Abstract
The phosphoglucomutase (PGM)-encoding gene of Bordetella bronchiseptica is required for lipopolysaccharide (LPS) biosynthesis. An insertion mutant of the wild-type B. bronchiseptica strain BB7865 which disrupted LPS biosynthesis was created and characterized (BB7865pgm). Genetic analysis of the mutated gene showed it shares high identity with PGM genes of various bacterial species and forms part of an operon which also encompasses the gene encoding phosphoglucose isomerase. Functional assays for PGM revealed that enzyme activity is expressed in both bvg-positive and bvg-negative strains of B. bronchiseptica and is substantially reduced in BB7865pgm. Complementation of the mutated PGM gene with that from BB7865 restored the wild-type condition for all phenotypes tested. The ability of the mutant BB7865pgm to survive within J774.A1 cells was significantly reduced at 2 h (40% reduction) and 24 h (56% reduction) postinfection. BB7865pgm was also significantly attenuated in its ability to survive in vivo following intranasal infection of mice, being effectively cleared from the lungs within 4 days, whereas the wild-type strain persisted at least 35 days. The activities of superoxide dismutase, urease, and acid phosphatase were unaffected in the PGM-deficient strain. In contrast, the inability to produce wild-type LPS resulted in a reduced bacterial resistance to oxidative stress and a higher susceptibility to the antimicrobial peptide cecropin P.
Of the Bordetella genus, Bordetella bronchiseptica is the principle effector of respiratory disease in a wide range of mammals (16). However, B. bronchiseptica only rarely infects humans (18, 46, 54). B. bronchiseptica is credited as being the primary etiological component of tracheobronchitis in dogs from as early as 1910 (14) and is known to be associated with atrophic rhinitis, a common bronchial affliction of swine (40). B. bronchiseptica infections are mediated by the controlled expression of a number of virulence factors, such as the adhesin filamentous hemagglutinin (10) and the toxins adenylate cyclase hemolysin and dermonecrotic toxin (7, 50). The regulation of these virulence determinants is under the control of a two-component signal transduction system known as the Bordetella virulence gene (bvg) locus. Genetic control is observed in response to environmental conditions such as temperature and chemical modulators, i.e., sulfate anions (1, 27).
Lipopolysaccharide (LPS) is a highly toxic and immunogenic molecule that constitutes a major component of the cell membranes in gram-negative bacteria (37). LPS has now emerged as having an integral role in the infection process, being responsible for resistance to serum, antibiotics (39), and naturally occurring antimicrobial peptides termed defensins (5). The role of LPS as an important adhesin molecule also seems likely; however, its role in the pathogenesis process still remains largely unknown. It has recently been stated that the LPS of some strains of B. bronchiseptica is regulated by the bvg system (48).
The LPS of the related bacterium Bordetella pertussis displays a structure that generally typifies that of nonenteric bacteria. This consists of a lipid A region anchored in the cell membrane, being linked to a branched oligosaccharide domain constituting the core, by a single keto-deoxyoctulosonic acid (2, 17, 48). These glycolipids lack long repeating oligosaccharide units as are found in the Enterobacteriaceae and are therefore sometimes termed lipooligosaccharides (37). B. bronchiseptica and Bordetella parapertussis do, however, produce an O-antigen of a single sugar polymer, consisting of 2,3-dideoxy-di-N-acetylgalactosaminuronic acid (4, 13). There is considerable variation in glycolipid structure within the genus Bordetella (48).
Two distinct bands, i.e., band A and band B (35), are evident upon electrophoresis of purified B. pertussis LPS. Band B consists of the core region of the molecule, whereas the slower-migrating band A is this same core with the addition of a distal trisaccharide comprised of N-acetylglucosamine, N-acetyl-N-methylfucosamine, and 2,3-di-deoxy-2,3-di-N-acetylmannosaminuronic acid (2). It is understood that all three elements of the LPS molecule, i.e., lipid A, core, and O-antigen, are required for virulence of Escherichia coli (23). Viability of the organism is not necessarily disrupted by the absence of the O-antigen or several of the core sugars, but the keto-deoxyoctulosonic residues of the core and the lipid A are essential for growth (23). So important is the lipid A component of LPS to the viability of the cell that it has been described as a suitable pharmaceutical target (34).
A gene cluster for LPS production in B. pertussis and B. bronchiseptica has been identified, and the probable functions of the gene products have been discussed (2, 36); however, the role of LPS in the B. bronchiseptica infection process remains largely undescribed. We describe here an LPS mutant of B. bronchiseptica, designated BB7865pgm, resulting from an insertion in the phosphoglucomutase (PGM)-encoding gene. This gene appears to be organized into an operon with the phosphoglucose isomerase (PGI)-encoding gene. Regulation of pgm is constitutive and is therefore controlled independently of the bvg system. Loss of PGM activity due to insertional mutation of the gene resulted in a truncation of the LPS. Resistance to oxidative stress was reduced in the mutants as was the ability to resist cecropin P. Finally, BB7865pgm was unable to survive within the mouse macrophage-like cell line J774.A1 or colonize mouse lungs following intranasal inoculation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
B. bronchiseptica wild-type strain BB7865 and its isogenic bvg-negative derivative BB7866 have been characterized in a previous study (33). E. coli strain SM10λpir (32) containing pUTmini-Tn5 lacZ1 (11) was used in mutagenesis experiments. E. coli JM109 (55) was used in standard cloning experiments while E. coli 294 Rifr (51) was used for cosmid cloning. B. bronchiseptica strains were grown on Bordet Gengou (BG) agar (Difco) containing defibrinated horse blood (10%, vol/vol) and Stainer and Scholte medium (SS-X) (45) or a modulating version of SS-X containing 40 mM MgSO4 replacing NaCl (SS-C). Liquid cultures were also grown in SS-X or SS-C. E. coli strains were grown on Z agar (51) or in LB broth (41). The following antibiotics were used at the indicated concentrations: cephalexin, 50 μg/ml; kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; rifampin, 100 μg/ml; nalidixic acid, 50 μg/ml; trimethoprim, 50 μg/ml. Isopropyl-β-d-galactopyranoside (IPTG) (0.04 mM) and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (0.004%, wt/vol) were used where appropriate.
Mini-transposon mutagenesis.
Conjugation between B. bronchiseptica BB7866 and the donor E. coli SM10λpir containing the suicide plasmid (pUTmini-Tn5 lacZ1) was performed. Equal amounts of each bacterial strain were mixed in 0.7% saline and plated onto BG agar. Transconjugants were selected from the resultant growth on SS-X at 37°C containing cephalexin, kanamycin, X-Gal, and IPTG.
In vivo chromosome transfer.
The method by which the mini-transposon was transferred from BB7866pgm to the homologous location on the wild-type chromosome (BB7865pgm) was that described by Smith and Walker (44). E. coli Q358(pR715::Tn813) was mated with BB7866pgm. This resulted in the plasmid being introduced into BB7866pgm. A permanent cointegration of the plasmid into the chromosome occurred, catalyzed by the transposase encoded by Tn813. This recombinant strain of BB7866pgm (Tpr Kmr) was then conjugally mated with BB7865 (Nalr Rifr). Chromosome transfer is then promoted by the integrated tra genes of pR751, and the recipient strain thus received the original Kmr cassette following homologous recombination.
DNA manipulations.
Plasmid DNA was extracted from host cells using midi-prep columns in accordance with manufacturer's instructions (Qiagen). Chromosomal DNA was extracted according to the method of Priefer et al. (38). Restriction endonuclease digestion and agarose gel electrophoresis were conducted using standard methods (41). Cosmid cloning was achieved via the packaging of recombinant cosmids (pHC79) (21) containing chromosomal DNA fragments into lambda phage Max Plax kits (Epicentre Technologies) and subsequent transduction into E. coli 294 Rifr cells. Desired clones were selected and screened by colony hybridization. Chromosomal DNA for Southern hybridization was transferred to positively charged nylon membranes by way of alkaline transfer. Probe DNA was either digoxigenin labeled (Roche) or radiolabeled with [α-32P]dATP using a nick translation kit (Gibco BRL). Blots were probed under stringent conditions (65°C hybridization). Automated DNA sequencing was performed on plasmid DNA with a Perkin Elmer ABI Prism 377 DNA sequencer. Reactions were performed with Perkin Elmer BigDye terminator cycle sequencing ready reaction mix. Contiguous sequences were constructed using AutoAssembler software (Perkin-Elmer) and aligned with sequences in the GenBank database located at the Australian National Genomic Information Service by utilizing the Blastp algorithm.
PGM and phosphomannomutase (PMM) assays.
Crude lysates were prepared from the strains to be tested for mutase activity using the modified method from Sandlin and Stein (42). Cultures of the strains were grown in either SS-X at 37°C or SS-C at 25°C to late logarithmic phase and then centrifuged. The resultant pellets were resuspended in 10 ml of sonication buffer (50 mM MOPS [morpholinepropanesulfonic acid], pH 7.0; dithiothreitol, 1 mM; EDTA, 3 mM). The cells were again pelleted and resuspended in 1 ml of sonication buffer and frozen at −80°C. The cells were immediately thawed and sonicated five times at maximum output for 15 s per burst (Branson Sonifier 250) and allowed to cool on ice between bursts. The sonicated sample was centrifuged at 68,000 × g for 20 min to remove cellular debris. The resulting supernatant is the crude lysate used in both mutase assays and was stored at −80°C until required. Protein concentration of the crude lysates were determined in triplicate with a standard Bradford reagent assay (Sigma). PGM and PMM activities were determined essentially as previously described (25, 42). Specific activities were expressed as milliunits per milligram of protein, where 1 μmol of NADP (substrate) is reduced to NADPH (product) in 1 min by 1 U of enzyme. NADPH production was calculated from its molar extinction coefficient of 6,220. Lysates were tested in duplicate, and three independent lysates were prepared.
LPS extraction.
Cells were grown to late log phase in SS-C at 25°C or SS-X at 37°C to an A560 of 1.0 and concentrated to A560 1.5 in phosphate-buffered saline (PBS). The resultant cell pellet from 500 μl of sample was resuspended in 100 μl of distilled H2O. An equal volume of 2× sample buffer (6% sodium dodecyl sulfate; 6% 2-mercaptoethanol; 10 mM dithiothreitol; 46% glycerol; 60 mM Tris, pH 8.0; 0.1% bromophenol blue) was added, and the samples were boiled for 10 min. Protein was digested by the addition of proteinase K to a final concentration of 50 μg/ml at 37°C overnight. Samples were again boiled for 10 min, and a second volume of proteinase K, equal to the first, was added and incubated at 55°C for 3 h. LPS samples were stored at −20°C until required. LPS profiles were determined by Tricine-sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Gels of 16.5% were assembled and electrophoresis was performed as described by Lesse et al. (28). LPS was visualized by oxidative silver staining (47).
Complementation of pgm.
The mini-transposon insertion into BB7865pgm was complemented by the conjugal transfer into BB7865pgm of plasmid pBBR1MCS-4 (26), which harbored a 1.5-kb SacII fragment containing the complete pgm gene of BB7865. This plasmid was designated pBBR1MCS-4/pgm+.
Resistance to oxidative stress.
A disk diffusion assay was utilized to determine sensitivity to paraquat (methyl viologen; Sigma) (22). Approximately 106 cells of BB7865, BB7866, BB7865pgm, and BB7866pgm were plated onto SS-X or SS-C. A filter with a pore size of 0.22 μm, presoaked in 10 mM paraquat, was placed onto each plate, and this was followed by incubation at 30 or 37°C. Sensitivity to paraquat was measured by the zone of inhibition surrounding the disk. The zone was measured in two axes, and the mean values were calculated.
SOD, acid phosphatase, and urease assays.
For superoxide dismutase (SOD) assays, sonicated cell samples (100% output; four 15-s bursts) were cleared by centrifugation (12,000 × g for 2 min). A total of 115 μg of protein from each sample was loaded onto an 8% native polyacrylamide gel and electrophoresed according to standard procedures (52). Nitroblue tetrazolium was used to reveal regions of enzyme activity as outlined by Beauchamp and Fridovich (6). Acid phosphatase and urease activities were determined as previously described (22, 24, 31).
Cecropin P radial diffusion and liquid killing assays.
The sensitivity of the mutant strains to cecropin P, a bioreactive peptide, was tested. Radial diffusion assays were performed essentially as described previously (5). Bacteria were cultured on BG agar before being resuspended to a final optical density of 0.2 at A600 in SS-C or SS-X. Low-gelling-temperature agarose (1%) in either SS-X or SS-C was prepared and when cool was supplemented with bovine serum albumin (final concentration, 0.15%). To 10-ml aliquots, 200-μl aliquots of the cell suspensions were added and allowed to set in a standard 90-mm-diameter petri dish. Cecropin P (5 μg; 1 μg/μl in H2O; Sigma) was added to 3-mm-diameter wells made in the agarose. Following incubation at room temperature for 4 h, the plates were transferred to 37°C (SS-X plates) until zones were clearly visible. For liquid killing assays, BB7865 and BB7865pgm were cultured on SS-X at 37°C and suspended in PBS. Equal volumes of the cell suspension and cecropin P were combined, giving a final peptide concentration of 50 μg/ml. Following 1.5 h of incubation at 37°C, serial dilutions were performed on BB7865 and BB7865pgm, with and without cecropin P; plated onto SS-X; and incubated at 37°C.
Tissue culture.
The mouse macrophage-like cell line J774.A1 (ATCC TIB 67) was maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum (vol/vol) and 5 mM glutamine in an atmosphere containing CO2 (5%, vol/vol) at 37°C. Approximately 5 × 104 cells were seeded per well in 24-well tissue culture plates, incubated for 18 h, and then washed twice with complete medium. For invasion assays the method essentially followed that described by Guzmán et al. (19). The results reported are mean values of three independent assays with standard deviations. Incubation of B. bronchiseptica bacteria, resuspended to an optical density equal to that used in the invasion assays in Dulbecco's modified Eagle medium supplemented with gentamicin at 50 mg/ml for 2 h resulted in >6 orders of magnitude of reduction in CFU.
Murine respiratory infection model.
Female BALB/c mice at 6 to 10 weeks of age were used as a model of in vivo respiratory infection by B. bronchiseptica. Following treatment with Ketamine (50 mg/kg) and Xylazine (10 mg/kg) in sterile PBS, two 12.5-μl aliquots of a bacterial suspension were delivered intranasally to each mouse via an air-displacement pipette and the mouse was allowed to recover. The total number of viable bacteria administered was approximately 105 cells. At each time point four mice from each group were sacrificed and their lungs were removed aseptically. Lungs were homogenized in sterile physiological saline, and appropriate dilutions were plated onto nutrient agar to determine the number of viable bacteria present in the lungs.
Statistics.
The results tested for statistical significance were subjected to Student's t test. Differences were considered significant if P was ≤0.05.
Nucleotide sequence accession number.
The nucleotide sequence data for PGM and PGI have been submitted to the GenBank database under the accession number AF171632.
RESULTS
Identification of a novel LPS genetic locus in B. bronchiseptica.
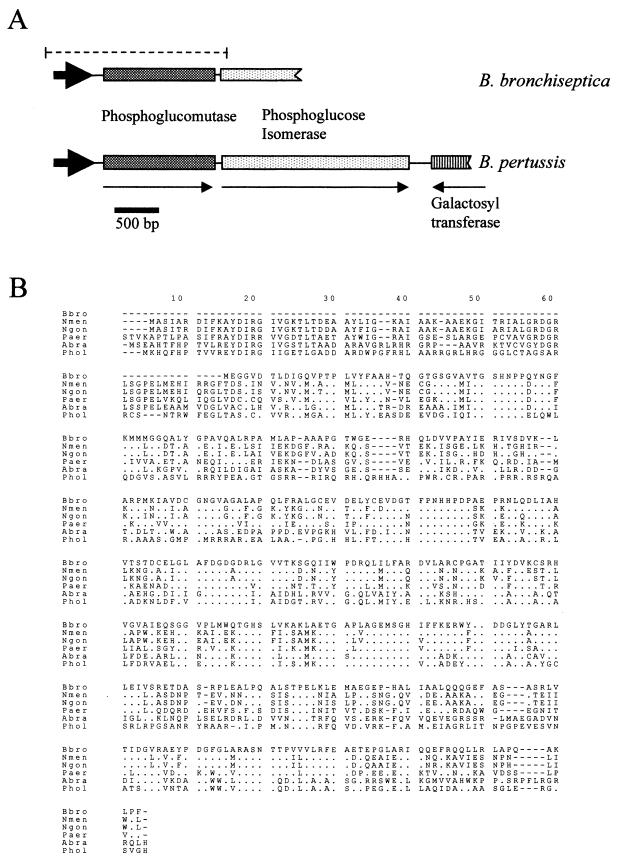
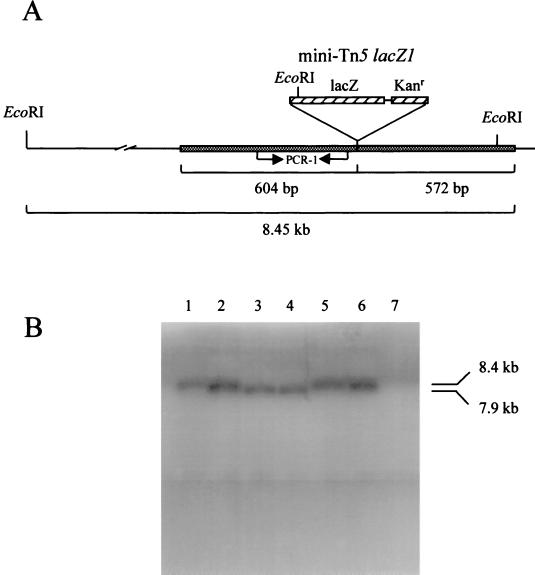
A mini-Tn5 mutant of the BvgS mutant strain BB7866 was produced (designated BB7866pgm) that demonstrated an inability to synthesize a complete LPS molecule, as demonstrated by oxidative silver staining of LPS extracts. Initial DNA sequence data obtained from a cosmid clone positive for the mini-Tn5 in Southern blots (data not shown) allowed the production of PCR primers adjacent to the insertion site. These primers enabled the amplification of a 300-bp gene fragment which was utilized to probe a BB7865 cosmid library. The result of these investigations, following cosmid cloning and subcloning, was a 3.4-kb NotI fragment shown to contain a complete gene (Fig. 1A) with an amino acid sequence identity of 55% (72% similarity) with the PGM gene from Neisseria gonorrhoeae (Fig. 1B). The PGM gene was shown via Southern blotting to hybridize with DNA fragments of B. pertussis and B. parapertussis but not with Bordetella avium (Fig. 2). Separated by just 10 bp and downstream of the PGM-encoding gene, a second open reading frame was identified which exhibits high homology with the PGI gene from several organisms. These two genes appear to be organized into an operon (Fig. 1A) based on their spatial organization and the absence of an intervening promoter-like sequence. PGI is also found in a similar genetic organization in B. pertussis and shares 99.3% nucleotide sequence homology with the B. bronchiseptica pgi gene as sequenced thus far (the B. pertussis genome is found on line at http://www.sanger.ac.uk/Projects/B_pertussis/). This operon has not been previously described in Bordetella. An open reading frame located downstream of the pgi gene from B. pertussis has been identified as encoding a possible galactosyl transferase; however, this gene is not included within the pgm operon. The identical gene was mutated in BB7865, the parental strain of BB7866, by way of an in vivo chromosome transfer technique (44). This allowed for the effects of such a mutation to be observed in a bvg-positive background. The resultant mutant was designated BB7865pgm.
FIG. 1.
Genetic organization and sequence homology of pgm. (A) Spatial orientation of the operon from B. bronchiseptica containing pgm and pgi. The equivalent genes were found to exist in B. pertussis in the same orientation and with high DNA sequence homology (98.6 and 99.3%, respectively). A gene downstream of B. pertussis pgi was located and shown to have the highest homology with other galactosyl transferases. The dashed line indicates the fragment of DNA cloned into pBBR1MCS-4 (26) and thereafter utilized for complementation of BB7865pgm. Arrows indicate the direction of transcription. Arrowheads indicate putative promoter sequences. (B) ClustalW alignment of B. bronchiseptica (Bbro) pgm gene and the following homologous sequences (pgm and pmm) (abbreviations and accession numbers are given in parentheses): Neisseria meningitidis pgm (Nmen; P40391), N. gonorrhoeae pgm (Ngon; P40390), P. aeruginosa pmm (Paer; P26276), Azospirillum brasilense pmm (Abra; P45632), and Prochlorothrix hollondica pmm (Phol; U23551). Identical residues are depicted by dots. Dashes indicate gaps introduced in the amino acid sequence for optimal alignment.
FIG. 2.
Characterization of the pgm insertion mutation. (A) The mini-Tn5 insertion (5.0 kb) was found to have occurred 604 bp downstream of the pgm methionine start codon. (B) Southern blot analysis of pgm mutants and other Bordetella spp. utilizing the 300-bp PCR fragment (PCR-1), amplified adjacent to the mini-transposon insertion (see panel A). The pgm gene exists in an EcoRI fragment of 8.4 kb. An EcoRI site located on the transposon results in a restriction fragment 539 bp shorter than that of the wild type. Lane 1, BB7865; lane 2, BB7866; lane 3, BB7865pgm; lane 4, BB7866pgm; lane 5, B. pertussis; lane 6, B. parapertussis; lane 7, B. avium.
Analysis of PGM enzyme activity from BB7865, BB7866, and their respective pgm-deficient mutants.
PGM assays were performed to determine the level of enzyme activity in parental and mutant strains. These assays demonstrate that the mutation eliminates the ability to produce functional enzyme, as BB7865pgm and BB7866pgm are shown to have a PGM activity below the detection limits of the assay (i.e., <2 mU/mg). This was shown to be statistically significant (P ≤0.01) for strains grown in either SS-C at 25°C or SS-X at 37°C (Table 1). PMM assays were also performed, as the mutated gene also shared high amino acid homology to PMM from Pseudomonas aeruginosa. Similar results were obtained for this enzyme, with PGM mutants again displaying low levels of activity, whereas the wild type expressed levels closer to 8.12 mU/mg. Complementation of BB7865pgm with the wild-type pgm gene restored PGM and PMM activity to levels observed in BB7865. It is of interest that PGM is known to be a bifunctional enzyme (42) capable of using either glucose or mannose as a substrate. Mannose, however, is not reported as a component of the B. pertussis LPS (3); it therefore seems likely that the affected enzyme acts as PGM in B. bronchiseptica.
TABLE 1.
Specific activities of PGM and PMM in B. bronchiseptica wild-type and mutant strainsa
| Strain | Sp act (mU/mg) of enzyme under condition:
|
|||
|---|---|---|---|---|
| PGM
|
PMM
|
|||
| SS-C, 25°C | SS-X, 37°C | SS-C, 25°C | SS-X, 37°C | |
| BB7866 | 7.37 ± 0.39 | 6.3 ± 0.32 | 10.9 ± 1.1 | 6.36 ± 0.3 |
| BB7866pgm | 1.9 ± 0.45 | 0.29 ± 0.13 | 1.75 ± 0.33 | 1.14 ± 0.06 |
| BB7865 | 7.17 ± 0.23 | 6.26 ± 0.28 | 8.12 ± 0.77 | 6.4 ± 0.4 |
| BB7865pgm | 0.46 ± 0.3 | 0.81 ± 0.23 | 1.31 ± 0.55 | 0.9 ± 0.36 |
| BB7865pgm (pBBR1MCS-4/pgm+) | 9.1 ± 0.83 | 7.75 ± 1.7 | 11.1 ± 0.42 | 7.2 ± 1.12 |
Assays were conducted in triplicate for cells grown under modulating and nonmodulating conditions. Assays were performed with independent lysates on three occasions. Values shown are means ± standard errors of the means.
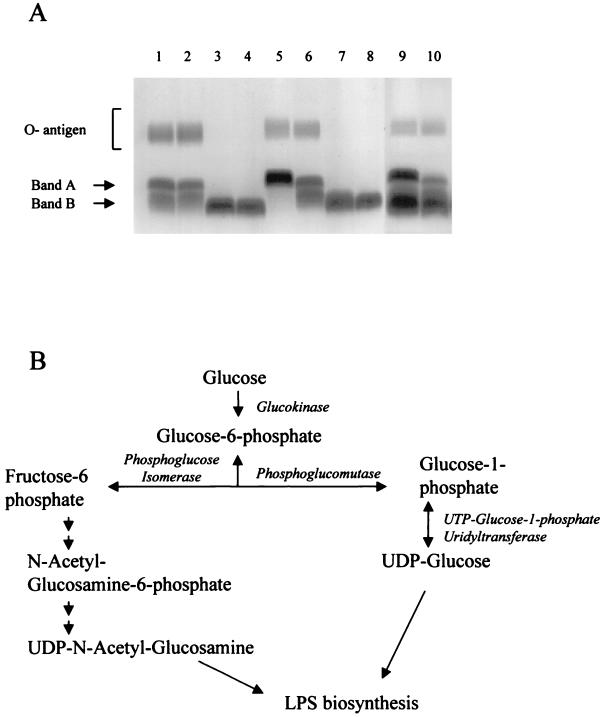
Electrophoretic profiles of LPS extractions clearly demonstrate the physical alterations caused by the mutation of PGM (Fig. 3A). The band A and band B core structures (35) and the O-antigen are all seen to be present in the parental strains (Fig. 3A). However, O-antigen is absent from BB7865pgm and BB7866pgm, and the core structure is considerably truncated, migrating faster than the band B of the parental strains. Complementation with pgm from the parental BB7865 strain restored the wild-type LPS phenotype to BB7865pgm (Fig. 3A). The influence of the bvg locus on the expression of PGM was analyzed. At 37°C and in the absence of chemical modulators such as MgSO4, virulence factors are expressed. There exists a separate class of genes that are repressed by the bvg locus, including the genes encoding flagellum biosynthesis (1). This occurs with growth in modulating medium, i.e., containing MgSO4, at lower temperatures (25°C). The size of the LPS molecule from the mutant strains is unaffected by growth under either modulating or nonmodulating conditions, as is the case for the wild type, indicating that the bvg locus elicits control only over the distal region of the molecule including the O-antigen. PGM and PGI are both required early in the pathway for production of sugar nucleotides destined for LPS synthesis. In fact they both catalyze the same substrate, glucose 1-phosphate, converting it to the respective precursors (Fig. 3B).
FIG. 3.
LPS phenotypes and biosynthesis pathway. (A) Electrophoretic profiles of LPS extracted from BB7865 (lanes 1 and 5), BB7866 (lanes 2 and 6), BB7865pgm (lanes 3 and 7), and BB7866pgm (lanes 4 and 8); lanes 9 and 10 contain the complemented mutant, BB7865pgm (pBBR1MCS-4/pgm+). Strains represented in lanes 1 to 4 and 9 were cultured in SS-C at 25°C, whereas those shown in lanes 5 to 8 and 10 were cultured in SS-X at 37°C. O-antigen and core band A and B are indicated to the left. (B) Biosynthesis of nucleotide sugars via pgm and pgi for production of LPS. (Modified from reference 56 with permission of the publisher.)
Intracellular survival of parental and PGM-deficient B. bronchiseptica.
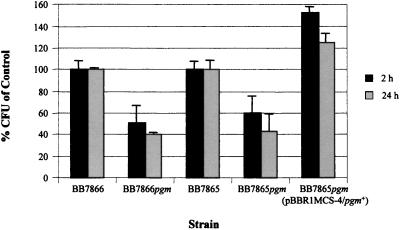
In vitro invasion and survival assays utilizing J774.A1 cells demonstrated a significantly reduced ability of the mutant strains to survive. Compared to the CFU of the respective parental strains, only 43% of BB7865pgm and 40% of BB7866pgm remained after 24 h (Fig. 4). Complementation of BB7865pgm with the wild-type pgm gene restored survival rates to levels above those observed in BB7865.
FIG. 4.
Intracellular survival of B. bronchiseptica strains in J774.A1 cells at 2 and 24 h. Results shown are represented as mean percentages of CFU recovered of the respective parental strains. Error bars indicate the standard error of the mean.
Highly reactive oxygen anion radicals are an effective intracellular defense mechanism employed by the host. Intracellular bacteria can utilize various enzymes such as SOD for the conversion of these radicals to more stable forms, i.e., O2− to H2O2 and O2. Of interest here is the effect of a compromised physical barrier to free radical attack; therefore, sensitivity of PGM mutants to oxidative stress was measured. BB7865pgm was notably more sensitive to paraquat (Table 2), a superoxide radical-generating compound, exhibiting a zone of inhibition of 8.5 mm on SS-C at 25°C and 7 mm on SS-X at 37°C compared to 0 mm for BB7865 under both conditions. Complementation of BB7865pgm with the wild-type pgm gene restored the wild-type phenotype. SOD activity levels remained unchanged for BB7865pgm (results not shown), suggesting a role for LPS as a physical barrier. Other phenotypes tested that are suggested to participate in intracellular survival within eukaryote cells are acid phosphatase (9) and urease (31). These phenotypes remained unaffected in the BB7865pgm mutant (results not shown).
TABLE 2.
Sensitivity of B. bronchiseptica to oxidative stress as measured by disk diffusion of paraquata
| Strain | Zone of inhibition (mm) under condition:
|
|||
|---|---|---|---|---|
| 30°C
|
37°C
|
|||
| SS-C | SS-X | SS-C | SS-X | |
| BB7866 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BB7866pgm | 4.5 ± 0.5 | 3.5 ± 0.5 | 3.5 ± 0.5 | 1.5 ± 1.5 |
| BB7865 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| BB7865pgm | 8.5 ± 1.5 | 3 ± 3 | 10.5 ± 0.5 | 7 ± 0 |
| BB7865pgm (pBBR1MCS-4/pgm+) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
Values shown are means for three separate assays ± standard errors of the means.
Resistance to the cationic peptide cecropin P.
LPS is credited as being responsible for conferring some protection against antibiotics and serum (53) as a mode of protection from host defenses. Another naturally occurring class of molecules are the defensins. These are cationic peptides found in a wide variety of vertebrate and invertebrate organisms (15) on the surface of skin, trachea, and tongue among others (20, 43). The sensitivity of BB7865pgm to the defensin cecropin P in a radial diffusion assay was shown to be significantly increased (P ≤ 0.01) compared to that of the wild-type strain (Table 3). Complementation of BB7865pgm with the wild-type pgm gene restored the wild-type phenotype in radial diffusion assays. It was also established that 90% of BB7865pgm, compared to BB7865 cells, were killed when incubated with a 50-μg/ml solution of cecropin P for 1.5 h in liquid killing assays (results not shown).
TABLE 3.
Sensitivity of B. bronchiseptica wild-type and mutant strains to cecropin P as measured by radial diffusion
| Strain | Inhibition zonea (mm) |
|---|---|
| BB7866 | 11 |
| BB7866pgm | 17 |
| BB7865 | 11.3 |
| BB7865pgm | 16.3 |
| BB7865pgm (pBBR1MCS-4/pgm+) | 10.6 |
Inhibition zone refers to the diameter of the region of growth inhibition.
Survival of BB7865pgm within the murine respiratory tract.
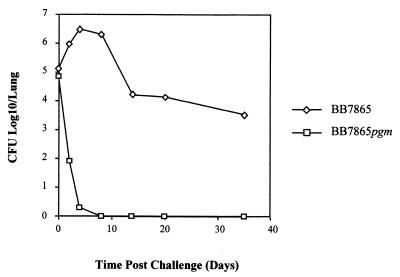
In vitro survival assays demonstrated a marked reduction in the ability of the PGM mutant to invade or persist within J774.A1 cells (Fig. 4). This result led to the investigation of the effect the B. bronchiseptica pgm mutation would have within a murine respiratory infection model. Nonlethal doses of BB7865 or BB7865pgm were administered to BALB/c mice intranasally, and the numbers of CFU present in the lungs were measured at various time points following infection. Although the wild-type strain showed a classic pattern of infection (22), the mutant strain was unable to survive, being effectively cleared within 4 days. This was the result observed in two independent trials. The reduction in BB7865pgm CFU was found to be consistently significant from day 2 (P ≤ 0.05). Mice infected with the wild-type strain still had an average of 1,000 CFU/lung persisting 35 days following inoculation (Fig. 5).
FIG. 5.
In vivo persistence of wild-type BB7865 and the pgm mutant, BB7865pgm, in a murine respiratory model. Lungs were taken at different time intervals, and the number of viable bacteria per lung was determined. The results are averages of values for four mice.
DISCUSSION
A new gene cluster critically involved in the production of LPS in B. bronchiseptica is described here. The operon includes genes identified as encoding PGM and PGI, which are utilized in the synthesis of nucleotide sugars for inclusion into growing LPS molecules. Abolition of PGM activity will lead to the loss of glucose and all other components distal to the heptose residue of the B. bronchiseptica inner core (i.e., band A, band B, and O-antigen). Of further interest is the fact that the abolition of PGM activity renders the bacteria more susceptible to antimicrobial peptides and oxidative stress, reduces bacterial ability to survive within J774.A1 cells, and results in an inability to colonize the murine respiratory tract. Complementation of the PGM mutant B. bronchiseptica pgm with the wild-type pgm gene restored the wild-type condition for all phenotypes tested, indicating that the mutation of pgm was responsible for the altered phenotypes observed in this study.
The role played by the bvg locus in regard to the regulation of the PGM operon was investigated and was not found to be required for expression. The bvg locus has been shown in this study and in others (48) to influence the expression of LPS in response to a modulating environment (i.e., reduced temperature and increased sulfate anions). This is exemplified by the identical LPS profile of the bvg-negative mutant BB7866 and BB7865 grown in SS-C at 25°C compared to the contrasting LPS profile seen in BB7865 when grown in SS-X at 37°C. It has been observed that LPS expressed by B. bronchiseptica can change during an infection (17). This type of expression has been suggested to be a mechanism of adaptation to the host environment in other mucosal pathogens such as P. aeruginosa and N. gonorrhoeae (12, 49). The length of the O-antigen has been demonstrated to be an important factor for resistance to complement (8). It is therefore feasible that regulation of the LPS composition includes one form required for colonization or invasion and another for survival in a particular niche protected from host humoral defenses. The bvg-repressed form of LPS may also be advantageous to the cell during transmission between hosts. Other evidence existing for bvg-regulated LPS expression includes monoclonal antibodies specific for band B LPS of B. pertussis, which only react with B. bronchiseptica LPS in a bvg-repressed state (30).
However, pgm is not regulated by bvg. There is not a significant difference in the activity of the enzyme produced by bvg-positive or bvg-negative parental strains under any growth condition. This result is mirrored in the LPS profiles of the mutant strains that indicate the same size LPS molecule is produced for both, irrespective of growth conditions. A possible explanation for this observation is that the early core biosynthesis is not bvg-regulated. These genes are likely to be expressed as housekeeping genes rather than being controlled by the virulence regulator. Genes utilized for the synthesis of the LPS molecule distal to the core glucose are not required for growth but are utilized for virulence and thus may be regulated by the bvg locus. This hypothesis also seems likely given that the genes of the PGM operon are distinct from the other LPS biosynthesis genes, such as the wlb genetic locus responsible for band A biosynthesis and also involved in O-antigen production. Strengthening this point is the existence of the waaA and waaC genes (required for synthesis of the deep inner core), which are not incorporated in the wlb locus (3, 36).
The importance of LPS to pathogenesis can be inferred by the B. bronchiseptica pgm mutant. The results obtained from the murine respiratory infection model demonstrate that although lipid A and the deep inner core are sufficient for viability, this truncated form of LPS is inadequate for colonization and survival of B. bronchiseptica in vivo. The absence of the O-antigen is likely to be a major factor in the behavior of BB7865pgm mutant in vivo. The increased sensitivity to defensins is probably due to the lack of the O-antigen and may offer some reason as to why bacterial clearance was so efficient. The antimicrobial peptide tested in this study was of the cecropin class. These, like the defensins, are cationic peptides and play an important role in the innate immunity of the host respiratory tract. The mode of action of the peptides is the destabilization of the cellular membrane by binding with anionic phospholipids (29). The O-antigen of B. bronchiseptica is thought to constitute a protective barrier, thereby concealing the negative charge of the membrane (5).
Resistance to superoxide anions is an important factor in terms of bacterial intracellular survival. Evidence to suggest that LPS is in some way responsible for a level of protection from intracellular superoxide radicals may come from the fact that the mutants were more susceptible to paraquat despite SOD levels remaining at wild-type levels (results not shown). Again, the high charge of B. bronchiseptica O-antigen may be able to shield the cell from the destructive O2− radicals, at least at the concentration tested in this study.
The bvg regulation of the distal portion of the LPS molecule is in response to the environment in which the bacterium finds itself. The pathogen must be capable of expressing an O-antigen of the correct length and composition. Sequential mutagenesis of the LPS molecule from the distal region would be required to highlight which portion of the molecule is specifically required for pathogenesis.
ACKNOWLEDGMENTS
We acknowledge support from the Australian Research Council (grant A09231658). N. P. West is the recipient of a University of Wollongong postgraduate research award.
REFERENCES
- 1.Akerley B J, Monack D M, Falkow S, Miller J F. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J Bacteriol. 1992;174:980–990. doi: 10.1128/jb.174.3.980-990.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 3.Allen A G, Isobe T, Maskell D J. Identification and cloning of waaF (rfaF) from Bordetella pertussis and use to generate mutants of Bordetella spp. with deep rough lipopolysaccharide. J Bacteriol. 1998;180:35–40. doi: 10.1128/jb.180.1.35-40.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen A G, Thomas R M, Cadisch J T, Maskell D J. Molecular and functional analysis of the lipopolysaccharide biosynthesis locus wlb from Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Mol Microbiol. 1998;29:27–38. doi: 10.1046/j.1365-2958.1998.00878.x. [DOI] [PubMed] [Google Scholar]
- 5.Banemann A, Deppisch H, Gross R. The lipopolysaccharide of Bordetella bronchiseptica acts as a protective shield against antimicrobial peptides. Infect Immun. 1998;66:5607–5612. doi: 10.1128/iai.66.12.5607-5612.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 7.Betsou F, Sismeiro O, Danchin A, Guiso N. Cloning and sequence of the Bordetella bronchiseptica adenylate cyclase-hemolysin-encoding gene—comparison with the Bordetella pertussis gene. Gene. 1995;162:165–166. doi: 10.1016/0378-1119(95)00339-8. [DOI] [PubMed] [Google Scholar]
- 8.Byrd D W, Roop R M, Veit H P, Schurig G G. Serum sensitivity and lipopolysaccharide characteristics in Bordetella bronchiseptica, B. pertussis and B. parapertussis. J Med Microbiol. 1991;34:159–165. doi: 10.1099/00222615-34-3-159. [DOI] [PubMed] [Google Scholar]
- 9.Chhatwal G S, Walker M J, Yan H R, Timmis K N, Guzman C A. Temperature dependent expression of an acid phosphatase by Bordetella bronchiseptica—role in intracellular survival. Microb Pathog. 1997;22:257–264. doi: 10.1006/mpat.1996.0118. [DOI] [PubMed] [Google Scholar]
- 10.Cotter P A, Yuk M H, Mattoo S, Akerley B J, Boschwitz J, Relman D A, Miller J F. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun. 1998;66:5921–5929. doi: 10.1128/iai.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deLorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deretic V, Schurr M, Yu H. Pseudomonas aeruginosa, mucoidy and the chronic infection phenotype in cystic fibrosis. Trends Microbiol. 1995;3:351–356. doi: 10.1016/s0966-842x(00)88974-x. [DOI] [PubMed] [Google Scholar]
- 13.Di Fabio J L, Caroff M, Karibian D, Richards J C, Perry M B. Characterization of the common antigenic lipopolysaccharide O-chains produced by Bordetella bronchiseptica and Bordetella parapertussis. FEMS Microbiol Lett. 1992;76:275–281. doi: 10.1016/0378-1097(92)90348-r. [DOI] [PubMed] [Google Scholar]
- 14.Ferry N S. A preliminary report of the bacterial findings in canine distemper. Am Vet Rev. 1910;37:499–504. [Google Scholar]
- 15.Ganz T, Lehrer R I. Defensins. Curr Opin Immunol. 1994;6:584–589. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 16.Goodnow R A. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980;44:722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gueirard P, Leblay K, Lecoustumier A, Chaby R, Guiso N. Variation in Bordetella bronchiseptica lipopolysaccharide during human infection. FEMS Microbiol Lett. 1998;162:331–337. doi: 10.1111/j.1574-6968.1998.tb13017.x. [DOI] [PubMed] [Google Scholar]
- 18.Gueirard P, Weber C, Lecoustumier A, Guiso N. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in host. J Clin Microbiol. 1995;33:2002–2006. doi: 10.1128/jcm.33.8.2002-2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzmán C A, Rohde M, Bock M, Timmis K N. Invasion and intracellular survival of Bordetella bronchiseptica in mouse dendritic cells. Infect Immun. 1994;62:5528–5537. doi: 10.1128/iai.62.12.5528-5537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harder J, Bartels J, Christophers E, Schroder J M. A peptide from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 21.Hohn B, Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980;11:291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 22.Jungnitz H, West N P, Walker M J, Chhatwal G S, Guzman C A. A second two-component regulatory system of Bordetella bronchiseptica required for bacterial resistance to oxidative stress, production of acid phosphatase, and in vivo persistence. Infect Immun. 1998;66:4640–4650. doi: 10.1128/iai.66.10.4640-4650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadrmas J L, Raetz C R H. Enzymatic synthesis of lipopolysaccharide in Escherichia coli—purification and properties of heptosyltransferase 1. J Biol Chem. 1998;273:2799–2807. doi: 10.1074/jbc.273.5.2799. [DOI] [PubMed] [Google Scholar]
- 24.Kaltwasser H, Schlegel H G. NADP-dependent coupled enzyme assay for urease and other ammonia producing systems. Anal Biochem. 1966;16:132–138. doi: 10.1016/0003-2697(66)90088-1. [DOI] [PubMed] [Google Scholar]
- 25.Köplin R, Arnold W, Hötte B, Simon R, Wang G, Pühler A. Genetics of xanthan production in Xanthomonas campestris: the xanA and xanB genes are involved in UDP-glucose and GDP-mannose biosynthesis. J Bacteriol. 1992;174:191–199. doi: 10.1128/jb.174.1.191-199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop R M, Peterson K M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 27.Lacey B W. Antigenic modulation of Bordetella pertussis. J Hyg. 1960;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesse A J, Campagnari A A, Bittner W E, Apicella M A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990;126:109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 29.Maloy W L, Kari U P. Structure-activity studies on magainin and other host defence peptides. Biopolymers. 1995;37:105–122. doi: 10.1002/bip.360370206. [DOI] [PubMed] [Google Scholar]
- 30.Martin D, Peppler M S, Brodeur B R. Immunological characterization of the lipooligosaccharide B band of Bordetella pertussis. Infect Immun. 1992;60:2718–2725. doi: 10.1128/iai.60.7.2718-2725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMillan D J, Shojaei M, Chhatwal G S, Guzman C A, Walker M J. Molecular analysis of the Bvg-repressed urease of Bordetella bronchiseptica. Microb Pathog. 1996;21:379–394. doi: 10.1006/mpat.1996.0069. [DOI] [PubMed] [Google Scholar]
- 32.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholorae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monack D, Arico B, Rappouli R, Falkow S. Phase variants of Bordetella bronchiseptica arise by spontaneous deletions in the vir locus. Mol Microbiol. 1989;3:1719–1728. doi: 10.1111/j.1365-2958.1989.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 34.Onishi H R, Pelak B A, Gerckens L S, Silver L L, Kahan F M, Chen M H, Patchett A A, Galloway S M, Hyland S A, Anderson M S, Raetz C R H. Antibacterial agents that inhibit lipid A biosynthesis. Science. 1996;274:980–982. doi: 10.1126/science.274.5289.980. [DOI] [PubMed] [Google Scholar]
- 35.Peppler M S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984;43:224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Preston A, Allen A G, Cadisch J, Thomas R, Stevens K, Churcher C M, Badcock K L, Parkhill J, Barrell B, Maskell D J. Genetic basis for lipopolysaccharide O-antigen biosynthesis in Bordetellae. Infect Immun. 1999;67:3763–3767. doi: 10.1128/iai.67.8.3763-3767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Preston A, Mandrell R E, Gibson B W, Apicella M A. The lipooligosaccharides of pathogenic Gram-negative bacteria. Crit Rev Microbiol. 1996;22:139–180. doi: 10.3109/10408419609106458. [DOI] [PubMed] [Google Scholar]
- 38.Priefer U, Simon R, Puhler A. Cloning with cosmids. In: Puhler A, Timmis K N, editors. Advanced molecular genetics. Berlin, Germany: Springer-Verlag; 1984. pp. 190–221. [Google Scholar]
- 39.Rietschel E T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zahringer U, Seydel U, Dipadova F, Schreier M, Brade H. Bacterial endotoxin—molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 40.Roop R M, II, Veit H P, Sinsky R J, Veit S P, Hewlett E L, Kornegay E T. Virulence factors of Bordetella bronchiseptica associated with the production of infectious atrophic rhinitis and pneumonia in experimentally infected neonatal swine. Infect Immun. 1987;55:217–222. doi: 10.1128/iai.55.1.217-222.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning. A laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 42.Sandlin R C, Stein D C. Role of phosphoglucomutase in lipooligosaccharide biosynthesis in Neisseria gonorrhoeae. J Bacteriol. 1994;176:2930–2937. doi: 10.1128/jb.176.10.2930-2937.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schonwetter B S, Stolzenberg E D, Zasloff M A. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 44.Smith A M, Walker M J. Transfer of a pertussis toxin expression locus to isogenic Bvg-positive and Bvg-negative strains of Bordetella bronchiseptica using an in vivo technique. Microb Pathog. 1996;20:263–273. doi: 10.1006/mpat.1996.0025. [DOI] [PubMed] [Google Scholar]
- 45.Stainer D, Scholte M. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1971;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 46.Tamion F, Girault C, Chevron V, Pestel M, Bonmarchand G. Bordetella bronchiseptica pneumonia with shock in an immunocompetent patient. Scand J Infect Dis. 1996;28:197–198. doi: 10.3109/00365549609049077. [DOI] [PubMed] [Google Scholar]
- 47.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 48.van den Akker W M R. Lipopolysaccharide expression within the genus Bordetella—influence of temperature and phase variation. Microbiology. 1998;144:1527–1535. doi: 10.1099/00221287-144-6-1527. [DOI] [PubMed] [Google Scholar]
- 49.Van Putten J P. Phase variation of lipopolysaccharide interconversion of invasive and immuno-resistant phenotypes of Neisseria gonorrhoeae. EMBO J. 1993;12:4043–4051. doi: 10.1002/j.1460-2075.1993.tb06088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker K E, Weiss A A. Characterization of the dermonecrotic toxin in members of the genus Bordetella. Infect Immun. 1994;62:3817–3828. doi: 10.1128/iai.62.9.3817-3828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker M J, Birch R G, Pemberton J M. Cloning and characterisation of an albicidin resistance gene from Klebsiella oxytoca. Mol Microbiol. 1988;2:443–454. doi: 10.1111/j.1365-2958.1988.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 52.Westermeier R. Electrophoresis in practice. Weinheim, Germany: VCH Verlagsgesellschaft; 1993. [Google Scholar]
- 53.Wood A C, Oldfield N J O, Dwyer C A, Ketley J M. Cloning, mutation and distribution of a putative lipopolysaccharide biosynthesis locus in Campylobacter jejuni. Microbiol. 1999;145:379–388. doi: 10.1099/13500872-145-2-379. [DOI] [PubMed] [Google Scholar]
- 54.Woolfrey B F, Moody J A. Human infections associated with Bordetella bronchiseptica. Clin Microbiol Rev. 1991;4:243–255. doi: 10.1128/cmr.4.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yanisch-Perron C, Vieiria J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 56.Zhou D G, Stephens D S, Gibson B W, Engstrom J J, Mcallister C F, Lee F K N, Apicella M A. Lipooligosaccharide biosynthesis in pathogenic Neisseria—cloning, identification, and characterization of the phosphoglucomutase gene. J Biol Chem. 1994;269:11162–11169. [PubMed] [Google Scholar]