Abstract
Background
Identifying the high recurrence group of patients with early-stage papillary thyroid cancer (PTC) is the greatest challenge in the management of this disease. It has been noted that B-type Rafkinase (BRAF) V600E mutation and programmed death ligand 1 (PD-L1) are associated in PTC and highly expressed in PTC, correlating in PTC as potential prognostic biomarkers. However, whether they can be used to predict the aggressiveness and recurrence of early PTC remains unclear.
Methods
Clinicopathological data of 137 patients with early PTC [tumor-node-metastasis (TNM) stage I–II] who underwent surgery in Zhejiang Cancer Hospital between 2008 and 2010 were retrospectively analyzed. BRAFV600E mutation and PD-L1 was detected by immunohistochemistry. The median follow-up time was 136 months (interquartile range 5.8). The presence of tumor confirmed by imaging or pathology or lymph node metastasis was considered as tumor recurrence. The association of both alone and in combination with clinicopathological features and recurrence was statistically analyzed respectively. The risk of recurrence was assessed using Cox regression models.
Results
Most of the 137 early PTC were female (78.1%). The mean age was 43.2±12.1 years. The median tumor size was 1.4 cm; 14 patients developed recurrence during follow-up period; 56 patients (40.9%) were detected positive for BRAFV600E mutation; 76 patients (55.5%) were detected positive for PD-L1. Patients with both BRAFV600E mutation and PD-L1 expression had larger tumors (P=0.038), were more likely to have extrathyroidal invasion (P=0.045), and had a lower rate of cervical lymph node metastasis (P=0.046). The recurrence rate was 17.5% (7/40) in patients with BRAFV600E mutation and PD-L1 double expression compared to 8.9% (4/45) in patients with BRAFV600E mutation and PD-L1 double negative [hazard ratio (HR) =1.267; 95% CI: 0.841–1.909; P=0.257]. Survival curves showed flatter recurrence-free survival (RFS) curves in positive BRAFV600E mutation only and PD-L1 expression only, whereas decreased sharply in positive expression of both BRAFV600E mutation and PD-L1; however, the differences were not significant (P>0.05).
Conclusions
The combination of BRAFV600E mutation and PD-L1 to identify group at higher risk of recurrence in early PTC has insufficient clinical evidence and should be used with caution in the clinical management of PTC.
Keywords: BRAFV600E mutation, programmed death-ligand 1 (PD-L1), early stage, papillary thyroid carcinoma prognosis
Highlight box.
Key findings
• BRAFV600E mutation and PD-L1 are significantly correlated in early PTC. The combined expression of BRAFV600E mutation and PD-L1 suggests a higher risk of invasiveness and recurrence.
What is known and what is new?
• BRAFV600E mutation in PTC is correlated with the PD-L1 protein. When considered separately, these two factors are often associated with higher aggressiveness and poor prognosis of PTC.
• The coexistence of the BRAFV600E mutation and PD-L1 suggests a higher risk of invasiveness and recurrence.
What is the implication, and what should change now?
• Using these two factors can help to identify patients with relatively high risk and prone to recurrence in the early stage of PTC, improve the risk stratification of PTC, and be used to develop individualized treatment plans and optimize follow-up plans. it is recommended to detect BRAFV600E mutation and PD-L1 in early PTC patients after surgery.
Introduction
In the past 30 years, the incidence of thyroid cancer has been steadily increasing, and it is expected to continue to increase in the next decade (1). According to statistics, thyroid cancer accounted for 93.8% of the new cases of endocrine system malignant tumors in 2021 (2). Papillary thyroid carcinoma (PTC) is the most common histological subtype of thyroid cancer. Improvements in diagnostic techniques, an increase in monitoring methods, and the screening of thyroid diseases under large-scale physical examination have led to the increasing incidence of PTC. Most of the new cases are early stage [tumor-node-metastasis (TNM) stage I–II] indolent PTC, with a high survival rate and good prognosis. After surgical treatment and regular postoperative follow-up, the 10-year survival rate is 95% (3,4). However, some patients still show invasive clinical processes, especially recurrence (5). Therefore, early detection of PTC patients with high rates of aggression and recurrence is particularly important. Early identification of patients with high recurrence in early PTC is the current clinical management dilemma of PTC. It has been suggested that new molecular-based management will help address this challenge.
With the in-depth study of the pathogenesis of PTC, the significance of molecular biomarkers in the occurrence and development of PTC has become more apparent. Studies have found that molecular indicators play important roles in assessing and predicting the biological behavior and prognosis of tumor invasion, helping to guide clinical treatment and determine prognosis (6-8). The murine sarcoma filtering viral oncogene homolog B1, known as BRAF, is a serine/threonine kinase that is usually activated by binding to RAS to affect the MAPK signaling pathway and has the ability to inhibit tumor cell proliferation and induce cell apoptosis. BRAF can cause oncogenic mutations in a variety of malignant tumors, especially malignant melanoma and colorectal cancer. The mutation frequency of BRAF is as high as 40–80% in PTC, and BRAF mutations mostly occur in the early tumor stage and are initiators of tumorigenesis (9). The most common BRAF mutation site in PTC is the T1799A point mutation, expressly, the replacement of A by T at position 1799 in exon 15 (T1799A), resulting in the substitution of valine with glutamic acid at position 600 in the encoded product, in other words, the BRAFV600E mutation. This mutation abnormally activates the RAF/MEK/MAPK signaling pathway, leading to the occurrence of PTC and affecting tumor progression (10-13). The BRAFV600E mutation can be used to guide the diagnosis of PTC. Preoperative fine-needle aspiration biopsy (FNAB) with detection of the BRAFV600E mutation facilitates the determination of benign or malignant thyroid nodules that cannot be diagnosed by FNAB alone, effectively improving the accuracy of the clinical diagnosis of thyroid nodules (14). Many studies have found that PTC patients with the BRAFV600E mutation often have high disease invasiveness and poor prognosis, thus making it a valuable prognostic marker of the disease (13,15-19). Given the high prevalence of BRAFV600E mutation, a universal recommendation for aggressive treatment for all positive PTC is unrealistic. More precise risk stratification strategies need to be developed in conjunction with other molecular markers to guide treatment. Meanwhile, some researchers have noted that for nonhigh-risk early (TNM stage I–II) PTC patients, the BRAFV600E mutation maybe cannot predict recurrence and tumor invasiveness after long-term follow-up. It has even been suggested that to avoid unwarranted anxiety, BRAFV600E should not be tested to determine prognosis in such patients (20-22).
Programmed death-ligand 1 (PD-L1) negatively regulates the immune response process, can mediate the immune escape of tumors, promote tumor growth, is often overexpressed in PTC, is associated with increased tumor invasiveness, and is associated with metastasis and poor prognosis (23,24). The association between the BRAFV600E mutation and PD-L1 expression has been reported in a variety of cancers. For example, the simultaneous expression of these two markers in colon cancer has been shown to have prognostic significance (25). Multiple current studies have shown that the BRAFV600E mutation in PTC is correlated with the PD-L1 protein (26,27). Therefore, this study aimed to examine the expression and correlation between the BRAFV600E mutation and PD-L1 in early PTC and to investigate their relationship, clinicopathological characteristics, and recurrence to further improve the risk stratification of early PTC, and more accurately identify high-risk patients who would benefit from aggressive treatment and surveillance. We present the following article in accordance with the REMARK reporting checklist (available at https://gs.amegroups.com/article/view/10.21037/gs-22-701/rc).
Methods
Patients and clinicopathological data
To explore the feasibility of combining BRAFV600E mutation and PD-L1 to predict recurrence of early PTC, this study preliminary retrospectively enrolled a total of 200 patients with early PTC who underwent surgical treatment in the Department of Head and Neck Surgery of Zhejiang Cancer Hospital between December 2008 and March 2010 in succession. The inclusion criteria were as follows: (I) 18–80 years of age; (II) routine postoperative pathological examination suggesting PTC; (III) complete clinical and pathological data; and (IV) no serious organic lesions in important organs. The exclusion criteria were as follows: (I) a history of thyroid surgery or treatment; (II) a history of other malignant tumors; and (III) a history of radiotherapy or chemotherapy or exposure to radioactive materials.
The surgical plan was developed based on the American Thyroid Association (ATA) guidelines for the management of patients with thyroid nodules and differentiated thyroid carcinoma (28). On the basis of each individual’s condition, total thyroidectomy or unilateral thyroid lobe combined with isthmus resection was performed. All patients underwent ipsilateral cervical lymph node dissection (CLND). Referencing guideline recommendations and considering individualized differences, the decision to perform lateral CLND and radioactive iodine treatment was determined based on each patient’s condition. Pathological examination of the resected lymph nodes was performed routinely after surgery. General demographic data were collected, such as age at diagnosis, sex and so on. Pathological data such as tumor size, number of lesions, capsule invasion, extraglandular invasion, lymphatic, vascular, and nerve invasion, cervical lymph node metastasis (CLNM) in the central region, Hashimoto’s thyroiditis (HT), and distant metastasis, were obtained through the routine pathological report after surgery. The recurrence of tumor was obtained through postoperative follow-up. Follow-up intervals were 3 months within 1 year, 6 months within 1 to 2 years, and annual follow-up thereafter. The patients in our hospital were mainly followed up through the outpatient department and electronic medical records, and the patients in other hospitals were mainly followed up by telephone. At each follow-up visit, routine physical examination, eight thyroid tests and neck ultrasound were performed, and if a suspected malignant thyroid mass or lymph nodes in the neck were present, fine needle aspiration pathology was performed. The starting point of follow-up is after the first thyroid cancer surgery, and the end point of follow-up is to detect tumor recurrence. Tumor recurrence refers to the presence of a PTC tumor or lymph node metastasis confirmed by cervical ultrasound, cervical computed tomography (CT), FNAB, or postoperative routine pathological examination. Recurrence-free survival (RFS) refers to the time from the time of recurrence of the tumor after the first surgery. The follow-up time refers to the time from the first thyroidectomy to tumor recurrence, and for no tumor recurrence, the follow-up time refers to the time interval to the end of follow-up. The last follow-up time was November 2020. Disease staging was determined using the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging manual (29). The analysis of molecular markers in this study was performed after surgery and radioactive iodine treatment and did not affect the clinical treatment decisions for patients. None of the enrolled patients received anti-PD-L1 immunotherapy or BRAF inhibitor treatment during the follow-up period. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Committee of Zhejiang Cancer Hospital (No. IRB-2020-64) and informed consent was taken from all the patients.
Immunohistochemistry
Immunohistochemistry (IHC) was used to detect the expression of the BRAFV600E mutation and PD-L1 protein in PTC tissue. All surgical specimens were fixed in 10% neutral formalin, embedded in paraffin, and prepared into 4 µm-thick serial tissue sections. Xylene (Chengdu Kelong Chemical Reagent Factory, Chengdu, China) was used to deparaffinize the sections (3 times, 5 minutes each), followed by dehydration in a gradient ethanol series (100%, 90%, 70%) (Shanghai Lingfeng Chemical Reagent Co., Ltd., Shanghai, China) (2 times, 5 minutes each) and a rinse with water (2 times, 5 minutes each). After soaking in 3% H2O2 solution for 5 minutes, phosphate buffer solution (PBS) composed of potassium chloride (GENERAL-REAGENT, Shanghai Titan Scientific Co., Ltd., Shanghai, China), sodium chloride (Guangdong Guanghua Sci Co., Ltd., Guangdong, China), disodium hydrogen phosphate, sodium orthovanadate dodecahydrate, and sodium dihydrogen phosphate (Aladdin Reagent Co., Ltd., Shanghai, China) was used to rinse the sections 3 times. Trisodium citrate (Shanghai Lingfeng Chemical Reagent Co., Ltd.) and citric acid monohydrate (Shanghai Lingfeng Chemical Reagent Co., Ltd.) were used to prepare antigen retrieval solution (10 mm citric acid with pH 6.0). After immersion in the solution, the sections were placed in a pressure cooker for 90 seconds and then allowed to cool to room temperature for approximately 30 minutes. The sections were blocked with blocking solution. The sections were incubated with either rabbit anti-human BRAFV600E monoclonal antibody EP152Y (1:100; Abcam, Cambridge, MA, USA) or mouse anti-human PD-L1 monoclonal antibody 66248-1-Ig (1:5,000; Proteintech Group, Wuhan, China). The sections were incubated with the primary antibody for 1 hour at room temperature and then washed with PBS 3 times. Sections were incubated for 20 minutes at room temperature after the dropwise addition of reaction enhancement solution and then rinsed 3 times with PBS; 3,3’-diaminobenzidine (DAB) reagent was added dropwise for color development, and color development was terminated by rinsing the sections with tap water. Hematoxylin (Ningbo Tongsheng Biological Technology Co., Ltd., Ningbo, China) was used as a counterstain, and the sections were rinsed with PBS and mounted with neutral gum. As a negative control, PBS was used instead of the primary antibody. The DAB chromogenic reagent kit was purchased from Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd. (Beijing, China).
Assessment of the BRAFV600E mutation and PD-L1 expression
The IHC-stained sections were observed under an optical microscope to assess the BRAFV600E mutation and PD-L1 expression; 2 experienced pathologists performed independent readings and interpretations in the absence of knowledge of the molecular results. When there was a disagreement, a consensus was reached through consultation. At least 5 areas with the strongest staining intensity were randomly selected for each section under high magnification (×200 magnification) as the observation area, and the necrotic area and marginal area of the section were avoided as much as possible during the observation.
BRAFV600E mutation: Mutated BRAFV600E is mainly located in the cytoplasm of tumor cells. The semiquantitative integration method was used to determine the mutation status. The percentage of positive cells and the staining intensity of positive cells in each section were scored as follows: staining intensity: no staining (−), light yellow (+), brown (++), and brown (+++), was counted as 0, 1, 2, and 3 points, respectively; the number of positive cells was scored based on the percentage of staining: 0%, 0 points; ≤25%, 1 point; 26–50%, 2 points; 51–75%, 3 points; >75%, 4 points. The score for the number of positive cells was multiplied by the score for staining intensity; scores <2 points were considered negative for the BRAFV600E mutation, and scores ≥2 were considered positive for the BRAFV600E mutation (+).
PD-L1 expression: PD-L1 protein expression in tumor cells is mainly located in the cell membrane and/or cytoplasm. When the cell membrane and/or cytoplasm was tan or brown, tumor cells were considered positive for PD-L1 expression; expression in the nucleus was considered meaningless. The number of positive cells in each field of view was manually counted, and the number of positive cells/total number of cells was used to calculate the average percentage of positive cells in each section. Judgment was based on the percentage of positive cells in tumor cells: if the percentage of positive cells was <1%, the cells were considered negative for PD-L1 expression (−), and if the percentage of positive cells was ≥1%, the cells were considered positive for PD-L1 expression (+).
After IHC and image analyses, 63 of the 200 PTC patients enrolled in this study were excluded due to IHC failure and the inability to read images. Therefore, a total of 137 PTC patients were included in this study.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software (IBM Corp., Armonk, NY, USA). Normally distributed measurement data are expressed as the mean ± standard deviation (mean ± SD), and the t-test was used for intergroup comparisons; skewed measurement data are expressed as the median (interquartile range) M (IQR), and the Wilcoxon Mann-Whitney test was used for intergroup comparisons. Count data and ranked data are expressed as frequency (percentage) [n (%)]. Intergroup comparisons were made using the χ2 test or Fisher’s exact probability method. The Kaplan-Meier method was used to plot recurrence-free survival curves and perform the log-rank test. Combining BRAFV600E mutation and PD-L1, early PTC cases were divided into 4 groups. Every group was compared with each other and Cox regression models were used to assess the risk of recurrence. When BRAFV600E mutation and PD-L1 were used alone to establish Cox regression model, factors such as the age, sex of the patients, multifocality, capsular invasion, extraglandular invasion, lymphatic vascular and nerve invasion, cervical lymph node metastasis, concomitant HT and 131I therapy were adjusted to confirm the independent influence of molecular markers on recurrence. A two-sided P value <0.05 indicated that a difference was statistically significant. The test level was α =0.05.
Results
Patient characteristics
Referencing the 8th edition of the AJCC TNM staging manual, the 137 patients with early-stage PTC enrolled in this study were all stage I and stage II patients (133 patients with stage I disease and 4 patients with stage II disease). The clinical data of all patients are shown in Tables 1,2. Most patients were female (78.1%), and the male to female ratio was approximately 1:3. Age ranged from 16 to 82 years, with an average age of 43.2±12.1 years. The median tumor size was 14 mm, ranging from 5 to 45 mm. There were 53 patients with papillary thyroid microcarcinoma (PTMC; tumor size ≤10 mm). At the time of the initial surgery, more than half of the patients had central lymph node metastasis (80 patients, 58.4%), and no patients had distant metastasis. As of the last follow-up in November 2020, a total of 14 patients had recurrence. The median follow-up time of this study was 136 months (IQR, 5.8), and the longest follow-up time was 144 months.
Table 1. Demographic characteristics of PTC patients with the BRAFV600E mutation or PD-L1 expression.
| Parameters | Total (n=137) | BRAFV600E mutation | PD-L1 expression | |||||
|---|---|---|---|---|---|---|---|---|
| Positive (n=56) | Negative (n=81) | P value | Positive (n=76) | Negative (n=61) | P value | |||
| Age at diagnosis (years) | 43.2±12.1 | 44.6±12.5 | 42.3±11.7 | 0.259 | 44.3±12.0 | 41.9±12.2 | 0.248 | |
| Sex | ||||||||
| Male | 30 (21.9) | 8 (14.3) | 22 (27.2) | 0.073 | 17 (22.4) | 13 (21.3) | 0.882 | |
| Female | 107 (78.1) | 48 (85.7) | 59 (72.8) | 59 (77.6) | 48 (78.7) | |||
| Tumor size | ||||||||
| ≤1.0 cm | 53 (38.7) | 18 (32.1) | 35 (43.2) | 0.191 | 24 (31.6) | 29 (47.5) | 0.057 | |
| >1.0 cm | 84 (61.3) | 38 (67.9) | 46 (56.8) | 52 (68.4) | 32 (52.5) | |||
| Multifocality | 45 (32.8) | 16 (28.6) | 29 (35.8) | 0.376 | 28 (36.8) | 17 (27.9) | 0.266 | |
| Envelope invasion | 77 (56.2) | 36 (64.3) | 41 (50.6) | 0.113 | 45 (59.2) | 32 (52.5) | 0.429 | |
| Extraglandular invasion | 40 (29.2) | 19 (33.9) | 21 (25.9) | 0.311 | 28 (36.8) | 12 (19.7) | 0.028 | |
| Lymphatic, vascular and nerve invasion | 12 (8.8) | 6 (10.7) | 6 (7.4) | 0.715 | 8 (10.5) | 4 (6.6) | 0.414 | |
| Cervical lymph node metastasis | 80 (58.4) | 30 (53.6) | 50 (61.7) | 0.341 | 38 (50.0) | 42 (68.9) | 0.026 | |
| Concomitant HT | 30 (21.9) | 17 (30.4) | 13 (16.0) | 0.047 | 17 (22.4) | 13 (21.3) | 0.882 | |
| 131I dose (mCi) | 0 [0] | 0 [75] | 0 [0] | 0.667 | 0 [100] | 0 [0] | 0.213 | |
| Follow-up time (months) | 136 [5.8] | 136 [7.5] | 136 [5.0] | 136 [6.9] | 135 [5.1] | |||
Normally distributed measurement data are expressed as the mean ± standard deviation, such as age at diagnosis. Skewed measurement data are expressed as the median [interquartile range], such as 131I dose and follow-up time. Categorical data are expressed as frequency (percentage of all cases) [n (%)], including sex, tumor size, multifocality, envelope invasion, extraglandular invasion, lymphatic, vascular and nerve invasion, cervical lymph node metastasis, concomitant HT. PTC, papillary thyroid carcinoma; PD-L1, programmed death-ligand 1; HT, Hashimoto’s thyroiditis.
Table 2. BRAFV600E mutation or PD-L1 expression in PTMC patients.
| Parameters | Total (n=53) | BRAFV600E mutation | PD-L1 expression | |||||
|---|---|---|---|---|---|---|---|---|
| Positive (n=18) | Negative (n=35) | P value | Positive (n=24) | Negative (n=29) | P value | |||
| Age at diagnosis (years) | 44.8±10.6 | 49.1±11.6 | 42.6±9.4 | 0.031 | 46.2±10.6 | 43.7±10.6 | 0.394 | |
| Sex | ||||||||
| Male | 9 (17.0) | 2 (11.1) | 7 (20.0) | 0.667 | 3 (12.5) | 6 (20.7) | 0.672 | |
| Female | 44 (83.0) | 16 (88.9) | 28 (80.0) | 21 (87.5) | 23 (79.3) | |||
| Multifocality | 20 (37.7) | 5 (27.8) | 15 (42.9) | 0.283 | 10 (41.7) | 10 (34.5) | 0.591 | |
| Envelope invasion | 20 (37.7) | 8 (44.4) | 12 (34.3) | 0.470 | 9 (37.5) | 11 (37.9) | 0.974 | |
| Extraglandular invasion | 9 (17.0) | 4 (22.2) | 5 (14.3) | 0.732 | 5 (20.8) | 4 (13.8) | 0.755 | |
| Lymphatic, vascular and nerve invasion | 1 (1.9) | 0 (0) | 1 (2.9) | 1.000 | 1 (4.2) | 0 (0) | 0.453 | |
| Cervical lymph node metastasis | 26 (49.1) | 10 (55.6) | 16 (45.7) | 0.497 | 9 (37.5) | 17 (58.6) | 0.126 | |
| Concomitant HT | 7 (13.2) | 3 (16.7) | 4 (11.4) | 0.916 | 4 (16.7) | 3 (10.3) | 0.788 | |
| 131I dose (mCi) | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] | |||
| Follow-up time (months) | 135 [5.1] | 134 [5.6] | 135 [4.8] | 133 [5.1] | 135 [5.1] | |||
Normally distributed measurement data are expressed as the mean ± standard deviation, such as age at diagnosis. Skewed measurement data are expressed as the median [interquartile range], such as 131I dose and follow-up time. Categorical data are expressed as frequency (percentage of all cases) [n (%)], including sex, multifocality, envelope invasion, extraglandular invasion, lymphatic, vascular and nerve invasion, cervical lymph node metastasis, concomitant HT. PD-L1, programmed death-ligand 1; PTMC, papillary thyroid microcarcinoma; HT, Hashimoto’s thyroiditis.
The BRAFV600E mutation and PD-L1 expression in early-stage PTC
IHC was used to detect the BRAFV600E mutation and PD-L1 expression in PTC patients. As seen in Table 3 and Figure 1, among the 137 patients with early PTC, 56 were positive for the BRAFV600E mutation, 81 were negative, for a positive rate of 40.9%. A total of 76 patients were positive for PD-L1, and 61 were negative, for a positive rate of 55.5%. The positivity rates for the BRAFV600E mutation and PD-L1 among the 53 PTMC patients were 34.0% (18/53) and 45.3% (24/53), respectively.
Table 3. Relationship between the BRAFV600E mutation and PD-L1 expression in 137 patients with early PTC.
| Expressing Status | PD-L1 expression | OR value | OR, 95% CI | P value | ||
|---|---|---|---|---|---|---|
| + | − | Total | ||||
| BRAFV600E mutation | ||||||
| + | 40 | 16 | 56 | 3.125 | (1.511, 6.464) | 0.002 |
| − | 36 | 45 | 81 | |||
| Total | 76 | 61 | 137 | |||
PD-L1, programmed death-ligand 1; PTC, papillary thyroid carcinoma; OR, odds ratio; CI, confidence interval.
Figure 1.
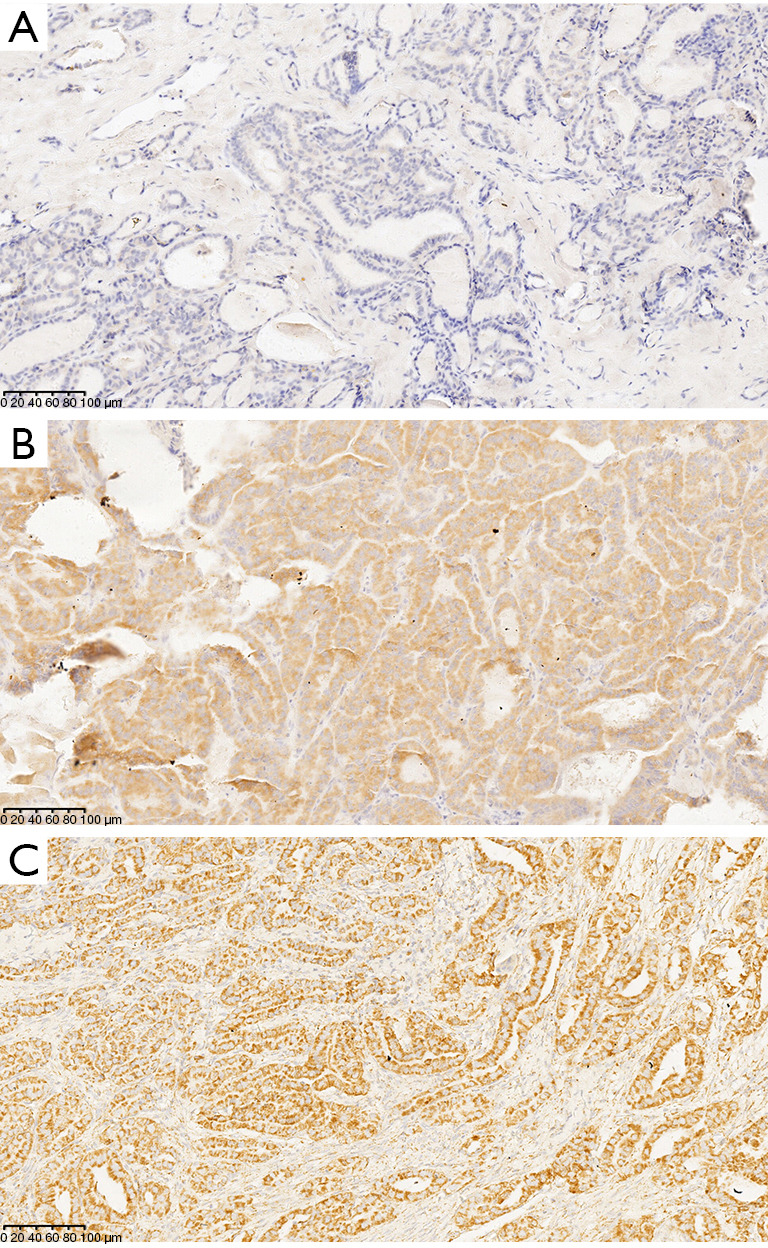
Detection of the BRAFV600E mutation and PD-L1 protein expression in early PTC tumor tissue using IHC. (A) BRAFV600E mutation and PD-L1 expression are negative; (B) BRAFV600E mutation is positive, diffuse cytoplasmic staining can be seen in tumor cells; (C) PD-L1 expression is positive, tumor cell membrane and/or cytoplasm are brown (+++). IHC staining, ×200. PD-L1, programmed death-ligand 1; PTC, papillary thyroid carcinoma; IHC, immunohistochemistry.
We further studied the correlation between BRAFV600E mutation and PD-L1 expression. Among the 56 patients with BRAFV600E mutation-positive PTC, 40 patients (71.4%) were positive for PD-L1 expression, and among the 81 PTC patients negative for the BRAFV600E mutation, only 36 (44.4%) were positive for PD-L1 expression. Similarly, among the 76 PTC patients who were positive for PD-L1 expression, 40 (52.6%) were positive for the BRAFV600E mutation; among the 61 PTC patients who were negative for PD-L1 expression, only 16 (26.2%) were positive for the BRAFV600E mutation (Figure 2). There was a high correlation between the BRAFV600E mutation and PD-L1 expression among PTC patients [odds ratio (OR): 3.125; 95% confidence interval (CI): 1.511–6.464; P=0.002]. Among all 137 PTC patients, 40 (29.2%) had both the BRAFV600E mutation and PD-L1 expression, and 45 were negative for both the BRAFV600E mutation and PD-L1 expression (32.8%), 16 (11.7%) carried the BRAFV600E mutation alone; 36 (26.3%) had PD-L1 alone.
Figure 2.
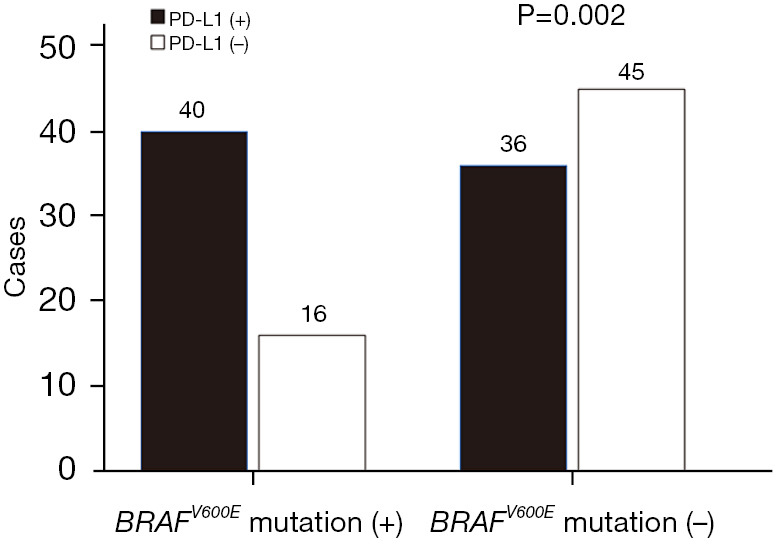
The BRAFV600E mutation and PD-L1 expression in early PTC. PD-L1, programmed death-ligand 1; PTC, papillary thyroid carcinoma.
Association of the BRAFV600E mutation and PD-L1 expression with clinicopathological features of early-stage PTC
The relationships of the BRAFV600E mutation and PD-L1 expression with clinicopathological characteristics of PTC are shown in Tables 1,2. In the early PTC cohort (Table 1), the BRAFV600E mutation was only associated with HT (P=0.047). Compared with BRAFV600E mutation-negative patients, early PTC patients with the BRAFV600E mutation more often had HT. There was no significant correlation between the BRAFV600E mutation and factors such as sex, age at diagnosis, tumor size, multifocal tumor, capsular invasion, extraglandular invasion, lymphatic, vascular, and nerve invasion, and CLNM (P>0.05). PD-L1 expression was significantly correlated with external thyroid invasion (P=0.028) and CLNM (P=0.026). Compared with patients without PD-L1 expression, PTC patients with PD-L1 expression were more susceptible to extrathyroid invasion, and the rate of CLNM was lower. The clinicopathological characteristics of PTC patients, such as age, sex, tumor size, multifocal tumor, capsular invasion, lymphatic, vascular and nerve invasion, and concomitant HT (P>0.05) were not significantly correlated with PD-L1 expression. In the PTMC cohort (Table 2), only age at diagnosis was significantly correlated with the BRAFV600E mutation (P=0.031), and the age of PTMC patients positive for the BRAFV600E mutation was older. There was no significant correlation between other clinicopathological factors and the BRAFV600E mutation and PD-L1 expression.
The association between the coexistence of the BRAFV600E mutation and PD-L1 expression and the clinicopathological factors of PTC was further analyzed (Table 4). When both molecular markers are considered simultaneously, in the early PTC cohort, compared with patients who were negative for the BRAFV600E mutation and PD-L1 expression, patients who were positive for the BRAFV600E mutation or PD-L1 expression alone failed to show significant risk effects. In contrast, patients with both the BRAFV600E mutation and PD-L1 expression had larger tumors (P=0.038), were more prone to extrathyroid invasion (P=0.045), and had a lower CLNM rate (P=0.046). However, the BRAFV600E mutation and PD-L1 expression were not correlated with age at diagnosis, sex, multifocality, capsular invasion, lymphatic, vascular and nerve invasion, or concomitant HT (P>0.05). These results indicated that the BRAFV600E mutation and PD-L1 expression had a partial synergistic effect on tumor invasiveness. Analysis of the PTMC cohort showed that those with only the BRAFV600E mutation were older; there were no significant differences in the coexistence of other clinicopathological characteristics with the BRAFV600E mutation and PD-L1 expression.
Table 4. Effect of the BRAFV600E mutation and PD-L1 expression alone or coexisting on the clinicopathological characteristics of PTC.
| Clinical pathological factors | BRAF-/PD-L1- (group 1) | BRAF+/PD-L1- (group 2) | P value (1 vs. 2) |
BRAF-/PD-L1+ (group 3) | P value (1 vs. 3) |
BRAF+/PD-L1+ (group 4) | P value (1 vs. 4) |
|---|---|---|---|---|---|---|---|
| Early PTC | |||||||
| Number of cases | 45 | 16 | 36 | 40 | |||
| Age at diagnosis (years) | 40.9±11.9 | 44.8±13.0 | 0.277 | 44.0±11.5 | 0.239 | 44.6±12.5 | 0.164 |
| Sex | |||||||
| Male | 12 (26.7) | 1 (6.3) | 0.175 | 10 (27.8) | 0.911 | 7 (17.5) | 0.311 |
| Female | 33 (73.3) | 15 (93.8) | 26 (72.2) | 33 (82.5) | |||
| Tumor size (cm) | |||||||
| ≤1.0 | 21 (46.7) | 8 (50.0) | 0.819 | 14 (38.9) | 0.483 | 10 (25.0) | 0.038 |
| >1.0 | 24 (53.3) | 8 (50.0) | 22 (61.1) | 30 (75.0) | |||
| Multifocality | 13 (28.9) | 4 (25.0) | 1.000 | 16 (44.4) | 0.147 | 12 (30.0) | 0.911 |
| Envelope invasion | 25 (55.6) | 7 (43.8) | 0.417 | 16 (44.4) | 0.320 | 29 (72.5) | 0.105 |
| Extraglandular invasion | 10 (22.2) | 2 (12.5) | 0.635 | 11 (30.6) | 0.395 | 17 (42.5) | 0.045 |
| Lymphatic, vascular and nerve invasion | 4 (8.9) | 0 (0.0) | 0.518 | 2 (5.6) | 0.887 | 6 (15.0) | 0.592 |
| Cervical lymph node metastasis | 32 (71.1) | 10 (62.5) | 0.746 | 18 (50.0) | 0.052 | 20 (50.0) | 0.046 |
| With HT | 7 (15.6) | 6 (37.5) | 0.137 | 6 (16.7) | 0.892 | 11 (27.5) | 0.179 |
| 131I dose (mCi) | 0 [0] | 0 [0] | 0 [0] | 0 [100] | |||
| Follow-up time (months) | 135 [4.9] | 135 [7.0] | 137 [6.0] | 136 [8.1] | |||
| Tumor recurrence | 4 (8.9) | 1 (6.3) | 2 (5.6) | 7 (17.5) | |||
| PTMC | |||||||
| Number of cases | 21 | 8 | 14 | 10 | |||
| Age at diagnosis (years) | 41.1±9.2 | 50.4±11.5 | 0.032 | 44.8±9.5 | 0.260 | 48.1±12.2 | 0.086 |
| Sex | |||||||
| Male | 5 (23.8) | 1 (12.5) | 0.874 | 2 (14.3) | 0.796 | 1 (10.0) | 0.672 |
| Female | 16 (76.2) | 7 (87.5) | 12 (85.7) | 9 (90.0) | |||
| Multifocal | 8 (38.1) | 2 (25.0) | 0.821 | 7 (50.0) | 0.486 | 3 (30.0) | 0.969 |
| Envelope invasion | 9 (42.9) | 2 (25.0) | 0.647 | 3 (21.4) | 0.345 | 3 (30.0) | 0.583 |
| Extraglandular invasion | 3 (14.3) | 1 (12.5) | 1.000 | 2 (14.3) | 1.000 | 6 (60.0) | 0.611 |
| Cervical lymph node metastasis | 12 (57.1) | 5 (62.5) | 1.000 | 4 (28.6) | 0.096 | 5 (50.0) | 1.000 |
| Concomitant HT | 2 (9.5) | 1 (12.5) | 1.000 | 2 (14.3) | 1.000 | 2 (20.0) | 0.810 |
| 131I dose (mCi) | 0 [0] | 0 [0] | 0 [0] | 0 [0] | |||
| Follow-up time (months) | 135 [5.3] | 136 [3.7] | 134 [4.7] | 133 [61.5] |
Normally distributed measurement data are summarized as the mean ± standard deviation, such as age at diagnosis. Skewed measurement data are expressed as the median [interquartile range], such as 131I dose and follow-up time. Categorical data are expressed as frequency (percentage of all cases) [n (%)], including sex, multifocality, envelope invasion, extraglandular invasion, lymphatic, vascular and nerve invasion, cervical lymph node metastasis, concomitant HT. The P values for each group were derived from comparisons with the BRAFV600E mutation-/PD-L1-group (Group 1). PD-L1, programmed death-ligand 1; PTC, papillary thyroid carcinoma; HT, Hashimoto’s thyroiditis; PTMC, papillary thyroid microcarcinoma.
Impact of the BRAFV600E mutation and PD-L1 expression on recurrence
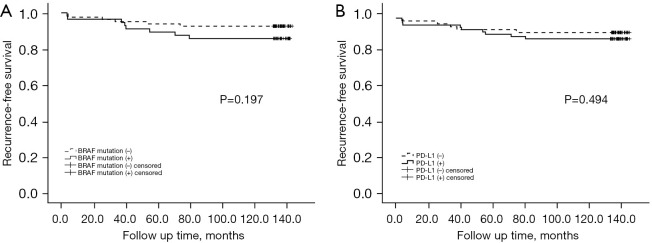
For early-stage PTC, the tumor recurrence rate for patients without the BRAFV600E mutation was 7.4%, and the tumor recurrence rate for patients with the BRAFV600E mutation was 14.3%. However, the correlation between the presence of the BRAFV600E mutation and the risk of tumor recurrence was not significant (log rank P=0.197; Figure 3A). We further performed Cox regression analysis, and the results indicated that after adjusting for age at diagnosis and sex, the correlation between the presence of the BRAFV600E mutation and the risk of tumor recurrence was still not significant; the hazard ratio (HR) was 2.552 (95% CI: 0.847–7.689; P=0.096). After further adjustment for tumor size, multifocality, capsular invasion, extrathyroidal invasion, CLNM, concomitant HT, and radioactive iodine 131 treatment, there was a significant correlation between the BRAFV600E mutation and the risk of recurrence; the HR was 3.915 (95% CI: 1.087–14.106; P=0.037) (Table 5).
Figure 3.
Survival curve for the BRAFV600E mutation and the recurrence of early-stage PTC (log rank P=0.197) (A); survival curve for PD-L1 expression and the recurrence of early-stage PTC (log rank P=0.494) (B). PD-L1, programmed death-ligand 1; PTC, papillary thyroid carcinoma.
Table 5. Relationship of the BRAFV600E mutation and PD-L1 expression with PTC recurrence.
| Grouping | Recurrence rate | Unadjusted | Adjusted *1 | Adjusted *2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Early PTC | 14/137 | 10.2 | |||||||||
| BRAFV600E mutation | |||||||||||
| Negative | 6/81 | 7.4 | 1.979 (0.687–5.705) | 0.206 | 2.552 (0.847–7.689) | 0.096 | 3.915 (1.087–14.106) | 0.037 | |||
| Positive | 8/56 | 14.3 | |||||||||
| PD-L1expression | |||||||||||
| Negative | 5/61 | 8.2 | 1.461 (0.490–4.360) | 0.497 | 1.502 (0.503–4.489) | 0.467 | 1.762 (0.537–5.778) | 0.350 | |||
| Positive | 9/76 | 11.8 | |||||||||
| PTMC | 5/53 | 9.4 | |||||||||
| BRAFV600E mutation | |||||||||||
| Positive | 2/35 | 5.7 | 2.899 (0.484–17.357) | 0.244 | 2.766 (0.406–18.867) | 0.299 | 5.206 (0.647–41.872) | 0.121 | |||
| Negative | 3/18 | 16.7 | |||||||||
| PD-L1 expression | |||||||||||
| Positive | 2/29 | 6.9 | 1.770 (0.296–10.593) | 0.532 | 1.548 (0.251–9.564) | 0.638 | 1.310 (0.131–13.091) | 0.818 | |||
| Negative | 3/24 | 12.5 | |||||||||
Adjusted *1: the age and sex of the patients were adjusted. Adjusted *2: the age at diagnosis, sex, tumor size, multifocality, capsular invasion, extraglandular invasion, lymphatic vascular and nerve invasion, cervical lymph node metastasis, concomitant HT, and 131I therapy were adjusted. PD-L1, programmed death-ligand 1; PTC, papillary thyroid carcinoma; HR, hazard ratio; CI, confidence interval; PTMC, papillary thyroid microcarcinoma; HT, Hashimoto’s thyroiditis.
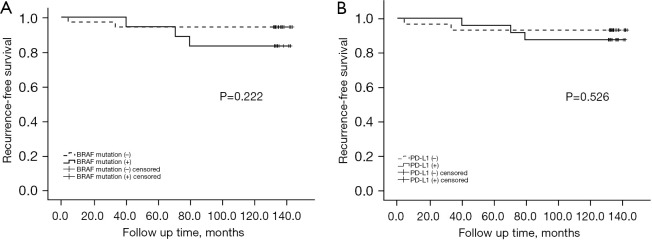
The tumor recurrence rate for patients who were negative for PD-L1 expression was 8.2%, and the tumor recurrence rate for patients who were positive for PD-L1 expression was 11.8%. Similarly, the correlation between the presence of PD-L1 and the risk of tumor recurrence was not significant (log rank P=0.222; Figure 3B). Further Cox regression analysis indicated that after adjusting for age at diagnosis and sex, the correlation between PD-L1 and the risk of tumor recurrence was still not statistically significant; the HR was 1.502 (95% CI: 0.503–4.489; P=0.467). After additional adjustments for tumor size, multifocality, capsular invasion, extrathyroid invasion, CLNM, concomitant HT, and radioactive iodine 131 treatment, there was still no significant correlation between PD-L1 expression and the risk of recurrence (HR =1.762; 95% CI: 0.537–5.778; P=0.350) (Table 5). The same results were also observed for patients with PTMC (Figure 4A,4B, Table 5).
Figure 4.
Survival curve for the BRAFV600E mutation and PTMC recurrence (log rank P=0.222) (A); survival curve for PD-L1 expression and PTMC recurrence (log rank P=0.526) (B). PD-L1, programmed death-ligand 1; PTMC, papillary thyroid microcarcinoma.
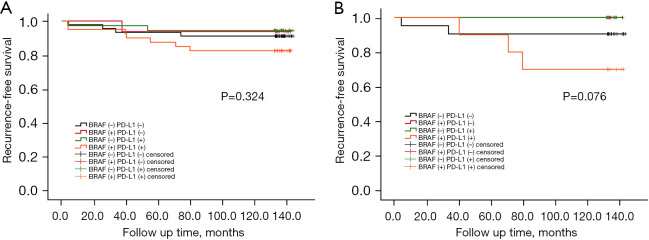
Subsequently, we evaluated whether the coexistence of the BRAFV600E mutation and PD-L1 affected the recurrence of early PTC and PTMC after long-term follow-up. We found that for both early PTC and PTMC, the BRAFV600E mutation or PD-L1 expression alone did not significantly change the risk of recurrence compared with that for patients without the BRAFV600E mutation and PD-L1 expression. Kaplan-Meier analysis showed that the RFS curves for double-negative and any single-positive PTC patients were flat and that the survival curves for PTC patients who were positive for the BRAFV600E mutation and PD-L1 expression decreased sharply. However, the significance of this effect is not yet statistically meaningful (P>0.05, Figure 5A,5B). Combining the two molecular markers for Cox regression, only 4 of 45 patients with BRAFV600E mutation and PD-L1 double negative showed recurrence (8.9%), while 7 of 40 patients (17.5%) with BRAFV600E mutation and PD-L1 double expression had the highest recurrence rate (HR =1.267; 95% CI: 0.841–1.909; P=0.257). Separate between-group comparisons revealed that the coexistence of the BRAFV600E mutation and PD-L1 had a greater effect on PTC recurrence than either mutation alone; 1 of 16 early PTC cases carrying only the BRAFV600E mutation developed recurrence (6.3%) (HR =1.704; 95% CI: 0.598–4.858; P=0.319) and 2 of 36 cases expressing PD-L1 only (5.6%) (HR =3.315; 95% CI: 0.689–15.958; P=0.135). However, the statistical significance of this effect was not significant (P>0.05) (Table 6).
Figure 5.
Survival curve for the BRAFV600E mutation & PD-L1 and the recurrence of early-stage PTC (log rank P=0.324) (A); survival curve for the BRAFV600E mutation & PD-L1 and PTMC recurrence (log rank P=0.076) (B). PD-L1, programmed death-ligand 1; PTMC, papillary thyroid microcarcinoma.
Table 6. Comparison of PTC recurrence between the BRAFV600E mutation and PD-L1 expression alone and combined.
| Group comparison | HR | 95% CI | P value |
|---|---|---|---|
| Early PTC | |||
| Group 1 vs. Group 2 | 0.683 | 0.076–6.115 | 0.733 |
| Group 1 vs. Group 3 | 0.786 | 0.337–1.837 | 0.579 |
| Group 1 vs. Group 4 | 1.267 | 0.841–1.909 | 0.257 |
| Group 2 vs. Group 4 | 1.704 | 0.598–4.858 | 0.319 |
| Group 3 vs. Group 4 | 3.315 | 0.689–15.958 | 0.135 |
| Group 2 vs. Group 3 | 0.887 | 0.080–9.779 | 0.922 |
| PTMC | |||
| Group 1’ vs. Group 2’ | 0.030 | 0–13655.040 | 0.597 |
| Group 1’ vs. Group 3’ | 0.146 | 0–49.834 | 0.518 |
| Group 1’ vs. Group 4’ | 1.464 | 0.806–2.659 | 0.211 |
| Group 2’ vs. Group 4’ | 7.674 | 0.073–807.719 | 0.391 |
| Group 3’ vs. Group 4’ | 112.910 | 0.007–NA | 0.341 |
| Group 2’ vs. Group 3’ | – | – | – |
PTC, papillary thyroid carcinoma; PD-L1, programmed death-ligand 1; HR, hazard ratio; CI, confidence interval; PTMC, papillary thyroid microcarcinoma; NA, unable to calculate.
Discussion
In recent decades, the incidence of thyroid cancer in China and globally has been increasing, of which the increase in new cases of PTC is the main reason. Improvements in diagnostic techniques, increased monitoring methods, and large-scale cancer screening have all led to an increase in the detection rate of small, low-risk, and indolent PTCs (3). Therefore, how to better deal with many early PTC and PTMC patients has become an urgent problem in China. For this part of the population, identifying patients with relatively high risk and excluding patients who do not need excessive active treatment is conducive to improving the current medical situation of the overtreatment of thyroid cancer in China.
The BRAFV600E mutation is considered one of the most common specific diagnostic markers of thyroid cancer. It plays an important role in the occurrence and development of PTC, mainly through the abnormal activation of the MAPK signaling pathway (11). The mutation frequency of BRAFV600E in PTC is generally higher in Asian countries than in Western countries; the mutation rate in China is 31–91%, with an average of 71.2% (30). The literature shows that (31) compared with methods such as gene sequencing and polymerase chain reaction (PCR), IHC using the VE1 antibody has higher specificity, sensitivity, and positive and negative predictive values and is a reliable and highly sensitive method for the detection of the BRAFV600E mutation in PTC. IHC has been widely applied in clinical practice. In this study, IHC was used to detect the BRAFV600E mutation in 137 PTC patients, and the positive detection rate was 40.9%. The positive rate in the PTMC cohort was 33.9%, slightly lower than the average. The included cases were TNM stage I to stage II, which are relatively early stages. A recent meta-analysis of 9,908 patients with PTC (16) indicated that late TNM staging (stage III and IV) was an important risk factor for the BRAFV600E mutation in PTC patients. Additionally, the results of this study also indicated that there was no association between or increased risk for tumor size >1 cm and the BRAFV600E mutation in PTC patients. However, some studies (32,33) show that the BRAFV600E mutation is associated with larger tumor size. This is consistent with the slightly lower BRAFV600E mutation rate in the PTMC cohort in this study, which was at the lower limit for China. The detection of the BRAFV600E mutation in PTMC indicates that this mutation may be an early event in the development of PTC. Fa et al. (30) conducted subgroup analyses and found that compared with studies that used formalin-fixed paraffin-embedded tissues, studies that used fresh frozen tissue and fine-needle aspirates showed a higher incidence of BRAFV600E mutations. This may also be one of the reasons for the relatively low positive rate of the BRAFV600E mutation in this study. In addition, the relationship between the BRAFV600E mutation and clinicopathological parameters is still controversial. In this study, in the early stage of PTC, the BRAFV600E mutation was only significantly correlated with HT but was not found to be significantly correlated with age at diagnosis, sex, tumor size, multifocality, capsular invasion, extrathyroidal invasion, lymphatic, vascular and nerve invasion, and CLNM. The BRAFV600E mutation in PTMC was only associated with older age at diagnosis and was not significantly correlated with other clinicopathological features. This is consistent with the results reported in several studies (34,35).
The BRAFV600E mutation is often regarded as a poor prognostic marker of PTC and is closely associated with PTC recurrence (18). Studies by Xing et al. (19,36) found that there was a significant association between the BRAFV600E mutation and disease relapse in patients with low-risk stage I or II PTC and PTMC and various subtypes of PTC. Consistent with the above viewpoint, Kim et al. (37) proposed that the BRAFV600E mutation can be used to predict clinical recurrence in patients with low-risk PTC. However, a single-center retrospective study (20) involving 461 patients with TNM stage I or II PTC reported that the BRAFV600E mutation could not predict recurrence in patients with TNM stage I–II PTC after initial treatment with total thyroidectomy (TTE) and radioactive iodine therapy (RRA). Our findings are consistent with the results of this study. In our study, after 136 months of long-term follow-up of patients with stage I–II PTC and PTMC who underwent surgical treatment, the correlation between the BRAFV600E mutation and PTC recurrence was not significant.
PD-L1 is expressed in a variety of tumor cells, such as malignant melanoma and non-small cell lung cancer. A series of studies (26,38) also proved that high PD-L1 expression in PTC patients is associated with tumor invasiveness and recurrence, different from the results for PD-L1 in this study. The reason may be that the included patients in our study were at an early stage; TNM stage has been shown to be one of the influencing factors of PD-L1 expression in PTC (39).
Several previous studies have reported an association between PD-L1 expression and the BRAFV600E mutation in PTC patients. However, the relevant results are controversial. Angell et al. (40) found that the positive status of PD-L1 in PTC patients was closely related to the BRAFV600E mutation. Bastman et al. (41) did not observe a significant correlation between PD-L1 expression and BRAFV600E mutation status in advanced PTC cases. Bai et al. (34) conducted a study of 110 cases of PTC with a diameter >1 cm and found that the correlation between PD-L1 and BRAFV600E was not statistically significant. However, after excluding PTC patients with a background of chronic lymphocytic thyroiditis (CLT) or Hashimoto’s disease, BRAFV600E was positively correlated with PD-L1 expression statistically. The association between the BRAFV600E mutation and PD-L1 has not been confirmed. A recent study (26) proposed that there is an association between PD-L1 overexpression and the BRAFV600E mutation. Compared with patients with the BRAF mutation alone, the patients with both PD-L1 overexpression and the BRAFV600E mutation had significantly less favorable disease-free survival (DFS). In addition, in vitro experiments confirmed that the overexpression of PD-L1 in PTC was significantly correlated with BRAF mutations. The results of this study showed that the BRAFV600E mutation and PD-L1 expression were highly correlated in early PTC. These results further support that the coexistence of PD-L1 and BRAFV600E mutations may be associated with the more invasive behavior of early PTC. This indicates that there may be a synergistic effect between the 2 in the development and progression of tumors.
Therefore, we further combined the BRAFV600E mutation and PD-L1 to identify patients with strong invasiveness and a high risk of recurrence among early PTC patients. We observed that only the BRAFV600E mutation or PD-L1 positivity alone had no significant effect on clinical pathological characteristics and the recurrence of PTC. However, when these 2 molecular markers coexist, it suggests that the tumor is larger and more prone to extraglandular invasion, and the RFS curve has a significant decreasing trend, indicating that the coexistence of the 2 may be related to more aggressive tumor behavior. Further Cox regression analysis also showed that patients with BRAFV600E mutation and PD-L1 double positive had a higher recurrence rate than patients with double negative and single factor positive. A previous study (40) suggested that the BRAFV600E mutation facilitates tumor immunosuppression through mechanisms such as PD-L1 and HLA-G expression and induces or recruits inhibitory immune cell populations to disrupt the immune surveillance and immune response of the host. In addition, in colon cancer, BRAFV600E can transcriptionally upregulate the expression of PD-L1 and induce cell apoptosis (25). This further supports that the combined use of the BRAFV600E mutation and PD-L1 has a predictive effect on the clinicopathological characteristics and prognosis of early PTC. However, the effect of the combination of the BRAFV600E mutation and PD-L1 on clinicopathological characteristics and prognosis in PTMC has not been confirmed. This indicates that the combination of the BRAFV600E mutation and PD-L1 serves as a poor indication for the prognosis of PTMC and should be interpreted with caution.
This study has some limitations. We used IHC to detect the BRAFV600E mutation and PD-L1, but the time frame of the cases included in this study, the preservation conditions of the paraffin blocks, and the IHC operation procedures all can affect the final results, which can lead to the exclusion of some cases, resulting in a small number of cases included in this study. In addition, there was no negative case control in this study, potentially causing bias in the study results. Further studies are needed to increase the number of early- or low-risk PTC cases to confirm the effect of the combination of the BRAFV600E mutation and PD-L1 on the clinicopathological characteristics and prognosis of early PTC. Therefore, the explanatory value of these data is limited, and the conclusion may lack credibility. Second, this study did not classify pathological subtypes because of the limitations of the conditions of the study. However, the median follow-up period of this study was 136 months, which to a certain extent effectively avoided the underestimation of the recurrence rate of PTC.
Conclusions
In summary, this study demonstrated a significant association between the BRAFV600E mutation and PD-L1 in early PTC, and when only one of these indicators was expressed alone, the effect on the aggressiveness and prognosis of early PTC was small. The coexistence of the BRAFV600E mutation and PD-L1 suggests a higher risk of invasiveness and recurrence but has insufficient clinical evidence. And there was no significant predictive effect for PTMC. To a certain extent, these results can assist identification of the relatively high-risk patients among the early PTC population in clinical practice, improve the risk stratification of PTC, and be used to develop individualized treatment plans and optimize follow-up plans. In the future, further prospective and larger-sample-size studies are needed to provide more reliable evidence to support this point of view.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank all the patients who participated in this study.
Funding: This work was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LY20H160007).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Review Committee of Zhejiang Cancer Hospital (approval ID: IRB-2020-64) and informed consent was taken from all the patients.
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://gs.amegroups.com/article/view/10.21037/gs-22-701/rc
Data Sharing Statement: Available at https://gs.amegroups.com/article/view/10.21037/gs-22-701/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://gs.amegroups.com/article/view/10.21037/gs-22-701/coif). The authors have no conflicts of interest to declare.
(English Language Editor: J. Jones)
References
- 1.Cheng F, Xiao J, Shao C, et al. Burden of Thyroid Cancer From 1990 to 2019 and Projections of Incidence and Mortality Until 2039 in China: Findings From Global Burden of Disease Study. Front Endocrinol (Lausanne) 2021;12:738213. 10.3389/fendo.2021.738213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 3.Seib CD, Sosa JA. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol Metab Clin North Am 2019;48:23-35. 10.1016/j.ecl.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 4.Kaliszewski K, Diakowska D, Wojtczak B, et al. Cancer screening activity results in overdiagnosis and overtreatment of papillary thyroid cancer: A 10-year experience at a single institution. PLoS One 2020;15:e0236257. 10.1371/journal.pone.0236257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krajewska J, Kukulska A, Oczko-Wojciechowska M, et al. Early Diagnosis of Low-Risk Papillary Thyroid Cancer Results Rather in Overtreatment Than a Better Survival. Front Endocrinol (Lausanne) 2020;11:571421. 10.3389/fendo.2020.571421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tirrò E, Martorana F, Romano C, et al. Molecular Alterations in Thyroid Cancer: From Bench to Clinical Practice. Genes (Basel) 2019;10:709. 10.3390/genes10090709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nylén C, Mechera R, Maréchal-Ross I, et al. Molecular Markers Guiding Thyroid Cancer Management. Cancers (Basel) 2020;12:2164. 10.3390/cancers12082164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdullah MI, Junit SM, Ng KL, et al. Papillary Thyroid Cancer: Genetic Alterations and Molecular Biomarker Investigations. Int J Med Sci 2019;16:450-60. 10.7150/ijms.29935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 2004;6:313-9. 10.1016/j.ccr.2004.09.022 [DOI] [PubMed] [Google Scholar]
- 10.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 2007;28:742-62. 10.1210/er.2007-0007 [DOI] [PubMed] [Google Scholar]
- 11.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer 2005;12:245-62. 10.1677/erc.1.0978 [DOI] [PubMed] [Google Scholar]
- 12.Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: pathogenic role and clinical implications. J Chin Med Assoc 2010;73:113-28. 10.1016/S1726-4901(10)70025-3 [DOI] [PubMed] [Google Scholar]
- 13.Leonardi GC, Candido S, Carbone M, et al. BRAF mutations in papillary thyroid carcinoma and emerging targeted therapies (review). Mol Med Rep 2012;6:687-94. 10.3892/mmr.2012.1016 [DOI] [PubMed] [Google Scholar]
- 14.Gimm O, Ivansson K, Beka E, et al. Increased diagnostic sensitivity of palpation-guided thyroid nodule fine-needle aspiration cytology by BRAF V600E-mutation analysis. J Pathol Clin Res 2021;7:556-64. 10.1002/cjp2.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 2013;309:1493-501. 10.1001/jama.2013.3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei X, Wang X, Xiong J, et al. Risk and Prognostic Factors for BRAF(V600E) Mutations in Papillary Thyroid Carcinoma. Biomed Res Int 2022;2022:9959649. 10.1155/2022/9959649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim TH, Park YJ, Lim JA, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 2012;118:1764-73. 10.1002/cncr.26500 [DOI] [PubMed] [Google Scholar]
- 18.Enumah S, Fingeret A, Parangi S, et al. BRAF(V600E) Mutation is Associated with an Increased Risk of Papillary Thyroid Cancer Recurrence. World J Surg 2020;44:2685-91. 10.1007/s00268-020-05521-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 2015;33:42-50. 10.1200/JCO.2014.56.8253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelttari H, Schalin-Jäntti C, Arola J, et al. BRAF V600E mutation does not predict recurrence after long-term follow-up in TNM stage I or II papillary thyroid carcinoma patients. APMIS 2012;120:380-6. 10.1111/j.1600-0463.2011.02844.x [DOI] [PubMed] [Google Scholar]
- 21.Ito Y, Yoshida H, Kihara M, et al. BRAF(V600E) mutation analysis in papillary thyroid carcinoma: is it useful for all patients? World J Surg 2014;38:679-87. 10.1007/s00268-013-2223-2 [DOI] [PubMed] [Google Scholar]
- 22.Cappola AR, Mandel SJ. Molecular testing in thyroid cancer: BRAF mutation status and mortality. JAMA 2013;309:1529-30. 10.1001/jama.2013.3620 [DOI] [PubMed] [Google Scholar]
- 23.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 24.Chowdhury S, Veyhl J, Jessa F, et al. Programmed death-ligand 1 overexpression is a prognostic marker for aggressive papillary thyroid cancer and its variants. Oncotarget 2016;7:32318-28. 10.18632/oncotarget.8698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng D, Qin B, Pal K, et al. BRAF(V600E)-induced, tumor intrinsic PD-L1 can regulate chemotherapy-induced apoptosis in human colon cancer cells and in tumor xenografts. Oncogene 2019;38:6752-66. 10.1038/s41388-019-0919-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siraj AK, Parvathareddy SK, Pratheeshkumar P, et al. PD-L1 Is an Independent Prognostic Marker in Middle Eastern PTC and Its Expression Is Upregulated by BRAFV600E Mutation. Cancers (Basel) 2021;13:555. 10.3390/cancers13030555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girolami I, Pantanowitz L, Mete O, et al. Programmed Death-Ligand 1 (PD-L1) Is a Potential Biomarker of Disease-Free Survival in Papillary Thyroid Carcinoma: a Systematic Review and Meta-Analysis of PD-L1 Immunoexpression in Follicular Epithelial Derived Thyroid Carcinoma. Endocr Pathol 2020;31:291-300. 10.1007/s12022-020-09630-5 [DOI] [PubMed] [Google Scholar]
- 28.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer ; Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. 10.1089/thy.2009.0110 [DOI] [PubMed] [Google Scholar]
- 29.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9. [DOI] [PubMed] [Google Scholar]
- 30.Rashid FA, Munkhdelger J, Fukuoka J, et al. Prevalence of BRAF(V600E) mutation in Asian series of papillary thyroid carcinoma-a contemporary systematic review. Gland Surg 2020;9:1878-900. 10.21037/gs-20-430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choden S, Keelawat S, Jung CK, et al. VE1 Immunohistochemistry Improves the Limit of Genotyping for Detecting BRAF(V600E) Mutation in Papillary Thyroid Cancer. Cancers (Basel) 2020;12:596. 10.3390/cancers12030596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czarniecka A, Kowal M, Rusinek D, et al. The Risk of Relapse in Papillary Thyroid Cancer (PTC) in the Context of BRAFV600E Mutation Status and Other Prognostic Factors. PLoS One 2015;10:e0132821. 10.1371/journal.pone.0132821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samà MT, Grosso E, Mele C, et al. Molecular characterisation and clinical correlation of papillary thyroid microcarcinoma. Endocrine 2021;71:149-57. 10.1007/s12020-020-02380-8 [DOI] [PubMed] [Google Scholar]
- 34.Bai Y, Guo T, Huang X, et al. In papillary thyroid carcinoma, expression by immunohistochemistry of BRAF V600E, PD-L1, and PD-1 is closely related. Virchows Arch 2018;472:779-87. 10.1007/s00428-018-2357-6 [DOI] [PubMed] [Google Scholar]
- 35.Ji W, Xie H, Wei B, et al. Relationship between BRAF V600E gene mutation and the clinical and pathologic characteristics of papillary thyroid microcarcinoma. Int J Clin Exp Pathol 2019;12:3492-9. [PMC free article] [PubMed] [Google Scholar]
- 36.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 2005;90:6373-9. 10.1210/jc.2005-0987 [DOI] [PubMed] [Google Scholar]
- 37.Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364-8. 10.1111/j.1365-2265.2006.02605.x [DOI] [PubMed] [Google Scholar]
- 38.Shi RL, Qu N, Luo TX, et al. Programmed Death-Ligand 1 Expression in Papillary Thyroid Cancer and Its Correlation with Clinicopathologic Factors and Recurrence. Thyroid 2017;27:537-45. 10.1089/thy.2016.0228 [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Zhang Z, Yan Z, et al. PD-L1, PDK-1 and p-Akt are correlated in patients with papillary thyroid carcinoma. Adv Clin Exp Med 2020;29:785-92. 10.17219/acem/121518 [DOI] [PubMed] [Google Scholar]
- 40.Angell TE, Lechner MG, Jang JK, et al. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid 2014;24:1385-93. 10.1089/thy.2014.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bastman JJ, Serracino HS, Zhu Y, et al. Tumor-Infiltrating T Cells and the PD-1 Checkpoint Pathway in Advanced Differentiated and Anaplastic Thyroid Cancer. J Clin Endocrinol Metab 2016;101:2863-73. 10.1210/jc.2015-4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as