and benefits should be discussed before electing to proceed with surgery, particularly for patients with advanced stage disease. Patients should be counseled that surgery might not improve OS when compared with nonsurgical therapy if gross total resection is not achieved, and that surgery bears inherent risks.
In clinically node negative disease, elective neck dissection (END) remains controversial. In a retrospective study of 1120 patients using the National Cancer Database (NCDB), 220 patients (19.6%) underwent END. The occult metastasis rate in the END cohort was 12.7% and in propensity score-matched cohorts END did not correlate with OS (HR 0.971, 95% CI 0.677–1.392). (41)
To achieve negative surgical margins, the use of induction chemotherapy has been investigated as part of multimodal treatment. In the past decade, multiple studies have shown promising results of induction chemotherapy by improving morbidity, function/organ preservation, and overall survival. (42–46) A large retrospective study by Abdelmeguid et al (47) included 123 locoregionally advanced SNSCC patients who were treated with platinum-based induction chemotherapy as part of their overall treatment. 88.6% of patients received platinum with taxanes with or without another agent, and the rest received platinum-based doublet regimens without a taxane. Seventy patients (56.9%) achieved at least a partial response, and stable disease was seen in 32 patients (26.0%), whereas 21 patients (17.0%) had progressive disease. The 2-year OS of patients who had at least stable disease after induction chemotherapy compared with those who had progressive disease was 68.2% vs 33.3%, a statistically significant difference. More importantly, before treatment, 66.7% of patients had orbital invasion, but only 18.3% required orbital exenteration at the time of surgery. These data support the hypothesis that induction chemotherapy is associated with an improved OS and better chance of organ preservation.
The data on neoadjuvant chemoradiation in SNSCCs is scarce. Robin et al (48) conducted a large retrospective multivariate analysis of 11,160 patients with sinonasal cancer using the National Cancer Data Base and found those who received neoadjuvant treatment had a higher likelihood of achieving a negative surgical margin and better OS than those who did not, but this group also included patients who received any form of neoadjuvant treatment (including chemotherapy or radiotherapy alone). Another retrospective study by Fernstrom et al (49) suggested that neoadjuvant chemoradiotherapy can be beneficial to patients with locally advanced disease to decrease surgical morbidity, but does not improve OS. However, prospective clinical studies studying the exact role and benefit of neoadjuvant chemoradiotherapy are needed.
Adjuvant intensity-modulated RT (IMRT) after complete resection is considered the standard treatment for pT2-T4 SNSCCs, aiming to decrease the incidence of local recurrence. A handful of retrospective studies have indicated that control rates with surgery and adjuvant radiotherapy ranged between 50 and 85%. (50–52) Compared to conventional 2D and 3D techniques, IMRT for the management of unresectable T4 paranasal sinus and skull base malignancy may help preserve organ function and minimize toxicity. (53) Intensity-modulated particle therapy (IMPT) such as protons and 12C-carbon ions are also being investigated for these patients and allows the design of a sharp dose gradient to a well-defined depth, leading to much higher radiobiological effectiveness and reduced dependence on tissue oxygenation. (54) A retrospective study by Zhang et al revealed that in comparing proton radiation therapy (PRT), IMRT, and carbon ion radiation therapy (CIRT), CIRT appeared to provide significant improvement in 3-year OS of 75.1% compared to 66.2% for PRT and 63.8% for IMRT in nasal cavity and paranasal sinuses malignancies. (55) However, no prospective studies have been published to confirm the superiority of CIRT. Concurrent platinum-based adjuvant therapy is often delivered to further optimize tumor control. The aim is two-fold, both radiosensitization and targeting of residual disease in cases of positive margins, with high-risk features, such as nodal, perineural or lymphovascular invasion. (29) The dose and schedule of cisplatin typically used is either 100mg/m2 every 3 weeks or 30–50 mg/m2 weekly. Carboplatin can be considered if the patient is ineligible for cisplatin. (56,57)
Definitive chemoradiation therapy is employed for patients with unresectable tumors or for those who do not choose to undergo surgery. The chemotherapy is platinum-based as above delivered using the same schedule described above in the adjuvant setting. Radiation is typically delivered in the form of IMRT, although IMPT can be used as an alternative to IMRT, concurrent with chemotherapy. Currently, there are no prospective data comparing these two forms of radiation. A phase II study investigating the efficacy and toxicity of intensity-modulated or proton radiation therapy for locally advanced sinonasal malignancy is ongoing. (58)
For locoregional recurrence, salvage surgery or re-radiation is generally recommended, if possible. Otherwise, palliative systemic chemotherapy is considered the treatment of choice. (59–61) Orlandi et al (62) reported that patients with locally advanced sinonasal cancers treated with palliative chemotherapy achieved significantly improved OS compared to those without. Palliative regimens were either platinum-, anthracycline-, taxane-, and/or alkylating-agent-based (e.g., temozolomide or ifosfamide) in different combinations. However, there was no treatment-related subgroup OS analysis in SNSCCs. The role of immunotherapy is still not defined as most of these patients are excluded from HNSCC immunotherapy clinical studies. (63) However, Riobello et al (64) have demonstrated membranous expression of PD-L1 by the cancer cells in 34% of SNSCC cases and by immune infiltrating cells in 45%, which might indicate a potential benefit from immunotherapy. Figure 3 demonstrates an algorithm for the management of SNSCC.
Figure 3:
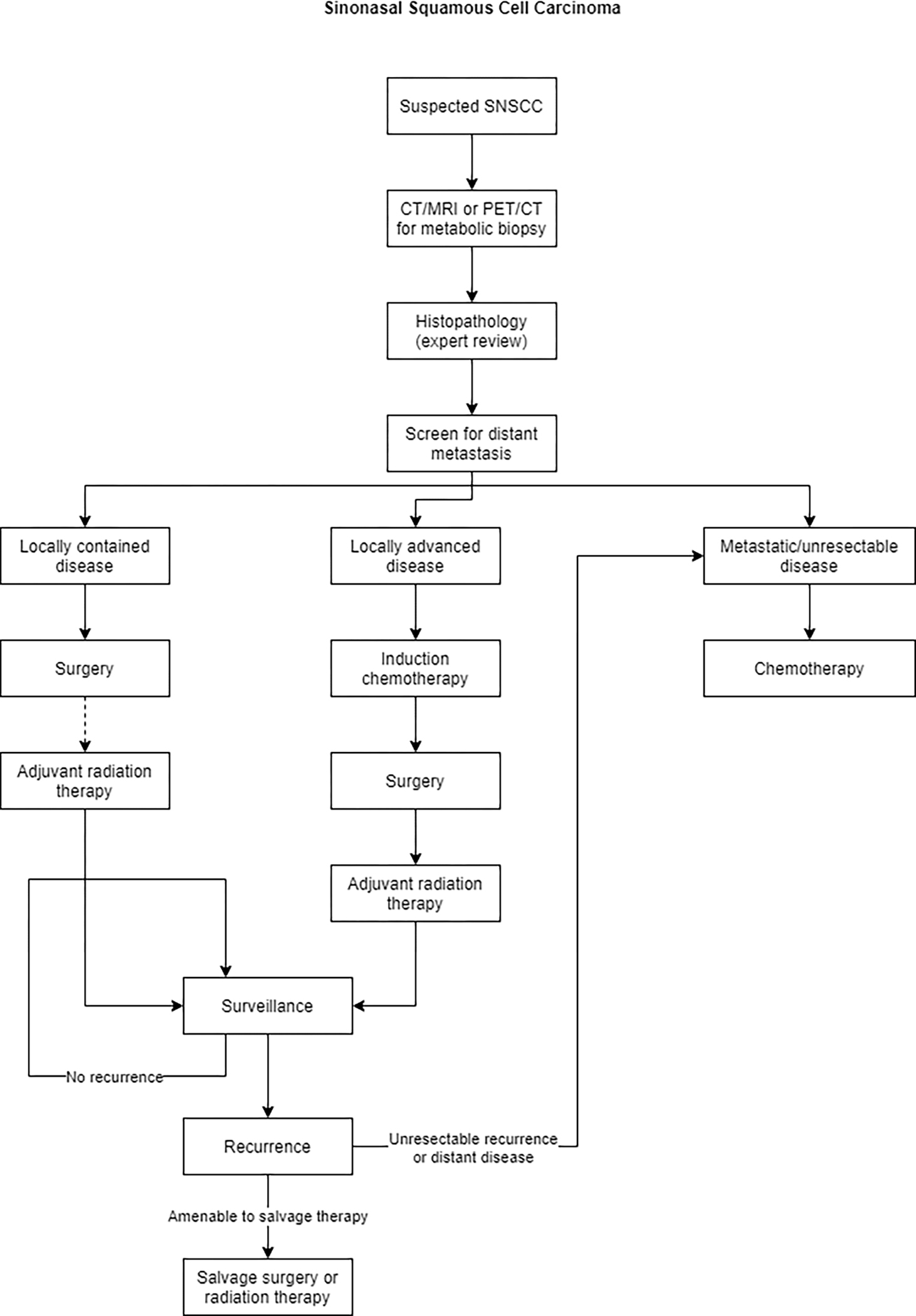
Authors algorithm summarizing management of Sinonasal Squamous Cell Carcinoma
Prognosis/Surveillance
The 5-year overall survival (OS) has been around 50% in the past three decades partly due to high rates of local recurrence. (65–67) Patients with nasal cavity SCC have increased 5-year relative survival rates compared to patients with SCC of the maxillary, ethmoid, frontal, and the sphenoid sinuses. (48,67) Additionally, male sex, older age, higher T and N stage, and poorer tumor grade portend a poor prognosis. (68) Recurrence is as high as 56% at 5 years and 5-year OS is 50%. (69) Patients who survive after multimodal treatment should continue to undergo clinical (endoscopy) and radiological (CT, MRI, PET) examinations for at least 5 years given the high recurrence risk, as per head and neck cancer guidelines. (70) Optimal timing of surveillance in sinonasal malignancy is ill defined and 70% of clinicians follow the NCCN guidelines for head and neck cancer. (70) The frequency and length of follow-up should be individualized based on histology and grade.
Recent or ongoing developments/research
Few clinical studies for HNSCC are actively enrolling patients with SNSCC. However, there are studies for SNSCC, specifically evaluating IMRT and PRT for sinonasal malignancies (NCT01586767). (58) In addition, a phase II study is underway evaluating the response of docetaxel, cisplatin, and fluorouracil (TPF) in previously untreated Stage II-IV SNSCC (NCT00707473). (71)
Sinonasal Adenocarcinoma
Incidence and patient demographics
Adenocarcinoma is the second most common type of sinonasal cancer after squamous cell carcinoma. International data from public cancer registries suggests that it accounts for approximately 27% of all sinonasal malignancies. (72) The incidence of sinonasal adenocarcinoma (SNAC) has remained stable over the 40 years from 1973 to 2013.
Overall incidence based on SEER data from 1973 to 2013 was 0.44 per million, with men making up 58.2% of the cohort. The median age was 64 at diagnosis. The widespread use of nasal endoscopy and radiologic studies may have allowed earlier diagnosis leading to improved survival. (2)
Presentation
The most common sites of origin are the ethmoid sinuses and the nasal cavity, which account for more than half of the cases. An MD Anderson cohort of 66 patients noted 37.9% originated from the ethmoid sinus, 36.4% from the nasal cavity and 19.7% from the maxillary sinus.(73) Tumor extension to the skull base, sphenoid sinus, dura, brain, orbit, or cribriform was seen at diagnosis causing related symptoms.
Pathophysiology
A wide range of histopathology findings is seen in SNAC, which can be divided into the salivary type and non-salivary type. The non-salivary type can be further subdivided into the intestinal type and non-intestinal type (low grade and high grade). (74) The risk factors for sinonasal adenocarcinoma have been identified based on reports of environmental exposure in affected patients. Low-grade non-intestinal SNAC has no known association with environmental exposures. For intestinal-type SNAC, there is a notable association with long-term exposure to wood dust. Workers with occupational exposure to hardwood dust show incidences that are 1,000 times those of the general population. Wood dust exposure was observed in 20% of SNAC cases within a historic cohort with an exposure period of 40–43 years. (74) In a more recent cohort of 66 patients seen at a single institution in the US, smoking and alcohol consumption were the most common exposures and reported in 30 (45%) and 45 (68%) patients respectively. Only three patients reported exposure to wood dust. (75) The carcinogenic compound from wood dust exposure has not been identified but a possible role of tannins is suspected. (74)
Pathology
Low-grade non-intestinal adenocarcinoma (LGNIA) encompasses a heterogeneous morphology with exophytic masses with crowded back-to-back glandular complex configurations in papillary or microcystic patterns. They also lack necrosis, increased mitotic activity and nuclear polymorphism, which differentiate them from high-grade non-intestinal adenocarcinoma. (76)Intestinal-type SNAC mimics the appearance of the normal or neoplastic large and small intestine and cannot be differentiated from metastatic colorectal carcinoma based on histology alone. Both intestinal-type SNAC and colorectal carcinoma express CK20, CDX-2, MUC2, and villin. The presence of CK7 may be suggestive of intestinal-type SNAC. (74) TP53 is the most common genetic alteration seen in 40–50% cases. (26) Other notable differences are seen at the molecular level, where SNAC is associated with MET gene amplification, and colorectal carcinoma is associated with KRAS and BRAF mutations. (77)
Two other rare entities are sinonasal renal cell-like adenocarcinoma and ETV6 rearranged SNAC. (76) The former is a clear cell variant of LGNIA and was included as a new tumor entity in the 4th edition of the WHO classification of sinonasal tumors. It expresses CK7, CAIX, DOG-1, and SOX10, and is negative for CK20 and PAX-8, findings that help to distinguish it from metastatic renal cell carcinoma. ETV6 rearranged sinonasal adenocarcinoma has tubular proliferations with rare apocrine features, and is strongly positive for CK7, GCDFP-15, DOG-1 and SOX-10, weakly and focally positive for S100 and negative for GATA-3 and mammaglobin. They are associated with ETV6/receptor tyrosine kinase gene fusions, like ETV6-NTRK3 or ETV6-RET. Both ETV6-rearranged and LGNIA have an indolent clinical course.
Imaging
Imaging and staging of SNAC are similar to SNSCC.
Current treatment
Local control and prevention of locoregional recurrence are the main treatment objectives in SNAC. Disease-related death is typically attributed to local recurrence, which occurs in approximately half of cases. Lymph node spread and distant metastases are uncommon and occur in approximately 8% and 13% of cases, respectively. Most published reports are retrospective studies with heterogeneous disease characteristics, making evidence-based conclusions difficult. (73,75,78,79)
The mainstay of treatment is surgical resection with curative intent. There are no prospective studies comparing surgery alone to other treatments. For tumors involving the ethmoid sinus, surgery with or without radiation had a survival advantage compared to radiotherapy alone in a retrospective study of 418 patients. (75) Different surgical approaches are reported with endoscopic resection having a survival advantage; however, bias is present with patient selection for this approach favoring smaller, lower stage tumors.(78) Prophylactic dissection of cervical lymph nodes is not recommended in SNAC. In a retrospective study of sinonasal malignancies diagnosed at two academic centers from 1975 to 1994, 5 of 386 patients had lymph node involvement at diagnosis.(79) None of the five lymph node positive patients had SNAC, with four of the five patients having squamous cell carcinoma and one patient having undifferentiated carcinoma.
Most case series report the use of preoperative or postoperative radiotherapy, but there are no prospective studies. In retrospective series, patients treated with radiotherapy alone are more likely to have locally advanced unresectable tumors and the outcomes are not comparable to surgery. Adjuvant radiotherapy is also more likely to be given in higher-grade tumors or cases with positive margins. In a review of 66 patients treated at MD Anderson Cancer Center from 1993 to 2009, 24 patients (36.4%) were treated with surgery alone and 26 (39.3%) were treated with surgery and radiation with or without chemotherapy.(73) The rest received either surgery with chemotherapy, only radiotherapy or no treatment at all.
Several chemotherapeutic agents are reported to be effective for SNAC. Cisplatin and 5-FU were associated with a 37% overall response rate in the neoadjuvant setting. The complete response rate was 15% and partial response was seen in 22%.(80)
Prognosis/Surveillance
SEER analyses suggest 5-year survival improved over time from 49.6% between years 1973 and 2006 to 63.8% between years 1973 to 2013. (65,81) SNAC appears to have improved survival outcomes compared with SNSCC. The 5-year disease-specific survival (DSS) is 63% for SNAC, which is higher than 53% for SNSCC. (73) Paranasal sinus involvement, age ≥ 75 years, black race, advanced stage, and high-grade tumors have been identified as poor prognostic factors for patients with SNAC. (73,82) Survival is variable from cure in early stages to poor prognosis in higher stage patients; specific factors that were found to be significant in a multicenter study by Choussy et al. (75) included the size of the lesion (specifically T4 disease), lymph node involvement, and extracranial extension.
Patients who are at high risk are more likely to have a local recurrence than distant metastases. Therefore patients who undergo treatment should have interval ENT evaluation examination.
Recent or ongoing developments/research
There is a single ongoing study evaluating IMRT and PRT for sinonasal malignancies irrespective of pathology. (58)
Sinonasal Neuroendocrine Carcinoma
Incidence and patient demographics
Like all sinonasal malignancies, sinonasal neuroendocrine carcinoma (SNEC) is exceedingly rare at 2% of sinonasal malignancies. (11) In an analysis of the SEER database, the incidence rate was 0.012 cases per 100,000 in 1986, and 0.0077 cases per 100,000 in 2011. (83) The mean age at diagnosis was 55.8 years, and males were predominant with 60% of cases.The most common site for SNEC in the SEER database was the nasal cavity followed by the ethmoid sinus. (83)
Presentation
Like most sinonasal malignancies, the symptoms at presentation are non-specific. Lower-grade SNEC can be locally aggressive and cause localized symptoms, while poorly differentiated SNECs can metastasize outside the sinonasal cavity and skull base. (84,85) Small cell NEC has been associated with paraneoplastic syndromes causing SIADH, SIADH, and Lambert- Eaton Syndrome. (85)
Pathophysiology
There is no information available about the etiology and pathophysiology of SNEC. This is likely due to the rarity of the disease.
Pathology
Typically, neuroendocrine carcinomas (NEC) show histologic features of neuroendocrine differentiation with patterns comprising nests, ribbons, festoons, glands and rosettes, and solid areas. They also are positive for broad-spectrum keratin antibodies and neuroendocrine markers like CD56/N-CAM (diffuse) with synaptophysin and/or chromogranin (focal/variable) and dense-core, small membrane-bound cytoplasmic vesicles by electron microscopy. (86,87) The 2017 WHO classification of neoplasms of the head and neck identified four different subtypes of SNECs - carcinoid tumor, atypical carcinoid tumor, poorly differentiated neuroendocrine carcinoma of small cell type and poorly differentiated neuroendocrine carcinoma of large cell type. (88)
There is likely a correlation between grade and prognosis in SNEC, though due to low incidence, the evidence is not definitive.(87) Small cell SNEC and large cell SNEC are aggressive with a 5-year survival of patients of 20.8 % and 21.7%, respectively. (89)
There is some similarity in the mutation profiles of aggressive sinonasal SNEC and small cell carcinoma of the lung with mutations of TP53 and RB1, up-regulation of BCL2 signaling, and activation of MYC and PI3K pathways. (90) This suggests that additional studies of this rare histologic type are required to better define the mutational landscape of SNEC.
Imaging
Imaging and a staging workup are similar to other sinonasal malignancies. There is no definitive recommendation about the use of Gallium-DOTATATE PET/CT in SNEC but there have been some studies evaluating their utility in low-grade SNEC where there was no radiotracer uptake on FDG PET/CT. (91)
Current treatment
The ideal treatment for SNEC is still unknown. Surgical management is likely appropriate for carcinoid. But there is no consensus among physicians treating higher grade SNEC about the sequencing of surgery, radiotherapy, and chemotherapy. Babin et al (92) developed a protocol using response to induction chemotherapy as a marker of tumor response, and this approach has been validated by other studies showing response to chemotherapy as a sign of favorable prognosis. (84) Chemotherapy utilized included platinum along with etoposide. If there was a good response, or surgery was not possible after 2 cycles, the protocol recommended radiotherapy followed by additional cycles of chemotherapy. If there was a poor response but resection was possible, the recommendation was to proceed with surgery followed by adjuvant radiation and complete 4–6 cycles of chemotherapy. Figure 4 outlines treatment algorithm for the SNEC.
Figure 4:
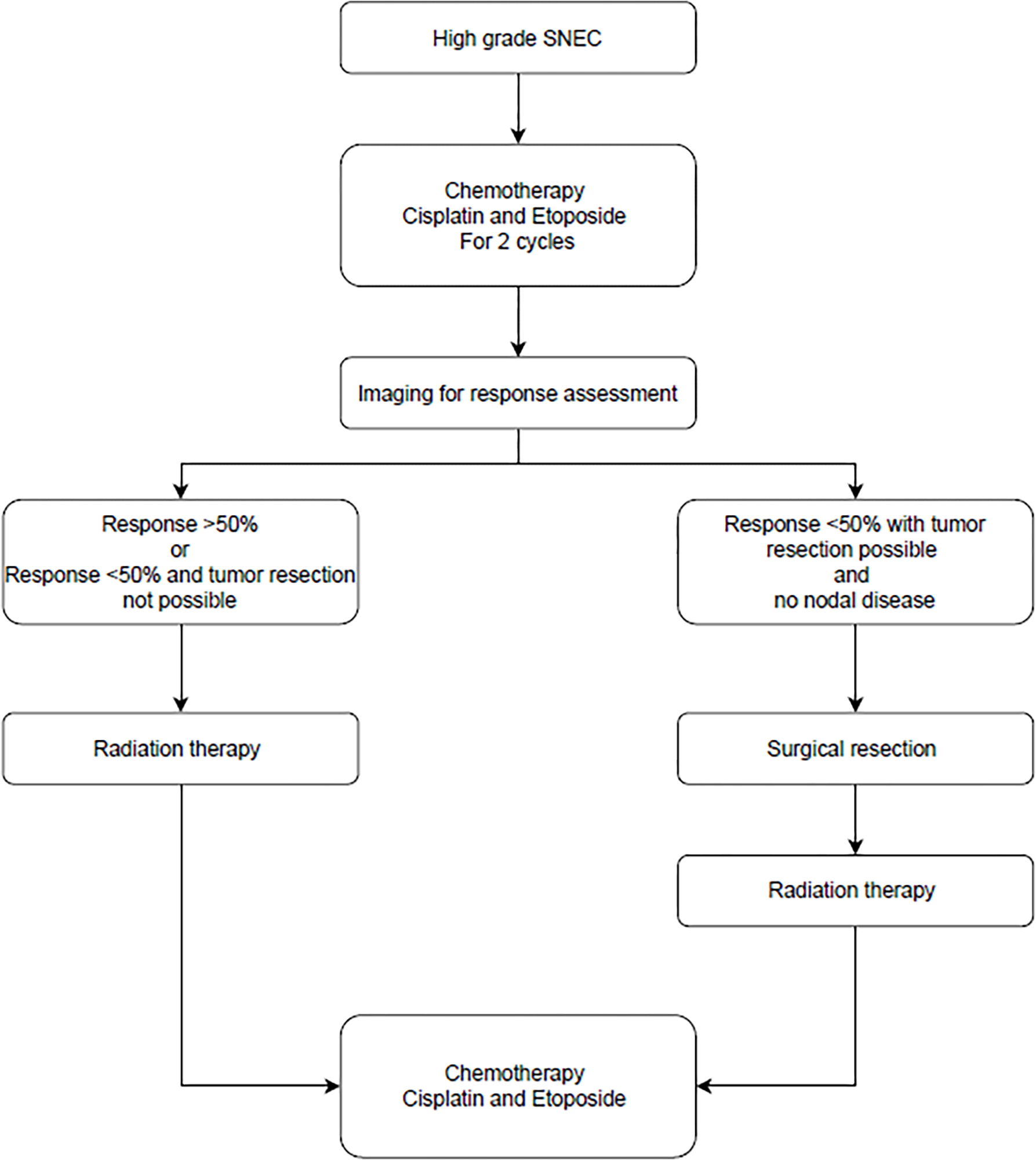
Authors algorithm summarizing management of Sinonasal Neuroendocrine Carcinoma
Prognosis and surveillance
The 5-year disease-specific survival (DSS) rate for SNEC ranges from 50 to 70%. Treatment failure can be a locoregional or distant in high-grade SNEC. (83,93) The median overall survival was 32 months. (83) As expected, survival was better for lower grade and stage SNEC. The DSS was longer for primary nasal cavity tumors, followed by maxillary sinus and ethmoid sinuses, possibly due to nasal cavity tumors being symptomatic earlier. (93) The best survival was noted for sphenoid sinuses SNEC, for unclear reasons; this observation may the result of random error due to the small sample size.
Surveillance of these patients depends on the grade of SNEC. Low-grade SNEC can be followed by ENT examinations, whereas higher-grade SNEC like small cell variant should undergo serial imaging to detect distant metastasis. This recommendation is based on recommendations for small cell carcinoma of the lung, due to a lack of prospective post-treatment surveillance data in patients with SNEC.
Recent or ongoing developments/research
There are no current active clinical studies for patients with SNEC.
Sinonasal undifferentiated carcinoma (SNUC)
Incidence and patient demographics
SNUC is an extremely rare carcinoma that makes up 5% of all sinonasal malignancies.(11) It was first described in 1986 as a highly aggressive carcinoma of the nasal cavity and paranasal sinuses that originates from the Schneiderian epithelium or nasal ectoderm. This pathologic diagnosis originated from a small case series of eight patients diagnosed between 1975 and 1985. (94) Since the original description, 318 total cases were documented from 1973 to 2010 in the SEER Program, with an approximate annual incidence rate of 0.02 per 100,000. There was a higher incidence in males than females (0.03 vs 0.01 per 100,000 per year). (95) The median age at which patients present is 50–60 years. (96) Overall, SNUC has become somewhat of a catchall diagnosis for all undifferentiated sinonasal malignancies. (97)
Pathophysiology
The risk factors and etiology of SNUC are still unknown. Possible associations with tobacco use and with certain environmental/work exposures have been reported. Seven of the eight patients in the original 1986 paper by Frierson et al. had prior exposure to tobacco (no median pack-year history available). One worked in the coal industry, and another worked in the chrome plating industry and was exposed to sulfuric acid, chromic acid, nickel, zinc, copper, and metallic dust. (94) Nickel refinery workers (98) and softwood exposed furniture workers may also be at a higher risk. (99)
There is controversy surrounding other potential risk factors associated with SNUC, particularly the role of Epstein-Barr virus (EBV). One study by Lopategui et al (100) identified EBV RNA in seven of the eleven SNUC patients from Asia but none of eleven Western SNUC patients. However, a subsequent study of 36 SNUC patients from Taiwan (where EBV is endemic) found no SNUC patients with EBV. (101) Additional studies failed to confirm any association between EBV and SNUC. (102)
Clinical presentation
One systematic review of 140 patients reported that patients presented with common symptoms like nasal obstruction (20.0%), epistaxis (17.1%), diplopia or other visual symptoms (15.0%), or headache (12.1%). Since these are generally aggressive tumors, orbital involvement was seen in 42.9% of patients. (103) In a retrospective study of TNM stage at diagnosis in 128 patients, the majority met the criteria for Stage IV disease (72.9%). Common sites of metastases include bone, cervical lymph nodes, lung, brain, and liver. (103–107)
Pathology
Grossly, SNUCs present as a large, fungating tumor with poorly defined margins. They are locally aggressive tumors invading surrounding structures and lead to bone destruction. There is a high risk of regional and distant metastasis – with the rate varying from 5–16% of regional and 20–30% of distant metastasis. (108)
Histologically, as their name implies, SNUCs are truly undifferentiated as they lack any clear lineage pattern by histology and immunophenotyping, and is a diagnosis of exclusion. (109) It lacks S-100 but expresses certain epithelial type cytokeratins like CK 7, 8 and 19. (110) The cells are small to medium-sized, pleomorphic, and arranged in ribbons, sheets, large nests, trabeculae, and an organoid pattern. There is scant cytoplasm, hyperchromatic nuclei, prominent nucleoli, and homogenous chromatin. Further, there may be necrosis, lymphovascular and perineural invasion, and a high mitotic rate. (110,111) There has been an evolution of the definition of SNUC from being a wastebasket for any unclassifiable sinonasal malignancy to a specific entity. (86,112) Figure 5 shows histopathology of SNUC.
Figure 5:
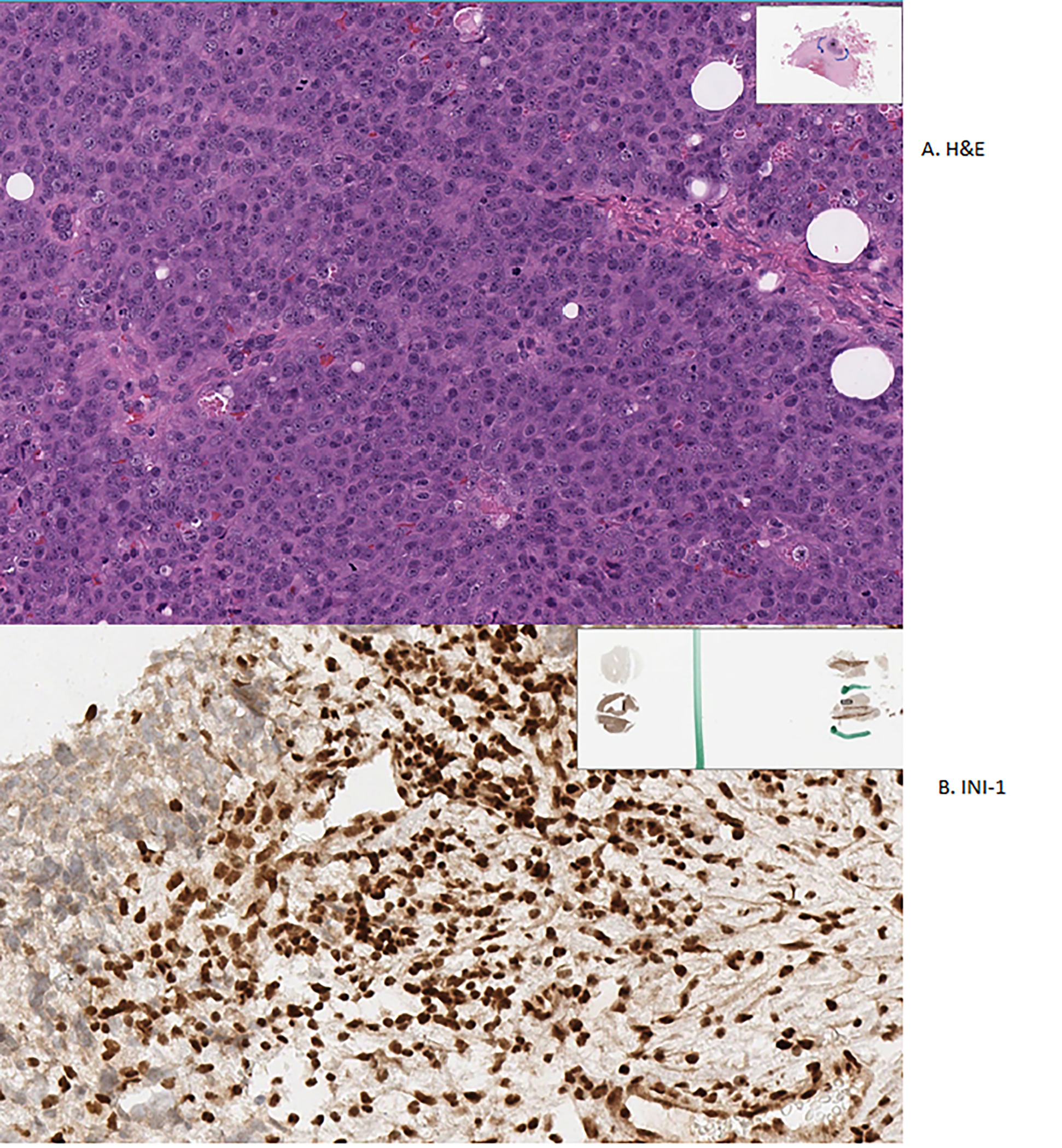
A. H&E stain of Sinonasal Undifferentiated Carcinoma showing undifferentiated cells B. Loss of INI-1 staining seen in Sinonasal Undifferentiated Carcinoma
Immunohistochemically, SNUC appears to have variable positivity for pan-cytokeratin (anti-cytokeratin monoclonal antibodies 1 and 3 (AE1/AE3)), low molecular weight cytokeratin (CAM 5.2), and cytokeratin 8 (CK8). There was a suggestion of association with p16 and KIT (CD117), however, this has been questioned. Wadsworth et al. (113) postulate that p16 may simply be the epithelial tract staining that can be seen normally in sinonasal tissue and not truly indicative of SNUC, as there was absence of HPV DNA expression. (114) Further, Chernock et al. (115) did not find any activating mutation nor gene amplification that led to the expression of KIT in SNUC. There was no reactivity seen with CK5/6, p40, HER2/neu, (116), lymphoma markers (CD45), sarcoma markers (CD99, desmin, and myogenin), and melanoma markers (MelanA and HMB45). Finally, there may be some expression of neuroendocrine markers like NSE, CD56, synaptophysin, and chromogranin; however, Thompson et al (117) states that there is no corresponding neuroendocrine morphology.
Takahashi et al. (118) performed a gene expression analysis between SNUC and SNSCC and found 7 genes related to DNA repair, synthesis, and cell division that completely distinguished between the two entities. The genes with greater expression in SNUC were CLCA2, ARID2, MAP1LC3A, SMAD4, HELLS, MAPKAPK5-AS1, and KRT16; CLCA was the most differentially expressed between the two groups. SNUC has been linked to somatic mutations in SMARCB1 (INI-1), SMARCA4 (BRG1), and isocitrate dehydrogenase 2 (IDH2). (119) Both SMARCB1 (on chromosome 22q11.2) and SMARCA4 (on chromosome 19) genes encode for subunits of the SWItch/Sucrose Non-Fermentable (SWI/SNF) protein complex, which regulates transcription and chromatin remodeling. (120) SMARCB1 deficiency was seen in nine of 142 (6%) cases in a study by Bishop et al. (121) and only a total of 89 cases of SMARCB1 deficiency have been reported thus far. (122) SMARCA4 deficiency was seen in ten patients in one study by immunostaining. (123) IDH2 R172X mutations have also been associated with SNUC. Mito et al. (124) found ten of sixteen (62.5%) SNUC cases with IDH2 mutations, Jo et al. (125) found six of the eleven cases (54.5%) on targeted next-generation sequencing, and Dogan et al (126) found variants in 14 of the 17 analyzed cases (82.4%) of SNUC. In their study, Dogan et al. studied 12 IDH2-mutated cases and found co-existing TP53 mutations in five cases (41.7%), CDKN2A/2B loss-of-function in four (33.3%), MYC amplification in four (33.3%), and SETD2 mutations in three (25%). (126,127)
Imaging
Imaging typically shows a large mass with local invasive growth. On CT imaging, SNUC tumors are noncalcified and can lead to sinus obstruction. On MRI, signal intensity was found to be isointense to muscle on T1- weighted images, iso- to hyperintense on T2, and heterogeneous enhancement was seen with gadolinium. (128) Figure 6 demonstrates an MRI with local invasion of SNUC into the base of the skull.
Figure 6:
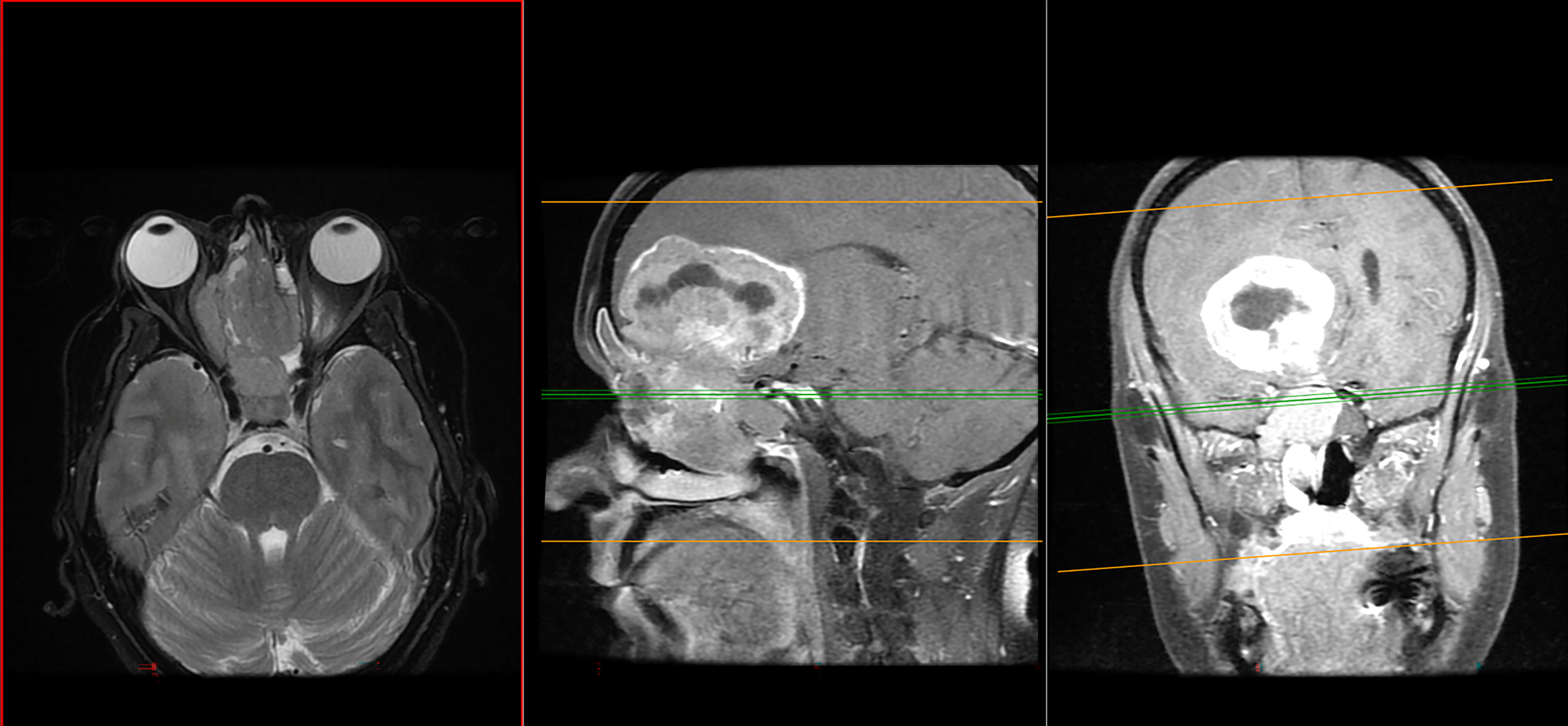
MRI with local invasion of Sinonasal Undifferentiated Carcinoma
Staging for SNUC uses either the American Joint Committee on Cancer orthe Modified Kadish staging system. The Modified Kadish system places patients in stages from A-D with A being limited to nasal cavity and D being regional or distant metastasis. (129)
Current treatment
Given the aggressive nature of SNUC, and involvement of the brain or eye, treatment can be quite difficult. A multimodal approach is typically favored incorporating surgery and radiation therapy with or without chemotherapy. (130) Patients treated with all three modalities had better outcomes when the dose of radiotherapy was ≥60 Gy). (131) In one meta-analysis, locoregional control with trimodality therapy was 63.9% as compared to bimodality regimens 49.2% and surgery alone at 31.3%. (132) However, in another meta-analysis comparing survival, trimodality was not superior to double modality treatment but combination therapy was still superior to single modality. (133)
There is no clear curative treatment for patients with SNUC. Case studies and retrospective studies help formulate the acceptable treatment options available for SNUC. Surgical resection remains a major component of treatment along with radiation therapy and chemotherapy. Previously, the gold standard was open craniofacial resection, with coronal or transfacial incisions and craniotomy, which has been replaced by endoscopic endonasal or craniofacial resection. (129) Radiation therapy is a critical component in the treatment of SNUC leading to improved survival. (134) Within radiation, intensity-modulated radiotherapy (IMRT) of at least 60 Gy as compared to conventional radiotherapy (CRT) was associated with improved overall survival, decreases toxicities, and improved 3- year recurrence-free survival. (130,135)
Finally, systemic chemotherapy is almost always included as part of the therapy regimen. Typically, cisplatin, etoposide, 5-FU, docetaxel, and paclitaxel are employed. The optimal sequence of these treatments is unclear but Amit et al (136) used induction chemotherapy to guide further management of SNUC in 95 treatment- naïve patients. In this study, patients first received platinum-based doublet regimen: cisplatin (60–80 mg/m2 on day one) followed by etoposide (100 to 120 mg/m2) or docetaxel (75 mg/m2) on days 1–3, every 21 days for one-five cycles (median three) prior to locoregional therapy. Response to this regimen was used to prognosticate patients, and further develop plans. As per their observation, patients with favorable responses to induction therapy did better than those with unfavorable responses. Patients who had a favorable response who were treated with combination chemoradiotherapy did better than patients who underwent surgery followed by adjuvant radiotherapy with or without chemotherapy. The hypothesis for this observation is that surgery might delay definitive radiotherapy which these patients need. Moreover, patients who had an unfavorable response to induction chemotherapy did better with surgery compared to definitive chemoradiation. Figure 7 summarizes recommendations on the management of SNUC.
Figure 7:
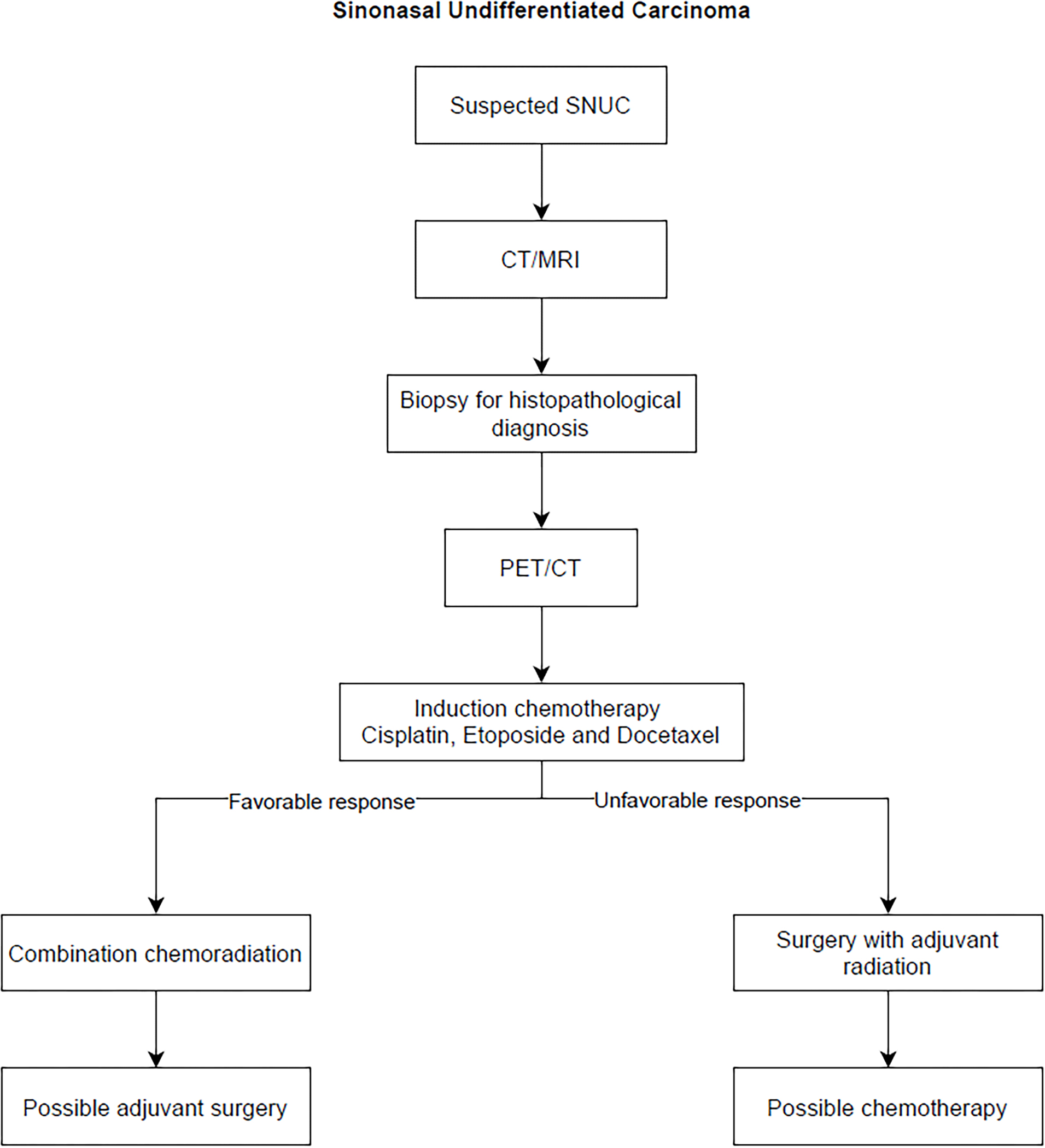
Algorithm summarizing management of Sinonasal Undifferentiated Carcinoma
Prognosis and surveillance
SNUC is an aggressive malignancy that confers a poor prognosis. These cancers tend to be locally recurrent and frequently metastasize leading to morbidity and mortality. The SEER database showed a median survival of 22.1 months, with a 5-year survival rate of 34.9%. (95)
Data on prognosis and survivorship varies considerably. 5-year survival estimates vary from6.25% to 74% in different series. Five-year survival rates following surgery, radiation, or combination of surgery and radiation were 38.7%, 36.0%, and 39.1%, respectively. (95)
The evidence-based recommendations for post-treatment surveillance are limited by the rarity of the disease and poor overall survival. We recommend surveillance with multidisciplinary follow-up for treated patients.
Recent or ongoing developments/research
Currently, ongoing studies are exploring the use of proton beam therapy for SNUC. In one multi-institutional study by Yu et al, (137) 69 patients underwent either uniform scanning or pencil-beam scanning PBT with a median dose per fraction of 2.0 Gy and found this to be a safe, equivalent, and possibly less toxic option than even IMRT.
Other strategies include the use of RADPLAT, which refers to radiation followed by intra-arterial infusion of cisplatin and thiosulfate. Noticewala et al (138) proposed this given the success of the strategy in other similar head and neck cancers (HNC). Elective neck treatment (irradiation or lymph node dissection) for patients with clinically node-negative disease leads to a lower risk of regional recurrence (OR 0.38 and 95% CI 0.25–0.58) compared to observation alone; however, the impact on overall survival is not clear. (139)
Primary Mucosal Melanoma
Incidence and patient demographics
Primary Mucosal Melanoma (PMM) is a rare disease and accounts for 0.03% of all cancer diagnoses. (140) Only 1.3% of all melanomas originate in the mucous membranes, but 70% of mucosal melanomas originate in the head and neck, with an increasing incidence over time. (141–143) The most common head and neck sites of origin for PMM are the nasal cavity and paranasal sinuses, followed by the oral cavity. (144) Between 1988 and 2010, the SEER database has 924 cases of head and neck PMM, of which 50% were nasal, 24% were sinus, 22% were oral and the rest were pharyngeal. (145)
For epidemiological purposes, most studies have considered all mucosal melanomas as one entity, including anogenital and vulvovaginal MM. The overall incidence is known to be 2.3 per million, which is significantly lower than for cutaneous or ocular melanoma. (145) The average age of patients diagnosed with PMM is 70 years, compared with 65 years for cutaneous melanoma. (146,147) The incidence increases exponentially with age without a plateau, and with no gender difference.(145) Like cutaneous melanoma, PMM is also more common in Caucasians, but these represent a smaller proportion of all melanomas than in other racial groups, due to a greater incidence of cutaneous melanoma in Caucasians.
Pathophysiology
The etiology and pathogenesis of PMM are unknown, and its association with pre-existing mucosal nevi is controversial. PMM is not related to racial pigmentation or sun exposure, unlike the more common cutaneous melanoma. (140,148) It has been conjectured that the close anatomic proximity of all the common sites in the head and neck region may reflect an embryological predisposition to PMMs. There is also speculation that PMM arises from pigment producing cells and Schwann cells found in the mucous membranes. (149) In addition, like other sinonasal malignancies, inhaled and ingested carcinogens may be linked to the pathogenesis of sinonasal, pharyngeal, and laryngeal PMMs, but these claims have never been confirmed with robust studies. (150–152)
Presentation
In addition to sinonasal symptoms, a pigmented lesion might be seen on ENT examination when patients present with symptoms. Rarely, a non-pigmented lesion may be seen. (153) These macules are asymmetric and irregular which differentiates them from melanosis.
Pathology
On histopathology, since PMMs usually present at advanced stages, they are seen as nodular neoplasms rather than as flat in situ lesions. (140) When PMM cells contain melanin, the histological diagnosis is straightforward, and can be confirmed using immunohistochemistry. When melanin is scarce or absent, then immunohistochemistry is required to make a diagnosis. PMMs commonly express S-100 protein and melanocytic markers, including MART-1/Melan-A, tyrosinase, HMB-45, and MITF. (154) In Figure 8, H&E-stained sections of a sinonasal melanoma show sheets of pigmented tumor cells, as well as intermixed lymphocytes.
Figure 8:
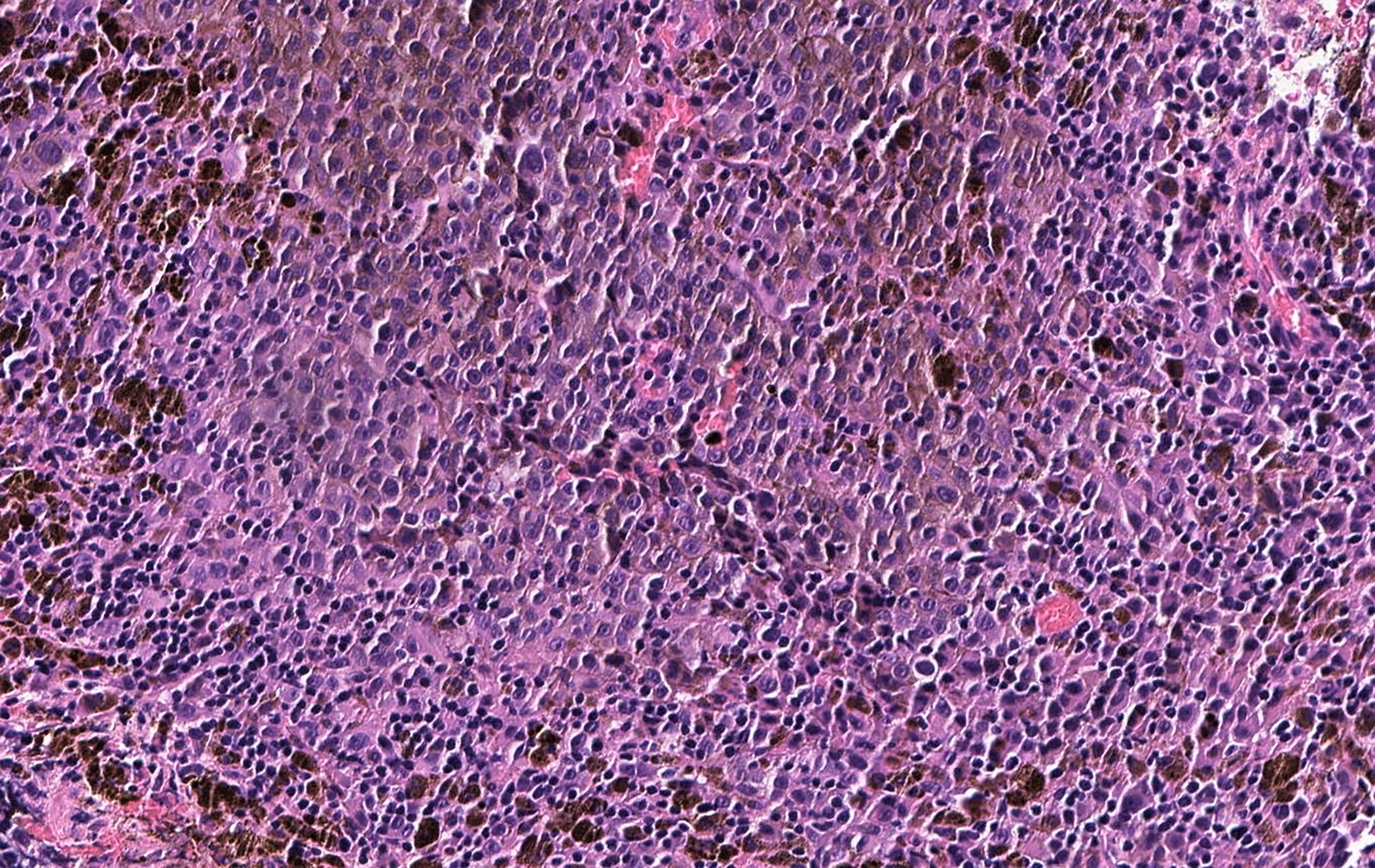
H&E of sinonasal melanoma with sheets of tumor cells, many of them loaded with melanin pigment, as well as intermixed lymphocytes
The molecular mechanisms that cause PMM are still largely unknown. The understanding of molecular pathways leading to malignant transformation of melanocytes will lead to more specific and successful therapies. (140) Notably, an increased frequency of c-KIT aberrations in PMM compared to cutaneous melanoma is shown in multiple studies. (155–160) These aberrations can be either somatic mutations or overexpression of unmutated KIT, which regulates the activity of MITF (microphthalmia-associated transcription factor) which is essential for melanogenesis and melanocyte function. (161) MITF can also be amplified in cutaneous melanomas, especially in the head and neck. (162)
While the discovery of BRAF mutations led to the successful development of BRAF inhibitors for the treatment of cutaneous melanoma, their role in PMM is limited due to a low prevalence of these mutations. BRAF mutations are seen in less than 10% of PMM cases, and while the incidence in sinonasal tract PMM is unavailable, the number is not believed to be high. (155,156,163,164) Another common genetic alteration in cutaneous melanoma is inactivation of the CDKN2A locus, which encodes the tumor suppressor protein p16/INK4A which is known to cause familial cases of melanoma, unrelated to sun exposure. (140,165) While 50% of PMMs were associated with loss of p16 expression, CDKN2A mutations, and/or loss of heterozygosity for CDKN2A, these abnormalities do not confer a worse prognosis in PMM, unlike in cutaneous melanoma. (158,166–170)
Imaging
MRI of PMMs exhibit low-signal intensity on T2-weighted images and enhancement on precontrast T1-weighted images. (140) In addition, since melanoma has high fluorodeoxyglucose (FDG) avidity, PET/CT plays an important role in staging these patients. In Figure 9, a PET-CT shows nodal metastasis in a patient after resection of primary sinonasal melanoma.
Figure 9:
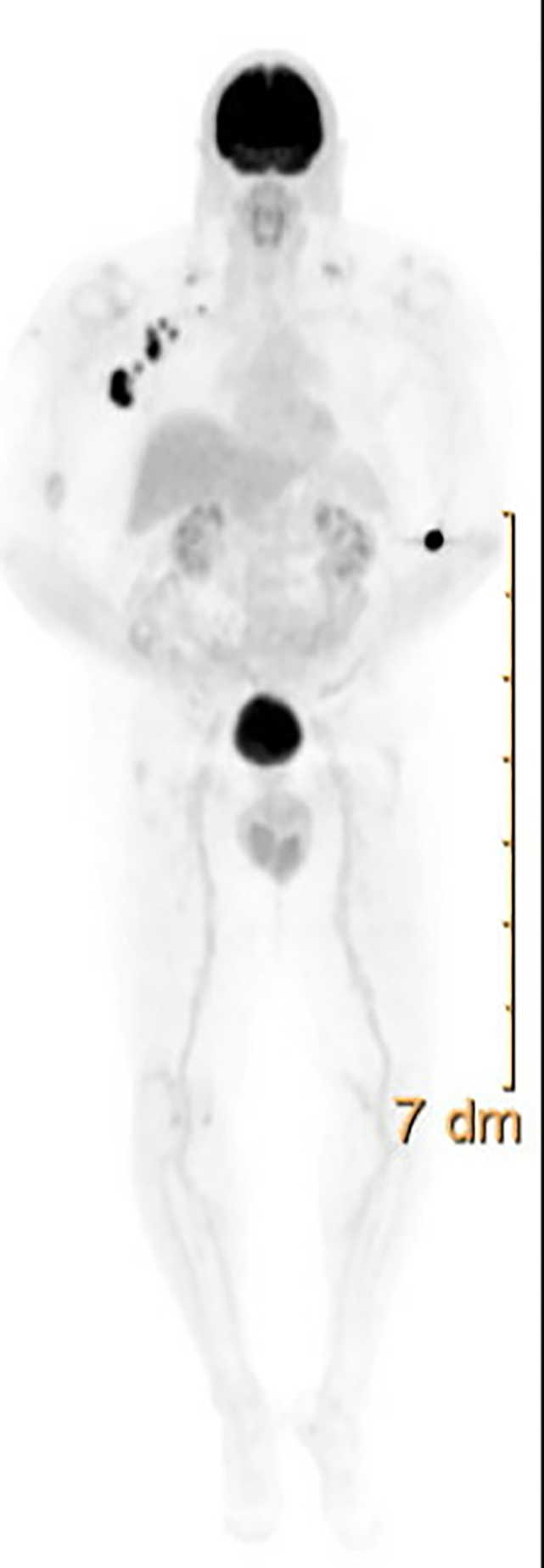
FDG-PET showing axillary lymphadenopathy in a patient with primary mucosal melanoma after primary resection
Staging of these tumors remains challenging, and there are multiple ways to stage the disease. (9,140) The American Joint Committee on Cancer (AJCC) staging for head and neck mucosal melanoma (mmTNM) is available and did not exist before 2010. To account for the aggressiveness of the disease, the primary tumor is designated either T3 or T4 based on the depth of invasion. This is a modification of the TNM staging developed by AJCC for carcinoma of the nasal cavity and sinuses (carTNM). (171,172) Ballantyne (173) described a method of classification where stage I includes localized lesion, stage II includes regional lymph node metastases, and stage III includes distant metastases. Even though this method is simple, it does not account for the depth of invasion of the disease. Prasad et al. (174) proposed another staging system based on the level of invasion of tissue compartments within the mucosa. Level 1 included in situ disease, level 2 included superficially invasive disease invading up to the lamina propria, and level 3 includes deeply invasive melanomas.
In a head-to-head comparison, Michel et al (175) compared survival between 3 staging systems for sinonasal PMMs -- the Ballantyne staging system modified by Prasad, the AJCC mmTNM, and the AJCC carTNM, and concluded that carTNM was the only staging system that significantly correlated with OS (p = .012) and DFS (p = .041). However, more recently, Gras-Cabrerizo et al. (176) performed a comparison of staging systems and concluded that the correlation between survival and tumor stage was better with mmTNM staging. The AJCC 8th edition recommends this classification system for staging PMM of the head and neck.
Current treatment
Complete surgical resection with uninvolved surgical margins is the mainstay of initial treatment. Patients with positive margins have a 21-fold increased risk of death and decreased survival. (177,178) Like most sinonasal malignancies, negative margins may not be achievable due to the need to preserve vital organs. If there is local recurrence without distant disease, salvage surgery is an option, but there is a significant chance of distant disease in a large majority of patients with local recurrence. Even though surgical resection with curative intent offers the best chance at survival, more than half of the patients ultimately develop distant metastases. (179) Elective dissection of the neck is recommended only in the treatment of non-sinonasal PMMs where lymph node metastases are more common. In oral PMM regional lymph node metastases is seen in 25% of cases at presentation and in 42% during the course of disease. The rate is only 6% at presentation and 20% during the course of the disease in sinonasal PMMs. (180)
Another topic of controversy is the role of sentinel lymph node biopsy in patients with clinically node-negative disease. Prinzen et al. (181), found sentinel lymph node (SLN) biopsy was an accurate staging tool, and their two SLN biopsy negative patients remained regional or distant disease-free, while two of three SLN biopsy positive patients developed distant metastases within the first year.
Radiation therapy may have a role in the adjuvant setting following surgery, or as primary treatment in patients who are not surgical candidates. In a study by Temam et al (182), postoperative radiotherapy was associated with better local disease-free survival in a historic dataset of head and neck PMMs treated between 1979 and 1997. However, patients who received postoperative radiotherapy developed distant metastasis more rapidly than patients who did not; although this observation is potentially confounded by the fact that patients who received postoperative radiotherapy had more locally advanced tumors than those who did not. This association was also noted in other retrospective studies. (183,184) A systematic review of the published literature showed a moderate survival benefit of postoperative radiation most likely due to reduced local recurrence. (185) Most experts agree that patients with a high risk of local recurrence, including patients with extracapsular disease, two or more lymph nodes involved, large nodes (3 cm or more), positive or close margins, or patients with residual disease, should receive postoperative radiotherapy. (186) A retrospective analysis by Moreno et al. (187) showed that patients with a total dose of 54 Gy or more had a lower recurrence rate compared to patients who had a total dose of 30–50 Gy. They also found that standard fractionation was associated with a lower locoregional failure rate compared to hypofractionation.
There have been very few clinical studies on systemic treatments for advanced head and neck mucosal melanoma patients, and the treatment of these patients is based on small case series. These few studies have failed to show a significant survival benefit with chemotherapy. (180) Older studies report response to chemotherapy in 7 of 15 patients with use of different combination regimens that included interferon alpha-2b, interleukin-2, cisplatin, vinblastine, dacarbazine, and/or temozolomide. The median time to progression for these patients was 10 months, and the median overall survival was 22 months. (188) A more recent study concluded that adjuvant chemotherapy with temozolomide and cisplatin resulted in lower risk of relapse and metastasis in resected mucosal melanoma when compared to high-dose IFN-a2b. (189)
Like cutaneous melanoma, immune checkpoint inhibitors are being used for the treatment of metastatic PMMs. Studies by Shoushtari et al (190) and Moya-Plana et al (183) have demonstrated an ORR of 23% and 20%, respectively. In another post hoc analysis of KEYNOTE-001, 002, and 006 evaluating the outcomes of pembrolizumab in mucosal versus non-mucosal melanomas, the ORR in mucosal melanoma patients was 19% in contrast to non-mucosal melanoma patients with an ORR of 33%. The median OS for these patients was 11.3 months. The lower response rate in PMMs could be because of lower PD-L1 expression. (192) CheckMate 067 explored 5-year outcomes of melanoma, and a subset analysis of 79 mucosal melanoma patients showed poorer long-term efficacy in comparison to patients with cutaneous melanoma. The study also noted that the combination of nivolumab and ipilimumab was superior to single agent nivolumab or ipilimumab. (193)
In addition, PMMs also have a lower prevalence of BRAF and NRAS (12%) mutations which limits the use of BRAF kinase inhibition treatment. PMMs have demonstrated increased c-KIT aberrations. Curtin et al (155) found 21% of patients with PMMs carry KIT mutations, and 61% overexpress c-KIT, supporting the use of imatinib as a therapeutic option in patients with c-KIT driven PMM. Beadling et al reported similar mutation profiles (194) with only 15.6% of patients with PMM having a c-KIT mutation and 26.3% having increases in copy number. Hodi et al (195) conducted a multicenter phase II study of imatinib in metastatic mucosal, acral, or chronically sun-damaged melanoma with c-KIT aberrations, and they concluded that imatinib is effective in tumors harboring KIT mutations, but not in tumors with KIT amplification only. They found a significantly different disease control rate between patients with wild type or mutant KIT, in favor of patients with KIT-mutant melanoma, but no statistical difference in overall survival. Current American Society of Clinical Oncology (ASCO) guidelines recommend enrolling patients in a clinical study if available, otherwise, using the treatment guidelines of cutaneous melanoma. (196) Figure 10 summarizes recommendations on the management of PMMs.
Figure 10:
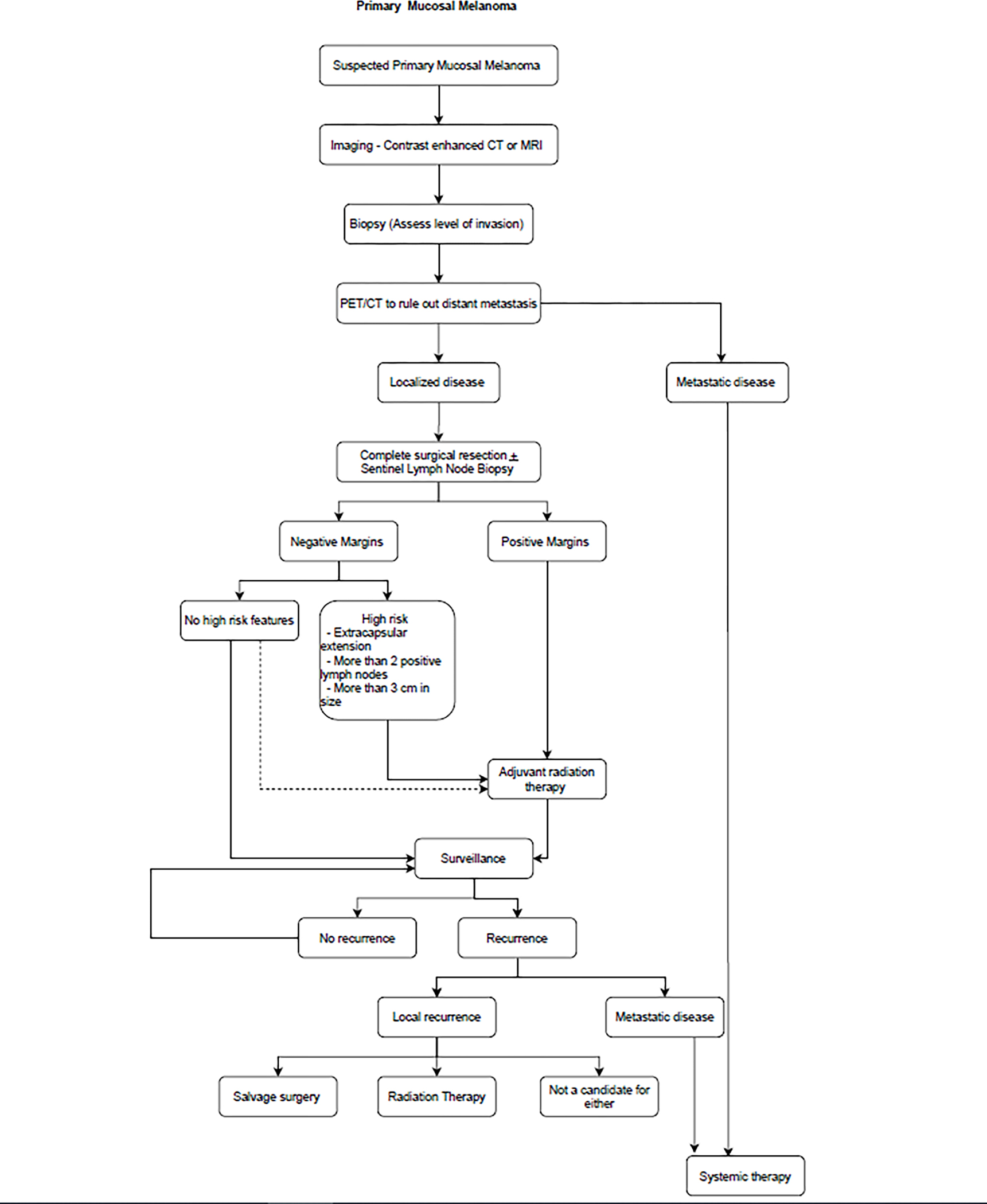
Authors algorithm summarizing management of Primary Mucosal Melanoma
Prognosis/Surveillance
The median survival for sinonasal PMMs is 26 months, with a 5-year survival rate of 22%. (197) Even when matched for stage at diagnosis, the prognosis of PMM is significantly worse than cutaneous melanoma. Most patients with PMMs of the nasal cavity have localized disease, whereas PMMs in the paranasal sinuses are usually more advanced, which confers a less favorable prognosis for the latter site. (187,198) Ethmoid and maxillary sinus PMMs have the worst prognosis amongst all sinonasal PMMs because of frequent infiltration into the skull base, orbit, or facial tissue and thus, the difficulty to obtaining clear surgical margins. (199)
Only 10–20% of sinonasal PMMs have cervical lymph node metastases at presentation, and less than 10% have distant metastases. (140) But over time, an additional 20% can develop nodal metastases, and 40–50% develop distant disease in the lungs, brain, bone, and liver. (200) PMMs are also characterized by early and repeated recurrences. (140) Stanimirov Rossi et al (201) described two different types of sinonasal PMMs- unilocular and multilocular, and the latter were associated with an unfavorable disease-free survival.
These patients need surveillance in accordance with patients with cutaneous melanoma, in addition to local examination of the ear, nose and throat.
Recent or ongoing developments/research
Most clinical studies that include patients with metastatic mucosal melanoma are basket studies in which patients with Stage IV disease are enrolled irrespective of primary tumor site, based on biomarkers suggesting response to a particular regimen. The role of neoadjuvant immunotherapy is being investigated due to the high rates of systemic relapse even when negative margins are achieved. (191) There is one trial investigating neoadjuvant chemotherapy in head and neck mucosal melanoma (NCT 03313206). (202)
The majority of clinical studies of mucosal melanoma are based in China due to the high prevalence of acral and mucosal melanoma in this region. Currently, several clinical studies are investigating neoadjuvant and adjuvant therapies in mucosal melanoma. (202–204)
Nuclear protein in the testis (NUT) Carcinoma
Incidence and patient demographics
Nuclear protein of the testis (NUT) carcinoma, previously known as NUT midline carcinoma (NMC) was first identified in 1991 as a thymic carcinoma with a unique chromosome translocation at t(15;19) (q15;p13) not seen in thymic carcinomas. (205) Initially, the name NMC was used given because that carcinoma was typically found in midline structures (mediastinum, head, and neck). (206) However, as more anatomic structures outside of the midline became involved, the nomenclature has shifted away from NMC to NUT carcinoma, as per the World Health Organization’s classification. (207)
The exact incidence of NUT carcinoma in the head and neck is unknown. Lee et al (208) have found a total of 105 reported cases of NUT since the first case was reported in 1991. Among head and neck cancers, which collectively refers to all cancers of the oral cavity, salivary glands, larynx and pharynx as well as nasal cavity and paranasal sinuses, NUT has an unclear prevalence rate, ranging from 1.9% to 17.9% of undifferentiated carcinomas of the head and neck. Lee et al (208) examined immunohistochemical and histological features in 362 cases previously classified as poorly differentiated or undifferentiated carcinomas of head and neck origin and found the prevalence of NUT carcinoma to be 2.9% among poorly differentiated carcinomas and 12.5% among undifferentiated carcinomas. The location was predominantly in the sinonasal tract (73.4%), followed by the larynx (7.3%), pharynx (4.6%), salivary gland (11.0%), and finally oral cavity (3.7%) (208) However, there have been reports of NUT carcinoma originating at other anatomical sites including the pelvis (209), kidney (210), larynx, maxillary gingiva, orbital cavity (211), lung (212), pancreas (213), and bone (214).
Patients can present with NUT at any age (0.1– 81.7 years) with a female predominance (1.5:1). (215) In one study of 119 patients, the median age was found to be 23 years (216) similar to other studies reporting a median age of 17.6– 25 years. (217,218)
Pathophysiology
These aerodigestive carcinomas, are associated with chromosomal rearrangement of the nuclear protein in testis midline carcinoma family member 1, also known as NUTMI1 or C15orf55, located on chromosome 15q14. Two case reports from 1991 (one in a 22-year-old and the other in an 11-year-old female) detailed the translocation at t(15;19)(q14;p13.1). (219) This translocation leads to the formation of a 6.4-kb fusion oncogene, between NUT and bromodomain protein family member 4 (BRD4) creating the chimeric-gene (BRD4-NUT) where exon 11 of BRD4 fused to exon 3 of NUT. This fusion is seen in nearly two-thirds of all NUT cases. NUT may also fuse to other bromodomain members, including BRD2, BRD3, and BRDT. About one-third of fusions will involve BRD3. (220) There are other (less frequently seen) fusion partners including the Zinc Finger- Containing Proteins (ZNF532 and ZNF592), MAX Dimerization Protein 4 and 1 (MGA, MXD4, and MXD1), CCL6 Corepressor Like 1 (BCORL1), Nuclear Receptor Binding SET Domain Protein 3 (NSD3), (221)and Capicua Transcriptional Repressor (CIC). (222)
NUT is expressed in the testis, but the exact function of the gene is unclear. Bromodomains are key players in transcription, histone acetylation, and chromatin remodeling. (223) The fusion of BRD and NUT leads to uncontrolled cell growth and restricts epithelial differentiation via transcription suppression and histone hypoacetylation. The NUT portion of the complex binds to p300, a specific histone acetyltransferase (HAT), to chromatin and propagates the formation of megadomains that are actively transcribed. These megadomains then express specific oncogenes. (224–226) There is also acetylation of p53 via this pathway (binding of p300), and thereby suppression of TP53. (227)
Unlike other HNCs that have possible associations with certain environmental factors, there are currently no identified exposures (including smoking) that have been linked with NUT. Furthermore, there has been no link seen between NUT and EBV or HPV. (227,228)
Presentation
The malignancy often affects midlines structures, and in the sinonasal cavity, it can present with sinonasal symptoms. (229) More local symptoms can occur are other structures are also involved. Typically, patients will present at more advanced stages and therefore will have metastases to lymph nodes, bone, or pleura. (230)
Pathology
Routine hematoxylin and eosin histopathology alone cannot reliably diagnose NUT carcinoma as there is no distinguishing feature and it is frequently mistaken for other undifferentiated or poorly differentiated carcinomas. (222) Cells are small to medium-sized and monomorphic, with indistinct to clear cytoplasm, prominent nucleoli, and areas of abrupt squamous differentiation. There may be areas of spindled cells and keratin pearls. Given the aggressive nature of NUT, there are also vast areas of necrosis. (231) Given the gene fusion, the diagnosis can be made using fluorescence in situ hybridization (FISH), reverse-transcriptase polymerase chain reaction (RT- PCR), or next-generation sequencing (NGS). FISH is superior to RT- PCR as it can detect all variants, whereas RT-PCR can only detect BRD-3 or BRD4-NUT tumors. (218)
Stelow (231) found most NUT carcinomas to be immunoreactive with antibodies to p63, CK20, and sometimes CD34. Finally, the diagnosis can also be made via immunohistochemistry with a novel rabbit monoclonal antibody (C52B1) to NUT, which has a sensitivity of 87% and a specificity of 100%. (232)
Imaging
Bair et al (233) outlined that NUT appears to be a hypoattenuating heterogeneously enhancing infiltrative mass with poorly defined margins on CT. It has also shown necrosis and calcification along with invasion into adjacent structures, with a predilection for central airways and vascular structures. On MRI, a heterogeneous mass with predominantly hypointense signal on T-1 weighted images and hyperintense on T-2 weighted images has been described. FDG PET/CT is recommended to evaluate for metastatic disease. Figure 11 shows MRI and PET imaging of the brain in a patient with NUT midline carcinoma with brain invasion and retropharyngeal adenopathy.
Figure 11:
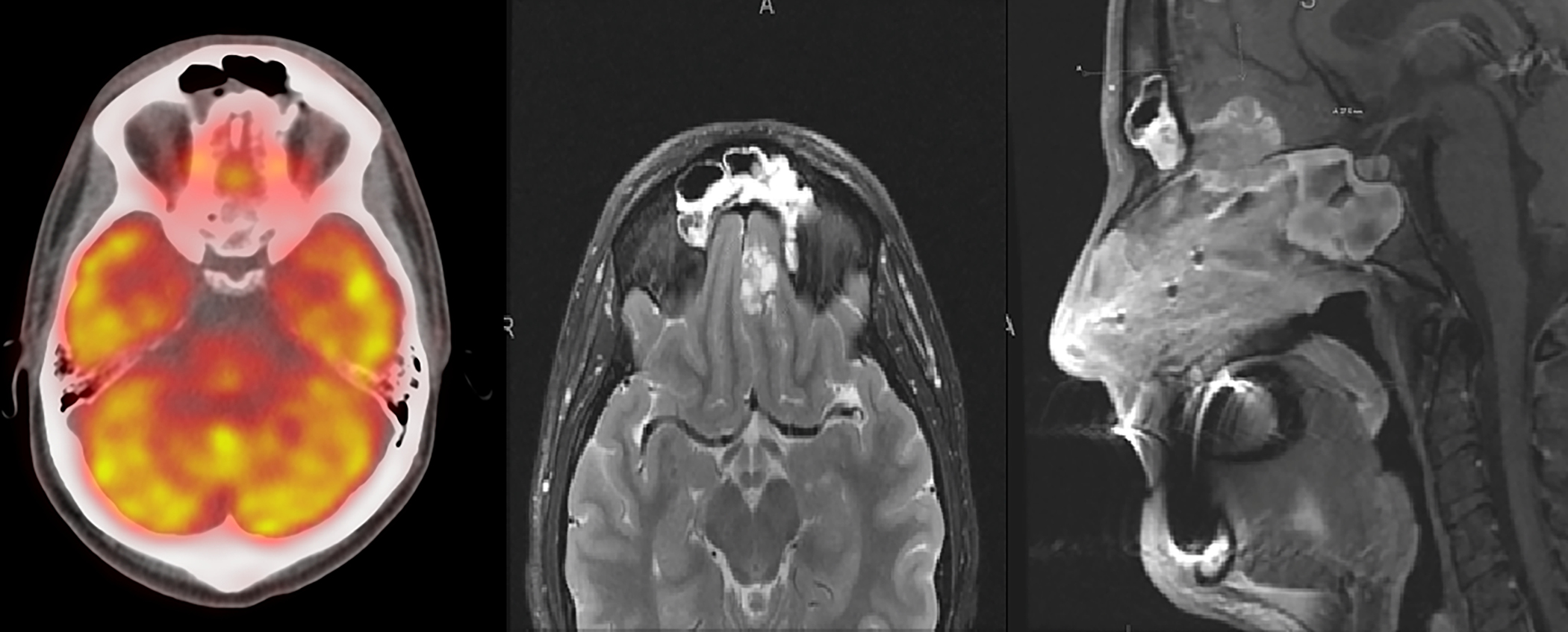
MRI and PET imaging of the brain in a patient with NUT midline carcinoma with brain invasion and retropharyngeal adenopathy.
Current treatment
Currently, there is no gold standard treatment for NUT carcinoma. Mainstay remains surgical resection, radiation therapy, and possibly chemotherapy. (234)
Surgery is frequently the first line for resectable disease as it is associated with both improved progression-free survival and overall survival. Chau et al. found that surgery with or without postoperative chemoradiation or radiation were critical components in overall survival. The study also found that chemotherapy or radiation therapy alone was insufficient. (215)
Chemotherapy options for NUT include monotherapy or combinations including 5-fluorouracil, actinomycin D, bleomycin, carboplatin, cisplatin, cyclophosphamide, docetaxel, doxorubicin, etoposide, gemcitabine, ifosfamide, S-1, vinblastine, vincristine, and vinorelbine. (216) Case reports have found some benefit when using alkylating agents, cisplatin, and taxanes. (220) There are no head to head comparisons between agent and combinations.
Finally, there has been some development in the utilization of targeted histone deacetylase inhibitor (HDACi) therapy. As previously mentioned, the BRD-NUT fusion protein leads to hypoacetylation and thereby transcription repression. HDACis work to reverse that process. Schwartz et al. have found promising results in xenograft models with growth inhibition and survival benefits. Two HDACi are approved by the Food and Drug Administration (FDA) - romidepsin and vorinostat. In one study, vorinostat induced squamous differentiation and growth arrest in a ten-year-old with NUT carcinoma. The therapy had to be discontinued due to thrombocytopenia.(225) In another case report, vorinostat induced a decrease in lymphadenopathy and tumor lesion, but again, was discontinued due to thrombocytopenia. (235)
Prognosis/Surveillance
Prognosis is poor with median survival time from diagnosis estimated at 6–7 months. (236) In one retrospective review of 48 cases, median progression-free survival was estimated to be 6.6 months (range of 4.7– 8.4) and median overall survival was 9.7 months (range of 6.6– 15.6). (215) Another review of 54 patients found median survival to be 6.7 months, with a two-year PFS of nine percent, and two-year overall survival to be nineteen percent. (237) Chau et al (238) performed a retrospective review of all known cases of head and neck NUT carcinoma and found 40 patients. They found no statistically significant difference in PFS or OS by age, gender, tumor location, size, histology, presence of neck lymph node involvement, or BRD4-NUT translocation. However, they did find that presence of distant metastasis was associated with 2-year PFS and 2-year OS of 0%. Contradicting the results though, French et al (217) found that individuals with non BRD4-NUTM1 fusions (BRD3-NUTM1 or NSD3-NUTM1 fusions) have a better prognosis, independent of the extent of metastatic disease.
Recent or ongoing developments/research
There has also been some discussion about the utility of BET inhibitors in NUT carcinoma. These work by binding to the acetyl-lysing binding pocket of BD1 and BD2, or the bromodomain that all BET proteins contain. JQ1, a thieno-triazolo-1,4-diazepine, is one of the earliest BETi that works to accelerate squamous differentiation and inhibit the cellular proliferation driven by BRD4. (239) Using mouse xenografts, Beesley et al. found that the efficacy of JQ1 on NUT carcinoma might depend on the presence of a BRD4 translocation. (240) Similar results have been seen for Birabresib in mice, with a success rate of 79% tumor growth inhibition in one study. (241) In one Phase Ib study using Birabresib, three of ten patients achieved a partial response to the therapy. These treatments carry toxicities including anorexia, diarrhea, headache, fatigue, nausea, and thrombocytopenia. (242) Another BRDi, Molibresib, was evaluated in 19 patients with NUT carcinoma in a phase I study and showed no complete responses and two (11%) confirmed partial responses. (243) Likely, these BET inhibitors will need to be used in combination with other agents.
There is one current study for patients with NUT where a novel BET inhibitor, ZEN003694, is being used in combination with etoposide and cisplatin (NCT05019716). (244)
Extranodal NK/T cell lymphoma
Incidence and patient demographics
Extranodal NK/T Cell Lymphoma (ENKTCL) is another rare malignancy, accounting for 3% of T-cell lymphomas in USA, Canada, and Europe with higher incidence seen among Asian Pacific Islanders or people of Hispanic ethnicity. (245) ENKTCL is even more common in East Asia and Latin America representing 15% and 13% of all T-cell lymphomas, respectively. (246)
Data from 797 patients with ENKTL patients from SEER was reported between 2001 to 2014, the median age at diagnosis was 53 years, and males tended to be younger at diagnosis. The annual incidence rate of the disease in the US was 0.07 per 100,000. (247) As expected from international data, the incidence was higher in US Hispanics with a recent incidence of 0.16 per 100,000. The malignancy most commonly presents with disease in the head and neck, with 52.6% cases in the nasal cavity and the sinuses, followed by 15.3% in the upper aerodigestive tract.
Pathophysiology
Although the exact pathogenesis of ENKTCL is unclear, EBV has been implicated in association with this disease entity across all ethnic groups. (245) Possible mechanisms include EBV-infected NK cells and their microenvironment producing IL-2, IL-9, and IL-10 to promote proliferation and overexpression of the latent membrane protein 1 (LMP1) which is oncogenic. (248,249) Another plausible mechanism is activation of NF-κB pathway that inhibits apoptosis and thereby increases the survival of cells. (246,250)
Presentation
The most common presentation is nasal obstruction due to chronic rhinosinusitis, followed by ulcerative necrosis of the upper aerodigestive tract and the face. Most patients have localized disease (Ann Arbor Stage I/II) with rare cases of disseminated disease, and the majority lack B symptoms. (251)
Pathology
The diagnosis of ENKTCL is confirmed by biopsy. Histopathology shows an atypical lymphoid proliferation with an angiocentric pattern, and angiodestruction. (246) Most cases are positive for CD2. Cytoplasmic CD3, CD56, cytotoxic granules (granzyme-B, TIA-1) indicate NK cell origin. In addition, CD4, CD8, T-cell receptors (TCR) are expressed variably. (251) Figure 12 shows H&E and several immunostains of a biopsy of from a patient with ENKTL. Positivity for LMP-1 immunostains is also necessary to confirm the diagnosis and may help to differentiate it from other T-cell lymphomas, which are EBV negative. (246,251) Plasma EBV DNA measured with RT-PCR is also associated with tumor load and serial EBV DNA monitoring is used to assess response and detect recurrence. (252,253)
Figure 12:
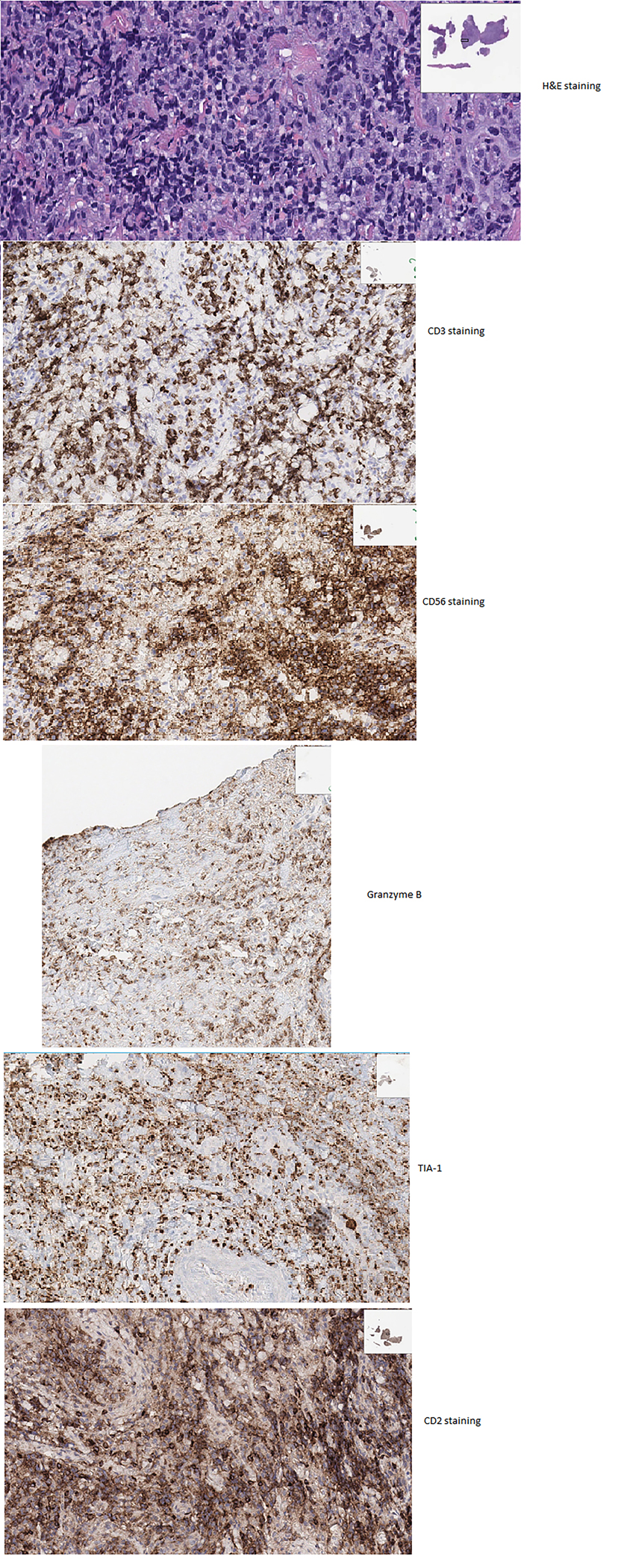
H&E and different immunostains in a biopsy sample with NK/T Cell Lymphoma
There likely is a role of somatic mutations in the pathogenesis of ENKTCL. Activating JAK3 mutations are seen in 35% cases, in addition to STAT3 and STAT5B mutations. (254,255) In addition, mutations of the DDXX3X gene have been identified which participates in RNA translation initiation and assembly in ribosome and spliceosome. (256) Deletions in tumor suppressor genes such as TP53, PRDM1, FOXO3, and HACE2 have been noted in some cases of ENKTCL. (256–258) Some studies have correlated environmental exposures and socioeconomic status with the risk of developing ENKTCL. (246)
Imaging
CT or MRI are acceptable to define the local extent of disease, but for sensitive detection of metastases, a PET/CT should be peformed. (252) Figure 13 below shows an MRI of nasal NK/T Cell lymphoma. Bone marrow involvement is uncommon, but aspirate and biopsy can be considered for evaluation, though PET/CT may also be able to detect bone marrow disease. (259,260)
Figure 13:
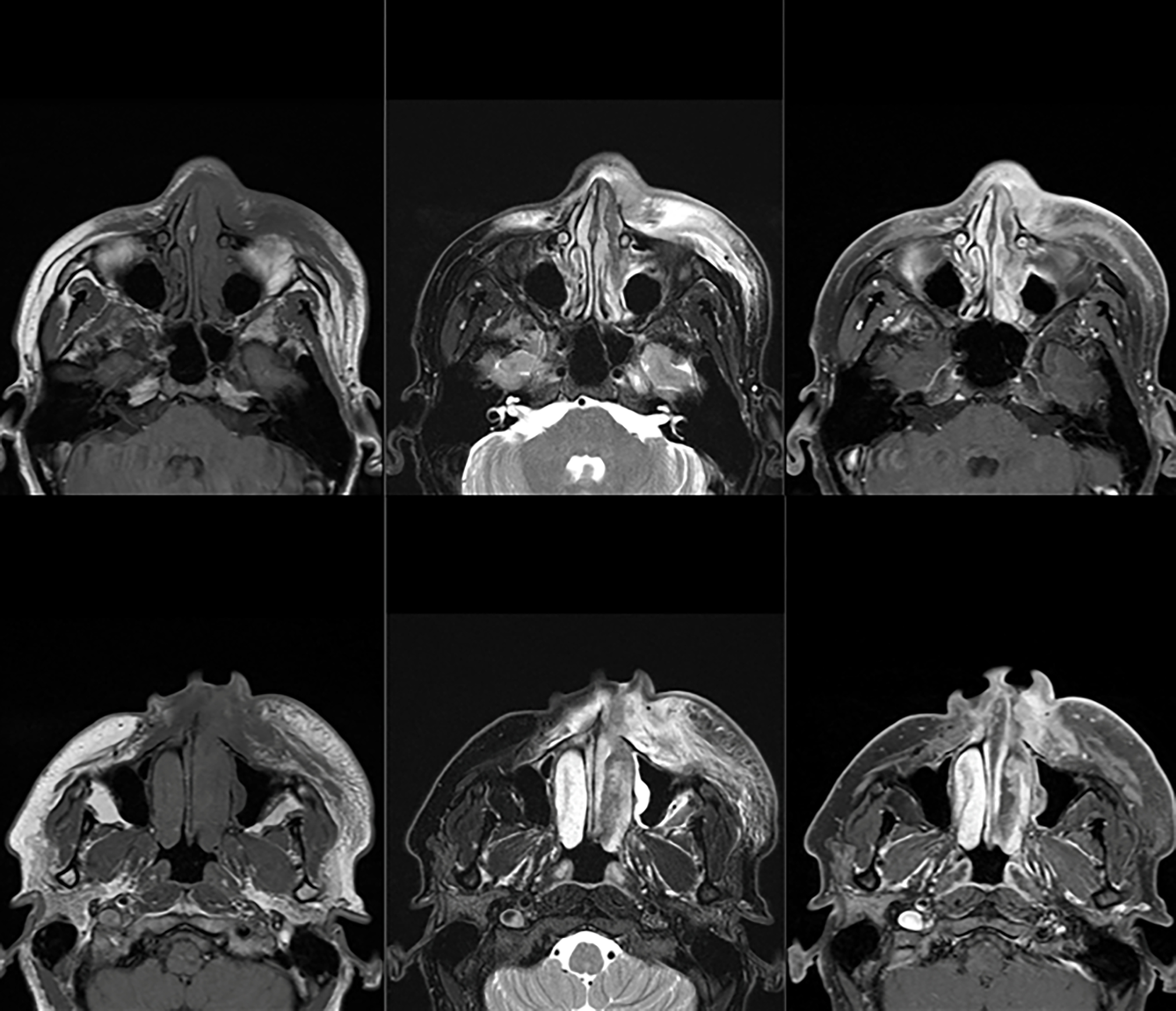
MRI with nasal NK/T Cell Lymphoma
Current treatment
The treatment of ENKTCL is based on retrospective studies and phase II studies. For localized disease, radiotherapy with chemotherapy is considered the standard of care. Radiotherapy by itself has been associated with a higher relapse rate, and the addition of anthracycline therapy to radiotherapy did not result in improvement of survival outcomes. (261,262) This poor response is thought to be due to NK-cells expressing very high P-glycoproteins concentrations causing a multidrug resistance (MDR) phenotype. (263) Therefore, non-MDR-dependent drugs have been used in treating NK/T cell lymphomas. Kim et al (264) compared concurrent chemoradiotherapy with cisplatin followed by VIDP chemotherapy to dose-intensified CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) followed by radiotherapy, and found significantly improved OS and PFS with the concurrent chemoradiotherapy regimen. This was also studied by Yamaguchi et al. (265) where they found that concurrent radiotherapy and 3 courses of dexamethasone, etoposide, ifosfamide, and carboplatin produced improved survival compared with CHOP. L-asparaginase was found to be effective for relapsed/refractory NK/T-cell lymphomas, after which its use up front was investigated in many studies, with radiotherapy sandwiched between infusions. (266) The different regimens studied were LVP (l-asparaginase, vincristine, and prednisolone), with an ORR of 89% and CR rate of 81%, GELOX (gemcitabine, l-asparaginase, and oxaliplatin) with an ORR of 96% and CR rate of 74%, and SMILE (dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide) with an ORR of 90% and CR rate of 69%. (267–269) SMILE was associated with treatment-related mortality of 7%. (269) L-asparaginase has been associated with allergic reactions including anaphylactic shock, and Erwinia asparaginase can be used as an alternative. (252)
For advanced-stage or relapsed/refractory NK/T-cell lymphoma, combination chemotherapy remains the treatment of choice. The SMILE regimen used for these patients showed an ORR of 80%, 93%, and 25% in stage IV, relapsed, and primary refractory diseases and CR rate of 40%, 64%, and 0% after 2 cycles. (270) Another regimen used for patients with refractory/relapsed disease was AspaMetDex (l-asparaginase, methotrexate, and dexamethasone) with an ORR and CR rate of 78% and 61%, respectively. Once remission is achieved, these patients might benefit from allogeneic hematopoietic stem cell transplant, but prospective data is not robust. (271) Figure 14 details a treatment algorithm for patients with ENKTL.
Figure 14:
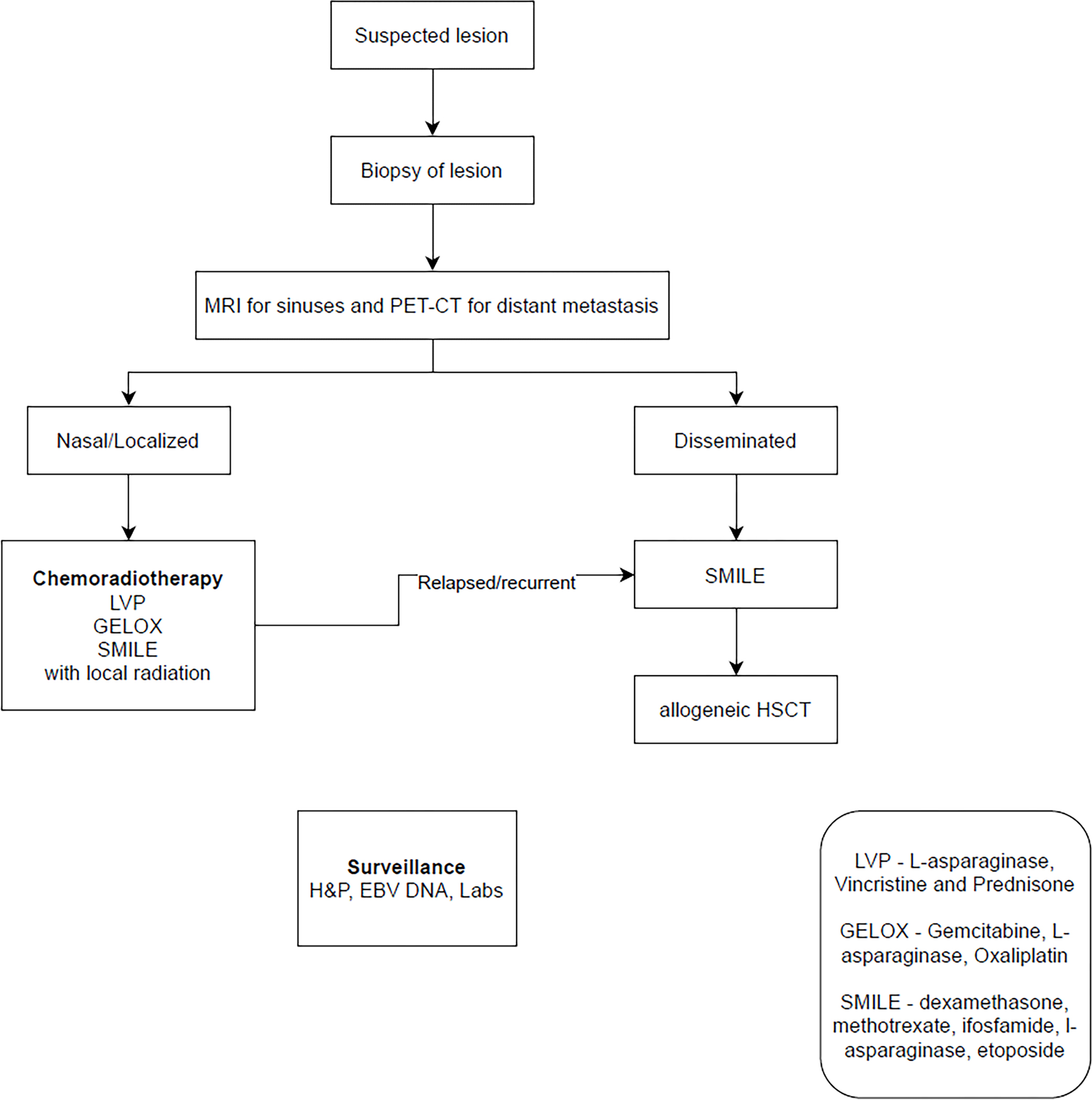
Algorithm summarizing management of NK/T Cell Lymphoma
Prognosis/Survivorship
The survival of patients with ENKTCL is relatively poor with a median OS of 20 months after diagnosis, but it varies significantly based on the stage of disease, age, and nodal disease site. (247) the overall survival (OS) was reported to be around 42% while the 5-year progression-free survival (PFS) was only 29%. (272)A prognostic index for patients with ENKTL was developed using retrospective data called prognostic index for natural killer cell lymphoma (PINK). (273) Four independent risk factors are incorporated: age greater than 60 years, stage III/IV disease, distant lymph-node involvement, and non-nasal type disease. The patients were stratified into low-risk (no risk factors), intermediate-risk (one risk factor), or high-risk (two or more risk factors) groups. Low, intermediate, and high-risk groups had a 3-year OS of 81% (95% CI 75–86), 62% (95% CI 55–70), and 25% (95% CI 20–34), respectively. In the same study, 328 patients had EBV DNA levels available, and a score called PINK-E was developed. Low (zero or one risk factor), intermediate (two risk factors), and high risk (three or more risk factors) were associated with an OS of 81%, 55%, and 28% respectively.
Disease surveillance uses plasma EBV DNA titers from RT-PCR, along with symptom-guided imaging. There are no guidelines on duration or frequency of surveillance but there have been late relapses reported up to 29 years after initial remission. (274) Thus, lifelong surveillance is recommended. These patients with late relapses have a prognosis similar to primary ENKTL, rather than relapsed/refractory disease. (275)
These patients face significant compromise in quality of life with radiation in the head and neck region, in addition to long-term toxicity from chemotherapeutic agents used in treatment. In addition, they are at increased risk of the development of treatment-related acute myeloid leukemia. (247)
Recent or ongoing developments/research
There are abundant clinical studies available for patients with ENKTL. Trials are studies investigating immune checkpoint inhibitors in early-stage disease, with or without other agents. (276–278) HDAC inhibitors such as Chidamide are also being investigated in several studies. (276,279) More studies are investigating newer regimens and combinations for relapsed/refractory ENKTL and advanced ENKTL. (280–285) Table 2 lists selected clinical studies for patients with ENKTL.
Table 2:
Selected clinical studies for patients with Extranodal NK/T Cell Lymphoma
| NCT # | Condition | Title | Intervention |
|---|---|---|---|
| NCT03671850 | VT-EBV-N for Treatment of Severe in EBV Positive Extranodal NK/T Cell Lymphoma Patients | VT-EBV-N (EBV-CTL) is a cytotoxicity T lymphocyte | |
| NCT03246750 | Newly diagnosed Extranodal NK/ T-cell Lymphoma | B-MAD Chemotherapy in Newly diagnosed Extranodal NK/ T-cell Lymphoma | Brentuximab vedotin and methotrexate/ L-asparaginase/ dexamethasone |
| NCT03728972 | Newly diagnosed Extranodal NK/ T-cell Lymphoma | Study of Pembrolizumab in Patients With Early-Stage NK/T-cell Lymphoma, Nasal Type | Pembrolizumab |
| NCT04602065 | Relapsed/Refractory Extranodal NK/T Cell Lymphoma (Nasal Type) | Evaluation of Safety and Efficacy of IBI318 Monotherapy for Relapsed/Refractory Extranodal NK/T Cell Lymphoma (Nasal Type) Study | IBI318(Recombinant human anti-PD1/PD-L1 bispecific antibody) |
| NCT04096690 | Advanced Stage NK/T-cell Lymphoma | Anti-PD-1 Antibody Combined With Pegaspargase in the Treatment of Advanced Stage NK/T-cell Lymphoma | Pegaspargase, Anti-PD-1 monoclonal antibody |
| NCT04279379 | Relapsed/Refractory or Advanced NK/T-cell Lymphoma | Sintilimab and Decitabine for Patients With Relapsed/Refractory or Advanced NK/T-cell Lymphoma | Sintilimab and Decitabine |
| NCT04484506 | Newly diagnosed Extranodal NK/ T-cell Lymphoma | Pegaspargase-COEP Chemotherapy Combined With Radiotherapy for Extra-nodal NK/T Cell Lymphoma | Pegaspargase, cyclophosphamide, vincristine, etoposide, prednisone |
| NCT04405375 | Relapsed/Refractory or Advanced ENKTCL | GPED Regimen for Relapsed/Refractory or Advanced ENKTCL | Gemcitabine, Pegaspargase, Etoposide, Dexamethasone |
| NCT04509466 | Any ENKTCL | Clinical Study of Liposomal Mitoxantrone Hydrochloride Injection Combined With Pegaspargase in the Treatment of NKTCL | Liposomal mitoxantrone hydrochloride and Pegaspargase |
| NCT04365036 | Newly diagnosed Extranodal NK/ T-cell Lymphoma | A Multicenter, Phase 3, Randomized Study of Sequencial Chemoradiotherapy With or Without Toripalimab (PD-1 Antibody) in Newly Diagnosed Early-Stage Extranodal Natural Killer/T Cell Lymphoma, Nasal Type (ENKTL) | Toripalimab, Pegaspargase, Gemcitabine, Oxaliplatin, Definitive intensity-modulated radiotherapy (IMRT) |
| NCT04127227 | Newly diagnosed advanced extranodal natural killer/T-cell lymphoma, nasal type | Sintilimab With P-GemOx Regimen for Newly Diagnosed Advanced Extranodal Natural Killer/T-cell Lymphoma, Nasal Type | Sintilimab, pegaspargase, gemcitabine, oxaliplatin |
| NCT04511351 | Stage I/II Extranodal Nasal NK/T-cell Lymphoma | Radiotherapy Combined With GDP With or Without Chidamide in Stage I/II Extranodal Nasal NK/T-cell Lymphoma | Chidamide, Gemcitabine, Cisplatin, Dexamethasone |
| NCT04414969 | Early Stage of NK/T Cell Lymphoma | Anti-PD-1 Antibody Combined With Peg-Asparaginase and Chidamide for the Early Stage of NK/T Cell Lymphoma | Anti-PD-1 Antibody, Peg-Asparaginase, Chidamide |
| NCT03936452 | Untreated stage I-II Extranodal NK/T Cell Lymphoma | Combined Treatment of Sintilimab, Peg-aspargase Plus Anlotinib in NK/T Cell Lymphoma | Sintilimab, Peg-aspargase, Anlotinib |
| NCT05058755 | Refractory Extranodal NK/T-cell Lymphoma | Tislelizumab Combined Treatment in Refractory Extranodal NK/T-cell Lymphoma | Tislelizumab, azacytidine, lenalidomide Or Tislelizumab, etoposide, pegaspargase |
| NCT04004637 | Relapsed refractory CD7+ NK/T Cell Lymphoma | CD7 CAR-T Cells for Patients With R/R CD7+ NK/T Cell Lymphoma,T-lymphoblastic Lymphoma and Acute Lymphocytic Leukemia | CD7 CAR-T cells infusion |
Esthesioneuroblastoma (Olfactory neuroblastoma, ONB)
Incidence and patient demographics
Esthesioneuroblastoma, or olfactory neuroblastoma (ONB), is a rare tumor arising from the olfactory neuroepithelium in the sinonasal cavity that extends to the anterior skull base. (286) ONB comprises 2–6% of all sinonasal tumors and affects four people per ten million in the population. (286–288) The estimated incidence is 0.04 per 100,000 people. (286) ONB affects men and women equally and has a bimodal age distribution with peaks in the second and sixth decades.
Pathophysiology
The etiology of ONB is unclear, and no clear genetic or environmental risk factors have been isolated. A case report described the association of ONB with chronic wood dust exposure. (289) Animal studies have correlated ONB development with nitrosamine compound exposure, (290,291) and retrovirus exposure, (292) however, these associations have not been described in humans.
Presentation
Patients with ONB often present with symptoms related to direct effects of the nasal mass. Tumors are often slow growing and local symptoms are most common. (293) Delayed diagnosis is common and is made on average six months after the onset of symptoms (15). (294) Cervical adenopathy due to nodal metastasis may be a presenting symptom in 9–22% of patients with a new ONB diagnosis. (295)
Paraneoplastic syndromes that have been described in ONB cases include Cushing syndrome from ectopic adrenocorticotropin syndrome, (296,297) syndrome of inappropriate anti-diuretic hormone,(297,298) catecholamine secretion induced hypertension, cerebellar degeneration, and clonus-myoclonus-ataxia and humoral hypercalcemia of malignancy. (297)
A nasal exam may not reveal the mass unless endoscopy or imaging is utilized as the tumor can be localized high in the nasal cavity. Upon viewing, the mass may appear as a vascular, gray or red polypoid lesion. Imaging prior to biopsy is recommended to avoid swelling or bleeding from the tumor that may impact imaging findings.
Pathology
Histopathological diagnosis usually follows imaging. There is no specific immunohistochemical marker; however, ONBs are typically positive for S-100, chromogranin, and synaptophysin, and negative for cytokeratin, desmin, vimentin, actin, glial fibrillary acidic protein, UMB45 and common leukocyte antigen. For poorly differentiated tumors, electron microscopy can visualize dense core neurosecretory granules and microtubules, neurofilaments and rare synapses although it is rarely used for diagnostic purposes. There is marked heterogeneity within esthesioneuroblastoma tumors. Pathology may range from low grade, reflecting a more indolent malignancy to rarely, a high-grade tumor representing a highly aggressive cancer. (286,299) Genomic sequencing of ONB tunors is limited, and mutations in TP53, c-kit, PDGFR-b, EGFR, FGF-FGFR1 signaling, Sonic hedgehog pathway; apoptosis-related pathways (Bcl-2, TRAIL) and neoangiogenesis (VEGF; KDR) have been identified. (300)
The Hyams grading system is used to grade tumors from I to IV based on pathologic findings such as mitotic activity, preservation or disruption of lobular architecture, necrosis, and presence of rosettes. (301) Grades I and II are considered low-grade lesions and grades III and IV are high grades. Light microscopy on low-grade tumors reveals well-preserved lobular architecture below an intact mucosa, with tumor cells arranged in nests. The tumor cells appear characteristically as small, blue, and round cells consistent with the neuroendocrine origin. In up to one-third of samples a rosette or pseudorosette pattern can be found.(286,302) However, in high-grade ONB, there is often a sheet-like pattern of growth with disrupted architecture. For this reason, high-grade ONB has been challenging to distinguish from other high-grade small cell sinonasal malignancies. The use of immunohistochemistry for high-grade ONB is helpful to distinguish it from neuroendocrine carcinoma, sinonasal undifferentiated carcinoma (SNUC), large cell neuroendocrine carcinoma, mucosal malignant melanoma, rhabdomyosarcoma, Ewing sarcoma/PNET, and other metastatic tumors.(286,302)
Hyams grade has been shown to correlate with prognosis. Median five-year overall survival for low-grade lesions ranges between 56–80% compared to 20–40% for high-grade lesions. (303,304) Analysis of cases from the SEER database data confirmed the prognostic impact based on tumor grade, where a 10-year overall survival of 64–67% was found in low-grade tumors compared to 34–40% in high-grade tumors. (305,306) Figure 15 shows a low-grade ONB with Hyams grade I.
Figure 15:
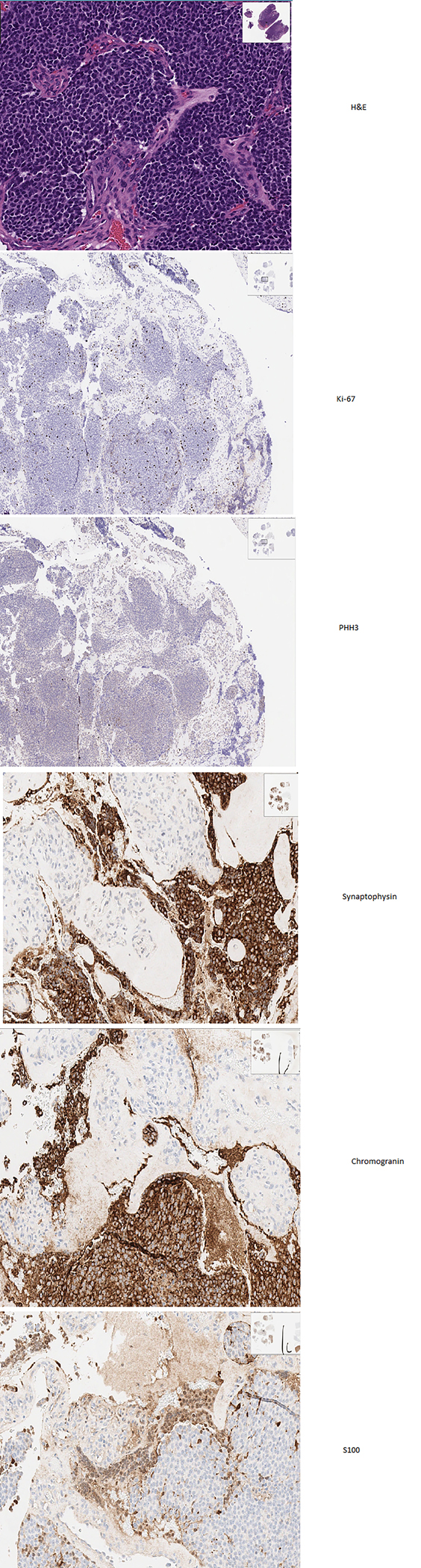
Esthesioneuroblastoma histology in H&E with Hyams grade I, with Ki67 7%, with PHH3, synaptophysin, chromogranin and S100 positivity.
Imaging
CT or MRI shows a characteristic radiographic appearance of a bilobed, or dumbbell-shaped mass at the cribriform plate. (286) Additional imaging is often not indicated unless there are symptoms of distant metastatic disease. If clinical suspicion is present or the biopsy reveals high-grade features, PET CT or somatostatin receptor-based imaging is used, given olfactory neuroblastomas typically express somatostatin receptors. (307,308) Figure 16 shows DOTATE PET and MRI in a patient with ONB in the right nasal cavity with extension into the right ethmoid air cells and epidural intracranial involvement along the cribriform plate.
Figure 16:
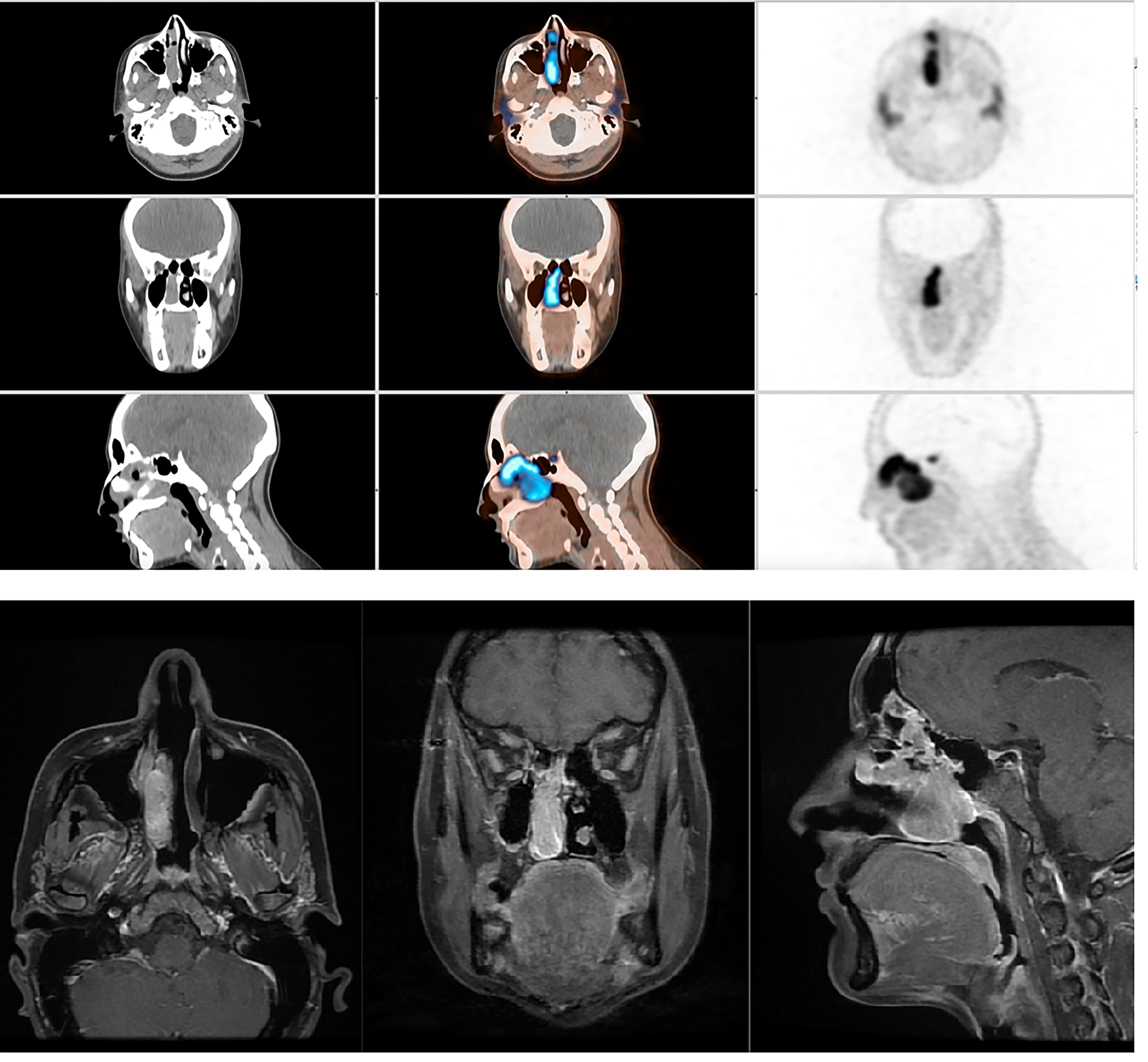
DOTATE PET and MRI in a patient with Esthesioneuroblastoma in the right nasal cavity mass with extension into the right ethmoid air cells and epidural intracranial involvement along the cribriform plate.
There are two staging systems for ONB: the Kadish system and Dulguerov system. The Kadish system was created in 1976 and originally had three stages, and has, since then, been modified to include four stages. In Stage A, the tumor remains in the nasal cavity; in stage B it extends into the paranasal sinuses; in stage C the tumor extends outside of the paranasal sinuses (involving the dura); and staged where regional lymph nodes or distant metastases are present. (304) Most patients present with stage C (53–61%) followed by stage A or B (34–39%), with only 8–9% presenting with stage D. (295,309) Later, the Dulguerov system was created to mirror the tumor, nodes, metastasis (TNM) system used in other malignancies. In this system, tumors range from T1 through T4 with advanced T stages representing more invasive tumors: T1 involves the nasal cavity and paranasal sinuses excluding the sphenoid, T2 extends into the cribriform plate, T3 extends to the orbit and anterior cranial fossa and T4 invades into the brain. Nodal staging is N0 or N1, if cervical lymph node metastasis is present. M0 or M1 reflect if distant metastatic disease is present. (299,310) The Kadish staging system is more commonly utilized given that it preceded the Dulguerov system in creation. (304) There is also no consensus regarding which staging system is a better prognostic tool. Data from two comparative studies have resulted in conflicting findings. (310)
An important consideration is within the Kadish stage C group that includes tumors with dural extension. This subgroup within Kadish stage C with dural invasion has a worse prognosis as dural invasion is a known predictor for recurrence and metastasis. Possibly, in those with dural involvement outcomes may be better predicted with the Dulguerov system. (311,312)
Current treatment
ONB is most often treated with surgery plus radiation therapy given the radiosensitive nature of the tumor and high risk of local recurrence.(313) Surgery alone or radiation alone is typically reserved for those who have a contraindication to combined treatment or, are a poor surgical or radiotherapy candidate. Further, nasal cavity and paranasal sinuses are reportedly difficult sites in which to ensure adequate surgical margins, and so adjuvant radiation is often recommended even after negative margins are obtained. (313) Craniofacial or transfacial approaches are most employed given cranial cavity extension or other local extension. Resection of the ipsilateral cribriform plate, crista galli, overlying dura and olfactory bulb are performed to help reduce recurrence rates. Less favored is the endoscopic rhinotomy approach given high rates of local recurrence but can be considered for a T1 tumor or Kadish A lesion, where the tumor is limited to the nasal cavity. (299,301,314)
In those patients presenting with N1 disease in cervical lymph nodes, treatment most commonly involves surgical resection of the primary tumor and cervical nodes, followed by radiation, if possible, to both sites. (295,315) In the absence of nodal positivity at diagnosis, elective neck surgery is usually not performed although it may be considered for high-grade disease or tumors that invade the dura. (316,317) If relapse involving the neck occurs later, salvage surgery with or without radiation therapy is performed and is successful in achieving remission in up to one-third of patients.(318)
Radiation therapy is administered adjuvantly after surgery. In theory, tumor shrinkage may be obtained with neoadjuvant radiation and result in a smaller resection, however similar to radiation effects in other malignancies, post radiation changes result in unclear tumor margins and resection becomes challenging. Adjuvant radiation to a total of 55–65 Gy, is used most often using a stereotactic approach to minimize damage to surrounding healthy structures. (319) Other current radiation techniques utilize proton beam and intensity-modulated radiotherapy approaches.(320–322)
Chemotherapy is not typically given outside of palliative intent in distant metastatic disease. Neoadjuvant chemotherapy has been explored, however with unclear benefit. This was described in a series of patients with Kadish stage C disease, defined as tumors extending beyond the paranasal sinuses. These patients were given neoadjuvant cyclophosphamide and vincristine, with or without doxorubicin, followed by neoadjuvant radiation to 50 Gy total then surgery. Five and 10-year overall survival outcomes were similar to other studies of similar stage C disease that did not incorporate chemotherapy. (323) Chemotherapy combinations such as cisplatin and etoposide for high-grade tumors, similar to regimens used in other small cell malignancies are often employed in the advanced stage setting. Other regimens have been used including irinotecan plus docetaxel, and single agent treatments with doxorubicin, ifosfamide, vincristine or temozolomide have also been reported.(324,325)
Systemic treatment for distant or recurrent disease with targeted treatments has been reported. Durable responses have been observed with tyrosine kinase inhibitors such as sunitinib and pazopanib, mammalian target of rapamycin inhibitors, and epidermal growth factor receptor (EGFR) inhibitors. (326) Peptide receptor radionuclide therapy (PRRT), based on somatostatin receptor expression by ONB cells, has been used in later line treatments for progressive disease and described in case reports. (327)
Prognosis/Surveillance
The overall 5- and 10-year survival rates were 62.1% and 45.6%, respectively. (293) Per staging, five-year overall survival rates for Kadish stage A, B and C tumors are 75–91%, 49–71%, and 38–47%. (286,328) Since the introduction of Kadish stage D, the overall mean survival time for patients with stage A disease was 145.6 months (95% CI, 129.0–162.2), 149.5 months (95% CI, 119.0–180.0) for those with stage B disease, 72.5 months (95% CI, 32.3–112.7) for those with stage C disease, and 53.7 months (95% CI, 27.3–80.0) for those with stage D disease. (293) Similarly trending, the overall survival rates at 10 years for patients with stage A disease was 83.4%, stage B disease was 49%; for stage C disease was 38.66%; and for those with stage D disease was 13.3%.
One report noted earlier Kadish stage A conferred a worse 5-year overall survival compared to Kadish stage B, however, this was thought primarily due to lack of adjuvant radiation therapy and higher number of positive surgical margins in the stage A group. (329) Similarly, outcomes using the Dulguerov system show a higher T category associated with worse five-year overall survival: T1 with 81%, T2 93%, T3 59% and T4 48%. (313) Local and locally advanced ONB without distant metastasis is curable. However, the presence of nodal metastasis, and using only radiotherapy was associated with a worse prognosis. (293)
The risk for local recurrence is up to 20–40% at ten years after initial treatment. This highlights the need for ongoing long-term surveillance. (313) Optimal surveillance is not defined, however one protocol described obtaining a baseline MRI brain and maxillofacial 2–4 months after treatment, then repeating at 4–6-month intervals for 5 years, then annually for a lifetime. History and physical exams should coincide with the above schedule and additional imaging should be guided by symptoms or exam findings. (330)
Recent or ongoing developments/research
Research is centered on developing a universal staging system, defining the role of nodal dissection in high-risk node-negative patients, and exploring the role of systemic treatments. Currently, a phase II study is evaluating the role of Bintrafusp Alfa in recurrent/metastatic ONB (NCT05012098). (331) In addition, there are studies on the use of DOTATATE based imaging in the diagnosis and management of SSR positive tumors, including ONB (NCT04081701). (332)
Survivorship issues in sinonasal malignancies
Survivorship and surveillance of patients with sinonasal malignancies is not defined clearly. They do require multidisciplinary involvement from diagnosis to follow-up. (333) Individual surveillance plans are discussed in every section, and includes serial imaging of the sinonasal area. (334) In addition to medical oncologists, radiation oncologists and head and neck surgeons, involvement from from speech-language pathologists, audiologists, dentists, and nutritionists is valuable. Follow up is essential to facilitate early diagnosis of recurrence and to help patients deal with survivorship issues. Immediate issues include rehabilitation after radiation and surgery to the sinonasal cavity. Comprehensive nutritional assessment is necessary to ensure recovery Delayed issues include development of radiation induced soft tissue sarcomas, hypothyroidism and damage to organs from surgery or radiation.
The risk factors for some sinonasal cancer types are also common with several other head and neck malignancies, and smoking cessation is essential to prevent additional primary malignancies. (334)
Future of sinonasal malignancies
Given the poor overall survival for a majority of patients with sinonasal malignancies these limitations, until most effective ways are available, increasing awareness for early detection is the only way to decrease morbidity and mortality from sinonasal malignancies. Some efforts include cancer registries for rare diseases to provide a large database with patient information, treatment variables, and outcomes available for providers to contribute to and learn from. (335) Another effort includes development of multi-national clinical studies for patients to allow accruing significant number of patients to allow for meaningful testing of treatment approaches. Therefore, initiatives like the International Rare Cancer Initiative can expand the reach of clinical studies for these rare diseases. A challenge for studies for such rare cancers is the comparison of treatment regime to standard of care that does not exist is always challenging. A possible solution to these problems with cancer registries and clinical studies for rare diseases is to have multi-national institutions maintain a well-constructed prospective registry. (335) One such group is the Trans-Atlantic Australasian Retroperitoneal Sarcoma Working Group, which is a collaborative group for Surgical Oncologists and Sarcoma professionals who aim to provide better evidence for patients with retroperitoneal sarcoma. The Head and Neck Cancer International Group is also one such group that aims to promote and conduct high quality clinical studies to improve outcomes in patients. At this point, they do not have clinical studies for rare cancers, but a platform like this is perfect for international cooperation for rare tumors, and it is one of the goals of the group. (336) While there are only a handful of studies for these patients, table 3 does list open clinical studies for patients with sinonasal malignancies.
Table 3:
Clinical studies for patients with sinonasal malignancies
| NCT # | Condition | Title | Intervention |
|---|---|---|---|
| NCT05151588 | Sinonasal Carcinoma | Induction Chemotherapy and Tazemetostat for Locally Advanced SMARCB1-deficient Sinonasal Carcinoma | docetaxel, cisplatin, 5-FU and tazemetostat followed by surgery or radical chemoradiation and maintenance tazemetostat |
| NCT00707473 | - Locally Advanced Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma - Nasal Cavity and Paranasal Sinus Poorly Differentiated Carcinoma - Sinonasal Undifferentiated Carcinoma |
Docetaxel, Cisplatin and Fluorouracil in Treating Patients With Previously Untreated Stage II-IV Nasal Cavity and Paranasal Sinus Cancer | Induction Therapy With Docetaxel, Cisplatin and Fluorouracil |
| NCT01586767 | Any sinonasal malignancy | Intensity-Modulated or Proton Radiation Therapy for Sinonasal Malignancy | Proton radiation therapy, Intensity-modulated radiotherapy |
| NCT04081701 | Esthesioneuroblastoma | 68-Ga DOTATATE PET/MRI in the Diagnosis and Management of Somatostatin Receptor Positive CNS Tumors. (DOMINO-START) | Ga68-DOTATATE-PET/MRI |
| NCT04318717 | Mucosal Melanoma of the Head and Neck | Pembrolizumab and Hypofractionated Radiation Therapy for the Treatment of Mucosal Melanoma | Pembrolizumab, Hypofractionated radiation therapy during first 2 cycles |
| NCT03241186 | Resected mucosal melanoma | Ipilimumab and Nivolumab as Adjuvant Treatment of Mucosal Melanoma | Ipilimumab and Nivolumab |
| NCT04622566 | Resected mucosal melanoma | Lenvatinib and Pembrolizumab in Resectable Mucosal Melanoma | Neoadjuvant Lenvatinib and Pembrolizumab, followed by maintenance Pembrolizumab |
| NCT03313206 | Resectable Head and Neck Mucosal Melanoma | Neoadjuvant Treatment Associated With Maintenance Therapy by Anti-PD1 Immunotherapy in Patients With Resectable Head and Neck Mucosal Melanoma (IMMUQ) | Pembrolizumab |
| NCT05019716 | Advanced, metastatic or unresectable NUT Carcinoma | Testing the Safety and Efficacy of the Addition of A New Anti-cancer Drug, ZEN003694, to Chemotherapy Treatment (Etoposide and Cisplatin) for Adult and Pediatric Patients (12–17 Years) With NUT Carcinoma | BET Bromodomain Inhibitor ZEN-3694, Cisplatin, Etoposide |
| NCT03313206 | Resectable head and neck mucosal melanomas | Phase II Multicentric Study: Efficacy Evaluation of Neoadjuvant Treatment Associated With Maintenance Therapy by Anti-PD1 Immunotherapy on Disease-free-survival (DFS) in Patients With Resectable Head and Neck Mucosal Melanoma | Pembrolizumab |
Figure 17:
H&E of paraganglioma showing pink epitheloid cells with round hyperchromatic nucleus.
Table 4:
Summary of incidence, molecular expression, prognosis and treatment of sinonasal malignancies
| Disease entity | Incidence | Molecular expression | Prognosis |
|---|---|---|---|
| Sinonasal Squamous Cell Carcinoma | 61% of sinonasal cancers | TP53, EGFR, FGFR1, SOX2 amplification, microsatellite instability, KRAS rarely | 5-year OS 50% |
| Sinonasal Adenocarcinoma | 27% of sinonasal cancers | ETV6 fusions rarely seen | 5-year OS 66.7% |
| Sinonasal Neuroendocrine Carcinoma | 2% of sinonasal cancers | mutations of TP53 and RB1, up-regulation of BCL2 signaling, and activation of MYC and PI3K pathways | 5-year DSS 70.2% |
| Sinonasal Undifferentiated Carcinoma | 5% of sinonasal cancers | IDH2 mutations, P53 mutations, CDKN2A/2B loss-of-, MYC amplification SETD2 | 5-year OS 34.9% |
| Primary Mucosal Melanoma | Rare (< 1%) | KIT, BRAF, loss of p16 expression, CDKN2A mutations, and/or loss of heterozygosity for CDKN2A | 5-year OS 22% |
| NUT Carcinoma | Rare (< 1%) | Translocations involving the NUT gene on 15q14, TP53 suppression | 2-year OS 0% |
| Extranodal NK/T Cell Lymphoma | Rare (< 1%) | JAK/STAT activation, mutations of DDX3X, dmutations in tumor suppressor genes such as TP53, PRDM1, FOXO3, and HACE2 | 5-year OS 42% |
| Esthesioneuroblastoma | 2–6% | TP53, c-kit, PDGFR-b, EGFR, FGF-FGFR1 signaling, Sonic hedgehog pathway; apoptosis-related pathways (Bcl-2, TRAIL) and neoangiogenesis (VEGF; KDR) | 5-year OS 62% |
Acknowledgement:
The authors would like to thank Dr. Christopher Corless for his contribution with pathology images for this manuscript.
Funding:
MCH received partial salary support from the following sources: a research grant from the Jonathan David Foundation, a VA Merit Review Grant (I01BX005358), and from an NCI R21 grant (R21CA263400).
Footnotes
Conflict of interest statement: Michael C Heinrich serves in a consultancy role for Blueprint Medicines, Deciphera Pharmaceuticals, and Novartis; receives royalties from Novartis; receives grant funding from Blueprint Medicines and Deciphera Pharmaceuticals.
References
- 1.Abdou R, Baredes S. Population-Based Results in the Management of Sinonasal and Ventral Skull Base Malignancies. Otolaryngol Clin North Am. 2017. Apr;50(2):481–97. [DOI] [PubMed] [Google Scholar]
- 2.Sharma RK, Irace AL, Schlosser RJ, Overdevest JB, Rowan NR, Troob SH, et al. Conditional and Overall Disease-Specific Survival in Patients With Paranasal Sinus and Nasal Cavity Cancer: Improved Outcomes in the Endoscopic Era. Am J Rhinol Allergy. 2022. Jan;36(1):57–64. [DOI] [PubMed] [Google Scholar]
- 3.Bossi P, Hermsen M, Lechner M, Franchi A. Precision medicine in rare tumors and the need for multicenter trials and international collaboratives: Sinonasal cancer as paradigm. Oral Oncol. 2020. Oct 1;109:104737. [DOI] [PubMed] [Google Scholar]
- 4.Wenig BM. Nasal Cavity and Paranasal Sinuses. In: Non-Neoplastic Diseases of the Head and Neck [Internet]. 2017. [cited 2022 Jan 29]. (1; vol. 11). Available from: https://lesterthompsonmd.com/non-neoplastic-diseases-of-the-head-and-neck-1st-series-vol-11/ [Google Scholar]
- 5.Cardesa A, Alos L, Nadal A, Franchi A. Nasal Cavity and Paranasal Sinuses. Pathol Head Neck. 2017. Feb 11;49–127. [Google Scholar]
- 6.Llorente JL, López F, Suárez C, Hermsen MA. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol. 2014. Aug;11(8):460–72. [DOI] [PubMed] [Google Scholar]
- 7.Mayr SI, Hafizovic K, Waldfahrer F, Iro H, Kütting B. Characterization of initial clinical symptoms and risk factors for sinonasal adenocarcinomas: results of a case-control study. Int Arch Occup Environ Health. 2010. Aug;83(6):631–8. [DOI] [PubMed] [Google Scholar]
- 8.Sanghvi S, Khan MN, Patel NR, Yeldandi S, Baredes S, Eloy JA. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. The Laryngoscope. 2014. Jan;124(1):76–83. [DOI] [PubMed] [Google Scholar]
- 9.Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and neck cancers—major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122–37. [DOI] [PubMed] [Google Scholar]
- 10.Ackall FY, Issa K, Barak I, Teitelbaum J, Jang DW, Jung SH, et al. Survival Outcomes in Sinonasal Poorly Differentiated Squamous Cell Carcinoma. The Laryngoscope. 2021. Apr;131(4):E1040–8. [DOI] [PubMed] [Google Scholar]
- 11.Sjöstedt S, Jensen DH, Jakobsen KK, Grønhøj C, Geneser C, Karnov K, et al. Incidence and survival in sinonasal carcinoma: a Danish population-based, nationwide study from 1980 to 2014. Acta Oncol 2018. Sep 2;57(9):1152–8. [DOI] [PubMed] [Google Scholar]
- 12.Elgart K, Faden DL. Sinonasal Squamous Cell Carcinoma: Etiology, Pathogenesis, and the Role of Human Papilloma Virus. Curr Otorhinolaryngol Rep. 2020;8(2):111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes RB, Kardaun JW, de Bruyn A. Tobacco use and sinonasal cancer: a case-control study. Br J Cancer. 1987. Dec;56(6):843–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda K, Shibata A. Exposure-response relationships between woodworking, smoking or passive smoking, and squamous cell neoplasms of the maxillary sinus. Cancer Causes Control CCC. 1990. Sep;1(2):165–8. [DOI] [PubMed] [Google Scholar]
- 15.Chang Sing Pang KJW, Mur T, Collins L, Rao SR, Faden DL. Human Papillomavirus in Sinonasal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2020. Dec 25;13(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kılıç S, Kılıç SS, Kim ES, Baredes S, Mahmoud O, Gray ST, et al. Significance of human papillomavirus positivity in sinonasal squamous cell carcinoma. Int Forum Allergy Rhinol. 2017. Oct;7(10):980–9. [DOI] [PubMed] [Google Scholar]
- 17.Oliver JR, Lieberman SM, Tam MM, Liu CZ, Li Z, Hu KS, et al. Human papillomavirus and survival of patients with sinonasal squamous cell carcinoma. Cancer. 2020. Apr 1;126(7):1413–23. [DOI] [PubMed] [Google Scholar]
- 18.Lawson W, Schlecht NF, Brandwein-Gensler M. The Role of the Human Papillomavirus in the Pathogenesis of Schneiderian Inverted Papillomas: An Analytic Overview of the Evidence. Head Neck Pathol. 2008. Jun;2(2):49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Human Papillomavirus Testing in Head and Neck Carcinomas [Internet]. College of American Pathologists. [cited 2022 May 12]. Available from: https://www.cap.org/protocols-and-guidelines/cap-guidelines/current-cap-guidelines/human-papillomavirus-testing-in-head-and-neck-carcinomas [Google Scholar]
- 20.Bishop JA, Ogawa T, Stelow EB, Moskaluk CA, Koch WM, Pai SI, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013. Jun;37(6):836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zupancic M, Näsman A. Human Papillomavirus-Related Multiphenotypic Sinonasal Carcinoma—An Even Broader Tumor Entity? Viruses. 2021. Sep;13(9):1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmila R, Bornholdt J, Suitiala T, Cyr D, Dictor M, Steiniche T, et al. Profile of TP53 gene mutations in sinonasal cancer. Mutat Res Mol Mech Mutagen. 2010. Apr 1;686(1):9–14. [DOI] [PubMed] [Google Scholar]
- 23.Doescher J, Piontek G, Wirth M, Bettstetter M, Schlegel J, Haller B, et al. Epstein-Barr virus infection is strictly associated with the metastatic spread of sinonasal squamous-cell carcinomas. Oral Oncol. 2015. Oct;51(10):929–34. [DOI] [PubMed] [Google Scholar]
- 24.Udager AM, McHugh JB, Betz BL, Montone KT, Livolsi VA, Seethala RR, et al. Activating KRAS mutations are characteristic of oncocytic sinonasal papilloma and associated sinonasal squamous cell carcinoma. J Pathol. 2016. Aug;239(4):394–8. [DOI] [PubMed] [Google Scholar]
- 25.Cabal VN, Menendez M, Vivanco B, Potes-Ares S, Riobello C, Suarez-Fernandez L, et al. EGFR mutation and HPV infection in sinonasal inverted papilloma and squamous cell carcinoma. Rhinology. 2020. Aug 1;58(4):368–76. [DOI] [PubMed] [Google Scholar]
- 26.Taverna C, Agaimy A, Franchi A. Towards a Molecular Classification of Sinonasal Carcinomas: Clinical Implications and Opportunities. Cancers. 2022. Jan;14(6):1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schröck A, Göke F, Wagner P, Bode M, Franzen A, Braun M, et al. Sex determining region Y-box 2 (SOX2) amplification is an independent indicator of disease recurrence in sinonasal cancer. PloS One. 2013;8(3):e59201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandoh N, Hayashi T, Takahara M, Kishibe K, Ogino T, Katayama A, et al. VEGF and bFGF expression and microvessel density of maxillary sinus squamous cell carcinoma in relation to p53 status, spontaneous apoptosis and prognosis. Cancer Lett. 2004. May 28;208(2):215–25. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari M, Taboni S, Carobbio ALC, Emanuelli E, Maroldi R, Bossi P, et al. Sinonasal Squamous Cell Carcinoma, a Narrative Reappraisal of the Current Evidence. Cancers. 2021. Jan;13(11):2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari M, Ioppi A, Schreiber A, Gualtieri T, Mattavelli D, Rampinelli V, et al. Malignant tumors of the maxillary sinus: Prognostic impact of neurovascular invasion in a series of 138 patients. Oral Oncol. 2020. Jul 1;106:104672. [DOI] [PubMed] [Google Scholar]
- 31.Dubal PM, Bhojwani A, Patel TD, Zuckerman O, Baredes S, Liu JK, et al. Squamous cell carcinoma of the maxillary sinus: A population-based analysis. The Laryngoscope. 2016. Feb;126(2):399–404. [DOI] [PubMed] [Google Scholar]
- 32.Haerle SK, Gullane PJ, Witterick IJ, Zweifel C, Gentili F. Sinonasal Carcinomas: Epidemiology, Pathology, and Management. Neurosurg Clin N Am. 2013. Jan 1;24(1):39–49. [DOI] [PubMed] [Google Scholar]
- 33.Ganti A, Tajudeen BA, Plitt MA, Rossi I, Gattuso P, Batra PS. Discordance in Preoperative and Postoperative Histopathology of Sinonasal Tumors. Am J Rhinol Allergy. 2018. Mar;32(2):101–5. [DOI] [PubMed] [Google Scholar]
- 34.Mehrad M, Chernock RD, El-Mofty SK, Lewis JS. Diagnostic Discrepancies in Mandatory Slide Review of Extradepartmental Head and Neck Cases: Experience at a Large Academic Center. Arch Pathol Lab Med. 2015. Dec;139(12):1539–45. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi M, Kato H, Tomita H, Mizuta K, Aoki M, Hara A, et al. Imaging Characteristics of Malignant Sinonasal Tumors. J Clin Med. 2017. Dec 6;6(12):E116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lloyd G, Lund VJ, Howard D, Savy L. Optimum imaging for sinonasal malignancy. J Laryngol Otol. 2000. Jul;114(7):557–62. [DOI] [PubMed] [Google Scholar]
- 37.Yan CH, Tong CCL, Penta M, Patel VS, Palmer JN, Adappa ND, et al. Imaging predictors for malignant transformation of inverted papilloma. The Laryngoscope. 2019. Apr;129(4):777–82. [DOI] [PubMed] [Google Scholar]
- 38.Ozturk K, Gencturk M, Caicedo-Granados E, Li F, Cayci Z. Utility of FDG PET/CT in the Characterization of Sinonasal Neoplasms: Analysis of Standardized Uptake Value Parameters. AJR Am J Roentgenol. 2018. Dec;211(6):1354–60. [DOI] [PubMed] [Google Scholar]
- 39.Abu-Ghanem S, Yafit D, Ghanayem M, Abergel A, Yehuda M, Fliss DM. Utility of first positron emission tomography-computed tomography scan as a prognostic tool following treatment of sinonasal and skull base malignancies. Head Neck. 2019. Mar;41(3):701–6. [DOI] [PubMed] [Google Scholar]
- 40.Jafari A, Shen SA, Qualliotine JR, Orosco RK, Califano JA, DeConde AS. Impact of margin status on survival after surgery for sinonasal squamous cell carcinoma. Int Forum Allergy Rhinol. 2019. Oct;9(10):1205–11. [DOI] [PubMed] [Google Scholar]
- 41.Crawford KL, Jafari A, Qualliotine JR, Stuart E, DeConde AS, Califano JA, et al. Elective neck dissection for T3/T4 cN0 sinonasal squamous cell carcinoma. Head Neck. 2020. Dec;42(12):3655–62. [DOI] [PubMed] [Google Scholar]
- 42.Noronha V, Patil VM, Joshi A, Krishna MV, Dhumal S, Juvekar S, et al. Induction chemotherapy in technically unresectable locally advanced carcinoma of maxillary sinus. Chemother Res Pract. 2014;2014:487872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirakawa H, Hanai N, Ozawa T, Suzuki H, Nishikawa D, Matayoshi S, et al. Prognostic impact of pathological response to neoadjuvant chemotherapy followed by definitive surgery in sinonasal squamous cell carcinoma. Head Neck. 2016. Apr;38 Suppl 1:E1305–1311. [DOI] [PubMed] [Google Scholar]
- 44.Ock CY, Keam B, Kim TM, Han DH, Won TB, Lee SH, et al. Induction chemotherapy in head and neck squamous cell carcinoma of the paranasal sinus and nasal cavity: a role in organ preservation. Korean J Intern Med. 2016. May;31(3):570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanna EY, Cardenas AD, DeMonte F, Roberts D, Kupferman M, Weber R, et al. Induction chemotherapy for advanced squamous cell carcinoma of the paranasal sinuses. Arch Otolaryngol Head Neck Surg. 2011. Jan;137(1):78–81. [DOI] [PubMed] [Google Scholar]
- 46.Farrell NF, Mace JC, Detwiller KY, Li R, Andersen PE, Smith TL, et al. Predictors of survival outcomes in sinonasal squamous cell carcinoma: an analysis of the National Cancer Database. Int Forum Allergy Rhinol. 2021. Jun;11(6):1001–11. [DOI] [PubMed] [Google Scholar]
- 47.Abdelmeguid AS, Teeramatwanich W, Roberts DB, Amit M, Ferraroto R, Glisson BS, et al. Neoadjuvant chemotherapy for locoregionally advanced squamous cell carcinoma of the paranasal sinuses. Cancer. 2021. Jun 1;127(11):1788–95. [DOI] [PubMed] [Google Scholar]
- 48.Robin TP, Jones BL, Gordon OM, Phan A, Abbott D, McDermott JD, et al. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer. 2017. Aug 15;123(16):3040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernström E, Nyman J, Hammerlid E, Holmberg E, Haugen-Cange H, Petruson K, et al. Results of preoperative chemoradiotherapy for patients with advanced cancer of the nasal cavity and paranasal sinuses. Acta Otolaryngol (Stockh). 2017. Dec;137(12):1292–300. [DOI] [PubMed] [Google Scholar]
- 50.Park SH, Lee JE, Ahn D. Outcome of definitive and postoperative radiotherapy in patients with sinonasal squamous cell carcinomas. Tumori. 2016. Aug 3;102(4):426–32. [DOI] [PubMed] [Google Scholar]
- 51.Duru Birgi S, Teo M, Dyker KE, Sen M, Prestwich RJD. Definitive and adjuvant radiotherapy for sinonasal squamous cell carcinomas: a single institutional experience. Radiat Oncol Lond Engl. 2015. Sep 17;10:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim JH, Lee YS, Chung YS, Jang YJ, Kim SB, Lee SW, et al. Treatment outcomes of concurrent chemoradiotherapy for locally advanced sinonasal squamous cell carcinoma: A single-institution study. Acta Otolaryngol (Stockh). 2015;135(11):1189–95. [DOI] [PubMed] [Google Scholar]
- 53.Liang ZG, Kusumawidjaja G, Kazmi F, Wee JTS, Chua MLK. Intensity-modulated radiotherapy for paranasal sinuses and base of skull tumors. Oral Oncol. 2018. Nov;86:61–8. [DOI] [PubMed] [Google Scholar]
- 54.Jensen AD. Particle Therapy: Protons and Heavy Ions. Adv Otorhinolaryngol. 2020;84:87–105. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Hu W, Hu J, Gao J, Yang J, Kong L, et al. Carbon ion radiation therapy for sinonasal malignancies: Promising results from 2282 cases from the real world. Cancer Sci. 2020. Dec;111(12):4465–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lasrado S, Moras K, Pinto GJO, Bhat M, Hegde S, Sathian B, et al. Role of concomitant chemoradiation in locally advanced head and neck cancers. Asian Pac J Cancer Prev APJCP. 2014;15(10):4147–52. [DOI] [PubMed] [Google Scholar]
- 57.Melotek JM, Cooper BT, Koshy M, Silverman JS, Spiotto MT. Weekly versus every-three-weeks platinum-based chemoradiation regimens for head and neck cancer. J Otolaryngol - Head Neck Surg J Oto-Rhino-Laryngol Chir Cervico-Faciale. 2016. Nov 24;45(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MD AWC. A Phase II Study of Intensity-Modulated or Proton Radiation Therapy for Locally Advanced Sinonasal Malignancy [Internet]. clinicaltrials.gov; 2021. Jun [cited 2021 Oct 11]. Report No.: NCT01586767. Available from: https://clinicaltrials.gov/ct2/show/NCT01586767 [Google Scholar]
- 59.Farber NI, Povolotskiy R, Bavier RD, Riccardi J, Eloy JA, Hsueh WD. Impact of palliative treatment on survival in sinonasal malignancies. Int Forum Allergy Rhinol. 2019. Dec;9(12):1499–507. [DOI] [PubMed] [Google Scholar]
- 60.Fossati P, Vavassori A, Deantonio L, Ferrara E, Krengli M, Orecchia R. Review of photon and proton radiotherapy for skull base tumours. Rep Pract Oncol Radiother J Gt Cancer Cent Poznan Pol Soc Radiat Oncol. 2016. Aug;21(4):336–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaplan DJ, Kim JH, Wang E, Snyderman C. Prognostic Indicators for Salvage Surgery of Recurrent Sinonasal Malignancy. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2016. Jan;154(1):104–12. [DOI] [PubMed] [Google Scholar]
- 62.Orlandi E, Cavalieri S, Granata R, Nicolai P, Castelnuovo P, Piazza C, et al. Locally advanced epithelial sinonasal tumors: The impact of multimodal approach. The Laryngoscope. 2020. Apr;130(4):857–65. [DOI] [PubMed] [Google Scholar]
- 63.Licitra L, Resteghini C, Bossi P. The Evolving Role of Systemic Therapy in the Primary Treatment of Sinonasal Cancer. Adv Otorhinolaryngol. 2020;84:78–86. [DOI] [PubMed] [Google Scholar]
- 64.Riobello C, Vivanco B, Reda S, López-Hernández A, García-Inclán C, Potes-Ares S, et al. Programmed death ligand-1 expression as immunotherapeutic target in sinonasal cancer. Head Neck. 2018. Apr;40(4):818–27. [DOI] [PubMed] [Google Scholar]
- 65.Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012. Jun;34(6):877–85. [DOI] [PubMed] [Google Scholar]
- 66.Jain S, Li Y, Kuan EC, Tajudeen BA, Batra PS. Prognostic Factors in Paranasal Sinus Squamous Cell Carcinoma and Adenocarcinoma: A SEER Database Analysis. J Neurol Surg Part B Skull Base. 2019. Jun;80(3):258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dutta R, Dubal PM, Svider PF, Liu JK, Baredes S, Eloy JA. Sinonasal malignancies: A population-based analysis of site-specific incidence and survival. The Laryngoscope. 2015. Nov;125(11):2491–7. [DOI] [PubMed] [Google Scholar]
- 68.Bhattacharyya N. Cancer of the nasal cavity: survival and factors influencing prognosis. Arch Otolaryngol Head Neck Surg. 2002. Sep;128(9):1079–83. [DOI] [PubMed] [Google Scholar]
- 69.Fornelli RA, Fedok FG, Wilson EP, Rodman SM. Squamous cell carcinoma of the anterior nasal cavity: a dual institution review. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2000. Sep;123(3):207–10. [DOI] [PubMed] [Google Scholar]
- 70.Workman AD, Palmer JN, Adappa ND. Posttreatment surveillance for sinonasal malignancy. Curr Opin Otolaryngol Head Neck Surg. 2017. Feb;25(1):86–92. [DOI] [PubMed] [Google Scholar]
- 71.Anderson Cancer Center MD. Phase II Trial of Induction Therapy With Docetaxel, Cisplatin and Fluorouracil in Previously Untreated Patients With Locally Advanced Squamous Cell Carcinoma and/or Poorly Differentiated Carcinoma of the Nasal Cavity and/or Paranasal Sinuses [Internet]. clinicaltrials.gov; 2021. Jul [cited 2021 Oct 11]. Report No.: NCT00707473. Available from: https://clinicaltrials.gov/ct2/show/NCT00707473 [Google Scholar]
- 72.Youlden DR, Cramb SM, Peters S, Porceddu SV, Møller H, Fritschi L, et al. International comparisons of the incidence and mortality of sinonasal cancer. Cancer Epidemiol. 2013. Dec;37(6):770–9. [DOI] [PubMed] [Google Scholar]
- 73.Bhayani MK, Yilmaz T, Sweeney A, Calzada G, Roberts DB, Levine NB, et al. Sinonasal adenocarcinoma: a 16-year experience at a single institution. Head Neck. 2014. Oct;36(10):1490–6. [DOI] [PubMed] [Google Scholar]
- 74.Leivo I. Sinonasal Adenocarcinoma: Update on Classification, Immunophenotype and Molecular Features. Head Neck Pathol. 2016. Feb 1;10(1):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choussy O, Ferron C, Védrine PO, Toussaint B, Liétin B, Marandas P, et al. Adenocarcinoma of Ethmoid: a GETTEC retrospective multicenter study of 418 cases. The Laryngoscope. 2008. Mar;118(3):437–43. [DOI] [PubMed] [Google Scholar]
- 76.Kak I, Perez-Ordoñez B. Sinonasal tract pathology: an updated review of select entities. Diagn Histopathol. 2019. Jul 1;25(7):265–73. [Google Scholar]
- 77.López-Hernández A, Pérez-Escuredo J, Vivanco B, García-Inclán C, Potes-Ares S, Cabal VN, et al. Genomic profiling of intestinal-type sinonasal adenocarcinoma reveals subgroups of patients with distinct clinical outcomes. Head Neck. 2018;40(2):259–73. [DOI] [PubMed] [Google Scholar]
- 78.Lund VJ, Chisholm EJ, Takes RP, Suárez C, Mendenhall WM, Rinaldo A, et al. Evidence for treatment strategies in sinonasal adenocarcinoma. Head Neck. 2012. Aug;34(8):1168–78. [DOI] [PubMed] [Google Scholar]
- 79.Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001. Dec 15;92(12):3012–29. [DOI] [PubMed] [Google Scholar]
- 80.Roux FX, Brasnu D, Devaux B, Chabardes E, Schwaab G, Laccourreye O, et al. Ethmoid sinus carcinomas: results and prognosis after neoadjuvant chemotherapy and combined surgery--a 10-year experience. Surg Neurol. 1994. Aug;42(2):98–104. [DOI] [PubMed] [Google Scholar]
- 81.Kılıç S, Samarrai R, Kılıç SS, Mikhael M, Baredes S, Eloy JA. Incidence and survival of sinonasal adenocarcinoma by site and histologic subtype. Acta Otolaryngol (Stockh). 2018. Apr 3;138(4):415–21. [DOI] [PubMed] [Google Scholar]
- 82.Chen MM, Roman SA, Sosa JA, Judson BL. Predictors of Survival in Sinonasal Adenocarcinoma. J Neurol Surg Part B Skull Base. 2015. Jun;76(3):208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel TD, Vazquez A, Dubal PM, Baredes S, Liu JK, Eloy JA. Sinonasal neuroendocrine carcinoma: a population-based analysis of incidence and survival. Int Forum Allergy Rhinol. 2015;5(5):448–53. [DOI] [PubMed] [Google Scholar]
- 84.Mitchell EH, Diaz A, Yilmaz T, Roberts D, Levine N, DeMonte F, et al. Multimodality treatment for sinonasal neuroendocrine carcinoma. Head Neck. 2012. Oct;34(10):1372–6. [DOI] [PubMed] [Google Scholar]
- 85.Mineta H, Miura K, Takebayashi S, Araki K, Ueda Y, Harada H, et al. Immunohistochemical analysis of small cell carcinoma of the head and neck: a report of four patients and a review of sixteen patients in the literature with ectopic hormone production. Ann Otol Rhinol Laryngol. 2001. Jan;110(1):76–82. [DOI] [PubMed] [Google Scholar]
- 86.Bell D, Hanna EY, Weber RS, DeMonte F, Triantafyllou A, Lewis JS, et al. Neuroendocrine neoplasms of the sinonasal region. Head Neck. 2016. Apr;38 Suppl 1:E2259–2266. [DOI] [PubMed] [Google Scholar]
- 87.Shah K, Perez-Ordóñez B. Neuroendocrine Neoplasms of the Sinonasal Tract: Neuroendocrine Carcinomas and Olfactory Neuroblastoma. Head Neck Pathol. 2016. Feb 1;10(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klöppel G, Franchi A, Matias-Guiu X. Neuroendocrine Neoplasms, Olfactory Neuroblastomas and Paragangliomas of the Head and Neck. In: Cardesa A, Slootweg PJ, Gale N, Franchi A, editors. Pathology of the Head and Neck [Internet]. Berlin, Heidelberg: Springer; 2016. [cited 2022 Feb 21]. p. 515–38. Available from: 10.1007/978-3-662-49672-5_11 [DOI] [Google Scholar]
- 89.Kao HL, Chang WC, Li WY, Chia-Heng Li A, Fen-Yau Li A. Head and neck large cell neuroendocrine carcinoma should be separated from atypical carcinoid on the basis of different clinical features, overall survival, and pathogenesis. Am J Surg Pathol. 2012. Feb;36(2):185–92. [DOI] [PubMed] [Google Scholar]
- 90.Dogan S, Chute DJ, Xu B, Ptashkin RN, Chandramohan R, Casanova-Murphy J, et al. Frequent IDH2 R172 Mutations in Undifferentiated and Poorly-Differentiated Sinonasal Carcinomas. J Pathol. 2017. Aug;242(4):400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu KY, Goldrich DY, Ninan SJ, Filimonov A, Lam H, Govindaraj S, et al. The value of 68Gallium-DOTATATE PET/CT in sinonasal neuroendocrine tumor management: A case series. Head Neck. 2021;43(6):E30–40. [DOI] [PubMed] [Google Scholar]
- 92.Babin E, Rouleau V, Vedrine PO, Toussaint B, Raucourt D de, Malard O, et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. J Laryngol Otol. 2006. Apr;120(4):289–97. [DOI] [PubMed] [Google Scholar]
- 93.van der Laan TP, Iepsma R, Witjes MJH, van der Laan BFAM, Plaat BEC, Halmos GB. Meta-analysis of 701 published cases of sinonasal neuroendocrine carcinoma: The importance of differentiation grade in determining treatment strategy. Oral Oncol. 2016. Dec 1;63:1–9. [DOI] [PubMed] [Google Scholar]
- 94.Frierson HF, Mills SE, Fechner RE, Taxy JB, Levine PA. Sinonasal undifferentiated carcinoma. An aggressive neoplasm derived from schneiderian epithelium and distinct from olfactory neuroblastoma. Am J Surg Pathol. 1986. Nov;10(11):771–9. [PubMed] [Google Scholar]
- 95.Chambers KJ, Lehmann AE, Remenschneider A, Dedmon M, Meier J, Gray ST, et al. Incidence and Survival Patterns of Sinonasal Undifferentiated Carcinoma in the United States. J Neurol Surg Part B Skull Base. 2015. Mar;76(2):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abdelmeguid AS, Bell D, Hanna EY. Sinonasal Undifferentiated Carcinoma. Curr Oncol Rep. 2019. Feb 26;21(3):26. [DOI] [PubMed] [Google Scholar]
- 97.Agaimy A, Franchi A, Lund VJ, Skálová A, Bishop JA, Triantafyllou A, et al. Sinonasal Undifferentiated Carcinoma (SNUC): From an Entity to Morphologic Pattern and Back Again-A Historical Perspective. Adv Anat Pathol. 2020. Mar;27(2):51–60. [DOI] [PubMed] [Google Scholar]
- 98.Sunderman FW, Morgan LG, Andersen A, Ashley D, Forouhar FA. Histopathology of sinonasal and lung cancers in nickel refinery workers. Ann Clin Lab Sci. 1989. Feb;19(1):44–50. [PubMed] [Google Scholar]
- 99.Boysen M, Voss R, Solberg LA. The nasal mucosa in softwood exposed furniture workers. Acta Otolaryngol (Stockh). 1986. Jun;101(5–6):501–8. [DOI] [PubMed] [Google Scholar]
- 100.Lopategui JR, Gaffey MJ, Frierson HF, Chan JK, Mills SE, Chang KL, et al. Detection of Epstein-Barr viral RNA in sinonasal undifferentiated carcinoma from Western and Asian patients. Am J Surg Pathol. 1994. Apr;18(4):391–8. [DOI] [PubMed] [Google Scholar]
- 101.Jeng YM, Sung MT, Fang CL, Huang HY, Mao TL, Cheng W, et al. Sinonasal undifferentiated carcinoma and nasopharyngeal-type undifferentiated carcinoma: two clinically, biologically, and histopathologically distinct entities. Am J Surg Pathol. 2002. Mar;26(3):371–6. [DOI] [PubMed] [Google Scholar]
- 102.Cerilli LA, Holst VA, Brandwein MS, Stoler MH, Mills SE. Sinonasal undifferentiated carcinoma: immunohistochemical profile and lack of EBV association. Am J Surg Pathol. 2001. Feb;25(2):156–63. [DOI] [PubMed] [Google Scholar]
- 103.Xu CC, Dziegielewski PT, McGaw WT, Seikaly H. Sinonasal Undifferentiated Carcinoma (SNUC): the Alberta experience and literature review. J Otolaryngol - Head Neck Surg. 2013. Jan 31;42(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Righi PD, Francis F, Aron BS, Weitzner S, Wilson KM, Gluckman J. Sinonasal undifferentiated carcinoma: a 10-year experience. Am J Otolaryngol. 1996. Jun;17(3):167–71. [DOI] [PubMed] [Google Scholar]
- 105.D’Aguanno V, Ralli M, Cerbelli B, Greco A, De Vincentiis M. Liver metastases from maxillary sinus sinonasal undifferentiated carcinoma: A case report. Oncol Lett. 2019. Jun;17(6):5811–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sarangi S, Khera S, Vishwajeet V, Meshram V, Setia P, Malik A. A rare presentation of Sinonasal Undifferentiated Carcinoma with brain metastasis and para-aortic mass. Autopsy Case Rep. 2020. Nov 20;10(4):e2020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sienna J, Nguyen NT, Arsenault J, Hodson I, Meyers B. A Case of Sinonasal Undifferentiated Carcinoma with Brain Metastases. Cureus. 2018. Mar 13;10(3):e2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reiersen DA, Pahilan ME, Devaiah AK. Meta-analysis of treatment outcomes for sinonasal undifferentiated carcinoma. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2012. Jul;147(1):7–14. [DOI] [PubMed] [Google Scholar]
- 109.Stelow EB, Bishop JA. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Nasal Cavity, Paranasal Sinuses and Skull Base. Head Neck Pathol. 2017. Feb 28;11(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Franchi A, Moroni M, Massi D, Paglierani M, Santucci M. Sinonasal Undifferentiated Carcinoma, Nasopharyngeal-Type Undifferentiated Carcinoma, and Keratinizing and Nonkeratinizing Squamous Cell Carcinoma Express Different Cytokeratin Patterns. Am J Surg Pathol. 2002. Dec;26(12):1597–604. [DOI] [PubMed] [Google Scholar]
- 111.Soldatova L, Campbell RG, Carrau RL, Prevedello DM, Wakely P, Otto BA, et al. Sinonasal Carcinomas with Neuroendocrine Features: Histopathological Differentiation and Treatment Outcomes. J Neurol Surg Part B Skull Base. 2016. Dec;77(6):456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Agaimy A, Franchi A, Lund VJ, Skálová A, Bishop JA, Triantafyllou A, et al. Sinonasal Undifferentiated Carcinoma (SNUC): From an Entity to Morphologic Pattern and Back Again—A Historical Perspective. Adv Anat Pathol. 2020. Mar;27(2):51–60. [DOI] [PubMed] [Google Scholar]
- 113.Wadsworth B, Bumpous JM, Martin AW, Nowacki MR, Jenson AB, Farghaly H. Expression of p16 in Sinonasal Undifferentiated Carcinoma (SNUC) Without Associated Human Papillomavirus (HPV). Head Neck Pathol. 2011. Jul 30;5(4):349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schwerer MJ, Sailer A, Kraft K, Baczako K, Maier H. Expression of retinoblastoma gene product in respiratory epithelium and sinonasal neoplasms: relationship with p16 and cyclin D1 expression. Histol Histopathol. 2003. Jan;18(1):143–51. [DOI] [PubMed] [Google Scholar]
- 115.Chernock RD, Perry A, Pfeifer JD, Holden JA, Lewis JS. Receptor tyrosine kinases in sinonasal undifferentiated carcinomas--evaluation for EGFR, c-KIT, and HER2/neu expression. Head Neck. 2009. Jul;31(7):919–27. [DOI] [PubMed] [Google Scholar]
- 116.Singh L, Ranjan R, Arava S, Singh MK. Role of p40 and cytokeratin 5/6 in the differential diagnosis of sinonasal undifferentiated carcinoma. Ann Diagn Pathol. 2014. Oct 1;18(5):261–5. [DOI] [PubMed] [Google Scholar]
- 117.Thompson LD. Small round blue cell tumors of the sinonasal tract: a differential diagnosis approach. Mod Pathol. 2017. Jan;30(1):S1–26. [DOI] [PubMed] [Google Scholar]
- 118.Takahashi Y, Gleber-Netto FO, Bell D, Roberts D, Xie TX, Abdelmeguid AS, et al. Identification of novel diagnostic markers for sinonasal undifferentiated carcinoma. Head Neck. 2019;41(8):2688–95. [DOI] [PubMed] [Google Scholar]
- 119.Libera L, Ottini G, Sahnane N, Pettenon F, Turri-Zanoni M, Lambertoni A, et al. Methylation Drivers and Prognostic Implications in Sinonasal Poorly Differentiated Carcinomas. Cancers. 2021. Oct 8;13(19):5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.SMARCA4 gene: MedlinePlus Genetics [Internet]. [cited 2021 Jul 14]. Available from: https://medlineplus.gov/genetics/gene/smarca4/
- 121.Bishop JA, Antonescu CR, Westra WH. SMARCB1 (INI-1)-deficient carcinomas of the sinonasal tract. Am J Surg Pathol. 2014. Sep;38(9):1282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ou CP, Ho SP. SMARCB1 (INI1)-deficient sinonasal carcinoma, basaloid subtype: First reported case in Taiwan and literature review. Hum Pathol Case Rep. 2020. Nov 1;22:200423. [Google Scholar]
- 123.Agaimy A, Jain D, Uddin N, Rooper LM, Bishop JA. SMARCA4-deficient sinonasal carcinoma: A series of 10 cases expanding the genetic spectrum of SWI/SNF-driven sinonasal malignancies. Am J Surg Pathol. 2020;44(5):703–10. [DOI] [PubMed] [Google Scholar]
- 124.Mito JK, Bishop JA, Sadow PM, Stelow EB, Faquin WC, Mills SE, et al. Immunohistochemical Detection and Molecular Characterization of IDH-mutant Sinonasal Undifferentiated Carcinomas. Am J Surg Pathol. 2018. Aug;42(8):1067–75. [DOI] [PubMed] [Google Scholar]
- 125.Jo VY, Chau NG, Hornick JL, Krane JF, Sholl LM. Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol Off J U S Can Acad Pathol Inc. 2017. May;30(5):650–9. [DOI] [PubMed] [Google Scholar]
- 126.Dogan S, Chute DJ, Xu B, Ptashkin RN, Chandramohan R, Casanova-Murphy J, et al. Frequent IDH2 R172 Mutations in Undifferentiated and Poorly-Differentiated Sinonasal Carcinomas. J Pathol. 2017. Aug;242(4):400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Agaimy A, Koch M, Lell M, Semrau S, Dudek W, Wachter DL, et al. SMARCB1(INI1)-deficient sinonasal basaloid carcinoma: a novel member of the expanding family of SMARCB1-deficient neoplasms. Am J Surg Pathol. 2014. Sep;38(9):1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Phillips CD, Futterer SF, Lipper MH, Levine PA. Sinonasal undifferentiated carcinoma: CT and MR imaging of an uncommon neoplasm of the nasal cavity. Radiology. 1997. Feb 1;202(2):477–80. [DOI] [PubMed] [Google Scholar]
- 129.Tyler MA, Holmes B, Patel ZM. Oncologic management of sinonasal undifferentiated carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2019. Feb;27(1):59–66. [DOI] [PubMed] [Google Scholar]
- 130.Al-Mamgani A, van Rooij P, Mehilal R, Tans L, Levendag PC. Combined-modality treatment improved outcome in sinonasal undifferentiated carcinoma: single-institutional experience of 21 patients and review of the literature. Eur Arch Otorhinolaryngol. 2013;270(1):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gamez ME, Lal D, Halyard MY, Wong WW, Vargas C, Ma D, et al. Outcomes and patterns of failure for sinonasal undifferentiated carcinoma (SNUC): The Mayo Clinic Experience. Head Neck. 2017. Sep 1;39(9):1819–24. [DOI] [PubMed] [Google Scholar]
- 132.Faisal M, Seemann R, Lill C, Hamzavi S, Wutzl A, Erovic BM, et al. Elective neck treatment in sinonasal undifferentiated carcinoma: Systematic review and meta-analysis. Head Neck. 2020;42(5):1057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morand GB, Anderegg N, Vital D, Ikenberg K, Huber GF, Soyka MB, et al. Outcome by treatment modality in sinonasal undifferentiated carcinoma (SNUC): A case-series, systematic review and meta-analysis. Oral Oncol. 2017. Dec;75:28–34. [DOI] [PubMed] [Google Scholar]
- 134.Kuan EC, Arshi A, Mallen-St Clair J, Tajudeen BA, Abemayor E, St John MA. Significance of Tumor Stage in Sinonasal Undifferentiated Carcinoma Survival: A Population-Based Analysis. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2016. Apr;154(4):667–73. [DOI] [PubMed] [Google Scholar]
- 135.de Bonnecaze G, Verillaud B, Chaltiel L, Fierens S, Chapelier M, Rumeau C, et al. Clinical characteristics and prognostic factors of sinonasal undifferentiated carcinoma: a multicenter study. Int Forum Allergy Rhinol. 2018. Sep;8(9):1065–72. [DOI] [PubMed] [Google Scholar]
- 136.Amit M, Abdelmeguid AS, Watcherporn T, Takahashi H, Tam S, Bell D, et al. Induction Chemotherapy Response as a Guide for Treatment Optimization in Sinonasal Undifferentiated Carcinoma. J Clin Oncol. 2019. Feb 20;37(6):504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu NY, Gamez ME, Hartsell WF, Tsai HK, Laramore GE, Larson GL, et al. A Multi-Institutional Experience of Proton Beam Therapy for Sinonasal Tumors. Adv Radiat Oncol. 2019. Oct 1;4(4):689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Noticewala SS, Mell LK, Olson SE, Read W. Survival in unresectable sinonasal undifferentiated carcinoma treated with concurrent intra-arterial cisplatin and radiation. World J Clin Cases WJCC. 2015. Feb 16;3(2):191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Galloni C, Locatello LG, Bruno C, Cannavicci A, Maggiore G, Gallo O. The Role of Elective Neck Treatment in the Management of Sinonasal Carcinomas: A Systematic Review of the Literature and a Meta-Analysis. Cancers. 2021. Apr 13;13(8):1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.López F, Rodrigo JP, Cardesa A, Triantafyllou A, Devaney KO, Mendenhall WM, et al. Update on primary head and neck mucosal melanoma. Head Neck. 2016. Jan;38(1):147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998. Oct 15;83(8):1664–78. [DOI] [PubMed] [Google Scholar]
- 142.Marcus DM, Marcus RP, Prabhu RS, Owonikoko TK, Lawson DH, Switchenko J, et al. Rising Incidence of Mucosal Melanoma of the Head and Neck in the United States. J Skin Cancer. 2012;2012:231693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Abt NB, Miller LE, Mokhtari TE, Lin DT, Richmon JD, Deschler DG, et al. Nasal and paranasal sinus mucosal melanoma: Long-term survival outcomes and prognostic factors. Am J Otolaryngol. 2021. Nov 1;42(6):103070. [DOI] [PubMed] [Google Scholar]
- 144.Hicks MJ, Flaitz CM. Oral mucosal melanoma: epidemiology and pathobiology. Oral Oncol. 2000. Mar;36(2):152–69. [DOI] [PubMed] [Google Scholar]
- 145.Bishop KD, Olszewski AJ. Epidemiology and survival outcomes of ocular and mucosal melanomas: A population-based analysis. Int J Cancer. 2014;134(12):2961–71. [DOI] [PubMed] [Google Scholar]
- 146.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base Report on Cutaneous and Noncutaneous Melanoma. :15. [DOI] [PubMed] [Google Scholar]
- 147.Tyrrell H, Payne M. Combatting mucosal melanoma: recent advances and future perspectives. Melanoma Manag. 2018. Sep 1;5(3):MMT11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Papaspyrou G, Garbe C, Schadendorf D, Werner JA, Hauschild A, Egberts F. Mucosal melanomas of the head and neck: new aspects of the clinical outcome, molecular pathology, and treatment with c-kit inhibitors. Melanoma Res. 2011. Dec;21(6):475–82. [DOI] [PubMed] [Google Scholar]
- 149.Gorsky M, Epstein JB. Melanoma arising from the mucosal surfaces of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. 1998. Dec 1;86(6):715–9. [DOI] [PubMed] [Google Scholar]
- 150.McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005. Mar 1;103(5):1000–7. [DOI] [PubMed] [Google Scholar]
- 151.Holmstrom M, Lund VJ. Malignant melanomas of the nasal cavity after occupational exposure to formaldehyde. Br J Ind Med. 1991. Jan;48(1):9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Reuter VE, Woodruff JM. Melanoma of the larynx. The Laryngoscope. 1986. Apr;96(4):389–93. [DOI] [PubMed] [Google Scholar]
- 153.Guevara-Canales JO, Gutiérrez-Morales MM, Sacsaquispe-Contreras SJ, Sánchez-Lihón J, Morales-Vadillo R. Malignant melanoma of the oral cavity. Review of the literature and experience in a Peruvian Population. Med Oral Patol Oral Cir Bucal. 2012. Mar;17(2):e206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Prasad ML, Jungbluth AA, Iversen K, Huvos AG, Busam KJ. Expression of Melanocytic Differentiation Markers in Malignant Melanomas of the Oral and Sinonasal Mucosa. Am J Surg Pathol. 2001. Jun;25(6):782–7. [DOI] [PubMed] [Google Scholar]
- 155.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol Off J Am Soc Clin Oncol. 2006. Sep 10;24(26):4340–6. [DOI] [PubMed] [Google Scholar]
- 156.Satzger I, Schaefer T, Kuettler U, Broecker V, Voelker B, Ostertag H, et al. Analysis of c-KIT expression and KIT gene mutation in human mucosal melanomas. Br J Cancer. 2008. Dec;99(12):2065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Omholt K, Grafström E, Kanter-Lewensohn L, Hansson J, Ragnarsson-Olding BK. KIT pathway alterations in mucosal melanomas of the vulva and other sites. Clin Cancer Res Off J Am Assoc Cancer Res. 2011. Jun 15;17(12):3933–42. [DOI] [PubMed] [Google Scholar]
- 158.Turri-Zanoni M, Medicina D, Lombardi D, Ungari M, Balzarini P, Rossini C, et al. Sinonasal mucosal melanoma: Molecular profile and therapeutic implications from a series of 32 cases. Head Neck. 2013. Aug;35(8):1066–77. [DOI] [PubMed] [Google Scholar]
- 159.Schoenewolf NL, Bull C, Belloni B, Holzmann D, Tonolla S, Lang R, et al. Sinonasal, genital and acrolentiginous melanomas show distinct characteristics of KIT expression and mutations. Eur J Cancer Oxf Engl 1990. 2012. Aug;48(12):1842–52. [DOI] [PubMed] [Google Scholar]
- 160.Rivera RS, Nagatsuka H, Gunduz M, Cengiz B, Gunduz E, Siar CH, et al. C-kit protein expression correlated with activating mutations in KIT gene in oral mucosal melanoma. Virchows Arch Int J Pathol. 2008. Jan;452(1):27–32. [DOI] [PubMed] [Google Scholar]
- 161.Lourenço SV, Fernandes JD, Hsieh R, Coutinho-Camillo CM, Bologna S, Sangueza M, et al. Head and Neck Mucosal Melanoma: A Review. Am J Dermatopathol. 2014. Jul;36(7):578–87. [DOI] [PubMed] [Google Scholar]
- 162.Alaeddini M, Etemad-Moghadam S. Immunohistochemical profile of oral mucosal and head and neck cutaneous melanoma. J Oral Pathol Med. 2015;44(3):234–8. [DOI] [PubMed] [Google Scholar]
- 163.Cohen Y, Rosenbaum E, Begum S, Goldenberg D, Esche C, Lavie O, et al. Exon 15 BRAF mutations are uncommon in melanomas arising in nonsun-exposed sites. Clin Cancer Res Off J Am Assoc Cancer Res. 2004. May 15;10(10):3444–7. [DOI] [PubMed] [Google Scholar]
- 164.Buery RR, Siar CH, Katase N, Gunduz M, Lefeuvre M, Fujii M, et al. NRAS and BRAF mutation frequency in primary oral mucosal melanoma. Oncol Rep. 2011. Oct;26(4):783–7. [DOI] [PubMed] [Google Scholar]
- 165.Richmond-Sinclair NM, Lee E, Cummings MC, Williamson R, Muller K, Green AC, et al. Histologic and epidemiologic correlates of P-MAPK, Brn-2, pRb, p53, and p16 immunostaining in cutaneous melanomas. Melanoma Res. 2008. Oct;18(5):336–45. [DOI] [PubMed] [Google Scholar]
- 166.Suzuki K, Li F, Sone S, Doi K. Computer-aided diagnostic scheme for distinction between benign and malignant nodules in thoracic low-dose CT by use of massive training artificial neural network. IEEE Trans Med Imaging. 2005. Sep;24(9):1138–50. [DOI] [PubMed] [Google Scholar]
- 167.Hsieh R, Nico MMS, Coutinho-Camillo CM, Buim ME, Sangueza M, Lourenço SV. The CDKN2A and MAP kinase pathways: molecular roads to primary oral mucosal melanoma. Am J Dermatopathol. 2013. Apr;35(2):167–75. [DOI] [PubMed] [Google Scholar]
- 168.Tanaka N, Odajima T, Mimura M, Ogi K, Dehari H, Kimijima Y, et al. Expression of Rb, pRb2/p130, p53, and p16 proteins in malignant melanoma of oral mucosa. Oral Oncol. 2001. Apr;37(3):308–14. [DOI] [PubMed] [Google Scholar]
- 169.Franchi A, Alos L, Gale N, Massi D, Paglierani M, Santucci M, et al. Expression of p16 in sinonasal malignant melanoma. Virchows Arch Int J Pathol. 2006. Dec;449(6):667–72. [DOI] [PubMed] [Google Scholar]
- 170.Gonzalgo ML, Bender CM, You EH, Glendening JM, Flores JF, Walker GJ, et al. Low Frequency of p16/CDKN2A Methylation in Sporadic Melanoma: Comparative Approaches for Methylation Analysis of Primary Tumors. Cancer Res. 1997. Dec 1;57(23):5336–47. [PubMed] [Google Scholar]
- 171.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. John Wiley & Sons; 2011. 209 p. [Google Scholar]
- 172.Koivunen P, Bäck L, Pukkila M, Laranne J, Kinnunen I, Grénman R, et al. Accuracy of the current TNM classification in predicting survival in patients with sinonasal mucosal melanoma. The Laryngoscope. 2012. Aug;122(8):1734–8. [DOI] [PubMed] [Google Scholar]
- 173.Ballantyne AJ. Malignant melanoma of the skin of the head and neck. An analysis of 405 cases. Am J Surg. 1970. Oct;120(4):425–31. [DOI] [PubMed] [Google Scholar]
- 174.Prasad ML, Patel SG, Huvos AG, Shah JP, Busam KJ. Primary mucosal melanoma of the head and neck. Cancer. 2004;100(8):1657–64. [DOI] [PubMed] [Google Scholar]
- 175.Michel J, Perret-Court A, Fakhry N, Braustein D, Monestier S, Richard MA, et al. Sinonasal mucosal melanomas: the prognostic value of tumor classifications. Head Neck. 2014. Mar;36(3):311–6. [DOI] [PubMed] [Google Scholar]
- 176.Gras-Cabrerizo JR, León-Vintró X, Tarruella MM, Sarria GP, Gonzalez CB, Montserrat-Gili JR, et al. Management of Sinonasal Mucosal Melanomas and Comparison of Classification Staging Systems. Am J Rhinol Allergy. 2015. Jan;29(1):e37–40. [DOI] [PubMed] [Google Scholar]
- 177.Penel N, Mallet Y, Mirabel X, Van JT, Lefebvre JL. Primary mucosal melanoma of head and neck: prognostic value of clear margins. The Laryngoscope. 2006. Jun;116(6):993–5. [DOI] [PubMed] [Google Scholar]
- 178.Lee SP, Shimizu KT, Tran LM, Juillard G, Calcaterra TC. Mucosal melanoma of the head and neck: the impact of local control on survival. The Laryngoscope. 1994. Feb;104(2):121–6. [DOI] [PubMed] [Google Scholar]
- 179.Manolidis S, Donald PJ. Malignant mucosal melanoma of the head and neck: review of the literature and report of 14 patients. Cancer. 1997. Oct 15;80(8):1373–86. [DOI] [PubMed] [Google Scholar]
- 180.Patel SG, Prasad ML, Escrig M, Singh B, Shaha AR, Kraus DH, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002. Mar;24(3):247–57. [DOI] [PubMed] [Google Scholar]
- 181.Prinzen T, Klein M, Hallermann C, Wermker K. Primary head and neck mucosal melanoma: Predictors of survival and a case series on sentinel node biopsy. J Cranio-Maxillofac Surg. 2019. Sep 1;47(9):1370–7. [DOI] [PubMed] [Google Scholar]
- 182.Temam S, Mamelle G, Marandas P, Wibault P, Avril MF, Janot F, et al. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer. 2005;103(2):313–9. [DOI] [PubMed] [Google Scholar]
- 183.Benlyazid A, Thariat J, Temam S, Malard O, Florescu C, Choussy O, et al. Postoperative radiotherapy in head and neck mucosal melanoma: a GETTEC study. Arch Otolaryngol Head Neck Surg. 2010. Dec;136(12):1219–25. [DOI] [PubMed] [Google Scholar]
- 184.Caspers CJI, Dronkers E a. C, Monserez D, Wieringa MH, Baatenburg de Jong RJ, Hardillo J a. U. Adjuvant radiotherapy in sinonasal mucosal melanoma: A retrospective analysis. Clin Otolaryngol Off J ENT-UK Off J Neth Soc Oto-Rhino-Laryngol Cervico-Facial Surg. 2018. Apr;43(2):617–23. [DOI] [PubMed] [Google Scholar]
- 185.Grant-Freemantle MC, Lane O’Neill B, Clover AJP. The effectiveness of radiotherapy in the treatment of head and neck mucosal melanoma: Systematic review and meta-analysis. Head Neck. 2021. Jan;43(1):323–33. [DOI] [PubMed] [Google Scholar]
- 186.Spencer KR, Mehnert JM. Mucosal Melanoma: Epidemiology, Biology and Treatment. In: Kaufman HL, Mehnert JM, editors. Melanoma [Internet]. Cham: Springer International Publishing; 2016. [cited 2021 May 22]. p. 295–320. (Cancer Treatment and Research). Available from: 10.1007/978-3-319-22539-5_13 [DOI] [PubMed] [Google Scholar]
- 187.Moreno MA, Roberts DB, Kupferman ME, DeMonte F, El-Naggar AK, Williams M, et al. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M. D. Anderson Cancer Center. Cancer. 2010. May 1;116(9):2215–23. [DOI] [PubMed] [Google Scholar]
- 188.Bartell HL, Bedikian AY, Papadopoulos NE, Dett TK, Ballo MT, Myers JN, et al. Biochemotherapy in patients with advanced head and neck mucosal melanoma. Head Neck. 2008. Dec;30(12):1592–8. [DOI] [PubMed] [Google Scholar]
- 189.Lian B, Cui C, Song X, Zhang X, Wu D, Si L, et al. Phase Ⅲ randomized, multicenter trial comparing high-dose IFN-a2b with temozolomide plus cisplatin as adjuvant therapy for resected mucosal melanoma. J Clin Oncol. 2018. May 20;36(15_suppl):9589–9589. [Google Scholar]
- 190.Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016. Nov 15;122(21):3354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moya-Plana A, Herrera Gómez RG, Rossoni C, Dercle L, Ammari S, Girault I, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019. Jul 1;68(7):1171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kaunitz GJ, Cottrell TR, Lilo M, Muthappan V, Esandrio J, Berry S, et al. Melanoma subtypes demonstrate distinct PD-L1 expression profiles. Lab Invest. 2017. Sep;97(9):1063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shoushtari AN, Wagstaff J, Ascierto PA, Butler MO, Lao CD, Marquez-Rodas I, et al. CheckMate 067: Long-term outcomes in patients with mucosal melanoma. J Clin Oncol. 2020. May 20;38(15_suppl):10019–10019. [Google Scholar]
- 194.Beadling C, Jacobson-Dunlop E, Hodi FS, Le C, Warrick A, Patterson J, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res Off J Am Assoc Cancer Res. 2008. Nov 1;14(21):6821–8. [DOI] [PubMed] [Google Scholar]
- 195.Hodi FS, Corless CL, Giobbie-Hurder A, Fletcher JA, Zhu M, Marino-Enriquez A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol Off J Am Soc Clin Oncol. 2013. Sep 10;31(26):3182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Seth R, Messersmith H, Kaur V, Kirkwood JM, Kudchadkar R, McQuade JL, et al. Systemic Therapy for Melanoma: ASCO Guideline. J Clin Oncol. 2020. Nov 20;38(33):3947–70. [DOI] [PubMed] [Google Scholar]
- 197.Mochel MC, Duncan LM, Piris A, Kraft S. Primary Mucosal Melanoma of the Sinonasal Tract: A Clinicopathologic and Immunohistochemical Study of Thirty-Two Cases. Head Neck Pathol. 2014. Oct 8;9(2):236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Loree TR, Mullins AP, Spellman J, North JH, Hicks WL. Head and neck mucosal melanoma: a 32-year review. Ear Nose Throat J. 1999. May;78(5):372–5. [PubMed] [Google Scholar]
- 199.Roth TN, Gengler C, Huber GF, Holzmann D. Outcome of sinonasal melanoma: clinical experience and review of the literature. Head Neck. 2010. Oct;32(10):1385–92. [DOI] [PubMed] [Google Scholar]
- 200.Medhi P, Biswas M, Das D, Amed S. Cytodiagnosis of mucosal malignant melanoma of nasal cavity: A case report with review of literature. J Cytol. 2012. Jul;29(3):208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stanimirov Rossi O, Vital D, Soyka MB, Roth TN, Huber GF, Holzmann D. Multilocular sinonasal malignant melanoma: a poor prognostic subgroup? Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2015. Jan;272(1):123–9. [DOI] [PubMed] [Google Scholar]
- 202.Roussy Gustave, Campus Cancer, Paris Grand. Phase II Multicentric Study: Efficacy Evaluation of Neoadjuvant Treatment Associated With Maintenance Therapy by Anti-PD1 Immunotherapy on Disease-free-survival (DFS) in Patients With Resectable Head and Neck Mucosal Melanoma [Internet]. clinicaltrials.gov; 2021. Jul [cited 2021 Aug 3]. Report No.: NCT03313206. Available from: https://clinicaltrials.gov/ct2/show/NCT03313206 [Google Scholar]
- 203.MD RRM. Single Arm Phase II Study of Ipilimumab and Nivolumab as Adjuvant Therapy for Resected Mucosal Melanoma (SALVO Study). HCRN: MEL16–252 [Internet]. clinicaltrials.gov; 2021. Apr [cited 2021 Aug 3]. Report No.: NCT03241186. Available from: https://clinicaltrials.gov/ct2/show/NCT03241186 [Google Scholar]
- 204.Guo J. A Phase II Study of Neoadjuvant Lenvatinib and Pembrolizumab in Resectable Mucosal Melanoma [Internet]. clinicaltrials.gov; 2020. Nov [cited 2021 Aug 3]. Report No.: NCT04622566. Available from: https://clinicaltrials.gov/ct2/show/NCT04622566 [Google Scholar]
- 205.Kubonishi I, Takehara N, Iwata J, Sonobe H, Ohtsuki Y, Abe T, et al. Novel t(15;19)(q15;p13) Chromosome Abnormality in a Thymic Carcinoma. Cancer Res. 1991. Jun 15;51(12):3327–8. [PubMed] [Google Scholar]
- 206.Al Diffalha S, Al Aukla N, Hasan S, Dickinson S, Khalil F. NUT Midline Carcinoma: A Rare Malignancy. Cancer Control. 2017. Apr 1;24(2):202–6. [DOI] [PubMed] [Google Scholar]
- 207.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2015. Sep;10(9):1243–60. [DOI] [PubMed] [Google Scholar]
- 208.Lee T, Cho J, Baek CH, Son YI, Jeong HS, Chung MK, et al. Prevalence of NUT carcinoma in head and neck: Analysis of 362 cases with literature review. Head Neck. 2020. May;42(5):924–38. [DOI] [PubMed] [Google Scholar]
- 209.Ball A, Bromley A, Glaze S, French CA, Ghatage P, Köbel M. A rare case of NUT midline carcinoma. Gynecol Oncol Case Rep. 2012. Oct 8;3:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yang F, Shen D, Shi J. Primary renal NUT carcinoma identified by next-generation sequencing: a case report and literature review. Int J Clin Exp Pathol. 2021. May 15;14(5):662–9. [PMC free article] [PubMed] [Google Scholar]
- 211.Zhou L, Yong X, Zhou J, Xu J, Wang C. Clinicopathological Analysis of Five Cases of NUT Midline Carcinoma, including One with the Gingiva. BioMed Res Int. 2020. Feb 14;2020:e9791208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tanaka M, Kato K, Gomi K, Yoshida M, Niwa T, Aida N, et al. NUT midline carcinoma: report of 2 cases suggestive of pulmonary origin. Am J Surg Pathol. 2012. Mar;36(3):381–8. [DOI] [PubMed] [Google Scholar]
- 213.Shehata BM, Steelman CK, Abramowsky CR, Olson TA, French CA, Saxe DF, et al. NUT midline carcinoma in a newborn with multiorgan disseminated tumor and a 2-year-old with a pancreatic/hepatic primary. Pediatr Dev Pathol Off J Soc Pediatr Pathol Paediatr Pathol Soc. 2010. Dec;13(6):481–5. [DOI] [PubMed] [Google Scholar]
- 214.Mertens F, Wiebe T, Adlercreutz C, Mandahl N, French CA. Successful treatment of a child with t(15;19)-positive tumor. Pediatr Blood Cancer. 2007;49(7):1015–7. [DOI] [PubMed] [Google Scholar]
- 215.Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, et al. Intensive treatment and survival outcomes in NUT midline carcinoma (NMC) of the head and neck (HN). Cancer. 2016. Dec 1;122(23):3632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Giridhar P, Mallick S, Kashyap L, Rath GK. Patterns of care and impact of prognostic factors in the outcome of NUT midline carcinoma: a systematic review and individual patient data analysis of 119 cases. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2018. Mar;275(3):815–21. [DOI] [PubMed] [Google Scholar]
- 217.French CA, Kutok JL, Faquin WC, Toretsky JA, Antonescu CR, Griffin CA, et al. Midline Carcinoma of Children and Young Adults With NUT Rearrangement. J Clin Oncol. 2004. Oct 15;22(20):4135–9. [DOI] [PubMed] [Google Scholar]
- 218.French CA. NUT Midline Carcinoma. Cancer Genet Cytogenet. 2010. Nov;203(1):16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kees UR, Mulcahy MT, Willoughby ML. Intrathoracic carcinoma in an 11-year-old girl showing a translocation t(15;19). Am J Pediatr Hematol Oncol. 1991. Jan 1;13(4):459–64. [DOI] [PubMed] [Google Scholar]
- 220.Napolitano M, Venturelli M, Molinaro E, Toss A. NUT midline carcinoma of the head and neck: current perspectives. OncoTargets Ther. 2019. Apr 30;12:3235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.French CA, Rahman S, Walsh EM, Kühnle S, Grayson AR, Lemieux ME, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov. 2014. Aug;4(8):928–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang QW, He LJ, Zheng S, Liu T, Peng BN. An Overview of Molecular Mechanism, Clinicopathological Factors, and Treatment in NUT Carcinoma. BioMed Res Int [Internet]. 2019. Nov 11 [cited 2021 May 19];2019. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6877965/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Josling GA, Selvarajah SA, Petter M, Duffy MF. The Role of Bromodomain Proteins in Regulating Gene Expression. Genes. 2012. May 29;3(2):320–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.French CA, Ramirez CL, Kolmakova J, Hickman TT, Cameron MJ, Thyne ME, et al. BRD–NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008. Apr;27(15):2237–42. [DOI] [PubMed] [Google Scholar]
- 225.Schwartz BE, Hofer MD, Lemieux ME, Bauer DE, Cameron MJ, West NH, et al. Differentiation of NUT Midline Carcinoma by Epigenomic Reprogramming. Cancer Res. 2011. Apr 1;71(7):2686–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Morrison-Smith CD, Knox TM, Filic I, Soroko KM, Eschle BK, Wilkens MK, et al. Combined Targeting of the BRD4–NUT–p300 Axis in NUT Midline Carcinoma by Dual Selective Bromodomain Inhibitor, NEO2734. Mol Cancer Ther. 2020. Jul 1;19(7):1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wang S, Li J, Tong W, Li H, Feng Q, Teng B. Advances in the pathogenesis and treatment of nut carcinoma: a narrative review. Transl Cancer Res [Internet]. 2020. Oct [cited 2021 May 20];9(10). Available from: https://tcr.amegroups.com/article/view/45461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.French CA. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol. 2010. Jun 1;63(6):492–6. [DOI] [PubMed] [Google Scholar]
- 229.Liu S, Ferzli G. NUT carcinoma: a rare and devastating neoplasm. Case Rep 2018. Sep 1;2018:bcr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Stelow EB, French CA. Carcinomas of the Upper Aerodigestive Tract With Rearrangement of the Nuclear Protein of the Testis (NUT) Gene (NUT Midline Carcinomas). Adv Anat Pathol. 2009. Mar;16(2):92–6. [DOI] [PubMed] [Google Scholar]
- 231.Stelow EB. A Review of NUT Midline Carcinoma. Head Neck Pathol. 2011. Jan 8;5(1):31–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Haack H, Johnson LA, Fry CJ, Crosby K, Polakiewicz RD, Stelow EB, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009. Jul;33(7):984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bair RJ, Chick JF, Chauhan NR, French C, Madan R. Demystifying NUT Midline Carcinoma: Radiologic and Pathologic Correlations of an Aggressive Malignancy. Am J Roentgenol. 2014. Oct;203(4):W391–9. [DOI] [PubMed] [Google Scholar]
- 234.NUT Carcinoma - National Cancer Institute [Internet]. 2020. [cited 2021 May 20]. Available from: https://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/rare-soft-tissue-tumors/nut-carcinoma
- 235.Maher OM, Christensen AM, Yedururi S, Bell D, Tarek N. Histone deacetylase inhibitor for NUT midline carcinoma. Pediatr Blood Cancer. 2015;62(4):715–7. [DOI] [PubMed] [Google Scholar]
- 236.About NUT Carcinoma - Dana-Farber Cancer Institute | Boston, MA [Internet]. [cited 2021 May 20]. Available from: https://www.dana-farber.org/nut-carcinoma/
- 237.Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, Lüer SC, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2012. Oct 15;18(20):5773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, et al. Intensive treatment and survival outcomes in NUT midline carcinoma (NMC) of the head and neck (HN). Cancer. 2016. Dec 1;122(23):3632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010. Dec 23;468(7327):1067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Beesley AH, Stirnweiss A, Ferrari E, Endersby R, Howlett M, Failes TW, et al. Comparative drug screening in NUT midline carcinoma. Br J Cancer. 2014. Mar;110(5):1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Noel JK, Iwata K, Ooike S, Sugahara K, Nakamura H, Daibata M. Abstract C244: Development of the BET bromodomain inhibitor OTX015. Mol Cancer Ther. 2013. Nov 1;12(11 Supplement):C244–C244. [Google Scholar]
- 242.Lewin J, Soria JC, Stathis A, Delord JP, Peters S, Awada A, et al. Phase Ib Trial With Birabresib, a Small-Molecule Inhibitor of Bromodomain and Extraterminal Proteins, in Patients With Selected Advanced Solid Tumors. J Clin Oncol. 2018. Oct 20;36(30):3007–14. [DOI] [PubMed] [Google Scholar]
- 243.Piha-Paul SA, Hann CL, French CA, Cousin S, Braña I, Cassier PA, et al. Phase 1 Study of Molibresib (GSK525762), a Bromodomain and Extra-Terminal Domain Protein Inhibitor, in NUT Carcinoma and Other Solid Tumors. JNCI Cancer Spectr. 2019. Nov 6;4(2):pkz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.National Cancer Institute (NCI). A Phase 1/2 Study of the Bromodomain Inhibitor ZEN003694 in Combination With Etoposide/Platinum in Patients With NUT Carcinoma [Internet]. clinicaltrials.gov; 2021. Oct [cited 2022 Jan 31]. Report No.: NCT05019716. Available from: https://clinicaltrials.gov/ct2/show/NCT05019716 [Google Scholar]
- 245.Haverkos BM, Pan Z, Gru AA, Freud AG, Rabinovitch R, Xu-Welliver M, et al. Extranodal NK/T Cell Lymphoma, Nasal Type (ENKTL-NT): An Update on Epidemiology, Clinical Presentation, and Natural History in North American and European Cases. Curr Hematol Malig Rep. 2016. Dec;11(6):514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sánchez-Romero C, Bologna-Molina R, Paes de Almeida O, Santos-Silva AR, Prado-Ribeiro AC, Brandão TB, et al. Extranodal NK/T cell lymphoma, nasal type: An updated overview. Crit Rev Oncol Hematol. 2021. Mar 1;159:103237. [DOI] [PubMed] [Google Scholar]
- 247.Kommalapati A, Tella SH, Ganti AK, Armitage JO. Natural Killer/T-cell Neoplasms: Analysis of Incidence, Patient Characteristics, and Survival Outcomes in the United States. Clin Lymphoma Myeloma Leuk. 2018. Jul 1;18(7):475–9. [DOI] [PubMed] [Google Scholar]
- 248.Mosialos G, Birkenbacht M, Yalamanchill R, Van Arsdale T, Ware C, Kleff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995. Feb 10;80(3):389–99. [DOI] [PubMed] [Google Scholar]
- 249.Kis LL, Takahara M, Nagy N, Klein G, Klein E. IL-10 can induce the expression of EBV-encoded latent membrane protein-1 (LMP-1) in the absence of EBNA-2 in B lymphocytes and in Burkitt lymphoma- and NK lymphoma-derived cell lines. Blood. 2006. Apr 1;107(7):2928–35. [DOI] [PubMed] [Google Scholar]
- 250.Takada H, Imadome KI, Shibayama H, Yoshimori M, Wang L, Saitoh Y, et al. EBV induces persistent NF-κB activation and contributes to survival of EBV-positive neoplastic T- or NK-cells. PLOS ONE. 2017. Mar 27;12(3):e0174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Su YJ, Wang PN, Chang H, Shih LY, Lin TL, Kuo MC, et al. Extranodal NK/T-cell lymphoma, nasal type: Clinical features, outcome, and prognostic factors in 101 cases. Eur J Haematol. 2018;101(3):379–88. [DOI] [PubMed] [Google Scholar]
- 252.Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013. Jun 20;121(25):4997–5005. [DOI] [PubMed] [Google Scholar]
- 253.Kwong YL, Anderson BO, Advani R, Kim WS, Levine AM, Lim ST. Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009. Nov 1;10(11):1093–101. [DOI] [PubMed] [Google Scholar]
- 254.Koo GC, Tan SY, Tang T, Poon SL, Allen GE, Tan L, et al. Janus kinase 3-activating mutations identified in natural killer/T-cell lymphoma. Cancer Discov. 2012. Jul;2(7):591–7. [DOI] [PubMed] [Google Scholar]
- 255.Küçük C, Jiang B, Hu X, Zhang W, Chan JKC, Xiao W, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat Commun. 2015. Jan 14;6:6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Jiang L, Gu ZH, Yan ZX, Zhao X, Xie YY, Zhang ZG, et al. Exome sequencing identifies somatic mutations of DDX3X in natural killer/T-cell lymphoma. Nat Genet. 2015. Sep;47(9):1061–6. [DOI] [PubMed] [Google Scholar]
- 257.Karube K, Nakagawa M, Tsuzuki S, Takeuchi I, Honma K, Nakashima Y, et al. Identification of FOXO3 and PRDM1 as tumor-suppressor gene candidates in NK-cell neoplasms by genomic and functional analyses. Blood. 2011. Sep 22;118(12):3195–204. [DOI] [PubMed] [Google Scholar]
- 258.Küçük C, Hu X, Iqbal J, Gaulard P, Klinkebiel D, Cornish A, et al. HACE1 Is a Tumor Suppressor Gene Candidate in Natural Killer Cell Neoplasms. Am J Pathol. 2013. Jan 1;182(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wong KF, Chan JKC, Cheung MMC, So JCC. Bone Marrow Involvement by Nasal NK Cell Lymphoma at Diagnosis Is Uncommon. Am J Clin Pathol. 2001. Feb 1;115(2):266–70. [DOI] [PubMed] [Google Scholar]
- 260.Wang Y, Xie L, Tian R, Deng Y, Zhang W, Zou L, et al. PET/CT-based bone-marrow assessment shows potential in replacing routine bone-marrow biopsy in part of patients newly diagnosed with extranodal natural killer/T-cell lymphoma. J Cancer Res Clin Oncol. 2019. Oct 1;145(10):2529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cheung MMC, Chan JKC, Lau W hon, Ngan RKC, Foo WWL. Early stage nasal NK/T-cell lymphoma: clinical outcome, prognostic factors, and the effect of treatment modality. Int J Radiat Oncol Biol Phys. 2002. Sep 1;54(1):182–90. [DOI] [PubMed] [Google Scholar]
- 262.Kim GE, Lee SW, Chang SK, Park HC, Pyo HR, Kim JH, et al. Combined chemotherapy and radiation versus radiation alone in the management of localized angiocentric lymphoma of the head and neck. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2001. Dec;61(3):261–9. [DOI] [PubMed] [Google Scholar]
- 263.Yamaguchi M, Kita K, Miwa H, Nishii K, Oka K, Ohno T, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995;76(11):2351–6. [DOI] [PubMed] [Google Scholar]
- 264.Kim SJ, Kim K, Kim BS, Kim CY, Suh C, Huh J, et al. Phase II Trial of Concurrent Radiation and Weekly Cisplatin Followed by VIPD Chemotherapy in Newly Diagnosed, Stage IE to IIE, Nasal, Extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma Study. J Clin Oncol. 2009. Dec 10;27(35):6027–32. [DOI] [PubMed] [Google Scholar]
- 265.Yamaguchi M, Tobinai K, Oguchi M, Ishizuka N, Kobayashi Y, Isobe Y, et al. Phase I/II Study of Concurrent Chemoradiotherapy for Localized Nasal Natural Killer/T-Cell Lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol Off J Am Soc Clin Oncol. 2009. Oct 1;27:5594–600. [DOI] [PubMed] [Google Scholar]
- 266.Yong W, Zheng W, Zhang Y, Zhu J, Wei Y, Zhu D, et al. L-Asparaginase—Based Regimen in the Treatment of Refractory Midline Nasal/Nasal-Type T/NK-Cell Lymphoma. Int J Hematol. 2003. Aug 1;78(2):163–7. [DOI] [PubMed] [Google Scholar]
- 267.Jiang M, Zhang H, Jiang Y, Yang Q, Xie L, Liu W, et al. Phase 2 trial of “Sandwich” L-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer. 2012;118(13):3294–301. [DOI] [PubMed] [Google Scholar]
- 268.Wang L, hui Wang Z, qin Chen X, jun Li Y, feng Wang K, fei Xia Y, et al. First-line combination of gemcitabine, oxaliplatin, and L-asparaginase (GELOX) followed by involved-field radiation therapy for patients with stage IE/IIE extranodal natural killer/T-cell lymphoma. Cancer. 2013;119(2):348–55. [DOI] [PubMed] [Google Scholar]
- 269.Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E, et al. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012. Oct 11;120(15):2973–80. [DOI] [PubMed] [Google Scholar]
- 270.Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol Off J Am Soc Clin Oncol. 2011. Nov 20;29(33):4410–6. [DOI] [PubMed] [Google Scholar]
- 271.Tse E, Kwong YL. NK/T-cell lymphomas. Best Pract Res Clin Haematol. 2019. Sep 1;32(3):253–61. [DOI] [PubMed] [Google Scholar]
- 272.Mei M, Wang Y, Zhang M. Causes of mortality in cases with extra nodal natural killer/T-cell lymphoma, nasal type: A cohort study. PLoS ONE. 2019. Apr 17;14(4):e0214860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016. Mar 1;17(3):389–400. [DOI] [PubMed] [Google Scholar]
- 274.Au WY, Kim SJ, Yiu HHY, Ngan RKC, Loong F, Kim WS, et al. Clinicopathological features and outcome of late relapses of natural killer cell lymphomas 10–29 years after initial remission. Am J Hematol. 2010. May;85(5):362–3. [DOI] [PubMed] [Google Scholar]
- 275.Kim SJ, Park Y, Kim BS, Kim I, Ko YH, Kim WS. Extranodal natural killer/T-cell lymphoma with long-term survival and repeated relapses: does it indicate the presence of indolent subtype? Korean J Hematol. 2012. Sep;47(3):202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Li YJ. Anti-PD-1 Antibody, Peg-Asparaginase, Chidamide Combined With Radiotherapy for the First-line Treatment of Patients With Stage I/II Extranodal Natural Killer/T Cell Lymphoma, Nasal Type (ENKTL): An Open-label, Multicenter, Phase II Trial [Internet]. clinicaltrials.gov; 2020. Jul [cited 2021 Aug 3]. Report No.: NCT04414969. Available from: https://clinicaltrials.gov/ct2/show/NCT04414969 [Google Scholar]
- 277.Memorial Sloan Kettering Cancer Center. Pilot Study of Pembrolizumab in Untreated Extranodal, NK/T Cell Lymphoma, Nasal Type [Internet]. clinicaltrials.gov; 2021. May [cited 2021 Aug 3]. Report No.: NCT03728972. Available from: https://clinicaltrials.gov/ct2/show/NCT03728972 [Google Scholar]
- 278.wanghua. Anti-PD-1 Antibody and Pegaspargase Combined With Radiotherapy As First-Line Treatment in Early-Stage Extranodal Natural Killer/T Cell Lymphoma, Nasal Type (ENKTL) [Internet]. clinicaltrials.gov; 2020. Dec [cited 2021 Aug 3]. Report No.: NCT04676789. Available from: https://clinicaltrials.gov/ct2/show/NCT04676789 [Google Scholar]
- 279.Dong M Radiotherapy Combined With GDP (Gemcitabine, Cisplatin, Dexamethasone) Chemotherapy With or Without Chidamide in High-risk Early-stage Extranodal Nasal NK/T-cell Lymphoma [Internet]. clinicaltrials.gov; 2020. Aug [cited 2021 Aug 3]. Report No.: NCT04511351. Available from: https://clinicaltrials.gov/ct2/show/NCT04511351 [Google Scholar]
- 280.ImmuneOncia Therapeutics Inc. An Open-label, Single-arm, Global Phase 2 Study to Investigate the Efficacy and Safety of IMC-001 in Patients With Relapsed or Refractory Extranodal NK/T Cell Lymphoma, Nasal Type [Internet]. clinicaltrials.gov; 2020. Oct [cited 2021 Aug 3]. Report No.: NCT04414163. Available from: https://clinicaltrials.gov/ct2/show/NCT04414163 [Google Scholar]
- 281.Weili Z The Efficacy and Safety of Anti-PD-1 Antibody in Combination With Pegaspargase in the Treatment of Newly Diagnosed, Stage III to IV Extranodal Natural Killer/T-Cell Lymphoma, Nasal Type [Internet]. clinicaltrials.gov; 2020. Mar [cited 2021 Aug 3]. Report No.: NCT04096690. Available from: https://clinicaltrials.gov/ct2/show/NCT04096690 [Google Scholar]
- 282.Cai Q Sintilimab With P-GemOx (Pegaspargase, Gemcitabine and Oxaliplatin) Regimen for Newly Diagnosed Advanced Extranodal Natural Killer/T-cell Lymphoma, Nasal Type (ENKTL): a Single Arm, Open, Multicenter, Phase II Study [Internet]. clinicaltrials.gov; 2019. Oct [cited 2021 Aug 3]. Report No.: NCT04127227. Available from: https://clinicaltrials.gov/ct2/show/NCT04127227 [Google Scholar]
- 283.Jiangsu HengRui Medicine Co., Ltd. An Open-Label, Single Arm, Multi-Center, Phase 2 Study of PD-1 Antibody SHR-1210 in Subjects With Relapsed or Refractory Extranodal NK/T Cell Lymphoma [Internet]. clinicaltrials.gov; 2020. Jun [cited 2021 Aug 3]. Report No.: NCT03363555. Available from: https://clinicaltrials.gov/ct2/show/NCT03363555 [Google Scholar]
- 284.WANG JW. Gemcitabine, Pegaspargase, Etoposide, and Dexamethasone (GPED) for Patients With Relapsed/Refractory or Advanced NK/T-cell Lymphoma : a Single Arm,Open-lable,Phase II Study [Internet]. clinicaltrials.gov; 2020. May [cited 2021 Aug 3]. Report No.: NCT04405375. Available from: https://clinicaltrials.gov/ct2/show/NCT04405375 [Google Scholar]
- 285.Innovent Biologics (Suzhou) Co. Ltd. Evaluation of Safety and Efficacy of IBI318 Monotherapy for Relapsed/Refractory Extranodal NK/T Cell Lymphoma (Nasal Type), a Multicenter, Open-label Phase Ib/II Trial [Internet]. clinicaltrials.gov; 2020. Dec [cited 2021 Aug 3]. Report No.: NCT04602065. Available from: https://clinicaltrials.gov/ct2/show/NCT04602065 [Google Scholar]
- 286.Thompson LDR. Olfactory neuroblastoma. Head Neck Pathol. 2009. Sep;3(3):252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Broich G, Pagliari A, Ottaviani F. Esthesioneuroblastoma: a general review of the cases published since the discovery of the tumour in 1924. Anticancer Res. 1997. Aug;17(4A):2683–706. [PubMed] [Google Scholar]
- 288.Bunch PM, Kelly HR. 45 - Esthesioneuroblastoma. In: Small JE, Noujaim DL, Ginat DT, Kelly HR, Schaefer PW, editors. Neuroradiology [Internet]. Philadelphia: Elsevier; 2019. [cited 2021 Jul 27]. p. 346–57. Available from: https://www.sciencedirect.com/science/article/pii/B9780323445498000456 [Google Scholar]
- 289.Magnavita N Aesthesioneuroblastoma in a woodworker. Occup Med. 2003. May 1;53(3):231–4. [DOI] [PubMed] [Google Scholar]
- 290.Ivankovic S, Seibel J, Komitowski D, Spiegelhalder B, Preussmann R, Siddiqi M. Caffeine-derived N-nitroso compounds. V. Carcinogenicity of mononitrosocaffeidine and dinitrosocaffeidine in bd-ix rats. Carcinogenesis. 1998. May;19(5):933–7. [DOI] [PubMed] [Google Scholar]
- 291.Reznik-Schüller H Ultrastructure of N-diethylnitrosamine induced tumours in the nasal olfactory region of the Syrian golden hamster. J Pathol. 1978. Mar;124(3):161–4. [DOI] [PubMed] [Google Scholar]
- 292.Koike K, Jay G, Hartley JW, Schrenzel MD, Higgins RJ, Hinrichs SH. Activation of retrovirus in transgenic mice: association with development of olfactory neuroblastoma. J Virol. 1990. Aug;64(8):3988–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Jethanamest D, Morris LG, Sikora AG, Kutler DI. Esthesioneuroblastoma: a population-based analysis of survival and prognostic factors. Arch Otolaryngol Head Neck Surg. 2007. Mar;133(3):276–80. [DOI] [PubMed] [Google Scholar]
- 294.Bak M, Wein RO. Esthesioneuroblastoma: a contemporary review of diagnosis and management. Hematol Oncol Clin North Am. 2012. Dec;26(6):1185–207. [DOI] [PubMed] [Google Scholar]
- 295.Kuan EC, Nasser HB, Carey RM, Workman AD, Alonso JE, Wang MB, et al. A Population-Based Analysis of Nodal Metastases in Esthesioneuroblastomas of the Sinonasal Tract. The Laryngoscope. 2019. May;129(5):1025–9. [DOI] [PubMed] [Google Scholar]
- 296.Galioto S, Di Petrillo A, Pastori M, Arecchi A. Metastatic esthesioneuroblastoma secreting adrenocorticotropic hormone in pediatric patients. J Craniofac Surg. 2011. Sep;22(5):1924–9. [DOI] [PubMed] [Google Scholar]
- 297.Kunc M, Gabrych A, Czapiewski P, Sworczak K. Paraneoplastic syndromes in olfactory neuroblastoma. Contemp Oncol Poznan Pol. 2015;19(1):6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Gabbay U, Leider-Trejo L, Marshak G, Gabbay M, Fliss DM. A case and a series of published cases of esthesioneuroblastoma (ENB) in which long-standing paraneoplastic SIADH had preceded ENB diagnosis. Ear Nose Throat J. 2013. Nov;92(10–11):E6. [PubMed] [Google Scholar]
- 299.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970–1990. The Laryngoscope. 1992. Aug;102(8):843–9. [DOI] [PubMed] [Google Scholar]
- 300.Czapiewski P, Kunc M, Haybaeck J. Genetic and molecular alterations in olfactory neuroblastoma: implications for pathogenesis, prognosis and treatment. Oncotarget. 2016. May 31;7(32):52584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Bradley PJ, Jones NS, Robertson I. Diagnosis and management of esthesioneuroblastoma. Curr Opin Otolaryngol Head Neck Surg. 2003. Apr;11(2):112–8. [DOI] [PubMed] [Google Scholar]
- 302.Hyams VJ, Batsakis JG, Michaels L. Tumors of the Upper Respiratory Tract and Ear. Armed Forces Institute of Pathology; 1988. 362 p. [Google Scholar]
- 303.Woods RSR, Subramaniam T, Leader M, McConn-Walsh R, O’Neill JP, Lacy PD. Changing Trends in the Management of Esthesioneuroblastoma: Irish and International Perspectives. J Neurol Surg Part B Skull Base. 2018. Jun;79(3):262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Morita A, Ebersold MJ, Olsen KD, Foote RL, Lewis JE, Quast LM. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993. May;32(5):706–14; discussion 714–715. [DOI] [PubMed] [Google Scholar]
- 305.Tajudeen BA, Arshi A, Suh JD, Palma-Diaz MF, Bergsneider M, Abemayor E, et al. Esthesioneuroblastoma: an update on the UCLA experience, 2002–2013. J Neurol Surg Part B Skull Base. 2015. Feb;76(1):43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Goshtasbi K, Abiri A, Abouzari M, Sahyouni R, Wang BY, Tajudeen BA, et al. Hyams Grading as a Predictor of Metastasis and Overall Survival in Esthesioneuroblastoma: A Meta-Analysis. Int Forum Allergy Rhinol. 2019. Sep;9(9):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Nowak B, Di Martino E, Jänicke S, Cremerius U, Adam G, Zimny M, et al. Diagnostic evaluation of malignant head and neck cancer by F-18-FDG PET compared to CT/MRI. Nukl Nucl Med. 1999;38(8):312–8. [PubMed] [Google Scholar]
- 308.Ramsay HA, Kairemo KJ, Jekunen AP. Somatostatin receptor imaging of olfactory neuroblastoma. J Laryngol Otol. 1996. Dec;110(12):1161–3. [DOI] [PubMed] [Google Scholar]
- 309.Konuthula N, Iloreta AM, Miles B, Rhome R, Ozbek U, Genden EM, et al. Prognostic significance of Kadish staging in esthesioneuroblastoma: An analysis of the National Cancer Database. Head Neck. 2017. Oct;39(10):1962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Joshi RR, Husain Q, Roman BR, Cracchiolo J, Yu Y, Tsai J, et al. Comparing Kadish, TNM, and the modified Dulguerov staging systems for esthesioneuroblastoma. J Surg Oncol. 2019. Jan;119(1):130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yu Y, El-Sayed IH, McDermott MW, Theodosopoulos PV, van Zante A, Kased N, et al. Dural recurrence among esthesioneuroblastoma patients presenting with intracranial extension. The Laryngoscope. 2018. Oct;128(10):2226–33. [DOI] [PubMed] [Google Scholar]
- 312.Marinelli JP, Janus JR, Van Gompel JJ, Link MJ, Moore EJ, Van Abel KM, et al. Dural Invasion Predicts the Laterality and Development of Neck Metastases in Esthesioneuroblastoma. J Neurol Surg Part B Skull Base. 2018. Oct;79(5):495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001. Nov;2(11):683–90. [DOI] [PubMed] [Google Scholar]
- 314.Spaulding CA, Kranyak MS, Constable WC, Stewart FM. Esthesioneuroblastoma: a comparison of two treatment eras. Int J Radiat Oncol Biol Phys. 1988. Sep;15(3):581–90. [DOI] [PubMed] [Google Scholar]
- 315.Banuchi VE, Dooley L, Lee NY, Pfister DG, McBride S, Riaz N, et al. Patterns of regional and distant metastasis in esthesioneuroblastoma. The Laryngoscope. 2016. Jul;126(7):1556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wang EW, Zanation AM, Gardner PA, Schwartz TH, Eloy JA, Adappa ND, et al. ICAR: endoscopic skull-base surgery. Int Forum Allergy Rhinol. 2019. Jul;9(S3):S145–365. [DOI] [PubMed] [Google Scholar]
- 317.Demiroz C, Gutfeld O, Aboziada M, Brown D, Marentette LJ, Eisbruch A. Esthesioneuroblastoma: is there a need for elective neck treatment? Int J Radiat Oncol Biol Phys. 2011. Nov 15;81(4):e255–261. [DOI] [PubMed] [Google Scholar]
- 318.Davis RE, Weissler MC. Esthesioneuroblastoma and neck metastasis. Head Neck. 1992. Dec;14(6):477–82. [DOI] [PubMed] [Google Scholar]
- 319.Zabel A, Thilmann C, Milker-Zabel S, Schlegel W, Zuna I, Wannenmacher M, et al. The role of stereotactically guided conformal radiotherapy for local tumor control of esthesioneuroblastoma. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 2002. Apr;178(4):187–91. [DOI] [PubMed] [Google Scholar]
- 320.Fitzek MM, Thornton AF, Varvares M, Ancukiewicz M, Mcintyre J, Adams J, et al. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer. 2002. May 15;94(10):2623–34. [DOI] [PubMed] [Google Scholar]
- 321.Levine PA, Scher RL, Jane JA, Persing JA, Newman SA, Miller J, et al. The craniofacial resection--eleven-year experience at the University of Virginia: problems and solutions. Otolaryngol--Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 1989. Dec;101(6):665–9. [DOI] [PubMed] [Google Scholar]
- 322.Madani I, Bonte K, Vakaet L, Boterberg T, De Neve W. Intensity-modulated radiotherapy for sinonasal tumors: Ghent University Hospital update. Int J Radiat Oncol Biol Phys. 2009. Feb 1;73(2):424–32. [DOI] [PubMed] [Google Scholar]
- 323.Loy AH, Reibel JF, Read PW, Thomas CY, Newman SA, Jane JA, et al. Esthesioneuroblastoma: continued follow-up of a single institution’s experience. Arch Otolaryngol Head Neck Surg. 2006. Feb;132(2):134–8. [DOI] [PubMed] [Google Scholar]
- 324.Turano S, Mastroianni C, Manfredi C, Biamonte R, Ceniti S, Liguori V, et al. Advanced adult esthesioneuroblastoma successfully treated with cisplatin and etoposide alternated with doxorubicin, ifosfamide and vincristine. J Neurooncol. 2010. May;98(1):131–5. [DOI] [PubMed] [Google Scholar]
- 325.McElroy EA, Buckner JC, Lewis JE. Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience. Neurosurgery. 1998. May;42(5):1023–7; discussion 1027–1028. [DOI] [PubMed] [Google Scholar]
- 326.Spengler M, Wheelden M, Mackley HB, Drabick JJ. Durable Major Response With Pazopanib in Recurrent, Heavily Pretreated Metastatic Esthesioneuroblastoma Harboring a Fumarate Hydratase Mutation. JCO Precis Oncol. 2021;5:PO.20.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Hasan OK, Ravi Kumar AS, Kong G, Oleinikov K, Ben-Haim S, Grozinsky-Glasberg S, et al. Efficacy of Peptide Receptor Radionuclide Therapy for Esthesioneuroblastoma. J Nucl Med. 2020. Sep;61(9):1326–30. [DOI] [PubMed] [Google Scholar]
- 328.Austin JR, Cebrun H, Kershisnik MM, El-Naggar AK, Garden AS, Demonte F, et al. Olfactory neuroblastoma and neuroendocrine carcinoma of the anterior skull base: treatment results at the m.d. Anderson cancer center. Skull Base Surg. 1996;6(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Barinsky GL, Azmy MC, Kilic S, Grube JG, Baredes S, Hsueh WD, et al. Comparison of Open and Endoscopic Approaches in the Resection of Esthesioneuroblastoma. Ann Otol Rhinol Laryngol. 2021. Feb;130(2):136–41. [DOI] [PubMed] [Google Scholar]
- 330.Girod D, Hanna E, Marentette L. Esthesioneuroblastoma. Head Neck. 2001. Jun;23(6):500–5. [DOI] [PubMed] [Google Scholar]
- 331.National Cancer Institute (NCI). Phase 2 Study of Bintrafusp Alfa in Recurrent/Metastatic Olfactory Neuroblastoma (BARON). [Internet]. clinicaltrials.gov; 2022. Jan [cited 2022 Jan 17]. Report No.: NCT05012098. Available from: https://clinicaltrials.gov/ct2/show/NCT05012098 [Google Scholar]
- 332.Weill Medical College of Cornell University. 68Ga(Gallium)-DOTATATE Positron Emission Tomography (PET)/MRI in the Diagnosis and Management of Somatostatin Receptor Positive Central Nervous System CNS Tumors. [Internet]. clinicaltrials.gov; 2021. Sep [cited 2022 Jan 31]. Report No.: NCT04081701. Available from: https://clinicaltrials.gov/ct2/show/NCT04081701 [Google Scholar]
- 333.Caudell JJ, Gillison ML, Maghami E, Spencer S, Pfister DG, Adkins D, et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2022. J Natl Compr Cancer Netw JNCCN. 2022. Mar;20(3):224–34. [DOI] [PubMed] [Google Scholar]
- 334.Paleri V, Roland N. Introduction to the United Kingdom National Multidisciplinary Guidelines for Head and Neck Cancer. J Laryngol Otol. 2016. May;130(S2):S3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Gronchi A, Haas RL, Bonvalot S. Cancer registries and randomised clinical trials in rare tumours: At the two extremes of daily clinical practice. Eur J Cancer. 2016. Sep 1;64:113–5. [DOI] [PubMed] [Google Scholar]
- 336.Welcome [Internet]. Head and Neck Cancer International Group. [cited 2021 Sep 28]. Available from: http://hnc.intergroup.info/ [Google Scholar]