Abstract
Background/Aims
The prevalence of nonalcoholic fatty liver disease (NAFLD) has increased rapidly as a consequence of more sedentary lifestyles and a Westernized diet. Fracture is a major clinical problem in older people, but few large-scale cohort studies have evaluated the relationship between NAFLD and fracture. Therefore, we aimed to determine whether the fatty liver index (FLI), which represents the severity of NAFLD, can predict fracture risk.
Methods
We analyzed the relationship between the FLI and incident fracture using multivariate Cox proportional hazards models and data for 180,519 individuals who underwent National Health check-ups in the Republic of Korea between 2009 and 2014.
Results
A total of 2,720 participants (1.5%) were newly diagnosed with fracture during the study period (median 4.6 years). The participants were grouped according to FLI quartiles (Q1, 0 to <5.653; Q2, 5.653 to <15.245; Q3, 15.245 to <37.199; and Q4 ≥37.199). The cumulative fracture incidence was significantly higher in the highest FLI group than in the lowest FLI group (Q4, 986 [2.2%] and Q1, 323 [0.7%]; p<0.001). The adjusted hazard ratio indicated that the highest FLI group was independently associated with a higher incidence of fracture (hazard ratio for Q4 vs Q1, 2.956; 95% confidence interval, 2.606 to 3.351; p<0.001). FLI was significantly associated with a higher incidence of fracture, independent of the baseline characteristics of the participants.
Conclusions
Our data imply that the higher the FLI of a Korean patient is, the higher their risk of osteoporotic fracture, independent of key confounding factors. (Gut Liver, Published online July 27, 2022)
Keywords: Non-alcoholic fatty liver disease, Osteoporotic fracture, Fatty liver index, Fatty liver
INTRODUCTION
The prevalence of nonalcoholic fatty liver disease (NAFLD) has been increasing alongside those of obesity and insulin resistance, as a consequence of more sedentary lifestyles and a Westernized diet.1,2 Positive associations have been demonstrated between NAFLD and features of the metabolic syndrome, including visceral adipose tissue accumulation, dyslipidemia, insulin resistance, and high blood pressure.3-5
Owing to the aging of the population worldwide and changing lifestyles, the prevalence of osteoporosis has risen significantly.6 In 2010, there were an estimated 158 million individuals at high risk of fracture, and it has been estimated that this figure will double by 2040, because of demographic shifts.7 Consistent with this, 22.4% and 47.9% of Korean adults aged ≥50 years have osteopenia or osteoporosis, respectively, and the number of osteoporotic fractures increased between 2008 and 2016, regardless of sex.8
Although seemingly of little significance, a positive epidemiologic association between metabolic syndrome and osteoporosis has been recently reported.9-13 Specifically, it has been shown that the incidence of osteoporotic fracture is higher in patients with metabolic syndrome.14-16 Moreover, it has been shown that mean lumbar bone mineral density (BMD) is lower in postmenopausal women with NAFLD than in those without.17-21 NAFLD is a leading cause of chronic liver disease. Most studies of adults have shown that the values of bone formation markers are lower in chronic liver disease, alongside abnormal metabolic indices.21 However, despite a number of previous studies having suggested an association between NAFLD and fracture, the relatively small sample sizes and cross-sectional nature of these studies mean that the quality of the evidence is relatively low.
Therefore, in the present study, we aimed to confirm an association between NAFLD and new-onset fracture by analyzing a large cohort comprising Korean adults without known conventional rick factors for fracture or comorbidities.
MATERIALS AND METHODS
1. Data sources
The National Health Insurance Service-National Sample Cohort 2.0 (NHIS-NSC 2.0) dataset was analyzed. All the participants (n=48,222,537) in the National Health Insurance Service (NHIS), which includes >97% of the Korean population, were classified into 2,142 classes according to their age, sex, region, eligibility, and income level. From 2006, 2.1% of each class (total n=1,021,208) have been randomly selected to comprise the NHIS-NSC 2.0 dataset. This also includes data collected retrospectively and prospectively between 2002 and 2015.22 Because the NHIS covers 97% of the Korean population, it can be assumed that the NHIS-NSC 2.0 is representative of the Korean population as a whole. The data collected consist of sociodemographic information; clinical information, including diagnoses made on the basis of the 10th revision of the International Classification of Disease (ICD-10) codes, and details of the admission and treatment; National Health Screening data; and information regarding the participating institutions. The free health check-up, which the Korean government recommends that all Korean adults attend biennially, includes the completion of questionnaires regarding medical history and health-related behavior, including smoking status and alcohol consumption; chest X-ray; physical examination; and blood tests. According to the 2013 NHIS statistics, ~72.1% of the eligible population underwent National Health check-ups. In addition, we collected mortality data from the Death Registration Database of Statistics Korea, a central government statistical organization.
The NHIS-NSC 2.0 is open to any researcher if the NHIS Review Committee approves the relevant study protocol. The present study was approved by the Institutional Review Board of the Chungnam National University Hospital, Daejeon, Korea (IRB number: 2019-10-053). The Institutional Review Board waived the requirement for informed consent.
2. Study sample
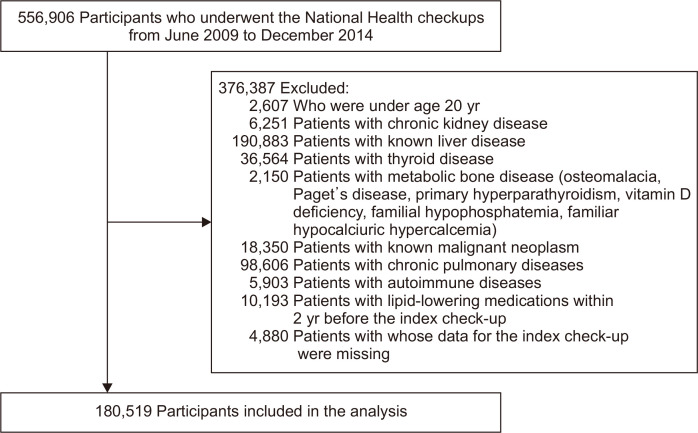
We included data from individuals of ≥20 years who had undergone at least one National Health check-up between 2009 and 2014. Data obtained during the first check-up were used as the index data, and the year in which the index check-up data were collected was regarded as the index year. All the participants for whom there were pre-specified exclusion criteria were excluded. To objectively evaluate the relationship between fatty liver index (FLI) and fracture, we used the following exclusion criteria: comorbidities that could affect the risk of fracture; previously diagnosed liver diseases that could affect FLI; chronic kidney disease; thyroid disease; rheumatoid disease; asthma or chronic obstructive pulmonary disease; neoplastic disease which might affect bone metabolism; primary metabolic bone diseases, such as osteomalacia, Paget’s disease, primary hyperparathyroidism, vitamin D deficiency, familial hypophosphatemia, and familial hypocalciuric hypercalcemia; use of prescribed medications, including lipid-lowering agents, within the 2 years preceding the index check-up; and missing data at the index check-up (Fig. 1, Supplementary Material).
Fig. 1.
A graphical representation of participants' selection for the study samples. Of 556,906 participants who underwent National Health checkups from June 2009 to December 2014, 180,519 were included in the analysis after the exclusion of 376,387 individuals according to the exclusion criteria.
3. Definition of incident fracture
The primary outcome of the study was the incidence of fracture, according to FLI. Incident fracture was defined as a new fracture occurring at typical sites of osteoporotic fracture, defined as the hip, pelvis, proximal humerus, forearm, and vertebrae, but not the head, hands, and feet.23
Fractures were classified according to the ICD-10 disease code in the claim dataset, as previously described.24 These included hip fractures (S72.0 [fracture of the femoral neck] and S72.1 [pertrochanteric fracture]); spinal fractures (S22.0 [fracture of the thoracic spine], S22.1 [multiple fractures of the thoracic spine], S32.0 [fracture of the lumbar spine], S32.7 [multiple fractures of the lumbar spine], S32.8 [other fractures of the lumbar spine], M48.4 [fatigue fracture of vertebra], and M48.5 [collapsed vertebra, not otherwise classified]); distal radial fractures (S52.5 [fracture of the distal radius] and S52.6 [combined fracture of the distal radius/ulna]); proximal humeral fractures (S42.2 [fracture of the proximal humerus] and S42.3 [fracture of shaft of the humerus]); and osteoporosis with pathologic fracture (M.80).
4. Definition and assessment of covariates
Covariates were identified on the basis of the ICD-10 disease code recorded in the claim dataset provided within the 2 years prior to the index year or using the index (NHIS) data. We calculated body mass index (BMI) by dividing body mass (kg) by height (m), squared. Individuals with a BMI of ≥25 kg/m2 were regarded as having obesity, according to the World Health Organization guideline for Asian populations.25
Individuals were classified into three categories according to their smoking status: non-smoker, ex-smoker, and current smoker. Alcohol consumption was assessed using standardized a self-reported questionnaire, which consisted of questions about the number of days per week alcohol was consumed and the amount of alcohol consumed on each day of consumption. The total amount of alcohol consumed was calculated by multiplying these two numbers. The questionnaire regarding physical activity was composed of questions about the number of days per week on which 30 minutes of light exercise, 30 minutes of moderate exercise, and 20 minutes of vigorous exercise were performed. Light exercise was estimated to be two metabolic equivalent of tasks, moderate exercise to be 3 metabolic equivalent of tasks, and vigorous exercise to be 6 metabolic equivalent of tasks. These values were multiplied by 30, 30, and 20 minutes, respectively, and the appropriate number of days per week.
Data were censored when fracture occurred, at disqualification from the NHIS, because of death or immigration, or at the end of the study (December 31, 2015), as previously described.26
5. Calculation of FLI
We used the well-validated index FLI to identify participants with NAFLD. FLI was calculated using the values of four variables (triglycerides [TG], BMI, gamma-glutamyl transferase [GGT] activity, and waist circumference [WC]) and the following equation:
The study that originally used FLI defined >60 as the cutoff value for the diagnosis of fatty liver, with a positive likelihood ratio of 4.3, in the general population.27 Although FLI is simple to calculate and represents an easy method of screening for fatty liver disease, there is insufficient evidence regarding the use of FLI for the diagnosis of fatty liver disease in Asian people because their BMIs and WCs are typically lower than those of people of other ethnicities.28 Therefore, we grouped the participants according to the quartile of FLI for the statistical analysis of the data.
6. Statistical analysis
Continuous data are reported as mean ± standard deviation and categorical data as number with percentage. We performed statistical analyses using R software, version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). The chi-square test and the one-way analysis of variance were used to compare the FLI quartiles. Cumulative event rates for each of the FLI quartiles were compared using the Kaplan-Meier method and the log-rank test. Adjusted hazard ratios and 95% confidence interval for the incidence of fracture were estimated using the Cox proportional hazard regression analysis. In the multivariate analysis, we adjusted for age and sex in model 1, and also for the clinical characteristics and the log-transformed covariates associated with incident fracture that were statistically significant (p<0.001) in models 2 and 3, respectively.
RESULTS
1. Baseline characteristics of the participants
We analyzed data from 180,519 participants after the exclusion of 376,387 individuals according to the exclusion criteria. The numbers of individuals who fulfilled each exclusion criterion are presented in Fig. 1. We grouped the study participants according to quartile of FLI: first quartile (Q1: 0 to <5.653), second quartile (Q2: 5.653 to <15.245), third quartile (Q3: 15.245 to <37.199) and fourth quartile (Q4: ≥37.199). Comparisons of the baseline clinical characteristics and laboratory findings among the FLI quartiles are shown in Table 1. Participants with higher FLIs tended to be older and male. BMI, WC, blood pressure, alcohol consumption, and the proportion of current smokers tended to increase from Q1 to Q4. Aspartate aminotransferase, alanine aminotransferase, and GGT activities; hemoglobin; and the fasting glucose, total cholesterol, triglyceride, low-density lipoprotein cholesterol, and creatinine concentrations increased with ascending FLI quartile. Consistent with previous reports,29,30 the prevalences of diabetes mellitus, dyslipidemia, hypertension, and coronary artery disease were higher in the high FLI groups.
Table 1.
Baseline Characteristics and Laboratory Data of the Participants
| Variable | FLI quartile* | p-value | p for trend | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Baseline characteristics | ||||||
| Age, yr | 36.5±12.1 | 42.0±13.5 | 44.5±13.4 | 44.0±12.1 | <0.001 | <0.001 |
| Male sex | 9,732 (21.6) | 22,843 (50.6) | 31,463 (69.7) | 38,226 (84.7) | <0.001 | <0.001 |
| Height, cm | 162.1±7.7 | 164.6±9.3 | 166.6±9.2 | 169.0±8.3 | <0.001 | <0.001 |
| Body mass, kg | 53.0±6.6 | 60.6±7.8 | 67.1±8.6 | 76.6±11.2 | <0.001 | <0.001 |
| Body mass index, kg/m2 | 20.1±1.8 | 22.3±1.9 | 24.1±2.1 | 26.8±3.1 | <0.001 | <0.001 |
| Waist circumference, cm | 68.5±5.1 | 76.1±4.9 | 81.8±5.1 | 89.0±7.0 | <0.001 | <0.001 |
| Waist/hip ratio | 0.3±0.0 | 0.4±0.0 | 0.4±0.0 | 0.5±0.1 | <0.001 | <0.001 |
| SBP, mm Hg | 112.8±12.6 | 118.9±13.5 | 123.2±14.0 | 128.2±14.6 | <0.001 | <0.001 |
| DBP, mm Hg | 70.5±8.8 | 74.1±9.3 | 76.8±9.5 | 80.5±10.2 | <0.001 | <0.001 |
| Smoking status | ||||||
| Non-smoker | 36,361 (80.6) | 28,327 (62.8) | 21,842 (48.4) | 15,023 (33.3) | <0.001 | <0.001 |
| Ex-smoker | 2,361 (5.2) | 4,447 (9.9) | 64,540 (15.0) | 8,114 (18.0) | <0.001 | <0.001 |
| Current smoker | 6,406 (14.2) | 12,354 (27.4) | 16,541 (36.6) | 21,993 (48.7) | <0.001 | <0.001 |
| Alcohol consumption, g/wk | 36.7±83.9 | 60.0±112.9 | 87.1±142.0 | 139.9±189.6 | <0.001 | <0.001 |
| Activity, MET-min/wk | 351.1±352.4 | 385.7±386.9 | 391.7±388.8 | 377.4±377.2 | <0.001 | <0.001 |
| Laboratory findings | ||||||
| AST, IU/L | 19.7±8.7 | 21.9±11.8 | 24.1±16.7 | 30.7±37.5 | <0.001 | <0.001 |
| ALT, IU/L | 14.5±10.3 | 18.4±11.6 | 23.7±20.8 | 37.8±48.4 | <0.001 | <0.001 |
| GGT, IU/L | 14.6±6.0 | 20.7±11.6 | 31.5±23.5 | 69.9±75.4 | <0.001 | <0.001 |
| Hemoglobin, g/dL | 13.1±1.5 | 13.8±1.6 | 14.4±1.5 | 15.0±1.4 | <0.001 | <0.001 |
| Fasting glucose, mg/dL | 88.9±12.4 | 92.4±16.5 | 96.2±21.4 | 102.4±28.7 | <0.001 | <0.001 |
| Total cholesterol, mg/dL | 176.9±33.2 | 187.3±36.2 | 196.6±38.5 | 208.0±40.7 | <0.001 | <0.001 |
| Triglyceride, mg/dL | 64.1±23.8 | 92.2±37.0 | 128.2±58.8 | 210.9±144.4 | <0.001 | <0.001 |
| HDL-cholesterol, mg/dL | 63.8±19.0 | 58.6±20.9 | 54.7±26.2 | 51.1±28.1 | <0.001 | <0.001 |
| LDL-cholesterol, mg/dL | 106.6±216.5 | 114.1±153.5 | 120.3±126.7 | 119.6±92.6 | <0.001 | <0.001 |
| Creatinine, mg/dL | 0.9±0.9 | 1.0±1.1 | 1.0±1.1 | 1.1±1.1 | <0.001 | <0.001 |
| Comorbidities | ||||||
| Diabetes mellitus | 639 (1.4) | 1,335 (3.0) | 2,122 (4.7) | 2,621 (5.8) | <0.001 | <0.001 |
| Dyslipidemia | 811 (1.8) | 1,371 (3.0) | 1,787 (4.0) | 1,910 (4.2) | <0.001 | <0.001 |
| Hypertension | 1,272 (2.8) | 3,233 (7.2) | 5,407 (12.0) | 6,749 (15.0) | <0.001 | <0.001 |
| Coronary artery disease | 358 (0.8) | 644 (1.4) | 929 (2.1) | 988 (2.2) | <0.001 | <0.001 |
| Arrhythmia | 273 (0.6) | 396 (0.9) | 416 (0.9) | 383 (0.8) | <0.001 | <0.001 |
| Valvular heart disease | 30 (0.1) | 46 (0.1) | 58 (0.1) | 50 (0.1) | 0.029 | 0.018 |
| Peripheral arterial disease | 1,234 (2.7) | 1,824 (4.0) | 2,276 (5.0) | 2,056 (4.6) | <0.001 | <0.001 |
| Cardiovascular disease | 594 (1.3) | 978 (2.2) | 1,215 (2.7) | 1,097 (2.4) | <0.001 | <0.001 |
| Osteoporosis | 1,101 (2.4) | 1,768 (3.9) | 1,635 (3.6) | 808 (1.8) | <0.001 | <0.001 |
| Use of steroid | 16,226 (36.0) | 14,659 (32.5) | 14,187 (31.4) | 13,363 (29.6) | <0.001 | <0.001 |
| Use of vitamin D | 70 (0.2) | 82 (0.2) | 71 (0.2) | 29 (0.1) | <0.001 | <0.001 |
| Fracture | <0.001 | 0.037 | ||||
| Femur | 17.5 (5.3) | 28 (5.0) | 46 (5.5) | 62 (6.3) | ||
| Vertebra | 112 (34.7) | 218 (37.5) | 343 (41.3) | 396 (40.2) | ||
| Distal radius | 141 (43.7) | 234 (40.2) | 320 (38.7) | 370 (37.5) | ||
| Humerus | 17 (5.3) | 28 (4.8) | 42 (5.0) | 95 (9.6) | ||
| Unknown | 36 (10.9) | 73 (12.5) | 79 (9.5) | 63 (6.4) | ||
Data are presented as mean±SD or number (%).
FLI, fatty liver index; SBP, systemic blood pressure; DBP, diastolic blood pressure; MET, metabolic equivalent of task; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
*The chi-square test and one-way analysis of variance were used to compare the FLI quartiles. Q1, 0 to <5.653; Q2, 5.653 to <15.245; Q3, 15.245 to <37.199; Q4, ≥37.199
2. Relationship between FLI and the incidence of fracture
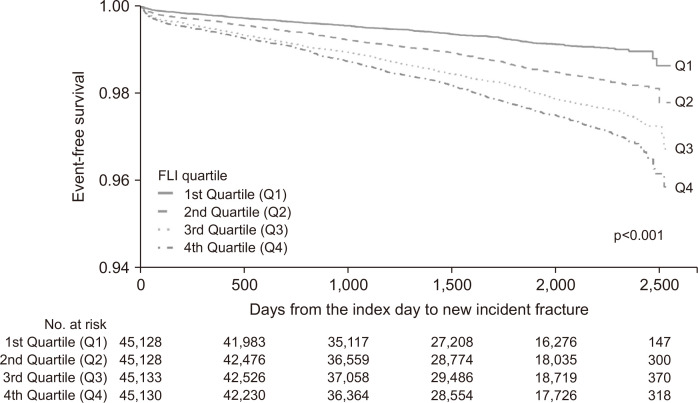
The cohort was followed for a median 4.6 years (interquartile range, 3.2 to 6.1 years) and 2,720 participants (1.5%) experienced a fracture during this period. The incidence of fracture was significantly higher in participants with higher FLIs than in those with lower FLIs (Q1, 323 [0.7%]; Q2, 581 [1.3%]; Q3, 830 [1.8%]; and Q4, 986 [2.2%]; p<0.001, log-rank test). The cumulative incidences of fracture, according to FLI quartile, are presented in Fig. 2.
Fig. 2.
Cumulative incidence of fracture, according to quartile of fatty liver index (FLI). Data were analyzed using the log-rank test.
Table 2 shows the results of the univariate analyses of incident fracture. Female sex, older age, higher BMI, higher total cholesterol and TG, and lower high-density lipoprotein cholesterol were significantly associated with incident fracture. Furthermore, comorbid diabetes mellitus, dyslipidemia, hypertension, coronary artery disease, arrhythmia, valvular heart disease, peripheral arterial disease, cerebrovascular disease, osteoporosis, and use of steroids and vitamin D supplements were associated with incident fracture.
Table 2.
Univariate Analyses of the Relationships between Clinical Parameters and Fracture
| Characteristics | Value | No. | Fracture (%) | HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Sex | Female | 78,255 | 1,237 (1.58) | Reference | |
| Male | 102,264 | 1,483 (1.45) | 0.871 (0.807–0.939) | <0.001 | |
| Age | Continuous | 1.089 (1.086–1.091) | <0.001 | ||
| Body mass index | Continuous | 1.060 (1.049–1.071) | <0.001 | ||
| Smoking | Non-smoker | 101,553 | 1,608 (1.58) | Reference | |
| Ex-smoker | 21,672 | 419 (1.93) | 1.169 (1.050–1.302) | 0.004 | |
| Current smoker | 57,294 | 693 (1.21) | 0.750 (0.686–0.820) | <0.001 | |
| Alcohol consumption | Continuous | 0.999 (0.999–1.000) | <0.001 | ||
| Systemic blood pressure | Continuous | 1.037 (1.035–1.039) | <0.001 | ||
| Diastolic blood pressure | Continuous | 1.037 (1.034–1.040) | <0.001 | ||
| Activity | Continuous | 0.9997 (0.9996–0.9998) | <0.001 | ||
| Waist circumference | Continuous | 1.041 (1.037–1.045) | <0.001 | ||
| Fasting blood glucose* | Continuous | 7.320 (6.427–8.337) | <0.001 | ||
| Total cholesterol* | Continuous | 2.465 (2.021–3.006) | <0.001 | ||
| Triglyceride* | Continuous | 1.604 (1.509–1.705) | <0.001 | ||
| HDL-cholesterol* | Continuous | 0.373 (0.326–0.426) | <0.001 | ||
| LDL-cholesterol* | Continuous | 1.386 (1.257–1.528) | <0.001 | ||
| Aspartate aminotransferase* | Continuous | 2.113 (1.950–2.291) | <0.001 | ||
| Alanine aminotransferase* | Continuous | 1.273 (1.192–1.359) | <0.001 | ||
| Gamma-glutamyl transferase* | Continuous | 1.338 (1.275–1.404) | <0.001 | ||
| Diabetes mellitus | No | 173,802 | 2,223 (1.28) | Reference | |
| Yes | 6,717 | 497 (7.40) | 5.511 (5.000–6.074) | <0.001 | |
| Dyslipidemia | No | 174,640 | 2,444 (1.40) | Reference | |
| Yes | 5,879 | 276 (4.69) | 3.092 (2.730–3.502) | <0.001 | |
| Hypertension | No | 163,858 | 1,327 (0.81) | Reference | |
| Yes | 16,661 | 1,393 (8.36) | 9.935 (9.215–10.711) | <0.001 | |
| Coronary artery disease | No | 177,600 | 2,340 (1.32) | Reference | |
| Yes | 2,919 | 380 (13.02) | 9.708 (8.711–10.820) | <0.001 | |
| Arrhythmia | No | 179,051 | 2,55 1 (1.42) | Reference | |
| Yes | 1,468 | 169 (11.51) | 7.825 (6.696–9.143) | <0.001 | |
| Valvular heart disease | No | 180,335 | 2,646 (1.47) | Reference | |
| Yes | 184 | 74 (40.22) | 33.701 (26.748–42.461) | <0.001 | |
| Peripheral arterial disease | No | 173,129 | 2,304 (1.33) | Reference | |
| Yes | 7,390 | 416 (5.63) | 3.870 (3.486–4.296) | <0.001 | |
| Cardiovascular disease | No | 176,635 | 2,401 (1.36) | Reference | |
| Yes | 3,884 | 319 (8.21) | 5.746 (5.112–6.459) | <0.001 | |
| Osteoporosis | No | 1,175,207 | 2,364 (1.35) | Reference | |
| Yes | 5,312 | 356 (6.7) | 4.451 (3.981–4.976) | <0.001 | |
| Use of steroid | No | 122,084 | 1,790 (1.47) | Reference | |
| Yes | 58,435 | 930 (1.59) | 1.128 (1.042–1.221) | 0.003 | |
| Use of vitamin D | No | 180,267 | 2,696 (1.5) | Reference | |
| Yes | 252 | 24 (9.52) | 5.437 (3.637–8.127) | <0.001 |
HR, hazard ratio; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
*Log-transformed data.
Next, multivariate proportional hazards analysis was performed. In model 1, which was adjusted for age and sex; in model 2, which was additionally adjusted for statistically significant clinical characteristics; and in model 3, which was additionally adjusted for log-transformed statistically significant laboratory covariates, the association between FLI quartile and fracture incidence remained statistically significant (Table 3). All the variance inflation factors were less than 10 in multicollinearity analysis (Supplementary Table 1). We re-analyzed the data based on two different FLI criteria, those of Bedogni et al.27 (Europe) and those of Yang et al.31 (Asia). The results were consistent with our initial results obtained using quartile-FLI (Supplementary Tables 2 and 3).
Table 3.
Relationship between the FLI and Incident Fracture
| FLI quartile* | No. | Incident fracture No. (%) |
Univariate | Model 1† | Model 2‡ | Model 3§ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||||||
| Q1 | 45,128 | 323 (0.7) | Reference | Reference | Reference | Reference | |||||||
| Q2 | 45,128 | 581 (1.3) | 1.732 (1.512–1.985) | <0.001 | 1.061 (0.925–1.217) | 0.399 | 0.962 (0.837–1.105) | 0.585 | 0.960 (0.835–1.104) | 0.568 | |||
| Q3 | 45,133 | 830 (1.8) | 2.438 (2.144–2.772) | <0.001 | 1.319 (1.157–1.505) | <0.001 | 1.035 (0.904-1.185) | 0.622 | 1.021 (0.889–1.173) | 0.770 | |||
| Q4 | 45,130 | 986 (2.2) | 2.956 (2.606–3.351) | <0.001 | 1.900 (1.667–2.167) | <0.001 | 1.292 (1.123–1.487) | <0.001 | 1.233 (1.060–1.433) | 0.007 | |||
FLI, fatty liver index; HR, hazard ratio; CI, confidence interval.
*Q1, 0 to <5.653; Q2, 5.653 to <15.245; Q3, 15.245 to <37.199; Q4, ≥37.199; †Cox proportional hazard model including age and sex as covariates; ‡Cox proportional hazard model including age, sex, smoking status, alcohol consumption, activity, systolic blood pressure, diastolic blood pressure, fasting blood glucose, total cholesterol, alanine aminotransferase activity, aspartate aminotransferase activity, diabetes mellitus, dyslipidemia, hypertension, coronary artery disease, arrhythmia, valvular heart disease, peripheral arterial disease, cerebrovascular disease, osteoporosis, use of steroids and use of vitamin D supplements as covariates; §Cox proportional hazard model including age, sex, smoking status, alcohol consumption, activity, systolic blood pressure, diastolic blood pressure diabetes mellitus, dyslipidemia, hypertension, coronary artery disease, arrhythmia, valvular heart disease, peripheral arterial disease, cerebrovascular disease, osteoporosis, use of steroid, use of vitamin D supplements as covariates, and log-transformed fasting blood glucose, total cholesterol, low-density lipoprotein cholesterol, alanine aminotransferase activity, and aspartate aminotransferase activity as covariates.
3. Subgroup analysis
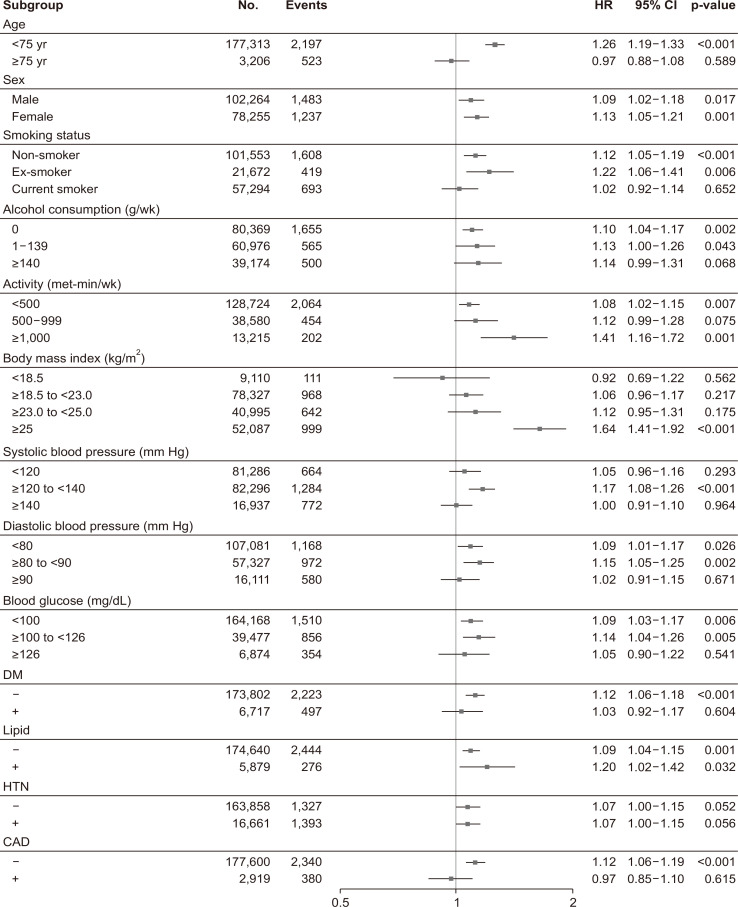
The adjusted hazard ratios for each subgroup are summarized in Fig. 3. FLI was a significant determinant of incident fracture, independently of sex, alcohol consumption, and physical activity, and it was more closely associated with incident fracture in younger participants (<75 years), non-smokers, individuals with high BMI (>25 kg/m2), those with normal blood pressure, and in those with a fasting glucose concentration <126 mg/dL (Fig. 3).
Fig. 3.
Forest plots of hazard ratios for incident fractures in participants categorized according to their clinical characteristics.
HR, hazard ratio; CI, confidence interval; DM, diabetes mellitus; HTN, hypertension; CAD, coronary artery disease.
DISCUSSION
In the present study, we have shown that FLI, a surrogate marker of NAFLD, is associated with the risk of incident fracture in Korean adults. To the best of our knowledge, this is one of the largest studies ever to have been conducted to characterize the relationship between FLI and incident fracture in healthy individuals. Patients with known metabolic bone disease and comorbid malignancies were excluded from the analyses. A positive association remained after adjustment for multiple confounding factors, including age, daily activity, smoking, and alcohol consumption.
A number of previous studies have shown an association between NAFLD and osteoporosis or fracture. It was previously shown that NAFLD was negatively associated with right-hip BMD and serum osteocalcin concentration in 859 Korean men.32 Similarly, in a cross-sectional cohort study using data from the National Health and Nutrition Examination Survey, which included 4,264 Koreans (1,908 men and 2,356 women), high FLI was significantly associated with low BMD in men, but not in women, irrespective of the presence of insulin resistance.33 In clinical practice, the risk of fracture does not seem to be directly related to BMD. For example, type 2 diabetes is associated with high BMD, but also a high fracture risk.13 Accordingly, it is important to evaluate fracture risk in the clinical setting. In addition, it has previously been shown that osteoporosis, as well as osteoporotic fracture, is more prevalent in individuals with metabolic syndrome. Moreover, a meta-analysis of 12 cross-sectional and case-control studies showed that despite no significant differences in BMD at specific skeletal sites between individuals with and without NAFLD, the risk of osteoporotic fracture appeared to be higher in individuals with NAFLD.34
In subgroup analysis (Fig. 3), the data did not show an association between FLI and risk of fracture in the elderly (≥75 years). It is likely that the number of elderly participants was relatively small and that the fracture rate due to frailty factors other than FLI might be higher.35 Koehler et al.36 validated FLI in elderly people (mean age, 76.3±6.0 years),36 and they showed the validation power of FLI was lower than that reported by Bedogni et al.,27 who first proposed FLI. The association between current smoker and FLI was likely not significant, since smoking itself is an overwhelming risk factor for fractures.37 Low BMI,38 high blood pressure,39 high glucose,40 diabetes,41 and coronary artery disease42 are also robust risk factors for fractures; thus, their association with FLI does not appear to be significant. Because FLI includes BMI, the association of high FLI with fracture was highly prominent in participants with high BMI. High FLI strongly correlated with fracture despite high physical activity. However, since this study was a cross-sectional retrospective study, each value should be validated in a well-designed case-control or randomized control study.
Although the pathophysiological mechanisms of these associations remain elusive, a number of plausible candidates have been suggested. A previous study using gene expression profiles of osteoporotic bone tissue revealed that TG metabolism is reduced in osteoporotic bones, and reduced TG metabolism impairs osteoblast differentiation.43 As TG metabolism is impaired, it can be inferred that patients with high serum TG levels are more prone to osteoporotic bone fractures. A previous large-scale study showed a negative association between hypertriglyceridemia and BMD.44
Furthermore, insulin resistance, in addition to truncal obesity, is associated with lower BMD.45 Fasting glucose concentration, BMI, and WC were higher in the high FLI groups in the present study (Table 1), which is consistent with these previous findings. In addition, it has been postulated that inflammation is responsible for bone loss.20 Because NAFLD also involves chronic low-grade inflammation, it is plausible that fracture would be more prevalent in individuals with higher FLI.46,47 Furthermore, a number of other characteristics of NAFLD, including hypercoagulability and hypofibrinolysis, high osteopontin, low osteoprotegerin, low osteocalcin, low leptin, low adiponectin, and low 25-hydroxyvitamin D3, could be responsible for poor bone health outcomes.48,49 Intriguingly, high baseline serum GGT activity, which is a characteristic of NAFLD, was shown to positively correlate with incident osteoporotic fracture in 16,036 Korean men >50 years old.24 In addition, it was shown that GGT-overexpressing mice exhibit more bone loss, which implies that GGT might be responsible for bone loss.50 Because GGT activity is a component of FLI, FLI is high in patients with high GGT activity, and the GGT activity increased with ascending FLI quartile in the present study (Table 1). Mechanistically, oxidative stress is reported to play an important role in the pathogenesis of osteoporosis after menopause.51 Upon oxidative stress, glutathione, an antioxidant, increases the activity of GGT.52 Therefore, it can be predicted that serum GGT, an indicator of oxidative stress, is related to bone density.
The main strength of the present study was that the data were obtained from the government-initiated NHIS-NSC 2.0 cohort, meaning that they should be highly reliable.22 Because the Korean government randomly selected approximately one million individuals from the NHIS, to which >97% of Korean adults are affiliated, the data are likely to be representative of the Korean adult population as a whole. In addition, the large sample size is in contrast to those of previous studies on the relationship between NAFLD and osteoporosis or fracture. However, there were also some limitations to the study. Firstly, none of BMD, or serum calcium, phosphorus, or vitamin D, was evaluated during the study. Secondly, although we recorded the ICD-10 codes for fractures, there was no information regarding whether the fractures were “osteoporotic” or “traumatic.” Thirdly, because more than 10,000 individuals who were using lipid-lowering agents and 190,000 who had chronic liver disease were excluded, it is likely that relatively few of the remaining participants would have had high FLI scores. Finally, because the cohort exclusively comprised Koreans, the findings cannot be generalized to other ethnicities.
In conclusion, the present findings obtained using the NHIS-NSC 2.0 cohort clearly demonstrate that Korean adults with high FLI are predisposed toward fractures. FLI can be easily calculated in the clinic, and more attention should be paid to individuals with a high FLI score with respect to the primary prevention of fracture. Further mechanistic insights regarding the liver-bone axis will be required in the future to explain the epidemiological observations made in the present study.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl210571.
ACKNOWLEDGEMENTS
This work was supported by a Biomedical Research Institute grant from Kyungpook National University Hospital (2016).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: J.H.R., J.H.J. Data acquisition: M.S.K., J.H.R. Data analysis and interpretation: M.J.K., M.S.K., H.B.L., J.H.R., J.H.J. Drafting of the manuscript: M.J.K., J.H.R., J.H.J. Critical revision of the manuscript for important intellectual content: M.J.K., J.H.R., J.H.J. Statistical analysis: H.B.L. Obtained funding: J.H.J Administrative, technical, or material support; study supervision: J.H.R., J.H.J. Approval of final manuscript: all authors.
REFERENCES
- 1.Murag S, Ahmed A, Kim D. Recent epidemiology of nonalcoholic fatty liver disease. Gut Liver. 2021;15:206–216. doi: 10.5009/gnl20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Lim S, Oh TJ, Koh KK. Mechanistic link between nonalcoholic fatty liver disease and cardiometabolic disorders. Int J Cardiol. 2015;201:408–414. doi: 10.1016/j.ijcard.2015.08.107. [DOI] [PubMed] [Google Scholar]
- 4.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 5.Li AA, Ahmed A, Kim D. Extrahepatic manifestations of nonalcoholic fatty liver disease. Gut Liver. 2020;14:168–178. doi: 10.5009/gnl19069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Real A, Riancho-Zarrabeitia L, Riancho JA. Epigenetic aging in osteoporosis. J Bone Miner Res. 2018;33:1902–1903. doi: 10.1002/jbmr.3567. [DOI] [PubMed] [Google Scholar]
- 7.Odén A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010-2040. Osteoporos Int. 2015;26:2243–2248. doi: 10.1007/s00198-015-3154-6. [DOI] [PubMed] [Google Scholar]
- 8.Ahn SH, Park SM, Park SY, et al. Osteoporosis and osteoporotic fracture fact sheet in Korea. J Bone Metab. 2020;27:281–290. doi: 10.11005/jbm.2020.27.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon YK, Lee JG, Kim SS, et al. Association between bone mineral density and metabolic syndrome in pre- and postmenopausal women. Endocr J. 2011;58:87–93. doi: 10.1507/endocrj.K10E-297. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Wang C, Hao J, Yin L, Wang Y, Li W. Association between metabolic syndrome and osteoporosis: a systematic review and meta-analysis. Int J Endocrinol. 2021;2021:6691487. doi: 10.1155/2021/6691487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 12.Wong SK, Chin KY, Suhaimi FH, Ahmad F, Jamil NA, Ima-Nirwana S. Osteoporosis is associated with metabolic syndrome induced by high-carbohydrate high-fat diet in a rat model. Biomed Pharmacother. 2018;98:191–200. doi: 10.1016/j.biopha.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 13.Chin KY, Wong SK, Ekeuku SO, Pang KL. Relationship between metabolic syndrome and bone health: an evaluation of epidemiological studies and mechanisms involved. Diabetes Metab Syndr Obes. 2020;13:3667–3690. doi: 10.2147/DMSO.S275560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin L, Yang Z, Zhang W, et al. Metabolic syndrome and osteoporotic fracture: a population-based study in China. BMC Endocr Disord. 2016;16:27. doi: 10.1186/s12902-016-0106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KC, Shin DH, Lee SY, Im JA, Lee DC. Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med J. 2010;51:857–863. doi: 10.3349/ymj.2010.51.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amouzegar A, Asgari S, Azizi F, Momenan AA, Bozorgmanesh M, Hadaegh F. The role of metabolic syndrome and its components in incident fracture: a 15-year follow-up among the Iranian population. J Clin Endocrinol Metab. 2021;106:e1968–e1983. doi: 10.1210/clinem/dgab023. [DOI] [PubMed] [Google Scholar]
- 17.Leslie WD, Bernstein CN, Leboff MS American Gastroenterological Association Clinical Practice Committee, author. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941–966. doi: 10.1016/S0016-5085(03)01062-X. [DOI] [PubMed] [Google Scholar]
- 18.Moon SS, Lee YS, Kim SW. Association of nonalcoholic fatty liver disease with low bone mass in postmenopausal women. Endocrine. 2012;42:423–429. doi: 10.1007/s12020-012-9639-6. [DOI] [PubMed] [Google Scholar]
- 19.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 20.Faulhaber GA, Premaor MO, Moser Filho HL, Silla LM, Furlanetto TW. Low bone mineral density is associated with insulin resistance in bone marrow transplant subjects. Bone Marrow Transplant. 2009;43:953–957. doi: 10.1038/bmt.2009.70. [DOI] [PubMed] [Google Scholar]
- 21.Guañabens N, Parés A. Osteoporosis in chronic liver disease. Liver Int. 2018;38:776–785. doi: 10.1111/liv.13730. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 23.Borhan S, Papaioannou A, Gajic-Veljanoski O, et al. Incident fragility fractures have a long-term negative impact on health-related quality of life of older people: the Canadian Multicentre Osteoporosis Study. J Bone Miner Res. 2019;34:838–848. doi: 10.1002/jbmr.3666. [DOI] [PubMed] [Google Scholar]
- 24.Kim BJ, Baek S, Ahn SH, et al. A higher serum gamma-glutamyl transferase level could be associated with an increased risk of incident osteoporotic fractures in Korean men aged 50 years or older. Endocr J. 2014;61:257–263. doi: 10.1507/endocrj.EJ13-0463. [DOI] [PubMed] [Google Scholar]
- 25.WHO Expert Consultation, author. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 26.Roh JH, Park JH, Lee H, et al. Higher fatty liver index is associated with increased risk of new onset heart failure in healthy adults: a nationwide population-based study in Korea. BMC Cardiovasc Disord. 2020;20:204. doi: 10.1186/s12872-020-01444-x.9afe914eceb94ffc86cb48cd833c3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 29.Olubamwo OO, Virtanen JK, Pihlajamaki J, Tuomainen TP. Association of fatty liver index with risk of incident type 2 diabetes by metabolic syndrome status in an Eastern Finland male cohort: a prospective study. BMJ Open. 2019;9:e026949. doi: 10.1136/bmjopen-2018-026949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozakova M, Palombo C, Eng MP, et al. Fatty liver index, gamma-glutamyltransferase, and early carotid plaques. Hepatology. 2012;55:1406–1415. doi: 10.1002/hep.25555. [DOI] [PubMed] [Google Scholar]
- 31.Yang BL, Wu WC, Fang KC, et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS One. 2015;10:e0120443. doi: 10.1371/journal.pone.0120443.f34507884ef74e37af775f02e3a9992f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang HJ, Shim SG, Ma BO, Kwak JY. Association of nonalcoholic fatty liver disease with bone mineral density and serum osteocalcin levels in Korean men. Eur J Gastroenterol Hepatol. 2016;28:338–344. doi: 10.1097/MEG.0000000000000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn SH, Seo DH, Kim SH, Nam MS, Hong S. The relationship between fatty liver index and bone mineral density in Koreans: KNHANES 2010-2011. Osteoporos Int. 2018;29:181–190. doi: 10.1007/s00198-017-4257-z. [DOI] [PubMed] [Google Scholar]
- 34.Mantovani A, Dauriz M, Gatti D, et al. Systematic review with meta-analysis: non-alcoholic fatty liver disease is associated with a history of osteoporotic fractures but not with low bone mineral density. Aliment Pharmacol Ther. 2019;49:375–388. doi: 10.1111/apt.15087. [DOI] [PubMed] [Google Scholar]
- 35.Ensrud KE, Ewing SK, Taylor BC, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 36.Koehler EM, Schouten JN, Hansen BE, Hofman A, Stricker BH, Janssen HL. External validation of the fatty liver index for identifying nonalcoholic fatty liver disease in a population-based study. Clin Gastroenterol Hepatol. 2013;11:1201–1204. doi: 10.1016/j.cgh.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 37.Hernigou J, Schuind F. Tobacco and bone fractures: a review of the facts and issues that every orthopaedic surgeon should know. Bone Joint Res. 2019;8:255–265. doi: 10.1302/2046-3758.86.BJR-2018-0344.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang HL, Pan CC, Hsiao YF, et al. Associations of body mass index and diabetes with hip fracture risk: a nationwide cohort study. BMC Public Health. 2018;18:1325. doi: 10.1186/s12889-018-6230-y.a1866570955348af881cd413c09e1f07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vestergaard P, Rejnmark L, Mosekilde L. Hypertension is a risk factor for fractures. Calcif Tissue Int. 2009;84:103–111. doi: 10.1007/s00223-008-9198-2. [DOI] [PubMed] [Google Scholar]
- 40.Holmberg AH, Nilsson PM, Nilsson JA, Akesson K. The association between hyperglycemia and fracture risk in middle age: a prospective, population-based study of 22,444 men and 10,902 women. J Clin Endocrinol Metab. 2008;93:815–822. doi: 10.1210/jc.2007-0843. [DOI] [PubMed] [Google Scholar]
- 41.Moseley KF. Type 2 diabetes and bone fractures. Curr Opin Endocrinol Diabetes Obes. 2012;19:128–135. doi: 10.1097/MED.0b013e328350a6e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.den Uyl D, Nurmohamed MT, van Tuyl LH, Raterman HG, Lems WF. (Sub)clinical cardiovascular disease is associated with increased bone loss and fracture risk; a systematic review of the association between cardiovascular disease and osteoporosis. Arthritis Res Ther. 2011;13:R5. doi: 10.1186/ar3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dragojevič J, Zupan J, Haring G, Herman S, Komadina R, Marc J. Triglyceride metabolism in bone tissue is associated with osteoblast and osteoclast differentiation: a gene expression study. J Bone Miner Metab. 2013;31:512–519. doi: 10.1007/s00774-013-0445-x. [DOI] [PubMed] [Google Scholar]
- 44.Chi JH, Shin MS, Lee BJ. Identification of hypertriglyceridemia based on bone density, body fat mass, and anthropometry in a Korean population. BMC Cardiovasc Disord. 2019;19:66. doi: 10.1186/s12872-019-1050-2.c1ea2ae3878c4ed9a948353e4f8dbf46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greco EA, Francomano D, Fornari R, et al. Negative association between trunk fat, insulin resistance and skeleton in obese women. World J Diabetes. 2013;4:31–39. doi: 10.4239/wjd.v4.i2.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 47.Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. World J Gastroenterol. 2010;16:4773–4783. doi: 10.3748/wjg.v16.i38.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Targher G, Lonardo A, Rossini M. Nonalcoholic fatty liver disease and decreased bone mineral density: is there a link? J Endocrinol Invest. 2015;38:817–825. doi: 10.1007/s40618-015-0315-6. [DOI] [PubMed] [Google Scholar]
- 49.Yilmaz Y. Review article: non-alcoholic fatty liver disease and osteoporosis: clinical and molecular crosstalk. Aliment Pharmacol Ther. 2012;36:345–352. doi: 10.1111/j.1365-2036.2012.05196.x. [DOI] [PubMed] [Google Scholar]
- 50.Hiramatsu K, Asaba Y, Takeshita S, et al. Overexpression of gamma-glutamyltransferase in transgenic mice accelerates bone resorption and causes osteoporosis. Endocrinology. 2007;148:2708–2715. doi: 10.1210/en.2007-0215. [DOI] [PubMed] [Google Scholar]
- 51.Maggio D, Barabani M, Pierandrei M, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88:1523–1527. doi: 10.1210/jc.2002-021496. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Forman HJ. Redox regulation of gamma-glutamyl transpeptidase. Am J Respir Cell Mol Biol. 2009;41:509–515. doi: 10.1165/rcmb.2009-0169TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.