Abstract
Background/Aims
There are no data regarding the association between sarcopenic obesity status and nonalcoholic fatty liver disease (NAFLD) and NAFLD-associated liver fibrosis. Therefore, we aimed to investigate the relationship between sarcopenic obesity status (sarcopenia only, obesity only, and sarcopenic obesity) and NAFLD and liver fibrosis in Korean adults.
Methods
In total, 2,191 subjects completed a health checkup program, including abdominal ultrasonography and FibroScan. Subjects were classified into the following four categories optimal body composition (nonobese and nonsarcopenic), sarcopenia only (nonobese), obesity only (nonsarcopenic), and sarcopenic obesity. Sarcopenic obesity was stratified by the skeletal muscle mass index and body fat using bioelectrical impedance analysis. NAFLD was diagnosed by ultrasonography, and liver fibrosis was assessed using transient elastography in subjects with NAFLD.
Results
The prevalence of NAFLD and liver fibrosis significantly increased according to the sarcopenic obesity status. In the logistic regression analysis, after adjusting for multiple risk factors, the odds ratio (OR) for the risk of NAFLD was largest in the sarcopenic obesity group (OR, 3.68; 95% confidence interval [CI], 2.94 to 4.60), followed by the obesity only (OR, 2.25; 95% CI, 1.67 to 3.03) and sarcopenia only (OR, 1.92; 95% CI, 1.30 to 2.84) groups, when compared with the optimal group. Additionally, liver fibrosis was independently associated with sarcopenic obesity status (OR 4.69, 95% CI 1.95 to 11.29; OR 4.17, 95% CI 1.56 to 11.17; OR 3.80, 95% CI 0.86 to 16.75, respectively).
Conclusions
These results demonstrated that sarcopenic obesity was independently associated with NAFLD and liver fibrosis and increased the risk of NAFLD and liver fibrosis more than obesity or sarcopenia alone.
Keywords: Obesity, Non-alcoholic fatty liver disease, Liver fibrosis
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the leading cause of liver disease worldwide. The worldwide prevalence of NAFLD has been steadily increasing and is relatively higher, particularly in Asians.1 NAFLD involves histological changes similar to those of alcoholic hepatitis without a history of alcohol abuse. This includes a wide range of diseases such as hepatic steatosis, nonalcoholic steatohepatitis, liver fibrosis, and cirrhosis.2 NAFLD refers to the accumulation of fat in hepatocytes, mainly due to insulin resistance; therefore, it often occurs because of changes in metabolic processes such as obesity.3-5
Sarcopenia refers to a decrease in the number of muscle fibers that form a muscle, resulting in decreased muscle strength and function.6 It can often be accompanied by an increase in visceral abdominal fat, which contributes to numerous metabolic diseases such as type 2 diabetes, dyslipidemia, and cardiovascular disease.7
Recently, several studies have shown an association between sarcopenia and NAFLD and fibrosis.8,9 However, to date, only one study in the United States has investigated the relationship among sarcopenic obesity, NAFLD, and NAFLD-associated liver fibrosis.9
Therefore, in this study, we investigated the association among sarcopenic obesity status (optimal, sarcopenia only, obesity only, and sarcopenic obesity), NAFLD, and liver fibrosis in Korean adults.
MATERIALS AND METHODS
1. Study population
We reviewed subjects who underwent abdominal ultrasonography and transient elastography, as part of a self-examination program, at the Hospital Health Promotion Center at Gangnam Severance in Seoul, Korea, from February 2007 to December 2018. A total of 2,399 subjects were enrolled. Patients with a history of liver disease was excluded based on the results of the questionnaire and laboratory tests. Cases that they had a history of liver disease (hepatitis, cirrhosis, or hepatoma) were basically excluded, and cases that were positive for hepatitis B surface antigen and anti-hepatitis C virus ins serology tests were further excluded. Alcohol consumption history was investigated on the questionnaire. Patients with excessive alcohol consumption (>30 g/day for males and >20 g/day for females), and patients with missing data were excluded. Finally, 2,191 subjects were enrolled in the study. This study was approved by the Institutional Review Board of the Yonsei University College of Medicine (IRB number: 3-2020-0239). The requirement for informed consent from the patient was waived.
2. Clinical measurement and laboratory assessment
The body mass index (BMI, kg/m2) was calculated using the height and weight of each subject. Using an automatic sphygmomanometer (HEM-7080IC; Omron Healthcare, Lake Forest, IL, USA), an experienced technician placed the subject’s arm flush with the heart and measured the systolic blood pressure (SBP) and diastolic blood pressure (DBP) after 5 minutes of rest.
Blood samples were collected from all subjects after 8 hours of fasting. Fasting plasma glucose (FPG), total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (γ-GT), and insulin levels were assessed according to enzymatic procedures, with an automated chemistry analyzer (Hitachi 7600-120, Tokyo, Japan). Levels of anti-hepatitis C virus antibody and hepatitis B surface antigen were measured using a Roche E-170 device (Roche Diagnostics, Mannheim, Germany).
Each subject’s social and medical history was obtained through a self-questionnaire that included questions about alcohol status, smoking, medications, and other medical history. Diabetes was diagnosed on the basis of a history of diabetes or the diagnostic criteria of the American Diabetes Association. Subjects who were currently on antihypertensive medication or had SBP and/or DBP ≥140/90 mm Hg were defined as having hypertension. Subjects who had smoked regularly in the past 6 months were considered current smokers. Regular exercise was defined as moderate-intensity exercise for at least 30 minutes at least twice a week.
3. Measurement of skeletal muscle mass index
Bioelectrical impedance analysis (BIA) was performed according to the manufacturer’s instructions (InBody 720; Biospace Inc., Seoul, Korea) to determine the appendicular skeletal muscle mass for each limb (kg) using a multifrequency BIA device. To increase the accuracy of the results, BIA was conducted after an overnight fast with water for at least 8 hours.
Skeletal muscle mass index was calculated by dividing the sum of the appendicular skeletal muscle mass values of both upper and lower extremities by the body weight (kg) according to the transformation formula presented in a previous study10,11 and expressed as a percentage (=total appendicular skeletal muscle mass/body weight×100%).
4. Definition of sarcopenia and obesity
According to previous studies on the Korean population, sarcopenia was defined as less than one standard deviation below the mean of the sex-specific skeletal muscle mass index value for a young reference group (18 to 40 years old).12,13 The threshold of sarcopenia was 30.0% for males and 26.8% for females.14 Obesity was defined as a significant body fat mass using BIA (≥25% for males and ≥35% for females).15,16
Subjects were divided into four categories according to body composition (sarcopenic obese status): (1) optimal body composition (i.e., nonsarcopenic and nonobese), (2) sarcopenia (i.e., nonobese), (3) obesity (i.e., nonsarcopenic), and (4) sarcopenic obesity.
5. Assessment of liver fibrosis and steatosis
Fatty liver disease was diagnosed on the basis of the results of an abdominal ultrasonography scan conducted using a 3.5-MHz transducer (HDI 5000; Philips, Bothell, WA, USA). One of the three experienced radiologists performed abdominal ultrasonography without knowing the subject’s clinical information. Any degree of fat accumulation in the liver was considered NAFLD.
Transient elastography was performed using FibroScan (Echosens, Paris, France), with a standard probe. Only liver stiffness (LS) values with at least 10 valid measurements, a success rate of at least 60%, and an interquartile range-to-median ratio of <30% were considered reliable, as suggested in previous studies.17-20 Quality controlprocedures ensure the collection and documentation ofaccurate and reliable controlled attenuation parameter (CAP) and LS measurement data.We definedNAFLD (≥S2) as a CAP score of ≥260 dB/m.21,22 Among subjects with NAFLD, an LS of ≥7.5 kPa (≥F2) was used todefine liver fibrosis.23,24
6. Statistical analysis
Continuous variables are expressed as mean±standard deviation. Between-group comparisons were conducted using the Student t-test or one-way analysis of variance. Categorical variables, expressed as percentages, were compared using the chi-square test. The association between NAFLD and liver fibrosis and sarcopenic obesity status was assessed using logistic regression. After adjusting for confounding variables, multivariate logistic regression was used to estimate the odds ratio (OR) and associated 95% confidence intervals for NAFLD and liver fibrosis based on the sarcopenic obesity status. Differences were considered statistically significant at p-values of less than 0.05. SPSS for Windows 25.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses in this study.
RESULTS
1. Baseline characteristics of study subjects according to sarcopenic obesity status and NAFLD and liver fibrosis
The subjects were divided into four categories stratified by sarcopenic obesity status (stratified by skeletal muscle mass index and fat mass). Table 1 shows the biochemical and clinical characteristics for each category. Patients with sarcopenic obesity had the highest levels of AST, ALT, and γ-GT and CAP score. In addition, BMI; SBP; DBP; FPG, low-density lipoprotein cholesterol, and insulin levels; and the prevalence of diabetes were higher than in the other groups.
Table 1.
Baseline Characteristics of the Study Subjects According to Their Sarcopenic Obesity Status
| Characteristics | Optimal (n=910) | Sarcopenia (n=158) | Obesity (n=278) | Sarcopenic obesity (n=845) | p-value |
|---|---|---|---|---|---|
| Age, yr | 50.72±10.60 | 55.73±10.14 | 52.61±10.35 | 54.68±10.75 | <0.001 |
| Sex, male/female | 578/332 | 21/137 | 256/22 | 513/332 | |
| Skeletal muscle mass index, % | 32.00±2.91 | 26.20±1.42 | 31.21±1.93 | 26.38±2.75 | <0.001 |
| Body fat mass, % | 22.80±5.33 | 31.43±3.92 | 27.98±3.43 | 34.80±5.95 | <0.001 |
| Body mass index, kg/m2 | 23.00±2.66 | 23.01±2.11 | 26.72±2.43 | 27.87±3.71 | <0.001 |
| Systolic blood pressure, mm Hg | 120.35±12.30 | 121.09±11.24 | 122.47±11.44 | 123.97±11.69 | <0.001 |
| Diastolic blood pressure, mm Hg | 71.39±8.65 | 70.92±7.79 | 72.20±9.27 | 72.8±8.31 | 0.002 |
| Fasting plasma glucose, mg/dL | 100.2±20.63 | 99.57±19.53 | 107.16±22.18 | 108.3±26.45 | <0.001 |
| Total cholesterol, mg/dL | 202.01±41.39 | 208.64±45.44 | 201.73±40.00 | 208.23±42.96 | 0.006 |
| Triglyceride, mg/dL | 124.2±101.81 | 125.09±70.00 | 163.16±81.13 | 161.69±106.17 | <0.001 |
| HDL-C, mg/dL | 57.56±14.29 | 60.52±14.52 | 50.38±10.60 | 52.36±11.95 | <0.001 |
| LDL-C, mg/dL | 125.56±33.50 | 128.39±33.89 | 131.58±32.35 | 131.87±33.75 | <0.001 |
| Aspartate aminotransferase, IU/L | 29.77±16.92 | 30.52±24.00 | 33.28±15.97 | 34.36±22.44 | 0.001 |
| Alanine aminotransferase, IU/L | 26.99±18.43 | 27.47±15.02 | 36.30±21.80 | 37.52±28.62 | <0.001 |
| γ-Glutamyl transferase, IU/L | 31.37±40.76 | 35.00±138.01 | 42.96±28.90 | 45.20±49.86 | <0.001 |
| Insulin, μIU/mL | 6.15±3.60 | 6.37±2.65 | 9.33±4.75 | 10.45±6.02 | <0.001 |
| Diabetes, % | 122 (13.4) | 28 (17.7) | 46 (16.5) | 168 (19.9) | <0.001 |
| Smoking, % | 182 (20.7) | 6 (4.2) | 75 (27.3) | 163 (20.4) | <0.001 |
| Exercise, % | 680 (76.2) | 111 (71.6) | 214 (78.1) | 519 (64.9) | <0.001 |
| CAP, dB/m | 235.15±49.64 | 236.66±53.17 | 267.87±47.43 | 278.71±54.35 | <0.001 |
| Liver stiffness, kPa | 4.08±3.12 | 3.83±1.88 | 4.39±1.46 | 4.69±1.93 | <0.001 |
Data are presented as mean±SD or number (%).
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CAP, controlled attenuation parameter.
Subjects with NAFLD were older, predominantly male, and had higher BMI; SBP; DBP; FPG, triglyceride, low-density lipoprotein cholesterol, AST, ALT, γ-GT, and insulin levels; prevalence of diabetes; and CAP score. However, they had a lower high-density lipoprotein cholesterol level and regular exercise ratio than subjects without NAFLD (Table 2). The baseline characteristics of subjects with NAFLD diagnosed by the CAP score were also analyzed (Supplementary Table 1).
Table 2.
Comparison of Baseline Characteristics According to the Presence of NAFLD Defined by Ultrasonography
| Characteristics | All (n=2,191) | No NAFLD (n=1,011) | NAFLD (n=1,180) | p-value |
|---|---|---|---|---|
| Age, yr | 52.85±10.76 | 52.36±11.23 | 53.27±10.33 | 0.049 |
| Sex, male/female | 1,368/823 | 524/487 | 844/336 | |
| Skeletal muscle mass index, % | 29.31±3.81 | 29.82±3.84 | 28.89±3.73 | <0.001 |
| Body fat mass, % | 28.71±7.58 | 27.10±7.45 | 30.08±7.41 | <0.001 |
| Body mass index, kg/m2 | 25.35±3.83 | 23.77±3.40 | 26.71±3.66 | <0.001 |
| Systolic blood pressure, mm Hg | 122.07±11.99 | 120.52±12.39 | 123.39±11.48 | <0.001 |
| Diastolic blood pressure, mm Hg | 72.00±8.57 | 71.31±8.43 | 72.59±8.65 | <0.001 |
| Fasting plasma glucose, mg/dL | 104.16±23.49 | 98.47±17.34 | 109.02±26.74 | <0.001 |
| Total cholesterol, mg/dL | 204.85±42.22 | 203.47±40.42 | 206.02±43.69 | 0.157 |
| Triglyceride, mg/dL | 143.66±100.89 | 110.41±55.94 | 172.05±120.22 | <0.001 |
| HDL-C, mg/dL | 54.86±13.40 | 58.85±14.20 | 51.45±11.64 | <0.001 |
| LDL-C, mg/dL | 128.96±33.59 | 125.87±32.18 | 131.60±34.54 | <0.001 |
| Aspartate aminotransferase, IU/L | 32.04±19.77 | 30.10±18.84 | 33.69±20.40 | <0.001 |
| Alanine aminotransferase, IU/L | 32.26±23.63 | 26.09±17.71 | 37.53±26.61 | <0.001 |
| γ-Glutamyl transferase, IU/L | 38.44±56.24 | 31.17±68.23 | 44.64±42.52 | <0.001 |
| Insulin, μIU/mL | 8.38±5.26 | 6.52±3.78 | 10.14±5.83 | <0.001 |
| Diabetes, % | 364 (16.7) | 120 (11.9) | 244 (20.7) | <0.001 |
| Smoking, % | 426 (20.7) | 159 (16.5) | 267 (23.6) | <0.001 |
| Exercise, % | 1,524 (69.6) | 748 (75.7) | 776 (68.5) | <0.001 |
| CAP, dB/m | 256.21±55.43 | 230.76±49.34 | 278.01±50.90 | <0.001 |
| Liver stiffness, kPa | 4.33±2.47 | 4.06±3.04 | 4.57±1.81 | <0.001 |
Data are presented as mean±SD or number (%).
NAFLD, nonalcoholic fatty liver disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CAP, controlled attenuation parameter.
As expected, among subjects with NAFLD (diagnosed by ultrasound), subjects with liver fibrosis were more likely to have low high-density lipoprotein cholesterol levels and higher BMI; FPG, triglyceride, AST, ALT, γ-GT, and insulin levels; prevalence of diabetes; current smoking; and CAP score than those without (Table 3).
Table 3.
Comparison of Baseline Characteristics According to the Presence of Liver Fibrosis
| Characteristics | All (n=1,180) | No fibrosis (n=1,105) | Fibrosis (n=75) | p-value |
|---|---|---|---|---|
| Age, yr | 53.27±10.33 | 53.19±10.26 | 54.45±11.26 | 0.347 |
| Sex, male/female | 844/336 | 783/322 | 61/14 | |
| Skeletal muscle mass index, % | 28.88±3.73 | 28.97±3.71 | 27.60±3.91 | 0.002 |
| Body fat mass, % | 30.08±7.41 | 29.85±7.29 | 33.52±8.40 | <0.001 |
| Body mass index, kg/m2 | 26.71±3.66 | 26.44±3.35 | 30.56±5.48 | <0.001 |
| Systolic blood pressure, mm Hg | 123.39±11.48 | 123.35±11.50 | 123.99±11.26 | 0.637 |
| Diastolic blood pressure, mm Hg | 72.59±8.65 | 72.60±8.69 | 72.49±8.06 | 0.912 |
| Fasting plasma glucose, mg/dL | 109.02±26.74 | 107.88±25.19 | 125.72±40.34 | <0.001 |
| Total cholesterol, mg/dL | 206.02±43.69 | 206.40±43.66 | 200.44±44.05 | 0.260 |
| Triglyceride, mg/dL | 172.05±120.22 | 169.11±118.22 | 215.31±140.51 | 0.007 |
| HDL-C, mg/dL | 51.45±11.64 | 51.79±11.68 | 46.43±9.82 | <0.001 |
| LDL-C, mg/dL | 131.6±34.54 | 131.75±34.66 | 129.41±32.91 | 0.555 |
| Aspartate aminotransferase, IU/L | 33.69±20.40 | 32.29±14.68 | 54.36±54.33 | 0.001 |
| Alanine aminotransferase, IU/L | 37.53±26.61 | 35.81±22.51 | 62.91±55.01 | <0.001 |
| γ-Glutamyl transferase, IU/L | 44.64±42.52 | 42.44±35.43 | 77.07±94.55 | 0.002 |
| Insulin, μIU/mL | 10.14±5.83 | 9.75±5.22 | 16.37±10.06 | <0.001 |
| Diabetes, % | 244 (20.7) | 217 (19.6) | 27 (36.0) | 0.001 |
| Smoking, % | 267 (22.6) | 243 (22.0) | 24 (32.0) | 0.001 |
| Exercise, % | 776 (65.8) | 732 (66.2) | 44 (58.7) | 0.113 |
| CAP, dB/m | 278.01±50.90 | 275.47±49.80 | 315.51±52.48 | <0.001 |
| Liver stiffness, kPa | 4.57±1.81 | 4.22±1.07 | 9.72±2.56 | <0.001 |
Data are presented as mean±SD or number (%).
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CAP, controlled attenuation parameter.
2. NAFLD and liver fibrosis according to sarcopenic obesity status
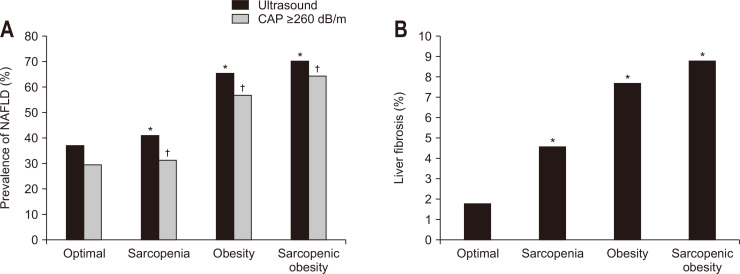
The prevalence of NAFLD based on ultrasound was 37.4% (optimal), 41.1% (sarcopenia), 65.5% (obesity), and 70.2% (sarcopenic obesity) according to sarcopenic obesity status. The fatty liver grade in ultrasound was also positively associated with sarcopenic obesity status and that the prevalence of moderate or severe fatty liver sequentially increased according to the sarcopenic obesity status (Supplementary Table 2). Moreover, when NAFLD was diagnosed using the CAP score, similar results were observed (Fig. 1A). The prevalence of liver fibrosis (LS ≥7.5) also significantly increased according to the sarcopenic obesity status (Fig. 1B).
Fig. 1.
Prevalence of NAFLD and liver fibrosis according to sarcopenic obesity status. (A) NAFLD according to sarcopenic obesity status. (B) Liver fibrosis according to sarcopenic obesity status.
NAFLD, nonalcoholic fatty liver disease; CAP, controlled attenuation parameter. *The prevalence of NAFLD and liver fibrosis based on ultrasound showed a statistically significant difference when compared with optimal (p<0.01); †The prevalence of NAFLD based on the CAP score also showed a statistically significant difference when compared with optimal (p<0.01).
3. Association between NAFLD and sarcopenic obesity status
The association between NAFLD (diagnosed by ultrasonography) and sarcopenic obesity status was investigated. When the optimal group (nonobese and nonsarcopenic categories) was set as the reference, unadjusted multivariate logistic regression analysis revealed that the sarcopenic obesity group had the highest OR for the presence of NAFLD. According to the logistic regression analysis after adjusting for multiple risk factors, including age, sex, SBP, FPG, presence of diabetes, presence of hypertension, smoking, and exercise, the OR of the risk of NAFLD was much higher in the sarcopenic obesity group, followed by the obesity only group and the sarcopenia only group, than in the optimal (nonobese, nonsarcopenic) group (Table 4). We further explored the association between NAFLD, defined by CAP score and sarcopenic obesity status (Supplementary Table 3). The association between NAFLD and sarcopenic obesity status, defined by BMI was also analyzed, and a significant association was observed (Supplementary Table 4).
Table 4.
Odds Ratio of Risk Factors for NAFLD Defined by Ultrasonography According to the Sarcopenic Obesity Status
| Odds ratio (95% CI) | p for trend | ||||
|---|---|---|---|---|---|
| Optimal | Sarcopenia | Obesity* | Sarcopenic obesity | ||
| Unadjusted | 1 | 1.17 (0.83–1.65) | 3.18 (2.40–4.21) | 3.95 (3.23–4.82) | <0.001 |
| Age and sex adjusted | 1 | 1.90 (1.31–2.75) | 2.51 (1.88–3.35) | 4.30 (3.49–5.30) | <0.001 |
| Multivariable adjusted† | 1 | 1.92 (1.30–2.84) | 2.25 (1.67–3.03) | 3.68 (2.94–4.60) | <0.001 |
NAFLD, nonalcoholic fatty liver disease; CI, confidence interval.
*Obesity was defined by fat mass; †Age, sex, systolic blood pressure, fasting plasma glucose, presence of diabetes, presence of hypertension, smoking, and exercise.
4. Association between liver fibrosis and sarcopenic obesity status
There was a significant relationship between sarcopenic obesity and liver fibrosis, even after adjusting for confounding variables. After adjusting for multiple risk factors, sarcopenic obesity for liver fibrosis was associated with the highest risk of liver fibrosis when compared with the other three categories of body composition (adjusted OR, 4.69; 95% confidence interval, 1.95 to 11.29) (Table 5). In the sensitivity analyses, the results did not change when BMI was substituted for fat mass in the model (Supplementary Table 5).
Table 5.
Odds Ratio of Risk Factors for Liver Fibrosis According to Sarcopenic Obesity Status
| Odds ratio (95% CI) | p for trend | ||||
|---|---|---|---|---|---|
| Optimal | Sarcopenia | Obesity* | Sarcopenic obesity | ||
| Unadjusted | 1 | 2.69 (0.66–11.06) | 4.64 (1.75–12.29) | 5.35 (2.27–12.60) | <0.001 |
| Age and sex adjusted | 1 | 3.99 (0.93–17.20) | 4.22 (1.59–11.19) | 5.74 (2.43–13.58) | <0.001 |
| Multivariable adjusted† | 1 | 3.80 (0.86–16.75) | 4.17 (1.56–11.17) | 4.69 (1.95–11.29) | <0.001 |
CI, confidence interval.
*Obesity was defined by fat mass; †Age, sex, systolic blood pressure, fasting plasma glucose, presence of hypertension, smoking, and exercise.
DISCUSSION
In this study, we showed an independent association between the prevalence of NAFLD and liver fibrosis and categorized the sarcopenic obesity status. Several studies have already shown an association between sarcopenia and NAFLD or liver fibrosis and between obesity and NAFLD or liver fibrosis.3,8,9,12-14,25,26 However, to the best of our knowledge, this is the first study to demonstrate that NAFLD diagnosed on the basis of ultrasound findings and CAP score was positively associated with sarcopenic obesity status and that the prevalence of liver fibrosis sequentially increased according to the sarcopenic obesity status.
The definition of sarcopenic obesity is a combination of sarcopenia and obesity, and a universally accepted definition has not been established and various diagnostic criteria exist.9,27,28 Sarcopenia refers to a decrease in skeletal muscle mass and strength, and the most commonly used method is a measure of muscle mass with a test of grip test.29 Methods for measuring skeletal muscle mass include dual-energy X-ray absorptiometry, BIA, and computed tomography, which have different advantages depending on validity and cost. BIA is widely used as an inexpensive, portable, and easy-to measure method, though it has sub-optimal validity.29 Although BMI is commonly used as a method of diagnosing obesity, it has a disadvantage in that it does not show specific body composition. When diagnosing sarcopenic obesity, a phenomenon in which muscle loss and body fat accumulation occur simultaneously, fat accumulation in BIA has been widely used to define obesity in several studies.27,28
Both obesity and sarcopenia have been shown to be associated with the prevalence of NAFLD and liver fibrosis, and it has been proposed that it is more closely related to sarcopenic obesity, NAFLD, and liver fibrosis. However, there are only few reports on the relationship between sarcopenic obesity and NAFLD and liver fibrosis, and the results are controversial.9,30 Sung et al.30 reported that sarcopenia was independently associated with liver fibrosis in patients with type 2 diabetes. In this study, liver fibrosis was assessed using the fibrosis-4 index and NAFLD fibrosis score, and obesity was defined as a BMI of >25 kg/m2. Additionally, the sample size was small, and only subjects with type 2 diabetes were included. Moreover, they failed to show statistically significant findings of sarcopenia and liver fibrosis in the obese group.
Recently, Wijarnpreecha et al.9 showed an association between sarcopenic obesity and NAFLD and NAFLD-associated liver fibrosis. They only showed that the association was statistically significant in subjects with sarcopenic obesity compared to those without sarcopenic obesity, while our study showed that the prevalence of NAFLD and liver fibrosis significantly increased according to the sarcopenic obesity status. Moreover, in the sensitivity analyses, the results did not change when BMI was substituted for fat mass.
Obesity is a well-known major factor in the pathogenesis and progression of NAFLD and liver fibrosis,31,32 however, the mechanism underlying the relationship between sarcopenic obesity and the increased risk of NAFLD and liver fibrosis has not been fully elucidated. There are several explanations for this association, which may be explained by the fact that obesity and sarcopenia share several pathophysiological processes. The skeletal muscle is the primary tissue responsible for insulin signaling, and loss of skeletal muscle mass in sarcopenia leads to decreased insulin signaling and insulin resistance.14 Thus, insulin resistance is an important mechanism underlying the development of NAFLD and liver fibrosis in subjects with sarcopenic obesity.33,34 Second, obesity and low muscle mass are related to inflammation, and chronic inflammation may also play a role in the increased risk of NAFLD and liver fibrosis.35,36 Wijarnpreecha et al.9 also explained that the mechanism underlying these relationships is primarily due to inter-organ interconnections by insulin resistance, chronic inflammation, oxidative stress, and secretion of cytokines.
Our study has several strengths. Our study used obesity defined as fat mass measured by BIA rather than BMI calculated simply by height and weight values. Fat mass is more correlated with an individual’s metabolic diseases, and fat mass and lean mass cannot be distinguished in individuals with similar BMI.28,37,38 In our study, the association between sarcopenic obesity status defined by BMI and NAFLD and fibrosis were analyzed as a part of sensitivity test. Interestingly, the trend was consistent with sarcopenic obesity status measured by BIA (Supplementary Tables 3 and 4).
Our study has several limitations. First, because this was a cross-sectional observational study, a causal relationship could not be established. Second, this study was limited to generalization, as it was conducted only on Koreans from a single institution and there is a possibility of inherent selection bias due to self-referred checkup tests. The ratio of males and females was not balanced in our study, which suggests that males were more interested in their health. Third, recall bias could occur because information on smoking history, drinking history, and exercise history was obtained through questionnaires. Fourth, we estimated skeletal muscle mass and fat mass using BIA; sarcopenia and obesity can be classified differently according to imaging studies or definition criteria, and we were unable to assess muscle strength and/or physical performance due to the lack of data. Lastly, NAFLD and liver fibrosis could not be assessed through histologic evaluation. Moreover, there is no universal cutoff value to determine NAFLD and liver fibrosis using CAP and LS scores; therefore, it is possible that NAFLD and liver fibrosis may have been misclassified. However, we defined various cutoff values for CAP and LS scores from previous studies.21-24
In conclusion, sarcopenic obesity is independently associated with NAFLD and liver fibrosis, and the risk of NAFLD and liver fibrosis increased according to the sarcopenic obesity status. Additional prospective longitudinal studies are needed to evaluate the impact of sarcopenic obesity on NAFLD and liver fibrosis over time.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl220041.
ACKNOWLEDGEMENTS
We would like to thank the Gangnam Severance Health Promotion Research team for supporting the construction of the registry of data from the Health Promotion Center of the Gangnam Severance Hospital.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: H.W.L, J.S.P. Data acquisition: W.S., S.H.Y., S.J.B., B.K.L. Data analysis and interpretation: W.S., S.H.Y. Drafting of the manuscript: W.S., S.H.Y., J.J. Critical revision of the manuscript for important intellectual content: H.W.L., J.S.P. Statistical analysis: J.S.P. Study supervision: H.W.L., J.S.P. Final approval of the manuscript: all authors.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev. 2007;65(6 Pt 2):S57–S63. doi: 10.1301/nr.2007.jun.S57-S63. [DOI] [PubMed] [Google Scholar]
- 4.Jung CH, Rhee EJ, Kwon H, Chang Y, Ryu S, Lee WY. Visceral-to-subcutaneous abdominal fat ratio is associated with nonalcoholic fatty liver disease and liver fibrosis. Endocrinol Metab (Seoul) 2020;35:165–176. doi: 10.3803/EnM.2020.35.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MK, Rhee EJ, Kim MC, et al. Metabolic health is more important than obesity in the development of nonalcoholic fatty liver disease: a 4-year retrospective study. Endocrinol Metab (Seoul) 2015;30:522–530. doi: 10.3803/EnM.2015.30.4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults: current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim TN, Choi KM. The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease. J Cell Biochem. 2015;116:1171–1178. doi: 10.1002/jcb.25077. [DOI] [PubMed] [Google Scholar]
- 8.Issa D, Alkhouri N, Tsien C, et al. Presence of sarcopenia (muscle wasting) in patients with nonalcoholic steatohepatitis. Hepatology. 2014;60:428–429. doi: 10.1002/hep.26908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wijarnpreecha K, Aby ES, Ahmed A, Kim D. Association between sarcopenic obesity and nonalcoholic fatty liver disease and fibrosis detected by Fibroscan. J Gastrointestin Liver Dis. 2021;30:227–232. doi: 10.15403/jgld-3323. [DOI] [PubMed] [Google Scholar]
- 10.Chen LK, Lee WJ, Peng LN, et al. Recent advances in sarcopenia research in Asia: 2016 update from the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2016;17:767. doi: 10.1016/j.jamda.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 12.Lim HS, Park YH, Suh K, et al. Association between sarcopenia, sarcopenic obesity, and chronic disease in Korean elderly. J Bone Metab. 2018;25:187–193. doi: 10.11005/jbm.2018.25.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim S, Kim JH, Yoon JW, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA) Diabetes Care. 2010;33:1652–1654. doi: 10.2337/dc10-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim TN, Park MS, Lim KI, et al. Relationships between sarcopenic obesity and insulin resistance, inflammation, and vitamin D status: the Korean Sarcopenic Obesity Study. Clin Endocrinol (Oxf) 2013;78:525–532. doi: 10.1111/j.1365-2265.2012.04433.x. [DOI] [PubMed] [Google Scholar]
- 15.Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601. doi: 10.1016/j.clnu.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Upadhya B, Haykowsky MJ, Eggebeen J, Kitzman DW. Sarcopenic obesity and the pathogenesis of exercise intolerance in heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2015;12:205–214. doi: 10.1007/s11897-015-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung KS, Kim SU, Ahn SH, et al. Risk assessment of hepatitis B virus-related hepatocellular carcinoma development using liver stiffness measurement (FibroScan) Hepatology. 2011;53:885–894. doi: 10.1002/hep.24121. [DOI] [PubMed] [Google Scholar]
- 18.Kim MN, Kim SU, Kim BK, et al. Increased risk of hepatocellular carcinoma in chronic hepatitis B patients with transient elastography-defined subclinical cirrhosis. Hepatology. 2015;61:1851–1859. doi: 10.1002/hep.27735. [DOI] [PubMed] [Google Scholar]
- 19.Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTETM guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Yen YH, Chen JB, Cheng BC, et al. Using controlled attenuation parameter combined with ultrasound to survey non-alcoholic fatty liver disease in hemodialysis patients: a prospective cohort study. PLoS One. 2017;12:e0176027. doi: 10.1371/journal.pone.0176027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lupșor-Platon M, Feier D, Stefănescu H, et al. Diagnostic accuracy of controlled attenuation parameter measured by transient elastography for the non-invasive assessment of liver steatosis: a prospective study. J Gastrointestin Liver Dis. 2015;24:35–42. doi: 10.15403/jgld.2014.1121.mlp. [DOI] [PubMed] [Google Scholar]
- 22.Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan(Ⓡ)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease: where do we stand? World J Gastroenterol. 2016;22:7236–7251. doi: 10.3748/wjg.v22.i32.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–1730. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 24.Imanirad D, Bozanich N, Vidyarthi G. Institutional review of utilization of vibration-controlled transient elastography (FibroScan) in evaluation of non alcoholic fatty liver disease. Am J Gastroenterol. 2018;113:S583–S584. doi: 10.14309/00000434-201810001-01025. [DOI] [Google Scholar]
- 25.Silva Neto LS, Karnikowiski MG, Tavares AB, Lima RM. Association between sarcopenia, sarcopenic obesity, muscle strength and quality of life variables in elderly women. Rev Bras Fisioter. 2012;16:360–367. doi: 10.1590/S1413-35552012005000044. [DOI] [PubMed] [Google Scholar]
- 26.Yang KC, Hung HF, Lu CW, Chang HH, Lee LT, Huang KC. Association of non-alcoholic fatty liver disease with metabolic syndrome independently of central obesity and insulin resistance. Sci Rep. 2016;6:27034. doi: 10.1038/srep27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong SH, Choi KM. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci. 2020;21:494. doi: 10.3390/ijms21020494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoo JH, Park SW, Jun JE, et al. Relationship between low skeletal muscle mass, sarcopenic obesity and left ventricular diastolic dysfunction in Korean adults. Diabetes Metab Res Rev. 2021;37:e3363. doi: 10.1002/dmrr.3363. [DOI] [PubMed] [Google Scholar]
- 29.Han A, Bokshan SL, Marcaccio SE, DePasse JM, Daniels AH. Diagnostic criteria and clinical outcomes in sarcopenia research: a literature review. J Clin Med. 2018;7:70. doi: 10.3390/jcm7040070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sung MJ, Lim TS, Jeon MY, et al. Sarcopenia is independently associated with the degree of liver fibrosis in patients with type 2 diabetes mellitus. Gut Liver. 2020;14:626–635. doi: 10.5009/gnl19126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 32.Talbot NA. Royal Veterinary College, University of London; London: 2014. Obesity, inflammation and insulin resistance in skeletal muscle [Ph.D. thesis] [Google Scholar]
- 33.Angulo P, Alba LM, Petrovic LM, Adams LA, Lindor KD, Jensen MD. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol. 2004;41:943–949. doi: 10.1016/j.jhep.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/S0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 35.Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20:2515–2532. doi: 10.3748/wjg.v20.i10.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duarte N, Coelho IC, Patarrão RS, Almeida JI, Penha-Gonçalves C, Macedo MP. How inflammation impinges on NAFLD: a role for Kupffer cells. Biomed Res Int. 2015;2015:984578. doi: 10.1155/2015/984578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. 2017;10:207–215. doi: 10.1159/000471488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17. doi: 10.1007/978-3-319-48382-5_1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.