Abstract
Background/Aims
There is increasing evidence that supplementation with pre- and probiotics appears to have positive effects on irritable bowel syndrome (IBS). The aim of this study was to determine the effects of a new synbiotic formulation on gastrointestinal symptoms in elderly patients with IBS.
Methods
Sixty-seven IBS patients aged ≥60 years were randomly assigned to either a placebo group (n=34) or a synbiotic group (n=33). During a 4-week intervention, subjects used a placebo or a synbiotic containing Lactobacillus paracasei DKGF1 and extracts of Opuntia humifusa once a day. Patients were evaluated with the subject global assessment, visual analog scale, and Bristol stool chart. The primary outcome was the overall responder rate and the secondary outcome was the responder rates for abdominal symptom reduction at week 4.
Results
Overall, responder rates were significantly higher in the synbiotic group (51.5%) than in the placebo group (23.5%) (p=0.017). Abdominal pain (58.8% vs 81.8%) and psychological well-being (26.4% vs 60.6%) were noticeably improved in the synbiotic group (p=0.038 and p=0.004, respectively). However, there were no significant differences in gas and bloating symptoms (p=0.88 and p=0.88, respectively). In patients with constipation-dominant and diarrhea-dominant IBS (n=16), the synbiotic significantly improved abdominal pain and defecation symptoms (responder rates for the placebo vs the synbiotic 22.2% vs 85.7%, p=0.04). There were no adverse events in either group.
Conclusions
The results indicate that this new synbiotic supplement can potentially relieve abdominal symptoms in elderly IBS patients.
Keywords: Irritable bowel syndrome, Synbiotics, Lactobacillus paracasei, Opuntia humifusa, Aged
INTRODUCTION
Irritable bowel syndrome (IBS) is a disease including complex of symptoms characterized by recurrent abdominal pain, bloating, and altered bowel habits in the absence of any structural or inflammatory abnormalities.1 IBS is estimated to affect approximately one in 10 people globally.2 The condition is common in women and young people but generally uncommon in elderly people. However, the impact of IBS on elderly patients is not negligible. The prevalence of IBS in elderly people has been estimated to be 9%–13%,3,4 suggesting that IBS is an important health problem in elderly people. As aging increases the risk of organic abnormalities, the management of IBS in elderly people is more difficult and complicated than in the younger population.5
The pathophysiology of IBS is complex. Abnormal stress response, infection, or inflammatory response may alter intestinal permeability and trigger a series of events (e.g., inflammatory cell infiltration, local edema, and cytokine or chemokine release) that result in the development of IBS symptoms.6 Recent data have demonstrated that changes in the gut microbiome may play an important role in IBS. There have been consistent results of lower concentrations of Lactobacilli7 and Bifidobacteria8 and of higher amounts of Enterobacteriaceae (coliforms) and Bacteroides in IBS patients.9 These observations led to the use of prebiotics, probiotics and synbiotics in the treatment of IBS.
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit to the host.10 Prebiotics are selectively fermented ingredients that induce specific changes in the composition and/or activity of the gastrointestinal microbiota, thus benefiting the health of the host. Synbiotics are formulations that contain both probiotics and prebiotics, with reported health benefits.11 Probiotics and synbiotics beneficially affect patients with IBS in terms of improvement in overall global symptom, abdominal pain, bloating, and flatulence scores.12 Lactobacilli and Bifidobacteria are the most commonly studied probiotics in patients with IBS.9,13 Lactobacillus paracasei DKGF1 has been isolated from kimchi, a traditional Korean food.14 We chose L. paracasei DKGF1 over several other Lactobacillus strains (L. casei, L. plantarum, and L. acidophilus) based on its in vitro activities (antioxidant effect, heat resistance, and intestinal adhesion). Clinical trials on probiotics or synbiotics of L. paracasei showed significant improvement in symptoms in patients with IBS.15,16 Among many sources of prebiotics, there is increasing interest in Opuntia, prickly pear cactus, with high-fiber source and antioxidants.17 Isorhamnetin, a flavonoid from Opuntia humifusa, possess potential anti-oxidative and anti-inflammatory activities.18 In addition, mucilage19 and pectin20 may be ingested by lactic acid microorganisms, which can increase the short-chain fatty acids production. We conducted an animal study with L. paracasei DKGF1 and extract of Opuntia. Wistar rats with induced IBS who consumed L. paracasei DKGF1 and Opuntia extract showed greater improvement in stool consistency compared to those who consumed placebo and the L. paracasei DKGF1 only group. These findings indicated that synbiotic supplements have therapeutic effects on IBS, and that the extract of Opuntia may enhance the effects of L. paracasei DKGF1.21
The treatment of IBS in elderly patients is similar to that in young patients. However, greater caution is needed when treating elderly patients. According to a systematic review, probiotics and synbiotics are well tolerated, and no significant events were reported in previous trials.12 Safe medication is needed for the treatment of elderly patients with IBS, and synbiotics may constitute one such treatment option. However, there is significant heterogeneity across previous studies that have examined this issue. Moreover, most studies were limited by suboptimal design or unqualified data. Furthermore, there is limited information on the effect of synbiotics on IBS symptoms in elderly patients. Thus, the aim of this study was to investigate the effects of a novel synbiotic on IBS symptoms in elderly patients.
MATERIALS AND METHODS
1. Study design, setting, and participants
The 4-week randomized, double-blind, placebo-controlled trial was conducted at the Samsung Medical Center, Seoul, South Korea. Elderly IBS patients, aged ≥60 years and who fulfilled the Rome IV criteria,22 were enrolled. Patients were excluded if they had previous abdominal surgery except appendectomy and caesarian section, history of inflammatory bowel disease, and evidence of other severe illnesses (liver cirrhosis, cancer, or psychological, cardiovascular, or pulmonary diseases). Additionally, patients who had used antibiotics and probiotics within the 2 weeks preceding the study were also excluded.
At baseline, eligible patients were instructed to complete the visual analog scale (VAS)23 and the Bristol stool chart (BSC).24 Sixty-eight patients were diagnosed with IBS according to the Rome IV criteria. One patient from the synbiotic group was excluded because of poor compliance. A total of 67 patients were randomized. During the intervention, subjects used a synbiotic mixture or placebo once daily with water or a beverage (except for acidic juices such as orange juice or soda). During the study period, all subjects were prohibited from consuming laxatives, antidiarrheal agents, antibiotics, and probiotics. The eligible patients recorded their IBS symptoms weekly using subject global assessment (SGA),25 VAS, and BSC. Signed informed consent was obtained prior to enrollment in the trial. The study protocol was reviewed and approved by the Institutional Review Board of Samsung Medical Center (IRB number: SMC IRB 2019-06-127). This study was registered with the Clinical Research Information Service after enrollment completion (KCT0005449, https://cris.nih.go.kr; date of registration: 10/7/2020).
2. Synbiotic and placebo preparation
The synbiotic mixture, packed in sachets, contained L. paracasei DKGF1 and extracts from O. humifusa (eastern prickly pear cactus). Each synbiotic sachet contained 1.0×1011 colony-forming units of L. paracasei DKGF1, 0.2 g of O. humifusa extract, and 1.59 g of maltodextrin. The placebo only contained maltodextrin (1.98 g) and its appearance was identical to that of the synbiotic preparation. The products were refrigerated at a temperature lower than 10℃.
3. Outcome measurement
During study periods, all patients recorded the degree of symptom improvement using a 5-point Likert scale on the SGA (0: unchanged, 1: somewhat relieved, 2: moderately relieved, 3: considerably relieved, and 4: completely relieved).25 They also provided VAS scores (0: very good to 10: very bad) for the severity of IBS symptoms including abdominal pain, gas, and bloating, as well as for psychological well-being.26 Stool form and consistency were evaluated using the BSC.24 The primary end point was overall responder rates of improvement in global IBS symptoms. Overall responders were patients who fulfilled improvements in overall symptoms assessed by SGA more than 2 of the 4 weeks. Weekly responders were patients who fulfilled improvements in SGA score of two or higher each week. The secondary outcome was the responder rates for abdominal symptom reduction (more than 30% decrease in the VAS score from the baseline) at week 4. Among patients with constipation-dominant IBS (IBS-C), those with improved abdominal pain and stool form or frequency (increase in the Bristol score or one or more bowel movements per week compared with baseline) were considered responders. Among patients with diarrhea-dominant IBS (IBS-D), those with improved abdominal pain and stool form or consistency (decrease in the Bristol score or number of bowel movements per week compared with baseline) were considered responders according to the U.S. Food and Drug Administration’s guidance for IBS. Adverse events were also recorded during the intervention period. Medication adherence was measured by pill counts and a compliance rate of more than 90% was set as the minimum.
4. Statistical analysis
The sample size was calculated to provide 80% power and to estimate at least 35% difference in symptom improvement between the two groups. It was estimated that at least 31 patients per group were required. Allowing for an 8% dropout rate, total of 68 patients (34 per group) were randomized. The random allocation sequence was conducted using a computer-generated, blocked randomization list, independent of the research group, and with a concealed block. All data were presented as median (interquartile range) or number (%). Analyses were performed with the Kolmogorov-Smirnov test to determine the normality of variable distribution; age was not distributed normally. Continuous variables were analyzed by the Student t-test or the Mann-Whitney U-test as appropriate. The Chi-square and the Fisher exact tests were used to compare the responder rates between the placebo and synbiotic groups. IBM SPSS Statistics for Windows, version 25.0 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
RESULTS
1. Baseline characteristics
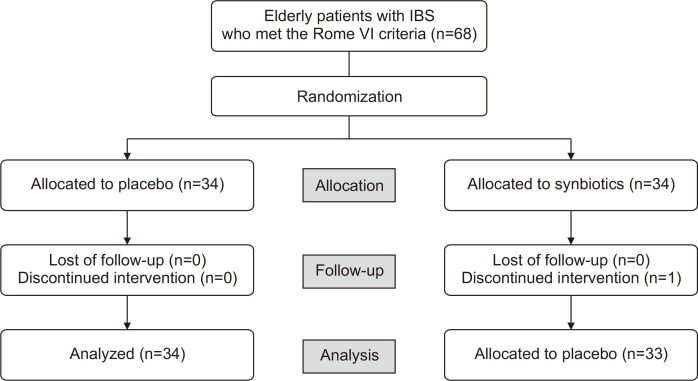
Sixty-eight elderly patients fulfilled the Rome IV criteria of IBS. One patient from the synbiotic group was excluded for poor compliance. Finally, 33 patients received the synbiotic and 34 received the placebo (Fig. 1).
Fig. 1.
Flowchart of participants.
IBS, irritable bowel syndrome.
The baseline characteristics are shown in Table 1. The mean age was 64.0 years (range, 60 to 76 years), and 68.6% of the subjects were female. The synbiotic and placebo groups were comparable with respect to age, sex, body mass index, and abdominal symptoms.
Table 1.
Baseline Characteristics of the Randomized Patients
| Characteristics | Placebo (n=34) | Synbiotic (n=33) |
|---|---|---|
| Age, yr* | 64.0 (61.0–66.0) | 63.0 (62.0–67.0) |
| Female sex | 25 (73.5) | 21 (66.6) |
| BMI, kg/m2 | 24.1 (22.7–25.7) | 24.2 (22.8–26.5) |
| Never smoker | 27 (79.4) | 28 (84.8) |
| Never drinker | 16 (48.4) | 17 (51.5) |
| IBS subtype | ||
| IBS-C | 3 (8.8) | 4 (12.1) |
| IBS-D | 6 (17.7) | 3 (9.1) |
| IBS-M | 2 (6.9) | 3 (9.1) |
| IBS-U | 23 (67.6) | 23 (69.7) |
| VAS | ||
| Abdominal pain | 3.0 (3.0–3.0) | 3.0 (3.0–4.0) |
| Gas | 4.0 (3.0–6.0) | 4.0 (2.0–6.0) |
| Bloating | 4.0 (3.0–5.8) | 4.0 (2.0–6.0) |
| Psychological well-being | 3.0 (1.0–3.0) | 3.0 (2.0–5.0) |
| Stool frequency,/day | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Stool form (BSC) | 5.0 (3.0–5.8) | 5.0 (3.0–6.0) |
Data are presented as median (interquartile range) or number (%).
BMI, body mass index; IBS, irritable bowel syndrome; IBS-C, constipation-dominant IBS; IBS-D, IBS with predominant diarrhea; IBS-M, IBS with mixed bowel habits; IBS-U, IBS unclassified; VAS, visual analog scale; BSC, Bristol stool chart.
*Age was not distributed normally.
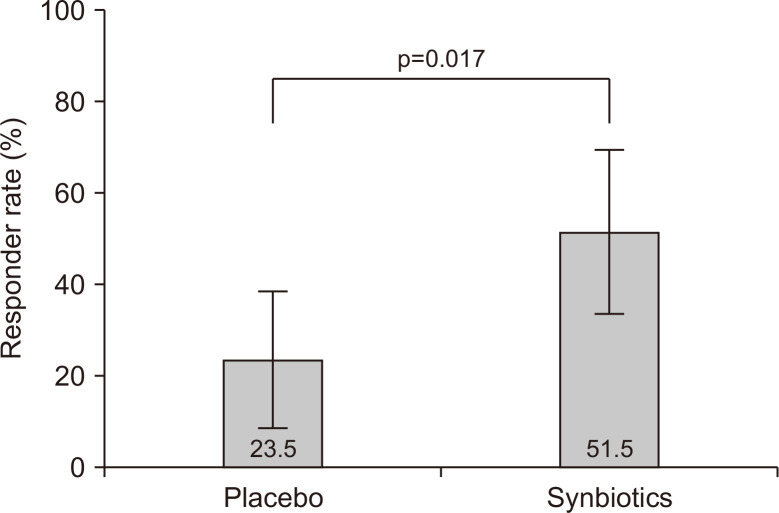
2. Primary outcomes
Overall responder rates for overall IBS symptom improvement evaluated using the SGA were significantly higher in the synbiotic group than in the placebo group (51.5% vs 23.5%, p=0.017) (Fig. 2). Additionally, the synbiotic group had consistently higher responder rates than those in the placebo group. The weekly responder rates for the SGA are presented in Supplementary Table 1.
Fig. 2.
Overall responder rates for overall irritable bowel syndrome symptom improvement assessed with the subject global assessment score. The overall responder rates were significantly higher in the synbiotic group than in the placebo group.
3. Secondary outcomes
The overall responder rates for the improvement of specific symptoms were assessed with the VAS. The proportions of subjects achieving VAS score reduction in abdominal pain and psychological well-being were significantly higher in the synbiotic group than in the placebo group. The responder rates for gas and bloating were slightly higher in the synbiotic group; however, the differences were not statistically significant (Table 2). After 4 weeks, the median VAS score for abdominal pain improved from 3.0 to 1.0 in the synbiotic group and from 3.0 to 2.0 in the placebo group (p=0.003). The VAS score for psychological well-being also significantly improved in the synbiotic group (from 3.0 to 1.0) compared to the placebo group (from 3.0 to 2.0) (p=0.005). No significant differences were observed between the groups for gas and bloating (Supplementary Fig. 1).
Table 2.
Overall Responder Rates for Improvement of Symptoms
| Variable | Placebo (n=34) | Synbiotics (n=33) | p-value |
|---|---|---|---|
| Abdominal pain | 20 (58.8) | 27 (81.8) | 0.038 |
| Gas | 20 (58.8) | 20 (60.6) | 0.880 |
| Bloating | 19 (55.8) | 19 (57.5) | 0.880 |
| Psychological well-being | 9 (26.4) | 20 (60.6) | 0.004 |
Data are presented as number (%).
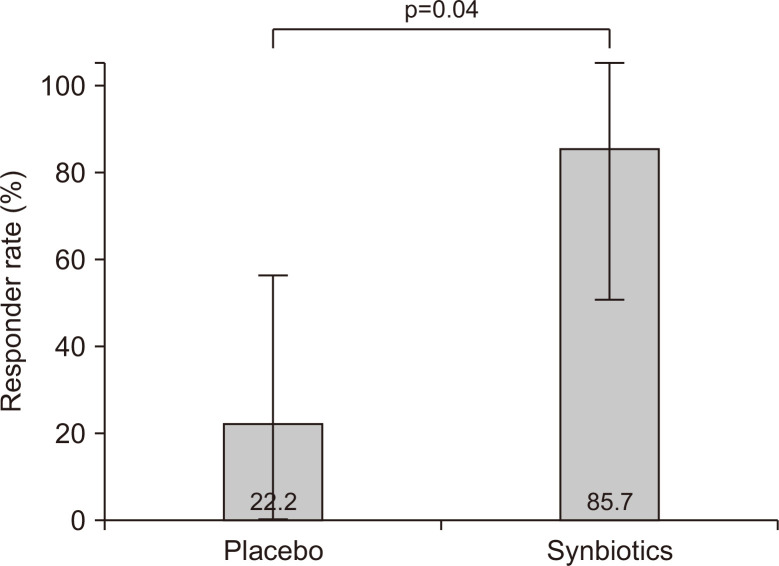
Sixteen patients were classified as having IBS-C or IBS-D. The rates of responders for whom both abdominal pain and stool form or consistency improved were significantly higher in the synbiotic group (85.7%) than in the placebo group (22.2%) (p=0.04) (Fig. 3). Among the patients with IBS-C, we noted a positive response in no patient in the placebo group and in all four patients in the synbiotic group (0% and 100%, respectively, p=0.029). Among the patients with IBS-D, two of six patients in the placebo group and two of three patients in the synbiotic group showed a response (33.3% and 66.6%, respectively, p=0.52).
Fig. 3.
Improvements in abdominal pain and defecation symptoms in patients with constipation- or diarrhea-dominant irritable bowel syndrome. The overall responder rates were significantly higher in the synbiotic group than in the placebo group.
4. Safety
No adverse events were reported for either group during the 4-week trial. The treatment was well-tolerated, and all of the study participants showed 100% drug compliance.
DISCUSSION
In this randomized, double-blind, placebo-controlled trial, consumption of a synbiotic combination, consisting of L. paracasei DKGF1 and prebiotics extracted from O. humifusa, was associated with overall relief of IBS symptoms in elderly patients. In particular, abdominal pain and psychological well-being noticeably improved. When stratified by the type of IBS, the synbiotic alleviated not only abdominal pain, but also defecation symptoms in patients with IBS-C or IBS-D. There were no adverse events in either group during the study period.
Several studies have examined the effect of synbiotics providing different results. A placebo-controlled trial of Lactobacillus, Bifidobacterium, and prebiotic short chain fructo-oligosaccharides with 68 patients with IBS showed reduced abdominal pain and bloating.27 A multicenter controlled trial of L. paracasei and prebiotic xylo-oligosaccharides showed beneficial effects in terms of global IBS symptoms and reduced stool frequency in patients with IBS-D.16 In contrast, in a trial with 132 patients with IBS, Lactobacillus, Bifidobacterium, Streptococcus, and prebiotic fructo-oligosaccharides showed no beneficial effects over placebo during a 2-week treatment.28 A systematic review also reported that synbiotics had no statistically significant effect on symptom reduction, although the involved trials were individually positive.29 However, there is considerable heterogeneity across the studies, and these studies were limited by their study designs and sample sizes. Furthermore, most studies lacked background on the mechanisms of synbiotics. In this study, we used validated parameters and instruments. The 5-point Likert scale of the SGA is a useful tool to identify IBS responders during pharmacologic studies.25 The VAS and BSC are required to interpret the results of IBS clinical trials as per Food and Drug Administration’s IBS guidance. Additionally, our previous animal model revealed the possible mechanisms of the synbiotic used in this study, including a reduction of serum corticosterone levels, low levels of tumor necrosis factor α in the colonic mucosa, and an increase in the expression of tight junction proteins.21 After using these validated parameters and based on our previously acquired in vivo data, the findings of the present study are consistent with those of previous studies, which reported the clinical benefits of synbiotics in patients with IBS.
The human microbiota changes in terms of microbial diversity and variation with age. The gut microbiota is established at birth, and its composition remains relatively stable throughout adulthood.30 However, the core microbiota groups including Lactobacilli decrease31 and their stability is reduced in old age.32 In this population, synbiotics may yield greater clinical benefits than in other age groups. Unfortunately, most clinical trials have excluded elderly patients, and it is uncertain whether synbiotic use is safe in the elderly. To meet the unmet needs, the present study focused on elderly patients with IBS. The synbiotic in this study was superior to placebo with respect to global IBS symptom improvement and showed a good safety profile.
Lactobacillus is commonly used to treat IBS symptoms. In line with the present findings, the effectiveness of L. paracasei in improving IBS symptoms (global IBS symptoms, stool frequency, and stool consistency) has been reported by other clinical studies.15,16 The plausible mechanisms are that L. paracasei exhibits broad-spectrum antimicrobial activity and can inhibit pathogenic bacteria by producing both D- and L-lactate.14,33 L. paracasei also induces a significant decrease of the pro-inflammatory cytokine interleukin-15 and significant increase in short-chain fatty acids.34 These mechanisms may provide effective symptomatic relief in patients with IBS. Moreover, additional prebiotics may provide synergistic benefits in combination with L. paracasei.
Carbohydrates from fruit, vegetables, and other edible plants may function as prebiotics.35 These carbohydrates have five properties: (1) resistance to digestion in the upper parts of the gastrointestinal tract, (2) fermentation by intestinal microbiota, (3) beneficial effects on host health, (4) selective stimulation of the growth of probiotics, and (5) stability in different food and feed processing.36 We found that O. humifusa extracts induced growth and survival of L. paracasei DKGF1 in an in vivo study. According to the animal model, the amount of fecal L. paracasei was increased in the treatment group, indicating that a sufficient number of live bacteria reached the intestine.21 The marker compound was 3-O-β-D-(6-O-α-L-rhamnosyl) glucoside. Flavonoids, metabolites of isorhamnetin 3-O-β-D-(6-O-α-L-rhamnosyl) glucoside, were reported to have anti-oxidative effects via both direct scavenging activity and the modulation of inflammatory cytokines.37 Additionally, through electrostatic interactions, the dietary fibers, mucilage, and pectin have the potential to protect probiotic bacteria. This enhances the survival of Lactobacillus species exposed to low pH, bile acids, and digestive enzymes.38,39 These substances may synthetically play a role as prebiotics.
The present study has several strengths. This study targeted elderly patients and the sample size was sufficient for analysis. We provided evidence of the efficacy of a new synbiotic combination for the treatment of IBS in elderly patients. This trial also has some limitations. We did not directly measure fecal microbial levels for the restoration of the normal flora following synbiotic supplementation. As we only included patients elderly than 60 years, it is unclear whether this synbiotic has similar effects in other age groups. Additionally, the distribution of subtypes of IBS was different from generally known. Although the reason for the higher prevalence of IBS-U is unclear, there are two plausible explanations. First, the prevalence of subtypes of IBS may be different in elderly IBS patients. Qumseya et al.40 conducted a population-based, cross-sectional study to determine the prevalence of IBS (Rome III) and its subtypes in middle-aged and elderly Palestinians. Among 1,352 participants, the overall prevalence of IBS was 30% and mixed IBS was the most common subtypes (55%). This study suggested the ratio of subtypes could be different depending on the age group. IBS subtypes may overlap considerably and vary over time in elderly patients. Moreover, not only IBS subtypes overlap but other functional gastrointestinal disorders may overlap in these patients’ group.41 The overlapping symptoms make it difficult to divide elderly patients into four subtypes. Second, patient-reported outcome is a validated assessment strategy for IBS symptoms. The challenge persists, however, because the researcher does not know which symptoms patients use as the basis for their judgement, and the symptoms might be confusing, especially in elderly patients.42 Since patient-reported outcomes may be subjective, it would be more accurate if researchers involve while patients filled out the questionnaires. Further studies involving various age groups and including microbial analysis are required.
In conclusion, this randomized controlled trial indicated that the synbiotic containing L. paracasei DKGF1 and O. humifusa extracts is effective and safe for the treatment of global IBS symptoms in elderly patients with IBS. This finding supports the use of synbiotics as a treatment option for elderly patients with IBS.
SUPPLEMENTARY MATERIALS
Supplementary materials can be accessed at https://doi.org/10.5009/gnl210478.
ACKNOWLEDGEMENTS
This research was supported by the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program for The Industries of Economic Cooperation Region (P0004697).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Study concept and design: Y.W.M. Data acquisition: Y.S.J. Data analysis and interpretation: J.H.O. Drafting of the manuscript: J.H.O. Critical revision of the manuscript for important intellectual content: Y.W.M., D.K.C. Statistical analysis: J.H.O., D.K. Obtained funding: Y.W.M. Administrative, technical, or material support; study supervision: H.S.K., E.J.K., S.Y.P., C.H.K. Approval of final manuscript: all authors.
REFERENCES
- 1.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 2.Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17:473–486. doi: 10.1038/s41575-020-0286-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Wu Z, Qiao H, Zhang Y. A genetic association study of single nucleotide polymorphisms in GNβ3 and COMT in elderly patients with irritable bowel syndrome. Med Sci Monit. 2014;20:1246–1254. doi: 10.12659/MSM.890315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharucha AE, Camilleri M. Functional abdominal pain in the elderly. Gastroenterol Clin North Am. 2001;30:517–529. doi: 10.1016/S0889-8553(05)70193-X. [DOI] [PubMed] [Google Scholar]
- 5.Kurniawan I, Kolopaking MS. Management of irritable bowel syndrome in the elderly. Acta Med Indones. 2014;46:138–147. [PubMed] [Google Scholar]
- 6.Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med. 2017;376:2566–2578. doi: 10.1056/NEJMra1607547. [DOI] [PubMed] [Google Scholar]
- 7.Malinen E, Rinttilä T, Kajander K, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 8.Si JM, Yu YC, Fan YJ, Chen SJ. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10:1802–1805. doi: 10.3748/wjg.v10.i12.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkes GC, Sanderson JD, Whelan K. Treating irritable bowel syndrome with probiotics: the evidence. Proc Nutr Soc. 2010;69:187–194. doi: 10.1017/S002966511000011X. [DOI] [PubMed] [Google Scholar]
- 10.Hill C, Guarner F, Reid G, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 11.Guarner F, Khan AG, Garisch J, et al. World Gastroenterology Organisation Global Guidelines: probiotics and prebiotics October 2011. J Clin Gastroenterol. 2012;46:468–481. doi: 10.1097/MCG.0b013e3182549092. [DOI] [PubMed] [Google Scholar]
- 12.Ford AC, Quigley EM, Lacy BE, et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547–1561. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 13.Spiller R. Review article: probiotics and prebiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28:385–396. doi: 10.1111/j.1365-2036.2008.03750.x. [DOI] [PubMed] [Google Scholar]
- 14.Oh JH, Jang YS, Kang D, Chang DK, Min YW. Efficacy and safety of new Lactobacilli probiotics for unconstipated irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Nutrients. 2019;11:2887. doi: 10.3390/nu11122887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis ED, Antony JM, Crowley DC, et al. Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in alleviating symptoms of irritable bowel syndrome (IBS): a randomized, placebo-controlled study. Nutrients. 2020;12:1159. doi: 10.3390/nu12041159.fa7a4e2a891c44b683537dd3c3018f4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andriulli A, Neri M, Loguercio C, et al. Clinical trial on the efficacy of a new symbiotic formulation, Flortec, in patients with irritable bowel syndrome: a multicenter, randomized study. J Clin Gastroenterol. 2008;42 Suppl 3 Pt 2:S218–S223. doi: 10.1097/MCG.0b013e31817fadd6. [DOI] [PubMed] [Google Scholar]
- 17.Guevara-Arauza JC, de Jesús Ornelas-Paz J, Pimentel-González DJ, Rosales Mendoza S, Soria Guerra RE, Paz Maldonado LM. Prebiotic effect of mucilage and pectic-derived oligosaccharides from nopal (Opuntia ficus-indica) Food Sci Biotechnol. 2012;21:997–1003. doi: 10.1007/s10068-012-0130-1. [DOI] [Google Scholar]
- 18.Avila-Nava A, Calderón-Oliver M, Medina-Campos ON, et al. Extract of cactus (Opuntia ficus indica) cladodes scavenges reactive oxygen species in vitro and enhances plasma antioxidant capacity in humans. J Funct Foods. 2014;10:13–24. doi: 10.1016/j.jff.2014.05.009. [DOI] [Google Scholar]
- 19.Gullón B, Gullón P, Tavaria F, Alonso JL, Pintado M. In vitro assessment of the prebiotic potential of Aloe vera mucilage and its impact on the human microbiota. Food Funct. 2015;6:525–531. doi: 10.1039/C4FO00857J. [DOI] [PubMed] [Google Scholar]
- 20.Chung WS, Meijerink M, Zeuner B, et al. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiol Ecol. 2017;93:fix127. doi: 10.1093/femsec/fix127. [DOI] [PubMed] [Google Scholar]
- 21.Seong G, Lee S, Min YW, et al. Effect of a synbiotic containing Lactobacillus paracasei and Opuntia humifusa on a murine model of irritable bowel syndrome. Nutrients. 2020;12:3205. doi: 10.3390/nu12103205.70ae045186674e76acca269f248d1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150:1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 23.Bengtsson M, Ohlsson B, Ulander K. Development and psychometric testing of the visual analogue scale for irritable bowel syndrome (VAS-IBS) BMC Gastroenterol. 2007;7:16. doi: 10.1186/1471-230X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blake MR, Raker JM, Whelan K. Validity and reliability of the Bristol stool form scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44:693–703. doi: 10.1111/apt.13746. [DOI] [PubMed] [Google Scholar]
- 25.Müller-Lissner S, Koch G, Talley NJ, et al. Subject's global assessment of relief: an appropriate method to assess the impact of treatment on irritable bowel syndrome-related symptoms in clinical trials. J Clin Epidemiol. 2003;56:310–316. doi: 10.1016/S0895-4356(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 26.Yoo HY, Park B, Joo J, et al. Validation of the Korean version of visual analogue scale for irritable bowel syndrome questionnaire for assessment of defecation pattern changes. Ann Surg Treat Res. 2018;94:254–261. doi: 10.4174/astr.2018.94.5.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuchiya J, Barreto R, Okura R, Kawakita S, Fesce E, Marotta F. Single-blind follow-up study on the effectiveness of a symbiotic preparation in irritable bowel syndrome. Chin J Dig Dis. 2004;5:169–174. doi: 10.1111/j.1443-9573.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 28.Shavakhi A, Minakari M, Farzamnia S, et al. The effects of multi-strain probiotic compound on symptoms and quality-of-life in patients with irritable bowel syndrome: a randomized placebo-controlled trial. Adv Biomed Res. 2014;3:140. doi: 10.4103/2277-9175.135157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford AC, Harris LA, Lacy BE, Quigley EM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044–1060. doi: 10.1111/apt.15001. [DOI] [PubMed] [Google Scholar]
- 30.Power SE, O'Toole PW, Stanton C, Ross RP, Fitzgerald GF. Intestinal microbiota, diet and health. Br J Nutr. 2014;111:387–402. doi: 10.1017/S0007114513002560. [DOI] [PubMed] [Google Scholar]
- 31.Saraswati S, Sitaraman R. Aging and the human gut microbiota-from correlation to causality. Front Microbiol. 2015;5:764. doi: 10.3389/fmicb.2014.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015;350:1214–1215. doi: 10.1126/science.aac8469. [DOI] [PubMed] [Google Scholar]
- 33.Damodharan K, Palaniyandi SA, Suh JW, Yang SH. Probiotic characterization of Lactobacillus paracasei subsp. paracasei KNI9 inhibiting adherence of Yersinia enterocolitica on Caco-2 cells in vitro. Probiotics Antimicrob Proteins. 2020;12:600–607. doi: 10.1007/s12602-019-09535-8. [DOI] [PubMed] [Google Scholar]
- 34.Cremon C, Guglielmetti S, Gargari G, et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: a pilot randomized clinical trial. United European Gastroenterol J. 2018;6:604–613. doi: 10.1177/2050640617736478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markowiak P, Śliżewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho JY, Park SC, Kim TW, et al. Radical scavenging and anti-inflammatory activity of extracts from Opuntia humifusa Raf. J Pharm Pharmacol. 2006;58:113–119. doi: 10.1211/jpp.58.1.0014. [DOI] [PubMed] [Google Scholar]
- 38.Larsen N, Cahú TB, Isay Saad SM, Blennow A, Jespersen L. The effect of pectins on survival of probiotic Lactobacillus spp. in gastrointestinal juices is related to their structure and physical properties. Food Microbiol. 2018;74:11–20. doi: 10.1016/j.fm.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Han SH, Park K, Kim EY, Ahn SH, Lee HS, Suh HJ. Cactus (Opuntia humifusa) water extract ameliorates loperamide-induced constipation in rats. BMC Complement Altern Med. 2017;17:49. doi: 10.1186/s12906-016-1552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qumseya BJ, Tayem Y, Almansa C, et al. Irritable bowel syndrome in middle-aged and elderly Palestinians: its prevalence and effect of location of residence. Am J Gastroenterol. 2014;109:723–739. doi: 10.1038/ajg.2014.27. [DOI] [PubMed] [Google Scholar]
- 41.Lackner J, Jaccard J, Baum C, et al. Patient-reported outcomes for irritable bowel syndrome are associated with patients' severity ratings of gastrointestinal symptoms and psychological factors. Clin Gastroenterol Hepatol. 2011;9:957–964. doi: 10.1016/j.cgh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.