Abstract
Background:
Choosing adequate topical antimicrobial agents in burn patients still represents a challenge. Therefore, this systematic review was conducted to compile and evaluate current recommendations in international clinical practice guidelines (CPGs) to develop more consistent clinical guidance.
Methods:
A systematic search for CPGs was conducted independently by two reviewers using PubMed, EMBASE, Google Scholar, and external citations. The quality of the selected CPGs was evaluated separately using the AGREE II instrument, and intraclass correlation coefficients were calculated. Statistical analysis was performed using R V 1.4.1 statistical software.
Results:
Eleven CPGs were included in the study. Most guidelines tend to recommend silver-containing dressings over antiseptics or antibiotics, regardless of the depth of the burn. Silver sulfadiazine is the most recommended topical antimicrobial in low-resource settings. An overall mean appraisal AGREE II score of 68.2% was obtained. The global intraclass correlation coefficient was 0.62 (95% confidence intervals 0.54-0.69), which corresponds to a substantial global concordance between both appraisers.
Conclusions:
Great heterogeneity was found between recommendations and CPGs. The three determining factors considered to issue a recommendation were the clinical scenario, burn-wound depth, and burn severity. There is consensus among the guidelines to use topical antimicrobials as a tool to prevent infection, and most of these recommend the use of silver-containing dressings for most scenarios. However, there is currently no ideal topical antimicrobial agent that can be recommended for all clinical scenarios. The development of more consistent recommendations is warranted to standardize clinical practice.
Key Words: Burn, Infection, Prevention, Antimicrobials, Topical
INTRODUCTION
Although just decades ago the adoption of topical antimicrobial agents led to a significant reduction in complications such as dissemination and sepsis, burn-wound infection still represents a potential challenge in burn care. According to the American Burn Association (ABA), two out of the five most common complications in burn patients are burn-wound infectious conditions (cellulitis in third place and burn-wound infection in the 5th 1,2.. What is more, current infection-related mortality rates still range from 50-75%.3-6. These numbers highlight the importance of preventing any type of infectious disease and inherent complications among burn-injured subjects. Furthermore, whereas nowadays solid evidence exists for the treatment of both burn-wound contamination and established infection 7,8 . There is a lack of consensus in the literature 9,10, and international clinical practice guidelines (CPGs) regarding the optimal use of topical antimicrobial agents to prevent burn-wound infection in different settings. Therefore, condensing the currently available recommendations is essential to develop more consistent guidance for practitioners in their clinical decisions.
Clinical practice guidelines represent a systematically developed link between scientific evidence and standardized clinical practice; thus, medical practitioners rely on their recommendations for evidence-based decisions 11. Nevertheless, the recommendations found in these resources may sometimes be unclear, ambiguous, or may contain gaps. Furthermore, recommendations may vary between international guidelines, making consensus difficult. Such is the case of the recommendations for optimal topical antimicrobial agents to prevent burn-wound infections. The reason for these contrasting recommendations may be that, as various authors conclude, the available studies in the area provide flawed, contradictory data and may be at high risk of bias 10, 12-14.. For the scope of this review, Supplementary table 6 provides a detailed definition and classification of topical antimicrobial agents used in burn care as defined by Cambiasio et al 7.
Given that infection is still one of the leading causes of morbidity and the main cause of mortality in burn patients 1,15 , selecting optimal topical agents for the prevention of burn-wound infection is essential, especially in the imminent era of antimicrobial resistance. While clear recommendations regarding topical antimicrobial agents exist in the therapeutic setting (i.e., established burn-wound infection), international recommendations for their use in infection prevention are contrasting and may be ambiguous. Therefore, we conducted a systematic review to evaluate the methodological quality of different published clinical practice guidelines using the AGREE II critical appraisal approach instrument 16 and compared their key recommendations on the use of topical antimicrobial agents for burn-wound infection prevention in partial-thickness and full-thickness wounds in adult patients. In the absence of a consistent consensus, a comprehensive systematic review that evaluates the variable recommendations for different settings and clinical scenarios in currently available guidelines was warranted. This manuscript condenses these recommendations and sheds light on the preferred topical management to prevent burn-wound infection in different situations.
METHODS
Data resources and search methodology
A detailed description of the search methodology can be found in Supplementary Tables 1 and 2. A systematic search was conducted in duplicate by two independent reviewers between July 24 and 25, 2021. Three databases (PubMed, EMBASE, and Google Scholar) were searched to identify clinical practice guidelines (CPGs) published between January 1, 2010, and July 25, 2021, using the search algorithms described in Supplementary Table 1. The database search results’ titles and abstracts were scrutinized for references to additional CPGs, which were also included. In addition, five international guideline repositories 17-21 and six websites of international burn associations and journals 22-27 were searched. Language restriction to English, Spanish, French, and German was applied.
Study selection and data extraction
Inclusion and exclusion criteria are outlined in Supplementary Table 2. CPG selection and data extraction were completed in duplicate. Both reviewers independently screened titles and abstracts of search results and selected guidelines that appeared to meet potentially standards for inclusion. Both reviewers obtained full-text guidelines and screened a second time using the pre-specified inclusion and exclusion criteria. Final inclusion and disagreements were resolved by mutual consensus. Both reviewers undertook guideline scoring independently, using a pre-designed data-pooling worksheet for AGREE II-scores. As for guideline contents, the following data were synthesized and documented: guideline title, author, year of publication, publishing society/organization or journal, country of origin, and a summary of topical antimicrobial agent recommendations with infection prevention as an outcome (burn-wound depth, minor/major burn, inpatient/outpatient management, clinical scenario, recommended agents, alternatives). The study selection process was documented using a flow diagram as per the PRISMA 2020 guideline for reporting systematic reviews 28 found in Supplementary Figure 1.
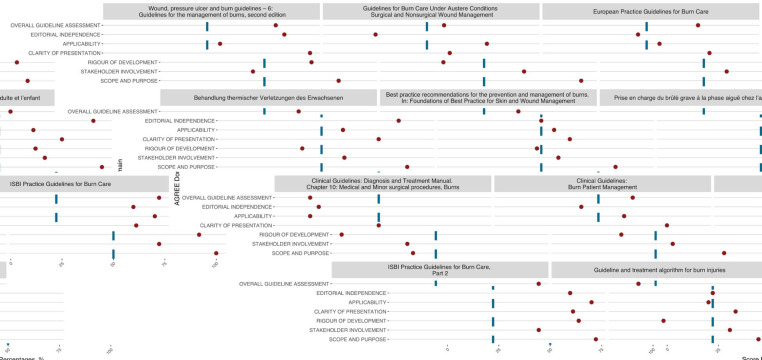
Figure 1.
AGREE II domain scores for each CPG
Assessment: Guideline Recommendations
Each guideline’s previously outlined key data were retrieved, synthesized, and tabulated by one reviewer. The tabulated results were revised and compared with the corresponding guidelines by a second reviewer to check for accuracy. Any discrepancies were resolved through consensus after a third revision. The recommendations and justifications were examined, and we designated the following domains to evaluate similarities and differences between CPG recommendations: burn-wound depth (full or partial-thickness), management according to burn severity (inpatient/outpatient), clinical scenario, and recommendations (general/specific). Gaps in recommendations in any of these criteria were noted in Table 1.
Table 1.
Summarized recommendations on topical antimicrobials with burn-wound infection prevention as an outcome
| Title, (PMID if available) | Quality assessment % (Mean appraisal score) % | Summary | ||||||
|---|---|---|---|---|---|---|---|---|
| Recommendations on topical antimicrobial agents to prevent burn-wound infection | ||||||||
| Burn wound depth (thickness) | Minor/ Major | Outpatient/ Inpatient | Clinical scenario | Addresses: AB, AS, ACD* | Recommended agents | Alternatives | ||
|
Wound, pressure ulcer and burn guidelines – 6: Guidelines for the management of burns, second edition
40
(PMID: 32343002) |
83.33 (75.79) | Partial | Major | Inpatient | Initial treatment | AB | Not specified. | Not specified. |
| Partial | Major | Inpatient | Not specified. | ACD | Silver-containing Hydrofiber (1A) | "Silver alginate and silver-containing polyurethane foam/soft silicone. (2A)" | ||
|
Guidelines for Burn Care Under Austere Conditions- Surgical and Nonsurgical Wound Management
39
(PMID: 27355660) |
58.33 (60.07) | Full | Major | Inpatient | Austere conditions, scarce supply, and delayed excision. | AB | Mafenide acetate 11% cream | Silver sulfadiazine as a sole agent, silver-containing dressings, aqueous 10% mafenide acetate solution, Dakin's solution, cerium-based products, honey. |
| Full | Major | Inpatient | AS | Silver sulfadiazine | ||||
| Full and Partial | Minor | Outpatient | Austere conditions | AS, AB | Silver sulfadiazine cream and Mafenide acetate, Silver impregnated dressings | Bacitracin, Polysporin®, and Neosporin® | ||
| Not specified | Minor | Outpatient | Austere conditions, facial burns | AB | Bacitracin, Polysporin®, and Neosporin® | Not specified. | ||
| European Practice Guidelines for Burn Care 38 | 75 (70.98) | Not specified | Not specified | Inpatient | Not specified. General recommendation. | ACD | Dressings containing nanocrystalline silver. | Not specified. |
| Behandlung thermischer Verletzungen des Erwachsenen (33) | 66.66 (69.39) | Superficial partial | Not specified | Inpatient | Not specified. | ACD | Silver-containing dressings. | Non-specified antimicrobial ointments, medicinal honey. |
| Partial (Unclear depth) | Not specified | Inpatient | Temporally unable to tell burn-wound depth. | ACD | Antiseptic dressings (Non-specific) | Not specified. | ||
| Best practice recommendations for the prevention and management of burns. In: Foundations of Best Practice for Skin and Wound Management. (32) | 66.66 (61.31) | Not specified | Not specified | Not specified | Not specified. General recommendation. | AS and AB | Honey, PMHB, Gentian Violet/Methylene Blue, Silver sulfadiazine (SSD) 1% cream, Mafenide acetate cream 11% or solution 5% | Not specified. |
| Not specified | Minor burns | Not specified | Not specified. | ACD | PVP-I dressings (Knitted viscose fabric impregnated with polyethylene glycose containing 1% povidone-iodine) | Not specified. | ||
| Deep-partial and full | Not specified | Inpatient | Burns awaiting surgery | ACD | Silver-containing dressings | Not specified. | ||
| Prise en charge du brûlé grave à la phase aiguë chez l’adulte et l’enfant (36) | 83.33 (80.90) | Full and Partial | Major | Inpatient | Emergency room. The patient cannot be transferred to a Burns Treatment Center within hours of the accident | ACD, AB, AS | Silver or antiseptic-containing dressings (Chlorhexidine, Povidone Iodine) | Not specified. |
| Clinical Guidelines: Diagnosis and Treatment Manual. Chapter 10: Medical and Minor surgical procedures, Burns. (31) | 16.66 (26.19) | Full and Partial | Major | Inpatient | Curative care at the dispensary and primary hospital in health care exclusion settings. | AS | Silver sulfadiazine | Not specified. |
| Not specified | Minor | Outpatient | AS | Silver sulfadiazine | ||||
| Clinical Guidelines: Burn Patient Management (30) | 66.66 (61.31) | Partial | Not specified | Not specified | Not specified. | ACD | Ionic silver dressings | Not specified. |
| Not specified | Not specified | Not specified. | ACD | Chlorhexidine impregnated paraffin gauze, Mesh gauze impregnated with 3% Xeroform® (Bismuth tribomophophenate) | ||||
| Full and Partial | Not specified | Not specified | Not specified. | ACD | Nanocrystalline silver dressings | |||
| Full and Partial | Major | Inpatient | Burn units | AS | Silver sulfadiazine 1% + Cerium nitrate 2.2% | |||
|
ISBI Practice Guidelines for Burn Care. (37) (PMID: 27542292) |
100 (91.17) | Superficial-partial | Not specified | Not specified | Fresh burns expected to heal spontaneously. | ACD | Iodine and silver-containing dressings. | Not specified. |
| Superficial-partial | Not specified | Not specified | Resource-limited settings with no available modern dressings to cover de-roofed or snipped blisters. | NSTA | Not specified. | Not specified. | ||
| Full and deep-partial | Not specified | Inpatient | Burn wounds awaiting excision. Closed technique. | NSTA | Not specified. | Not specified. | ||
| Full and deep-partial | Not specified | Inpatient | Burn wounds treated with open (after eschar removal) and semi-open techniques in resource-limited settings | NSTA | Not specified. | Not specified. | ||
|
ISBI Practice Guidelines for Burn Care, Part 2 (35) (PMID: 30343831) |
100 (91.17) | Not specified | Not specified | Not specified | In general, for most burn wounds, weigh the risk of delayed wound healing | NSTA | Not specified | Not specified |
| Full and deep-partial | Major | Inpatient | Especially applies to burn wounds prior to surgical debridement | AS, ACD | Silver-based topical agents and dressings. | Not specified | ||
| Superficial-partial thickness | Minor | Outpatient | More superficial burns expected to heal on their own, treated as outpatients. | ACD | Silver-containing dressings (Hydrofiber and activated charcoal dressings) | Not specified | ||
| Full and deep-partial | Minor and major | Inpatient | Deep burns of the ear. Deep burns. | AB | Mafenide acetate 11% cream + *topical nystatin | Mafenide acetate 5% aqueous solution | ||
| Full | Major | Inpatient | It may have a role in for deeper burn wounds (that are not expected to heal spontaneously) prior to surgical excision. | AS | Dakin's solution (0.5% buffered NaOCl) | Not specified | ||
| Superficial-partial | Not specified | Not specified | Resource-limited settings with no available topical antimicrobial ointments or creams | AS | Honey | Not specified | ||
| Full | Major | Inpatient | Early surgical excision and wound closure cannot be performed | AS | Cerium nitrate (2.2% CN with 1% SSD) | Not specified | ||
|
Guideline and treatment algorithm for burn injuries (34) (PMID: 25904267) |
41.66 (51.03) | Superficial-partial | Minor | Outpatient | Minor burns | ACD | Nitrofurazone 0.2% pomade | Not specified |
| Full | Major | Inpatient | Eschar and large burns | ACD | 1% silver sulfadiazine (SSD) | Mupirocin, Nitrofurazone | ||
*Abbreviations: AB= Antibiotics, AS= Antiseptics, ACD= Antimicrobial-containing dressings, NSTA= Not specified topical antimicrobials
Assessment: AGREE II
Formal methodological assessment of the guidelines’ development process was evaluated using the AGREE II (APPRAISAL OF GUIDELINES FOR RESEARCH & EVALUATION II) tool, which uses a Likert-scale scoring system to evaluate each of its 23 items. Concomitantly, these items are grouped within six main domains: scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence 16. As indicated by the AGREE II User’s Manual, for each guideline, both evaluators worked independently to assign a score to each of the tool’s items, and domain scores were later calculated and tabulated. (Supplementary Table 3) In addition, mean appraisal scores (arithmetic mean for all the domains in one guideline, including overall assessment) were calculated. There is no fixed AGREE II cutoff score at present, so we established a value for mean appraisal scores of >70% as adequate quality, 60-69.99% as intermediate quality, and <60% for low quality.
We used intraclass correlation coefficients (ICC) with a two-way random-effects model 29 and the corresponding 95% confidence intervals to estimate the global inter-rater agreement. Additionally, we calculated ICC values for each AGREE-II domain score and each CPG. Our models were built under the assumption that both evaluators were unbiased now of scoring, both representing a random variable. To test the null hypothesis that the AGREE II appraisal scores were assigned by random chance, we used a two-way random-effects ANOVA model, with P-values <0.05 considered statistically significant. The level of agreement of ICC’s was classified as poor (ICC < 0.40), moderate (ICC 0.40–0.59), substantial (ICC 0.60–0.79), or excellent (ICC 0.80–1.00). Statistical analyses were performed using R (The R Project for Statistical Computing Version 1.4.1).
RESULTS
Key data and recommendations
Overall, 635 relevant titles were identified through database searches and 14 through Google Scholar and citation searches. After abstract screening and a full-text review of potential titles, 11 CPGs that met the inclusion criteria were selected 11, 30-39. Table 1 summarizes the key data that was extracted from each CPG. The included CPGs notably originated from a heterogeneous background of countries and geographic regions, with seven different countries identified, one continental CPG 38 and three international CPGs 31,35,37. It is worth noting that all the selected guidelines were developed by diverse medical societies focused on different fields in burn care.
Only two of the selected CPGs provide explicit recommendations in favor of the general use of topical antimicrobial agents in most burn wounds to prevent infection, regardless of other specific factors (burn-wound depth, extension, clinical scenario) 32,35 . Notably, the recommendations vary between most guidelines because they consider these factors for their formulation. In this way, all the included CPGs recommend preferred topical antimicrobial agents to prevent burn-wound infection in specific situations. Nonetheless, only five CPGs suggested alternative agents in the same specific scenarios 33–35,39,40 . In general, the majority of guidelines tend toward recommending antimicrobia–containing modern dressings over conventional antiseptics/antibiotics 30,32–38,40 . As for more conventional agents, silver sulfadiazine (SSD) is the most commonly reported; however, recommendations tend to favor its use in low-resource settings and in deeper wounds (Full and deep-partial thickness).
Among the most commonly identified clinical scenarios in CPGs were low-resource settings and scenarios with austere conditions 31–33,35,37,39. Interestingly, among the alternative agents recommended in these scenarios are honey 32,33,35,39 and SSD 31,39 for partial and full-thickness wounds, and Dakin’s solution for full-thickness wounds39 . Other commonly identified clinical scenarios were deeper wounds in settings where early excision is not possible 32,35,37 or prior to excision 32,35,39 . Likewise, these recommendations showed a preference for silver compounds and silver-containing dressings. Other reported scenarios include facial burns39, deep ear burns35, uncertainty about wound depth 33., emergency room (inability to transfer the patient to burn unit) 36, and burn unit-specific topical management 30.
Finally, categorizing burn wounds and considering wound depth is paramount in identifying key recommendations for their management. Five CPGs provide at least one recommendation in favor of using topical antimicrobials without explicitly specifying burn-wound depth31,32,35,38,39. Contrarywise, in most recommendations, burn-wound depth was clearly identified and addressed. As for superficial-partial thickness wounds, silver-containing dressings were the most recommended 33,35,37, but other preferred agents included iodine-containing dressings 37, honey 35, and nitrofurazone34. Of note, recommendations for deeper wounds often incorporate deep-partial and full-thickness burn wounds. For these categories, silver-containing dressings 32,35,36,39, silver sulfadiazine 1% 31,34,35,39, mafenide acetate 11% cream35,39 were the most recommended agents. Overall, most guidelines have listed silver compounds as the preferred strategy for burn-wound infection prevention in terms of burn-wound depth.
Methodological assessment
The AGREE II domain scores for each CPG are presented in Figure 1. Of the 11 CPGs, only five guidelines reached a mean appraisal score and overall assessment of >70% 35–38,40, while two guidelines obtained mean appraisal scores and overall assessments <60% 31,34. The overall mean appraisal score (%) of all the included CPGs was 67.20%, and similarly, the mean overall assessment was 68.9%. The domain that obtained the highest mean score for all guidelines was “1. Scope and purpose” with 86% (SD=17.87%), which assesses the guideline’s aim, formulation of specific health questions, and target population 16. On the contrary, the domain with the lowest mean score for all guidelines was “3. Rigor of development” with 53.87% (SD=29.85%), which evaluates the systematic methods for collecting information, formulating recommendations, and updates 16. The global ICC was 0.62 (95% confidence intervals 0.54-0.69), which corresponds to a substantial global concordance between both appraisers. The P-value for the global ICC was <0.001, qualified as statistically significant. A detailed description of the domain scores for each CPG and intraclass correlation (ICC) calculations can be found in Supplementary tables 3, 4, and 5.
DISCUSSION
The key strength of this systematic review is that, to the extent of our knowledge, a study that evaluates the methodological quality of burn-care CPGs and assesses their recommendations on this subject has not been previously conducted. In this way, including AGREE II appraisal scores provides insight into the recommendations included herein and the methodological standards under which they were developed. While other studies have used the AGREE II appraisal tool to evaluate CPGs in burn care, they have focused on areas other than burn-wound management.41–43. Moreover, we did not restrict our search to English or databases only, broadening the possibilities of finding as many available international CPGs as possible. We also included guidelines from various societies and disciplines that address different clinical scenarios, which further enriches the compilation of recommendations in different settings and allows for comparison and contrast.
Given that there is no apparent consensus on the optimal use of topical antimicrobial agents to prevent burn-wound infection, the objective of the present systematic review was to identify, compare and condense the recommendations that current international CPGs provide in different clinical scenarios. Notably, while all the comprised CPGs provide recommendations in favor of topical antimicrobial agents to prevent burn-wound infection in specific situations, the recommendation on preferred topical agents remains dependent on assessing factors such as clinical scenario, burn-wound depth, and burn severity. Still, it is necessary to emphasize that there is currently no ideal topical antimicrobial agent that can be recommended in all clinical scenarios.
The most noteworthy finding is perhaps that eneralds and their broad range of alternatives are still the most recommended first-choice agents in partial and full-thickness burn wounds. Remarkably, whereas most guidelines will endorse more modern silver-containing dressings for most clinical scenarios, silver sulfadiazine is typically considered for deeper wounds and burn wounds treated in low-resource settings. Extensive evidence exists in favor of recommending the use of silver compounds as the mainstay in burn wounds. Its bactericidal activity, low incidence of antimicrobial resistance 44 and broad antimicrobial spectrum make it useful against gram-positive and gram-negative organisms 45, particularly the most common pathogens infecting burn wounds (Staphylococcus aureus and Pseudomonas aeuriginosa) 2,46,47,48. In contrast, the recommendations for using topical antibiotics are unsurprisingly limited to situations like superficial or facial wounds. This can be explained by the findings of a systematic review conducted by Norman et al., who suggest that “antimicrobial resistance is transmitted even more frequently by topical application of antibiotics” 10. Additionally, a recent meta-analysis conducted in Iran that compared antibiotic-resistance profiles in Iranian burn patients, linked antibiotic overuse and misuse to higher resistance in pathogenic organisms 48. Hence, in the imminent era of antimicrobial resistance, achieving burn-wound infection prevention while also counteracting the development of resistant organisms is essential.
As to the methodological quality of the CPGs, we used the AGREE II appraisal tool to evaluate the process of formulating these recommendations. We hypothesized that the scores assigned by each appraiser would not be attributed to random chance. In that matter, our appraisal scores yielded an estimated ICC of 0.62 (95% confidence intervals 0.54-0.69, with a statistically significant P-value <0.001). This corresponds to a substantial global concordance between both appraisers, thus, rejecting our null hypothesis. Of note, levels of agreement that qualify as excellent are usually highly suggestive of bias.
The results of this systematic review indicate that there are currently few guidelines to guide wound care that satisfactorily meet this tool’s evaluating criteria. Consequently, poor methodology in developing recommendations may favor non-standardized practice. Therefore, methodological development of more consistent recommendations identifying a clear question to address, a specific clinical scenario, and explicit recommended agents and alternatives is essential to better guide practitioners in their clinical decisions.
Despite its strengths, certain caveats should be noted regarding the present systematic review. Firstly, a small number of available CPGs met inclusion criteria (n=11), mostly from developed countries. We were unable to retrieve guidelines from geographic regions like Latin America or Africa using our search methods (which would further broaden our compilation of recommendations). However, some of the included guidelines addressed or were aimed at low-resource settings explicitly. Furthermore, the findings in the methodological appraisal may be somewhat limited by the subjective nature of the evaluating scale and the nonideal number of reviewers (minimum two, preferably four) 16. Despite well-established evaluations and criteria, Likert scores are prone to subjectivity. Nonetheless, the AGREE II has been widely used as an available tool to evaluate CPGs since the creation of its first version, and it has undergone extensive testing, with results showing that it is a valid and reliable instrument49.
CONCLUSION
Burn-wound infection prevention is a priority issue due to the numerous undesirable consequences in the patient, such as alterations in healing, sepsis, and death. Therefore, the use of topical agents with antimicrobial properties is considered a standard intervention. The present systematic review aimed to compile the available recommendations in international CPGs on topical antimicrobial agents to prevent burn-wound infection. Although there is a trend in CPGs towards recommending antimicrobial-containing dressings (specifically silver compounds) for partial and full-thickness burn wounds, most guidelines fail to issue recommendations with specific agents or for specific, different clinical scenarios. Herein, we have developed an integrative approach that approximates guidance that is more consistent by compiling and evaluating available recommendations. Future development of CPGs that abide by methodological criteria is essential for achieving a more standardized practice.
CONFLICT OF INTEREST
None declared.
SUPPLEMENTARY FILES
ACKNOWLEDGMENTS
Systematic review was conducted under the supervision of Harvard Medical School Postgraduate Education’s Effective Writing for Healthcare faculty. No financial support was received for this study.
References
- 1.American Burn Association. National Burn Repository: 2019 Update, Report of data from 2009–2018. 2019. [Google Scholar]
- 2.Greenhalgh DG, Saffle JR, Holmes JH 4th, Gamelli RL, Palmieri TL, Horton JW, et al. American Burn Association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28(6):776–90. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 3.Corcione S, Pensa A, Castiglione A, Lupia T, Bortolaso B, Romeo MR, et al. Epidemiology, prevalence and risk factors for infections in burn patients: results from a regional burn centre’s analysis. J Chemother. 2021;33(1):62–6. doi: 10.1080/1120009X.2020.1780776. [DOI] [PubMed] [Google Scholar]
- 4.Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, et al. Burn wound healing and treatment: review and advancements. Crit Care. 2015;19(1):243. doi: 10.1186/s13054-015-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norbury W, Herndon DN, Tanksley J, Jeschke MG, Finnerty CC. Infection in burns. Surg Infect (Larchmt) 2016;17(2):250–5. doi: 10.1089/sur.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mir MA, Khurram MF, Khan AH. What should be the antibiotic prescription protocol for burn patients admitted in the department of burns, plastic and reconstructive surgery: Antibiotic prescription protocol for burn patients. Int Wound J. 2017;14(1):194–7. doi: 10.1111/iwj.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cambiaso-Daniel J, Boukovalas S, Bitz GH, Branski LK, Herndon DN, Culnan DM. Topical antimicrobials in burn care: Part 1-topical antiseptics. Ann Plast Surg. 2018 doi: 10.1097/SAP.0000000000001297. doi: 10.1097/SAP.0000000000001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merchant N, Smith K, Jeschke MG. An ounce of prevention saves tons of lives: Infection in burns. Surg Infect (Larchmt) 2015;16(4):380–7. doi: 10.1089/sur.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers. 2020;6(1):11. doi: 10.1038/s41572-020-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norman G, Christie J, Liu Z, Westby MJ, Jefferies JM, Hudson T. Antiseptics for burns. Cochrane Database of Systematic Reviews . 2017 doi: 10.1002/14651858.CD011821.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster K. Clinical guidelines in the management of burn injury: a review and recommendations from the organization and delivery of burn care committee. J Burn Care Res. 2014;35(4):271–83. doi: 10.1097/BCR.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 12.Barajas-Nava LA, López-Alcalde J, Roqué I Figuls M, Solà I, Bonfill Cosp X. Antibiotic prophylaxis for preventing burn wound infection. Cochrane Database of Systematic Reviews. 2013 doi: 10.1002/14651858.CD008738.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heyneman A, Hoeksema H, Vandekerckhove D, Pirayesh A, Monstrey S. The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: A systematic review. Burns. 2016;42(7):1377–86. doi: 10.1016/j.burns.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd ECO, Rodgers BC, Michener M, Williams MS. Outpatient burns: prevention and care. Am Fam Physician. 2012;85(1):25–32. [PubMed] [Google Scholar]
- 15.Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Advanced Drug Delivery Reviews . 2018 doi: 10.1016/j.addr.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–42. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Publications. Ministry of Health NZ. [[cited 2022 Sep 15]]. Available from: https://www.health.govt.nz/publications?f%5B0%5D=im_field_publication_type%3A26.
- 18.CPG infobase: Clinical practice guidelines. CMA Joule. [[cited 2022 Sep 15]]. Available from: https://joulecma.ca/cpg/homepage.
- 19.Evidence search service closure information | NICE. [.[cited 2022 Sep 15]]. Available from: https://www.evidence.nhs.uk.
- 20.Gov.au. [[cited 2022 Sep 15]]. Available from: https://www.clinicalguidelines.gov.au.
- 21.Guidelines international network. Z Evid Fortbild Qual Gesundhwes [Internet]. 2008 [cited 2022 Sep 15];102(2):119–20. Available from: https://guidelines.ebmportal.com.
- 22.American Burn Association. Journal of Burn Care and Research [Internet] Practice Guidelines Collection. 2021. 2021. [ [cited 2021 Jul 24]]. Available from: https://academic.oup.com/jbcr/pages/practice_guidelines_collection.
- 23.Asia pacific burn association Apburn.org. [ [cited 2022 Sep 15]]. Available from: https://www.apburn.org.
- 24.DGV. [cited 2022 Sep 15] Verbrennungsmedizin.de. Available from: https://verbrennungsmedizin.de.
- 25.British Burn Association. Britishburnassociation.org. [ [cited 2022 Sep 15]]. Available from: https://www.britishburnassociation.org.
- 26.American Burn Association. American Burn Association 2017 [cited 2022 Sep 15] Available from: https://ameriburn.org.
- 27.European Burns Association (EBA) European Burns Association (EBA) 2016 . [[cited 2022 Sep 15]]. Available from: https://www.euroburn.org.
- 28.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–63. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NSW Statewide Burn Injury Service. Clinical Guidelines: Burn Patient Management. Agency for Clinical Innovation; 2019. [Google Scholar]
- 31.Médecins Sans Frontières. Clinical Guidelines: Diagnosis and Treatment Manual. Chapter 10: Medical and Minor surgical procedures. 2021 [Google Scholar]
- 32.Jeschke M, McCallum C, Baron D, Godleski M, Knighton J, Shahrokhi S. Foundations of Best Practice for Skin and Wound Management. 2018. Best practice recommendations for the prevention and management of burns. [Google Scholar]
- 33.Deutsche Gesellschaft für Verbrennungsmedizin (DGV) Behandlung thermischer Verletzungen des Erwachsenen. [Google Scholar]
- 34.Yastı AÇ, Şenel E, Saydam M, Özok G, Çoruh A, Yorgancı K. Guideline and treatment algorithm for burn injuries. Ulus Travma Acil Cerrahi Derg. 2015;21(2):79–89. doi: 10.5505/tjtes.2015.88261. [DOI] [PubMed] [Google Scholar]
- 35.Allorto N, Atieh B, Bolgiani A, Chatterjee P, Cioffi W, Dziewulski P, et al. ISBI practice guidelines for burn care, part 2. Burns. 2018;44(7):1617–706. doi: 10.1016/j.burns.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Vaittinada Ayar P, Benyamina M. Prise en charge du patient brûlé en préhospitalier Première partie : cas eneral et inhalation de fumées. Annals of burns and fire disasters. 2019;32(1):22–9. [PMC free article] [PubMed] [Google Scholar]
- 37.ISBI Practice Guidelines Committee, Ahuja RB, Gibran N, Greenhalgh D, Jeng J, Mackie D, et al. ISBI practice guidelines for burn care. Burns. 2016;42(5):953–1021. doi: 10.1016/j.burns.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 38.European Burns Association. European Practice Guidelines for Burn Care: Minimum Level of Burn Care Provision in Europe. [Google Scholar]
- 39.Cancio LC, Barillo DJ, Kearns RD, Holmes JH IV, Conlon KM, Matherly AF, et al. Guidelines for burn care under austere conditions: Surgical and nonsurgical wound management. J Burn Care Res. 2017;38(4):203–14. doi: 10.1097/BCR.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 40.Yoshino Y, Hashimoto A, Ikegami R, Irisawa R, Kanoh H, Sakurai E, et al. Wound, pressure ulcer and burn guidelines – 6: Guidelines for the management of burns, second edition. J Dermatol. 2020;47(11):1207–35. doi: 10.1111/1346-8138.15335. [DOI] [PubMed] [Google Scholar]
- 41.Grammatikopoulou MG, Theodoridis X, Gkiouras K, Stamouli E-M, Mavrantoni M-E, Dardavessis T, et al. AGREEing on guidelines for nutrition management of adult severe burn patients. JPEN J Parenter Enteral Nutr. 2019;43(4):490–6. doi: 10.1002/jpen.1452. [DOI] [PubMed] [Google Scholar]
- 42.Gamst-Jensen H, Vedel PN, Lindberg-Larsen VO, Egerod I. Acute pain management in burn patients: appraisal and thematic analysis of four clinical guidelines. Burns. 2014;40(8):1463–9. doi: 10.1016/j.burns.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Kis E, Szegesdi I, Dobos E, Nagy E, Boda K, Kemény L, et al. Quality assessment of clinical practice guidelines for adaptation in burn injury. Burns. 2010;36(5):606–15. doi: 10.1016/j.burns.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 44.Marx DE, Barillo DJ. Silver in medicine: the basic science. Burns. 2014;40 Suppl 1:S9–18. doi: 10.1016/j.burns.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Patel PP, Vasquez SA, Granick MS, Rhee ST. Topical antimicrobials in pediatric burn wound management. J Craniofac Surg. 2008;19(4):913–22. doi: 10.1097/SCS.0b013e318175b516. [DOI] [PubMed] [Google Scholar]
- 46.D’Abbondanza JA, Shahrokhi S. Burn infection and burn sepsis. Surg Infect (Larchmt). 2021;22(1):58–64. doi: 10.1089/sur.2020.102. [DOI] [PubMed] [Google Scholar]
- 47.Corcione S, Pensa A, Castiglione A, Lupia T, Bortolaso B, Romeo MR, et al. Epidemiology, prevalence and risk factors for infections in burn patients: results from a regional burn centre’s analysis. J Chemother. 2021;33(1):62–6. doi: 10.1080/1120009X.2020.1780776. [DOI] [PubMed] [Google Scholar]
- 48.Javanmardi F. A Study of multidrug resistance in prevalent gram-negative bacteria in burn patients in Iran: A systematic review and meta-analysis. Journal of Global Antimicrobial Resistance. 2019; 19:64–72. doi: 10.1016/j.jgar.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 49.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. Development of the AGREE II, part 2: assessment of validity of items and tools to support application. CMAJ. 2010;182(10):E472 –8. doi: 10.1503/cmaj.091716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.