Abstract
Myocardial ischemia with no obstructive coronary arteries (INOCA) is a chronic coronary syndrome condition that is increasingly being recognized as a substantial contributor to adverse cardiovascular mortality and outcomes, including myocardial infarction and heart failure with preserved ejection fraction (HFpEF). While INOCA occurs in both women and men, women are more likely to have the finding of INOCA and are more adversely impacted by angina, with recurrent hospitalizations and a lower quality of life with this condition. Abnormal epicardial coronary vascular function and coronary microvascular dysfunction (CMD) have been identified in a majority of INOCA patients on invasive coronary function testing. CMD can co-exist with obstructive epicardial CAD, diffuse non-obstructive epicardial CAD, and with coronary vasospasm. Epicardial vasospasm can also occur with normal coronary arteries that have no atherosclerotic plaque on intravascular imaging. While all predisposing factors are not clearly understood, cardiometabolic risk factors, and endothelium dependent and independent mechanisms that increase oxidative stress and inflammation are associated with microvascular injury, CMD and INOCA. Cardiac autonomic dysfunction has also been implicated in abnormal vasoreactivity and persistent symptoms. INOCA is under-recognized and under-diagnosed, partly due to the heterogenous patient populations and mechanisms. However, diagnostic testing methods are available to guide INOCA management. Treatment of INOCA is evolving, and focuses on cardiac risk factor control, improving ischemia, reducing atherosclerosis progression, and improving angina and quality of life. This review focuses on INOCA, relations to HFpEF, available diagnostics, current and investigational therapeutic strategies, and knowledge gaps in this condition.
Keywords: coronary microvascular, coronary vasospasm, myocardial ischemia, endothelial dysfunction
Graphical Abstract
1. Introduction
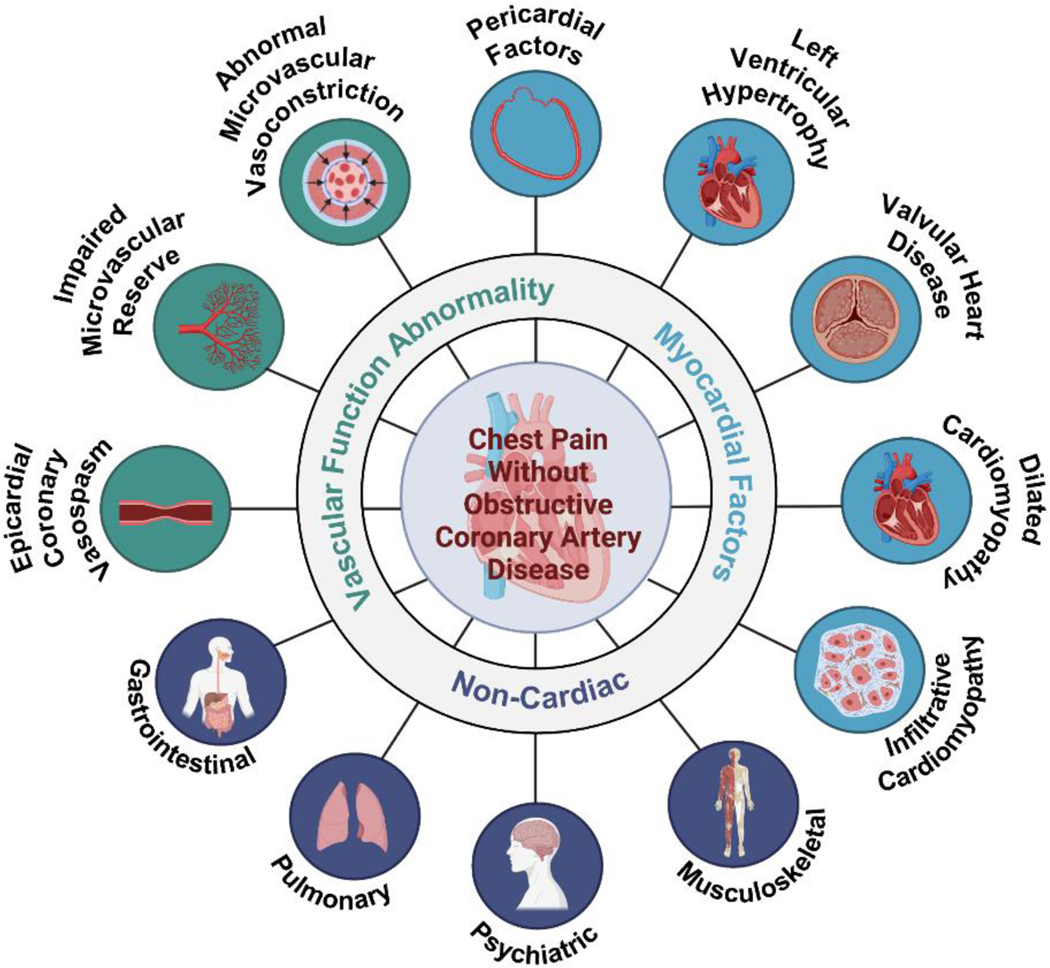
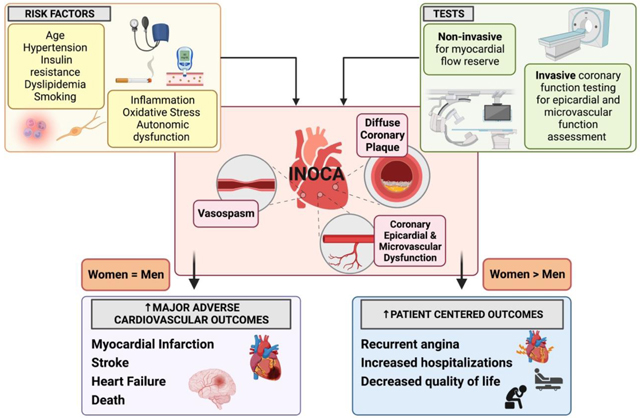
Approximately 50% of patients with chest pain and objective evidence of myocardial ischemia are found to have non-obstructive coronary artery disease (CAD) on angiography, which is defined as <50% stenosis in any major epicardial vessel.1–4 Evidence of ischemia usually refers to abnormal stress electrocardiogram, abnormal cardiac stress imaging test, or biomarker evidence (i.e. troponin elevation). This chest pain syndrome, termed ischemia with no obstructive coronary arteries (INOCA), is increasingly being recognized as a significant contributor to adverse cardiovascular outcomes such as myocardial infarction and heart failure with preserved ejection fraction (HFpEF) at long-term follow-up.1,5,6 While there is considerable heterogeneity in INOCA patients, mounting evidence over the past two decades indicates that INOCA patients are at risk for adverse cardiovascular morbidity and mortality.1,2,4 While patients with obstructive CAD and high CAD burden are the highest MACE risk group, having non-obstructive CAD is not benign and portends an intermediate prognosis, with a MACE risk that is higher than those with minimal to no atherosclerosis.7–9 Furthermore, there is significant symptom-related disability, recurrent hospitalizations for chest pain, and poor quality of life (QoL) in patients with INOCA, who often do not receive appropriate medical management because of the false perception that they are a low-risk group.1,10–13 Angina symptoms and functional disability in women does not seem to be associated with extent of atherosclerotic burden.10 INOCA is considered as a diagnosis when there is no obstructive CAD, no overt cardiomyopathy or heart failure, no valvular or structural heart disease, and no pericardial disease as an explanation of patient’s anginal symptoms (Figure 1). This syndrome is more common in women compared to men for reasons that are not clear, although INOCA also occurs in men and associated with adverse prognosis.14
Figure 1.
Etiology of chest pain without obstructive coronary artery disease.
The differential diagnosis of chest pain with no obstructive coronary artery disease is broad. If signs and symptoms point to angina, then abnormal coronary vascular dysfunction should be considered. Vascular mechanisms include coronary vasospasm (macro and microvascular spasm), coronary endothelial dysfunction, coronary microvascular dysfunction, capillary obstruction, or rarefaction. Image created using Biorender.com.
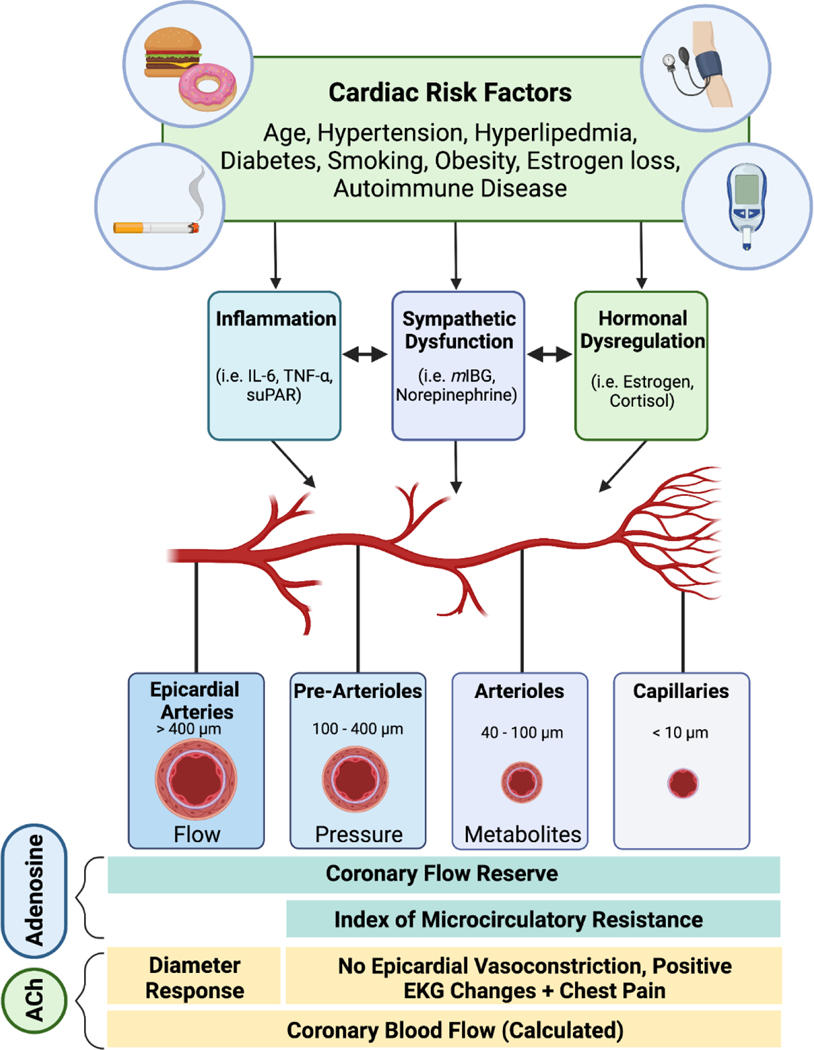
Studies such as the Women’s Ischemia Syndrome Evaluation (WISE) and others have found that a vast majority of patients with INOCA have coronary vascular dysfunction, which can be present in the epicardial vessels or the smaller arteries and the microcirculation.15–17 In this narrative review, we focus on these observations from the WISE and other recent studies that offer mechanistic insights in INOCA. Underlying cardiac risk factors such as hypertension, hyperlipidemia, diabetes, obesity, and menopause contribute to inflammation and oxidative stress that are associated with endothelial dysfunction and coronary microvascular dysfunction (CMD). CMD can lead to myocardial supply-demand mismatch and is defined as abnormal myocardial blood flow reserve in response to vasodilatory stimuli. Abnormalities in myocardial blood flow can occur due to both endothelium-dependent and endothelium-independent vascular dysfunction due to a variety of factors such as inflammation, capillary obliteration/obstruction, autonomic dysregulation, as well as local metabolic disruptions (e.g. hyperglycemia) (Figure 2). Over time this can lead to perivascular inflammation, microvascular injury, arteriolar remodeling, microthrombi, or capillary obliteration, which all contribute to CMD.
Figure 2.
Coronary vascular dysfunction.
Cardiac risk factors such as age, hypertension, insulin resistance, hyperlipidemia, smoking, obesity, menopause, and chronic autoimmune inflammatory disorders, lead to abnormal coronary vascular function (functional abnormalities as well as structural abnormalities from arteriolar remodeling) that impair myocardial blood flow and cause microvascular ischemia even in the absence of epicardial obstructive stenosis. The main stimulus for vascular reactivity varies along the coronary vascular tree depending on the vessel caliber and the surrounding myocardial matrix; for example, while changes in sheer stress and pressure stimulate periarteriolar vessels, the micro vessels are under metabolic control (pH, adenosine, hypoxia, K+, etc). Comprehensive coronary function testing using adenosine and acetylcholine interrogates coronary epicardial and microcirculatory function. Image created using Biorender.com.
A majority of women who present with signs and symptoms of ischemia but no obstructive CAD at coronary angiography continue to have chest pain at 1 year and 5 years.1,18 INOCA is also associated with anxiety, depression, and poor QoL in this group of patients.18 While CMD occurs in both men and women, it is unclear why women tend to have more angina and lower QoL compared to men. CMD can also be present in the setting of obstructive CAD, but usually once CAD is identified, anti-anginal and anti-atherosclerotic medications are deployed. The risk is higher in the presence of CMD and epicardial obstructive CAD.19 There are patients who have persistent angina even after percutaneous coronary angiography, where coronary vasospasm or CMD may be an explanation. A recent study noted a high prevalence of CMD in patients with recurrent angina post-PCI, and increased BMI and LDL correlated with CMD.20 A definitive diagnosis of CMD requires access to advanced imaging modalities and coronary function testing, which is not widely available in most centers. Pharmacologic and nonpharmacologic angina management strategies are currently used to treat INOCA. This review focuses on our current understanding of INOCA and addresses knowledge gaps (Table 2) that remain to reduce the morbidity and mortality caused by this underappreciated condition.
Table 2.
Knowledge gaps and future directions in INOCA
| Mechanistic research |
| ➢ Are there sex differences in human coronary artery innervation and blood flow regulation |
| ➢ What is the impact on microvascular function with premature estrogen loss? |
| ➢ Sex-differences in myocardial energy utilization |
| ➢ Investigation of pathways that lead to diastolic dysfunction and fibrosis in cardiometabolic inflammation |
| ➢ Chronic psychological stress, coronary inflammation, and immune dysfunction |
| ➢ Is CMD a systemic process: brain, renal, cardiac, retinal, skeletal muscle |
| ➢ Sex-differences in Central pain processing in INOCA syndrome |
| ➢ Are mechanisms of stress-induced abnormal microvascular reactivity in INOCA different than in obstructive CAD? |
| identification of at risk subsets |
| ➢ Using precision medicine methods – i.e. proteomic profiling |
| ➢ Leveraging electronic health record to better characterize INOCA phenotypes for risk stratification |
| ➢ Clarifying race/ethnic differences in CMD as a contributor to adverse outcomes |
| Improving diagnostic strategies |
| ➢ Utility & safety of functional testing in the setting of ACS/MINOCA |
| ➢ Improved imaging techniques/cost for radiotracers to detect coronary inflammation |
| ➢ Utility of cardiac sympathetic imaging in INOCA |
| Improving therapeutics/management |
| ➢ Can microvascular function be restored with angiogenic factors or stem cells? |
| ➢ Can nutraceuticals with flavonoids/polyphenols in berries, beets, dark chocolate, etc. impact microvascular flow reserve? |
| ➢ Will anti-atherosclerotic medications alter outcomes in INOCA? |
| ➢ GLP-1A and impact of weight loss on CMD-ischemia and INOCA symptoms |
| ➢ Use of endothelin receptor antagonists in INOCA |
| ➢ Testing autonomic modulation strategies for improvement in CFR and angina |
1.1. Terminology
Harvey Kemp used the term “Cardiac Syndrome X” (CSX) in 1973 to describe patients who had exercise induced angina and normal coronary angiograms.21 This general definition did not necessarily include objective evidence of ischemia and represented a broad heterogenous group. However, a stricter definition of CSX included not only exercise-induced, angina-like chest discomfort and normal epicardial coronary arteries, but also ST-segment depression during angina, and exclusion of coronary artery vasospasm (inducible or spontaneous), and also absence of myocardial disease that is associated with CMD such as hypertrophic cardiomyopathy.22–24 There are patients who have angina-like chest pain and no obstructive CAD on angiography but fail to meet the above CSX criteria. For example, patients who have angina predominantly at rest or those without ST changes noted during angina do not meet the strict definition of CSX. Chest pain in these cases is often attributed to non-cardiac, psychological, or gastrointestinal causes. The broad and strict definitions of CSX are used variably in the literature, and the historical heterogenous CSX cohorts lacked objective measures of coronary blood flow reserve and coronary vascular reactivity assessments, resulting in low ischemic burden and therefore relatively low MACE risk.24 As advanced imaging techniques such as cardiac PET and cardiac MRI have become more routine in clinical practice, along with evolution of invasive coronary function testing to interrogate various vascular dysfunction pathways, the term INOCA is increasing being used. INOCA was coined during an NHLBI-ACC sponsored think tank and includes all objective evidence of ischemia (whether it be electrocardiographic or by imaging).1 While INOCA improves on the CSX definitions, it still refers to a heterogenous group, and therefore specific criteria are proposed to standardize definitions of vasomotor disorders.25–27 There are many knowledge gaps and conundrums in INOCA, and the field of vasomotor disorders continues to evolve as our pathophysiologic understanding improves and newer imaging/interventional techniques emerge to assess coronary vascular function and its impact on the myocardium.
2. Myocardial Infarction with No Obstructive Coronary Artery Disease (MINOCA)
Myocardial Infarction with Non-Obstructive Coronary Artery (MINOCA) characterized by evidence of myocardial infarction (MI) in the absence of obstructive CAD. MINOCA accounts for up to 14% of patients with acute MI. MINOCA patients are more likely to be younger and female compared to patients with obstructive CAD.28,29 The pathogenesis of MINOCA is wide and includes vascular and non-vascular etiologies. Vascular causes include plaque rupture/erosion, coronary embolism/thrombosis, vasospasm, spontaneous coronary artery dissection and CMD. Takotsubo syndrome and myocarditis should be considered in the differential when a patient has a troponin elevation that is unexplained by obstructive CAD.30 Of note, per the International Takotsubo Registry (InterTAK) diagnostic criteria, a diagnosis of Takotsubo can coexist with obstructive CAD.31 To confirm a diagnosis of typical Takotsubo syndrome of apical ballooning and akinesia, obstructive CAD in the left anterior descending artery must be excluded. Nonetheless, obstructive CAD in other coronary arteries can coexist. The wide umbrella of diverse etiologies may contribute to confusion and misnomer and it has recently been proposed to use the term MINOCA exclusively to describe patients with evidence of ischemia-related myocardial necrosis.32 Understanding and identifying the underlying etiology of MINOCA is important to implement specific therapeutic plans and target the underlying pathogenesis. In the setting of MINOCA, use of advanced imaging (e.g., optical coherence tomography [OCT]) and cardiac magnetic resonance imaging [CMR]) is may be helpful beyond coronary angiography alone; in fact, multimodality imaging helps better characterize the mechanism of MINOCA.33,34 The Women’s Heart Attack Research Program (HARP), a prospective, multicenter, international, observational study, showed that multimodality imaging in women with MINOCA (N=170) identified potential mechanisms in 84.5% of patients (ischemic: 63.8%, myocarditis: 14.7%, takotsubo 3.4%, non-ischemic cardiomyopathy 2.6%, no cause identified: 15.5%).34 The ongoing HARP-2 study will further build on this work of multi-modality imaging by including both men and women with MINOCA to study sex-differences in pathophysiologic mechanisms of MINOCA (NCT02905357). Coronary artery spasm provocation and coronary function testing to diagnose CMD was not done in the HARP study. The ESC and AHA have provided diagnostic algorithms to aid in management of MINOCA.30,35
In a recent metanalysis of 14 studies with 30,733 MINOCA patients, the all-cause mortality and major cardiovascular events at 1 year were 3.4% and 9.6% respectively.36 A population-level Alberta COPAT study reported that 5-year mortality was 10.9% in patients with MINOCA.37 In addition, medical therapy was lower in the MINOCA group compared to the obstructive CAD group at 6 months. In another metanalysis, comparison showed that MINOCA patients (N=12,983) have more favorable prognosis than MI-CAD patients (N=133,247) (12-month all-cause mortality: 3.3% [95% CI, 2.5–4.1%] vs 5.6% [95% CI, 4.1–7.0%]; odds ratio, 0.60 [95% CI,0.520.70], p<0.001).28 When compared to patients with no MI, there was a trend that did not reach statistical significance toward a worse prognosis in the MINOCA patients. 28 In an observational study, Biere et al. reported that in 131 MINOCA patients with a normal left ventricular systolic function, ventricular arrythmia was noted in 13.8% of patients.38 Furthermore, 25% of MINOCA patients suffer from a high angina burden in 1 year similar to those with obstructive disease.39
MINOCA patients are less likely to receive secondary prevention treatment compared to patients with obstructive CAD.40,41 In addition, there is a lack of prospective randomized controlled trials and limited evidence-based literature on the management of patients with MINOCA.42–44 The SWEDHEART (Swedish Web-system for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapy) registry showed that after 4.1 years mean follow up of MINOCA patients, there were lower adverse cardiac events rates (all-cause mortality, hospitalization for MI, ischemic stroke, and heart failure) with angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin-receptor blockers, and a trend for lower event rate with beta-blockers. However, the use of dual antiplatelet agents was not associated with a lower cardiac event rate. 42
3. CMD and heart failure with preserved ejection fraction (HFpEF)
HFpEF is a heterogenous syndrome that is clinically characterized by symptoms and signs of heart failure (HF) with a normal or near-normal left ventricular ejection fraction. 45 HFpEF accounts for 50% of all diagnosed HF. 45 Myocardial overload secondary to hypertension has been traditionally considered as the mechanism of HFpEF development.46 Emerging data points towards relations between CMD and HFpEF, and cardiometabolic risk factors of obesity, insulin resistance, and related hypertension are all associated with both CMD and HFpEF. There is a high prevalence of CMD in HFpEF patients. 75%−81% of patients hospitalized with HFpEF were found to have CMD without obstructive epicardial CAD.47,48 Furthermore, women with CMD have more diastolic dysfunction and more fibrosis detected by (cardiac magnetic resonance) CMR imaging compared to non-CMD women.49,50 In 208 women with INOCA in the WISE study, a majority were found to have elevated resting left ventricular end diastolic pressure (LVEDP) with an elevated, and NT-pro BNP levels in this group correlated directly with age and inversely with BMI, but not coronary flow reserve.51 Compared to reference controls, women with INOCA were found to have greater aortic stiffness and lower early diastolic circumferential strain rate on CMR imaging.49
Early in vivo data in animal models has shown that HFpEF is associated with coronary microvascular endothelial activation from oxidative stress. A reduction in vascular nitic oxide (NO)-dependent signaling to myocardial tissue, which in turn could contribute to the elevated myocardial stiffness and hypertrophy that are characteristic of HFpEF.52 In an autopsy study patients with HFpEF had more cardiac hypertrophy, myocardial fibrosis, and coronary microvascular rarefaction than controls. These pathologic findings demonstrate an association between microvascular damage and LV diastolic dysfunction and impairment in cardiac reserve function.53
An exercise hemodynamics study by Van Empel et al. evaluated the role of impaired myocardial oxygen delivery in the pathophysiology of HFpEF. They found significantly lower transcardiac oxygen gradient in HFpEF patients compared to controls. This finding suggests that the abnormal diastolic reserve observed during exertion in HFpEF patients could be explained by impaired myocardial oxygen delivery, possibly explained by microvascular dysfunction. 54 Alterations in endothelial function of the peripheral vasculature have also been demonstrated in patients with HFpEF. Lee et al. found that reactive hyperemia, an index of microvascular function, was significantly reduced in patients with HFpEF, demonstrating that maladaptation at the microvascular level is present in HFpEF patients.55 Maréchaux et al. found that patients with HFpEF had abnormal endothelial function in the forearm microvasculature.56
Moreover, evidence of low vasodilatory reserve is an independent predictor of prognosis. Srivaratharajah et al. used Rb-82 positron emission tomography (PET) to evaluate global myocardial flow reserve (MFR) and found that a HFpEF diagnosis was associated with 2.6-fold greater risk of having low global MFR (<2.0). HFpEF remained a significant predictor of reduced global MFR after adjusting for comorbidities.57 Taqueti et al. reported that in patients with angina without epicardial CAD, impaired MFR was associated with diastolic dysfunction and increased hospitalization rates. The presence of CMD was associated with a markedly increased risk of HFpEF events.58 A similar result was observed in a recent prospective observational study where the presence of microvascular disease (defined as MFR < 2.0) was present in 70% of patients with HFpEF and was an independent predictor of prognosis.59 Dryer et al. evaluated coronary flow reserve (CFR) and the index of microvascular resistance (IMR) during cardiac catheterization and found that patients with HFpEF had more abnormalities of CFR and microvascular resistance, implicating CMD in HFpEF.60 In a recent study by Ahmad et al., participants underwent coronary function testing and exercise right heart catheterization to determine the relationship between CMD and filling pressures.61 Those with HFpEF were found to have a significantly lower CFR, and CFR was inversely correlated with pulmonary wedge pressure at rest and with exercise.
PROMIS-HFpEF (Prevalence of Microvascular Dysfunction in Heart Failure With Preserved Ejection Fraction) was a multicenter, prospective study that also recently demonstrated a high prevalence of CMD in HFpEF (75% of patients) in the absence of macrovascular obstructive CAD. HFpEF patients with CMD had markers of systemic endothelial dysfunction and markers of HF severity (elevated NTproBNP and right ventricular dysfunction) after multivariate adjustment. These findings further suggest that HFpEF is associated with endothelial and microvascular dysfunction.47 CMD-related HFpEF may represent a distinct subgroup of HFpEF patients where recurrent CMD-related microvascular ischemia causes myocardial shifts in energy metabolism, myocardial fibrosis, and diastolic dysfunction.62 A deeper understanding of the interrelatedness of CMD and HFpEF pathophysiology is needed to develop strategies that may halt the progression to HFpEF in the setting of cardiac risk factors, and to develop novel therapeutics in HFpEF.
4. INOCA, recurrent angina hospitalizations, and symptom disability
Patients with INOCA have recurrent angina hospitalizations and a reduced quality of life.10–13 In the WISE study of 883 women who were followed for a mean of 5 years, those with non-obstructive CAD (62% of the cohort) were compared to those with obstructive CAD. Myocardial infarction and cardiovascular death rates were higher in the obstructive CAD group. However, there was almost a two-fold increase in recurrent angina hospitalizations and repeat catheterizations in the non-obstructive group at one year follow-up.10 In another subsequent study from the WISE that focused on 224 women with INOCA, abnormal coronary endothelium-dependent vasoreactivity was associated with angina hospitalization at 5 year follow-up.63 In a large meta-analysis including 35,039 patients (49% women) with no obstructive CAD, while the prognosis was heterogenous and depended on presence of atherosclerosis, there was a high incidence of recurrent hospitalizations and testing in this cohort.12
COVADIS, which was an international cohort study consisting of 14 medical centers in seven countries, showed that patients with microvascular angina also reported having more physical limitations.64 A recent study evaluated quality of life measures in INOCA patients with either definite CMD (MPR<2.0) or borderline CMD (MPR 2.0–2.4).65 These patients reported greater physical limitations, angina frequency, and reduced QoL compared to stable CAD and acute MI patients. The exercise capacity for patients with and without definite CMD was also compared: no patients with CMD were able to achieve ≥10 METs. As a complement to these findings, Bechsgaard et al. found that although there was no difference in self-reported physical activity levels in patients with CMD compared to controls, patients with angina and CMD had severely reduced exercise capacity compared with sex-matched controls according to cardiopulmonary exercise testing results.66 In this study, women with CMD had lower peak VO2 values, with a median peak of 17.3 (IQR 15.5–21.3, p = 0.001) ml/kg/min compared to 21.8 (18.5–25.4) ml/kg/min in patients with normal coronary flow reserve. A study conducted in Poland investigated the role of exercise as a treatment for CMD. Fifty-five women with microvascular angina completed a cardiac rehabilitation program. Baseline and post-program exercise treadmill testing, myocardial perfusion imaging, and QoL surveys were administered. Regular exercise was shown to lead to improvement in patient symptoms and QoL (median score of 156 for baseline vs 159 after cardiac rehabilitation, p<0.05).67
5. INOCA pathophysiologic mechanisms and contributors
5.1. CVD risk factors
Traditional atherosclerotic risk factors of age, hypertension, insulin resistance, smoking, hyperlipidemia, and obesity are associated with INOCA. Furthermore, women have unique sex-specific risk factors68 such as preeclampsia/eclampsia and gestational diabetes, and whether these and other adverse pregnancy outcomes are associated with increased future CMD risk is unknown. Effects of aging and CVD risk factor burden increase during the menopause transition can all contribute to endothelial dysfunction. Polycystic ovarian syndrome (PCOS) is also associated with increased metabolic syndrome risk, endothelial dysfunction, and premature carotid atherosclerosis.69 Impaired arginine metabolism was reported in PCOS compared to women with normal menstrual cycles, along with reduced plasma nitric oxide levels, due to reduced inducible nitric oxide synthase (iNOS)/endothelial NOS (eNOS) transcript expression, low hydrogen peroxide levels, and high Asymmetric Dimethyl Arginine (ADMA) synthesis.70 INOCA studies have found significant coronary plaque burden,71,72 and it should be noted that extent of CAD is prognostic, even if it is non-obstructive. In the WISE study of symptomatic women with no obstructive CAD, 70–80% were found to have coronary plaque and positive remodeling detected by intra-vascular ultrasound study (IVUS) of the left coronary artery.73 Although studies have also shown that epithelial dysfunction and epicardial vasospasm can cause INOCA in the absence plaque demonstrated on IVUS as normal coronary arteries.74
Cardiometabolic risk factors of obesity, insulin resistance, and related hypertension are all associated with CMD and HFpEF. Emerging data has demonstrated the presence of CMD in obese individuals and CMD has been shown to be independently associated with higher BMI.75 Recently, Bajaj et al. reported a correlation between BMI and decreased CFR. A higher BMI in obese patients was associated with worsening coronary microvascular function, independent from other clinical risk factors. Interestingly, in obese patients, only those with impaired CFR demonstrated a significantly increased risk of major adverse cardiovascular events.76 A potential mechanism could be that obesity triggers systemic inflammation and, as adipose tissue is infiltrated with macrophages, the release of pro-inflammatory cytokines stimulates myocardial fibrosis and endothelial dysfunction at the microvasculature level.77
The relationship between diabetes and CMD has been explored extensively. Two major mechanisms, altered coronary autoregulation and impaired microvascular vasodilatory function, have been proposed to contribute to diabetes related CMD. Jaskanwal et al. reported an association between poor glycemic control and CMD among female patients with diabetes who presented with chest pain and non-obstructive CAD. These findings also highlight the importance of sex stratification models.78 Gallinoro et al. found that CFR and microvascular resistance reserve by coronary angiography were lower in patients with DM with no obstructive CAD compared with non-diabetic controls. Left atrial reservoir strain, an early marker of diastolic dysfunction, was decreased in diabetic patients, suggesting an early subtle diastolic dysfunction associated with microcirculatory impairment.79 Hyperglycemia has adverse effects on vascular endothelium.80 Diabetes also impairs autoregulatory responses, thereby exposing coronary microcirculation to higher resting flow.81 With time, diabetes leads to a significant structural microvascular remodeling with increased microvascular resistance in combination with reduced coronary flow velocity, which may be a consequence of prior exposure to continuously increased blood flow.82
5.2. Inflammation
The role of inflammation in endothelial dysfunction, atherosclerotic plaque initiation, progression, and destabilization of plaque is established and has been recently reviewed.83,84 Chronic underlying inflammatory and autoimmune disorders are considered risk enhancing factors that are not only associated with premature and accelerated atherosclerosis, but also CMD, even in the absence of obstructive CAD.85 Patients with conditions such as lupus, psoriasis, and rheumatoid arthritis are found to have evidence of low MFR, not explained by traditional CAD risk factors.86–89 In the WISE study of women with INOCA, an elevated IL-6 level predicted heart failure hospitalization and all-cause mortality at 6 years.90 Soluble urokinase plasminogen activator receptor (suPAR) is a circulating biomarker of inflammation and immune regulation that is released from various cell types including leukocytes and endothelial cells and is an independent predictor of outcomes in patients with CAD. In men and women with no obstructive CAD, plasma suPAR level was an independent predictor of CMD and predictive of MACE along with high sensitivity troponin I (hs-TnI).91,92 Interestingly, cross-coronary suPAR and plasminogen activator inhibitor (PAI-1) production rates were higher in those with coronary microvascular endothelial dysfunction compared to those without, as assessed by simultaneous measurements of these markers from the left main and the coronary sinus.93 Understanding the relationships between local vascular inflammatory cytokines, immune dysregulation, and impaired fibrinolytic pathways in contributing to CMD remains to be investigated for potential therapeutic options.
5.3. Autonomic nervous system
In addition to metabolic autoregulatory mechanisms that control vasomotor function, the cardiac autonomic nervous system (ANS) plays a critical role in myocardial blood flow regulation.94 It is hypothesized that the disruption of adventitial baroreceptors and chemoreceptors by advancing plaque may interfere with homeostatic autonomic regulation.95 There is also evidence implicating sympathetic dysfunction in vascular remodeling and microvascular complications.96 Prior cardiac syndrome X (CSX) literature has investigated the role of ANS and found impaired parasympathetic tone as well as sympathetic predominance.97 Low heart rate variability has been shown to precede episodes of myocardial ischemia, implicating the ANS in the pathophysiology of angina.98
In the Cardiac Autonomic Nervous System (CANS) study, women with INOCA (mean age 58 ± 9 years) had greater peripheral microvascular vasoconstriction in response to arithmetic mental stress compared to age-matched asymptomatic reference control women.99 Both groups had similar mean increases in blood pressures and heart rates with mental stress, so while hemodynamic reactivity was similar, the INOCA group experienced more chest pain and greater microvascular reactivity.99 In the CANS substudy over a quarter of women with INOCA had abnormal cardiac sympathetic activity, detected by 123I-meta-iodobenzylguanidine (mIBG) cardiac imaging, compared to reference controls.100 Cardiac sympathetic function has been investigated using the radiotracer mIBG in a variety of settings, including heart failure, to assess for potential of developing lethal arrhythmias101 and diabetic autonomic neuropathy.102–104 Low cardiac retention of mIBG at 4 hours after injection, measured by the heart to mediastinal ratio (HMR), is associated with ventricular tachyarrhythmias and adverse outcomes in patients with heart failure.101,105 Di Monaco et al..106 assessed whether mIBG uptake predicts symptomatic outcome in 40 participants with CSX with an average follow up of 79 months. Participants with worsening angina status, with hospital readmission for recurrent chest pain, and those who had repeat angiography had lower MIBG uptake, indicating that abnormal cardiac adrenergic nerve function predicts worse clinical outcomes. The role of heightend sympathetic activation in INOCA as a contributor to symptoms and microcirculatory dysfunction warrants further investigation.
Advancing age in women is marked by decreased vagal tonic modulation of heart rate and predominance of sympathetic tone.107 Furthermore, there is a reduction in β-receptor responsiveness and increase in α1-receptor responsiveness. One clinical syndrome related to INOCA where there is a clear sex difference is in Takotsubo Syndrome or stress cardiomyopathy.108 The prevalence is highest in post-menopausal women and is characterized by an abnormal response to a catecholamine surge due to emotional or a physical stressor, which leads to troponin elevation and acute heart failure, irrespective of obstructive CAD.109 The most common variant of apical ballooning may be secondary to the regional variation in the density of β-adrenergic receptors and cardiac sympathetic hyperactivity has been demonstrated based on abnormal cardiac mIBG uptake.110 It remains unclear why a majority of cases present in women but in addition to multi-vessel spasm, coronary endothelial and microvascular dysfunction are implicated.111,112 A possible mechanism for post-menopausal female prevalence may include the age-specific decrease in vagal tone and baroreflex sensitivity from reduced estrogen levels. Increased sympathetic activity and impaired baroreflex sensitivity were shown in women with takotsubo compared to women with chronic heart failure.113
6. Coronary microvascular dysfunction diagnosis
6.1. Non-invasive methods
Our currently used traditional diagnostic tests are designed to detect ischemia due to obstructive flow-limiting lesions. When a patient has a positive stress ECG but is found to have no obstructive CAD on angiography, the EKG ST segment depressions are dismissed as being “false positive”. Previously, this group of patients who were more likely to be female were referred to as having CSX which was believed to be benign. However, this term is now no longer used since a majority of these patients likely had CMD-related ischemia.114 In the CANS study, on 24-hour ambulatory monitoring of women with INOCA who were diagnosed with CMD by invasive evaluation and were compared to age-matched, asymptomatic reference controls, more than one-third of INOCA women had ST-segment depression, while control group had no depressions detected.115 If there are EKG changes indicative of ischemia or symptoms during exercise epicardial endothelial dysfunction or CMD should be suspected, even in the absence of wall motion or prefusion abnormalities (Table 1).116 Doppler echocardiography is used mainly in Europe to detect impaired coronary flow velocity reserve in response to hyperemia.117,118
Table 1.
Differences between INOCA and MINOCA.
| INOCA | MINOCA | |
|---|---|---|
| Symptoms | Yes | Yes |
| Resting EKG abnormalities | Possible | Possible |
| Exercise EKG abnormalities | Usually | N/A* |
| Abnormal wall motion on stress echo | Possible, but usually not | N/A |
| Abnormal perfusion on SPECT | Possible, but usually “breast artifact” or “probably normal” | N/A |
| Abnormal PET-derived myocardial flow reserve | If yes, diagnose CMD If no, could still be CMD due to a vasocontrictor problem; definitive diagnosis requires invasive coronary function testing |
N/A |
| Troponin elevation | May have a prior history of troponin elevation, and now recurrent non-MI chest pain | Yes |
Not applicable in acute coronary syndrome
Cardiac positron emission tomography (PET) is a non-invasive method for CMD diagnosis because of its ability to quantify rest and stress myocardial blood flow per gram of tissue and it provides myocardial flow reserve (MFR). A low MFR is associated with adverse outcomes regardless of CAD severity and in both men and women.19,58,119 Cardiac magnetic resonance (CMR) imaging detects semi-quantitative myocardial perfusion reserve index and may detect CMD, but is only available at select academic centers.120–123 Rahman et al. reported that highresolution CMR techniques using fully quantitative perfusion had a good accuracy and were better than visual assessment, for detecting CMD.124 Cardiac imaging modalities to detect abnormal vasodilator response to diagnose CMD have been recently reviewed.125
None of the non-invasive stress testing modalities can reliably detect microvascular spasm or coronary endothelial dysfunction, and a negative noninvasive stress test does not rule out coronary vasomotor dysfunction, especially in symptomatic patients. Invasive coronary function testing (CFT) is often needed to assess coronary vascular function pathways. Cassar et al. reported on limited accuracy of stress testing in patients with non-obstructive CAD who underwent both non-invasive stress testing (stress ECG, echocardiogram, or nuclear imaging) and invasive CFT within 6 months.126 Exercise ECG was found to be more specific but least sensitive for detecting vascular dysfunction compared to imaging tests, although only 9% had a cardiac PET in this study. A comprehensive CFT that includes testing for impaired vasodilatory reserve as well as heightened vasoreactivity is often needed for an accurate diagnosis.
6.2. Coronary function testing (CFT)
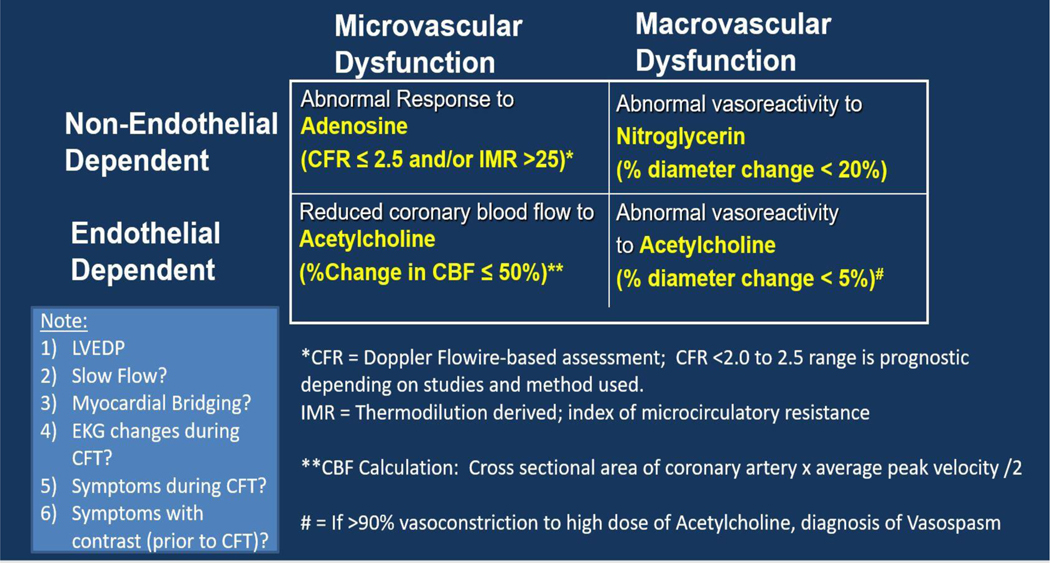
Invasive CFT, also referred to as coronary reactivity testing (CRT) or interventional diagnostic procedure (IDP) in the literature, is pursued to diagnose abnormal coronary vascular function and to guide therapy as well as for prognostication. In patients with persistent symptoms with INOCA, the differential includes epicardial coronary endothelial dysfunction, CMD, coronary vasospasm, myocardial bridging, and/or cardiac nociceptive abnormality. Invasive CFT can help diagnose micro- and macro-vascular dysfunction due to endothelial and nonendothelial pathways, and one example is shown (Figure 3), although there are variations in the methods and protocols among centers.15,127 Lack of standardized methods makes it difficult to combine results from various studies, but data point to the benefit of CFT to clarify diagnosis, for prognosis, and for improved patient-reported outcomes of angina and QoL.63,128 Safety of CFT has been published and it is typically done in the stable ischemic heart disease patients in the United States since it requires withholding vasoactive medications prior to testing, although testing in unstable angina and MI population has been reported.15,16 The European Society of Cardiology 2019 guidelines on management of chronic coronary syndromes give “guidewirebased CFR and/or microcirculatory resistance measurements” a IIa (level of evidence B) indication in those suspected of CMD, while intracoronary acetylcholine testing to assess microvascular spasm is a Class IIb (level of evidence B) recommendation.129
Figure 3.
Coronary Function Testing (CFT) components.
Vasoactive agents such as adenosine (that causes hyperemia) and acetylcholine (which tests NO-dependent pathway) are used in a comprehensive evaluation to diagnose coronary vasomotor disorders. Spasm testing is done using high dose acetylcholine.
There are four main vascular pathway abnormalities that are discussed in the literature: (1) CMD, assessed using vasodilatory agents such as adenosine5,14,15,64 (2) coronary microvascular spasm, diagnosed when acetylcholine testing induces ischemic ST changes and/or symptoms, but there is no visible epicardial spasm130; (3) epicardial endothelial dysfunction, typically diagnosed as abnormal decrease in epicardial coronary artery diameter to intracoronary acetylcholine;15 (4) coronary vasospasm (i.e. abnormal smooth muscle hyperactivity) with acetylcholine or ergonovine with >90% narrowing of the epicardial artery.25,26 Of note, testing protocols vary among various countries. For example, in the United States, endothelium-dependent CMD is also diagnosed based on percent change in coronary blood flow (CBF) in response to acetylcholine, which is calculated based on change in coronary artery diameter and flow velocity change in response to acetylcholine.15,131 EKG changes and symptoms are assessed during CFT, as well as pain with contrast and catheter is also noted to diagnose a nociceptive abnormality. Prior studies in CSX have reported abnormal pain sensitivity in women who present with persistent chest pain and are found to have no obstructive CAD.132,133
Abnormal hyperemic response to adenosine with a diminished CFR is associated with adverse CV outcomes.5 CFR is a combined measure that interrogates the flow through the epicardial artery and the downstream microvasculature, and is dependent on hemodynamic factors such as blood pressure; CFR ranges less than 2.0 – 2.5 are considered abnormal depending on the method used.134,135 In order to independently assess the microcirculatory function, the index of microcirculatory resistance (IMR) was developed.136 IMR is defined as the distal coronary pressure divided by the inverse of the hyperemic mean transit time and is a reproducible measure of microcirculatory function.136,137 An elevated IMR predicts major adverse cardiac events in patients with both stable and unstable angina.138,139 In addition, abnormal coronary endothelial function response to acetylcholine testing is also associated with adverse prognosis.25,63,140 While low doses of acetylcholine are used to test for endothelial dysfunction, high doses are used to test for coronary vasospasm, since at higher doses of acetylcholine, muscarinic receptors on vascular smooth muscle cells are activated.26
7. INOCA management
Management of INOCA centers around 3 main strategies: 1) Treatment of CVD risk factors, such as hypertension, diabetes, obesity, hyperlipidemia, and smoking since they contribute to endothelial dysfunction, CMD, and atherosclerosis; 2) Use of therapies to improve angina burden and QoL; 3) Use of therapies that would potentially improve MACE (trials are on-going). Given that a majority of INOCA patients do have coronary plaque on imaging, treatment of risk factors is imperative in this condition. The ongoing multicenter Women’s Ischemia Trial to Reduce Events in Non-Obstructive CAD (WARRIOR) trial is testing whether optimal medical therapy with maximally tolerated statin (atorvastatin or rosuvastatin), ACE-I (lisinopril)/ARB (losartan), and low dose aspirin leads to a reduction in MACE in women with INOCA (NCT03417388).141 The KAMIR‐NIH (Korean Acute Myocardial Infarction‐National Institutes of Health) registry demonstrated that the use of renin‐angiotensin system blockers and statins was associated with lower mortality in patients with MINOCA.43 An international, randomized trial in patients with Myocardial Infarction with No Obstructive Coronary Artery testing Beta Blocker and ACEI/ARB Treatment (MINOCA-BAT) is ongoing (NCT03686696). One important consideration for management is that information from invasive testing improves patient-oriented outcomes. The CORMICA trial enrolled 157 patients with angina and non-obstructive CAD and used invasive CFT to guide treatment compared to standard of care according to provider preference (with provider’s blinded to results of CFT),128 and found that therapy guided by CFT improved angina at 6 months and sustained improvement in angina and QoL at 1 year.
7.1. Traditional anti-anginals
Traditional anti-anginal therapy with beta-blockers and calcium channel blockers are usually the first line agents used in INOCA. Of note, published reports indicate that beta-blockers may be more beneficial than calcium channel blockers in Cardiac Syndrome X population;142,143 however, this has not been adequately investigated in CMD. Traditional, first and second generation beta-blockers (such as atenolol, metoprolol, and propranolol) are non-vasodilating, do not impact vascular resistance significantly, and have variable effects on flow reserve. In contrast, third generation vasodilating beta-blockers such as carvedilol and nebivolol improve vascular resistance and have shown to improve CFR in patients with dilated and hypertrophic cardiomyopathy.144,145 Carvedilol is a commonly prescribed lipophilic, non-selective β1/β2 receptor antagonist and a selective α1 adrenergic receptor antagonist that improves mortality in heart failure and reduces events after myocardial infarction.146 It has anti-oxidant properties,147,148 promotes nitric oxide release, improves endothelial function,149 induces vasodilation150 and inhibits vascular smooth muscle proliferation.151 Carvedilol has a stronger inhibitory effect on sympathetic nervous system compared to traditional beta-blockers and lacks sympathomimetic activity. It lowers coronary sinus norepinephrine levels, decreases muscle sympathetic nerve activity, and improves HRV (by both decreasing adrenergic activity and increasing vagal tone) in congestive heart failure.152–154 In contrast, calcium channel blockade does not affect plasma catecholamine levels. Studies with calcium channel blockers have shown variable results on INOCA patients.155–157 Patient with abnormal vasodilator reserve taking either verapamil or nifedipine may have improved angina, less nitrate use, and improved exercise tolerance.156 The ENCORE I (Evaluation of Nifedipine and Cerivastatin On Recovery of coronary Endothelial function) trial showed improved coronary endothelial function in patients taking nifedipine and cerivastatin for at least 6 months.158 However, a recent study testing Efficacy of Diltiazem to Improve Coronary Microvascular Dysfunction (EDIT-CMD) found that diltiazem did not improve angina or QoL in patients with CMD,159 although the subgroup of patients with coronary epicardial vasospasm improved. Limitations of this trial are notable for being underpowered, and including a heterogenous INOCA endo-type population, with several study dropouts who did not undergo a repeat CFT. There are no head-to-head contemporary clinical trials testing the impact of beta-blockers vs. calcium channel blockers in patients with INOCA based on specific vascular dysfunction pathways. Often there are inter-related mixed vascular pathway abnormalities in CMD patients, and a subset have concomitant coronary vasospasm in addition abnormal CFR and endothelial dysfunction. Use of late sodium channel blocker, ranolazine, has been established as an anti-anginal, with the advantage of not having significant hemodynamic effects on blood pressure, which enables its use in patients who do not tolerate other agents due to low blood pressures.160 Short acting nitrates are helpful in patients with vasospastic angina and stable angina from epicardial CAD. While nitrates do not have an effect on the microcirculation, since many patients with INOCA have a mixed pattern of spasm and abnormal vasodilatory reserve, nitrates are commonly used in management. In a study of 99 patients with CSX161 nitrates were effective in 40–50% of the patients. Long-acting nitrates did not improve exercise stress testing in patients with CMD compared to those with stable epicardial disease in one study.162 The vasodilator, Nicorandil, was found to improve exercise ischemia, without altering autonomic activity,163 and is another anti-anginal option but is not available in the United States. Nicorandil has nitrate-like properties and is available in Europe but not available in the United States.164 L-arginine, a nitric oxide precursor, showed an improvement in endothelial function and symptoms after 6 months of treatment.165 However, in the setting of post-myocardial infarction, this supplement had an adverse effect,166 and therefore, we recommend caution in those with a prior history of MI.
7.2. Other agents and strategies
Ivabradine is a selective inhibitor of the If current of the sinoatrial node and it is mainly used for heart rate control. In patients with stable CAD, treatment with Ivabradine has shown to significantly improve hyperemic coronary flow velocity and CFR.167 Compared to Bisoprolol, Ivabradine improved hyperemic peak coronary flow velocity and coronary flow velocity reserve in CAD patients more than Bisoprolol despite similar decrease in heart rate.168 When studied in patients with CMD, Ivabradine had no effect on the coronary microvascular function assessed by coronary blood flow nor on the peripheral endothelial function. However, Ivabradine was associated with improvement in angina and QoL.169 The Rho-kinase inhibitor, fasudil, is available in Japan to treat epicardial and microvascular vasospasm but is also not available in the United States.170 Trimetazidine is a metabolic modulator that inhibits beta-oxidation of fatty acids and improves myocardial ischemia and may be useful in some CMD patients.171 It is however only available in Europe, and not available in the United States.
In the Precision Medicine with Zibotentan in Microvascular Angina (PRIZE trial, NCT04097314), oral endothelin A (ET-A) receptor blockade is being tested. In the ESCAPE-CMD pilot feasibility study, injection of autologous CD34+ stem cells intracoronary demonstrated an improvement in CFR, angina, and QoL. CD34+ cells may repair the microcirculation via their ability to differentiate into endothelial cells and promote angiogenesis and improve microvascular ischemia.172 In another study, Corban et al. showed significant improvement in microvascular endothelial function with autologous intracoronary CD34+ cells.173 This strategy was being tested in a larger randomized multicenter FREEDOM trial (NCT04614467), which was terminated prematurely. Implantation of a device to create narrowing in the coronary sinus (Neovasc Reducer™) and increase back pressure in the venous system, leading to vasodilation of arterioles, is also being tested for treatment of microvascular angina (NCT04523168 and NCT04606459).174
Enhanced External Counterpulsation (EECP) is an FDA approved therapy that is used to manage refractory angina from either CAD or microvascular angina (typically 35 hours of total therapy, delivered over 3.5 to 7 weeks). It has been shown to improve angina class, functional capacity, and time to ST-segment depression during exercise.175–177 Augmentation of myocardial perfusion during diastole by inflation of pneumatic cuffs on lower extremities is thought to improve endothelial function and also improve collateral blood flow circulation.
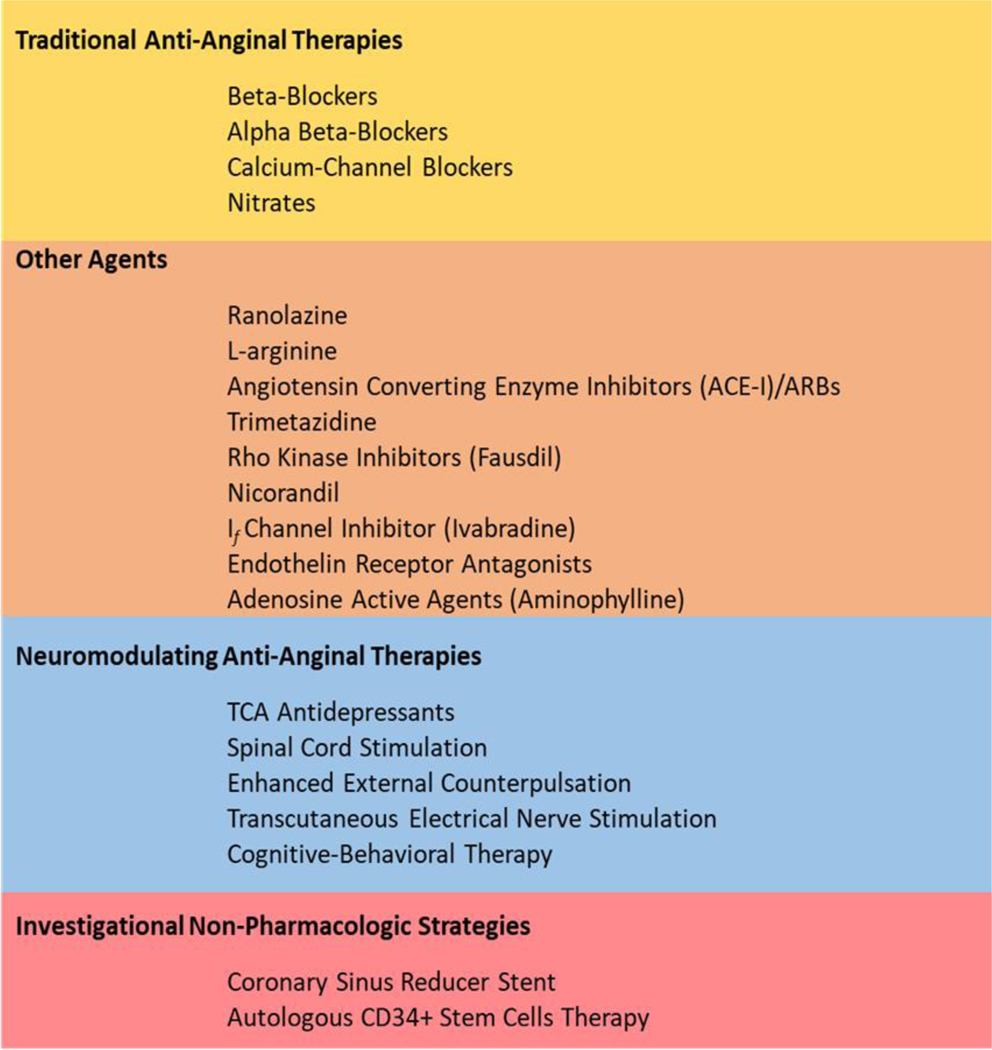
SGLT2 inhibitors have been shown to improve endothelial function by multiple mechanisms including its anti-inflammatory effect, improving flow-mediated dilation, reducing oxidative stress, increasing nitric oxide bioavailability, regulating vascular repair and angiogenesis, promoting endothelial cells viability and suppressing endothelial cells senescence.178 For example, Dapagliflozin has been shown to reduce oxidative stress, improves flow-mediated vasodilation and decreases arterial stiffness in type 2 DM.179 Canagliflozin and Tofogliflozin have also been shown to ameliorate endothelial function.180,181 Interestingly, Empagliflozin was not associated with improvement in endothelial function in relatively well-controlled type 2 diabetic patients.182 Given the key role of inflammation in INOCA, agents that attenuate inflammation (IL-6 signaling, IL-1a, IL-1b, TNF-α inhibitors) are of interest but whether they have impact on angina and outcomes is needed. Role of these and other agents in INOCA population remain to be tested. Mechanistic trials with pharmacotherapy for CMD has been comprehensively reviewed recently, and a selected list is shown in Figure 4.160
Figure 4.
INOCA anti-anginal management strategies.
INOCA patients have a variable response to traditional anti-anginal therapies. Optimal medical therapy with statins, ACE-I, and Aspirin as anti-atherosclerotic medications are used in INOCA, and the results of the WARRIOR trial are pending.
7.3. Cardiac nociceptive and neuromodulatory agents
Aminophylline is a non-selective adenosine receptor antagonist, which was shown to improve effort angina and ischemia by ECG in patients with CSX.183 This is a xanthine derivative which may also have an effect on cardiac nociception but is reserved in refractory cases.184 In some INOCA patients, abnormal cardiac nociceptive abnormality with enhanced pain sensitivity is the dominant cause of pain. Prior work has shown that catheter manipulation in the right heart, atrial pacing, or intracoronary contrast injection causes chest pain in a large percentage of CSX patients.133,185 Whether enhanced pain is due to abnormal myocardial pain fiber signaling or abnormality with central pain processing is unclear and remains to be more fully investigated in INOCA patients with objective measures of microvascular dysfunction.
Low dose tri-cyclic anti-depressant medications, such as imipramine or amitriptyline can be used in patients with refractory cases where abnormal cardiac nociception is suspected.132 They may have an analgesic effect via modulation of norepinephrine uptake as well as anticholinergic effects. In patients who have considerable anxiety related to chest pain syndrome, it is important to refer to a mental health provider who can help with anti-anxiety medications such as selective serotonin-receptor antagonists.132,186 Neuromodulation is an alternative treatment strategy for those with refractory angina and while the mechanisms are not clear, it reduces duration and frequency of angina, and is thought to reduce myocardial oxygen consumption.187 Other strategies such as left stellate ganglion blockade are considered in refractory cases.188,189
8. Conclusions
Our current understanding of INOCA places it as a major concerning health treat that is associated with MACE in both men and women, although women are more often diagnosed with this condition and are more adversely impacted by angina. Coronary endothelial dysfunction and CMD found in a majority of INOCA patients. Heart disease risk factors, increased inflammation, oxidative stress, and autonomic dysregulation are implicated pathophysiologic mechanisms in INOCA. Invasive CFT is often needed to diagnose vascular dysfunction (epicardial and/or microvascular) and is helpful for guiding management of INOCA. Given the heterogenous nature of INOCA patients, strategies for improved identification of high-risk INOCA subgroups are needed. Clinical trials are ongoing to determine optimal medical therapy in INOCA.
Highlights.
Myocardial ischemia and no obstructive coronary arteries (INOCA) is a chronic coronary syndrome that predominates in women and is associated with adverse cardiovascular mortality and outcomes, including myocardial infarction and heart failure with preserved ejection fraction (HFpEF).
INOCA occurs in both men and women and portends an adverse prognosis in both sexes; however, women are more impacted by angina, with recurrent hospitalizations, and report a lower quality of life compared to men with INOCA.
In addition to diffuse coronary plaque and epicardial coronary endothelial dysfunction that are prevalent in INOCA, coronary microvascular dysfunction (CMD) is implicated as a key contributor to microvascular ischemia and symptoms in this INOCA. Epicardial vasospasm can also coexist with normal coronary arteries that have no visible plaque on intravascular imaging.
In the setting of risk factors that lead to oxidative stress and inflammation, CMD occurs due to structural and/or functional causes, resulting in impaired blood flow supply to match myocardial demand.
While non-invasive stress testing modalities detect abnormal flow reserve, invasive coronary function testing is needed to assess abnormal coronary vasoreactivity (epicardial and microcirculatory dysfunction) as well as vasospasm.
In addition to pharmacologic anti-anginal and anti-ischemic strategies, nonpharmacologic treatments are used to manage INOCA, while large, outcomes-based trials are ongoing.
Acknowledgement:
We acknowledge Esha Dave, MS for her assistance with figures and tables.
Financial support:
This work was supported by the National Institutes of Health R01HL124649, U54 AG065141, 1U54AG062334-01, 1R01HL157311, the Edythe L. Broad and the Constance Austin Women’s Heart Research Fellowships, Cedars-Sinai Medical Center, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, The Society for Women’s Health Research (SWHR), Washington, DC, the Linda Joy Pollin Women’s Heart Health Program, the Erika Glazer Women’s Heart Health Project, and the Adelson Family Foundation, Cedars-Sinai Medical Center, Los Angeles, California.
Footnotes
Declaration of competing interests: Mehta, Levit, Malas, Huang, Waheed: none; Bairey Merz: iRhythm and fees paid to CSMC - Abbott and Sanofi.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–744. doi: 10.1093/eurheartj/ehr331 [DOI] [PubMed] [Google Scholar]
- 3.Wang ZJ, Zhang LL, Elmariah S, Han HY, Zhou YJ. Prevalence and Prognosis of Nonobstructive Coronary Artery Disease in Patients Undergoing Coronary Angiography or Coronary Computed Tomography Angiography: A Meta-Analysis. Mayo Clin Proc. 2017;92:329–346. doi: 10.1016/j.mayocp.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 4.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, et al. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. doi: 10.1001/jama.2014.14681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169:843–850. doi: 10.1001/archinternmed.2009.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta PK, Johnson BD, Kenkre TS, Eteiba W, Sharaf B, Pepine CJ, Reis SE, Rogers WJ, Kelsey SF, Thompson DV, et al. Sudden Cardiac Death in Women With Suspected Ischemic Heart Disease, Preserved Ejection Fraction, and No Obstructive Coronary Artery Disease: A Report From the Women’s Ischemia Syndrome Evaluation Study. J Am Heart Assoc. 2017;6. doi: 10.1161/JAHA.117.005501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow BJW, Yam Y, Small G, Wells GA, Crean AM, Ruddy TD, Hossain A. Prognostic durability of coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. 2021;22:331–338. doi: 10.1093/ehjci/jeaa196 [DOI] [PubMed] [Google Scholar]
- 9.van Rosendael AR, Bax AM, Smit JM, van den Hoogen IJ, Ma X, Al’Aref S, Achenbach S, Al-Mallah MH, Andreini D, Berman DS, et al. Clinical risk factors and atherosclerotic plaque extent to define risk for major events in patients without obstructive coronary artery disease: the long-term coronary computed tomography angiography CONFIRM registry. Eur Heart J Cardiovasc Imaging. 2020;21:479–488. doi: 10.1093/ehjci/jez322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990 [DOI] [PubMed] [Google Scholar]
- 11.Jespersen L, Abildstrom SZ, Hvelplund A, Madsen JK, Galatius S, Pedersen F, Hojberg S, Prescott E. Burden of hospital admission and repeat angiography in angina pectoris patients with and without coronary artery disease: a registry-based cohort study. PLoS One. 2014;9:e93170. doi: 10.1371/journal.pone.0093170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radico F, Zimarino M, Fulgenzi F, Ricci F, Di Nicola M, Jespersen L, Chang SM, Humphries KH, Marzilli M, De Caterina R. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: a systematic review and meta-analysis. Eur Heart J. 2018;39:2135–2146. doi: 10.1093/eurheartj/ehy185 [DOI] [PubMed] [Google Scholar]
- 13.Johnson BD, Shaw LJ, Buchthal SD, Bairey Merz CN, Kim HW, Scott KN, Doyle M, Olson MB, Pepine CJ, den Hollander J, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:2993–2999. doi: 10.1161/01.CIR.0000130642.79868.B2 [DOI] [PubMed] [Google Scholar]
- 14.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. doi: 10.1161/CIRCULATIONAHA.113.008507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv. 2012;5:646–653. doi: 10.1016/j.jcin.2012.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S, Mehta PK, Eshtehardi P, Hung OY, Koh JS, Kumar A, Al-Badri A, Rabah R, D’Souza M, Gupta S, et al. Functional coronary angiography in symptomatic patients with no obstructive coronary artery disease. Catheter Cardiovasc Interv. 2020. doi: 10.1002/ccd.29237 [DOI] [PubMed]
- 17.Ford TJ, Ong P, Sechtem U, Beltrame J, Camici PG, Crea F, Kaski JC, Bairey Merz CN, Pepine CJ, Shimokawa H, et al. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc Interv. 2020;13:1847–1864. doi: 10.1016/j.jcin.2020.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jespersen L, Abildstrom SZ, Hvelplund A, Prescott E. Persistent angina: highly prevalent and associated with long-term anxiety, depression, low physical functioning, and quality of life in stable angina pectoris. Clin Res Cardiol. 2013;102:571–581. doi: 10.1007/s00392-013-0568-z [DOI] [PubMed] [Google Scholar]
- 19.Taqueti VR, Di Carli MF. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:2625–2641. doi: 10.1016/j.jacc.2018.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui L, Han L, Wang J, Huang P, Tian G, Wang Y, Li J. Prevalence and characteristics of coronary microvascular dysfunction in post-percutaneous coronary intervention patients with recurrent chest pain. Cardiovasc Diagn Ther. 2022;12:166–176. doi: 10.21037/cdt-21-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kemp HG Jr. Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol. 1973;32:375–376. doi: 10.1016/s0002-9149(73)80150-x [DOI] [PubMed] [Google Scholar]
- 22.Melikian N, De Bruyne B, Fearon WF, MacCarthy PA . The pathophysiology and clinical course of the normal coronary angina syndrome (cardiac syndrome X). Prog Cardiovasc Dis. 2008;50:294–310. doi: 10.1016/j.pcad.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 23.Parsyan A, Pilote L. Cardiac syndrome X: mystery continues. Can J Cardiol. 2012;28:S3–6. doi: 10.1016/j.cjca.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 24.Crea F, Lanza GA. Angina pectoris and normal coronary arteries: cardiac syndrome X. Heart. 2004;90:457–463. doi: 10.1136/hrt.2003.020594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN, Coronary Vasomotion Disorders International Study G. International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068 [DOI] [PubMed] [Google Scholar]
- 26.Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN, Coronary Vasomotion Disorders International Study G. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017;38:2565–2568. doi: 10.1093/eurheartj/ehv351 [DOI] [PubMed] [Google Scholar]
- 27.Mehta PK, Quesada O, Al-Badri A, Fleg JL, Volgman AS, Pepine CJ, Merz CNB, Shaw LJ. Ischemia and no obstructive coronary arteries in patients with stable ischemic heart disease. Int J Cardiol. 2022;348:1–8. doi: 10.1016/j.ijcard.2021.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. doi: 10.1161/CIRCULATIONAHA.114.011201 [DOI] [PubMed] [Google Scholar]
- 29.Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, et al. Presentation, Clinical Profile, and Prognosis of Young Patients With Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Results From the VIRGO Study. J Am Heart Assoc. 2018;7. doi: 10.1161/JAHA.118.009174 [DOI] [PMC free article] [PubMed]
- 30.Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian-Engoren C, et al. Contemporary Diagnosis and Management of Patients With Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement From the American Heart Association. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670 [DOI] [PubMed] [Google Scholar]
- 31.Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur Heart J. 2018;39:2047–2062. doi: 10.1093/eurheartj/ehy077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasupathy S, Tavella R, Beltrame JF. Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): The Past, Present, and Future Management. Circulation. 2017;135:1490–1493. doi: 10.1161/CIRCULATIONAHA.117.027666 [DOI] [PubMed] [Google Scholar]
- 33.Pacheco Claudio C, Quesada O, Pepine CJ, Noel Bairey Merz C. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin Cardiol. 2018;41:185–193. doi: 10.1002/clc.22894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reynolds HR, Maehara A, Kwong RY, Sedlak T, Saw J, Smilowitz NR, Mahmud E, Wei J, Marzo K, Matsumura M, et al. Coronary Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging to Determine Underlying Causes of Myocardial Infarction With Nonobstructive Coronary Arteries in Women. Circulation. 2021;143:624–640. doi: 10.1161/CIRCULATIONAHA.120.052008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, et al. ESC working group position paper on myocardial infarction with nonobstructive coronary arteries. Eur Heart J. 2017;38:143–153. doi: 10.1093/eurheartj/ehw149 [DOI] [PubMed] [Google Scholar]
- 36.Pasupathy S, Lindahl B, Litwin P, Tavella R, Williams MJA, Air T, Zeitz C, Smilowitz NR, Reynolds HR, Eggers KM, et al. Survival in Patients With Suspected Myocardial Infarction With Nonobstructive Coronary Arteries: A Comprehensive Systematic Review and Meta-Analysis From the MINOCA Global Collaboration. Circ Cardiovasc Qual Outcomes. 2021;14:e007880. doi: 10.1161/CIRCOUTCOMES.121.007880 [DOI] [PubMed] [Google Scholar]
- 37.Bainey KR, Welsh RC, Alemayehu W, Westerhout CM, Traboulsi D, Anderson T, Brass N, Armstrong PW, Kaul P. Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): Insights from the Alberta contemporary acute coronary syndrome patients invasive treatment strategies (COAPT) study. Int J Cardiol. 2018;264:12–17. doi: 10.1016/j.ijcard.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 38.Biere L, Niro M, Pouliquen H, Gourraud JB, Prunier F, Furber A, Probst V. Risk of ventricular arrhythmia in patients with myocardial infarction and non-obstructive coronary arteries and normal ejection fraction. World J Cardiol. 2017;9:268–276. doi: 10.4330/wjc.v9.i3.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grodzinsky A, Arnold SV, Gosch K, Spertus JA, Foody JM, Beltrame J, Maddox TM, Parashar S, Kosiborod M. Angina Frequency After Acute Myocardial Infarction In Patients Without Obstructive Coronary Artery Disease. Eur Heart J Qual Care Clin Outcomes. 2015;1:92–99. doi: 10.1093/ehjqcco/qcv014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maddox TM, Ho PM, Roe M, Dai D, Tsai TT, Rumsfeld JS. Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization: insights from the National Cardiovascular Data Registry Cath-PCI Registry. Circ Cardiovasc Qual Outcomes. 2010;3:632–641. doi: 10.1161/CIRCOUTCOMES.109.906214 [DOI] [PubMed] [Google Scholar]
- 41.Smilowitz NR, Dubner R, Hellkamp AS, Widmer RJ, Reynolds HR. Variability of discharge medical therapy for secondary prevention among patients with myocardial infarction with non-obstructive coronary arteries (MINOCA) in the United States. PLoS One. 2021;16:e0255462. doi: 10.1371/journal.pone.0255462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjold A, Gard A, Jernberg T. Medical Therapy for Secondary Prevention and Long-Term Outcome in Patients With Myocardial Infarction With Nonobstructive Coronary Artery Disease. Circulation. 2017;135:1481–1489. doi: 10.1161/CIRCULATIONAHA.116.026336 [DOI] [PubMed] [Google Scholar]
- 43.Choo EH, Chang K, Lee KY, Lee D, Kim JG, Ahn Y, Kim YJ, Chae SC, Cho MC, Kim CJ, et al. Prognosis and Predictors of Mortality in Patients Suffering Myocardial Infarction With Non-Obstructive Coronary Arteries. J Am Heart Assoc. 2019;8:e011990. doi: 10.1161/JAHA.119.011990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordenskjold AM, Agewall S, Atar D, Baron T, Beltrame J, Bergstrom O, Erlinge D, Gale CP, LopezPais J, Jernberg T, et al. Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA-BAT): Rationale and design. Am Heart J. 2021;231:96–104. doi: 10.1016/j.ahj.2020.10.059 [DOI] [PubMed] [Google Scholar]
- 45.Savarese G, Lund LH. Global Public Health Burden of Heart Failure. Card Fail Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redfield MM. Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2016;375:1868–1877. doi: 10.1056/NEJMcp1511175 [DOI] [PubMed] [Google Scholar]
- 47.Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, Beussink-Nelson L, Ljung Faxen U, Fermer ML, Broberg MA, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J. 2018;39:3439–3450. doi: 10.1093/eurheartj/ehy531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rush CJ, Berry C, Oldroyd KG, Rocchiccioli JP, Lindsay MM, Touyz RM, Murphy CL, Ford TJ, Sidik N, McEntegart MB, et al. Prevalence of Coronary Artery Disease and Coronary Microvascular Dysfunction in Patients With Heart Failure With Preserved Ejection Fraction. JAMA Cardiol. 2021;6:1130–1143. doi: 10.1001/jamacardio.2021.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuel TJ, Wei J, Sharif B, Tamarappoo BK, Pattisapu V, Maughan J, Cipher DJ, Suppogu N, Aldiwani H, Thomson LEJ, et al. Diastolic dysfunction in women with ischemia and no obstructive coronary artery disease: Mechanistic insight from magnetic resonance imaging. Int J Cardiol. 2021;331:1–7. doi: 10.1016/j.ijcard.2021.01.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw JL, Nelson MD, Wei J, Motwani M, Landes S, Mehta PK, Thomson LEJ, Berman DS, Li D, Bairey Merz CN, et al. Inverse association of MRI-derived native myocardial T1 and perfusion reserve index in women with evidence of ischemia and no obstructive CAD: A pilot study. Int J Cardiol. 2018;270:48–53. doi: 10.1016/j.ijcard.2018.06.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones E, Wei J, Nelson MD, Bakir M, Mehta PK, Shufelt C, Minissian M, Sharif B, Pepine CJ, Handberg E, et al. N-Terminal pro-B-type natriuretic peptide and coronary microvascular dysfunction in women with preserved ejection fraction: A report from the Women’s Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD) study. PLoS One. 2020;15:e0243213. doi: 10.1371/journal.pone.0243213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschope C, Leite-Moreira AF, Musters R, Niessen HW, Linke WA, et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016;4:312–324. doi: 10.1016/j.jchf.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 53.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Empel VP, Mariani J, Borlaug BA, Kaye DM. Impaired myocardial oxygen availability contributes to abnormal exercise hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2014;3:e001293. doi: 10.1161/JAHA.114.001293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JF, Barrett-O’Keefe Z, Garten RS, Nelson AD, Ryan JJ, Nativi JN, Richardson RS, Wray DW. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart. 2016;102:278–284. doi: 10.1136/heartjnl-2015-308403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marechaux S, Samson R, van Belle E, Breyne J, de Monte J, Dedrie C, Chebai N, Menet A, Banfi C, Bouabdallaoui N, et al. Vascular and Microvascular Endothelial Function in Heart Failure With Preserved Ejection Fraction. J Card Fail. 2016;22:3–11. doi: 10.1016/j.cardfail.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 57.Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, et al. Reduced Myocardial Flow in Heart Failure Patients With Preserved Ejection Fraction. Circ Heart Fail. 2016;9. doi: 10.1161/CIRCHEARTFAILURE.115.002562 [DOI] [PubMed] [Google Scholar]
- 58.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39:840–849. doi: 10.1093/eurheartj/ehx721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arnold JR, Kanagala P, Budgeon CA, Jerosch-Herold M, Gulsin GS, Singh A, Khan JN, Chan DCS, Squire IB, Ng LL, et al. Prevalence and Prognostic Significance of Microvascular Dysfunction in Heart Failure With Preserved Ejection Fraction. JACC Cardiovasc Imaging. 2022. doi: 10.1016/j.jcmg.2021.11.022 [DOI] [PubMed]
- 60.Dryer K, Gajjar M, Narang N, Lee M, Paul J, Shah AP, Nathan S, Butler J, Davidson CJ, Fearon WF, et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2018;314:H1033–H1042. doi: 10.1152/ajpheart.00680.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmad A, Corban MT, Toya T, Verbrugge FH, Sara JD, Lerman LO, Borlaug BA, Lerman A. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:765–772. doi: 10.1002/ejhf.2010 [DOI] [PubMed] [Google Scholar]
- 62.Wei J, Nelson MD, Sharif B, Shufelt C, Bairey Merz CN. Why do we care about coronary microvascular dysfunction and heart failure with preserved ejection fraction: addressing knowledge gaps for evidence-based guidelines. Eur Heart J. 2018;39:3451–3453. doi: 10.1093/eurheartj/ehy558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, et al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J Am Coll Cardiol. 2019;73:684–693. doi: 10.1016/j.jacc.2018.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimokawa H, Suda A, Takahashi J, Berry C, Camici PG, Crea F, Escaned J, Ford T, Yii E, Kaski JC, et al. Clinical characteristics and prognosis of patients with microvascular angina: an international and prospective cohort study by the Coronary Vasomotor Disorders International Study (COVADIS) Group. Eur Heart J. 2021;42:4592–4600. doi: 10.1093/eurheartj/ehab282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schumann CL, Mathew RC, Dean JL, Yang Y, Balfour PC Jr., Shaw PW, Robinson AA, Salerno M, Kramer CM, Bourque JM. Functional and Economic Impact of INOCA and Influence of Coronary Microvascular Dysfunction. JACC Cardiovasc Imaging. 2021;14:1369–1379. doi: 10.1016/j.jcmg.2021.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bechsgaard DF, Hove JD, Suhrs HE, Bove KB, Shahriari P, Gustafsson I, Prescott E. Women with coronary microvascular dysfunction and no obstructive coronary artery disease have reduced exercise capacity. Int J Cardiol. 2019;293:1–9. doi: 10.1016/j.ijcard.2019.07.048 [DOI] [PubMed] [Google Scholar]
- 67.Szot W, Zajac J, Kostkiewicz M, Owoc J, Bojar I. Cardiac rehabilitation: a good measure to improve quality of life in peri- and postmenopausal women with microvascular angina. Ann Agric Environ Med. 2015;22:390–395. doi: 10.5604/12321966.1152100 [DOI] [PubMed] [Google Scholar]
- 68.Mehta PK, Gaignard S, Schwartz A, Manson JE. Traditional and Emerging Sex-Specific Risk Factors for Cardiovascular Disease in Women. Reviews in Cardiovascular Medicine. 2022;23. doi: 10.31083/j.rcm2308288 [DOI] [PMC free article] [PubMed]
- 69.Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, Kuller LH. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20:2414–2421. doi: 10.1161/01.atv.20.11.2414 [DOI] [PubMed] [Google Scholar]
- 70.Krishna MB, Joseph A, Thomas PL, Dsilva B, Pillai SM, Laloraya M. Impaired Arginine Metabolism Coupled to a Defective Redox Conduit Contributes to Low Plasma Nitric Oxide in Polycystic Ovary Syndrome. Cell Physiol Biochem. 2017;43:1880–1892. doi: 10.1159/000484107 [DOI] [PubMed] [Google Scholar]
- 71.AlBadri A, Eshtehardi P, Hung OY, Bouchi Y, Khawaja S, Mercado K, Corban MT, Mehta PK, Shaw LJ, Samady H. Coronary Microvascular Dysfunction Is Associated With Significant Plaque Burden and Diffuse Epicardial Atherosclerotic Disease. JACC Cardiovasc Interv. 2019;12:1519–1520. doi: 10.1016/j.jcin.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 72.Dhawan SS, Corban MT, Nanjundappa RA, Eshtehardi P, McDaniel MC, Kwarteng CA, Samady H. Coronary microvascular dysfunction is associated with higher frequency of thin-cap fibroatheroma. Atherosclerosis. 2012;223:384–388. doi: 10.1016/j.atherosclerosis.2012.05.034 [DOI] [PubMed] [Google Scholar]
- 73.Khuddus MA, Pepine CJ, Handberg EM, Bairey Merz CN, Sopko G, Bavry AA, Denardo SJ, McGorray SP, Smith KM, Sharaf BL, et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). J Interv Cardiol. 2010;23:511–519. doi: 10.1111/j.1540-8183.2010.00598.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishimura RA, Lerman A, Chesebro JH, Ilstrup DM, Hodge DO, Higano ST, Holmes DR, Jr., Tajik AJ. Epicardial vasomotor responses to acetylcholine are not predicted by coronary atherosclerosis as assessed by intracoronary ultrasound. J Am Coll Cardiol. 1995;26:41–49. doi: 10.1016/0735-1097(95)00142-m [DOI] [PubMed] [Google Scholar]
- 75.Tona F, Serra R, Di Ascenzo L, Osto E, Scarda A, Fabris R, Montisci R, Famoso G, Tellatin S, Foletto M, et al. Systemic inflammation is related to coronary microvascular dysfunction in obese patients without obstructive coronary disease. Nutr Metab Cardiovasc Dis. 2014;24:447–453. doi: 10.1016/j.numecd.2013.09.021 [DOI] [PubMed] [Google Scholar]
- 76.Bajaj NS, Osborne MT, Gupta A, Tavakkoli A, Bravo PE, Vita T, Bibbo CF, Hainer J, Dorbala S, Blankstein R, et al. Coronary Microvascular Dysfunction and Cardiovascular Risk in Obese Patients. J Am Coll Cardiol. 2018;72:707–717. doi: 10.1016/j.jacc.2018.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taube A, Schlich R, Sell H, Eckardt K, Eckel J. Inflammation and metabolic dysfunction: links to cardiovascular diseases. Am J Physiol Heart Circ Physiol. 2012;302:H2148–2165. doi: 10.1152/ajpheart.00907.2011 [DOI] [PubMed] [Google Scholar]
- 78.Sara JD, Taher R, Kolluri N, Vella A, Lerman LO, Lerman A. Coronary microvascular dysfunction is associated with poor glycemic control amongst female diabetics with chest pain and non-obstructive coronary artery disease. Cardiovasc Diabetol. 2019;18:22. doi: 10.1186/s12933-019-0833-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gallinoro E, Paolisso P, Candreva A, Bermpeis K, Fabbricatore D, Esposito G, Bertolone D, Fernandez Peregrina E, Munhoz D, Mileva N, et al. Microvascular Dysfunction in Patients With Type II Diabetes Mellitus: Invasive Assessment of Absolute Coronary Blood Flow and Microvascular Resistance Reserve. Front Cardiovasc Med. 2021;8:765071. doi: 10.3389/fcvm.2021.765071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41:1387–1393. doi: 10.1016/s0735-1097(03)00166-9 [DOI] [PubMed] [Google Scholar]
- 81.van de Hoef TP, Bax M, Damman P, Delewi R, Hassell ME, Piek MA, Chamuleau SA, Voskuil M, van Eck-Smit BL, Verberne HJ, et al. Impaired Coronary Autoregulation Is Associated With Long-term Fatal Events in Patients With Stable Coronary Artery Disease. Circ Cardiovasc Interv. 2013;6:329–335. doi: 10.1161/CIRCINTERVENTIONS.113.000378 [DOI] [PubMed] [Google Scholar]
- 82.Sezer M, Kocaaga M, Aslanger E, Atici A, Demirkiran A, Bugra Z, Umman S, Umman B. Bimodal Pattern of Coronary Microvascular Involvement in Diabetes Mellitus. J Am Heart Assoc. 2016;5. doi: 10.1161/JAHA.116.003995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raggi P, Genest J, Giles JT, Rayner KJ, Dwivedi G, Beanlands RS, Gupta M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108. doi: 10.1016/j.atherosclerosis.2018.07.014 [DOI] [PubMed] [Google Scholar]
- 84.Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. 2021;117:2525–2536. doi: 10.1093/cvr/cvab303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women−-2011 update: a guideline from the american heart association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Faccini A, Kaski JC, Camici PG. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur Heart J. 2016;37:1799–1806. doi: 10.1093/eurheartj/ehw018 [DOI] [PubMed] [Google Scholar]
- 87.Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging. 2013;6:660–667. doi: 10.1016/j.jcmg.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 88.Weber BN, Stevens E, Barrett L, Bay C, Sinnette C, Brown JM, Divakaran S, Bibbo C, Hainer J, Dorbala S, et al. Coronary Microvascular Dysfunction in Systemic Lupus Erythematosus. J Am Heart Assoc. 2021;10:e018555. doi: 10.1161/JAHA.120.018555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin Rheumatol. 2007;26:1228–1233. doi: 10.1007/s10067-007-0548-7 [DOI] [PubMed] [Google Scholar]
- 90.AlBadri A, Lai K, Wei J, Landes S, Mehta PK, Li Q, Johnson D, Reis SE, Kelsey SF, Bittner V, et al. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: A report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS One. 2017;12:e0177684. doi: 10.1371/journal.pone.0177684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mekonnen G, Corban MT, Hung OY, Eshtehardi P, Eapen DJ, Al-Kassem H, Rasoul-Arzrumly E, Gogas BD, McDaniel MC, Pielak T, et al. Plasma soluble urokinase-type plasminogen activator receptor level is independently associated with coronary microvascular function in patients with non-obstructive coronary artery disease. Atherosclerosis. 2015;239:55–60. doi: 10.1016/j.atherosclerosis.2014.12.025 [DOI] [PubMed] [Google Scholar]
- 92.Al-Badri A, Tahhan AS, Sabbak N, Alkhoder A, Liu C, Ko YA, Vaccarino V, Martini A, Sidoti A, Goodwin C, et al. Soluble Urokinase-Type Plasminogen Activator Receptor and High-Sensitivity Troponin Levels Predict Outcomes in Nonobstructive Coronary Artery Disease. J Am Heart Assoc. 2020;9:e015515. doi: 10.1161/JAHA.119.015515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Corban MT, Prasad A, Nesbitt L, Loeffler D, Herrmann J, Lerman LO, Lerman A. Local Production of Soluble Urokinase Plasminogen Activator Receptor and Plasminogen Activator Inhibitor-1 in the Coronary Circulation Is Associated With Coronary Endothelial Dysfunction in Humans. J Am Heart Assoc. 2018;7:e009881. doi: 10.1161/JAHA.118.009881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Di Carli MF, Tobes MC, Mangner T, Levine AB, Muzik O, Chakroborty P, Levine TB. Effects of cardiac sympathetic innervation on coronary blood flow. N Engl J Med. 1997;336:1208–1215. [DOI] [PubMed] [Google Scholar]
- 95.Yun AJ, Doux JD, Bazar KA, Lee PY. Adventitial dysfunction: an evolutionary model for understanding atherosclerosis. Medical hypotheses. 2005;65:962–965. doi: 10.1016/j.mehy.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 96.von Scholten BJ, Hansen CS, Hasbak P, Kjaer A, Rossing P, Hansen TW. Cardiac Autonomic Function Is Associated With the Coronary Microcirculatory Function in Patients With Type 2 Diabetes. Diabetes. 2016;65:3129–3138. doi: 10.2337/db16-0437 [DOI] [PubMed] [Google Scholar]
- 97.Cemin R, Erlicher A, Fattor B, Pitscheider W, Cevese A. Reduced coronary flow reserve and parasympathetic dysfunction in patients with cardiovascular syndrome X. Coronary artery disease. 2008;19:1–7. doi: 10.1097/MCA.0b013e3282f18e8d [DOI] [PubMed] [Google Scholar]
- 98.Goseki Y, Matsubara T, Takahashi N, Takeuchi T, Ibukiyama C. Heart rate variability before the occurrence of silent myocardial ischemia during ambulatory monitoring. Am J Cardiol. 1994;73:845–849. [DOI] [PubMed] [Google Scholar]
- 99.Mehta PK, Hermel M, Nelson MD, Cook-Wiens G, Martin EA, Alkhoder AA, Wei J, Minissian M, Shufelt CL, Marpuri S, et al. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction: Results from the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) study. Int J Cardiol. 2018;251:8–13. doi: 10.1016/j.ijcard.2017.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehta PK, Thomson L, Friedman J, Hayes S, Slomka P, Swift A, Nelson MD, Hermel D, Michaelson M, Motwani M, et al. Abnormal Cardiac Sympathetic Activity Detected by 123-I-Meta-Iodobenzylguanidine Imaging in Women with Signs and Symptoms of Ischemia and No Obstructive Coronary Artery Disease. JACC Cardiovasc Imaging. 2016;67:1619. [Google Scholar]
- 101.Agostini D, Verberne HJ, Burchert W, Knuuti J, Povinec P, Sambuceti G, Unlu M, Estorch M, Banerjee G, Jacobson AF. I-123-mIBG myocardial imaging for assessment of risk for a major cardiac event in heart failure patients: insights from a retrospective European multicenter study. Eur J Nucl Med Mol Imaging. 2008;35:535–546. doi: 10.1007/s00259-007-0639-3 [DOI] [PubMed] [Google Scholar]
- 102.Henneman MM, Bengel FM, van der Wall EE, Knuuti J, Bax JJ. Cardiac neuronal imaging: application in the evaluation of cardiac disease. J Nucl Cardiol. 2008;15:442–455. doi: S1071-3581(08)00062-7[pii] 10.1016/j.nuclcard.2008.02.023 [DOI] [PubMed] [Google Scholar]
- 103.Travin MI, Matsunari I, Thomas GS, Nakajima K, Yoshinaga K. How do we establish cardiac sympathetic nervous system imaging with (123)I-mIBG in clinical practice? Perspectives and lessons from Japan and the US. J Nucl Cardiol. 2019;26:1434–1451. doi: 10.1007/s12350-018-1394-5 [DOI] [PubMed] [Google Scholar]
- 104.Sciammarella MG, Gerson M, Buxton AE, Bartley SC, Doukky R, Merlino DA, Tandon S, Thompson R, Travin MI. ASNC/SNMMI Model Coverage Policy: Myocardial sympathetic innervation imaging: Iodine-123 meta-iodobenzylguanidine ((123)I-mIBG). J Nucl Cardiol. 2015;22:804–811. doi: 10.1007/s12350-015-0202-8 [DOI] [PubMed] [Google Scholar]
- 105.Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–2221. doi: 10.1016/j.jacc.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 106.Di Monaco A, Lanza GA, Bruno I, Careri G, Pinnacchio G, Tarzia P, Battipaglia I, Giordano A, Crea F. Usefulness of impairment of cardiac adrenergic nerve function to predict outcome in patients with cardiac syndrome X. Am J Cardiol. 2010;106:1813–1818. doi: 10.1016/j.amjcard.2010.07.052 [DOI] [PubMed] [Google Scholar]
- 107.Lavi S, Nevo O, Thaler I, Rosenfeld R, Dayan L, Hirshoren N, Gepstein L, Jacob G. Effect of aging on the cardiovascular regulatory systems in healthy women. American journal of physiology Regulatory, integrative and comparative physiology. 2007;292:R788–793. doi: 10.1152/ajpregu.00352.2006 [DOI] [PubMed] [Google Scholar]
- 108.Lyon AR, Citro R, Schneider B, Morel O, Ghadri JR, Templin C, Omerovic E. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77:902–921. doi: 10.1016/j.jacc.2020.10.060 [DOI] [PubMed] [Google Scholar]
- 109.Sharkey SW, Maron BJ. Epidemiology and clinical profile of Takotsubo cardiomyopathy. Circulation journal : official journal of the Japanese Circulation Society. 2014;78:2119–2128. [DOI] [PubMed] [Google Scholar]
- 110.Prasad A, Madhavan M, Chareonthaitawee P. Cardiac sympathetic activity in stress-induced (Takotsubo) cardiomyopathy. Nature reviews Cardiology. 2009;6:430–434. doi: 10.1038/nrcardio.2009.51 [DOI] [PubMed] [Google Scholar]
- 111.Kume T, Akasaka T, Kawamoto T, Yoshitani H, Watanabe N, Neishi Y, Wada N, Yoshida K. Assessment of coronary microcirculation in patients with takotsubo-like left ventricular dysfunction. Circulation journal : official journal of the Japanese Circulation Society. 2005;69:934–939. [DOI] [PubMed] [Google Scholar]
- 112.Bybee KA, Prasad A, Barsness GW, Lerman A, Jaffe AS, Murphy JG, Wright RS, Rihal CS. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94:343–346. doi: 10.1016/j.amjcard.2004.04.030 [DOI] [PubMed] [Google Scholar]
- 113.Vaccaro A, Despas F, Delmas C, Lairez O, Lambert E, Lambert G, Labrunee M, Guiraud T, Esler M, Galinier M, et al. Direct evidences for sympathetic hyperactivity and baroreflex impairment in Tako Tsubo cardiopathy. PLoS One. 2014;9:e93278. doi: 10.1371/journal.pone.0093278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pepine CJ, Ferdinand KC, Shaw LJ, Light-McGroary KA, Shah RU, Gulati M, Duvernoy C, Walsh MN, Bairey Merz CN, Committee ACiW. Emergence of Nonobstructive Coronary Artery Disease: A Woman’s Problem and Need for Change in Definition on Angiography. J Am Coll Cardiol. 2015;66:1918–1933. doi: 10.1016/j.jacc.2015.08.876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roy R, Aldiwani H, Darouian N, Sharma S, Torbati T, Wei J, Nelson MD, Shufelt C, Minissian MB, Li L, et al. Ambulatory and silent myocardial ischemia in women with coronary microvascular dysfunction: Results from the Cardiac Autonomic Nervous System study (CANS). Int J Cardiol. 2020;316:1–6. doi: 10.1016/j.ijcard.2020.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Galiuto L, Sestito A, Barchetta S, Sgueglia GA, Infusino F, La Rosa C, Lanza G, Crea F. Noninvasive evaluation of flow reserve in the left anterior descending coronary artery in patients with cardiac syndrome X. Am J Cardiol. 2007;99:1378–1383. doi: 10.1016/j.amjcard.2006.12.070 [DOI] [PubMed] [Google Scholar]
- 118.Mygind ND, Michelsen MM, Pena A, Frestad D, Dose N, Aziz A, Faber R, Host N, Gustafsson I, Hansen PR, et al. Coronary Microvascular Function and Cardiovascular Risk Factors in Women With Angina Pectoris and No Obstructive Coronary Artery Disease: The iPOWER Study. J Am Heart Assoc. 2016;5:e003064. doi: 10.1161/JAHA.115.003064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. doi: 10.1161/CIRCULATIONAHA.114.011939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jalnapurkar S, Zarrini P, Mehta PK, Thomson LEJ, Agarwal M, Samuels BA, Shufelt CL, Eastwood JA, Berman D, Merz NB, et al. Role of Stress Cardiac Magnetic Resonance Imaging in Women with Suspected Ischemia but No Obstructive Coronary Artery Disease. J Radiol Nurs. 2017;36:180–183. doi: 10.1016/j.jradnu.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, Gill EB, Johnson BD, Kenkre T, Handberg EM, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging. 2015;8. doi: 10.1161/CIRCIMAGING.114.002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goykhman P, Mehta PK, Agarwal M, Shufelt C, Slomka PJ, Yang Y, Xu Y, Shaw LJ, Berman DS, Merz NB, et al. Reproducibility of myocardial perfusion reserve - variations in measurements from post processing using commercially available software. Cardiovasc Diagn Ther. 2012;2:268–277. doi: 10.3978/j.issn.2223-3652.2012.10.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Agarwal M, Shufelt C, Mehta PK, Gill E, Berman DS, Li D, Sharif B, Li N, Bairey Merz CN, Thomson LE. Cardiac risk factors and myocardial perfusion reserve in women with microvascular coronary dysfunction. Cardiovasc Diagn Ther. 2013;3:146–152. doi: 10.3978/j.issn.2223-3652.2013.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rahman H, Scannell CM, Demir OM, Ryan M, McConkey H, Ellis H, Masci PG, Perera D, Chiribiri A. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc Imaging. 2021;14:978–986. doi: 10.1016/j.jcmg.2020.10.015 [DOI] [PubMed] [Google Scholar]
- 125.Mathew RC, Bourque JM, Salerno M, Kramer CM. Cardiovascular Imaging Techniques to Assess Microvascular Dysfunction. JACC Cardiovasc Imaging. 2020;13:1577–1590. doi: 10.1016/j.jcmg.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cassar A, Chareonthaitawee P, Rihal CS, Prasad A, Lennon RJ, Lerman LO, Lerman A. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2:237–244. doi: 10.1161/CIRCINTERVENTIONS.108.841056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Feenstra RGT, Seitz A, Boerhout CKM, Bukkems LH, Stegehuis VE, Teeuwisse PJI, de Winter RJ, Sechtem U, Piek JJ, van de Hoef TP, et al. Principles and pitfalls in coronary vasomotor function testing. EuroIntervention. 2022;17:1271–1280. doi: 10.4244/EIJ-D-21-00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ford TJ, Stanley B, Good R, Rocchiccioli P, McEntegart M, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, et al. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J Am Coll Cardiol. 2018;72:2841–2855. doi: 10.1016/j.jacc.2018.09.006 [DOI] [PubMed] [Google Scholar]
- 129.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 130.Ong P, Athanasiadis A, Hill S, Schaufele T, Mahrholdt H, Sechtem U. Coronary microvascular dysfunction assessed by intracoronary acetylcholine provocation testing is a frequent cause of ischemia and angina in patients with exercise-induced electrocardiographic changes and unobstructed coronary arteries. Clin Cardiol. 2014;37:462–467. doi: 10.1002/clc.22282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Widmer RJ, Samuels B, Samady H, Price MJ, Jeremias A, Anderson RD, Jaffer FA, Escaned J, Davies J, Prasad M, et al. The functional assessment of patients with non-obstructive coronary artery disease: expert review from an international microcirculation working group. EuroIntervention. 2019;14:1694–1702. doi: 10.4244/EIJ-D-18-00982 [DOI] [PubMed] [Google Scholar]
- 132.Cannon RO 3rd, Quyyumi AA, Mincemoyer R, Stine AM, Gracely RH, Smith WB, Geraci MF, Black BC, Uhde TW, Waclawiw MA, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330:1411–1417. doi: 10.1056/NEJM199405193302003 [DOI] [PubMed] [Google Scholar]
- 133.Cannon RO 3rd, Quyyumi AA, Schenke WH, Fananapazir L, Tucker EE, Gaughan AM, Gracely RH, Cattau EL, Jr., Epstein SE. Abnormal cardiac sensitivity in patients with chest pain and normal coronary arteries. J Am Coll Cardiol. 1990;16:1359–1366. [DOI] [PubMed] [Google Scholar]
- 134.Kern MJ. Coronary physiology revisited : practical insights from the cardiac catheterization laboratory. Circulation. 2000;101:1344–1351. doi: 10.1161/01.cir.101.11.1344 [DOI] [PubMed] [Google Scholar]
- 135.de Bruyne B, Bartunek J, Sys SU, Pijls NH, Heyndrickx GR, Wijns W. Simultaneous coronary pressure and flow velocity measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve. Circulation. 1996;94:1842–1849. doi: 10.1161/01.cir.94.8.1842 [DOI] [PubMed] [Google Scholar]
- 136.Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. doi: 10.1161/01.CIR.0000080700.98607.D1 [DOI] [PubMed] [Google Scholar]
- 137.Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113:2054–2061. doi: 10.1161/CIRCULATIONAHA.105.603522 [DOI] [PubMed] [Google Scholar]
- 138.Nishi T, Murai T, Ciccarelli G, Shah SV, Kobayashi Y, Derimay F, Waseda K, Moonen A, Hoshino M, Hirohata A, et al. Prognostic Value of Coronary Microvascular Function Measured Immediately After Percutaneous Coronary Intervention in Stable Coronary Artery Disease: An International Multicenter Study. Circ Cardiovasc Interv. 2019;12:e007889. doi: 10.1161/CIRCINTERVENTIONS.119.007889 [DOI] [PubMed] [Google Scholar]
- 139.Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J Am Coll Cardiol. 2016;67:1158–1169. doi: 10.1016/j.jacc.2015.12.053 [DOI] [PubMed] [Google Scholar]
- 140.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16 [DOI] [PubMed] [Google Scholar]
- 141.Handberg EM, Merz CNB, Cooper-Dehoff RM, Wei J, Conlon M, Lo MC, Boden W, Frayne SM, Villines T, Spertus JA, et al. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am Heart J. 2021;237:90–103. doi: 10.1016/j.ahj.2021.03.011 [DOI] [PubMed] [Google Scholar]
- 142.Heidenreich PA, McDonald KM, Hastie T, Fadel B, Hagan V, Lee BK, Hlatky MA. Meta-analysis of trials comparing beta-blockers, calcium antagonists, and nitrates for stable angina. JAMA. 1999;281:1927–1936. [DOI] [PubMed] [Google Scholar]
- 143.Kayaalti F, Kalay N, Basar E, Mavili E, Duran M, Ozdogru I, Dogan A, Inanc MT, Kaya MG, Topsakal R, et al. Effects of nebivolol therapy on endothelial functions in cardiac syndrome X. Heart and vessels. 2010;25:92–96. doi: 10.1007/s00380-009-1170-1 [DOI] [PubMed] [Google Scholar]
- 144.Sugioka K, Hozumi T, Takemoto Y, Ujino K, Matsumura Y, Watanabe H, Fujimoto K, Yoshiyama M, Yoshikawa J. Early recovery of impaired coronary flow reserve by carvedilol therapy in patients with idiopathic dilated cardiomyopathy: a serial transthoracic Doppler echocardiographic study. J Am Coll Cardiol. 2005;45:318–319. doi: 10.1016/j.jacc.2004.10.019 [DOI] [PubMed] [Google Scholar]
- 145.Cortigiani L, Rigo F, Gherardi S, Galderisi M, Sicari R, Picano E. Prognostic implications of coronary flow reserve on left anterior descending coronary artery in hypertrophic cardiomyopathy. Am J Cardiol. 2008;102:1718–1723. doi: 10.1016/j.amjcard.2008.08.023 [DOI] [PubMed] [Google Scholar]
- 146.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7 [DOI] [PubMed] [Google Scholar]
- 147.Cargnoni A, Ceconi C, Bernocchi P, Boraso A, Parrinello G, Curello S, Ferrari R. Reduction of oxidative stress by carvedilol: role in maintenance of ischaemic myocardium viability. Cardiovasc Res. 2000;47:556–566. [DOI] [PubMed] [Google Scholar]
- 148.Jonsson G, Abdelnoor M, Seljeflot I, Arnesen H, Hostmark AT, Kjeldsen SE, Os I, Westheim AS. The antioxidative effects of long-term treatment are more pronounced for carvedilol than for atenolol in post-myocardial infarction patients. Journal of cardiovascular pharmacology. 2007;49:27–32. doi: 10.1097/FJC.0b013e31802bdd8c [DOI] [PubMed] [Google Scholar]
- 149.Vyssoulis GP, Marinakis AG, Aznaouridis KA, Karpanou EA, Arapogianni AN, Cokkinos DV, Stefanadis CI. The impact of third-generation beta-blocker antihypertensive treatment on endothelial function and the prothrombotic state: effects of smoking. American journal of hypertension. 2004;17:582–589. doi: 10.1016/j.amjhyper.2004.03.668 [DOI] [PubMed] [Google Scholar]
- 150.Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S, Malinski T. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 2003;107:2747–2752. doi: 10.1161/01.CIR.0000066912.58385.DE [DOI] [PubMed] [Google Scholar]
- 151.Nakajima T, Ma J, Iida H, Iwasawa K, Jo T, Omata M, Nagai R. Inhibitory effects of carvedilol on calcium channels in vascular smooth muscle cells. Japanese heart journal. 2003;44:963–978. [DOI] [PubMed] [Google Scholar]
- 152.De Matos LD, Gardenghi G, Rondon MU, Soufen HN, Tirone AP, Barretto AC, Brum PC, Middlekauff HR, Negrao CE. Impact of 6 months of therapy with carvedilol on muscle sympathetic nerve activity in heart failure patients. J Card Fail. 2004;10:496–502. [DOI] [PubMed] [Google Scholar]
- 153.Goldsmith RL, Bigger JT, Bloomfield DM, Krum H, Steinman RC, Sackner-Bernstein J, Packer M. Long-term carvedilol therapy increases parasympathetic nervous system activity in chronic congestive heart failure. Am J Cardiol. 1997;80:1101–1104. [DOI] [PubMed] [Google Scholar]
- 154.Mortara A, La Rovere MT, Pinna GD, Maestri R, Capomolla S, Cobelli F. Nonselective betaadrenergic blocking agent, carvedilol, improves arterial baroflex gain and heart rate variability in patients with stable chronic heart failure. J Am Coll Cardiol. 2000;36:1612–1618. [DOI] [PubMed] [Google Scholar]
- 155.Sutsch G, Oechslin E, Mayer I, Hess OM. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int J Cardiol. 1995;52:135–143. [DOI] [PubMed] [Google Scholar]
- 156.Cannon RO 3rd, Watson RM, Rosing DR, Epstein SE. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol. 1985;56:242–246. doi: 10.1016/0002-9149(85)90842-2 [DOI] [PubMed] [Google Scholar]
- 157.Bugiardini R, Borghi A, Biagetti L, Puddu P. Comparison of verapamil versus propranolol therapy in syndrome X. Am J Cardiol. 1989;63:286–290. doi: 10.1016/0002-9149(89)90332-9 [DOI] [PubMed] [Google Scholar]
- 158.Investigators E. Effect of nifedipine and cerivastatin on coronary endothelial function in patients with coronary artery disease: the ENCORE I Study (Evaluation of Nifedipine and Cerivastatin On Recovery of coronary Endothelial function). Circulation. 2003;107:422–428. doi: 10.1161/01.cir.0000046488.52939.bf [DOI] [PubMed] [Google Scholar]
- 159.Tea Jansen. Efficacy of diltiazem to improve coronary vasomotor dysfunction in angina and nonosbtructive coronary arteries (ANOCA): Results of the EDIT-CMD randomized clinical trial. J Am Coll Cardiol Img 2022. doi: DOI: 10.1016/j.cmg.2022.03.012 [DOI] [PubMed]
- 160.Bairey Merz CN, Pepine CJ, Shimokawa H, Berry C. Treatment of coronary microvascular dysfunction. Cardiovasc Res. 2020;116:856–870. doi: 10.1093/cvr/cvaa006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995;25:807–814. doi: 10.1016/0735-1097(94)00507-M [DOI] [PubMed] [Google Scholar]
- 162.Russo G, Di Franco A, Lamendola P, Tarzia P, Nerla R, Stazi A, Villano A, Sestito A, Lanza GA, Crea F. Lack of effect of nitrates on exercise stress test results in patients with microvascular angina. Cardiovasc Drugs Ther. 2013;27:229–234. doi: 10.1007/s10557-013-6439-z [DOI] [PubMed] [Google Scholar]
- 163.Rogers GE, Taylor LD. The enzymic derivation of citrulline residues from arginine residues in situ during the biosynthesis of hair proteins that are cross-linked by isopeptide bonds. Adv Exp Med Biol. 1977;86A:283–294. doi: 10.1007/978-1-4684-3282-4_17 [DOI] [PubMed] [Google Scholar]
- 164.Chen JW, Lee WL, Hsu NW, Lin SJ, Ting CT, Wang SP, Chang MS. Effects of short-term treatment of nicorandil on exercise-induced myocardial ischemia and abnormal cardiac autonomic activity in microvascular angina. Am J Cardiol. 1997;80:32–38. doi: 10.1016/s0002-9149(97)00279–8 [DOI] [PubMed] [Google Scholar]
- 165.Lerman A, Burnett JC Jr., Higano ST, McKinley LJ, Holmes DR, Jr. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97:2123–2128. doi: 10.1161/01.cir.97.21.2123 [DOI] [PubMed] [Google Scholar]
- 166.Dzavik V, Cotter G, Reynolds HR, Alexander JH, Ramanathan K, Stebbins AL, Hathaway D, Farkouh ME, Ohman EM, Baran DA, et al. Effect of nitric oxide synthase inhibition on haemodynamics and outcome of patients with persistent cardiogenic shock complicating acute myocardial infarction: a phase II dose-ranging study. Eur Heart J. 2007;28:1109–1116. doi: 10.1093/eurheartj/ehm075 [DOI] [PubMed] [Google Scholar]
- 167.Skalidis EI, Hamilos MI, Chlouverakis G, Zacharis EA, Vardas PE. Ivabradine improves coronary flow reserve in patients with stable coronary artery disease. Atherosclerosis. 2011;215:160–165. doi: 10.1016/j.atherosclerosis.2010.11.035 [DOI] [PubMed] [Google Scholar]
- 168.Tagliamonte E, Cirillo T, Rigo F, Astarita C, Coppola A, Romano C, Capuano N. Ivabradine and Bisoprolol on Doppler-derived Coronary Flow Velocity Reserve in Patients with Stable Coronary Artery Disease: Beyond the Heart Rate. Adv Ther. 2015;32:757–767. doi: 10.1007/s12325-015-0237-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Villano A, Di Franco A, Nerla R, Sestito A, Tarzia P, Lamendola P, Di Monaco A, Sarullo FM, Lanza GA, Crea F. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol. 2013;112:8–13. doi: 10.1016/j.amjcard.2013.02.045 [DOI] [PubMed] [Google Scholar]
- 170.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003;41:15–19. doi: 10.1016/s0735-1097(02)02632-3 [DOI] [PubMed] [Google Scholar]
- 171.Nalbantgil S, Altinti gbreve, Yilmaz H, Nalbantgil II, Onder R. The Effect of Trimetazidine in the Treatment of Microvascular Angina. Int J Angiol. 1999;8:40–43. doi: 10.1007/BF01616842 [DOI] [PubMed] [Google Scholar]
- 172.Rai B, Shukla J, Henry TD, Quesada O. Angiogenic CD34 Stem Cell Therapy in Coronary Microvascular Repair-A Systematic Review. Cells. 2021;10. doi: 10.3390/cells10051137 [DOI] [PMC free article] [PubMed]
- 173.Corban MT, Toya T, Albers D, Sebaali F, Lewis BR, Bois J, Gulati R, Prasad A, Best PJM, Bell MR, et al. IMPROvE-CED Trial: Intracoronary Autologous CD34+ Cell Therapy for Treatment of Coronary Endothelial Dysfunction in Patients With Angina and Nonobstructive Coronary Arteries. Circ Res. 2022;130:326–338. doi: 10.1161/CIRCRESAHA.121.319644 [DOI] [PubMed] [Google Scholar]
- 174.Gallone G, Baldetti L, Tzanis G, Gramegna M, Latib A, Colombo A, Henry TD, Giannini F. Refractory Angina: From Pathophysiology to New Therapeutic Nonpharmacological Technologies. JACC Cardiovasc Interv. 2020;13:1–19. doi: 10.1016/j.jcin.2019.08.055 [DOI] [PubMed] [Google Scholar]
- 175.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, Nesto RW. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33:1833–1840. doi: 10.1016/s0735-1097(99)00140-0 [DOI] [PubMed] [Google Scholar]
- 176.Urano H, Ikeda H, Ueno T, Matsumoto T, Murohara T, Imaizumi T. Enhanced external counterpulsation improves exercise tolerance, reduces exercise-induced myocardial ischemia and improves left ventricular diastolic filling in patients with coronary artery disease. J Am Coll Cardiol. 2001;37:93–99. doi: 10.1016/s0735-1097(00)01095-0 [DOI] [PubMed] [Google Scholar]
- 177.Kronhaus KD, Lawson WE. Enhanced external counterpulsation is an effective treatment for Syndrome X. Int J Cardiol. 2009;135:256–257. doi: 10.1016/j.ijcard.2008.03.022 [DOI] [PubMed] [Google Scholar]
- 178.Durante W, Behnammanesh G, Peyton KJ. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Vascular Cell Function and Arterial Remodeling. Int J Mol Sci. 2021;22. doi: 10.3390/ijms22168786 [DOI] [PMC free article] [PubMed]
- 179.Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H, Hirose T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16:84. doi: 10.1186/s12933-017-0564-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sezai A, Sekino H, Unosawa S, Taoka M, Osaka S, Tanaka M. Canagliflozin for Japanese patients with chronic heart failure and type II diabetes. Cardiovasc Diabetol. 2019;18:76. doi: 10.1186/s12933-019-0877-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tochiya M, Makino H, Tamanaha T, Matsuo M, Hishida A, Koezuka R, Ohata Y, Tomita T, Son C, Miyamoto Y, et al. Effect of tofogliflozin on cardiac and vascular endothelial function in patients with type 2 diabetes and heart diseases: A pilot study. J Diabetes Investig. 2020;11:400–404. doi: 10.1111/jdi.13122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tanaka A, Shimabukuro M, Okada Y, Taguchi I, Yamaoka-Tojo M, Tomiyama H, Teragawa H, Sugiyama S, Yoshida H, Sato Y, et al.. Rationale and design of a multicenter placebo-controlled double-blind randomized trial to evaluate the effect of empagliflozin on endothelial function: the EMBLEM trial. Cardiovasc Diabetol. 2017;16:48. doi: 10.1186/s12933-017-0532-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Emdin M, Picano E, Lattanzi F, L’Abbate A. Improved exercise capacity with acute aminophylline administration in patients with syndrome X. J Am Coll Cardiol. 1989;14:1450–1453. doi: 10.1016/0735-1097(89)90380-x [DOI] [PubMed] [Google Scholar]
- 184.Ong P, Athanasiadis A, Sechtem U. Pharmacotherapy for coronary microvascular dysfunction. Eur Heart J Cardiovasc Pharmacother. 2015;1:65–71. doi: 10.1093/ehjcvp/pvu020 [DOI] [PubMed] [Google Scholar]
- 185.Chauhan A, Mullins PA, Thuraisingham SI, Taylor G, Petch MC, Schofield PM. Abnormal cardiac pain perception in syndrome X. J Am Coll Cardiol. 1994;24:329–335. doi: 10.1016/07351097(94)90284-4 [DOI] [PubMed] [Google Scholar]
- 186.Jiang W, Velazquez EJ, Kuchibhatla M, Samad Z, Boyle SH, Kuhn C, Becker RC, Ortel TL, Williams RB, Rogers JG, et al. Effect of escitalopram on mental stress-induced myocardial ischemia: results of the REMIT trial. JAMA. 2013;309:2139–2149. doi: 10.1001/jama.2013.5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lanza GA, Sestito A, Sgueglia GA, Infusino F, Papacci F, Visocchi M, Ierardi C, Meglio M, Bellocci F, Crea F. Effect of spinal cord stimulation on spontaneous and stress-induced angina and ‘ischemialike’ ST-segment depression in patients with cardiac syndrome X. Eur Heart J. 2005;26:983–989. doi: 10.1093/eurheartj/ehi089 [DOI] [PubMed] [Google Scholar]
- 188.Chester M, Hammond C, Leach A. Long-term benefits of stellate ganglion block in severe chronic refractory angina. Pain. 2000;87:103–105. doi: 10.1016/S0304-3959(00)00270-0 [DOI] [PubMed] [Google Scholar]
- 189.Wiener L, Cox JW. Influence of stellate ganglion block on angina pectoris and the post-exercise electrocardiogram. Am J Med Sci. 1966;252:289–295. doi: 10.1097/00000441-196609000-00007 [DOI] [PubMed] [Google Scholar]