Highlights
-
•
Current genotype-phenotype correlations in GNAO1 disorders are insufficiently understood.
-
•
In a novel series of 15 patients, reported epilepsy phenotype and medication response is highly variable.
-
•
A review of the literature shows GNAO1-related neurodevelopmental disorders to be heterogeneous with variable manifestations of hypotonia (91%), movement disorders (89%), and epilepsy (53%).
Abbreviations: GNAO1, G protein subunit alpha o1; ASM, antiseizure medications; DEE, developmental and epileptic encephalopathy; cAMP, cyclic adenosine monophosphate; GPCR, G protein coupled receptor; MRI, magnetic resonance imaging; EEG, electroencephalogram
Keywords: GNAO1, Developmental and epileptic encephalopathy, Epilepsy, Movement disorder, Neurodevelopmental disorder, Genetic
Abstract
Background
GNAO1-related neurodevelopmental disorder is a heterogeneous condition characterized by hypotonia, developmental delay, epilepsy, and movement disorder. This study aims to better understand the spectrum of epilepsy associated with GNAO1 variants and experience with anti-seizure medications, and to review published epilepsy phenotypes in GNAO1.
Methods
An online survey was distributed to caregivers of individuals diagnosed with GNAO1 pathogenic variants, and a literature review was conducted.
Results
Fifteen respondents completed the survey with the median age of 39 months, including a novel variant p.Q52P. Nine had epilepsy – six had onset in the first week of life, three in the first year of life – but two reported no ongoing seizures. Seizure types varied. Individuals were taking a median of 3 seizure medications without a single best treatment. Our cohort was compared to a literature review of epilepsy in GNAO1. In 86 cases, 38 discrete variants were described; epilepsy is reported in 53 % cases, and a developmental and epileptic encephalopathy in 36 %.
Conclusions
While GNAO1-related epilepsy is most often early-onset and severe, seizures may not always be drug resistant or lifelong. Experience with anti-seizure medications is varied. Certain variant “hotspots” may correlate with epilepsy phenotype though genotype-phenotype correlation is poorly understood.
1. Introduction
Heterozygous de novo pathogenic variants in GNAO1 cause a neurodevelopmental disorder (GNAO1-NDD). Four individuals with variants in GNAO1 and severe developmental epileptic encephalopathy (DEE) were first described in 2013[1]. Three children had a diagnosis of Ohtahara Syndrome with suppression-burst pattern on EEG at less than one month of age and two had abnormal movements (chorea, dystonia). In 2016, multiple case reports of individuals with GNAO1 variants described a severe movement disorder with progressive chorea and athetosis in individuals without epilepsy, several of whom share the same variant p.R209H[2], [3], [4]. Movement disorders have been subsequently described as strikingly severe with up to 46 % reporting chorea “storms”, medically refractory exacerbations of hyperkinesia that can result in injuries (such as joint dislocations), hyperthermia, and rhabdomyolysis requiring ICU-level care[5]. Increasingly, deep brain stimulation has been used to reduce the hyperkinetic, choreoathetotic movements in a sustained manner, preventing life-threatening exacerbations in subsequent years[6]. Thus, it is now understood that pathogenic variants in GNAO1 cause a spectrum of early-onset DEE, a severe progressive movement disorder, or an overlapping disorder with both epilepsy and a movement disorder.
While movement disorders in GNAO1-NDD are well described given the high acuity and severity, the epilepsy phenotype is less well characterized. To better understand the spectrum of epilepsy associated with GNAO1 variants, we surveyed caregivers for seizure types and epilepsy therapies utilized and performed a literature review of the currently identified pathogenic variants.
1.1. Gαo function and genotype-phenotype correlations
The GNAO1 gene encodes the alpha o1 (αo) subunit of guanosine nucleotide-binding proteins, also known as G-proteins. Gαo serves as an intracellular modulator for many neurotransmitters regulating a wide array of cell signaling, neuronal growth, and development. Gαo is abundantly expressed in the brain, comprising up to 0.5 % of membrane protein with greater expression in the hippocampus, striatum and cerebellum[7]. The αo subunit joins with β and γsubunits to form a heterotrimeric G-protein complex that participates in intracellular signaling with G-protein coupled receptors (GPCRs)[1]. Following ligand binding, Gαo dissociates from both the G-protein coupled receptor and the β-γ dimer. GTP-Gαo complex is formed and interacts with downstream intracellular second messenger systems such as cyclic AMP (cAMP). Pathogenic variants have been primarily in or adjacent to the GTP-binding domain[8].
Assays of GNAO1 variants show evidence for both loss-of-function and gain-of-function activity. Nakamura (2013) showed that two variants p.T191_F197 del and p.G203R had aberrant localization to the cytosol rather than cell membrane in N2A cells, which was thought to result in loss of function effect on intracellular calcium signaling[1]. Subsequently, a biochemical functional assay assessing the ability of Gαo mutants to inhibit intracellular cAMP production showed a variety of gain-of-function, loss-of-function and normal function in clinical variants[9]. A potential dichotomous genotype-phenotype correlation was postulated based on these findings: loss-of-function in epilepsy phenotypes and gain-of-function in movement disorder phenotypes. This hypothesis does not account for the spectrum of patients (approximately 1/3) who have an overlapping disorder with both seizures and abnormal movements. Some variants, including the frequently reported movement disorder predominant R209 alleles, did not show alterations in this cAMP assay. The cAMP assay, which primarily assesses the effect of the de-coupled Gαo subunit, is unlikely to reflect a complete assessment of the in vivo effect of all pathogenic GNAO1 variants, particularly the β -γ complex’s postulated signaling effects[10], [11].
Recently, Muntean (2021) further described the role of Gαo in regulating cAMP production by controlling the availability of Gβ -γ for adenylyl cyclase binding. Pathogenic variants demonstrated neuron-type-specific dominant-negative or loss of function effects disrupting the cAMP pathway, which may contribute to clinical variability[12]. Complex and numerous signaling roles attributed to Gαo underscores the challenges in interpreting various assays and extending these findings to clinical use.
2. Materials and Methods
In the caregiver survey, subjects were recruited via the Bow Foundation (GNAO1-NDD family foundation, GNAO1.org) website and Facebook page between October 2018 and May 2019. Families accessed a link to a secure REDCap database. QY developed and BEP reviewed questions about seizures, movement disorder, and medications trialed. This descriptive study was approved by the Stanford University Institutional Review Board. In conducting the literature review, ETA queried PubMed database for search term “GNAO1”.
3. Results
3.1. Caregiver survey
Fifteen patients were included with characteristics described in Table 1. Eight were male, and the median age was 39 months (IQR 23–56). Nine had epilepsy, with multiple seizure types including infantile (epileptic) spasms, focal, tonic, and generalized tonic-clonic (GTC) seizures. Six developed seizures in the first week of life. At the time of study participation, seizure burden was one or more seizures daily (1), one or more seizures weekly (4), or seizure free (1). Three developed seizures in the first year of life. At the time of study participation, seizure burden was one or more seizures daily (2), or seizure free (1). Six reported no seizures; however, all had movement disorders.
Table 1.
Demographic, genetic, seizure, medication, and movement disorder data from caregiver report.
| Patient ID | Age (months) | Sex | GNAO1 Variant | Age of seizure onset | Seizure frequency | Types of seizures | Current seizure medications | Movement disorder | Movement disorder medications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | Female | p.G40W | First week of life | 2 to 3 per week | Focal, GTC | Lacosamide, oxcarbazepine, gabapentin | Yes | None |
| 2 | 150 | Male | NR | My child has never had seizures | NA | NA | NA | Yes | Levetiracetam, trazodone, tetrabenazine, clonazepam |
| 3 | 32 | Female | NR | My child has never had seizures | NA | NA | NA | Yes | None |
| 4 | 31 | Male | p.E246K | My child has never had seizures | NA | NA | NA | Yes | Tetrabenazine, diazepam |
| 5 | 38 | Female | p.R209H | My child has never had seizures | NA | NA | NA | Yes | None |
| 6 | 14 | Male | p.Q52P *Novel variant in this study |
First week of life | 20 per day | Infantile spasms, tonic, GTC | Levetiracetam, phenytoin, diazepam, clobazam | No | NA |
| 7 | 22 | Male | p.G203R | Less than 1 year of age | Seizure free | Infantile spasms, focal, GTC | Vigabatrin | Not sure | None |
| 8 | 60 | Female | NR | First week of life | Seizure free | Focal, tonic, GTC | Gabapentin, perampanel | Yes | Chlorprothixene |
| 9 | 48 | Male | p.G203R | Less than 1 year of age | 10 per day | Infantile spasms | Clobazam, clonazepam, vigabatrin, oxcarbazepine, valproic acid, levetiracetam | Yes | Clobazam, clonazepam |
| 10 | 72 | Female | p.G203R | First week of life | 1 per week | Tonic, GTC | Gabapentin, lacosamide, clobazam | Yes | Clobazam |
| 11 | 72 | Male | p.G203R | Less than 1 year of age | 30 per day | Absence, focal, tonic, GTC | Valproic acid, lamotrigine, clobazam | Yes | Baclofen |
| 12 | 51 | Female | p.G203R | First week of life | Varies significantly | Not sure | Clonazepam, topiramate | Yes | Clonazepam |
| 13 | 20 | Male | p.G203R | First week of life | 1 per week | Focal, GTC | Valproic acid, ketogenic diet | Yes | None |
| 14 | 20 | Female | p.E237K | My child has never had seizures | NA | NA | NA | Yes | None |
| 15 | 24 | Female | NR | My child has never had seizures | NA | NA | NA | Yes | Valproic acid |
Abbreviations: NR, not reported; NA, not applicable; GTC, generalized tonic clonic seizure.
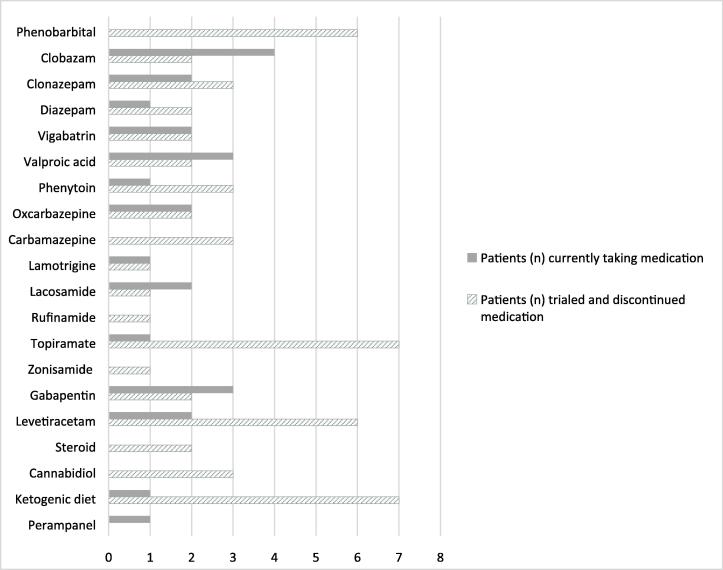
Patients with epilepsy were taking a median of three ASMs. Fig. 1 demonstrates seizure medications used at the time of study participation, and seizure medications trialed previously. While there was a range of drug mechanisms, most participants were currently taking GABAergic medications (including benzodiazepines) (8/9) or sodium channel blockers (5/9). Each participant identified different medications as “most helpful” for their child including lacosamide, topiramate, clobazam, lamotrigine, vigabatrin, phenytoin, and benzobarbital. No patients had vagal nerve stimulators. Also notable was the high rate of discontinuation for certain therapies: ketogenic diet (7), topiramate (7), phenobarbital (6), levetiracetam (6), carbamazepine (3) and cannabidiol (3). One individual reported a significant worsening of seizure frequency with pharmaceutical grade cannabidiol.
Fig. 1.
Anti-seizure medication usage.
Nearly all (13) reported a movement disorder, one without movement disorder, and one was unsure. Six reported they had never used a medication targeting their abnormal movements and none had deep brain stimulators.
Participants reported specific variants if this information was available, however genetic testing reports were not collected. Six reported the same variant p.G203R and all of these had epilepsy. One reported a novel variant p.Q52P (Patient ID #6) with DEE, onset first week of life, continuing to have 20 seizures per day on 4 ASMs.
3.2. Literature review
The search yielded 86 cases including 38 variants across 15 publications from 2013 to 2021 (Supplementary Table)[1], [2], [3], [4], [5], [6], [8], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]. Table 2 summarizes the clinical characteristics. Most reported hypotonia (91 %) and all reported developmental delay and/or intellectual disability, nearly all with some abnormality noted before age 1. Seventy-one (89 %) had movement disorders, 46 (53 %) had epilepsy and 35 (41 %) had both.
Table 2.
Basic demographics from literature review.
| Total cases described | 86 | |
|---|---|---|
| Unique variants described | 38% | |
| Sex∋ | Female | 53 (0.63) |
| Male | 31 (0.37) | |
| Deceased at time of publication | 4 (5 %) | |
| Epilepsy | Total | 46 (0.53) |
| DEE | 31 (0.36) | |
| Single seizure | 2 (0.02) | |
| Movement disorder * | Total | 71 (0.89) |
| Both seizures and movement disorder | 35 (0.41) | |
| MRI brain # | Normal | 36 (0.46) |
| Diffuse atrophy | 22 (0.28) | |
| Corpus callosum abnormality (thin or hypoplastic) | 12 (0.15) | |
| Basal Ganglia abnormalities | 8 (0.10) | |
| Abnormal myelination | 7 (0.09) | |
| Hypotonia§ | 66 (0.92) | |
| DD/ID* | 80 (1.00) |
DEE developmental and epileptic encephalopathy, DD/ID diagnosed with developmental delay and/or intellectual disability.
, see Supplementary Table, ∋ 84 reported, *80 reported, #78 reported, §72 reported.
In those with epilepsy, seizure onset was typically in the first 3 months of life; 31 (36 %) individuals were described as DEE. Seizure types varied widely and include both focal-onset and generalized seizures (generalized tonic-clonic, myoclonic, tonic, atonic, absence) and epileptic spasms. Subtypes of DEE included early-onset DEE (formerly known as Ohtahara Syndrome), Infantile Spasms, and Lennox Gastaut Syndrome. Several individuals reported only a single seizure without recurrent seizures[8], [19]. One individual with an initial presentation of DEE was reported to be off ASMs and seizure free for 9 years [29]. MRI brain was often normal (41 %) or findings were nonspecific such as diffuse atrophy (25 %), and thin corpus callosum, (12 %). Similarly, no unifying EEG pattern has been described, rather features are concordant with epilepsy phenotype (e.g. hypsarrythmia in infantile spasms or focal epileptiform discharges in those with focal seizures). Older patients (over the age of 10) have a mixture of ongoing or resolved seizures and movement disorders and intellectual disability.
The diagnosis was made via whole exome sequencing or more recently via targeted gene panels. Variants were primarily missense mutations, though an in-frame deletion and a splicing site mutation were reported. Most were de novo, though parental mosaicism was reported in multiple individuals including a pair of affected siblings with parents who lacked the variant[1], [8], [24].
Frequently reported amino acid residues were identified as variant “hotspots,” including AA209 (n = 21), AA246 (n = 12), AA203 (n = 8), AA40 (n = 7) and AA237 (n = 5). Notably, AA209 comprised 24 % of all cases described in the literature (p.R209C (n = 14), p.R209H (n = 5), p.R209G (n = 1), and p.R209L (n = 1)). While all individuals with AA209 variants had prominent, typically severe movement disorders, significant heterogeneity was seen in the epilepsy (7/21) ranging from DEE to a single seizure. The second most frequently reported variant p.E246K (n = 11), all reported a movement disorder, with one reporting seizures. In contrast, AA40 is a variant “hotspot” wherein all individuals had epilepsy (p. G40R (n = 4), p.G40E (n = 2), p.G40W (n = 1)), nearly all consistent with DEE. Movement disorders were not well-described in this variant but at least half had abnormal movements.
4. Discussion
Our survey results of 15 individuals with GNAO1-NDD demonstrate the variability in epilepsy phenotype with age of seizure onset, seizure control, and types of seizures. All 9 with epilepsy had onset within the first year of life, and 2 were seizure free on ASMs. Six individuals reported never having a seizure. It is important to note that the median age of this cohort at 39 months of and the median age of those six individuals without epilepsy at 31 months limits conclusions to be drawn regarding whether they do not have epilepsy, as they could develop seizures later in life. In comparison, the literature review (Supplementary Table) includes individuals in mid- to late teens. Similarly, the GNAO1 Natural History Study led by Axeen and colleagues (2021) included 82 participants with a median age of 6.5 years; 43 with a history of epilepsy, and 21 had no ongoing seizures[39]. We also report a novel variant p.Q52P in a child with early-onset DEE with poorly controlled seizures at age 15 months. However, genetic testing and transcript data were not available to confirm the variant reported.
We affirm prior reports indicating heterogeneity in ASM usage and response in those with drug resistant epilepsy[39]. Most patients remained on GABAergic medications, which may be in part to the dual benefit of benzodiazepines on GNAO1-related seizures and movement disorders. Most study participants had ongoing seizures, suggesting discontinued medications were either ineffective or had intolerable side effects. High rates of discontinuation were seen in topiramate and phenobarbital, which may be due to prohibitive side effects. While the dietary therapy has previously been reported as effective in one child, 7/8 of our participants discontinued the ketogenic diet suggesting a more muted benefit[27]. Additionally, none of the participants who trialed cannabidiol (3) remained on it long-term. Additional retrospective and prospective data are needed to clarify best therapeutic strategies for specific seizure types.
The literature review demonstrates a distribution of epilepsy (53 %) vs movement disorder (89 %) vs combined (41 %) similar to the recently published GNAO1 Natural History Study caregiver survey demonstrating 52 % with seizures or epilepsy, 76 % with a movement disorder, and 33 % with both[39]. Aggregate analysis of all variants reported in the literature yields interesting yet incomplete correlations between genotype and phenotype (Supplementary Table). Certain variant “hot spots” are associated with movement disorder more so than epilepsy, and vice versa. Variants in AA40 and AA203 have epilepsy-predominant or combined phenotype whereas AA209 and AA237 have a movement disorder-predominant presentation with either milder or no epilepsy.
This study has several limitations. It is a descriptive and retrospective study with data provided by caregiver report via an online survey, therefore subject to recall bias and inaccuracies. Medical records and genetic testing were not verified. There are limitations to the accuracy of genetic testing and the potential for misclassification of patients with GNAO1-NDD. There is the possibility of duplicate representation of patients described in the literature participating in our survey. Of note, our survey includes participants on the milder end of the spectrum for movement disorders as none had deep brain stimulators and 6/13 individuals. This likely reflects the limitations of our small sample size and a generally young age of the participants. These individuals may develop seizures and/or more severe movement disorder phenotype in later years, thus underscoring the importance of longitudinal natural history data.
5. Future therapy
Because the Gαo subunit is part of a heterotrimeric G-protein complex, which interacts with many families of G protein-coupled receptors (GPCRs) – including acetylcholine, adenosine, calcium-sensing, cannabinoid, dopamine, GABA, metabotropic glutamate, and serotonin receptors, modulation of these common neurotransmitter pathways may represent an opportunity for future therapies [9]. For example, a recently described C. elegans model of GNAO1 suggests caffeine, a widely available pharmacological treatment given orally for apnea in premature infants, may have a beneficial effect on aberrant movements, potentially through adenosine receptor antagonism [40]. At present, we are limited by our lack of understanding of the full scope of Gαo function and the natural history of the GNAO1 disease. The broad clinical heterogeneity is incompletely explained by our understanding of GNAO1 molecular mechanisms though hinted at by the large number of neurotransmitter pathways that GPCRs regulate. Further research is needed to understand the function of normal and aberrant Gαo function to permit precision therapies, including gene therapies, with the goal of normalizing Gαo function.
6. Patient support organizations
Families and advocates for GNAO1-NDD have formed organizations to provide support for patients impacted by GNAO1-NDD and advocate for further disease-specific research. The Bow Foundation (United States) has provided numerous grants and was instrumental in the launch of the GNAO1 Natural History Study. Other international family groups include Famiglie GNAO1 (Italy), Mondo GNAO1 (United Kingdom), Stichting GNAO1 (Netherlands), GNAO1 Tuki Ry (Finland), and GNAO1 Espana (Spain). The development of successful therapies will require international cooperation by patient organizations and funders for GNAO1 and other rare epilepsy syndromes.
7. Conclusion
GNAO1-related NDD is clinically heterogeneous, with epilepsy variable in onset and severity, and remains poorly understood. While genotype-phenotype correlation in GNAO1 remains incomplete, variant “hotspots” in amino acid residue AA40 and AA203 may correlate with epilepsy-predominant phenotypes. We provide further data that GNAO1-related epilepsy can be drug resistant and most commonly starts in the first year of life, but may not be lifelong.
Ethical Statement:
The authors attest to the nature of this manuscript in adherence with ethical guidelines in human subjects research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to acknowledge the Bow Foundation and thank the families who generously participated in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebr.2022.100582.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nakamura K., Kodera H., Akita T., Shiina M., Kato M., Hoshino H., et al. De Novo mutations in GNAO1, encoding a Galphao subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am J Hum Genet. 2013;93:496–505. doi: 10.1016/j.ajhg.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ananth A.L., Robichaux-Viehoever A., Kim Y.M., Hanson-Kahn A., Cox R., Enns G.M., et al. Clinical Course of Six Children With GNAO1 Mutations Causing a Severe and Distinctive Movement Disorder. Pediatr Neurol. 2016;59:81–84. doi: 10.1016/j.pediatrneurol.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 3.Dhamija R., Mink J.W., Shah B.B., Goodkin H.P. GNAO1-Associated Movement Disorder. Mov Disord Clin Pract. 2016;3(6):615–617. doi: 10.1002/mdc3.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni N., Tang S., Bhardwaj R., Bernes S., Grebe T.A. Progressive Movement Disorder in Brothers Carrying a GNAO1 Mutation Responsive to Deep Brain Stimulation. J Child Neurol. 2016;31(2):211–214. doi: 10.1177/0883073815587945. [DOI] [PubMed] [Google Scholar]
- 5.Waak M., Mohammad S.S., Coman D., Sinclair K., Copeland L., Silburn P., et al. GNAO1-related movement disorder with life-threatening exacerbations: movement phenomenology and response to DBS. J Neurol Neurosurg Psychiatry. 2018;89(2):221–222. doi: 10.1136/jnnp-2017-315653. [DOI] [PubMed] [Google Scholar]
- 6.Koy A., Cirak S., Gonzalez V., Becker K., Roujeau T., Milesi C., et al. Deep brain stimulation is effective in pediatric patients with GNAO1 associated severe hyperkinesia. J Neurol Sci. 2018;391:31–39. doi: 10.1016/j.jns.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Worley P.F., Baraban J.M., Van Dop C., Neer E.J., Snyder S.H. Go, a guanine nucleotide-binding protein: immunohistochemical localization in rat brain resembles distribution of second messenger systems. Proc Natl Acad Sci U S A. 1986;83(12):4561–4565. doi: 10.1073/pnas.83.12.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly M., Park M., Mihalek I., Rochtus A., Gramm M., Perez-Palma E., et al. Spectrum of neurodevelopmental disease associated with the GNAO1 guanosine triphosphate-binding region. Epilepsia. 2019;60:406–418. doi: 10.1111/epi.14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng H., Sjögren B., Karaj B., Shaw V., Gezer A., Neubig R.R. Movement disorder in GNAO1 encephalopathy associated with gain-of-function mutations. Neurology. 2017;89(8):762–770. doi: 10.1212/WNL.0000000000004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson T.S., Helbig I. Epileptic encephalopathy, movement disorder, and the yin and yang of GNAO1 function. Neurology. 2017;89(8):754–755. doi: 10.1212/WNL.0000000000004277. [DOI] [PubMed] [Google Scholar]
- 11.Wettschureck N., Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85(4):1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 12.Muntean B.S., Masuho I., Dao M., Sutton L.P., Zucca S., Iwamoto H., et al. Galphao is a major determinant of cAMP signaling in the pathophysiology of movement disorders. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akasaka M., Kamei A., Tanifuji S., Asami M., Ito J., Mizuma K., et al. GNAO1 mutation-related severe involuntary movements treated with gabapentin. Brain Dev. 2021;43(4):576–579. doi: 10.1016/j.braindev.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Arisaka A., Nakashima M., Kumada S., Inoue K., Nishida H., Mashimo H., et al. Association of early-onset epileptic encephalopathy with involuntary movements - Case series and literature review. Epilepsy Behav Rep. 2021;15:100417. doi: 10.1016/j.ebr.2020.100417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arya R., Spaeth C., Gilbert D.L., Leach J.L., Holland K.D. GNAO1-associated epileptic encephalopathy and movement disorders: c.607G>A variant represents a probable mutation hotspot with a distinct phenotype. Epileptic Disord. 2017;19:67–75. doi: 10.1684/epd.2017.0888. [DOI] [PubMed] [Google Scholar]
- 16.Benato A., Carecchio M., Burlina A., Paoloni F., Sartori S., Nosadini M., et al. Long-term effect of subthalamic and pallidal deep brain stimulation for status dystonicus in children with methylmalonic acidemia and GNAO1 mutation. J Neural Transm (Vienna) 2019;126(6):739–757. doi: 10.1007/s00702-019-02010-2. [DOI] [PubMed] [Google Scholar]
- 17.Bruun T.U.J., DesRoches C.-L., Wilson D., Chau V., Nakagawa T., Yamasaki M., et al. Prospective cohort study for identification of underlying genetic causes in neonatal encephalopathy using whole-exome sequencing. Genet Med. 2018;20(5):486–494. doi: 10.1038/gim.2017.129. [DOI] [PubMed] [Google Scholar]
- 18.Danti F.R., Galosi S., Romani M., Montomoli M., Carss K.J., Raymond F.L., et al. GNAO1 encephalopathy: Broadening the phenotype and evaluating treatment and outcome. Neurol Genet. 2017;3(2):e143. doi: 10.1212/NXG.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzinovic I., Skorvanek M., Necpal J., Boesch S., Svantnerova J., Wagner M., et al. Dystonia as a prominent presenting feature in developmental and epileptic encephalopathies: A case series. Parkinsonism Relat Disord. 2021;90:73–78. doi: 10.1016/j.parkreldis.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Myers C., McMahon J., Schneider A., Petrovski S., Allen A., Carvill G., et al. De Novo Mutations in SLC1A2 and CACNA1A Are Important Causes of Epileptic Encephalopathies. Am J Hum Genet. 2016;99(2):287–298. doi: 10.1016/j.ajhg.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gawlinski P., Posmyk R., Gambin T., Sielicka D., Chorazy M., Nowakowska B., et al. PEHO Syndrome May Represent Phenotypic Expansion at the Severe End of the Early-Onset Encephalopathies. Pediatr Neurol. 2016;60:83–87. doi: 10.1016/j.pediatrneurol.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerald B., Ramsey K., Belnap N., Szelinger S., Siniard A.L., Balak C., et al. Neonatal epileptic encephalopathy caused by de novo GNAO1 mutation misdiagnosed as atypical Rett syndrome: Cautions in interpretation of genomic test results. Semin Pediatr Neurol. 2018;26:28–32. doi: 10.1016/j.spen.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Honey C.M., Malhotra A.K., Tarailo-Graovac M., van Karnebeek C.D.M., Horvath G., Sulistyanto A. GNAO1 Mutation-Induced Pediatric Dystonic Storm Rescue With Pallidal Deep Brain Stimulation. J Child Neurol. 2018;33(6):413–416. doi: 10.1177/0883073818756134. [DOI] [PubMed] [Google Scholar]
- 24.Kim S.Y., Shim Y., Ko Y.J., Park S., Jang S.S., Lim B.C., et al. Spectrum of movement disorders in GNAO1 encephalopathy: in-depth phenotyping and case-by-case analysis. Orphanet J Rare Dis. 2020;15:343. doi: 10.1186/s13023-020-01594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law C.Y., Chang S.T., Cho S.Y., Yau E.K., Ng G.S., Fong N.C., et al. Clinical whole-exome sequencing reveals a novel missense pathogenic variant of GNAO1 in a patient with infantile-onset epilepsy. Clin Chim Acta. 2015;451:292–296. doi: 10.1016/j.cca.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Malaquias M.J., Fineza I., Loureiro L., Cardoso L., Alonso I., Magalhães M. GNAO1 mutation presenting as dyskinetic cerebral palsy. Neurol Sci. 2019;40(10):2213–2216. doi: 10.1007/s10072-019-03964-7. [DOI] [PubMed] [Google Scholar]
- 27.Marce-Grau A., Dalton J., Lopez-Pison J., Garcia-Jimenez M.C., Monge-Galindo L., Cuenca-Leon E., et al. GNAO1 encephalopathy: further delineation of a severe neurodevelopmental syndrome affecting females. Orphanet J Rare Dis. 2016;11:38. doi: 10.1186/s13023-016-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menke L.A., Engelen M., Alders M., Odekerken V.J.J., Baas F., Cobben J.M. Recurrent GNAO1 Mutations Associated With Developmental Delay and a Movement Disorder. J Child Neurol. 2016;31(14):1598–1601. doi: 10.1177/0883073816666474. [DOI] [PubMed] [Google Scholar]
- 29.Muir A.M., Myers C.T., Nguyen N.T., Saykally J., Craiu D., De Jonghe P., et al. EuroEpinomics-Res Nles working group SW. Genetic heterogeneity in infantile spasms. Epilepsy Res. 2019;156:106181. doi: 10.1016/j.eplepsyres.2019.106181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okumura A., Maruyama K., Shibata M., Kurahashi H., Ishii A., Numoto S., et al. A patient with a GNAO1 mutation with decreased spontaneous movements, hypotonia, and dystonic features. Brain Dev. 2018;40(10):926–930. doi: 10.1016/j.braindev.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Saitsu H., Fukai R., Ben-Zeev B., Sakai Y., Mimaki M., Okamoto N., et al. Phenotypic spectrum of GNAO1 variants: epileptic encephalopathy to involuntary movements with severe developmental delay. Eur J Hum Genet. 2016;24(1):129–134. doi: 10.1038/ejhg.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schirinzi T., Garone G., Travaglini L., Vasco G., Galosi S., Rios L., et al. Phenomenology and clinical course of movement disorder in GNAO1 variants: Results from an analytical review. Parkinsonism Relat Disord. 2019;61:19–25. doi: 10.1016/j.parkreldis.2018.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Schorling D., Dietel T., Evers C., Hinderhofer K., Korinthenberg R., Ezzo D., et al. Expanding Phenotype of De Novo Mutations in GNAO1: Four New Cases and Review of Literature. Neuropediatrics. 2017;48(05):371–377. doi: 10.1055/s-0037-1603977. [DOI] [PubMed] [Google Scholar]
- 34.Talvik I., Møller R.S., Vaher M., Vaher U., Larsen L.HG., Dahl H.A., et al. Clinical Phenotype of De Novo GNAO1 Mutation: Case Report and Review of Literature. Child Neurol Open. 2015;2(2):232. doi: 10.1177/2329048X15583717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita Y., Ogawa T., Ogaki K., Kamo H., Sukigara T., Kitahara E., et al. Neuroimaging evaluation and successful treatment by using directional deep brain stimulation and levodopa in a patient with GNAO1-associated movement disorder: A case report. J Neurol Sci. 2020;411:116710. doi: 10.1016/j.jns.2020.116710. [DOI] [PubMed] [Google Scholar]
- 36.Yilmaz S., Turhan T., Ceylaner S., Gökben S., Tekgul H., Serdaroglu G. Excellent response to deep brain stimulation in a young girl with GNAO1-related progressive choreoathetosis. Childs Nerv Syst. 2016;32(9):1567–1568. doi: 10.1007/s00381-016-3139-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhu X., Petrovski S., Xie P., Ruzzo E.K., Lu Y.-F., McSweeney K.M., et al. Whole-exome sequencing in undiagnosed genetic diseases: interpreting 119 trios. Genet Med. 2015;17(10):774–781. doi: 10.1038/gim.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appenzeller S., Balling R., Barisic N., Baulac S., Caglayan H., Craiu D., et al. De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am J Hum Genet. 2014;95(4):360–370. doi: 10.1016/j.ajhg.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Axeen E., Bell E., Robichaux Viehoever A., Schreiber J.M., Sidiropoulos C., Goodkin H.P. Results of the first GNAO1-related neurodevelopmental disorders caregiver survey. Pediatr Neurol. 2021;121:28–32. doi: 10.1016/j.pediatrneurol.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Di Rocco M., Galosi S., Lanza E., Tosato F., Caprini D., Folli V., et al. Caenorhabditis elegans provides an efficient drug screening platform for GNAO1-related disorders and highlights the potential role of caffeine in controlling dyskinesia. Hum Mol Genet. 2022;31(6):929–941. doi: 10.1093/hmg/ddab296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.