Abstract
Enteroendocrine cells (EECs) are specialized sensors of luminal forces and chemicals in the gastrointestinal (GI) epithelium that respond to stimulation with a release of signaling molecules, such as serotonin (5-HT). For mechano-sensitive EECs, force activates Piezo2 channels, which generate a very rapidly activating and inactivating (~10 msec) cationic (Na+, K+, Ca2+) receptor current. Piezo2 receptor currents lead to a large and persistent increase in intracellular calcium (Ca2+) that lasts many seconds to sometimes minutes, suggesting signal amplification. However, intracellular calcium dynamics in EEC mechanotransduction remain poorly understood. The aim of this paper was to determine the role of Ca2+ stores in EEC mechano-transduction. Mechanical stimulation of a human EEC cell model (QGP-1) resulted in a rapid increase in cytoplasmic Ca2+ and a slower decrease in ER stores Ca2+, suggesting the involvement of intracellular Ca2+ stores. Comparing murine primary colonic EECs with colonocytes showed expression of intercellular Ca2+ store receptors, a similar expression of IP3 receptors, but a >30-fold enriched expression of Ryr3 in EECs. In mechanically stimulated primary EECs, Ca2+ responses decreased dramatically by emptying stores and pharmacologically blocking IP3 and RyR1/3 receptors. RyR3 genetic knockdown by siRNA led to a significant decrease in mechanosensitive Ca2+ responses and 5-HT release. In tissue, pressure-induced increase in the Ussing short circuit current was significantly decreased by ryanodine receptor blockade. Our data show that mechanosensitive EECs use intracellular Ca2+ stores to amplify mechanically induced Ca2+ entry, with RyR3 receptors selectively expressed in EECs and involved in Ca2+ signaling, 5-HT release, and epithelial secretion.
Keywords: Enteroendocrine Cells, Intracellular Stores, Mechanotransduction
Graphical Abstract
Mechano-sensitive enteroendocrine cells (EECs) found in the intestinal epithelium express the mechanosensitive ion channel Piezo2. Force activates Piezo2 channels, which upon activation generate a very rapidly activating and inactivating (~10 msec) cationic receptor current. Yet, rapid Piezo2 receptor currents lead to a large and persistent increase in intracellular calcium (Ca2+) that lasts many seconds to sometimes minutes, suggesting signal amplification. This study has found that the signal amplification involves calcium induced calcium release (CICR) mechanisms activated through intracellular stores receptors, RyR3 and IP3Rs. Activation of these receptors leads to large and sustained increase in Ca2+ that results in the release of serotonin (5-HT) that plays an important role in many local and systemic processes.
Introduction
The gastrointestinal (GI) tract lumen is home to a vast range of environmental stimuli. Enteroendocrine cells (EECs) are epithelial cells that are specialized sensors of luminal stimuli, including mechanical forces (Linan-Rico et al., 2016; Wang et al., 2017; Alcaino et al., 2018; Jones et al., 2022) and chemicals, like nutrients (Reimann & Gribble, 2002) or bacterial metabolites (Bellono et al., 2017). While EECs make up ~1% of the intestinal epithelium, they produce, and in response to stimulation, release large amounts of physiologically relevant signaling molecules – neurotransmitters and hormones, like serotonin (5-HT) (Mawe & Hoffman, 2013; Gribble & Reimann, 2015; Alcaino et al., 2018).
Primary EECs (Racke et al., 1996; Braun et al., 2007; Reimann et al., 2008) and EEC cell lines (Sidhu et al., 2000; Zhou & Pestka, 2015) use cytoplasmic Ca2+ as a central regulator that drives signaling molecule release in response to chemical stimuli that include food components like sugars (Reimann et al., 2008; Martin et al., 2017), amino acids (Young et al., 2010), fatty acids (Sidhu et al., 2000), spices, odorants (Braun et al., 2007), tastants (Kidd et al., 2008; Vegezzi et al., 2014) and toxins (Zhou & Pestka, 2015), as well as metabolites produced in the gut by microbial metabolism (Bellono et al., 2017). Upon stimulation, receptors set off a Ca2+ cascade in the EEC signal transduction through Ca2+ conducting ion channels such as non-selective cation channels, including transient receptor potential ankyrin-1 (TRPA1) (Nozawa et al., 2009; Zhou & Pestka, 2015; Bellono et al., 2017) and voltage-gated L-type Ca2+ channels (Lomax et al., 1999; Young et al., 2010). In the case of mechanical stimulation, the increase in intracellular Ca2+ is due to the activation of the mechanosensitive non-selective cationic channel Piezo2 (Wang et al., 2017; Alcaino et al., 2018; Treichel et al., 2022). Ca2+ influx through Piezo2 is critical for initiating Ca2+ increase in EC cells (Alcaino et al., 2018). However, Piezo2 ionic current amplitude is small, and kinetics are quick, leading to inactivation in current in milliseconds even with a persistent stimulus (Coste et al., 2010; Alcaino et al., 2017), so Piezo2 accounts for a small fraction of the overall Ca2+ response. Nevertheless, the cytoplasmic Ca2+ elevation (Kim et al., 2001; Alcaino et al., 2018) and 5-HT release that follow a single stimulation last many seconds (Bertrand et al., 2008), suggesting a currently unknown Ca2+ amplification mechanism.
Neuroepithelial mechanosensory cells in other organs have developmental and functional similarities to mechanosensitive EECs. For example, Merkel cells, which are epithelial skin touch sensors, have previously been suggested to be a model system for sensory transduction in enteroendocrine cells (Treichel et al., 2018). Indeed, similar to EECs, Merkel cells use Piezo2 to convert force into signaling molecule release (Woo et al., 2014; Chang et al., 2016; Hoffman et al., 2018). Intracellular stores are essential regulators of the cytoplasmic Ca2+ through calcium-induced calcium release (CICR) in Merkel cells (Senok & Baumann, 1997; Piskorowski et al., 2008b). Another type of specialized mechanosensory epithelial cells is inner hair cells, which are critical for auditory processing and rely on CICR through ryanodine receptors for mechanotransduction (Beurg et al., 2005).
Transcripts for Ca2+ store receptors, like IP3 receptors, are abundantly expressed in EECs (Schafermeyer et al., 2004). Ca2+ stores appear to be involved in EEC signal transduction, but we derive much of our current knowledge of the topic from EE cell models, like STC-1 (Sidhu et al., 2000) and BON-1 (Linan-Rico et al., 2017) cells. These studies show that intracellular stores regulate the timing and amplitude of Ca2+ efflux in response to fatty acid stimulation (STC-1 and GLUTag) (Sidhu et al., 2000), bitter tastants (Chen et al., 2006), and toxins (Zhou & Pestka, 2015). Ca2+ stores are also involved in signal transduction by endogenous signaling molecules, like ATP, UTP (Linan-Rico et al., 2017), and angiotensin II (Pais et al., 2016). For example, circulating angiotensin II sensing by primary L-cells involves IP3 receptors for signal transduction and can act independently of extracellular Ca2+ (Pais et al., 2016). We know much less about intracellular Ca2+ signaling during mechanotransduction. Mechanical stimulation in BON-1 cells involves IP3 receptor-mediated Ca2+ increase (Kim et al., 2001; Hagbom et al., 2011; Linan-Rico et al., 2016). Additionally, in the KRJ-1 cell line and human EC cells, PKA/cAMP pathways are involved in mechanotransduction (Chin et al., 2012). These studies demonstrate that intracellular Ca2+ amplification plays a role in EEC signal transduction, but intracellular stores’ nature and functions in EEC mechanotransduction remain unclear.
We tested the hypothesis that intracellular Ca2+ stores participate in EEC mechanotransduction. We determined the identities of the intracellular Ca2+ receptors involved in regulating mechanosensitive increase in cytoplasmic Ca2+ and 5-HT release. Our results show that EECs express a range of intracellular Ca2+ signaling molecules. Compared to other epithelial cells, EECs are enriched in RyR3 receptors, and RyR3 are critical regulators of the intracellular Ca2+ increase, 5-HT release, and epithelial secretion in response to force.
Methods
Ethical Approval.
All experimental procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC) under protocol A00003261–18-R21. All experimental procedures comply with Journal of Physiology ethical principles and animal ethics checklist.
Drugs.
The dantrolene sodium salt, 2-Aminoethyl dipheylborinate (2-APB), thapsigargin, caffeine, and ondansetron (Sigma- Aldrich) were prepared as 1mM stock solutions. Dantrolene and 2-APB were dissolved in DMSO. Ondansetron and KCl were dissolved in water. Working solutions of dantrolene (10μM), 2-APB (50μM), thapsigargin (10μM), xestospongin C (5μM) ondansetron (10μM), KCl (50mM), GsMTx4 (10μM) and caffeine (2mM) were prepared fresh on the day of the experiments.
Animals.
NeuroD1-Cre mice were a kind gift of Dr. Andrew Leiter (University of Massachusetts, Worcester, MA). Mouse lines obtained from The Jackson Laboratories were: Wild Type C57BL/6J (Jax 000664) and Polr2aTn(pb-CAG-GCaMP5g,-tdTomato)Tvrd/J (Jax 024477). Mice had ad libitum access to food and water while housed. Mice were sacrificed according to ethical guidelines using a fatal dose of rising levels of carbon dioxide with a secondary measure of cervical dislocation.
QGP-1 Cells.
QGP‐1 (passages 17–23) were a kind gift of Dr. Valeria Giandomenico (Uppsala University, Uppsala, Sweden) and were cultured in DMEM (Sigma) with 10% heat-inactivated fetal bovine serum (Invitrogen), 1% penicillin–streptomycin (Invitrogen) and 1% L‐glutamine (Invitrogen). Cells were grown to 50–60% confluence in T25 flasks and transfected with calcium indicators R-GECO (Zhao et al., 2011) (Addgene #32444) and G-CEPIA (Suzuki et al., 2014) (Addgene #58215) (gifts from Dr. Fouad Chebib, Mayo Clinic, Rochester, MN, USA) for 48 hours with Lipofectamine 3000 (Invitrogen). On the night before experiments, cells were lifted with trypsin‐EDTA (0.5%) and plated on MatTek dishes (MatTek Co.) coated with 2.5% (wt/vol) Matrigel (Corning).
Primary Cultures.
Primary murine cultures were prepared from NeuroD1-GCaMP5 (Neurod1Cre/+::Polr2aTn(pb-CAG-GCaMP5g,-tdTomato)Tvrd/J) mice sacrificed at 5–7 weeks following our published culture methods (Strege et al., 2017; Knutson et al., 2018). Briefly, 10cm of the colon was inverted and minced. The tissue was washed 3 times in PBS then digested at 37°C in DMEM (Sigma), 0.1% BSA (Sigma), and 0.6mg/mL Collagenase Type XI (C9407; Sigma). Digestion solution was changed four times over 40 minutes, and the supernatant from digestions 3 and 4 was kept. Supernatants were spun down at 100rcf for 5 minutes. The pellet was re-suspended in DMEM, 5% heat-inactivated FBS (F4135; Sigma), 1% Pen strep (Invitrogen), 1% L-glutamine (Invitrogen) and plated in dishes (MatTek Co.) coated with 5% (wt/vol) Matrigel (Corning). Cells were maintained at 37°C for 24–48 hours (controls) and 48–72 hours for siRNA treatment.
siRNA.
Cultures were transfected with 20nM of Accell Mouse RyR3 siRNA SMARTpool (E-047308-00-0005; Dharmacon) or 20nM of Accell Non-Target control siRNA (D-001910-10-05; Dharmacon) 48–72 hours before the experiments.
Intestinal epithelial single-cell isolation and cell sorting.
Single murine intestinal epithelial cells were harvested from NeuroD1-GCaMP5 mice sacrificed at 8–12 weeks as previously described with modifications (Gracz et al., 2012; Treichel et al., 2021). Murine full-length colon tissue was extracted, flushed, opened lengthwise, and placed in an EDTA (30mM)/ DTT (1.5mM) solution for 20 min on ice. Y-27632, RHO/ROCK pathway inhibitor (72304, 10uM, StemCell Technologies) was supplemented to all enzyme solutions. The tissue was then placed in an EDTA (30mM) solution for 10 min at 37°C. The solution was vigorously pipetted ten times with a transfer pipet and then shook by hand for 1 min. The supernatant was collected, spun at 1000 × g, and resuspended in a DMEM/F12 (Sigma) supplemented with Collagenase XI (0.6mg/mL; Sigma) for 10 min at 37°C. The suspension was then shaken by hand for 1 min, pipetted 25 times with p1000 and p100 pipettes, and spun at 1000 × g. The resulting pellet was resuspended in 1 mL of 1% BSA (Bioworld) HBSS (Gibco) solution supplemented with RNAse inhibitor (2U, Promega) and Y-27632 (10uM). tdTomato+ and negative cells were sorted using fluorescence-activated cell sorting using a FACSAria IIu SORP flow cytometer (BD Biosciences). Cells were doublet discriminated and sorted on a plot of tdTomato versus side scatter. Gating was set using wild-type cells as a gating control. tdTomato positive and negative cells were collected into 1.5 mL Eppendorf tubes pre-filled with Buffer RLT (Qiagen) supplemented with RNAse inhibitor (2U, Promega). Cells were immediately processed to isolate RNA according to the RNeasy Plus Micro Kit (Qiagen).
Gene Expression
qRT-PCR.
RNA was collected from sorted NeuroD1-GCaMP5 cells or siRNA-treated primary cell cultures. RNA reverse transcription was completed using SuperScript VILO cDNA Synthesis Kit (Invitrogen) and a PCR of 10 min at 25 °C, a 60 min cycle at 42 °C, and 5 min at 85 °C. For RyR3 siRNA knockdown analysis, cDNA was diluted and analyzed for GAPDH, Bactin, Tph1, Chga Ryr3, and Piezo2 (Qiagen). NeuroD1-GCaMP5 cell cDNA was analyzed for GAPDH, HPRT, Piezo2, Vil1, Tph1, Chga, Ryr1, Ryr2, Ryr3, Itpr1, Itpr2, Itpr3 (Table 1). All experiments were done according to LightCycler 480 SYBR Green I Master (Roche) instructions.
Table 1:
Mus musculus RT-qPCR Primers Used in This Study
| Gen Bank Number | |||||
|---|---|---|---|---|---|
| Beta Actin | - | - | 154 | Qiagen: PPM02945B-200 | NM_007393 |
| Hprt | TGGATACAGGCCAGACTTTGTT | CAGATTCAACTTGCGCTCATC | 124 | (Wang et al., 2010) | NM_013556 |
| tdTomato | ACATCCCCGATTACAAGAAGC | TTGTAGATCAGCGTGCCGTC | 130 | Lukas Valihrach, The Czech Academy of Sciences | AY678269.1 |
| Vil1 | TCAAAGGCTCTCTCAACATCAC | AGCAGTCACCATCGAAGAAGC | 114 | Joyce Li, University of Massachusetts | |
| Chga | - | - | 161 | Qiagen: PPM03709A-200 | NM_007693 |
| Tph1 | - | - | 160 | Qiagen: PPM34957A-200 | NM_009414 |
| Ryr1 | AAGTCCCACAACTTTAAGCG | TCTTCTTGGTGCGTTCCTG | 151 | (Kurokawa et al., 2013) | NM_009109.2 |
| Ryr2 | AGCTTGAAAGACACCGAGGA | TAGAGAGCCATCTGCCACCT | 104 | (Kurokawa et al., 2013) | NM_023868.2 |
| Ryr3 | ACCAGCAGGAGCAAGTACG | GGGGTCGTGTCAAAGTAGTCA | 82 | (Martinez et al., 2016) | XM_017316712.2 |
| Itpr1 | - | - | 61 | Qiagen: PPM34085F-200 | NM_010585 |
| Itpr2 | CCTGACGGTGAACAAGAGGT | GCATTCACATTATCACCCTCGC | 141 | (Sankar et al., 2014) | NM_010586.2 |
| Itpr3 | GCAACCACATCTGGACGCTCTT | AGAAGGCACTGATGGTGTCCAG | 138 | Origene | NM_080553 |
Immunohistochemistry.
Cross-sectional of mouse colon from NeuroD1-GCaMP5 were fixed in 4% PFA-PB overnight at 4°. The following day tissue was transferred to 15% sucrose for 2hr, then 30% sucrose (Sigma) overnight at 4°. The next day tissue was frozen into blocks in the OCT compound (Sakura Finetek). Tissues were frozen at −80°C until ready to section into 20-micron sections. Cryosectioned tissues were directly mounted on slides (Cardinal Health Superfrost Plus, M6146-PLUS), washed in PBS × 10min, blocked with PBS based solution of 2% BSA/0.5% Triton-X/5% Normal Donkey Serum (NDS) for 1hr, then transferred to primary (Anti-TdTomato, Origene, AB8181–200, 1:600) solution of 2% BSA/0.3% Triton-X/2% NDS overnight. The next day, following 3 × 10min PBS washes, tissue sections were incubated in secondary (Cy3, Jackson Immuno, 705-165-147, 1:300 stored in glycerol) solution of 2% BSA/0.3% Triton-X/2% NDS. Slides were coverslipped in slowfade gold with 4′,6-diamidino-2-phenylindole (DAPI, Life Technologies) mounting buffer. All staining occurred at room temperature in a humidified chamber.
Calcium Imaging.
Calcium imaging experiments were done as before (Alcaino et al., 2018; Treichel et al., 2021). NeuroD1-GCaMP5 cultures and transfected QGP-1 cells were visualized on an inverted Olympus IX70 epifluorescence microscope (Olympus) and imaged with a 16-bit high-speed camera (ORCA-Flash4.0, Hamamatsu), a CoolLED pE-300Ultra illumination system (CoolLED limited, UK), and Metamorph Software (Molecular Devices) for acquisition, driven by pCLAMP 10 (Molecular Devices). Bath solution: 150 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM glucose, and 10 mM Hepes, pH 7.3 (adjusted with NaOH). EECs were identified by tdTomato fluorescence [ex/em 554/581 nm] and studied functionally using GCaMP5 [ex/em 480–505/525 nm]. MetaMorph and pClamp10.6 software (Molecular Devices) were used to acquire GCaMP5 fluorescence, and images were captured at a frame rate of 5 Hz. Mechanical stimulation was delivered using a piezoelectric-driven fire-polished glass probe (1–3-μm indentation, 50-ms duration) driven by a piezotransducer P-621.1CD attached to an E-625.CR controller (PI, Physik Instrumente) as described previously (Alcaino et al., 2018; Treichel et al., 2021). Previous work in our lab has shown ~60% of primary colonic EECs are responsive to mechanical stimuli (Alcaino et al., 2018). Cells were selected for small molecule experiments only if they had a robust response to initial mechanical stimulation (> 50% GCamP5 fluoresce increase from baseline). Fresh bath solution with inhibitors was perfused in the dish for 5–10 minutes prior to recording, and high KCl (50mM) was used as a positive control at the end of experiments. All experiments were performed at room temperature (25°C). Analysis of imaging data was performed within MetaMorph. Graphing and statistical analysis were performed in Prism 8 (GraphPad).
5-HT Biosensor assay.
HEK293 cells were co-transfected using CaCl2 with high-conductance non-desensitizing 5-HT3R (gift from Dr. Cecilia Bouzat, Instituto de Investigaciones Bioquímicas de Bahía Blanca, CONICET Bahía Blanca, Argentina) and GCaMP5G (1 μg/μL; Addgene #31788). HEK293 cells were cultured with primary EEC cells for 24 hours before the experiments (Alcaino et al., 2018). Calcium imaging acquisition and experimental conditions were described in the previous section. Only fields of view containing both transfected HEK293 cells and EECs were imaged. HEK293 cells respond specifically to 5-HT release with a diffusion limited increase in intracellular calcium which was quantified in the same way as EEC calcium increase. A caveat of this approach is that the fluorescence response is dependent on the distance between the EEC and biosensor cell.
Ussing Chamber.
Ussing chamber was setup as previously described (Wang et al., 2017; Alcaino et al., 2018; Treichel et al., 2021). Solutions. The Krebs-Ringer solution consisted of: 120 mM NaCl, 5.9 mM KCl, 15 mM NaH2CO3, 1.6 mM NaH2PO4, 1.3 mM CaCl2, 2.4 mM MgCl2, pH = 7.4, gassed with 95/5 mixture of O2/CO2. Glucose (10 mM) was added to the serosa bath, and mannitol (10 mM) was added to the mucosa bath to maintain osmotic balance. Preparation. 5–12 week old mice were sacrificed then 4 cm segments of the proximal colon were removed and bathed in ice-cold Krebs Ringer solution. Tissue was cut along the mesenteric border, and luminal contents were gently removed. Tissue was cut into 2-cm segments, and a cross-sectional area of 0.3 cm2 was mounted in 4-mL Ussing chambers (Physiologic Instruments). Upon mounting, the mucosal surface was bathed in 4mL of mannitol Krebs-Ringer solution, and the serosal surface was bathed in glucose Krebs-Ringer solution. Solutions and tissue were maintained at 37°C during the experiment. The transepithelial potential difference was measured using paired Ag-AgCl electrodes via 3% Agar with 3-M KCl bridges and clamped at 0 mV by another pair of Ag-AgCl electrodes. Tissue was equilibrated to attain stable basal short-circuit current (Isc) and tissue conductance (Gt) for 30 min before experiments. Thirty mmHg × 10-s pressure stimuli were applied from rest (atmospheric pressure) using a DPM-1 pneumatic transducer (Bio-Tek Instruments) in a sealed mucosal chamber. KCl (50 mM) was applied to the mucosal side at the end of the experiment as a positive control and to ensure tissue viability.
Data analysis.
For immunohistochemistry, a Nikon Ti2 AXR confocal was used to take single-plane images at a magnification of 100x. Images were processed using denoise.ai and background subtraction to reduce noise and correct imbalanced illumination. For calcium imaging, raw data were exported into MS Excel, background fluorescence was subtracted, and averaged fluorescence intensity over time was converted to ΔF/F0 = (F − F0)/F0, where F0 is the baseline fluorescence immediately before stimulation. Ussing data were recorded and analyzed using Acquire and Analyze 2.3 (Physiologic Instruments). Pressure-induced peak short-circuit current (Isc) was measured ΔIsc = Isc peak – Isc baseline.
Statistics.
Statistical significance was assigned for p<0.05 (*) using paired or unpaired two-tailed t-test, or one-way ANOVA as stated in the text. Statistical analyses, outlier identification, and graphs were generated using GraphPad Prism 7.02 (GraphPad Software).
Results
Mechanical stimulation of an EEC model leads to Ca2+ release from the endoplasmic reticulum (ER).
We wanted to know whether Ca2+ stores play a functional role in EEC mechanotransduction. To do so, we examined both cytoplasmic and ER Ca2+ during mechanical stimulation. QGP-1 cells are an established human EEC model which expresses EEC receptors (Kojima et al., 2014; Schulze et al., 2014), release 5-HT (Doihara et al., 2009), and are mechanosensitive due to the expression of the mechanosensitive ion channel Piezo2 (Alcaino et al., 2017; Wang et al., 2017). We transfected QGP-1 cells with the previous verified cytoplasmic (R-GECO) (Zhao et al., 2011) and ER (CEPIA-ER or G-CEPIA) (Suzuki et al., 2014) Ca2+ reporters and used them to study both compartments in the same cell (Fig. 1A). Partial localization of G-CEPIA to the ER stores in QGP-1 cells was verified by immunofluorescence staining using Calnexin, however we did see additional signal from G-GEPIA that could be from localization to the secretory granules in EECs. We then mechanically stimulated single QGP-1 cells using a finely polished glass probe for 50 ms (Wang et al., 2017; Alcaino et al., 2018). We found an increase in peak cytoplasmic Ca2+ response (R-GECO ΔF/F0 = 4.4±2.2, n=7) and a decrease in ER Ca2+ (CEPIA-ER ΔF/F0 = −0.54±0.22, n=7 *P=0.0011, paired t test) (Fig. 1B–C). The cytoplasmic response (Fig. 1D) peaked within seconds (time to peak = 5.1±3.2, n=7)(Fig. 1D–E) and persisted for nearly one minute (τ=56±20s, n=7) (not shown). The same stimulation led to a decrease in ER Ca2+ with a slower onset (time to peak 220±96s, n=7, *P<0.0001, paired t test)(Fig. 1D–E) These results in the EEC cell model suggest that intracellular Ca2+ stores contribute to the prolonged increase in cytoplasmic Ca2+ following mechanical stimulation.
Figure 1. EEC cell model (QGP-1) responds to mechanical stimulation with a rapid increase in cytoplasmic Ca2+ and concurrent slower decrease in ER Ca2+.
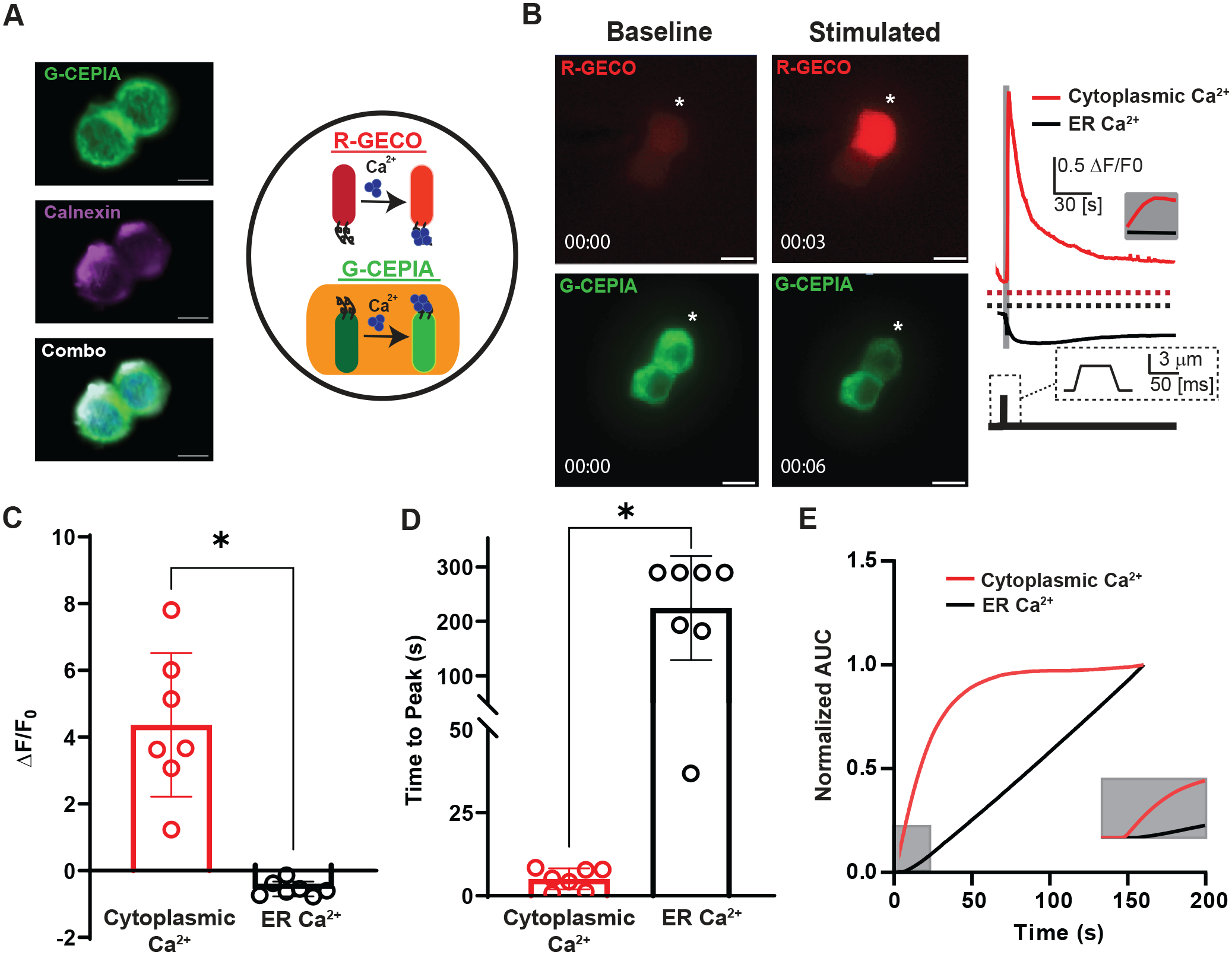
A) Left panel shows confocal images of QGP-1 cell transfected with G-CEPIA (green), stained with Calnexin (magenta), and combined together with Dapi (blue) to show partial overlap G-CEPIA to ER stores [Scale bar: 10 μm]. Right panel depicts a cartoon showing experimental design of a cell with dual genetically encoded Ca2+ indicators in the cytoplasm (R-GECO, red) and the ER (G-CEPIA, green) for concurrent Ca2+ reporting.
B) Left panel shows two QGP-1 cells transfected with R-GECO (red) and G-CEPIA (green) prior to mechanical stimulation by 50 ms pulse of 3 um membrane displacement. Post-stimulation images taken 3 or 6 seconds following displacement. * denotes cell being displaced. [Scale bar: 10 μm]. Right panel shows representative fluorescence responses (ΔF/F0) of mechanically stimulated QGP-1 cell in R-GECO (cytoplasmic Ca2+, red) and G-CEPIA (ER Ca2+, black). Grey bar and inset show the rapid increase in cytoplasmic Ca2+.
C) Individual (circles) and mean ± SD (bars) data for peak fluorescence responses reported increase in cytoplasmic Ca2+ (red, ΔF/F0 = 4.4±2.2, n=7) and decrease in ER Ca2+ (black, ΔF/F0 = −0.54±0.22, n=7)(*P=0.0011, paired t test)
D) Individual (circles) and mean ± SD (bars) data for time to peak for cytoplasmic Ca2+ (red, ΔF/F0 = 5.1±3.2 s, n=7) and ER Ca2+ (black, ΔF/F0 = 220±96 s, n=7)(*P<0.0001, paired t test)
E) Normalized area under the curve (AUC) showing kinetics of Ca2+ changes in the stores and the cytoplasm. The compartments are functioning under different time scales. Grey inset showing first 25 seconds after membrane displacement.
Intracellular Ca2+ store receptors in murine EECs.
We then asked whether Ca2+ store receptors are enriched in primary EECs. To isolate EECs, we used a validated mouse model (Alcaino et al., 2018) of NeuroD1-tdTomato/GCaMP5 mice (NeuroD1cre/+::Polr2apb-CAG-GCaMP5g,-tdTomato/+). In this model, all EECs are lineage traced using NeuroD1, a late transcription factor involved in EEC differentiation (Fig. 2A) (Haber et al., 2017; Billing et al., 2019). We used fluorescence-activated cell sorting (FACS) to collect populations of tdTomato+ (NeuroD1) and tdTomato− cells from dissociated colon epithelium, separating these cells from debris and eliminating doublets (Fig. 2B). FACS analysis showed that NeuroD1+ cells made up 0.4 to 1.6%, averaging 0.95±0.52% (n=6, later pooled together) of all epithelial cells (Fig. 2B). We collected 10,000 tdTomato+ cells per mouse and pooled together collections from two mice for a total of 20,000 cells per run. We then isolated RNA from each group of 20,000 cells and used RT-qPCR to compare both cell populations. We found that when compared to tdTomato− cells, the tdTomato+ population was indeed epithelial (Vil1 expressed by both fractions, 1.0±0.12-fold, n=3, P=0.990, unpaired t test), highly enriched for tdTomato (140±29-fold, n=3, *P=0.000559, unpaired t test), and the pan-EEC marker chromogranin A (Chga, 97±28-fold, n=3, *P<0.0001, unpaired t test) (Fig. 2C). Since the largest subpopulation of EECs are enterochromaffin (EC) cells, we also checked and found that the EC cell marker, tryptophan hydroxylase 1 (Tph1), the rate-limiting enzyme to produce serotonin (5-hydroxytryptamine, 5-HT) was enriched (200±47-fold, n=3, *P=0.000231, unpaired t test) (Fig. 2C). After validating that our tdTomato+ were composed of EECs, we checked for the classic intracellular Ca2+ store receptors: ryanodine (Ryr1-3) and IP3 (Itpr1-3) receptors. We found that both populations expressed select Ca2+ receptor subtypes, most notably of Ryr3 and Itpr3 (Fig. 2D and 2E), but when compared to EEo− population, the EEC+ population was significantly enriched in ryanodine receptor 3 (Ryr3) (32±25-fold, n=3,*P=0.00847, unpaired t test) and less so in Itpr3 (1.1±0.26-fold, n=3, P=0.921, unpaired t test).
Figure 2. EECs express select intracellular Ca2+ store receptors.
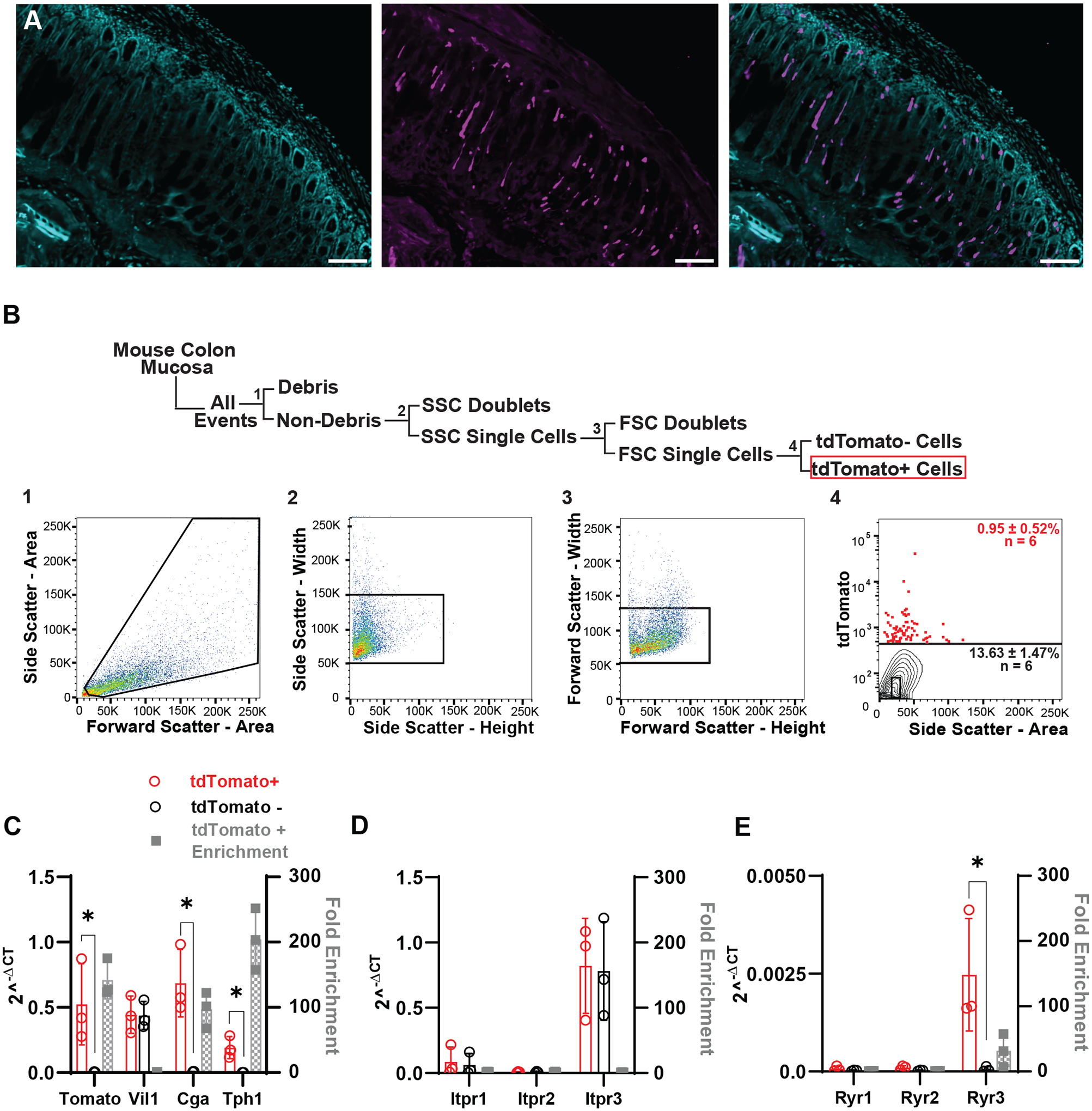
A) Confocal images of a NeuroD1-GCaMP5 colon cross-section using NeuroD1 Cre to drive tdTomato signal in lineage traced EEC (magenta) and nuclei counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (cyan). [Scale bar: 100 μm].
B) Schematic depicting fluorescence activated cell sorting (FACS) gating strategies and representative dot plots for dissociated cells from NeuroD1-GCaMP5 colonic mucosa. Cells were gated on forward and side light scatter to remove debris (1), and doublets (2,3), and gated on tdTomato fluorescence (4) to select tomato+ cells. tdTomato gating was based on unstained controls. After gating out debris and doublets, the average tomato+ cell density was 0.95±0.52% (n=6, 10,000 cells per run).
C) RNA expression from FACS sorted tdTomato+ cells were compared to the tdTomato− cells using RT-qPCR. Mean ± SD reported for 2−ΔCt values for tomato+ (red circles) and tomato− (black circles) correspond to the left y-axis, and fold enrichment (2−ΔΔCt, gray squares) comparing the two populations correspond to the right y-axis. tdTomato+ cells were enriched for tdTomato (140±29-fold, n=3, *P=0.000559, unpaired t test) and EEC markers, Chga (97±28-fold, n=3, *P<0.0001, unpaired t test) and Tph1 (200±47-fold, n=3, *P=0.000231, unpaired t test). Both cell populations had equal amounts of epithelial cells (Vil1, 1.0±0.12-fold, n=3, P=0.990, unpaired t test). Asterisk (*) used to denote P <0.05.
D) Mean ± SD of tdTomato+ and tdTomato− cells that were analyzed for IP3 receptors. qPCR analysis showed expression of all receptors in both populations, but tdTomato+ cells were not found to be enriched for any particular Itpr1-3 isoform. (Itpr1: 1.7±0.37-fold, n=3; Itpr2: 1.4±0.65-fold, n=3; Itpr3:1.07±0.26-fold, n=3)(P= 0.741, 0.709, 0.921, unpaired t test).
E) Mean ± SD showing tdTomato+ and tdTomato− cells that were analyzed for ryanodine receptors. tdTomato+ were found to only be enriched for the RyR3 isoform. (Ryr1: 0.86±0.25-fold, n=3; Ryr2: 0.86±0.25-fold, n=3; Ryr3: 32±25-fold, n=3)(P=0.250, 0.0810, 0.00847, unpaired t test). Asterisk (*) used to denote P <0.05.
Intracellular Ca2+ release from ER is essential for primary EEC mechanotransduction.
We wanted to determine whether intracellular Ca2+ stores play a functional role in primary EEC mechanotransduction. NeuroD1-GCaMP5 primary cultures (Knutson et al., 2018) allowed us to identify EECs as tdTomato+/GCaMP5+ cells (Fig. 3A) (Alcaino et al., 2018). We used a pharmacological approach to dissect the identities of the stores involved in EEC mechanotransduction (Fig. 3B). Similarly to our previous work, we found that under control conditions 66.6% (4/6) of primary colonic EECs were responsive to mechanical stimulation (Alcaino et al., 2018) (Fig. 3C). We selected cells with a robust response to initial stimulation for small molecule experiments. The sarco/endoplasmic reticulum ATPase (SERCA) is a Ca2+-ATPase that transfers Ca2+ from the cytosol to the ER lumen. Hence, SERCA inhibition by thapsigargin (10 μM) is a common approach to empty the intracellular Ca2+ stores (Acsai et al., 2011), and we found that thapsigargin diminished EEC response to direct mechanical stimulation by 62±40% (ΔF/F0 3.1±1.4 vs. 1.2±1.5, n=6 cells from 4 cultures, *P= 0.0379, paired t test) (Fig. 3D and F). We used IP3 receptor blocker 2-APB (50 μM) and found that it decreased the mechanosensitive Ca2+ response by 79±39% (ΔF/F0 3.7±1.5 vs. 0.48±0.81, n=8 cells from 3 cultures, *P= 0.0035, paired t test) (Fig. 3D and F). Although 2-APB is a blocker of IP3 receptors, it has been reported to have off-target effects on other ion channels (Xu et al., 2005), so we applied a more specific IP3 receptor blocker, Xestospongin C, to verify our results. Consistent with our 2-APB data, initial studies done with the application of Xestospongin C (5 μM) resulted in a significant decrease in the mechanically induced calcium increase by 65±29% (ΔF/F0 4.2±1.4 vs. 1.4±0.88, n=6 cells from 1 culture, *P= 0.0096, paired t test) (Fig. 3D and F). These data show that Ca2+ stores and specifically IP3 sensitive Ca2+ stores play a role in primary EEC mechanotransduction.
Figure 3. Mechanically induced primary EEC intracellular Ca2+ changes are modified by an agonist and blockers of intracellular Ca2+ receptors.
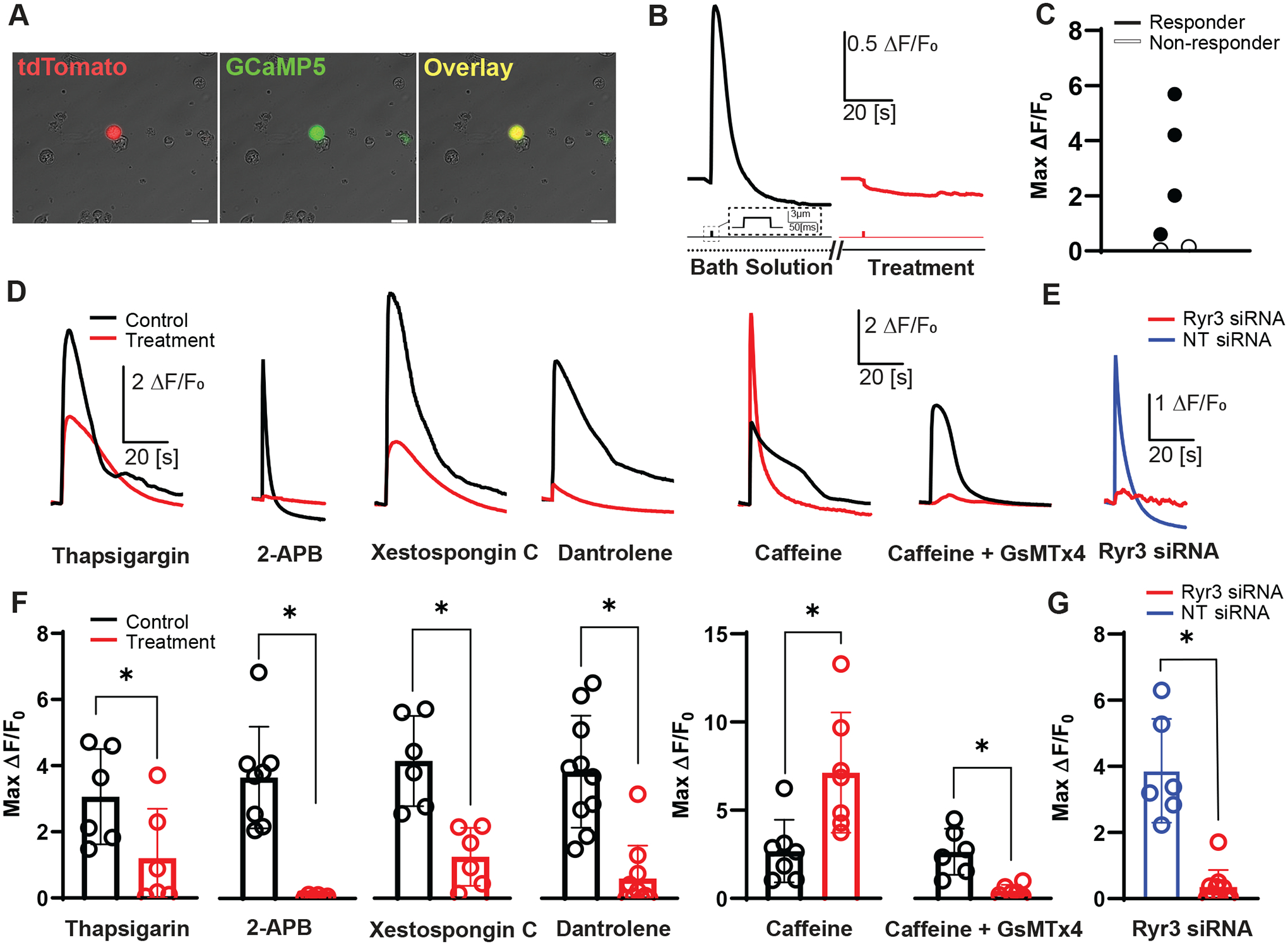
A) Images of a primary colonic EEC culture dissociated from a NeuroD1-GCaMP5 mouse. EECs from model express both tdTomato (red) and GCaMP5 (green) in same cell (yellow). [Scale bar: 20 μm].
B) Representative traces of mechanically induced intracellular calcium changes (ΔF/F0) before (black) and during treatment (red) with a putative intracellular Ca2+ receptor blocker [Scale bar: 0.5 ΔF/F0, 20 s]. Corresponding mechanical stimulation period shown in the line below trace [Scale bar: 3 μm, 50 ms]. Bath solution or treatment duration is depicted in line under mechanical stimulation. Double slashed lines represent gaps in timeline depicting a drug incubation (5–10 min).
C) Peak intracellular calcium responses (ΔF/F0) of individual cells (circles) depicting typical range of responsive (black filled) and non-responsive (open) EECs after mechanical stimulation during calcium imaging. 4/6 cells qualified as responsive to mechanical stimulation (ΔF/F0 ≥ 0.5).
D) Representative traces of GCaMP5 fluorescence changes showing paired primary EEC responses to a single brief mechanical stimulation by membrane displacement (3 μm, 50 ms duration) for control (black), with pharmacologic modulators (red): 10 μM thapsigargin, 50 μM 2-APB, 5 μM Xestospongin C, 10 μM dantrolene, 2 mM caffeine or 10 μM GsMTx4. [Scale bars: 2ΔF/F0, 20 s]
E) Representative traces of EECs mechanically stimulated after transfection with 20 nM RyR3 siRNA (red), or 20 nM non-target (NT) siRNA (blue). [Scale bars: 1ΔF/F0, 20 s]
F) Peak intracellular calcium responses (ΔF/F0) of individual cells (circles) and mean ± SD (bars) showing primary EEC intracellular Ca2+ changes in response to mechanical stimulation (3 μm, 50 ms duration) during paired experiments with: Thapsigargin (ΔF/F0 = 3.1±1.4 vs. 1.2±1.5, n=6 cells)(*P=0.0379, paired t test), 2-APB (ΔF/F0 = 3.7±1.5 vs. 0.48±0.81, n=8 cells)(*P=0.0035, paired t test), Xestospongin C (ΔF/F0 = 4.2±1.4 vs. 1.4±0.88, n=6 cells)(*P=0.0096, paired t test), Dantrolene (ΔF/F0 = 3.8±1.7 vs. 0.60±0.99, n=10 cells)(*P<0.0001, paired t test), Caffeine (ΔF/F0 = 2.7±1.8 vs. 7.1±3.4, n=7 cells)(*P=0.0276, paired t test), and Caffeine with GsMTx4 (ΔF/F0 = 2.6±1.3 vs. 0.45±0.32, n=6 cells)(*P=0.0079, paired t test). Asterisk (*) used to denote P <0.05.
G) Peak intracellular calcium responses (ΔF/F0) of individual cells (circles) and mean ± SD (bars) showing primary EEC intracellular Ca2+ changes in response to mechanical stimulation (3 μm, 50 ms duration) after RyR3 siRNA and NT siRNA (ΔF/F0 = 3.9±1.6 (NT) vs. 0.35±0.51 (Ryr3 siRNA), n=6 and 10 cells)(*P<0.0001, unpaired t test). Asterisk (*) used to denote P <0.05.
Our expression data point to RyR3 being enriched in EECs compared to other epithelial cells, making RyR3 potentially an exciting target for EEC modulation. We first used the RyR1/3 blocker, dantrolene (10 μM), since there is no selective RyR3 blocker, and RyR1 did not express in EECs (Fig. 2E). We found a significant decrease in the EEC intracellular Ca2+ increase in response to mechanical stimulation by 89±15% (ΔF/F0 3.8± 1.7 vs. 0.60±0.99, n=10 cells from 4 cultures, *P<0.0001, paired t test) (Fig. 3D and F). Caffeine, which at 2mM sensitizes the ryanodine receptors (Herrmann-Frank et al., 1999), increased the mechanosensitive Ca2+ response by 250±206% (ΔF/F0 2.7±1.8 vs. 7.1±3.4, n=7 cells from 2 cultures, *P=0.0276, paired t test) (Fig. 3D and F), suggesting that ryanodine receptors have a functional role in EEC mechanotransduction. We then wanted to validate a direct link between Piezo2 activation and CICR. We once again sensitized ryanodine receptors through caffeine, but did so in the presence of the specific Piezo2 channel blocker, GsMTx4 (Alcaino et al., 2018). Application of GsMTx4 almost completely abolished the caffeine driven increase in the mechanically induced calcium response with a 81±15% decrease, suggesting again that ryanodine’s role in EEC mechanotransduction is dependent on Piezo2 (ΔF/F0 2.6±1.3 vs. 0.45±0.32, n=6 cells from 1 culture, *P=0.0079, paired t test) (Fig. 3D and F). We pursued the RyR3 role using genetic knockdown. RyR3 siRNA decreased RyR3 mRNA by 60±24% (n=4 cultures treated with NT and Ryr3 siRNA). We found that compared to non-targeted siRNA, RyR3 siRNA substantially decreased the mechanosensitive Ca2+ responses by 88±23% (ΔF/F0 3.9±1.6 NT siRNA vs. 0.35±0.51 RyR3 siRNA, n=6 cells and 10 cells, from 2 and 3 cultures respectively, *P<0.0001, unpaired t test) (Fig. 3E and G). These data point to intracellular Ca2+ stores’ role in EEC mechanotransduction, and RyR3 stands alone as a selectively enriched receptor in EECs compared to other epithelial cells.
RyR3 is necessary for 5-HT release in response to mechanical stimulation.
Next, we focused on RyR3 and asked if it contributes to the mechanically induced release of 5-HT from EECs using biosensors as we (Alcaino et al., 2018) and others did (Bellono et al., 2017) previously. We co-cultured primary EECs with 5-HT biosensors – HEK-293 cells transfected with GCaMP5 and non-desensitizing 5-HT3R (Corradi et al., 2009)(Fig. 4A). Each dish of primary EECs was either treated with Ryr3 siRNA, non-targeted siRNA, the 5-HT3R blocker, ondansetron, or left as a control. Membrane displacement of control, NT siRNA, or ondansetron treated EECs resulted in a Ca2+ increase in primary EECs (Control ΔF/F0=4.8±2.5, NT siRNA ΔF/F0=3.1±1.8, Ondansetron ΔF/F0=3.1±1.6, n=9 cells from 2 cultures, 14 cells from 4 cultures, and 6 cells from 3 cultures) (Fig. 4B top traces and C). In the Ryr3 siRNA treated cells, mechanically evoked responses were significantly decreased compared to control and NT siRNA treated EECs (ΔF/F0=0.72±0.94, n=9 cells from 3 cultures, *P=0.0001 (control), one-way ANOVA with Dunnett’s post test) (Fig. 4B and C). We then wanted to test if membrane displacement of EECs resulted in the release of 5-HT and if this response could be blocked through the inhibition of RyR3. Our HEK-293 5-HT biosensors were able to detect 5-HT release from neighboring EECs, as EEC membrane displacement resulted in an equal Ca2+ increase in biosensors from both dishes that contained our control and NT siRNA EECs (control biosensor ΔF/F0=7.3±6.6, NT siRNA biosensor ΔF/F0=3.4±3.5, n=9 cells and 12 cells, from 2 and 4 cultures respectively, P=0.0966, one-way ANOVA with Dunnett’s post test) (Fig. 4B bottom trace and D). Application of ondansetron inhibited our 5-HT derived responses by 99.6±2.8% compared to our control cells (Biosensor ΔF/F0=0.029±0.028, n=5 cells from 2 cultures, *P=0.0091, one-way ANOVA with Dunnett’s post test) (Fig. 4B and D). Mechanical displacement of RyR3 knockdown EECs resulted in an 98±25% decrease in the 5-HT biosensor cell response compared to control cells (siRNA ΔF/F0=0.15±0.25, n=7 cells from 3 cultures, *P=0.0044, one-way ANOVA with Dunnett’s post test) (Fig. 4D). These data show that RyR3 is important for mechanosensitive 5-HT release from EECs.
Figure 4. Genetic RyR3 knockdown inhibits primary EEC cytoplasmic Ca2+ increase in response to mechanical stimulation and inhibits 5-HT release.
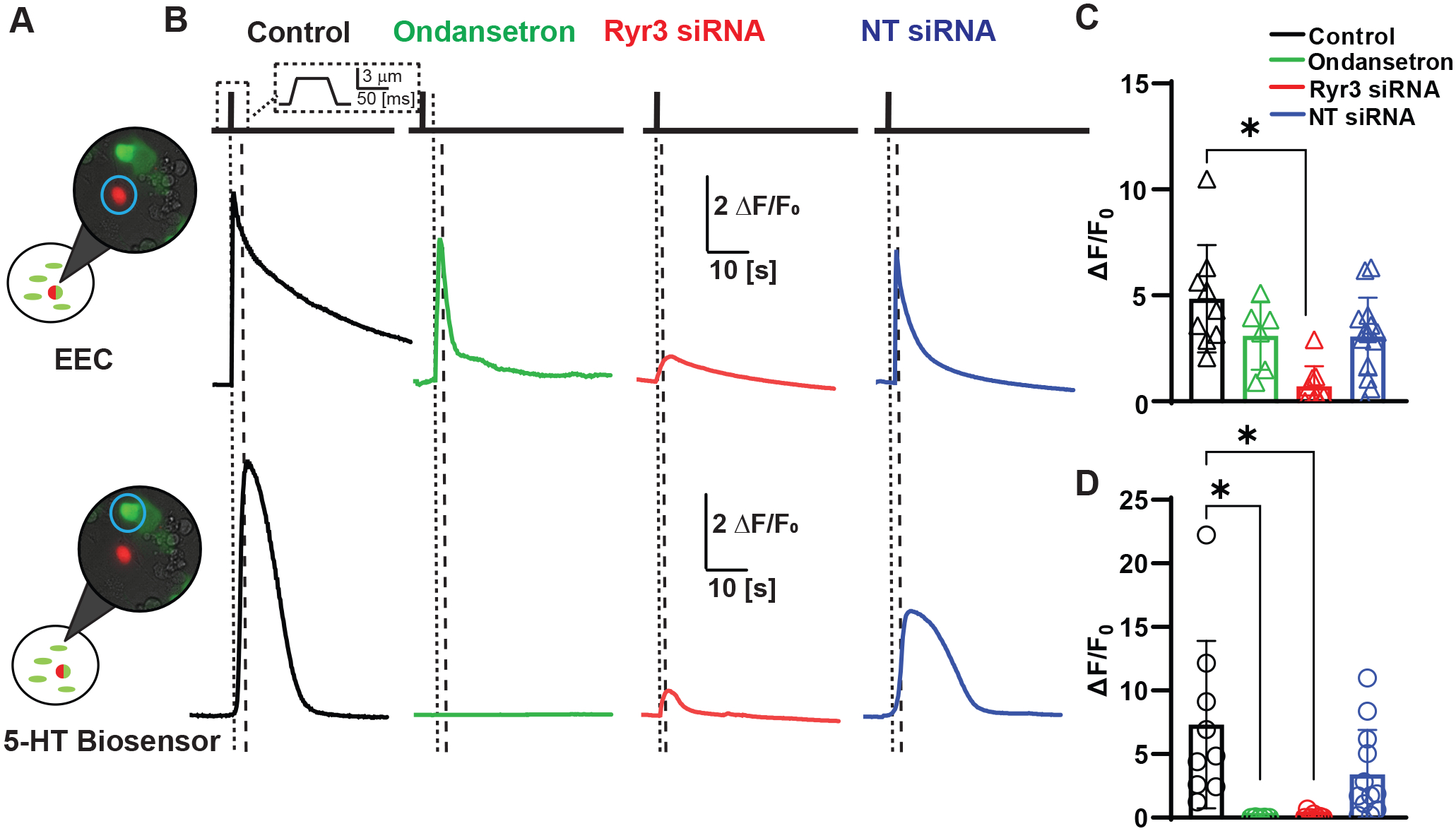
A) 5-HT biosensor experimental set-up showing overlays of tdTomato/DIC/GCaMP5 images with an EEC (red/green) and Ca2+ activated HEK 5HT3R biosensors (green).
B) Representative fluorescence traces of GCaMP5 fluorescence changes for mechanical stimulation (3 μm, 50 ms) of NeuroD1-GCaMP5 cells (top) and HEK293 biosensors transfected with 5HT3R and GCaMP5 (bottom) in control (black), 5HT3R blocked (Ondansetron, green), RyR3 siRNA treated (red), and non-targeting (NT) siRNA treated (blue) conditions. Vertical dotted lines represent stimulation of EEC with poker, thicker dotted lines mark the start of biosensor sensing 5-HT.
C) Peak intracellular calcium responses (ΔF/F0) of individual cells (circles) and mean ± SD (bars) for EECs showing block of intracellular Ca2+ changes by RyR3 siRNA (red), but not NT siRNA (blue) or ondansetron (green) when compared to control (black) conditions. (Control ΔF/F0 = 4.8±2.5, n=9 cells vs. NT siRNA = ΔF/F0 3.1±1.8, Ondansetron ΔF/F0 = 3.1±1.6, and Ryr3 siRNA ΔF/F0 = 0.72±0.94, n=14, 6 and 9 cells)(P= 0.0745 (NT siRNA), 0.1865 (Ondansetron), and 0.0001 (Ryr3 siRNA), one-way ANOVA with Dunnett’s post-test). Asterisk (*) used to denote P <0.05.
D) Peak ΔF/F0 responses in individual cells (circles) and mean ± SD (bars) data for biosensor cells showing significant decrease in 5-HT release in EEC treated with RyR3 siRNA and ondansetron when compared to control. (Control ΔF/F0 = 7.3±6.6, n=9 cells vs. NT siRNA = ΔF/F0 3.4±3.5, Ondansetron ΔF/F0 = 0.029±0.028, and Ryr3 siRNA ΔF/F0 = 0.15±0.25, n=12, 5 and 7 cells)(P= 0.0966 (NT siRNA), 0.0091 (Ondansetron), and 0.0044 (Ryr3 siRNA), one-way ANOVA with Dunnett’s post-test). Asterisk (*) used to denote P <0.05.
RyR3 contributes to colonic pressure-induced epithelial secretion.
Mechanical activation of EECs contributes to mechanically induced epithelial fluid secretion (Wang et al., 2017; Alcaino et al., 2018). To test whether RyR3 contributes to this process, we used a custom Ussing chamber setup with an integrated pressure-clamp on the epithelial side (Fig. 5A). We measured pressure-induced epithelial short circuit current (Isc), a correlate of epithelial secretion (Fig. 5A and 5B) (Alcaino et al., 2018). We used the proximal colon and found a robust pressure-induced Isc (Fig. 5C). After epithelial incubation with dantrolene (20 μM), ΔIsc for pressure-induced secretions decreased by 44±14% (Control ΔIsc 27±13 vs Dantrolene ΔIsc 15±6.6, n=6 from 6 mice, *P=0.01, paired t test), and was reversible after washout and application of KCl (KCl ΔIsc 20±10) (Fig. 5C and D). Incubation with vehicle (DMSO) did not significantly affect ΔIsc (control ΔIsc from 24±14 vs DMSO ΔIsc 30±27, n=5 from 5 mice, P=0.421, paired t test) (Fig. 5E and 5F). These data show that EEC RyR3 receptors contribute to mechanically induced epithelial fluid secretion.
Figure 5. Colonic epithelial pressure induced mucosal Isc is inhibited by RyR1/RyR3 blocker dantrolene.
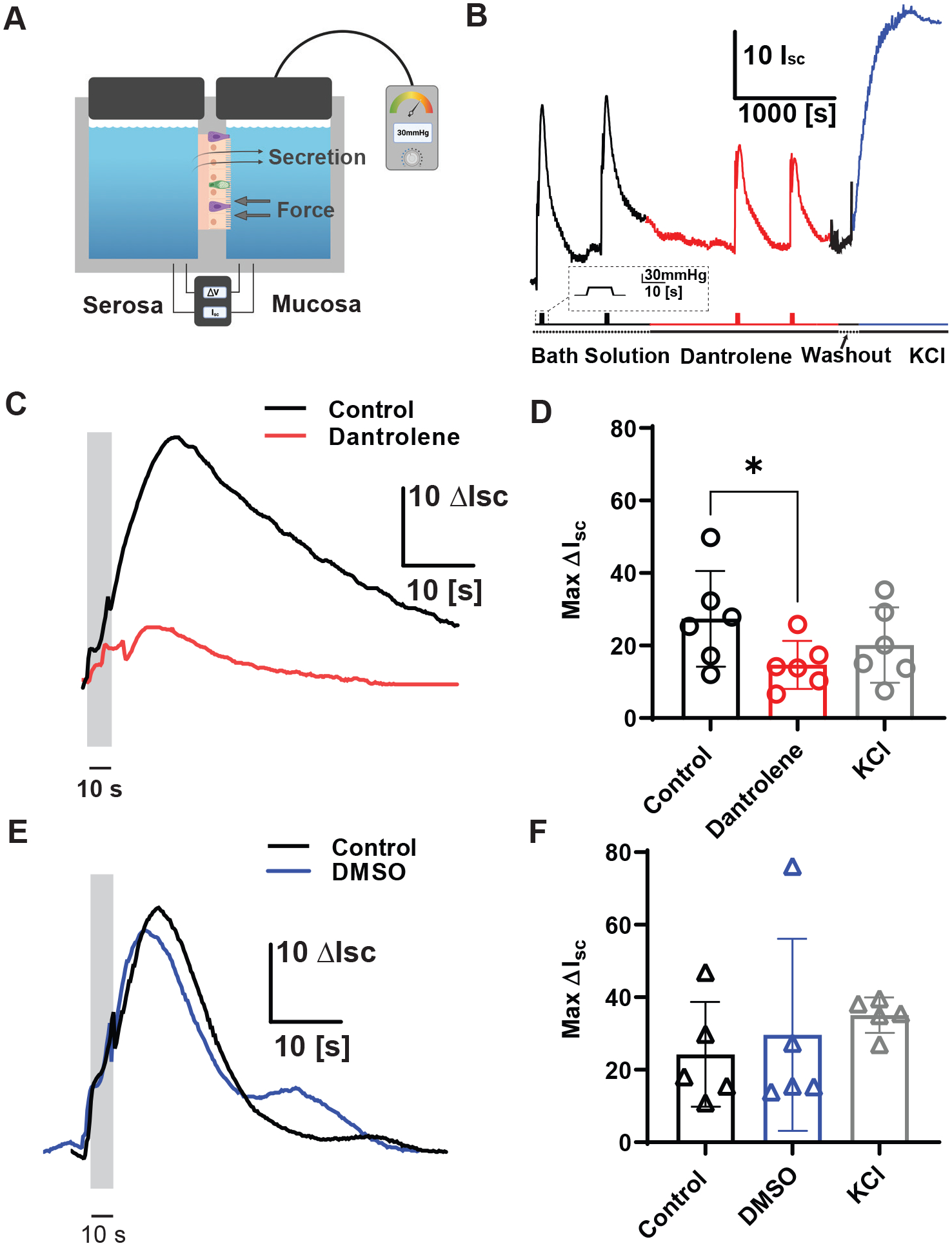
A) Cartoon showing experimental setup of mucosal pressure-clamped Ussing chamber with whole-thickness colon prep. Thick gray arrows represent force applied to mucosal tissue and thin black arrows represent ΔIsc as a measurement of secretion.
B) Typical experimental setup of pressurized Ussing experiments and representative traces of mechanically induced changes in short circuit current (Isc) before (black), during dantrolene treatment (red), washout (black) and during KCl (blue) treatment [Scale bar: 10Isc, 1000s]. Corresponding mechanical stimulation period shown in the line below trace [Scale bar: 30 mmHg, 10 s]. Bath solution (dotted line) or treatment (solid line) duration is depicted in line under mechanical stimulation.
C) Representative short circuit current (Isc) traces showing transient pressure application (30 mmHg, 10s) control response (black) and 20μM dantrolene in the same prep (red). [Scale bar: 10 Isc, 10s].
D) Individual (circles) and mean ± SD (bars) showing short-circuit current (Isc) in response to pressure (ΔP) steps in control, then dantrolene and 50mM KCl controls. Upon mechanical stimulation, tissues treated with dantrolene had a significantly decreased Isc compared to control tissue (Control ΔIsc = 27±13, Dantrolene ΔIsc 15±6.6, KCl ΔIsc 20±10, n=6 tissues)(*P = 0.01, paired t test comparing control to dantrolene). Asterisk (*) used to denote P <0.05.
E) Representative short circuit current (Isc) traces showing control response to 30 mmHg (black) compared to paired DMSO traces (blue). [Scale bar: 10 Isc, 10s].
F) Individual (circles) and mean ± SD (bars) showing short-circuit current (Isc) in response to pressure (ΔP) in control and DMSO incubation with 30mmHg pressure and KCl applied as positive control. Tissues treated with DMSO did not significantly differ from control tissues. (Control ΔIsc = 24±14, DMSO ΔIsc 30±27, KCl ΔIsc 35±5, n=5 tissues)(P = 0.421, paired t test comparing control to DMSO).
Discussion
Luminal forces stimulate a population of mechanosensitive EECs, which respond by an intracellular Ca2+ increase (Wang et al., 2017; Alcaino et al., 2018). EECs and other specialized mechanosensory cells are built using mechanical switches, like Piezo2 channels, which provide a receptor current upon mechanical stimulation (Mercado-Perez & Beyder, 2022). Piezo2 receptor current is a non-selective inward cationic current composed mainly of Ca2+ and Na+ (Coste et al., 2010; Szczot et al., 2017). However, Piezo2 dynamics are rapid, and channels inactivate within milliseconds, even in the presence of a persistent stimulus (Coste et al., 2010), while mechanically stimulated Ca2+ increase and 5-HT release last many seconds and sometimes minutes, suggesting signal amplification (Bertrand, 2004; Wang et al., 2017; Alcaino et al., 2018). In this study, we show that EECs express a range of intracellular Ca2+ receptors, but only ryanodine receptor 3 (RyR3) was significantly enriched in EECs compared to other epithelial cells. We show that mechanical stimulation of a human EEC cell model (Alcaino et al., 2017; Wang et al., 2017) led to a dynamic relationship between an increase in cytoplasmic Ca2+ with a corresponding decrease in Ca2+ within the stores. In primary EECs, Ca2+ stores, and specifically RyR3 stores, were critical for mechanically induced cytoplasmic Ca2+ increase and 5-HT release. Finally, we found that the increase in short circuit current by a brief mechanical stimulation of colonic mucosa can be modulated by RyR3 pharmacological blockers, suggesting that epithelial secretion relies on RyR3 receptors.
Cytoplasmic Ca2+ concentration is highly regulated and usually kept in the sub-micromolar range, and it can increase a thousand-fold from nM to mM range during signal transduction – these properties make Ca2+ an optimal signaling molecule. Ca2+ surges can come from activation of receptors or ion channels bringing ions from the extracellular compartment or from intracellular Ca2+ stores, which Ca2+ can directly activate in a process known as Ca2+-induced-Ca2+ release (CICR). Endoplasmic reticulum (ER) or sarcoplasmic reticulum (SR, muscle) express ryanodine (RyRs) and inositol 1,4,5-trisphosphate (IP3Rs) receptors, where they regulate Ca2+ concentrations. Most cell types express IP3Rs and rely on them for important cellular processes (Parys & Vervliet, 2020). They are activated through not only the presence of Ca2+, but also require the IP3 ligand. In contrast to the widely expressed and homeostatically important IP3Rs, RyRs are typically found in lower amounts, and often specifically in excitable cell types (Sorrentino & Volpe, 1993; Lanner et al., 2010). The RyR isoform is also highly specialized based on the function of the tissue type. Much of our knowledge about these SR or ER receptors has been gathered through ryanodine receptor type 1 (RyR1), which plays a critical role in the excitation-contraction coupling of skeletal muscle (Takeshima et al., 1989). The ryanodine receptor type 2 (RyR2) specializes in cardiac and smooth muscle function (Perez et al., 2005). The third ryanodine isoform, RyR3 is most abundant within the central nervous system (Giannini et al., 1995). All three isoforms of ryanodine receptors are expressed in the CNS, but the different RyRs were localized to a distinct location, with RyR3 most abundantly expressed in the neurons of the hippocampus (Giannini et al., 1995). Recent work shows that RyR3 in neurons mediates neurotransmitter release and dendritic action potentials (Del Prete et al., 2014).
Both types of receptors, RyRs and IP3Rs, play critical roles in specialized epithelial mechanosensory cells. There are two notable examples – skin touch sensors (Merkel cells) and ear’s hair cells. The skin epithelia specialized cells known as Merkel cells sense mechanical forces by activating the mechanosensitive ion channel Piezo2 (Ranade et al., 2014; Woo et al., 2014). Direct mechanical stimulation in Merkel cells induces an increase in intracellular Ca2+ (Haeberle & Lumpkin, 2008; Cha et al., 2011), serotonergic (Chang et al., 2016), and adrenergic signaling (Hoffman et al., 2018). In Merkel cells, intracellular stores are important regulators of Ca2+ dynamics, as emptying the endoplasmic reticulum using thapsigargin significantly reduced the Ca2+ transients induced by cell depolarization (Piskorowski et al., 2008a). Ryanodine potentiates Merkel cells’ mechanically evoked intracellular Ca2+ responses and was blocked by procaine (Senok & Baumann, 1997), a local anesthetic shown to be a blocker of CICR (Pike et al., 1989). Merkel cell expression studies found transcripts for two isoforms of IP3Rs (type I and II) (Tachibana et al., 2003; Haeberle & Lumpkin, 2008), and more recently a comparison between Merkel cells and basal keratinocytes and found upregulated expression of all three IP3Rs and RyR3 (Haber et al., 2017; Hoffman et al., 2018). All together, these studies suggest a role for RyR3 and IP3Rs in CICR mediated mechanical responses in Merkel cells. In the inner ear, hair cells that convert sound waves into chemo-electrical signaling, are another class of specialized epithelial mechanosensory cells. Interestingly, outer hair cells (OHCs) and a subset of inner hair cells (IHCs) express Piezo2, in which the channel function is tightly regulated by intracellular Ca2+ levels (Wu et al., 2017). The ability of hair cells to continuously detect and transmit changes in sound and balance to the central nervous systems depends on CICR dependent mechanisms that allow for rapid release of synaptic vesicles from IHCs (Zhang et al., 1999; Beutner et al., 2001) and efferent responses in OHCs (Evans et al., 2000; Lioudyno et al., 2004; Fettiplace & Hackney, 2006). RyR isoforms are the primary CICR receptors in hair cells, and indeed rat OHCs express RyR1 (Lioudyno et al., 2004; Grant et al., 2006), while mouse IHCs express both RyR1 and RyR2 (Grant et al., 2006; Morton-Jones et al., 2006; Liang et al., 2009). Treatment of OHCs in the cochlea with an agonist dose of ryanodine enhanced fast and slow responses to acoustic stimulation (Sridhar et al., 1997). Depolarization of murine IHCs led to increased intracellular Ca2+ that could be slowed and partially blocked by applying the antagonist dose of ryanodine (Kennedy & Meech, 2002). Another study verified RyR’s role in cochlear transduction role in guinea pig IHCs and OHCs (Beurg et al., 2005). Although CICR has been linked to the release of synaptic vesicles in IHCs, RyRs are also critical for vesicle recruitment and synaptic transmission (Castellano-Munoz et al., 2016). In all, many lines of evidence show that CICR channels, and specifically RyRs, are functionally relevant for specialized mechanosensing epithelial cells in the skin and ear.
Our expression and functional experiments show that in EECs, intracellular Ca2+ maintenance utilizes both RyR3 and IP3 receptors. A longitudinal study of mouse embryo development showed that IP3Rs were the dominant intracellular Ca2+ receptor in most tissue types during embryogenesis and played a critical role in cellular regulation and apoptosis. As the mouse embryo matured, many tissue types switched to the specialized RyRs as their intracellular Ca2+ needs became complex but often maintained IP3Rs for regulatory roles (Rosemblit et al., 1999). Interestingly, while both classes of receptors were expressed in EEC, RyR3 was the only receptor exclusively found in our EEC population, albeit at lower levels than IP3Rs. However, ryanodine channels have a 10-fold higher conductance; therefore, EECs should need fewer copies of the RyR3 channel to have a functional impact (Sorrentino & Volpe, 1993). In our functional primary EEC data, mechanically induced increases in intracellular Ca2+ were partially blocked but not completely abolished by applying IP3 receptor blocker and almost entirely inhibited by RyR3 pharmacological inhibition and genetic knockdown. While IP3Rs are playing a role in EEC mechanotransduction, their ubiquitous nature suggests roles beyond mechanotransduction and may have relevance given the multimodal sensing nature of EECs (Bellono et al., 2017).
RyR3 is highly enriched in EECs and appears to be essential in EEC mechanotransduction. In atrial myocytes that express both types of receptors, IP3R Ca2+ release events had slower kinetics and lower amplitude compared to the faster and larger Ca2+ spark response from RyR channels (Zima & Blatter, 2004). These fast kinetics of RyR complement the fast kinetics of Piezo2 (Coste et al., 2010), and may work to quickly mobilize Ca2+ that aids in EEC mechanotransduction and subsequent 5-HT release. Initial studies of RyR3 knockout mice showed no apparent physical abnormalities, but behavioral studies have found a more vibrant locomotor activity, decreased social contact, decreased hippocampus synaptic plasticity, and a deficiency in spatial learning (Takeshima et al., 1996; Balschun et al., 1999; Matsuo et al., 2009). These findings were mainly attributed to RyR3’s strong neuronal presence. A recent study looking at extraocular muscles in RyR3 knockout mice found altered mechanical properties and calcium dynamics, and implicated RyR3 in rapid Ca2+ oscillations found in myotubes, as knockout mice had slower oscillations and Ca2+ release, and less force that resulted in impaired vision (Eckhardt et al., 2019). Fast signaling is a critical component for normal physiologic function in these cell types and as such, EECs may also utilize RyR3’s fast kinetics in mechanotransduction. Specifically, mechanosensitive EECs, with high amounts of Piezo2, are similar to neurons and critical for fast sensing of luminal contents (Treichel et al., 2021; Treichel et al., 2022), making mechanosensitive EECs a crucial sensory component of the GI tract. RyR3’s fast kinetics might indeed be critical for amplification of rapid currents generated by Piezo2 channels in EECs.
Our study suggests that the presence of RyR3 in EEC may aid in a neuronal-like fast response to mechanical stimulation that allows for rapid increase of Ca2+, which may be further amplified by IP3Rs, and play a role in subsequent neurotransmitter and hormone release (Shaaban et al., 2021). Intriguingly, recent protein interaction study of Piezo2 in dorsal root ganglia (DRGs) found enrichment of both Itpr3 and RyR3, suggesting that these molecules are not only close to but physically interact with Piezo2 (Narayanan et al., 2016). Further work can help clarify other components involved in the EEC mechanotransduction pathway. For example, in the heart and skeletal muscle, RyR channel function is dependent on proximity to voltage-gated calcium channels (VGCC), to mediate calcium flow necessary to activate skeletal fibers (Meissner & Lu, 1995; Bers, 2002). In neurons, there is a functional coupling between L-type VGCCs and ryanodine receptors (Chavis et al., 1996). EECs also densely express L-, P/Q-, and T-Type VGCCs (Rogers et al., 2011; Bellono et al., 2017), but their function has not yet been explored in EEC mechanotransduction
In summary, our study shows that mechano-sensing EECs rely on intracellular Ca2+ stores, including IP3Rs and RyR3, which is selectively expressed in EECs, for cytoplasmic Ca2+ amplification mechanisms that are critical for mechanosensation and mechanically activated release 5-HT.
Supplementary Material
Key Points.
A population of enteroendocrine cells (EEC) are specialized mechanosensors of the gastrointestinal (GI) epithelium that respond to mechanical stimulation with the release of important signaling molecules, such as serotonin.
Mechanical activation of these EECs leads to an increase in intracellular calcium (Ca2+) with a longer duration than the stimulus, suggesting intracellular Ca2+ signal amplification.
In this study, we profiled the expression of intracellular Ca2+ store receptors and found an enriched expression of the intracellular Ca2+ receptor Ryr3, which contributed to the mechanically evoked increases in intracellular calcium, 5-HT release, and epithelial secretion.
Our data suggests mechanosensitive EEC rely on intracellular Ca2+ stores and are selective in their use of Ryr3 for amplification of intracellular Ca2+.
This work advances our understanding of EEC mechanotransduction and may provide novel diagnostic and therapeutic targets for GI motility disorders.
Acknowledgements
We thank Mrs. Lyndsay Busby for administrative assistance, Peter Strege for help with the graphics, Mayo Clinic Microscopy and Cell Analysis Core for help with the flow cytometry experiments.
Funding
This work was supported by NIH, including Mayo Center for Cell Signaling in Gastroenterology (DK084567), DP2AT10875, DK123549, and DK52766.
Biographies
Kaitlyn Knutson graduated from Gustavus Adolphus College with a BA in Biology in 2010. She is currently a senior research technologist in the Enteric Neuroscience Program (ENSP) within the Division of Gastroenterology and Hepatology at the Mayo Clinic in Rochester, MN. Kaitlyn’s main research focus is studying the cellular physiology of the gastrointestinal tract at the molecular level in hopes of discovering treatments for functional GI disorders and research interests involve approaches to study cellular transcriptomics.

Sara Whiteman graduated from Concordia College with a BA in Neuroscience in 2018. Sara conducted post-baccalaureate research with Dr. Arthur Beyder at the Mayo Clinic’s Enteric Neuroscience Program (ENSP). Sara is broadly interested in gut-brain connection’s role in central physiology and pathology, neurodegenerative diseases, and novel diagnostics.

Footnotes
Competing Interests
The authors declare that they have no competing interests.
Data Availability Statement
All data supporting the results has been presented in the manuscript figures.
References
- Acsai K, Antoons G, Livshitz L, Rudy Y & Sipido KR. (2011). Microdomain [Ca(2)(+)] near ryanodine receptors as reported by L-type Ca(2)(+) and Na+/Ca(2)(+) exchange currents. J Physiol 589, 2569–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaino C, Knutson K, Gottlieb PA, Farrugia G & Beyder A. (2017). Mechanosensitive ion channel Piezo2 is inhibited by D-GsMTx4 Channels 11, 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaino C, Knutson K, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G & Beyder A. (2018). A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proceedings of the National Academy of Sciences of the United States of America Sciences 115, E7632–7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Wolfer DP, Bertocchini F, Barone V, Conti A, Zuschratter W, Missiaen L, Lipp HP, Frey JU & Sorrentino V. (1999). Deletion of the ryanodine receptor type 3 (RyR3) impairs forms of synaptic plasticity and spatial learning. EMBO J 18, 5264–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellono NW, Bayrer JR, Leitch DB, Castro J, Zhang C, O’Donnell TA, Brierley SM, Ingraham HA & Julius D. (2017). Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 170, 185–198 e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM. (2002). Cardiac excitation-contraction coupling. Nature 415, 198–205. [DOI] [PubMed] [Google Scholar]
- Bertrand PP. (2004). Real-time detection of serotonin release from enterochromaffin cells of the guinea-pig ileum. Neurogastroenterol Motil 16, 511–514. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Hu X, Mach J & Bertrand RL. (2008). Serotonin (5-HT) release and uptake measured by real-time electrochemical techniques in the rat ileum. Am J Physiol Gastrointest Liver Physiol 295, G1228–1236. [DOI] [PubMed] [Google Scholar]
- Beurg M, Hafidi A, Skinner LJ, Ruel J, Nouvian R, Henaff M, Puel JL, Aran JM & Dulon D. (2005). Ryanodine receptors and BK channels act as a presynaptic depressor of neurotransmission in cochlear inner hair cells. Eur J Neurosci 22, 1109–1119. [DOI] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E & Moser T. (2001). Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron 29, 681–690. [DOI] [PubMed] [Google Scholar]
- Billing LJ, Larraufie P, Lewis J, Leiter A, Li J, Lam B, Yeo GS, Goldspink DA, Kay RG, Gribble FM & Reimann F. (2019). Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice - Identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Mol Metab 29, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T, Voland P, Kunz L, Prinz C & Gratzl M. (2007). Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology 132, 1890–1901. [DOI] [PubMed] [Google Scholar]
- Castellano-Munoz M, Schnee ME & Ricci AJ. (2016). Calcium-induced calcium release supports recruitment of synaptic vesicles in auditory hair cells. J Neurophysiol 115, 226–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha M, Ling J, Xu GY & Gu JG. (2011). Shear mechanical force induces an increase of intracellular Ca2+ in cultured Merkel cells prepared from rat vibrissal hair follicles. J Neurophysiol 106, 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Kanda H, Ikeda R, Ling J, DeBerry JJ & Gu JG. (2016). Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc Natl Acad Sci U S A 113, E5491–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Fagni L, Lansman JB & Bockaert J. (1996). Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature 382, 719–722. [DOI] [PubMed] [Google Scholar]
- Chen MC, Wu SV, Reeve JR Jr. & Rozengurt E. (2006). Bitter stimuli induce Ca2+ signaling and CCK release in enteroendocrine STC-1 cells: role of L-type voltage-sensitive Ca2+ channels. Am J Physiol Cell Physiol 291, C726–739. [DOI] [PubMed] [Google Scholar]
- Corradi J, Gumilar F & Bouzat C. (2009). Single-channel kinetic analysis for activation and desensitization of homomeric 5-HT(3)A receptors. Biophys J 97, 1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE & Patapoutian A. (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete D, Checler F & Chami M. (2014). Ryanodine receptors: physiological function and deregulation in Alzheimer disease. Mol Neurodegener 9, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doihara H, Nozawa K, Kojima R, Kawabata-Shoda E, Yokoyama T & Ito H. (2009). QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol Cell Biochem 331, 239–245. [DOI] [PubMed] [Google Scholar]
- Eckhardt J, Bachmann C, Sekulic-Jablanovic M, Enzmann V, Park KH, Ma J, Takeshima H, Zorzato F & Treves S. (2019). Extraocular muscle function is impaired in ryr3 (−/−) mice. J Gen Physiol 151, 929–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MG, Lagostena L, Darbon P & Mammano F. (2000). Cholinergic control of membrane conductance and intracellular free Ca2+ in outer hair cells of the guinea pig cochlea. Cell Calcium 28, 195–203. [DOI] [PubMed] [Google Scholar]
- Fettiplace R & Hackney CM. (2006). The sensory and motor roles of auditory hair cells. Nat Rev Neurosci 7, 19–29. [DOI] [PubMed] [Google Scholar]
- Giannini G, Conti A, Mammarella S, Scrobogna M & Sorrentino V. (1995). The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol 128, 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracz AD, Puthoff BJ & Magness ST. (2012). Identification, isolation, and culture of intestinal epithelial stem cells from murine intestine. Methods Mol Biol 879, 89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant L, Slapnick S, Kennedy H & Hackney C. (2006). Ryanodine receptor localisation in the mammalian cochlea: an ultrastructural study. Hear Res 219, 101–109. [DOI] [PubMed] [Google Scholar]
- Gribble FM & Reimann F. (2015). Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu Rev Physiol. [DOI] [PubMed] [Google Scholar]
- Haber AL, Biton M, Rogel N, Herbst RH, Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, Tirosh I, Beyaz S, Dionne D, Zhang M, Raychowdhury R, Garrett WS, Rozenblatt-Rosen O, Shi HN, Yilmaz O, Xavier RJ & Regev A. (2017). A single-cell survey of the small intestinal epithelium. Nature 551, 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle H & Lumpkin EA. (2008). Merkel Cells in Somatosensation. Chemosens Percept 1, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, Buesa J, Taylor JA, Loitto VM, Magnusson KE, Ahlman H, Lundgren O & Svensson L. (2011). Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLoS Pathog 7, e1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann-Frank A, Luttgau HC & Stephenson DG. (1999). Caffeine and excitation-contraction coupling in skeletal muscle: a stimulating story. J Muscle Res Cell Motil 20, 223–237. [DOI] [PubMed] [Google Scholar]
- Hoffman BU, Baba Y, Griffith TN, Mosharov EV, Woo SH, Roybal DD, Karsenty G, Patapoutian A, Sulzer D & Lumpkin EA. (2018). Merkel Cells Activate Sensory Neural Pathways through Adrenergic Synapses. Neuron 100, 1401–1413 e1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA, Jin B, Martin AM, Wei L, Flinders Gastrointestinal C, Ro S & Keating DJ. (2022). Diminished Piezo2-Dependent Tactile Sensitivity Occurs in Aging Human Gut and Slows Gastrointestinal Transit in Mice. Gastroenterology. [DOI] [PubMed] [Google Scholar]
- Kennedy HJ & Meech RW. (2002). Fast Ca2+ signals at mouse inner hair cell synapse: a role for Ca2+-induced Ca2+ release. J Physiol 539, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O & Pfragner R. (2008). Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol 295, G260–272. [DOI] [PubMed] [Google Scholar]
- Kim M, Javed NH, Yu JG, Christofi F & Cooke HJ. (2001). Mechanical stimulation activates Galphaq signaling pathways and 5-hydroxytryptamine release from human carcinoid BON cells. J Clin Invest 108, 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson K, Strege PR, Li J, Leiter AB, Farrugia G & Beyder A. (2018). Whole cell electrophysiology of primary cultured murine enterochromaffin (EC) cells. JoVE 139, e58112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima R, Nozawa K, Doihara H, Keto Y, Kaku H, Yokoyama T & Itou H. (2014). Effects of novel TRPA1 receptor agonist ASP7663 in models of drug-induced constipation and visceral pain. Eur J Pharmacol 723, 288–293. [DOI] [PubMed] [Google Scholar]
- Kurokawa K, Mizuno K & Ohkuma S. (2013). Dopamine D1 receptor signaling system regulates ryanodine receptor expression in ethanol physical dependence. Alcohol Clin Exp Res 37, 771–783. [DOI] [PubMed] [Google Scholar]
- Lanner JT, Georgiou DK, Joshi AD & Hamilton SL. (2010). Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2, a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Huang L & Yang J. (2009). Differential expression of ryanodine receptor in the developing rat cochlea. Eur J Histochem 53, e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linan-Rico A, Ochoa-Cortes F, Beyder A, Soghomonyan S, Zuleta-Alarcon A, Coppola V & Christofi FL. (2016). Mechanosensory Signaling in Enterochromaffin Cells and 5-HT Release: Potential Implications for Gut Inflammation. Front Neurosci 10, 564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linan-Rico A, Ochoa-Cortes F, Zuleta-Alarcon A, Alhaj M, Tili E, Enneking J, Harzman A, Grants I, Bergese S & Christofi FL. (2017). UTP - Gated Signaling Pathways of 5-HT Release from BON Cells as a Model of Human Enterochromaffin Cells. Front Pharmacol 8, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioudyno M, Hiel H, Kong JH, Katz E, Waldman E, Parameshwaran-Iyer S, Glowatzki E & Fuchs PA. (2004). A “synaptoplasmic cistern” mediates rapid inhibition of cochlear hair cells. J Neurosci 24, 11160–11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax RB, Gallego S, Novalbos J, Garcia AG & Warhurst G. (1999). L-Type calcium channels in enterochromaffin cells from guinea pig and human duodenal crypts: an in situ study. Gastroenterology 117, 1363–1369. [DOI] [PubMed] [Google Scholar]
- Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ & Keating DJ. (2017). Regional differences in nutrient-induced secretion of gut serotonin. Physiol Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez G, Vidal RL, Mardones P, Serrano FG, Ardiles AO, Wirth C, Valdes P, Thielen P, Schneider BL, Kerr B, Valdes JL, Palacios AG, Inestrosa NC, Glimcher LH & Hetz C. (2016). Regulation of Memory Formation by the Transcription Factor XBP1. Cell Rep 14, 1382–1394. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Tanda K, Nakanishi K, Yamasaki N, Toyama K, Takao K, Takeshima H & Miyakawa T. (2009). Comprehensive behavioral phenotyping of ryanodine receptor type 3 (RyR3) knockout mice: decreased social contact duration in two social interaction tests. Front Behav Neurosci 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe GM & Hoffman JM. (2013). Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nature Reviews: Gastroenterology and Hepatology 10, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G & Lu X. (1995). Dihydropyridine receptor-ryanodine receptor interactions in skeletal muscle excitation-contraction coupling. Biosci Rep 15, 399–408. [DOI] [PubMed] [Google Scholar]
- Mercado-Perez A & Beyder A. (2022). Gut feelings: mechanosensing in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 19, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton-Jones RT, Cannell MB, Jeyakumar LH, Fleischer S & Housley GD. (2006). Differential expression of ryanodine receptors in the rat cochlea. Neuroscience 137, 275–286. [DOI] [PubMed] [Google Scholar]
- Narayanan P, Sondermann J, Rouwette T, Karaca S, Urlaub H, Mitkovski M, Gomez-Varela D & Schmidt M. (2016). Native Piezo2 Interactomics Identifies Pericentrin as a Novel Regulator of Piezo2 in Somatosensory Neurons. J Proteome Res 15, 2676–2687. [DOI] [PubMed] [Google Scholar]
- Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Sano Y, Inamura K, Matsushime H, Koizumi T, Yokoyama T & Ito H. (2009). TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci U S A 106, 3408–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais R, Rievaj J, Meek C, De Costa G, Jayamaha S, Alexander RT, Reimann F & Gribble F. (2016). Role of enteroendocrine L-cells in arginine vasopressin-mediated inhibition of colonic anion secretion. J Physiol 594, 4865–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys JB & Vervliet T. (2020). New Insights in the IP3 Receptor and Its Regulation. Adv Exp Med Biol 1131, 243–270. [DOI] [PubMed] [Google Scholar]
- Perez CF, Lopez JR & Allen PD. (2005). Expression levels of RyR1 and RyR3 control resting free Ca2+ in skeletal muscle. Am J Physiol Cell Physiol 288, C640–649. [DOI] [PubMed] [Google Scholar]
- Pike GK, Abramson JJ & Salama G. (1989). Effects of tetracaine and procaine on skinned muscle fibres depend on free calcium. J Muscle Res Cell Motil 10, 337–349. [DOI] [PubMed] [Google Scholar]
- Piskorowski R, Haeberle H, Panditrao MV & Lumpkin EA. (2008a). Voltage-activated ion channels and Ca(2+)-induced Ca (2+) release shape Ca (2+) signaling in Merkel cells. Pflugers Arch 457, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskorowski R, Haeberle H, Panditrao MV & Lumpkin EA. (2008b). Voltage-activated ion channels and Ca(2+)-induced Ca (2+) release shape Ca (2+) signaling in Merkel cells. Pflugers Archiv : European journal of physiology 457, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke K, Reimann A, Schworer H & Kilbinger H. (1996). Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res 73, 83–87. [DOI] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR & Patapoutian A. (2014). Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ & Gribble FM. (2008). Glucose sensing in L cells: a primary cell study. Cell Metab 8, 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GJ, Tolhurst G, Ramzan A, Habib AM, Parker HE, Gribble FM & Reimann F. (2011). Electrical activity-triggered glucagon-like peptide-1 secretion from primary murine L-cells. J Physiol 589, 1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemblit N, Moschella MC, Ondriasova E, Gutstein DE, Ondrias K & Marks AR. (1999). Intracellular calcium release channel expression during embryogenesis. Dev Biol 206, 163–177. [DOI] [PubMed] [Google Scholar]
- Sankar N, deTombe PP & Mignery GA. (2014). Calcineurin-NFATc regulates type 2 inositol 1,4,5-trisphosphate receptor (InsP3R2) expression during cardiac remodeling. J Biol Chem 289, 6188–6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafermeyer A, Gratzl M, Rad R, Dossumbekova A, Sachs G & Prinz C. (2004). Isolation and receptor profiling of ileal enterochromaffin cells. Acta Physiol Scand 182, 53–62. [DOI] [PubMed] [Google Scholar]
- Schulze A, Hartung P, Schaefer M & Hill K. (2014). Transient receptor potential ankyrin 1 (TRPA1) channel activation by the thienopyridine-type drugs ticlopidine, clopidogrel, and prasugrel. Cell Calcium. [DOI] [PubMed] [Google Scholar]
- Senok SS & Baumann KI. (1997). Functional evidence for calcium-induced calcium release in isolated rat vibrissal Merkel cell mechanoreceptors. J Physiol 500 (Pt 1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban A, Maaß F, Schwarze V, Lund ML, Beuermann S, Chan M, Harenberg C, Bewick GA, Keating DJ, Benseler F, Cooper BH & Imig C. (2021). Dissecting Functional, Structural, and Molecular Requirements for Serotonin Release from Mouse Enterochromaffin Cells. bioRxiv, 2021.2005.2028.446100. [Google Scholar]
- Sidhu SS, Thompson DG, Warhurst G, Case RM & Benson RS. (2000). Fatty acid-induced cholecystokinin secretion and changes in intracellular Ca2+ in two enteroendocrine cell lines, STC-1 and GLUTag. J Physiol 528 Pt 1, 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino V & Volpe P. (1993). Ryanodine receptors: how many, where and why? Trends Pharmacol Sci 14, 98–103. [DOI] [PubMed] [Google Scholar]
- Sridhar TS, Brown MC & Sewell WF. (1997). Unique postsynaptic signaling at the hair cell efferent synapse permits calcium to evoke changes on two time scales. J Neurosci 17, 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y & Iino M. (2014). Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat Commun 5, 4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczot M, Pogorzala LA, Solinski HJ, Young L, Yee P, Le Pichon CE, Chesler AT & Hoon MA. (2017). Cell-Type-Specific Splicing of Piezo2 Regulates Mechanotransduction. Cell Reports 21, 2760–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana T, Endoh M, Kumakami R & Nawa T. (2003). Immunohistochemical expressions of mGluR5, P2Y2 receptor, PLC-beta1, and IP3R-I and -II in Merkel cells in rat sinus hair follicles. Histochem Cell Biol 120, 13–21. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Ikemoto T, Nishi M, Nishiyama N, Shimuta M, Sugitani Y, Kuno J, Saito I, Saito H, Endo M, Iino M & Noda T. (1996). Generation and characterization of mutant mice lacking ryanodine receptor type 3. J Biol Chem 271, 19649–19652. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T & et al. (1989). Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339, 439–445. [DOI] [PubMed] [Google Scholar]
- Treichel AJ, Farrugia G & Beyder A. (2018). The touchy business of gastrointestinal (GI) mechanosensitivity. Brain Res 1693, 197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treichel AJ, Finholm I, Knutson KR, Alcaino C, Whiteman ST, Brown MR, Matveyenko A, Wegner A, Kacmaz H, Mercado-Perez A, Gajdos GB, Ordog T, Grover M, Szurszewski J, Linden DR, Farrugia G & Beyder A. (2022). Specialized Mechanosensory Epithelial Cells in Mouse Gut Intrinsic Tactile Sensitivity. Gastroenterology 162, 535–547 e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treichel AJ, Finholm I, Knutson KR, Alcaino C, Whiteman ST, Brown MR, Matveyenko A, Wegner A, Kacmaz H, Mercado-Perez A, Gajdos GB, Ordog T, Grover M, Szurzewski J, Linden DR, Farrugia G & Beyder A. (2021). Specialized mechanosensory epithelial cells in mouse gut intrinsic tactile sensitivity. Gastroenterology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegezzi G, Anselmi L, Huynh J, Barocelli E, Rozengurt E, Raybould H & Sternini C. (2014). Diet-induced regulation of bitter taste receptor subtypes in the mouse gastrointestinal tract. PLoS One 9, e107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap PK, Grover M, Oeckler R, Gottlieb PA, Li HJ, Leiter AB, Farrugia G & Beyder A. (2017). Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. The Journal of Physiology 595, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang J, Liu D & Su Y. (2010). Normalizing genes for real-time polymerase chain reaction in epithelial and nonepithelial cells of mouse small intestine. Anal Biochem 399, 211–217. [DOI] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL & Patapoutian A. (2014). Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Grillet N, Zhao B, Cunningham C, Harkins-Perry S, Coste B, Ranade S, Zebarjadi N, Beurg M, Fettiplace R, Patapoutian A & Mueller U. (2017). Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nat Neurosci 20, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C & Beech DJ. (2005). Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol 145, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SH, Rey O, Sternini C & Rozengurt E. (2010). Amino acid sensing by enteroendocrine STC-1 cells: role of the Na+-coupled neutral amino acid transporter 2. Am J Physiol Cell Physiol 298, C1401–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Robertson D, Yates G & Everett A. (1999). Role of L-type Ca(2+) channels in transmitter release from mammalian inner hair cells I. Gross sound-evoked potentials. J Neurophysiol 82, 3307–3315. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T & Campbell RE. (2011). An expanded palette of genetically encoded Ca(2)(+) indicators. Science 333, 1888–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HR & Pestka JJ. (2015). Deoxynivalenol (Vomitoxin)-Induced Cholecystokinin and Glucagon-Like Peptide-1 Release in the STC-1 Enteroendocrine Cell Model is Mediated by Calcium-Sensing Receptor and Transient Receptor Potential Ankyrin-1 Channel. Toxicol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima AV & Blatter LA. (2004). Inositol-1,4,5-trisphosphate-dependent Ca(2+) signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol 555, 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the results has been presented in the manuscript figures.


