Abstract
Regdanvimab is the only monoclonal antibody available in Korea that targets severe acute respiratory syndrome coronavirus 2. We retrospectively evaluated the clinical characteristics of 374 adults hospitalized with coronavirus disease 2019 (COVID-19) who were treated with regdanvimab from September through December 2021. In total, 322 (86.1%) patients exhibited risk factors for disease progression. Most patients (91.4%) improved without additional treatment. No patient died or was transferred to intensive care. This study shows that regdanvimab prevented disease progression in high-risk patients with mild to moderate COVID-19 infections during Delta variant predominance.
Keywords: COVID-19, Regdanvimab, Delta variant
Monoclonal antibodies that target the spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reduce hospitalization, viral titers, and clinical symptoms in patients with coronavirus disease 2019 (COVID-19) [1]. The effectiveness of different monoclonal antibodies varies dramatically depending on the circulating variant, and the roles played by monoclonal antibodies as treatments for COVID-19 thus remain unclear [2].
Regdanvimab (CT-P59, Celltrion, Incheon, Korea) is the only recombinant monoclonal antibody available to treat COVID-19 in Korea [3,4]. Regdanvimab received conditional approval as an emergency treatment for patients with mild to moderate COVID-19 in February 2021 [5]. Regdanvimab is effective against the original and Beta variant viruses [6–9], but it remains unclear whether it has any therapeutic effect on the Delta variant in real-world situations [10]. Thus, we evaluated the clinical characteristics and outcomes of patients hospitalized with COVID-19 who were treated with regdanvimab during Delta variant predominance in Korea.
This retrospective study was conducted at two university-affiliated teaching hospitals located in Seoul and Goyang, Korea. In September 2021, the respective hospitals designated 30 and 32 general ward beds for the exclusive care of patients with COVID-19; this was mandated by an Executive Order of the Korean health authority. We reviewed the records of all patients with COVID-19 confirmed via real-time reverse transcriptase polymerase chain reaction from September through December 2021. The monthly proportions of the Delta variant in Korea were 96.1 – 100.0% during the study period [11].
Of patients hospitalized with COVID-19 during the study period, only those who received regdanvimab were included. Regdanvimab was given to patients who did not require oxygen therapy, were within 7 days of symptom onset, and who met at least one of the following criteria: age >50 years, pneumonia evident via chest X-ray or chest computed tomography (CT), or an underlying comorbidity. Underlying comorbidities were diabetes mellitus, obesity (body mass index >30 kg/m2), immunosuppression, and cardiovascular, chronic respiratory, chronic renal, or chronic liver disease [12]. Patients received a single intravenous infusion of 40 mg/kg regdanvimab over 60 - 90 min. We excluded patients who required oxygen therapy or other therapeutic agents within 24 h of regdanvimab treatment and in whom the illness progressed rapidly, making it difficult to evaluate the effect of regdanvimab [13]. Patients whose medical records were incomplete or who were transferred to tertiary care centers within 24 h of regdanvimab administration were also excluded.
We collected data on patients’ sex and age; dates of symptom onset, admission and discharge; COVID-19 disease severity at admission [14]; underlying diseases; COVID-19 vaccination status; body mass index; regdanvimab administration date; lowest oxygen saturation level on the day of regdanvimab infusion; oxygen therapy or any other treatment during hospitalization; and lung infiltration evident via chest X-ray or CT. We also collected data on the fever pattern after regdanvimab administration and adverse reactions associated with regdanvimab. Transfers to other hospitals for intensive care, and deaths during hospitalization were noted.
In total, 764 COVID-19 patients were hospitalized at the two facilities during the study period, of whom 426 (55.8%) received regdanvimab. Fifty-one were excluded because of administration of other therapeutics within 24 h of regdanvimab infusion. One patient who was transferred to another hospital for maintenance hemodialysis immediately after regdanvimab treatment was also excluded. Ultimately, 374 patients who received regdanvimab infusions were included (Table 1). The median age was 65 (range: 17 - 92) years and 196 (52.4%) were female. Of the 374 patients, 79.9% had one or more medical comorbidities. The most common underlying diseases were cardiovascular disease (52.9%), diabetes mellitus (25.4%), cancer (10.7%), and chronic lung disease (9.1%). Although 26 (7.0%) patients evidenced asymptomatic infections, 172 (46.0%) and 176 (47.0%) had mild and moderate COVID-19 disease at admission, respectively. The median time from symptom onset to regdanvimab treatment was 4 (interquartile range [IQR]: 2 - 6) days. Although 226 (60.4%) patients were fully vaccinated, 109 (29.1%) were not vaccinated at all. The median oxygen saturation on the day of regdanvimab treatment was 96% (IQR: 95 - 97%) and 185 (49.5%) patients evidenced pneumonia via chest X-ray or CT. Adverse reactions to regdanvimab were noted in 15 (4.0%).
Table 1. Demographic and clinical characteristics of patients hospitalized with COVID-19 who received regdanvimab from September through December 2021.
| Characteristics | Number (n = 374) | |
|---|---|---|
| Age (years) | 65 (53 - 75) | |
| Age ≥60 years | 245 (65.5) | |
| Female | 196 (52.4) | |
| Body mass index ≥30 kg/m2 | 43 (11.5) | |
| Medical comorbidities | 299 (79.9) | |
| Cardiovascular disease or hypertension | 198 (52.9) | |
| Diabetes mellitus | 95 (25.4) | |
| Cancer | 40 (10.7) | |
| Chronic lung disease | 34 (9.1) | |
| Immunocompromised condition | 17 (4.5) | |
| Chronic liver disease | 6 (1.6) | |
| Neurodevelopmental disorder | 6 (1.6) | |
| Chronic kidney disease | 12 (3.2) | |
| Other | 37 (17.9) | |
| Number of risk factors | ||
| 0 | 52 (13.9) | |
| 1 | 66 (17.6) | |
| 2 | 126 (33.7) | |
| 3 | 88 (23.5) | |
| 4 | 35 (9.4) | |
| 5 | 7 (1.9) | |
| Severity of disease [15] | ||
| Asymptomatic | 26 (7.0) | |
| Mild | 172 (46.0) | |
| Moderate | 176 (47.0) | |
| Days from symptom onset to admission | 3 (2 - 5) | |
| Days from symptom onset to regdanvimab infusion | 4 (2 - 6) | |
| Days from admission to regdanvimab infusion | 1 (0 - 1) | |
| Duration of hospitalization (days) | 8 (6 - 9) | |
| Vaccination statusa | ||
| Fully vaccinated | 226 (60.4) | |
| Partially vaccinated | 39 (10.5) | |
| Unvaccinated | 109 (29.1) | |
| SpO2 (%) on the day of regdanvimab administration (median, range) | 96 (95 - 97) | |
| Radiological evidence of pneumonia (chest X-ray or CT) | 185 (49.5) | |
| Chest CT performed | 242 (64.7) | |
| Adverse reaction after regdanvimab infusion | 15 (4.0) | |
| Rash | 5 (1.3) | |
| Itching | 5 (1.3) | |
| Nausea/vomiting | 3 (0.8) | |
| Fever | 2 (0.5) | |
| Diarrhea | 1 (0.3) | |
| Use of additional therapeutic agents | 32 (8.6) | |
| Oxygen support during admission | 15 (4.0) | |
| Dexamethasone and other glucocorticoids | 28 (7.5) | |
| Remdesivir | 27 (7.2) | |
| Time from regdanvimab treatment to administration of additional therapeutic agents (days) | 5 (3 - 7) | |
| Outcome | ||
| Referral to tertiary care center for intensive care | 0 | |
| In-hospital mortality | 0 | |
Data are nos. (%) or medians (interquartile ranges) unless otherwise indicated.
aVaccination status was divided by vaccine administration before the date of symptom onset for symptomatic patients and by the date of diagnosis for asymptomatic patients. Patients were considered fully vaccinated at least 14 days after they received the second dose of ChAdOx1 nCoV-19 (Oxford/AstraZeneca, Cambridge, UK), BNT162b2 (Pfizer/BioNTech, New York, NY, USA and Mainz, Germany), or mRNA-1273 (Moderna, Cambridge, MA, USA); or at least 14 days after receipt of a single dose of Ad26.COV2.S (Janssen, Beerse, Belgium).
SpO2, peripheral oxygen saturation; CT, computed tomography.
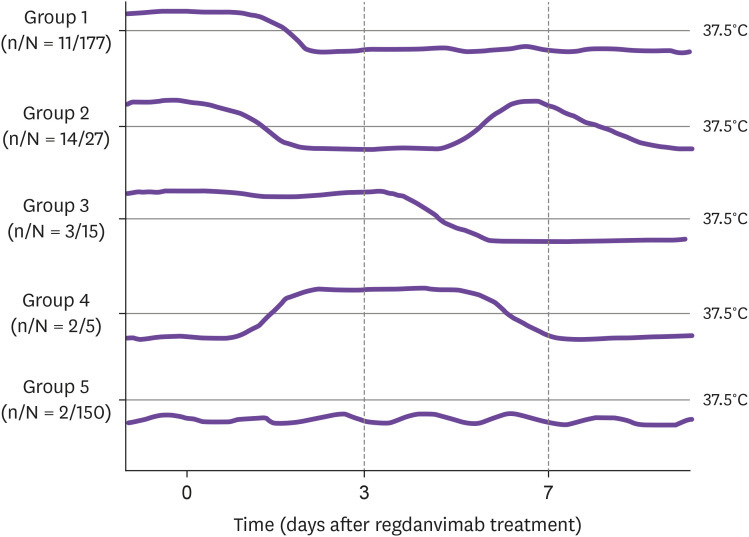
When the clinical course was classified by the fever pattern, fever resolved within 3 days after administration of regdanvimab in 177 patients (Group 1 of Fig. 1) but 11 (6.2%) required additional agents to treat ongoing dyspnea or pneumonia progression. Of the 47 patients whose fever initially resolved but recurred, who improved late, or who developed late fever (Groups 2 - 4), 19 (40.4%) required additional therapeutic agents such as a corticosteroid and/or remdesivir or oxygen. Of the 150 patients who lacked fever during hospitalization (Group 5), 2 (1.3%) received additional therapeutic agents because of dyspnea development or pneumonia progression. Pneumonia was more common in Group 2 - 4 (29/47 [61.7%]) when compared with Group 1 (99/177 [55.9%]) and Group 5 (57/150 [38.0%]) (P = 0.001 by the Chi square test). Overall, of the 32 (8.6%) patients who required additional therapeutic agents, the median time from regdanvimab treatment to the addition of such agents was 5 (IQR: 3 - 7) days. No study patients died or were transferred to another hospital for intensive care. When the 342 patients treated with regdanvimab only were compared to the 32 patients who received additional therapeutic agents, the pneumonia rate differed significantly (158/342 [46.2%] vs. 27/32 [84.4%], P <0.001).
Figure 1. Body temperature patterns of COVID-19 patients treated with regdanvimab.
In Group 1, the fever resolved within 3 days after regdanvimab treatment. In Group 2, the fever resolved within 3 days but relapsed. In Group 3, the fever resolved more than 3 days after regdanvimab treatment. In Group 4, no fever was evident on the day of regdanvimab treatment but fever developed later. In Group 5, no fever was apparent during hospitalization.
n, number of patients who received additional therapeutic agents.
N, total number in each fever pattern group.
Of patients treated with regdanvimab, 91.4% improved without other therapeutics but 8.6% required additional treatments because dyspnea developed or the pneumonia worsened after regdanvimab treatment. Our findings are consistent with those of previous reports; 4.7 - 9.8% of COVID-19 patients treated with regdanvimab progress to severe disease or require additional therapy [6–10]. A retrospective cohort study of regdanvimab treatment for COVID-19 patients showed that systemic inflammatory response syndrome was associated with an increased risk of progression to severe disease [7]. Another study found that older age and abnormalities detected via chest X-ray were associated with a significantly higher risk for a need for supplemental oxygen [6]. The fever patterns reflect the treatment responses of patients with many infectious diseases. Among our COVID-19 patients, the fever patterns varied considerably after regdanvimab treatment. Some patients (Groups 2 - 4) experienced relapse or persistence of fever for more than 3 days after treatment or fever development after regdanvimab treatment. We cannot explain the cause of these phenomena, but about 60% (28/47) of such patients improved without additional treatment as presented numerically in Figure 1. We think that relapse or persistence of fever after treatment with regdanvimab may not always indicate progression of COVID-19. Whether such findings reflect regdanvimab treatment failure or natural patterns of individual COVID-19 infection remains to be elucidated.
Regdanvimab has been reported to show antiviral activity and clinical efficacy in patients with mild to moderate SARS-CoV-2 infection, including high-risk patients [15,16]. However, these studies were performed before development of the Delta variants. In one study, although the antibody exhibited reduced binding affinity to Delta variants in vitro, and lower neutralizing activity, regdanvimab decreased the viral loads of the Delta variant in the upper and lower respiratory tracts and ameliorated weight loss in mice infected with this variant [17]. In other words, treated (real-world) patients may have remained susceptible to the virus. Some real-world reports have appeared, but the study patients were infected with mixtures of virus variants [6,7,8,9,10]. Only a few studies have been conducted during the period when the Delta variant predominated [18]. As we write, regdanvimab is no longer recommended in Korea; the Omicron variant has become dominant. In vitro studies have shown that regdanvimab is likely ineffective against that variant [19].
Our work had certain limitations. First, any retrospective study is susceptible to selection and information bias. Second, we did not genetically analyze the viruses. However, the Delta variant was overwhelmingly dominant in Korea during our study period [11]. Third, viral seropositivity was not checked prior to regdanvimab treatment. About 60.0% of patients were fully vaccinated at that time; the possibility that vaccine-induced antibody formation may have affected the clinical course after regdanvimab treatment cannot be excluded.
In summary, we found that regdanvimab was therapeutic in patients with mild to moderate COVID-19 during Delta variant predominance. The monoclonal antibody prevented progression to severe disease in most high-risk patients with mild to moderate COVID-19.
Footnotes
Funding: None.
Ethics Statement: This study was approved by our institutional review board (IRB no. ISPAIK 2022–04–015); the board waived the need for informed patient consent.
Conflict of Interest: BNK is an associate editor of the Infect Chemother. However, he did not involve in the reviewer selection, evaluation, and decision making of this article. Otherwise, no conflicts of interest were reported.
- Conceptualization: YGK, BNK.
- Data curation: YGK, BNK.
- Formal analysis: YGK, BNK.
- Investigation: YGK, BNK.
- Supervision: YGK, BNK.
- Writing – original draft: YGK, BNK.
- Writing – review & editing: YGK, BNK, JES, JK, JK, HKK, HKK, HKP, SBC, HPL, MJL.
References
- 1.Miguez-Rey E, Choi D, Kim S, Yoon S, Săndulescu O. Monoclonal antibody therapies in the management of SARS-CoV-2 infection. Expert Opin Investig Drugs. 2022;31:41–58. doi: 10.1080/13543784.2022.2030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health (NIH) COVID-19 treatment guidelines. Anti-SARS-CoV-2 monoclonal antibodies. [Accessed 2 May 2022]. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/?utm_source=site&utm_medium=home&utm_campaign=highlights.
- 3.Ryu DK, Song R, Kim M, Kim YI, Kim C, Kim JI, Kwon KS, Tijsma AS, Nuijten PM, van Baalen CA, Hermanus T, Kgagudi P, Moyo-Gwete T, Moore PL, Choi YK, Lee SY. Therapeutic effect of CT-P59 against SARS-CoV-2 South African variant. Biochem Biophys Res Commun. 2021;566:135–140. doi: 10.1016/j.bbrc.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C, Ryu DK, Lee J, Kim YI, Seo JM, Kim YG, Jeong JH, Kim M, Kim JI, Kim P, Bae JS, Shim EY, Lee MS, Kim MS, Noh H, Park GS, Park JS, Son D, An Y, Lee JN, Kwon KS, Lee JY, Lee H, Yang JS, Kim KC, Kim SS, Woo HM, Kim JW, Park MS, Yu KM, Kim SM, Kim EH, Park SJ, Jeong ST, Yu CH, Song Y, Gu SH, Oh H, Koo BS, Hong JJ, Ryu CM, Park WB, Oh MD, Choi YK, Lee SY. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat Commun. 2021;12:288. doi: 10.1038/s41467-020-20602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syed YY. Regdanvimab: First Approval. Drugs. 2021;81:2133–2137. doi: 10.1007/s40265-021-01626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi SJ, Park SW, Lee E. Effectiveness of regdanvimab at preventing the need for oxygen therapy in patients with mild-to-moderate COVID-19: a retrospective cohort study. Infect Chemother. 2022;54:91–101. doi: 10.3947/ic.2021.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JY, Lee JY, Ko JH, Hyun M, Kim HA, Cho S, Lee YD, Song J, Shin S, Peck KR. Effectiveness of regdanvimab treatment in high-risk COVID-19 patients to prevent progression to severe disease. Front Immunol. 2021;12:772320. doi: 10.3389/fimmu.2021.772320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S, Lee SO, Lee JE, Kim KH, Lee SH, Hwang S, Kim SW, Chang HH, Kim Y, Bae S, Kim AS, Kwon KT. Regdanvimab in patients with mild-to-moderate SARS-CoV-2 infection: A propensity score-matched retrospective cohort study. Int Immunopharmacol. 2022;106:108570. doi: 10.1016/j.intimp.2022.108570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S, Je NK, Kim DW, Park M, Heo J. Effectiveness and safety of regdanvimab in patients with mild-to-moderate COVID-19: a retrospective cohort study. J Korean Med Sci. 2022;37:e102. doi: 10.3346/jkms.2022.37.e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong SI, Ryu BH, Hong KW, Bae IG, Cho OH. Real world experience with regdanvimab treatment of mild-to-moderate coronavirus disease-19 in a COVID-19 designated hospital of Korea. Infect Chemother. 2022;54:114–124. doi: 10.3947/ic.2021.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim IH, Park AK, Lee H, Kim HM, Kim J, Kim JA, No JS, Lee CY, Rhee JE, Kim EJ. Status and characteristics of the SARS-CoV-2 variant outbreak in the Republic of Korea in January 2021. [Accessed 2 May 2022]. Available at: https://www.kdca.go.kr/board/board.es?mid=a30501000000&bid=0031&list_no=718804&act=view.
- 12.Ministry of Food and Drug Safety (MFDS) Regdanivmab full Korean approval. 2021. [Accessed 2 May 2022]. Available at: https://www.mfds.go.kr/brd/m_99/view.do?seq=45778.
- 13.Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8:ofab315. doi: 10.1093/ofid/ofab315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health (NIH) COVID-19 treatment guidelines. Clinical spectrum of SARS-CoV-2 infection. [Accessed 2 May 2022]. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum.
- 15.Streinu-Cercel A, Săndulescu O, Preotescu LL, Kim JY, Kim YS, Cheon S, Jang YR, Lee SJ, Kim SH, Chang I, Suh JH, Lee SG, Kim MR, Chung DR, Kim HN, Streinu-Cercel A, Eom JS. Efficacy and safety of regdanvimab (CT-P59): a phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate coronavirus disease 2019. Open Forum Infect Dis. 2022;9:ofac053. doi: 10.1093/ofid/ofac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Jang YR, Hong JH, Jung JG, Park JH, Streinu-Cercel A, Streinu-Cercel A, Săndulescu O, Lee SJ, Kim SH, Jung NH, Lee SG, Park JE, Kim MK, Jeon DB, Lee YJ, Kim BS, Lee YM, Kim YS. Safety, virologic efficacy, and pharmacokinetics of CT-P59, a neutralizing monoclonal antibody against SARS-CoV-2 spike receptor-binding protein: two randomized, placebo-controlled, phase I studies in healthy individuals and patients with mild SARS-CoV-2 infection. Clin Ther. 2021;43:1706–1727. doi: 10.1016/j.clinthera.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu DK, Kang B, Noh H, Woo SJ, Lee MH, Nuijten PM, Kim JI, Seo JM, Kim C, Kim M, Yang E, Lim G, Kim SG, Eo SK, Choi JA, Song M, Oh SS, Chung HY, Tijsma AS, van Baalen CA, Kwon KS, Lee SY. The in vitro and in vivo efficacy of CT-P59 against Gamma, Delta and its associated variants of SARS-CoV-2. Biochem Biophys Res Commun. 2021;578:91–96. doi: 10.1016/j.bbrc.2021.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim T, Joo DH, Lee SW, Lee J, Lee SJ, Kang J. Real-world efficacy of regdanvimab on clinical outcomes in patients with mild to moderate COVID-19. J Clin Med. 2022;11:1412. doi: 10.3390/jcm11051412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, Bolland WH, Porrot F, Staropoli I, Lemoine F, Péré H, Veyer D, Puech J, Rodary J, Baele G, Dellicour S, Raymenants J, Gorissen S, Geenen C, Vanmechelen B, Wawina-Bokalanga T, Martí-Carreras J, Cuypers L, Sève A, Hocqueloux L, Prazuck T, Rey FA, Simon-Loriere E, Bruel T, Mouquet H, André E, Schwartz O. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]