Abstract
Background:
Low quantities of circulating progenitor cells (CPCs), specifically CD34+ populations, reflect impairment of intrinsic regenerative capacity. This study investigates the relationship between subsets of CPCs and adverse outcomes.
Methods:
1366 individuals undergoing angiography for evaluation of coronary artery disease (CAD) were enrolled into the Emory Cardiovascular Biobank. Flow cytometry identified CPCs as CD45med blood mononuclear cells expressing the CD34 epitope, with further enumeration of hematopoietic CPCs as CD133+/CXCR4+ cells and endothelial CPCs as vascular endothelial growth factor receptor-2 (VEGFR2+) cells. Adjusted Cox or Fine and Gray’s sub-distribution hazard regression models analyzed the relationship between CPCs and 1) all-cause death and 2) a composite of cardiovascular death and non-fatal myocardial infarction (MI).
Results:
Over a median 3.1-year follow-up period (IQR 1.3–4.9), there were 221 (16.6%) all-cause deaths and 172 (12.9%) cardiovascular deaths/MIs. Hematopoietic CPCs were highly correlated, and the CD34+/CXCR4+ subset was the best independent predictor. Lower counts (≤median) of CD34+/CXCR4+ and CD34+/VEGFR2+ cells independently predicted all-cause mortality (HR 1.46 [95% CI 1.06–2.01], p=0.02 and 1.59 [95% CI 1.15–2.18], p=0.004) and cardiovascular death/MI (HR 1.50 [95% CI 1.04–2.17], p=0.03 and 1.47 [95% CI 1.01–2.03], p=0.04). A combination of low CD34+/CXCR4+ and CD34+/VEGFR2+ CPCs predicted all-cause death (HR 2.1, 95% CI 1.4–3.0; p=0.0002) and cardiovascular death/MI (HR 2.0, 95% CI 1.3–3.2; p=0.002) compared to those with both lineages above the cut-offs.
Conclusions:
Lower levels of hematopoietic and endothelial CPCs indicate diminished endogenous regenerative capacity and independently correlate with greater mortality and cardiovascular risk in patients with CAD.
Keywords: progenitor cells, CD34, coronary artery disease, biomarkers, risk assessment
INTRODUCTION
Cardiovascular risk factor-mediated injury of the vascular endothelium ultimately leads to development of hypertension, atherosclerosis and their associated adverse outcomes.1–3 Recent evidence suggests that progenitor cells (PCs) play a critical role in vascular repair and regeneration, largely through paracrine mechanisms.1,2,4,5 As mononuclear cells that primarily originate in the bone marrow, circulating progenitor cells (CPCs) can differentiate into several distinct lineages including hematopoietic progenitors, identified by expression of the CD34 (Cluster of Differentiation) epitope on hematopoietic CD45med cells, and non-hematopoietic or mesenchymal progenitors, that lack CD45 expression.6 CD34+ cells are associated with greater myocardial and endothelial regenerative potential and several sub-populations of CD34+ cells co-expressing CD133 and CXCR4 (chemokine (C-X-C Motif) receptor 4) epitopes have previously been studied.7 CD133 is a 5-transmembrane antigen marker on primitive stem cells that is subsequently lost during maturation, and dual expression of CD34+ and CD133+ identifies an early CPC subpopulation.8 Co-expression of CXCR4 on CD34+ cells promotes homing of CPCs to stromal-derived factor-rich hypoxic environments and potentially further characterizes CPCs with a capacity for vascular repair.9 Co-expression of the vascular endothelial growth factor receptor 2 (VEGFR2) on CD34+ cells identifies a rarer subpopulation that is enriched for endothelial PCs.
Lower levels and activity of CPCs are associated with endothelial dysfunction, atherosclerosis, and adverse cardiovascular events in patients with coronary and peripheral artery disease.10–13 Previous studies from our and other groups have shown that subjects with lower levels of CPCs enriched for hematopoietic progenitors (CD34+/CD133+ or CD34+/CXCR4+ cells) have worse prognosis, however, data regarding the prognostic value of endothelial CPC (CD34+/VEGFR2+) populations have been conflicting.12 Most previous studies have been small in size and the duration of follow-up has been limited.8,14–22 Whether deficiency of CPCs from multiple lineages is associated with worse outcomes over the longer term remains unknown.
Herein, we examined whether reduced regenerative capacity, estimated as lower levels of hematopoietic and/or endothelial CPCs, is associated with worse long-term prognosis among patients with coronary artery disease (CAD). We hypothesized that lower CPCs in both lineages will be additively associated with increased risk of adverse cardiovascular events in CAD.
METHODS
Study Population
The study population was derived from the Emory Cardiovascular Biobank, an ongoing prospective cohort of patients aged 20 to 90 years recruited from Emory Healthcare facilities.23 Patients were enrolled at the time of coronary angiography that had been ordered for the evaluation and management of suspected or known CAD. All participants provided written informed consent and this study was approved by the Emory University Institutional Review Board. Participants were interviewed to collect information about demographic characteristics, smoking history, medical history, and medication use as previously described. The prevalence of diabetes, hypertension, hypercholesterolemia, and established cardiovascular disease subtypes (CAD, heart failure [HF], and peripheral artery disease [PAD]) was determined by physician diagnosis and/or treatment.23 Medical records were reviewed to confirm self-reported medical history. Weight and height were measured at enrollment and body mass index (BMI) was calculated by dividing weight (in kilogram) by height (in meters)-square. Serum creatinine was measured at enrollment, and estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.24 Patients were excluded from enrollment in the biobank if they had congenital heart disease, severe valvular heart disease, severe anemia, a recent blood transfusion, myocarditis, history of active inflammatory disease, cancer or could not provide consent (approximately 5%). In addition, patients with a history of cardiac transplantation, those presenting with acute myocardial infarction, infection, or with advanced renal disease (eGFR <15 mL/min/1.73m2) were excluded as these factors influence CPC counts.
Follow-Up and End Points
Follow-up was performed by personnel blinded to the CPC data through telephone interviews, chart review, social security death index, and state records to determine end points of interest.8 Medical records were accessed or requested to validate all self-reported events including MI, which was adjudicated using the third universal definition of MI. The end points assessed were all-cause death (primary) and the composite end-point of cardiovascular death and non-fatal MI (secondary). Cardiovascular death was defined as death attributable to an ischemic cardiovascular cause (fatal MI, stroke, peripheral arterial disease) or sudden death due to an unknown but presumed cardiovascular cause in high-risk patients. Events were adjudicated by two cardiologists with a third arbitrator in case of disagreement. Medical records were accessed or requested to validate all self-reported events including MI.
Flow Cytometry/Progenitor cell assay
CPCs were measured in peripheral arterial blood samples collected in EDTA tubes before contrast administration for cardiac catheterization.25 Blood samples were prepared within 4 hours of collection and incubated with fluorochrome-labeled monoclonal antihuman mouse antibodies to identify surface markers expressed on mononuclear cells before quantification using flow cytometry. Three-hundred microL of peripheral blood was incubated with 7 microL of FITC-CD34 (BD Biosciences), PerCP-CD45 (BD Biosciences), PE-VEGFR2 (R&D system), 5 microL APC-CD133 (Miltenyi), and 3 microL PE-Cy7-conjugated anti-CXCR4 (EBioscience, clone 12G5) in the dark for 15 minutes.26 Then 1.5 mL ammonium chloride lysing buffer was added to lyse red blood cells, following which 1.5 mL staining medium (PBS with 3% heat-inactivated serum and 0.1% sodium azide) was added to stop the lysing reaction.26 Prior to flow cytometry, 100 microL of AccuCheck Counting Beads (Invitrogen, Cat#: PCB100) were added to act as an internal standard for direct estimation of the concentration of target cell subsets.26 At least 2.5 million events were acquired from the cytometer. Mononuclear cells enriched for CPCs were enumerated using flow cytometry as CD45med cells coexpressing CD34, CD133, VEGFR2, and CXCR4 epitopes. Flow cytometry data were analyzed with Flowjo software (Treestar, Inc.) and circulating PC populations (CD34+, CD34+/CD133+, CD34+/CXCR4+, and CD34+/VEGFR2+) were reported as cell counts per mL.26 The selection of CD45med cells excluded CD45bright and CD45− cells. Exclusion of the rare CD45bright cells helped to eliminate lymphoblasts, and exclusion of CD45− cells helped to eliminate nonhematopoietic progenitors, such as mesenchymal or osteoprogenitor cells, as these cells are typically CD45−. Twenty samples were analyzed on two occasions by two technicians. Percent repeatability coefficients (%) were calculated as the standard deviation (SD) of differences between pairs of measurements/mean of measurements × 100. The repeatability coefficients were 2.9%, 4.8%, 6.5%, and 21.6% for CD34+, CD34+/CD133+, CD34+/CXCR4+, CD34+/CD133+/CXCR4+, and CD34+/VEGFR2+, respectively.27
Statistical methods
Subject characteristics were reported as descriptive statistics with continuous adjusted variables presented as means (SD) or as medians [interquartile range] and with categorical variables as proportions. Differences among groups were studied using the analysis of variance (ANOVA) for normally distributed continuous variables, Kruskal–Wallis test for non-normally distributed continuous variables and Chi-square or Fisher’s exact tests for categorical variables where appropriate. CPC counts were analyzed as non-normally distributed continuous variables and as categorical variables as previously described.8 In multivariable analyses, CPC counts were examined as continuous variables after log-transformation (log2[cell count]), and hazard ratios and confidence intervals for continuous variables were reported as inverses. Using median and receiver operating characteristic (ROC) analysis to derive the optimal cut-point for prediction of all-cause death, CPC counts were categorized and analyzed in the multivariable analyses.
The relationships between CPC counts and all-cause death were examined in Cox proportional hazard regression models adjusted for age, sex, race, BMI, hypertension, hyperlipidemia, diabetes, low-density lipoprotein levels, current smoking, statin use, estimated glomerular filtration rate, left ventricular ejection fraction, and obstructive CAD (defined as >50% obstruction in one or more epicardial vessels).28 Fine and Gray’s sub-distribution hazard models adjusting for the same covariates were used for cardiovascular death and non-fatal MI, treating the non-cardiovascular deaths as competing risks.29
A progenitor cell score was created using optimal cut-points of CD34+/CXCR4+ and CD34+/VEGFR2+ CPC counts with a score of 0 assigned to those with levels of both cells above the ROC-derived cut-points, a score of 1 assigned to subjects with low levels of one cell but levels above the cut-point for the other cell, and a score of 2 assigned to those subjects with levels of both cell population below the respective cut-points. A similar progenitor cell score was created using optimal cut-points of CD34+ and CD34+/VEGFR2+ CPC counts, with point aggregation analogous to the scoring system above for cell counts below the respective cut points.
The incremental value of CPC counts to risk prediction was tested before and after their addition (using high v low categorization described above) to model with covariates of traditional risk factors for both scoring systems. The C-statistic (area under curve [AUC]) was calculated as an index of risk discrimination. Discrimination testing was also performed using the continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) metrics. Analyses were performed using IBM SPSS Statistics Version 22 (Armonk, NY, USA), SAS software (version 9.4, Cary, North Carolina) and R software version 3.6.1 (http://www.R-project.org). P values of <0.05 from two-sided tests were considered to indicate statistical significance.
RESULTS
A total of 1366 patients, 59.3% male, 79.9% white, were enrolled. Forty one percent had diabetes, 89% hypertension, 34% had heart failure, 77% had obstructive CAD, 15% had non-obstructive CAD, and 8% had angiographically normal coronary arteries, Table 1.
Table 1: Baseline Demographics of Total Cohort and Stratified by Progenitor Cell Score.
Mean (SD) shown unless stated. BMI indicates body mass index; CABG, coronary artery
| Baseline characteristics | All n=1366 | Progenitor Cell Score* | P value | ||
|---|---|---|---|---|---|
| 0 (N=494) | 1 (N=522) | 2 (N=350) | |||
| Age, y | 65.2 +/− 13.1 | 63.9 +/− 13.5 | 66.6 +/− 12.5 | 67.9 +/− 12.0 | <0.001 |
| Male, N (%) | 810 (59.3) | 325 (65.8) | 304 (58.2) | 181 (51.7) | <0.001 |
| Black, N (%) | 275 (20.1) | 106 (21.5) | 105 (20.1) | 64 (18.3) | 0.527 |
| BMI, kg/m2 | 29.4 +/− 6.4 | 30.1 +/− 6.4 | 28.9 +/− 6.4 | 29.4 +/− 6.7 | 0.002 |
| History of MI, N (%) | 276 (20.1) | 105 (21.3) | 95 (18.4) | 76 (22.0) | 0.343 |
| Diabetes, N (%) | 535 (41.4) | 176 (35.9) | 202 (38.8) | 157 (45.2) | 0.023 |
| Hypertension, N (%) | 1213 (88.8) | 437 (89.2) | 464 (88.9) | 312 (90.2) | 0.829 |
| Dyslipidemia, N (%) | 1014 (74.2) | 378 (76.8) | 383 (73.4) | 253 (72.9) | 0.330 |
| Current smoking, N (%) | 63 (4.6) | 19 (4.3) | 28 (5.9) | 16 (4.7) | 0.479 |
| History of CABG, N (%) | 324 (23.7) | 116 (23.5) | 127 (24.3) | 81 (23.1) | 0.911 |
| History of PCI, N (%) | 610 (44.7) | 168 (45.0) | 326 (40.9) | 116 (39.9) | 0.627 |
| Obstructive CAD**, N (%) | 1055 (77.2) | 347 (70.2) | 404 (77.4) | 304 (86.9) | <0.001 |
| History of heart failure, N (%) | 465 (34.0) | 157 (31.8) | 173 (33.1) | 135 (38.6) | 0.105 |
| eGFR, mL/min/1.73 m2 | 69.1 +/− 26.5 | 75.4 +/− 22.5 | 71.2 +/− 22.1 | 71.7 +/− 22.4 | 0.006 |
| History of peripheral vascular disease, N (%) | 251 (18.9) | 69 (14) | 94 (18.0) | 88 (25.4) | <0.001 |
| History of stroke, N (%) | 158 (11.7) | 49 (10.1) | 60 (12.0) | 49 (14.3) | 0.183 |
| Left ventricular ejection fraction (LVEF %) | 53 +/− 12.6 | 52.5 +/− 13.7 | 53.5 +/− 12.2 | 53.1 +/− 11.7 | 0.664 |
| PC populations (cells/mL) *** | |||||
| CD34+ | 1679 (1048–2514) | 2470 (850–10480) | 1470 (60–11940) | 1060 (0–3310) | <0.001 |
| CD34+/CD133+ | 760 (456–1207) | 1100 (100–9290) | 690 (0–6330) | 500 (0–2550) | <0.001 |
| CD34+/VEGFR2+ | 41 (21–138) | 240 (30–3660) | 40 (0–610) | 10 (0–20) | <0.001 |
| CD34+/CXCR4+ | 820 (487–1360) | 1680 (80–6280) | 710 (60–10470) | 440 (0–790) | <0.001 |
| Outcomes (3.1 year follow-up) | |||||
| Death, N (%) | 221 (16.6) | 66 (13.4) | 80 (15.3) | 75 (23.0) | 0.007 |
| CV death, N (%) | 143 (10.8) | 43 (8.7) | 55(10.5) | 45 (12.9) | 0.023 |
| MI, N (%) | 29 (2.1) | 8 (1.6) | 10 (1.9) | 11 (3.1) | 0.013 |
bypass grafting; CAD, coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; GFR, glomerular filtration rate; CXCR4, chemokine (C-X-C motif) receptor 4; VEGFR2, vascular endothelial growth factor receptor 2.
Stratified by Progenitor Cell (PC) Score (0,1,2). PC Score of 2 indicates both CD34+/CXCR4+ and CD34+/VEGFR2+ cells are below the ROC-derived cutoff, PC Score 1 indicates either CD34+/CXCR4+ or CD34+/VEGFR2+ are below the ROC-derived cutoff, and PC Score 0, with both CD34+/CXCR4+ cells and CD34+/VEGF2R+ cells above the ROC-derived cutoff. Cutoffs used were 25 cells/milliliter for CD34+/VEGFR2+ cells and 794 cells/milliliter for CD34+/CXCR+ cells (see Methods section of this article).
Obstructive coronary artery disease (CAD) defined as ≥50% stenosis in ≥1 epicardial coronary artery
Cell counts shown as median [interquartile range]
Median cell counts for CD34+, CD34+/CD133+, CD34+/CXCR4+, and CD34+/VEGFR2+ CPCs were 1679, 760, 820, and 41 cells/ml, respectively. CPCs enriched for hematopoietic progenitors, including CD34+, CD34+/CD133+ and CD34+/CXCR4+ cells were highly correlated with each other, but only modestly with CD34+/VEGFR2+ CPCs, enriched for endothelial progenitors (Supplemental Table 1).
Relationship between PCs and Outcomes
During a median 3.1 (IQR 1.3–4.9)-year follow-up, there were 221 all-cause deaths, 143 cardiovascular deaths, and 29 non-fatal MI events.
Association between hematopoietic progenitor-enriched CPC counts and adverse cardiovascular outcomes:
In unadjusted Cox regression analysis, CD34+/CD133+ and CD34+/CXCR4+ CPC counts as continuous variables were associated with all-cause mortality and the composite of cardiovascular death/MI (Table 2). Patients with low CD34+ or CD34+/CXCR4+ CPC counts (below median value) had a 53% (p=0.001) and 46% (p=0.02) greater risk, respectively, of all cause death. Low CD34+/CXCR4+ cell counts were associated with a 50% higher risk of cardiovascular death/MI, than those with these CPC counts above their respective cut-offs, after adjustment for demographic and clinical covariates (Table 2). Survival curves are shown in Figures 1 and 2. Results did not differ significantly when subjects with angiographically normal coronaries were removed from the analysis.
Table 2.
Association Between Progenitor Cells and Outcomes (n=1366)
| Outcome HR (95% CI), P Value | Cell Type | Continuous unadjusted* | Continuous adjusted* | Median unadjusted | Median adjusted | Threshold cutoff, Using ROC unadjusted ** | Threshold cutoff, Using ROC adjusted ** |
|---|---|---|---|---|---|---|---|
| Model 1: Individual PC subtypes | |||||||
| Death | CD34+ | 1.03 (1.00–1.07), 0.07 | 1.03 (0.99 – 1.08), 0.16 | 1.42 (1.08–1.85), 0.01 | 1.53 (1.10–2.10), 0.001 | 1.70 (1.28–2.25), 0.0002 | 1.67 (1.21–2.31), 0.002 |
| CD34+/CD133+ | 1.04 (1.01–1.07), 0.01 | 1.04 (1.00– 1.07), 0.03 | 1.28 (0.98–1.67), 0.07 | 1.14 (0.83–1.56), 0.42 | 1.48 (1.14–1.92), 0.004 | 1.38 (1.01–1.89), 0.04 | |
| CD34+/CXCR4+ | 1.04 (1.02–1.07), 0.0007 | 1.05 (1.00– 1.08), 0.001 | 1.53 (1.17–2.00), 0.002 | 1.46 (1.06–2.01), 0.02 | 1.52 (1.17–1.99), 0.002 | 1.47 (1.08–2.02), 0.01 | |
| CD34+/VEGFR2+ | 1.01 (0.99–1.01), 0.11 | 1.01 (0.99–1.02), 0.12 | 1.53 (1.17–2.01), 0.0019 | 1.59 (1.15–2.18), 0.004 | 1.58 (1.2–2.06), 0.0009 | 1.70 (1.25–2.31), 0.0008 | |
| CV death/MI | CD34+ | 1.03 (1.00–1.07), 0.07 | 1.04 (0.99–1.06), 0.08 | 1.17 (0.87–1.57), 0.29 | 1.20 (0.85–1.71), 0.30 | 1.61 (1.16–2.18), 0.003 | 1.64 (1.14–2.38), 0.008 |
| CD34+/CD133+ | 1.04 (1.02–1.06), 0.001 | 1.04 (1.02–1.06), 0.0002 | 1.11 (0.83–1.50), 0.48 | 1.01 (0.71–1.41), 0.98 | 1.34 (1.00–1.81), 0.05 | 1.30 (0.92–1.83), 0.13 | |
| CD34+/CXCR4+ | 1.04 (1.02–1.08), 0.003 | 1.06 (1.02–1.10), 0.005 | 1.44 (1.07–1.95), 0.02 | 1.50 (1.04–2.17), 0.03 | 1.46 (1.08–1.97), 0.01 | 1.51 (1.08–2.11), 0.02 | |
| CD34+/VEGFR2+ | 1.01 (0.99–1.02), 0.16 | 1.01 (0.99–1.02), 0.17 | 1.36 (1.01–1.84), 0.04 | 1.47 (1.01–2.13), 0.04 | 1.48 (1.10–2.00), 0.01 | 1.54 (1.07–2.21), 0.02 | |
Adjusted for age, body mass index, sex, race, smoking history, history of myocardial infarction, hypertension, diabetes, hyperlipidemia, renal function (GFR), obstructive coronary artery disease, left ventricular ejection fraction and statin use.
Log-transformed cell counts (log2[cells]). Values reported = 1/HR (1/upper CI–1/lower CI), P
Cutoffs used were 1032 cells/milliliter for CD34+ cells, 625 cells/milliliter for CD34+/CD133+ cells, 25 cells/milliliter for CD34+/VEGFR2+ cells and 794 cells/milliliter for CD34+/CXCR+ cells (see Methods section of this article).
Figure 1: Kaplan-Meier survival curves for all-cause death using ROC cutoff.
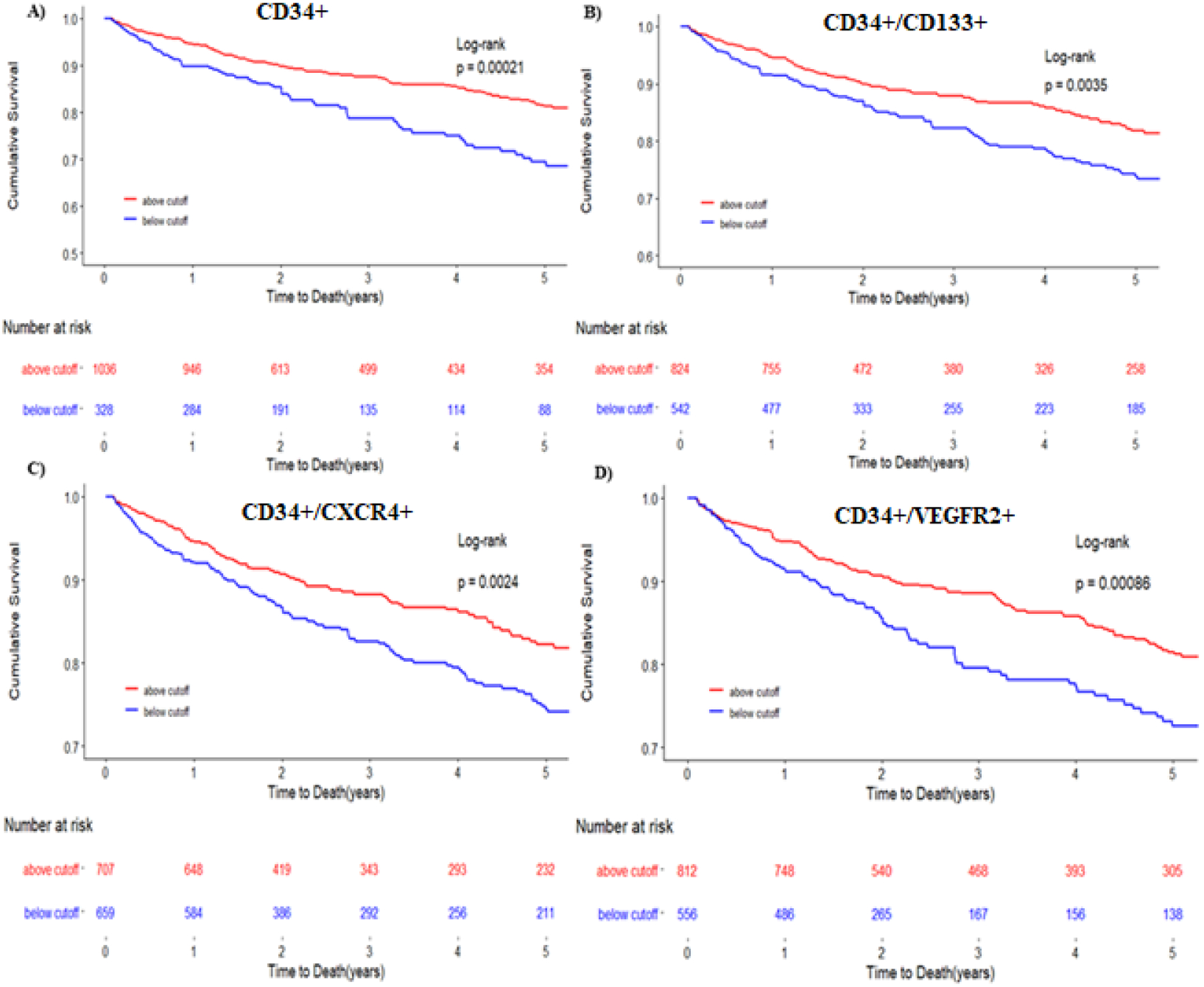
Kaplan-Meier survival curves for the primary end point of death for CD34+ (A), CD34+/CD133+ (B), CD34+/CXCR4+ (C), CD34+/VEGFR2+ (D) The blue line represents cells below the ROC derived cutoff. The red line represents progenitor cell levels below the ROC derived cutoff. Cutoffs used were 1032 cells/milliliter for CD34+ cells, 625 cells/milliliter for CD34+/CD133+ cells, 25 cells/milliliter for CD34+/VEGF2R+ cells and 794 cells/milliliter for CD34+/CXCR+ cells.
Figure 2: Kaplan-Meier survival curves for cardiovascular death/MI using ROC cutoff.
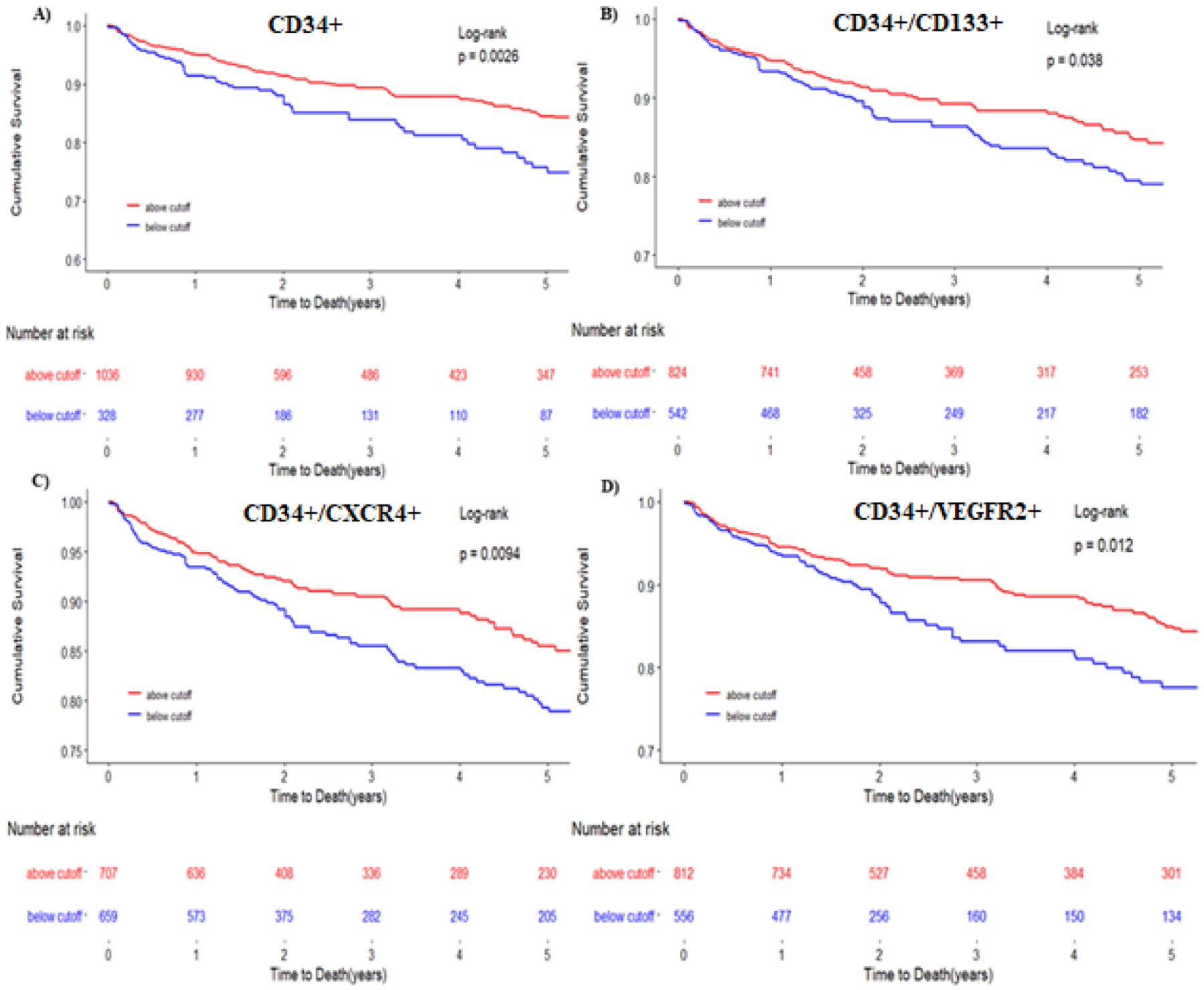
Kaplan-Meier survival curves for the composite end point of cardiovascular death/MI for CD34+ (A), CD34+/CD133+ (B), CD34+/CXCR4+ (C), CD34+/VEGFR2+ (D). The blue line represents cells below the ROC derived cutoff. The red line represents progenitor cell levels below the ROC derived cutoff. Cutoffs used were 1032 cells/milliliter for CD34+ cells, 625 cells/milliliter for CD34+/CD133+ cells, 25 cells/milliliter for CD34+/VEGF2R+ cells and 794 cells/milliliter for CD34+/CXCR+ cells.
To determine if there was a difference between predictive capacity of CD34+ cells depending on whether CD133+ or CXCR4+ epitopes were co-expressed, each PC subset was tested individually. CD34+ cells co-expressing CD133 without CXCR4 (CD34+/CD133+/CXCR4−) had no association with incident events. However, CD34+ CPCs coexpressing CXCR4 in the absence of CD133 (CD34+/CXCR4+/CD133−) had significant associations with incident adverse events, indicating that the CD34+/CXCR4+ CPC subset was most predictive of incident adverse events (Supplementary Table 2). In sensitivity analyses, there was no significant interaction between covariates and the association of CD34+/CXCR4+ cells with outcomes (Supplemental Figure 2A).
Association between endothelial progenitor-enriched CPC (CD34+/VEGFR2+) counts and adverse cardiovascular outcomes:
In unadjusted Cox regression models, lower CD34+/VEGFR2+ counts were associated with an increased risk of incident all-cause death and cardiovascular death/MI when stratified by median and at the ROC-derived cutoff value of 25 cells/mL (Table 2, Figure 1 and 2), findings that persisted after adjustment for the aforementioned clinical covariates. Patients with low CD34+/VEGFR2+ CPC counts (below median) had a 59% greater risk of all cause death and a 47% greater risk of cardiovascular death/MI than those above the median value, (Table 2, Figures 1 and 2). These findings persisted after removing patients with angiographically normal coronaries. In sensitivity analyses, there was a significant interaction between gender as well as diabetes mellitus and the association of CD34+/VEGFR2+ CPCs with adverse events (Supplementary Figure 2B), with a stronger association in men and those without diabetes.
Association between Progenitor Cell Score (PC Score) comprised of both CPC lineages and adverse cardiovascular outcomes:
We examined the association between the combination of both hematopoietic-enriched CPCs, represented by CD34+/CXCR4+ cell counts, and endothelial-enriched CD34+/VEGFR2+ CPCs and the risk of adverse outcomes in both unadjusted and adjusted models. (Table 3). To demonstrate the additive value of evaluating CPCs enriched for two lineages, patients were divided into 3 groups (PC Score 0, 1, 2) using optimal cut-points of CD34+/CXCR4+ and CD34+/VEGFR2+ CPC counts. Patients with both CD34+/CXCR4+ and CD34+/VEGFR2+ counts below cut-off values derived from ROC curves were assigned a score of 2, subjects with either low CD34+/VEGFR2+ or low CD34+/CXCR4+ cells were assigned a score of 1, and subjects with both CPCs levels above the ROC cut-off values were assigned a group of 0. Individuals with low CPCs in both lineages (PC Score of 2) were more likely to be older, and female with a greater number of comorbidities, including diabetes, peripheral vascular disease, and heart failure (Table 1). There was a graded increase in event risk with patients in the group with low levels of CPCs from both lineages (PC Score 2) experiencing the highest event risk, those with high levels of both CPCs with the lowest event risk (PC Score 0), and those with low CPCs in only one lineage with an intermediate event risk (PC Score 1), Figure 3. Thus, the all-cause mortality and cardiovascular death/MI risks in the group with low CPC counts in both lineages were 108% and 103% higher, respectively, than those with high counts in both lineages, Table 3, Figure 3.
Table 3.
Association Between both CD34+/CXCR4+ and CD34+/VEGFR2+ Cells and Outcomes (n=1366)
| Outcome HR (95% CI), P Value | Cell Type | Continuous Adjusted* | Median Adjusted | Threshold cutoff, Using ROC adjusted ** |
|---|---|---|---|---|
| Death | CD34+/CXCR4+ | 1.05 (1.01–1.08), 0.007 | 1.27 (0.93–1.74), 0.13 | 1.32 (0.97–1.80), 0.08 |
| CD34+/VEGFR2+ | 1.01 (1.00–1.01), 0.23 | 1.51 (1.09–2.11), 0.01 | 1.61 (1.17–2.21), 0.003 | |
| CV death/MI | CD34+/CXCR4+ | 1.05 (1.01–1.10), 0.01 | 1.35 (0.95–1.92), 0.10 | 1.46 (1.03–2.06), 0.03 |
| CD34+/VEGFR2+ | 1.00 (0.99–1.01), 0.42 | 1.44 (0.97–2.12), 0.07 | 1.41 (0.98–2.03), 0.07 | |
| Outcome | Categorical PC Score*** | Threshold cutoff, Unadjusted | Threshold cutoff, Adjusted | |
| Death | 1 vs. 0 | 1.61 (1.17–2.21), 0.003 | 1.80 (1.27–2.55), 0.001 | |
| 2 vs. 0 | 2.06 (1.48–2.88), <0.0001 | 2.08 (1.42–3.03), 0.0002 | ||
| CV Death/MI | 1 vs. 0 | 1.38 (0.97–1.96), 0.08 | 1.55 (1.05–2.28), 0.03 | |
| 2 vs. 0 | 1.82 (1.24–2.66), 0.002 | 2.03 (1.30–3.17), 0.002 |
Model includes clinical covariates as above plus CD34+/VEGFR2+ and CD34+/CXCR4+. Adjusted for age, body mass index, sex, race, smoking history, history of myocardial infarction, hypertension, diabetes, hyperlipidemia, renal function (GFR), obstructive coronary artery disease, left ventricular ejection fraction and statin use.
Log-transformed cell counts (log2[cells]). Values reported = 1/HR (1/upper CI–1/lower CI), P
Cutoffs used were 25 cells/milliliter for CD34+/VEGFR2+ cells and 794 cells/milliliter for CD34+/CXCR+ cells (see Methods section of this article).
Progenitor Cell score based on number of PC subtypes (CD34+/CXCR4+ or CD34+/VEGF2R+) below threshold ROC cutoff (score of 2) compared to reference group with both above threshold cutoff (score of 0). Cell counts based on threshold cutoffs (794 cells/mL for CD34+/CXCR4+ cells and 25 cells/mL for CD34+/VEGF2R+ cells)
Figure 3: Adjusted survival curves for all-cause death and cardiovascular death/MI.
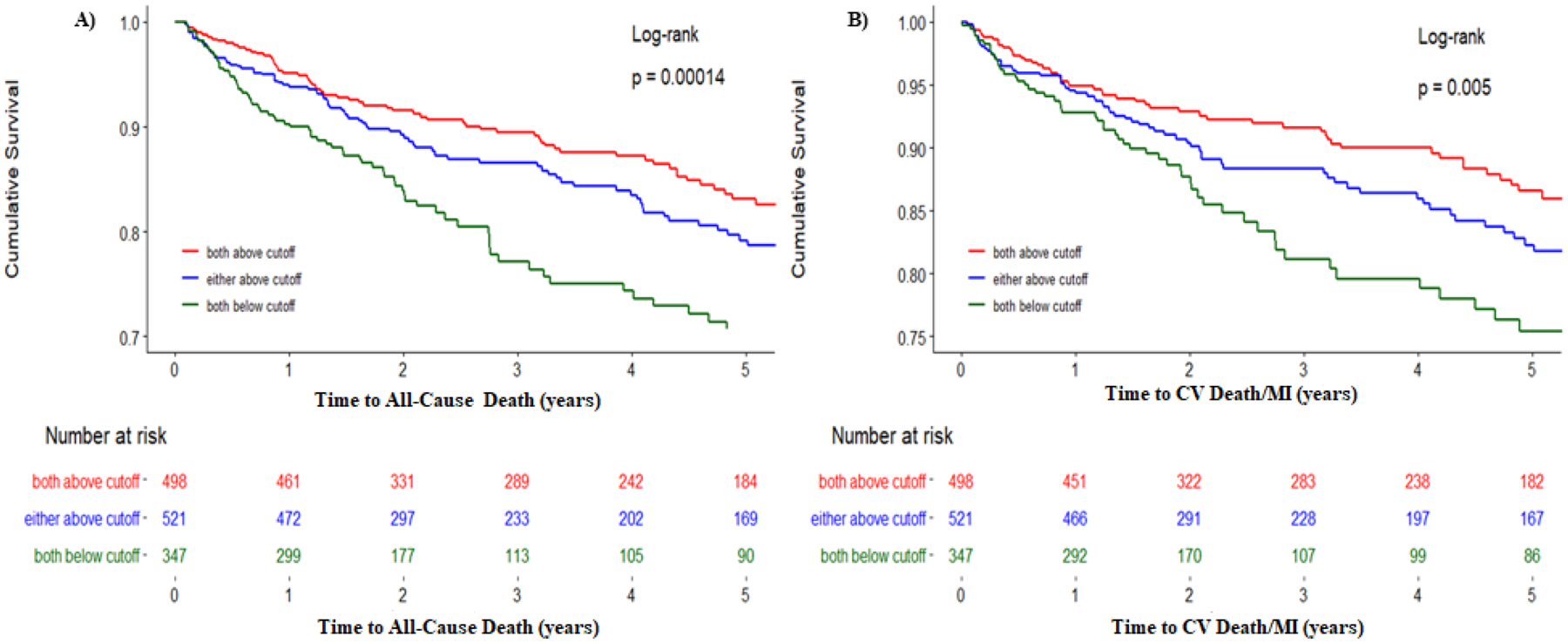
Kaplan-Meier survival curves for the primary end point of A) death, B) composite of cardiovascular death and myocardial infarction (MI) stratified by Progenitor Cell Score. The green line represents a Progenitor Cell Score of 2, indicating both CD34+/CXCR4+ and CD34+/VEGFR2+ cells are below the ROC-derived cutoff. Blue line represents progenitor cell score of 1, indicating either CD34+/CXCR4+ or CD34+/VEGFR2+ are below the ROC-derived cutoff. The red line represents progenitor cell score of 0, with both CD34+/CXCR4+ cells and CD34+/VEGF2R+ cells above the ROC-derived cutoff. Cutoffs used were 25 cells/milliliter for CD34+/VEGFR2+ cells and 794 cells/milliliter for CD34+/CXCR+ cells (see Methods section of this article)
An analogous analysis was performed examining the association between CD34+, CD34+/VEGFR2+ CPC counts and the risk of adverse outcomes in continuous, median-based, and score-based models using ROC-derived cutoffs (Supplemental Table 3). When both CPC counts were entered into the same model, both were independently predictive of all-cause mortality and cardiovascular death/MI using ROC-derived cutoffs. An analogous PC scoring system summarizing cutoff-based decreases in CD34+ and CD34+/VEGFR2+ cell counts exhibited a similar graded increase in event risk. Those with deficiencies in both were at 147% and 116% greater risk of all-cause mortality and cardiovascular death/MI, respectively (Supplemental Table 3).
Discrimination testing:
We tested the incremental value of this threshold driven CPC count. We compared the c-statistic of a model with traditional risk factors only (Model 1) and models incorporating CPC counts (Supplemental Table 4). Addition of either CD34+/CXCR4+ (Model 2) or CD34+/VEGFR2+ (Model 3) CPC counts was associated with modest improvements in the C-statistic. The largest improvement was noted when both CD34+/CXCR4+ and CD34+/VEGFR2+ CPC counts were added to the model together. There was no improvement in continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI).
DISCUSSION
In the largest study conducted to date examining the role of circulating progenitor cells on cardiovascular outcomes, we demonstrate that low circulating levels of both hematopoietic and endothelial PC-enriched populations are associated with higher risk of adverse events in patients with CAD. Importantly, participants with low levels of both CPC lineages were at the highest risk of adverse events. We and others have previously shown an association between various PC subsets and cardiovascular outcomes in smaller studies with limited follow-up.25,30–32 Our present study with 1366 participants represents the largest cohort of patients with CAD phenotyped for CPCs and prospectively followed for over 3 years. We now show definitively that deficiencies in both hematopoietic and endothelial progenitor-enriched cells are independent and additive predictors of adverse cardiovascular outcomes and mortality, and that the combined deficiencies of these two lineages of PCs identifies the highest risk subgroup.
CPCs provide unique insights into the importance of endogenous regenerative and reparative capacity.1,2,4,33 The content of CD34+ progenitor cells in blood have consistently been shown to be predictive of atherosclerotic disease progression and cardiovascular events.8,32,34–38 CPCs contribute to endothelial repair following vascular injury39–41 and lower content of these cells is associated with worsening of vascular function, thus highlighting the importance of these cells in vascular homeostasis.41–43 Experimental studies have shown that in response to endogenous and exogenous stimuli, including exposure to cardiovascular risk factors, tissue ischemia or damage, CD34+ PCs are mobilized from the bone marrow. Mobilization of progenitors from bone marrow niches is in response to higher circulating levels of VEGF and stromal cell-derived factor 1 (SDF-1) that are ligands for VEGFR2 and CXCR4, respectively, receptors that are expressed on CPCs.44 This capacity to mobilize PCs that home to areas of hypoxia and injury leading to accelerated repair and regeneration appears to be limited and may exhaust with higher exposure to these injurious stimuli and with aging.1,41,45–49 We have shown a mild decline in CPCs with age that is accelerated in those aging with multiple risk factors or CVD.27 Similarly, in patients with transient ischemia or infarction, there is mobilization of CPCs.50–55 High-risk subjects within our cohort, particularly those who were older, female, diabetic and with greater severity of CAD and PAD had fewer CPCs than participants without these risk factors. Our study shows that lower levels of CPCs, and presumed loss of regenerative capacity, regardless of underlying risk factors, was an independent determinant of incident cardiovascular outcomes and mortality.33,56–58
We have previously shown that low numbers of endothelial-enriched CPCs expressing VEGFR2 in blood samples to be an important marker of peripheral arterial disease (PAD), suggesting a role in the development and progression of diffuse atherosclerotic disease.11 A meta-analysis examining 12 studies on CD34+/VEGFR2+ cells had previously been inconclusive regarding their predictive role, likely due to heterogeneity of populations studied, outcomes measured and size of studies.12 Moreover, because CD34+/VEGFR2+ cells are rare, they are subject to higher variability during measurement.12 In our large cohort with high event rate and longer duration of follow-up, CD34+/VEGFR2+ PCs were significantly and independently predictive of outcomes. Other reports in patients with aortic stenosis and heart failure with preserved ejection fraction, CD34+/VEGFR2+ were also reported to be predictive of adverse cardiovascular events.35,59,60
CXCR4, a receptor for stromal derived factor-1 (SDF-1), is required for homing of PCs to areas of ischemia and serves to identify cells that have higher migratory capacity and potential for tissue repair and neo-vascularization.9 In contrast to a recent meta-analysis in which CD34+/CD133+ and CD34+ cells were shown to be highly predictive of adverse outcomes,61 low levels of CD34+/CD133+ in the absence of CXCR4 expression was not associated with worse outcomes in this population. Due to our ability for advanced phenotyping, we are able to identify the subpopulation of CD34+/CD133+/CXCR4− cells, specifically, and show that levels of the CXCR4− subset of CD34+/CD133+ was not prognostic for cardiovascular events. The CXCR4− subset may represent cells that mechanistically have diminished ability to migrate to areas of ischemia, representing an advancement from what has been previously shown.
Importantly, CPC levels are modifiable by lifestyle and therapeutic interventions that are also associated with improved cardiovascular outcomes. For example, treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and statins increase CPC counts and function.62–65 Exercise modulates CPC levels and in animal models, physical activity improves replacement of the dysfunctional endothelium by bone marrow-derived cells.66,67 Data in animal models and some early human studies have shown benefit from cell-based therapies in those with advanced CVD, while recent clinical trials in patients with non-obstructive coronary disease and microvascular angina have shown beneficial effects of CD34+ based therapy on anginal severity and markers of coronary endothelial dysfunction.68–71 The therapeutic impact of targeted treatment with hematopoietic and endothelial progenitor subsets remains to be determined.
Important implications of our findings include: (1) numbers of circulating CD34+ cells that are enriched for bone marrow-derived hematopoietic and endothelial progenitors are predictors of outcomes in patients with CAD, similar to observations in settings of acute lung injury, renal failure, and stroke;72–75 (2) low levels of CD34+/CXCR4+ and CD34+/VEGFR2+ cells could be considered as modifiable risk factors and thus targets for therapeutic interventions; (3) both CD34+/CXCR4+ and CD34+/VEGFR2+ cell counts may be considered ‘biomarkers’ of regenerative capacity and as independent predictors of adverse outcomes; and (4) these findings could influence cell based therapy by supporting targeted selection of patients with impaired regenerative capacity.
Strengths of our study include (1) a large cohort study design to limit heterogeneity, (2) use of commonly used high-throughput technology (flow cytometry) for quantification of PCs by standardized and reproducible assay techniques, (3) exploration of several CD34+ cell subpopulations enriched for both hematopoietic and endothelial PCs, and (4) the association with incident cardiac and vascular events.
Study limitations
Limitations are that we only examined a high-risk population enriched with CAD, and therefore our conclusions may not be applicable to the general population. We also have not measured cell functionality. Finally, the observational nature of this analysis does not imply causation and thus further interventional studies directly influencing PC levels are required. Studies demonstrating improvements in intermediate phenotypes, such as endothelial function with PC mobilization suggest that this could be feasible.76 Further research is needed to investigate whether age-specific cut-points would improve risk determination.
CONCLUSIONS
Lower CPC counts are associated with mortality and incident cardiovascular death and MI risk in individuals with CAD, independent of all other risk factors. Moreover, lower levels of both CD34+/CXCR4+ and CD34+/VEGFR2+ PCs, representing reduced numbers of hematopoietic and endothelial PCs have an independent and additive prognostic impact. Our findings suggest that impaired endogenous regenerative capacity in multiple PC lineages is associated with higher mortality and has important implications for biological understanding of cardiovascular risk, for risk prediction and potentially for selection for PC-modifying therapies.
Supplementary Material
Highlights.
Circulating progenitor cells (CPCs), which play a critical role in vascular repair and regeneration, can differentiate into several distinct lineages including hematopoietic progenitors, identified by expression of the CD34 (Cluster of Differentiation) epitope on hematopoietic CD45med cells, and non-hematopoietic or mesenchymal progenitors that lack CD45 expression.
Previous studies from our and other groups have shown that subjects with lower levels of CPCs enriched for hematopoietic progenitors (CD34+/CD133+ or CD34+/CXCR4+ cells) have worse prognosis, however, data regarding the prognostic value of endothelial CPC (CD34+/VEGFR2+) populations have been conflicting.
In the largest study conducted to date examining the role of endogenous regenerative capacity on cardiovascular outcomes, we demonstrate that low circulating levels of both hematopoietic and endothelial PC-enriched populations are associated with higher risk of adverse events in patients with CAD.
Deficiencies in both hematopoietic and endothelial progenitor-enriched cells are independent and additive predictors of adverse cardiovascular outcomes and mortality, and the combined deficiencies of these two lineages of PCs identifies the highest risk subgroup.
Additionally, our study specifically identifies and demonstrates that low levels of CD34+/CD133+/CXCR4− cell population are not prognostic for cardiovascular events, indicating that the CXCR4− subset may have a diminished ability to migrate to areas of ischemia, representing an advancement from what has been previously shown.
Sources of Funding:
Dhindsa, Sandesara, Mehta, and Tahhan have been supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA). Mehta is supported by American Heart Association grant 19POST34400057. Quyyumi is supported by NIH grants 1P20HL113451-01, 1R61HL138657-02, 1P30DK111024-03S1, 5R01HL095479-08, 3RF1AG051633-01S2, 5R01AG042127-06, 2P01HL086773-08, U54AG062334-01, 1R01HL141205-01, 5P01HL101398-02, 1P20HL113451-01, 5P01HL086773-09 1RF1AG051633-01, R01 NS064162-01, R01 HL89650-01, HL095479-01, 1DP3DK094346-01, 2P01HL086773, and American Heart Association grant 15SFCRN23910003.
Role of the Funder/Sponsor:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest to report.
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
REFERENCES
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964 [DOI] [PubMed] [Google Scholar]
- 2.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/jci8071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med. 1998;105:32s–39s. doi: 10.1016/s0002-9343(98)00209-5 [DOI] [PubMed] [Google Scholar]
- 4.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221 [DOI] [PubMed] [Google Scholar]
- 5.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.Res.0000137877.89448.78 [DOI] [PubMed] [Google Scholar]
- 6.Waller EK, Olweus J, Lund-Johansen F, Huang S, Nguyen M, Guo GR, Terstappen L. The “common stem cell” hypothesis reevaluated: human fetal bone marrow contains separate populations of hematopoietic and stromal progenitors. Blood. 1995;85:2422–2435. doi: [PubMed] [Google Scholar]
- 7.Yang J, Ii M, Kamei N, Alev C, Kwon SM, Kawamoto A, Akimaru H, Masuda H, Sawa Y, Asahara T. CD34+ cells represent highly functional endothelial progenitor cells in murine bone marrow. PloS one. 2011;6:e20219. doi: 10.1371/journal.pone.0020219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RS, Li Q, Ghasemzadeh N, Eapen DJ, Moss LD, Janjua AU, Manocha P, Al Kassem H, Veledar E, Samady H, et al. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116:289–297. doi: 10.1161/CIRCRESAHA.116.304187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. CXCR4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1802–1809. doi: 10.1161/atvbaha.109.194688 [DOI] [PubMed] [Google Scholar]
- 10.Patel RS, Su S, Neeland IJ, Ahuja A, Veledar E, Zhao J, Helgadottir A, Holm H, Gulcher JR, Stefansson K, et al. The chromosome 9p21 risk locus is associated with angiographic severity and progression of coronary artery disease. Eur Heart J. 2010;31:3017–3023. doi: 10.1093/eurheartj/ehq272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayek SS, MacNamara J, Tahhan AS, Awad M, Yadalam A, Ko YA, Healy S, Hesaroieh I, Ahmed H, Gray B, et al. Circulating Progenitor Cells Identify Peripheral Arterial Disease in Patients With Coronary Artery Disease. Circulation research. 2016;119:564–571. doi: 10.1161/circresaha.116.308802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigato M, Avogaro A, Fadini GP. Levels of Circulating Progenitor Cells, Cardiovascular Outcomes and Death: A Meta-Analysis of Prospective Observational Studies. Circ Res. 2016;118:1930–1939. doi: 10.1161/CIRCRESAHA.116.308366 [DOI] [PubMed] [Google Scholar]
- 13.Fadini GP, Mehta A, Dhindsa DS, Bonora BM, Sreejit G, Nagareddy P, Quyyumi AA. Circulating stem cells and cardiovascular outcomes: from basic science to the clinic. European heart journal. 2019. doi: 10.1093/eurheartj/ehz923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelliccia F, Pasceri V, Rosano G, Pristipino C, Roncella A, Speciale G, Pannarale G, Schiariti M, Greco C, Gaudio C. Endothelial Progenitor Cells Predict Long-Term Prognosis in Patients With Stable Angina Treated With Percutaneous Coronary Intervention – Five-Year Follow-up of the PROCREATION Study &ndash. Circulation Journal. 2013;77:1728–1735. doi: 10.1253/circj.CJ-12-1608 [DOI] [PubMed] [Google Scholar]
- 15.Briguori C, Testa U, Riccioni R, Colombo A, Petrucci E, Condorelli G, Mariani G, D’Andrea D, Micco FD, Rivera NV, et al. Correlations between progression of coronary artery disease and circulating endothelial progenitor cells. 2010;24:1981–1988. doi: 10.1096/fj.09-138198 [DOI] [PubMed] [Google Scholar]
- 16.Lee HJ, Kim W, Kim WS, Woo JS, Kim YG, Moon JY, Lee SH, Ihm CG, Lee TW, Jeong KH. Circulating Endothelial Progenitor Cell Levels Predict Cardiovascular Events in End-Stage Renal Disease Patients on Maintenance Hemodialysis. Nephron. 2015;130:151–158. doi: 10.1159/000430471 [DOI] [PubMed] [Google Scholar]
- 17.Maruyama S, Taguchi A, Iwashima S, Ozaki T, Yasuda K, Kikuchi-Taura A, Soma T, Ishii H, Murohara T, Takahashi H, et al. Low circulating CD34+ cell count is associated with poor prognosis in chronic hemodialysis patients. Kidney International. 2008;74:1603–1609. doi: 10.1038/ki.2008.495 [DOI] [PubMed] [Google Scholar]
- 18.Lorenzen J, David S, Bahlmann FH, de Groot K, Bahlmann E, Kielstein JT, Haller H, Fliser D. Endothelial progenitor cells and cardiovascular events in patients with chronic kidney disease--a prospective follow-up study. PloS one. 2010;5:e11477. doi: 10.1371/journal.pone.0011477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu C-L, Leu J-G, Liu W-C, Zheng C-M, Lin Y-F, Shyu J-F, Wu C-C, Lu K-C. Endothelial Progenitor Cells Predict Long-Term Mortality in Hemodialysis Patients. Int J Med Sci. 2016;13:240–247. doi: 10.7150/ijms.14209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating Endothelial Progenitor Cells and Cardiovascular Outcomes. 2005;353:999–1007. doi: 10.1056/NEJMoa043814 [DOI] [PubMed] [Google Scholar]
- 21.Alba AC, Lalonde SD, Rao V, Walter SD, Guyatt GH, Ross HJ. Changes in Circulating Progenitor Cells Are Associated With Outcome in Heart Failure Patients: A Longitudinal Study. Canadian Journal of Cardiology. 2013;29:1657–1664. doi: 10.1016/j.cjca.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 22.Fadini GP, de Kreutzenberg S, Agostini C, Boscaro E, Tiengo A, Dimmeler S, Avogaro A. Low CD34+ cell count and metabolic syndrome synergistically increase the risk of adverse outcomes. Atherosclerosis. 2009;207:213–219. doi: 10.1016/j.atherosclerosis.2009.03.040 [DOI] [PubMed] [Google Scholar]
- 23.Ko YA, Hayek S, Sandesara P, Samman Tahhan A, Quyyumi A. Cohort profile: the Emory Cardiovascular Biobank (EmCAB). BMJ Open. 2017;7:e018753. doi: 10.1136/bmjopen-2017-018753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel RS, Li Q, Ghasemzadeh N, Eapen DJ, Moss LD, Janjua AU, Manocha P, Kassem HA, Veledar E, Samady H, et al. Circulating CD34+ progenitor cells and risk of mortality in a population with coronary artery disease. Circ Res. 2015;116:289–297. doi: 10.1161/circresaha.116.304187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahar EA, Mou L, Hayek SS, Quyyumi AA, Waller EK. Flow cytometric data analysis of circulating progenitor cell stability. Data Brief. 2017;10:346–348. doi: 10.1016/j.dib.2016.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Mheid I, Hayek SS, Ko YA, Akbik F, Li Q, Ghasemzadeh N, Martin GS, Long Q, Hammadah M, Maziar Zafari A, et al. Age and Human Regenerative Capacity Impact of Cardiovascular Risk Factors. Circulation research. 2016;119:801–809. doi: 10.1161/circresaha.116.308461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinning C, Lillpopp L, Appelbaum S, Ojeda F, Zeller T, Schnabel R, Lubos E, Jagodzinski A, Keller T, Munzel T, et al. Angiographic score assessment improves cardiovascular risk prediction: the clinical value of SYNTAX and Gensini application. Clin Res Cardiol. 2013;102:495–503. doi: 10.1007/s00392-013-0555-4 [DOI] [PubMed] [Google Scholar]
- 29.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 30.Fadini GP, de Kreutzenberg S, Agostini C, Boscaro E, Tiengo A, Dimmeler S, Avogaro A. Low CD34+ cell count and metabolic syndrome synergistically increase the risk of adverse outcomes. Atherosclerosis. 2009;207:213–219. doi: 10.1016/j.atherosclerosis.2009.03.040 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/circulationaha.104.504340 [DOI] [PubMed] [Google Scholar]
- 32.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. The New England journal of medicine. 2005;353:999–1007. doi: 10.1056/NEJMoa043814 [DOI] [PubMed] [Google Scholar]
- 33.Urbich C, Dimmeler S. Endothelial Progenitor Cells. 2004;95:343–353. doi: doi: 10.1161/01.RES.0000137877.89448.78 [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Lucke C, Rössig L, Fichtlscherer S, Vasa M, Britten M, Kämper U, Dimmeler S, Zeiher AM. Reduced Number of Circulating Endothelial Progenitor Cells Predicts Future Cardiovascular Events. 2005;111:2981–2987. doi: doi: 10.1161/CIRCULATIONAHA.104.504340 [DOI] [PubMed] [Google Scholar]
- 35.Shimoni S, Bar I, Meledin V, Derazne E, Gandelman G, George J. Circulating Endothelial Progenitor Cells and Clinical Outcome in Patients with Aortic Stenosis. PLoS One. 2016;11:e0148766. doi: 10.1371/journal.pone.0148766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fadini GP, Rigato M, Cappellari R, Bonora BM, Avogaro A. Long-term Prediction of Cardiovascular Outcomes by Circulating CD34+ and CD34+CD133+ Stem Cells in Patients With Type 2 Diabetes. Diabetes care. 2017;40:125–131. doi: 10.2337/dc16-1755 [DOI] [PubMed] [Google Scholar]
- 37.Maruyama S, Taguchi A, Iwashima S, Ozaki T, Yasuda K, Kikuchi-Taura A, Soma T, Ishii H, Murohara T, Takahashi H, et al. Low circulating CD34+ cell count is associated with poor prognosis in chronic hemodialysis patients. Kidney international. 2008;74:1603–1609. doi: 10.1038/ki.2008.495 [DOI] [PubMed] [Google Scholar]
- 38.Lu CL, Leu JG, Liu WC, Zheng CM, Lin YF, Shyu JF, Wu CC, Lu KC. Endothelial Progenitor Cells Predict Long-Term Mortality in Hemodialysis Patients. International journal of medical sciences. 2016;13:240–247. doi: 10.7150/ijms.14209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin Therapy Accelerates Reendothelialization. 2002;105:3017–3024. doi: doi: 10.1161/01.CIR.0000018166.84319.55 [DOI] [PubMed] [Google Scholar]
- 40.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and Transplantation of Autologous Circulating Endothelial Cells Into Denuded Vessels and Prosthetic Grafts. 2003;108:2710–2715. doi: doi: 10.1161/01.CIR.0000096490.16596.A6 [DOI] [PubMed] [Google Scholar]
- 41.Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. 2003;348:593–600. doi: 10.1056/NEJMoa022287 [DOI] [PubMed] [Google Scholar]
- 42.Werner N, Wassmann S, Ahlers P, Schiegl T, Kosiol S, Link A, Walenta K, Nickenig G. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic research in cardiology. 2007;102:565–571. doi: 10.1007/s00395-007-0680-1 [DOI] [PubMed] [Google Scholar]
- 43.Witkowski S, Lockard MM, Jenkins NT, Obisesan TO, Spangenburg EE, Hagberg JM. Relationship between circulating progenitor cells, vascular function and oxidative stress with long-term training and short-term detraining in older men. Clinical science (London, England : 1979). 2010;118:303–311. doi: 10.1042/cs20090253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann BR, Wagner JR, Prisco AR, Janiak A, Greene AS. Vascular endothelial growth factor-A signaling in bone marrow-derived endothelial progenitor cells exposed to hypoxic stress. Physiological genomics. 2013;45:1021–1034. doi: 10.1152/physiolgenomics.00070.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and Migratory Activity of Circulating Endothelial Progenitor Cells Inversely Correlate With Risk Factors for Coronary Artery Disease. 2001;89:e1–e7. doi: doi: 10.1161/hh1301.093953 [DOI] [PubMed] [Google Scholar]
- 46.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a [DOI] [PubMed] [Google Scholar]
- 47.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. Journal of the American College of Cardiology. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074 [DOI] [PubMed] [Google Scholar]
- 48.Głowińska-Olszewska B, Moniuszko M, Hryniewicz A, Jeznach M, Rusak M, Dąbrowska M, Łuczyński W, Bodzenta-Łukaszyk A, Bossowski A. Relationship between circulating endothelial progenitor cells and endothelial dysfunction in children with type 1 diabetes: a novel paradigm of early atherosclerosis in high-risk young patients. 2013;168:153. doi: 10.1530/eje-12-0857 [DOI] [PubMed] [Google Scholar]
- 49.Masuda H, Alev C, Akimaru H, Ito R, Shizuno T, Kobori M, Horii M, Ishihara T, Isobe K, Isozaki M, et al. Methodological development of a clonogenic assay to determine endothelial progenitor cell potential. Circulation research. 2011;109:20–37. doi: 10.1161/circresaha.110.231837 [DOI] [PubMed] [Google Scholar]
- 50.Leone AM, Rutella S, Bonanno G, Contemi AM, de Ritis DG, Giannico MB, Rebuzzi AG, Leone G, Crea F. Endogenous G-CSF and CD34+ cell mobilization after acute myocardial infarction. International journal of cardiology. 2006;111:202–208. doi: 10.1016/j.ijcard.2005.06.043 [DOI] [PubMed] [Google Scholar]
- 51.Wojakowski W, Tendera M, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Ochala A, Ratajczak MZ. Mobilization of CD34/CXCR4+, CD34/CD117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.Cir.0000147609.39780.02 [DOI] [PubMed] [Google Scholar]
- 52.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122 [DOI] [PubMed] [Google Scholar]
- 53.Roberts N, Xiao Q, Weir G, Xu Q, Jahangiri M. Endothelial progenitor cells are mobilized after cardiac surgery. The Annals of thoracic surgery. 2007;83:598–605. doi: 10.1016/j.athoracsur.2006.09.087 [DOI] [PubMed] [Google Scholar]
- 54.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circulation research. 2001;88:167–174. doi: 10.1161/01.res.88.2.167 [DOI] [PubMed] [Google Scholar]
- 55.Sepp D, Franz D, Triftshaeuser N, Ott I, Esposito-Bauer L, Feurer R, Seifert CL, Thaler M, Hemmer B, Poppert H. Mobilization of CD133+ progenitor cells in patients with acute cerebral infarction. PLoS One. 2014;9:e70796. doi: 10.1371/journal.pone.0070796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spinetti G, Cordella D, Fortunato O, Sangalli E, Losa S, Gotti A, Carnelli F, Rosa F, Riboldi S, Sessa F, et al. Global remodeling of the vascular stem cell niche in bone marrow of diabetic patients: implication of the microRNA-155/FOXO3a signaling pathway. Circulation research. 2013;112:510–522. doi: 10.1161/circresaha.112.300598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teraa M, Fledderus JO, Rozbeh RI, Leguit RJ, Verhaar MC. Bone marrow microvascular and neuropathic alterations in patients with critical limb ischemia. Circulation research. 2014;114:311–314. doi: 10.1161/circresaha.114.302791 [DOI] [PubMed] [Google Scholar]
- 58.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone Marrow Origin of Endothelial Progenitor Cells Responsible for Postnatal Vasculogenesis in Physiological and Pathological Neovascularization. 1999;85:221–228. doi: doi: 10.1161/01.RES.85.3.221 [DOI] [PubMed] [Google Scholar]
- 59.Koller L, Hohensinner P, Sulzgruber P, Blum S, Maurer G, Wojta J, Hulsmann M, Niessner A. Prognostic relevance of circulating endothelial progenitor cells in patients with chronic heart failure. Thrombosis and haemostasis. 2016;116:309–316. doi: 10.1160/th16-01-0051 [DOI] [PubMed] [Google Scholar]
- 60.Samman Tahhan A, Hammadah M, Sandesara PB, Hayek SS, Kalogeropoulos AP, Alkhoder A, Mohamed Kelli H, Topel M, Ghasemzadeh N, Chivukula K, et al. Progenitor Cells and Clinical Outcomes in Patients With Heart Failure. Circulation Heart failure. 2017;10. doi: 10.1161/circheartfailure.117.004106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rigato M, Avogaro A, Fadini GP. Levels of Circulating Progenitor Cells, Cardiovascular Outcomes and Death. 2016;118:1930–1939. doi: doi: 10.1161/CIRCRESAHA.116.308366 [DOI] [PubMed] [Google Scholar]
- 62.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816 [DOI] [PubMed] [Google Scholar]
- 63.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, Nishimura H, Losordo DW, Asahara T, Isner JM. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105:3017–3024. doi: 10.1161/01.cir.0000018166.84319.55 [DOI] [PubMed] [Google Scholar]
- 64.Bahlmann FH, de Groot K, Mueller O, Hertel B, Haller H, Fliser D. Stimulation of endothelial progenitor cells: a new putative therapeutic effect of angiotensin II receptor antagonists. Hypertension (Dallas, Tex : 1979). 2005;45:526–529. doi: 10.1161/01.Hyp.0000159191.98140.89 [DOI] [PubMed] [Google Scholar]
- 65.Wang CH, Verma S, Hsieh IC, Chen YJ, Kuo LT, Yang NI, Wang SY, Wu MY, Hsu CM, Cheng CW, et al. Enalapril increases ischemia-induced endothelial progenitor cell mobilization through manipulation of the CD26 system. Journal of molecular and cellular cardiology. 2006;41:34–43. doi: 10.1016/j.yjmcc.2006.03.006 [DOI] [PubMed] [Google Scholar]
- 66.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.Cir.0000082924.75945.48 [DOI] [PubMed] [Google Scholar]
- 67.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.Cir.0000109141.48980.37 [DOI] [PubMed] [Google Scholar]
- 68.Choi JH, Hur J, Yoon CH, Kim JH, Lee CS, Youn SW, Oh IY, Skurk C, Murohara T, Park YB, et al. Augmentation of therapeutic angiogenesis using genetically modified human endothelial progenitor cells with altered glycogen synthase kinase-3beta activity. The Journal of biological chemistry. 2004;279:49430–49438. doi: 10.1074/jbc.M402088200 [DOI] [PubMed] [Google Scholar]
- 69.Henry TD, Merz CNB, Wei J, Corban MT, Quesada O, Joung S, Kotynski CL, Wang J, Lewis M, Schumacher AM, et al. Autologous CD34+ Stem Cell Therapy Increases Coronary Flow Reserve and Reduces Angina in Patients With Coronary Microvascular Dysfunction. Circulation: Cardiovascular Interventions. 2022;15:e010802. doi: doi: 10.1161/CIRCINTERVENTIONS.121.010802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corban MT, Toya T, Albers D, Sebaali F, Lewis BR, Bois J, Gulati R, Prasad A, Best PJM, Bell MR, et al. IMPROvE-CED Trial: Intracoronary Autologous CD34+ Cell Therapy for Treatment of Coronary Endothelial Dysfunction in Patients With Angina and Nonobstructive Coronary Arteries. Circulation Research. 2022;130:326–338. doi: doi: 10.1161/CIRCRESAHA.121.319644 [DOI] [PubMed] [Google Scholar]
- 71.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50 [DOI] [PubMed] [Google Scholar]
- 72.Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. doi: [PubMed] [Google Scholar]
- 73.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:2804–2808. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rafat N, Tonshoff B, Bierhaus A, Beck GC. Endothelial progenitor cells in regeneration after acute lung injury: do they play a role? American journal of respiratory cell and molecular biology. 2013;48:399–405. doi: 10.1165/rcmb.2011-0132TR [DOI] [PubMed] [Google Scholar]
- 75.Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Sher F, O’Rourke F, Nasser AM, Schwindt B, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36:151–153. doi: 10.1161/01.str.0000149944.15406.16 [DOI] [PubMed] [Google Scholar]
- 76.Subramaniyam V, Waller EK, Murrow JR, Manatunga A, Lonial S, Kasirajan K, Sutcliffe D, Harris W, Taylor WR, Alexander RW, et al. Bone marrow mobilization with granulocyte macrophage colony-stimulating factor improves endothelial dysfunction and exercise capacity in patients with peripheral arterial disease. American heart journal. 2009;158:53–60.e51. doi: 10.1016/j.ahj.2009.04.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


