Abstract
Bone and joint infections (BJI) caused by vancomycin-resistant Enterococcus spp. (VRE) are difficult to treat due to limited antibiotic options. Although linezolid can be used for VRE treatment, it is often discontinued due to time-dependent bone marrow suppression. Daptomycin, a lipopeptide antibiotic agent with rapid bactericidal activity, is another available therapeutic option for VRE infections. We report a case of VRE BJI successfully treated with a high dose of daptomycin plus β-lactam agents. An 84-year-old man received linezolid for the treatment of VRE BJI. After 2 weeks of therapy, the patient experienced bleeding events associated with linezolid-induced bone marrow toxicity and linezolid was discontinued. Next, high-dose daptomycin therapy combined with a β-lactam agent was selected to treat the remaining VRE BJI. During daptomycin treatment, microbiological eradication was achieved, and the patient clinically improved without evidence of adverse events. We highlight the need for daptomycin use for the treatment of VRE infections, especially in cases where linezolid is ineffective.
Keywords: Enterococcus spp, Daptomycin, Bone and joint infection
Introduction
Bone and joint infections (BJIs) are difficult to treat due to poor penetration of antibiotics at the infection site, resulting in treatment failure [1,2]. BJIs are also associated with recurrence or poor outcomes, especially in cases caused by multidrug-resistant pathogens [3]. In particular, BJIs caused by vancomycin-resistant Enterococcus spp. (VRE) are more challenging to treat because of limited antibiotic options. Although linezolid is available for VRE treatment, it is often discontinued because it can cause bone marrow suppression in a time-dependent manner in BJIs that require long-term treatment. In addition, the bacteriostatic activity of linezolid has raised concerns for its use in the treatment of severe BJIs. In such cases, daptomycin (DAP) can be considered an effective therapeutic option for BJIs caused by VRE [4].
DAP is a cyclic lipopeptide that exhibits rapid bactericidal activity through the inhibition of DNA, RNA, and protein synthesis. It is effective against most Gram-positive bacteria including Enterococcus spp. [5]. Due to the high DAP minimal inhibitory concentration (MIC) of Enterococcus faecium, in particular vancomycin-resistant isolates, a high dose of DAP is required to treat infections caused by E. faecium according to the clinical and laboratory standards institute (CLSI) guidelines [6]. With the use of high-dose DAP for infections caused by isolates with high DAP MIC, β-lactam combination therapy can be an effective strategy as it reduces the MIC of DAP [7].
Currently, DAP usage in Korea is limited. Here, we report the treatment of a patient with BJIs, caused by VRE, with high-dose DAP plus β-lactam agents.
Case report
An 84-year-old man was transferred to our hospital in February 2021 with hematuria and oral bleeding of 7 days due to pancytopenia. The patient had a history of hypertension, diabetes mellitus, and end stage renal disease and was on hemodialysis. He underwent right hip replacement surgery in July 2020 for a traumatic hip fracture. Subsequently, prosthetic joint infection occurred in December 2020, resulting in implant removal and antibiotic-loaded cement insertion surgery. He received antibiotic therapy with meropenem and linezolid for more than 2 weeks in February 2021 because of isolation of multidrug-resistant bacteria, including VRE, in a hemovac mounted at the surgical site.
Upon his arrival at the hospital, laboratory test results were as follows: white blood cell count, 3,740/mm3; hemoglobin level, 7.3 g/dL; platelet count, 23,000/mm3; and C-reactive protein (CRP) level, 85.9 mg/L. As linezolid-induced thrombocytopenia was suspected and there was no gross evidence of surgical site infection at the initial examination, we discontinued linezolid. However, pneumonia that required mechanical ventilation was observed. Therefore, we selected another antibiotic agent to treat hospital-acquired pneumonia. Platelet counts returned to normal levels after linezolid discontinuation, and the pneumonia improved. However, localized swelling and heating sensation were noted in the right hip (previous surgical site), with gradually increasing CRP levels on hospital day 21.
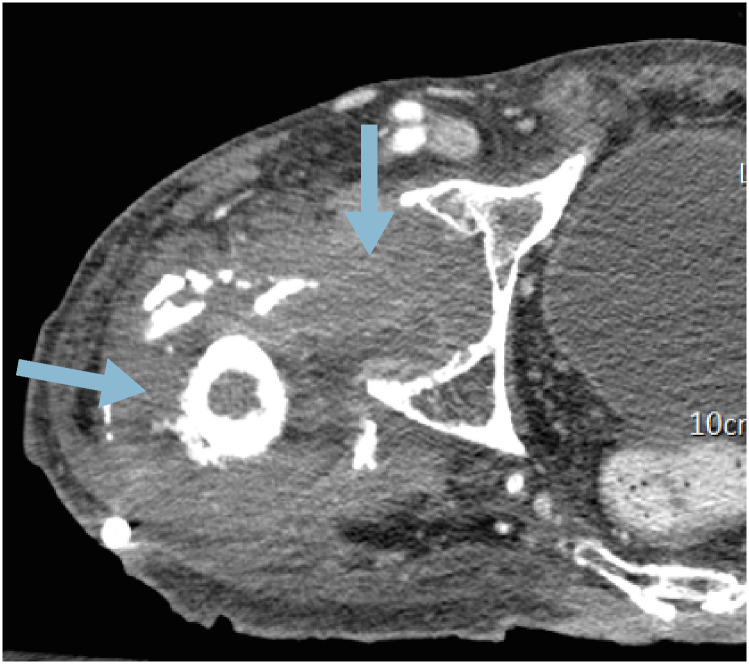
We performed computed tomography (CT) of the hip joint after suspecting a surgical site infection (Fig. 1). A CT scan demonstrated complicated fluid collection around the previous surgical site, consistent with deep-seated surgical site infection. Consequently, foreign body removal and debridement were performed on hospital day 34. Vancomycin-resistant E. faecium was identified on abscess aspiration and tissue culture performed at the time of surgery. According to these results, linezolid was re-administered with close monitoring of bone marrow toxicity. The CRP level decreased slightly after linezolid administration; however, thrombocytopenia developed and aggravated during linezolid use. Since there was no definite evidence of the cause of thrombocytopenia other than linezolid use, we discontinued linezolid. The platelet count increased 5 days after linezolid discontinuation (Supplementary Fig. 1).
Figure 1. Computer tomography scan of abscess formation around the right hip joint indicating deep seated surgical site infection (blue arrows) with foreign body (red arrow) on hospital day 28.
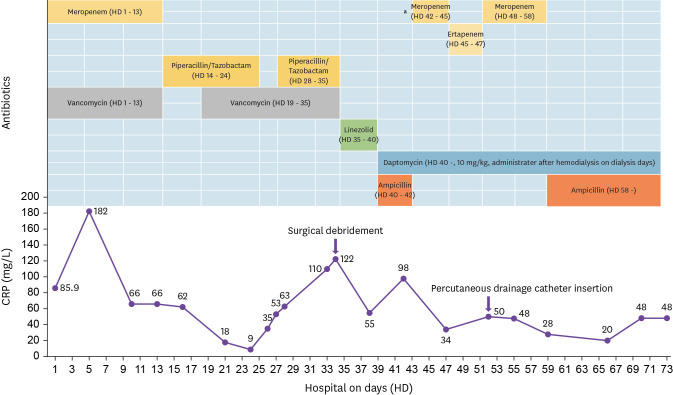
We administered DAP for the treatment of VRE infection. A DAP susceptibility test for VRE was conducted and a DAP MIC of 2.0 µg/ml was obtained. In 2020 CLSI guidelines for E. faecium, a susceptible dose-dependent breakpoint of ≤4 µg/ml was established based on a dosage of 8 - 12 mg/kg/day [6]. Therefore, a high-dose DAP (10 mg/kg) was administered to the patient. In addition, β-lactam agents were used in combination therapy to improve the therapeutic effect. Subsequently, the CRP levels continued to decrease. The specimen obtained through the drainage tube at the surgical site revealed microbiological eradication, and a follow-up CT scan showed an improvement in fluid collection on hospital day 76 (Fig. 2). No further recurrence was noted after a high dose of DAP plus β-lactam combination therapy (Fig. 3).
Figure 2. Computer tomography scan of the hip joint showing reduced abscess and soft tissue swelling (blue arrows) in the right hip joint on hospital day 76.
Figure 3. Clinical course of bone and joint infection caused by vancomycin-resistant Enterococcus faecium according to antibiotic use and intervention.
aCarbapenem was used for the treatment of central line-associated bloodstream infection caused by extended spectrum β-lactamase-producing Klebsiella pneumoniae.
Discussion
In this case, linezolid was discontinued because its long-term use resulted in bone marrow toxicity. We then selected DAP for VRE BJI treatment, considering previous studies showing that DAP is active against VRE, penetrates the bone tissue, and maintains an effective concentration in the synovial fluid [8,9]. In addition, the characteristics of E. faecium with a high DAP MIC and biofilm-forming ability led us to use a high DAP dose (10 mg/kg) combined with β-lactam agents. As a result, microbiological eradication was achieved and the patient improved without evidence of adverse therapy events.
In Korea, linezolid is mostly used to treat VRE infections. However, approximately 6.6% of patients experience bone marrow toxicity during linezolid use, which usually occurs within 10 - 14 days of treatment initiation [10]. This notable adverse effect of linezolid results from its myelosuppression and immune-mediated platelet destruction mechanism [11]. If symptoms or signs associated with bone marrow suppression (e.g., bleeding) occur, linezolid should be replaced with another active drug for VRE treatment. Currently, DAP can be an excellent option for VRE treatment. Several studies have already identified that patients treated with DAP for VRE infection had favorable outcomes in terms of efficacy and safety compared to linezolid treatment [8,12,13]. With regard to pharmacodynamics, DAP has dose-dependent bactericidal activity [14]. Although the FDA-approved DAP dosage is 4 - 6 mg/kg/day, higher DAP dosages are recommended for severe infections. For example, a DAP dose of 6 mg/kg/day is recommended for Staphylococcus aureus bacteremia (including right-sided endocarditis), but 8 - 10 mg/kg/day is recommended only for complicated cases [15]. A higher dose of DAP is also required to treat infections caused by E. faecium. Indeed, E. faecium has a higher DAP MIC than S. aureus or E. faecalis according to the 2020 CLSI guidelines [6]. In addition, E. faecium is often associated with biofilm-producing infections, which reduces the efficacy of antibiotics [16]. Therefore, it is effective to use DAP at 8 - 12 mg/kg based on MIC for E. faecium infection [6]. When high doses are used, clinicians should carefully monitor creatinine phosphokinase (CPK) levels and related symptoms, especially in patients who have impaired renal function or are taking statins as high-dose therapy that sometimes results in myopathy, including rhabdomyolysis [13]. Although data on the use of high-dose DAP in patients receiving hemodialysis are not sufficient, some studies demonstrated that high-dose DAP can be used safely when administered after the dialysis sessions, with close monitoring of CPK level [17,18].
DAP can be used in combination with β-lactams, rifampin, trimethoprim-sulfamethoxazole, fosfomycin, tigecycline, and linezolid. Of these drugs, the large amount of data on DAP combined with β-lactam agents allows β-lactam agents to be selected for combination therapy [5,19]. The possible mechanisms by which β-lactam agents are effective in DAP combination therapy is the see-saw effect, synergism, increased killing by various cationic host defense peptides, and prevention of resistance emergence [5]. One retrospective study of 114 patients who received DAP for VRE bacteremia demonstrated that patients treated with high-dose DAP with β-lactam had improved survival compared to those treated with only high-dose DAP [7]. In another recent study, DAP with β-lactam was associated with a reduced risk of mortality and recurrence in patients with methicillin-resistant S. aureus bacteremia compared with DAP monotherapy [20].
In conclusion, we report a case of VRE BJI that was successfully treated with a high dose of DAP plus β-lactam agents in a situation where it was difficult to use linezolid due to the occurrence of linezolid-related thrombocytopenia.
Footnotes
Funding: None.
Ethics statement: Written informed consent for the publication of this report was obtained from the patient.
Conflict of Interest: All authors report no potential conflicts of interest.
- Conceptualization: Yong Chan Kim.
- Data curation: Yong Chan Kim, Yeon Ju La.
- Methodology: Yong Chan Kim.
- Project administration: Yong Chan Kim, Yeon Ju La.
- Supervision: Yong Chan Kim.
- Writing - original draft: Yong Chan Kim, Yeon Ju La.
- Writing - review & editing: Yong Chan Kim, Yeon Ju La.
SUPPLEMENTARY MATERIAL
Platelet (PLT) count and white blood count (WBC) with differential count trends related to linezolid administration on hospital days.
References
- 1.Tande AJ, Osmon DR, Greenwood-Quaintance KE, Mabry TM, Hanssen AD, Patel R. Clinical characteristics and outcomes of prosthetic joint infection caused by small colony variant staphylococci. mBio. 2014;5:e01910–e01914. doi: 10.1128/mBio.01910-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trouillet-Assant S, Lelièvre L, Martins-Simões P, Gonzaga L, Tasse J, Valour F, Rasigade JP, Vandenesch F, Muniz Guedes RL, Ribeiro de Vasconcelos AT, Caillon J, Lustig S, Ferry T, Jacqueline C, Loss de Morais G, Laurent F. Adaptive processes of Staphylococcus aureus isolates during the progression from acute to chronic bone and joint infections in patients. Cell Microbiol. 2016;18:1405–1414. doi: 10.1111/cmi.12582. [DOI] [PubMed] [Google Scholar]
- 3.Korean Society for Chemotherapy; Korean Society of Infectious Diseases; Korean Orthopaedic Association. Clinical guidelines for the antimicrobial treatment of bone and joint infections in Korea. Infect Chemother. 2014;46:125–138. doi: 10.3947/ic.2014.46.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther. 2018;12:1759–1767. doi: 10.2147/DDDT.S164515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhand A, Sakoulas G. Daptomycin in combination with other antibiotics for the treatment of complicated methicillin-resistant Staphylococcus aureus bacteremia. Clin Ther. 2014;36:1303–1316. doi: 10.1016/j.clinthera.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Satlin MJ, Nicolau DP, Humphries RM, Kuti JL, Campeau SA, Lewis Ii JS, Weinstein MP, Jorgensen JH. Development of daptomycin susceptibility breakpoints for Enterococcus faecium and revision of the breakpoints for other enterococcal species by the Clinical and Laboratory Standards Institute. Clin Infect Dis. 2020;70:1240–1246. doi: 10.1093/cid/ciz845. [DOI] [PubMed] [Google Scholar]
- 7.Chuang YC, Chen PY, Lin CY, Chen YC, Wang JT, Chang SC. A retrospective clinical comparison of daptomycin vs daptomycin and a beta-lactam antibiotic for treating vancomycin-resistant Enterococcus faecium bloodstream infections. Sci Rep. 2018;8:1632. doi: 10.1038/s41598-018-19986-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O’Gara P, Taubert KA American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 9.Montange D, Berthier F, Leclerc G, Serre A, Jeunet L, Berard M, Muret P, Vettoretti L, Leroy J, Hoen B, Chirouze C. Penetration of daptomycin into bone and synovial fluid in joint replacement. Antimicrob Agents Chemother. 2014;58:3991–3996. doi: 10.1128/AAC.02344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerson SL, Kaplan SL, Bruss JB, Le V, Arellano FM, Hafkin B, Kuter DJ. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother. 2002;46:2723–2726. doi: 10.1128/AAC.46.8.2723-2726.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein WB, Trotta RF, Rector JT, Tjaden JA, Barile AJ. Mechanisms for linezolid-induced anemia and thrombocytopenia. Ann Pharmacother. 2003;37:517–520. doi: 10.1345/aph.1C361. [DOI] [PubMed] [Google Scholar]
- 12.Britt NS, Potter EM, Patel N, Steed ME. Comparison of the effectiveness and safety of linezolid and daptomycin in vancomycin-resistant enterococcal bloodstream infection: a national cohort study of veterans affairs patients. Clin Infect Dis. 2015;61:871–878. doi: 10.1093/cid/civ444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi C, Jin W, Xie Y, Zhou D, Xu S, Li Q, Lin N. Efficacy and safety of daptomycin versus linezolid treatment in patients with vancomycin-resistant enterococcal bacteraemia: an updated systematic review and meta-analysis. J Glob Antimicrob Resist. 2020;21:235–245. doi: 10.1016/j.jgar.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Avery LM, Kuti JL, Weisser M, Egli A, Rybak MJ, Zasowski EJ, Arias CA, Contreras GA, Chong PP, Aitken SL, DiPippo AJ, Wang JT, Britt NS, Nicolau DP. Pharmacodynamic analysis of daptomycin-treated enterococcal bacteremia: It is time to change the breakpoint. Clin Infect Dis. 2019;68:1650–1657. doi: 10.1093/cid/ciy749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF Infectious Diseases Society of America. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 16.Beenken KE, Blevins JS, Smeltzer MS. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect Immun. 2003;71:4206–4211. doi: 10.1128/IAI.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butterfield JM, Mueller BA, Patel N, Cardone KE, Grabe DW, Salama NN, Lodise TP. Daptomycin pharmacokinetics and pharmacodynamics in a pooled sample of patients receiving thrice-weekly hemodialysis. Antimicrob Agents Chemother. 2013;57:864–872. doi: 10.1128/AAC.02000-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilay AM, Grio M, Depestel DD, Sowinski KM, Gao L, Heung M, Salama NN, Mueller BA. Daptomycin pharmacokinetics in critically ill patients receiving continuous venovenous hemodialysis. Crit Care Med. 2011;39:19–25. doi: 10.1097/CCM.0b013e3181fa36fb. [DOI] [PubMed] [Google Scholar]
- 19.Jahanbakhsh S, Singh NB, Yim J, Kebriaei R, Smith JR, Lev K, Tran TT, Rose WE, Arias CA, Rybak MJ. Impact of daptomycin dose exposure alone or in combination with β-lactams or rifampin against vancomycin-resistant enterococci in an in vitro biofilm model. Antimicrob Agents Chemother. 2020;64:e02074–e02019. doi: 10.1128/AAC.02074-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorgensen SCJ, Zasowski EJ, Trinh TD, Lagnf AM, Bhatia S, Sabagha N, Abdul-Mutakabbir JC, Alosaimy S, Mynatt RP, Davis SL, Rybak MJ. Daptomycin plus β-lactam combination therapy for methicillin-resistant Staphylococcus aureus bloodstream infections: a retrospective, comparative cohort study. Clin Infect Dis. 2020;71:1–10. doi: 10.1093/cid/ciz746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Platelet (PLT) count and white blood count (WBC) with differential count trends related to linezolid administration on hospital days.