Summary
Peripersonal space (PPS) is a highly plastic “invisible bubble” surrounding the body whose boundaries are mapped through multisensory integration. Yet, it is unclear how the spatial proximity to others alters PPS boundaries. Across five experiments (N = 80), by recording behavioral and electrophysiological responses to visuo-tactile stimuli, we demonstrate that the proximity to others induces plastic changes in the neural PPS representation. The spatial proximity to someone else’s hand shrinks the portion of space within which multisensory responses occur, thus reducing the PPS boundaries. This suggests that PPS representation, built from bodily and multisensory signals, plastically adapts to the presence of conspecifics to define the self-other boundaries, so that what is usually coded as “my space” is recoded as “your space”. When the space is shared with conspecifics, it seems adaptive to move the other-space away from the self-space to discriminate whether external events pertain to the self-body or to other-bodies.
Subject areas: Behavioral neuroscience, Sensory neuroscience, Cognitive neuroscience
Graphical abstract

Highlights
-
•
We tested whether proximity to others changes peripersonal space (PPS) boundaries
-
•
Behavioral and EEG multisensory responses are collected as a proxy of PPS coding
-
•
Proximity to others shrinks PPS boundaries at behavioral and physiological level
-
•
In presence of conspecifics “my space” is recoded as “your space”
Behavioral neuroscience; Sensory neuroscience; Cognitive neuroscience.
Introduction
Influential views in neuroscience describe the concept of peripersonal space (PPS) as an “invisible bubble” surrounding the body,1 whose boundaries are coded through the integration of multisensory stimuli arising from the body and from the space around it. This portion of space has the adaptive function of discriminating external stimuli occurring close to our body from those occurring far from it, thus orienting goal-directed actions2 and supporting the body protection.3,4,5 In non-human primates, there is extensive evidence that multimodal PPS neurons are able to map the body boundaries, as their firing rate decreases whereas the distance between the stimulated body district and the concurrent (visual or auditory) stimuli increases.3,6 Analogously, in humans, visual or auditory stimuli occurring at a limited distance from the body speed up the behavioral responses to tactile stimuli7,8,9 and boost the neural activity (e.g.,10,11,12,13). In both human and non-human primates, the PPS representation is known to be highly plastic, changing with experience (e.g.,14,15,16,17,18,19). Accordingly, it is well known that PPS can widen if a tool is employed to interact with objects in the far space, as proven by converging evidence in monkeys14 and humans, including several examples in clinical (e.g.,20,21,22) and normal populations (e.g.,7,15,16,23,24,25,26,27). Furthermore, the size of PPS varies depending on contextual factors, such as the emotional valence of the external stimuli, so that a threatening stimulus expands it whereas a safe one reduces it.18,28,29,30,31 By contrast, how the PPS size varies depending on a social factor, such as the presence of another individual, is controversial. Some studies suggest that viewing another’s body fosters multisensory responses,32,33,34 thus enlarging the space for integration to create a “shared mapping of own and others’ bodies”.35 Conversely, other studies propose that the presence of someone else reduces multisensory responses,36,37,38,39 thus inducing a sort of PPS shrinkage.
Here, in a series of five experiments, we focus on the hand-centered PPS representation because the hand plays a crucial role in human social life, being the medium through which we come in contact with others.40 In particular, we ask whether the mere spatial proximity to someone else’s hand alters the size of the hand-centered PPS and, if so, which is the underlying mechanism that modulates the direction of this change (widening versus shrinking). To this aim, we manipulate (1) the occurrence of multisensory stimuli, so that they can appear at either shorter (ultra-near) or larger (near) distance from the participants’ hand (hereinafter “self-hand”), and (2) the presence/absence of someone else’s hand (hereinafter “other-hand”). In each experiment, participants actively respond to (psychophysics) or passively attend (electroencephalography - EEG) tactile stimuli delivered to the self-hand, either in isolation or simultaneously with visual stimuli. A multisensory enhancement occurs when the presence of visual stimuli induces faster reaction times (RTs) to tactile stimuli (e.g.,8,41,42,43,44) and sub- or super-additive responses in event related potentials (ERPs) (e.g.,12,13,45,46,47,48). Such effect is expected to be greater when visual stimuli occur at a shorter (ultra-near) than a larger (near) distance from the self-hand receiving tactile stimuli. This spatial modulation can be considered as a proxy of an effective coding of the hand-centered PPS, thus representing a reliable variable to investigate whether and how the size of PPS varies depending on the spatial proximity to others. For details, see STAR Methods.
In Experiment 1 (preliminary study), starting from electrophysiological evidence in non-human primates suggesting that visual receptive fields of visuo-tactile neurons are anchored to the hand and move with it,49 we initially verify whether our experimental paradigm is able to map the hand-centered PPS regardless the self-hand position in space, by recording both psychophysical and EEG measures. In Experiment 2 (discovery sample), we ask whether the spatial proximity to others alters the hand-centered PPS representation, by comparing the multisensory enhancement on psychophysical measures in two different scenarios, differing from the presence/absence of the other-hand. Significant differences between the two scenarios should support the view that the proximity to others is able to modulate the PPS extent, also showing the directionality of this effect (i.e., widening versus shrinking). In Experiment 3 (position control experiment), we control whether the modulation induced by the other-hand is present irrespective of its position in space. In Experiment 4 (replicating sample), we replicate the design of Experiment 2, also collecting EEG measures. The modulation induced by the other-hand proximity is expected not only when participants actively respond to the tactile stimulus (psychophysics), but also when they passively attend it (EEG), thus revealing the electrophysiological correlate of this effect. Finally, in Experiment 5 (perspective control experiment), we test whether the modulation observed when the other-hand is presented in first-person perspective (as in Experiment 2, 3, 4) persists when the other-hand is presented in third-person-perspective, from which we typically observe other people in social context.
Results
Experiment 1 (preliminary study; N = 16): Comparing internal and external position of the self-hand
This experiment is designed to map the hand-centered PPS irrespective of the self-hand position in space. To this aim we compared two scenarios wherein the self-hand could be either close to the participants’ trunk (i.e., internally aligned to the participants’ shoulder) or farther from it (i.e., externally misaligned to the shoulder) (Figure 1A). Both RTs and ERPs are collected (in separate sessions).
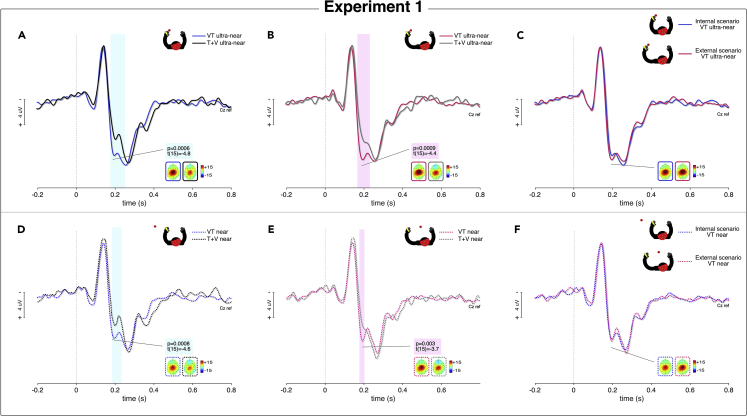
Figure 1.
Experiment 1
(A) The figure depicts the experimental setting and conditions. Tactile and visual stimuli are respectively represented with a flash and a red dot. The self-hand is depicted in black.
(B) The figure represents the RTs (ms) modulation as shown by the main effect of Condition, while (C) represents the Scenario∗Condition interaction. The empty histograms represent the unimodal T condition, the solid ones represent the VT ultra-near condition and the dotted ones the VT near condition. Error bars represent the standard error of the mean (SEM) and the asterisks the significant differences (∗p<0.05; ∗p<0.05; ∗∗p<0.005; ∗∗∗p<0.0005). Dots represent individual values.
Psychophysical results
The 2x3 ANOVA, with ‘Scenario’ (two levels: Internal; External) and ‘Condition’ (three levels: T; VT near; VT ultra-near) as within-subject factors, shows a significant main effect of Condition (F2,28 = 17.4910; p = 0.000012; η2p = 0.5555428) (Figure 1B), while neither a main effect of Scenario (F1,14 = 0.1741; p = 0.682849) nor a Scenario∗Condition interaction (F2,28 = 0.8599; p = 0.434063) is found, suggesting the presence of similar effects in both scenarios (Figure 1C). Post hoc comparisons exploring the main effect of Condition reveal (1) a significant multisensory enhancement (i.e., faster RTs compared to unimodal T condition) in both bimodal VT ultra-near (p = 0.000129) and VT near conditions (p = 0.002493) and (2) a significant difference between the two bimodal conditions, with faster RTs in VT ultra-near than in VT near conditions (p = 0.016617).
Electrophysiological results
In both internal and external scenarios, the analysis reveals significant time-periods of super-additivity in both bimodal conditions (VT ultra-near; VT near), meaning that the amplitude of somatosensory evoked potentials (SEPs) elicited by each bimodal condition is significantly different from the sum of the two respective unimodal conditions (V ultra-near + T; V near + T). Such super-additivity represents the electrophysiological signature of the multisensory enhancement.10,50 This effect is found in long-latency SEPs components, with super-additive effects within a time-window coinciding with the P2 latency. See Figures 2A, 2B, 2D, and 2E). Furthermore, in both internal and external scenarios, the difference between bimodal conditions (VT) and the sum of unimodal conditions (V + T), calculated as an integration index [i.e., VT-(V + T)], is significantly greater in ultra-near as compared to near position, in a time-window corresponding to the P2 latency. See Figure S1 in SI. Importantly, when internal and external scenarios are compared within each position, no significant difference between scenarios is found neither in ultra-near nor in near positions. Figures 2C–2G.
Figure 2.
ERPs results of Experiment 1
The figure represents the ERPs waveforms at Cz electrode (referenced to the nose). The x axis represents time (s) and the y axis represents the voltage amplitude (μV), the vertical dashed line represents the stimulus onset. In the top panels the ultra-near conditions are shown, whereas the near conditions are shown in the bottom panels. The time window wherein the ERPs waveforms are significantly different between conditions are highlighted by color bars, and the t and p values reported within the colored box are collected from Cz. Super-additivity was significant in (A) within 183–241 ms after stimulus onset; in (B) within 175–213 ms after stimulus onset; in (D) within 188–225 ms post stimulus onset; in (E) within 183–199 ms after stimulus onset. No significant differences emerged in (C) and (F). Topographical CSD maps for peaks latency within the significant range are plotted too. The scalp topography of the positive peak and negative peaks is symmetrically distributed over central and parietal regions. See also Figure S1.
Taken together, these preliminary results suggest that, at both psychophysical and electrophysiological level, our experimental paradigm is able to map the hand-centered PPS, with greater multisensory enhancement on both RTs and ERPs in ultranear than in near condition, irrespective of the self-hand position.
Experiment 2 (discovery sample; N = 16): Exploring the other-dependent modulation of PPS
This experiment is designed to investigate whether the spatial proximity to others alters the hand-centered PPS representation. The multisensory enhancement is tested in two scenarios differing because of the presence/absence of the other-hand, so that visual stimuli occurring at a larger distance from the self-hand are presented either close to the other-hand or in an empty portion of the table (Figure 3A). Only RTs are collected.
Figure 3.
Experiment 2
(A) The figure depicts the experimental setting and conditions. Tactile and visual stimuli are respectively represented with a flash and a red dot. The self-hand is depicted in black, whereas the other-hand in green.
(B) The figure represents the RTs (ms) modulation as shown by the main effect of Condition, while (C) represents the Scenario∗Condition interaction. The empty histograms represent the unimodal T condition, the solid ones represent the VT ultra-near condition and the dotted ones the VT near condition. Error bars represent the SEM and the asterisks the significant differences (∗p<0.05; ∗∗p<0.005; ∗∗∗p<0.0005). Dots represent individual values.
Psychophysical results
The 2x3 ANOVA, with ‘Scenario’ (two levels: Without other-hand; With other-hand) and ‘Condition’ (three levels: T; VT near; VT ultra-near) as within-subject factors, shows a significant main effect of Condition (F2,30 = 25.9415; p = 0.000001; η2p = 0.633623) with post hoc comparisons revealing significantly faster RTs in VT ultra-near as compared to both T (p = 0.0001) and VT near conditions (p = 0.0001), but no significant difference between VT near and T condition (p = 0.1376) (Figure 3B). Crucially, a significant Scenario∗Condition interaction (F2,30 = 4.31; p = 0.02; η2p = 0.22) better explain this result (Figure 3C). Post hoc comparisons exploring the interaction reveal, in both scenarios, a significant difference between bimodal conditions, with faster RTs in VT ultra-near than in VT near condition (without other-hand p = 0.00092; with-other-hand p = 0.00013). More importantly, although in both scenarios a significant multisensory enhancement (i.e., faster RTs compared to unimodal T condition) is found in bimodal VT ultra-near condition (without-other-hand p = 0.00013; with other-hand p = 0.00012), a significant multisensory enhancement in bimodal VT near condition is found only in the without other-hand scenario (p = 0.00323), when visual stimuli appear in an empty portion of the table, and not in the with other-hand scenario (p = 0.89115), when visual stimuli appear close to the other-hand. Coherently, a significant difference between the two scenarios is found when comparing the two VT near conditions, with significantly slower RTs in the with other-hand scenario as compared to the without other-hand scenario (p = 0.00482). Importantly, no difference between scenarios is found in both T (p = 0.711534) and VT ultra-near (p = 0.37054) conditions.
These results suggest that the presence of someone else’s hand induces a shrinkage of the hand-centered PPS representation (i.e., other-dependent effect), abolishing the multisensory enhancement when visual stimuli occurring at a larger distance from the self-hand are delivered close to the other-hand instead of in an empty portion of the table.
Experiment 3 (position control experiment; N = 16): Comparing internal and external position of the self-hand and the other-hand
Once we demonstrated that the presence of the other-hand affects the PPS boundaries, in Experiment 3 we test whether the reciprocal position of the self-hand and the other-hand can impact on the other-dependent effect (Figure 4A). Only RTs are collected.
Figure 4.
Experiment 3
(A) The figure depicts the experimental setting and conditions. Tactile and visual stimuli are respectively represented with a flash and a red dot. The self-hand is depicted in black, whereas the other-hand in green.
(B) The figure represents the RTs (ms) modulation as shown by the main effect of Condition, while (C) represents the Scenario∗Condition interaction. The empty histograms represent the unimodal T condition, the solid ones represent the VT ultra-near condition and the dotted ones the VT near condition. Error bars represent the SEM and the asterisks the significant differences (∗p<0.05; ∗∗p<0.005; ∗∗∗p<0.0005). Dots represent individual values.
Psychophysical results
The 2x3 ANOVA, with ‘Scenario’ (two levels: Internal; External) and ‘Condition’ (three levels: T; VT near; VT ultra-near) as within-subject factors, shows a significant main effect of Condition (F2,30 = 33.12392; p = 0.00001; η2p = 0.688305) (Figure 4B), whereas neither a main effect of Scenario (F1,15 = 0.75850; p = 0.397524) nor a Scenario∗Condition interaction (F2,30 = 1.26387; p = 0.297175) is found, suggesting a presence of similar effects in both internal and external scenarios (Figure 4C). Post hoc comparisons exploring the main effect of Condition reveal a significant multisensory enhancement (i.e., faster RTs compared to unimodal T condition) in VT ultra-near (p = 0.000121) but not in VT near conditions (p = 0.256085) and a significant difference between bimodal conditions, with faster RTs in VT ultra-near than in VT near condition (p = 0.000138).
These results suggest that the reciprocal position of the self-hand and the other-hand has no impact on the other-dependent modulation of PPS. In both scenarios, the multisensory enhancement is abolished when visual stimuli are delivered close to the other-hand, irrespective of its location.
Experiment 4 (replicating sample; N = 16): Replicating the other-dependent modulation of PPS and exploring its electrophysiological correlate
This experiment is designed to replicate psychophysical data (RTs) of Experiment 2 (also controlling for the presence of a neutral object instead of the empty portion of the table) and to explore the electrophysiological correlate of the other-dependent modulation of PPS, by collecting ERPs (Figure 5A).
Figure 5.
Experiment 4
(A) The figure depicts the experimental setting and conditions. Tactile and visual stimuli are respectively represented with a flash and a red dot. The self-hand is depicted in black, whereas the other-hand in green. The pink box represents the neutral object.
(B) The figure represents the RTs (ms) modulation as shown by the main effect of Condition, while (C) represents the Scenario∗Condition interaction. The empty histograms represent the unimodal T condition, the solid ones represent the VT ultra-near condition and the dotted ones the VT near condition. Error bars represent the SEM and the asterisks the significant differences (∗p<0.05; ∗∗p<0.005; ∗∗∗p<0.0005). Dots represent individual values.
Psychophysical results
Analyses on RTs fully parallel the results of Experiment 2. The 2x3 ANOVA shows a significant main effect of Condition (F2,30 = 12.40027; p = 0.0001; η2p = 0.452560) with post hoc comparisons revealing significantly faster RTs in VT ultra-near condition as compared to both T (p = 0.000208) and VT near conditions (p = 0.002765), but no significant difference between VT near and T condition (p = 0.119257) (Figure 5B). Crucially, a significant Scenario∗Condition interaction (F2,30 = 4.37564; p = 0.021504; η2p = 0.225832) better explain this result (Figure 5C). Post hoc comparisons exploring the interaction reveal significant differences between bimodal conditions in both scenarios, with faster RTs in VT ultra-near than in VT near conditions (with other-hand scenario: p = 0.000121; with object scenario: p = 0.000336). More importantly, although in both scenarios a significant the multisensory enhancement (i.e., faster RTs compared to unimodal T condition) is found in bimodal VT ultra-near condition (with other-hand scenario: p = 0.000160; with-object scenario: p = 0.000134), a significant multisensory enhancement in bimodal VT near condition is found only in the with object scenario (p = 0.000300), when visual stimuli appear close to a neutral object, and not in the with-other-hand scenario (p = 0.443706), when visual stimuli appear close to the other-hand. Coherently, a significant difference between the two scenarios is found in VT near condition, with significantly slower RTs in the with other-hand scenario as compared to the with object scenario (p = 0.006826). Importantly, no difference between scenarios was found in both T (p = 0.226172) and VT ultra-near (p = 0.703754) conditions.
Electrophysiological results
In both with-object and with-other-hand scenarios, the analysis reveals significant time-periods of super-additivity in each bimodal condition (VT ultra-near; VT near) as compared to the sum of two respective unimodal conditions (V ultra-near + T; V near + T), representing the electrophysiological signature of the multisensory enhancement. This effect is found in long-latency SEPs components, with super-additive effect within a time-window coinciding with and P2 latency. Figures 6A, 6B, and 6D. Note that in near position of the with-other-hand scenario the multisensory enhancement effect is confined to the P2 latency (Figure 6E).
Figure 6.
ERPs results of Experiment 4
The figure represents the ERPs waveforms at Cz electrode (referenced to the nose). The x axis represents time (s) and the y axis represents the voltage amplitude (μV), the vertical dashed line represents the stimulus onset. In the top panels the ultra-near conditions are shown, while the near conditions are shown in the bottom panels. The time window wherein the ERPs waveforms are significantly different between conditions are highlighted by color bars, and the t and p values reported within the colored box are collected from Cz. Super-additivity was significant in (A) within 181–238 ms after stimulus onset; in (B) within 162-262 ms; in (D) within 156-200 ms after stimulus onset; in (E) within 181–209 ms after stimulus onset; in (F) within 163-191 ms after stimulus onset. No significant difference emerged in (C). Topographical CSD maps for peaks latency within the significant range are plotted too. The scalp topography of the positive and negative peaks is symmetrically distributed over central and parietal regions. See also Figure S2.
Furthermore, in both with-object and with-other-hand scenarios, a significantly greater difference between bimodal conditions (VT) and the sum of unimodal conditions (V + T), calculated as an integration index [i.e., VT-(V + T)], is found in ultra-near as compared to near position in a time-window corresponding to the P2 latency. See Figure S2 in SI. More interestingly, when with-object and with-other-hand scenarios are compared within each position, a significant difference between scenarios is found in near positions only, where the presence of the other-hand significantly reduces the multisensory enhancement at the P2 latency (Figure 6F). In ultra-near position, when the two scenarios are compared, no significant differences emerge (Figure 6C).
These results fully replicate the other-dependent modulation of PPS in psychophysical measures (see experiment 2), also controlling for the presence of a neutral object instead of the empty portion of the table. Importantly, here, the EEG correlate of this effect is described, with a reduced P2 when visual stimuli appear close to the other-hand instead of a neutral object.
Experiment 5 (perspective control experiment; N = 16): Exploring the other-dependent modulation of PPS in third-person-perspective
This experiment is designed to investigate whether the other-dependent modulation of PPS persists when the other hand is presented in third-person-perspective, from which we typically observe other people in social context (Figure 7A). Only RTs are collected.
Figure 7.
Experiment 5
(A) The figure depicts the experimental setting and conditions. Tactile and visual stimuli are respectively represented with a flash and a red dot. The self-hand is depicted in black, whereas the other-hand in green.
(B) The figure represents the RTs (ms) modulation as shown by the main effect of Condition, while (C) represents the Scenario∗Condition interaction. The empty histograms represent the unimodal T condition, the solid ones represent the VT ultra-near condition and the dotted ones the VT near condition. Error bars represent the SEM and the asterisks the significant differences (∗p<0.05; ∗∗p<0.005; ∗∗∗p<0.0005). Dots represent individual values.
Psychophysical results
The 2x3 ANOVA, with ‘Scenario’ (two levels: With object; With other-hand) and ‘Condition’ (three levels: T; VT near; VT ultra-near) as within-subject factors, shows a significant main effect of Condition (F2,30 = 21.45; p = 0.000002; η2p = 0.59) with post hoc comparisons revealing significantly faster RTs in VT ultra-near as compared to both T (p = 0.0001) and VT near conditions (p = 0.000689), and between VT near and T condition (p = 0.012587) (Figure 7B). Crucially, a significant Scenario∗Condition interaction (F2,30 = 3.57; p = 0.04; η2p = 0.19) better explains this result (Figure 7C). Post hoc comparisons exploring the interaction reveal, in both scenarios, a significant difference between bimodal conditions, with faster RTs in VT ultra-near than in VT near condition (with object: p = 0.032513; with other-hand: p = 0.000312). More importantly, although in both scenarios a significant multisensory enhancement (i.e., faster RTs compared to unimodal T condition) is found in bimodal VT ultra-near condition (with object: p = 0.000135; with other-hand: p = 0.000217), a significant multisensory enhancement in bimodal VT near condition is found only in the without-other-hand scenario (p = 0.000825), when visual stimuli appear close to the neutral object, and not in the with-other-hand scenario (p = 0.541375), when visual stimuli appear close to the other-hand. Coherently, a significant difference between the two scenarios is found when comparing the two VT near conditions, with significantly slower RTs in the with-other-hand scenario as compared to the without-other-hand scenario (p = 0.002031). Importantly, no difference between scenarios is found in both T (p = 0.712928) and VT ultra-near (p = 0.155524) conditions.
These results fully replicate the other-dependent modulation of PPS in psychophysical measures already observed when the other hand was presented in first-person perspective (see experiment 2, 3, 4), thus suggesting that this effect also pertains to the third-person-perspective that is typical of real-world social interaction with other people.
Discussion
The PPS has been argued to map the body boundary and to distinguish it from the environment and from other bodies, by exploiting multisensory integration phenomena. Here, we asked whether the proximity to others induces plastic changes in the PPS representation. In a series of five experiments, we recorded RTs and ERPs during a visuo-tactile paradigm, able to elicit multisensory integration, and we manipulated the presence/absence of someone else’s hand. Our results confirm the previously described multisensory enhancement within the hand-centered PPS, also providing conclusive evidence about plastic changes in the PPS extent induced by the simple presence of a conspecific. In particular, the proximity to others induces a shrinkage of the PPS boundaries, by reducing the space where multisensory responses occur.
Multisensory enhancement in the hand-centered PPS
The multisensory enhancement observed here confirms previous findings with our novel setting, where the azimuthal instead of radial direction is investigated (see STAR Methods). At the behavioral level, in five experiments (Experiment 1–5), faster RTs are observed in both bimodal ultra-near and bimodal near conditions, as compared to unimodal tactile condition (Figures 1, 3, 4, 5, 7B, and 7C), thus supporting the presence of multisensory facilitation within the hand-centered PPS.51 Importantly, the enhanced responses in bimodal conditions are spatially organized with faster RTs in ultra-near than in near condition. This confirms the view of PPS as a continuous field graded with the proximity to the body, whereby the amplitude of multisensory responses, when engaging the somatosensory system, decreases as the distance between the location of touch and external (visual) stimulation increases.52,53,54 As revealed by our preliminary Experiment 1, this multisensory enhancement, as well as its spatial organization within the PPS, is independent of the self-hand position, either close to the trunk (internal scenario) or farther from it (external scenario). This suggests a genuine coding of the hand-centered PPS with faster responses being graded with the proximity of visual stimuli to the self-hand (and not to the trunk or the head). Our results are in line with electrophysiological studies in monkeys demonstrating that visual receptive field is anchored to the hand and moves with it.49
Electrophysiological findings (Experiment 1 & 4) fully parallel these behavioral results, highlighting the physiological signature of multisensory enhancement. The point-by-point analysis indicates a multisensory non-linearity in both bimodal ultra-near and bimodal near conditions that are significantly different from their respective sum in a large time window encompassing long-latency SEP component,55 with super-additive responses at P212,15,48,50 latency (Figures 2, 6A, 6B, 6D, and 6E). Such a modulation of long-latency SEP component is in agreement with converging evidence from both scalp12,15,48,50,56 and intracranial EEG10,46,57 recordings showing that multisensory responses occur at relatively long latencies, likely reflecting processing from multimodal associative areas. Furthermore, the additional analyses, based on the multisensory integration index [i.e., VT-(V + T);50,58,59], highlight a time-window coinciding with the P2 latency, where the super-additivity is significantly greater in ultra-near than in near position (Figures S1 and S2 in SI), thus supporting the view of a spatial organization of multisensory integration within the PPS.
Multisensory enhancement is modulated by the proximity to the other-hand
In Experiment 2 (discovery sample), we find that the presence of someone else’s hand induces a shrinkage of the hand-centered PPS representation, abolishing multisensory facilitation when visual stimuli are delivered close to the other-hand instead of in an empty portion of the table. Experiment 4 (replicating sample) fully replicates this result, also controlling for the presence of a neutral object instead of the empty portion of the table. Hence, as systematically replicated among experiments, when the other-hand is absent a multisensory facilitation on RTs is observed in both bimodal ultra-near and bimodal near conditions, with faster responses as compared to unimodal tactile condition. Conversely, when the other-hand is present, the multisensory facilitation on RTs is abolished in bimodal near conditions, when visual stimuli occur close to the other-hand (Figures 3C and 5C). Coherently, in both Experiment 2 and 4, when the two scenarios are directly compared, significantly slower RTs are found in bimodal near condition when visual stimuli occur close to the other-hand as compared to when they occur in an empty portion of the table (Experiment 2) or close to a neutral object (Experiment 4). Of interest, as suggested by Experiment 3 (position control experiment), the reciprocal position of the self-hand and the other-hand has no impact on this effect (i.e., irrespective of the self-hand position, either internal or external, multisensory facilitation is abolished when visual stimuli are delivered close to the other-hand). Furthermore, as suggested by Experiment 5 (perspective control experiment), the multisensory enhancement is abolished also when the other-hand is presented in third-person perspective, from which we typically perceive the others’ body parts in social context, thus supporting the idea that the abolishment of multisensory enhancement at the other-hand location can be related to a social recoding of the PPS.
These behavioral findings highlight the presence of a specific mechanism modulating the PPS extent as a function of the proximity to others, by constantly updating the multisensory representation of the body in space (Figure 8). Electrophysiological findings (Experiment 4) fully parallel these behavioral results. When bimodal conditions are directly compared between scenarios, no difference is found in bimodal ultra-near condition (Figure 6C), although in bimodal near condition significantly different responses are found within a time-window corresponding to the P2 latency (Figure 6F). In particular, the multisensory facilitation at the P2 latency is significantly reduced when visual stimuli occur close to the other-hand (with-other-hand scenario) as compared to when they occur close to a neutral object (with-object scenario). Of interest, according to both previous12,50 and present data, the spatial modulation of multisensory integration, with greater multisensory enhancement in ultra-near than in near condition (see above), occurs within the P2 latency. This means that the same multisensory component is enhanced by the proximity to the self-hand and reduced by the proximity to the other-hand, thus suggesting that the neural mechanism for multisensory enhancement is diametrically modulated by the body identity.
Figure 8.
Other-related plastic changes in the PPS representation
The blue bubble represents the hand-centered PPS, whose gradual tonality represents the PPS as a continuous field graded with the proximity to the body when only the self-hand is present, as in (A). The proximity to someone else’s hand, irrespective of the other-hand position in space and regardless the perspective from which it is presented, as in (B, C, and D) induces a shrinkage of the hand-centered PPS boundary so that the portion of space previously coded as the self-hand PPS is recoded as the other-hand PPS (i.e., the orange bubble).
Taken together, these behavioral and electrophysiological data provide evidence for a PPS shrinkage when someone else’ body is close to us, regardless of the perspective from which the body is perceived. The abolishment of the multisensory enhancement suggests that the presence of other’s body boosts the self-other discrimination mechanism by inducing a shrinkage of the PPS boundary. This suggests that, in line with some previous studies,36,37,38 when the space is shared with conspecifics, it seems adaptive to move the other-space away from the self-space to distinguish between external events pertaining to the self-body or to the other-body. Of interest, an enhanced PPS segregation between individuals has been described in post-versus pre-pandemic context as a consequence of social distancing measurements,60 thus supporting the existence of an adaptive discrimination mechanism that plastically modulates the boundary between self and others according to contextual factors.
The presence of the other-hand could have alternatively induced an enlargement of the PPS, to include the other-space in the self-space. In agreement with this (not verified) hypothesis, previous studies suggest the existence of an “interpersonal visual enhancement of touch”.61,62 Viewing the body of another person significantly enhances the spatial resolution of touch on the corresponding body part, so that tactile discrimination is significantly more accurate when viewing both one’s own and another’s body compared to when viewing a neutral object.33,63 However, a crucial difference in our experimental setting can account for different results. In these previous studies, participants could see only one hand (or hand image) at a time, so that, when they saw the other-hand, the self-hand (receiving tactile stimulation) was hidden from view. This induces a dissociation between tactile and visual signals that immediately reminds us of the well-known embodiment mechanism of the rubber hand illusion (RHI; e.g.,42,64), which may have led to an enlargement of the PPS to include the (embodied) other-hand space into the self-hand space. Contrarily, in our design, both hands are visible on the table, and this may have enhanced the relevance of self-other discrimination mechanisms,65 because it is crucial to differentiate whether the external event occurs close to the self-body or to the other-body.
Furthermore, the (not verified) enlargement of the PPS could have been expected also on the base of previous studies proposing that, during an attentional task, the view of either the participant’s hand or a fake hand (presented one at a time) close to the visual target may induce attentional prioritization of the occupied portion of space,66,67,68 thereby increasing the potential relevance of cues and targets appearing within that space. Nevertheless, in our data, the abolishment of multisensory enhancement when visual stimuli occur close to the other-hand makes unlikely that attentional prioritization around the other-hand might explain our results.
Conclusions
In summary, the present behavioral and EEG data fully confirm the existence of a multisensory representation of the PPS as the radius immediately surrounding the body within which stimuli are perceived as physically relevant, thus boosting multisensory integration mechanisms. More interestingly, we show that the proximity to others induces shrinkage of the PPS boundaries, by modulating multisensory integration responses within the PPS. This means that the same portion of space, usually coded as “my space”, is recoded as “your space” (Figure 8). Overall, these findings suggest that the PPS representation, built from bodily and multisensory signals, plastically adapts to the presence of a conspecific, to define the self-other body boundaries.
Limitations of the study
Even though the present study provides compelling evidence for a shrinkage of the PPS boundaries in the presence of others, some limitations can be acknowledged. For instance, our study focused only on the hand-centered PPS and tested only two distances from the hand (ultra-near and near). Future studies should establish whether similar other-dependent plastic changes can be generalized to other body districts and whether looming visual stimuli might allow more precise mapping of the PPS boundaries recoding. Furthermore, the other hand was always of a stranger in our study. Thus, further studies should establish whether different results can be found with a familiar other-hand. Finally, future applications of our protocol in brain-damaged patients with selective impairment of the self-other discrimination mechanism (see e.g.,69,70) could improve our understanding of this clinical condition.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| Arduino UNO | Arduino | RRID:SCR_017284 |
| E-prime 2.0 | E-prime | RRID:SCR_009567 |
| Statistica Software release 7 | StatSoft | RRID:SCR_014213 |
| Matlab | MathWorks | RRID:SCR_001622 |
| Letswave 6 | NOCIONS | RRID:SCR_016414 |
| Other | ||
| Datasets and program codes | This paper | https://data.mendeley.com/datasets/8zvfr55754/draft?a=97ada005-043f-43f4-9007-5790009a79d6 |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Francesca Garbarini (francesca.garbarini@unito.it).
Materials availability
This study did not generate new materials.
Experimental model and subject details
A total of eighty healthy volunteers were recruited for the study. Five independent groups of sixteen participants took part in different experiments. The first group of healthy participants (mean ± SD: years= 25.6 ± 3.2, 8 females) took part in Experiment 1, the second group (mean ± SD: years= 24.4 ± 5.9, 9 females) took part in Experiment 2, the third group (mean ± SD: years= 23.2 ± 2.6, 11 females) took part in Experiment 3, the fourth one (mean ± SD: years= 23.5 ± 1.8, 10 females) took part in Experiment 4, and the fifth one (mean ± SD: years= 27.3 ± 4.0, 11 females) took part in Experiment 5. The sample size of sixteen subjects for each experiment was based on previous studies,7,71 wherein the effect-sizes (Cohen’s d = 0.91 and 0.92 respectively) indicated that 16 participants provided 95% statistical power to detect the effect with alpha = 0.05. All participants were right-handed, as assessed with the Edinburgh Handedness Inventory,72 naïve to the experimental procedure and they gave written informed consent before taking part in the study. None of them had history of neurological, major medical or psychiatric disorders. The experimental procedure was approved by local Ethics Committee of the University of Turin (prot. n. 122571, 11/07/2016). Since it is known that the PPS extent is modulated post-pandemic,60 it is important to note that the present data have been collected before the pandemic, except for the Experiment 5 that has been collected post-pandemic, based on the suggestion of an anonymous reviewer.
Method details
Study design and experimental procedures
Participants were comfortably seated in a silent and dimly lit room, resting their left arm on a table placed in front of them. Before starting the data recording, we measured the sensory threshold to electrical non-painful somatosensory stimuli for each participant in order to set tactile stimulation intensity according to subjective perception (see electrical stimulation details below). Then, subjects underwent a visuo-tactile multisensory paradigm: tactile stimuli were delivered to their left hand dorsum, while visual stimuli (i.e., colored-LEDs; see visual stimulation details below) could appear within the hand-centered PPS, at a shorter (ultra-near) or larger (near) distance from the self-hand receiving tactile stimuli. Usually, the PPS extent in the horizontal plane is tested in depth (i.e., in the radial direction relative to the observer), thus the furthest stimulus position is more distant from both the hand and the trunk (e.g.,15,73). Here, instead, we chose to explore the azimuthal direction (i.e., orthogonal to the radial direction), to be focused on the hand-centered PPS, rather than on the whole-body PPS.
In the ultra-near position, visual stimuli appeared close to participants’ stimulated hand, in front of the index finger. In the near position, visual stimuli appeared 20 cm away from the participants’ index finger (Figure 1A). The distance between ultra-near and near positions was chosen in agreement with previous literature suggesting that the multisensory facilitation is still present up to 24 cm from the hand,51 thus ensuring that both positions were inside the hand-centered PPS. Note that, in those experiments where the other-hand is present (i.e., Experiment 2-5), a co-experimenter (matched with the participant’s gender and always a stranger for the participants) put their left hand on the table in the position required by each experimental design (i.e., either internally aligned or externally misaligned with the participants’ shoulder; see details in Figures 3, 4, 5, and 7 panel A). Note that we focused on the left hand, instead of the right hand, for two reasons. A first, more practical, reason was that during RTs collection we preferred that participants used the contralateral foot with respect to the stimulated hand, and using the right foot is easier for right-handed participants. The second, more theoretical, reason was that a previous study showed a compatibility effect during a multisensory visuo-tactile paradigm only when concomitant visual stimuli occurred simultaneously to tactile ones delivered on the left (and not on the right) hand.56 Importantly, in experiments 2-4, the other-hand was presented in a first-person (egocentric) perspective in order to isolate the identity variable, thus presenting the self-hand and the other-hand in the same perspective. However, in Experiment 5, we presented the other-hand in third-person (allocentric) perspective that is typical of real-world social interaction (Figure 7A).
During the experiments, RTs to tactile stimuli and ERPs were recorded in separate sessions to avoid ERPs contamination due to the movement. RTs and EEG sessions took place within the same day in a counterbalanced order between participants, with half of the subjects starting with the RTs recording and the other half with the EEG recording. Each scenario was recorded in two different days at one week of distance in a counterbalanced order across participants. During the experimental task participants were asked to look at a fixation point, which was placed between ultra-near and near positions (i.e., at a distance of 10 cm from both positions) to ensure that the participants’ attentional focus was equally distant from both portions of space (Figure 1A). During the RTs recording, participants were instructed to respond to tactile stimuli (ignoring the presence of visual stimuli) by pressing a pedal with their right foot as soon as they felt a tactile stimulus on their left hand. ERPs recording details are explained below in EEG recording and EEG pre-processing sessions.
All experiments share the methods and procedures described above, except for specific details described in the ad-hoc paragraphs below.
Experiment 1 (preliminary experiment): Comparing internal and external position of the self-hand
This experiment was specifically designed to investigate whether our experimental paradigm was able to capture a multisensory enhancement in both psychophysical and electrophysiological measures, irrespective of the self-hand position in space, which could be either close to or distant from the trunk. To this aim we compared two scenarios (see Figure 1A). In the ‘Internal’ scenario, the self-hand was aligned with the participants’ shoulder, thus being closer to the trunk, and visual stimuli occurred at a shorter (ultra-near) or at a larger (near) distance from both the self-hand and the trunk (and the head). In the ‘external’ scenario, the participants’ left hand was externally misaligned with the participants’ shoulder thus being more distant from the trunk. Therefore, visual stimuli occurring at the shorter (ultra-near) distance from the stimulated hand resulted at a larger distance from the trunk (and the head), while visual stimuli occurring at the larger (near) distance from the stimulated hand resulted at a shorter distance from the trunk (and the head). In this experiment, RTs and ERPs were recorded. Note that, due to a technical problem during the RTs recording, data from one participant were missing, thus the behavioral analysis was performed on 15 participants, but all participants were included in the EEG analysis.
In bimodal conditions, faster RTs to tactile stimuli and super-additive EEG activity, should confirm the presence of a multisensory enhancement. Comparable effects in both scenarios should suggest that our experimental paradigm is able to map the hand-centered PPS irrespective of the self-hand position, either close to the trunk aligned (internal scenario) or farther from it misaligned (external scenario) with respect to the shoulder.
Experiment 2 (discovery sample): Exploring the other-dependent modulation of PPS
This experiment aimed at investigating whether the hand-centered PPS boundary was modulated by the proximity to someone else’s hand. More specifically, we aimed at verifying whether significant differences were present when visual stimuli in near position occurred either in an empty portion of the table (as in Experiment 1) or close to the other-hand (i.e., in front of the co-experimenter’s index finger). To this aim, participants underwent the very same paradigm described above, in two different scenarios (see Figure 3A). In the ‘without-other-hand’ scenario, as in the ‘external’ scenario of the Experiment 1, the participants’ left hand was misaligned with respect to the shoulder, thus leaving an empty portion of the table between the body midline and the self-hand. In the ‘with-other-hand’ scenario, the co-experimenter (matched with the participants’ gender and always a stranger) was behind the participants without being in physical contact with them and the co-experimenter’s arm was extended from the participants’ armpit. The other-hand (the co-experimenter’s one,) was aligned with the participants’ left shoulder, thus being placed between the participants’ body midline and their (externally misaligned) self-hand. The participants’ right hand was always placed on the table aligned with the participants’ shoulder and was never stimulated. In the ultra-near position of both scenarios, visual stimuli appeared in front of the index finger of the participants’ stimulated hand. Conversely, in the near position, important differences between the two scenarios were present; in the ‘without-other-hand’ scenario, visual stimuli appeared in the empty portion of the table included between the participants’ left hand and their body midline; while in the ‘with-other-hand’ scenario, they appeared close to the other-hand (i.e., in front of the co-experimenter’s index finger). Note that to create continuity with the participants body, the participants’ trunk and arms were covered by a black towel in both scenarios. In this experiment, RTs were recorded.
Significant differences between the two scenarios in the near positions, when visual stimuli occur either in an empty portion of the table or close to the other-hand, should support the view that the proximity to the other-hand is able to modify the hand-centered PPS representation.
Experiment 3 (control experiment): Comparing internal and external position of the self-hand and the other-hand
This experiment was designed to explore whether the reciprocal position of the self-hand and the other-hand can impact on the other-dependent multisensory enhancement observed in Experiment 2. To this aim, we manipulated the position wherein the other-hand was placed, that is in internal or external portion of space with respect to the participants’ stimulated hand. The experimental procedure was identical to that employed in Experiment 1 with two different scenarios (see Figure 4A). In the ‘external’ scenario, the self-hand was externally misaligned to the shoulder while the other-hand was located internally, between the participant’s hand and the trunk. In the ‘internal’ scenario, the co-experimenter is always behind the participants extending the arm laterally, next to the participants’ arm so that the other-hand was placed externally (on the left) whereas the self-hand was aligned with the shoulder, resulting in an internal position between the participant’s trunk. Note that to create continuity with the participants body, the participants’ trunk and arms were covered by a black towel in both scenarios. RTs were recorded.
Comparable effects in both scenarios should confirm the view that the proximity to the other-hand is able to shape the hand-centered PPS representation, irrespective of the other-hand position, either externally or internally located with respect to the self-hand.
Experiment 4 (replicating sample): Replicating the other-dependent modulation of PPS and exploring its electrophysiological correlate
This experiment was designed to replicate the other-dependent multisensory enhancement on RTs observed in Experiment 2 (also controlling for the presence of a neutral object, instead of the empty portion of the table, in the ‘without-other-hand’ scenario) and to explore the electrophysiological counterpart of the other-dependent modulation of PPS by recording ERPs. We opted for a neutral object as control condition based on previous studies that successfully employed a neutral object as control condition in visuo-tactile experiment (see e.g.,61,62,74,75,76,77). The procedure was identical to that employed in Experiment 2, except for the near position wherein visual stimuli occurred close to a neutral object (i.e., a wooden parallelepiped with approximatively the same dimension of the other-hand, i.e., 12 × 18 × 2 cm for females and 17 × 21 × 2 cm for males) instead of in an empty space (see Figure 5A). Note that to create continuity with the participants body, the participants’ trunk and arms were covered by a black towel that covered the discontinuity between the own body and the other hand or the neutral object. In this experiment, both RTs and ERPs were recorded.
Significant differences between the two scenarios in the near position, when visual stimuli occur either close to a neutral object or to the other-hand, should confirm the view that the proximity to the other-hand is able to shape the hand-centered PPS representation, not only when participants actively respond to the tactile stimulus (psychophysics), but also when they passively attend it (electroencephalography).
Experiment 5 (control experiment): Exploring the other-dependent effect in third-person-perspective
This experiment was designed to verify whether the other-dependent modulation of PPS was maintained also when the other-hand was presented in a third-person perspective. To this aim RTs were collected. The procedure was identical to that employed in Experiment 2, except for the perspective from which the other-hand was presented, that is allocentric (see Figure 7A).
Significant differences between the two scenarios in the near position, when visual stimuli occur either close to a neutral object or to the other-hand, should confirm the view that the proximity to the other-hand shapes the hand-centered PPS representation irrespective of the perspective from which the other-hand was presented.
Experimental protocol
Each experimental session comprises 4 blocks, two for each scenario. In each experimental block a total of 60 trials was presented at a jittering inter-stimulus interval (ISI) between 7000 and 9000 ms. Each block consisted of 12 trials of unimodal visual stimuli appearing at a shorter distance from the stimulated hand (V ultra-near); 12 trials of unimodal visual stimuli appearing at a larger distance from the stimulated hand (V near; i.e., either in an empty portion of the table in Experiment 1 and Experiment 2, or close to an object in Experiment 4 and 5, or close to the other-hand in Experiment 2, 3, 4, and 5); 12 trials of unimodal tactile stimuli delivered to the hand dorsum of the participants’ hand (T); 12 trials of bimodal visuo-tactile stimuli with the tactile stimuli delivered to the self-hand and the visual ones appearing at a shorter distance from it (VT ultra-near); 12 trials of bimodal visuo-tactile stimuli with the tactile stimuli delivered to the self-hand and the visual ones appearing at a larger distance from it (VT near). Overall, for each scenario, a total of 24 trials per condition (i.e., T, V near; V ultra-near; VT near; VT ultra-near) was collected. The order of trials presentation was pseudorandomized to avoid that more than two identical trials appeared in sequence. We acknowledge that including only 24 trial per condition may lower the signal-to-ratio of the resulting ERPs. However, to maximize the signal-to-noise ratio, in accordance with previous literature employing a similar number of trials,25,78,79,80,81 we employed a very long inter-trial interval (on average 8000 ms). Importantly, in previous EEG studies exploiting multisensory paradigm12,25 a similar number of trials demonstrated to be enough to highlight a significant modulation of the audio-tactile responses between the near and the far space. Note that, in Experiment 1 and 4, two experimental sessions comprising 4 blocks each were collected (within the same day) in order to separately record psychophysical and electrophysiological data. The order of the two sessions was counterbalanced between participants.
Stimulus presentation, synchronization and EEG and RTs recording were controlled by a combined Arduino (RRID:SCR_017284, https://www.arduino.cc/en/Guide/ArduinoUno) and E-prime 2.0 (RRID:SCR_009567, http://www.pstnet.com/eprime.cfm) script.
Stimuli
Somatosensory stimuli were transcutaneous electrical stimuli consisting in constant current square-wave pulses (DS7A, Digitimer) delivered to the left hand dorsum, using surface bipolar electrodes (1 cm between electrodes). The stimulus duration was 200 μs and the stimulation intensity was adjusted according to the individual sensory threshold level (estimated using the methods of limits,82 as in previous studies83,84,85,86). The mean intensity threshold was in Experiment 1: 1.7 ± 0.5, range 0.7-2.4 mA; in Experiment 2: 1.7 ± 0.4 mA, range 1.2-2.6 mA; in Experiment 3: 1.7 ± 0.4, range 1.1-2.9 mA; in Experiment 4: 1.6 ± 0.5, range 0.8-2.4 mA, and in Experiment 5: 1.8 ± 0.6, range 1.3-3.3 mA. During all experiments the stimulation intensity was set slightly above the perceptual threshold (stimulation intensity = threshold intensity∗2), so that participants always perceived the tactile stimulation, which was never felt as painful and respected the law of inverse effectiveness (i.e., stating that multisensory stimuli are more likely or effectively integrated when the best unisensory response is relatively weak87,88,89,90,91). Finally, to prevent habituation, three electrodes were placed at a constant distance between each other (i.e., about 1 cm) and connected to the electrical stimulator so that the one with the negative polarity was kept always active, while the other two with positive polarity were activated one at a time. In this way, participants might perceive the stimulation arising from two distinct sites of the hand dorsum, thus preventing habituation phenomena.
Visual stimuli were brief flashes (50 ms duration) delivered through two red light emitting diodes (5 mm) mounted in front of the index finger of the participant’s hand, in the empty portion of the table, as in Experiment 1 (Figure 1A), close to the index finger of the other-hand as in Experiment 2 (Figure 3A), Experiment 3 (Figure 4A), Experiment 4 (Figure 5A), Experiment 5 (Figure 7A), or close to a neutral object as in Experiment 4 (Figure 5A) and Experiment 5 (Figure 7A).
EEG recording
EEG was recorded using 32 Ag-AgCl electrodes placed on the scalp according to the International 10–20 system and referenced to the nose. Electrode impedances were kept lower than 5 kΩ. In addition, the electrooculogram (EOG) activity was recorded from two surface electrodes, one placed over the right lower eyelid and the other placed lateral to the outer canthus of the right eye. Signal was amplified and digitized at a sampling rate of 1024 Hz (Handy EEG, System Plus Evolution; Micromed, Treviso, IT).
Quantification and statistical analysis
Psychophysical data
Statistical analyses were performed using Statistica Software release 7 (RRID:SCR_014213, http://www.statsoft.com/Products/STATISTICA/Product-Index). In all experiments, behavioral data are analyzed by means of 2∗3 repeated-measures ANOVAs to explore the effect of the within-subjects factors ‘Scenario’ (two levels: Internal; External, in Experiment 1&3; With-other-hand; Without-other-hand, in Experiment 2; With-other-hand; With-object in Experiment 4) and ‘Condition’ (three levels: T; VT near; VT ultra-near), as well as their possible interaction. Newman-Keuls tests were performed as post-hoc comparisons. For each parametrical analysis, we performed Shapiro Wilk test (p always >0.05) to detect violation of normality. We reported p values and, when a significant effect was found (the level of significance was set at p<0.05), the effect size (η2p) was reported as well.
EEG pre-processing
EEG data were pre-processed and analyzed offline using Letswave 6 (RRID:SCR_016414; https://www.letswave.org), an open-source Matlab (RRID:SCR_001622; http://www.mathworks.com/products/matlab/) toolbox. Continuous EEG data were segmented into epochs using a time window ranging from 500 ms before the stimulus onset (either tactile, visual or visuo-tactile) to 1000 ms after the stimulus onset (total epoch duration: 1500 ms) and band-pass filtered (0.5-30 Hz) using a fast Fourier transform filter. Each epoch was baseline corrected using the interval from −200 ms to 0 ms as reference. Artifacts due to eye blinks or eye movements were removed using a validated method based on an Independent Component Analysis (ICA).92 Blinks were found to be the most frequent cause of rejection. Epochs belonging to the same experimental condition were averaged time-locked to the onset of the stimulus. Thus, five average waveforms (T; V ultra-near; V near; VT ultra-near; VT near) were obtained for each subject, separately for each scenario of each experiment. To assess the topography distribution of the relevant effects, current source density (CSD) maps (Figures 3, 6, S1, and S2) were obtained using spherical spline interpolation.93,94
ERPs statistical analysis
In EEG analyses of Experiment 1 and 4, we focused on super-additive responses in bimodal conditions that represents the electrophysiological signature of multisensory enhancement.10,12,46,50,87,95,96 Thus, to verify the presence/absence of an electrophysiological multisensory enhancement, we compared the bimodal conditions with the sum of the corresponding unimodal conditions [i.e., VT ultra-near vs V ultra-near + T; VT near vs V near + T;12,50,58,97]. This approach allows us to verify the presence/absence of an electrophysiological multisensory enhancement in each experiment where ERPs are recorded (Experiment 1 and 4). Then, we calculated an index of multisensory integration by subtracting the sum of the corresponding unimodal condition from each bimodal condition [i.e., VT ultra-near - (V ultra-near + T); VT near - (V near + T)13,50,58,59,97]. A gradual modulation of the multisensory enhancement within the hand-centered PPS should be proved by a greater index in ultra-near than in near positions. More crucially, to test the other-dependent modulation of the multisensory enhancement, with other-hand and with object scenarios were compared within each position (i.e., VT ultra-near: with object vs with other-hand; VT near: with object vs with other-hand). In all analyses, for each contrast of interest, we performed point-by-point, paired T-tests. Point-by-point analyses represent a statistical approach common in EEG studies,98,99,100 directed to highlight significantly different ERP time windows among experimental conditions, without any a-priori assumption. Point-by-point statistic, indeed, performs one statistical comparison for each time point composing a waveform. This statistic approach allows capturing significant amplitude differences and latency shifts at the same time, since both are displayed as discrepant waveform distributions across time. In order to correct for the resulting multiple comparisons, cluster-based permutation testing approach (1000 random permutations across all 32 channels) was always employed to each point-by-point analysis (cluster threshold was set at the 95th percentile of the cluster magnitude distribution; i.e., p<0.05101). The same statistical approach (point-by-point analyses combined with permutation testing as a correction for multiple comparisons) has been repeatedly employed in recent EEG studies.65,99 Importantly, in this statistical approach, the analysis is performed on each electrode separately. Note that only effects present in at least three adjacent electrodes (i.e., frontal, central, and parietal clusters98,99,100) are reported.
Acknowledgments
The authors are grateful to all of the participants involved in the study and to Werner Gaiotto for his technical support. This work has been funded by the San Paolo Foundation 2016 grant (CSTO165140) to F.G and by the Bial Foundation grant (311/2020) to C.F.
Author contributions
Conceptualization, C.F. and F.G.; Methodology, C.F. and F.G.; Software, C.F., M.G., and V.B.; Formal analysis, C.F. and M.G.; Investigation, C.F., M.G., A.R.S., V.B., and I.R.; Writing – Original Draft, C.F. and M.G.; Writing – Review and Editing, C.F., M.G., A.R.S., V.B., I.R., and F.G.; Visualization, C.F.; Supervision, F.G.; Funding Acquisition, F.G. and C.F.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: January 20, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105879.
Supplemental information
Data and code availability
The behavioral and EEG data have been deposited at Mendeley and are publicly available as of the date of publication. DOIs are listed in the key resources table.
All original code has been deposited at Mendeley and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
References
- 1.Graziano M.S.A., Cooke D.F. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia. 2006;44:2621–2635. doi: 10.1016/j.neuropsychologia.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Rizzolatti G., Scandolara C., Matelli M., Gentilucci M. Afferent properties of periarcuate neurons in macaque monkeys. II. Visual responses. Behav. Brain Res. 1981;2:147–163. doi: 10.1016/0166-4328(81)90053-X. [DOI] [PubMed] [Google Scholar]
- 3.Graziano M.S., Reiss L.A., Gross C.G. A neuronal representation of the location of nearby sounds. Nature. 1999;397:428–430. doi: 10.1038/17115. [DOI] [PubMed] [Google Scholar]
- 4.de Vignemont F., Iannetti G.D. How many peripersonal spaces? Neuropsychologia. 2015;70:327–334. doi: 10.1016/j.neuropsychologia.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Serino A., Annella L., Avenanti A. Motor properties of peripersonal space in humans. PLoS One. 2009;4:e6582. doi: 10.1371/journal.pone.0006582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maravita A., Iriki A. Tools for the body (schema) Trends Cogn. Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Serino A., Bassolino M., Farnè A., Làdavas E. Extended multisensory space in blind cane users. Psychol. Sci. 2007;18:642–648. doi: 10.1111/j.1467-9280.2007.01952.x. [DOI] [PubMed] [Google Scholar]
- 8.Fossataro C., Rossi Sebastiano A., Tieri G., Poles K., Galigani M., Pyasik M., Bruno V., Bertoni T., Garbarini F. Immersive virtual reality reveals that visuo-proprioceptive discrepancy enlarges the hand-centred peripersonal space. Neuropsychologia. 2020;146:107540. doi: 10.1016/j.neuropsychologia.2020.107540. [DOI] [PubMed] [Google Scholar]
- 9.Rossi Sebastiano A., Ronga I., Fossataro C., Galigani M., Poles K., Garbarini F. Multisensory-driven facilitation within the peripersonal space is modulated by the expectations about stimulus location on the body. Sci. Rep. 2022;12:20061. doi: 10.1038/s41598-022-21469-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernasconi F., Noel J.P., Park H.D., Faivre N., Seeck M., Spinelli L., Schaller K., Blanke O., Serino A. Audio-tactile and peripersonal space processing around the trunk in human parietal and temporal cortex: an intracranial EEG study. Cereb. Cortex. 2018;28:3385–3397. doi: 10.1093/cercor/bhy156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noel J.P., Blanke O., Serino A. From multisensory integration in peripersonal space to bodily self-consciousness: from statistical regularities to statistical inference. Ann. N. Y. Acad. Sci. 2018;1426:146–165. doi: 10.1111/nyas.13867. [DOI] [PubMed] [Google Scholar]
- 12.Ronga I., Galigani M., Bruno V., Noel J.P., Gazzin A., Perathoner C., Serino A., Garbarini F. Spatial tuning of electrophysiological responses to multisensory stimuli reveals a primitive coding of the body boundaries in newborns. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2024548118. e2024548118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahoney J.R., Molholm S., Butler J.S., Sehatpour P., Gomez-Ramirez M., Ritter W., Foxe J.J. Keeping in touch with the visual system: spatial alignment and multisensory integration of visual-somatosensory inputs. Front. Psychol. 2015;6:1068. doi: 10.3389/fpsyg.2015.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iriki A., Tanaka M., Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport. 1996;7:2325–2330. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- 15.Forsberg A., O’Dowd A., Gherri E. Tool use modulates early stages of visuo-tactile integration in far space: evidence from event-related potentials. Biol. Psychol. 2019;145:42–54. doi: 10.1016/j.biopsycho.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Galigani M., Castellani N., Donno B., Franza M., Zuber C., Allet L., Garbarini F., Bassolino M. Effect of tool-use observation on metric body representation and peripersonal space. Neuropsychologia. 2020;148:107622. doi: 10.1016/j.neuropsychologia.2020.107622. [DOI] [PubMed] [Google Scholar]
- 17.de Borst A.W., de Gelder B. Threat detection in nearby space mobilizes human ventral premotor cortex, intraparietal sulcus, and amygdala. Brain Sci. 2022;12:391. doi: 10.3390/brainsci12030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vagnoni E., Lourenco S.F., Longo M.R. Threat modulates perception of looming visual stimuli. Curr. Biol. 2012;22:826–R827. doi: 10.1016/J.CUB.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 19.Brendel E., DeLucia P.R., Hecht H., Stacy R.L., Larsen J.T. Threatening pictures induce shortened time-to-contact estimates. Atten. Percept. Psychophys. 2012;74:979–987. doi: 10.3758/S13414-012-0285-0/TABLES/3. [DOI] [PubMed] [Google Scholar]
- 20.Berti A., Frassinetti F. When far becomes near: remapping of space by tool use. J. Cogn. Neurosci. 2000;12:415–420. doi: 10.1162/089892900562237. [DOI] [PubMed] [Google Scholar]
- 21.Garbarini F., Fossataro C., Berti A., Gindri P., Romano D., Pia L., della Gatta F., Maravita A., Neppi-Modona M. When your arm becomes mine: pathological embodiment of alien limbs using tools modulates own body representation. Neuropsychologia. 2015;70:402–413. doi: 10.1016/j.neuropsychologia.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Farnè A., Làdavas E. Dynamic size-change of hand peripersonal space following tool use. Neuroreport. 2000;11:1645–1649. doi: 10.1097/00001756-200006050-00010. [DOI] [PubMed] [Google Scholar]
- 23.Costantini M., Ambrosini E., Sinigaglia C., Gallese V. Tool-use observation makes far objects ready-to-hand. Neuropsychologia. 2011;49:2658–2663. doi: 10.1016/j.neuropsychologia.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Miller L.E., Montroni L., Koun E., Salemme R., Hayward V., Farnè A. Sensing with tools extends somatosensory processing beyond the body. Nature. 2018;561:239–242. doi: 10.1038/s41586-018-0460-0. [DOI] [PubMed] [Google Scholar]
- 25.Ronga I., Galigani M., Bruno V., Castellani N., Rossi Sebastiano A., Valentini E., Fossataro C., Neppi-Modona M., Garbarini F. Seeming confines: electrophysiological evidence of peripersonal space remapping following tool-use in humans. Cortex. 2021;144:133–150. doi: 10.1016/j.cortex.2021.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Thibault S., Py R., Gervasi A.M., Salemme R., Koun E., Lövden M., Boulenger V., Roy A.C., Brozzoli C. Tool use and language share syntactic processes and neural patterns in the basal ganglia. Science. 2021;374:eabe0874. doi: 10.1126/science.abe0874. [DOI] [PubMed] [Google Scholar]
- 27.Fabio C., Salemme R., Koun E., Farnè A., Miller L.E. Alpha oscillations are involved in localizing touch on handheld tools. J. Cogn. Neurosci. 2022;34:675–686. doi: 10.1162/JOCN_A_01820. [DOI] [PubMed] [Google Scholar]
- 28.Ferri F., Tajadura-Jiménez A., Väljamäe A., Vastano R., Costantini M. Emotion-inducing approaching sounds shape the boundaries of multisensory peripersonal space. Neuropsychologia. 2015;70:468–475. doi: 10.1016/J.NEUROPSYCHOLOGIA.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Spaccasassi C., Romano D., Maravita A. Everything is worth when it is close to my body: how spatial proximity and stimulus valence affect visuo-tactile integration. Acta Psychol. 2019;192:42–51. doi: 10.1016/j.actpsy.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 30.de Haan A.M., Smit M., Van der Stigchel S., Dijkerman H.C. Approaching threat modulates visuotactile interactions in peripersonal space. Exp. Brain Res. 2016;234:1875–1884. doi: 10.1007/S00221-016-4571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambo C.F., Iannetti G.D. Better safe than sorry? The safety margin surrounding the body is increased by anxiety. J. Neurosci. 2013;33:14225–14230. doi: 10.1523/JNEUROSCI.0706-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brozzoli C., Gentile G., Bergouignan L., Ehrsson H.H. A shared representation of the space near oneself and others in the human premotor cortex. Curr. Biol. 2013;23:1764–1768. doi: 10.1016/j.cub.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Haggard P. Just seeing you makes me feel better: interpersonal enhancement of touch. Soc. Neurosci. 2006;1:104–110. doi: 10.1080/17470910600976596. [DOI] [PubMed] [Google Scholar]
- 34.Teramoto W. A behavioral approach to shared mapping of peripersonal space between oneself and others. Sci. Rep. 2018;8:5432. doi: 10.1038/s41598-018-23815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishida H., Nakajima K., Inase M., Murata A. Shared mapping of own and others’ bodies in visuotactile bimodal area of monkey parietal cortex. J. Cogn. Neurosci. 2010;22:83–96. doi: 10.1162/jocn.2009.21185. [DOI] [PubMed] [Google Scholar]
- 36.Teneggi C., Canzoneri E., di Pellegrino G., Serino A. Social modulation of peripersonal space boundaries. Curr. Biol. 2013;23:406–411. doi: 10.1016/j.cub.2013.01.043. [DOI] [PubMed] [Google Scholar]
- 37.Noel J.-P., Paredes R., Terrebonne E., Feldman J.I., Woynaroski T., Cascio C.J., Seriès P., Wallace M.T. Inflexible updating of the self-other divide during a social context in autism; psychophysical, electrophysiological, and neural network modeling evidence. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging. 2022;7:756–764. doi: 10.1016/j.bpsc.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heed T., Habets B., Sebanz N., Knoblich G. Others’ actions reduce crossmodal integration in peripersonal space. Curr. Biol. 2010;20:1345–1349. doi: 10.1016/j.cub.2010.05.068. [DOI] [PubMed] [Google Scholar]
- 39.Pellencin E., Paladino M.P., Herbelin B., Serino A. Social perception of others shapes one’s own multisensory peripersonal space. Cortex. 2018;104:163–179. doi: 10.1016/j.cortex.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 40.Brozzoli C., Ehrsson H.H., Farnè A. Multisensory representation of the space near the hand from perception to action and interindividual interactions. Neuroscientist. 2014;20:122–135. doi: 10.1177/1073858413511153. [DOI] [PubMed] [Google Scholar]
- 41.Stone K.D., Kandula M., Keizer A., Dijkerman H.C. Peripersonal space boundaries around the lower limbs. Exp. Brain Res. 2018;236:161–173. doi: 10.1007/s00221-017-5115-0. [DOI] [PubMed] [Google Scholar]
- 42.Bertoni T., Magosso E., Serino A. From statistical regularities in multisensory inputs to peripersonal space representation and body ownership: insights from a neural network model. Eur. J. Neurosci. 2021;53:611–636. doi: 10.1111/ejn.14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noel J.P., Samad M., Doxon A., Clark J., Keller S., Di Luca M. Peri-personal space as a prior in coupling visual and proprioceptive signals. Sci. Rep. 2018;8:15819. doi: 10.1038/s41598-018-33961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanini A., Patané I., Blini E., Salemme R., Koun E., Farnè A., Brozzoli C. Peripersonal and reaching space differ: evidence from their spatial extent and multisensory facilitation pattern. Psychon. Bull. Rev. 2021;28:1894–1905. doi: 10.3758/s13423-021-01942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kayser C., Petkov C.I., Augath M., Logothetis N.K. Functional imaging reveals visual modulation of specific fields in auditory cortex. J. Neurosci. 2007;27:1824–1835. doi: 10.1523/JNEUROSCI.4737-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quinn B.T., Carlson C., Doyle W., Cash S.S., Devinsky O., Spence C., Halgren E., Thesen T. Intracranial cortical responses during visual–tactile integration in humans. J. Neurosci. 2014;34:171–181. doi: 10.1523/JNEUROSCI.0532-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noel J.P., Serino A., Wallace M.T. Increased neural strength and reliability to audiovisual stimuli at the boundary of peripersonal space. J. Cogn. Neurosci. 2019;31:1155–1172. doi: 10.1162/jocn_a_01334. [DOI] [PubMed] [Google Scholar]
- 48.Noel J.-P., Bertoni T., Terrebonne E., Pellencin E., Herbelin B., Cascio C., Blanke O., Magosso E., Wallace M.T., Serino A. Rapid recalibration of peri-personal space: psychophysical, electrophysiological, and neural network modeling evidence. Cereb. Cortex. 2020;30:5088–5106. doi: 10.1093/cercor/bhaa103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graziano M.S., Cooke D.F., Taylor C.S. Coding the location of the arm by sight. Science. 2000;290:1782–1786. doi: 10.1126/science.290.5497.1782. [DOI] [PubMed] [Google Scholar]
- 50.Noel J.P., Chatelle C., Perdikis S., Jöhr J., Lopes Da Silva M., Ryvlin P., De Lucia M., Millán J.D.R., Diserens K., Serino A. Peri-personal space encoding in patients with disorders of consciousness and cognitive-motor dissociation. Neuroimage. Clin. 2019;24:101940. doi: 10.1016/j.nicl.2019.101940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serino A., Noel J.P., Galli G., Canzoneri E., Marmaroli P., Lissek H., Blanke O. Body part-centered and full body-centered peripersonal space representations. Sci. Rep. 2015;5:18603. doi: 10.1038/srep18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noel J.P., Serino A. High action values occur near our body. Trends Cogn. Sci. 2019;23:269–270. doi: 10.1016/j.tics.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Bufacchi R.J., Iannetti G.D. An action field theory of peripersonal space. Trends Cogn. Sci. 2018;22:1076–1090. doi: 10.1016/j.tics.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cléry J., Guipponi O., Odouard S., Wardak C., Ben Hamed S. Cortical networks for encoding near and far space in the non-human primate. Neuroimage. 2018;176:164–178. doi: 10.1016/J.NEUROIMAGE.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 55.Allison T., McCarthy G., Wood C.C., Williamson P.D., Spencer D.D. Human cortical potentials evoked by stimulation of the median nerve. II. Cytoarchitectonic areas generating long-latency activity. J. Neurophysiol. 1989;62:711–722. doi: 10.1152/jn.1989.62.3.711. [DOI] [PubMed] [Google Scholar]
- 56.Longo M.R., Musil J.J., Haggard P. Visuo-tactile integration in personal space. J. Cogn. Neurosci. 2012;24:543–552. doi: 10.1162/jocn. [DOI] [PubMed] [Google Scholar]
- 57.Del Vecchio M., Fossataro C., Zauli F.M., Sartori I., Pigorini A., D’Orio P., Abarrategui B., Russo S., Mikulan E.P., Caruana F., et al. Tonic somatosensory responses and deficits of tactile awareness converge in the parietal operculum. Brain. 2021;144:3779–3787. doi: 10.1093/brain/awab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foxe J.J., Morocz I.A., Murray M.M., Higgins B.A., Javitt D.C., Schroeder C.E. Multisensory auditory-somatosensory interactions in early cortical processing revealed by high-density electrical mapping. Brain Res. Cogn. Brain Res. 2000;10:77–83. doi: 10.1016/S0926-6410(00)00024-0. [DOI] [PubMed] [Google Scholar]
- 59.Giard M.H., Peronnet F. Auditory-visual integration during multimodal object recognition in humans: a behavioral and electrophysiological study. J. Cogn. Neurosci. 1999;11:473–490. doi: 10.1162/089892999563544. [DOI] [PubMed] [Google Scholar]
- 60.Serino S., Trabanelli S., Jandus C., Fellrath J., Grivaz P., Paladino M.P., Serino A. Sharpening of peripersonal space during the COVID-19 pandemic. Curr. Biol. 2021;31:R889–R890. doi: 10.1016/j.cub.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kennett S., Taylor-Clarke M., Haggard P. Noninformative vision improves the spatial resolution of touch in humans. Curr. Biol. 2001;11:1188–1191. doi: 10.1016/S0960-9822(01)00327-X. [DOI] [PubMed] [Google Scholar]
- 62.Taylor-Clarke M., Kennett S., Haggard P. Vision modulates somatosensory cortical processing. Curr. Biol. 2002;12:233–236. doi: 10.1016/S0960-9822(01)00681-9. [DOI] [PubMed] [Google Scholar]
- 63.Beck B., Bertini C., Haggard P., Làdavas E. Dissociable routes for personal and interpersonal visual enhancement of touch. Cortex. 2015;73:289–297. doi: 10.1016/j.cortex.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Brozzoli C., Gentile G., Ehrsson H.H. That’s near my hand! Parietal and premotor coding of hand-centered space contributes to localization and self-attribution of the hand. J. Neurosci. 2012;32:14573–14582. doi: 10.1523/JNEUROSCI.2660-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galigani M., Ronga I., Fossataro C., Bruno V., Castellani N., Rossi Sebastiano A., Forster B., Garbarini F. Like the back of my hand: visual ERPs reveal a specific change detection mechanism for the bodily self. Cortex. 2021;134:239–252. doi: 10.1016/j.cortex.2020.10.014. [DOI] [PubMed] [Google Scholar]
- 66.Limanowski J. Precision control for a flexible body representation. Neurosci. Biobehav. Rev. 2022;134:104401. doi: 10.1016/J.NEUBIOREV.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 67.Reed C.L., Leland D.S., Brekke B., Hartley A.A. Attention’s grasp: early and late hand proximity effects on visual evoked potentials. Front. Psychol. 2013;4:420. doi: 10.3389/fpsyg.2013.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reed C.L., Grubb J.D., Steele C. Hands up: attentional priorization of space near the hand. J. Exp. Psychol. Hum. Percept. Perform. 2006;32:166–177. doi: 10.1037/0096-1523.32.1.166. [DOI] [PubMed] [Google Scholar]
- 69.Errante A., Rossi Sebastiano A., Ziccarelli S., Bruno V., Rozzi S., Pia L., Fogassi L., Garbarini F. Structural connectivity associated with the sense of body ownership: a diffusion tensor imaging and disconnection study in patients with bodily awareness disorder. Brain Commun. 2022;4:fcac032. doi: 10.1093/braincomms/fcac032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pia L., Fossataro C., Burin D., Bruno V., Spinazzola L., Gindri P., Fotopoulou K., Berti A., Garbarini F. The anatomo-clinical picture of the pathological embodiment over someone else’s body part after stroke. Cortex. 2020;130:203–219. doi: 10.1016/j.cortex.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 71.Bassolino M., Serino A., Ubaldi S., Làdavas E. Everyday use of the computer mouse extends peripersonal space representation. Neuropsychologia. 2010;48:803–811. doi: 10.1016/j.neuropsychologia.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 73.Sambo C.F., Forster B. An ERP investigation on visuotactile interactions in peripersonal and extrapersonal space: evidence for the spatial rule. J. Cogn. Neurosci. 2009;21:1550–1559. doi: 10.1162/jocn.2009.21109. [DOI] [PubMed] [Google Scholar]
- 74.Cardini F., Haggard P., Ladavas E. Seeing and feeling for self and other: proprioceptive spatial location determines multisensory enhancement of touch. Cognition. 2013;127:84–92. doi: 10.1016/j.cognition.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 75.Longo M.R., Cardozo S., Haggard P. Visual enhancement of touch and the bodily self. Conscious. Cogn. 2008;17:1181–1191. doi: 10.1016/j.concog.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 76.Serino A., Farnè A., Rinaldesi M.L., Haggard P., Làdavas E. Can vision of the body ameliorate impaired somatosensory function? Neuropsychologia. 2007;45:1101–1107. doi: 10.1016/j.neuropsychologia.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 77.Fiorio M., Haggard P. Viewing the body prepares the brain for touch: effects of TMS over somatosensory cortex. Eur. J. Neurosci. 2005;22:773–777. doi: 10.1111/j.1460-9568.2005.04267.x. [DOI] [PubMed] [Google Scholar]
- 78.Iannetti G.D., Hughes N.P., Lee M.C., Mouraux A. Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J. Neurophysiol. 2008;100:815–828. doi: 10.1152/jn.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ronga I., Valentini E., Mouraux A., Iannetti G.D. Novelty is not enough: laser-evoked potentials are determined by stimulus saliency, not absolute novelty. J. Neurophysiol. 2013;109:692–701. doi: 10.1152/jn.00464.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torta D.M., Liang M., Valentini E., Mouraux A., Iannetti G.D. Dishabituation of laser-evoked EEG responses: dissecting the effect of certain and uncertain changes in stimulus spatial location. Exp. Brain Res. 2012;218:361–372. doi: 10.1007/s00221-012-3019-6. [DOI] [PubMed] [Google Scholar]
- 81.Valentini E., Torta D.M.E., Mouraux A., Iannetti G.D. Dishabituation of laser-evoked EEG responses: dissecting the effect of certain and uncertain changes in stimulus modality. J. Cogn. Neurosci. 2011;23:2822–2837. doi: 10.1162/jocn.2011.21609. [DOI] [PubMed] [Google Scholar]
- 82.Gescheider G.A. Psychophysics: The Fundamentals. Lawrence Erlbaum Associates; 1997. The classical psychofisical methods; pp. 45–72. [Google Scholar]
- 83.Fossataro C., Bucchioni G., D’Agata F., Bruno V., Morese R., Krystkowiak P., Garbarini F. Anxiety-dependent modulation of motor responses to pain expectancy. Soc. Cogn. Affect. Neurosci. 2018;13:321–330. doi: 10.1093/scan/nsx146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Colle L., Hilviu D., Rossi R., Garbarini F., Fossataro C. Self-harming and sense of agency in patients with borderline personality disorder. Front. Psychiatry. 2020;11:449. doi: 10.3389/fpsyt.2020.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fossataro C., Burin D., Ronga I., Galigani M., Rossi Sebastiano A., Pia L., Garbarini F. Agent-dependent modulation of corticospinal excitability during painful transcutaneous electrical stimulation. Neuroimage. 2020;217:116897. doi: 10.1016/j.neuroimage.2020.116897. [DOI] [PubMed] [Google Scholar]
- 86.Dell’Anna A., Rosso M., Bruno V., Garbarini F., Leman M., Berti A. Does musical interaction in a jazz duet modulate peripersonal space? Psychol. Res. 2021;85:2107–2118. doi: 10.1007/s00426-020-01365-6. [DOI] [PubMed] [Google Scholar]
- 87.Senkowski D., Saint-Amour D., Höfle M., Foxe J.J. Multisensory interactions in early evoked brain activity follow the principle of inverse effectiveness. Neuroimage. 2011;56:2200–2208. doi: 10.1016/j.neuroimage.2011.03.075. [DOI] [PubMed] [Google Scholar]
- 88.Stein B.E., Stanford T.R. Multisensory integration: current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 2008;9:255–266. doi: 10.1038/nrn2331. [DOI] [PubMed] [Google Scholar]
- 89.Chandrasekaran C. Computational principles and models of multisensory integration. Curr. Opin. Neurobiol. 2017;43:25–34. doi: 10.1016/J.CONB.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holmes N.P. The law of inverse effectiveness in neurons and behaviour: multisensory integration versus normal variability. Neuropsychologia. 2007;45:3340–3345. doi: 10.1016/j.neuropsychologia.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 91.Meijer G.T., Mertens P.E.C., Pennartz C.M.A., Olcese U., Lansink C.S. The circuit architecture of cortical multisensory processing: distinct functions jointly operating within a common anatomical network. Prog. Neurobiol. 2019;174:1–15. doi: 10.1016/j.pneurobio.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 92.Jung T.-P., Makeig S., Humphries C., Lee T.-W., McKeown M.J., Iragui V., Sejnowski T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. doi: 10.1111/1469-8986.3720163. [DOI] [PubMed] [Google Scholar]
- 93.Perrin F., Pernier J., Bertrand O., Echallier J. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- 94.Kayser J., Tenke C.E. Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: a tutorial review. Int. J. Psychophysiol. 2015;97:189–209. doi: 10.1016/j.ijpsycho.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schürmann M., Kolev V., Menzel K., Yordanova J. Spatial coincidence modulates interaction between visual and somatosensory evoked potentials. Neuroreport. 2002;13:779–783. doi: 10.1097/00001756-200205070-00009. [DOI] [PubMed] [Google Scholar]
- 96.Murray M.M., Molholm S., Michel C.M., Heslenfeld D.J., Ritter W., Javitt D.C., Schroeder C.E., Foxe J.J. Grabbing your ear: rapid auditory-somatosensory multisensory interactions in low-level sensory cortices are not constrained by stimulus alignment. Cereb. Cortex. 2005;15:963–974. doi: 10.1093/cercor/bhh197. [DOI] [PubMed] [Google Scholar]
- 97.Cappe C., Thut G., Romei V., Murray M.M. Auditory-visual multisensory interactions in humans: timing, topography, directionality, and sources. J. Neurosci. 2010;30:12572–12580. doi: 10.1523/JNEUROSCI.1099-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Novembre G., Pawar V.M., Bufacchi R.J., Kilintari M., Srinivasan M., Rothwell J.C., Haggard P., Iannetti G.D. Saliency detection as a reactive process: unexpected sensory events evoke corticomuscular coupling. J. Neurosci. 2018;38:2385–2397. doi: 10.1523/JNEUROSCI.2474-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bruno V., Ronga I., Fossataro C., Galigani M., Sacco K., Garbarini F. Long-term limb immobilization modulates inhibition-related electrophysiological brain activity. Neuroimage. 2020;218:116911. doi: 10.1016/j.neuroimage.2020.116911. [DOI] [PubMed] [Google Scholar]
- 100.Galigani M., Ronga I., Bruno V., Castellani N., Rossi Sebastiano A., Fossataro C., Garbarini F. Face-like configurations modulate electrophysiological mismatch responses. Eur. J. Neurosci. 2021;53:1869–1884. doi: 10.1111/ejn.15088. [DOI] [PubMed] [Google Scholar]
- 101.Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/J.JNEUMETH.2007.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The behavioral and EEG data have been deposited at Mendeley and are publicly available as of the date of publication. DOIs are listed in the key resources table.
All original code has been deposited at Mendeley and is publicly available as of the date of publication. DOIs are listed in the key resources table.
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.