Abstract
Background/Aims
Tegoprazan, a novel potassium-competitive acid blocker, is expected to overcome the limitations of proton pump inhibitors and effectively control nocturnal acid breakthrough. To evaluate the pharmacodynamics of tegoprazan versus dexlansoprazole regarding nocturnal acid breakthrough in healthy subjects.
Methods
In a randomized, open-label, single-dose, balanced incomplete block crossover study, 24 healthy male volunteers were enrolled and randomized to receive oral tegoprazan (50, 100, or 200 mg) or dexlansoprazole (60 mg) during each of two administration periods, separated by a 7- to 10-day washout period. Blood samples were collected for pharmacokinetic parameter analysis; gastric monitoring was performed for pharmacodynamic parameter evaluation.
Results
All 24 subjects completed the study. Average maximum plasma concentration, area under the plasma concentration–time curve, and mean time with gastric pH >4 and pH >6 for tegoprazan demonstrated dose-dependent incremental increases. All the tegoprazan groups reached mean pH ≥4 within 2 hours, whereas the dexlansoprazole group required 7 hours after drug administration. Based on pharmacodynamic parameters up to 12 hours after evening dosing, 50, 100, and 200 mg of tegoprazan presented a stronger acid-suppressive effect than 60 mg of dexlansoprazole. Moreover, the dexlansoprazole group presented a comparable acid-suppressive effect with the tegoprazan groups 12 hours after dosing.
Conclusions
All the tegoprazan groups demonstrated a significantly faster onset of gastric pH increase and longer holding times above pH >4 and pH >6 up to 12 hours after evening dosing than the dexlansoprazole group.
Keywords: Tegoprazan, Dexlansoprazole, Healthy subjects, Potassium-competitive acid blocker, Nocturnal acid breakthrough
INTRODUCTION
Acid-related gastrointestinal diseases such as gastroesophageal reflux disease (GERD) and non-erosive reflux disease are the most common diseases among gastrointestinal disorders.1 Proton pump inhibitors (PPIs) and histamine 2 receptor antagonists have been used as treatment regimens for acid-related diseases and are expected to improve the quality of life of patients.2 However, although PPIs have advantages over histamine 2 receptor antagonists with their greater and longer-lasting acid-suppressive potentials, some unmet clinical needs still exist beyond current acid-suppressive therapy.3 In particular, the nocturnal acid reflux from nocturnal acid breakthrough (NAB) poses a significant challenge for the successful control of GERD under PPI treatment, and patients with NAB have a significantly higher risk of complications with GERD.4,5 Owing to the inadequate control of NAB with PPIs, an additional bedtime dose of histamine 2 receptor antagonists has been suggested, but the long-term use of these agents eventually leads to the development of tolerance.6,7 Dexlansoprazole, a second-generation PPI, with some advantages in the pharmacological control of proton pumps, has been developed, but it still falls short of achieving the ideal control of NAB.8
Tegoprazan is a novel potassium-competitive acid blocker (P-CAB) showing promising in vitro and in vivo animal pharmacology activities;9 moreover, clinical studies showed favorable tolerability and safety as well as potent and long-lasting efficacy.10,11 P-CABs represent a new class of drugs; they exhibit rapid and effective anti-secretory activity by competitively and reversibly binding with H+/K+-ATPase on the parietal cell.12 PPIs are pro-drugs that require activation in acidic conditions, and it takes 3 to 5 days of repeated administration to achieve a steady anti-secretory effect for symptom relief.13 Moreover, since PPIs should be ingested before a meal as these drugs irreversibly inhibit only activated proton pumps, they have limitations in the control of NAB.14 Unlike conventional PPIs, tegoprazan offers a rapid onset of action and full effect from the first dose as it can immediately inhibit proton pumps.10 Furthermore, since P-CABs do not require activation in the presence of gastric acid, they can be administered regardless of meals.15 Therefore, P-CABs are expected to have improved NAB control, overcoming the limitations of current PPIs. This study was conducted to evaluate the efficacy of the night-time dosing of tegoprazan in terms of NAB control compared to dexlansoprazole, which is known to be the most effective in controlling NAB among the currently available PPIs,16,17 in healthy male subjects.
MATERIALS AND METHODS
1. Subjects
Healthy male volunteers aged 20 to 45 years, with a body mass index of 19 to 28 kg/m2 and negative on 13C-urea breath test and Helicobacter pylori, participated in this study. Written informed consent was obtained from each subject before enrolment. The subjects had no history of clinically significant diseases, including symptomatic GERD, erosive esophagitis, and duodenal ulcer, or allergy to any study drugs. The protocol of this study was approved by the Institutional Review Board of Asan Medical Center (IRB number: 2015-0356; ClinicalTrials.gov registry number: NCT03043521), and the study was conducted at the same institute in accordance with the Declaration of Helsinki and the International Congress on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use–Good Clinical Practice guidelines.
2. Study design
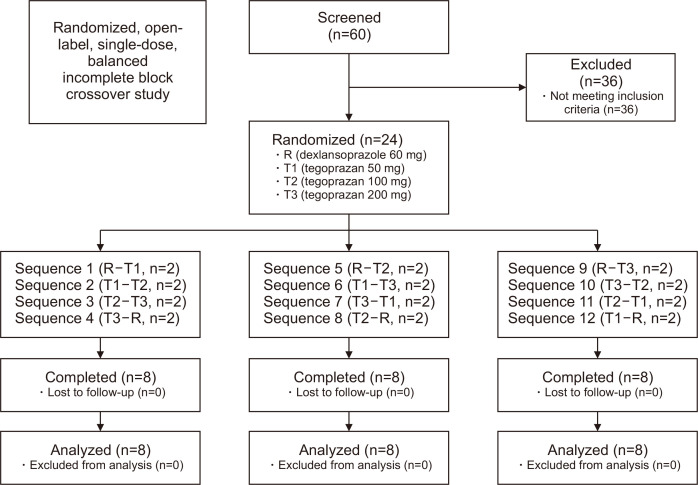
This study was a randomized, open-label, single-dose, balanced incomplete block crossover design, which involved a total of 24 subjects who were paired and assigned to 1 of 12 sequences (Fig. 1). The subjects were randomly assigned to receive 60 mg dexlansoprazole or 50, 100, or 200 mg tegoprazan orally during each of two administration periods, separated by a washout period of 7 to 10 days. Water intake was restricted 1 hour before study drug administration, and the assigned study drug was administered between 8:30 p.m. and 10:30 p.m. with 200 mL of water. The subjects were instructed to refrain from lying down for 3 hours after study drug administration, but this was exempted in cases when the subjects had to be in a supine or semi-reclined position as part of study procedure.
Fig. 1.
Subject disposition.
3. Pharmacodynamic evaluation
Gastric pH monitoring was performed for 24 hours on Day –1 (pre-dose) and for 24 hours on Day 1 and Day 7 (post-dose) in period 1 and Day –1 (pre-dose) and Day 1 (post-dose) in period 2. Gastric pH was measured and recorded using a disposable single-use pH probe (ComforTecTM plus antimony pH catheter, single pH channel; Sandhill Scientific, Inc., Highlands Ranch, CO, USA). Before insertion, the disposable pH probe was calibrated using standard buffer (pH 4 and pH 7) at 25°C. Using the pH step-up method, the pH probe was passed through the nose and down the esophagus; it was positioned about 5 to 10 cm below the lower esophageal sphincter. The probe was inserted slowly to minimize subject discomfort, and once the insertion was completed, the position of the probe was fixed using paper or cotton plaster. After insertion, the probe was connected to a gastric pH logger (ZepHrTM impedance/pH reflux monitoring system, Sandhill Scientific). Gastric pH was measured every five seconds and saved automatically. Food and water were restricted until 4 hours after pH probe insertion. After the gastric pH monitoring was completed, the probe was gently removed from the subjects.
All gastric pH data was transferred and extracted using the exclusive pH data management program (ZepHr BioVIEW analysis system, Sandhill Scientific). These gastric pH data were then adjusted for the difference between pH calibration (room temperature, 21℃) and pH recording (body temperature, about 37℃) using a predefined regression equation (Y=0.9132X–0.2511). If the adjusted pH data was beyond the calibration range, i.e., <0.5 or >7.5, these data were excluded as per the protocol.
The obtained gastric pH data was used to calculate the pharmacodynamic (PD) parameters using RⓇ version 3.0.2. The PD parameters included time pH >4 (percentage of values greater than pH 4 during the course of the gastric pH monitoring), time pH >6 (percentage of values greater than pH 6 during the course of the gastric pH monitoring), integrated acidity (IA) (the acid concentration [mM] was calculated as 1,000×10‐pH; this parameter is the time‐weighted average of the acid concentration expressed as mmol·hr/L and corresponds to the area under the acid concentration–time curve from time 0 to the last measurable acid concentration), percent inhibition of IA (this parameter is calculated by 100×[baseline IA–treatment IA]/baseline IA), percent inhibition of time pH ≤4, and median pH value during the monitoring period.
4. Pharmacokinetic evaluation
Blood samples for the assessment of pharmacokinetic (PK) parameters were collected at 0 (pre-dose), 1, 2, 3, 12, and 20 hours post-dose in each period after drug administration. Tubes containing the collected blood samples were immediately placed in an icebox and centrifuged at 1,800 ×g for 8 minutes at 4°C to separate the plasma supernatant; then, the plasma samples were aliquoted and stored at ≤–70°C.
The plasma concentrations of tegoprazan and M1, which is an active metabolite of tegoprazan, were determined by BioInfra (Suwon, Korea) using a pre-validated method based on liquid-chromatographic separation (column: Waters ACQUITY UPLCⓇBEH C18) coupled with tandem mass spectrometry detection (Waters Micromass Quattro PermierTM XE Mass Spectrometer). The bioanalytical method was confirmed to be reliable over the concentration ranges of 20–7,500 ng/mL and 10–3,750 ng/mL for tegoprazan and M1 in plasma, respectively.
Phoenix WinNonlinⓇ version 6.4 (Certara USA, Inc., Princeton, NJ, USA) was used for PK analysis. PK parameters were determined using a non-compartmental method. PK parameters included area under the plasma concentration–time curve to the last sampling time point at which quantifiable blood was drawn (AUClast), maximum drug concentration in plasma (Cmax), and time to peak concentration (tmax) of plasma tegoprazan and M1.
5. Safety and tolerability assessment
Adverse events (AEs) were recorded through questionnaires by investigators or spontaneous reports from subjects. Physical examinations, vital signs, 12-lead electrocardiograms, and laboratory tests were performed throughout the study at intervals predefined in the study protocol. All abnormal clinically significant changes from the tests were reported as AEs. The investigators assessed the AEs with regard to severity, course, outcome, seriousness, and relationship to the study drug and recorded all AEs regardless of the suspected relationship to the study drug.
6. Statistical analyses
All statistical analyses used SASⓇ version 9.4 (SAS Institute Inc., Cary, NC, USA). PK parameters were summarized using descriptive statistics by dose groups. The concentration–time curve for tegoprazan was presented as the means for each treatment group. Key PK parameters were summarized and presented as treatment groups using descriptive statistics. The PD parameters were summarized using descriptive statistics by treatment groups. Where possible, the gastric pH–time curve was presented as the mean for each treatment group. The subjects that were administered at least one dose of the investigational drugs were included in the safety analysis. Clinically significant results were summarized according to treatment group. For AEs, severity and causality were summarized according to the treatment group and presented using descriptive statistics.
RESULTS
1. Demographics
Twenty-four healthy male volunteers aged 20 to 45 years (mean±standard deviation: 26.92±4.48 years), with a body mass index of 19 to 28 kg/m2 (22.7±1.97 kg/m2) and negative on the 13C-urea breath test and H. pylori, were enrolled in the study. In total, the 24 subjects enrolled in this study completed the study in accordance with the protocol (Fig. 1).
2. Pharmacodynamics
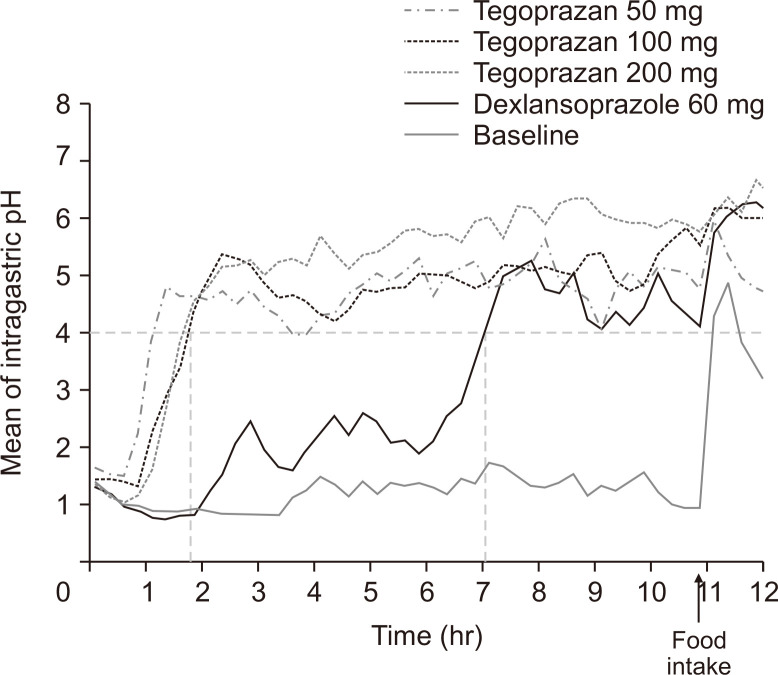
The tegoprazan groups reached mean pH ≥4 within 2 hours for all doses, whereas the dexlansoprazole group required about 7 hours to reach pH ≥4 after drug administration (Fig. 2). The dose-dependent pattern of mean time pH >4 (%) and pH >6 (%) was observed in the tegoprazan groups with values of 58.55%, 70.07%, and 81.73% for time pH >4, and 25.97%, 31.91%, and 48.44% for time pH >6 in the 50, 100, and 200 mg groups, respectively (Table 1). Holding times above pH >4 (60.55%) and pH >6 (25.85%) for the dexlansoprazole group were comparable to those of the 50 mg tegoprazan group.
Fig. 2.
Mean intragastric pH–time values over 12 hours after a single, evening oral dose of 50, 100, or 200 mg of tegoprazan or 60 mg of dexlansoprazole compared to the baseline. A breakfast meal was provided at the indicated time point (black arrow). A horizontal dashed line: pH=4, vertical dashed lines: time to reach pH 4 for tegoprazan group (left) and the dexlansoprazole group (right).
Table 1.
Pharmacodynamic Parameters Following a Single Oral Dose of the Study Drugs
| Parameter | Tegoprazan | Dexlansoprazole 60 mg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg | 100 mg | 200 mg | |||||||||
| Baseline (n=12) |
Post-dose (n=12) |
Baseline (n=12) |
Post-dose (n=12) |
Baseline (n=12) |
Post-dose (n=12) |
Baseline (n=12) |
Post-dose (n=12) |
||||
| 0–12 hr (night-time) | |||||||||||
| Time pH >4, % | 8.48±5.38 | 65.11±21.13 | 9.76±10.21 | 65.61±20.83 | 8.54±4.53 | 78.15±9.21 | 9.79±9.89 | 37.78 ±1.08 | |||
| Time pH >6, % | 2.16±1.88 | 30.41±24.00 | 4.44±5.99 | 33.85±19.04 | 3.39±2.74 | 53.63±18.78 | 4.32±6.06 | 17.76±9.11 | |||
| IA, mmol∙hr/L | 1,415±617 | 171±143 | 1,523±592 | 162±138 | 1,390±616 | 116±66 | 1,399±562 | 697±257 | |||
| % inhibition of IA | - | 83.98±17.58 | - | 88.68±9.14 | - | 91.17±4.44 | - | 44.06±25.77 | |||
| % inhibition of time pH ≤4 | - | 61.41±24.16 | - | 62.53±20.93 | - | 76.00±10.41 | - | 29.98±15.62 | |||
| Median pH | 1.00±0.24 | 4.98±1.23 | 0.99±0.29 | 5.01±1.33 | 0.98±0.18 | 6.00±0.44 | 1.02±0.31 | 2.55±1.16 | |||
| 0–24 hr | |||||||||||
| Time pH >4, % | 13.33±9.80 | 58.55±19.41 | 13.12±9.92 | 70.07±18.31 | 14.09±8.15 | 81.73±8.29 | 13.36±8.75 | 60.55±5.86 | |||
| Time pH >6, % | 1.84±1.79 | 25.97±19.69 | 3.49±3.52 | 31.91±16.37 | 3.72±3.55 | 48.44±15.39 | 3.56±3.99 | 25.85±14.82 | |||
| IA, mmol∙hr/L | 2,132±832 | 355±290 | 2,181±737 | 230±189 | 2,191±989 | 142±88 | 2,268±956 | 720±243 | |||
| % inhibition of IA | - | 78.18±26.97 | - | 88.78±9.80 | - | 93.46±3.47 | - | 64.66±15.92 | |||
| % inhibition of time pH ≤4 | - | 51.35±24.95 | - | 66.22±18.04 | - | 78.87±8.73 | - | 53.96±8.57 | |||
| Median pH | 1.34±0.59 | 4.57±1.25 | 1.29±0.58 | 5.10±1.07 | 1.13±0.23 | 5.90±0.49 | 1.13±0.27 | 4.98±0.60 | |||
Data are presented as mean±SD.
Time pH >4 (%), percentage of values greater than pH 4 during the course of gastric pH monitoring; Time pH >6 (%), percentage of values greater than pH 6 during the course of gastric pH monitoring; IA, integrated acidity.
Among the tegoprazan groups, the 50 mg group required the shortest time to reach mean pH ≥4 but did not reach pH ≥6 within 12 hours after administration. In contrast, the mean pH for the 100 mg and 200 mg groups increased above pH >6 at some point before food intake. Moreover, the rate of pH decreases after food intake was more gradual in the 100 and 200 mg groups than in the 50 mg group, resulting in a longer time phase for mean pH ≥6. The pattern of pH change after food intake in the dexlansoprazole group was similar to that in the 100 and 200 mg tegoprazan groups (Fig. 2).
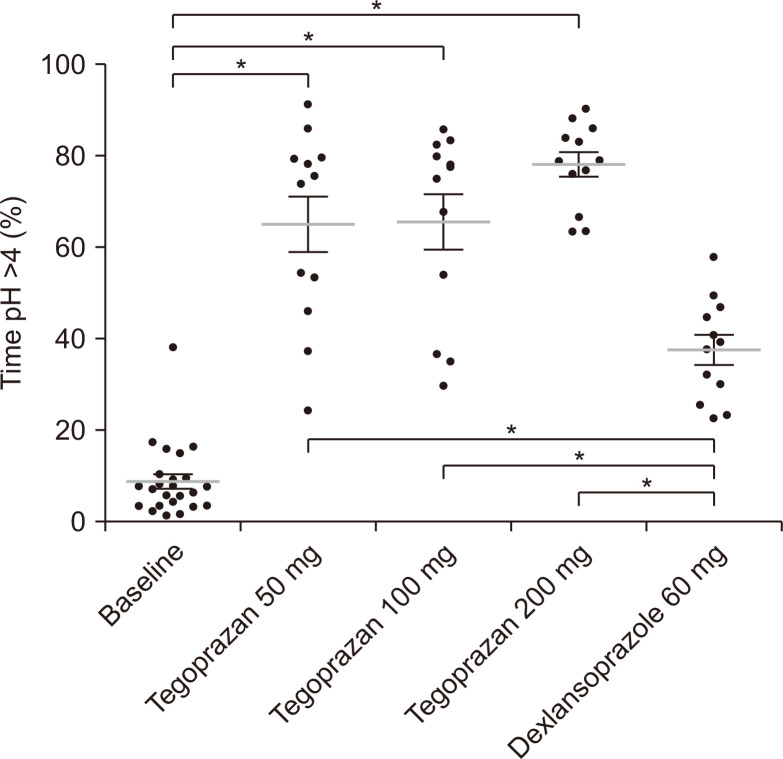
The PD parameters up to 12 and 24 hours after evening dosing were also assessed (Table 1). The mean time with gastric pH >4 (%) (65.11% and 65.61% for 50 and 100 mg, respectively) and IA values (171 and 162 mmol∙hr/L for 50 and 100 mg, respectively) were similar between the 50 mg and 100 mg tegoprazan groups. The acid-suppressive effect was the most apparent in the 200 mg group as the mean time with gastric pH >4 (%) was 78.15% and IA value was 116 mmol∙hr/L, whereas the dexlansoprazole group had time pH >4 (%) of 37.78% and IA value of 697 mmol∙hr/L (Fig. 3).
Fig. 3.
Time (% of the total time, 12 hours) that gastric pH was >4, measured at baseline and subsequent to completion of the four treatment regimens in healthy volunteers. *p<0.001 of Tukey post hoc test, one-way analysis of variance, gray lines represent means, and error bars represent SDs.
The PD parameters from 12 to 24 hours post-dose also showed a dose-dependent pattern in the tegoprazan groups, as the mean time with gastric pH >4 (%) of the 50, 100, and 200 mg groups were 52.06%, 74.57%, and 85.34%, and IA values were 184.29, 67.72, and 25.51 mmol∙hr/L, respectively.
The IA value of the dexlansoprazole group was the highest among all groups (Table 1). Especially, the values of holding times above pH >4 (37.78%) and pH >6 (17.76%) up to 12 hours post-dose were the lowest among all the groups. The dexlansoprazole group demonstrated the weakest acid-suppressive effect up to 12 hours after evening dosing, and the overall average values were comparable to those of the 50 mg tegoprazan group.
3. Pharmacokinetics
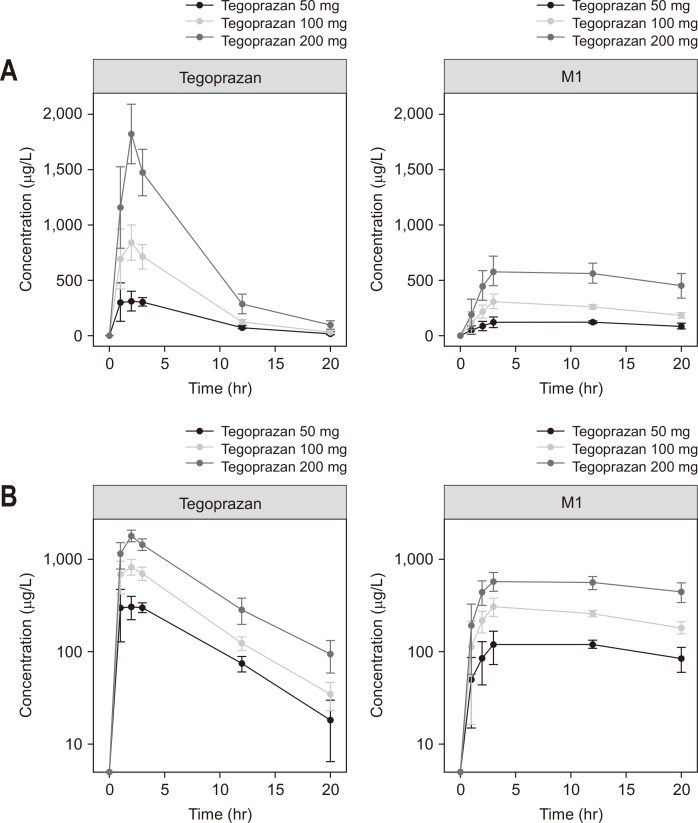
A dose-dependent increase was observed in the plasma drug concentrations (observed as average Cmax: 383, 970, and 1,859 ng/mL), whereas the AUClast was 2,469, 5,385, and 11,512 ng∙hr/mL for the 50, 100, and 200 mg tegoprazan groups, respectively (Fig. 4). The time to reach the maximum plasma concentration was between 1.42 and 1.84 hours for tegoprazan (range of dose group medians). A similar pattern of dose-dependency was also observed in M1 (Fig. 4). The results of the PK parameters are summarized in Table 2.
Fig. 4.
Mean±standard deviation plasma concentrations of tegoprazan and M1 versus time following a single oral dose of 50, 100, or 200 mg tegoprazan in (A) linear and (B) semilogarithmic scales.
Table 2.
Pharmacokinetic Parameters of Tegoprazan and M1 Following a Single Oral Dose of 50, 100, or 200 mg of Tegoprazan
| Parameter | Tegoprazan | M1 | |||||
|---|---|---|---|---|---|---|---|
| 50 mg (n=12) | 100 mg (n=12) | 200 mg (n=12) | 50 mg (n=12) | 100 mg (n=12) | 200 mg (n=12) | ||
| Cmax, ng/mL | 383±112 | 970±185 | 1,859±461 | 141±47 | 319±99 | 660±222 | |
| AUClast, ng∙hr/mL | 2,469±616 | 5,385±1,190 | 11,512±2,647 | 2,104±704 | 4,790±963 | 10,113±2,787 | |
| tmax, hr | 1.84 (0.83–3.00) | 1.42 (0.83–3.00) | 1.83 (0.87–2.00) | 11.90 (1.83–12.02) | 2.85 (2.83–12.00) | 7.42 (2.83–12.00) | |
Data are presented as mean±SD or median (range).
Cmax, maximum concentration; AUClast, area under the plasma concentration versus time curve from time 0 to the last measurable concentration; tmax, time of the maximum measured plasma concentration.
4. Safety and tolerability
Clinically significant results were not observed in the physical examinations, vital signs, electrocardiogram, or laboratory tests. Five instances of AEs were reported, but all were mild in severity and transient. This included three cases (headache, neutrophil count decreased, skin abrasion) in the tegoprazan 50 mg group, one case (epigastric discomfort) in the tegoprazan 200 mg group, and one case (blood creatine phosphokinase increased) in the dexlansoprazole group. Among these events, four cases were unlikely related or unrelated AEs, and there was one AE (headache) that had a possible causality with tegoprazan 50 mg; however, this AE was resolved after 1 day without any specific treatment.
DISCUSSION
Although there is a contentious debate whether esophageal acid exposure is increased during NAB episodes,18 NAB may increase a risk of complications with GERD4,5 and cause night-time heartburn and/or wakening by cough or stuffiness, ultimately affecting sleep quality and daytime functioning.19,20 Therefore, it is important to pharmacologically control the night-time gastric acid secretion in patients with GERD and this study was designed to compare and evaluate PD characteristics following evening dosing of tegoprazan 50, 100, or 200 mg or dexlansoprazole 60 mg in healthy male volunteers.
Evening dosing of 50, 100, and 200 mg of tegoprazan showed a dose-dependent pattern in the PD parameters as the mean of time pH >4 (%, of 24 hours) was 58.55 (50 mg), 70.07 (100 mg), and 81.73 (200 mg), respectively (Table 1), which were similar to the results from a previous phase I study.10 The mean of time pH >4 (%, of 24 hours) was much smaller in the dexlansoprazole 60 mg group (60.55%, in the present study) and esomeprazole 40 mg group (54.3%, in a previous study10), suggesting higher efficacy of tegoprazan in gastric acid suppression over dexlansoprazole or esomeprazole. All tegoprazan dose groups reached mean pH ≥4 within 2 hours, whereas dexlansoprazole 60 mg group required 7 hours to reach pH ≥4 after drug administration, indicating a faster onset of pH increase of tegoprazan than dexlansoprazole, which is selected as one of the most effective PPIs controlling NAB. This property also supports the differentiated therapeutic potential of tegoprazan for on-demand symptom relief.10
Dexlansoprazole, a delayed-release formulation of the R-enantiomer of lansoprazole, was selected as the reference drug because this second-generation PPI is known to be the most effective in controlling NAB among currently available PPIs.16,17 A previous study reported that dexlansoprazole is more effective than esomeprazole because time pH >4 (%) was 58% and 48% after a single dose of dexlansoprazole 60 mg and esomeprazole 40 mg, respectively.17 Patients are advised to take dexlansoprazole, which has a delayed-release formulation, with the final meal of the day, and it effectively suppresses acid secretion during the night. Howden et al.21 confirmed that dexlansoprazole 60 mg and 90 mg were superior to placebo for nocturnal heartburn (median days without heartburn during treatment: 98.3% and 97.1%, respectively, vs 50.0%). The nocturnal pattern of mean pH change and PD parameter (% of time pH >4) of dexlansoprazole 60 mg in the present study was highly comparable with the two study results reported by Kukulka et al.17,22 PD effect on NAB was significantly lower in before-snack regimen than before-breakfast regimen on repeated dosing of Day 5.23
Overall, the 24-hour PD parameters showed similar patterns between the 50 mg tegoprazan and 60 mg dexlansoprazole group (Table 1). The dexlansoprazole group demonstrated the weakest acid-suppressive effect up to 12 hours after evening dosing compared to all dose groups of tegoprazan, but the pattern became similar to the 200 mg tegoprazan group after 12 hours. We strongly believe that this observation results from the differences in pharmacological properties between PPIs and tegoprazan. Whereas PPIs, which are pro-drugs, require activation in acidic condition, and it takes several days to fully reach anti-secretory effects, tegoprazan immediately inhibits both activated and inactivated proton pumps and shows the full effect from the first dose. This is evident that the recent study also showed the rapid and well-sustained night-time gastric acid suppression by the single oral administration of tegoprazan compared to that by vonoprazan or esomeprazole.24
The evening dose of tegoprazan 50, 100, and 200 mg shows comparable PK profiles with the previous first-in-human phase 1 study results10 with a morning dose of tegoprazan 50, 100, 200, and 400 mg, which also showed similar dose-dependent PKs. The current formulation of tegoprazan is slightly different from the formulation of the previous study; however, the two formulations of tegoprazan showed similar PK profiles, fulfilling the regulatory criteria for bioequivalence in the independent bioequivalence study.25
Single oral doses of tegoprazan were considered well tolerated by healthy male subjects when administered at doses of up to 200 mg, and the safety profile of tegoprazan was comparable with that of dexlansoprazole 60 mg. One AE (headache) has possible causality related to tegoprazan, but it was resolved after 1 day without any specific treatment. Tegoprazan was approved in the Republic of Korea in 2018, and the drug label of tegoprazan reports headache as a known AE.
This study has several limitations. First, the saturated or full potential of gastric acid inhibition by repeated dosing was not evaluated. because the on-demand inhibitory potential for NAB, is more related to the single evening dosing regimen. Second, nocturnal PD effects may be different in other dosing timing relative to a meal (i.e., before-breakfast, before-lunch, or before-dinner dosing).
In conclusion, all examined doses of tegoprazan demonstrated a significantly faster onset of gastric pH increase and longer holding times above pH >4 and pH >6 up to 12 hours after evening dosing compared to dexlansoprazole.
ACKNOWLEDGEMENTS
This study was funded in full by HK inno.N Corp, Seoul, Korea.
Footnotes
CONFLICTS OF INTEREST
S.K., J.Y.N., B.K., and G.S.S. are employees of HK inno.N Corp, Seoul, Korea. None of the other authors have any intellectual property rights or significant financial interest in tegoprazan, the test product.
AUTHOR CONTRIBUTIONS
Study concept and design: H.Y.C., Y.H.K, H.S.L., K.S.B. Data acquisition: H.Y.C., Y.H.K., H.S.L., K.S.B. Data analysis and interpretation: S.H., H.Y.C., Y.H.K., K.S.B., G.S.S. Drafting of the manuscript: S.H. Critical revision of the manuscript for important intellectual content: S.C.C., S.K., J.Y.N., B.K. Statistical analysis: H.Y.C., Y.H.K., S.H. Obtained funding: K.S.B. Administrative, technical, or material support; study supervision: K.S.B. Approval of final manuscript: all authors.
REFERENCES
- 1.Gold BD, Pilmer B, Kierkuś J, Hunt B, Perez MC, Gremse D. Dexlansoprazole for heartburn relief in adolescents with symptomatic, nonerosive gastro-esophageal reflux disease. Dig Dis Sci. 2017;62:3059–3068. doi: 10.1007/s10620-017-4743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilotto A, Franceschi M, Paris F. Recent advances in the treatment of GERD in the elderly: focus on proton pump inhibitors. Int J Clin Pract. 2005;59:1204–1209. doi: 10.1111/j.1368-5031.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 3.Tytgat GN. Are there unmet needs in acid suppression? Best Pract Res Clin Gastroenterol. 2004;18 Suppl:67–72. doi: 10.1016/j.bpg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Peghini PL, Katz PO, Bracy NA, Castell DO. Nocturnal recovery of gastric acid secretion with twice-daily dosing of proton pump inhibitors. Am J Gastroenterol. 1998;93:763–767. doi: 10.1111/j.1572-0241.1998.221_a.x. [DOI] [PubMed] [Google Scholar]
- 5.Pehlivanov ND, Olyaee M, Sarosiek I, McCallum RW. Comparison of morning and evening administration of rabeprazole for gastro-oesophageal reflux and nocturnal gastric acid breakthrough in patients with reflux disease: a double-blind, cross-over study. Aliment Pharmacol Ther. 2003;18:883–890. doi: 10.1046/j.1365-2036.2003.01776.x. [DOI] [PubMed] [Google Scholar]
- 6.Xue S, Katz PO, Banerjee P, Tutuian R, Castell DO. Bedtime H2 blockers improve nocturnal gastric acid control in GERD patients on proton pump inhibitors. Aliment Pharmacol Ther. 2001;15:1351–1356. doi: 10.1046/j.1365-2036.2001.01050.x. [DOI] [PubMed] [Google Scholar]
- 7.Fackler WK, Ours TM, Vaezi MF, Richter JE. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology. 2002;122:625–632. doi: 10.1053/gast.2002.31876. [DOI] [PubMed] [Google Scholar]
- 8.Oshima T, Miwa H. Potent potassium-competitive acid blockers: a new era for the treatment of acid-related diseases. J Neurogastroenterol Motil. 2018;24:334–344. doi: 10.5056/jnm18029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi N, Take Y. Tegoprazan, a novel potassium-competitive acid blocker to control gastric acid secretion and motility. J Pharmacol Exp Ther. 2018;364:275–286. doi: 10.1124/jpet.117.244202. [DOI] [PubMed] [Google Scholar]
- 10.Han S, Choi HY, Kim YH, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ-12420), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2019;50:751–759. doi: 10.1111/apt.15438. [DOI] [PubMed] [Google Scholar]
- 11.Lee KJ, Son BK, Kim GH, et al. Randomised phase 3 trial: tegoprazan, a novel potassium-competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment Pharmacol Ther. 2019;49:864–872. doi: 10.1111/apt.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarpignato C, Hunt RH. Editorial: towards extended acid suppression: the search continues. Aliment Pharmacol Ther. 2015;42:1027–1029. doi: 10.1111/apt.13384. [DOI] [PubMed] [Google Scholar]
- 13.Hunt R. Acid suppression for reflux disease: "off-the-peg" or a tailored approach? Clin Gastroenterol Hepatol. 2012;10:210–213. doi: 10.1016/j.cgh.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Skrzydło-Radomańska B, Radwan P. Dexlansoprazole: a new-generation proton pump inhibitor. Prz Gastroenterol. 2015;10:191–196. doi: 10.5114/pg.2015.56109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han S, Choi HY, Kim YH, et al. Pharmacokinetics, pharmacodynamics and food-effect of single oral dose of tegoprazan (CJ-12420), a novel potassium-competitive acid blocker (P-CAB) in healthy male subjects. Paper presented at: 2017 Korea Digestive Disease Week; 2017 Nov 23-25; Seoul, Korea. [DOI] [Google Scholar]
- 16.Wallace JL, Ferraz JG. New pharmacologic therapies in gastrointestinal disease. Gastroenterol Clin North Am. 2010;39:709–720. doi: 10.1016/j.gtc.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Kukulka M, Eisenberg C, Nudurupati S. Comparator pH study to evaluate the single-dose pharmacodynamics of dual delayed-release dexlansoprazole 60 mg and delayed-release esomeprazole 40 mg. Clin Exp Gastroenterol. 2011;4:213–220. doi: 10.2147/CEG.S24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weigt J, Kandulski A, Büsch F, Malfertheiner P. Nocturnal gastric acid breakthrough is not associated with night-time gastroesophageal reflux in GERD patients. Dig Dis. 2009;27:68–73. doi: 10.1159/000210107. [DOI] [PubMed] [Google Scholar]
- 19.Farup C, Kleinman L, Sloan S, et al. The impact of nocturnal symptoms associated with gastroesophageal reflux disease on health-related quality of life. Arch Intern Med. 2001;161:45–52. doi: 10.1001/archinte.161.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Shaker R, Castell DO, Schoenfeld PS, Spechler SJ. Nighttime heartburn is an under-appreciated clinical problem that impacts sleep and daytime function: the results of a Gallup survey conducted on behalf of the American Gastroenterological Association. Am J Gastroenterol. 2003;98:1487–1493. doi: 10.1111/j.1572-0241.2003.07531.x. [DOI] [PubMed] [Google Scholar]
- 21.Howden CW, Larsen LM, Perez MC, Palmer R, Atkinson SN. Clinical trial: efficacy and safety of dexlansoprazole MR 60 and 90 mg in healed erosive oesophagitis: maintenance of healing and symptom relief. Aliment Pharmacol Ther. 2009;30:895–907. doi: 10.1111/j.1365-2036.2009.04119.x. [DOI] [PubMed] [Google Scholar]
- 22.Kukulka M, Nudurupati S, Perez MC. Pharmacokinetics and pharmacodynamics of an orally disintegrating tablet formulation of dexlansoprazole. Therap Adv Gastroenterol. 2016;9:759–769. doi: 10.1177/1756283X16670073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee RD, Mulford D, Wu J, Atkinson SN. The effect of time-of-day dosing on the pharmacokinetics and pharmacodynamics of dexlansoprazole MR: evidence for dosing flexibility with a Dual Delayed Release proton pump inhibitor. Aliment Pharmacol Ther. 2010;31:1001–1011. doi: 10.1111/j.1365-2036.2010.04272.x. [DOI] [PubMed] [Google Scholar]
- 24.Yang E, Kim S, Kim B, et al. Night-time gastric acid suppression by tegoprazan compared to vonoprazan or esomeprazole. Br J Clin Pharmacol. 2022;88:3288–3296. doi: 10.1111/bcp.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang JG, Yoo H, Lee JW, Song GS, Lee S, Kim MG. Comparison of pharmacokinetic characteristics of two Tegoprazan (CJ-12420) formulations in healthy male subjects. Transl Clin Pharmacol. 2019;27:80–85. doi: 10.12793/tcp.2019.27.2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]